Summary
Curcumin, a polyphenolic antioxidant derived from the turmeric root has undergone extensive preclinical development, showing remarkable efficacy in wound repair, cancer and inflammatory disorders. This review addresses the rationale for its use in neurodegenerative disease, particularly Alzheimer’s disease (AD). Curcumin is a pleiotropic molecule, which not only directly binds to and limits aggregation of the β-sheet conformations of amyloid characteristic of many neurodegenerative diseases but also restores homeostasis of the inflammatory system, boosts the heat shock system to enhance clearance of toxic aggregates, scavenges free radicals, chelates iron and induces anti-oxidant response elements. Although curcumin corrects dysregulation of multiple pathways, it may exert many effects via a few molecular targets. Pharmaceutical development of natural compounds like curcumin and synthetic derivatives have strong scientific rationale, but will require overcoming various hurdles including; high cost of trials, concern about profitability and misconceptions about drug specificity, stability and bioavailability.
Keywords: Alzheimer’s Disease, amyloid, amyloid-binding, antioxidant, Activator Protein 1, brain derived neurotrophic factor, c-jun N terminal Kinase, Heat shock proteins, misfolded proteins, Neurodegeneraton, neuroinflammation, Non-steroidal anti-inflammatory drugs, Nuclear Factor Kappa B, Polyphenolic antioxidants
I. Introduction
Here we will review the rationale for the use of curcumin relevant to AD and related neurodegenerative diseases, focusing on curcumin’s amyloid binding effects, antioxidant and anti-inflammatory activities, effects on neurogenesis and neuroprotection as well as its effects on the heat shock protein system important for removal of misfolded proteins. It is also referred to as a ‘hormetic phytochemical’ because it activates adaptive stress response signaling pathways that increase cellular resistance to injury and disease [1]. Prior reviews summarize some of the key details related to Alzheimer’s in more depth [2-5].
For over 4,000 years, practitioners of Ayurvedic and traditional Chinese and Southeast Asian medicine have identified and used turmeric root extracts as a food preservative and for culinary, religious and medical conditions. Curcumin, the major active component of turmeric, is a biphenolic anti-inflammatory and antioxidant molecule that has shown exceptional promise as a chemopreventive and chemosensitizer in many cancers, inflammatory disorders and in models of Alzheimer’s (AD) and other neurodegenerative disorders.
Asian countries typically ingest the highest intake of turmeric, with up 1 gram of turmeric, which can contain from 60 to 200 mg of curcumin. A cross national epidemiological (Indo-US) study comparing rural populations in Northern India (n = 4,450) and southwestern Pennsylvania (n = 886), showed a lower incidence and prevalence of AD in India [6,7] even after correction for E4 isoform [8], despite a greater than 25% prevalence of metabolic syndrome a major AD risk factor. The Delphi Consensus study similarly showed a low prevalence in South Asia [9], but more detailed analysis showed that this protection is limited to rural areas, as urban populations in India showed considerable increased risk relative to rural Indian populations and no differences in incidence [10].
We discuss current problems in drug development, including proprietary formulations and conflicting information about its bioavailability, stability and activity of parent versus conjugated forms.
II. Curcumin binds to the β-pleated sheet of Aβ, tau and other amyloid proteins in the brain and retina
A prominent feature of neurodegenerative diseases is protein dyshomeostasis characterized by accumulation of misfolded protein aggregates within (e.g. tau from neurofibrillary tangles) and outside of cells (e.g. neuritic β-amyloid plaques). Curcumin’s ability to bind to the β-pleated structure of amyloids is one mechanism by which it reduces plaque burden in most AD models of plaque pathogenesis [11-13]. Structurally, curcumin is similar to amyloid binding probes like Congo Red and Chrysamine G, which bind to positive charges on amino acid chains of every 4 of beta-pleated sheets (spanning 19 A°or distance between four beta pleated sheets). Curcumin can bind Aβ and reduce toxic aggregates by modulating aggregation [13-15]. Curcumin’s diketone bridge is essential for amyloid binding and plaque reduction, but is not essential for curcumin’s anti-inflammatory effects since reduced curcumin (tetrahydrocurcumin) retains potent anti-inflammatory effects [16]. Curcumin also reduces Aβ aggregates in APPsw models with the dominant mutation Presenilin 1, but not necessarily amyloid burden, but novel curcumin derivatives with substitutions at C4 can reduce amyloid deposition in these aggressive models [17].
Because of curcumin’s natural fluorescence, it may be useful to monitor disease progression, particularly via analysis of retinal plaques, which may mirror brain plaque deposition. After oral gavage with curcumin, Curcumin fluorescence is apparent in the retinal plaques of APP transgenic mice, which may serve as a non-invasive clinical diagnostic or surrogate biomarker [18], now being testing in the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL) study [19]. The binding affinity of curcumin for Aβ aggregates is as high or higher than successful molecular imaging probes such as PIB in FDG PET, with a Ki of 0.07 nM for F18 labeled curcumin binding for fibrillar Aβ [20]. Curcumin analogues such as CRANAD-17 and CRANAD-58 are being developed that may be particularly useful in live fluorescence imaging of plaques [21].
X-ray microcrystallography of fiber forming segments of tau with small molecule binders identify curcumin as a pharmacophore, binding to the β-pleated sheet in the paired helical filaments of tau [22]. Curcumin binds to neurofibrillary tangles (NFTs) in human AD brain and animals [23,24]. Some high throughput screens have identified curcumin and related polyphenols as significant tau aggregation inhibitors [25]. In pure tauopathy models, oral curcumin reduces soluble pTau oligomers without impacting insoluble tau and corrects synaptic deficits [26]. Curcumin binds other β-pleated sheet structures including aggregates of huntingtin [27] prion [28] and α-synuclein [29-31]. In Caenorhabditis elegans with human tau mutations, curcumin improves coordinative movement, reduces neuritic abnormalities, while other compounds reported to clear tau (trehalose and methylene blue) had no favorable effect [32].
III. Neurodegeneration related HSPs defects are corrected by curcumin
Major routes for the removal of toxic protein aggregate are through the ubiquitin proteasome system and the autophagy-lysosomal pathways. Heat shock proteins (HSPs) are molecular chaperones that are important for refolding or degrading misfolded proteins, including amyloidogenic proteins or reducing the risk of formation of toxic oligomeric assemblies of Aβ, tau, Huntingtin or α-synuclein etc., in addition to promoting ubiquitination and degradation of those aggregates. Efficiency of this system is associated with longevity and the associated genes have been coined the Vitagene system, which includes the cytoprotective heat shock proteins (Hsp) Hsp70 and or Hsp32, also known as heme oxygenase-1 (see Section VI) [33].
Curcumin directly interacts with HSP90 and increase HSP70 and other HSPs and their client proteins in Aβ-infused rats and in human tau transgenic mice where it reduces tau oligomers [26]. We show that across multiple amyloid models, curcumin corrects transgene-dependent reductions in levels of Hsp90, Hsp70, Hsc70, Hsp60, Hsp40, and their client proteins such as FKBP51, Cdc37, P23 (unpublished observations Frautschy, Cole and Maiti). These effects were observed across multiple amyloid models, suggesting high specificity of curcumin for molecular chaperones involved in protein homeostasis.
IV. Stimulation of phagocytic Aβ clearance (M2 activation and attenuation of aberrant chronic inflammation (M1 activation)
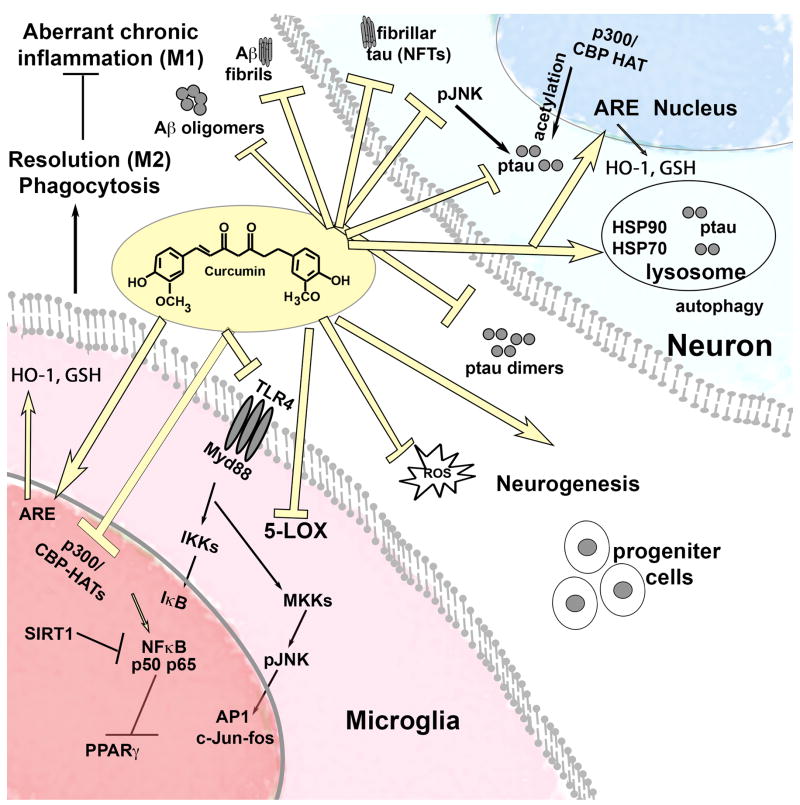
Turmeric has been used for thousands of years to treat inflammatory disorders and for wound repair. It is a homeostatic regulator of inflammation, attenuating chronic aberrant inflammation (M1 activation) and stimulating resolution of inflammation (M2 activation, Figure 1), but whether it acts on one or multiple transcription factors, receptors or oxygenases is unknown.
Figure 1. Curcumin is a pleiotropic molecule correcting dysregulation of pathways in Alzheimer’ models.
Pleiotropic actions of curcumin that could exert neuroprotection, neural repair, remove misfolded proteins in multiple neurodegenerative diseases.
Similar to the amyloid vaccine, curcumin increases the association of phagocytic cells with plaque structures and stimulates clearance of Aβ aggregates in various human cell and rodent AD models [34-36]. The amyloid vaccine can be associated with microhemorrhages and vasogenic edema, which can be visualized clinically in MRI as amyloid-related imaging abnormalities (ARIA) [37], but it has not been demonstrated if curcumin has this effect. Although curcumin is protective in mouse models of intracerebral hemorrhage curcumin [38], potential adverse effects need to be examined in a vascular amyloid model.
Curcumin also reduces chronic inflammation while promoting resolution of inflammation including in AD models [34,36,39]. Curcumin reduces elevations in the inflammatory cytokines Interleukin 1β and TNFα in the Tg2576 AD mouse model [40].
Curcumin is an inhibitor of NFκB and the Activator Protein 1 (AP1) [41-43]. Pathways associated with these transcription factors are dysregulated in AD, limiting inflammatory resolution. Curcumin reduces AP1 transcription by reducing the upstream hyperactivation of the tau kinase JNK that will also reduce hyperphosphorylated tau.
Data suggest curcumin interferes with the homodimerization of the TLR4 receptor complex that is responsible for NFκB activation [44], which limits PPARγ activation. Curcumin also increases PPARγ expression [45], but it is not clear if it can do so directly. PPARγ forms heterodimers with RXR alpha to regulate microglial activation and phagocytosis [46]. PPARγ will downregulate inflammatory cytokines that contribute to tau kinase hyperactivity, ptau accumulation and oxidative damage.
The mechanism of curcumin in the homeostatic regulation of inflammation likely includes inhibition of p300 histone acetyltransferase (HAT) activity, which it does so at picomolar levels, far below levels needed to inhibit NFκB activation. This acetyltransferase acetylates and stabilizes NFκB to further contribute to chronic inflammation, and counteracts deacetylases like sirtuins that can resolve inflammation. Since hyperacetylation plays a role in pathogenesis of many disorders, P300 HAT inhibitors are being developed for heart myopathy, HIV, cancer and other disorders. In addition to the effects of hyperacetylation in inflammatory disease, hyperacetylation of tau contributes to tau aggregation [47]. Therefore p300 HAT inhibition may be one of the significant targets of curcumin against both tau and inflammation pathways in AD pathogenesis.
Curcumin limits arachidonic acid (AA) substrates and aberrant inflammatory cytokine production. Curcumin does not directly inhibit cyclooxygenase except at high concentrations (50-100 μM) [48]. However, it alters multiple AA metabolites by reducing the induction of cyclooxygenase-2 and inhibits 5-lipoxygenase (IC50 ~ 0.7 μM), while suppressing the phosphorylation and activation of cytosolic phospholipase A2 [49].
Curcumin can directly reduce Aβ production by inhibiting the activity of β-secretase BACE, the N terminal cleavage of the transmembrane amyloid precursor protein (APP) [50], but may also indirectly reduce BACE by attenuating its proinflammatory upregulation [51]. It also may reduce Aβ production by inhibiting GSK3β-mediated PS1 activation [52] or lowering Aβ levels by attenuating the maturation of APP in the secretory pathway [53].
V. Modulating of aberrant tau phosphorylation and acetylation
Curcumin reduces accumulation of soluble tau aggregates, a major cause of synapse loss. It does so via multiple actions. It reduces ptau by directly reducing the activation of tau kinase such as c-Jun N terminal kinase (JNK) and GSK3β in neurons and by reducing glial produced inflammatory cytokines that activate neuronal tau kinases. Further it reduces oxidation of tau that promotes its activation and can reduce acetylation of tau via effects on CBP/p300 HAT. It can promote HSPs responses that selectively remove soluble tau oligomers associated with cognitive and synapse loss.
VI. Curcumin’s antioxidant activity and induction of antioxidant response elements (AREs)
Structurally, curcumin’s two methoxyphenol groups linked by a β diketone bridge confers both metal chelation and Michael acceptor activities [54]. Curcumin scavenges superoxide and hydroxyl radicals, conserves glutathione (GSH) levels and decreases the concentration of circulating free radical end products in mouse models and humans [55,56]. Increased oxidative stress in AD by Aβ, tau or inflammation may cause protein oxidation, lipid peroxidation, DNA oxidation and neuronal cell death. We have shown that curcumin effectively protects against DNA oxidative damage in the Tg2576 mouse [57] and lipid peroxidation in an Aβ-infusion rat model [34].
Like many phytochemicals, curcumin is a phase II inducer [58] that upregulates defense enzymes. Curcumin is a selective Nrf2-Keap1-ARE activator, providing protection against various forms of stress, leading to the increase in activity of heme oxygenase-1 (HO-1), a redox-sensitive inducible protein [59]. Curcumin increases HO-1 in astrocytes and neurons where it mediates neuroprotection against oxidative stress [60]. Increases in astrocytes can improve outcome after stroke [61]. HO-1 is oxidized in AD and MCI brain and has been implicated in insulin resistance, a major AD risk factor [62]. Induction of ARE [63] also increases glutathione biosynthesis [64], and the activity of c-glutamyl-cysteinyl synthetase and other GSH-linked detoxifying enzymes [65] as well as human glutathione S-transferase P1 expression [66].
Curcumin can also limit oxidation by its action as a copper and iron chelator [54,67]. Reports shows that the transition metals iron and copper, which accumulate excessively in the brains of AD, PD, and other neurodegenerative diseases induce genotoxic ROS and inhibit DNA damage repair. Curcumin with both chelating and reducing activities reverses their binding [68]. Curcumin also attenuates aluminum-induced oxidative stress and mitochondrial dysfunction in rat brain [69].
VII. Insulin signaling “type III diabetes”
Insulin signaling defects are observed in AD brain and insulin resistance associated with type II diabetes mellitus is a risk factor for AD [70]. In AD subjects, there are deficits in glucose metabolism [71] corresponding to reductions in insulin signaling [72,73]. Curcumin can enhance insulin signaling by stimulating Akt and reducing GSK3β activation [26]. It can also regulate insulin signaling in part by inhibition of JNK that phosphorylates Insulin Receptor Substrate (IRS) [74]. IRS is an adaptor protein linked to insulin resistance in AD and in diabetes animal models [75]. Treatment of 3xTg-AD mice with curcumin significantly suppressed a JNK/IRS-1/tau signaling pathway that leads to AD-like p-IRS-1/insulin signaling defects, providing an additional mechanism for efficacy in an AD model with tau pathology, particularly in combination with fish oil [74].
VIII. BDNF and Neurogenesis
Curcumin at low but not high concentrations stimulated neurogenesis and the proliferation of adult hippocampal progenitor cells [76]. It has been demonstrated that curcumin nanoparticles induced neurogenesis in an AD model by suppression of the Wnt/beta catenin signaling pathway regulating GSK3β activity [77]. A related potential mechanism is via promoting brain-derived neurotrophic factor (BDNF) expression which regulates neurogenesis [78]. Curcumin has been shown to restore BDNF function after brain trauma [79] and a curcumin pyrazole derivative CNB-001 stimulated BDNF and improved memory [80].
IX. Reliability of pharmacokinetic Studies in predicting efficacy
Curcumin’s development clinically has been slow, likely related to concerns over poor bioavailability, poor water solubility at neutral or acid pH, instability at basic pH and rapid intestinal and first pass glucuronidation. Initial attempts to evaluate curcumin levels in humans demonstrated no detectable levels of the parent compound in the plasma unless patients ingested more than 8 g [81]. Other clinical pharmacokinetic studies confirmed nondetectable plasma levels of parent compound and its extensive glucuronidation and sulfation [5,82-84]. These and other studies show that high levels of glucuronidated or sulfated curcumin are achieved without formulation [83,85,86], and even though these conjugates will not readily penetrate the brain, they may exerts beneficial effects on peripheral inflammation, such has been reported with the Meriva formulation [87]. However, Theracurmin is being tested to examine effect on biomarkers in MCI (NCT01383161) and did not show any effect on inflammatory cytokines in a Phase I cancer trial [88].
Curcumin molecules that bypass intestinal conjugation, are still likely to quickly be conjugated by first pass metabolism in the liver within 3 to 6 hours, yet this may be sufficient to allow delivery to lipid tissue like the brain where it remains stable and can even accumulate with frequent ingestion. Traditionally curcumin pharmacokinetic studies have used one-compartment model (V1) and show rapid first pass metabolism, but because curcumin, like other lipophilic drugs, can accumulate at different rates in different tissues and have different stability in different tissues, two (V2) or three (V3) compartment models are likely to yield more clinically useful information.
IX. Commercial Formulations
New formulations being developed for improved bioavailability rely on measurement of plasma levels of glucuronidated curcumin to assess quality, but it is unclear if this meaningfully reflects brain tissue levels, particularly since high levels of parent compound accumulating in the brain can occur in the absence of detectable levels in the blood [16]. Although we have established that curcumin is more stable in the red blood cell (RBC) compartment than plasma and that levels of curcumin in the RBC parallel levels in the brain (unpublished observations), standardizing better methods are paramount to predicting neuroprotection in humans.
Although several new formulations have been developed, there has not been an adequate side-by-side comparative efficacy and PK studies comparing formulations. Meta-analyses are unlikely to yield reliable comparisons, since each study uses different methods of analysis and different experimental design and different methods of delivery. One major problem in comparing formulations done at different times and institutions is differences in the blood storage and preparation, since there is still rapid ex vivo metabolism, glucuronidation and/or hydrolysis of curcumin, so methods of sample preparation need to be standardized, such as the need to add 5% phosphoric acid to plasma to prevent hydrolysis [89]. Another major problem in extending rodent studies to humans is the diluents for new formulation gavage studies are in themselves a form of formulation (e.g. cyclodextrins, deoxycholates, Chremophor, olive oil) making them confounders to assess new formulations. Further, in addition to fasting that increases absorption, effects of high protein, fat or carbohydrate and gender on absorption
The first curcumin formulation developed was Curcumin Bioperine (Sabinsa, [90] which included a molecule in black pepper, piperine, a glucuronidation inhibitor. Since alkalinity increases absorption [91], piperine’s alkalinity may instead or also contribute to the improved absorption. Biocurcumax or BCM-95 is a curcumin extract, with essential oils added back in and proposed to increase bioavailability [92]. Theracurmin [88] is another novel formulation with 10% curcumin, 38% water, 46% glycerin, 4% gum ghatti, which is a polysaccharide emulsifier and thickener.
Our group was involved in development of Curcumin Longvida, a solid lipid nanoparticle, which did show detectable levels of parent compound “free or unconjugated curcumin” in a human PK study [93], but whether this formulation is superior to other formulations on the market has not established. NIH funded the development of this curcumin formulation to improve efficacy in AD models. The final product was then licensed by UC Regents to Verdure Sciences (Indianapolis, IN) as Longvida Curcumin. It has undergone extensive safety testing in animals which showed that the Observed-Adverse-Effect Level (NOAEL) for this standardized novel curcumin preparation given for 90 days was 720 mg/kg bw/day (5760 ppm), the highest dose tested [94]. There were small significant and gender-dependent changes in eosinophils, bilirubin, urea and neutrophils, but all changes were still in the mid-range of normal.
X. Curcumin Safety and Trials for cognition
Curcumin has undergone extensive toxicology testing because of its chemopreventive activities showing that long term use of doses 5,000 ppm or below are safe [95]. Further turmeric oleoresin (85% curcumin) is on the FDA GRAS list. Although none of the trials have shown any evidence of significant toxicity, some changes were observed leading to concerns of potential toxicity. One 6-month trial demonstrated no evidence of liver toxicity, since although ALT/GPT (alanine aminotransferase /glutamic-pyruvic transaminase levels) were higher at baseline in subjects to be given low dose curcumin neither dose of curcumin showed any changes from baseline [96]. In this same study curcumin did not increase triacylglycerols, total, LDL or cholesterol over 1 month or 6 months. Although total cholesterol correlated positively with plasma glucuronidated curcumin, this corresponded to increases in HDL, particularly in the 1 g group where HDL increase 0.27 mM and LDL reduced 0.12 mM in response to curcumin. Importantly these patients were not hypercholesterolemic to begin with nor did they become hypercholesterolemic. Similarly no toxicity was observed in a similar AD trial [85].
Despite the excellent safety profiles of curcumin, neither study showed any changes on cognitive endpoints [83,85]. However in the Baum study there was a trend (p < 0.11) for increased plasma levels of Abeta40, which could indicate clearance out of the brain. That lack of efficacy could be due to 1-that high conjugated curcumin but not the parent compound was detected in plasma, suggesting levels are too low for adequate brain delivery or 2- that the treatment of 6 months was of too short duration or 3-curcumin is not effective after the onset of disease.
In another study three patients with advanced stage dementia were given turmeric capsules with bioavailable curcumin (equivalent to 764 mg turmeric and 100 mg/day of curcumin) over a one-year period, and patients showed improvement in neuropsychiatric inventory scores and cognition and reduction in caregiver burden [97].
Current approaches are to intervene earlier prior to disease onset. There are two ongoing trials for subjects with MCI at University of California, Los Angeles and the Veterans Administration to be completed in 2016 or 2017. First, Theracurmin is being tested in an 18-month study at 30 mg curcumin/day (465 mg Theracurmin) (NCT01383161). Second, Longvida is tested in a 12-month study in MCI subjects, using a dose of 4 g per day (equivalent to 800 mg/day curcumin) for effects on and plasma inflammatory biomarkers and cerebral metabolism using FDG PET imaging (NCT01811381).
Curcumin is also being considered for prevention. To date there have been two completed trials in the cognitively normal geriatric population, using Longvida, at 400 mg per day (equivalent to 80 mg/day curcumin) showing positive but small effects on plasma biomarkers in a middle aged population [98] and on cognition and mood in an elderly population [99].
Finally acute dosing of Longvida is being used in the AIBL program as a non-invasive biomarker for retinal plaques to determine if it parallels the PET amyloid probes [19].
Expert commentary
Alzheimer’s disease is a complicated syndrome of aging with a decades-long prodromal period of accumulation of Aβ and tau pathology accompanied by oxidative damage and inflammation, which develops before an insidious clinical onset. These factors can also act independently to cause neuronal damage and cognitive deficits, arguing against benefits of single drug targets for neurodegenerative disease. Curcumin is a safe and inexpensive pleiotropic agent that is a promising candidate to act upon multiple aspects of the disease process. Although the most relevant molecular targets are uncertain, it’s effect on binding amyloid and maintaining inflammatory and protein homeostasis are critical for observed benefits in AD models. A comparison of current formulations in clinical trials is warranted, and short trials that can determine doses to achieve efficacious target blood levels.
Five-year view
Many obstacles limiting the clinical development of curcumin (and other promising natural compounds) are likely to be overcome with foundation or government funding for comparative efficacy studies. As we come to terms with decades of research and failed trials with single molecular target strategies, finding therapies based on genetic triggers are likely to take a back burner, particularly for prevention or early stage therapeutic drug development. Taking a front seat will be multiple targets to correct dysregulation of multiple signaling pathways, extensive amyloid and tau seeding and ] stimulate repair mechanisms with both pharmacological as well as non-pharmacological methods (cognitive and exercise therapy). These therapies will be designed to both target precipitating factors as well as downstream signaling hubs that can remain autonomously dysregulated. Pleiotropic compounds like curcumin that affect multiple targets in the disease can simplify trial design, minimizing the number of drugs needed to alter the disease course. The overhaul of current trial designs will reduce their enormous cost, including incorporation of adaptive trial designs, and more rigorous shorter trials designed to improve strategies to optimize dosage and identify whether drug efficacy may be dependent on ApoE isoform- or gender. Hopefully, these shorter trials will use newly identified surrogate plasma biomarkers as primary outcomes that can be used to increase likelihood of success in prevention trials. Congress will approve increased funding for translational research in Alzheimer’s, and pharmaceutical companies will devise strategies to make development of natural compounds profitable. Pleiotropic compounds, are likely to become an important component of treatment and prevention paradigms for AD, but like CVD, a cocktail of drugs and natural supplements like docosahexaenoic acid and fisetin, a cognitive enhancer will be needed to dramatically change the disease course.
Key issues.
Curcumin affects both causal and downstream mediators in Alzheimer’s disease pathogenesis and stimulates repair mechanisms but has not been adequately developed clinically.
Curcumin is an amyloid-binding probe, which reduces chronic inflammation and facilitates resolution of inflammation and reduces lipid peroxidation that correlates with synapse loss.
For age related diseases like Alzheimer’s, which have long prodromal periods and multiple dysregulated signaling pathways, pleiotropic molecules may be more effective than highly specific drugs.
Although medicinal chemistry and high throughput screening using multiple culture or C. elegans or Drosophila model systems will likely lead to better treatments in upcoming decades, there needs to be a concerted effort to identify current FDA approved or natural GRAS list compounds that can be quickly and affordably brought to the clinic.
Government and private funds dedicated to translational research are insufficient, which make it virtually impossible to identify dose, gender, and ApoE isoform dependent effects of drugs with limited patent protection, including curcumin.
Increase funding for translational research in Alzheimer’s is necessary, and for the pharmaceutical industry to envision development of natural compounds as a profitable approach, as has been the case for many natural compounds beginning with aspirin.
Acknowledgments
The paper was supported by Veterans Affairs RX000669, BX001257, NIH RO1AG021975, NIH U01AG28583. SA Frautschy and G Cole are co-inventors of a UCLA and Veterans Affairs patent on a curcumin formulation.
Abbreviations
- AIBL
Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing
- AA
arachidonic acid
- AD
Alzheimer’s Disease
- Aβ
β-amyloid peptide
- AP1
Activator Protein 1
- APP
Amyloid precursor protein
- ARE
antioxidant response element
- BDNF
brain derived neurotrophic factor
- CNS
central nervous system
- CREB
cyclic adenosine monophosphate response element-binding protein
- FDG PET
Fluorodeoxyglucose (18F) positron emission tomography
- GSH
glutathione
- GSK3β
Glycogen Synthase Kinase 3β
- Hsp
Heat Shock Protein
- HAT
p300 histone acetyl transferase
- HO
Heme-oxygenase, Hsp32
- IRS
Insulin Receptor Substrate
- JNK
c-jun N terminal kinase
- MCI
Mild Cognitive Impairment
- Nrf2
Nuclear Factor-Erythroid 2-related factor 2
- NFκB
nuclear factor κ-light-chain-enhancer of activated B cells, transcription factor
- PK
Pharmacokinetics
- PPAR
Peroxisome proliferator-activator receptor
- ROS
reactive oxygen species
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Murugaiyah V, Mattson MP. Neurohormetic phytochemicals: An evolutionary-bioenergetic perspective. Neurochemistry international. 2015 doi: 10.1016/j.neuint.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkacemi A, Doggui S, Dao L, Ramassamy C. Challenges associated with curcumin therapy in Alzheimer disease. Expert reviews in molecular medicine. 2011;13:e34. doi: 10.1017/S1462399411002055. [DOI] [PubMed] [Google Scholar]
- 3.Ray B, Lahiri DK. Neuroinflammation in Alzheimer’s disease: different molecular targets and potential therapeutic agents including curcumin. Current opinion in pharmacology. 2009;9(4):434–444. doi: 10.1016/j.coph.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Frautschy SA, Cole GM. Why pleiotropic interventions are Needed for Alzheimer’s disease. Mol Neurobiol. 2010;41(2-3):392–409. doi: 10.1007/s12035-010-8137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancuso C, Siciliano R, Barone E, Preziosi P. Natural substances and Alzheimer’s disease: from preclinical studies to evidence based medicine. Biochimica et biophysica acta. 2012;1822(5):616–624. doi: 10.1016/j.bbadis.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Chandra V, Pandav R, Dodge HH, et al. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology. 2001;57(6):985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- 7.Chandra V, Ganguli M, Pandav R, Johnston J, Belle S, DeKosky ST. Prevalence of Alzheimer’s disease and other dementias in rural India: the Indo-US study. Neurology. 1998;51(4):1000–1008. doi: 10.1212/wnl.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 8.Ganguli M, Chandra V, Kamboh M, et al. Apolipoprotein e polymorphism and Alzheimer disease: the Indo-US cross-national dementia study. Arch Neurol. 2000;57(6):824–830. doi: 10.1001/archneur.57.6.824. [DOI] [PubMed] [Google Scholar]
- 9.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tripathi M, Vibha D, Gupta P, et al. Risk factors of dementia in North India: a case-control study. Aging & mental health. 2012;16(2):228–235. doi: 10.1080/13607863.2011.583632. [DOI] [PubMed] [Google Scholar]
- 11.Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AH, Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. The AAPS journal. 2013;15(2):324–336. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. Journal of neurochemistry. 2007;102(4):1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 13.Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. The Journal of biological chemistry. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Park BS, Lee KG, et al. Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J Agric Food Chem. 2005;53(22):8537–8541. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 15.Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. Journal of neuroscience research. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 16.Begum AN, Jones MR, Lim GP, et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. The Journal of pharmacology and experimental therapeutics. 2008;326(1):196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanagisawa D, Ibrahim NF, Taguchi H, et al. Curcumin derivative with the substitution at C-4 position, but not curcumin, is effective against amyloid pathology in APP/PS1 mice. Neurobiology of aging. 2015;36(1):201–210. doi: 10.1016/j.neurobiolaging.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 18.Koronyo Y, Salumbides BC, Black KL, Koronyo-Hamaoui M. Alzheimer’s Disease in the Retina: Imaging Retinal Abeta Plaques for Early Diagnosis and Therapy Assessment. Neurodegener Dis. 2012 doi: 10.1159/000335154. [DOI] [PubMed] [Google Scholar]
- 19.Frost S, Kanagasingam Y, Macaulay L, et al. Retinal Amyloid Fluorescence Imaging predicts cerebral amyloid burden and Alzheimer’s Disease. Alzheimer’s & Dementia. 2015;10(4 Supplement) 33-13-01. [Google Scholar]
- 20.Ryu EK, Choe YS, Lee KH, Choi Y, Kim BT. Curcumin and dehydrozingerone derivatives: synthesis, radiolabeling, and evaluation for beta-amyloid plaque imaging. Journal of medicinal chemistry. 2006;49(20):6111–6119. doi: 10.1021/jm0607193. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Tian Y, Li Z, et al. Design and synthesis of curcumin analogues for in vivo fluorescence imaging and inhibiting copper-induced cross-linking of amyloid beta species in Alzheimer’s disease. Journal of the American Chemical Society. 2013;135(44):16397–16409. doi: 10.1021/ja405239v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landau M, Sawaya MR, Faull KF, et al. Towards a pharmacophore for amyloid. PLoS Biol. 2011;9(6):e1001080. doi: 10.1371/journal.pbio.1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohorko N, Repovs G, Popovic M, Kovacs GG, Bresjanac M. Curcumin labeling of neuronal fibrillar tau inclusions in human brain samples. Journal of neuropathology and experimental neurology. 2010;69(4):405–414. doi: 10.1097/NEN.0b013e3181d709eb. [DOI] [PubMed] [Google Scholar]
- 24.Mutsuga M, Chambers JK, Uchida K, et al. Binding of curcumin to senile plaques and cerebral amyloid angiopathy in the aged brain of various animals and to neurofibrillary tangles in Alzheimer’s brain. J Vet Med Sci. 2012;74(1):51–57. doi: 10.1292/jvms.11-0307. [DOI] [PubMed] [Google Scholar]
- 25.Brunden KR, Ballatore C, Crowe A, Smith AB, 3rd, Lee VM, Trojanowski JQ. Tau-directed drug discovery for Alzheimer’s disease and related tauopathies: A focus on tau assembly inhibitors. Exp Neurol. 2010;223(2):304–310. doi: 10.1016/j.expneurol.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma QL, Zuo X, Yang F, et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. The Journal of biological chemistry. 2013;288(6):4056–4065. doi: 10.1074/jbc.M112.393751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickey MA, Gallant K, Zhu C, DeLima M, Levine MS, Chesselet MF. A preclinical trial of curcumin in a knock-in mouse model (CAG140) of Huntington’s disease. Society for Neuroscience. 2005;31:90–95. [Google Scholar]
- 28.Caughey B, Raymond LD, Raymond GJ, Maxson L, Silveira J, Baron GS. Inhibition of protease-resistant prion protein accumulation in vitro by curcumin. J Virol. 2003;77(9):5499–5502. doi: 10.1128/JVI.77.9.5499-5502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey N, Strider J, Nolan WC, Yan SX, Galvin JE. Curcumin inhibits aggregation of alpha-synuclein. Acta Neuropathol. 2008;115(4):479–489. doi: 10.1007/s00401-007-0332-4. [DOI] [PubMed] [Google Scholar]
- 30.Ono K, Yamada M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. Journal of neurochemistry. 2006;97(1):105–115. doi: 10.1111/j.1471-4159.2006.03707.x. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad B, Lapidus LJ. Curcumin prevents aggregation in alpha-synuclein by increasing the reconfiguration rate. The Journal of biological chemistry. 2012 doi: 10.1074/jbc.M111.325548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyasaka T, Yoshimura S, Saka A, et al. Curcumin Improves Tau-mediated Neuronal Dysfunction in Nematode. Alzheimer’s & Dementia. 2010;6(4 supplement):e27–e28. [Google Scholar]
- 33.Calabrese V, Cornelius C, Mancuso C, et al. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33(12):2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- 34.Frautschy SA, Hu W, Kim P, et al. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiology of aging. 2001;22(6):993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 35.Cole GM, Morihara T, Lim GP, Yang F, Begum A, Frautschy SA. NSAID and Antioxidant Prevention of Alzheimer’s Disease: Lessons from In Vitro and Animal Models. Annals of the New York Academy of Sciences. 2004;1035:68–84. doi: 10.1196/annals.1332.005. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Fiala M, Cashman J, et al. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2006;10(1):1–7. doi: 10.3233/jad-2006-10101. [DOI] [PubMed] [Google Scholar]
- 37.Morgan D. Immunotherapy for Alzheimer’s disease. J Intern Med. 2011;269(1):54–63. doi: 10.1111/j.1365-2796.2010.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King MD, McCracken DJ, Wade FM, Meiler SE, Alleyne CH, Jr, Dhandapani KM. Attenuation of hematoma size and neurological injury with curcumin following intracerebral hemorrhage in mice. J Neurosurg. 2011;115(1):116–123. doi: 10.3171/2011.2.JNS10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim GP, Calon F, Morihara T, et al. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25(12):3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu YX, Pindolia KR, Janakiraman N, Chapman RA, Gautam SC. Curcumin inhibits IL1 alpha and TNF-alpha induction of AP-1 and NF-kB DNA-binding activity in bone marrow stromal cells. Hematopathol Mol Hematol. 1997;11(1):49–62. [PubMed] [Google Scholar]
- 42.Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med. 2007;19(3):469–474. [PubMed] [Google Scholar]
- 43.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] The Journal of biological chemistry. 1995;270(42):24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 44.Youn HS, Saitoh SI, Miyake K, Hwang DH. Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem Pharmacol. 2006;72(1):62–69. doi: 10.1016/j.bcp.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 45.Wang HM, Zhao YX, Zhang S, et al. PPARgamma agonist curcumin reduces the amyloid-beta-stimulated inflammatory responses in primary astrocytes. J Alzheimers Dis. 2010;20(4):1189–1199. doi: 10.3233/JAD-2010-091336. [DOI] [PubMed] [Google Scholar]
- 46.Heneka MT, Sastre M, Dumitrescu-Ozimek L, et al. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta 1-42 levels in APPV717I transgenic mice. Brain. 2005;128(Pt 6):1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 47.Min SW, Cho SH, Zhou Y, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67(6):953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer research. 1991;51(3):813–819. [PubMed] [Google Scholar]
- 49.Hong J, Bose M, Ju J, et al. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25(9):1671–1679. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- 50.Lin R, Chen X, Li W, Han Y, Liu P, Pi R. Exposure to metal ions regulates mRNA levels of APP and BACE1 in PC12 cells: blockage by curcumin. Neurosci Lett. 2008;440(3):344–347. doi: 10.1016/j.neulet.2008.05.070. [DOI] [PubMed] [Google Scholar]
- 51.Morihara T, Teter B, Yang F, et al. Ibuprofen suppresses interleukin-1beta induction of pro-amyloidogenic alpha1-antichymotrypsin to ameliorate beta-amyloid (Abeta) pathology in Alzheimer’s models. Neuropsychopharmacology. 2005;30(6):1111–1120. doi: 10.1038/sj.npp.1300668. [DOI] [PubMed] [Google Scholar]
- 52.Xiong Z, Hongmei Z, Lu S, Yu L. Curcumin mediates presenilin-1 activity to reduce beta-amyloid production in a model of Alzheimer’s Disease. Pharmacol Rep. 2011;63(5):1101–1108. doi: 10.1016/s1734-1140(11)70629-6. [DOI] [PubMed] [Google Scholar]
- 53.Zhang C, Browne A, Child D, Tanzi RE. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. The Journal of biological chemistry. 2010;285(37):28472–28480. doi: 10.1074/jbc.M110.133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J Alzheimers Dis. 2004;6(4):367–377. doi: 10.3233/jad-2004-6403. discussion 443-369. [DOI] [PubMed] [Google Scholar]
- 55.Soudamini KK, Unnikrishnan MC, Soni KB, Kuttan R. Inhibition of lipid peroxidation and cholesterol levels in mice by curcumin. Indian J Physiol Pharmacol. 1992;36(4):239–243. [PubMed] [Google Scholar]
- 56.Soni KB, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol. 1992;36(4):273–275. [PubMed] [Google Scholar]
- 57.Lim GP, Yang F, Chu T, et al. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiology of aging. 2001;22:983–981. doi: 10.1016/s0197-4580(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 58.Dinkova-Kostova AT, Talalay P. Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxification enzymes. Carcinogenesis. 1999;20(5):911–914. doi: 10.1093/carcin/20.5.911. [DOI] [PubMed] [Google Scholar]
- 59.Balogun E, Hoque M, Gong P, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. The Biochemical journal. 2003;371(Pt 3):887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scapagnini G, Colombrita C, Amadio M, et al. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxidants & redox signaling. 2006;8(3-4):395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- 61.Chen-Roetling J, Song W, Schipper HM, Regan CS, Regan RF. Astrocyte overexpression of heme oxygenase-1 improves outcome after intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2015;46(4):1093–1098. doi: 10.1161/STROKEAHA.115.008686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barone E, Butterfield AD. Insulin resistance in Alzheimer disease: Is heme oxygenase-1 an Achille’s heel? Neurobiology of disease. 2015 doi: 10.1016/j.nbd.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 63.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2 dependent induction of proteasome and Pa28alphabeta regulator is required for adaptation to oxidative stress. The Journal of biological chemistry. 2012 doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stridh MH, Correa F, Nodin C, et al. Enhanced glutathione efflux from astrocytes in culture by low extracellular Ca2+ and curcumin. Neurochem Res. 2010;35(8):1231–1238. doi: 10.1007/s11064-010-0179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singhal SS, Awasthi S, Pandya U, et al. The effect of curcumin on glutathione-linked enzymes in K562 human leukemia cells. Toxicol Lett. 1999;109(1-2):87–95. doi: 10.1016/s0378-4274(99)00124-1. [DOI] [PubMed] [Google Scholar]
- 66.Nishinaka T, Ichijo Y, Ito M, et al. Curcumin activates human glutathione S-transferase P1 expression through antioxidant response element. Toxicol Lett. 2007;170(3):238–247. doi: 10.1016/j.toxlet.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 67.Jiao Y, Wilkinson Jt, Christine Pietsch E, et al. Iron chelation in the biological activity of curcumin. Free Radic Biol Med. 2006;40(7):1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Hegde ML, Hegde PM, Holthauzen LM, Hazra TK, Rao KS, Mitra S. Specific Inhibition of NEIL-initiated repair of oxidized base damage in human genome by copper and iron: potential etiological linkage to neurodegenerative diseases. The Journal of biological chemistry. 2010;285(37):28812–28825. doi: 10.1074/jbc.M110.126664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sood PK, Nahar U, Nehru B. Curcumin attenuates aluminum-induced oxidative stress and mitochondrial dysfunction in rat brain. Neurotox Res. 2011;20(4):351–361. doi: 10.1007/s12640-011-9249-8. [DOI] [PubMed] [Google Scholar]
- 70.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Current Alzheimer research. 2007;4(2):147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 71.Cherbuin N, Leach LS, Christensen H, Anstey KJ. Neuroimaging and APOE genotype: a systematic qualitative review. Dement Geriatr Cogn Disord. 2007;24(5):348–362. doi: 10.1159/000109150. [DOI] [PubMed] [Google Scholar]
- 72.Craft S, Asthana S, Cook DG, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28(6):809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 73.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8(3):247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 74.Ma QL, Yang F, Rosario ER, et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci. 2009;29(28):9078–9089. doi: 10.1523/JNEUROSCI.1071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiology of aging. 2008 doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Kim SJ, Son TG, Park HR, et al. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. The Journal of biological chemistry. 2008;283(21):14497–14505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tiwari SK, Agarwal S, Seth B, et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/beta-catenin pathway. ACS nano. 2014;8(1):76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- 78.Xu Y, Ku B, Cui L, et al. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain research. 2007;1162:9–18. doi: 10.1016/j.brainres.2007.05.071. [DOI] [PubMed] [Google Scholar]
- 79.Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197(2):309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y, Dargusch R, Maher P, Schubert D. A broadly neuroprotective derivative of curcumin. Journal of neurochemistry. 2008;105(4):1336–1345. doi: 10.1111/j.1471-4159.2008.05236.x. [DOI] [PubMed] [Google Scholar]
- 81.Lao CD, Ruffin MTt, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 83.Baum L, Lam CW, Cheung SK, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. Journal of clinical psychopharmacology. 2008;28(1):110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 84.Garcea G, Jones DJ, Singh R, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90(5):1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ringman JM, Frautschy SA, Teng E, et al. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s research & therapy. 2012;4(5):43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vareed SK, Kakarala M, Ruffin MT, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(6):1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drobnic F, Riera J, Appendino G, et al. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva(R)): a randomised, placebo-controlled trial. Journal of the International Society of Sports Nutrition. 2014;11:31. doi: 10.1186/1550-2783-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanai M, Otsuka Y, Otsuka K, et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer chemotherapy and pharmacology. 2013;71(6):1521–1530. doi: 10.1007/s00280-013-2151-8. [DOI] [PubMed] [Google Scholar]
- 89.Wang YY, Pan MH, Cheng AL, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15(12):1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 90.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 91.Chan MM, Huang HI, Fenton MR, Fong D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol. 1998;55:1955–1962. doi: 10.1016/s0006-2952(98)00114-2. [DOI] [PubMed] [Google Scholar]
- 92.Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian journal of pharmaceutical sciences. 2008;70(4):445–449. doi: 10.4103/0250-474X.44591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem. 2010;58(4):2095–2099. doi: 10.1021/jf9024807. [DOI] [PubMed] [Google Scholar]
- 94.Dadhaniya P, Patel C, Muchhara J, et al. Safety assessment of a solid lipid curcumin particle preparation: Acute and subchronic toxicity studies. Food Chem Toxicol. 2011;49(8):1834–1842. doi: 10.1016/j.fct.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 95.National Toxicology P. NTP Toxicology and Carcinogenesis Studies of Turmeric Oleoresin (CAS No. 8024-37-1) (Major Component 79%-85% Curcumin, CAS No. 458-37-7) in F344/N Rats and B6C3F1 Mice (Feed Studies) National Toxicology Program technical report series. 1993;427:1–275. [PubMed] [Google Scholar]
- 96.Baum L, Cheung SK, Mok VC, et al. Curcumin effects on blood lipid profile in a 6-month human study. Pharmacol Res. 2007;56(6):509–514. doi: 10.1016/j.phrs.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 97.Hishikawa N, Takahashi Y, Amakusa Y, et al. Effects of turmeric on Alzheimer’s disease with behavioral and psychological symptoms of dementia. Ayu. 2012;33(4):499–504. doi: 10.4103/0974-8520.110524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Disilvestro RA, Joseph E, Zhao S, Joshua B. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J. 2012;11(1):79. doi: 10.1186/1475-2891-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cox KH, Pipingas A, Scholey AB. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. Journal of psychopharmacology. 2014 doi: 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]