Abstract
BACKGROUND
The feasibility of reducing the population-level incidence of human immunodeficiency virus (HIV) infection by increasing community coverage of antiretroviral therapy (ART) and male circumcision is unknown.
METHODS
We conducted a pair-matched, community-randomized trial in 30 rural or periurban communities in Botswana from 2013 to 2018. Participants in 15 villages in the intervention group received HIV testing and counseling, linkage to care, ART (started at a higher CD4 count than in standard care), and increased access to male circumcision services. The standard-care group also consisted of 15 villages. Universal ART became available in both groups in mid-2016. We enrolled a random sample of participants from approximately 20% of households in each community and measured the incidence of HIV infection through testing performed approximately once per year. The prespecified primary analysis was a permutation test of HIV incidence ratios. Pair-stratified Cox models were used to calculate 95% confidence intervals.
RESULTS
Of 12,610 enrollees (81% of eligible household members), 29% were HIV-positive. Of the 8974 HIV-negative persons (4487 per group), 95% were retested for HIV infection over a median of 29 months. A total of 57 participants in the intervention group and 90 participants in the standard-care group acquired HIV infection (annualized HIV incidence, 0.59% and 0.92%, respectively). The unadjusted HIV incidence ratio in the intervention group as compared with the standard-care group was 0.69 (P=0.09) by permutation test (95% confidence interval [CI], 0.46 to 0.90 by pair-stratified Cox model). An end-of-trial survey in six communities (three per group) showed a significantly greater increase in the percentage of HIV-positive participants with an HIV-1 RNA level of 400 copies per milliliter or less in the intervention group (18 percentage points, from 70% to 88%) than in the standard-care group (8 percentage points, from 75% to 83%) (relative risk, 1.12; 95% CI, 1.09 to 1.16). The percentage of men who underwent circumcision increased by 10 percentage points in the intervention group and 2 percentage points in the standard-care group (relative risk, 1.26; 95% CI, 1.17 to 1.35).
CONCLUSIONS
Expanded HIV testing, linkage to care, and ART coverage were associated with increased population viral suppression. (Funded by the President’s Emergency Plan for AIDS Relief and others; Ya Tsie ClinicalTrials.gov number, .)
Antiretroviral treatment (art) improves health outcomes in persons with human immunodeficiency virus (HIV) infection1 and reduces the risk of HIV transmission in discordant couples.2–5 Expanding ART coverage is thus expected to affect the population-level incidence of HIV infection.6 With the goal of ending the acquired immunodeficiency syndrome (AIDS) epidemic by 2030, the Joint United Nations Program on HIV and AIDS (UNAIDS) promotes “90-90-90” targets: namely, that by the year 2020, 90% of all HIV-positive persons will know their HIV status, 90% of those with a diagnosis of HIV infection will receive sustained ART, and 90% of persons receiving ART will have viral suppression. However, factors other than ART coverage — such as sexual networks or transmission from acutely infected persons — can affect the incidence of HIV infection. Thus, the feasibility of meeting these ambitious UNAIDS targets and whether doing so will reduce the population-level incidence of HIV infection are unknown. An understanding of the feasibility and effect of these measures is critical to inform policymakers’ decisions regarding resource allocation.7,8
Botswana is a middle-income country with a population of 2.3 million and a severe generalized HIV epidemic (23% of adults were reported to have HIV infection in 2017).9 Botswana was the first African country to offer free ART to its citizens (in 2002); routine “opt out” HIV testing was added in 2004, and medical male circumcision programs were added in 2009. However, the annual HIV incidence has remained high; UNAIDS estimated that 1.3% of adults 15 to 49 years of age were newly infected in 2017.9 We sought to determine whether and to what degree a community-based intervention to maximize HIV testing and case identification, linkage to care, early (expanded) ART, and male circumcision could reduce the population-level incidence of HIV infection in Botswana.
METHODS
TRIAL DESIGN OVERVIEW
The Ya Tsie trial (also called the Botswana Combination Prevention Project) was a pair-matched, community-randomized trial that tested whether a strategy of treatment and interventions to prevent HIV infection would reduce the population-level cumulative incidence of HIV infection over 29 months.10,11 The trial was conducted from 2013 to 2018 in 30 rural or periurban communities with an average population of 6000 (Fig. 1). Fifteen community pairs were matched according to population size and age structure, health services, and geographic location. The community pairs were then assigned, by random draw witnessed by persons who were unaffiliated with the trial, to the intervention group or the standard-care group. After community mobilization (e.g., provision of information about the trial to community leaders, open discussions and question-and-answer sessions about the trial with residents in public meetings, and announcements over a loudspeaker throughout the village before initiation of trial activities), we enrolled residents from a random sample of approximately 20% of households in each community in a prospective cohort to assess the incidence of HIV infection and the intervention uptake. The interventions were scaled up in the 15 communities in the intervention group shortly after enrollment of the cohort. The planned follow-up was shortened from 36 months to 29 months because of budgetary constraints.
Figure 1.
Map of the 15 Matched Community Pairs in Botswana.
INTERVENTIONS AND ELIGIBLE POPULATIONS
The intervention strategy included communitywide, standardized, home-based and mobile HIV testing campaigns, enhancement of routine testing in health facilities (e.g., with placement of additional staff members for HIV testing and an emphasis on the importance of routine testing), and targeted outreach testing of all men (regardless of age) and young persons (men and women ≤25 years of age), as well as active linkage to care at local clinics for HIV-positive persons who were not receiving ART (with an appointment scheduled within 1 week, a text message reminder before the appointment, and active tracing if the appointment was missed). In addition, until August 2015, expanded ART was provided for participants with an HIV type 1 (HIV-1) RNA level of 10,000 copies per milliliter or more (if the CD4 count was >350 cells per cubic millimeter); from August 2015 through May 2016, ART was provided for participants with a CD4 count of 500 cells per cubic millimeter or less or an HIV-1 RNA level of 10,000 copies per milliliter or more (if the CD4 count was >500 cells per cubic millimeter). Starting in June 2016, universal ART (treatment eligibility for all persons with a diagnosis of HIV infection, regardless of the CD4 count or HIV-1 RNA level) was initiated at the first clinic visit. Increased access to male circumcision services (mobilization campaigns, mobile clinics, and peer linkage with scheduled appointments, reminders, and transportation) was also provided.
In all 30 communities, from the initiation of the trial until June 2016, HIV-positive persons who had a CD4 count of 350 cells per cubic millimeter or less or World Health Organization (WHO) stage III or IV disease, as well as those who were pregnant or breast-feeding, were eligible to receive ART. After June 2016, universal ART was offered in both the intervention and standard-care groups.
All the communities implemented the relevant changes in the ART eligibility criteria simultaneously. In the communities in the intervention group, participants who were eligible to receive ART according to the revised criteria were contacted and referred. Beginning in June 2016, the first-line ART regimen provided by the government to all trial communities was switched from efavirenz–tenofovir disoproxil fumarate (TDF)–emtricitabine (FTC) to dolutegravir–TDF-FTC. Persons who moved into communities in the intervention group were eligible for all interventions. Noncitizens were eligible only for free HIV testing. Interventions were provided by cadres of staff who were typically employed in the region, and ART was provided in public clinics by government staff.
SURVEY COHORT POPULATIONS AND PROCEDURES
Prospective cohort participants were identified with the use of a household-based probabilistic sampling strategy at the community level. Within all 30 communities, every plot of land with a houselike structure was geocoded with satellite imagery (Google Earth). A simple random sample of approximately 20% of households in each community was selected from the list of geocoded plots. Residents of houses on selected plots were enumerated, and research staff approached all potentially eligible residents for participation. Plots were visited up to three times for enumeration; up to three additional visits were made to enroll each potentially eligible resident. Eligibility criteria included the following: age of 16 to 64 years, an average of 3 or more nights per month spent in the community over the preceding 12 months, more nights spent in this household than any other household in the same community, documented Botswana citizenship or marriage to a citizen of Botswana, and the ability to provide informed consent. Both HIV-positive and HIV-negative residents were enrolled; HIV-negative residents constituted the HIV-incidence cohort. The household survey included questions about sociodemographic information, health, and HIV-risk behavior. Participants without documented HIV-positive status were offered counseling and parallel HIV rapid tests (KHB, Shanghai Kehua Bio-Engineering; Uni-Gold, Trinity Biotech). HIV-1 RNA was tested (Abbott RealTime HIV-1 assay) in all HIV-positive participants at baseline and at the final surveys, irrespective of ART use. An undetectable HIV-1 RNA level was defined as 400 copies per milliliter or less. If they were not receiving ART, persons with an undetectable HIV-1 RNA level underwent testing with dual enzyme immunoassay. A point-of-care CD4 count (Alere Pima) was obtained for HIV-positive participants who were not receiving ART at enrollment. Documentation of treatment with ART was required in order to classify a participant as receiving ART. During follow-up visits at 12 months and approximately 29 months, we administered questionnaires and (in HIV-negative participants) repeat HIV testing.
Three community pairs (six communities), one per geographic region, were selected to participate in an end-of-trial survey to assess coverage of intervention components (including HIV testing, ART initiation, viral suppression, and male circumcision) according to group. Selection of these communities was made without knowledge of the intervention uptake. End-of-trial survey participants in these six communities were consenting members of the remaining 80% of the community residents who did not take part in the longitudinal cohort. The same survey procedures as described above were followed. Persons who moved into communities during the trial were eligible to participate in the end-of-trial survey.
In both groups, results of testing to determine HIV, HIV-1 RNA, and CD4 status were shared with the participants, and HIV-infected participants who were not yet receiving ART were referred to their local clinic. According to the protocol, adverse events that were both unexpected and possibly related to trial participation were to be reported.
TRIAL OVERSIGHT
The trial was approved by the Botswana Health Research and Development Committee and the institutional review board of the Centers for Disease Control and Prevention and was monitored by a data and safety monitoring board and Westat. All cohort participants provided written informed consent. Participants who were 16 or 17 years of age provided written assent (with written permission from their parents or guardians).
STATISTICAL ANALYSIS
All analyses were prespecified and performed in accordance with the protocol and statistical analysis plan, available with the full text of this article at NEJM.org. Statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
An agent-based network epidemic model was used to estimate trial power.12 We estimated that 15 clusters per group and an average of 300 participants in the HIV-incidence cohort per community would provide at least 85% power to detect a 40% lower incidence of HIV infection in the intervention group than in the standard-care group (between-cluster coefficient of variation, 0.26). In 2017, on the basis of conditional power calculations reflecting high baseline ART coverage, a changing standard of ART, and shortened follow-up, we estimated greater than 60% power to detect a 34% lower incidence of HIV infection in the intervention group than in the standard-care group.
The primary end point was the incidence of HIV infection, measured as the time to HIV infection from enrollment in the HIV-incidence cohort. Person-time for incident cases was interval censored between the most recent negative HIV test and the first positive test; a time interval in which the infection occurred was determined since the actual time of infection was not observable. Data for participants without a positive HIV test were right censored at the time of the last available negative test. Data for participants who died were censored at the time of death.
For the prespecified unadjusted primary analysis, we used a permutation test with the statistic defined as the inverse-variance weighted average of pair-specific incidence ratios estimated under a Cox proportional-hazards model for the matched pair, accounting for interval censoring. This permutation test is fully robust to clustering but does not yield a confidence interval. To obtain a 95% confidence interval for the intervention effect, we fit a pair-stratified interval-censored Cox proportional-hazards model that accounted for between-pair variability in baseline incidence. To assess sensitivity to within-pair clustering, we fit an unmatched Cox proportional-hazards mixed model with random effect for community. P values were two-sided. We generated Kaplan–Meier survival curves and pointwise confidence intervals using interval-midpoint imputation of event times.13 To assess the joint effect of nonparticipation and dependent censoring due to death, we implemented inverse probability weighting.14
Covariate-adjusted versions of these analyses were also performed, as prespecified, with backward variable selection with a pair-stratified interval-censored Cox proportional-hazards model with an inclusion P value cutoff of 0.20; the intervention group was omitted from the model in this selection process and was added only to the final model. In post hoc analyses, we examined the interaction between the intervention and the participants’ sex, age, and geographic region.
Comparison of coverage of intervention components according to group was a prespecified end point, analyzed with the use of baseline data from the cohort of approximately 20% of residents and postintervention data from the remaining 80% of the population involving six communities that participated in the end-of-trial survey. Population viral suppression was defined as the number of HIV-positive persons receiving ART who had an HIV-1 RNA level of 400 copies per milliliter or less divided by the number of all HIV-positive persons who had an HIV-1 RNA result, regardless of ART status (overall 90-90-90 cascade). We used Poisson regression with log-link, fixed effects for matched-pair, time, and their interaction (accounting for community-level clustering) to assess the effect of randomized group on coverage of intervention components.
Secondary, exploratory, and subgroup analyses were not adjusted for multiple comparisons. Results are reported with point estimates and 95% confidence intervals, without P values. The confidence intervals were not adjusted for multiple comparisons, and inferences drawn from them may not be reproducible.
All the authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. The trial investigators had access to the data, but the sponsors did not.
RESULTS
PARTICIPANTS
We enrolled 12,610 participants in the cohort from the random sample of approximately 20% of households in each community at baseline, representing 81% of eligible residents in the enumerated households (Fig. 2). At baseline, 3596 participants (29%) were HIV-positive, and 83% of these participants were aware of their positive HIV status.10 At enrollment, 87% of participants who were known to be HIV-positive were receiving ART, and 96% of these participants had viral suppression. Thus, at baseline, 70% of all HIV-positive persons enrolled in the cohort (in all 30 communities) had an undetectable viral load. No trial-related adverse events that met the criteria of being both unexpected and possibly related to trial participation were reported.
Figure 2. Trial Randomization and Outcomes in the HIV-Incidence Cohort.
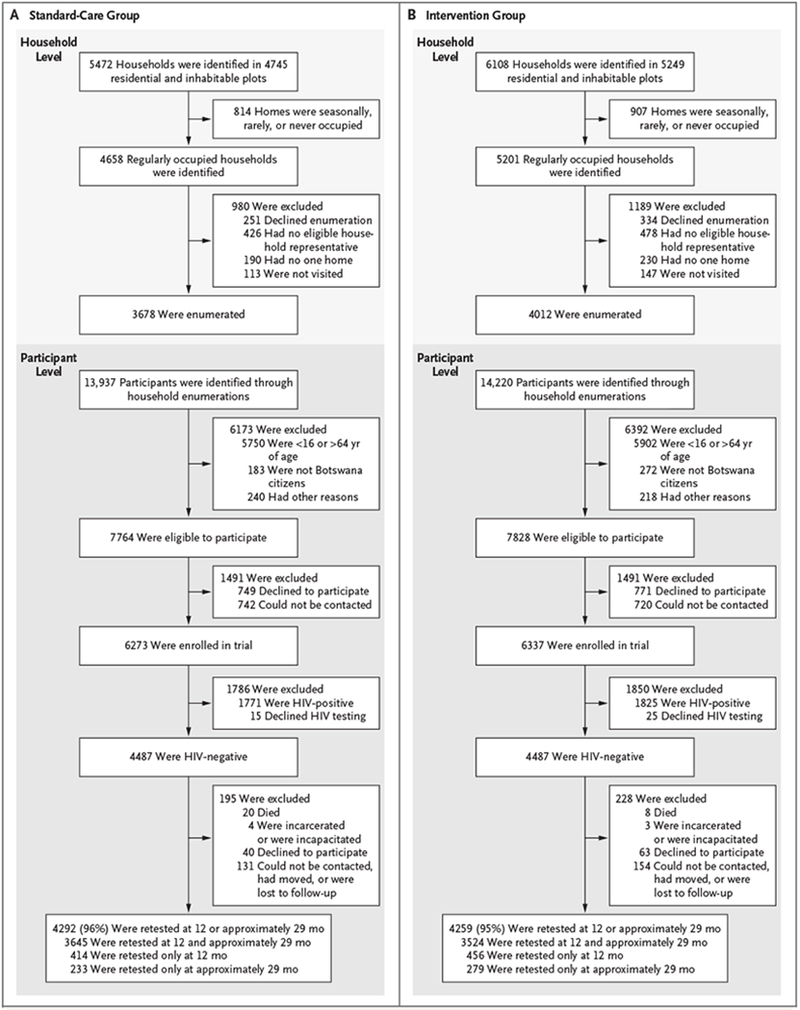
Shown are data on randomization and outcomes in the standard-care group (Panel A) and the intervention group (Panel B).
We enrolled 4487 HIV-negative participants in the HIV-incidence cohort in each group. The baseline characteristics of participants in the HIV-incidence cohort were similar in the two groups (Table 1); 60% of these participants, as well as those in the eligible enumerated population, were female (Table S1 in the Supplementary Appendix, available at NEJM.org). The median duration of follow-up was 29 months (with follow-up visits at a median of 12.3 and 29.4 months), and 95 to 96% of the participants were retested for HIV at least once (Fig. 2).
Table 1.
Baseline Characteristics of the Trial Participants with an HIV-Negative Test Result at Enrollment, According to Randomization Group, 2013–2015.*
| Variable | Standard-Care Group (N = 4487) | Intervention Group (N = 4487) |
|---|---|---|
| Age — no. (%) | ||
| 16–24 yr | 1619 (36) | 1562 (35) |
| 25–34 yr | 1293 (29) | 1313 (29) |
| 35–44 yr | 567 (13) | 583 (13) |
| 45–54 yr | 495 (11) | 510 (11) |
| 55–64 yr | 513 (11) | 519 (12) |
| Female sex — no. (%) | 2698 (60) | 2694 (60) |
| Marital status — no./total no. (%) | ||
| Single or never married | 3592/4485 (80) | 3494/4484 (78) |
| Married | 724/4485 (16) | 810/4484 (18) |
| Widowed, divorced, or separated | 169/4485 (4) | 180/4484 (4) |
| Education level — no./total no. (%) | ||
| No schooling, informal schooling, or primary schooling | 1151/4452 (26) | 1067/4467 (24) |
| Junior secondary schooling | 1694/4452 (38) | 1546/4467 (35) |
| Senior secondary schooling | 905/4452 (20) | 967/4467 (22) |
| Postsecondary schooling | 702/4452 (16) | 887/4467 (20) |
| Income — no./total no. (%) | ||
| None | 2651/4464 (59) | 2390/4453 (54) |
| <$96 per mo | 691/4464 (15) | 715/4453 (16) |
| $96 to $477 per mo | 844/4464 (19) | 977/4453 (22) |
| >$477 per mo | 278/4464 (6) | 371/4453 (8) |
| Alcohol consumption in past 1 mo — no./total no. (%) | ||
| Never | 3375/4483 (75) | 3292/4477 (74) |
| <1 time a wk | 293/4483 (7) | 328/4477 (7) |
| 1 time a wk | 317/4483 (7) | 357/4477 (8) |
| 2–3 times a wk | 315/4483 (7) | 328/4477 (7) |
| >3 times a wk | 183/4483 (4) | 172/4477 (4) |
| No. of sexual partners in past 12 mo — no./total no. (%)† | ||
| 0 | 462/3745 (12) | 457/3745 (12) |
| 1 | 2226/3745 (59) | 2213/3745 (59) |
| 2 | 888/3745 (24) | 868/3745 (23) |
| 3 | 101/3745 (3) | 114/3745 (3) |
| ≥4 | 68/3745 (2) | 93/3745 (2) |
| Participants with concurrent sexual partners in past 12 mo — no./total no. (%)‡ | 1023/3283 (31) | 1022/3287 (31) |
| Alcohol use by participant, partner, or both during last sexual encounter — no./total no. (%)§ | 425/3893 (11) | 335/3850 (9) |
| Participant-reported circumcision — no./total no. (%)¶ | 490/1538 (32) | 593/1536 (39) |
| HIV infection — no./total no. (%)‖ | 1771/6258 (28) | 1825/6312 (29) |
Some percentages may not sum to 100 because of rounding. Percentages were calculated according to the number of available responses.
Of the 7773 participants reporting any lifetime sexual activity, 283 declined to provide information about sexual activity during the past 12 months and were counted as missing for status with regard to the number sexual partners in the past 12 months.
A total of 6571 participants reported any sexual activity during the past 12 months. One participant reported having had two sexual partners in the past year but then declined to answer all subsequent questions about them. Therefore, the participant was counted as missing for concurrency status.
Of the 7773 participants reporting any lifetime sexual activity, 30 declined to provide information about alcohol use during the most recent sexual encounter and were counted as missing for status with regard to alcohol use by participant, partner, or both during the last sexual encounter.
Of the 3092 HIV-negative men who were 16 to 49 years of age, 18 declined to provide information about circumcision and were counted as missing for circumcision status.
Of the 12,610 enrolled participants, 40 declined HIV testing and were counted as missing for HIV status.
HIV INCIDENCE
In the HIV-incidence cohort, 57 participants in the intervention group acquired HIV infection (annualized HIV incidence, 0.59%) and 90 participants in the standard-care group acquired HIV infection (annualized HIV incidence, 0.92%). The incidence of HIV infection varied according to community, from 0.23% to 1.81% (Table 2). Figure 3A shows the estimated incidence curves for time to seroconversion. Table S2 and Figure S1 in the Supplementary Appendix provide details on participants who had seroconversion (96% of whom had HIV-1 RNA measured).
Table 2.
Observed Incidence of HIV Infection and Incidence Ratios among Participants in the HIV-lncidence Cohort, According to Community Pair and Overall.
| Community Pair No. | Standard-Care Group | Intervention Group | Overall Incidence Ratio (95% CI)* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants | Person-yr of Follow-up | Incident HIV Infections | Annualized Incidence | Participants | Person-yr of Follow-up | Incident HIV Infections | Annualized Incidence | ||
| no. | no. | event rate/100 person-yr | no. | no. | no. | event rate/100 person-yr | |||
| 1 | 131 | 269.1 | 1 | 0.37 | 182 | 367.3 | 1 | 0.27 | 0.73 (0.05–11.72) |
| 2 | 325 | 620.3 | 7 | 1.13 | 347 | 658.3 | 4 | 0.61 | 0.54 (0.16–1.83) |
| 3 | 392 | 939.8 | 11 | 1.17 | 302 | 733.4 | 3 | 0.41 | 0.35 (0.10–1.25) |
| 4 | 349 | 824.3 | 3 | 0.36 | 355 | 837.3 | 5 | 0.60 | 1.64 (0.39–6.87) |
| 5 | 259 | 594.7 | 3 | 0.50 | 336 | 771.8 | 6 | 0.78 | 1.54 (0.39–6.17) |
| 6 | 284 | 678.8 | 9 | 1.33 | 304 | 687.3 | 8 | 1.16 | 0.89 (0.34–2.30) |
| 7 | 236 | 542.0 | 6 | 1.11 | 156 | 345.7 | 1 | 0.29 | 0.26 (0.03–2.13) |
| 8 | 288 | 651.5 | 4 | 0.61 | 311 | 727.0 | 4 | 0.55 | 0.90 (0.22–3.60) |
| 9 | 368 | 819.5 | 2 | 0.24 | 320 | 723.5 | 4 | 0.55 | 2.29 (0.42–12.49) |
| 10 | 185 | 415.0 | 6 | 1.45 | 106 | 234.6 | 1 | 0.43 | 0.29 (0.04–2.43) |
| 11 | 216 | 511.5 | 5 | 0.98 | 235 | 545.3 | 5 | 0.92 | 0.94 (0.27–3.25) |
| 12 | 344 | 847.8 | 3 | 0.35 | 337 | 820.9 | 7 | 0.85 | 2.43 (0.63–9.38) |
| 13 | 365 | 853.5 | 10 | 1.17 | 381 | 882.4 | 2 | 0.23 | 0.19 (0.04–0.88) |
| 14 | 224 | 516.2 | 7 | 1.36 | 267 | 624.5 | 3 | 0.48 | 0.35 (0.09–1.37) |
| 15 | 326 | 717.6 | 13 | 1.81 | 320 | 708.4 | 3 | 0.42 | 0.23 (0.07–0.81) |
| All | 4292 | 9801.5 | 90 | 0.92 | 4259 | 9667.7 | 57 | 0.59 | 0.65 (0.46–0.90)† |
Overall incidence ratios and 95% confidence intervals were calculated with the use of a pair-stratified Cox proportional-hazards model accounting for interval censoring.
A formal test of homogeneity in the pair-specific incidence ratios did not reject the null hypothesis of equal incidence ratios across pairs (P=0.50 by Cochran’s Q test).
Figure 3. Kaplan–Meier Estimates of the Time to Seroconversion among Participants without HIV Infection and Subgroup Analysis of HIV Incidence.
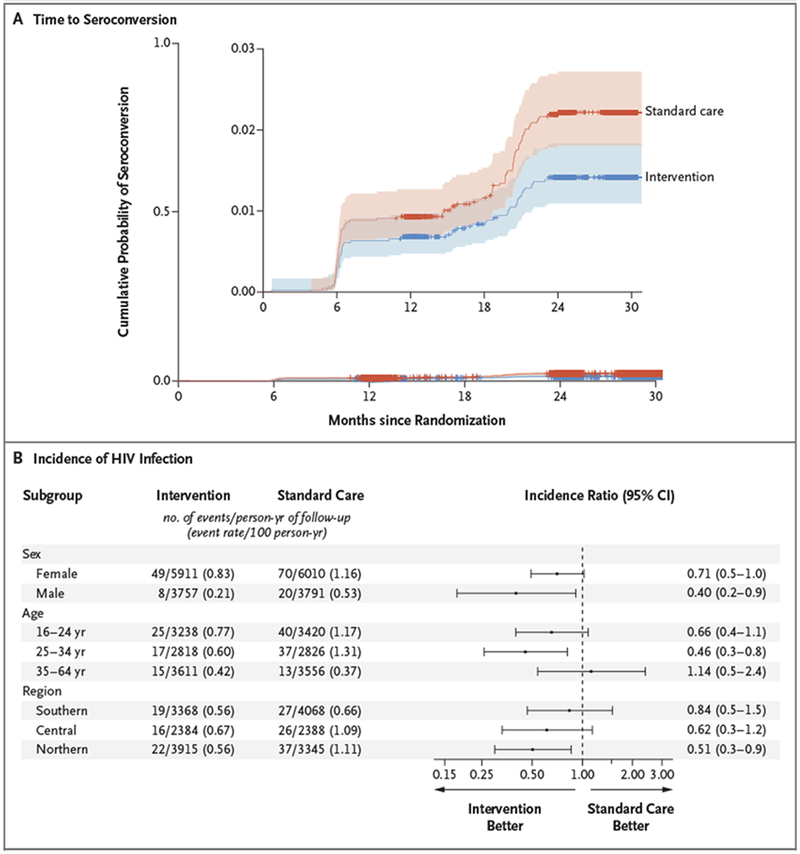
Panel A shows the estimated cumulative probability of seroconversion among 8974 HIV-negative participants. The inset shows the same data on an enlarged y axis. The shaded areas indicate 95% confidence intervals, and tick marks indicate censored data. Panel B shows a forest plot of HIV incidence ratios for key subgroups. I bars indicate 95% confidence intervals.
In the unadjusted primary analysis, the HIV incidence ratio was 0.69 (P = 0.09) in the intervention communities as compared with the standard-care communities. Calculation with an unadjusted pair-stratified Cox model resulted in an HIV incidence ratio of 0.65 (95% confidence interval [CI], 0.46 to 0.90). Sensitivity analyses accounting for within-pair clustering for the incidence end point yielded similar results (incidence ratio, 0.64; 95% CI, 0.43 to 0.95), as did adjustment for nonparticipation and dependent censoring due to death incidence ratio, 0.67; 95% CI, 0.46 to 0.98) (Tables S3 through S5 in the Supplementary Appendix).
In prespecified multivariate analyses, the adjusted HIV incidence ratio in the intervention group as compared with the standard-care group was 0.63 (P=0.05). The pair-stratified Cox proportional-hazards model showed an adjusted incidence ratio of 0.70 (95% CI, 0.50 to 0.98). Factors predictive of HIV incidence that were included in the final multivariate model were sex, age, marital status, education, sexual partners in the past 12 months, alcohol consumption in the past month, and alcohol use during the most recent sexual encounter (Table S6 in the Supplementary Appendix). Figure 3B shows forest plots of the incidence of HIV infection according to sex, age, and geographic region. We observed a greater, although nonsignificant, effect of the intervention than standard care on the incidence of HIV infection among men, persons younger than 35 years of age, and communities in northern Botswana.
VIRAL SUPPRESSION AND MALE CIRCUMCISION IN THE END-OF-TRIAL SURVEY
HIV-1 RNA results were available for nearly 100% of all HIV-positive participants at baseline and for 98% at the trial end (Fig. S2 in the Supplementary Appendix). We observed a significantly greater increase in the percentage of all HIV-positive participants (regardless of previous HIV diagnosis or ART status) with an HIV-1 RNA level of 400 copies per milliliter or less in the communities in the intervention group (absolute increase of 18 percentage points, from 70% to 88%) than in the communities in the standard-care group (absolute increase of 8 percentage points, from 75% to 83%) (relative risk, 1.12; 95% CI, 1.09 to 1.16). Among HIV-positive participants who were not receiving ART at enrollment, the median time to initiation of ART was 81% shorter in the intervention group than in the standard-care group (69 days vs. 367 days) (Table S7 in the Supplementary Appendix). The percentage of HIV-negative men who were 16 to 49 years of age and who reported being circumcised increased from 30% at baseline to 40% at trial end in the intervention group, as compared with an increase from 33% to 35% in the standard-care group (relative risk, 1.26; 95% CI, 1.17 to 1.35).
DISCUSSION
By the end of this community-randomized HIV-prevention trial that offered HIV testing, linkage to care, and early ART, 88% of all HIV-positive persons in the intervention group had viral suppression, and the incidence of HIV infection in the intervention group was 30% lower than the incidence in the standard-care group. Gains in viral suppression and a possible effect on HIV incidence were observed even in a country with a high prevalence of HIV infection and viral suppression at baseline.
Our unadjusted primary analysis of the effect of the intervention on HIV incidence did not reach significance with a P value threshold of 0.05 (P = 0.09; P = 0.05 for adjusted analysis). Sensitivity analyses and models used to construct 95% confidence intervals resulted in incidence ratios that were very similar to those of the primary analysis and 95% confidence intervals that all excluded the null. Taken in totality and in the context of significantly higher (and earlier) levels of viral suppression in communities in the intervention group, these results suggest that the observed approximately 30% lower incidence of HIV infection may be related to the intervention.
By the end of the trial, 88% of all HIV-positive participants in the intervention communities had undetectable HIV-1 RNA (regardless of previous diagnosis or ART status). This percentage exceeds the prevalence of viral suppression of 73% in the UNAIDS 90-90-90 targets and the 86% suppression achieved by “95-95-95,” and it is one of the highest reported levels of population viral suppression globally. However, although the percentage of men who underwent circumcision was greater in the intervention group than in the standard-care group in our trial, it was lower than expected.
We were unable to directly determine which specific intervention or interventions were most important in reducing the incidence of HIV infection. However, we identified large numbers of HIV-positive persons who were not receiving ART15 and successfully linked them to ART,16 with a notably shorter time to ART initiation in the intervention group than in the standard-care group (69 days vs. 367 days). Expanded ART eligibility, in the absence of effective HIV testing and linkage activities, would probably not yield as great a reduction in the incidence of HIV infection. The more rapid viral suppression observed after fast-track and dolutegravir-based ART initiation starting in June 2016 may also have contributed to the reduction in incidence.17 Given the small number of men who underwent circumcision and the low prevalence of circumcision (approximately 50%) by trial end, it is unlikely that male circumcision contributed substantially.
Our findings suggest that even in populations with high viral suppression, it is feasible to find persons who are not yet receiving ART (including many who have not yet received a diagnosis of HIV infection), and these persons are willing to start and adhere to universal ART. Because our interventions were delivered within community and programmatic settings by cadres of staff that are typical in African countries, the feasibility of implementation may be generalizable. As WHO guidance on HIV testing and treatment evolves,18–20 we think that key elements of the Ya Tsie interventions can be locally adapted. Planned costing and cost-effectiveness analyses will be critical to inform policy formulation.
In our trial, potential remaining sources of HIV transmission included noncommunity residents, residents who were not receiving ART, noncitizens (who cannot access free ART), and persons who had acute HIV infection before they began to receive ART. Women remained at high risk for HIV acquisition, even in the intervention group. Additional interventions for prevention such as preexposure prophylaxis remain important, particularly for women.
Other community-randomized trials evaluating the effect of universal test-and-treat interventions on the incidence of HIV infection have been completed.21–23 The Agence Nationale de Recherche sur le Sida (ANRS) 12249 TasP study in South Africa showed high rates of HIV testing, but low and similar rates (approximately 53%) of the initiation of ART in both groups probably contributed to an absence of an observed effect on the incidence of HIV infection.21 The SEARCH (Sustainable East Africa Research in Community Health) trial in Uganda and Kenya showed high rates of HIV testing, linkage to care, and viral suppression; as in our trial, the incidence of HIV decreased over time by approximately 30%, but the decrease was similar in the intervention group and the control group. The absence of a difference in the incidence of HIV between the two SEARCH trial groups may have been due to community-wide HIV testing at baseline in both trial groups (a key component of the intervention package in all three trials) and a rapidly improving standard of ART care.22 In the PopART (Population Effects of Antiretroviral Therapy to Reduce HIV Transmission) trial in South Africa and Zambia, the incidence of HIV infection in the group that received the intervention (which included HIV testing and linkage to care) in combination with standard ART was 30% lower than in the standard-care group, but the incidence in the group that received the intervention in combination with universal ART was not lower than in the standard-care group.23
Our trial had several limitations. The improving standard of care in Botswana, shortened follow-up period, and population movement probably reduced the effect of interventions on the incidence of HIV infection. Despite efforts to reach and test all community residents, approximately one of five enumerated residents did not participate in the survey (and some households could not be enumerated). This could have reduced the effect on the incidence of HIV infection or affected generalizability, but a sensitivity analysis for nonparticipation did not alter our findings. Our estimates of population viral suppression at the end of the trial included persons who moved into the communities but not those who moved away during the trial, and therefore these estimates incorporate population mobility. ART coverage may have been higher in a stable population or if interventions were scaled up nationwide. We present intervention uptake results from only the 6 communities that participated in the end-of-trial survey, which may not be fully representative of all 30 communities. Although we made targeted efforts to reach men and youth, we did not design interventions specifically for key populations and did not include noncitizens (approximately 3% of the enumerated population).24 The participating trial communities, which were small and rural or periurban, may not be fully representative of urban areas or other environments or cultures. Finally, the HIV testing and clinic referral that we offered to the cohort of participants in each community in which participants were receiving standard care may have decreased the incidence of HIV infection, which would lead to a smaller estimated effect of the intervention on the incidence of HIV infection.
In conclusion, we found that a community-wide intervention to test for and treat HIV infection was efficacious in increasing viral suppression. This intervention probably contributed to a reduction in the incidence of HIV infection in a high-prevalence, generalized epidemic area where baseline ART coverage was high.
Supplementary Material
Acknowledgments
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official positions of the funding agencies.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Supported by the President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention (CDC) (cooperative agreements U01 GH000447 and U2G GH001911); the National Institutes of Health (grant R37 AI051164, to Drs. De-Gruttola, Wang, and Wirth, grant K23 AI091434, to Dr. Dryden-Peterson, grants D43 TW000004 and D43 TW009610, to Drs. Gaseitsiwe, Moyo, and Okui, grant K24 AI131928, to Dr. Lockman, grant K23 HD070774, to Dr. Powis, grant R01 CA222147, to Dr. Tchetgen Tchetgen, and grants R01 AI104459 and R01 AI127271, to Drs. Tchetgen Tchetgen and Wirth); the Oak Foundation; and the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a Developing Excellence in Leadership, Training, and Science (DELTAS) Africa initiative (grant DEL-15-006, through Wellcome Trust 107752/Z/15/Z, to Drs. Moyo and Gaseitsiwe).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the trial participants, dikgosi and other community leaders, and the clinic staff, district health management teams, and community health facilities at trial sites for their participation in this project; the Ya Tsie Study Team at the Botswana-Harvard AIDS Institute Partnership, the Harvard T.H. Chan School of Public Health, the CDC, and the Botswana Ministry of Health and Wellness for trial conduct and oversight; Ria Madison and Lars Colson for administrative support; our implementing partners, Tebelopele Voluntary Counseling and Testing Centers, and Jhpiego-Botswana for implementing components of the intervention; the National Institute of Allergy and Infectious Diseases Prevention-Africa data and safety monitoring board for reviewing the trial; and persons who served on the Ya Tsie community advisory board: Masego Baipidi, Bashingi Boingoto, Matlhogonolo Gocha, Kago Ikaneng, Dolly Joubert, Oemetswe Kelebogile, Gaotlhobogwe Kgari, Gobona B. Khupe, Tshegofatso Lebakeng, Goitsemodimo Lepate, Dorcas Mababa, Sethunya Magudu, Katlego Maruapula, Kgosi Emanuel Mathambo, Royal Mathumo, Clement Mmope, Keabetswe Mogale, Tirelo Moganetsi, Omphemetse Mokopi, Victoria Molatlhegi, Kgosi Moitshepi Molelwa, Kgosi Thabo Monaga, Maikgodiso Moshe, Utlwanang Mothibi, Olebogeng Nkwe, Thero Oki, Janet Ranko, Mingi Sesotho, Kgomotso Sethomolane, and Sophinah Tafila.
REFERENCES
- 1.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016;316:171–81. [DOI] [PubMed] [Google Scholar]
- 4.Bavinton BR, Pinto AN, Phanuphak N, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV 2018;5(8):e438–e447. [DOI] [PubMed] [Google Scholar]
- 5.Rodger A, Cambiano V, Bruun T, et al. HIV transmission risk through condomless sex in gay couples with suppressive ART: the PARTNER2 Study extended results in gay men. Presented at the 22nd International AIDS Conference, Amsterdam, July 23–27, 2018 (slide presentation). [Google Scholar]
- 6.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009;373:48–57. [DOI] [PubMed] [Google Scholar]
- 7.Barnhart S PEPFAR: is 90-90-90 magical thinking? Lancet 2016;387:943–4. [DOI] [PubMed] [Google Scholar]
- 8.Williams BG, Granich R. Ending AIDS: myth or reality? Lancet 2017;390:357. [DOI] [PubMed] [Google Scholar]
- 9.Botswana. Geneva: UNAIDS, 2018. (http://www.unaids.org/en/regionscountries/countries/botswana). [Google Scholar]
- 10.Gaolathe T, Wirth KE, Holme MP, et al. Botswana’s progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV 2016; 3(5):e221–e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perriat D, Balzer L, Hayes R, et al. Comparative assessment of five trials of universal HIV testing and treatment in sub-Saharan Africa. J Int AIDS Soc 2018; 21(1):e25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Goyal R, Lei Q, Essex M, De Gruttola V. Sample size considerations in the design of cluster randomized trials of combination HIV prevention. Clin Trials 2014;11:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1997;53:457–81. [Google Scholar]
- 14.Robins JM, Rotnitzky A. Recovery of information and adjustment for dependent censoring using surrogate markers In: Jewell N, Dietz K, Farewell V, eds. AIDS epidemiology: methodological issues. Boston: Birkhäuser, 1992:297–331. [Google Scholar]
- 15.Roland M, Block L, Bachanas P, et al. Mobile and home testing identifies previously diagnosed HIV infected men and women who are not taking ART in Botswana. Presented at the 22nd International AIDS Conference, Amsterdam, July 23–27, 2018 abstract. [Google Scholar]
- 16.Bachanas PJ, Lebelonyane R, Alwano MG, et al. Linkage, treatment, and suppression in the Botswana Combination Prevention Project. Presented at the Conference on Retroviruses and Opportunistic Infections, Boston, March 4–7, 2018 abstract. [Google Scholar]
- 17.Lebelonyane R, Bachanas P, Abrams W, et al. Fast-track ART initiation in Botswana is associated with high rates of ART initiation, retention in care, and virological suppression. Presented at the 22nd International AIDS Conference, Amsterdam, July 23–27, 2018 abstract. [Google Scholar]
- 18.March 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization, 2014. (http://www.who.int/hiv/pub/guidelines/arv2013/arvs2013upplement_march2014/en/). [PubMed] [Google Scholar]
- 19.Consolidated guidelines on HIV testing services. Geneva: World Health Organization, 2015. (http://www.who.int/hiv/pub/guidelines/hiv-testing-services/en/). [PubMed] [Google Scholar]
- 20.Guidelines on HIV self-testing and partner notification: supplement to consolidated guidelines on HIV testing services. Geneva: World Health Organization, 2016. (http://www.who.int/hiv/pub/self-testing/hiv-self-testing-guidelines/en/). [PubMed] [Google Scholar]
- 21.Iwuji CC, Orne-Gliemann J, Larma-range J, et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. Lancet HIV 2018;5(3): e116–e125. [DOI] [PubMed] [Google Scholar]
- 22.Havlir DV, Balzer LB, Charlebois ED, et al. HIV testing and treatment with the use of a community health approach in rural Africa. N Engl J Med 2019;381:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes RJ, Donnell D, Floyd S, et al. Effect of universal testing and treatment on HIV incidence — HPTN 071 (PopART). N Engl J Med 2019;381:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marukutira T, Alwano MG, Behel S, et al. Immigrants and Botswana’s ART program: potential barriers to epidemic control. Presented at the Conference on Retroviruses and Opportunistic Infections, Seattle, February 13–16, 2017 abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


