Abstract
Recent advances in tissue engineering highlight biomaterial designs with context-specific growth factors, cytokines and various small molecules to better mimic the natural extracellular matrix (ECM) microenvironments. These efforts have led to direct improvements in cell-cell and cell-ECM interactions while mitigating undesirable cellular and immunogenic responses. In this short review, we focus on the crucial roles and regulation of transforming growth factor β (TGF-β) signaling in biomaterial applications during tissue repair and regeneration.
Keywords: Biomaterials, Tissue engineering, Regenerative medicine, TGF beta, Inflammation
1. Introduction
Biomaterial scaffolds based on natural and synthetic polymers are now widely employed in regenerative medicine [1–3]. Collagen, fibrins and decellularized extracellular matrices (ECMs) are examples of natural polymers often used for the repair or reconstruction of skin and other soft-tissues because of their superior biocompatibility, functionality and degradation characteristics. In contrast, synthetic polymers tend to be less biocompatible but easier to formulate with greater consistency in mechanochemical properties. Indeed, a variety of macromolecules such as polycaprolactone (PCL) [4], poly-lactic acid (PLA) [5,6] and polymethyl methacrylate (PMMA) have been used in the replacement, repair and regeneration of bone, vessel or other organs [1,7,8].
But despite significant progress, the most prominent technical challenges faced in tissue engineering still relate to long-term cell retention following transplantation or mitigating immunologic responses triggered by the biomimetic ECM scaffolds (Fig. 1). Since physical contact with biomaterial surfaces can alter cell behavior and signaling, biomaterial designs have incorporated growth factors, cytokines and other small molecules to better mimic the natural ECM environment for specific cell and tissue types. This review highlights our current understanding of how transforming growth factor β (TGF-β) signaling is influenced by and modulates cell behavior in different bioactive implant materials.
Fig. 1.
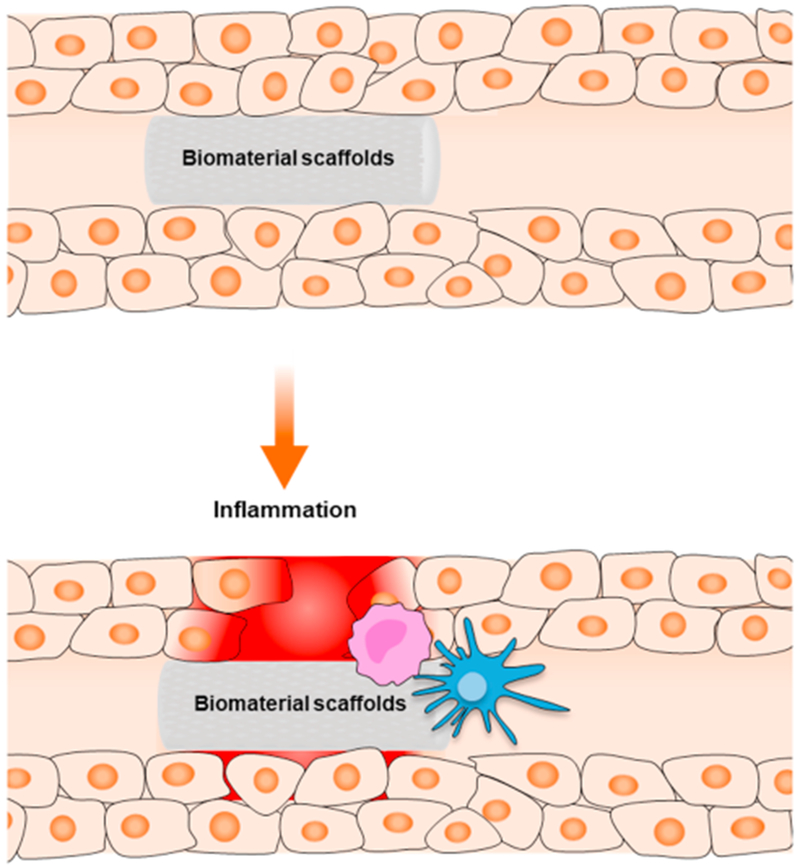
Overview of inflammatory responses commonly faced by biomaterials and transplanted tissues. Both acute innate (e.g., resident macrophages) and adaptive immune responses (e.g., T-cells) can be triggered by tissue injury and the presence of foreign materials.
1.1. TGF-β as a mediator of cell adhesion, growth and ECM deposition
The multifunctional TGF-β superfamily ligands comprise over 40 members including TGF-β [1–3], activin and bone morphogenetic proteins (BMP), many of which have diverse roles in embryonic development, tissue homeostasis and various disease states [9–11]. The prototype ligand TGF-β1 signals through the ubiquitous type I (TβRI/ALK5) and type II (TβRII) serine/threonine kinase receptors to transcriptionally regulate numerous genes related to growth, differentiation and wound healing. Besides gene expression, TGF-β signaling is regulated heavily through its bioavailability, which in most cell types is coordinated by a multi-step proteolytic processing and release from the ECM [12,13].
To mimic these physiologic checkpoints, exogenous TGF-β ligands are incorporated into layered synthetic biomaterials to modulate their local delivery and signaling (Fig. 2). These biomimetic cues have proven crucial for adhesion of tissue scaffolds with transplanted cells, but also for their proper migration, survival and differentiation. For instance, silk fibroin and decellularized cartilage extracellular matrix have demonstrated exceptional biochemical and mechanical properties with a well-controlled TGF-β3 release system that support cell adhesion, proliferation and differentiation of adipose-derived stem cells (ADSCs) [14].
Fig. 2. Enhanced TGF-β signaling mediated by various biomaterial platforms.
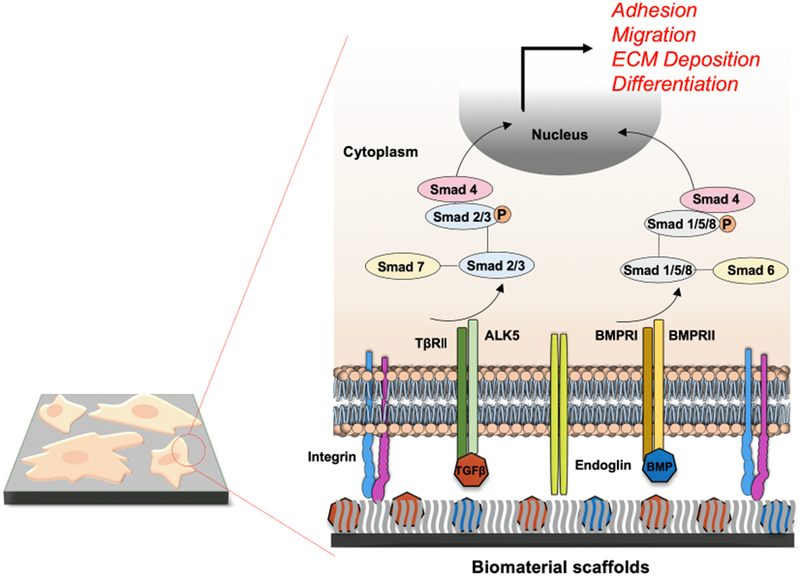
Exogenous TGF-β family ligands including BMPs are integrated into layers of synthetic polymers to promote TGF-β signaling through ALK5 and TβRII and BMPRI/II receptors for Smad2/3 and Smad1/5/8 pathways, respectively. Smads control the adhesion, migration and differentiation of various cell/tissue implants while further augmenting ECM deposition surrounding the biomaterial scaffolds.
Likewise, hyaluronic acid (HyA) derivatives are also highly capable biomimetic systems that enhance retention and survival of transplanted cells. In a recent study, HyA-based hydrogels co-decorated with RGD peptides and TGF-β1 were shown to promote the formation of vascular-like networks by human cardiosphere-derived cells (hCDC) [15]. These hydrogel-encapsulated cells notably demonstrated improved cell survival, proliferation and endothelial differentiation through the canonical TGF-β1/endoglin/TβRII pathway that normally mediates proangiogenic responses [16]. TGF-β can also enhance ECM production itself in many cases, as reported by numerous studies demonstrating the dramatic increase in matrix synthesis and deposition by vascular smooth muscle and endothelial cells when grown on PEG hydrogels with tethered TGF-β [15,17]. Hence, considering its ease of modification, bioactivity and biodegradable characteristics, these and other semi-synthetic HyA hydrogels represent viable therapeutic strategies for many ischemic injuries.
Other TGF-β family ligands such as BMPs are also widely used in biomaterial applications for tissue regeneration [18,19]. In one study, implanted collagen sponges containing both TGF-β1 and BMP2 were shown to strongly induce osteoinductive activity and markedly accelerate bone regeneration than by BMP2 alone [20]. Indeed, there exist many variations of BMP-based matrices demonstrating similarly promising results toward improving bone and joint repairs-including a study in which surface delivery of BMP2 at tunable doses from polymeric scaffolds allowed enhanced bone regeneration, while in another study BMP-2 and TGF-β3 were covalently linked on polycaprolactone (PCL) scaffold surfaces to help stimulate the neighboring human mesenchymal stromal cells (hMSCs), thereby resulting in osteogenic and chondrogenic differentiation [21–25].
1.2. Role of TGF-β signaling in modulating the inflammatory response
Despite numerous advances in applied biomaterials, complications still arise in tissue engineering particularly from host inflammatory responses [26]. Indeed, immunologic responses remain one of the primary factors affecting tissue regeneration, and accordingly, considerable efforts have aimed at identifying new immunomodulatory materials to improve clinical outcomes.
In particular, how the physicochemical properties of a biomaterial surface affect host immune cell behavior is a key consideration in maintaining tissue homeostasis and long-term implant functions [19]. TGF-β is a potent regulator of both innate and adaptive immunity, as it inhibits chemotactic migration and proliferation of neutrophils, macrophages as well as suppression of T cell maturation [27,28]. The molecular bases for these immunosuppressive effects primarily involve the transcriptional inactivation of a number of proinflammatory cytokine genes such as interleukin 2 (IL-2) that are necessary for T cell growth and differentiation. In addition, TGF-β can either regulate cell growth by increasing the expression of cell cycle inhibitors such as p21 and p27, or conversely, repress key mitogenic factors including c-Myc, Cyclin D2, CDK2, Cyclin E[29]. To further inactivate gene targets related to inflammation, Smad6 combined with Pellinos E3 ubiquitin ligase can regulate the Toll-like receptor/interleukin receptor (TIR) while its homolog, Smad7, blocks IL-6 expression and impair NF-κB signaling [30–32].
Recent efforts have exploited these immunosuppressive properties of TGF-β and similar immune modulators to resolve inflammation within hybrid biomaterials (Fig. 3) [29,33,34]. One such study by Liu et al. effectively demonstrated the role of TGF-β in regulating inflammation surrounding the transplanted microporous polylactide-coglycolide (PLG) scaffold [35]. Here these TGF-β1-embedded immune-modulator scaffolds reduced inflammation by curtailing the local production of proinflammatory cytokines and leukocyte infiltration. In another study, McHugh et al., investigated a nanocarrier-based approach using polylacticglycolic acid (PLGA) loaded with TGF-β and IL-2 as a means of directly targeting the CD4+ cell surface for immunosuppression [36]. Similar studies involving nanoparticles and even TGF-β1 affinity peptides (HSNGLPL) have now been reported to improve the biocompatibility of various biomaterials in several contexts including orthotopic cartilage regeneration[37] and skeletal muscle repair [38].
Fig. 3. TGF-β-based immunosuppression in the implant microenvironment.
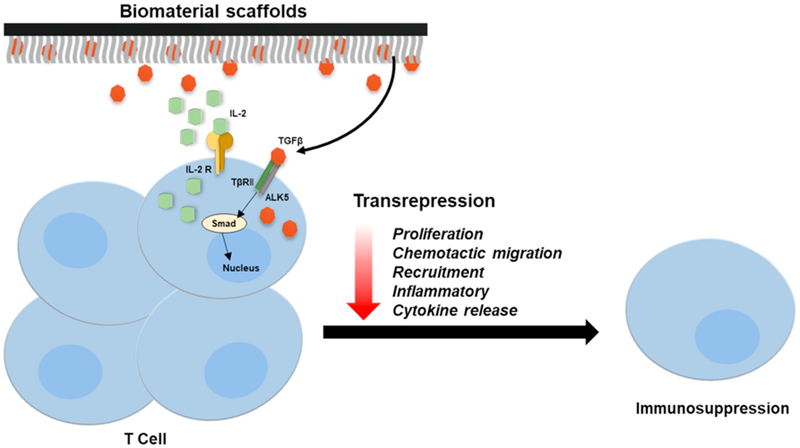
Biomaterial-based delivery of exogenous TGF-β initiates the transcriptional inhibition of inflammatory cytokines, recruitment and proliferation of various resident macrophages and T-cells to attenuate host immune responses and inflammation.
2. Conclusion
TGF-β family ligands have now been used in a variety of tissue engineering applications with the purpose of modulating important functions related to growth, adhesion and survival of implanted cells as well as their local environments. But like most tissue engineering studies to date, our understanding largely derives from animal models that have yet to be fully characterized and validated in clinical settings. One of the more challenging aspects deal with the highly diverse and often context-specific actions of TGF-β ligands which, at least for the time being, can only translate to their variable success in humans. Indeed, the dichotomous role of TGF-β in the immune system is well recognized in that, while predominantly immunosuppressive, in certain contexts these cytokines can exert precisely the opposite effects. As the next generation of biomaterial platforms begins to take shape, these risk factors must be into account while aiming to better recapitulate the release kinetics and the overall efficiency of TGF-β ligands in physiologically-relevant manner.
Acknowledgments
This work was supported in part by NIH CA178443 awarded to N.Y.L., and University of Arizona Cancer Center and University of Arizona Department of Pharmacology and Department of Chemistry and Biochemistry for internal funding.
Footnotes
Declaration of interest statement
The authors have no competing financial or non-financial interests.
References
- [1].Kundu J, Shim J-H, Jang J, Kim S-W, Cho D-W, An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering, J. Tissue Eng. Regenerative Med 9 (2015) 1286–1297. [DOI] [PubMed] [Google Scholar]
- [2].Mao AS, Mooney DJ, Regenerative medicine: current therapies and future directions, Proc. Natl. Acad. Sci 112 (2015) 14452–14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Oshima M, Inoue K, Nakajima K, Tachikawa T, Yamazaki H, Isobe T, Sugawara A, Ogawa M, Tanaka C, Saito M, Kasugai S, Takano-Yamamoto T, Inoue T, Tezuka K, Kuboki T, Yamaguchi A, Tsuji T, Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy, Sci. Reports 4 (2014) 6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen S-H, Chen C-H, Fong YT, Chen J-P, Prevention of peritendinous adhesions with electrospun chitosan-grafted polycaprolactone nanofibrous membranes, Acta Biomater. 10 (2014) 4971–4982. [DOI] [PubMed] [Google Scholar]
- [5].Kessler M, Esser E, Groll J, Tessmar J, Bilateral PLA/alginate membranes for the prevention of postsurgical adhesions, J. Biomed. Mater. Res. Part B: Appl. Biomater 104 (2016) 1563–1570. [DOI] [PubMed] [Google Scholar]
- [6].Yamaoka T, Njatawidjaja E, Kasai A, Agudelo CA, Ehashi T, Kakinoki S, Kato S, Mahara A, Elastic/adhesive double-layered PLA-PEG multiblock copolymer membranes for postoperative adhesion prevention, Polym. Degrad. Stab 98 (2013) 2168–2176. [Google Scholar]
- [7].Serra T, Planell JA, Navarro M, High-resolution PLA-based composite scaffolds via 3-D printing technology, Acta Biomater. 9 (2013) 5521–5530. [DOI] [PubMed] [Google Scholar]
- [8].Seyednejad H, Gawlitta D, Kuiper RV, de Bruin A, van Nostrum CF, Vermonden T, Dhert WJA, Hennink WE, In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(ε-caprolactone), Biomaterials 33 (2012) 4309–4318. [DOI] [PubMed] [Google Scholar]
- [9].Derynck R, Zhang YE, Smad-dependent and Smad-independent pathways in TGF-β family signalling, Nature 425 (2003) 577. [DOI] [PubMed] [Google Scholar]
- [10].Guo X, Wang X-F, Signaling cross-talk between TGF-β/BMP and other pathways, Cell Res. 19 (2008) 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Massagué J, TGFβ signalling in context, Nat. Rev. Mol. Cell Biol 13 (2012) 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY, Latent transforming growth factor β-binding Protein 1 interacts with fibrillin and is a microfibril-associated protein, J. Biol. Chem 278 (2003) 2750–2757. [DOI] [PubMed] [Google Scholar]
- [13].Taipale J, Miyazono K, Heldin C, Keski-Oja J, Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein, J. Cell Biol 124 (1994) 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang Q, Teng B-H, Wang L-N, Li K, Xu C, Ma X-L, Zhang Y, Kong D-L, L-Y. Wang, Y.-H. Zhao, Silk fibroin/cartilage extracellular matrix scaffolds with sequential delivery of TGF-β3 for chondrogenic differentiation of adipose-derived stem cells, Int. J. Nanomed 12 (2017) 6721–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Browne S, Jha AK, Ameri K, Marcus SG, Yeghiazarians Y, Healy KE, TGF-β1/CD105 signaling controls vascular network formation within growth factor sequestering hyaluronic acid hydrogels, Plos One 13 (2018) eOl94679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sridhar BV, Doyle NR, Randolph MA, Anseth KS, Covalently tethered TGF-β 1 with encapsulated chondrocytes in a PEG hydrogel system enhances extracellular matrix production, J. Biomed. Mater. Res. Part A. 102 (2014) 4464–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mann BK, Schmedlen RH, West JL, Tethered-TGF-β increases extracellular matrix production of vascular smooth muscle cells, Biomaterials 22 (2001) 439–444. [DOI] [PubMed] [Google Scholar]
- [18].Chen G, Deng C, Li Y-P, TGF-β and BMP signaling in osteoblast differentiation and bone formation, Int. J. Biol. Sci 8 (2012) 272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, Idowu 0, Li M, Shen C, Hu A, Haydon RC, Kang R, Mok J, Lee MJ, Luu HL, Shi LL, Bone Morphogenetic Protein (BMP) signaling in development and human diseases, Genes Diseases 1 (2014) 87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tachi K, Takami M, Sato H, Mochizuki A, Zhao B, Miyamoto Y, Tsukasaki H, Inoue T, Shintani S, Koike T, Honda Y, Suzuki 0, Baba K, Kamijo R, Enhancement of bone morphogenetic protein-2-induced ectopic bone formation by transforming growth factor-β1, Tissue Eng. Part A 17 (2011) 597–606. [DOI] [PubMed] [Google Scholar]
- [21].Bouyer M, Guillot R, Lavaud J, Plettinx C, Olivier C, Curry V, Boutonnat J, Coll J-L, Peyrin F, Josserand V, Bettega G, Picart C, Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration, Biomaterials 104 (2016) 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Di Luca A, Klein-Gunnewiek M, Vancso JG, van Blitterswijk CA, Benetti EM, Moroni L, Covalent binding of bone morphogenetic protein-2 and transforming growth factor-β3 to 3D plotted scaffolds for osteochondral tissue regeneration, Biotechnol. J 12 (2017) 1700072. [DOI] [PubMed] [Google Scholar]
- [23].Liu H, Peng H, Wu Y, Zhang C, Cai Y, Xu G, Li Q, Chen X, Ji J, Zhang Y, OuYang HW, The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs, Biomaterials 34 (2013) 4404–4417. [DOI] [PubMed] [Google Scholar]
- [24].Lowery JW, Intini G, Gamer L, Lotinun S, Salazar VS, Ote S, Cox K, Baron R, Rosen V, Loss of type 2 bone morphogenetic protein receptor (BMPR2) leads to high bone mass due to increased osteoblast activity, J. Cell Sci (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhu J, Huang B, Ding S, Zhang W, Ma X, Niu H, Yuan Y, Liu C, Tethering of rhBMP-2 upon calcium phosphate cement via alendronate/heparin for localized, sustained and enhanced osteoactivity, RSC Adv. 7 (2017) 20281–20292. [Google Scholar]
- [26].Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, de Gramont A, Targeting the TGFβ pathway for cancer therapy, Pharmacol. Ther 147 (2015) 22–31. [DOI] [PubMed] [Google Scholar]
- [27].Li MO, Wan YY, Sanjabi S, Robertson A-KL, Flavell RA, Transforming growth factor-B regulation of immune responses, Annu. Rev. Immunol 24 (2006) 99–146. [DOI] [PubMed] [Google Scholar]
- [28].Sanjabi S, Oh SA, Li MO, Regulation of the immune response by TGF-β: from conception to autoimmunity and infection, Cold Spring Harbor Perspect. Biol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang L, Pang Y, Moses HL, TGF-β and immune cells: an important regulatory axis in the tumor microenvironment and progression, Trends Immunol. 31 (2010) 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Choi K-C, Lee YS, Lim S, Choi HK, Lee C-H, Lee E-K, Hong S, Kim I-H, Kim S-J, Park SH, Smad6 negatively regulates interleukin 1-receptor-Toll-like receptor signaling through direct interaction with the adaptor Pellino-1, Nat. Immunol 7 (2006) 1057. [DOI] [PubMed] [Google Scholar]
- [31].Hong S, Lim S, Li AG, Lee C, Lee YS, Lee E-K, Park SH, Wang X-J, Kim S-J, Smad7 binds to the adaptors TAB2 and TAB3 to block recruitment of the kinase TAK1 to the adaptor TRAF2, Nat. Immunol 8 (2007) 504. [DOI] [PubMed] [Google Scholar]
- [32].Lee YS, Park JS, Kim JH, Jung SM, Lee JY, Kim S-J, Park SH, Smad6-specific recruitment of Smurf E3 ligases mediates TGF-β 1-induced degradation of MyD88 in TLR4 signalling, Nat. Commun 2 (2011) 460. [DOI] [PubMed] [Google Scholar]
- [33].McHugh MD, Park J, Uhrich R, Gao W, Horwitz DA, Fahmy TM, Paracrine co-delivery of TGF-β and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells, Biomaterials 59 (2015) 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Trompouki E, Bowman Teresa V., Lawton Lee N., Fan Zi P., Wu D-C, DiBiase A, Martin Corey S., Cech Jennifer N., Sessa Anna K., Leblanc Jocelyn L., Li P, Durand Ellen M., Mosimann C, Heffner Garrett C., Daley George Q., Paulson Robert F., Young Richard A., Zon Leonard I., Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration, Cell 147 (2011) 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu JMH, Zhang J, Zhang X, Hlavaty KA, Ricci CF, Leonard JN, Shea LD, Gower RM, Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets, Biomaterials 80 (2016) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McHugh MD, Park J, Uhrich R, Gao W, Horwitz DA, Fahmy TM, Paracrine co-delivery of TGF-beta and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells, Biomaterials 59 (2015) 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen J, Li Y, Wang B, Yang J, Heng BC, Yang Z, Ge Z, Lin J, TGF-β1 affinity peptides incorporated within a chitosan sponge scaffold can significantly enhance cartilage regeneration, J. Mater. Chem. B 6 (2018) 675–687. [DOI] [PubMed] [Google Scholar]
- [38].Shi D, Xiao J, Gu R, Wu G, Liao H, Polyurethane conjugating TGF-β on surface impacts local inflammation and endoplasmic reticulum stress in skeletal muscle, J. Biomed. Mater. Res. Part A 105 (2017) 1156–1165. [DOI] [PubMed] [Google Scholar]


