Abstract
Development of the axial skeleton is a complex, stepwise process that relies on intricate signaling and coordinated cellular differentiation. Disruptions to this process can result in a myriad of skeletal malformations that range in severity. The notochord and the sclerotome are embryonic tissues that give rise to the major components of the intervertebral discs and the vertebral bodies of the spinal column. Through a number of mouse models and characterization of congenital abnormalities in human patients, various growth factors, transcription factors, and other signaling proteins have been demonstrated to have critical roles in the development of the axial skeleton. Balance between opposing growth factors as well as other environmental cues allows for cell fate specification and divergence of tissue types during development. Furthermore, characterization of progenitor cells for specific cell lineages has furthered the understanding of specific spatiotemporal cues that cells need in order to initiate and complete development of distinct tissues. Identifying specific marker genes that can distinguish between the various embryonic and mature cell types is also of importance. Clinically, understanding developmental clues can aid in the generation of therapeutics for musculoskeletal disease through the process of developmental engineering. Studies into potential stem cell therapies are based on knowledge of the normal processes that occur in the embryo, which can then be applied to stepwise tissue engineering strategies.
Keywords: spine, notochord, sclerotome, somite, vertebrae, intervertebral disc, annulus fibrosus, nucleus pulposus
Introduction
The vertebrate axial skeleton was an evolutionary development that provided support for the body and protection of the spinal cord (Alkhatib, 2018; Cox, 2014). The spine consists of two major components, the bony vertebrae derived from cartilage models through endochondral bone formation and fibrous connective tissues including the intervertebral discs (IVDs), ligaments and tendons. The IVD is the shock absorber of the spine and consists of two compartments, the nucleus pulposus (NP) and the annulus fibrosus (AF). The spine has a segmented structure, with the IVDs positioned between neighboring vertebral bodies (VBs). This organization is key for proper function, since the individual parts of the spinal column have discrete roles. The rigid, bony vertebrae provide support and protection. The IVDs distribute weight and other mechanical loads. The NP of the IVD consists of a gelatinous core that gives the IVD its weight and load distribution properties. The AF surrounds the NP and provides structural support and integrity (Adams and Roughley, 2006).
The NP and the AF cohesively form the mature IVD but are derived from very different embryonic structures (Paavola et al., 1980; Rufai et al., 1995; Theiler, 1988). The NP is derived from the notochord, while the AF is derived from the sclerotome of the somites (Christ et al., 2004, 2007; Christ et al., 2000). Somites are transient structures that determine the segmented nature of the vertebral column and further differentiate into the sclerotome, which eventually forms most of the connective tissues of the axial skeleton, including the vertebral body, AF, ligaments and tendons (Figure 1). The signaling mechanisms involved in the development of these embryonic tissues into the terminal structures of the spine are critical for its proper development. Since the spine is derived from the notochord, somites, and sclerotome, alterations in the signaling pathways important for the formation of any of these tissues can result in severe developmental disorders (Cox, 2014). Therefore, insight into the mechanisms of how the axial skeleton develops and the important factors involved in its development can help to address pathology of the spine. An understanding of the basic mechanisms of how the axial skeleton developments can provide a basis for treatment, repair, or regeneration strategies for injury or damage to the spine (Alkhatib, 2018; Cox, 2014; Gadjanski et al., 2012; Lenas et al., 2011; Lenas et al., 2009a, b). This concept of “developmental engineering” could be used in the future for cell and/or tissue replacement therapy. This chapter will cover the signaling mechanisms governing the major steps of development of the axial skeleton, human diseases associated with dysregulation of these signals and the current advancements in tissue engineering strategies.
Figure 1. Illustration of a brief summary of IVD development and matrix organization.
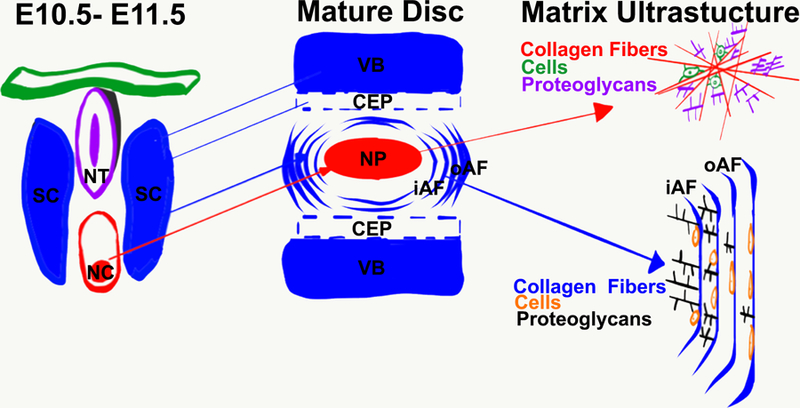
At E10.5–11.5, the neural tube (NT) and the notochord (NC) are located in between the sclerotome (SC). The NC is positioned ventral to the NT. The nucleus pulposus (NP) is derived from the NC (red arrow between E10.5–11.5 and Mature Disc) and the inner and outer annulus fibrosus (iAF and oAF), the cartilaginous endplate (CEP), and the vertebral body (VB) are derived from the SC (blue arrows between E10.5–11.5 and Mature Disc). The matrix ultrastructure of the NP is composed of randomly aligned collagen fibers with cells entrapped. A high abundance of proteoglycans helps keep the collagen ultrastructure together and helps NP cells communicate with the extracellular matrix. The AF is composed of organized, aligned collagen fibers. Cells are aligned parallel to the collagen fibers and are more round in the iAF and more elongated in the oAF. Proteoglycans are more abundant in the iAF than the oAF. NT-neural tube; NC-notochord; SC-sclerotome; VB-vertebral body; CEP-cartilaginous endplate; NP-nucleus pulposus; iAF-inner annulus fibrosus; oAF-outer annulus fibrosus.
Development of Somite Derived Structures
Somitogenesis
Somites are transient, spherical shaped structures that pattern and segment the embryo along the anterior to posterior axis (Christ et al., 2004; Christ et al., 2000; Stockdale et al., 2000). The process of somitogenesis begins with a dynamic reorganization of the embryo due to the formation of the primitive streak, which marks the beginning of gastrulation at E6.0 in mice (Arendt and Nubler-Jung, 1999; Mikawa et al., 2004). Gastrulation establishes the three germ layers of the embryo; the ectoderm, which forms terminal structures such as epidermis and neurons, the endoderm, which forms the digestive and respiratory tract, and the mesoderm, which forms structures in much of the connective tissues in the body (Mikawa et al., 2004; Tam and Behringer, 1997). Most of the skeleton is formed from mesoderm although many components of the head and face are derived from neural crest. Trunk mesoderm is further divided into three compartments, lateral plate mesoderm (LPM), intermediate mesoderm (IM), and paraxial mesoderm (PM). Somites are derived specifically from the PM, also known as presomitic mesoderm, and eventually differentiate into muscle, dermis, and the fibrous and cartilaginous tissues of the spine (Brand-Saberi and Christ, 2000; Pourquie, 2011). Follistatin, an Activin antagonist, has been shown to be required for the specification of the PM. Follistatin works in conjunction with Noggin to inhibit BMP signaling from the LPM and thus permit PM formation (Stafford et al., 2014).
Much research has been devoted to stimulating the in vitro differentiation of stem cells into PM using small molecule inhibitors that target growth factors previously shown to be involved PM differentiation. A recent study derived PM-like cells from pluripotent embryonic stem cells (ESCs) by utilizing such small molecule inhibitors. Formation of the PM is dependent upon Wnt3a and Noggin signaling (Aulehla and Pourquie, 2010; Yamaguchi et al., 1999). By using a GSK3 inhibitor to mimic Wnt3a signaling and an inhibitor of BMP type 1 receptors to replace Noggin, the ESCs began to express PM markers, Tcf15 and Meox1 (Zhao et al., 2014). Another study stimulated the differentiation of 12 human mesodermal cell lineages from induced pluripotent stem cells using extrinsic factors previously shown to be critical during mesoderm formation and differentiation (Loh et al., 2016). These studies highlight the advancements in regenerative science by using “developmental engineering” strategies to potentially repair damaged connective tissues in the spine (Gadjanski et al., 2012; Lenas et al., 2011; Lenas et al., 2009a, b). Engineered PM is important because it can be used as a starting point to engineer all of the musculoskeletal derivatives of the somite. For example, a recent study showed stimulation of chondrocyte differentiation from mouse ESCs by first generating Flk-1−/Pdgfrα-positive PM cells with Activin, Wnt, and VEGF and subsequently treating those cells with BMP4 or GDF5 to stimulate chondrogenesis (Craft et al., 2013). Table 1 contains a list of known factors essential for somitogenesis. These factors can be potential targets for future studies of developmental engineering strategies to generate PM.
Table 1:
Proteins Involved in Important Signaling Pathways during Somitogenesis
| Protein Namea | Abbreviationa | Protein Fucntiona | Skeletal Disordersbc |
|---|---|---|---|
| Activin | ACTIVIN | TGFß superfamily ligand | Osteogenesis imperfecta type 1: OMIM #166200 |
| Ephrin | EPHRIN | Cell surface transmembrane ligand | n/a |
| EPH Receptor A4 | EPHA4 | Receptor tyrosine kinase | n/a |
| Fibroblast Growth Factor 8 | FGF8 | Ligand for FGF receptors | n/a |
| Fibronectin | FN1 | Glycoprotein | Spondylometaphyseal Dysplasia, Corner Fracture Type: OMIM #184255 |
| Follistatin | FST | Activin antagonist | Spinal muscular atrophy, type iv : OMIM #271150 |
| Hes Family BHLH Transcription Factor 1 | HES1 | Basic helix loop helix transcription factor | n/a |
| Mesenchyme Homeobox 1 | ME0X1 | Homeobox transcription factor | Klippel-feil syndrome, OMIM #613702; Diaphanospondylodysostosis, OMIM #608022 |
| Neural Cadherin | NCAD | Calcium dependent cell adhesion protein | n/a |
| Neural Cell Adhesion Molecule | NCAM | Cell adhesion | n/a |
| Noggin | NOG | BMP inhibitor | Multiple synostoses syndrome 1, OMIM #186500 |
| Notch | NOTCH | Type l transmembrane protein | Adams-Oliver syndrome 5, OMIM #616028 |
| Paired Box 3 | PAX3 | PAX family transcription factor | Craniofacial-deafness-hand syndrome, OMIM #122880 |
| Platelet Derived Growth Factor Receptor Alpha | PDGFRα | Cell surface tyrosine kinase receptor | n/a |
| Rentinoic Acid | RA | Ligand for nuclear RA receptors | n/a |
| Transcription Factor 15 (Paraxis) | TCF15 | Basic helix loop helix transcription factor | n/a |
| Vascular Endothelial Growth Factor | VEGF | Growth factor | n/a |
| Vascular Endothelial Growth Factor Receptor 2 | VEGFR2 (FLK-1) | Cell surface tyrosine kinase receptor | n/a |
| Wnt Family Member 3A | WNT3a | Secreted ligand | Craniodiaphyseal Dysplasia, OMIM #122860 |
The mesenchymal, rod-like structure of the PM begins to undergo somitogenesis in an anterior to posterior fashion along the embryo axis at E8.0 in mice. The spherical somites are pinched off from the PM in a fashion that is highly regulated temporally and spatially (Brand-Saberi and Christ, 2000; Pourquie, 2011). The current model for somitogenesis is the Clock and Wavefront Model where Notch and Wnt signaling act as the “clock” that provides the temporal, permissive signal, while FGF and Retinoic Acid signaling acts as the “wave” that provides spatial specificity. The wave is responsible for the anterior to posterior formation of somites due to FGF and Retinoic Acid. These signals provide the directionality and spatial regulation needed to stimulate the mesenchymal to epithelial transition (MET) of the mesenchymal cells of the PM to the epithelial outer layer of the newly formed somite (Bellairs et al., 1978; Brand-Saberi and Christ, 2000; Cooke and Zeeman, 1976; Pourquie, 2011). The MET causes somites to consist of two cell types, an outer layer composed of epithelial cells and an inner cell mass composed of mesenchyme called the somitocoel (Ferrer-Vaquer et al., 2010; Mittapalli et al., 2005). Various studies have identified key factors and signaling pathways salient for proper epithelization during somitogenesis. Adhesion molecules such as N-cadherin, fibronectin, cytoactin, and neural cell adhesion molecules are expressed in the developing somite and are important for epithelization (Crossin et al., 1986; Duband et al., 1987). Two transcription factors involved in MET are Pax3 and Paraxis. Studies have shown that overexpression of Pax3 is sufficient to stimulate epithelialization of mesenchymal cell lines in vitro (Wiggan et al., 2002), and Pax3 expression in the PSM is also required to maintain the epithelial integrity later in somites (Mansouri et al., 2001). Similarly, loss of Paraxis expression disrupts epithelialization (Burgess et al., 1996). The tyrosine kinase EphA4 is also required for proper epithelialization of the somite. Attenuation of EphA4/Ephrin signaling results in somite boundaries, but no epithelial layer formation (Barrios et al., 2003). The mechanism of how the Clock and Wavefront Model fully translates into MET has yet to be determined, but Notch regulates the expression of transcription factor Hes1, which regulates Ephrin expression (Glazier et al., 2008).
Sclerotome Specification
Shortly after MET, the somite begins to differentiate into its respective tissues: the dermatome, the myotome, and the sclerotome. The dermatome forms the derms of the back, the myotome forms all the skeletal muscle of the body, and the sclerotome forms the connective tissues of the axial skeleton: vertebrae (VB), cartilaginous end plates, annulus fibrosus (AF), tendon and ligament (Brand-Saberi and Christ, 2000; Kalcheim and Ben-Yair, 2005). The sclerotome is a transient, embryonic tissue composed of pluripotent, mesenchymal stem cells located in the ventromedial region of the somite. The localization and specification of the sclerotome is a tightly controlled and highly dynamic process induced by Shh signaling from the floor plate of the neural tube and notochord, which induces expression of early sclerotome markers Pax1, Pax 9, and Mfh1 (Borycki et al., 1998; Brand-Saberi and Christ, 2000; Chiang et al., 1996; Dockter, 2000; Fan and Tessier-Lavigne, 1994). The embryonic knock out of both Pax1 and Pax9 causes the complete loss of the VB and AF (Peters et al., 1999). This defect can be caused by two alternative possibilities. Pax 1/9 loss can result in a failure of the initial formation of the sclerotome. Alternatively, sclerotome formation may occur, but the VB and AF do not form due to defects in proliferation and subsequent differentiation. Nkx3.2, also known as Bapx1, is another early marker of sclerotome formation. Its expression is regulated by Shh and it initially has the same temporal and spatial expression pattern as Pax1/9; however, Nkx3.2 remains expressed throughout the sclerotome while Pax expression later becomes restricted to more fibrous tissues (Murtaugh et al., 2001). BMP from the LPM has the capacity to disrupt sclerotomal specification by interfering with Shh signaling. However, several BMP antagonists are expressed regionally that carefully restrict BMP activity, such as Noggin and Gremlin1 (Rider and Mulloy, 2010; Stafford et al., 2011). Noggin and Gremlin1 cooperate to antagonize BMP signaling, allowing sclerotome differentiation in the presence of Shh (Stafford et al., 2011). Noggin and Gremlin1 are required for sclerotome differentiation in mice, since their deletion results in a lack of sclerotome cells, while the dermomyotome remains completely unaffected. Inhibition of BMP alone is not sufficient to specify sclerotome or expand sclerotome differentiation in vivo or in vitro suggesting that antagonism of BMP is a permissive factor for sclerotome differentiation (Rider and Mulloy, 2010). Sclerotome formation is coupled with an epithelial to mesenchymal transition to permit migration and further differentiation of the mesenchymal cells (Christ and Ordahl, 1995; Dockter and Ordahl, 2000). More information about the important factors that contribute to sclerotome specification can be found in Table 2.
Table 2:
Proteins Involved in Important Signaling Pathways during Sclerotome Specification
| Protein Namea | Abbreviationa | Protein Fucntiona | Skeletal Disordersbc |
|---|---|---|---|
| Forkhead Box C2 | F0XC2 (MFH1) | Forkhead family transcription factor | n/a |
| Gremlin | GREM1 | BMP antagonist | Sclerosteosis, OMIM #269500 |
| Noggin | NOG | BMP inhibitor | Multiple synostoses syndrome 1, OMIM #186500 |
| NK3 Homeobox 2 | NXK3.2 (BAXP1) | Transcriptional repressor | Spondylo-megaepiphyseal-metaphyseal dysplasia, OMIM #613330 |
| Paired Box 1 | PAX1 | PAX family transcription factor | Otofaciocervica syndrome 2, OMIM #615560; Klippel-feil syndrome, OMIM #613702; Diaphanospondyiodysostosis, OMIM #608022 |
| Paired Box 9 | PAX9 | PAX family transcription factor | Tooth agenesis, OMIM #604625 |
| Sonic Hedgehog | SHH | Secreted ligand | Laurin-Sandrow syndrome, OMIM #135750; Hypoplasia or aplasia of tibia with polydactyly, OMIM #188740; Solitary median maxillary central incisor, OMIM #147250 |
Resegmentation
After the sclerotome has been specified, the process of resegmentation begins. Resegmentation is the formation of rostral and caudal domains within the sclerotome that separate and recombine with the adjacent domain in the neighboring sclerotomal segment. Reorganization of the rostral and caudal domains creates a spatial organization where the myotome is staggered one half segment to the developing VB to allow the nerves projecting from the spinal cord to innervate the developing muscles (Bagnall et al., 1988; Goldstein and Kalcheim, 1992; Huang et al., 2000; Tanaka and Uhthoff, 1981). Resegmentation is necessary for the proper formation of the Intervertebral Disc (IVD) and VB. Therefore, deviations to this process have clinical implications. Rare diseases that occur in humans called Proatlas Segmentation Anomalies OMIM: 109500 are thought to be caused by failure in the proatlas sclerotome, which will partially go on to form the C1 VB, to undergo resegmentation. These disorders result in dysplasia within the craniovertebral junction and can manifest in various ways clinically, such as cervical VB fusion and ventral brain stem compression (Muthukumar, 2016; Spittank et al., 2016; Umegaki et al., 2017). Klippel-Feil Syndrome (OMIM: 613702), where patients suffer from cervical vertebral fusion due to lack of IVD formation, has also been associated with a disruption in resegmentation and mutations in the GDF6 gene (Tassabehji et al., 2008). For more information concerning skeletal disorders and the associated proteins that cause these disorders see Table 3.
Table 3:
Proteins Involved in Important Signaling Pathways during Resegmentation
| Protein Namea | Abbreviationa | Protein Fucntiona | Skeletal Disordersbc |
|---|---|---|---|
| Growth Differentiation Factor 6 | GDF6 | TGFß superfamily ligand | Klippel-feil syndrome, OMIM #613702; Multiple Synostoses Syndrome 4, OMIM #617898 |
| Mesoderm Posterior BHLH Transcription Factor 2 | MESP2 | Basic helix loop helix transcription factor | Spondylocosta dysostosis 2, OMIM #608681 |
| Paired Box 1 | PAX1 | PAX family transcription factor | Otofaciocervica syndrome 2, OMIM #615560; Klippel-feil syndrome, OMIM #613702; Diaphanospondyiodysostosis, OMIM #608022 |
| Paired Box 9 | PAX 9 | PAX family transcription factor | Tooth agenesis, OMIM #604625 |
| Ripply Transcriptional Repressor | RIPPLY1/2 | Transcriptio nal repressor | Klippel-feil syndrome, OMIM #613702;Spondyiocosta dysostosis 6, autosoma recessive, OMIM #616566 |
| T-box transcription factor TBX1S | TBX18 | Transcriptio nal repressor | n/a |
| UNC Homeobox | UNCX4.1 | Homeobox transcription factor | n/a |
Rostral-caudal polarity is established in the sclerotome by the expression of rostral markers such as Mesp2 and Tbx18 and caudal markers such as Ripply 1 and 2, Uncx4.1, and Pax1/9 (Kawamura et al., 2008; Leitges et al., 2000; Morimoto et al., 2007; Neubuser et al., 1995). The sclerotome migrates ventrally to surround the notochord, splits into two separate halves along the rostral-caudal border called von Ebner’s fissure and combines with half of the adjacent sclerotome (Bagnall et al., 1988; Christ et al., 2000). As a result, in mice, the IVD is derived from the cells in the caudal half of the sclerotome near von Ebner’s fissure. The recombined sclerotomal halves form the VB, while the AF of the IVD differentiates from cells in the caudal half in mice and the rostral half in chicks near the fissure (Bruggeman et al., 2012). Some of the resegmented cells will then migrate dorsally to surround the neural tube and form the neural arches (Monsoro-Burq, 2005). A new model coined the “resegmentation-shift” model suggests that after the sclerotome resegments the cells that migrate dorsally to surround the neural tube do so in a non-linear fashion. The model suggests that as sclerotomal cells migrate dorsally to form the neural arches, there is a cranial shift in their migration pattern. This cranial shift was visualized in vivo by using lipophilic dyes to label the migrating sclerotome in the chick model and was only observed in the lumbo-sacral region of the spine. It is believed this cranial shift is necessary to establish the slant of the neural arch pedicle specifically in the lumbo-sacral region (Ward et al., 2017).
Sclerotome Derivatives
Vertebra
Cells destined to undergo chondrogenesis to form the VB proliferate, condense, and differentiate after sclerotome resegmentation. Sclerotome cells organize to form many structures within the spine, including hyaline cartilage that will form the VB and Cartilaginous End Plate (CEP) via chondrogenesis (Figure 1). A list of signaling molecules involved in specification of the vertebrae are provided in Table 4. Defects in chondrogenesis results in improper formation of the VB and leads to severe spinal disorders such as kyphosis and scoliosis (Sivakamasundari et al., 2017). Vertebral chondrocyte cell fate is specified early when the sclerotome first forms (Murtaugh et al., 1999). Shh secreted from the notochord, primes the cells to respond to the chondrogenic actions of BMP signaling. Pax1 and Pax9, which are regulated by Shh, are early sclerotome markers but they also regulate condensation of the sclerotome mesenchyme and the initiation of chondrogenesis (Sivakamasundari et al., 2017). Pax1/9 regulate the transcription factor Sox 5, which in conjunction with Sox 6 and Sox 9, is necessary for the activation of early chondrocyte differentiation markers, Col2a1 and Aggrecan (Han and Lefebvre, 2008; Lefebvre et al., 2001). Pax 1/9 and Shh also regulate Nkx3.2, another early marker of sclerotome formation and a required factor in stimulating chondrogenic differentiation. Nkx3.2 is one of the earliest markers of prechondrogenic cells in the axial skeleton and induces the expression of Sox9, a master regulator of chondrogenesis (Tribioli and Lufkin, 1999; Zeng et al., 2002). Nkx3.2 acts as a transcriptional repressor by inhibiting repression of Sox9 and thus allowing the sclerotome to be competent to undergo chondrogenesis by responding to BMP signals, while simultaneously inhibiting anti-prechondrogenic factors in the prospective VB. The embryonic deletion of Nkx3.2 causes perinatal lethality in mice and results in severe spinal dysplasia due to failure of the sclerotome to undergo chondrogenesis (Murtaugh et al., 2001).
Table 4:
Proteins Involved in Important Signaling Pathways to form Sclerotomal Derivatives
| Vertebra and Cartilaginous End Plate | |||
|---|---|---|---|
| Protein Namea | Abbreviationa | Protein Fucntiona | Skeletal Disordersbc |
| Aggrecan | ACAN | Proteoglycan | Spondyloepimetaphyseal dysplasia, aggrecan type, OMIM #612813; Osteochondritis Dissecans, OMIM# 165800 |
| Bone Morphogenetic Protein | BMP | TGFß superfamily ligand | Brachydactyly, Type A2, OMIM#112600, Osteonecrosis, OMIM #608805, Short Stature, Facial Dysmorphism, and Skeletal Anomalies with or Without Cardiac Anomalies, OMIM #617877 |
| Bone Morphogenetic Protein Receptor Type 1A | BMPR1a | Serine/Threonine kinase transmembrane receptor | n/a |
| Bone Morphogenetic Protein Receptor Type 1B | BMPR1b | Serine/Threonine kinase transmembrane receptor | Brachydactyly, type a1, d, OMIM # 616849, Acromesomelic dysplasia, Demirhan type, OMIM #609441 |
| Chordin | CHRD | BMP antagonist | |
| Collagen Type II Alpha 1 Chain | COL2a1 | Extracellular matrix protein | Kniest Dysplasia, OMIM #156550; Spondyloepiphyseal dysplasia congenital type, OMIM #83900; Achondrogenesis 2, OMIM #200610; Legg-Calve-Perthes disease, OMIM #150600 |
| Crossveinless | CV-2 | BMP inhibitor | Diaphanospondylodysostosis, OMIM #608022; Ischio-Vertebral Syndrome, OMIM #n/a |
| Dachsous Cadherin-Related 1 | DCHS1 | Calcium-dependent cadherin | Van Maldergem syndrome 1, OMIM #601390; Hennekam Syndrome, OMIM #235510 |
| FAT Atypical Cadherin 4 | FAT4 | Protocadherin | Van maldergem syndrome 2, OMIM #615546; Hennekam Syndrome, OMIM #235510 |
| Growth and Differentiation Factor 5 | GDF5 | TGFß superfamily ligand | Acromesomelic chondrodysplasia, Grebe type, OMIM #200700; Brachydactyly C, OMIM #113100; Du Pan syndrome, OMIM #228900; Multiple synostoses syndrome 2, OMIM #610017; Symphalangism, proximal 1B, OMIM #615298 |
| NK3 Homeobox 2 | NKX3.2 (BAXP1) | Transcriptional repressor | Spondylo-megaepiphysealmetaphyseal dysplasia, OMIM #613330 |
| Notch | NOTCH | Type I transmembrane protein | Adams-Oliver syndrome 5, OMIM #616028 |
| Paired Box 1 | PAX1 | PAX family transcription factor | Otofaciocervical syndrome 2, OMIM #615560; Klippel-feil syndrome, OMIM #613702; Diaphanospondylodysostosis, OMIM #608022 |
| Paired Box 9 | PAX9 | PAX family transcription factor | Tooth agenesis, OMIM #604625 |
| Recombination Signal Binding Protein For Immunoglobulin Kappa J | RBPJ | Transcriptional Repressor | Adams-Oliver syndrome , OMIM #100300 |
| SMAD Family Member 1 | SMAD 1 | Transcriptional coactivators | Buschke-Ollendorff Syndrome, OMIM #166700; Osteopoikilosis, OMIM #166705 |
| SMAD Family Member 5 | SMAD 5 | Transcriptional coactivator | n/a |
| Sonic Hedgehog | SHH | Secreted ligand | Laurin-Sandrow syndrome, OMIM #135750; Hypoplasia or aplasia of |
| SRY-Box 5 | SOX5 | SOX family transcription factor | Lamb-Shaffer syndrome, OMIM #616803 |
| SRY-Box 6 | SOX6 | SOX family transcription factor | Multiple synostoses syndrome 1, OMIM #186500 |
| SRY-Box 9 | SOX9 | SOX family transcription factor | Campomelic dysplasia, OMIM #114290 |
| Transforming Growth Factor Beta | TGFBs | TGFR superfamily ligand | Camurati-Engelmann disease, OMIM #131300; Loeys-Dietz syndrome 5, OMIM #615582; Holt-Oram Syndrome, OMIM #142900 |
| Transforming Growth Factor Beta Receptor 2 | TGFBR2 | Serine/Threonine kinase transmembrane receptor | Loeys-Dietz syndrome 2, OMIM #610168 |
| Annulus Fibrosus | |||
|---|---|---|---|
| Protein Namea | Abbreviationa | Protein Fucntiona | Skeletal Disordersbc |
| Bone Morphogenetic Protein | BMP | TGFß superfamily ligand | Brachydactyly, Type A2, OMIM#112600, Osteonecrosis, OMIM #608805, Short Stature, Facial Dysmorphism, and Skeletal Anomalies with or Without Cardiac Anomalies, OMIM #617877 |
| Collagen Type I A | COLla | Extracellular matrix protein | Caffey disease, OMIM #114000; Ehlers-Danlos syndrome, classic type, OMIM #130000; Osteogenesis imperfecta 1, OMIM #166200; Osteoporosis, OMIM #166710 |
| Fibromodulin | FMOD | Proteoglycan | Hypochondrogenesis, OMIM #200610; Pseudoachondroplasia, OMIM #177170 |
| Filamin B | FLNB | Cytoskeleton protein | Atelosteogenesis 1, OMIM #108720; Boomerang dysplasia, OMIM #112310; Larsen syndrome, OMIM #150250; pondylocarpotarsal synostosis syndrome; OMIM #272460 |
| Kelch Like Family Member 14 | Klhl14 | Long non-coding RNA | n/a |
| Mohawk | MKX | IRX family-related homeobox protein | Cleft Palate, Isolated, OMIM #119540 |
| Noggin | NOG | BMP inhibitor | Multiple synostoses syndrome 1, OMIM #186500 |
| NK3 Homeobox 2 | NKX3.2 (BAXP1) | Transcriptional repressor | Spondylo-megaepiphysealmetaphyseal dysplasia, OMIM #613330 |
| Paired Box 1 | PAX1 | PAX family transcription factor | Otofaciocervical syndrome 2, OMIM #615560; Klippel-feil syndrome, OMIM #613702; |
| Paired Box 9 | PAX9 | PAX family transcription factor | Tooth agenesis, OMIM #604625 |
| Scleraxis | SCX | Basic helix loop helix transcription factor | Acrocallosal Syndrome, OMIM #200990; Wilson-Turner X-Linked Mental Retardation Syndrome, OMIM #309585 |
| SMAD Family Member 2 | SMAD 2 | Transcriptional coactivator | Buschke-Ollendorff Syndrome, OMIM #166700; Melorheostosis, OMIM #155950 |
| SMAD Family Member 3 | SMAD 3 | Transcriptional coactivator | Loeys-Dietz syndrome 3, OMIM #613795 |
| Sonic Hedgehog | SHH | Secreted ligand | Laurin-Sandrow syndrome, OMIM #135750; Hypoplasia or aplasia of tibia with polydactyly, OMIM #188740; Solitary median maxillary central incisor, OMIM #147250 |
| SRY-Box6 | SOX6 | SOX family transcription factor | Multiple synostoses syndrome 1, OMIM #186500 |
| SRY-Box9 | SOX9 | SOX family transcription factor | Campomelic dysplasia, OMIM #114290 |
| Syndecan 4 | SDC4 | transmembrane (type I) heparan sulfate proteoglycan | n/a |
| Transforming Growth Factor Beta | TGFß | TGFfc superfamily ligand | Camurati-Engelmann disease, OMIM #131300; Loeys-Dietz syndrome 5, OMIM #615582; Holt-Oram Syndrome, OMIM #142900 |
| Transforming Growth Factor Beta Receptor 2 | TGFßR2 | Serine/Threonine kinase transmembrane receptor | Loeys-Dietz syndrome 2, OMIM #610168 |
BMP signaling is essential for chondrocyte specification and differentiation and is a potent regulator upstream of Sox 5, 6, and 9 transcription factors (Lefebvre et al., 2001; Ohba et al., 2015; Yoon et al., 2005). Research has established the requirement of BMP signaling for chondrogenesis by creating an embryonic knockout of BMPR1b and conditional knockout of BMPR1a in Collagen Type 2-expressing cells in mice. This study demonstrated that progenitor cells can condense but do not further differentiate into chondrocytes (Yoon et al., 2005). In vitro studies have shown that mesenchymal stem cells exogenously treated with BMP ligands 2, 4, and 6 begin to undergo chondrogenic differentiation and express chondrocyte markers such as Col2a and Aggrecan (Sekiya et al., 2005). Once chondrogenesis is initiated in the axial skeleton, chondrocyte differentiation and endochondral bone formation occur in the vertebra.
During development the sclerotome is organized into a patterned structure of alternating loose and dense mesenchyme. The dense mesenchyme will differentiate into the AF of the IVD, and the loose mesenchyme will differentiate into the VB. A sharp boundary exists between the two compartments due to the differential expression of certain factors. High activity levels of BMP signaling are required for differentiation of the vertebral cartilage; however, BMP mRNA is synthesized in the presumptive IVD cells (Zakin et al., 2010; Zakin et al., 2008). To get BMP from the tissue of production, the IVD, to the responsive tissues, the VB, BMP expression must be relocalized and concentrated. The BMP interacting proteins, Crossveinless-2 (Cv-2) and Chordin (Chd), have been shown to be required for this translocation. Cv-2 mRNA is made in cartilaginous cells of the presumptive VB, and Chd mRNA is made in the presumptive IVD; however, most of the protein is localized to the vertebral compartment. Chd binds to and inactivates BMP in the developing IVD, and subsequently moves BMP to the developing VB. Once there, Chd binds to Cv-2, and is cleaved by a protease, thus releasing BMP (Zakin et al., 2010; Zakin et al., 2008). The deletion of Cv-2 or Chd results in small vertebral bodies and a slightly expanded intervertebral space. This movement and relocation of BMP helps define the discrete boundaries within the developing spinal column. In addition, knock out of Tgfbr2 in collagen type II expressing cells indicated that Tgfbr2 is required to maintain the sharp boundary between the developing VB and AF (Baffi et al., 2006). TGFß signaling is a known antagonist of BMP signaling; therefore, it is possible that loss of TGFß results in inappropriate BMP activity in the presumptive IVD where BMP mRNA is synthesized (Candia et al., 1997; Li et al., 2006).
The protocadherins Fat4 and Dchs1 act as a receptor-ligand pair to establish planar cell polarity and have also been shown to be required for proper formation of the VB. The double knockout of Fat4 and Dchs1 shows a reduced number of chondrogenic cells within the developing VB and malformed VB that is split along the midline (Kuta et al., 2016). These results suggest that cell adhesion and polarity are important in chondrocyte differentiation and formation of the vertebrae.
Annulus Fibrosus
The compartment of the sclerotome that will form the AF of the IVD can be traced back to the somitocoel cells of the somite (Mittapalli et al., 2005). As described above, somites are transient structures that create the segmented organization of the embryo. They consist of an outer epithelial ball with a central core of mesenchymal cells called the somitocoel. After sclerotome formation, Shh from the notochord causes an epithelial-to-mesenchymal transition in the ventral-lateral portion of the somite, causing these cells along with the mesenchymal cells of the somitocoel to migrate ventrally to surround the notochord (Monsoro-Burq, 2005) (Figure 1). After migration, the sclerotome reorganizes during resegmentation so that cells in the caudal boundary near von Ebner’s fissure will end up in between the VB as the AF. This area has been termed the arthrotome. The importance of the somitocoel in IVD formation was shown in the chick model by surgically removing the somitocoel and replacing the space with an inert bead (Mittapalli et al., 2005). This resulted in about half of the operated embryos lacking IVDs and the fusion of adjacent articular processes. Studies have shown that although somitocoel cells can contribute to the AF, they are not committed to AF differentiation and can be driven towards another cell fate if moved to a different environment (Senthinathan et al., 2012).
The developing AF is further differentiated into two compartments, the inner AF (iAF) and outer AF (oAF) (Figure 1). iAF is fibrocartilage that exhibits characteristics of both fibrous tissues and cartilage. oAF is more fibrous and resembles ligament and tendon (van den Akker et al., 2017). Pax1/9 expression becomes enriched in the caudal region of the sclerotome where the AF will form, even though expression is initially throughout the entire sclerotome and is required for the initiation of chondrogenesis. Expression is eventually solely restricted to the developing AF and marks the boundary between the IVD and developing VB by E12.5 in mice (Sivakamasundari et al., 2017). This restricted expression pattern may be due to a feed-back loop in which Pax1/9 induced expression of Sox proteins, which in turn inhibit further expression of Pax1(Sivakamasundari et al., 2017). The transcriptome of Pax1/9-GFP cells was recently characterized using a GFP tag to isolate and sort fluorescent cells. Pax1/9-GFP expressing cells were enriched for genes involved in salient AF developmental processes, such as cell proliferation, condensation, and collagen fibrillogenesis, and had reduced expression of genes associated with cartilage development when compared to GFP-negative controls. Pax1/9 were also shown to promote both iAF and oAF expression profiles in differentiating cells; however, Pax1/9 in combination with TGFß and BMP was primarily involved in promoting iAF (Sivakamasundari et al., 2017; Sohn et al., 2010). Additionally, Pax1/9 was recently shown to regulate a long non-coding RNA, Klhl14, during IVD development. In Pax1/9 knock out mice, Klhl14 was the most down regulated transcript in the vertebral column and seemed to be regulated by Pax1/9, Sox6/9 and Nxk.2, specifically in the iAF. These data suggest a potential role for Klhl14 in AF development that has yet to be elucidated (Kraus et al., 2018).
The cells in the developing AF are fibroblast-like and elongate to organize into concentric circles around the developing NP (Hayes and Ralphs, 2011; Peacock, 1951; Rufai et al., 1995). This orientation is caused by the organization of an intracellular network of actin stress fibers and is thought to provide a template for the deposition of collagen, the protein responsible for the trademark lamellar structure of the AF (Hayes et al., 1999; Hayes et al., 2011a; Hayes et al., 2011b). Fibromodulin is strongly expressed in the AF relative to the vertebral cartilage and plays an important role in collagen cross-linking, packing, and fibril diameter (Hayes and Ralphs, 2011; Kalamajski et al., 2016; Smits and Lefebvre, 2003; Sohn et al., 2010). Recent work suggests heparan sulfate proteoglycans (HSPG) may be important in the formation of lamellar structure in the AF as well. A recent study showed that Syndecan-4, a co-receptor for integrin signaling in formation of focal adhesion complexes, is localized to the lamellar structures in the oAF of rats (Beckett et al., 2015; Choi et al., 2011). Syndecan 4 is expressed very early on the cell surface of developing AF cells and becomes restricted to the oAF as the AF begins to organize. The co-receptor has an expression pattern very similar to that of Fibronectin, an extracellular matrix component known to be critical for the initiation of lamellar structures. This suggests a potential role for Syndecan 4 in promoting lamellar structure in AF as well.
TGFß signaling is an important regulator of spinal column development and is required for proper development of the AF. Mice with targeted deletion of the Tgfbr2 gene in the sclerotome manifested severe defects in the development of the AF, which were reduced or completely missing (Baffi et al., 2006; Baffi et al., 2004). The annulus was most affected with the expression of Fibromodulin highly reduced or altogether missing. Vertebral markers were ectopically expressed in the presumptive AF and peanut agglutinin, which normally only stains the presumptive VB, stained the length of the developing spine. Microarray analysis comparing wild-type and Tgfbr2 mutant IVDs showed that the AF from Tgfbr2 mutant mice resembled wild-type VB more so than AF (Sohn et al., 2010). Additionally, a significant number of BMP and TGFß regulated genes are also regulated by Pax1/9, suggesting that Pax1/9 cooperates with BMP and TGFß signaling pathways during axial skeleton development (Sivakamasundari et al., 2017).
A balance between TGFβ and BMP signaling is also important in maintaining cell fate and maintenance of postnatal AF. A study showed that Filamin B knock out mice demonstrate an increase in both canonical and non-canonical TGFβ signaling, as measured by increased phosphorylated Smad2/3, activated ERK and increase in target genes CTGF and P21. Similarly, both canonical and non-canonical BMP signaling were increased as measured by phosphorylated Smad1/5, activation of P38, and an increase in the target gene Msx2 (Zieba et al., 2016). A recent study showed that mice with disrupted Tgfbr2 signaling, whether through dominant-negative interference of TGFß signaling or deletion of Tgfbr2 in Aggrecan Cre expressing cells, cannot maintain postnatal AF (Alkhatib et al. 2018). Filamin B knock out mice also display Spondylocarpotarsal Synostosis Syndrome (OMIM: 272460), characterized by vertebral fusion, lordosis, and scoliosis (Mitter et al., 2008). The AF cells showed characteristic hyaline chondrocyte morphology and began to overtake the NP space. Collagen deposition was disrupted, and Collagen type II expression was inappropriately localized in the oAF, which normally expresses Collagen type I, a more fibrous collagen. The oAF also expressed the hypertrophic marker Collagen type X, indicating inappropriate endochondral bone formation. Taken together the data suggest that Filamin B is important to maintain cell types of the AF in the postnatal IVD (Mitter et al., 2008; Zieba et al., 2016).
Scleraxis (Scx) is a transcription factor that is required for tendon formation but has been shown through lineage tracing of Scx positive cells to be localized in both the developing and mature oAF (Sugimoto et al., 2013; Yoshimoto et al., 2017). However, Scx does not appear to be required for formation of the AF since IVD is formed in Scx knock out mice (Murchison et al., 2007; Schweitzer et al., 2001). When Sox9, an initiator of chondrogenesis, is deleted in Scx positive cells, mice demonstrate an expanded oAF and a decreased iAF size (Blitz et al., 2013; Sugimoto et al., 2013). The results indicate an important relationship between Sox 9 and Scx positive cells in the division between iAF and oAF. These data also suggest that although Scx may not be required for development of the AF, the Scx positive population of cells does contribute to its formation. Another tendon-associated transcription factor, Mohawk (Mkx), is involved in AF development (Nakamichi et al., 2016). A recent study showed that Mkx is expressed in the oAF in embryonic and adult stages in mice and has a role in collagen fiber formation in oAF by affecting expression of collagens and small leucine-rich proteoglycans (SLRPs). Collagens and SLRPS have an established, essential role in forming the lamellar structure in oAF (Aszodi et al., 1998; Furukawa et al., 2009). Mkx has also been shown to promote regeneration of oAF when expressed in mesenchymal cells from degenerated IVD, thus suggesting Mkx is a critical regulator of cell fate in mesenchymal cells (Nakamichi et al., 2016). Together the results indicate a close relationship between AF and tendon/ligament differentiation. A list of regulators of AF differentiation is provided in Table 4.
Tendon/ligament
Axial tendons develop from the syndetome, a compartment of the sclerotome (Brent et al., 2003). Since tendons link the muscle to the vertebrae, localization during development is critical. The epithelial dermomyotome is formed from the most dorsal part of the somite and the sclerotome forms the ventral and medial parts. The myotome is immediately dorsal and adjacent to the sclerotome. It expresses master regulators of muscle development MyoD and Myf5 and secretes fibroblast growth factors (FGF) 4 and 6. Receptors for FGF are located on the sclerotome cells and FGF signaling stimulates expression of Scx, inducing their differentiation to tendon cells. The ventral side of the sclerotome sees high levels of Shh from the notochord, which prevents tendon formation and promotes formation of the vertebral bodies. Thus, the tendon forms in between the vertebra and muscle (Brent et al., 2005; Brent et al., 2003). More recently it was shown that TGF-ß is also an important regulator of tendon development and maintenance. Conditional deletion of Tgfbr2 or deletion of both Tgfb1 and Tgfb3 ligands in mice results in loss of most tendons (Pryce et al., 2009). Other signals that regulate formation of tendons and ligaments are now beginning to be elucidated (Huang et al., 2015)
Development of the Nucleus Pulposus from Notochord.
Formation and Function of the Notochord
The embryonic notochord is a rod-like structure that forms at the midline of vertebrates (Beddington, 1994; Salisbury, 1993; Sulik et al., 1994). It is a transient structure that serves as a preliminary axial skeleton and a signaling center during development (Salisbury, 1993). The notochord gives rise to the nucleus pulposus (NP) of the intervertebral disc (IVD). During mouse development, the dorsal organizer gives rise to the notochord during gastrulation at around E6 (Stemple, 2005). Subsequent to the dorsal organizer giving rise to the chordamesoderm, cells undergo convergent extension and migrate towards the midline to lengthen the presumptive notochord (E8.5 to E10.5 in mice) (Stemple, 2005). Chordamesodermal cells then acquire a thick sheath and become vacuolated (Stemple, 2005). Osmotic pressure acts against the notochoral sheath to give the notochord its final rod-like appearance (Stemple, 2005).
A recent study attempted to decipher the notochord’s role in vertebral column segmentation (Ward et al., 2018). In chick embryos, ablation of the notochord caused loss of segmentation of the vertebral bodies (VB) and IVDs. However, without the surrounding sclerotome, the notochord was not segmented. To examine whether the notochord dictates the segmentation of the sclerotome, ectopic notochords were grafted into chick embryos. The data indicated that intrinsic segmentation of the sclerotome is dominant over any segmental information that the notochord may possess. No evidence was found to suggest that the chick notochord was intrinsically segmented. Therefore, it was concluded that the segmental pattern of the VBs and IVDs in the chick is dictated by the sclerotome. Furthermore, the sclerotome must first signal to the notochord to ensure that the NP develops in register with the annulus fibrosus (AF), and the notochord is required for the maintenance of sclerotome segmentation and formation of mature VBs and IVDs.
Mechanical forces are known to play a role in the formation of the notochord and are hypothesized to also play a role in the notochord-to-NP transition. A recent study asserted that mechanical forces, rather than the alignment of the notochordal sheath, define the collagen architecture of the IVDs (Ghazanfari et al., 2018). This study compared the development of the mammal and avian IVDs. The authors postulate that upon bulging of the notochord to become the NP in mammals, a bi-axial strain is applied on the sclerotome between condensations which induces the criss-cross aligned structure of the collagen fibers within the AF. However, in birds, notochord bulging does not occur and the notochord is not squeezed (birds do not form an NP); therefore, the notochord persists and collagen fibers are concentrically organized.
Aside from mechanical forces, the matrix environment of a tissue can greatly impact cell behavior and differentiation. Cells have the ability to take cues from their environment and adapt to create a functional tissue during development and maintain that tissue into adulthood. A recent study investigated whether naïve stem cells (human induced pluripotent stem cells, hiPSCs) could differentiate into notochordal cells through direct contact with porcine NP matrix (Liu et al., 2014). Contact and non-contact cultures both yielded functional notochordal-like cells; however, contact cultures had a significantly higher differentiation yield. Generated notochordal-like cells were highly homogenous in their expression of notochordal marker genes from both contact and non-contact cultures. Culture medium containing FGF, EGF, VEGF, and IGF-1 further supported notochordal-like cell differentiation in the presence of porcine NP matrix. Notochordal-like cells from these cultures produced aggrecan and collagen type II and had a proteoglycan-to-collagen ratio that was similar to native NP. Overall, native NP matrix could be used to differentiate hiPSCs to functional notochordal-like cells that could be implanted to treat disc degeneration.
Gene mutations have identified molecular components critical to the development and maintenance of the notochord. Of these, the T gene (brachyury), Danforth’s short-tail (Sd), Sickle tail gene (Skt), Sonic hedgehog (Shh), Sox5/6, and collagen II mutations are among the best characterized. The T gene has been identified as a notochordal marker and its expression is restricted and maintained within the notochord (Kispert et al., 1994). Homozygous mutations in the T gene in mice resulted in failure to establish the trunk notochord. Further mutations in the spine and allantois led to early embryonic lethality (Dobrovolskaia-Zavadskaia, 1927). Mice with a dominant-negative mutation in the T gene survived but demonstrated abnormal NP morphology (Stott et al., 1993).
While notochord formation is a vital first step, notochord maintenance is also important and is affected by Danforth’s short-tail (Sd) mutation. The gene in which this mutation occurs is still unidentified. Sd mice with a homozygous mutation had a discontinuous, fragmented notochord (Paavola et al., 1980). The notochord eventually disappeared and NP morphology was severely affected. In mice with a heterozygous mutation, the NP was absent and was replaced by fibrous tissue resembling the annulus fibrosus (AF) (Semba et al., 2006). Sd is located on the same chromosome as the Sickle tail gene (Skt). In mice with mutations in the Skt gene, the NP did form but its position was shifted to the periphery of the disc (Semba et al., 2006). Furthermore, the boundary between the NP and AF was altered. AF collagen layers were also thin compared to wild-type mice. Polymorphisms in the SKT gene have been identified in Japanese and Finnish populations and are associated with lumbar disc herniation (Karasugi et al., 2009, OMIM#617367).
A Sktcre mouse has been generated. Cre expression from the Sktcre allele was used to activate β-galactosidase and expression in the notochord from E9.5 onwards was characterized. (Abe et al., 2012). When crossed to lacZ reporter (R26R) mice, reporter activity was detected at E15.5 in the NP and a portion of the AF. Mice from this study could be used to fate map notochordal cells or to develop mouse models for disc degeneration.
Notochord Sheath
The notochordal sheath is composed of extracellular matrix (ECM) proteins such as collagens and proteoglycans (Gotz et al., 1995). It functions to contain hydrostatic pressure within the notochord (Adams et al., 1990). Previous studies have demonstrated that mutations in genes that encode proteins within the notochordal sheath lead to aberrant development and maintenance of the notochord and the prospective NP. Sox 5/6 and Shh are both expressed in the notochord while collagen II is expressed in the notochordal sheath. Sox 5/6-null mice form a rod-like notochord but it fails to be surrounded by a sheath (Smits and Lefebvre, 2003). Extracellular matrix protein genes are downregulated in notochordal cells and eventually the notochord is dismantled. This leads to formation of an irregular NP tissue. Shh is also expressed in the notochord and postnatally in the NP (Dahia et al., 2009; DiPaola et al., 2005). Similarly, Shh-null mice form a notochord but it is not maintained (Chiang et al., 1996). Early lethality of these mice precluded further study of the NP. However, when Smoothened (Smo) is conditionally deleted in Shh-expressing cells, the notochordal sheath is missing and the NP does not expand into the IVD region (Choi and Harfe, 2011). Notochordal cells are scattered throughout the vertebral column. Deletion of Smo after formation of the notochordal sheath does not affect the formation of the NP. Disruption of the ECM components of the sheath can also lead to notochord and NP aberrations. Collagen II is a major structural component of the notochordal sheath (Gotz et al., 1995; Swiderski and Solursh, 1992). In Col2a1-null mice, the notochord is not removed from the vertebral bodies (VBs) and the IVDs do not form (Aszodi et al., 1998). Presumably, structural weakening of the notochordal sheath leads to loss of the ability to contain osmotic pressure (Adams et al., 1990). As a result, the notochord is not removed from the VBs and does not expand in to the IVD and the NP does not form. Together, these results demonstrate an important role for the notochordal sheath in the development and maintenance of the notochord and eventually formation of the NP.
Identification of Notochordal and NP Markers
In order to more clearly distinguish between notochordal and NP cells, various studies have employed advanced techniques to identify marker genes for both populations. One aim is to confirm whether NP cells are in fact derived solely from the notochordal cell population. Another impetus for studies of this nature is the desire to use this knowledge to develop gene therapies to regenerate diseased NP tissue. Previously, Sonic hedgehog (Shh) was investigated in an attempt to fate map notochordal cells and discern whether NP cells are indeed derived from the notochord. In mice, Shh is highly expressed in notochordal cells and later in NP cells into adulthood. Inducible-Cre mice with a promoter under the control of Shh were utilized to track notochordal cells as they transition to NP cells (Choi et al., 2008). Cre recombination was activated (β-galactosidase staining indicated activation of the ROSA26-lacZ locus) at E8.0 in mice and IVD tissue was examined at E13.5 and 19 months. At both ages, all NP cells were labeled with the reporter and no AF cells were labeled. A subsequent study attempted to corroborate these results by studying a different marker for notochordal cells (McCann et al., 2012). Noto is a highly conserved transcription factor with restricted expression limited to the node and early notochord. Reporter activity, as judged by activation of the ROSA26-lacZ locus by the Noto-cre promoter, was observed in all mouse NP cells even into adulthood. The results of these studies indicate that NP cells are derived from notochordal cells in mice (Figure 1). A list of genes and signaling molecules involved in notochord and NP development is provided in Table 5.
Table 5:
Genes and Proteins Involved in Nucleus Pulposus development
| Brachyury | T | Transcription Factor | N/A |
| Collagen II Type 1A | Col2A1 | Protein | Diastrophic dysplasia, OMIM#222600 |
| CYR61, CTGF, and NOV | CCN | Protein | Childhood Progressive Pseudorheumatoid Arthropathy, OMIM#208230 |
| Forkhead Box A | FOXA | Transcription Factor | N/A |
| Forkhead Box O | FOXO | Transcription Factor | N/A |
| Hypoxia Inducible Factor 1 Subunit Alpha | HIF-1α | Transcription Factor | N/A |
| Sickle Tail Gene | Skt | Protein | Lumbar Disc Disease, OMIM#617367 |
| Smoothened | Smo | G Protein-Coupled Receptor | Curry-Jones Syndrome, OMIM#601707 |
| Sonic Hedgehog | Shh | Secreted Ligand | Brachydactyly Type A1, OMIM#112500 |
| Sox 5 | Sox 5 | Transcription Factor | Multiple synostoses syndrome, OMIM#186500 |
Another group of extensive studies is focused on identifying specific marker genes for both notochordal and NP cells. Previous work has harnessed the power of the microarray technique to determine gene expression profiles of NP, AF and articular cartilage cells from various species, including human, bovine, canine, and rodent cells (Lee et al., 2007; Minogue et al., 2010; Sakai et al., 2009). Cumulatively, the T (Brachyury) gene, K8, K18, and K19 were all identified to be more highly expressed in NP than in AF cells or in articular cartilage. The data from these studies suggest that certain marker genes can clearly distinguish NP cells from other cartilaginous and fibrocartilaginous cell types. More recent studies, however, have focused on delineating specific markers for notochordal and NP cells with the aim of further understanding the developmental mechanisms and open the door for therapeutic strategies. Rodrigues-Pinto et al. performed a unique, detailed transcriptomic profiling of human embryonic and fetal spine cells (Rodrigues-Pinto et al., 2018). CD24-positive notochordal cells were isolated using FACS and microarray analysis along with qPCR validation and utilized for identification of specific marker genes. CD24, STMN2, RTN1, PRPH, CXCL12, IGF1, MAP1B, ISL1, CLDN1, and THBS2 were all identified as notochord-specific marker genes. Expression of these genes was confirmed in NP cells from aging and degenerated IVDs. Furthermore, Ingenuity pathway analysis (analysis tool that can identify new targets or candidate biomarkers within RNA-seq or microarray data) demonstrated that genes encoding molecules involved in inhibition of vascularization (WISP2, NOGGIN, EDN2) and inflammation (IL1-RN) were master regulators of notochordal genes. Altogether, the results confirmed data from previous studies and established putative markers for the notochord and NP. However, effective markers for a specific cell type must be continually expressed even through tissue changes in adulthood. Another study performed by Richardson et al. examined NP tissue at various ages and stages of degeneration to determine whether notochordal markers continue to be expressed (Richardson et al., 2017). Gene expression and immunohistochemistry data from 116 individual tissue samples indicated that expression of the NP markers FOXF1, PAX1, KRT8/18, CA12 and notochordal markers TBXT, LGALS3, and CD24 are maintained in NP cells irrespective of age or stage of degeneration.
Determining useful markers is only one step towards the understanding of development and development of therapeutics. Profiling gene expression at different stages of development leading up to the formation of the NP is also important. To this end, a distinctive study was performed in mice to identify genes that are critical in the notochord-to-NP transition (Peck et al., 2017). Notochordal cells were sorted from Shh-cre;ROSA:YFP mice at E12.5 and P0. Shh is specifically expressed in the developing notochord. Expression of Sonic hedgehog signaling molecules along with Wnt pathway components were significantly reduced at P0 compared to E12.5. Conversely, TGF-β pathway components along with IGF pathway components were significantly increased at P0 compared to E12.5. As expected, mRNA of several ECM components was increased at P0 (Acan, Bcan, Bgn, Dcn, Col1a1 and Col6a1) when compared to E12.5. NP marker genes (Tbxt, Krt8/18, Hif1α) were comparable at both E12.5 and P0. These results indicate that signaling and biosynthesis of notochordal-derived cells can change depending on the stage of development. This could provide insights into the developmental process and identify gene targets for therapeutics.
Notochord-to-Nucleus Pulposus Transition
Much debate has surrounded the process of how the notochord transitions to the NP. At this point, two central models exist, the ‘pressure’ model and the ‘repulsion/attraction’ model (Lawson and Harfe, 2015). In the ‘pressure model’ mouse histological sections revealed that no major cell death occurred in the region where the prospective vertebrae form. Furthermore, cell proliferation was not observed in the region where the prospective NP is formed. Therefore, it was hypothesized that notochordal cells were ‘squeezed into’ or ‘pushed into’ the regions where the IVDs are forming. The ‘repulsion/attraction’ model suggests that cell movement is dictated by repulsive or attractant molecules. It is hypothesized that attractant molecules lure notochordal cells to aggregate within the region where the prospective IVD will form. Conversely, it is also possible that repulsive molecules within the prospective vertebrae regions exclude notochordal cells in these regions.
Regardless of the mechanism underlying the transition from notochord to NP, several mouse models have been used to investigate genes that are important in the formation of the NP. Foxa genes are required for embryonic development and postnatal life in mice. Foxa1 and Foxa2 are expressed in the notochord (Besnard et al., 2004; Kaestner et al., 1994; Monaghan et al., 1993; Sasaki and Hogan, 1993). One study performed by Maier et al. used a double knockout model to investigate whether Foxa1 and Foxa2 genes are required for the formation of the IVDs (Maier et al., 2013). On a Foxa1 null background, Foxa2 was conditionally knocked out in Shh-expressing cells and these were termed double knockouts (dKOs). Massive cell death was observed at E11.5 in the posterior somites and midline of the tail in dKO mice. At E19.5, NPs of dKO mice were compressed and small. IVD defects were more severe posteriorly than anteriorly and dKOs had shorter tails that lacked IVD and VB structures. The notochordal sheath and the notochord-toNP transition were both abnormal in dKO mice. The dKO notochordal sheath was very faint at E11.5 compared to the control when visualized using alcian blue and fast green staining. β-galactosidase treatment of dKO mice that contained the R26R allele revealed that notochordal cells were dispersed throughout the vertebral column.
Expression of genes involved in notochordal development and formation of the IVDs were affected by the knockout of the Foxa genes (Maier et al., 2013). Evidence from a Shh enhancer element suggests that FOXA proteins can regulate Shh within the notochord. Shh expression and signaling was decreased in the notochord of dKO tails and floorplates. In contrast, expression of Pax1 and Tbx18 within the sclerotome of dKO mice was indistinguishable from control mice. This indicated that Foxa1 and Foxa2 expression within the notochord are not required for the formation of the sclerotome. Dorsal-ventral patterning of the neural tube, however, was greatly affected in dKO mice. Expression of markers such as Nkx6.1, Nkx2.2, and Pax3 was irregular and indicated that Foxa expression within the notochord is critical for dorsal-ventral patterning of the neural tube. Altogether, the results suggest that Foxa genes are critical for the development and formation of the IVDs.
Shh is secreted from the notochord and NP and is required for the formation and post-natal maintenance of the IVD (Choi and Harfe, 2011; Dahia et al., 2012). A recent study took an interesting approach to further confirm the importance of Shh in the development of the NP. The sacrum in vertebrates is formed by collapse of the IVDs between the vertebrae and their fusion into a single bone. Bonavita et al. demonstrated that, in mice, the collapse of these sacral discs is associated with a down-regulation of Shh signaling in the NP (Bonavita et al., 2018). Furthermore, experimental postnatal reactivation of Shh signaling in dormant NP cells can reactivate sacral discs of the mouse spine. This evidence suggests that loss of Shh signaling is pathological in one region of the spine but part of normal turnover in another.
The CCN family of secreted matricellular proteins serves as multifunctional signaling mediator that regulates interactions between cells, growth factors and the ECM (Perbal, 2004). Mouse knockout models have demonstrated that these proteins are involved in angiogenesis, embryonic cartilage and bone formation, as well as inflammation and fibrosis in adults (Brigstock, 2003; Moussad and Brigstock, 2000). CCN2 has been demonstrated to be expressed in the embryonic node and notochord (Tamplin et al., 2011). In the IVD, CCN2 was first discovered as an anabolic factor secreted by notochordal cells that induces NP cell proliferation and aggrecan production in vitro (Aguiar et al., 1999; Erwin et al., 2006; Erwin and Inman, 2006). In a recent study, Bedore et al. conditionally knocked out CCN2 to study the effects on the mouse IVD at various timepoints (Bedore et al., 2013). Expression of the Cre recombinase was under the control of a notochord-specific promoter (Notocre) which allowed for conditional deletion of CCN2 in notochordal cells. Notochord segmentation and IVD patterning was not affected by the conditional CCN2 knockout. At E15.5, CCN3 expression was increased in the prospective NP; however, aggrecan and collagen II expression was decreased. ECM perturbations were more pronounced in P1 mice. Levels of aggrecan and collagen II were significantly decreased and collagen I expression was increased. No appreciable differences were observed between conditional knockout mice and wild-type littermates at P28. However, at 12 months of age, conditional knockout IVDs had lost distinct NP-AF boundaries. The cellularity of the NP had significantly decreased and the IVD structure was disorganized. This was accompanied by a decrease in aggrecan levels within the NP and decreased signal intensity on a T2/T1-weighted MRI. Conditional knockout mice at 17 months of age had more pronounced intervertebral disc degeneration. IVDs were herniated and osteophytes had formed within the IVD tissue. These IVDs also had low signal intensities on T2/T1-weighted MRI scans. Overall, these results demonstrated that CCN2 expression in the notochord is essential for the regulation of IVD development and age-associated maintenance of the NP.
Animal modeling has provided valuable information pertaining to the developmental process of the IVD. Nevertheless, recent in vitro experimentation has yielded vital results in the investigation of the transition process from notochord to NP. Many of these studies, with proper follow-ups, could have significant clinical implications. One such study investigated whether mechanical load would induce maturation of notochordal cell (NC)-rich porcine NP tissue (6 to 8-week old) (Purmessur et al., 2013). NC-rich NP tissue was loaded with hydrostatic pressure (0.5 to 2 MPa at 0.1 Hz for 2 hours) and analyzed after eight days. Three conditions were examined: Control (no pressurization), one single dose of loading, and daily loading. The percentage of cells that had transitioned from large notochordal cells to small NP-like cells was higher in daily (73.8%) and single dose (44%) than in control (28%) cultures. No cell death was observed in any of the conditions. Furthermore, loading increased metabolic activity and levels of safranin-O stained matrix, indicative of maturation of loaded tissues. These results suggested that mechanical loading is an integral component of the notochord transition to NP.
Although the study of notochordal cells and their transition to NP cells is essential for understanding the processes of aging and degeneration, regenerative medicine requires more translational ideas and methodologies. Currently, there is no consistent, high-quality source of notochordal cells that can be used for implantation to regenerate NP tissue. For this reason, the study of stem cell differentiation to NP-like cells has become paramount for the treatment of IVD degeneration. One study isolated induced pluripotent stem cells (iPSCs) from mice and sorted them using magnetic activated cell sorting (MACS) to further isolate a CD24+ iPSC subpopulation (Chen et al., 2013). CD24+ sorted cells exhibited an increase in expression of notochordal gene markers (Noto, Shh, Foxa2, and T (brachyury)) when compared with presorted cells. CD24+ iPSCs were cultured on a laminin-rich culture system for 28 days and the phenotype of the cells was assessed using various biochemical methods. Post-culture, CD24+ iPSCs had formed three-dimensional cell clusters that were rich in sulfated glycosaminoglycans (sGAG) and type II collagen. The cells also expressed integrin α6, vimentin and cytokeratin 5/8, markers of the immature NP cell phenotype. CD24- cells were also cultured in the laminin-rich system but under different conditions to mimic in situ oxygen tension. Cells were cultured in 2% O2 and in media containing factors secreted by notochordal cell-containing NP tissue (NCCM). The results were similar to those observed with CD24+ iPSC cultures. A follow-up study attempted to differentiate human iPSCs using a novel stepwise protocol (Tang et al., 2018). When colonies of human iPSCs were stimulated with BMP4/GFG2 and Wnt-3a/Activin A, the cells were found to express early mesodermal and notochordal markers. Human iPSCs were also cultured in monolayers with the addition of GDF-5 and in pellet cultures with TGF-β3 and L-Proline to stimulate matrix synthesis. This protocol eventually led to the formation of larger, proteoglycan-containing pellets and the vacuole-structure characteristic of NP-like cells. These results demonstrated that iPSCs could be used for cellular therapy to treat IVD degeneration.
Subsequent to the notochord transitioning to the NP, much debate has surrounded the true identity of the adult NP cell. It is thought that the NP cell population is a heterogenous one that consists of cells which are more prone to either anabolic or catabolic stimulation. A recent study generated 54 immortal clones of non-degenerate healthy human NP cells (van den Akker et al., 2014). Subclones were profiled using a novel set of NP markers (CD24, CA12, PAX1, PTN, FOXF1, and KRT19 mRNA) and expression of these markers confirmed their NP origin. Two predominant clones were identified based on their ability to induce SOX9 and COL2A1 in a Matrigel hydrogel culture system. In a follow-up study, clones that were able to induce SOX9 and COL2A1 were termed responders (NP-R) and those that could not were termed non-responders (NP-nR) (van den Akker et al., 2016). The authors hypothesized that NP-R cells could represent undifferentiated cells and that NP-nR cells are differentiated cells. When challenged with catabolic stimuli, NP-nR clones were more responsive (higher expression of catabolic genes) than NP-R clones. These results support the hypothesis that the adult NP population is not homogenous and different subpopulations could be more susceptible to catabolic stimuli leading to IVD degeneration.
Maintenance of the Nucleus Pulposus
Recent studies have begun to investigate genes that are critical for the maintenance of NP tissue. Mouse modeling has provided the most evidence for genes required for homeostasis of the NP. FOXO proteins are transcription factors that are primarily involved in development, aging, and longevity (Kahn, 2015; Martins et al., 2016). One study investigated the effects of conditional deletion of all Foxo isoforms (1, 3, and 4) using either the Col2a1 promoter (Col2a1cre, germ-line deletion) or the aggrecan promoter (AcancreER, deletion after skeletal maturity) to drive cre- or tamoxifen-inducible cre-dependent recombination (Alvarez-Garcia et al., 2018). In 6-month old Col2a1cre-FOXO KO mice, a significant loss of cellularity was observed in the NP and cartilaginous endplate (EP). Furthermore, the boundary between the NP and AF was disrupted and AF lamellae were disorganized. The presence of hypertrophic cells was observed in the inner AF. FOXO deficiency led to severe spinal deformities with abnormal curvature of the spine and kyphosis in 6-month old mice. To study the role of FOXO proteins in the maintenance of the mature IVD, four-month old AcancreER-FOXO KO mice were injected with tamoxifen and vertebral column tissue was analyzed at 12 months of age. AcancreER-FOXO KO mice had a significant reduction in NP and EP cellularity and disc height at 12 months of age. NP cell clustering, loss of NP/AF boundary demarcation, and disorganization of AF lamellae were all observed in AcancreER-FOXO KO mice. GStudies of gene expression in both mouse models revealed that FOXO-deficient mice had impaired autophagy and reduced antioxidant defense. Human primary NP cells were also studied in vitro to further discern the function of FOXO proteins by gene expression analysis. FOXO directly regulates autophagy, adaptation to hypoxia, and resistance to oxidative and inflammatory stress in human primary NP cells. Overall, FOXO proteins are involved in both the maturation and maintenance of the NP.
Another study investigated the role of HIF-1α in the development and maintenance of the NP (Merceron et al., 2014). Notochordal cells were targeted using a Foxa2-cre line and HIF-1α was conditionally deleted in these cells. HIF-1α is stabilized within the prospective NP and is used as an adaptive response protein under a variety of stresses, including low oxygen tension within the NP. Notochord development was not affected in this study. However, at E15.5, mutant NPs appeared smaller than control NPs and lacked large vacuoles indicative of residual notochordal cells. By 1 month, NPs had completely disappeared in mutants and were replaced by fibrocartilaginous tissue that stained intensely with safranin-O. Lineage studies revealed that NP cells did not transdifferentiate into chondrocyte-like cells. Alternatively, NP cells underwent massive cell death as confirmed by TUNEL assay. Mutant IVDs were functionally inferior and had an impaired ability to absorb axial energy and distribute it. This study identified HIF-1α as a critical protein in the development and maintenance of the NP.
Conclusions and implications
Understanding how the axial skeleton develops will provide the basis for future repair, regeneration, and tissue engineering strategies for spine disease. In addition, defining signals involved in the embryonic development of the axial skeleton provides insights into mechanisms of spinal pathology. Using what is known about the normal developmental processes to generate new tissues in vitro has been termed developmental engineering (Gadjanski et al., 2012; Lenas et al., 2011; Lenas et al., 2009a, b). The focus on developmental mechanisms will provide a rational step-wise process for tissue engineering of skeletal tissues. Cartilage and bone organs have already been generated using the principles of developmental engineering (Loh et al., 2016; Oldershaw et al., 2010; Scotti et al., 2010). The spine is obviously more complex than cartilage or bone tissue alone. Nevertheless, mimicking embryonic development is likely to facilitate tissue engineering of complex tissues like the IVD, and difficult problems, such as the integration of the IVD with the surrounding end plates and ligaments, may be solved.
Acknowledgements
The authors would like to thank Ga I Ban for help with text and figure for this chapter.
Spine research in RS laboratory is funded by a grant from the National Institutes of Health, National Institute of Arthritis, Musculoskeletal and Skin Diseases, R01 AR053860. BA was funded through NIH/NIDCR T90 DE022736. SW was funded by T32 AR069516, from NIH/NIAMS.
Abbreviations
- AF
Annulus Fibrosus
- CEP
Cartilage End Plate
- ECM
Extracellular Matrix
- ESC
Embryonic Stem Cell
- hiPSCs
human induced pluripotent stem cells
- iAF
inner Annulus Fibrosus
- IM
Intermediate Mesoderm
- iPSCs
induced pluripotent stem cells
- IVD
Intervertebral Disc
- LPM
Lateral Plate Mesoderm
- MET
Mesenchymal to Epithelial Transition
- MRI
Magnetic resonance imaging
- NCCN
Notochordal Cell Containing NP tissue
- NP
Nucleus Pulposus
- oAF
outer Annulus Fibrosus
- P
Postnatal day
- PM
Paraxial Mesoderm
- PSM
Presomitic Mesoderm
- SLRPs
Small Leucine Rich Proteoglycans
- VB
Vertebral Body
References
- Abe K, Araki K, Tanigawa M, Semba K, Ando T, Sato M, Sakai D, Hiyama A, Mochida J, Yamamura K, 2012. A Cre knock-in mouse line on the Sickle tail locus induces recombination in the notochord and intervertebral disks. Genesis 50, 758–765. [DOI] [PubMed] [Google Scholar]
- Adams DS, Keller R, Koehl MA, 1990. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development 110, 115–130. [DOI] [PubMed] [Google Scholar]
- Adams MA, Roughley PJ, 2006. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 31, 2151–2161. [DOI] [PubMed] [Google Scholar]
- Aguiar DJ, Johnson SL, Oegema TR, 1999. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res 246, 129–137. [DOI] [PubMed] [Google Scholar]
- Alkhatib B, Ban G, Williams S Serra R, 2018. IVD Development: Nucleus Pulposus Development and Sclerotome Specification. Current Molecular Biology Reports 4, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Garcia O, Matsuzaki T, Olmer M, Miyata K, Mokuda S, Sakai D, Masuda K, Asahara H, Lotz MK, 2018. FOXO are required for intervertebral disk homeostasis during aging and their deficiency promotes disk degeneration. Aging Cell 17, e12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D, Nubler-Jung K, 1999. Rearranging gastrulation in the name of yolk: evolution of gastrulation in yolk-rich amniote eggs. Mechanisms of development 81, 3–22. [DOI] [PubMed] [Google Scholar]
- Aszodi A, Chan D, Hunziker E, Bateman JF, Fassler R, 1998. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol 143, 1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulehla A, Pourquie O, 2010. Signaling gradients during paraxial mesoderm development. Cold Spring Harbor perspectives in biology 2, a000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffi MO, Moran MA, Serra R, 2006. Tgfbr2 regulates the maintenance of boundaries in the axial skeleton. Dev Biol 296, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R, 2004. Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol 276, 124–142. [DOI] [PubMed] [Google Scholar]
- Bagnall KM, Higgins SJ, Sanders EJ, 1988. The contribution made by a single somite to the vertebral column: experimental evidence in support of resegmentation using the chick-quail chimaera model. Development (Cambridge, England) 103, 69–85. [DOI] [PubMed] [Google Scholar]
- Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW, 2003. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Current biology : CB 13, 1571–1582. [DOI] [PubMed] [Google Scholar]
- Beckett MC, Ralphs JR, Caterson B, Hayes AJ, 2015. The transmembrane heparan sulphate proteoglycan syndecan-4 is involved in establishment of the lamellar structure of the annulus fibrosus of the intervertebral disc. European cells & materials 30, 69–88; discussion 88. [DOI] [PubMed] [Google Scholar]
- Beddington RS, 1994. Induction of a second neural axis by the mouse node. Development 120, 613–620. [DOI] [PubMed] [Google Scholar]
- Bedore J, Sha W, McCann MR, Liu S, Leask A, Seguin CA, 2013. Impaired intervertebral disc development and premature disc degeneration in mice with notochord-specific deletion of CCN2. Arthritis Rheum 65, 2634–2644. [DOI] [PubMed] [Google Scholar]
- Bellairs R, Curtis AS, Sanders EJ, 1978. Cell adhesiveness and embryonic differentiation. Journal of embryology and experimental morphology 46, 207–213. [PubMed] [Google Scholar]
- Besnard V, Wert SE, Hull WM, Whitsett JA, 2004. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns 5, 193–208. [DOI] [PubMed] [Google Scholar]
- Blitz E, Sharir A, Akiyama H, Zelzer E, 2013. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development (Cambridge, England) 140, 2680–2690. [DOI] [PubMed] [Google Scholar]
- Bonavita R, Vincent K, Pinelli R, Dahia CL, 2018. Formation of the sacrum requires down-regulation of sonic hedgehog signaling in the sacral intervertebral discs. Biol Open 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borycki AG, Mendham L, Emerson CP Jr., 1998. Control of somite patterning by Sonic hedgehog and its downstream signal response genes. Development (Cambridge, England) 125, 777–790. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Christ B, 2000. Evolution and development of distinct cell lineages derived from somites. Current topics in developmental biology 48, 1–42. [DOI] [PubMed] [Google Scholar]
- Brent AE, Braun T, Tabin CJ, 2005. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development 132, 515–528. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ, 2003. A somitic compartment of tendon progenitors. Cell 113, 235–248. [DOI] [PubMed] [Google Scholar]
- Brigstock DR, 2003. The CCN family: a new stimulus package. J Endocrinol 178, 169–175. [DOI] [PubMed] [Google Scholar]
- Bruggeman BJ, Maier JA, Mohiuddin YS, Powers R, Lo Y, Guimaraes-Camboa N, Evans SM, Harfe BD, 2012. Avian intervertebral disc arises from rostral sclerotome and lacks a nucleus pulposus: implications for evolution of the vertebrate disc. Developmental dynamics : an official publication of the American Association of Anatomists 241, 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R, Rawls A, Brown D, Bradley A, Olson EN, 1996. Requirement of the paraxis gene for somite formation and musculoskeletal patterning. Nature 384, 570–573. [DOI] [PubMed] [Google Scholar]
- Candia AF, Watabe T, Hawley SH, Onichtchouk D, Zhang Y, Derynck R, Niehrs C, Cho KW, 1997. Cellular interpretation of multiple TGF-beta signals: intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development (Cambridge, England) 124, 4467–4480. [DOI] [PubMed] [Google Scholar]
- Chen J, Lee EJ, Jing L, Christoforou N, Leong KW, Setton LA, 2013. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PLoS One 8, e75548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA, 1996. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413. [DOI] [PubMed] [Google Scholar]
- Choi KS, Cohn MJ, Harfe BD, 2008. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn 237, 3953–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Harfe BD, 2011. Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc Natl Acad Sci U S A 108, 9484–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Chung H, Jung H, Couchman JR, Oh ES, 2011. Syndecans as cell surface receptors: Unique structure equates with functional diversity. Matrix biology : journal of the International Society for Matrix Biology 30, 93–99. [DOI] [PubMed] [Google Scholar]
- Christ B, Huang R, Scaal M, 2004. Formation and differentiation of the avian sclerotome. Anat Embryol (Berl) 208, 333–350. [DOI] [PubMed] [Google Scholar]
- Christ B, Huang R, Scaal M, 2007. Amniote somite derivatives. Dev Dyn 236, 2382–2396. [DOI] [PubMed] [Google Scholar]
- Christ B, Huang R, Wilting J, 2000. The development of the avian vertebral column. Anat Embryol (Berl) 202, 179–194. [DOI] [PubMed] [Google Scholar]
- Christ B, Ordahl CP, 1995. Early stages of chick somite development. Anatomy and embryology 191, 381–396. [DOI] [PubMed] [Google Scholar]
- Cooke J, Zeeman EC, 1976. A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. Journal of theoretical biology 58, 455–476. [DOI] [PubMed] [Google Scholar]
- Cox MK, Serra R, 2014. Development of the Intervertebral Disc, in: Shapiro IM, Risbud MV (Ed.), The Intervertebral Disc. Springer-Verlag Wien, Heidelberg New York Dordrecht London, pp. 33–53. [Google Scholar]
- Craft AM, Ahmed N, Rockel JS, Baht GS, Alman BA, Kandel RA, Grigoriadis AE, Keller GM, 2013. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development (Cambridge, England) 140, 2597–2610. [DOI] [PubMed] [Google Scholar]
- Crossin KL, Hoffman S, Grumet M, Thiery JP, Edelman GM, 1986. Site-restricted expression of cytotactin during development of the chicken embryo. The Journal of cell biology 102, 1917–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahia CL, Mahoney E, Wylie C, 2012. Shh signaling from the nucleus pulposus is required for the postnatal growth and differentiation of the mouse intervertebral disc. PLoS One 7, e35944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahia CL, Mahoney EJ, Durrani AA, Wylie C, 2009. Intercellular signaling pathways active during intervertebral disc growth, differentiation, and aging. Spine (Phila Pa 1976) 34, 456–462. [DOI] [PubMed] [Google Scholar]
- DiPaola CP, Farmer JC, Manova K, Niswander LA, 2005. Molecular signaling in intervertebral disk development. J Orthop Res 23, 1112–1119. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia-Zavadskaia N, 1927. Sur la mortification spotanee de la chez la souris nouvau-nee et sur l’existence d’un caractere (facteur) herededitaire, non-viable. CR Soc Biol 97, 114–116. [Google Scholar]
- Dockter J, Ordahl CP, 2000. Dorsoventral axis determination in the somite: a re-examination. Development (Cambridge, England) 127, 2201–2206. [DOI] [PubMed] [Google Scholar]
- Dockter JL, 2000. Sclerotome induction and differentiation. Current topics in developmental biology 48, 77–127. [DOI] [PubMed] [Google Scholar]
- Duband JL, Dufour S, Hatta K, Takeichi M, Edelman GM, Thiery JP, 1987. Adhesion molecules during somitogenesis in the avian embryo. The Journal of cell biology 104, 1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin WM, Ashman K, O’Donnel P, Inman RD, 2006. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum 54, 3859–3867. [DOI] [PubMed] [Google Scholar]
- Erwin WM, Inman RD, 2006. Notochord cells regulate intervertebral disc chondrocyte proteoglycan production and cell proliferation. Spine (Phila Pa 1976) 31, 1094–1099. [DOI] [PubMed] [Google Scholar]
- Fan CM, Tessier-Lavigne M, 1994. Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell 79, 1175–1186. [DOI] [PubMed] [Google Scholar]
- Ferrer-Vaquer A, Viotti M, Hadjantonakis AK, 2010. Transitions between epithelial and mesenchymal states and the morphogenesis of the early mouse embryo. Cell adhesion & migration 4, 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Ito K, Nuka S, Hashimoto J, Takei H, Takahara M, Ogino T, Young MF, Shinomura T, 2009. Absence of biglycan accelerates the degenerative process in mouse intervertebral disc. Spine 34, E911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadjanski I, Spiller K, Vunjak-Novakovic G, 2012. Time-dependent processes in stem cell-based tissue engineering of articular cartilage. Stem Cell Rev 8, 863–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfari S, Werner A, Ghazanfari S, Weaver JC, Smit TH, 2018. Morphogenesis of aligned collagen fibers in the annulus fibrosus: Mammals versus avians. Biochem Biophys Res Commun 503, 1168–1173. [DOI] [PubMed] [Google Scholar]
- Glazier JA, Zhang Y, Swat M, Zaitlen B, Schnell S, 2008. Coordinated action of N-CAM, N-cadherin, EphA4, and ephrinB2 translates genetic prepatterns into structure during somitogenesis in chick. Current topics in developmental biology 81, 205–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RS, Kalcheim C, 1992. Determination of epithelial half-somites in skeletal morphogenesis. Development (Cambridge, England) 116, 441–445. [DOI] [PubMed] [Google Scholar]
- Gotz W, Osmers R, Herken R, 1995. Localisation of extracellular matrix components in the embryonic human notochord and axial mesenchyme. J Anat 186 (Pt 1), 111–121. [PMC free article] [PubMed] [Google Scholar]
- Han Y, Lefebvre V, 2008. L-Sox5 and Sox6 drive expression of the aggrecan gene in cartilage by securing binding of Sox9 to a far-upstream enhancer. Molecular and cellular biology 28, 4999–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, Benjamin M, Ralphs JR, 1999. Role of actin stress fibres in the development of the intervertebral disc: cytoskeletal control of extracellular matrix assembly. Developmental dynamics : an official publication of the American Association of Anatomists 215, 179–189. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Hughes CE, Ralphs JR, Caterson B, 2011a. Chondroitin sulphate sulphation motif expression in the ontogeny of the intervertebral disc. European cells & materials 21, 1–14. [PubMed] [Google Scholar]
- Hayes AJ, Isaacs MD, Hughes C, Caterson B, Ralphs JR, 2011b. Collagen fibrillogenesis in the development of the annulus fibrosus of the intervertebral disc. European cells & materials 22, 226–241. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Ralphs JR, 2011. The response of foetal annulus fibrosus cells to growth factors: modulation of matrix synthesis by TGF-beta1 and IGF-1. Histochemistry and cell biology 136, 163–175. [DOI] [PubMed] [Google Scholar]
- Huang AH, Lu HH, Schweitzer R, 2015. Molecular regulation of tendon cell fate during development. J Orthop Res 33, 800–812. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Brand-Saberi B, Christ B, 2000. New experimental evidence for somite resegmentation. Anatomy and embryology 202, 195–200. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Hiemisch H, Luckow B, Schutz G, 1994. The HNF-3 gene family of transcription factors in mice: gene structure, cDNA sequence, and mRNA distribution. Genomics 20, 377–385. [DOI] [PubMed] [Google Scholar]
- Kahn AJ, 2015. FOXO3 and related transcription factors in development, aging, and exceptional longevity. J Gerontol A Biol Sci Med Sci 70, 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamajski S, Bihan D, Bonna A, Rubin K, Farndale RW, 2016. Fibromodulin Interacts with Collagen Cross-linking Sites and Activates Lysyl Oxidase. The Journal of biological chemistry 291, 7951–7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcheim C, Ben-Yair R, 2005. Cell rearrangements during development of the somite and its derivatives. Current opinion in genetics & development 15, 371–380. [DOI] [PubMed] [Google Scholar]
- Karasugi T, Semba K, Hirose Y, Kelempisioti A, Nakajima M, Miyake A, Furuichi T, Kawaguchi Y, Mikami Y, Chiba K, Kamata M, Ozaki K, Takahashi A, Makela P, Karppinen J, Kimura T, Kubo T, Toyama Y, Yamamura K, Mannikko M, Mizuta H, Ikegawa S, 2009. Association of the tag SNPs in the human SKT gene (KIAA1217) with lumbar disc herniation. J Bone Miner Res 24, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura A, Koshida S, Takada S, 2008. Activator-to-repressor conversion of T-box transcription factors by the Ripply family of Groucho/TLE-associated mediators. Molecular and cellular biology 28, 3236–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A, Herrmann BG, Leptin M, Reuter R, 1994. Homologs of the mouse Brachyury gene are involved in the specification of posterior terminal structures in Drosophila, Tribolium, and Locusta. Genes Dev 8, 2137–2150. [DOI] [PubMed] [Google Scholar]
- Kraus P, Sivakamasundari V, Olsen V, Villeneuve V, Hinds A, Lufkin T, 2018. Klhl14 Antisense RNA is a Target of Key Skeletogenic Transcription Factors in the Developing Intervertebral Disc. Spine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuta A, Mao Y, Martin T, Ferreira de Sousa C, Whiting D, Zakaria S, Crespo-Enriquez I, Evans P, Balczerski B, Mankoo B, Irvine KD, Francis-West PH, 2016. Fat4-Dchs1 signalling controls cell proliferation in developing vertebrae. Development (Cambridge, England) 143, 2367–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson L, Harfe BD, 2015. Notochord to Nucleus Pulposus Transition. Curr Osteoporos Rep 13, 336–341. [DOI] [PubMed] [Google Scholar]
- Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, Grad S, 2007. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J 16, 2174–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Behringer RR, de Crombrugghe B, 2001. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis and cartilage 9 Suppl A, S69–75. [DOI] [PubMed] [Google Scholar]
- Leitges M, Neidhardt L, Haenig B, Herrmann BG, Kispert A, 2000. The paired homeobox gene Uncx4.1 specifies pedicles, transverse processes and proximal ribs of the vertebral column. Development (Cambridge, England) 127, 2259–2267. [DOI] [PubMed] [Google Scholar]
- Lenas P, Luyten FP, Doblare M, Nicodemou-Lena E, Lanzara AE, 2011. Modularity in developmental biology and artificial organs: a missing concept in tissue engineering. Artif Organs 35, 656–662. [DOI] [PubMed] [Google Scholar]
- Lenas P, Moos M, Luyten FP, 2009a. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Part B Rev 15, 381–394. [DOI] [PubMed] [Google Scholar]
- Lenas P, Moos M, Luyten FP, 2009b. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part II: from genes to networks: tissue engineering from the viewpoint of systems biology and network science. Tissue Eng Part B Rev 15, 395–422. [DOI] [PubMed] [Google Scholar]
- Li TF, Darowish M, Zuscik MJ, Chen D, Schwarz EM, Rosier RN, Drissi H, O’Keefe RJ, 2006. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 21, 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rahaman MN, Bal BS, 2014. Modulating notochordal differentiation of human induced pluripotent stem cells using natural nucleus pulposus tissue matrix. PLoS One 9, e100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KM, Chen A, Koh PW, Deng TZ, Sinha R, Tsai JM, Barkal AA, Shen KY, Jain R, Morganti RM, Shyh-Chang N, Fernhoff NB, George BM, Wernig G, Salomon REA, Chen Z, Vogel H, Epstein JA, Kundaje A, Talbot WS, Beachy PA, Ang LT, Weissman IL, 2016. Mapping the Pairwise Choices Leading from Pluripotency to Human Bone, Heart, and Other Mesoderm Cell Types. Cell 166, 451–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JA, Lo Y, Harfe BD, 2013. Foxa1 and Foxa2 are required for formation of the intervertebral discs. PLoS One 8, e55528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A, Pla P, Larue L, Gruss P, 2001. Pax3 acts cell autonomously in the neural tube and somites by controlling cell surface properties. Development (Cambridge, England) 128, 1995–2005. [DOI] [PubMed] [Google Scholar]
- Martins R, Lithgow GJ, Link W, 2016. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell 15, 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MR, Tamplin OJ, Rossant J, Seguin CA, 2012. Tracing notochord-derived cells using a Noto-cre mouse: implications for intervertebral disc development. Dis Model Mech 5, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merceron C, Mangiavini L, Robling A, Wilson TL, Giaccia AJ, Shapiro IM, Schipani E, Risbud MV, 2014. Loss of HIF-1alpha in the notochord results in cell death and complete disappearance of the nucleus pulposus. PLoS One 9, e110768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Poh AM, Kelly KA, Ishii Y, Reese DE, 2004. Induction and patterning of the primitive streak, an organizing center of gastrulation in the amniote. Developmental dynamics : an official publication of the American Association of Anatomists 229, 422–432. [DOI] [PubMed] [Google Scholar]
- Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA, 2010. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum 62, 3695–3705. [DOI] [PubMed] [Google Scholar]
- Mittapalli VR, Huang R, Patel K, Christ B, Scaal M, 2005. Arthrotome: a specific joint forming compartment in the avian somite. Developmental dynamics : an official publication of the American Association of Anatomists 234, 48–53. [DOI] [PubMed] [Google Scholar]
- Mitter D, Krakow D, Farrington-Rock C, Meinecke P, 2008. Expanded clinical spectrum of spondylocarpotarsal synostosis syndrome and possible manifestation in a heterozygous father. American journal of medical genetics. Part A 146a, 779–783. [DOI] [PubMed] [Google Scholar]
- Monaghan AP, Kaestner KH, Grau E, Schutz G, 1993. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development 119, 567–578. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, 2005. Sclerotome development and morphogenesis: when experimental embryology meets genetics. The International journal of developmental biology 49, 301–308. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Sasaki N, Oginuma M, Kiso M, Igarashi K, Aizaki K, Kanno J, Saga Y, 2007. The negative regulation of Mesp2 by mouse Ripply2 is required to establish the rostro-caudal patterning within a somite. Development (Cambridge, England) 134, 1561–1569. [DOI] [PubMed] [Google Scholar]
- Moussad EE, Brigstock DR, 2000. Connective tissue growth factor: what’s in a name? Mol Genet Metab 71, 276–292. [DOI] [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R, 2007. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development (Cambridge, England) 134, 2697–2708. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Chyung JH, Lassar AB, 1999. Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes & development 13, 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Zeng L, Chyung JH, Lassar AB, 2001. The chick transcriptional repressor Nkx3.2 acts downstream of Shh to promote BMP-dependent axial chondrogenesis. Developmental cell 1, 411–422. [DOI] [PubMed] [Google Scholar]
- Muthukumar N, 2016. Proatlas segmentation anomalies: Surgical management of five cases and review of the literature. Journal of pediatric neurosciences 11, 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamichi R, Ito Y, Inui M, Onizuka N, Kayama T, Kataoka K, Suzuki H, Mori M, Inagawa M, Ichinose S, Lotz MK, Sakai D, Masuda K, Ozaki T, Asahara H, 2016. Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nature communications 7, 12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubuser A, Koseki H, Balling R, 1995. Characterization and developmental expression of Pax9, a paired-box-containing gene related to Pax1. Dev Biol 170, 701–716. [DOI] [PubMed] [Google Scholar]
- Ohba S, He X, Hojo H, McMahon AP, 2015. Distinct Transcriptional Programs Underlie Sox9 Regulation of the Mammalian Chondrocyte. Cell reports 12, 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE, Kimber SJ, 2010. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol 28, 1187–1194. [DOI] [PubMed] [Google Scholar]
- Paavola LG, Wilson DB, Center EM, 1980. Histochemistry of the developing notochord, perichordal sheath and vertebrae in Danforth’s short-tail (sd) and normal C57BL/6 mice. J Embryol Exp Morphol 55, 227–245. [PubMed] [Google Scholar]
- Peacock A, 1951. Observations on the prenatal development of the intervertebral disc in man. Journal of anatomy 85, 260–274. [PMC free article] [PubMed] [Google Scholar]
- Peck SH, McKee KK, Tobias JW, Malhotra NR, Harfe BD, Smith LJ, 2017. Whole Transcriptome Analysis of Notochord-Derived Cells during Embryonic Formation of the Nucleus Pulposus. Sci Rep 7, 10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B, 2004. CCN proteins: multifunctional signalling regulators. Lancet 363, 62–64. [DOI] [PubMed] [Google Scholar]
- Peters H, Wilm B, Sakai N, Imai K, Maas R, Balling R, 1999. Pax1 and Pax9 synergistically regulate vertebral column development. Development (Cambridge, England) 126, 5399–5408. [DOI] [PubMed] [Google Scholar]
- Pourquie O, 2011. Vertebrate segmentation: from cyclic gene networks to scoliosis. Cell 145, 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R, 2009. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development 136, 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purmessur D, Guterl CC, Cho SK, Cornejo MC, Lam YW, Ballif BA, Laudier JC, Iatridis JC, 2013. Dynamic pressurization induces transition of notochordal cells to a mature phenotype while retaining production of important patterning ligands from development. Arthritis Res Ther 15, R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SM, Ludwinski FE, Gnanalingham KK, Atkinson RA, Freemont AJ, Hoyland JA, 2017. Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human nucleus pulposus cells through aging and degeneration. Sci Rep 7, 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CC, Mulloy B, 2010. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. The Biochemical journal 429, 1–12. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Pinto R, Ward L, Humphreys M, Zeef LAH, Berry A, Hanley KP, Hanley N, Richardson SM, Hoyland JA, 2018. Human notochordal cell transcriptome unveils potential regulators of cell function in the developing intervertebral disc. Sci Rep 8, 12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufai A, Benjamin M, Ralphs JR, 1995. The development of fibrocartilage in the rat intervertebral disc. Anat Embryol (Berl) 192, 53–62. [DOI] [PubMed] [Google Scholar]
- Sakai D, Nakai T, Mochida J, Alini M, Grad S, 2009. Differential phenotype of intervertebral disc cells: microarray and immunohistochemical analysis of canine nucleus pulposus and anulus fibrosus. Spine (Phila Pa 1976) 34, 1448–1456. [DOI] [PubMed] [Google Scholar]
- Salisbury JR, 1993. The pathology of the human notochord. J Pathol 171, 253–255. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hogan BL, 1993. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development 118, 47–59. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ, 2001. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development (Cambridge, England) 128, 3855–3866. [DOI] [PubMed] [Google Scholar]
- Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, Lopez-Rios J, Zeller R, Barbero A, Martin I, 2010. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A 107, 7251–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ, 2005. Comparison of effect of BMP-2, −4, and −6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell and tissue research 320, 269–276. [DOI] [PubMed] [Google Scholar]
- Semba K, Araki K, Li Z, Matsumoto K, Suzuki M, Nakagata N, Takagi K, Takeya M, Yoshinobu K, Araki M, Imai K, Abe K, Yamamura K, 2006. A novel murine gene, Sickle tail, linked to the Danforth’s short tail locus, is required for normal development of the intervertebral disc. Genetics 172, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthinathan B, Sousa C, Tannahill D, Keynes R, 2012. The generation of vertebral segmental patterning in the chick embryo. Journal of anatomy 220, 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakamasundari V, Kraus P, Sun W, Hu X, Lim SL, Prabhakar S, Lufkin T, 2017. A developmental transcriptomic analysis of Pax1 and Pax9 in embryonic intervertebral disc development. Biol Open 6, 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P, Lefebvre V, 2003. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development 130, 1135–1148. [DOI] [PubMed] [Google Scholar]
- Sohn P, Cox M, Chen D, Serra R, 2010. Molecular profiling of the developing mouse axial skeleton: a role for Tgfbr2 in the development of the intervertebral disc. BMC Dev Biol 10, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spittank H, Goehmann U, Hage H, Sacher R, 2016. Persistent proatlas with additional segmentation of the craniovertebral junction - The Tsuang-Goehmann-Malformation. Journal of radiology case reports 10, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford DA, Brunet LJ, Khokha MK, Economides AN, Harland RM, 2011. Cooperative activity of noggin and gremlin 1 in axial skeleton development. Development (Cambridge, England) 138, 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford DA, Monica SD, Harland RM, 2014. Follistatin interacts with Noggin in the development of the axial skeleton. Mechanisms of development 131, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL, 2005. Structure and function of the notochord: an essential organ for chordate development. Development 132, 2503–2512. [DOI] [PubMed] [Google Scholar]
- Stockdale FE, Nikovits W Jr., Christ B, 2000. Molecular and cellular biology of avian somite development. Developmental dynamics : an official publication of the American Association of Anatomists 219, 304–321. [DOI] [PubMed] [Google Scholar]
- Stott D, Kispert A, Herrmann BG, 1993. Rescue of the tail defect of Brachyury mice. Genes Dev 7, 197–203. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Takimoto A, Akiyama H, Kist R, Scherer G, Nakamura T, Hiraki Y, Shukunami C, 2013. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development (Cambridge, England) 140, 2280–2288. [DOI] [PubMed] [Google Scholar]
- Sulik K, Dehart DB, Iangaki T, Carson JL, Vrablic T, Gesteland K, Schoenwolf GC, 1994. Morphogenesis of the murine node and notochordal plate. Dev Dyn 201, 260–278. [DOI] [PubMed] [Google Scholar]
- Swiderski RE, Solursh M, 1992. Localization of type II collagen, long form alpha 1(IX) collagen, and short form alpha 1(IX) collagen transcripts in the developing chick notochord and axial skeleton. Dev Dyn 194, 118–127. [DOI] [PubMed] [Google Scholar]
- Tam PP, Behringer RR, 1997. Mouse gastrulation: the formation of a mammalian body plan. Mechanisms of development 68, 3–25. [DOI] [PubMed] [Google Scholar]
- Tamplin OJ, Cox BJ, Rossant J, 2011. Integrated microarray and ChIP analysis identifies multiple Foxa2 dependent target genes in the notochord. Dev Biol 360, 415–425. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Uhthoff HK, 1981. Significance of resegmentation in the pathogenesis of vertebral body malformation. Acta orthopaedica Scandinavica 52, 331–338. [DOI] [PubMed] [Google Scholar]
- Tang R, Jing L, Willard VP, Wu CL, Guilak F, Chen J, Setton LA, 2018. Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells. Stem Cell Res Ther 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassabehji M, Fang ZM, Hilton EN, McGaughran J, Zhao Z, de Bock CE, Howard E, Malass M, Donnai D, Diwan A, Manson FD, Murrell D, Clarke RA, 2008. Mutations in GDF6 are associated with vertebral segmentation defects in Klippel-Feil syndrome. Human mutation 29, 1017–1027. [DOI] [PubMed] [Google Scholar]
- Theiler K, 1988. Vertebral malformations. Adv Anat Embryol Cell Biol 112, 1–99. [DOI] [PubMed] [Google Scholar]
- Tribioli C, Lufkin T, 1999. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development (Cambridge, England) 126, 5699–5711. [DOI] [PubMed] [Google Scholar]
- Umegaki M, Miyao Y, Sasaki M, Yoshimura K, Iwatsuki K, Yoshimine T, 2017. Report of a familial case of proatlas segmentation abnormality with late clinical onset. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 39, 79–81. [DOI] [PubMed] [Google Scholar]
- van den Akker GG, Surtel DA, Cremers A, Hoes MF, Caron MM, Richardson SM, Rodrigues-Pinto R, van Rhijn LW, Hoyland JA, Welting TJ, Voncken JW, 2016. EGR1 controls divergent cellular responses of distinctive nucleus pulposus cell types. BMC Musculoskelet Disord 17, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker GG, Surtel DA, Cremers A, Rodrigues-Pinto R, Richardson SM, Hoyland JA, van Rhijn LW, Welting TJ, Voncken JW, 2014. Novel immortal human cell lines reveal subpopulations in the nucleus pulposus. Arthritis Res Ther 16, R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker GGH, Koenders MI, van de Loo FAJ, van Lent P, Blaney Davidson E, van der Kraan PM, 2017. Transcriptional profiling distinguishes inner and outer annulus fibrosus from nucleus pulposus in the bovine intervertebral disc. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 26, 2053–2062. [DOI] [PubMed] [Google Scholar]
- Ward L, Evans SE, Stern CD, 2017. A resegmentation-shift model for vertebral patterning. Journal of anatomy 230, 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L, Pang ASW, Evans SE, Stern CD, 2018. The role of the notochord in amniote vertebral column segmentation. Dev Biol 439, 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggan O, Fadel MP, Hamel PA, 2002. Pax3 induces cell aggregation and regulates phenotypic mesenchymal-epithelial interconversion. Journal of cell science 115, 517–529. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP, 1999. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes & development 13, 3185–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM, 2005. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proceedings of the National Academy of Sciences of the United States of America 102, 5062–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin L, Chang EY, Plouhinec JL, De Robertis EM, 2010. Crossveinless-2 is required for the relocalization of Chordin protein within the vertebral field in mouse embryos. Dev Biol 347, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin L, Metzinger CA, Chang EY, Coffinier C, De Robertis EM, 2008. Development of the vertebral morphogenetic field in the mouse: interactions between Crossveinless-2 and Twisted Gastrulation. Dev Biol 323, 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Kempf H, Murtaugh LC, Sato ME, Lassar AB, 2002. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes & development 16, 1990–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Li S, Trilok S, Tanaka M, Jokubaitis-Jameson V, Wang B, Niwa H, Nakayama N, 2014. Small molecule-directed specification of sclerotome-like chondroprogenitors and induction of a somitic chondrogenesis program from embryonic stem cells. Development (Cambridge, England) 141, 3848–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieba J, Forlenza KN, Khatra JS, Sarukhanov A, Duran I, Rigueur D, Lyons KM, Cohn DH, Merrill AE, Krakow D, 2016. TGFbeta and BMP Dependent Cell Fate Changes Due to Loss of Filamin B Produces Disc Degeneration and Progressive Vertebral Fusions. PLoS genetics 12, e1005936. [DOI] [PMC free article] [PubMed] [Google Scholar]


