Abstract
Introduction:
Administration of cholic acid, or its synthetic derivative, 6-alpha-ethyl-23(S)-methylcholic acid (INT-777), activates the membrane GPCR, TGR5, influences whole body metabolism, reduces atherosclerosis, and benefits the cardiovascular physiology in mice. Direct effects of TGR5 agonists, and the role for TGR5, on myocardial cell biology and stress response are unknown.
Methods:
Mice were fed chow supplemented with 0.5% cholic acid (CA) or 0.025% INT-777, a specific TGR5 agonist, or regular chow for 3 weeks. Anthropometric, biochemical, physiologic (electrocardiography and echocardiography), and molecular analysis was performed at baseline. CA and INT-777 fed mice were challenged with acute exercise-induced stress, acute catecholamine-induced stress, and hemodynamic stress induced by transverse aortic constriction (TAC) for a period of 8 weeks. In separate experiments, mice born with constitutive deletion of TGR5 in cardiomyocytes (CM- TGR5del) were exposed to exercise, inotropic, and TAC-induced stress.
Results:
Administration of CA and INT-777 supplemented diets upregulated TGR5 expression and activated Akt, PKA, and ERK1/2 in the heart. CA and INT-777 fed mice showed improved exercise tolerance, improved sensitivity to catecholamine and attenuation in pathologic remodeling of the heart under hemodynamic stress. In contrast, CM-TGR5del showed poor response to exercise and catecholamine challenge as well as higher mortality and signs of accelerated cardiomyopathy under hemodynamic stress.
Conclusions:
Bile acids, specifically TGR5 agonists, induce cytoprotective changes in the heart and improve myocardial response to physiologic, inotropic, and hemodynamic stress in mice. TGR5 plays a critical role in myocardial adaptability, and TGR5 activation may represent a potentially attractive treatment option in heart failure.
Keywords: cholic acid, INT-777, myocardial adaptation, preconditioning, TGR5
1 |. INTRODUCTION
Heart failure is a growing epidemic in the USA, costing ~$35 billion each year. Despite significant advances in its treatments, the 5-year mortality for this disease remains high at ~59% and has remained unchanged over a decade.1–3 Current treatments have focused more on rate control and afterload reduction. For outcomes to improve beyond this current status quo, newer strategies must target the heart muscle and enhance cardiomyocyte response to stress.
Bile acids, known merely as detergents aiding in digestion of lipids in the intestine, are now seen as hormones with important regulatory function.4–6 Recent observations suggest benefits of modest increases in bile acid levels on host cardiovascular status. A direct correlation has been made between adaptive cardiac metabolic changes and elevated levels of serum bile acids7 in a rat model of gastric bypass. Ursodeoxychlic acid, a secondary hydrophilic bile acid, has been shown to decrease ER stress in diabetic cardiomyopathy in rats, while secondary hydrophobic bile acid-lithocholic acid has been shown to attenuate apoptosis in isolated cardiomyocytes.8 Cholic acid, a primary bile acid in humans, and its semi- synthetic derivative INT-777,9 have recently been shown to reverse diet- induced obesity, reduce atherosclerosis, and improve metabolic syndrome in mice.10-12 These effects are attributed to the ability of cholic acid and INT-777 to activate the membrane bile acid receptor TGR513 expressed in the brown adipose tissue, skeletal muscles, and macrophages. TGR5 has also been found in rodent and human hearts11,14 and is amenable to be targeted by bile acids, both in health and in disease.
It has been known for over a century that pathologic elevations of circulating bile acids (~200–300 μmol/L), as seen in liver diseases, are toxic to the heart.14–18 However, less is known about the effects of modest elevations of bile acids on myocardial physiology, function, and stress response. The effects of natural endogenous TGR5 agonist, such as cholic acid, or its semi-synthetic derivative and specific TGR5 agonist INT-777 on the heart remains untested. Also unexplored is the role of the new GPCR- TGR5, which is present in the heart in myocardial health and disease. We hypothesize that upregulation of TGR5 in the heart, achieved by feeding cholic acid or INT-777 supplemented diet, will induce cyto-protectective changes in the heart and enhance its adaptability to physiologic, inotropic, and hemodynamic stress. In contrast, genetic deletion of TGR5 in cardiomyocytes will impair host response to stress.
2 |. MATERIALS AND METHODS
2.1 |. Animals and diet
Six-to 8-week-old male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) were fed with 0.5% cholic acid (CA) supplemented chow (Harlan-Teklad Inc., Madison, WI, USA) for 3 weeks. Alternatively, mice were fed with the specific TGR5 agonist INT-7779 (0.025%; provided by Intercept Pharmaceuticals Inc. New York, NY, USA) supplemented chow for 3 weeks. CA and INT-777 doses were calculated based on previously published data.10,11 Farnesoid-X-receptor (FXR) is present in the heart and the vascular endothelial cells and belongs to the family of metabolic nuclear receptor that is responsive to bile acids.19 To evaluate a role for FXR agonists in myocardial function and stress response, we separately fed mice INT-747 (obeticholic acid), a specific FXR agonist,20 (0.025%; provided by Intercept Pharmaceuticals Inc. New York, NY, USA) supplemented chow for 3 weeks. Age-matched male C57BL/6J mice fed isocaloric chow were used as controls. Mice were weighed twice per week. Feeds and bedding were weighed and changed twice per week to provide an estimate of food intake. Mice were fed ad libitum and had free access to water. Food was withdrawn 4 hours before experiments, and all experiments were carried out in accordance with Institutional Animal Care and Use Committee–approved protocols at Baylor College of Medicine (BCM).
Generation of mice with cardiomyocyte specific deletion of TGR5 (CM-TGR5del): CM- TGR5del mice with constitutive cardiomyocyte-specific deletion of TGR5—were generated by crossing TGR5 floxed mice (TGR5flox/flox) on C57BL/6 background, a gift of Dr. Johan Auwerx,10 to mice transgenic for cre under control of the MYH6 promoter, also on C57BL/6 background.21 Cre mediated excision of exon 1 of the floxed TGR5 allele resulted in CM- TGR5del mice (TGR5flox/flox/cre+). TGR5 deletion was confirmed using PCR and Western blot techniques. Littermates with the absence of cre were used as wild-type (WT) controls.
Anthropometric Measurements: Dual energy X-ray absorptiometry scanning (GE-Lunar PIXImus, Madison, WI, USA) to evaluate lean mass, fat mass, bone mineral density, bone mineral content, and percent fat content (Mouse Phenotype Core [MPC] facility, BCM, Houston, TX, USA) as performed before.14
2.2 |. Cardiac parameters
Two- dimensional echocardiography (2DE) was performed in the Mouse Phenotyping Core (BCM) on sedated mice (Vevo 770 Digital RF; VisualSonic Inc., Toronto, CN, USA) as performed before.14,16,18
2.3 |. Catecholamine challenge and stress echocardiography
To evaluate the effects of catecholamine on hearts in vivo, mice were anesthetized and challenged with 20 μg/kg of isoprenaline, i.p. (Sigma-Aldrich; St. Louis, MO, USA). Cardiac parameters were then evaluated pre- and postinjection by echocardiography as described previously.18
2.4 |. Treadmill exercise protocol and energy expenditure
Mice were challenged with acute submaximal stress in the form of exercise on a treadmill integrated with metabolic chamber (Oxymax Deluxe VO2/VCO2 System Columbus Instruments, Columbus, OH), as previously described.14,18
2.5 |. Transverse aortic constriction
To evaluate response to chronic pressure overload induced heart failure, mice underwent transverse aortic constriction (TAC).18,22 Tightness of the band was assessed by carotid Doppler 1 week post-TAC. Contractile function was evaluated every 2 weeks by echocardiography for a period of 8 weeks. Findings were then compared between TAC groups and SHAM groups, where mice underwent all procedures except aortic banding.
2.6 |. Isolation of neonatal mouse cardiomyocytes
Cardiomyocytes were isolated from 1–2-day old C57BL/6 pups (n = 8–10 each) using the isolation kit from Cellutron Life Technologies as described before.18 Fibroblasts were removed by pre-plating the culture for 90 minutes at 37°C in the complete medium. The cardiomyocytes suspension was then seeded on the plates coated with Sure Coat (1 mL per well) (Cellutron Life Technologies, Baltimore, MD, USA). Twelve-to 16-hour postseeding, the medium was changed to the non-serum medium. All the media were purchased from Cellutron Life Technologies.
2.7 |. Serum analyses
Sera were collected from either retro-orbital or inferior vena cava (IVC) and analyzed for Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), total, and conjugated bilirubin levels (Cobas Integra 400+; Cobas-Roche, Waltham, MA, USA) at the Center of Comparative Medicine (BCM). Serum bile acid levels were evaluated by colorimetric methods (BioQuant Inc, SanDiego, CA, USA & Roche).
2.8 |. Histology
Liver and heart was stained with routine H/E. All histological studies were performed by the Texas Gulf Coast Digestive Disease Center.
2.9 |. Quantitative real- time PCR (qRTPCR)
RNA quantification of genes was conducted using standard protocol, using probes and primers as previously published.14 Relative RNA expression was calculated by delta Ct method. Gene expression of target genes was normalized to an internal standard (GAPDH/18S) and the difference expressed as amount of gene expressed per internal standard.
2.10 |. Immunoblotting
Proteins from whole homogenized hearts and membranes were extracted using standard procedures.23 Protein concentrations were measured using the Pierce BCA kit (Thermo Scientific, Rockford, IL, USA). After gel electrophoresis and immunoblotting, the gels were analyzed for expression of various proteins with specific antibodies as described before.14 Equal protein loading was confirmed by α-tubulin/GAPDH (Sigma-Aldrich. St. Louis, MO, USA). Results were analyzed by densitometry (Kodak software) and reported as fold change compared to chow fed hearts.
2.11 |. Statistical analysis
Data between 2 groups was compared using t test and Mann–Whitney, as specified. Data involving 4 groups (TAC experiments) were compared using ANOVA. All statistical calculations were performed using the PRISM 3.0 software program (Graph-Pad Prism, San Diego, CA, USA). P < 0.05 was selected as the level of significance.
3 |. RESULTS
3.1 |. CA feeding induces modest increases in bile acid levels and decreased fat mass in mice
When compared to chow fed mice, whole body weights of mice fed isocaloric 0.5% cholic acid supplemented diet were found to be significantly lower at 1-, 2-and 3-week intervals (Figure S1A). Chow fed mice showed ~10% increase, while CA fed mice showed ~5% decrease in body weight when compared to their individual weights at the start of the experiment (Δ in weight from baseline +2.7 ± 0.5 g in chow vs −1 ± 0.6 g in CA group). On analyzing body composition by DEXA, the decrease in body weight in the CA fed mice, was explained by a 15% decrease in body fat content, with no changes in lean mass (Figure S1B). Biochemical analysis of the serum shows a modest increase in serum ALT levels (Figure S1D), but without any evidence of hepatocyte damage on routine hematoxylin and eosin staining (Figure S1C) with CA feeding. There was a mild (3X) increase in circulating bile acid levels, without evidence of cholestasis (Figure S1D). There was no mortality or morbidity noted in the CA fed group, in line with observations made by us and others.11,24
3.2 |. CA feeding induces cytoprotective changes in the heart at a molecular level
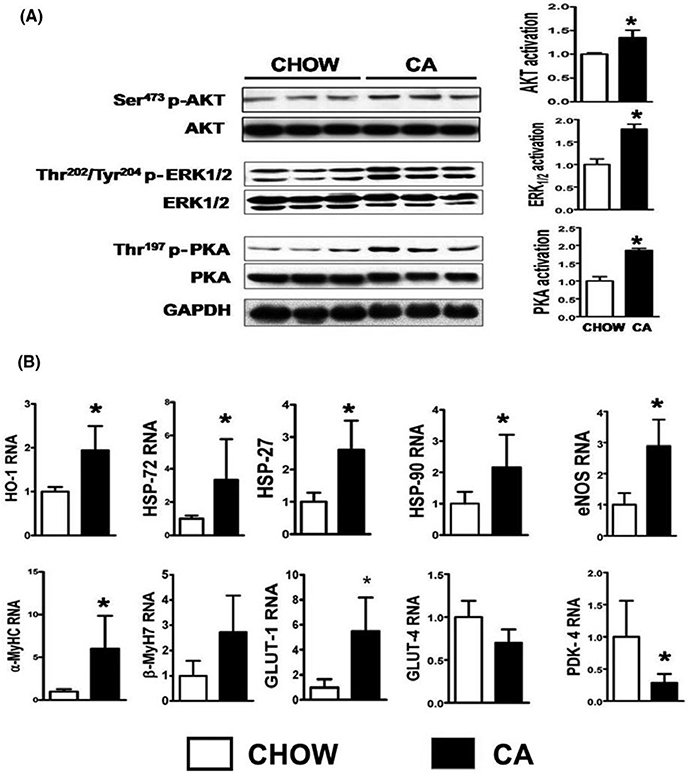
Short- term activation of stress induced kinases such as Akt, p42/p44 extra-cellular signal- regulated kinases (ERK 1/2) and PKA enhance cardiomyocyte survival,25,26 while certain heat shock proteins, such as HSP-32 (HO- 1), HSP 27, HSP 72, confer protection in heart failure.27 We examined whether bile acids activated these pro- survival kinases and heat shock proteins in the heart and found that hearts of CA fed mice demonstrate a ~2× increases in Ser473-phospho- Akt (activation of Akt), Thr202/Tyr204—phospho-ERK (activation of ERK), and Thr197-phospho-PKA (activation of PKA) (Figure 1A), along with upregulation of HSP32 [HO- 1], HSP27, HSP72, HSP90, and eNOS (Figure 1B).
FIGURE 1.
CA induces cytoprotective changes in the heart: (A) Hearts of mice fed CA for 3 wk demonstrate activation of cytoprotective survival kinases such as Akt, ERK, and PKA. Note ~2× increase in phosphorylated Akt, ERK, and PKA (activation) when divided by their unphosphorylated band. GAPDH band shows equal loading of samples. (B) shows key cytoprotective genes (HO-1, HSP 72, 27, and 90) as well as eNOS are upregulated in the CA fed hearts compared to chow fed. Note upregulation of GLUT-1 which determines basal glucose uptake and downregulation of PDK4, which negatively regulates glucose oxidation in CA fed mouse hearts. n = 5 per group; Results: Mean ± SD; *P < 0.05; Stats: t test
3.3 |. CA feeding improves chronotropic response to catecholamine and enhances whole body oxygen consumption during exercise
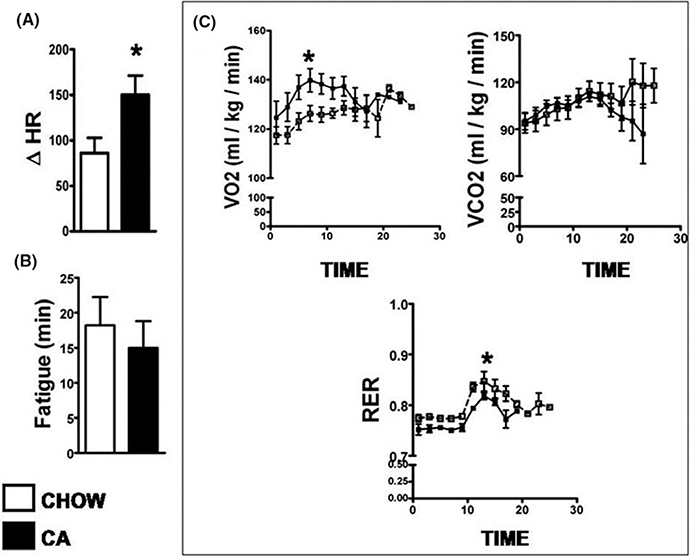
We examined if alterations at the cellular level translated into changes in the physiologic parameters of the heart. Under basal conditions, CA feeding did not affect heart rate, rhythm, contractility, stroke volume, cardiac output, or LV geometry (Figure S3). When mice were injected with a single dose of 20 mcg/kg isoprenaline, both chow fed and CA fed mice showed the expected increase in heart rates from baseline. However, the chronotropic effect was enhanced in CA fed mice, as evidenced by a significantly higher peak heart rate achieved in the CA fed mice compared to chow (Figure 2A). On treadmill challenge, although exhaustion times (time to fatigue) were comparable between the two groups (Figure 2B), the CA fed mice had a higher VO2 during exercise (Figure 3C). VCO2 was similar, and the respiratory exchange ratio (RER), which is a ratio of VCO2 and VO2, was lower in the CA fed than the chow fed mice (Figure 2C).
FIGURE 2.
CA improves chronotropic response to catecholamine and enhances whole body oxygen consumption during exercise: (A) shows change in heart rate after intraperitoneal injection of 20 mcg/kg of single dose of isoprenaline. Note significant higher response to catecholamine as evidenced by a higher change in heart rate postisoprenaline. (B) denotes time taken by the mouse to get exhausted on the treadmill. Although the exhaustion times were comparable, there was a significant increase in VO2 (oxygen consumed-a surrogate marker for cardiac output at peak of exercise and decrease in respiratory exchange ratio during peak exercise in CA fed mice compared to chow fed mice (C)). n = 5 per group; Results: Mean ± SD for A and B, SEM for C; *P < 0.05; Stats: t test for A and B. Mann–Whitney across time-points for C
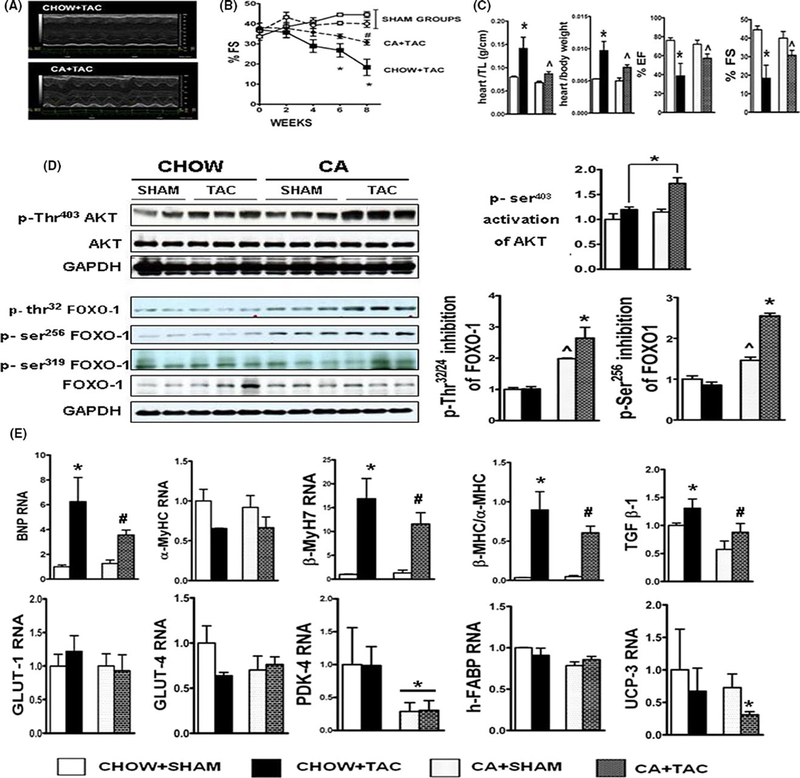
FIGURE 3.
CA attenuates transverse aortic constriction (TAC) induced hypertrophy and contractile failure: (A) shows M-Mode 2DE images of chow fed and CA fed hearts post- TAC. Note attenuation in contractile dysfunction in CA fed post- TAC hearts. (B) shows serial shortening fractions (%FS) of C57BL6 mice either fed CHOW or 0.5% cholic acid (CA) supplemented chow, when challenged with TAC or SHAM. Note significant attenuation of %FS in CA fed TAC (CA + TAC) mice compared to CHOW+ TAC mice. (n = 5– 10/grp; Stats: Multiple comparison ANOVA; *P < 0.05 compared to all groups; # P < 0.05 vs SHAM, Results: Mean ± SEM). (C) denotes quantification of heart weights indexed to body weight and tibial length at end of 8 wk. Note attenuation in TAC induced pathologic hypertrophy of hearts in CA fed mice (n = 5/sham groups, 10/TAC group); Stats: ANOVA with Tukey’s post hoc comparison between groups; *P < 0.05 when CHOW + TAC was compared to all groups; ^P < 0.05 when CA + TAC is compared to SHAM groups and CHOW + TAC group. vs SHAM, Results: Mean ± SEM). (D) shows images of Ser 473 phospho- AKT, total AKT, Thr32, Ser 256 and Ser 319 phospho-FOXO-1 and total FOXO-1. Activation (phosphorylation) of AKT, and inhibition (phosphorylation) of FOXO-1 was evaluated by dividing the phosphorylated to unphosphorylated forms of AKT and FOXO- 1, respectively. GAPDH blot is depicted to show equal loading. CA+TAC mice show enhanced activation of AKT and increased inhibition of FOXO-1 than CHOW+TAC mice. Also note significantly increased inhibition of FOXO-1 in the CA + SHAM mice, compared to CHOW+SHAM.(Results: Mean ± SEM; *P < 0.05 when CA + TAC was compared to all groups; ^P < 0.05 when CA+SHAM is compared to all groups, Stats: ANOVA with Tukey’s post hoc). (E) denotes BNP, βMYH7 RNA along with βMHC:αMHC ratio as measured by QRTPCR, in the 4 experimental groups. RNA has been standardized to 18S subunit and expressed as fold change compared to CHOW fed SHAM mice. Note attenuation of BNP, βMYH7 and βMHC:αMHC ratio at RNA level in the CA + TAC group (#P < 0.05 comparing CA+TAC with CHOW+TAC and SHAM groups; *P < 0.05 compared to all groups). Also note downregulation of PDK4 (*P < 0.05 when CA fed groups are compared to CHOW fed groups), and downregulation of UCP3 (*P < 0.05 when CA+TAC is compared to rest of the groups) (n = 5 per group; Results: Mean ± SD; Stats: ANOVA with Tukey’s post hoc)
3.4 |. CA feeding attenuates transverse aortic constriction (TAC) induced hypertrophy and contractile failure
Short-term activation of AKT, ERK, and PKA as well as expression of heat shock proteins have been associated with improved contractile function in mouse model of pressure overload. We examined whether CA fed hearts were protected from pressure overload induced by transverse aortic constriction (TAC). Both chow fed and CA fed sham mice had comparable ECHO findings throughout the 8 weeks of the experimental period (Figure 3B). TAC induced a decrease in ejection and shortening fractions in both CA and chow fed mice. However, contractile dysfunction in the CA fed group was significantly attenuated at 6-and 8-week interval, when compared to chow fed mice (Figure 3B). At necropsy heart weights indexed to both body weight and tibial length increased post TAC, but the increase was attenuated in CA fed mice compared to chow fed mice (Figure 3C). CA fed TAC mice also showed an attenuated increase in key markers of LV stress (BNP), pathologic hypertrophy (β-myosin/α-myosin ratio), and fibrosis (TGFβ) when compared to chow fed TAC mice (Figure 3E). In addition, we noted that CA fed TAC mice demonstrated modestly (~50%) higher levels of Ser403 phosphorylated (activated) AKT when compared to chow fed TAC mice, suggesting that CA feeding enhanced activation of AKT post- TAC (Figure 3D). One of the many downstream targets of AKT is the Forkhead box subfamily O (FOXO) proteins, and particularly FOXO-1, which is involved with modulation of cardiovascular biology and stress response.28–30 AKT mediated inhibition of FOXO-1 promotes cell survival.28 AKT directly phosphorylates FOXO-1 at Thr32/24, Ser319 or Ser256 sites and inactivates FOXO-1 by expulsion of FOXO-1 from nucleus to cytoplasm. This leads to decreased transcription of FOXO-1-mediated genes responsible for cell apoptosis and cell death.28 Hearts of CA fed TAC mice show increased Thr32 and Ser256 phosphorylation of FOXO-1, suggesting inhibition of FOXO-1 activity (Figure 3D). A key effect of Akt activation and FOXO1 inhibition is the regulation of PDK4 transcription. PDK4 is a key regulator of myocardial glucose oxidation and determines the metabolic flexibility of the heart.31,32 Downregulation of PDK4 is essential for adaptability of the heart to withstand stress in the form of injury (ischemic/metabolic/hemodynamic).33,34 We found ~50% downregulation of PDK4, a key inhibitor of pyruvate dehydrogenase complex in CA fed TAC hearts (Figure 3E).
3.5 |. NT-777 supplementation is well tolerated in mice
CA has multiple on and off target effects, including effects on FXR. TGR5 as well as FXR was upregulated in CA fed mouse hearts (Figure S3). In order to test if the molecular and functional effects of CA are secondary through its effects on TGR5, we evaluated whether CA effects can be mimicked using INT-777, a well-studied semisynthetic derivative of CA, which specifically targets TGR5. Mice fed INT-777 supplemented diet for 3 weeks showed comparable weight gain throughout the course of feeding, with similar increases in weights as isocaloric chow fed mice (Figures S4A and B). These findings are in line with previously published data from other laboratories.10,35 To assess the effects of INT-777 feeding on physiologic parameters of the heart, we performed echocardiography on mice fed INT-777 supplemented diet for 3 weeks and found that basal myocardial function was comparable to chow fed counterparts (Figures S4C).
3.6 |. INT-777 feeding induces cytoprotective changes in the heart at a molecular level like CA fed mice
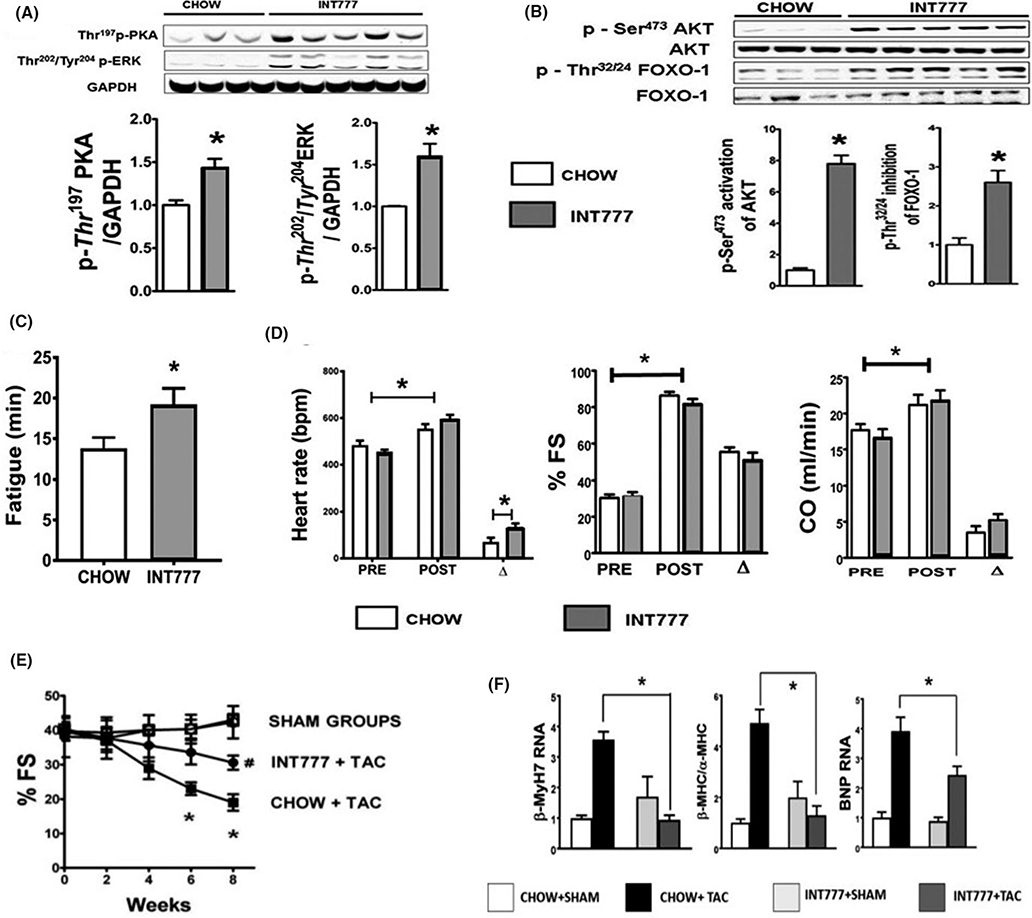
INT-777 administration positively influences whole body metabolism, tissue function, and improves the metabolic syndrome by enhancing glycemic control, all of which indirectly benefit the host cardiovascular status.10,11,35 INT-777 activates Akt, p42/p44 extra-cellular signal-regulated kinases (Erk 1/2) and PKA in the liver and extra- hepatic organs such as brown adipose tissue, skeletal muscles, vascular endothelial cells, and gastro-intestinal tract, through pathways mediated by TGR5.11,36,37 Effects of INT-777 administration on the heart have never been studied, despite its presence in the heart. We hence investigated whether administration of INT-777 could activate Akt, ERK, and PKA in the heart. The results show significant increases in Ser473-phospho-AKT (activation of Akt), Thr202/Tyr204—phospho-ERK (activation of ERK) and Thr197-phospho-PKA (activation of PKA) (Figures 4A and B). Similar to CA, INT-777 fed hearts showed a significant downregulation of PDK4.
FIGURE 4.
INT-777 induces cytoprotective changes in the hearts at a molecular level, improves exercise tolerance, chronotropic response to catecholamine, and attenuates TAC-induced contractile failure. Hearts of mice fed INT-777 showed activation (phosphorylation) of AKT, ERK, and PKA (A) These are pro-survival kinases and are part of the Reperfusion Injury Salvage Kinase (RISK) pathway. Bar graph shows phosphorylation (activation), with GAPDH as loading control and compared to chow (fold activation) (n = 3–5; Statistics: t test, *P < 0.05). (B) shows images of Ser473 phospho-Akt, total Akt, Thr 32/34 phospho-FOXO1 and total FOXO1. Activation (phosphorylation) of Akt and inhibition (phosphorylation) of FOXO1 was evaluated with densitometry by dividing the phosphorylated form with its un-phosphorylated form. INT-777 fed mice show a robust activation of Akt and inhibition of FOXO1 compared to chow fed mice. (n = 3–5 per group; Stats: t test). (C) When challenged with acute physiologic stress in the form of treadmill exercise, INT-777 fed mice ran longer (higher time to fatigue) than chow fed mice. (n = 8/group; Statistics: t test; *P < 0.05 when INT-777 is compared to CHOW). (D) When mice were challenged with acute i.p injection of isoprenaline, hearts from INT-777 fed mice showed a stronger chronotropic response compared to chow fed mice. There was no significant difference in the effect of INT-777 on shortening fraction or the cardiac output. (n = 8/group; Statistics: t test; *P < 0.05 pre-isoprenaline vs. post-isoprenaline. Also, * P < 0.05 with change in heart rate (Δ) between CHOW fed and INT-777 fed groups). (E) Figure shows serial shortening fractions (%FS) of C57BL6 mice either fed CHOW or 0.025% INT-777 supplemented diet, when challenged with TAC or SHAM. Note significant attenuation of %FS in INT-777 fed TAC (INT-777 + TAC) mice compared to CHOW+TAC mice. (n = 5–8/grp; Stats: Multiple comparison ANOVA, *P < 0.05 compared to all groups; #P < 0.05vs SHAM, Results: Mean ± SEM). (F) shows INT-777 feeding attenuates key genes involved in TAC induced pathologic remodeling as evidenced by attenuated increase in TAC induced BNP, βMYHC RNA along with βMHC:αMHC ratio in INT-777 fed TAC mice compared with chow fed controls. RNA has been standardized to GAPDH and expressed as fold change compared to chow fed SHAM mice. (*P < 0.05, n = 5–10 per group; Stats: ANOVA with Tukey’s post hoc)
3.7 |. INT-777 feeding improves exercise tolerance, chronotropic response to catecholamine, and attenuates transverse aortic constriction (TAC)-induced contractile failure
INT-777 fed mice showed improved exercise tolerance, as evidenced by longer time for exhaustion on the treadmill compared to chow fed controls (Figure 4C). On isoprenaline challenge, both chow fed and INT-777 fed mice showed an expected increase in heart rates, shortening fraction and cardiac output from baseline. However, the chronotropic (heart rate) effect of isoprenaline was enhanced in INT-777 fed mice, as evidenced by the significantly higher peak heart rate achieved compared to chow fed mice (Figure 4D). Effect of isoprenaline on shortening fractions, ejection fractions, and cardiac output were similar in chow and INT-777 fed groups (Figure 4D). We next examined the effects of INT-777 fed hearts to TAC induced pressure overload. Both chow fed and INT-777 fed sham mice had comparable ECHO findings throughout the 8 weeks after TAC. TAC induced the expected decrease in shortening fractions in both INT-777 and chow fed mice. However, contractile dysfunction in the INT-777 fed group was significantly attenuated at 6-and 8-week interval, compared to chow fed mice (Figure 4E). In line with the echocardiography, INT-777 fed TAC mice also showed an attenuated increase in key markers of LV stress (BNP) and pathologic hypertrophy (β-myosin/α-myosin ratio) (Figure 4F).
3.8 |. Direct effects of bile acids on isolated neonatal mouse cardiomyocytes
To assess direct effects of bile acids on the heart, we isolated cardiomyocytes from neonatal pups as described before,14,18 and incubated them with either 50 μmol/L of tauro-cholic acid (TCA) or 10 μmol/L of INT-777 along with vehicle control for 4 hours (for protein analysis) and 24 hours for RNA analysis. Taurocholic acid (TCA) was chosen for these experiments because TCA, being a conjugated form of cholic acid does not cross the plasma membrane, and is ideal to study effects of extracellular bile acids on cardiomyocyte biology. Also, TCA, apart from being a strong TGR5 agonist,38 is the most predominant circulating conjugated bile acid in mice fed 0.5% CA diet.11 We have already shown that TCA activates Akt in vitro.14 Here we show that TCA upregulates TGR5 and downregulates PDK4 at RNA level (Figure S5A) in line with our in vivo data. Similarly, INT-777 activates Akt in vitro (Figure S5C) upregulates TGR5 and downregulates PDK4 (Figure S5B).
3.9 |. Mice with cardiomyocyte-specific deletion of TGR5 (CM-TGR5del), demonstrate increased mortality and exaggerated contractile dysfunction in response to TAC, compared to littermates
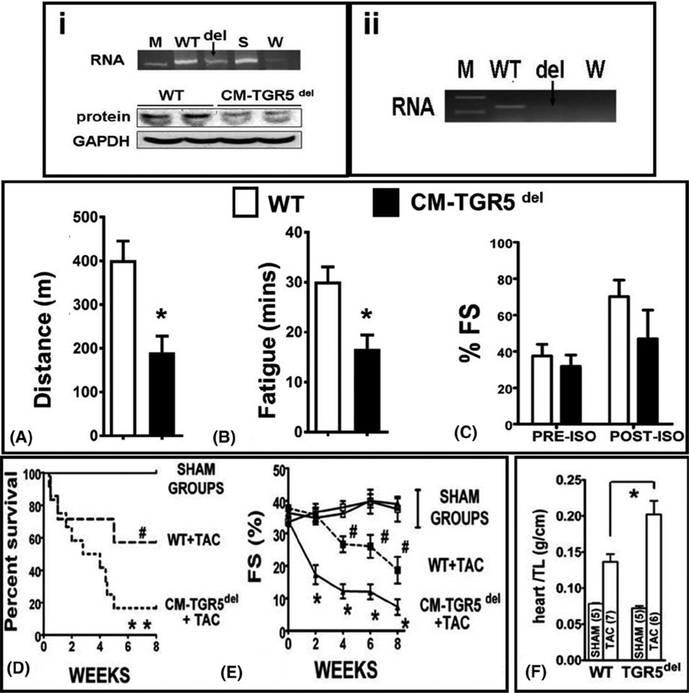
Once TGR5 deletion was confirmed (Figure 5 i and ii), CM- TGR5del and their littermate WT controls (TGR5f/f/cre-) were challenged with exercise, catecholamine, and TAC to evaluate the critical role of TGR5 in the heart. CM-TGR5 del mice ran shorter distance (Figure 5A) and showed early fatigue (Figure 5B) compared to WT littermates. Catecholamine response was similar between the groups (Figure 5C). We then randomized WT and CM-TGR5del mice to TAC/SHAM. In SHAM animals, ECHO was comparable between WT and CM-TGR5 del mice (Figure 5E). However, when challenged with hemodynamic stress, CM-TGR5 del mice showed increased mortality (Figure 5D), exaggerated contractile dysfunction (Figure 5E) and increased heart weight-to-tibial length ratio (Figure 5F) compared to WT littermates, suggesting that absence of TGR5 is detrimental to mice under conditions of pressure overload. The presence of cre recombinase in the hearts did not affect contractility in SHAM or TAC mice (data not shown).
FIGURE 5.
Cardiomyocyte specific deletion of TGR5 (CM-TGR5del) in mice, demonstrate exercise intolerance, increased mortality and exaggerated contractile dysfunction in response to TAC, compared to littermates: (i) and (ii) shows proof of TGR5 deletion from the whole heart and adult isolated cardiomyocyte in CM-TGR5del mice. (i) shows knockdown of TGR5 from the whole hearts in CM-TGR5del mice at both RNA and protein level using primers and antibodies used and tested by us before. As this is a cardiomyocyte specific deletion, weak bands seen on PCR and Western blots are a result of presence of TGR5 in the vascular endothelium which is not deleted. A ~60% deletion was achieved from the whole heart when quantified objectively by qRTPCR and densitometry (data not shown). [M] is marker, [WT] is whole hearts of TGR5 flox/flox/cre− littermates, deletion (del) shown as arrow [CM-TGR5del hearts], with spleen(S) as positive control and water (W) as negative control. (ii) shows complete deletion of TGR5 from adult cardiomyocytes. We isolated cardiomyocytes from the hearts of adult CM-TGR5del mice and probed them for the presence of TGR5 using primers as before. We found a near complete deletion of TGR5 RNA (arrow). [WT] is whole hearts of TGR5 flox/flox/cre− mice, deletion (del) shown as arrow [CM-TGR5del hearts], water [W] as negative control. When challenged with acute physiologic stress in the form of treadmill exercise, CM- TGR5del mice ran shorter distance (A) and fatigued (B) earlier than WT mice. (n = 5/group; Statistics: t test; *P < 0.05; RESULTS: Mean ± SEM). There was no significance in shortening fraction between the two groups on isoprenaline (C). When CM-TGR5del mice were randomized to SHAM or TAC, increased mortality was noted in CM-TGR5del mice compared to WT mice post-TAC (D). No mortality noted in sham groups. (*P = 0.013 compared to all groups; # P < 0.05 compared to sham (Stats: Mantell-Cox test, n = 3/grp in SHAM and 10/grp in TAC). (E) shows serial analysis of shortening fractions (%FS) as evaluated by ECHOs. Note significant decrease in FS in CM- TGR5del mice post- TAC over 8 wk, starting as early as 2 wk (n = 3–5/grp; Stats: Multiple comparison ANOVA, P < 0.05). (F) shows bar graphs of heart weight/tibial length ratios of WT and CM-TGR5del mice who undergo either SHAM or TAC. CM- TGR5del mice had ~60% higher heart weight/TL ratio suggesting increased hypertrophy compared to WT+TAC mice. (*P < 0.05; ANOVA; Results: Mean ± SD; number of animals = WT + SHAM (n = 3); WT+TAC (n = 7 survived of 10); CM- TGR5del + SHAM (n = 3); CM- TGR5del (n = 6 survived of 20)
3.10 |. Effects of INT-747 feeding on mouse hearts
INT-747, specific agonist for FXR, was well tolerated in mice as evidenced by comparative weight gain in both groups (Figure S6). There was no difference in myocardial parameters as evaluated by echocardiography (data not shown), when compared to chow fed mice. Similarly exercise tolerance on treadmill challenge and heart rate response to isoprenaline (20 mcg/kg) were similar in the INT-747 fed mice compared to chow fed counterparts (Figure S6).
4 |. DISCUSSION
In this study, we show for the first time that a modest increment in circulating serum bile acid level, achieved by supplementation of CA in diet, induces cytoprotective changes in the heart characterized by activation of pro-survival kinases and upregulation of heat shock proteins. CA fed hearts showed improved chronotropic response to catecholamine stimulation, and whole body oxygen consumption during exercise, and more importantly attenuated contractile failure and hypertrophy in the TAC model of heart failure. Our study also shows that cholic acid supplementation enhances activation of AKT, inhibition of FOXO-1 when exposed to pressure overload and induces downregulation of PDK4, a key inactivator of pyruvate dehydrogenase complex, and a determinant of metabolic flexibility in the heart. The critical role of TGR5 present in the heart is emphasized by cardioprotective effects of pharmacological activation of TGR5 by CA and INT-777 and deleterious effects of genetic deletion of TGR5 under stress. These data further advance our scientific understanding of bile acid–myocardial interaction and proposes a novel role for bile acids, both natural and endogenous like cholic acid and semi-synthetic like INT-777, as well as their membrane GPCR-TGR5 in myocardial cell biology, function, metabolism, and cell signaling pathways, with potential pharmacologic application in treatment of heart failure.
Short-term modest elevations of bile acid levels, achieved by cholic acid supplementation, alter the molecular makeup of the heart and its response to stress. At a protein level, we found activation of three classic pro- survival kinases, Akt, PKA, and ERK. These kinases have a special significance in the heart, as they are collectively known as Reperfusion Injury Salvage Kinases (RISK), and are now being pharmacologically activated to preserve cardiomyocyte function and prevent cell death in ischemia and reperfusion.26,39,40 At the RNA level, we found in cholic acid fed mice an upregulation of heat shock proteins 32 (HO- 1), 27, 72, and 90, and eNOS, in the heart, all of which are known to be cardioprotective.27 These changes at the molecular level translated to enhanced host response to myocardial stress in the form of exercise, inotropy, and pressure overload. CA fed mice, albeit showing comparable times to exhaustion, had a higher rate of oxygen consumption (VO2)—a surrogate marker for cardiac output than their chow fed counterparts. The most significant response to priming of the heart with CA feeding was seen when mice were subjected to pressure overload by aortic banding. In this well-established model of heart failure, we found that the CA feeding delayed the onset of contractile failure. Collectively this introduces cholic acid as a novel endogenous metabolite that has the potential to induce preconditioning of the heart. An important finding of our study, which could be potentially mechanistic is the effect of CA feeding on the Akt- FOXO-1 pathway in the heart. A well-studied mechanism behind short- term AKT activation and cardiomyocyte survival is its effect on regulating translocation and activity of the forkhead transcription factors (FOXO) subfamily, which includes FOXO-1. FOXO-1 is directly phosphorylated at Thr32 or Ser256 sites by Akt, resulting in export from the nucleus and hence inactivation of FOXO1. One of the many effects of inactivation of FOXO-1 is suppression of genes that regulate apoptosis and cell death and genes regulating cardiac metabolism and substrate utilization, primarily Pyruvate Dehydrogenase Kinase 4 (PDK4).41,42 Suppression of PDK4 increases the activity of Pyruvate Dehydrogenase Complex (PDHc), which leads to the production of acetyl CoA for oxidation in the Krebs cycle,43–45 and thereby optimizing glucose utilization and improving energy efficiency under stress. Whether the observed cardioprotective effects of cholic acid are mediated by activation of Akt, inhibition of FOXO-1, and downregulation of PDK4, thus inducing a tighter coupling of glucose uptake and oxidation remains to be investigated further.
Our study also proposes a critical yet hitherto unrecognized role for TGR5 and TGR5 agonists in myocardial cell biology and heart failure. This G-protein coupled receptor is encoded by a single exon gene and is well conserved among vertebrates, emphasizing its translational and physiologic significance.13,46 This study is the first to characterize the effects of activation and deletion of TGR5 on myocardial function and stress response. Irrespective of etiology, heart failure is associated with disturbances in myocardial energy metabolism.47,48 Modulation of cardiac energy metabolism to preserve cardiac health remains a promising but unachieved goal, as the ideal agent remains elusive. Studies have shown that in response to cholic acid and INT-777,38 TGR5 regulates energy metabolism in brown adipose tissue and skeletal muscles,11 prevents metabolic syndrome,10,46,49,50 and reduces atherosclerosis in mice,35 all of which improve the overall cardiovascular status of the host.
Mice tolerated INT-777 supplemented diet. Remarkably, 3 weeks of feeding induced a robust activation of Akt-FOXO-1-PDK4 pathway. INT-777 enhanced mouse ability to exercise on a treadmill, and similar to CA fed mice, INT-777 primed hearts demonstrated increased sensitivity to catecholamines and showed an attenuated response to pressure overload both at a functional and molecular level. INT-777 which is currently being tested in preclinical studies to cure metabolic syndrome and diabesity could be an interesting agent to be tested in various models of heart failure, with considerable clinical potential.
The critical role for TGR5 in myocardial physiology and adaptation to stress is perhaps best highlighted by our findings in mice with constitutive deletion of TGR5 in cardiomyocytes. In the CM-TGR5del hearts, because the deletion is driven by alpha myosin cre, TGR5 is still expressed in the vascular endothelium. Nevertheless, genetic lack of TGR5 in cardiomyocytes significantly and surprisingly impaired myocardial ability to adapt to the three stressors tested. These studies now introduce a new GPCR that may be pharmacologically or genetically targeted for a healthier heart. TGR5 can potentially be easily targeted not only by bile acids, but also by a wide array of readily available synthetic analogues and natural products38,46 to activate the Akt signaling pathway, to modulate myocardial expression of PDK4 and optimize myocardial glucose metabolism to benefit patients suffering from different forms of heart diseases.
Our previous studies have shown that mice with pathologically high circulating bile acid levels have abnormal heart rate, rhythm, contractility, and catecholamine resistance, along with exercise intolerance. High levels of bile acids disrupt fatty acid oxidation and impair ability of a cholestatic myocardium to adapt to stress, a concept we termed “cholecardia”.18 In this report, we observe that modest increases in bile acids are cardio protective. These diametric opposite observations are intriguing and the mechanisms need to be elucidated and will be the next steps in our research. It is, however, worth speculating why there are such contrasting effects. Bile acids are signaling metabolites with effects beyond the hepatobiliary tract. As with all metabolites, dose determines its toxicity, and we believe that levels of circulating bile acids determine cardio-toxic effects versus cardio-protective effects. Bile acids are known to be ligands for TGR5 as well as muscarinic M2 cholinergic receptors, (M2), both of which are present on the cardiomyocyte cell membrane.12,14,51 TGR5 is a Gαs (stimulatory) protein coupled receptor, while M2 receptors are Gi (inhibitory) receptors.12,51 These receptors have contrasting effects on signaling and function. It has already been shown that at toxic concentrations, bile acids stimulate the M2 receptors, which cause negative effects on myocardial contractility and heart rate.52 A study recently published by the same group shows that bile acid effect on TGR5 and M2 is dose and species (type of bile acid) dependent, whereby TGR5 effects are seen at lower concentration, whereas muscarinic effects are seen at higher concentrations.12 This study further adds credence to the theory that dose determines cardio- toxicity vs. cardio-protection. It is our speculation, that cardio-protective effects are mediated by functional activation of TGR5, induced by modest elevation in circulating bile acid levels, and that that low dose of bile acids improves metabolic flexibility through TGR5 mediated downregulation of PDK4 by activating the Akt-FOXO-1 pathway. In reverse, cardio-toxic effects seen with bile acid overload are potentially mediated by M2 receptors. Bile acid excess leads to downregulation of PGC1α and impaired fatty acid metabolism and loss of myocardial adaptive capacity to stress. We would like to acknowledge that these are merely observations and associations and do not show cause and effect. This major limitation will need to be addressed by exhaustive studies to dissect the mechanisms to convincingly answer this important question.
In conclusion, we propose a unique role for TGR5 receptor and its agonists in enhancing adaptive responses of the heart to stress. Future studies should test TGR5 agonists as rational adjuncts to current therapy to prevent rapid progression of heart failure.
Supplementary Material
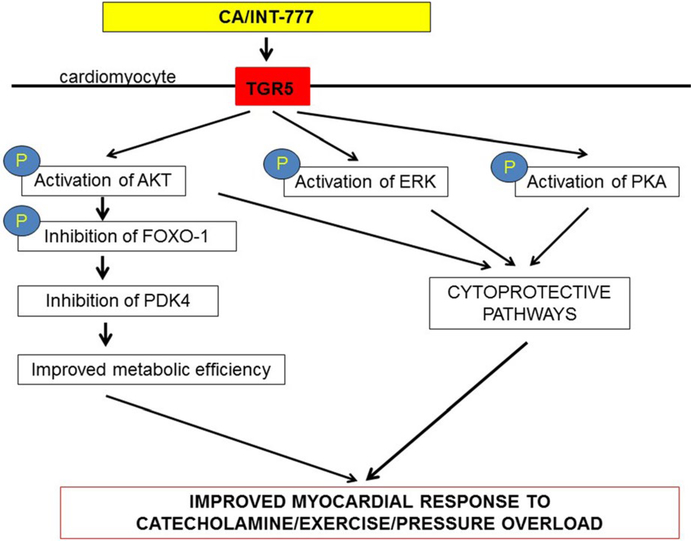
FIGURE 6.
Proposed mechanisms of cardioprotection: Cholic acid and INT-777, acting as TGR5 agonists, activate Akt, ERK and PKA pathways which are cytoprotective and improve myocardial response to stress. Activation of Akt, may inhibit FOXO-1 and suppress PDK4, which improves the metabolic efficiency and adaptability of the heart to catecholamine, exercise, and pressure overload mediated stress
Acknowledgments
Funding information
[P30 DK056338(MD)]; [Unrestricted grant from Intercept Pharmaceuticals (MD)]; [Texas Children’s Hospital Pediatric Pilot Award- 2017 (MD), US Public Health Service R01- HL 061483 (HT)].
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. [DOI] [PubMed] [Google Scholar]
- 4.Groen AK. The emerging role of bile acids as integrators of intermediary metabolism. J Hepatol. 2006;45(2):337–338. [DOI] [PubMed] [Google Scholar]
- 5.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25(7):1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50(8):1509–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashrafian H, Li JV, Spagou K, et al. Bariatric surgery modulates circulating and cardiac metabolites. J Proteome Res. 2014;13(2):570–580. [DOI] [PubMed] [Google Scholar]
- 8.Jehle J, Staudacher I, Wiedmann F, et al. Regulation of apoptosis in HL- 1 cardiomyocytes by phosphorylation of the receptor tyrosine kinase EphA2 and protection by lithocholic acid. Br J Pharmacol. 2012;167(7):1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellicciari R, Gioiello A, Macchiarulo A, et al. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S- EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J Med Chem. 2009;52(24):7958–7961. [DOI] [PubMed] [Google Scholar]
- 10.Thomas C, Gioiello A, Noriega L, et al. TGR5- mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim E, Diakonov I, Arunthavarajah D, et al. Bile acids and their respective conjugates elicit different responses in neonatal cardiomyocytes: role of Gi protein, muscarinic receptors and TGR5. Sci Rep. 2018;8(1):7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamata Y, Fujii R, Hosoya M, et al. A G protein- coupled receptor responsive to bile acids. J Biol Chem. 2003;278(11):9435–9440. [DOI] [PubMed] [Google Scholar]
- 14.Desai MS, Shabier Z, Taylor M, et al. Hypertrophic cardiomyopathy and dysregulation of cardiac energetics in a mouse model of biliary fibrosis. Hepatology. 2010;51(6):2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai MS, Penny DJ. Bile acids induce arrhythmias: old metabolite, new tricks. Heart 2013;99(22):1629–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai MS, Eblimit Z, Thevananther S, et al. Cardiomyopathy reverses with recovery of liver injury, cholestasis and cholanemia in mouse model of biliary fibrosis. Liver Int 2015;35(4):1464–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira M, Coxito PM, Sardao VA, Palmeira CM, Oliveira PJ. Bile acids are toxic for isolated cardiac mitochondria: a possible cause for hepatic- derived cardiomyopathies? Cardiovasc Toxicol. 2005;5(1):63–73. [DOI] [PubMed] [Google Scholar]
- 18.Desai MS, Mathur B, Eblimit Z, et al. Bile acid excess induces cardiomyopathy and metabolic dysfunctions in the heart. Hepatology. 2017;65(1):189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126(4):789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baghdasaryan A, Claudel T, Gumhold J, et al. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO(−)(3) output. Hepatology. 2011;54(4):1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Oort RJ, Garbino A, Wang W, et al. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123(9):979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Oort RJ, Respress JL, Li N, et al. Accelerated development of pressure overload- induced cardiac hypertrophy and dysfunction in an RyR2- R176Q knockin mouse model. Hypertension. 2010;55(4):932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. Endotoxin leads to rapid subcellular re- localization of hepatic RXRalpha: A novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept. 2004;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai MS, Mariscalco MM, Tawil A, Vallejo JG, Smith CW. Atherogenic diet- induced hepatitis is partially dependent on murine TLR4. J Leukoc Biol. 2008;83(6):1336–1344. [DOI] [PubMed] [Google Scholar]
- 25.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15(2):69–75. [DOI] [PubMed] [Google Scholar]
- 26.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12(3–4):217–234. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83(2):117–132. [DOI] [PubMed] [Google Scholar]
- 28.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. [DOI] [PubMed] [Google Scholar]
- 29.Chaanine AH, Hajjar RJ. AKT signalling in the failing heart. Eur J Heart Fail. 2011;13(8):825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puthanveetil P, Wan A, Rodrigues B. FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell survival. Cardiovasc Res. 2013;97(3):393–403. [DOI] [PubMed] [Google Scholar]
- 31.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284(5):E855–E862. [DOI] [PubMed] [Google Scholar]
- 32.Sugden MC, Bulmer K, Holness MJ. Fuel-sensing mechanisms integrating lipid and carbohydrate utilization. Biochem Soc Trans. 2001;29(Pt 2):272–278. [DOI] [PubMed] [Google Scholar]
- 33.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104(24):2923–2931. [DOI] [PubMed] [Google Scholar]
- 34.Taegtmeyer H, Razeghi P, Young ME. Mitochondrial proteins in hypertrophy and atrophy: a transcript analysis in rat heart. Clin Exp Pharmacol Physiol. 2002;29(4):346–350. [DOI] [PubMed] [Google Scholar]
- 35.Pols TW, Nomura M, Harach T, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14(6):747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon- like peptide- 1 secretion through TGR5 in a murine enteroendocrine cell line STC- 1. Biochem Biophys Res Commun. 2005;329(1):386–390. [DOI] [PubMed] [Google Scholar]
- 37.Kida T, Tsubosaka Y, Hori M, Ozaki H, Murata T. Bile acid receptor TGR5 agonism induces NO production and reduces monocyte adhesion in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(7):1663–1669. [DOI] [PubMed] [Google Scholar]
- 38.Sato H, Macchiarulo A, Thomas C, et al. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure- activity relationships, and molecular modeling studies. J Med Chem. 2008;51(6):1831–1841. [DOI] [PubMed] [Google Scholar]
- 39.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288(2):H971–H976. [DOI] [PubMed] [Google Scholar]
- 40.Hausenloy DJ, Garcia-Dorado D, Botker HE, et al. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res. 2017;113(6):564–585. [DOI] [PubMed] [Google Scholar]
- 41.Piao L, Sidhu VK, Fang YH, et al. FOXO1- mediated upregulation of pyruvate dehydrogenase kinase-4 (PDK4) decreases glucose oxidation and impairs right ventricular function in pulmonary hypertension: therapeutic benefits of dichloroacetate. J Mol Med (Berl). 2013;91(3):333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puthanveetil P, Wang Y, Wang F, Kim MS, Abrahani A, Rodrigues B. The increase in cardiac pyruvate dehydrogenase kinase-4 after short-term dexamethasone is controlled by an Akt-p38-forkhead box other factor-1 signaling axis. Endocrinology. 2010;151(5):2306–2318. [DOI] [PubMed] [Google Scholar]
- 43.Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY. ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol Heart Circ Physiol. 2013;304(8):H1103–H1113. [DOI] [PubMed] [Google Scholar]
- 44.Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab (Lond). 2014;11(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao G, Jeoung NH, Burgess SC, et al. Overexpression of pyruvate dehydrogenase kinase 4 in heart perturbs metabolism and exacerbates calcineurin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;294(2):H936–H943. [DOI] [PubMed] [Google Scholar]
- 46.Tiwari A, Maiti P. TGR5: an emerging bile acid G- protein- coupled receptor target for the potential treatment of metabolic disorders. Drug Discov Today. 2009;14(9–10):523–530. [DOI] [PubMed] [Google Scholar]
- 47.Wallhaus TR, Taylor M, DeGrado TR, et al. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2001;103(20):2441–2446. [DOI] [PubMed] [Google Scholar]
- 48.Taylor M, Wallhaus TR, DeGrado TR, et al. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in Patients with Congestive Heart Failure. J Nucl Med. 2001;42(1):55–62. [PubMed] [Google Scholar]
- 49.Pols TW. TGR5 in inflammation and cardiovascular disease. Biochem Soc Trans. 2014;42(2):244–249. [DOI] [PubMed] [Google Scholar]
- 50.Thomas C, Auwerx J, Schoonjans K. Bile acids and the membrane bile acid receptor TGR5–connecting nutrition and metabolism. Thyroid. 2008;18(2):167–174. [DOI] [PubMed] [Google Scholar]
- 51.Vasavan T, Ferraro E, Ibrahim E, Dixon P, Gorelik J, Williamson C. Heart and bile acids - Clinical consequences of altered bile acid metabolism. Biochim Biophys Acta. 2018;1864(4 Pt B):1345–1355. [DOI] [PubMed] [Google Scholar]
- 52.Sheikh Abdul Kadir SH, Miragoli M, Bu-Hayyeh S, et al. Bile acid-induced arrhythmia is mediated by muscarinic M2 receptors in neonatal rat cardiomyocytes. PLoS One. 2010;5(3):e9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.