Abstract
Objective:
To investigate the neural mechanisms of food motivation in children and adolescents, and examine brain activation differences between healthy weight (HW) and obese participants.
Subjects:
Ten HW children (ages 11–16; BMI < 85%ile) and 10 obese children (ages 10–17; BMI > 95%ile) matched for age, gender and years of education.
Measurements:
Functional magnetic resonance imaging (fMRI) scans were conducted twice: when participants were hungry (pre-meal) and immediately after a standardized meal (post-meal). During the fMRI scans, the participants passively viewed blocked images of food, non-food (animals) and blurred baseline control.
Results:
Both groups of children showed brain activation to food images in the limbic and paralimbic regions (PFC/OFC). The obese group showed significantly greater activation to food pictures in the PFC (pre-meal) and OFC (post-meal) than the HW group. In addition, the obese group showed less post-meal reduction of activation (vs pre-meal) in the PFC, limbic and the reward-processing regions, including the nucleus accumbens.
Conclusion:
Limbic and paralimbic activation in high food motivation states was noted in both groups of participants. However, obese children were hyper-responsive to food stimuli as compared with HW children. In addition, unlike HW children, brain activations in response to food stimuli in obese children failed to diminish significantly after eating. This study provides initial evidence that obesity, even among children, is associated with abnormalities in neural networks involved in food motivation, and that the origins of neural circuitry dysfunction associated with obesity may begin early in life.
Keywords: children, adolescent, neuroimaging, functional MRI, neural mechanism
Introduction
The prevalence of obese and overweight children in the United States is increasing rapidly.1 Since the late 1970’s, the prevalence of overweight has doubled among children aged 6–11 and tripled among youth aged 12–17.2 Recent estimates show that approximately one in three children are over-weight (body mass index (BMI) 85–95% for age and sex) or obese (BMI >95% for age and sex).3 Obese youth are increasingly diagnosed with impaired glucose tolerance, type-2 diabetes and show symptoms of insulin resistance syndrome and cardiovascular risk factors.4–6 Childhood obesity is a significant concern, as many believe it will reverse the trend in increasing life expectancy.7
Several factors contribute to obesity, but disruption in energy balance between calorie consumption and energy expenditure is a principal source of weight gain. The brain has a major role in modulating hunger and regulating motivated behaviors such as eating. Neural mechanisms are believed to have an integral role in mediating eating behaviors through regulation of food motivation and behavior control.8 Neuroimaging studies using positron emission tomography and functional magnetic resonance imaging (fMRI) have examined neural mechanisms involved in food motivation.8–10 The brain regions most commonly associated with food motivation in healthy weight (HW) adults are the limbic and paralimbic brain areas associated with taste, motivation, reward and behavioral control. The most consistently implicated regions include the orbitofrontal cortex (OFC) and medial prefrontal cortex, the amygdala, insula, striatum, anterior cingulate cortex and the hippocampal formation.11–19
To our knowledge only three published studies have examined food motivation in HW youths. One fMRI study examined brain activations in HW youth and found that brain activation in the insula, amygdala and the medial frontal cortex and OFC in response to appetizing food images was greater when hungry than when satiated.20 These are similar to patterns found in adults. Another study compared adolescent and adult brain activation to food images and corroborated earlier results finding brain activation in the OFC and hippocampus.21 A recent study reported that obese children show higher activation of the dorsolateral PFC than HW children, which they hypothesize to be associated with increased inhibitory control in the obese group.22 These studies suggest that neural networks of food motivation are active in childhood and continue throughout the lifespan, although longitudinal studies are needed to better characterize this process.
Although studies have examined brain activation differences between HW and obese adults, no published studies have examined differences in brain activation between HW and obese children. Thus, little is known regarding the potential brain mechanisms of overeating and obesity in children or adolescents. This study examined brain activation in response to food images and compared the same across hunger states and weight category using previously published methods.10,20,23,24 We hypothesized increased activity in the limbic, paralimbic and prefrontal brain regions in obese vs HW children in both pre- and post-meal states.
Materials and methods
Participants and clinical measurements
Subjects included 10 obese but otherwise healthy children (5 males; mean age = 13 [11–16]) and 10 HW children (5 males; mean age = 13 [10–17]), matched for age (P = 0.99), education years (P = 1.0) (all subjects were in age-appropriate grade) and gender (both groups 5 males, 5 females). The obese children were recruited from the community by flyers and email advertisements to medical center employees. None of the children were enrolled in a weight loss intervention or were trying to lose weight. BMI was calculated as the weight-to-height ratio for age and sex as determined by growth curve charts from the CDC. The obese participants’ mean BMI was 31.3 [27.0–41.6] and were all >95%ile for age and sex. The HW controls’ mean BMI was 18.8 [15.9–22.3] and all were <85%ile. The exclusion criteria included major psychiatric diagnoses and neurological illness (parental interview), left-handedness and impaired, uncorrected vision. The study was approved by the Human Subjects Committee at the University of Kansas Medical Center. Written informed consent was obtained from a parent of each participant and written assent was obtained from subjects.
fMRI acquisition
Scanning was performed using a 3T Siemens Allegra scanner (Siemens Medical Solutions, Erlangen, Germany) fitted with a quadrature head coil. The participants’ heads were immobilized with cushions. T1-weighted anatomic images were acquired with a three-dimensional (3-D) MP-RAGE sequence (TR/TE = 23/4 ms, flip angle = 8 degrees, field of view = 256 mm, matrix = 256 × 192, slice thickness =1 mm). Two gradient echo BOLD scans were acquired in 43 contiguous coronal slices perpendicular to the AC-PC line (repetition time/echo time = 3000/30 ms, flip angle = 90 degrees, field of view = 192 mm, matrix 64 × 64, slice thickness = 3 mm (0.5 mm skip), in-plane resolution = 3 × 3 mm, 130 data points). Slice acquisition was prescribed to provide full brain coverage, from the frontal pole to the posterior regions of the occipital lobe. One anatomical and two functional sequences were run in each session (pre- and post-meal). Functional scans were 6.5 min each.
Experimental paradigm
The experimental paradigm was based on the study by LaBar et al. (2001)14 and is described in more detail in previous reports.10,20,23,24 The participants viewed pictures of food, animals and Gaussian-blurred control images during two scanning sessions: one after fasting for 4 h (pre-meal) and one immediately after eating a uniform meal (post-meal) standardized for calories (kcal = 500) and macro- and micro-nutrient content. The meal was typical lunch food consisting of a weighed turkey or ham sandwich with cheese, a piece of fruit, one vegetable and skimmed milk. This paradigm was designed to parallel normal hunger and eating cycles, and to examine the brain response to a healthy meal. The participants were instructed to consume the entire meal. The order of sessions (pre-meal, post-meal) was counter-balanced across subjects so that half of them started with the pre-meal session. All scans were conducted over the traditional lunch time (noon) to eliminate any effects related to the time of the day.
The stimuli for the two categories (food and blurred control images) were obtained from LaBar et al.14 The food images consisted of a wide range of low- and high-energy foods from fresh fruits/vegetables to steak and desserts. All images were rated as appetizing. Animal images were chosen for contrast to food images to increase the young participants’ interest in the task and to control for general familiarity. The overall goal was to generate two sets of images that were matched for valence and arousal,25 but differed on appetite generation. The animal images were obtained from professional CD-ROMs and matched to food and blurred control images on brightness, resolution and size. In addition, by applying a Gaussian kernel to a subset of the animal images (so that the objects were not identifiable), approximately 150 new blurred control images were obtained. Animals reminiscent of food (that is, fish) were removed from the stimuli pool to the best degree possible, preventing confusion between animal/food categorizations. Blurred objects were included as a low-level control comparison. Each image was presented once only to each subject.
Functional scans involved three repetitions of each block of each stimulus type (that is, food), alternated between blocks of blurred images. Visual stimuli were projected through 3-D limited-view goggles (Resonance Technology Inc., Northridge, CA, USA) connected to the stimulus-generating computer program (NeuroSTIM; Neuroscan, ElPaso, TX, USA). The stimulus presentation time was 2.5 s with a between-stimulus interval of 0.5 s. The two functional scans consisted of 13 blocks of stimuli presentation, with 10 images in each block. The order of category presentation was counterbalanced across subjects. To ensure participants were attending to the stimuli, they were instructed to remember images for a recognition memory test outside the scanner, immediately after each scanning session. From each of the food and animal groups, 50% of the images used in the scanning session (30 images) were chosen for recall (old) and interspersed with 15 new distracter images from the same category (new). The participants were instructed to press the left or right mouse button if they had seen the image in the scanner (old) or if they had not seen the image (new).
fMRI data analysis
fMRI data were analyzed using the Brain Voyager QX statistical package (Brain Innovation, Maastricht, the Netherlands). The preprocessing steps included trilinear 3-D motion correction, sinc-interpolated slice scan time correction, 3-D spatial smoothing (4 mm Gaussian filter) and high-pass filter temporal smoothing. Functional images were realigned to the anatomic images obtained within each session and normalized to the BrainVoyager template image, which conforms to the space defined by the Talairach and Tournoux26 stereotaxic atlas. Motion in any run of more than 3 mm along any axis (x, y, or z) resulted in that run getting discarded. Out of 80 total runs, three runs were discarded due to excessive motion and two runs were discarded because the subject fell asleep in the scanner.
Activation maps were generated using statistical parametric methods27 and random effects (in Brain Voyager QX). Statistical contrasts were conducted using multiple regression analysis with the general linear model, allowing multiple predictors to be built into the model. Regressors representing experimental conditions of interest were modeled with a hemodynamic response filter and entered into the multiple regression analysis using a random-effects model. Contrasts between conditions of interest were assessed with t-statistics. Statistical parametric maps were overlaid on 3-D renderings of averaged structural scans.
Three sets of analyses were completed. First, an interaction analysis was performed, focusing on the group (obese, HW) × stimulus type (food, non-food) interaction, separately for the pre- and post-meal conditions. The regions of activation resulting from these analyses identify brain areas in which the obese group showed disproportionately greater activation to food pictures than to non-food pictures, in comparison with the HW group (random effects).
Second, to determine the difference in the brain response with the hunger condition (pre-meal vs post-meal), interaction analyses were performed, focusing on the stimulus type (both food vs non-food; food vs control) × motivational state (pre-meal, post-meal) interaction, separately for the obese and HW groups.
Finally, analyses were performed targeting the difference in the brain response with the hunger condition, this time focusing on the between-group × stimulus type (food vs control) and motivational state (pre-meal, post-meal) contrasts.
On the basis of previous research, a priori regions of interest for the primary analyses included the limbic, paralimbic and prefrontal brain regions. Specifically, the following regions were examined, bilaterally: amygdala, hippocampal formation, OFC, medial PFC, lateral PFC, anterior cingulate cortex and insular cortex. For all region of interest analyses, voxels in each contrast were considered significant if the activation survived a statistical threshold of P<0.001, uncorrected (minimum cluster size: three contiguous voxels). Other areas were considered significant if they exceeded a threshold of P<0.0001, uncorrected (minimum cluster size: six contiguous voxels). For anatomical verification of a priori region activation, the Talairach coordinates for each maximum pixel were confirmed by examination of an additional anatomical brain atlas.28
Results
Behavioral data
The memory test scores for both pre- and post-meal sessions for all children were significantly higher than chance (using discriminability index). The HW group’s recognition memory for food items was 87.5% pre- and 81.9% post-meal. The obese group’s memory for food items was 82.9% pre- and 85.2% post-meal. The HW group’s memory for non-food items was 92% pre- and 91% post-meal. The obese group’s memory for non-food items was 89.8% pre- and 90.3% post-meal. The analysis of variance results for group (obese vs HW) recognition memory was not significant (F(1, 18) = 0.30, P = 0.59); the analysis of variance results for motivational state (pre- vs post-meal) were not significant (F(2, 18) = 0.001, P = 0.97). There was a significant main effect for picture type (non-food pictures were more easily recognized than food pictures; F(2, 18) = 23.14, P<0.001). The analysis of variance interaction results for the group × motivation state interaction were not significant (F(2, 18) = 2.13, P = 0.16). Finally, mixed-factor analysis of variance showed no significant between-group differences for pre- vs post-meal recognition memory for food pictures (F(2, 18) = 1.61, P = 0.22).
fMRI data
The group (obese vs HW) × stimulus type (food vs non-food; food vs control) interaction.
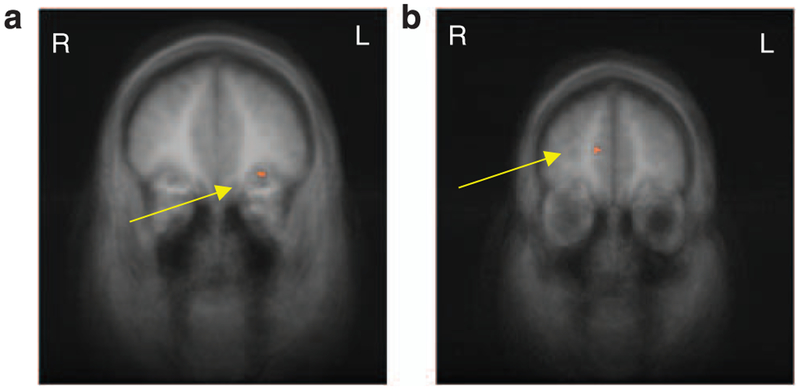
In the group (obese, HW) × stimulus type (food, non-food) interaction pre-meal condition, the obese group showed greater activation than the HW group to food vs non-food pictures in three regions of frontal/PFC (Table 1). After eating (post-meal condition), the obese group showed greater activation than the HW group to food vs non-food pictures in the OFC. Findings from the post-meal analysis are illustrated in Figure 1, highlighting activation differences in OFC between obese and HW children. In the group × stimulus type (food vs baseline control) interaction, there were several significant areas of activation between obese and HW children in our a priori region of interest in the frontal/PFC and hippocampal regions (Table 1).
Table 1.
Regions reaching significance for the contrasts between food vs non-food and food vs baseline contrasts between groups during pre-meal and post-meal states (P<0.001, random effects)
| Contrast and region | Coordinates | |||
|---|---|---|---|---|
| x | y | z | t | |
| Pre-meal: Obese > lean | ||||
| Food vs non-food | ||||
| Superior frontal gyrus | 24 | 8 | 58 | 4.25 |
| Middle frontal gyrus | −42 | 8 | 40 | 3.95 |
| Inferior frontal gyrus | −54 | 29 | 16 | 3.84 |
| Food vs baseline | ||||
| Superior frontal gyrus | −3 | 44 | 40 | 4.14 |
| −3 | 50 | 42 | 4.32 | |
| −18 | −11 | 55 | −4.19 | |
| Medial frontal gyrus | −21 | 41 | 19 | 4.28 |
| −15 | 32 | 37 | 4.47 | |
| 15 | 14 | 34 | −4.56 | |
| Lateral geniculate body | 21 | −22 | −5 | −4.43 |
| CA1 field of hippocampus | −15 | −43 | 1 | −4.68 |
| Parahippocampal gyrus | −15 | −46 | −8 | −4.29 |
| Post-meal: Obese > lean | ||||
| Food vs non-food | ||||
| Orbitofrontal cortex | −30 | 35 | −8 | 3.92 |
| Food vs baseline | ||||
| Superior frontal gyrus | 18 | 32 | 43 | 4.20 |
| 12 | 20 | 58 | 4.13 | |
| −12 | 26 | 34 | 4.39 | |
| 12 | 17 | 61 | 4.11 | |
| 15 | −4 | 70 | 5.11 | |
| 33 | 2 | 64 | 4.16 | |
| 24 | 8 | 61 | 4.64 | |
| −15 | 2 | 67 | 4.56 | |
| Inferior frontal gyrus | 51 | 11 | 10 | −5.03 |
Abbreviation: CA1, cornu ammonis 1.
Figure 1.
(a) fMRI results from the between-group food vs non-food contrasts, co-registered with average structural MRI data from the participants. The maps are presented in the coronal perspective. The significance thresholds for display are set at P<0.001, uncorrected. The arrow highlights greater activation in the left OFC in the obese group than the HW group in the post-meal condition (x = −30, y = 35, z = −8; t = 3.92). (b) fMRI results from the direct group comparison (obese, HW) of the condition (pre-meal, post-meal) and stimulus type (food, control) co-registered with average structural MRI data from the participants. The maps are presented in the coronal perspective. The significance thresholds for display are set at P<0.001, uncorrected. The arrow highlights greater reduction of activation in the medial PFC for HW vs obese groups (x = 12, y = 47, z = 16; t = 4.64). fMRI, functional MRI; HW, healthy wright; OFC, orbitofrontal cortex; PFC, prefrontal cortex.
The stimulus type (food vs non-food) × motivational state (pre-meal vs post-meal) interaction.
Second, to determine the difference in the brain response with the hunger condition, interaction analyses were performed, contrasting pre- and post-meal sessions separately for the obese and HW groups, to identify regions in which the food vs non-food contrast was greater pre-meal than post-meal. These analyses identify areas showing decreased brain activation after eating. In the HW group, the medial PFC, PFC and posterior cingulate regions responded to food images to a greater extent before eating than after eating (Table 2). Similarly, obese subjects showed significant reduction in brain activation between pre- and post-meal conditions only in the superior and medial frontal gyri and thalamus. No changes were observed in the a priori limbic and paralimbic brain regions.
Table 2.
Regions reaching significance for the contrasts between food and non-food stimuli categories within groups comparing pre- to post-meal states (P<0.001, random effects)
| Contrast and region | Coordinates | |||
|---|---|---|---|---|
| x | y | z | t | |
| Obese: Pre-meal> post-meal | ||||
| Food vs non-food | ||||
| Superior frontal gyrus | −24 | −13 | 46 | 5.48 |
| Thalamus | 18 | −25 | 7 | 6.17 |
| Medial frontal gyrus | −15 | 5 | 49 | 6.76 |
| Lean: Pre-meal> post-meal | ||||
| Food vs non-food | ||||
| Superior frontal gyrus | 0 | 47 | 19 | 5.50 |
| −21 | 32 | 55 | 7.33 | |
| Posterior cingulate gyrus | −12 | −46 | 34 | 5.52 |
| Food vs baseline | ||||
| Insular cortex | −30 | −28 | 13 | 5.03 |
| 30 | −1 | −2 | 6.17 | |
| 33 | −10 | −5 | 5.90 | |
| 42 | −13 | −5 | 6.85 | |
| 45 | 5 | 1 | 6.00 | |
| Nucleus accumbens | 12 | 2 | −5 | 6.03 |
| Ventral putamen | −21 | 2 | −5 | 8.23 |
| Amygdala | 18 | −4 | −14 | 6.06 |
| Parahippocampal cortex | 21 | 5 | −8 | 6.70 |
| −12 | −46 | 1 | 6.34 | |
| 12 | −43 | −2 | 5.43 | |
| Cingulate gyrus | 0 | −9 | 40 | 6.95 |
| 6 | 44 | 7 | 4.96 | |
| Medial frontal gyrus | 42 | −4 | 49 | 5.86 |
| 30 | −10 | 49 | 5.09 | |
| Superior frontal gyrus | −6 | 23 | 64 | 5.19 |
| 0 | 38 | 31 | 5.10 | |
| 3 | 38 | 28 | 4.84 | |
| 6 | 17 | 61 | 4.93 | |
| Inferior frontal gyrus | −33 | 32 | −5 | 5.33 |
| Precentral gyrus | −45 | −43 | 13 | 4.90 |
| Superior temporal gyrus | 54 | −40 | 19 | 6.19 |
| Thalamus | −15 | −31 | 1 | 6.86 |
| −3 | −4 | −5 | 5.44 | |
| Precuneus | −12 | −40 | 40 | 5.15 |
| 3 | −40 | 46 | 5.73 | |
| Lingual gyrus | 12 | −55 | −5 | 6.99 |
| Cerebellum | −21 | −67 | −32 | 6.20 |
These contrasts are examining reduction of brain activity after eating a meal.
The stimulus type (food vs control) × motivational state (pre-meal vs post-meal) interaction.
Similar analyses were performed, contrasting pre- and post-meal sessions separately for the obese and HW groups, using the food vs control contrast, identifying regions of reduction after eating using baseline images. Once again, these analyses identify areas exhibiting less brain activity after eating. In the HW group, the PFC, medial prefrontal cortex, anterior cingulate cortex, insula, nucleus accumbens, ventral putamen and parahippocampal gyrus, amygdala showed greater response to food images before eating than after eating (Table 2). In the obese group, only the PFC and insula responded more to food images as compared with control images before eating than after eating. Even when the statistical threshold was relaxed (P<0.05; uncorrected), there were no other significant areas of reduced brain activity in the obese group.
The group (OB vs HW) × stimulus (food vs control) × motivation (pre-meal vs. post-meal) interaction.
Finally, we conducted a direct comparison of the obese and HW groups, contrasting pre- and post-meal sessions for the food vs control stimuli, identifying regions in which HW children exhibited greater reduction (modulation) of brain activation than obese children. Figure 1b illustrates the significantly greater reduction in brain activation in the medial PFC in the HW group than in the obese group.
Discussion
This study extends the childhood obesity and food motivation literature by providing preliminary evidence of the neural mechanisms of food motivation in obese and HW children. We hypothesized that there would be increased activation in the limbic, paralimbic and PFC regions of the brain in obese vs lean children in both pre-meal and post-meal states. We further expected that the obese group would show less reduction (modulation) of brain activation between the pre- and post-meal states.
Initial findings in adults indicate that many areas implicated in normal food motivation are hyper-responsive in obese groups. Differential activations (hyperactivation) have been found in the hypothalamus,29,30 OFC,19,30 dorsal striatum,17 insula31,32 and hippocampus31 in obese individuals. Obese adults also show a smaller decrease in hypothalamic activity after a meal when compared with HW individuals.29 The implicated regions have a central role in processing motivation, reward and cognitive control, and contribute to behavioral problems such as impulsivity and addiction.33
Consistent with past studies using adults and the few published child studies, the food images in our study produced significant brain activations in the limbic and paralimbic regions for both obese and HW groups. The obese group showed greater activation to food pictures than the HW group in the frontal and paralimbic cortex (PFC, OFC) under both pre- and post-meal conditions. These findings corroborate and extend on a recent fMRI study of obese and HW children as we included two food motivation conditions (pre and post-meal) and a standardized scan time to remove any confounding of time of day.23 Our study adds to the mounting evidence that obese children are hyper-responsive to visual food stimuli when compared with HW children. Their hyper-responsiveness to food stimuli might contribute to increased food motivation, increased intake and poor health-related behaviors. Also, as Davids et al.22 suggested, increased activity in the PFC may reflect attempts to control behavior in the context of increased food motivation. Another important distinction between the HW and obese groups was that obese subjects failed to show significant post-meal reduction of activation in the prefrontal, limbic and reward processing (ventral striatum) regions, whereas the HW subjects showed a significant reduction in prefrontal and limbic activity after eating. These results indicate that the neural networks of the HW subjects are more modulated after eating a meal. These data provide evidence that obesity, even among children, is associated with abnormalities in the networks involved in motivation and regulating food selection and intake.
There are some differences between the previously published reports, with some studies identifying areas of increased activity and others decreased activity.17,34 For example, Stice et al.34 found decreased activity in the dopaminergic brain pathways (striatum) in obese individuals as compared with that in HW individuals. They proposed that obese individuals have both reduced dopamine receptor density and impaired dopamine signaling in the reward-processing areas of the brain. It is significant to note that Stice et al. scanned young adults during feeding, whereas this study examined children and adolescents while viewing food images. Future studies are needed to more fully explore differences between children and adults, and between different activation paradigms.
There are several potential limitations to this study. Some researchers have expressed some doubt about using transformation to stereotaxic space in children.35,36 Others, however, have shown that differences due to age are beneath the resolution provided by the current imaging technology.37 In addition, functional analysis maps projected onto averaged anatomical images appear not to produce false variance between age groups.37 Nonetheless, because the subjects’ ages in this study ranged from 10 to 17 years, with some pre-pubertal and some post-pubertal, we cannot rule out the effects of anatomical variability in these developing brains. Another potential limitation is the likelihood of signal loss in the ventromedial regions of the PFC due to susceptibility artifact. While we do report activation in some regions of the OFC and have verified that these activations come from regions of good signal coverage, we cannot rule out that other regions were missed. This study is also limited by the relatively small sample size and the use of uncorrected statistics. Future studies should replicate these findings with larger groups. In addition, the inclusion of psychophysiological measurements of satiety could be an important behavioral measurement to examine in conjunction with the imaging data. Finally, on the basis of the cross-sectional study design, it is impossible to determine causal relationships between brain activity and obesity. Specifically, we cannot determine whether increased brain activations cause overeating and obesity, or are the result of overeating and obesity. Longitudinal studies of individuals at risk of obesity are needed to further our understanding of the relationship between brain function and obesity. For all of these reasons, results from this study should be considered preliminary. While our study is not without limitations, our preliminary findings are intriguing and will lead to further investigation of the brain’s role in childhood obesity.
This study provides preliminary evidence that obese children are hyper-responsive to food stimuli and fail to modulate brain activation after a meal as compared with HW children. These findings have potential clinical implications, including clarifying the relationships between the neural mechanisms of obesity and weight loss interventions. This area of research is at an early stage and future studies will increase our limited understanding of the neural correlates of obesity, food motivation and other health behaviors in children. Eventually, a better understanding of the brain’s role in food motivation, reward and cognitive control might lead to specific, targeted obesity interventions and the ability to better place individuals into tailored health programs.
Acknowledgements
This project was supported by grants from the Hall Family Foundation and Hoglund Pilot Funds. The Hoglund Brain Imaging Center is supported by the Health Research and Services Administration C76HF00201. WM Brooks is supported in part by R01NS39123.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ritchie LD, Ivey SL, Woodward-Lopez G, Crawford PB. Alarming trends in pediatric overweight in the United States. Soc Prevent Med 2003; 48: 168–177. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. J Am Med Assoc 2002; 288: 1723–1727. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. J Am Med Assoc 2006; 295: 1549–1555. [DOI] [PubMed] [Google Scholar]
- 4.Baker JL, Olsen LW, Sorensen TIA. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 2007; 357: 2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med 2007; 357: 2371–2379. [DOI] [PubMed] [Google Scholar]
- 6.Goran MI, Ball GDC, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab 2002; 88: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 7.Daniels SR. The consequences of childhood overweight and obesity. Future Children 2006; 16: 47–67. [DOI] [PubMed] [Google Scholar]
- 8.DelParigi A, Pannacciulli N, Le D, Tataranni PA. In pursuit of neural risk factors for weight gain in humans. Neurobiol Aging 2005; 26S: S50–S55. [DOI] [PubMed] [Google Scholar]
- 9.DelParigi A, Gautier JF, Chen K, Salbe AD, Ravussin E, Reiman E et al. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann NY Acad Sci 2002; 967: 389–397. [PubMed] [Google Scholar]
- 10.Martin LE, Holsen LM, Chambers R, Bruce AS, Brooks WM, Zarcone JR et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity 2010; 18: 254–260. [DOI] [PubMed] [Google Scholar]
- 11.Gordon CM, Dougherty DD, Rauch SL, Emans SJ, Grace E, Lamm R et al. Neuroanatomy of human appetitive function: a positron emission tomography investigation. Int J Eating Disord 2000; 27: 163–171. [DOI] [PubMed] [Google Scholar]
- 12.Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. Eur J Neurosci 2004; 20: 1411–1418. [DOI] [PubMed] [Google Scholar]
- 13.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high-versus low-calorie foods. NeuroImage 2003; 19: 1381–1394. [DOI] [PubMed] [Google Scholar]
- 14.LaBar KS, Gitelman DR, Parrish TB, Kim Y, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci 2001; 115: 493–500. [DOI] [PubMed] [Google Scholar]
- 15.Morris JS, Dolan RJ. Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. J Neurosci 2001; 21: 5304–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron 2002; 28: 668–671. [DOI] [PubMed] [Google Scholar]
- 17.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage 2007; 27: 410–421. [DOI] [PubMed] [Google Scholar]
- 18.Tataranni PA, Gautier G, Chen K, Uecker A, Bandy D, Salbe AD et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 1999; 96: 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volkow ND, Wang G, Fowler JS, Logan J, Jayne M, Franceschi D et al. ‘Nonhedonic’ food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 2002; 44: 175–180. [DOI] [PubMed] [Google Scholar]
- 20.Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS et al. Neural mechanisms underlying food motivation in children and adolescents. NeuroImage 2005; 27: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Killgore WD, Yurgelun-Todd DA. Developmental changes in the functional brain responses of adolescents to images of high and low-calorie foods. Dev Neurobiol 2005; 47: 377–397. [DOI] [PubMed] [Google Scholar]
- 22.Davids S, Lauffer H, Thoms K, Jagdhun M, Hirschfeld H, Domin M et al. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obesity 2010; 34: 94–104. [DOI] [PubMed] [Google Scholar]
- 23.Holsen LM, Zarcone JR, Brooks WM, Butler MB, Thompson TI, Ahluwalia JS et al. Neural mechanisms underlying hyperphagia in Prader–Willi syndrome. Obesity 2006; 14: 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holsen LM, Zarcone JR, Chambers RJ, Butler MG, Bittel D, Brooks WM et al. Genetic subtype differences in neural circuitry of food motivation in Prader–Willi syndrome. Int J Obesity 2009; 33: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida: Gainsville, FL, 2008. [Google Scholar]
- 26.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers Inc.: New York, 1988. [Google Scholar]
- 27.Friston KJ, Holmes AP, Worsely JJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping 1995; 2: 189–210. [Google Scholar]
- 28.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain, 2nd edn Academic Press: San Diego, 2003. [Google Scholar]
- 29.Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999; 48: 1801–1806. [DOI] [PubMed] [Google Scholar]
- 30.Tataranni PA, DelParigi A. Functional neuroimaging: a new generation of human brain studies in obesity research. Obesity Rev 2003; 4: 229–238. [DOI] [PubMed] [Google Scholar]
- 31.Gautier J, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M et al. Differential brain responses to satiation in obese and lean men. Diabetes 2000; 49: 838–846. [DOI] [PubMed] [Google Scholar]
- 32.Gautier J, DelParigi A, Chen K, Salbe AD, Bandy D, Pratley RE et al. Effect of satiation on brain activity in obese and lean women. Obesity Res 2001; 9: 676–684. [DOI] [PubMed] [Google Scholar]
- 33.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005; 162: 1403–1413. [DOI] [PubMed] [Google Scholar]
- 34.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA1 gene. Science 2008; 322: 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaillard WD, Grandin CB, Xu B. Developmental aspects of pediatric fMRI: considerations for image acquisition, analysis, and interpretation. NeuroImage 2001; 13: 239–249. [DOI] [PubMed] [Google Scholar]
- 36.Yoon U, Fonov VS, Perusse D, Evans AC. The effect of template choice on morphometric analysis of pediatric brain data. Neuroimage 2009; 45: 769–777. [DOI] [PubMed] [Google Scholar]
- 37.Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE et al. Feasibility of a common stereotactic space for children and adults in fMRI studies of development. NeuroImage 2002; 17: 184–200. [DOI] [PubMed] [Google Scholar]