Abstract
The recently discovered glymphatic system, which supports brain-wide clearance of metabolic waste, has become the subject of intense research within the past few years. Its nomenclature arose due to its functionally analogous nature to the lymphatic system in combination with glial cells that are part of its anatomical boundaries. The influx of cerebrospinal fluid (CSF) from perivascular spaces into the brain interstitium acts to clear intraparenchymal solutes. CSF is produced by the choroid plexus and flows from the ventricles to the sub-arachnoid space via the cisterna magna, and as such the injection of tracer molecules into any one of these spaces could be used for studying CSF movement through the glymphatic system. Of these options, the cisterna magna is most favorable as it offers a route of entry that does not involve craniotomy. Herein we describe the cisterna magna (CM) injection procedure carried out in rats, essential for studying glymphatic influx and efflux dynamics.
1. Introduction
The glymphatic system is an exciting new, systems-level, discovery in the mammalian brain. Except for the brain, the body possesses a lymphatic system, acting to pick up excess fluid and solutes from the various parenchymal interstitia, and return it back to cardiovascular circulation, furthermore, sampling and delivering antigens to sentinel lymph nodes, thereby acting as an immune surveillance and delivery system [1]. Until the discovery of the glymphatic system, it was a paradox that the organ responsible for some of the highest rates of energy metabolism in the mammalian body seemingly lacked efficient means by which to remove extracellular fluid and solutes [2, 3]. Prior to the discovery of the glymphatic system it was speculated that metabolic waste was cleared from the central nervous system by diffusion.
In 2012 the first description of the glymphatic system was put forward by Iliff et al. (2012). The physiological processes underlying glymphatic function rests in the bulk flow of CSF driven into perivascular spaces wherefrom it can exchange solutes with the interstitial fluids of the brain parenchyma, the CSF thereby acting as a global, waste-removing, filtrate [4–6]. Literature is currently divided on which precise force drives the movement of CSF in the brain parenchyma, whether it be convection, diffusion, or even a combination of both (advection), however, it is known that the aquaporin-4 (AQP4) water channel is of utmost importance for normal glymphatic function [7–10]. The location of APQ4 is polarized to the astrocytic endfeet in the plasma membrane that contributes to the formation of the outer barrier of the perivascular space [7, 8, 10, 11]. The necessity of AQP4 channels for normal glymphatic function is believed to lie in their capacity to reduce the resistance of movement of H2O from the perivascular space and into the brain parenchyma [12, 13].
As with the discovery of any new field of research, so too do methods arise by which to study it. The method used to study the glymphatic system in vivo and ex vivo imaging involves the injection of tracers into the CSF at the cisterna magna, however, the method sections in the published articles may not provide sufficient information to get started easily.
Previously, injections into the CSF have not uncommonly been done in the ventricles, which have the capacity to confound results due to its invasive nature of penetrating the skull and the entire depth of cortex before reaching the ventricles. For glymphatic system analysis it is therefore preferable to cannulate the cisterna magna and this approach has proven to be an efficient and relatively non-invasive way of injecting [10, 14]. This minimally invasive procedure involves the insertion of a small cannula into the CM, through which small volumes of tracer may be injected into the CSF, at a controlled rate, thus making fluctuations in ICP negligible and preventing trauma to the brain parenchyma [15]. Furthermore, this method permits the injection of tracer and study of fluid motion dynamics in awake animals, allowing for glymphatic study in different arousal states.
In this chapter, we will describe an adapted method by which the CM cannulation procedure can be performed in the larger rat model, expanding on the tools, resources and procedures one could exploit in order to carry out experiments that aim to analyse the glymphatic system.
2. Materials
2.1. Animals
Sprague Dawley Rats (adult, 10 weeks).
2.2. Disposables
1 mL and 2 mL syringes.
100 μL Hamilton syringe.
Polyethylene (PE) tubing (PE10, internal diameter: 0.28 mm).
30G dental needles.
30G and 18G hypodermic needles.
Alcohol pads.
Cotton swabs.
Glue accelerator.
Super glue (cyanoacrylate glue).
2.3. Fixed Equipment
Surgical tools (scalpel, blade, tweezers, forceps, haemostat, needle holder, fine scissors).
Micro-infusion pump.
High-temperature cautery pen.
Stereotaxic frame with rat adaptor.
Heating pad.
2.4. Solutions
Anaesthesia: prepare the KX anaesthetic cocktail containing 10 mg/mL of Ketamine and 2 mg/mL of Xylazine in sterile sodium chloride (NaCl, veterinary grade) 0.9 %; suggested dose of KX cocktail for anaesthesia is 10 μL/gram body weight. In addition, prepare a 10 % ketamine solution in saline. The 10 % ketamine without xylazine is used for re-dosing animals, if the length of the procedure requires re-dosing.
CSF tracer dissolved in artificial CSF (126 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 2 mM CaCl2, 10 mM glucose, 26 mM NaHCO3; pH 7.4 when gassed with 95% O2 and 5% CO2).
NaCl solution 0.9 % in water.
De-ionized water.
Phosphate buffered saline (PBS) 1X (pH 7.4): prepare 1L of PBS 1X, add in a suitable recipient 100 mL of PBS 10X, and 900 mL of ultrapure water; store at 4 C.
Paraformaldehyde (PFA) 4% in PBS: to prepare 1L of PFA 4%, add 980 mL of PBS 1X (pH 7.4) in a 1 L bottle, and heat to 60 C on a hot plate (or in microwave oven); weigh 40 g of PFA powder in the fume hood; under the hood, dissolve PFA powder in PBS 1X, stirring and keeping the temperature at approximately 60 C; add NaOH, until the solution clears; adjust the pH to 7.4, and volume to 1 L with PBS 1X; let the solution cool down, filter it; prepare aliquots of 50 mL and store at 4C for short term storage or −20 C for long-term storage.
3. Methods
The following protocol describes acute cannulation of the cisterna magna (CM) for immediate injection of tracer molecules or drugs into the cistern. Although this is a non-recovery surgery, sterile conditions are recommended. Chronic CM cannulation can also be performed and the CSF tracers injected later. This approach is thus useful for studies of glymphatic activity in awake animals.
3.1. Preparation of the cannula
Prepare the cannula by breaking off the metal tip of a 30G dental needle (break off a tip of approximately 5–10 mm) with a haemostat and inserting the tip into one end of a ca. 30 cm PE10 tubing.
Attach the other end of the tubing to a 100 μL Hamilton syringe filled with saline using a 30G needle and fill the tubing with saline. (see Note 1). Avoid air bubbles in the tubing.
Fit the syringe onto the micro-infusion pump (see Note 2).
Using the micro-infusion pump (but can also be done by hand), first withdraw a small air bubble and then fill the cannula with up to 30 μL of tracer, avoiding that the tracer mixes with and gets diluted in the saline (see Note 3). This step can be done immediately before the injection (see Note 4). Remember to check that the diameter setting on the micro-infusion pump corresponds to the diameter of the Hamilton syringe.
3.2. Surgical procedure
Weigh the rat (Sprague Dawley, both sexes, adult stage) and anaesthetise it by injecting a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg) intraperitoneally (i.p.) (see Note 5). Wait approximately 5–15 minutes, check for toe and tail pinch reflexes and if necessary re-administer 20% of the original dose of the anaesthetic.
When the rat is deeply anaesthetised and breathing steadily, shave the fur around the posterior end of the head to expose the skin.
Place the rat in the stereotaxic frame and fix the head to the frame with the nose of the rat slightly pointing downwards.
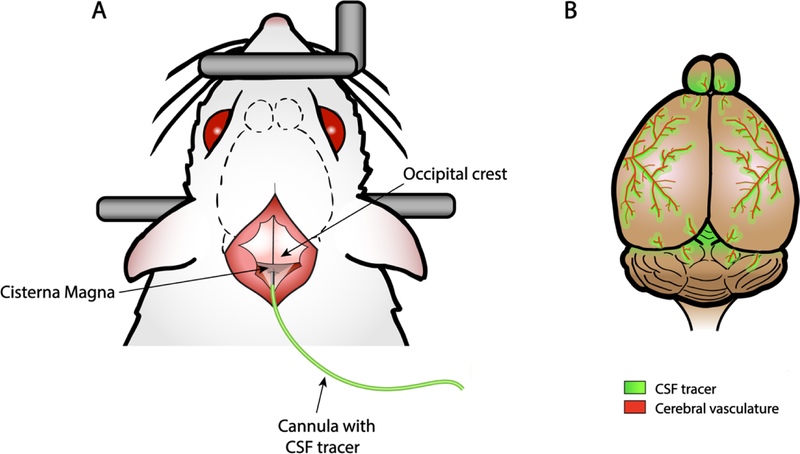
Locate the posterior end of the skull at the level of the ears and open the skin by cutting approximately 1 cm along the midline using fine scissors. The occipital crest can be used as a reference point (Figure 1).
Pull apart the superficial connective tissue using fine, curved forceps to expose the neck muscles. Use cotton swabs to control any resulting bleeding. It is important to only pull or tear tissue gently using curved forceps or cotton swabs and only use scissors for the skin. Using scissors to cut connective tissue or muscle will cause excessive bleeding.
Using the tip of curved forceps to carefully separate the muscles of the neck at the midline in the anterior-to posterior axis. The CM can be exposed by pulling the muscles aside. The CM can be recognised as an inverted grey triangle covered by the transparent dural membrane between the cerebellum and the medulla, at the base of the skull (Figure 1).
Keep the muscles apart with help of retractors and wipe the dural membrane above the CM using a cotton swab. If bleeding from connective tissue occurs, use saline and cotton swabs to clean and dry the dura covering the CM (see Note 6).
Hold the cannula, filled with CSF tracer, by the tubing-covered dental needle with a pair of curved serrated tweezers.
Insert the bevelled end of the needle of the cannula in the centre of the CM, perpendicular to the dura. Only the needle tip should be inserted, as shown in Figure 1 (approximately 1–2 mm, see Note 7). It is recommended to dry off any CSF leakage resulting from the insertion using cotton swabs before the next step.
Apply a few drops of cyanoacrylate glue, or dental cement mixed with cyanoacrylate glue, onto the needle insertion site and spray glue accelerator to immediately cure the glue. Hold the needle in place until the glue is dry.
Fig. 1. Schematic representation of the cisterna magna cannulation procedure and expected outcome.
A) Surgical procedure for the cannulation of the cisterna magna (CM). The occipital crest (posterior end of the skull) can be used as a reference point. Pulling the neck muscles aside, the CM can be recognised as an inverted grey triangle covered by the transparent dural membrane. The bevelled end of the needle of the cannula is injected in the centre of the CM, perpendicular to the dura. B) Rat brain showing the perivascular influx of the CSF tracer injected (30 minutes after injection).
3.2. CSF tracer injection
Start the injection of CSF tracer into the CM using the micro-injection syringe pump at a rate of 1–2 μL per minute for 10–20 minutes, resulting in a total volume injected of 20 μL.
After injection, stop the pump and seal the PE10 tubing (approximately 3–4 cm in length from the cannula insertion site) by cauterizing the tubing connected to the cannula, using a high-temperature cautery, in order to avoid CSF tracer reflux when cutting the tubing (see Note 8).
Carefully place the rat on a heating pad with the same angle between head and body used during injection, and allow time for the CSF tracer to circulate through the brain. The standard procedure for the study of glymphatic influx requires 30–60 minutes of tracer circulation. To study glymphatic efflux, the tracer should instead be allowed to circulate for approximately 3 hours. Experiments with 30–60 min and 3 hour time points provide different information, but the 3 hour time point is only meaningful when comparing to a shorter time point.
Under deep anaesthesia, euthanize the animal by decapitation or transcardial perfusion (see Note 9), dissect the intact brain with olfactory bulbs and immersion fix it in a solution of 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS; 0.01 M; pH 7.4) overnight at 4°C.
4. Notes
In the case of chronic cannulation, given that the liquid from the cannula will directly access the CM at the moment of the injection, the use of artificial CSF is preferred over saline.
A smaller Hamilton syringe can be used as well, the maximum size being 100 μL. In this case, the Hamilton syringe should only be filled up to a maximum volume of 70 μL of ddH2O in order to enable the withdrawal of up to 30 μL of CSF tracer.
The presence of an air bubble separating the saline from the tracer is pivotal to control the delivery of pure tracer and not saline-diluted tracer. To make sure that the exact amount of tracer is injected in the CM, it is useful to mark with a pen on the tube the line separating the tracer and the air bubble before and after injection. At the end of the injection, the amount of tracer injected can be calculated based on the length of the tracer injected and the internal diameter of the tubing. This allows for adjustments of the tracer volume injected, if needed.
The CSF tracer can be withdrawn from the vial at any time after cannula preparation. However, it is optimal to withdraw the tracer from the vial immediately before injection, or leave the needle tip in the tracer solution until immediately before the injection. This serves two purposes, 1) the tracer can be kept in the dark for a longer time and 2) keeping the needle tip in the tracer solution prevents the dye from drying out and crystals forming at the tip of the needle. In any case, smooth movement of the tracer in the cannula should be checked before injection by running the micro-infusion pump immediately before the tracer injection.
When assessing glymphatic function, it should be taken into consideration that glymphatic activity is enhanced in ketamine/xylazine anesthetized rats compared to the awake state, closely resembling natural sleep state [16]. The type of anaesthesia for glymphatic function plays a critical role, e.g. glymphatic function is decreased under pentobarbital and isoflurane anaesthesia [17, 18]. If the awake state is to be studied, the cannula may be implanted during isoflurane anaesthesia (induction at 2.5–3 %, thereafter 2 % isoflurane, in circa 1 L/min O2) and a closed tubing with artificial CSF may be implanted in connection with the needle. The tubing can then be cut and the tracer inserted into the implanted tubing.
The point of injection should be clean and dry, in order to avoid blood clotting at the needle tip, and fluid-bridging between the inside and outside of the cisterna magna, respectively. In addition, retractors or hooks can be used to keep the skin and the muscles apart. Eye ointment should be applied to prevent corneal drying.
When inserting the needle, a little pressure needs to be applied to be able to break through the dura membrane covering the CM. The penetration depth should only be of approximately 1–2 mm, until the needle tip is inside, to avoid perforation of the cerebellum or the medulla.
The method of euthanasia best suited depends upon the experimental purpose. Perfusion or decapitation under anaesthesia may be the preferred options.
References
- 1.Liao S, Padera TP (2013) Lymphatic Function and Immune Regulation in Health and Disease. Lymphat Res Biol 11:136–143. doi: 10.1089/lrb.2013.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cumming P, Ase A, Diksic M, et al. (1995) Metabolism and blood-brain clearance of l-3,4-dihydroxy-[3H]phenylalanine ([3H]DOPA) and 6-[18F]fluoro-l-DOPA in the rat. Biochem Pharmacol 50:943–946. doi: 10.1016/0006-2952(95)00216-M [DOI] [PubMed] [Google Scholar]
- 3.Shram N, Netchiporouk L, Cespuglio R (2002) Lactate in the brain of the freely moving rat: Voltammetric monitoring of the changes related to the sleep-wake states. Eur J Neurosci 16:461–466. doi: 10.1046/j.1460-9568.2002.02081.x [DOI] [PubMed] [Google Scholar]
- 4.Nedergaard M (2013) Garbage truck of the brain. Science (80-) 340:1529–1530. doi: 10.1126/science.1240514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen NA, Munk ASF, Lundgaard I, Nedergaard M (2015) The Glymphatic System: A Beginner’s Guide. Neurochem Res 40:2583–2599. doi: 10.1007/s11064-015-1581-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boespflug EL, Iliff JJ (2018) The Emerging Relationship Between Interstitial Fluid–Cerebrospinal Fluid Exchange, Amyloid-β, and Sleep. Biol Psychiatry 83:328–336. doi: 10.1016/j.biopsych.2017.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mestre H, Kress BT, Zou W, et al. (2017) Aquaporin-4 dependent glymphatic solute transport in rodent brain. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kress BT, Iliff JJ, Xia M, et al. (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76:845–861. doi: 10.1002/ana.24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundgaard I, Lu ML, Yang E, et al. (2017) Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab 37:2112–2124. doi: 10.1177/0271678X16661202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iliff JJ, Wang M, Liao Y, et al. (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4:147ra111. doi: 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagelhus EA, Ottersen OP (2013) Physiological Roles of Aquaporin-4 in Brain. Physiol Rev 93:1543–1562. doi: 10.1152/physrev.00011.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solenov E (2004) Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. AJP Cell Physiol 286:426C–432. doi: 10.1152/ajpcell.00298.2003 [DOI] [PubMed] [Google Scholar]
- 13.Huber VJ, Igarashi H, Ueki S, et al. (2018) Aquaporin-4 facilitator TGN-073 promotes interstitial fluid circulation within the blood–brain barrier. Neuroreport 29:697–703. doi: 10.1097/WNR.0000000000000990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xavier ALR, Hauglund NL, von Holstein-Rathlou S, et al. (2018) Cannula Implantation into the Cisterna Magna of Rodents. J Vis Exp e57378–e57378. doi: 10.3791/57378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Kress BT, Weber HJ, et al. (2013) Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med 11:1. doi: 10.1186/1479-5876-11-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie L, Xie L, Kang H, et al. (2013) from the Adult Brain. 373:373–378. doi: 10.1126/science.1241224 [DOI] [Google Scholar]
- 17.Groothuis DR, Vavra MW, Schlageter KE, et al. (2007) Efflux of drugs and solutes from brain: The interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters. J Cereb Blood Flow Metab 27:43–56. doi: 10.1038/sj.jcbfm.9600315 [DOI] [PubMed] [Google Scholar]
- 18.Benveniste H, Lee H, Ding F, et al. (2017) Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology 127:976–988 [DOI] [PMC free article] [PubMed] [Google Scholar]