To the Editor:
Prader–Willi syndrome (PWS) is a classic genomic imprinting disorder in which affected individuals display hypotonia, failure to thrive, feeding difficulties, developmental delays and hypogenitalism/hypogonadism in infancy. At approximately 2–3 years of age, hyperphagia, central obesity, short stature, small hands and feet, behavioral problems and characteristic facial features are present [Cassidy, 1984; Butler, 1990; Bittel and Butler, 2005; Butler et al., 2006]. The genetic causes are generally due to a paternal deletion of the chromosome 15q11–q13 region (about 70%), maternal disomy 15 (UPD) (about 25%), or imprinting defects or translocations involving chromosome 15 (about 5%) [Bittel and Butler, 2005]. The characteristic findings of hyperphagia, central obesity, short stature, cryptorchidism and hypogonadism are consistent with endocrine and/or metabolic abnormalities involving the hypothalamic–pituitary axis. While some studies have examined specific hormonal levels in Prader–Willi syndrome [Nagai et al., 1998; Burman et al., 2001; Eiholzer and Lee, 2006; Eiholzer et al., 2006; Butler et al., 2007], a comprehensive examination of follicle stimulating hormone (FSH), leutinizing hormone (LH), estradiol and testosterone levels is lacking in individuals with Prader–Willi syndrome with known genetic subtypes. Thus, we report FSH, LH, estradiol and testosterone levels in a relatively large cohort of males and females (16 years of age and older) with Prader–Willi syndrome as a function of genetic subtype and obesity status.
The regulation of FSH and LH secretion and subsequent estradiol (female) and testosterone (male) secretion is highly integrated involving multiple signaling pathways, receptor systems and signaling molecules such as inhibin and ghrelin [Ojeda et al., 2006]. In females, FSH levels vary throughout the menstrual cycle peaking at mid-cycle while LH levels peak prior to ovulation. Both FSH and LH levels increase dramatically after menopause. Hypothalamic hypogonadism subjects are characterized by low FSH, LH and sex hormone levels while normal or elevated FSH levels indicate a gonadal origin. Elevated LH levels are seen in females with ovarian failure while low levels are seen in pituitary failure.
In males, there is a prominent neonatal surge of LH, FSH and testosterone that begins shortly after birth and peaks at 6–8 weeks of age before gradually waning over 4–6 months [Veldhuis et al., 2006]. This early rise in FSH and LH is not observed in females. At 10 years of age for females and at 11 years of age in males, adrenarche usually occurs as defined by the appearance of pubic and axillary hair and increased apocrine sweat gland activity. This event is triggered by increased adrenal production of dehydroepiandrosterone, dehydroepiandrosterone sulfate and androstenedione but not cortisol [Veldhuis et al., 2006]. However, maturation of the testes and ovaries (gonadarche) requires the orderly activation of the hypothalamic–pituitary–gonadal axis. The initiation of this process remains poorly defined but involves increased pulsatile secretion of gonadotropin releasing hormone (GnRH) that subsequently controls FSH and LH secretion from the anterior pituitary [Veldhuis et al., 2006]. This orderly process of maturation is somehow lost resulting in the commonly observed hypogenitalism/ hypogonadism seen in PWS. Most studies in Prader–Willi syndrome suggest that abnormal regulation of the hypothalamic–pituitary–gonadal axis results from abnormalities within the hypothalamus [Burman et al., 2001; Eiholzer and Lee, 2006]. Other studies suggest that gonadal failure may be due to a combined hypothalamic and peripheral hypogonadism, the latter potentially due in part to cryptorchidism in males [Eiholzer et al., 2006].
In addition to the common finding of hypogonadism in Prader–Willi syndrome, several reports of precocious puberty have been reported [Vanelli et al., 1984; Hockey et al., 1987; Linnenmann et al., 1999]. At least three documented pregnancies in females with PWS have been reported further complicating the underlying mechanisms responsible for the observed gonadal dysfunction in PWS [Akefeldt et al., 1999; Schulze et al., 2001].
Herein, we present gonadal hormone data (fasting FSH, LH, estradiol and testosterone levels) from 26 females (age 16–50 years) and 24 males (age 16–45 years) with genetically confirmed PWS. Genetic testing included DNA methylation studies, cytogenetic analysis with FISH using the SNRPN probe and/or genotyping informative DNA microsatellites from the 15q11–q13 region and genomic DNA from the parents and individuals with PWS to identify the deletion type (type I or type II) or UPD status as previously described [Bittel and Butler, 2005]. There were 7 females with the typical larger type I deletion (mean age 29.4 years), 11 females with the smaller typical type II deletion (mean age 22.0 years) and 8 females with UPD having a mean age of 26.3 years (Table I). There were 8 males with the type I deletion (mean age 26.8 years), 6 males with the type II deletion (mean age 26.7 years) and 10 males with UPD having a mean age of 26.2 years (Table II). The overall female to male ratio was 26:24 and their ages ranged from 13 to 50 years with a combined mean age of 25.9 years. Ninety percent of our subjects were Caucasian. The study was approved by the local institutional review board and informed consent obtained from the participants and/or legal guardians. Our study examined several variables including age, obesity status, gender and genetic subtype that may influence hormone levels in PWS.
TABLE I.
Age, BMI, Gonadotropin and Sex Hormone Levels in Females With Prader–Willi Syndrome (PWS)
| PWS total |
PWS deletion type I |
PWS deletion type II |
PWS UPD |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | N | Mean±SD | N | Mean±SD | N | Mean±SD | N | Mean±SD | P-value |
| Age (years) | 26 | 25.3±8.0 | 7 | 29.4±10.8 | 11 | 22.0±6.2 | 8 | 26.3±6.2 | 0.147* |
| BMI (kg/m2) | 26 | 33.9±9.4 | 7 | 36.2±13.2 | 11 | 33.6±7.9 | 8 | 32.4±8.3 | 0.739* |
| FSH (mIU/ml) | 25 | 3.5±3.3 | 7 | 4.4±4.8 | 11 | 2.2±2.2 | 7 | 4.6±2.6 | 0.354** |
| LH (mIU/ml) | 26 | 1.6±1.4 | 7 | 1.7±1.3 | 11 | 1.0±0.9 | 8 | 2.3±1.7 | 0.177** |
| Estradiol (pg/ml) | 25 | 25.5±17.6 | 6 | 28.7±13.1 | 11 | 24.4±14.5 | 8 | 24.8±25.1 | 0.983** |
Normal reference range: follicular stimulating hormone (FSH) for ≥16-year-old females (follicular phase)—2.8–11.3 mIU/ml; lutenizing hormone (LH) for ≥16-year-old females (follicular phase)—1.1–11.6 mIU/ml; estradiol for ≥16-year-old females (follicular phase)—30–100 pg/ml, estradiol (luteal phase)—70–300 pg/ml, estradiol (postmenopausal)—<15 pg/ml.
P-values calculated by using one-way ANOVA.
P-values calculated by using univariate analysis of variance among all three genetic subtype groups (deletion type I, deletion type II and UPD) adjusting for age and BMI.
TABLE II.
Age, BMI, Gonadotropin and Sex Hormone Levels in Males With Prader–Willi Syndrome (PWS)
| PWS total |
PWS deletion type I |
PWS deletion type II |
PWS UPD |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | N | Mean±SD | N | Mean±SD | N | Mean±SD | N | Mean±SD | P-value |
| Age (years) | 24 | 26.5±8.7 | 8 | 26.8±4.1 | 6 | 26.7±11.5 | 10 | 26.2±10.3 | 0.989* |
| BMI (kg/m2) | 24 | 35.0±9.0 | 8 | 35.2±9.6 | 6 | 36.3±9.3 | 10 | 34.0±9.1 | 0.889* |
| FSH (mIU/ml) | 24 | 13.4±14.8 | 8 | 12.3±11.0 | 6 | 27.1±22.0 | 10 | 7.0±4.9 | 0.010** |
| LH (mIU/ml) | 24 | 2.9±2.4 | 8 | 1.7±2.0 | 6 | 4.9±3.3 | 10 | 2.6±1.1 | 0.019** |
| Testosterone (ng/ml) | 23 | 1.5±1.4 | 8 | 1.1±1.0 | 5 | 1.4±1.6 | 10 | 1.9±1.7 | 0.543** |
Normal reference range: follicular stimulating hormone (FSH) for ≥16-year-old males—0.7–11.1 mIU/ml; lutenizing hormone (LH) for ≥16-year-old males—0.8–7.6 mIU/ml; testosterone for ≥16-year-old males—2.45–18.4 ng/ml.
P-values calculated by using one-way ANOVA.
P-values calculated by using univariate analysis among all three genetic subtype groups (deletion type I, deletion type II and UPD) adjusting for age and BMI.
None of the PWS subjects were on growth, thyroid or sex hormone therapy. Eleven of the 50 individuals with PWS (7 deletion and 4 UPD) had a history of diabetes mellitus, nonetheless, only 4 individuals were being treated with insulin at the time of the study. Height to the nearest 0.1 cm and weight to the nearest 0.1 kg were obtained at baseline for each subject in the clinical setting. Body mass index (BMI) was calculated (kg/m2) for each subject and obesity defined as BMI ≥30 for adults (≥18 years) and a BMI ≥95% using published standards for sex for subjects less than 18 years [Kuczmarski et al., 2000].
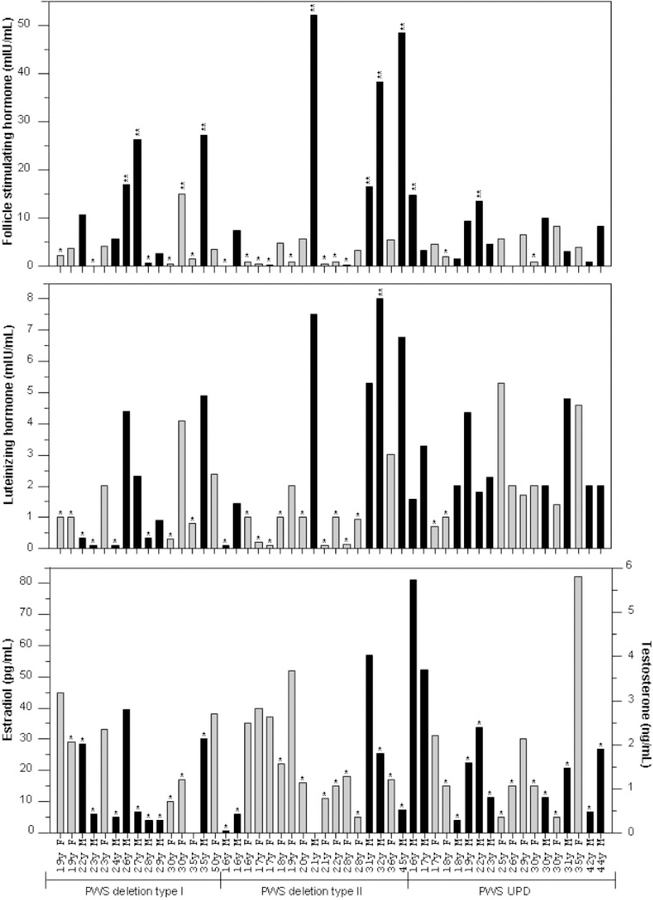
Plasma FSH, LH, estradiol and testosterone levels were measured in fasting blood samples drawn between 7:00 and 10:00 AM after a 12 hr overnight fast. FSH and LH were measured using Immulite® 2000 chemiluminescence assay kits by Siemans (Los Angeles, CA). Estradiol and total testosterone levels were determined by radioimmunoassays (RIA) using kits also purchased from Siemans. Standard laboratory methods were followed for all assays. Reference ranges were provided in the Immulite® assays kits and were comparable to published standards [e.g., Robertson and Shilkofski, 2005] or available commercially (e.g., Esoterix, Austin, TX). All participants were consuming a weight-maintaining diet appropriate for Prader–Willi syndrome for at least 2 days before blood sampling. Individual values for FSH, LH, estradiol and testosterone are shown in Figure 1.
Fig. 1.
Histograms of follicular stimulating hormone (FSH), lutenizing hormone (LH), estradiol and testosterone levels for each subject with Prader–Willi syndrome. * and ** denote below and above the normal range, respectively. Males are represented by black bars and females by gray bars.
Experimental data (age and BMI) were analyzed by one-way ANOVA and Pearsonian correlation coefficients were calculated. The three genetic subgroups were analyzed by univariate analysis of variance with age and BMI correction. Male and female data were analyzed separately. Statistical significance was set at 5% for all tests. Statistical analyses were performed using SPSS version 12 for Windows (SPSS, Inc., Chicago IL).
For PWS females, the average values for FSH and LH were within the low normal range while the average estradiol levels were below the normal range and equivalent to the postmenopausal range for all genetic subtypes. Twelve of 25 PWS females had FSH levels below the age related normative range and one PWS female (30 years old with type I deletion) had an elevated FSH level. Fifteen of the 26 PWS females had low LH levels. Fifteen of 25 PWS females had low estradiol levels. Low normal FSH and LH levels and low estradiol levels found in the PWS females would support the hypogonadism (particularly hypothalamic hypogonadism) and infertility reported in PWS. In addition, there was no significant correlation among the individual female hormone levels and BMI values but a significant positive correlation (r = 0.44, P = 0.026) was found for only age and LH levels in the females.
Analysis of hormone levels among subtypes was analyzed by univariate analysis of variance with correction for age and BMI. There were no significant differences among the three genetic subtypes for any of the measured values (Table I); however, the mean LH level for PWS females with UPD was almost twice the value for PWS females with a deletion subtype (type I and type II) but not significantly different (e.g., 2.3–1.0 mIU/ml, P = 0.133 for UPD vs. type II deletion). This finding does suggest possible subtle differences in hypothalamic function in PWS individuals with UPD, although if present, the differences appear not to be clinically significant. The low normal values in this study are somewhat surprising in light of the common finding of hypogonadism in PWS females. These results further suggest other abnormal components in the sexual maturation process of PWS females which requires a pulsatile release of GnRH [Veldhuis et al., 2006]. Alternatively, the observed hypogonadism in PWS could result from abnormalities in the serotonin signaling pathways. Several studies suggest that release of FSH and LH is controlled through serotonin pathways [Ulrich et al., 1994; Quirk and Siegel, 2005]. In addition, work in our laboratory and by others has shown abnormalities in serotonin receptor synthesis in PWS individuals [Kishore and Stamm, 2006; Bittel et al., 2007]. It is also possible that alterations in other mediators, such as leptin and/or ghrelin, may induce central effects at the hypothalamus through the neuropeptide Y receptor pathway and further contribute to the observed hypogonadism seen in PWS females [Chanoine et al., 2002; Butler and Bittel, 2007].
For the PWS males, 19 of the 23 testosterone values were below the referenced normative range and four values were normal. Eighteen of the 24 PWS males had normal LH levels while five values were below the age related normative range. Twelve of the 24 FSH measurements were within the age related normative reference range for males; three values were below and nine were elevated. Data analysis for the PWS males did reveal significant differences for mean FSH and LH levels among the genetic subgroups. For example, PWS males with type II deletions had significantly higher levels of both FSH and LH when compared to other subgroups by univariate analysis of variance (P = 0.010 for FSH and P = 0.019 for LH). Interestingly, the mean testosterone levels were below the normative range for all PWS males regardless of genetic subtype whereas the mean FSH level was above the normative range for the combined genetic subgroups. However, the type II deletion males had FSH levels nearly double the upper reference value (27.3 mIU/ml for type II deletion subjects compared to 11.1 mIU/ml for the upper reference value). The elevated FSH levels in the type II deletion males are similar to those observed in Klineflelter syndrome. However, in Klinefelter syndrome the LH levels are also elevated [Kamischke et al., 2003]. Although the mean LH value of 4.9 mIU/ml for type II deletion PWS males in our study is within the normal range (0.8–7.6 mIU/ml); this mean value was significantly elevated in comparison to the values seen for type I deletion and UPD subjects. The finding of low testosterone levels and elevated to normal levels of FSH and LH is consistent with the report by Eiholzer et al. [2006] that hypogonadism and hypogenitalism may result in part from a primary defect in steroid hormone production in the gonads of PWS males. In addition, there was a significant negative correlation between testosterone levels and BMI values (r = −0.50, P = 0.016); however, there was no significant correlation between BMI and FSH or LH.
In conclusion, our study of FSH, LH and estradiol levels in PWS females greater than 16 years of age showed LH values for PWS females with UPD at almost twice the values for those with deletion subtypes. However, values for FSH and LH were found to be within the low normal (follicular) range for all PWS females while the estradiol levels were low, thus implying that the observed hypogonadism in PWS females may involve mechanism(s) other than decreased gonadotropin production. In PWS males with type II deletions, higher levels were found for both FSH and LH compared with other genetic subtypes. This observation suggests differences in the hypothalamic–pituitary–gonadal axis among the genetic subtypes in PWS males. However, due to the small sample size, further work is required to elicit these differences.
REFERENCES
- Akefeldt A, Tornhage CJ, Gillberg C. 1999. A woman with Prader–Willi syndrome gives birth to a healthy baby girl. Dev Med Child Neurol 41:789–790. [DOI] [PubMed] [Google Scholar]
- Bittel DC, Butler MG. 2005. Prader–Willi syndrome: Clinical genetics, cytogenetics, and molecular biology. Expert Rev Mol Med 7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Sell SM, Strong TV, Butler MG. 2007. Whole genome microarray analysis of gene expression in Prader–Willi syndrome. Am J Med Genet Part A 143A:430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman P, Ritzen EM, Lindgren C. 2001. Endocrine dysfunction in Prader–Willi syndrome: A review with special reference to GH. Endocr Rev 22:787–799. [DOI] [PubMed] [Google Scholar]
- Butler MG. 1990. Prader–Willi syndrome: Current understanding of cause and diagnosis. Am J Med Genet 35:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Bittel DC. 2007. Plasma obestatin and ghrelin levels in subjects with Prader–Willi syndrome. Am J Med Genet Part A 143A:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Hanchett JM, Thompson T. 2006. Clinical findings and natural history of Prader–Willi syndrome In: Butler MG, Lee P, Whitman B, editors. Management of Prader–Willi Syndrome. 3rd edition. New York, NY: Springer; p 3–48. [Google Scholar]
- Cassidy SB. 1984. Prader-Willi syndrome. Curr Probl Pediatr 14: 1–55. [DOI] [PubMed] [Google Scholar]
- Chanoine JP, Yeung LP, Wong AC, Birmingham CL. 2002. Immunoreactive ghrelin in human cord blood: Relation to anthropometry, leptin, and growth hormone. J Pediatr Gastroenterol Nutr 35:282–286. [DOI] [PubMed] [Google Scholar]
- Eiholzer U, Lee PDK. 2006. Medical considerations in Prader–Willi syndrome In: Butler MG, Lee P, Whitman B, editors. Management of Prader–Willi Syndrome. 3rd edition. New York, NY: Springer; p 97–152. [Google Scholar]
- Eiholzer U, l’Allemand D, Rousson V, Schumpf M, Gasser T, Girard J, Gruters A, Simoni M. 2006. Hypothalamic and gonadal components of hypogonaadism in boys with Prader–Willi syndrome. J Clin Endocrinol Metab 91:892–898. [DOI] [PubMed] [Google Scholar]
- Hockey A, Byrne G, Cohen A. 1987. Precocious puberty in the male offspring of a mother and daughter with the Prader–Willi syndrome. Am J Med Genet 26:749. [DOI] [PubMed] [Google Scholar]
- Kamischke A, Baumgardt A, Horst J, Nieschlag E. 2003. Clinical and diagnostic features of patients with suspected Klinefelter syndrome. J Androl 24:41–448. [PubMed] [Google Scholar]
- Kishore S, Stamm S. 2006. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science 311:230–232. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. 2000. CDC Growth Charts: United Stated. Adv Data 8:1–27. [PubMed] [Google Scholar]
- Linnenmann K, Schroder C, Mix M, Kruger G, Fusch C. 1999. Prader–Labhart–Willi syndrome with central precocious puberty and empty sellica syndrome. Acta Paediatr 88:1295–1297. [DOI] [PubMed] [Google Scholar]
- Nagai T, Mimura N, Tomizawa T, Monden T, Mori M. 1998. Prader–Willi syndrome with elevated follicle stimulating hormone levels with diabetes mellitus. Int Med 37:1039–1041. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE. 2006. Minireview: The neuroendocrine regulation of puberty: Is the time ripe for a systems biology approach. Endocrinology 147:1166–1174. [DOI] [PubMed] [Google Scholar]
- Quirk PL, Siegel RE. 2005. The serotonin type 3A receptor facilitates luteinizing hormone release and LHbeta promoter activity in immortalized pituitary gonadotropes. Endocrine 27: 37–43. [DOI] [PubMed] [Google Scholar]
- Robertson J, Shilkofski N. 2005. The Johns Hopkins Hospital Harriet Lane Handbook. 17th edition. Philadelphia, PA: Mosby; p 271. [Google Scholar]
- Schulze A, Morgensen H, Hamborg-Petersen B, Graem N, Ostergaard J, Brondum-Nielsen K. 2001. Fertility in Prader–Willi syndrome: A case report with Angelman syndrome in the offspring. Acta Paediatr 90:455–459. [PubMed] [Google Scholar]
- Ulrich U, Nowara I, Rossmanith WG. 1994. Serotoninergic control of gonadotrophin and prolactin secretion in women. Clin Endocrinol 41:779–785. [DOI] [PubMed] [Google Scholar]
- Vanelli M, Bernasconi S, Caronna N, Virdis R, Terzi C, Giovannelli G. 1984. Precocious puberty in a male with Prader–Labhart–Willi syndrome. Helv Paediatr 39:373–377. [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. 2006. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27: 101–140. [DOI] [PubMed] [Google Scholar]