Abstract
Skin disease alters cutaneous lipid mediator metabolism, and if skin secretions contain evidence of these changes, they may constitute useful clinical matrices with low associated subject burden. The influences of skin diseases on sebum lipid mediators are understudied. Here, sebum oxylipins, endocannabinoids, sphingolipids, and fatty acids were quantified from the non-lesional bilateral cheeks of subjects with and without quiescent atopic dermatitis (AD) using LC-MS/MS and GC-MS. AD decreased C36 [NS] and [NdS] ceramide concentrations. Compared to males, females demonstrated increased concentrations of oxylipin alcohols and ketones, and saturated and monounsaturated non-esterified fatty acids, as well as decreased concentrations of C42 [NS] and [NdS] ceramides. Additionally, contemporaneously collected sweat lipid mediator profiles were distinct, with sebum showing higher concentrations of most targets, but fewer highly polar lipids. Therefore, AD and gender appear to alter sebum lipid metabolism even in non-lesional skin of quiescent subjects.
Keywords: Oxygenated Lipids, Endocannabinoids, Sphingolipids, Fatty Acids, Metabolic Profiling, Non-invasive Sampling
1. INTRODUCTION
Sebum is an oily and waxy substance secreted by the sebaceous glands of the skin, and represents one of the two main cutaneous secretions (the other being sweat). Though sebum is secreted throughout the body (with the exception of the palms of the hand and soles of the feet), sebum production is most prominent at the forehead, nose and chin (the so-called “t-zone” of the face), where sebaceous gland density is the highest [1]. However, other sites such as the cheeks and upper torso also contain a high sebaceous gland density (and therefore produce appreciable amounts of sebum), and in general, sebaceous gland density decreases towards the extremities of the body [1]. The specific functions of sebum are yet to be fully elucidated, but the composition of sebum suggests that it (along with other epidermal surface lipids) plays a role in skin barrier formation, moderation of cutaneous inflammation, and anti-microbial defense [2].
Preliminary characterizations of sebum have indicated that it is a lipid-rich matrix, with its major components being glycerolipids (30–50%), free fatty acids (15–30%), cholesterol (1.5–2.5%), cholesterol esters (3–6%), squalene (12–20%), and wax esters (26–30%) [3]. Changes in the relative abundances of these sebum components have been associated with cutaneous diseases such as acne, papulopustular rosacea, atopic dermatitis and seborrheic dermatitis [4–7], though specific mechanisms explaining the role of sebum in these diseases have yet to be elucidated. More comprehensive characterizations of sebum content have recently been attempted, and the relative abundances and concentrations of individual species in each of the sebum lipid classes are now reported [4, 6, 8]. These recent reports have led to more nuanced understanding of the mechanism of diseases such as acne and papulopustular rosacea, as we now know that individuals with acne demonstrate an increased proportion of sebum diacylglycerol species that is correlated to disease severity [7], and the sebum of individuals with papulopustular rosacea contains a greater proportion of saturated fatty acids compared to controls [6].
In addition to the lipids described above, there has been some indication that lipid-derivatives such as prostaglandins (PGs), endocannabinoids such as anandamide (AEA) and 2-arachidonylglycerol (2-AG), ceramides and other sphingolipids all play roles in cutaneous disease [9]. These and other related compounds are all members of the bioactive lipid mediator family, a super-class of fatty acid-derivatives that display biological activity and are capable of regulating a variety of processes, including inflammation, cell growth and differentiation, and vascular homeostasis [10]. With respect to sebum and sebum-associated diseases, 15-deoxy PGJ2, AEA and 2-AG are known to enhance lipid synthesis in sebocyte culture models in a manner resembling acne vulgaris [11, 12], and several lipid mediator-generating enzyme systems such as cyclooxygenase (COX)-2, 5-lipoxygenase (LOX) and leukotriene (LT) A4 hydrolase have increased expression in sebocytes cultured from acne lesions [13]. However, to the best of our knowledge bioactive lipid mediators in sebum have yet to be characterized.
In an effort to further the understanding of sebum composition and its relevance to cutaneous disease, the present study aims to quantitatively characterize the presence of 38 fatty acids in both their non-esterified (“NEFA”) and aggregate esterified (“total fatty acid”, TFA) forms, as well as >150 bioactive lipid mediators including oxygenated lipids (“oxylipins”), nitrolipids, endocannabinoids and endocannabinoid-like compounds, and sphingolipids in subjects with and without atopic dermatitis (AD) from a single sebum sample extraction. Secondarily, we compare these profiles to those recently characterized in the sweat of many of the same subjects [14], to identify similarities and differences in the lipid mediator content of the two major cutaneous secretions. Characterization and quantitation of these compounds in sebum will enhance our understanding of cutaneous biochemistry, and may suggest novel biomarker and therapeutic targets for cutaneous diseases.
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
Methanol, acetonitrile, isopropanol, toluene and hexane used during sample preparation, ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis and/or gas chromatography-mass spectrometry (GC-MS) analysis were of UPLC-grade or better and were purchased from Fisher Scientific (Waltham, MA). Sodium methoxide (0.5 M), methanolic hydrochloric acid (3 N) and trimethylsilyl-diazomethane (2 M) used during sample preparation and glacial acetic acid, formic acid, ammonium hydroxide (10 M) used during UPLC-MS/MS analysis were purchased from either Fisher Scientific or Sigma Aldrich (St Louis, MO). Fatty acid and lipid mediator standards, analytical surrogates, and internal standards were synthesized or purchased from Cayman Chemicals (Ann Arbor, MI), Avanti Polar Lipids, Inc. (Alabaster, AL), Larodan (Malmo, Sweden), Nu-Chek Prep, Inc. (Elysian, MN), or CDN Isotopes (Pointe-Claire, Canada).
2.2. Subject Recruitment and Study Design
This study uses the same study population as our previous work examining the sweat lipid mediator profile, and details on subject recruitment and inclusion and exclusion criteria can be found in the associated manuscript [14]. Briefly, 26 subjects (n = 13 each with and without AD) were recruited from the greater Sacramento, CA metropolitan area for this study between February 2015 and February 2016. Inclusion criteria included either a diagnosis of AD by a board-certified dermatologist or the absence of any inflammatory skin conditions, and exclusion criteria included current use of systemic immunosuppressive medications. All subjects with AD were sampled while they were in the quiescent state. Written informed consent was obtained from all subjects prior to participation in the study, and all study protocols were approved by the Institutional Review Board of the University of California-Davis (Protocol #605131).
Subjects participated in a single study visit that lasted approximately one hour. Prior to their study visit, subjects were asked to refrain from use of any topical moisturizers or medications for at least 12 h. Sebum was collected from approximately 5.5 cm2 areas located at non-lesional sites on the bilateral cheeks using Sebutape® Adhesive Patches (CuDerm Corporation, Dallas, TX). An image of the Sebutape® patch placed on the bilateral cheeks is available in Supplemental Fig. S1. Prior to sebum collection, subjects were acclimated to the ambient environment for 15 min, the collection area was wiped with a 70% isopropanol swab (Covidien, Minneapolis, MN) and one Sebutape® patch was placed on each cheek using a pair of methanol-rinsed forceps. Sebutape® patches were left in place for 1 h, after which they were removed using a pair of methanol-rinsed forceps and placed on Sebutape® Clear View PRO Storage Cards (CuDerm Corporation) that were stored at −80 °C in commercially available re-sealable zipper storage bags until analysis.
Skin sebum levels were measured at a non-lesional site immediately adjacent to each collection site using a Sebumeter® SM 815 (Courage and Khazaka Electronic GmbH, Cologne, Germany), and transepidermal water loss was measured in triplicate at the same sites using a Tewameter® TM 300 (Courage and Khazaka Electronic GmbH), both in accordance with manufacturer instructions. Sebum levels were reported in units of micrograms of sebum per square centimeter of skin (μg/cm2) and transepidermal water loss was reported in units of grams of water lost per hour per square meter of skin (g/h/m2). Sebum levels and transepidermal water loss measurements are available in Table 1.
Table 1.
Sampling and storage parameters of sebum collected from subjects with and without atopic dermatitis. All data reported as geometric mean [range].
| Parameter | AD Male (n = 6) | AD Female (n = 5) | Control Male (n = 5) | Control Female (n = 4) |
|---|---|---|---|---|
| Age (yr) | 30.3 [23.1 – 38.5] | 42.0 [26.6 – 62.2]a | 32.0 [29.0 – 38.1] | 28.8 [26.7 – 30.9] |
| Sebumeter® Reading (μg/cm2)b | 33.1 [6 – 73] | 8.8 [1 – 28] | 36.1 [3 – 123] | 39 [20 – 61] |
| Transepidermal Water Loss (g/h/m2)c | 18.6 [8.4 – 34.8] | 9.5 [2.8 – 17.5] | 13.6 [3.9 – 38.2] | 9.5 [5.3 – 21.6] |
These data include two female subjects with AD who were outliers with respect to age (62.2yr and 57.6yr vs. ADn=9 = 31.2 [23.1 – 46.3]), but not with respect to observed lipid mediators.
P > 0.05 for all fixed effects when analyzed using a full factorial MANOVA approach, testing for the effects of disease, gender, and the interaction between them. Disease, gender and their interaction were included as fixed effects, and subject was included as a random effect.
P = 0.048 for gender when analyzed using a full factorial MANOVA approach, testing for the effects of disease, gender, and the interaction between them. Disease, gender and their interaction were included as fixed effects, and subject was included as a random effect. P > 0.05 for all other fixed effects.
Of the 26 subjects recruited, six were excluded. Two subjects with and three subjects without AD did not have measurable sebum levels as measured using the Sebumeter®, and one subject without AD had flared acne vulgaris at the time of sampling. Therefore, the study proceeded with 11 subjects with and 9 subjects without AD. Group characteristics are shown in Table 1.
2.3. Analysis of Sebum Lipid Mediators and Fatty Acids
2.3.1. Preparation of the Total Lipid Extract (TLE)
Oxylipins, nitrolipids, endocannabinoids and endocannabinoid-like compounds, ceramides, sphingoid bases and fatty acids were initially isolated from sebum collected from the cheeks by isopropanol extraction. Prior to extraction, Sebutape® patches from each cheek were combined in a methanol- and hexane-rinsed 2-mL Eppendorf Tube™ (Fisher Scientific) using methanol-rinsed forceps, and enriched with 5 μL of anti-oxidant solution (0.2 mg/ml solution butylated hydroxytoluene/EDTA in 1:1 methanol:water), 5 μL of 500 nM deuterated oxylipin/endocannabinoid analytical surrogate solution in methanol, 10 μL of 1000 nM C17-analog sphingolipid analytical surrogate solution in methanol, and 5 μL of a fatty acid surrogate solution (MSS-D2) containing 1.4 mM C16:0-d31, 0.7 mM C18:0-d35, 2.9 mM C22:1n9 and 1.7 mM C22:3n3. Isopropanol (1 mL) was added to each tube, and samples were shaken at 1200 rpm for 5 min using a Geno/Grinder (SPEX SamplePrep, Metuchen, NJ). The isopropanol layer was then transferred to a methanol- and hexane-rinsed 2-mL amber vial (Waters Corporation, Milford, MA) and the samples were evaporated to dryness under vacuum (GeneVac EZ-2 Personal Evaporator, SP Scientific, Warminster, PA). Samples were reconstituted in 50 μL toluene followed by 50 μL methanol, and aliquoted for further analysis as described below.
2.3.2. Analysis of Lipid Mediators
A 50 μL aliquot of the TLE was transferred to a methanol-rinsed 2-mL amber vial and evaporated to dryness under vacuum. Samples were reconstituted in 50 μL of internal standard solution containing 50 nM each of 1-cyclohexyl-3-ureido dodecanoic acid (Sigma Aldrich) and 1-phenyl,3-ureido hexanoic acid (gift from B.D. Hammock, University of California-Davis) in 1:1 (v/v) methanol:acetonitrile, and the reconstituted sample was filtered by centrifugation using Amicon® Ultrafree-MC Durapore PVDF 0.1 μm filters (Merck Millipore, Billerica, MA) prior to analysis.
UPLC-MS/MS analysis of target analytes generally followed a previously published protocol [15]. Briefly, two 7 μL aliquots of the reconstituted TLE aliquot was injected onto a Shimadzu Nexera X2 series UPLC system (Columbia, MD). Oxylipins, nitrolipids, endocannabinoids and endocannabinoid-like compounds were separated on a 2.1 × 150 mm, 1.7 μm BEH C18 column (Waters Corporation) and ceramides and sphingoid bases were separated on a 2.1 × 100, 1.7 μm BEH C8 column (Waters Corporation). Analytes were detected using an API 6500 QTrap MS/MS system (Sciex, Framingham, MA) with positive-negative mode switching electrospray ionization. Chromatographic solvent gradients, analyte retention times and MS/MS parameters are available in Supplemental Tables S1–S2. Analytes were quantified by internal standard methodology using 5–7 point calibration curves (r ≥ 0.997) and data were processed using AB Sciex MultiQuant version 3.0.2. Lipid mediators were reported in units of picomoles of lipid mediator per Sebutape® patch analyzed (i.e. pmol/tape).
2.3.3. Analysis of NEFAs and TFAs
NEFAs were analyzed as their fatty acid methyl esters (FAMEs) using modifications of a previously published protocol [16]. Briefly, a 10 μL aliquot of the TLE was transferred to a hexane-rinsed 2-mL amber vial, and samples were enriched with 20 μL of a 60 μM C15:1n5 fatty acid surrogate solution. Samples were diluted with 150 μL of a 1:1 (v/v) methanol:toluene solution and 80 μL of methanol, and 45 μL of a 2 M trimethylsilyl-diazomethane solution in hexane was added to derivatize samples. Samples were allowed to react for 30 min at room temperature (~ 23 °C) and evaporated to dryness under vacuum. Residues were reconstituted in 400 μL of hexane and 40 μL of a 44 μM C23:0 internal standard solution in hexane was added to samples prior to analysis.
TFAs were analyzed as their FAME-derivatives using modifications of previously published protocols [16, 17]. Briefly, a 5 μL aliquot of the TLE was transferred to a hexane-rinsed 2-mL amber vial, and samples were enriched with 20 μL of a 60 μM C15:1n5 fatty acid surrogate solution. Samples were diluted with 35 μL of a 1:1 (v/v) methanol:toluene solution and 140 μL of methanol, and 100 μL of a 0.5 M sodium methoxide solution was added to trans-esterify samples. Samples were allowed to react at 60 °C for 30 min after which 100 μL of a 3 N methanolic hydrochloric acid solution was added to samples to form the methyl esters. Samples were allowed to react at 60 °C for a further 30 min after which they were neutralized by the addition of 400 μL of a solution containing 0.25 M potassium bicarbonate and 0.5 M potassium carbonate. FAMEs were back-extracted using 400 μL of hexane and 400 μL of a saturated saline solution, and a 50 μL aliquot of the hexane phase was transferred to a 100 μL spring-footed glass insert (Agilent Technologies) and 5 μL of a 44 μM C23:0 internal standard solution in hexane was added to all samples.
GC-MS analysis of a 1 μL aliquot of the samples was performed in splitless mode using an HP6890 GC coupled to a 5973N MS detector (Agilent Technologies, Santa Clara, CA) that was equipped with a 30 m × 0.25 mm i.d. × 0.25 μm DB-225ms column (Agilent Technologies). Helium gas was used as the mobile phase with an initial flow rate of 1.1 mL/min which was held for 12 min before being increased at a rate of 0.2 mL/min until a flow rate of 2.1 mL/min was achieved, and then held at 2.1 mL/min for 7 min. The GC inlet was maintained at 230 °C, and the column oven was initially maintained at 64 °C for 1 min, after which the temperature was ramped up at a rate of 32 °C/min until a temperature of 192 °C was achieved, subsequently ramped up at 4 °C/min until a final temperature of 240 °C was achieved, and the final temperature was held for 13 min before returning to initial conditions. The overall method runtime was 30 min. Data were acquired by electron impact ionization in simultaneous selected ion monitoring/full scan mode with m/z 67.1, 69.1, 74.1, 77.1, 79.1 and 368.4 being the ions monitored along with 50–400 m/z scans. Analytes were quantified by internal standard methodology using 5–7 point calibration curves (r ≥ 0.997) and data were processed using MassHunter version b.07.01. NEFAs and TFAs were reported in units of micromoles of fatty acid per Sebutape® patch analyzed (i.e. μmol/tape).
2.3.4. Lipid Mediator and Fatty Acid Nomenclature
The abbreviations used to describe the lipid mediators quantified in this study follow standard conventions in the field, and the rationale behind the choice of abbreviations are fully described in our previous manuscript [14]. The abbreviations used to describe the fatty acids quantified in this study follow the standard conventions of the LIPID MAPS initiative [18, 19]. All analyte abbreviations used in this study are fully expanded in Supplemental Table S3, and common database identifiers for all analytes are also provided.
2.4. Data Quality Assurance and Quality Control
Data quality assurance and quality control consisted of several steps executed during sample preparation and post-analysis to determine acceptable data for reporting purposes. During sample preparation, deuterated or analog surrogates were introduced to all samples prior to extraction to compensate for procedural losses and matrix effects. Post-extraction, all samples were reconstituted in solutions containing instrument-specific internal standards to account for variations in final volume, allow sample-specific monitoring of instrument performance, and in the case of electrospray ionization, discriminate between sample-specific surrogate loss and ion suppression. During data processing, the relative retention time (i.e. ratio of analyte tR to surrogate tR) were evaluated and a coefficient of variance (%CV) ≤ 1% was considered acceptable. Additionally, to assess matrix effects and analytical losses due to extraction, sample surrogates and internal standard peak areas and surrogate to internal standard area ratios were compared to those of the calibration standards. All calibration standards were prepared from commercial materials in methanol and not subjected to extraction.
Each sample extraction batch also contained two unexposed Sebutape® patches as method blanks to evaluate potential confounding contributions from the Sebutape® patches. Analytes for which the peak area in the method blank was > 25% of the average peak area of the subject samples were excluded from reporting. Fatty acid analysis batches also contained derivatization blanks, consisting of 100 μL of 1:1 methanol:toluene (v/v) spiked with 5 μL MSS-D2. Derivatization blanks were not subject to extractions allowing discrimination of extraction losses from methylation failure, and contaminating sources of the Sebutape® patches versus the derivatization reagents. Sample analysis batches also contained two replicates of an archived sebum sample (“replicates”) to evaluate batch reproducibility. Correlations between analyte responses in the replicates were evaluated, and correlations for which r ≥ 0.985 and slope was between 0.8 and 1.2 were considered acceptable. Additionally, the %CV for each analyte greater than the method detection limit in the replicate pair was determined, and a batch was considered reproducible if > 80% of analytes had %CV ≤ 30%.
2.5. Statistical Analysis
Statistical analyses generally followed the approach previous reported [14]. Briefly, data were blank-corrected by subtraction of the average concentrations of the respective analytes in simultaneously processed method blanks, and analytes observed in >70% of the samples were considered for further analysis. Curated data were screened for outliers using the Cauchy distribution to estimate the center and spread of the data, missing data were imputed by probabilistic principal components analysis [20], and data were transformed to normal using the Johnson system [21]. Data imputation was performed using MetaboAnalyst 3.0 [22], and all other data pretreatment was performed using JMP Pro 13 (SAS Institute, Inc., Cary, NC).
Data were analyzed using a full factorial MANOVA approach, testing for the effects of disease, gender, and the interaction between them on sebum lipid mediators and fatty acids. Disease, gender, their interaction and metabolite were included as fixed effects, and subject was included as a random effect. One-way MANOVA was used for variables with no interaction between gender and disease. Prior to analysis, variables were subjected to hierarchical cluster analysis using Ward’s method [23], and clusters were assigned using imputed data that was auto-scaled to promote the identification of biological effects and ensure that all metabolites were considered equally important in the statistical models [24]. The hierarchical cluster dendrogram was pruned into 32 clusters such that the resulting clusters separated the treatment and intra-individual variability associated variance (Supplemental Fig. S2). Specifically, tree pruning (i.e. the cluster number selection) was established such that compounds with significant false discovery rate-adjusted ANOVA p-values were maintained in clusters showing significant changes by MANOVA. Results from the MANOVA analysis were adjusted for multiple comparisons using the Benjamini-Hochberg procedure at q = 0.2 [25]. All procedures were performed using JMP Pro 13.
Statistical comparisons of sweat and sebum lipid mediators were conducted using only data from those subjects that were able to produce sweat and sebum during their study visits (n = 19) and considering only analytes detected in both matrices. Sweat data was originally reported in our study examining the sweat lipid mediator profiles of the same subject population [14]. Data were analyzed as absolute amounts of lipid mediator quantified in each matrix as no common standardizing factor exists between the two matrices, and we analyzed the entire sample collected during the study visit. Data pre-treatment was conducted as described above, and univariate comparisons were conducted using the paired heteroscedastic Student’s t-test algorithm in MetaboAnalyst 3.0, with data adjusted for multiple comparisons as described above. Correlations between the imputed raw lipid mediator data in the two matrices were also estimated using Spearman’s rank correlation coefficient, and this analysis was performed in JMP Pro 13.
Though all lipid mediator and fatty acid data in this manuscript are reported as either pmol or μmol of analyte per Sebutape® patch, other potential denominators to standardize detected analyte concentrations include per μg of sebum (as estimated by the Sebumeter®), per μmol of total NEFA, or per μmol of total TFA. In order to compare these alternative denominators to the per tape denominator used in this study, the %CV of the lipid mediator data standardized by these various denominators were compared using the method of Feltz and Miller [26], which was performed manually in Microsoft Excel 2016 (Redmond, WA). Standardization was performed on the imputed lipid mediator data (following appropriate adjustments to ensure only the total amount of lipid mediator quantified was being standardized). Results of the Feltz and Miller test were adjusted for multiple comparisons by the Benjamini-Hochberg procedure at q = 0.2 [25]. A χ2 test was used to compare the %CV of the Sebutape® number normalization to that of each other evaluated standardizing factor, with the null hypothesis being that 50% of analytes would have a lower %CV if normalization approaches are not different.
3. RESULTS
3.1. Lipid Mediators and Fatty Acids in Sebum
A total of 58 lipid mediators, 20 NEFAs and 29 TFAs were quantified in the sebum of subjects with and without AD. Quantified lipid mediators included 35 oxylipins, 1 nitrolipid, 13 endocannabinoids and endocannabinoid-like compounds, 8 [NS] or [NdS] ceramides, and 1 sphingosine (Supplemental Table S4). Quantified NEFAs included 11 saturated fatty acids (SFAs), 4 monounsaturated fatty acids (MUFAs), 2 n-6 polyunsaturated fatty acids (PUFAs) and 3 n-3 PUFAs, whereas quantified TFAs included 12 SFAs, 7 MUFAs, 8 n-6 PUFAs and 2 n-3 PUFAs (Supplemental Table S4). Interestingly, the total sebum TFA content was correlated to skin sebum levels as measured by the Sebumeter® (Spearman’s ρ = 0.45, P = 0.048) whereas the total sebum NEFA content was not (Spearman’s ρ = 0.43, P = 0.062), suggesting that sebum TFA content may be an acceptable proxy for skin sebum content.
For both the lipid mediators and fatty acids, substantial concentrations of the analytes were noted in blank Sebutapes® (Supplemental Table S4), and lower concentrations of the fatty acids were noted in the derivatization blanks, which suggested that the Sebutape® patches were contributing to the observed lipid profiles. Therefore, all data was blank-corrected, which possibly precluded identification of additional analytes. The blank-corrected replicate samples demonstrated good correlation for all analyte panels (r ≥ 0.996, slope = 0.87 to 1.16) and 80% of targets greater than the method detection limit demonstrated %CV ≤ 30%, suggesting acceptable batch reproducibility. It should be noted that while two female subjects with AD were substantially older than the other subjects with AD sampled in this study (62.2yr and 57.6yr vs. ADn=9 = 32.0 ± 7.4yr), these extreme ages did not influence the detected lipid mediators and fatty acids or their concentrations.
The choice of standardizing factor (number of Sebutape® patches analyzed, skin sebum content, total sebum TFA content, or total sebum NEFA content) affected the variance structure of the data as estimated by %CV. In subjects with and without AD, 56 out of 58 analytes and 57 out of 58 analytes respectively (i.e. 97% and 98% respectively) demonstrated statistically significant differences between the various standardizing factors tested (Supplemental Table S5A–B). Standardizing to number of Sebutape® patches analyzed produced the lowest %CV for the most analytes in both groups (Table 2), and pairwise comparisons indicated that the %CV for analytes standardized to the number of Sebutape® patches was significantly lower than the other standardizing factors in all cases for the AD group and in one out of three cases for the controls (Table 3).
Table 2.
Number of analytes where each standardizing factor demonstrated the lowest %CV when reported lipid mediator concentrations were standardized to that factor.
| Standardizing Factor | AD | Control |
|---|---|---|
| Number of Sebutape® patches | 38 (66%) | 21 (36%) |
| Sebumeter® reading | 9 (16%) | 5 (9%) |
| Total TFA | 6 (10%) | 16 (28%) |
| Total NEFA | 5 (9%) | 16 (28%) |
Table 3. Evaluation of analyte standardization approach variance minimization using the chi-square (χ2) statistic.
The variance in each of the 58 reported analytes processed through the four standardization factors were compared, with the per Sebutape® standardization set as the reference. If equivalent, 50% of analytes would have a lower %CV than the reference approach.
| Standardizing Factor | AD | Control | ||||
|---|---|---|---|---|---|---|
| n > 1 (%)a | χ2 | P | n > 1 (%)a | χ2 | P | |
| Sebumeter® reading | 48 (83%) | 12.45 | 0.0004 | 48 (83%) | 12.45 | 0.0004 |
| Total TFA | 51 (88%) | 16.69 | 0.00004 | 22 (38%) | 1.69 | 0.2 |
| Total NEFA | 49 (84%) | 13.79 | 0.0002 | 37 (64%) | 2.21 | 0.1 |
The number (and percentage) of reported analytes with variance greater than the reference per Sebutape® standardization approach.
3.2. AD and Gender Affect Sebum Lipid Mediator and Fatty Acid Profiles
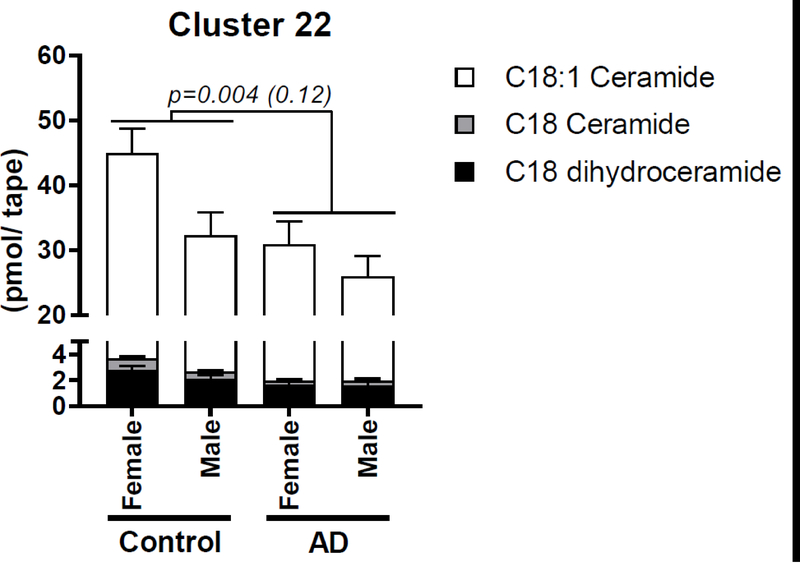
Subjects with AD demonstrated decreased sebum concentrations of C18 ceramide, C18:1 ceramide and C18 dihydroceramide i.e. C36 [NS] and [NdS] ceramides (Fig. 1). No interactions were observed between AD status and gender for the cluster containing these analytes, suggesting a gender-independent effect (Supplemental Table S4). Clusters containing other short-chain (i.e. 30–40 total carbon) [NS] and [NdS] ceramides also demonstrated a similar trend, though these changes did not meet the threshold for statistical significance (Padj = 0.3, Supplemental Table S4).
Fig. 1. Subjects with atopic dermatitis demonstrate decreased sebum concentrations of ceramides and dihydroceramides with 18-carbon fatty acid chains and 18-carbon sphingosine or dihydrosphingosine chains.
Differences were tested by one-way MANOVA with adjustments for gender, and both the raw P value and the P value adjusted for multiple comparisons by the Benjamini-Hochberg procedure (q = 0.2) are reported. Analytes were clustered by hierarchical clustering using Ward’s method. Data are reported as Mean ± SEM.
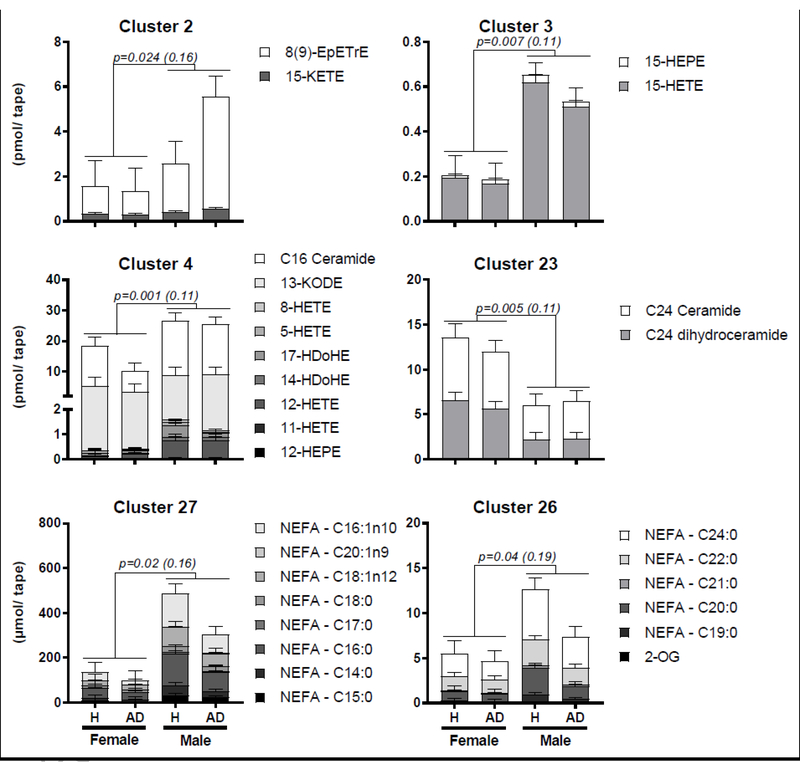
With respect to gender, female subjects demonstrated decreased sebum concentrations of LOX-derived alcohol and ketone metabolites of linoleate, arachidonate, eicosapentanoate and docosahexanoate, as well as all detected saturated and monounsaturated NEFAs (Fig. 2). Additionally, female subjects demonstrated increased sebum concentrations of the C24 ceramide and dihydroceramide i.e. C42 [NS] and [NdS] ceramides (Fig. 2). No interactions between AD status and gender were observed for the clusters containing any of these analytes, suggesting an AD-independent gender effect (Supplemental Table S4).
Fig. 2. Gender differences exist in sebum lipid mediator and fatty acid profiles.
Female subjects demonstrate decreased sebum concentrations of all detected saturated and monounsaturated non-esterified fatty acids, as well as lipoxygenase-derived alcohol and ketone metabolites of linoleate, arachidonate, eicosapentanoate and docosahexanoate. Female subjects also demonstrate increased sebum concentrations of C24 ceramide and dihydroceramide. Differences were tested by one-way MANOVA with adjustments for AD status, and both the raw P value and the P value adjusted for multiple comparisons by the Benjamini-Hochberg procedure (q = 0.2) are reported. Analytes were clustered by hierarchical clustering using Ward’s method. Data are reported as Mean ± SEM.
3.3. Sweat and Sebum Display Distinct Lipid Mediator Profiles
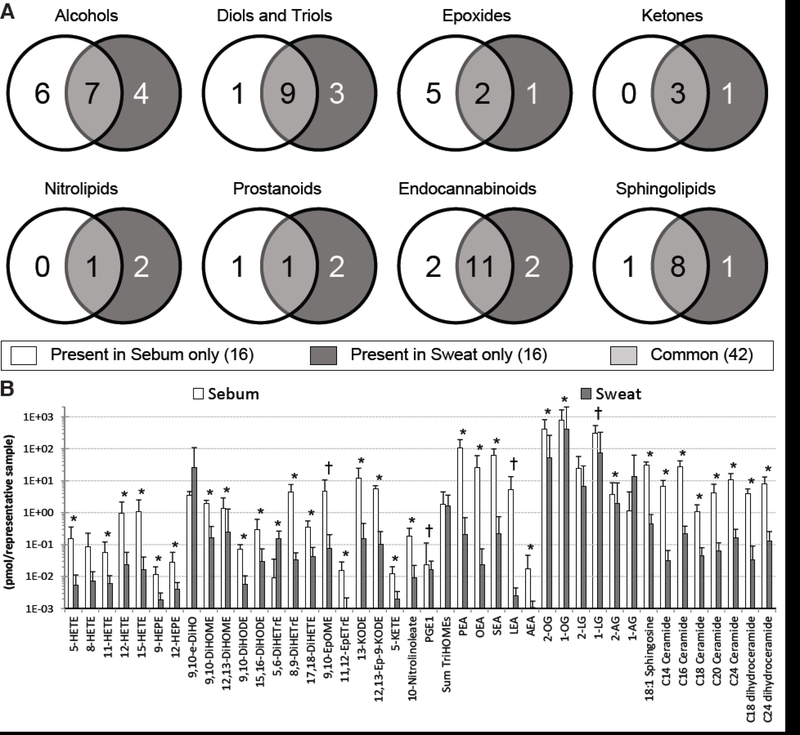
Of the 20 subjects sampled in this study, and the 22 subjects sampled in our characterization of the sweat lipid mediator profile [14], we were able to collect both sweat and sebum from 19 subjects, and only these results are discussed here. Of the 58 lipid mediators detected in each matrix, 42 analytes were common to both matrices, and this is represented diagrammatically in Fig. 3A. Concentrations of almost all analytes were elevated in the sebum, with only concentrations of 8-HETE, 9,10-e-DiHO, total linoleate-derived triols, 2-LG and 1-AG not being different between the two matrices, and concentrations of 5,6-DiHETrE being higher in sweat (Fig. 3B). Individual sweat and sebum lipid mediators were not correlated (Supplemental Table S6).
Fig. 3. Sebum and sweat demonstrate different lipid mediator profiles.
A: Of the 58 lipid mediators detected in each matrix, 42 are common to both matrices and 16 are unique. B: On a per sample basis, sebum contains larger amounts of almost all detected lipid mediators. *Padj < 0.01; †Padj < 0.2 (q = 0.2).
4. DISCUSSION
As one of the two main cutaneous secretions, sebum is a matrix whose composition is being increasingly characterized in an effort to understand its specific functions. Several comprehensive and quantitative characterizations exist of the various sebum lipid super-classes [4, 6–8, 27–29], and more recently, quantitative and relative abundance estimates are available of individual lipid or fatty acid species in sebum [4, 6, 8]. However, bioactive lipid mediators, which have demonstrated relevance in cutaneous disease [9, 14], have not been previously quantified in sebum, yet represent potential biomarker targets to evaluate skin disease. The present study represents for the first time a simultaneous comprehensive quantitative characterization of sebum fatty acids and lipid mediators from a single extraction of a Sebutape® patch, and attempts to evaluate the utility of this approach in an atopic dermatitis disease model.
The fatty acid profile of sebum, both as NEFA and TFA, has been previously published [4, 6, 8], and our measures of the relative abundances of sebum fatty acids generally agree with published findings. Compared to a previously published report detecting 28 TFA species in sebum [6], we were able to detect 29 TFA species, of which 19 species were common to both studies. The relative abundances of the 10 fatty acids present at > 1% in sebum in both studies were not statistically different. Similarly, compared to a previously published report detecting 45 NEFA species in sebum [8], we were able to detect 20 NEFA species, of which 14 were common to both studies. The relative abundances of the 9 fatty acids present at > 1% in sebum in both studies were not statistically different. Finally, compared to the only study we were able to find that provided quantitative estimates of sebum NEFAs [4], 16 analytes out of the 22 reported were common to our study, and the absolute amount of all of these fatty acids were four-fold lower compared to our study. A possible explanation for this discrepancy is differences in sample processing. Pappas et al. extracted lipids, then separated them by thin layer chromatography before NEFA isolation and quantification by GC with aflame ionization detector [4]. It is unclear whether an internal standard was added to samples prior to the initial extraction, and such an extensive sample preparation process could conceivably cause a decrease in fatty acid recovery from the samples. By contrast, our sample preparation contained fewer material transfers, and surrogates were added to samples prior to extraction, allowing accurate correction for analytical losses.
Sebum fatty acids are known to contain unique species with double bond distributions that differ from the common fatty acids found in most other tissues. Our calibration solutions are tailored to quantify the more common fatty acid species, and we noted that several common sebaceous lipids apparently co-eluted with our analytical standards. We performed additional GC-MS experiments using a 100 m × 0.25 mm i.d. × 0.2 μm SP-2380 capillary column (Varian, Inc., Walnut Creek, CA) which confirmed that sapienic acid (i.e. 16:1n10) co-eluted with palmiteliadic acid (i.e. 16:1n7t) on the DB-225ms column used in this study (Supplemental Fig. S3). Additionally, it also appears that the peak quantified as 18:1n9 is the equivalent elongation product petroselinic acid (i.e. 18:1n12). Sebaleic acid (i.e. 18:2n10), a known component of sebum was not definitively identified but may correspond to the peak identified as linoleic acid (i.e. 18:2n6) (Supplemental Fig. S3). Therefore, the choice of analytical column used in sebum analysis may affect positive identification of sebum fatty acids, and future researchers are cautioned about potential coeluters which may confound identification of sebum fatty acids. For the purposes of this study, we have indicated where we may have potentially identified a coeluting fatty acid.
To the best of our knowledge, this is the first report of bioactive lipid mediators in sebum. While the implications of quantifying these analytes in this matrix remain unclear for now, some interesting observations do arise from the obtained data. The relative abundances of lipid mediators within their individual classes (e.g. oxylipin alcohols, endocannabinoid monoacylglycerols, [NS] ceramides, etc.) generally track the relative abundances of their respective fatty acid precursors, which would suggest that sebocytes are able to convert fatty acids to lipid mediators de novo. This observation appears to be supported by literature indicating that cultured sebocytes express common lipid mediator-forming enzymes such as COX and LOX [30], and the fact that the majority of sebum content is synthesized de novo by sebocytes prior to holocrine secretion (Sivamani et al., unpublished results). It would thus appear that any changes observed in sebocyte lipid mediator content are more reflective of the local microenvironment rather than the system and therefore sebum lipid mediator analysis may be useful in testing for diseases or interventions that directly affect sebocytes.
The speculation that sebum lipid mediators are synthesized de novo by sebocytes rather than simply being excreted in the sebum may also explain the differences observed in the sweat and sebum lipid mediator profiles in the same subjects. We have previously indicated that sweat appears to be primarily an excretory matrix, and that lipid mediators observed in sweat could originate from the systemic circulation or surrounding cutaneous tissue [14, 15]. Thus, it is not surprising that the sweat and sebum lipid mediator profiles are not correlated. The observation that sebum contains greater amounts of lipid mediators compared to sweat is also not surprising, since sebum is a lipid-rich matrix whereas sweat appears to be predominantly aqueous. Sebocytes, which have been previously shown to contain lipid mediator forming enzymes such as COX and LOX, appear to synthesize lipid mediators de novo from available fatty acid substrates. Overall, differences between the sweat and sebum lipid mediator profiles suggest that lipid mediator results from sweat or sebum collected from area of “mixed secretion”, i.e. areas where both sweat and sebaceous glands are present in high abundance such as the chest, upper back or upper arm should be interpreted with caution as it may not be clear which matrix is dominating the observed lipid mediator profile (or if both matrices are contributing equally), which in turn could impact biological interpretation of the results. If collections are to be done from sites that potentially have mixed secretion, it is even more imperative that participants are allowed to acclimate to ambient room temperature prior to collection to minimize sweat collection when sebum collection is intended.
The observed decreases in C36 [NS] and [NdS] ceramide content in sebum collected from non-lesional sites on the cheeks of subjects with quiescent AD is in contrast to previous studies examining the effects of AD on C30–40 [NS] and [NdS] ceramides [14, 31]. Subjects with AD have previously been reported to demonstrate increased levels of all even chain C30–40 [NS] and [NdS] ceramides in their skin when in a flared state [31], as well as in their sweat when in an quiescent state [14], and these changes have been associated with increased ceramide synthase 4 activity in the skin of subjects with AD [32]. The study examining sweat changes in ceramides due to AD was our previous work on these same subjects, and it is also interesting to observe that not only was the directionality of the ceramide changes different between the two matrices, but the magnitude of observed changes was much lower in the sebum (~ 1–2-fold) compared to the sweat (~ 3–7-fold). Furthermore, while AD had a larger impact on sweat ceramides in male versus female subjects, AD increased sebum ceramides in females more than males, despite a lack of significant gender × AD interactions. On the other hand, sebum had 10- to 100-fold higher concentrations of many of lipid mediators compared to sweat (Fig. 3), suggesting that sebum is a much larger source of lipid mediators to the skin surface. The mechanistic rationale behind these contradictory changes in ceramides in the sweat and sebum of subjects with AD is unclear at this time, particularly as very little is known about the formation and secretion of these metabolites into both matrices. Further investigation into the sources of sebum and sweat lipid mediators, evaluation of sebaceous ceramide synthase 4 activity in subjects with and without AD, and correlations of the sebaceous ceramide profile to systemic circulation and surrounding cutaneous tissue is needed in order to fully appreciate these observed changes. Regardless, the finding that AD alters sebum lipids from non-lesional skin is interesting. In particular, the decrease in C18 based ceramides in sebum is notable. While ceramide deficiencies in the keratinocytes are well described [28, 33–36], our findings now expand the pathophysiology to include the sebaceous glands and sebum and suggest that sebum delivery of ceramides to the skin surface is deficient in individuals with AD compared to those without this disorder.
Sebum is likely to be altered in those that have actively flared AD. At least one report describes differences in facial sebaceous squalenes, cholesterols, wax esters and total lipid content in adults and children with AD compared to controls when collected from lesional sites when the subjects were in a flared state [29]. Therefore, it is likely that differences in sebaceous lipid mediators will also be observed between subjects with and without AD when the sebum is collected from a lesional site, or in fact when comparing other conditions that involve the sebaceous gland. One report delineated differences in sebaceous lipids between subjects with and without seborrheic dermatitis when sampling from a lesional site on the forehead, but no differences in the same subjects when simultaneously sampling from a non-lesional site on the forehead [27]. Future studies should consider sampling sebum in those with AD before, during, and after a flare to understand how sebaceous gland activity and lipid composition change.
In general, sebaceous gland activity and sebum composition has largely been the focus of acne and seborrheic dermatitis but has largely been understudied for AD [37]. Skin barrier dysfunction is one of the main pathogenic factors in AD and the sebaceous glands contribute significantly to the skin barrier. Our findings suggest that there is sebaceous gland dysfunction at baseline in AD and would be worthy of further study and clinical evaluation. Future strategies may require therapies that incorporate normalization of sebum as part of the strategy for controlling AD but further studies are needed to better delineate how sebum is altered in lesional skin in addition to our presented findings in non-lesional skin.
Previous studies examining gender differences in sebum lipid composition report no gender differences in sebum lipid super-classes [38–40], though one study reported differences in nine TFAs when combining subjects with and without papulopustular rosacea [6]. With respect to the latter study, it is unclear whether the univariate analyses were adjusted for multiple comparisons, and therefore, it may be the case that these reported gender differences are in fact underpowered. Therefore, our findings that female subjects demonstrate decreased concentrations of sebum non-esterified fatty acids and lipoxygenase metabolites, as well as increased concentrations of sebum C42 [NS] and [NdS] ceramides is surprising. The significance of these findings is unclear at present, but future studies examining the sebum lipid mediator and fatty acid profile are advised to control for gender in their analyses.
To obtain accurate and precise results from any analytical study, standardization of obtained results is a critical step [41]. In the case of homeostatically regulated matrices such as blood, standardization by sample amount is most appropriate, whereas for other matrices such as urine where factors such as hydration status or diet can significantly alter metabolite concentrations, standardization to a stable metabolite with a constant rate of production (e.g. creatinine) is frequently used to control for variable rates of matrix production [41]. Our comparisons of four different standardizing factors suggest that in both subjects with and without AD, standardizing to number of Sebutape® patches analyzed appears to produce the lowest inter-individual variance for the most analytes. However, inter-individual variance is only one aspect to consider in determining an appropriate standardizing factor. The ideal standardizing factor is unbiased to gender, disease state, diet and other potential confounders, such that any biological impacts of these factors is observable in the composition of the matrix under study. This study has already demonstrated that gender has an impact on sebum NEFA content, and others have shown that disease can affect sebum NEFA and TFA content [6, 7, 29]. Therefore, it would seem these are not appropriate standardizing factors, and we have shown that standardizing to total sebum NEFA or TFA content increases variance in the data. While we are unaware of any studies examining the impact of disease on sebum excretion rate, others have suggested that Sebumeter® outputs may not be an appropriate method to standardize sebum analyte measurements, particularly as the amount of sebum collected by a Sebutape® depends on both sebaceous duct output and the lateral spread of sebum across the Sebutape® surface, whereas the Sebumeter® reading represents only sebaceous duct output [42]. Therefore, of the factors we have evaluated, it would appear that standardizing to the number of Sebutapes® analyzed makes the least assumptions about the effects of potential confounders on sebum composition and excretion, and provides an unbiased estimate of sebum content provided the collection time is uniform across all subjects. Additionally, several independent studies collectively suggest that collected sebum can be quantified by assessing the transparency of the Sebutape® patch either visually or by photo densitometry [42–45], and that photo densitometry may be the most robust method to estimate amount of sebum collected [46]. However, photo densitometric measurements of Sebutapes® frequently requires specialized equipment or software that is beyond the scope of most analytical laboratories.
Our study has several limitations. Our sebum analysis was limited to the face. AD does not typically involve the face. However, we sampled non-lesional skin so that our findings may more truly reflect inherent alterations in sebaceous gland lipid mediators. Secondly, we recruited adults with AD in this study and our findings may not be relevant for pediatric AD. Sebum synthesis increases during puberty and our study was not designed to understand how sebum compositions change prior to and after puberty. Nevertheless, adults with AD tend to have persistent disease and many of the participants had AD as a child that extended into adulthood. Therefore, while not directly relatable to pediatric AD, our findings may serve as an initial step toward understanding the sebaceous contribution to AD. Finally, while we attempted to minimize collection of epidermal lipids by wiping the collection site with a 70% isopropanol swab prior to sebum collection, we cannot entirely rule out an epidermal contribution to our observed lipid and lipid mediator profiles, particularly in the case of the sphingolipids. However, the analysis of sebocyte conditioned media indicates that this cell type can produce ceramides and sphingosines in similar proportions to those observed in the present study (data not shown). Therefore, we are confident that we are predominantly reporting on sebaceous lipids and lipid mediators in this study.
Overall this study presents a comprehensive quantitative characterization of lipid mediators and fatty acids in sebum collected from the bilateral cheeks, and demonstrates the ability of the matrix to report on non-lesional skin in AD. Future studies should continue to evaluate the potential applications of the sebum lipid mediator and fatty acid profile in the context of cutaneous diseases that directly affect the sebaceous gland, such as acne or seborrheic dermatitis, as well as characterize the impact of sampling strategy on these analytes by evaluating factors such as collection site, temporal stability, and inflammatory state. We hope to continue the characterization of this apparently robust and noninvasive tool that can be used to support future cutaneous research studies.
Supplementary Material
Highlights.
Sebum contains oxygenated lipids (oxylipins), endocannabinoids and sphingolipids
Quiescent atopic dermatitis decreases sebum C36 [NS] and [NdS] ceramide content
Atopic dermatitis-associated changes were observed at non-lesional sites on cheeks
Gender affects sebum oxylipin, ceramide and free fatty acid concentrations
Sweat and sebum lipid mediator profiles are dissimilar
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the assistance and advice provided by R.E. Bosviel, I.J. Gray, W.R. Keyes, M.V. La, R.H. Lester and B. Rust during sample preparation and data analysis, and the assistance provided by M. Notay during subject recruitment.
Funding: This work was supported by the National Institutes of General Medical Sciences [Grant No. T32-GM008799] (K.A.), and the University of California-Davis Medical Student Research Fellowship (L.A.H.). Additional support was provided by the National Institutes of Diabetes and Digestive and Kidney Diseases [Grant No. U24DK097154-01] (J.W.N.), and the United States Department of Agriculture [Project No. 2032-51530-022-00D] (J.W.N.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS, NIH or USDA. The USDA is an equal opportunity provider and employer.
Abbreviations
- AD
atopic dermatitis
- AEA
arachidonoylethanolamide (anandamide
- AG
arachidonoylglycerol
- COX
cyclooxygenase
- %CV
coefficient of variance
- FAME
fatty acid methyl ester
- LOX
lipoxygenase
- LT
leukotriene
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- MUFA
mono-unsaturated fatty acid
- NEFA
non-esterified fatty acid
- PG
prostaglandin
- PLS-DA
partial least squares discriminant analysis
- PUFA
poly-unsaturated fatty acid
- QC
quality control
- TFA
total (aggregate-esterified) fatty acid
- TLE
total lipid extract
- UPLC
ultra-performance liquid chromatography
Footnotes
CONFLICTS OF INTEREST
R.K.S. serves as a scientific advisor for Dermveda and reports no financial conflict of interest. All other authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Strauss JS, Downing DT, Ebling JF, Stewart ME, Sebaceous glands, in: La G (Ed.), Physiology, Biochemistry and Molecular Biology of the Skin, Oxford University Press, New York, 1991, pp. 712–740. [Google Scholar]
- [2].Zouboulis CC, Baron JM, Bohm M, Kippenberger S, Kurzen H, Reichrath J, Thielitz A, Frontiers in sebaceous gland biology and pathology, Exp Dermatol 17 (2008) 542–551. [DOI] [PubMed] [Google Scholar]
- [3].Picardo M, Ottaviani M, Camera E, Mastrofrancesco A, Sebaceous gland lipids, Dermatoendocrinol 1 (2009) 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pappas A, Johnsen S, Liu JC, Eisinger M, Sebum analysis of individuals with and without acne, Dermatoendocrinol 1 (2009) 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].De Luca C, Valacchi G, Surface lipids as multifunctional mediators of skin responses to environmental stimuli, Mediators Inflamm 2010 (2010) 321494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ni Raghallaigh S, Bender K, Lacey N, Brennan L, Powell FC, The fatty acid profile of the skin surface lipid layer in papulopustular rosacea, Br J Dermatol 166 (2012) 279–287. [DOI] [PubMed] [Google Scholar]
- [7].Camera E, Ludovici M, Tortorella S, Sinagra JL, Capitanio B, Goracci L, Picardo M, Use of lipidomics to investigate sebum dysfunction in juvenile acne, J Lipid Res 57 (2016) 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Camera E, Ludovici M, Galante M, Sinagra JL, Picardo M, Comprehensive analysis of the major lipid classes in sebum by rapid resolution high-performance liquid chromatography and electrospray mass spectrometry, J Lipid Res 51 (2010) 3377–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kendall AC, Nicolaou A, Bioactive lipid mediators in skin inflammation and immunity, Prog Lipid Res 52 (2013) 141–164. [DOI] [PubMed] [Google Scholar]
- [10].Murakami M, Lipid mediators in life science, Exp Anim 60 (2011) 7–20. [DOI] [PubMed] [Google Scholar]
- [11].Iwata C, Akimoto N, Sato T, Morokuma Y, Ito A, Augmentation of lipogenesis by 15-deoxy-δ12,14-prostaglandin J2 in hamster sebaceous glands: Identification of cytochrome P-450-mediated 15-deoxy-δ12,14-prostaglandin J2 production, J Invest Dermatol 125 (2005) 865–872. [DOI] [PubMed] [Google Scholar]
- [12].Dobrosi N, Toth BI, Nagy G, et al. , Endocannabinoids enhance lipid synthesis and apoptosis of human sebocytes via cannabinoid receptor-2-mediated signaling, FASEB J 22 (2008) 3685–3695. [DOI] [PubMed] [Google Scholar]
- [13].Alestas T, Ganceviciene R, Fimmel S, Muller-Decker K, Zouboulis CC 2006. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J. Mol. Med. (Berl.) 84: 75–87. [DOI] [PubMed] [Google Scholar]
- [14].Agrawal K, Hassoun LA, Foolad N, Pedersen TL, Sivamani RK, Newman JW, Sweat lipid mediator profiling: a noninvasive approach for cutaneous research, J Lipid Res 58 (2017) 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Agrawal K, Waller JD, Pedersen TL, Newman JW, Effects of stimulation technique, anatomical region, and time on human sweat lipid mediator profiles, Prostaglandins Other Lipid Mediat 134 (2018) 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun C, Alkhoury K, Wang Y, et al. , IRF-1 and miRNA126 modulate VCAM-1 expression in response to a high fat meal, Circ Res 111 (2012) 1054–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tepaamorndech S, Kirschke CP, Pedersen TL, Keyes WR, Newman JW, Huang L, Zinc transporter 7 deficiency affects lipid synthesis in adipocytes by inhibiting insulin-dependent Akt activation and glucose uptake, FEBS J 283 (2016) 378–394. [DOI] [PubMed] [Google Scholar]
- [18].Fahy E, Subramaniam S, Brown HA, et al. , A comprehensive classification system for lipids, J Lipid Res 46 (2005) 839–861. [DOI] [PubMed] [Google Scholar]
- [19].Fahy E, Subramaniam S, Murphy RC, et al. , Update of the LIPID MAPS comprehensive classification system for lipids, J Lipid Res 50 (2009) S9–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stacklies W, Redestig H, Scholz M, Walther D, Selbig J, pcaMethods--a bioconductor package providing PCA methods for incomplete data, Bioinformatics 23 (2007) 1164–1167. [DOI] [PubMed] [Google Scholar]
- [21].Slifker JF, Shapiro SS, The Johnson system: Selection and parameter estimation, Technometrics 22 (1980) 239–246. [Google Scholar]
- [22].Xia J, Sinelnikov IV, Han B, Wishart DS, MetaboAnalyst 3.0—making metabolomics more meaningful, Nucleic Acids Res 43 (2015) W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ward JH, Hierarchical grouping to optimize an objective function, J Am Stat Assoc 58 (1963) 236–244. [Google Scholar]
- [24].van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ, Centering, scaling, and transformations: improving the biological information content of metabolomics data, BMC Genomics 7 (2006) 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Benjamini Y, Hochberg Y, Controlling the false discovery rate: A practical and powerful approach to multiple testing, J R Stat Soc Series B Stat Methodol 57 (1995) 289–300. [Google Scholar]
- [26].Feltz CJ, Miller GE, An asymptotic test for the equality of coefficients of variation from k populations, Stat Med 15 (1996) 646–658. [DOI] [PubMed] [Google Scholar]
- [27].Passi S, Picardo M, Morrone A, De Luca C, Ippolito F, Skin surface lipids in HIV sero-positive and HIV sero-negative patients affected with seborrheic dermatitis. J Dermatol Sci 2 (1991) 84–91. [DOI] [PubMed] [Google Scholar]
- [28].Yamamoto A, Serizawa S, Ito M, Sato Y, Stratum corneum lipid abnormalities in atopic dermatitis, Arch Dermatol Res 283 (1991) 219–223. [DOI] [PubMed] [Google Scholar]
- [29].Picardo M, Passi S, De Luca C, Morrone A, Bartoli F, Ippolito F, Skin surface lipids in patients affected with atopic dermatitis, in: Czernielewski JM (Ed.), Immunological and Pharmacological Aspects of Atopic and Contact Eczema, Karger, Basel, 1991, pp. 173–174. [Google Scholar]
- [30].Makrantonaki E, Ganceviciene R, Zouboulis C An update on the role of the sebaceous gland in the pathogenesis of acne, Dermatoendocrinol 3 (2011) 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ishikawa J, Narita H, Kondo N, et al. , Changes in the ceramide profile of atopic dermatitis patients, J Invest Dermatol 130 (2010) 2511–2514. [DOI] [PubMed] [Google Scholar]
- [32].Ito S, Ishikawa J, Naoe A, et al. , Ceramide synthase 4 is highly expressed in involved skin of patients with atopic dermatitis, J Eur Acad Dermatol Venereol 31 (2017) 135–141. [DOI] [PubMed] [Google Scholar]
- [33].Matsumoto M, Umemoto N, Sugiura H, Uehara M, Difference in ceramide composition between “dry” and “normal” skin in patients with atopic dermatitis, Acta Derm Venereol 79 (1999) 246–247. [DOI] [PubMed] [Google Scholar]
- [34].Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A, Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin?, J Invest Dermatol 96 (1991) 523–526. [DOI] [PubMed] [Google Scholar]
- [35].Bleck O, Abeck D, Ring J, et al. , Two ceramide subfractions detectable in Cer(AS) position by HPTLC in skin surface lipids of non-lesional skin of atopic eczema, J Invest Dermatol 113 (1999) 894–900. [DOI] [PubMed] [Google Scholar]
- [36].Di Nardo A, Wertz P, Giannetti A, Seidenari S, Ceramide and cholesterol composition of the skin of patients with atopic dermatitis, Acta Derm Venereol 78 (1998) 27–30. [DOI] [PubMed] [Google Scholar]
- [37].Shi VY, Leo M, Hassoun L, Chahal DS, Maibach HI, Sivamani RK, Role of sebaceous glands in inflammatory dermatoses, J Am Acad Dermatol 73 (1972) 856–863. [DOI] [PubMed] [Google Scholar]
- [38].Shetage SS, Traynor MJ, Brown MB, Raji M, Graham-Kalio D, Chilcott RP, Effect of ethnicity, gender and age on the amount and composition of residual skin surface components derived from sebum, sweat and epidermal lipids, Skin Res Tech 20 (2014) 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jacobi U, Gautier J, Sterry W, Lademann J, Gender-related differences in the physiology of the stratum corneum, Dermatology 211 (2005) 312–317. [DOI] [PubMed] [Google Scholar]
- [40].Cotterill JA, Cunliffe WJ, Williamson B, Bulusu L, Age and sex variation in skin surface lipid composition and sebum excretion rate, Br J Dermatol 87 (1972) 333–340. [DOI] [PubMed] [Google Scholar]
- [41].Wu Y, Li L, Sample normalization methods in quantitative metabolomics, J Chromatogr A 1430 (2016) 80–95. [DOI] [PubMed] [Google Scholar]
- [42].Serup J, Formation of oiliness and sebum output--comparison of a lipid-absorbant and occlusive-tape method with photometry, Clin Exp Dermatol 16 (1991) 258–263. [DOI] [PubMed] [Google Scholar]
- [43].Pierard GE, Pierard-Franchimont C, Kligman AM, Kinetics of sebum excretion evaluated by the Sebutape--Chromameter technique, Skin Pharmacol 6 (1993) 38–44. [DOI] [PubMed] [Google Scholar]
- [44].Pierard GE, Follicule to follicule heterogeneity of sebum excretion, Dermatologica 173 (1986): 61–65. [DOI] [PubMed] [Google Scholar]
- [45].Clarys P, Barel A, Quantitative evaluation of sebaceous secretion on the forehead: Comparison between the Sebumeter® and a microporus film (Sebutape®), in: Proceedings of the 9th International Symposium on Bioengineering and the Skin, Sendai, Japan, 1992. [Google Scholar]
- [46].Clarys P, Barel A, Quantitative evaluation of skin surface lipids, Clin Dermatol 13 (1995) 307–321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.