Abstract
The immunologic paradigm of pregnancy led to the conceptualization of pregnancy as an organ transplant which requires, for its success, a systemic immune suppression of the maternal immune system. Growing scientific evidence suggests that in many ways the placenta functions as a tumor rather than a transplant and the immune regulation of the maternal-fetal interface is the result of the coordinated interaction between all its cellular components, including bacteria. The role of microbiota in reproduction is in its infancy, but there is growing literature that supports its relevance. We discuss a potential normal function of bacteria in the establishment of immune-tolerance and the compelling evidence that a viral infection might be the underlying cause of perturbation of homeostasis. There is compelling evidence that many infectious diseases of humans are caused by more than one microorganism and are defined as polymicrobial infections. We propose that pregnancy complications, such as preterm birth, are the result of polymicrobial infections. We examine the potential cellular and molecular mechanisms by which a viral infection of the placenta might disrupt the normal interaction between the cellular component of the implantation site and bacteria. As we better understand the normal homeostasis between the maternal immune system, placenta and commensal, we will be able to elucidate the pathogenic conditions and design better approaches to treat pregnancy complications associated with infection.
Keywords: Trophoblast, bacteria, virus, inflammation, preterm birth
From Transplantation to tumor Immunology
The immunologic paradigm of pregnancy was described for the first time by a transplant immunologist, Sir Peter Medawar, who observed the antigenic mixture of the fetus containing paternal antigens that are not rejected by the maternal immune system1, 2. This unique observation led to the conceptualization of pregnancy as a “semiallograft” and it was proposed that pregnancy represents a natural model of transplantation that could help to understand the immunologic bases for transplant rejection and acceptance2. Consequently, the study of the immunology of pregnancy followed the immunological models associated with organ transplant. Over the years several mechanisms have been proposed to explain the immune privileged state of the fetus, all of them with the basic understanding that the maternal immune system is antagonistic to the fetus/placental unit 3. Since the success of an organ transplant is obtained by inducing immune suppression of the host, it was postulated that pregnancy may have a natural mechanism to induce systemic suppression of the maternal immune system. This concept has been studied by numerous investigators and over many years has become the conventional wisdom 4. Indeed, a wide array of factors in human serum have been found to possess profound in vitro immunosuppressive activities 5. However, if we carefully analyze this hypothesis it is difficult to imagine how, from an evolutionary point of view, pregnancy involves a stage of profound immune suppression. Early humans were not able to wash their hands or clean their food and, with the absence of antiseptics, were continually exposed to bacteria, parasites and other microorganisms. If pregnant women were systemically immunologically suppressed they would not have survived and the human species would have become extinct. Even today, in many parts of the world, pregnant women are continually exposed to harsh, unsanitary conditions and a suppressed immune system would make it impossible for the mother and fetus to survive. Furthermore, in countries such as Africa where HIV is pandemic, HIV-positive women do not develop AIDS during pregnancy 6. In fact, there are recent studies clearly demonstrating that the maternal antiviral immunity is not affected by pregnancy 7,8. Together, these observations argue against the existence of such non-specific immune suppression.
Medawar’s original observation was based on the assumption that the placenta was akin to a “piece of skin” with paternal antigens, which under normal immunological conditions, should be rejected. However, the placenta is more than just a transplanted organ. Our knowledge of placental biology has significantly increased over the last 50 years. We now know that the placenta is a complex organ, which has evolved from the original “egg cover”. Pregnancy and implantation, contrary to “graft implants”, has been taking place for more than 1,000,000 years. Therefore, from an evolutionary point of view it is difficult to conceive that the placenta and the maternal immune system still maintain an antagonistic status. Thus, while there should be an active mechanism preventing the potential recognition of paternal antigens by the maternal immune system, the trophoblast and the maternal immune system have evolved and established a cooperative status, helping each other against common enemies: infectious microorganisms 9–11.
Therefore, it is important to evaluate the immunologic aspects associated with pregnancy in order to further understand the potential biological reasons associated with the risk of pregnancy complications potentially triggered by infection. One wonders why the model of transplantation may not represent the correct immunological situation of pregnancy: During organ transplantation there is a major influx of foreign antigens as a result of the introduction of a fully foreign organ. Under this circumstance the host immune system acutely reacts to the foreign antigens and mounts an immunologic response to reject the source of foreign antigens. During pregnancy the process is different. Pregnancy is a slow and gradual process where paternal/fetal antigens are released in a gradual and increasing manner as the blastocyst grows into an embryo and then into a fetus. The exposure of small amounts of foreign antigens during this process may actually induce tolerance rather than rejection 12. Consequently, pregnancy, contrary to transplantation, does not require systemic immune suppression.
A second aspect that has been poorly evaluated for many years is the active role of the placenta in the modulation of the maternal immune system. Pregnant women represent an immunologically unique population because their immune system is influenced by signals originating from the placenta 13, 14. The presence of the fetus and placenta alters maternal immunity and physiology to sustain and protect the pregnancy. We and others have shown that the placenta may function as an immune modulatory organ that regulates the immune responses of cells present both at the implantation site as well as systemically15,10, 16, 17. However, this modulation is not suppressive, but protective. The placenta together with the decidua are responsible for establishing a unique microenvironment at the implantation site that i) prevents a pro-inflammatory cytokine storm; ii) inhibits the recruitment of T cells with cytolytic function, iii) educates the local immune system to facilitate the fetal development, iv) control bacterial growth and v) protect the fetus from viral infections.
In many ways, the placenta functions as a tumor rather than a transplant. Tumors secrete an array of factors that establish a local immunosuppressive microenvironment in which dysfunction and even death of tumor-specific T-cells can occur. This immune-regulatory effect occurs at two sites: 1) locally at the tumor-host interface where cancer cells condition the tumor stroma, and 2) systemically where cells and/or factors mediate suppression of anti-tumoral T-cells in the blood and lymphoid organs. The placenta has also two regulatory sites that are 1) locally, at the decidua where immune cells are differentiated towards a pregnancy supporting function, and 2) systemically, affecting the maternal immune system and preventing the expansion of T-cell clones that recognize paternal antigens.
The placenta and bacteria, friends or foes?
Bacterial infections are thought to pose a significant threat to a pregnancy and to the well-being of the fetus, by gaining access to gestational tissues, such as the decidua, the placenta, and the fetal membranes, through one of three major routes: By ascending into the uterus from the lower tract; by descending into the uterus from the peritoneal cavity; or via the maternal circulation 18,19–22,23. There are strong clinical links between bacterial infection and preterm birth 24,23,25, 26,27. Indeed, infections have been reported as responsible for up to 40% of preterm birth cases28, 29. Furthermore, 80% of preterm deliveries occurring at less than 30 weeks of gestation have evidence of infection 26,27. While many of the pathways involved are still largely undefined, growing literature suggests that the way in which a microorganism can induce a pregnancy complication, such as preterm birth, involves innate immune responses towards the pathogen, leading to excessive inflammation and/or apoptosis at the maternal-fetal interface 30,31, 32. Indeed, experimental in vivo models have demonstrated that delivery of infectious components (bacteria and bacterial products) to a variety of animals triggers preterm delivery 33, 34,35,36. Clinical studies have correlated placental infection/inflammation with prematurity 37–39 and this is supported by experimental studies 14, 34, 40, 41. In spite of the strong literature linking bacterial infection and pregnancy complications, targeting bacterial infections have failed to prevent pregnancy complications 42, 43. Furthermore, growing evidence suggests bacteria are a normal component of the pregnant and non-pregnant uterus 44,45,46, 47,48. These observations suggest that in most cases bacteria alone may not be sufficient to induce an inflammatory event leading to parturition and that the immune response to commensal bacterial product at the maternal-fetal unit is tightly controlled by regulatory mechanisms 17, 48. When and why bacteria become detrimental for pregnancy has not been defined.
Trophoblast cells as a component of innate immune system
The placenta is in direct contact with maternal component, thus it is imperative that a high level of immune protection is present at the maternal-fetal interface to protect the fetus against any infectious agent that reaches the placenta49. It is well known that classical immune cells, such as macrophages and natural killer cells, are present at the interface to facilitate innate immune responses. In addition to these immune cells, previous studies demonstrated that trophoblasts, the major constituents of the placenta, are also able to sense and respond to pathogen-associated molecular patterns (PAMPs) such as LPS or peptidoglycan 41. We have confirmed the expression of Toll-like receptors (TLRs), membranous receptors known to recognize microbial products and have demonstrated that via TLR4 trophoblasts elicit an immune response 50–53. Similar to other innate immune cells, ligation of TLR4 in trophoblast with LPS are able to activate both the classical myeloid differentiation factor 88 (MyD88) -dependent and the MyD88-independent signaling pathways leading to the production of cytokines and chemokines 52. This finding strongly supports that trophoblast can indeed recognize pathogen and initiate an immune response. The immune interaction between the trophoblast and bacteria will be further discussed in the later part of the review.
The placenta is not a sterile organ
Human body harbors multiple microorganisms termed “microbiota” and they are known to interact with the host and play various important roles to benefit the host. Bacterial colonization in the placenta from the patients who delivered preterm due to intrauterine inflammation or chorioamnionitis has been well reported in numerous studies 30, 54, 55. Based on these findings, a general belief among obstetricians was that the placenta should remain a sterile organ to maintain pregnancy through term. Recent study by Aagaard et al. 56 on placental microbiome provided a turning point for this dogma. By application of culture-independent whole genome shotgun metagenomic technology, they were able to sequence various bacterial species in the placenta obtained following normal term pregnancy, the dominant bacteria identified being Escherichia coli and the genus Escherichia species. Furthermore, Provotella tannerae, Bacteriodes species, Fusobacterium species, Streptomyces avermitilis, Neisseria polysaccharea, Neisseria lactamica, and Fusobacterium species, though low in abundance were also sequenced. In line with this, presence of bacteria in the placenta has been reported by others substantiating the concept that placenta is inhabited by commensal bacteria 43, 57, 58. Of particular interest is the finding that microbiota composition of placenta shared a great similarity, not with vagina or gut in the proximity, but with that of the oral cavity.
Although still controversial, there is growing evidences suggesting that the implantation site might not represent a sterile environment and the commensal bacterial might play a significant role during fetal development. Nonetheless, where and how the commensal bacteria migrate from and colonize in the placenta still remains a mystery.
‘Immune regulatory’ crosstalk between the trophoblast and commensal bacteria
What is the role of microbiota in the context of immune modulation? Among various functions, such as metabolizing nutrients and enforcing mucosal barrier, microbiota have been found to take part in modulating the host immune response as a way to prevent undesired inflammatory response to commensal bacteria or end-products, thereby, maintaining tissue homeostasis 59. Stimulation of TLR with commensal bacteria in gut epithelium has shown to suppress NF-κB pathway and persistent nucleotide-binding oligomerisation domain (NOD) 2 receptor ligation with peptidoglycan, a bacterial wall product induced down-regulation of proinflammatory cytokines 60, 61. And the disruption of host immunological tolerance to microbiota has been implicated in the pathogenesis of proinflammatory disease entities, such as inflammatory bowel disease, pulmonary asthma, chronic hepatitis, autoimmune disease, and others 62, 63.
The placenta cannot be an exception. If bacterium is present in proximity or at the maternal-fetal interface during a normal pregnancy, it might play a physiological role by contributing to the normal tissue homeostasis as disruption of the placental homeostasis may adversely affect not only the fetus but also the mother. This fundamental process relies on a complex and coordinated set of innate and adaptive responses that selects and calibrates responses against self, commensals and pathogens in the most appropriate manner. At the implantation site, specialized cell populations have to integrate local signals, such as cytokines, chemokines and microbial factors, allowing the induction of responses in a way that preserves the integrity of the tissue and its function (Fig 1A).
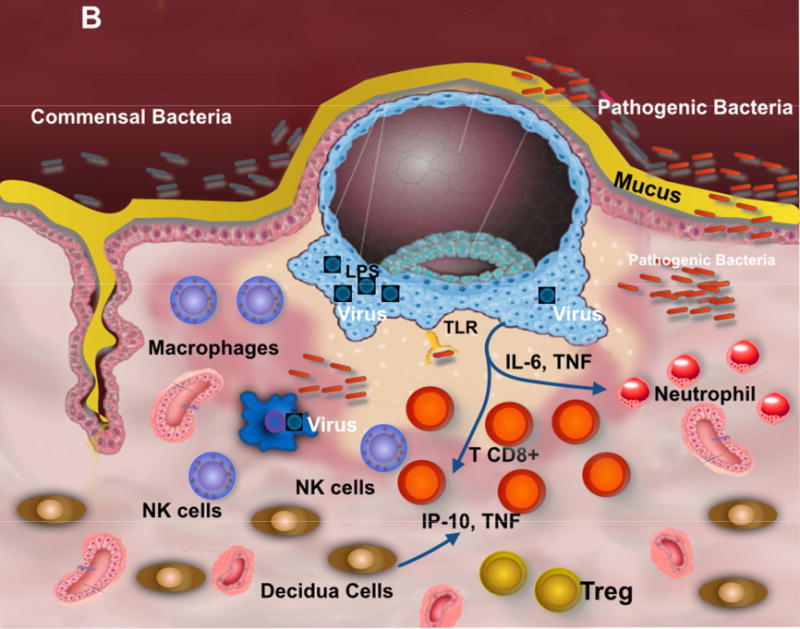
Figure 1. Contribution of the Microbiota to trophoblast-immune regulation at the implantation site.
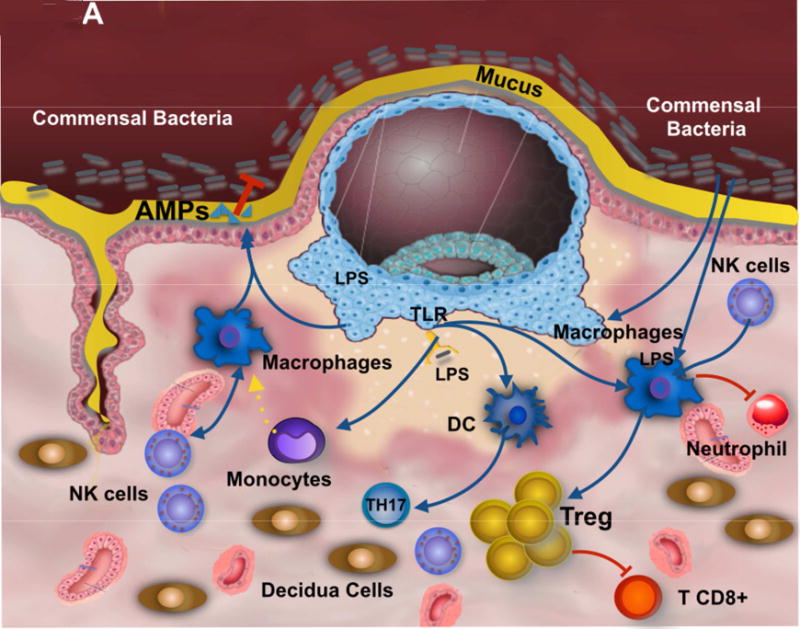
A. Commensal present at the mucus covering the epithelium of the uterus promotes the induction of regulatory factors by the trophoblast and decidual macrophages. Further, macrophages secrete anti-microbial products that control commensal overgrowth and prevent the invasion of pathogenic bacteria. The recognition of bacterial products by trophoblast enhances the expression of anti-inflammatory factors expanding T regs and promoting tolerance.
B. Viral infection at the implantation site inhibits macrophage capacity to control bacterial growth, disturbing the symbiosis between the microbiota, trophoblast and immune cells at the implantation site; leading to an inflammatory condition responsible for preterm birth.
However, as the presence of microbes at the maternal-fetal interface is just beginning to unravel, their contribution to the placental innate immune system is unknown. Our studies have focused on determining how constitutive sensing of commensal by the trophoblast, plays an important homeostatic role during pregnancy, whereas active responses against the flora might be associated with pregnancy complications. As mentioned earlier, expression of all 10 Toll Like Receptors (TLRs) as well as various co-receptors and accessory proteins, such as CD14, has been described in the human and mouse placenta 36, 41, 64, which provides the trophoblast the capacity to recognize and respond to bacteria and viral products 16, 65. Trophoblast express TLR4 and are able to recognize the bacterial product LPS; however, TLR4 ligation by LPS in the trophoblast did not induce the classical NF-κB-inflammatory response characterized by the production of inflammatory mediators, but promotes the secretion of regulatory cytokines, such as IL-10 and type I interferon-associated chemokines (Racicot et al 2015 in preparation) 14. It is plausible that bacteria, through the induction of regulatory cytokines by trophoblast, might stimulate the maternal-fetal interface to a tolerogenic microenvironment (Fig. 1A). How the trophoblast integrates microbial-derived signals remains unclear, but new data from others and us suggests that epigenetic modifications or alternative pathways mediating the response to TLRs are responsible for this unique type of responses 66, 67.
Consequently, we postulate that, when trophoblast operate properly with the commensals, their durable homeostatic relationship contributes to the regulatory tone of the maternal fetal interface and alterations in this relationship might be the base for pro-inflammatory conditions, such as preterm labor and delivery 68.
Preterm birth as a polymicrobial disease
There is compelling evidence that many infections of humans and animals are caused by more than one microorganism 69, 70. The mixed microbial nature of these diseases has been recognized since the early 1920s71; however, only more recently we have we begun to understand the mechanisms associated with this type of disease. Polymicrobial diseases can be caused by the synergistic or sequential action of infectious agents from either the same or different kingdoms, genera, species, strains or by different phenotypic variants of a single species 70, 72–74. A growing body of evidence suggests that preterm birth is a manifestation of polymicrobial disease 14, 48. Recently, Payne et al. 75 thoroughly reviewed multiple types of microorganisms, including bacteria, virus, and fungus, detected in the maternal-fetal unit and their association with preterm birth.
We have developed an animal model of polymicrobial disease that leads to preterm birth76. The model consists of a Herpes Viral infection of the placenta and decidua leading to increased sensitivity towards bacterial products, which then triggers a pro-inflammatory “cytokine storm” and preterm birth. Herpes Simplex Virus (HSV) is the most common sexually-transmitted infection among the adult female population worldwide 77. Although HSV-2 is the main cause of genital herpes and is almost always sexually transmitted, HSV-1 has emerged as a principle causative agent of genital herpes and its importance is increasing in college students78. From the late 1970s, HSV-2 seroprevalence in the US has increased by 30%, resulting that one out of five adults is infected79. The greatest incidence of HSV infection occurs in women of reproductive age and the potential transmission to the fetus during pregnancy has become a major health concern 80–83. In the US, approximately 22% of pregnant women are infected with HSV, 10% are at risk of acquiring infection from their partners and 2% are infected during pregnancy. In addition to the risk of placing the newborn at risk of infection, acquisition of genital herpes during pregnancy has been associated with spontaneous abortion, intrauterine growth retardation and preterm labor84. Primary HSV1/2 during pregnancy may have detrimental effects for the mother and the fetus85,86. However, the mechanism of how HSV1/2 infection during pregnancy may lead to preterm birth is poorly understood.
In our animal model, we use the murine gammaherpesvirus 68 (Murid herpesvirus 4 (NC_001826.2); MHV68) which is a rodents herpes virus that shares significant genomic co-linearity with human pathogens, Epstein-Barr virus (EBV) and HSV 87. Maternal infection with MHV68 alone had no effect on pregnancy outcome including litter size, weight or gestational age at delivery17, 76, similar to that observed in human infection with HSV. However, it had a significant effect on the response to microbial products such as LPS. Although, high doses of LPS administered to pregnant mice have been shown to trigger preterm birth, low doses have no detrimental effect or a mild effect on pregnancy outcome 88, 89. When we infected pregnant C57BL/6 mice with MHV68 on day E8.5 followed by low dose LPS (20ug/kg) on day E15.5 we observed preterm delivery in 100% of the mice in less than 24 hours. A similar dose of LPS in control mice (who did not receive MHV68) had a mild effect on pregnancy outcome (Fig. 2). Preterm birth observed in the combination virus-LPS was characterized by a cytokine storm consisting of high levels of pro-inflammatory cytokines associated with parturition 76,72;73;74. Furthermore, trophoblast cells predisposed to MHV infection demonstrated hyper-responsive proinflammatory cytokine production when challenged with LPS.
Figure 2. Murine model for preterm delivery as polymicrobial disease.
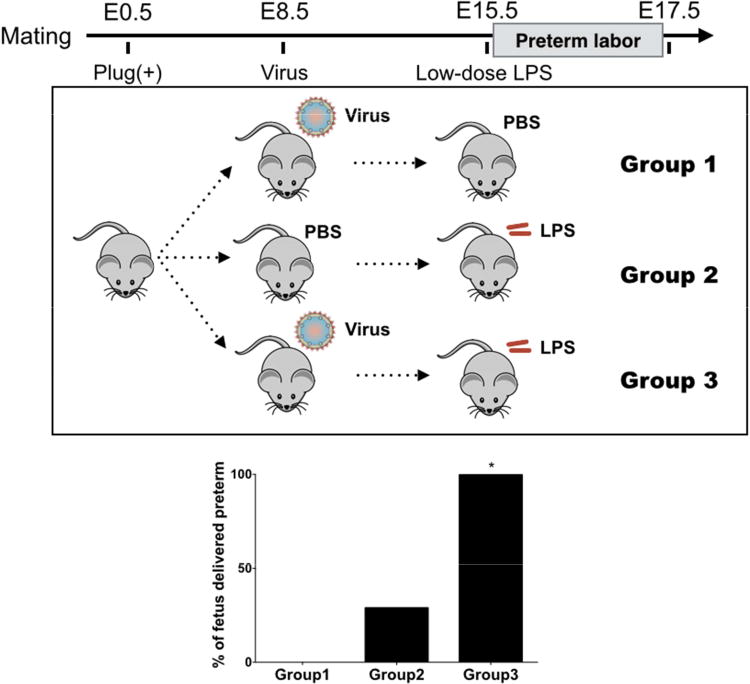
C57BL/6 mice infected with murine gammaherpesvirus 68 (MHV68) followed by low dose LPS demonstrated significantly higher rate of preterm birth in comparison to MHV68 or LPS alone. (Modified from Cardenas I et al. AJRI 201114)
These findings suggest that herpes virus infection at the implantation site has the potential to modify TLR4 response to LPS, disturbing the optimal trophoblast-microbiota interaction that was originally set at a point towards immune tolerance and predisposes the placenta to an inflammatory response leading to preterm birth (Fig 1B).
Conclusion
The role of microbiota in reproduction is in its infancy, but there is a growing literature that supports its relevance. As we understand the normal homeostasis between the maternal immune system of the host, placenta and commensal, we will be able to elucidate the pathogenic conditions and design better approaches to treat pregnancy complications associated with infection.
Acknowledgments
Support: This study is in part funded by grant P01HD054713 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors report no conflict of interest.
References
- 1.Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp SocExp Biol. 1952;7:320–38. [Google Scholar]
- 2.Medawar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. British Journal of Experimental Pathology. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 3.Mor G. Inflammation and pregnancy: the role of toll-like receptors in trophoblast-immune interaction. Ann N Y Acad Sci. 2008;1127:121–8. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- 4.PrabhuDas M, Bonney E, Caron K, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328–34. doi: 10.1038/ni.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Formby B. Immunologic response in pregnancy Its role in endocrine disorders of pregnancy. and influence on the course of maternal autoimmune diseases. Endocrinol Metab Clin North Am. 1995;24:187–205. [PubMed] [Google Scholar]
- 6.Wira CR, Veronese F. Mucosal immunity in the male and female reproductive tract and prevention of HIV transmission. Am J Reprod Immunol. 2011;65:182–5. doi: 10.1111/j.1600-0897.2010.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Seminars in fetal & neonatal medicine. 2006;11:317–26. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Read JS, Cahn P, Losso M, et al. Management of human immunodeficiency virus-infected pregnant women at latin american and Caribbean sites. Obstet Gynecol. 2007;109:1358–67. doi: 10.1097/01.AOG.0000265211.76196.ac. [DOI] [PubMed] [Google Scholar]
- 9.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 10.Mor G, Cardenas I. The immune system in pregnancy: A unique complexity. Am J Reprod Immunol. 2010;63:425–33. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–94. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 12.Sabatos-Peyton CA, Verhagen J, Wraith DC. Antigen-specific immunotherapy of autoimmune and allergic diseases. Curr Opin Immunol. 2010;22:609–15. doi: 10.1016/j.coi.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon JY, Romero R, Mor G. New Insights into the Relationship between Viral Infection and Pregnancy Complications. Am J Reprod Immunol. 2014 doi: 10.1111/aji.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardenas I, Mor G, Aldo P, et al. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol. 2011;65:110–7. doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naccasha N, Gervasi MT, Chaiworapongsa T, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185:1118–23. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 16.Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Human Reprod. 2004;10:55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 17.Cardenas I, Means RE, Aldo P, et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–57. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell CM, Haick A, Nkwopara E, et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol. 2015;212:611 e1–9. doi: 10.1016/j.ajog.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod. 2003;69:1957–63. doi: 10.1095/biolreprod.103.019620. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal V, Smart K, Jilling T, Hirsch E. Surfactant protein (SP)-A suppresses preterm delivery and inflammation via TLR2. PLoS One. 2013;8:e63990. doi: 10.1371/journal.pone.0063990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayraktar M, Peltier M, Vetrano A, et al. IL-10 modulates placental responses to TLR ligands. Am J Reprod Immunol. 2009;62:390–9. doi: 10.1111/j.1600-0897.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiefer DG, Keeler SM, Rust O, et al. Amniotic fluid inflammatory score is associated with pregnancy outcome in patients with mid trimester short cervix. Am J Obstet Gynecol. 2012;206:68.e1–6. doi: 10.1016/j.ajog.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol. 2006;194:630–7. doi: 10.1016/j.ajog.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 24.Romero R, Chaiworapongsa T, Espinoza J. Micronutrients and intrauterine infection, preterm birth and the fetal inflammatory response syndrome. J Nutr. 2003;133:1668S–73S. doi: 10.1093/jn/133.5.1668S. [DOI] [PubMed] [Google Scholar]
- 25.Andrews WW, Copper R, Hauth JC, Goldenberg RL, Neely C, Dubard M. Second-trimester cervical ultrasound: associations with increased risk for recurrent early spontaneous delivery. Obstetrics and gynecology. 2000;95:222–6. doi: 10.1016/s0029-7844(99)00483-4. [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. NEJM. 2000;342:1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 27.Lamont RF. The role of infection in preterm labour and birth. Hosp Med. 2003;64:644–7. doi: 10.12968/hosp.2003.64.11.2343. [DOI] [PubMed] [Google Scholar]
- 28.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200:374.e1–5. doi: 10.1016/j.ajog.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 29.Peltier MR, Klimova NG, Arita Y, et al. Polybrominated diphenyl ethers enhance the production of proinflammatory cytokines by the placenta. Placenta. 2012;33:745–9. doi: 10.1016/j.placenta.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Straszewski-Chavez SL, Abrahams VM, Aldo PB, Mor G. Isolation and characterization of a novel telomerase-immortalized human first trimester trophoblast cell line. Placenta. 2005;26:A.62. doi: 10.1016/j.placenta.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Straszewski-Chavez SL, Abrahams VM, Mor G. The Role of Apoptosis in the Regulation of Trophoblast Survival and Differentiation During Pregnancy. Endocr Rev. 2005 doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez JM, Xu H, Ofori E, Elovitz MA. Toll-like receptors in the uterus, cervix, and placenta: is pregnancy an immunosuppressed state? Am J Obstet Gynecol. 2007;197:296 e1–6. doi: 10.1016/j.ajog.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Srinivas SK, Ma Y, Sammel MD, et al. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol. 2006;195:797–802. doi: 10.1016/j.ajog.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 35.Taglauer ES, Adams Waldorf KM, Petroff MG. The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance. Int J Dev Biol. 2010;54:421–30. doi: 10.1387/ijdb.082800et. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63:587–600. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero R. Novel aspects of neutrophil biology in human pregnancy. Am J Reprod Immunol. 2005;53:275. [Google Scholar]
- 38.Romero R, Kadar N, Vaisbuch E, Hassan SS. Maternal Death Following Cardiopulmonary Collapse After Delivery: Amniotic Fluid Embolism or Septic Shock Due to Intrauterine Infection? Am J Reprod Immunol. 2010 doi: 10.1111/j.1600-0897.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero AI, Lagging M, Westin J, et al. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006;194:895–903. doi: 10.1086/507307. [DOI] [PubMed] [Google Scholar]
- 40.Golos TG, Bondarenko GI, Dambaeva SV, Breburda EE, Durning M. On the role of placental Major Histocompatibility Complex and decidual leukocytes in implantation and pregnancy success using non-human primate models. Int J Dev Biol. 2010;54:431–43. doi: 10.1387/ijdb.082797tg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koga K, Cardenas I, Aldo P, et al. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61:196–212. doi: 10.1111/j.1600-0897.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Digiulio DB, Gervasi M, Romero R, et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med. 2010 doi: 10.1515/JPM.2010.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril. 2004;82:799–804. doi: 10.1016/j.fertnstert.2004.05.076. [DOI] [PubMed] [Google Scholar]
- 45.!!! INVALID CITATION !!! {Digiulio, 2010 #3125};{Romero, 2004 #5215}.
- 46.Cao B, Mysorekar IU. Intracellular bacteria in placental basal plate localize to extravillous trophoblasts. Placenta. 2014;35:139–42. doi: 10.1016/j.placenta.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Stout MJ, Conlon B, Landeau M, et al. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 2013;208:226 e1–7. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Racicot K, Cardenas I, Wunsche V, et al. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013;191:934–41. doi: 10.4049/jimmunol.1300661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madsen-Bouterse SA, Romero R, Tarca AL, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abrahams VM, Romero R, Mor G. TLR-3 and TLR-4 mediate differential chemokine production and immune cell recruitment by first trimester trophoblast cells. Am J Reprod Immunol. 2005;53:279. -ASRI05-02. [Google Scholar]
- 51.Aldo PB, Mulla MJ, Romero R, Mor G, Abrahams VM. Viral ssRNA Induces First Trimester Trophoblast Apoptosis through an Inflammatory Mechanism. Am J Reprod Immunol. 2010 doi: 10.1111/j.1600-0897.2010.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abrahams VM, Bole-Aldo P, Kim YM, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–96. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 53.Abrahams VM, Schaefer TM, Fahey JV, et al. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I: C) Hum Reprod. 2006;21:2432–9. doi: 10.1093/humrep/del178. [DOI] [PubMed] [Google Scholar]
- 54.Vaisbuch E, Romero R, Erez O, et al. Activation of the alternative pathway of complement is a feature of pre-term parturition but not of spontaneous labor at term. Am J Reprod Immunol. 2010;63:318–30. doi: 10.1111/j.1600-0897.2009.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–74. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 56.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Science translational medicine. 2014;6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SE, Romero R, Lee SM, Yoon BH. Amniotic fluid volume in intra-amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med. 2010;38:39–44. doi: 10.1515/JPM.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romero R, Hassan SS, Gajer P, et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014;2:18. doi: 10.1186/2049-2618-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–41. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mortha A, Chudnovskiy A, Hashimoto D, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ridaura V, Belkaid Y. Gut microbiota: the link to your second brain. Cell. 2015;161:193–4. doi: 10.1016/j.cell.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 62.Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346:954–9. doi: 10.1126/science.1260144. [DOI] [PubMed] [Google Scholar]
- 63.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–70. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koga K, Aldo PB, Mor G. Toll-like receptors and pregnancy: trophoblast as modulators of the immune response. J Obstet Gynaecol Res. 2009;35:191–202. doi: 10.1111/j.1447-0756.2008.00963.x. [DOI] [PubMed] [Google Scholar]
- 65.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;175:8096–104. doi: 10.4049/jimmunol.175.12.8096. [DOI] [PubMed] [Google Scholar]
- 66.Alenghat T, Osborne LC, Saenz SA, et al. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature. 2013;504:153–7. doi: 10.1038/nature12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silasi M, Mor G. Decidual stromal cells as regulators of T-cell access to the maternal-fetal interface. Am J Reprod Immunol. 2012;68:279–81. doi: 10.1111/aji.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Racicot K, Kwon JY, Aldo P, Silasi M, Mor G. Understanding the Complexity of the Immune System during Pregnancy. Am J Reprod Immunol. 2014;72:107–16. doi: 10.1111/aji.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bakaletz LO. Developing animal models for polymicrobial diseases. Nature reviews Microbiology. 2004;2:552–68. doi: 10.1038/nrmicro928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith H. The role of microbial interactions in infectious disease. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1982;297:551–61. doi: 10.1098/rstb.1982.0060. [DOI] [PubMed] [Google Scholar]
- 72.Geyer AI, Kraus T, Roberts M, et al. Plasma level of interferon gamma induced protein 10 is a marker of sarcoidosis disease activity. Cytokine. 2013;64:152–7. doi: 10.1016/j.cyto.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 73.Li W, Moltedo B, Moran TM. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J Virol. 2012;86:12304–12. doi: 10.1128/JVI.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pazos M, Sperling RS, Moran TM, Kraus TA. The influence of pregnancy on systemic immunity. Immunologic research. 2012;54:254–61. doi: 10.1007/s12026-012-8303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Payne MS, Goss KC, Connett GJ, Legg JP, Bruce KD, Chalker V. A quantitative analysis of Ureaplasma urealyticum and Ureaplasma parvum compared with host immune response in preterm neonates at risk of developing bronchopulmonary dysplasia. J Clin Microbiol. 2012;50:909–14. doi: 10.1128/JCM.06625-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cardenas I, Aldo P, Koga K, Means R, Lang SH, Mor G. Subclinicla viral infection in pregnancy lead to inflammatoruy process at the placenta with non-lethal fetal damage. Am J Reprod Immunol. 2010;61:397–S12. [Google Scholar]
- 77.Anzivino E, Fioriti D, Mischitelli M, et al. Herpes simplex virus infection in pregnancy and in neonate: status of art of epidemiology, diagnosis, therapy and prevention. Virol J. 2009;6:40. doi: 10.1186/1743-422X-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith PD, Roberts CM. American College Health Association annual Pap test and sexually transmitted infection survey: 2006. Journal of American college health: J of ACH. 2009;57:389–94. doi: 10.3200/JACH.57.4.389-394. [DOI] [PubMed] [Google Scholar]
- 79.Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 80.Desselberger U. Herpes simplex virus infection in pregnancy: diagnosis and significance. Intervirology. 1998;41:185–90. doi: 10.1159/000024934. [DOI] [PubMed] [Google Scholar]
- 81.Harding-Esch EM, Edwards T, Sillah A, et al. Active trachoma and ocular Chlamydia trachomatis infection in two Gambian regions: on course for elimination by 2020? PLoS neglected tropical diseases. 2009;3:e573. doi: 10.1371/journal.pntd.0000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henriquez SA, Brett R, Alexander J, Pratt J, Roberts CW. Neuropsychiatric disease and Toxoplasma gondii infection. Neuroimmunomodulation. 2009;16:122–33. doi: 10.1159/000180267. [DOI] [PubMed] [Google Scholar]
- 83.Kawana K, Yoshikawa H, Sata T. Post-partum detection of varicella-zoster virus DNA in the placenta. Int J Gynaecol Obstet. 1996;55:165–6. doi: 10.1016/s0020-7292(96)02732-4. [DOI] [PubMed] [Google Scholar]
- 84.Brown ZA, Benedetti J, Selke S, Ashley R, Watts DH, Corey L. Asymptomatic maternal shedding of herpes simplex virus at the onset of labor: relationship to preterm labor. Obstet Gynecol. 1996;87:483–8. doi: 10.1016/0029-7844(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 85.Eskild A, Bruu AL, Stray-Pedersen B, Jenum P. Epstein-Barr virus infection during pregnancy and the risk of adverse pregnancy outcome. Bjog. 2005;112:1620–4. doi: 10.1111/j.1471-0528.2005.00764.x. [DOI] [PubMed] [Google Scholar]
- 86.Avgil M, Ornoy A. Herpes simplex virus and Epstein-Barr virus infections in pregnancy: consequences of neonatal or intrauterine infection. Reprod Toxicol. 2006;21:436–45. doi: 10.1016/j.reprotox.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 87.Olivadoti M, Toth LA, Weinberg J, Opp MR. Murine gammaherpesvirus 68: a model for the study of Epstein-Barr virus infections and related diseases. Comp Med. 2007;57:44–50. [PubMed] [Google Scholar]
- 88.Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol. 2006;177:4888–96. doi: 10.4049/jimmunol.177.7.4888. [DOI] [PubMed] [Google Scholar]
- 89.Murphy SP, Hanna NN, Fast LD, et al. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obstet Gynecol. 2009;200:308 e1–9. doi: 10.1016/j.ajog.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]


