Abstract
Epithelial-mesenchymal transition (EMT) is a critical process involved in cancer metastasis and chemo-resistance. Twist1 is a key EMT-inducing transcription factor, which is upregulated in multiple types of cancers and has been shown to promote tumor cell invasiveness and support tumor progression. Conversely, p53 is a tumor suppressor gene that is frequently mutated in cancers. This study demonstrates the ability of wild-type (wt) p53 to promote the degradation of Twist1 protein. By forming a complex with Twist1 and the E3 ligase Pirh2, wt p53 promotes the ubiquitination and proteasomal degradation of Twist1, thus inhibiting EMT and maintaining the epithelial phenotype. The ability of p53 to induce Twist1 degradation is abrogated when p53 is mutated. Consequently, the loss of p53-induced Twist1 degradation leads to EMT and the acquisition of a more invasive cancer phenotype.
Introduction
The developmental process termed epithelial-mesenchymal transition (EMT) plays a critical role in embryonic morphogenesis as well as in pathologic processes such as carcinoma progression and metastasis formation (1). Activation of EMT process produces changes in multiple aspects of cellular physiology including alterations in the cytoskeleton organization, changes in cell morphology, dissolution of epithelial cell-cell junctions, loss of apical-basal polarity and acquisition of motility (2,3). At the molecular level, EMT can be induced by the upregulation of transcriptional factors, such as Twist1, Snail, and Slug (4). These factors inhibit the expression of E-cadherin and cause epithelial cells to lose cell-cell contact thus leading to their enhanced motility and dissemination (5,6).
Twist1 is a basic helix-loop-helix (bHLH) transcription factor, whose expression is required for normal embryogenesis, but is largely absent in adult tissues. Upon dimerization with other bHLH proteins, Twist1 directly binds to DNA and regulates the transcription of EMT-related genes (7,8). Mutations affecting dimerization and activity of Twist1 have been linked to Saethre-Chotzen syndrome (9,10) and pathological expression and activation of Twist1 outside the time of embryonic organogenesis play an essential role in tumor initiation (11), stemness (12,13), angiogenesis, invasion, metastasis, and chemo-resistance (14–17) in a variety of carcinomas, sarcomas, and hematological malignances (4,18). In cancer, the upregulation of Twist1 has been associated with the initiation of the EMT process, increased migration, and enhanced tumor-initiating capacity (11,19–22). Although a great number of studies have demonstrated the role of Twist1 in cancer initiation and progression, the molecular mechanisms controlling or inhibiting its expression and function are not well understood.
Mutation of the tumor suppressor TP53 has been associated with tumorigenesis (23). In addition to the classical role of the p53 protein in regulating cell cycle and apoptosis (24), accumulating evidence has shown other novel roles (25). One of the most promising findings is the role of wild-type (wt) p53 in inhibiting EMT and tumor metastasis. As a transcription factor, wt p53 can downregulate the transcription of EMT inducers and/or increase the expression of microRNAs (i.e. miR-200c and miR-205), which in turn inhibit the EMT inducers Zeb1, Zeb2, and Snail (26,27). wt p53 can also facilitate MDM2-mediated ubiquitination and degradation of Snail and Slug (28,29). Acquisition of mutation in p53 may therefore abrogate this inhibitory effect on EMT (11).
Epithelial ovarian cancers are characterized by disseminated disease throughout the peritoneal cavity, which precludes the complete removal of the tumors and is a major cause for the high mortality associated with the disease (30). Therefore, in order to prevent or treat the carcinomatosis observed in these patients and consequently improve their survival, it is necessary to understand the mechanisms regulating the process of EMT in ovarian cancer cells. In previous studies, we and others have shown the critical role of Twist1 in the EMT process in ovarian cancer cells (16) (4,14). Specifically, our group previously demonstrated that Twist1 was constitutively targeted and degraded by the ubiquitin-proteasome system in the epithelial ovarian cancer cells (16). Other molecular mechanisms controlling Twist1 expression have revealed that Twist1 is regulated at the transcription level by ERK signaling and inhibited by the androgen-regulated homeobox gene NKX3–1 (31,32). Its translation is repressed by microRNAs (miRNAs), such as miR-145a-5p and miR-151–5p (33). Twist1 protein stability is mediated by post-translational modifications, such as phosphorylation by MAPKs or IKKβ and ubiquitination by FBXL14 or β-TRCP (34–36). However, whether these mechanisms are responsible for Twist1 regulation in ovarian cancer, is unknown. Since a high percentage of ovarian tumors are characterized by mutations on TP53, we explored a potential link between p53 and Twist1 and early EMT progression.
In this study, we assessed whether wt p53 maintains an epithelial phenotype by promoting the proteasome-dependent degradation of Twist1 and consequently prevents EMT. Furthermore, we evaluated whether the loss of p53 function due to mutation or inhibition of p53 can lead to increased expression of Twist1 protein and consequently to trigger the early stage of mesenchymal transformation. Using in vitro models, we demonstrate the ability of wt p53 to promote the proteasome-dependent degradation of Twist1 by forming a complex with Twist1 and E3 ligase Pirh2. Furthermore, we show that this function is lost in cancer cells bearing specific TP53 mutations. In this model, the inability of mutant p53 to degrade Twist1 leads to EMT.
Materials and Methods
Cell lines, culture conditions, and patient samples
Sample collection was performed with patient consent and approved by the Human Investigation Committee of Yale University School of Medicine. Ovarian cancer cells were isolated from patients diagnosed with stage III/IV serous ovarian carcinoma. These in-house derived ovarian cancer cells (R182, R2615, OCSC1-F2, 01–28, and Tara R182) have been previously described (30,37–40). Ovarian cancer cell lines (OVCAR3, OVCA432, SKOV3) were purchased from ATCC. Cells were cultured in RPMI media supplemented in 10% FBS and grown at 37oC with 5% CO2. All the cell lines have been tested negative for mycoplasma, authenticated in May 2016 by STR profiling, and used within 6 passages between experiments.
Sample collection from various ovarian and intra-peritoneal cancers was also carried out with patient consent and approved by the Human Investigations Committee of Yale University School of Medicine. Tumor tissues were flash-frozen and stored in liquid nitrogen until protein was extracted by homogenization.
TP53 sequencing
p53 cDNA was used as a template for a PCR reaction using the following primers: Fwd, 5′-ATGGAGGAGCCGCAG-3′; Rev, 5′-GTCTGAGTCAGGCCCTTC-3′. Genomic DNA was extracted with a DNeasy Kit (Qiagen). Exons 5–8 were amplified by PCR and sequenced using the same primers. Primer sequences are in Supplementary Table 1.
Plasmids, antibodies, and reagents
pCMV-Neo-Bam plasmids containing wild-type p53, R175H, R248W, and R273H were gifts from Bert Vogelstein (Addgene #16434, 16436, 16437, and 16439). pcDNA3 MDM2 WT was a gift from Mien-Chie Hung (Addgene #16233). shp53 pLKO.1 puro was a gift from Bob Weinberg (Addgene #19119). pcDNA3-Pirh2 plasmid was constructed by PCR amplification of Pirh2 gene from pGEX-4T-1-Pirh2 plasmid and cloning it into pcDNA3 vector. pGEX-4T-1-Pirh2 plasmid was a gift from Cheryl Arrowsmith (Addgene #24878). Silencing RNA (siRNA) against Pirh2 and scramble siRNA were purchased from Santa Cruz Biotechnology (Dallas, TX). Transfections with plasmid vectors were performed using X-tremeGENE9 (Millipore, Burlington, MA) according to manufacturer’s instructions.
Antibodies used were Anti-Twist1 (1:500, CS-81417, Santa Cruz Biotechnology, Dallas, TX, USA), anti-p53 (1:2000, OP43, EMD Millipore, Billerica, MA, USA), anti-MDM2 (1:1000, OP115, EMD Millipore, Billerica, MA, USA), anti-Pirh2 (1:2000, A300–357A, Bethyl Laboratories, Montgomery, TX, USA), anti-GAPDH (1:10000, G8795, Sigma, St. Louis, MO, USA), peroxidase-conjugated anti-rabbit IgG (1:10000), and anti-mouse (1:10000) IgG (Dako, Glostrup, Denmark). TrueBlot Ultra anti-mouse and anti-rabbit IgG HRP were used for detection in the western blots of Co-IP tests (#18–8817-31 and 18–8816-31, Rockland Immumochemicals Inc, Limerick, PA, USA).
Immunoblotting and immunoprecipitation
For western blot analysis, cell or tissue lysates were prepared with cell lysis buffer (1% Triton X-100, 0.05% SDS, 100 mM Na2HPO4, and 150 mM NaCl). Then, 10 μg of each protein lysate was electrophoresed on a 12% SDS-polyacrylamide gel. The proteins were transferred onto PVDF membranes (EMD Millipore, Burlington, MA, USA). After blocking with PBS-0.05% Tween 20 and 5% milk, the membranes were probed with primary antibodies at 4ºC overnight, and then secondary antibodies for 1 hour at room temperature. The blots were developed using the enhanced chemiluminescence (ECL) system (NEN Life Sciences, Waltham, MA, USA). Bands were quantified using the ROI tool.
500 ug of total protein was used for immunoprecipitation (IP). After an overnight incubation of antibody and protein lysate, the IP product was incubated with Protein-A-Sepharose beads and then isolated for Western blot detection (Life technologies, Carlsbad, CA).
Sequential IP is the IP of at least two antigens one after the other in a sequential order. We performed Sequential IP as described (41). Thus, we ran sequential IP of Twist1 followed by Pirh2 or Pirh2 followed by Twist1. In the first case (Twist1-Pirh2), we first IP Twist1 and check for the presence of Pirh2. Afterwards, from this Twist1 IP complex we did a second IP with anti-Pirh2 and determine the presence of p53. In the second case, (Pirh2-Twist1) we first IP Pirh2 and check for the presence of Twist1. Afterwards, from this Pirh2 IP complex we did a second IP with anti-Twist1 and determine the presence of p53.
qPCR analysis
Total RNA was extracted using RNeasy Mini Kits (Qiagen, Austin, TX, USA) according to the manufacturer’s instructions. cDNA was synthesized with iScript cDNA Kit (Bio-Rad, Hercules, CA, USA). Quantitative PCR was performed using SYBR Green Supermix (Bio-Rad) followed by detection with the CFX96TM PCR detection system (Bio-Rad). GAPDH was used as reference gene. Relative expression was calculated using the comparative ΔΔCT method. All reactions were performed in triplicate. Primer sequences are included in Supplementary Table 2.
Lenti-virus production and transduction
Lenti-virus was produced as previously described (42). The control virus and shRNA lentivirus were introduced into the cells by adding virus to cell growth medium. Stable knockdown or control cells were selected by medium containing 1 μg/mL puromycin (Sigma-Aldrich, St. Louis, MO). The control and Δb-Twist1 overexpressing virus were produced and used to infect cells following the same protocol.
Tumor spheroid formation assay
One thousand cells were re-suspended in RPMI medium containing 5% FBS, 10μg/ml EGF, 5 μM Y-27326, and 5% Matrigel (Corning, Corning, New York, USA), and then plated in each well of a 96-well plate. Seven days later well formed, tightly organized, and round-shaped spheres >100 um in diameter were manually counted.
Trans-well migration assay
Trans-well migration assay was performed using trans-well chambers with bottom filters of 8-μm pore size. Cells (4 × 104) suspended in 200 μl RPMI were plated in the top chamber. The bottom chambers were filled with 500 μl of RPMI containing 10% FBS. Cells were allowed to migrate for 24 h. The migrated cells in the bottom chamber were counted manually. Images were taken using an inverted microscope (Olympus).
Structural Analysis
Existing structure 4QO1 was obtained from the RCSB Protein Data Bank, courtesy of De Gieter et al.(43). Images illustrating positions of residues of interest were produced using the UCSF Chimera package. Chimera is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS P41-GM103311, http://www.rbvi.ucsf.edu/chimera).
Statistical analysis
The unpaired two-tailed Student’s t-test was used for comparing between different groups. Data of each group were collected with at least 3 biological repeats. The variances in each group were calculated to make sure they are similar between the compared groups. p-values of 0.05 or less were considered statistically significant.
Results
The loss of wt p53 is associated with Twist1 overexpression in intra-peritoneal tumors
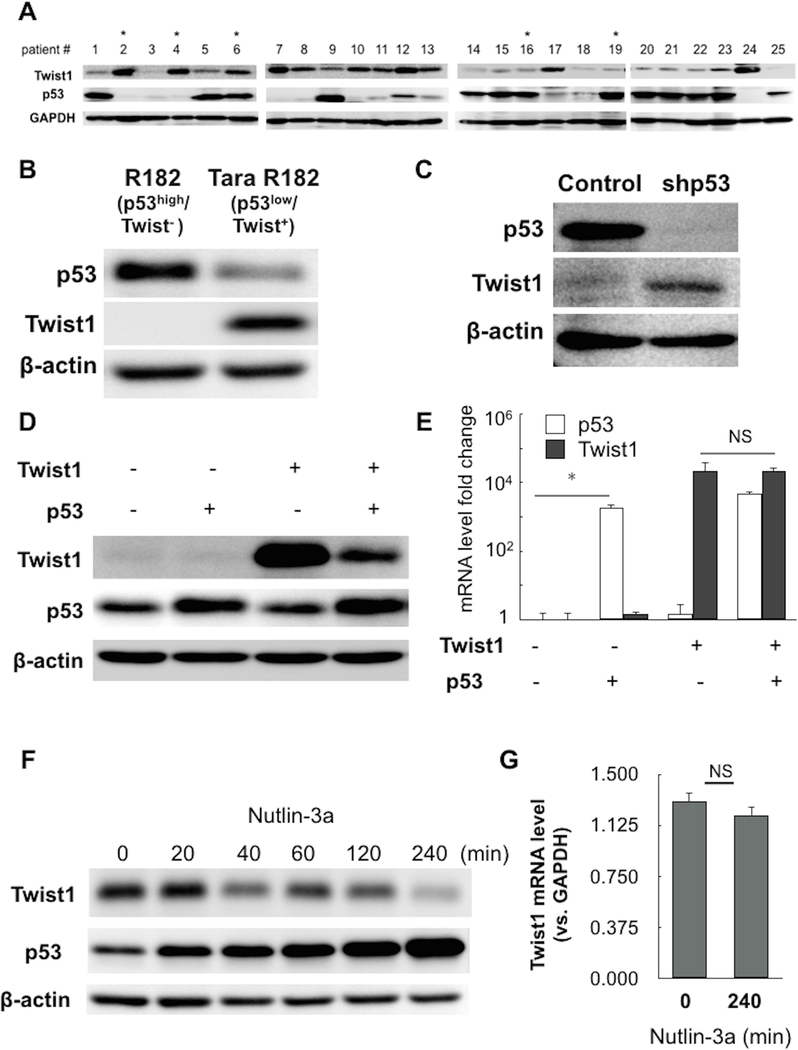
To assess the extent of association between p53 mutation and Twist1 expression we analyzed the expression of p53 and Twist1 in 25 flash-frozen samples from various ovarian and other intraperitoneal cancers (Supplementary Table 3). Interestingly, using western blot analysis we observed a great variation of tumor protein expression for both Twist1 and p53. However, we did observe a correlation between p53 and Twist1 protein expression levels. We found that tumors with high protein levels of Twist1 (11/25) had either a significantly lower level of wt p53 protein or a mutation in TP53 gene (Fig. 1A and Supplementary Figure 1). Conversely, tumors with undetectable or low Twist1 expression (9/25) showed high levels of wt p53 (Fig. 1A). Although the sample number is limited, these results demonstrate a trend that suggests a negative correlation between wt p53 and Twist1 expression (Supplementary Figure 1). In light of known p53 regulatory functions in cancer formation and progression we then further determined the molecular mechanism by which wt p53 might function as a negative regulator of Twist1 expression.
Figure 1. Protein expression of p53 and Twist1 is inversely correlated.
(A) Protein expression level of p53 and Twist1 in intra-peritoneal cancers was detected by western blot. GAPDH was used as loading control. Asterisks denote samples with p53 mutation. (B) Protein expression level of p53 and Twist1 in R182 cell line (representative p53-high/Twist1-) and Tara R182 cell line (representative p53-low/Twist1+). (C) p53 was knocked-down by shRNA in R182 cell line by lenti-virus transduction and effect on Twist1 protein was detected by western blot. Control shown is empty-vector control. (D,E) wild-type p53 and/or Twist1 were transiently overexpressed in R182 cell line; effect on protein and RNA were detected by western blot and qPCR, respectively. Control shown is empty-vector control. GAPDH was used as a reference gene in qPCR; *= p<0.05, NS= non-significant. (F,G) Tara R182 cells (p53-low/Twist1+) were treated with Nutlin-3a and harvested at indicated time points. Time shown is in minutes. Effect on protein and RNA were detected by western blot and qPCR, respectively. GAPDH was used as a reference gene in qPCR; NS= non-significant.
wt p53 promotes proteasome-dependent degradation of Twist1
To determine the molecular mechanism by which wt p53 affects Twist1 protein expression levels we first screened several ovarian cancer cell lines developed in our laboratory isolated from epithelial ovarian cancer tumors (37,44) or ovarian cancer cell lines obtained from ATCC. The p53 and Twist1 protein levels in these cells are shown in Supplementary Figure 2. Similar as observed in the whole tumor samples, we were able to identify cell cultures that are either p53-high and Twist1-negative (p53-high/Twist1) or conversely, p53-low and Twist1-positive (p53-low/Twist1+) (Fig. 1B). We also identified cell lines, which have either wt or mutated Tp53 (Supplementary Figure 2). In the succeeding mechanistic studies, we first focused on the cells that harbor a wt p53 status (Supplementary Table 4) to determine whether p53 affects Twist1 expression.
Thus, our first objective was to determine whether inhibition of p53 could affect Twist1 expression. Therefore, we knocked down p53 using small hairpin RNA specific for p53 (shp53) in p53-high/Twist1- ovarian cancer cells and evaluated Twist1 protein expression by western blot analysis. As shown in Figure 1C, the knockdown of p53 leads to an increase in Twist1 protein expression, demonstrating a direct regulatory function of p53 on Twist1 expression.
To further determine if the inverse correlation between wt p53 and Twist1 observed in these cells was indeed due to p53 expression, we use an overexpression system where we transfected plasmids expressing wt p53 or Twist1. Compared to the overexpression of Twist1 alone, the co-transfection of Twist1 and p53 led to significantly lower levels of Twist1 protein (Fig. 1D). Interestingly, the level of Twist1 mRNA was not altered by the overexpression of wt p53 (Fig. 1E) suggesting that the observed inhibitory effect on Twist expression is not at the transcriptional level but is exerted at the protein level.
MDM2 regulates p53 expression levels by promoting its proteasome degradation and inhibition of MDM2 leads to increased p53 levels (45). To further demonstrate that the expression of p53 is the factor associated with decrease in Twist1 protein we used the MDM2 antagonist Nutlin-3a, which promotes the increase in p53 protein expression. Thus, we treated p53-low/Twist1+ cells with Nutlin-3a and evaluated p53 and Twist1 protein expression by western blot analysis. As shown in Figure 1F, treatment with Nutlin-3a, which enhanced p53 protein levels, correlated with a decrease in Twist1 protein expression (Fig. 1F). Furthermore, we observed a time dependent decrease in Twist1 protein that inversely correlates with p53. Similar to what was observed in Figure 1E, the change in Twist1 protein was not a consequence of changes at the mRNA level (Fig. 1G). These results demonstrate that the effect of p53 on Twist1 expression is exerted at the post-translational level.
In view of our previous reported findings showing that Twist1 is targeted by the proteasome (16), we tested whether the effect of p53 on Twist1 is also due to proteasome degradation. Thus, we treated p53-low/Twist1+ cells with Nutlin-3a (to increase p53 protein levels) in the presence or absence of the proteasome inhibitor, MG132. Our results showed that while treatment with Nutlin-3a alone increased p53 levels and decreased Twist1, the presence of MG132 abrogated Nutlin-3a/p53-induced decrease in Twist1 protein expression (Fig. 2A); suggesting that p53 is able to enhance the proteasome degradation of Twist1.
Figure 2. p53-induced Twist1 degradation depends on the ubiquitin-proteasome system.
(A) Tara R182 cells (p53-low/Twist1+) were treated with Nutlin-3a alone for 8 hours or Nutlin-3a plus MG132. For the co-treatment, MG132 was added during the last 3h of treatment. Control shown is no treatment control. Twist1 and p53 protein levels were evaluated by western blot. β-actin was used as loading control. Note the inhibition of Twist1 degradation by MG132. (B) R182 cells (p53-high/Twist1-) were transfected with Twist1 and wt p53 plasmids in the presence or absence of MG132 and effect on protein expression was determined by western blot analysis. (C) Twist1 was overexpressed in HEK293T cells in the presence or absence of p53 and/or HA-tagged ubiquitin as indicated. Cells were then treated with MG132 and Twist1 was immunoprecipitated. The IP complex was blotted for HA-ubiquitin to determine the level of ubiquitinated Twist1.
We further confirmed the role of the proteasome in p53-induced Twist degradation in p53high/Twist1- cells. The co-transfection of wt p53 and Twist1 in these cells induced a significant decrease in Twist1 protein expression compared to transfection with Twist1 alone but this effect was reversed by treatment with the proteasome inhibitor, MG132 (Fig. 2B). The p53-induced proteasome-dependent degradation of Twist1 was abrogated in the presence of MG132.
To further establish the role of the proteasome system in p53-dependent Twist1 degradation in the absence of potential additional factors present in the cancer cells, we used a “clean” system utilizing HEK293T cells and co-transfected wt p53, Twist1, and HA-tagged ubiquitin. Following transfection, we performed IP of Twist1, and determined the presence of ubiquitin in the Twist1 IP complex by western blotting against the HA tag in the ubiquitin. Indeed, when HA-tagged ubiquitin was co-transfected with p53 and Twist1, the presence of p53 enhanced the ubiquitination of Twist1 (Fig. 2C). These data demonstrate the ability of wt p53 to promote Twist1 ubiquitination. Taken together, our data thus far show that wt p53 can promote Twist1 degradation and it is mediated through the ubiquitin-proteasome pathway.
Pirh2 E3 ligase promotes wt p53-induced Twist1 degradation
Since p53 does not have ligase activity, we hypothesized that p53 might recruit a specific ligase in order to induce Twist1 degradation. We first evaluated the role of MDM2, an E3 ubiquitin ligase that is known to bind to p53 (46) and has been shown to be involved in p53-induced protein degradation (28). To prevent the potential effect of endogenous proteins, we again used the “clean” system and utilized HEK293T cells. We found that co-transfection of MDM2 and Twist1 in HEK293T cells did not induce a decrease in Twist1 expression (Supplementary Figure. 3A), and further, IP of MDM2 showed co-precipitation of p53 but not Twist1 (Supplementary Figure. 3B). These findings are in line with data obtained in p53-low/Twist1+ cells showing that Nutlin-3a, which is a strong inhibitor of MDM2, was able to induce Twist1 degradation (Fig. 1F). Thus, taken together, these results demonstrate that MDM2 is not the ligase involved in p53-induced Twist1 degradation.
Since we have excluded MDM2 as the possible ligase that is involved in p53-induced Twist1 degradation, we performed a differential expression array comparing p53-high/Twist1- cells with p53-low/Twist1+ cells and identified eight E3 ligases in p53-high/Twist1- cells known to bind p53: Mdmx, Pirh2, E6-AP, Cop1, Topors, CHIP, RFWD3, and JFK (47–52). Since we observed higher mRNA levels of Pirh2 compared to other ligases (Supplementary Figure. 4A) we validated whether Pirh2 protein is also present in p53-high/Twist1- cells. As shown in Supplementary Figure 4B, Pirh2 protein is expressed in the ovarian cancer cell lines.
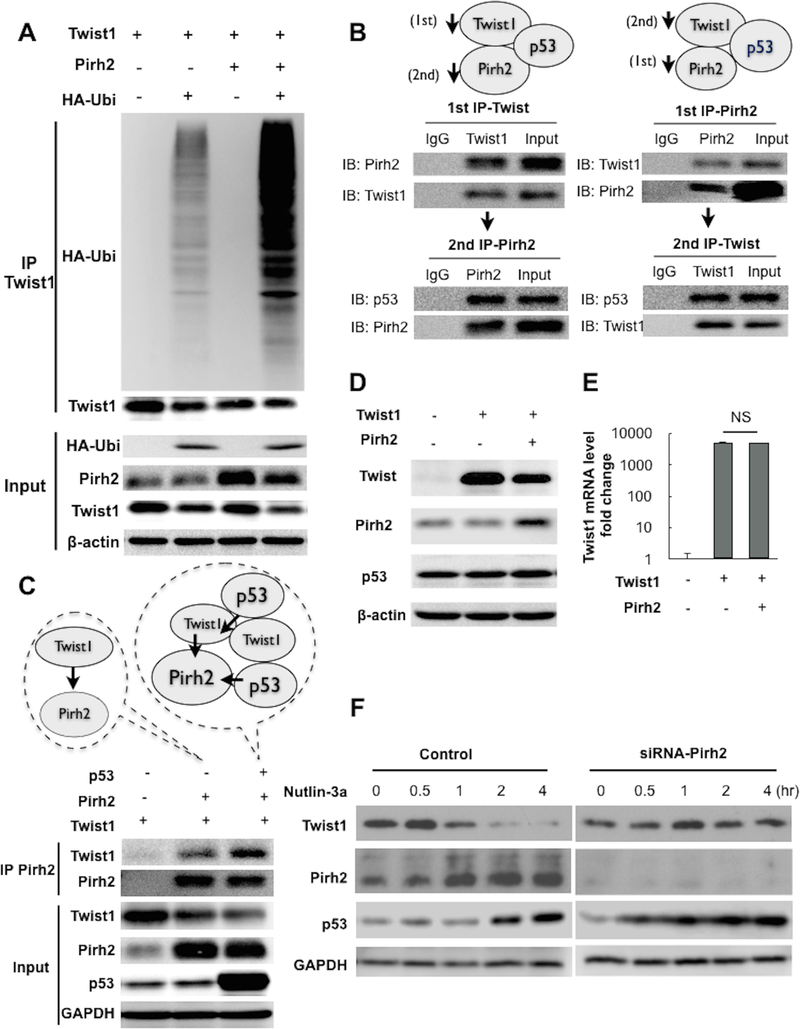
Next, we elucidated whether Pirh2 can promote ubiquitination of Twist1. Thus Pirh2, Twist1, and HA-tagged ubiquitin were by co-transfected in HEK293T cells and Twist1 was IP followed by western blotting for the HA-tag in ubiquitin. We observed that the presence of Pirh2 significantly enhanced the ubiquitination of Twist1 (Fig. 3A).
Figure 3. p53 forms a complex with Twist1 and Pirh2 to facilitate Twist1 degradation.
(A) Twist1, Pirh2, and HA-ubiquitin were transfected in HEK293T cells as indicated. Ubiquitination of Twist1 was determined by immunoblotting for HA-ubiquitin in the Twist1 IP product. Whole cell lysate was used as input control. (B) Twist1, Pirh2, and wild-type p53 were transfected in HEK293T cells. Sequential IP was performed as described in the Materials and Methods section and as depicted in the figure. Resulting IP product was used to immunoblot for p53. (C) Twist1, Pirh2, and p53 were transfected in HEK293T as indicated. Pirh2 was immunoprecipitated and Twist1 was detected in the IP product by immunoblotting. Note that the presence of p53 enhanced Twist1-Pirh2 binding. Whole cell lysate was used as input control. GAPDH was used as loading control for the input. (D) Twist1 was transfected in R182 cells (p53-high/Twist1-) in the presence or absence of Pirh2. Protein expression was detected by western blot. Note the decrease in Twist1 protein levels in the presence of Pirh2. (E) Twist1 mRNA was detected by qPCR in samples from D; NS= non-significant. (F) A2780 cells (p53-low/Twist1+) were transfected with siRNA specific for Pirh2 then treated with Nutlin-3a at indicated time-points. Protein levels of Twist1, Pirh2, and p53 were detected by western blot. Note that in the absence of Pirh2, Twist1 protein levels are not affected by Nutlin-3a treatment. Control cells are transfected with scramble siRNA.
We then tested the direct binding between Pirh2 and Twist1. Thus, we performed sequential IP of Twist1/Pirh2 or Pirh2/Twist1 in p53-low/Twist1+ cells (41). In the first case (Twist1/Pirh2), we first IP Twist1 and determine the presence of Pirh2 in the IP complex. Afterwards, we used the same IP complex to perform a second IP with anti-Pirh2 antibody. It is from this second IP complex that we determined the presence of p53. In the second case (Pirh2/Twist1), we first IP Pirh2 and check for the presence of Twist1. Afterwards, from this Pirh2 IPcomplex we did a second IP with anti-Twist1 antibody and determine the presence of p53. Analysis of the IP complexes did show the presence of p53 in the Pirh2-Twist1 complex (Fig. 3B) proving that indeed, p53, Twist1, and Pirh2 are able to form a complex.
The binding interaction between wt p53, Twist1, and Pirh2 was further evaluated in the HEK293T clean system. Following co-transfection with Twist1 and Pirh2 in the presence or absence of wt p53, we IP Pirh2 and observed that the binding of Twist1 to Pirh2 was enhanced in the presence of wt 53 (Fig. 3C).
To functionally validate the capacity of Pirh2 to promote p53-induced Twist1 degradation, Twist1 was transfected in p53-high/Twist- cells in the presence or absence of Pirh2. Our results show that the presence Pirh2 resulted in lower levels of Twist1 compared to the transfection of Twist1 alone and absence of Pirh2 (Fig. 3D). Likewise, the difference in Twist1 protein was not reflected at the mRNA level (Fig. 3E) demonstrating that Pirh2 regulates Twist1 mainly at the protein level.
Finally, to conclusively establish the role of Pirh2 in p53-induced Twist1 degradation, we knocked-down Pirh2 in p53-low/Twist+ cells using Pirh2 specific siRNA (siRNA-Pirh2). After knocking down Pirh2 we treated the cells with Nutlin-3a to upregulate p53. In control cells (transfected with scramble siRNA), treatment with Nutlin-3a upregulated p53 expression as well as Pirh2 expression since Pirh2 is a p53 target gene (Fig. 3F). In these cells, Twist1 expression is decreased as expected. In cells wherein Pirh2 was knocked down however, Nutlin-3a treatment was still able to upregulate p53 but not able to decrease Twist1 (Fig. 3F). Thus, although Nutlin-3a increased p53 levels, we did not observe changes on Twist1 expression in the cells lacking Pirh2. Taken together, our data provide evidence that wt p53 forms a complex with Pirh2 and Twist1 and facilitates Pirh2-mediated ubiquitination and degradation of Twist1.
Hotspot mutations abolish p53’s ability to enhance Twist1 degradation.
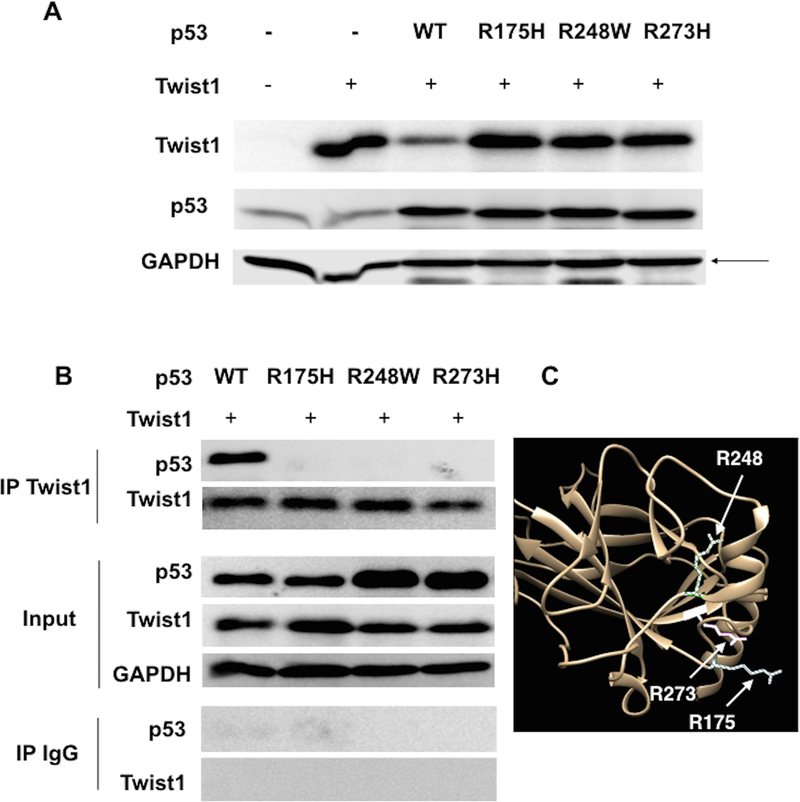
TP53 is frequently mutated in multiple solid tumors and is mutated in about 96% of high-grade serous ovarian carcinoma (23,53,54). Moreover, about 40% of ovarian cancer patients exhibit loss of heterozygosity or loss of the wt p53 allele (55). To determine the consequence of p53 mutation in p53-induced Twist1 degradation, we characterized three common hotspot p53 mutations: R175H, R248W and R273H. Thus, we established plasmid constructs expressing different p53 mutations and each p53 mutation plasmid was co-transfected with wt Twist1 in HEK293T cells. Whereas co-transfection of wt p53 and Twist1 resulted in lower Twist1 protein levels compared to expression of Twist1 alone, this decrease in Twist1 protein was not observed when Twist1 was co-transfected with the p53 mutants R175H, R248W and R273H (Fig. 4A).
Figure 4. Hotspot mutation on p53 abrogates its ability to promote Twist1 degradation.
(A) Twist1 was transfected in HEK293T cells with either wild-type p53 or p53 mutants (R175H, R248W, or R273H). Twist1 and p53 protein levels were detected by western blot. Note that only wt p53, and not the mutants tested, can promote the decrease in Twist1 protein. Control cells were transfected with empty-vector controls. (B) Twist1 was transfected in HEK293T cells with either wild-type p53 or p53 mutants. Afterwards Twist1 was immunoprecipitated and the presence of Twist1 and p53 was detected in the IP product. IgG was used as control for the immunoprecipitation. Whole cell lysate was used as input control and GAPDH was used as loading control. Note the absence of mutant p53 in the co-IP products compared to the presence of wt p53. (C) Analysis of the 3D structure of p53 (as described in the Materials and Methods section) demonstrates that R175 (striped), R248 (gridded), and R273 (solid) lie in the same region of the folded structure, suggesting this area may represent a binding interface with Twist1 and Pirh2.
Next, we determined whether the presence of hotspot p53 mutations could affect the interaction between p53 and Twist1. Thus after co-transfection of Twist1 with wt p53 or the three mutants we performed IP for Twist1. Analysis of the Twist1 IP complex showed the presence of p53 only when Twist1 was co-expressed with wt p53 and not when it is co-expressed with any of the p53 mutants (Fig. 4B). To determine if there was a structural basis for the effect of these hotspot mutations, we analyzed an existing crystal structure of p53 (43). We found that these commonly mutated residues lie in the same region of the protein, suggesting that this region may be a binding interface for Twist1 and/or Pirh2 (Fig. 4C). Taken together, these data strongly demonstrate that p53 mutation, especially in these hotspots, abolishes p53/Twist1 interaction and p53-induced Twist1 degradation.
Loss of p53-induced Twist1 degradation promotes EMT in ovarian cancer cells
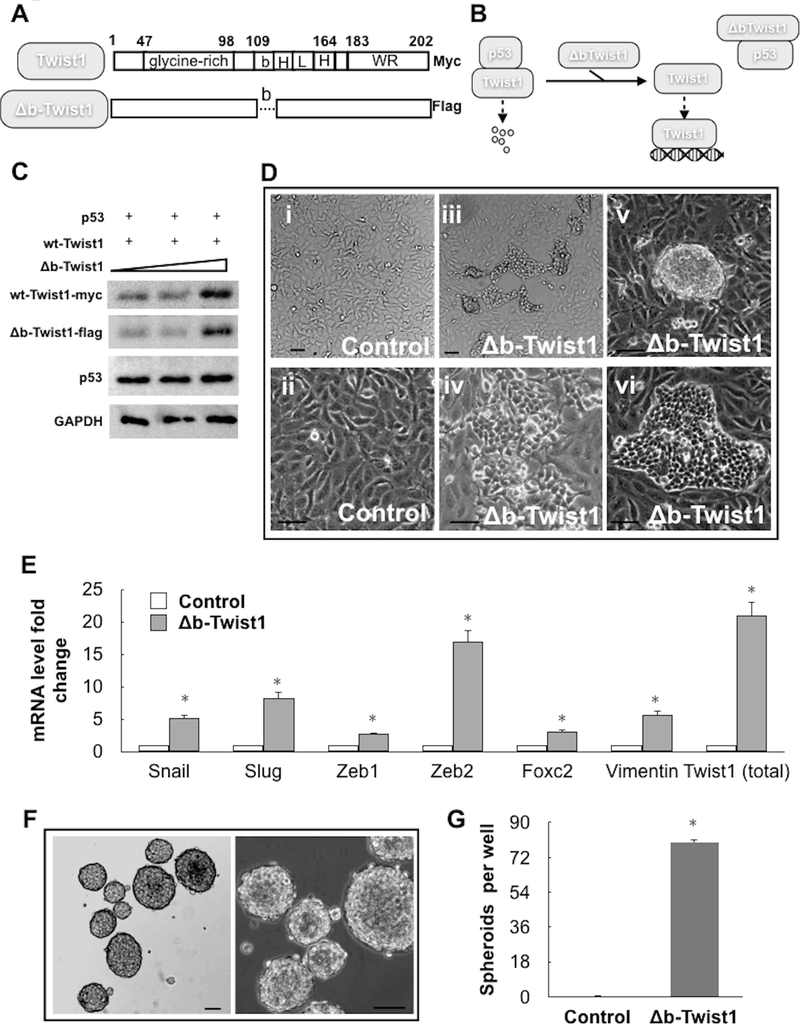
Having demonstrated the outcome of p53/Twist1 protein-protein interaction, we investigated its biological relevance. Since Twist1 is an essential regulator of EMT, we determined if we could promote EMT by protecting Twist1 from p53-dependent degradation. We utilized a construct of mutant Twist1 (Δb-Twist1; Fig. 5A) where the DNA binding domain has been removed (thus precluding the protein from binding to the DNA) but maintaining its ability to interact with other proteins such as p53 and therefore function as a dominant-negative inhibitor of Twist1-p53 interaction (9,56,57). As such, Δb-Twist1 can capture and sequester p53, thus functioning as a “sink” to rescue endogenous wt Twist1 (Fig. 5B). Thus, wt Twist1 (with myc-tag) and wt p53 were transfected into HEK293T cells with increasing concentrations of Δb-Twist1 (with flag-tag). Our data showed that Δb-Twist1 could inhibit the ability of p53 to degrade Twist1 in a dose dependent manner (Fig. 5C). An increase in wt Twist1 is specifically determined by western blotting fo the myc tag.
Figure 5. Inhibition of p53 and Twist1 interaction induces EMT in ovarian cancer cells.
(A) Schematic illustration showing the structure of the dominant negative Δb-Twist1 construct used compared to wild-type Twist1. Note difference in C-terminal tags. (B) Schematic illustration explaining how Δb-Twist1 competes with wild-type Twist1 to interact with p53, thus releasing and stabilizing wild-type Twist1. (C) HEK293T cells were transiently transfected with wild-type p53 and wild-type Twist1 in the presence of increasing concentrations of Δb-Twist1 and effect on protein expression determined by western blot. Anti-myc-tag and anti-flag-tag antibodies were used to distinguish wild-type Twist1 and Δb-Twist1, respectively. The amount of vectors are the same for each condition and equalized using empty-vector controls (D) Δb-Twist1 was stably overexpressed in R182 cells (p53-high/Twist1-) and its effect on morphology was determined by light microscopy. Control cells were transfected with empty-vector controls. Note that Δb-Twist1 is able to induce morphological changes associated with a fibroblastic/mesenchymal phenotype; i, iii, v taken at 4x; ii, iv, vi taken at 10x. (E) Expression of mesenchymal markers in samples from D was determined by QPCR; * = p<0.05 compared to empty-vector control. (G) R182 cells stably expressing Δb-Twist1 as in D were grown in ultra-low attachment plates and formed compact spheroid. (F) Quantification of spheroid formation in G. Data is presented as mean (+/− SEM). Note that the control cells transfected with empty vector did not form spheroids. *= p<0.05 compared to control. Representative of at least 3 independent experiments.
When we generated p53-high/Twist1- ovarian cancer cells stably expressing Δb-Twist1 using lenti-viral vector (Supplementary Figure 5), we found that the presence of Δb-Twist1 was able to induce morphological changes in these cells characterized by the acquisition of mesenchymal morphology (Fig. 5D). Molecular analysis conclusively demonstrated that cells expressing Δb-Twist1 underwent EMT and acquired the mesenchymal markers Snail, Slug, Zeb1/2, FOXC2, and Vimentin (Fig. 5E). Furthermore, when cells expressing Δb-Twist1 were cultured in ultra-low attachment plates, they form 3D spheroids, which is another characteristic of EMT (Fig. 5F,G). The presence of Δb-Twist1 also enhanced the migratory capacity of these cells in a trans-well migration assay (Supplementary Figure 6). These results demonstrate that p53-induced Twist1 degradation is critical in the maintenance of an epithelial phenotype and that its inhibition can lead to EMT.
Discussion
In the present study, we describe a novel mechanism of p53 as a modulator of Twist1 protein stabilization and consequently as a regulator of EMT. We demonstrate that p53 is able to create a complex with the ligase Pirh2 and Twist1 leading to the ubiquitination and proteasome degradation of Twist1. Notably, specific mutations in p53 abrogate binding to Twist1 leading to Twist1 stabilization and promotion of EMT.
The transition of epithelial cancer cells to a mesenchymal state has proved to be a key biological process in multiple steps of tumorigenesis, including pre-malignant transformation (58), metastasis formation (59), as well as in the establishment of recurrent disease (1,5,60). By gaining mesenchymal features, cancer cells become more motile, more invasive, and more chemoresistant (16,61). As such, targeting EMT by inhibiting EMT drivers has been an on-going anti-tumoral approach. In ovarian cancer, the EMT process is critical during the intra-abdominal dissemination of the disease. Therefore, understanding the molecular mechanisms involved in this process will provide new insights on how to treat or prevent carcinomatosis.
Twist1 is a crucial EMT driver that can be regulated by multiple pathways (7,8,16,19,31–36). The direct protein-protein interaction between p53 and Twist1 has been reported by several groups (62,63). However, the focus has mainly been on how Twist1 inhibited the DNA-binding ability of p53 and blocked key posttranslational modifications on p53 leading to p53 degradation (63). Our study demonstrates that this interaction also significantly affects Twist1 stability. Moreover, the demonstration that this is dependent on a wt p53 further highlights a targetable pathway that can prevent cancer invasiveness in tumors with mutated p53.
To our knowledge, this is the first study to demonstrate the role of Pirh2 as an E3 ligase in mediating the ubiquitination and degradation of Twist1. During proteasome-regulated degradation an E3 ubiquitin ligase is required for targeting specific protein substrates and attaching ubiquitin to a lysine on the target protein. MDM2 is a well-known p53-specific E3 ubiquitin ligase that can coordinate with p53 to degrade Snail and Slug (28,29). We show in this study that Twist1 degradation is an MDM2-independent mechanism of p53-induced protein degradation, which specifically engages Pirh2. Like MDM2, Pirh2 is also a p53 target gene (48) (64). The activation of p53 induces Pirh2 transcription and further enhances complex formation and Twist1 degradation.
Considering the critical multi-functionality of p53 in suppressing EMT, it is conceivable that dysfunctional mutant p53 is associated with EMT and the aggressiveness of cancers. p53 mutation is correlated with resistance to platinum-based chemotherapy, early relapse, tumor progression, and shortened overall survival. Our data demonstrate that hotspot p53 mutants failed to promote Twist1 degradation, which supports the hypothesis that p53 mutations attenuate its ability to suppress Twist1, leading to its stabilization and EMT.
A growing amount of evidence suggests that mutant p53 not only loses the ability to inhibit EMT, but also gains new functions to induce EMT (26,65,66). In terms of the regulation of Twist1, it has been shown that mutant p53 (R175H) could induce Twist1 expression in a prostate cancer-derived cell line and immortalized lung fibroblasts, possibly through the alleviation of epigenetic repression (67). As our data showed, wt p53 could downregulate Twist1 protein without affecting the Twist1 mRNA levels. Therefore, our study focused on the post-translational regulation of Twist1 by p53. However, it is plausible that in tumors in vivo, the mutant p53 (R175H), in addition to losing the ability to promote Twist1 protein degradation, could gain the ability to increase Twist1 transcription.
Our findings show that specific p53 mutations (i.e. R175H, R248Q, and R273H) can stabilize Twist1 protein but not surprisingly, can not cause its expression when Twist1 mRNA is absent. This demonstrates that p53 mutation can synergize with cues from the microenvironment (i.e. TGF-beta) in the induction of EMT. Thus whereas p53 mutation alone is not sufficient to promote Twist1 protein expression when Twist mRNA is absent; together with the signals from the microenvironment like TGF-beta, which can induce the transcription of Twist1 mRNA, the presence of p53 mutation can support a more rapid accumulation of Twist1 protein. Collectively, this evidence supports the association of p53 mutation and poor patient outcome.
In the same vein, genetic alterations and expression levels of Pirh2 is a subject that warrants further investigation. Due to its role in regulating p53 protein and tumorigenesis, Pirh2 is an emerging biomarker and potential therapeutic target. Pirh2 expression was found to be upregulated in multiple cancers (64,68–70). On the other hand, the reduced expression of Pirh2 is correlated with poor survival in ovarian, lung, breast, and bladder cancers (71–75). Combining the characterization of Pirh2 with the status of p53 and Twist1 to create a comprehensive profile of the p53-Pirh2-Twist1 pathway may provide a novel prognostic biomarker for cancer invasiveness.
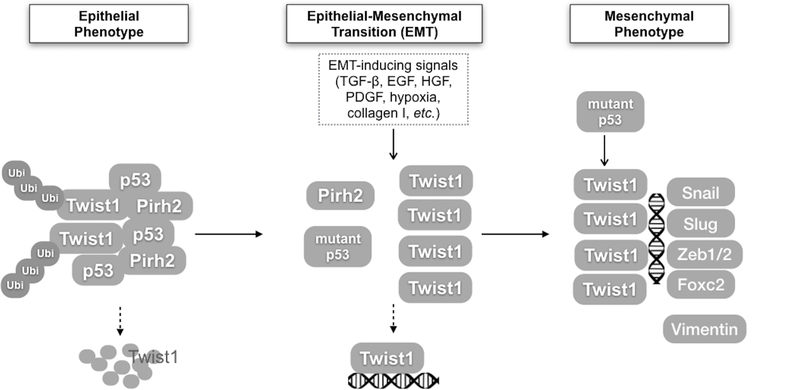
In this study, we propose a model by which wt p53, by inducing Twist1 degradation, inhibits EMT and consequently maintains a differentiated-epithelial phenotype (Fig. 6). A limitation of the study, which is the case for many of these types of molecular studies, is the fact that we are studying cellular/molecular aspects, which are impossible to elucidate in humans. However, these types of molecular research provide insights for the development of new therapeutic approaches.
Figure 6.
A schematic model of p53 mediating EMT via the regulation of Twist1 degradation. (1) In epithelial cells, wt p53 promotes the degradation of Twist1 by forming a complex with Twist1 and E3 ligase Pirh2, which keeps the level of Twist1 protein low and maintains the epithelial status of cancer cells. (2) When p53 protein is mutated or downregulated, the continuous or intensified stimuli of EMT-inducing signals lead to the stabilization of Twist1 protein. Twist1 protein binds to its target gene promoters. (3) The mutation or inhibition of p53 can induce Twist1 and EMT.
The mutation, downregulation, and/or inhibition of p53 is therefore a critical step towards the transformation of these epithelial cancer cells into a poorly differentiated, aggressive, mesenchymal phenotype. This finding is important in understanding the process of EMT and tumor progression. The newly discovered role of Pirh2 as an E3 ligase in mediating Twist1 ubiquitination and degradation has expanded the known spectrum of Pirh2 function. In addition to providing new insights into the metastatic process at the molecular level, our data suggest a signaling pathway that can potentially be used to develop new prognostic markers and therapeutic targets to curtail cancer progression.
Supplementary Material
Implication:
This data provide new insight into the metastatic process at the molecular level and suggest a signaling pathway that can potentially be used to develop new prognostic markers and therapeutic targets to curtail cancer progression.
Acknowledgement
This study is supported in part by NIH NCI RO1CA199004 (GM and ABA), The Sands Family Foundation (GM and ABA), Discovery to Cure Program (GM, ABA, and RT), Debra Levine Endowment (GM), Rivkin Center Cookie Laughlin Bridge Award (YYH), OCRF Ann Schreiber Mentored Investigator Award (YYH and GM), and the Office of the Assistant Secretary of Defense for Health Affairs through the Ovarian Cancer Research Program under Award No. W81XWH-15–1-0221 (YYH).
Footnotes
Conflict of Interest Disclosure: The authors declare no conflict of interest.
References
- 1.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13(2):97–110 doi 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 2.Cheng W, Ma Y, Gong F, Hu C, Qian L, Huang Q, et al. Cross-reactivity of autoreactive T cells with MBP and viral antigens in patients with MS. Frontiers in bioscience : a journal and virtual library 2012;17:1648–58. [DOI] [PubMed] [Google Scholar]
- 3.Arthur A, Cakouros D, Cooper L, Nguyen T, Isenmann S, Zannettino AC, et al. Twist-1 Enhances Bone Marrow Mesenchymal Stromal Cell Support of Hematopoiesis by Modulating CXCL12 Expression. Stem Cells 2016;34(2):504–9 doi 10.1002/stem.2265. [DOI] [PubMed] [Google Scholar]
- 4.Nuti SV, Mor G, Li P, Yin G. TWIST and Ovarian Cancer Stem Cells: Implications for Chemoresistance and Metastasis. Oncotarget 2014;5(17):7260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119(6):1420–8 doi 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008;68(10):3645–54 doi 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 7.Ansieau S, Morel AP, Hinkal G, Bastid J, Puisieux A. TWISTing an embryonic transcription factor into an oncoprotein. Oncogene 2010;29(22):3173–84 doi 10.1038/onc.2010.92. [DOI] [PubMed] [Google Scholar]
- 8.Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res 2012;22(1):90–106 doi 10.1038/cr.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard TD, Paznekas WA, Green ED, Chiang LC, Ma N, Ortiz de Luna RI, et al. Mutations in TWIST, a basic helix-loop-helix transcription factor, in Saethre-Chotzen syndrome. Nat Genet 1997;15(1):36–41 doi 10.1038/ng0197-36. [DOI] [PubMed] [Google Scholar]
- 10.El Ghouzzi V, Legeai-Mallet L, Aresta S, Benoist C, Munnich A, de Gunzburg J, et al. Saethre-Chotzen mutations cause TWIST protein degradation or impaired nuclear location. Hum Mol Genet 2000;9(5):813–9 doi ddd076 [pii]. [DOI] [PubMed] [Google Scholar]
- 11.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 2008;14(1):79–89 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Wu KJ, Yang MH. Epithelial-mesenchymal transition and cancer stemness: the Twist1-Bmi1 connection. Biosci Rep 2011;31(6):449–55 doi 10.1042/BSR20100114. [DOI] [PubMed] [Google Scholar]
- 13.Jung HY, Yang J. Unraveling the TWIST between EMT and cancer stemness. Cell Stem Cell 2015;16(1):1–2 doi 10.1016/j.stem.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Roberts CM, Tran MA, Pitruzzello MC, Wen W, Loeza J, Dellinger TH, et al. TWIST1 drives cisplatin resistance and cell survival in an ovarian cancer model, via upregulation of GAS6, L1CAM, and Akt signalling. Sci Rep 2016;6:37652 doi 10.1038/srep37652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res 2005;65(12):5153–62 doi 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 16.Yin G, Alvero AB, Craveiro V, Holmberg JC, Fu HH, Montagna MK, et al. Constitutive proteasomal degradation of TWIST-1 in epithelial-ovarian cancer stem cells impacts differentiation and metastatic potential. Oncogene 2013;32(1):39–49 doi 10.1038/onc.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin G, Chen R, Alvero AB, Fu HH, Holmberg J, Glackin C, et al. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene 2010;29(24):3545–53. 10.1038/onc.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridges RS, Kass D, Loh K, Glackin C, Borczuk AC, Greenberg S. Gene expression profiling of pulmonary fibrosis identifies Twist1 as an antiapoptotic molecular “rectifier” of growth factor signaling. Am J Pathol 2009;175(6):2351–61 doi 10.2353/ajpath.2009.080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terauchi M, Kajiyama H, Yamashita M, Kato M, Tsukamoto H, Umezu T, et al. Possible involvement of TWIST in enhanced peritoneal metastasis of epithelial ovarian carcinoma. Clin Exp Metastasis 2007;24(5):329–39 doi 10.1007/s10585-007-9070-1. [DOI] [PubMed] [Google Scholar]
- 20.Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 2002;359(9321):1877–90. [DOI] [PubMed] [Google Scholar]
- 21.Glackin CA. Targeting the Twist and Wnt signaling pathways in metastatic breast cancer. Maturitas 2014;79(1):48–51 doi 10.1016/j.maturitas.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Glackin CA, Murray EJ, Murray SS. Doxorubicin inhibits differentiation and enhances expression of the helix-loop-helix genes Id and mTwi in mouse osteoblastic cells. Biochemistry international 1992;28(1):67–75. [PubMed] [Google Scholar]
- 23.Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell 2014;25(3):304–17 doi 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs E, McKenna K, Bedi A. P53-Dependent DNA Damage-Induced Apoptosis Requires Fas/Apo-1-Independent Activation of Cpp32-Beta. Cancer Research 1997;57(13):2550–4. [PubMed] [Google Scholar]
- 25.Bonizzi G, Cicalese A, Insinga A, Pelicci PG. The emerging role of p53 in stem cells. Trends Mol Med 2011. doi 10.1016/j.molmed.2011.08.002. [DOI] [PubMed]
- 26.Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol 2011;13(3):317–23 doi 10.1038/ncb2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piovan C, Palmieri D, Di Leva G, Braccioli L, Casalini P, Nuovo G, et al. Oncosuppressive role of p53-induced miR-205 in triple negative breast cancer. Mol Oncol 2012;6(4):458–72 doi 10.1016/j.molonc.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang SP, Wang WL, Chang YL, Wu CT, Chao YC, Kao SH, et al. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat Cell Biol 2009;11(6):694–704 doi 10.1038/ncb1875. [DOI] [PubMed] [Google Scholar]
- 29.Lim SO, Kim H, Jung G. p53 inhibits tumor cell invasion via the degradation of snail protein in hepatocellular carcinoma. FEBS Lett 2010;584(11):2231–6 doi 10.1016/j.febslet.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Cardenas C, Montagna MK, Pitruzzello M, Lima E, Mor G, Alvero AB. Adipocyte microenvironment promotes Bclxl expression and confers chemoresistance in ovarian cancer cells. Apoptosis 2017;22(4):558–69 doi 10.1007/s10495-016-1339-x. [DOI] [PubMed] [Google Scholar]
- 31.Weiss MB, Abel EV, Mayberry MM, Basile KJ, Berger AC, Aplin AE. TWIST1 is an ERK1/2 effector that promotes invasion and regulates MMP-1 expression in human melanoma cells. Cancer Res 2012;72(24):6382–92 10.1158/0008-5472.CAN-12-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eide T, Ramberg H, Glackin C, Tindall D, Tasken KA. TWIST1, A novel androgen-regulated gene, is a target for NKX3–1 in prostate cancer cells. Cancer Cell Int 2013;13(1):4 10.1186/1475-2867-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nairismagi ML, Fuchtbauer A, Labouriau R, Bramsen JB, Fuchtbauer EM. The proto-oncogene TWIST1 is regulated by microRNAs. PLoS One 2013;8(5):e66070 doi 10.1371/journal.pone.0066070PONE-D-12-13500 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong J, Zhou J, Fu J, He T, Qin J, Wang L, et al. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res 2011;71(11):3980–90 10.1158/0008-5472.CAN-10-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lander R, Nordin K, LaBonne C. The F-box protein Ppa is a common regulator of core EMT factors Twist, Snail, Slug, and Sip1. J Cell Biol 2011;194(1):17–25 10.1083/jcb.201012085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong J, Ogura K, Wang Z, Inuzuka H. Degradation of the transcription factor Twist, an oncoprotein that promotes cancer metastasis. Discov Med 2013;15(80):7–15. [PMC free article] [PubMed] [Google Scholar]
- 37.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle 2009;8(1):158–66 doi 7533 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvero AB, Montagna MK, Chen R, Kim KH, Kyungjin K, Visintin I, et al. NV-128, a novel isoflavone derivative, induces caspase-independent cell death through the Akt/mammalian target of rapamycin pathway. Cancer 2009. doi 10.1002/cncr.24397. [DOI] [PMC free article] [PubMed]
- 39.Alvero AB, O’Malley D, Brown D, Kelly G, Garg M, Chen W, et al. Molecular mechanism of phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer 2006;106(3):599–608 doi 10.1002/cncr.21633. [DOI] [PubMed] [Google Scholar]
- 40.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res 2006;66(7):3859–68. [DOI] [PubMed] [Google Scholar]
- 41.Lu T, Stark GR. Using sequential immunoprecipitation and mass spectrometry to identify methylation of NF-kappaB. Methods Mol Biol 2015;1280:383–93 doi 10.1007/978-1-4939-2422-6_23. [DOI] [PubMed] [Google Scholar]
- 42.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc 2006;1(1):241–5 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 43.Bethuyne J, De Gieter S, Zwaenepoel O, Garcia-Pino A, Durinck K, Verhelle A, et al. A nanobody modulates the p53 transcriptional program without perturbing its functional architecture. Nucleic Acids Res 2014;42(20):12928–38 doi 10.1093/nar/gku962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvero AB, Montagna MK, Holmberg JC, Craveiro V, Brown D, Mor G. Targeting the mitochondria activates two independent cell death pathways in ovarian cancer stem cells. Mol Cancer Ther 2011;10(8):1385–93 doi 10.1158/1535-7163.MCT-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baliou E, Nonni A, Keramopoulos D, Ragos V, Tsiambas E, Patsouris E, et al. Deregulation of p53-MDM2 auto-regulatory pathway in breast carcinoma. Journal of BUON : official journal of the Balkan Union of Oncology 2016;21(5):1099–103. [PubMed] [Google Scholar]
- 46.Stommel JM, Wahl GM. A new twist in the feedback loop: stress-activated MDM2 destabilization is required for p53 activation. Cell Cycle 2005;4(3):411–7 doi 1522 [pii]. [DOI] [PubMed] [Google Scholar]
- 47.Shadfan M, Lopez-Pajares V, Yuan ZM. MDM2 and MDMX: Alone and together in regulation of p53. Transl Cancer Res 2012;1(2):88–9. [PMC free article] [PubMed] [Google Scholar]
- 48.Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, et al. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 2003;112(6):779–91. [DOI] [PubMed] [Google Scholar]
- 49.Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, et al. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature 2004;429(6987):86–92 doi 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- 50.Weger S, Hammer E, Heilbronn R. Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett 2005;579(22):5007–12 doi 10.1016/j.febslet.2005.07.088. [DOI] [PubMed] [Google Scholar]
- 51.Fu X, Yucer N, Liu S, Li M, Yi P, Mu JJ, et al. RFWD3-Mdm2 ubiquitin ligase complex positively regulates p53 stability in response to DNA damage. Proc Natl Acad Sci U S A 2010;107(10):4579–84 doi 10.1073/pnas.0912094107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain AK, Barton MC. Making sense of ubiquitin ligases that regulate p53. Cancer Biol Ther 2010;10(7):665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol 2013;15(1):2–8 doi 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 54.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474(7353):609–15 doi 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobel M, Piskorz AM, Lee S, Lui S, LePage C, Marass F, et al. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J Pathol Clin Res 2016;2(4):247–58 doi 10.1002/cjp2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bourgeois P, Stoetzel C, Bolcato-Bellemin AL, Mattei MG, Perrin-Schmitt F. The human H-twist gene is located at 7p21 and encodes a B-HLH protein that is 96% similar to its murine M-twist counterpart. Mammalian genome : official journal of the International Mammalian Genome Society 1996;7(12):915–7. [DOI] [PubMed] [Google Scholar]
- 57.Glackin C, Winters K, Murray E, Murray S. Transcripts encoding the basic-helix-loop-helix factor twist are expressed in mouse embryos, cell lines, and adult tissues. Mol Cell Differ 1994;2:309–28. [Google Scholar]
- 58.Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, et al. Systemic spread is an early step in breast cancer. Cancer Cell 2008;13(1):58–68 doi 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 2015;525(7568):256–60 doi 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133(4):704–15 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Craveiro V, Yang-Hartwich Y, Holmberg JC, Sumi NJ, Pizzonia J, Griffin B, et al. Phenotypic modifications in ovarian cancer stem cells following Paclitaxel treatment. Cancer Medicine 2013;2(6):751–62 doi 10.1002/cam4.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piccinin S, Tonin E, Sessa S, Demontis S, Rossi S, Pecciarini L, et al. A “twist box” code of p53 inactivation: twist box: p53 interaction promotes p53 degradation. Cancer Cell 2012;22(3):404–15 doi 10.1016/j.ccr.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Shiota M, Izumi H, Onitsuka T, Miyamoto N, Kashiwagi E, Kidani A, et al. Twist and p53 reciprocally regulate target genes via direct interaction. Oncogene 2008;27(42):5543–53 doi 10.1038/onc.2008.176. [DOI] [PubMed] [Google Scholar]
- 64.Duan W, Gao L, Druhan LJ, Zhu WG, Morrison C, Otterson GA, et al. Expression of Pirh2, a newly identified ubiquitin protein ligase, in lung cancer. J Natl Cancer Inst 2004;96(22):1718–21 doi 10.1093/jnci/djh292. [DOI] [PubMed] [Google Scholar]
- 65.Dong P, Karaayvaz M, Jia N, Kaneuchi M, Hamada J, Watari H, et al. Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene 2013;32(27):3286–95 doi 10.1038/onc.2012.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coradini D, Fornili M, Ambrogi F, Boracchi P, Biganzoli E. TP53 mutation, epithelial-mesenchymal transition, and stemlike features in breast cancer subtypes. J Biomed Biotechnol 2012;2012:254085 doi 10.1155/2012/254085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kogan-Sakin I, Tabach Y, Buganim Y, Molchadsky A, Solomon H, Madar S, et al. Mutant p53(R175H) upregulates Twist1 expression and promotes epithelial-mesenchymal transition in immortalized prostate cells. Cell Death Differ 2011;18(2):271–81 doi 10.1038/cdd.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Logan IR, Gaughan L, McCracken SR, Sapountzi V, Leung HY, Robson CN. Human PIRH2 enhances androgen receptor signaling through inhibition of histone deacetylase 1 and is overexpressed in prostate cancer. Mol Cell Biol 2006;26(17):6502–10 doi 10.1128/MCB.00147-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimada M, Kitagawa K, Dobashi Y, Isobe T, Hattori T, Uchida C, et al. High expression of Pirh2, an E3 ligase for p27, is associated with low expression of p27 and poor prognosis in head and neck cancers. Cancer Sci 2009;100(5):866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang XM, Yang LY, Guo L, Fan C, Wu F. p53-induced RING-H2 protein, a novel marker for poor survival in hepatocellular carcinoma after hepatic resection. Cancer 2009;115(19):4554–63 doi 10.1002/cncr.24494. [DOI] [PubMed] [Google Scholar]
- 71.Berchuck A, Iversen ES, Lancaster JM, Pittman J, Luo J, Lee P, et al. Patterns of gene expression that characterize long-term survival in advanced stage serous ovarian cancers. Clin Cancer Res 2005;11(10):3686–96 doi 10.1158/1078-0432.CCR-04-2398. [DOI] [PubMed] [Google Scholar]
- 72.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006;439(7074):353–7 doi 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 73.Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell 2006;10(6):529–41 doi 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 74.Hakem A, Bohgaki M, Lemmers B, Tai E, Salmena L, Matysiak-Zablocki E, et al. Role of Pirh2 in mediating the regulation of p53 and c-Myc. PLoS Genet 2011;7(11):e1002360 doi 10.1371/journal.pgen.1002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raponi M, Zhang Y, Yu J, Chen G, Lee G, Taylor JM, et al. Gene expression signatures for predicting prognosis of squamous cell and adenocarcinomas of the lung. Cancer Res 2006;66(15):7466–72 doi 10.1158/0008-5472.CAN-06-1191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.