Abstract
Aldo-keto reductase 1B10 (AKR1B10) is upregulated in breast cancer and promotes tumor growth and metastasis. However, little is known of the molecular mechanisms of action. Herein we report that AKR1B10 activates lipid second messengers to stimulate cell proliferation. Our data showed that ectopic expression of AKR1B10 in breast cancer cells MCF-7 promoted lipogenesis and enhanced levels of lipid second messengers, including phosphatidylinositol bisphosphate (PIP2), diacylglycerol (DAG) and inositol triphosphate (IP3). In contrast, silencing of AKR1B10 in breast cancer cells BT-20 and colon cancer cells HCT-8 led to decrease of these lipid messengers. Qualitative analyses by liquid chromatography-mass spectrum (LC-MS) revealed that AKR1B10 regulated the cellular levels of total DAG and majority of subspecies. This in turn modulated the phosphorylation of protein kinase C (PKC) isoforms PKCδ (Thr505), PKCμ (Ser744/748) and PKCα/βII (Thr638/641) and activity of the PKC-mediated c-Raf/MEK/ERK signaling cascade. A pan inhibitor of PKC (Go6983) blocked ERK1/2 activation by AKR1B10. In these cells, phospho-p90RSK, phospho-MSK and Cyclin D1 expression was increased by AKR1B10, and pharmacological inhibition of the ERK signaling cascade with MEK1/2 inhibitors U0126 and PD98059 eradicated induction of phospho-p90RSK, phospho-MSK and Cyclin D1. In breast cancer cells, AKR1B10 promoted the clonogenic growth and proliferation of breast cancer cells in two-dimension (2D) and three-dimension (3D) cultures and tumor growth in immunodeficient female nude mice through activation of the PKC/ERK pathway. These data suggest that AKR1B10 stimulates breast cancer cell growth and proliferation through activation of DAG-mediated PKC/ERK signaling pathway.
Keywords: AKR1B10, breast cancer, lipid second messengers, phosphatidylinositol bisphosphate, diacylglycerol, PKC/ERK cascade
INTRODUCTION
Lipids are a group of water-insoluble intracellular molecules, such as phosphoglycerides, triglycerides, sphingolipids and sterols. Phospholipids are essential components of bio-membranes and important second messengers in cellular signaling transduction, regulating various pathophysiological processes [1-3]. Fatty acids are essential components of various lipids, such as triglycerides and phosphoglycerides. Lipogenesis is increased in tumors and contributes to various aspects of tumorigenesis and progression, such as cell proliferation, differentiation and invasion [1,4]. The high proliferation of cancer cells depends on lipid synthesis to meet both the needs of biomembrane synthesis in cell division and function as signal molecules, stimulating cell growth and proliferation [5]. G-protein coupled receptors (GPCRs) and tyrosine kinase receptors (TKRs) are important upstream activators of lipid second messengers. Phosphoinositide 3-kinase (PI3K) and phospholipase C (PLC) are two major mediators of cellular lipid messengers. PI3K phosphorylates phosphatidylinositol bisphosphate (PIP2) to produce phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which activates protein kinase B (PKB, also named AKT); PLC hydrolyzes PIP2 to form inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 diffuses freely into cytoplasm, triggering endoplasmic reticulum (ER)-mediated Ca2+ signaling; DAG remains in cell membrane and activates protein kinase C (PKC) [5-7]. PKC is an important oncogenic kinase that activates ERK1/2 signaling to promote cancer growth and progression [8,9]. The phosphorylation-activated ERK1/2 translocates into the nucleus where it phosphorylates and activates MSK, p90RSK and transcription factors (e.g., c-Myc, Elk-1 and Ets-1) to stimulate cell growth and proliferation [10]. Therefore, lipid second messengers are important cellular signal molecules that regulate cell growth, proliferation and survival. Interestingly, this study found that aldo-keto reductase 1B10 is a novel regulator of intracellular lipid messengers.
Aldo-keto reductase 1B10 (AKR1B10), also referred as aldose reductase-like-1 (ARL-1) [11], is a monomeric enzyme with strong catalytic activity to α, β-unsaturated carbonyl compounds [12-14] and protects the host cells from carbonyl lesions [15-17]. This has important biological significance as the human beings are constantly exposed to the carbonyls derived from daily diet consumption, microbes inhabited within digestive tract, cellular metabolism and lipid peroxidation in oxidative stress [18-21]. As a carbonyl reductase, AKR1B10 also works as an efficient retinal reduction enzyme to convert retinals to retinols, thus mediating homeostasis of retinoid acid and cell differentiation [22,23]. AKR1B10 also mediates cancer cell sensitivity to anthracyclines [24,25].
More importantly, AKR1B10 is a mediator of long chain fatty acid synthesis. AKR1B10 associates with acetyl-CoA carboxylase-α (ACCA) and prevents its ubiquitin-dependent degradation [26]. ACCA is a rate-limiting enzyme in de novo fatty acid synthesis, and thus AKR1B10 drives biosynthesis of long chain fatty acids [26]. Long chain fatty acids are precursors of lipids and are the main components of biomembrane phospholipids. Increased lipogenesis is an essential feature of cancer cells to meet the needs of phospholipids for biomembrane synthesis and cell division. Cancer cells preferentially use the newly synthesized fatty acids for phospholipid synthesis and biomembrane construction [27,28]. Therefore, AKR1B10-induced lipogenesis may have critical impact in cancer development and progression. In fact, AKR1B10 is upregulated in multiple solid cancers, such as liver, breast, lung and pancreatic cancers, being a potential prognostic biomarker [29-32]. In breast cancer, AKR1B10 is upregulated in ductal carcinoma in situ (DCIS) and invasive, metastatic and recurrent tumors and correlates with tumor size, lymph node metastasis, and disease-related death. Very recently, we found that AKR1B10 stimulates metastasis of breast cancer through integrin α5/δ-catenin mediated FAK/Src/Rac1 signaling pathway [33]. In normal tissues, AKR1B10 is primarily expressed in the colon and small intestine [11,34], where it regulates proliferation and self-renewal of cryptic epithelial cells [35].
AKR1B10 is an oncoprotein that promotes growth and progression of breast cancer. Biologically, AKR1B10 promotes fatty acid/lipid synthesis. The gap of knowledge of AKR1B10 is how the AKR1B10-induced lipogenesis leads to growth and metastasis of breast cancer. Herein we found that AKR1B10 activates the cellular lipid second messengers and thus triggers the lipid-mediated cell proliferative signal transduction. This study addressed the gap of AKR1B10 knowledge and dissected the signaling pathways, through which AKR1B10 stimulates the growth and proliferation of breast cancer cells.
MATERIALS AND METHODS
Cell culture:
MCF-7, BT-20, HCT-8 and 293T cells purchased from American Type Culture Collection (ATCC, VA) were maintained in indicated medium at 37°C, 5% CO2. For 2D culture, cells were seeded at 200 cells per 60-mm culture dish and incubated in indicated medium for 14 days; colonies were fixed by methanol (cooled at −20°C) for 10 min and visualized by 0.1% crystal violet. Plating efficiency was calculated as: Colony number/seeded cell number. The 3D culture was done in growth factor-reduced Matrigel (BD Biosciences, CA) [36]. Cells (4000/well) were seeded. Acini were photographed by a phase contrast microscopy (Carl Zeiss, CA).
AKR1B10 ectopic expression and silencing:
Full-length AKR1B10 cDNA was inserted into pCDH lentiviral expression vector with a GFP reporter (System Biosciences, CA). After packaging in 293T cells, AKR1B10 and empty pCDH lentiviral particles were introduced into cells with standard procedures. GFP-labeled cells were sorted for a homogeneous population. Scrambled and AKR1B10 siRNAs were chemically synthesized (Ambion, TX) and delivered into cells as previously described [37]. Two siRNAs that target encoding (siRNA 1: 5` GCAAGUUGUGGCCCACUUUtt) and 3` untranslational (siRNA 2: 5` CGAGAAUCGAGGUGCUGUUtt) regions of AKR1B10 were used for silencing. A scrambled siRNA with random RNA sequences was used as a control. AKR1B10 knockdown was examined using Western blot.
Growth rate:
Growth rate of cells was measured using Alamar blue (ABD Serotec, UK) reduction assay. Briefly, MCF-7 cells (3,000 cells/well) were plated in 96-well plates. Medium was added with 1/10 (v/v) Alamar blue and changed regularly every 24hr. At indicated time points, reduced Alamar blue was detected at 590 nm with a fluorescent spectrum (Thermo, CA). Viable cells correlate with the magnitude of Alamar blue reduction (%) following the manufacture’s instructions. For cell number counting, cells (0.1×106) were plated in 6-well plate. At indicated time points, cells were trypsinized, stained with trypan blue and counted by a Vi-cell counter (Beckman coulter, CA).
Western blot:
Protein lysates, SDS-PAGE and Western blotting were performed as previously described [26].
Lipid synthesis and PIP2 and IP3 separation:
Cells were pulsed with 1µCi of [2-14C] acetate (53 mCi/mmol; Amersham Biosciences) per well of 12-well plates for 4h at 37 °C, 5% CO2 in complete medium. Total lipids were extracted as described [15,38]. Briefly, after being washed with PBS, cells were harvested by trypsinization and suspended in 40 μl of PBS. An aliquot (10 μl) of the cell suspension was used for protein quantitation, and the remainder was mixed vigorously with 20 volumes of chloroform/ methanol (2:1, v/v). After incubation on ice for 10 min, debris was removed at 21,000 xg for 10 min, and the supernatant was washed with 0.2 volume of distilled water. The organic phase was collected and dried by speed vacuum. Lipids were dissolved in 50 μl of chloroform/methanol (2:1, v/v). An aliquot (10 μl) of extracts was subjected to radioactivity measurements to determine the total fatty acid synthesis. Acetate incorporation into lipid species, including phosphatidylinositol-4,5-bisphosphate (PIP2), inositol-1,4,5- trisphosphate (IP3) and diacylglycerol (DAG), was analyzed by thin layer chromatography (TLC). Lipid extracts and appropriate lipid standards (Sigma, MO) were spotted on silica gel (60Å; Sigma, MO). After being air-dried, plates were developed in hexane/diethyl ether/acetic acid (70/30/1, v/v) to separate neutral lipids, such as DAG, or in chloroform/methanol/acetic acid (65/25/10, v/v) to separate phospholipids, such as PIP2 and IP3. Lipid samples and standards were visualized by coloration in staining solution (0.12 M NaCl, 20% methanol, and 300 mg/liter Coomassie Blue). Lipid fractions were re-suspended into 50% methanol solution, and radioactivity were measured by scintillation counter (Beckman Instruments). Values were normalized for protein contents (CPM/mg of protein).
LC-MS analyses of diacylglycerol:
Diacylglycerol (DAG) and subspecies were quantitatively and qualitatively detected by LC-MS through Washington University Mass Spec Facility. In brief, lipids were extracted from 5×106 cells using a modified Bligh-Dyer method in the presence of an internal standard DG15:0-15:0 (0.5 µg per sample). Measurement of DAGs were performed with a Shimadzu 10A HPLC system and a Shimadzu SIL-20AC HT auto-sampler coupled to a Thermo Scientific TSQ Quantum Ultra triple quadrupole mass spectrometer operated in SRM mode under ESI(+). Data processing was conducted with X calibur (Thermo). Quality control (QC) samples were prepared by pooling the aliquots of study samples and were used to monitor instrument stability. The QC was injected seven times at the beginning to stabilize the instrument, and was injected again every five study samples. Only the lipid species with CV < 15% in QC sample were reported. Relative quantification of lipids was provided, and data were reported as the peak area ratios of the analytes to the internal standard. Relative quantification data generated in same batches are appropriate to compare the change of an analyte in AKR1B10 expression or AKR1B10 silencing samples to the corresponding control.
Tumorigenesis in female nude mice:
MCF-7 cells were labeled with luciferase expression vector. Each nude mouse was implanted with a 1.7 mg of 17β-estradiol pellet (Innovative Research of America, CA) to support MCF-7 proliferation. Cells were trypsinized and suspended in mix of medium and equal volume of Matrigel (BD Bioscience, CA) at 5×106/50 ml. Cell suspensions (50 ml/inoculation) were subcutaneously injected with a 25-gauge needle into mammary fat pads of female nude mice at 5 weeks old. Tumor volume was measured using in vivo bioluminescent imaging with an IVIS VR imaging system (Xenogen, CA) and also measured by Vernier caliper. Briefly, mice were given (i.p.) luciferase substrate D-luciferin at 100 mg/kg in PBS and anesthetized by isoflurane. Mice were placed onto a warm stage inside a light-tight camera box with continuous exposure to isoflurane. Imaging times were controlled equally at 15 sec, and signal quantification was performed by an acquisition and analysis software (Living ImageVR, Xenogen, CA) and expressed as amount of Flux (photons per second).
Statistical analysis:
Statistical analyses were performed using Student`s t tests or ANOVA with INSTAT statistical analysis package (Graph Pad software, CA). Significance was defined as p<0.05. Correlation analyses were conducted using Spearman correlation and multivariate canonical correlation.
RESULTS
AKR1B10 promotes lipogenesis and enhances cellular levels of lipid second messengers in breast cancer cells.
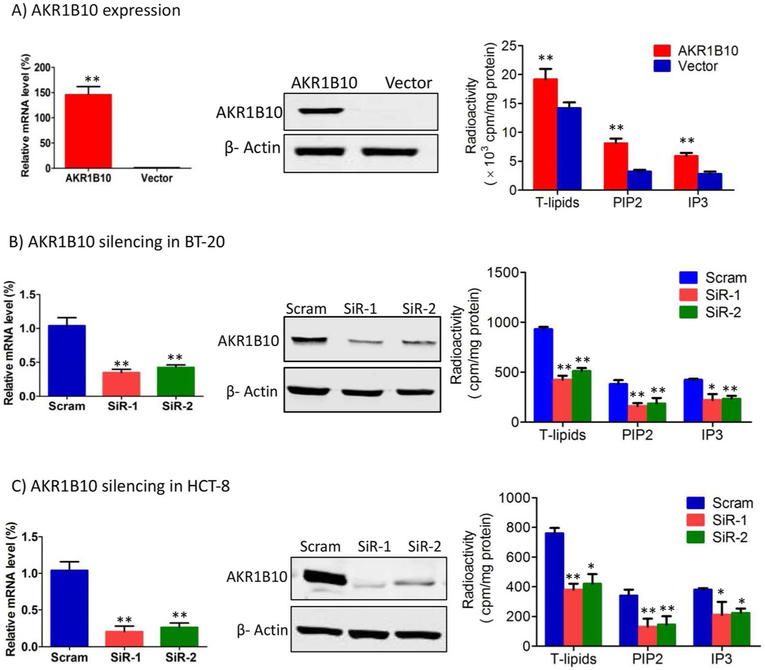
AKR1B10 promotes biosynthesis of long chain fatty acids by stabilizing ACCA [26]. Long chain fatty acids are important components of membrane phospholipids and lipid second messengers which mediate cellular signaling transduction and thus cell survival and proliferation [5-7]. Therefore, AKR1B10 may activate lipid second messenger-mediated signaling cascades to affect growth and proliferation of breast cancer cells. By targeted expression in MCF-7 cells, we examined cellular lipids, and our results showed that AKR1B10 significantly enhanced the levels of total lipids and lipid second messengers, phosphatidylinositol 4,5-bisphosphate (PIP2) and inositol 1,4,5-trisphosphate (IP3) (Fig. 1A). Oppositely, silencing of AKR1B10 in BT-20 cells led to decrease in total lipids, PIP2 and IP3 (Fig. 1B). This result was confirmed in HCT-8 cells derived from colon cancer (Fig. 1C). These data suggest that AKR1B10 participates in regulation of lipid second messengers in breast cancer cells.
Figure 1. AKR1B10 promotes lipogenesis of cancer cells.
AKR1B10 expression and lipid synthesis in the MCF-7 (A), BT-20 (B) and HCT-8 (C) cells. Left: AKR1B10 mRNA by qRT-PCR, GAPDH mRNA was used as an internal control; middle: AKR1B10 protein by Western blot; and right: Lipogenesis, showing total lipid (T-lipid), PIP2 and IP3 levels influenced by AKR1B10. Scram, scrambled siRNA; SiR-1, AKR1B10 siRNA-1; and SiR-2, AKR1B10 siRNA-2. *, p<0.05 and **, p<0.01 when compared to vector control.
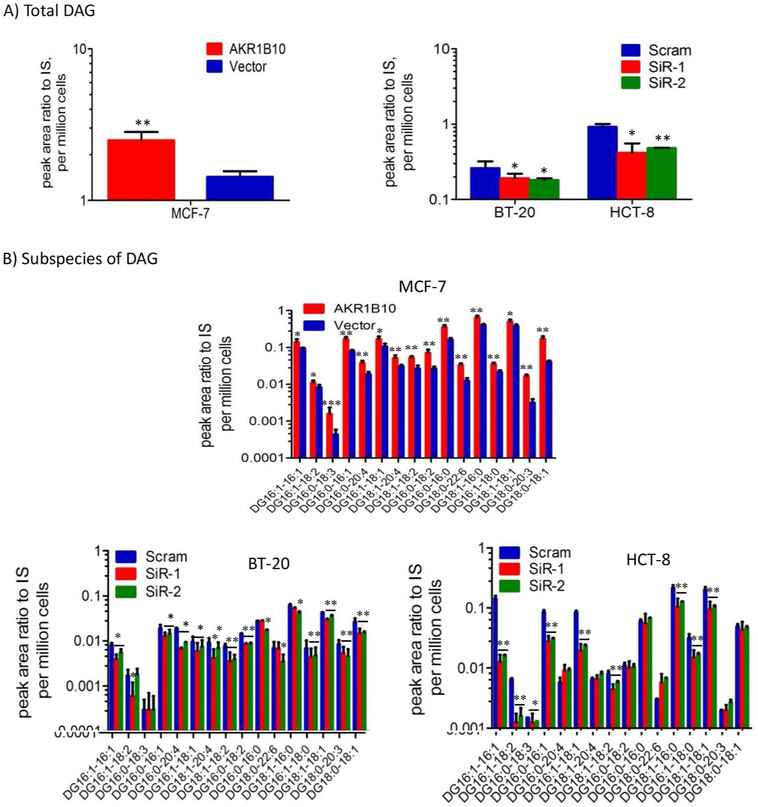
PIP2 is a precursor of the important lipid second messenger diacylglycerol (DAG). By catalysis of phospholipase C (PLC), PIP2 is hydrolyzed into DAG and IP3 [9]. Increased PIP2 and IP3 levels in breast cancer cells encouraged an extended study on DAG and the DAG-mediated signaling transduction. We used the LC-Mass Core facility of Washington University at St Louis to quantitatively measure total DAG and 16 subspecies in cells with targeted expression or silencing of AKR1B10 (Supplemental Figure S1). The results showed that targeted AKR1B10 expression in MCF-7 cells increased total cellular DAG levels, but oppositely, silencing of AKR1B10 led to decrease of DAG in both BT-20 and HCT-8 cells (Fig. 2A). Analytical data of DAG subspecies demonstrated that targeted expression of AKR1B10 markedly increased the levels of majority of 16 DAG subspecies, but AKR1B10 silencing led to decrease of DAG subspecies (Fig. 2B). Together these data suggest that in breast cancer cells, AKR1B10 promotes lipogenesis and increases the cellular levels of important lipid second messengers, PIP2, IP3 and DAG.
Figure 2. AKR1B10 increases DAG levels in breast cancer cells.
DAG and subspecies were measured quantitatively by liquid chromatography-mass spectrum as described in Methods and Materials. (A) Total DAG levels in MCF-7 cells with AKR1B10 expression (left) and BT-20 cells and HCT-8 cells with AKR1B10 silencing (right). (B) Levels of various subspecies of DAG in MCF-7 (upper), BT-20 (lower left) and HCT-8 (lower right) cells. Y axis is in log10. IS, internal standard. *, p<0.05 and **, p<0.01 when compared to vector control.
PKC/ERK signaling cascade is activated by AKR1B10 in breast cancer cells.
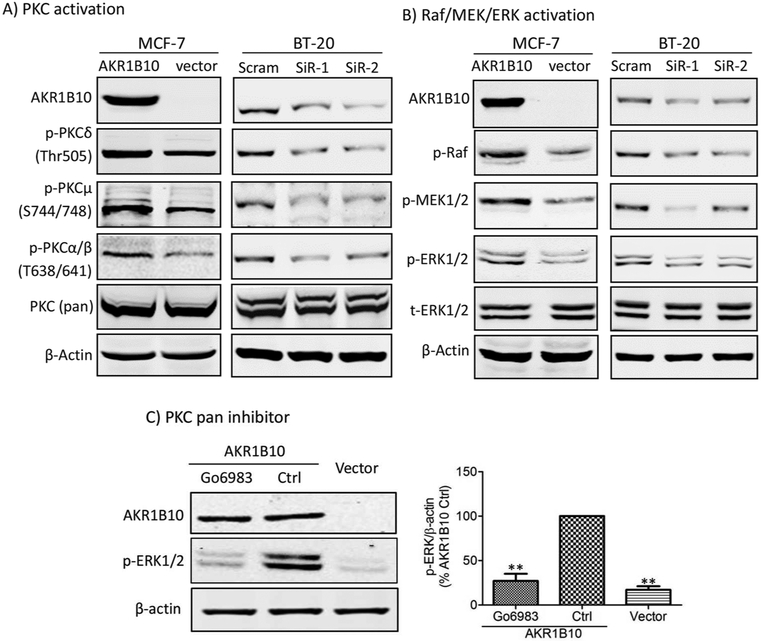
DAG is an activator of protein kinase C (PKC) family members [9]. Therefore, we examined phosphorylation activation of PKC isoforms. As shown in Fig. 3A, AKR1B10 expression in MCF-7 cells enhanced the level of phosphorylated PKCδ (Thr505), PKCμ (Ser744/748) and PKCα/βII (Thr638/641). In contrast, phosphorylation of these PKC isoforms decreased in BT-20 cells with AKR1B10 silencing by two different siRNAs that target different regions of mRNA.
Figure 3. AKR1B10 activates PKC/ERK signaling pathway in breast cancer cells.
(A) PKC activation by AKR1B10, showing p-PKCδ (Thr505), p-PKCμ (Ser744/748) and p-PKCα/βII (Thr638/641) levels in the MCF-7 with ectopic expression of AKR1B10 and BT-20 cells with silencing of AKR1B10. (B) Raf/MER/ERK activation by AKR1B10, showing p-Raf, p-MEK and p-ERK1/2 levels in the MCF-7 with ectopic expression of AKR1B10 and BT-20 cells with silencing of AKR1B10. (C) ERK inhibition by a broad PKC inhibitor, Go6893 (10μM), showing decreased p-ERK1/2 level by Go6893 in the MCF-7 cells with AKR1B10 expression. Left panel: Quantitative analysis of band intensity. **, p<0.01 when compared to AKR1B10 Control cells. Ctrl: control.
PKC isoforms are master kinases of the important signaling cascade, Raf/MEK/ERK, regulating a variety of cellular events, such as cell survival and proliferation. The activated PKCs phosphorylates and activates c-Raf, which in turn phosphorylates and activates MEK, provoking the ERK1/2 signaling [8]. As shown in Fig. 3B, phosphorylated c-Raf, MEK1/2 and ERK1/2 all increased in MCF-7 cells with ectopic expression of AKR1B10, but decreased in BT-20 cells with AKR1B10 knockdown. We further confirmed the activation of ERK1/2 signaling by PKC using a broad PKC inhibitor, Go6983. Western blot results showed that exposure of MCF-7 cells to Go6983 (10 µM) abolished phosphorylation activation of ERK1/2 induced by AKR1B10 (Fig. 3C).
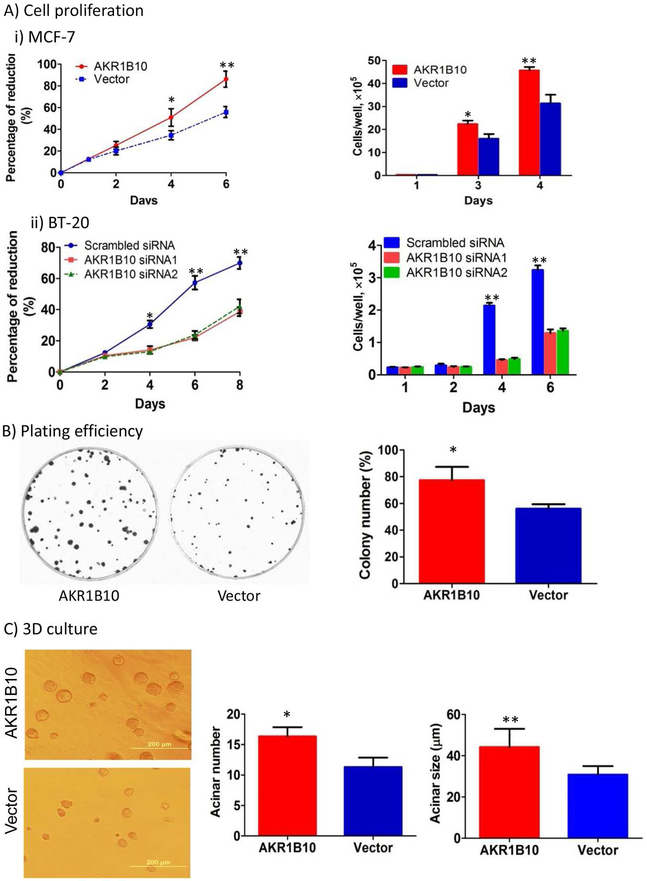
AKR1B10 promotes clonogenic growth of breast cancer cells.
AKR1B10 is upregulated in human breast cancer and correlates with tumor size and lymph node metastasis [31], but the underlying molecular mechanisms are unclear. This study showed that AKR1B10 promoted lipogenesis and activated DAG-mediated PKC/ERK signaling cascade. This may explain the functional role of AKR1B10 in breast cancer growth and progression. We thus assessed the effect of targeted AKR1B10 expression on growth and proliferation of breast cancer cells. As shown in Fig. 4A, ectopic expression of AKR1B10 in MCF-7 increased the cell growth and proliferation compared to the empty vector control, whereas silencing of AKR1B10 in BT-20 cells decreased the cell proliferation. This result was confirmed by both Alamar blue (Fig. 4A left) and cell number counting (Fig. 4A right) assays. More impressively, AKR1B10 promoted clonogenic growth of MCF-7 cells, a feature of cancer cells. In 2D plating efficiency assays, the colony formation rate of MCF-7 cells with AKR1B10 expression was at an average of 78.0±4.8% (n=3), compared to 56.0±1.6% in the vector control cells. The colony size of AKR1B10 expression cells was notably larger than that of vector control cells (Fig. 4B). We further assessed the effect of AKR1B10 on clonogenic growth of MCF-7 cells in three-dimensional (3D) culture in growth factor-reduced extracellular matrix that mimics in vivo environment of mammary epithelial cells. The results showed that MCF-7 cells with AKR1B10 expression formed more and larger acini than vector control cells (Fig. 4C). Together these data suggest that AKR1B10 promotes clonogenic growth and proliferation of breast cancer cells.
Figure 4. AKR1B10 promotes growth and proliferation of breast cancer cells.
(A) Proliferation of MCF-7 and BT-20 cells measured by Alamar blue assays (left) and cell counting assays (right). (B) Plating efficiency of MCF-7 cells in cell culture dishes. (C) Acinar formation and growth of MCF-7 cells in the Matrigel-based 3D culture. *, p<0.05 and **, p<0.01 when compared to vector control.
p90RSK, MSK and cyclin D1 function as downstream effectors of the ERK1/2 signaling in breast cancer cells.
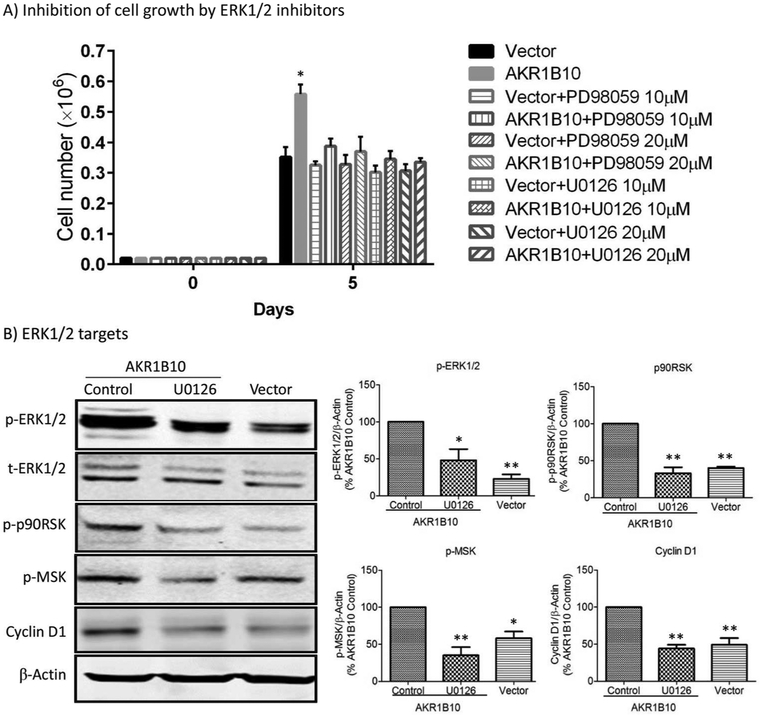
We next tested if the ERK1/2 signaling activation is responsible for increased cell proliferation by AKR1B10. U0126 and PD98059 are MEK1/2 inhibitors that inhibit ERK1/2 signaling activity. As shown in Fig. 5A, exposure of AKR1B10 expression MCF-7 cells to either U0126 or PD98059 suppressed cell growth and proliferation. We further explored downstream effectors of the ERK1/2 signaling in MCF-7 cells. The results showed that AKR1B10 expression and resulting activation of the MEK/ERK signaling increased the phosphorylation of p90RSK and MSK and cyclin D1 expression; and the MEK1/2 inhibitor U0126 abolished the p90RSK and MSK phosphorylation and cyclin D1 expression stimulated by AKR1B10 (Fig. 5B). These data suggest that the ERK1/2 signaling activated by AKR1B10 promotes cell growth and proliferation through the targets p90RSK, MSK and cyclin D1 in the breast cancer cells.
Figure 5. ERK signaling and target genes that are involved in cell proliferation enhanced by AKR1B10.
(A) Inhibition of MCF-7 cell proliferation by MEK inhibitors, PD98059 and U0126 at 10 µM or 20 µM. **, p<0.01 when compared to the vector control or PD98059/U0126-treated cells. (B) ERK targeted genes in MCF-7 cell. The p-ERK1/2, p-p90RSK and p-MSK levels and Cyclin D1 expression were increased by AKR1B10 in the cells, and the increased p-p90RSK, p-MSK and Cyclin D1 levels were eradicated by MEK inhibitor, U0126 (10 µM). Right panel: Quantitative analyses of indicated proteins. *, p<0.05 and **, p<0.01 when compared to AKR1B10 Control cells.
AKR1B10 promotes growth of MCF-7 tumor xenografts in female nude mice.
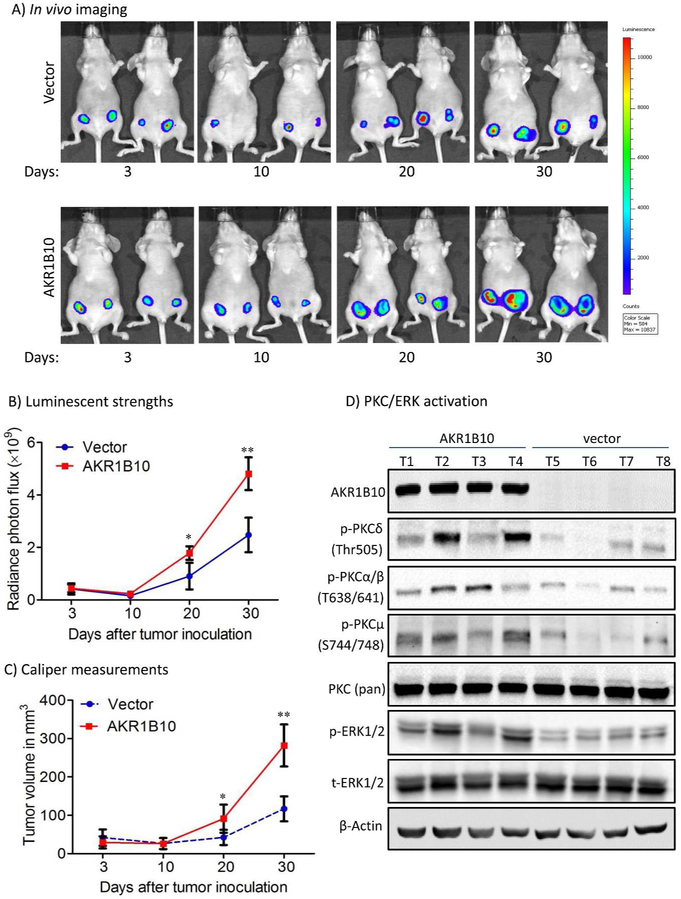
To confirm the cancer-promoting role of AKR1B10, we further evaluated its effects on growth of tumor xenografts in vivo. Luciferase-labelled MCF-7 cells with targeted AKR1B10 expression or a vector control were injected subcutaneously into the mammary fat pads of female nude mice (n=5 per group). Tumor volume was monitored by both in vivo imaging and caliper measurements. As shown in Fig. 6, MCF-7 tumors with AKR1B10 expression grew faster than vector control tumors. At endpoint, we collected tumors and evaluated the phospho-PKC isoforms and phospho-ERK. As shown in Figure 6D, the PKC/ERK signaling pathway was activated by AKR1B10 in tumors. These data suggest that AKR1B10 promotes breast cancer growth in vivo by activating the PKC/ERK signaling pathway, being an oncogenic protein in breast cancer growth and progression.
Figure 6. AKR1B10 promotes tumor growth in female nude mice.
MCF-7 cells (5 × 106) labeled with luciferase were implanted in the mammary fat pads (orthotopic) of immunodeficient female mice at 4-6 weeks old. A pellet of 17β-estradiol (0.72mg/pellet) was implanted beneath the neck skin. (A) Representative in vivo bioluminescent images at days 3, 10, 20 and 30 post the cell injections. (B) Tumor volumes by in vivo bioluminescence at photon/second. (C) Caliper measurements of the tumor size. *, p<0.05 and **, p<0.01 when compared to vector control. (D) PKC/ERK signaling activity in tumors. *, p<0.05 and **, p<0.01 when compared to vector control.
DISCUSSION
AKR1B10 is not expressed in normal breast, but up-regulated in breast cancer, indicating a poor prognosis. However, little is known of the molecular mechanisms of action. This study showed that AKR1B10 promoted clonogenic growth of breast cancer cells by increasing lipogenesis and thus activating lipid second messengers PIP2 and DAG-mediated PKC/ERK signaling cascade. In vivo studies in immunodeficient female nude mice confirmed the tumor-promoting role of AKR1B10 in breast cancer. These results define an oncogenic role of AKR1B10 in growth and progression of breast cancer and clarify the mechanisms of action.
AKR1B10 increases fatty acid synthesis by stabilizing ACCA, enhancing cellular phospholipids and sterols. Phospholipid, sphingolipids and sterols are important building blocks of biological membranes. Increased lipogenesis meets the needs of biomembrane synthesis for proliferation of cancer cells. More importantly, membrane phospholipids are a major group of lipid second messengers, mediating cellular signaling pathways of cancer cell growth and proliferation [1]. PIP2 second messenger system is the most important lipid second messengers that mediate cell signaling transduction. Hydrolysis by PLC, PIP2 is broken down into DAG and IP3. DAG activates important oncogenic signaling molecule PKC, and IP3 provokes ER-mediated calcium signaling, further activating PKC. Our results demonstrated that AKR1B10 increased lipid synthesis, enhanced cellular levels of PIP2, IP3 and DAG, and thus activated the PKC-mediated Raf/MEK/ERK signaling cascade in breast cancer cells. In particular, we quantitatively measured total cellular DAG and all 16 subspecies using LC-MS through the core facility in Washington University at St Louis, and the results strengthened the quality and accuracy of our data.
Activated ERK1/2 phosphorylates several substrates, including p90RSK and MSK. ERK1/2 can phosphorylate T369 and T573 residues of p90RSK to enhance cell survival signals [10,39]. ERK1/2 also induces cyclin D1 expression through an AP-1 mediated mechanism, stimulating G1/S progression and cell proliferation [10,40]. In breast cancer cells, the activated ERK signaling by AKR1B10 enhanced cell growth and proliferation through phosphorylation activation of p90RSK and MSK and expression of cyclin D1, and this was proven by pharmacological inhibition of ERK1/2 activity using MEK1/2 inhibitors U0126 and PD98059.
ERK1/2 is an important oncogenic signaling that regulates cell proliferation and survival [41]. Thus we further observed the effect of AKR1B10 on proliferation and tumorigenesis of breast cancer cells. The results showed that ectopic expression of AKR1B10 promoted clonogenic growth in both 2D and 3D cultures and tumorigenesis in nude mice, but silencing of AKR1B10 suppressed growth and proliferation of breast cancer cells. These results are in consistence with clinical settings that AKR1B10 expression in breast cancer correlates with tumor size and worse disease-free survival [31].
It is noteworthy to note that AKR1B10 can promote cell survival through protection from reactive carbonyl lesions [13,17,37]. Although repetitive observations were not conducted in these breast cancer cells with targeted expression or silencing of AKR1B10, we believe that the carbonyl detoxification mechanism of AKR1B10 may also contribute to the cell growth and proliferation.
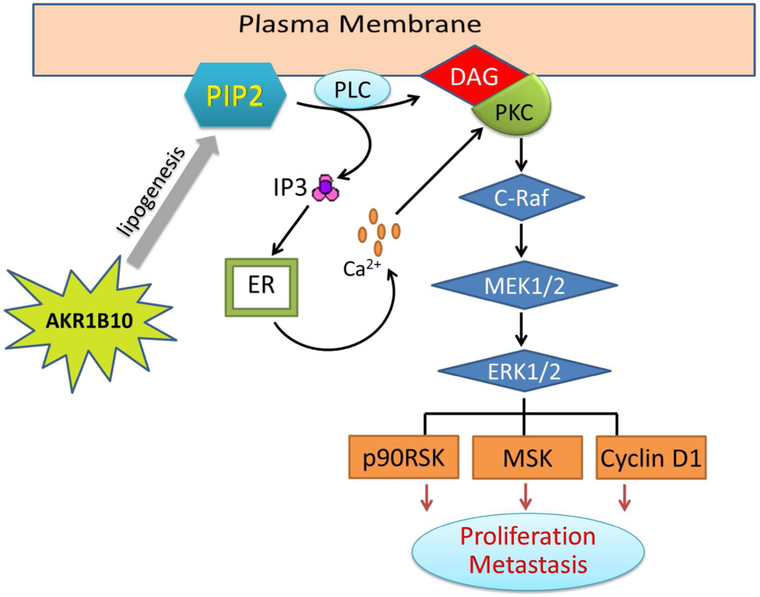
In summary, this study obtained a series of data that mechanistically address the oncogenic role of AKR1B10 in growth and progression of breast cancer. AKR1B10 promotes lipogenesis and enhances cellular PIP2, DAG and IP3 levels. These lipid second messengers activate PKC and thus the PKC-mediated ERK signaling cascade, promoting growth and proliferation of breast cancer cells (Fig. 7). AKR1B10 is a novel oncogenic protein in breast cancer.
Figure 7. Hypothetic model of cell growth and proliferation promoted by AKR1B10.
In the breast cancer cells, AKR1B10 promotes lipogenesis and increases cellular lipid second messengers PIP2, IP3 and DAG, which activates the PKC/ERK signaling cascade. The p90RSK, MSK and cyclin D1 functions as downstream targets of the ERK1/2 in this process.
Supplementary Material
Acknowledgment:
Mass spectrometry was performed in the Metabolomics Facility at Washington University School of Medicine (P30 DK020579), Campus Box 8086, 660 South Euclid Avenue, St Louis, Missouri 63110. Tel: (314) 747-0677.
Grant support: This work was supported in part by National Natural Science Foundation of China (81472465 and 81772842) and Natural Science Foundation of Guangxi (2015GXNSFEA139003).
Abbreviations used are:
- AKR1B10
aldo-keto reductase 1B10
- ACCA
acetyl-CoA carboxylase α
- DAG
diacylglycerol
- DCIS
ductal carcinoma in situ
- GPCR
G-protein coupled receptor
- IP3
inositol triphosphate
- PIP2
phosphatidylinositol bisphosphate
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate
- PKC
protein kinase C
- PLC
phospholipase C
- siRNA
small interfering RNA
Footnotes
Conflict of interest: Authors declare no conflict of interest with the contents of this article.
References
- 1.Santos CR, Schulze A. Lipid metabolism in cancer. The FEBS journal 2012;279(15):2610–2623. [DOI] [PubMed] [Google Scholar]
- 2.Ghaffari P, Mardinoglu A, Nielsen J. Cancer Metabolism: A Modeling Perspective. Frontiers in physiology 2015;6:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiegel S, Foster D, Kolesnick R. Signal transduction through lipid second messengers. Current opinion in cell biology 1996;8(2):159–167. [DOI] [PubMed] [Google Scholar]
- 4.Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell metabolism 2013;18(2):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nature cell biology 2015;17(4):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nature reviews Molecular cell biology 2008;9(2):112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blazer-Yost BL, Nofziger C. Phosphoinositide lipid second messengers: new paradigms for transepithelial signal transduction. Pflugers Archiv : European journal of physiology 2005;450(2):75–82. [DOI] [PubMed] [Google Scholar]
- 8.Koivunen J, Aaltonen V, Peltonen J. Protein kinase C (PKC) family in cancer progression. Cancer letters 2006;235(1):1–10. [DOI] [PubMed] [Google Scholar]
- 9.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nature reviews Cancer 2007;7(4):281–294. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell research 2002;12(1):9–18. [DOI] [PubMed] [Google Scholar]
- 11.Cao D, Fan ST, Chung SS. Identification and characterization of a novel human aldose reductase-like gene. The Journal of biological chemistry 1998;273(19):11429–11435. [DOI] [PubMed] [Google Scholar]
- 12.Spite M, Baba SP, Ahmed Y et al. Substrate specificity and catalytic efficiency of aldo-keto reductases with phospholipid aldehydes. The Biochemical journal 2007;405(1):95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong L, Liu Z, Yan R et al. Aldo-keto reductase family 1 B10 protein detoxifies dietary and lipid-derived alpha, beta-unsaturated carbonyls at physiological levels. Biochemical and biophysical research communications 2009;387(2):245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y, Zhong L, Johnson S, Cao D. Human aldo-keto reductases 1B1 and 1B10: A comparative study on their enzyme activity toward electrophilic carbonyl compounds. Chemico-biological interactions 2011;191(1-3):192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Yan R, Luo D, Watabe K, Liao DF, Cao D. Aldo-keto reductase family 1 member B10 promotes cell survival by regulating lipid synthesis and eliminating carbonyls. The Journal of biological chemistry 2009;284(39):26742–26748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zu X, Yan R, Pan J et al. Aldo-keto reductase 1B10 protects human colon cells from DNA damage induced by electrophilic carbonyl compounds. Molecular carcinogenesis 2016. [DOI] [PubMed] [Google Scholar]
- 17.Zu X, Yan R, Robbins S, Krishack PA, Liao DF, Cao D. Reduced 293T cell susceptibility to acrolein due to aldose reductase-like-1 protein expression. Toxicological sciences : an official journal of the Society of Toxicology 2007;97(2):562–568. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Li S, Cao Y et al. Oxidative Stress and Carbonyl Lesions in Ulcerative Colitis and Associated Colorectal Cancer. Oxidative medicine and cellular longevity 2016;2016:9875298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasai H, Kumeno K, Yamaizumi Z et al. Mutagenicity of methylglyoxal in coffee. Gann 1982;73(5):681–683. [PubMed] [Google Scholar]
- 20.Schuler BD, Eder E. Development of a 32P-postlabelling method for the detection of 1,N2-propanodeoxyguanosine adducts of crotonaldehyde in vivo. Archives of toxicology 2000;74(7):404–414. [DOI] [PubMed] [Google Scholar]
- 21.Homann N, Tillonen J, Salaspuro M. Microbially produced acetaldehyde from ethanol may increase the risk of colon cancer via folate deficiency. International journal of cancer Journal international du cancer 2000;86(2):169–173. [DOI] [PubMed] [Google Scholar]
- 22.Gallego O, Ruiz FX, Ardevol A et al. Structural basis for the high all-trans-retinaldehyde reductase activity of the tumor marker AKR1B10. Proceedings of the National Academy of Sciences of the United States of America 2007;104(52):20764–20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz FX, Gallego O, Ardevol A et al. Aldo-keto reductases from the AKR1B subfamily: retinoid specificity and control of cellular retinoic acid levels. Chemico-biological interactions 2009;178(1-3):171–177. [DOI] [PubMed] [Google Scholar]
- 24.Zhong L, Shen H, Huang C, Jing H, Cao D. AKR1B10 induces cell resistance to daunorubicin and idarubicin by reducing C13 ketonic group. Toxicology and applied pharmacology 2011;255(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin HJ, Breyer-Pfaff U, Wsol V, Venz S, Block S, Maser E. Purification and characterization of akr1b10 from human liver: role in carbonyl reduction of xenobiotics. Drug metabolism and disposition: the biological fate of chemicals 2006;34(3):464–470. [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Yan R, Zu X et al. Aldo-keto reductase family 1 B10 affects fatty acid synthesis by regulating the stability of acetyl-CoA carboxylase-alpha in breast cancer cells. The Journal of biological chemistry 2008;283(6):3418–3423. [DOI] [PubMed] [Google Scholar]
- 27.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care 2006;9(4):358–365. [DOI] [PubMed] [Google Scholar]
- 28.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature reviews Cancer 2007;7(10):763–777. [DOI] [PubMed] [Google Scholar]
- 29.Chung YT, Matkowskyj KA, Li H et al. Overexpression and oncogenic function of aldo-keto reductase family 1B10 (AKR1B10) in pancreatic carcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2012;25(5):758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukumoto S, Yamauchi N, Moriguchi H et al. Overexpression of the aldo-keto reductase family protein AKR1B10 is highly correlated with smokers' non-small cell lung carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research 2005;11(5):1776–1785. [DOI] [PubMed] [Google Scholar]
- 31.Ma J, Luo DX, Huang C et al. AKR1B10 overexpression in breast cancer: association with tumor size, lymph node metastasis and patient survival and its potential as a novel serum marker. International journal of cancer Journal international du cancer 2012;131(6):E862–871. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Yan R, Al-Salman A et al. Epidermal growth factor induces tumour marker AKR1B10 expression through activator protein-1 signalling in hepatocellular carcinoma cells. The Biochemical journal 2012;442(2):273–282. [DOI] [PubMed] [Google Scholar]
- 33.Huang C, Verhulst S, Shen Y et al. AKR1B10 promotes breast cancer metastasis through integrin alpha5/delta-catenin mediated FAK/Src/Rac1 signaling pathway. Oncotarget 2016;7(28):43779–43791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo DX, Huang MC, Ma J, Gao Z, Liao DF, Cao D. Aldo-keto reductase family 1, member B10 is secreted through a lysosome-mediated non-classical pathway. The Biochemical journal 2011;438(1):71–80. [DOI] [PubMed] [Google Scholar]
- 35.Shen Y, Ma J, Yan R et al. Impaired self-renewal and increased colitis and dysplastic lesions in colonic mucosa of AKR1B8-deficient mice. Clinical cancer research : an official journal of the American Association for Cancer Research 2015;21(6):1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003;30(3):256–268. [DOI] [PubMed] [Google Scholar]
- 37.Yan R, Zu X, Ma J, Liu Z, Adeyanju M, Cao D. Aldo-keto reductase family 1 B10 gene silencing results in growth inhibition of colorectal cancer cells: Implication for cancer intervention. International journal of cancer Journal international du cancer 2007;121(10):2301–2306. [DOI] [PubMed] [Google Scholar]
- 38.Medh JD, Weigel PH. Separation of phosphatidylinositols and other phospholipids by two-step one-dimensional thin-layer chromatography. Journal of lipid research 1989;30(5):761–764. [PubMed] [Google Scholar]
- 39.Lu Z, Cox-Hipkin MA, Windsor WT, Boyapati A. 3-phosphoinositide-dependent protein kinase-1 regulates proliferation and survival of cancer cells with an activated mitogen-activated protein kinase pathway. Molecular cancer research : MCR 2010;8(3):421–432. [DOI] [PubMed] [Google Scholar]
- 40.Balmanno K, Cook SJ. Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene 1999;18(20):3085–3097. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. Journal of receptor and signal transduction research 2015;35(6):600–604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.