Abstract
One of the most common inflammatory markers examined in depression is C-reactive protein (CRP). However, the magnitude of the association between CRP and depression when controlling for potentially confounding factors such as age, sex, socio-economic status, body mass index, medication and other substance use, and medical illness, is unclear. Inconsistencies in other methodological practices, such as sample collection, assaying, and data cleaning and transformation, may contribute to variations in results. We aggregate studies that examined the association between CRP and depression in two ways. First, a systematic review summarizes how studies of CRP and depression have reported on methodological issues. Second, a tiered meta-analysis aggregates studies that have adhered to various levels of methodological rigor. Findings from the systematic review indicate a lack of protocol detail provided. The effect between depression and CRP was small, but highly significant across all stages of the meta-analysis (p < 0.01). The effect size in the most methodologically rigorous stage of the meta-analysis, which included studies controlling for age, sex, obesity, medical conditions and substance, medication, or psychosocial factors, was small (r = 0.05). There were also only 26 articles in this stage (13% of studies from the systematic review), suggesting that more studies that consistently account for these confounding factors are needed. Additionally, an a priori quality score of methodological rigor was a significant moderator in this stage of the meta-analysis. The effect size was strikingly attenuated (r = 0.005) and non-significant in studies with higher quality scores. We describe a set of recommended guidelines for future research to consider, including sample collection and assaying procedures, data cleaning and statistical methods, and control variables to assess.
Keywords: C-reactive protein, Depression, Systematic review, Meta-analysis, Reproducibility
Major Depressive Disorder (MDD) is a disabling and prevalent mental health condition associated with significant mental and physical health comorbidity (Ferrari et al., 2013; Kessler et al., 2003; Vancampfort et al., 2014). Although several pathways have been implicated in the relationship between depression and physical health, inflammatory processes have garnered considerable attention in psychological research as potential biological mechanisms (Kiecolt-Glaser and Glaser, 2002; Miller, 1998; Miller et al., 2009; Raison et al., 2006). The macrophage theory of depression postulates that pro-inflammatory cytokines are secreted by activated macrophages, contributing to the onset or exacerbation of depression (Smith, 1991). Further, depressive symptoms (e.g., depressed mood, anhedonia, loss of appetite) are associated with acute infections and elevated cytokine levels (Dantzer and Kelley, 2007; Maier and Watkins, 1998). Notably, common physical health comorbidities observed in patients with depression, such as cardiovascular disease (CVD) (Elderon and Whooley, 2013), diabetes mellitus (DM) (Anderson et al., 2001), and autoimmune disorders (e.g., rheumatoid arthritis and lupus; (Benros et al., 2013) are characterized by robust disruptions in immunological functioning.
One of the most frequently studied inflammatory biomarkers is C-reactive protein (CRP), a pentameric protein that increases in level during the acute phase of inflammation (Du Clos, 2000). In 2009, Howren and colleagues published the first meta-analysis on depression and CRP, demonstrating that depression and CRP were positively associated in clinical and community samples (Howren et al., 2009). These results were substantiated with further meta-analytic studies (Haapakoski et al., 2015; Valkanova et al., 2013). Despite the preliminary evidence for the role of CRP in depression, effect sizes in these meta-analyses were attenuated after controlling for body mass index (BMI) or medication use (Haapakoski et al., 2015; Howren et al., 2009). Furthermore, some large-scale studies have failed to establish an association between CRP and depression (de Menezes et al., 2017; Steptoe et al., 2003), especially after controlling for covariates such as medical illness (Almeida et al., 2007), antidepressant use (White et al., 2017), and BMI (Shelton et al., 2015). Additionally, akin to many psychological disorders, MDD is highly heterogeneous (Fried and Randolph, 2015) and a growing area of research indicates that only certain symptoms, or subtypes, of depression may be associated with inflammatory dysregulation (e.g., Jokela et al., 2016). Taken together, such findings call into question if MDD, as a case level diagnosis, is reliably and robustly associated with CRP. Substantial heterogeneity in the handling of confounding variables exists across studies in prior meta-analyses (Haapakoski et al., 2015; Howren et al., 2009), suggesting that the relationship of immune dysregulation to depression may be strongly influenced by lifestyle, disease, and other variables. Such inconsistencies limit our ability to reliably determine if depression, either as a DSM diagnosis or a discreet symptom profile within the diagnosis, is associated with CRP.
In 2009, O’Connor and colleagues published a review on associations between biobehavioral factors and peripheral inflammatory biomarkers with specific suggestions regarding which variables to assess, control, and exclude in analyses (O’Connor et al., 2009). Variables that were considered necessary to account for included well-established factors in the association between CRP and depression, such as age, sex, and BMI or waist circumference. Additionally, substance use-related factors (e.g., nicotine use, alcohol use, and caffeine use), medication-related factors (e.g., antidepressant use, and statin/antihypertensive use), and psychosocial factors (e.g., socioeconomic status (SES) and racial and ethnic differences) were identified as important confounding variables (O’Connor et al., 2009). However, it remains unclear how widespread the adoption of such methodological guidelines for addressing confounds have been across the field. Equally important, other key methodological inconsistencies exist in the literature, including variations in sample collection and assaying procedures and data cleaning techniques (e.g., the handling of outliers and data transformation decisions). As such, without a comprehensive synthesis of data that has adhered to rigorous and appropriate methodological techniques (and a comparison of results across techniques), future progress and replication in this area of research will be hindered.
Several prior reviews have discussed biopsychosocial theories underlying the link between depression and inflammation (e.g., Dantzer and Kelley, 2007; Dantzer and Capuron, 2017; Miller et al., 2009). However, to facilitate valid and productive efforts to interpret and replicate the association between CRP and case-level depression, extend our findings to explore potential cytokine-induced discrete symptom profiles, and ultimately enhance our theoretical knowledge of the link between immunology and depression, an understanding of the current state of the literature is necessary. In the present paper, a systematic review enables an examination of how studies have reported and handled key methodological quandaries. From these studies, results are presented of a meta-analysis that probes how varying methodological approaches, such as confounding variables and data handling techniques, influence the strength of the association between CRP and depression. The meta-analysis is designed to advance knowledge about the individual effects of depression and other covariates on levels of CRP. Overall, the aim of the paper is produce a set of recommendations that can serve as guidelines for the field and a starting place for future replication and reproducibility efforts.
1. Review of methodological practices
1.1. Sample collection procedures
Recommendations for the proper collection and assaying of CRP samples vary in the literature. Assay manuals recommend that blood samples of CRP are tested within the same run to reduce inter-assay variability, and that samples are tested in duplicate (Aziz et al., 2003). However, there is no gold standard for behavioral studies about various collection and assaying issues, such as if the participant should be resting or in the supine position prior to sample collection. Given the established role of immune markers in common infections (e.g., influenza), and findings that have shown that inflammatory markers are elevated in individuals with a fever or the common cold (van den Broek et al., 1990; Whicher et al., 1985), it is recommended that subjects are free of acute infection and that vital signs (e.g., body temperature, blood pressure) are within normal limits.
1.2. Data cleaning and statistical methods
As the precision of immunoassays improves, the field has benefited from lower detection limits and heightened sensitivity (Vashist et al., 2016). However, particularly in healthy controls or community populations, inevitably there are samples where the concentration of CRP is too far below the lower limit to be detected by the assay reader (i.e., “nondetects”). To date, there is no consensus on the proper handling of such participants’ samples, even though they may account for nearly ¾ of participants (e.g., Ford and Erlinger, 2004). Studies may dichotomize or sort CRP levels into quartiles to include nondetects (Ford and Erlinger, 2004) or assign nondetects to the value of the lower detection limit (e.g., Kling et al., 2007) or equal to half of the lower detection limit (e.g., Gimeno et al., 2009). On the other hand, high values of CRP (e.g., > 10mg/L) are often excluded from analyses as they may be indicative of an acute infection (Pearson et al., 2003). Given that certain variables (e.g., SES and alcohol) have a dose-dependent relationship with CRP (Alley et al., 2006; Bell et al., 2017), the exclusion of high values may inadvertently result in the loss of valuable information on individual differences. Further, the adoption of excluding high values, and at what threshold, is inconsistent across studies, impeding replication and reproducibility efforts.
Values that are out of range of the assay, either “nondetects” or values representing extreme high levels, are considered a distinct form of missing data. The recommended techniques for how to deal with such values varies widely and can include listwise deletion (removing all cases with missing data from analyses; e.g., Lucas et al., 2016), pairwise deletion (calculating summary statistics for only detected observations; recommended by the United States Environmental Protection Agency for “nondetects”; (EPA, 2000), multiple imputation (estimating of the missing data m times and using covariate information to estimate missing data; (Chen et al., 2013; Little and Rubin, 1987), and winsorization (replacement of extreme data values with the next highest of lowest value; (Danner et al., 2003)). As mentioned previously, other researchers have substituted values for “nondetects” and excluded extreme high values, or most often, do not report data management procedures at all. Additionally, CRP levels are typically non-normally distributed and positively skewed (Woloshin and Schwartz, 2005). Many studies employ log-transformations, or comparable techniques to normalize the data, and run parametric statistical tests while others utilize non-parametric tests on the raw data. Further, some studies do not report skewness or kurtosis statistics, which limits transparency and the ability to successfully replicate findings.
Overall, studies fluctuate in methods for handling and cleaning data, which carries strong implications for the statistical models applied. The variance of the datasets has significant effects on the results and interpretations, adding to inconsistencies in the field. Coupled with a dearth of papers that report transparent data management techniques for the handling of “nondetects” and high value outliers, replication and reproducibility efforts are likely to be hindered. For example, with a single dataset, different researchers could theoretically reproduce distinct findings hinging primarily on the data management techniques they utilize. Without clear instruction or consensus in the field, this will obstruct future replication efforts.
2. Review of confounding variables
While Howren et al. (2009) established that the overall effect size between depression and CRP was moderate (d = 0.15; 95% CI = 0.10, 0.21), they also found that when restricting to studies that adjusted for BMI, the effect size was reduced (d = 0.11, CI = 0.06–0.17) (Howren et al., 2009). The role of medication use was unclear in this metaanalysis; when medications were not controlled for, the association decreased, but this finding was inconsistent and depended largely on the medication type. Notably, it is unclear how adjusting for BMI and medication use would have influenced the effect size in this metaanalysis. Further, variables that are conceptualized as confounders may actually be key mechanisms in the pathophysiology of inflammatory depression. A primary step for the field is to first determine the independent relationship between inflammation on depression to examine their impact above and beyond other factors. Despite the wide array of biobehavioral factors implicated in the relationship between depression and CRP, no meta-analysis to date has examined both community and clinical samples while simultaneously investigating the role of concurrent covariates beyond age, sex, BMI, and antidepressant use.
2.1. Age and sex
Inflammatory biology changes across the lifespan, with extensive research supporting that CRP levels increase with age in both men and women, even after statistical adjustment for key covariates, such as BMI, medication use, and race and ethnicity (Harris et al., 1999; Lowe, 2005; Puzianowska-Kuźnicka et al., 2016; Woloshin and Schwartz, 2005; Yamada et al., 2001). Population-based studies have also consistently reported elevated CRP levels in women compared to men, even after controlling for BMI (Khera et al., 2005, 2009; Lakoski et al., 2006; Nazmi et al., 2008). However, several studies specifically investigating CRP and depression have found that CRP levels were higher in men but not women (e.g., Danner et al., 2003; Elovainio et al., 2009; Häfner et al., 2011; Liu et al., 2014; Song et al., 2015), while others have found the opposite (e.g., Duivis et al., 2013), suggesting that the role of sex on CRP levels may be more complex in the context of depressive symptomatology.
Further, age and sex influence the frequency and severity of depressive symptomatology. Research has supported a consistent, nonlinear association between age and depressive symptoms (Kessler et al., 1992), with an increase after puberty, especially for girls (Angold et al., 1998). A wide gender gap emerges in adolescence, in which women are significantly more likely to develop MDD (Angold and Rutter, 1992), a trend that continues into adulthood (Weissman et al., 1993). Several factors, including childhood adversity, sociocultural factors (e.g., increased discrimination and placing less value on traditionally female roles), and coping styles, may underlie this gender disparity (Piccinelli and Wilkinson, 2000). Following puberty, depressive symptoms appear to increase modestly with age across both sexes. Health status (Fiske et al., 2003), social isolation (Cacioppo et al., 2006; Glass et al., 2006), and physical activity (Kim et al., 2017; Sin et al., 2016) are key factors potentially related to elevated depressive symptoms in older populations and have also been independently linked to elevated CRP levels (Albert et al., 2004; Benros et al., 2013; Elderon and Whooley, 2013; Ford et al., 2006; Heffner et al., 2011; Kasapis and Thompson, 2005).
2.2. Obesity
Indices of obesity, such as BMI, waist circumference, and waist-to-hip ratio, are dependably and robustly correlated with elevated CRP (Brooks et al., 2010; Panagiotakos et al., 2005; Rexrode et al., 2003). Evidence suggests that the relationship between CRP and BMI is likely driven primarily by obesity (Timpson et al., 2011). Adipocytes and tissue-resident macrophages produce a wide range of inflammatory biomarkers. While the typical measure of obesity is BMI, several investigations have also established that waist circumference and waist-to-hip ratio are also significantly associated with circulating levels of CRP (e.g., Choi et al., 2013; Forouhi et al., 2001; Panagiotakos et al., 2005; Saijo et al., 2004).
Despite the establishment of a strong link between obesity and depression (Luppino et al., 2010; Moreira et al., 2007; Rosmond et al., 1996), the mechanisms underlying this relationship have yet to be fully elucidated. Growing evidence suggests that abdominal obesity is a stronger risk factor for depressive disorders than general obesity (Greenfield et al., 2004; Vogelzangs et al., 2008; Zhao et al., 2011). Visceral adipose tissue is hypothesized to play a key role and high levels of inflammatory markers have been observed in visceral obesity and depression (Penninx et al., 2003; van Reedt Dortland et al., 2013). Understanding if inflammation and depression are significantly related above and beyond the effects of obesity (and other confounders) is critical to determine next steps for interventions and treatments.
2.3. Chronic medical health conditions
Given the high rates of comorbidity between depression and physical health conditions (e.g., CVD, DM, autoimmune disorders; Smith et al., 2014), chronic medical health conditions likely play a significant role in the relationship between CRP and depression. A methodological challenge for researchers investigating the link between depression and CRP is the proper measurement and statistical adjustment for chronic health conditions. Medical health conditions with strong immunological disruptions, such as CVD, DM, rheumatoid arthritis, and metabolic syndrome, may be the most significant medical conditions to consider. However, studies vary widely in which medical conditions they assess and adjust for (e.g., Copeland et al., 2012; Ford and Erlinger, 2004). To combat limitations related to self-report assessment, studies can also assess and control for biological factors such as cholesterol, triglycerides, blood pressure, and fasting glucose levels (e.g., Ford and Erlinger, 2004).
3. Substance-use related variables
Several substances, notably nicotine, alcohol, and caffeine, are related to dysregulated CRP levels (O’Connor et al., 2009).
3.1. Nicotine
The relationship between nicotine and circulating CRP is complex, but has been established in large, well-controlled studies (Nanri et al., 2007; Yanbaeva et al., 2007). Cigarette smoking weakens innate immune defenses, promotes autoimmune disease progression, moderates antigen presentation (Lee et al., 2012), and is associated with elevated CRP levels (Gonçalves et al., 2011). Elevated CRP levels have even been observed among former smokers (Bazzano et al., 2003; Hastie et al., 2008; Tracy et al., 1997). Notably, the effects of smoking on CRP have not been documented in all studies; however, this may be due partially to sex differences, with higher nicotine levels disproportionately linked to higher CRP in men (Bo et al., 2005; Fröhlich et al., 2003; Nazmi et al., 2008). Furthermore, smoking and depression are often comorbid, though there is debate if they share etiological vulnerabilities (Dierker et al., 2002).
3.2. Alcohol
Peripheral levels of CRP are typically lower in moderate drinkers, which has been roughly defined as 1–7 alcoholic beverages per week, or around 15–30 g of alcohol/day (O’Connor et al., 2009), compared to non-drinkers and heavy drinkers (Bell et al., 2017; Imhof et al., 2004; Pai et al., 2006; Raum et al., 2007; Wang et al., 2008), who have the highest levels of CRP even after adjustment for several covariates (Xu et al., 2016).
One of the most prevalent psychiatric comorbidities for individuals with alcohol use disorder is depression (Grant et al., 2004) with research suggesting the two disorders share common genetic factors (Prescott et al., 2000; Procopio et al., 2013) and several risk factors (e.g., childhood adversity, SES, race and ethnicity; (Swendsen and Merikangas, 2000).
3.3. Caffeine use
Caffeine use, in the form of coffee, soft drinks, and energy drinks, is the most widely consumed central nervous system stimulant (Heckman et al., 2010) with 85% of the United States population consuming at least one caffeinated beverage per day (Mitchell et al., 2014). Coffee may have anti-inflammatory properties as animal studies have indicated that caffeine intake prevents metabolites from inducing inflammation (Swirski and Nahrendorf, 2017), while cross-sectional studies have found an inverse relationship between coffee intake and CRP (Furman et al., 2017; Williams et al., 2008); however, this observation was more robust in healthy subjects (Lopez-Garcia et al., 2006). There are also contradictory findings, which may be due to type of caffeine intake. For example, boiled coffee, which produces higher caffeine levels, was associated with increased CRP levels in a study of 3032 individuals (Zampelas et al., 2004). The interactions between CRP and caffeine intake are complex and results are inconclusive. While caffeine modulates inflammatory markers, there is limited research on the neurobiological mechanisms and the overall impact of caffeine intake on CRP (Bonita et al., 2007). A recent meta-analysis of observational studies on the association between coffee, caffeine, and tea consumption and depression suggests a protective effect of coffee intake on depression, but was inconclusive regarding the effects of tea or other forms of caffeine (Grosso et al., 2016).
4. Medication-related confounding factors
Several medication types, including antidepressant use, NSAIDs, and statins and anti-hypertensive medications, are associated with both CRP and depression (O’Connor et al., 2009).
4.1. Antidepressant medication
Antidepressant medications, most notably selective serotonin uptake inhibitors (SSRIs), potentially modulate inflammatory processes, though the mechanisms behind this phenomenon are not clear. A longitudinal population-based study found that antidepressant use was associated with elevated levels of CRP, independent of mental health symptomatology and cardiovascular risk factors (Hamer et al., 2011). Several studies have found that CRP levels decrease significantly after SSRI treatment in patients with depression (Lanquillon et al., 2000; O’Brien et al., 2006; Tuglu et al., 2003; Uher et al., 2014) and that baseline CRP levels may predict treatment response in SSRIs and norepinephrine reuptake inhibitors (Uher et al., 2014). However, other studies did not observe a significant change in CRP levels following SSRI treatment (Chang et al., 2012) or, conversely, that CRP levels actually increased during SSRI treatment, even if patients had a therapeutic response (Dawood et al., 2007). Overall, the potential inflammatory mechanisms activated by antidepressants have not been thoroughly elucidated.
Antidepressant medications are considered a frontline treatment option for patients with depressive symptoms (Hollon et al., 2002) and are being prescribed at increasing rates across the world (Abbing-Karahagopian et al., 2014; Uchida et al., 2007; Zhong et al., 2014). Antidepressants may also be prescribed for individuals without a depressive disorder – a study that reviewed approximately one million health plan members filling an antidepressant prescription found that 39% of the sample did not have a mental health disorder (Simon et al., 2014). Patients with more severe or chronic depressive symptoms may benefit the most from antidepressant therapy (Fournier et al., 2010), and are likely to be on a higher dose and be prescribed adjunctive antidepressants (Cleare et al., 2015). Given the prevalence of antidepressant use in both community and clinical samples, and the variation in dosage, use of antidepressants should be considered as an important covariate in associations between CRP and depression.
4.2. NSAID use
The use of NSAIDs is widespread, with increasing use for the management of a range of physical health condition that are commonly comorbid with depression (e.g., CVD, arthritis) (Zhou et al., 2014). In healthy samples, NSAID use may not affect CRP levels (Azar et al., 2003; Feldman et al., 2001; Vaucher et al., 2014). However, in populations with physical health conditions, NSAID use does appear to reduce CRP levels (Ikonomidis et al., 1999; Solheim et al., 2003). Recently, clinical trials have investigated the potential of NSAIDs, such as celecoxib, as an adjunctive treatment option for MDD, with a small number studies demonstrating preliminary support, though the studies heretofore are very heterogeneous and demonstrate a high risk of bias (Köhler et al., 2014; Na et al., 2014).
4.3. Statins and anti-hypertensive medications
Hypertension (HTN) and hypercholesterolemia are both prevalent conditions, with HTN estimated to affect roughly 29% of the US adult population (Nwankwo et al., 2013). Cholesterol-lowering statins are prescribed as treatment options for both HTN and hypercholesterolemia (Wierzbicki, 2006). Statins have been shown to decrease CRP levels, though the majority of these studies have been conducted in individuals with physical health conditions (Prasad, 2006); however, statins may also reduce CRP levels in healthy individuals (Ridker et al., 2001). Angiotensin-converting enzyme (ACE) and beta-blockers are the most common antihypertensive medication prescribed (Gu et al., 2006) and appear to lower CRP levels in individuals with medical conditions (Di Napoli and Papa, 2003; Joynt et al., 2004; Palmas et al., 2007). In individuals with HTN, depressive symptoms may predict noncompliance with medication regimens (Bautista et al., 2012; Krousel-Wood et al., 2010). There does not appear to be a substantial link between the use of antihypertensive medication and elevated risk for depression when controlling for HTN (Ko et al., 2002).
5. Potentially confounding psychosocial factors
5.1. SES and education
Socioeconomic status (SES) has been reliably inversely correlated with circulating levels of CRP, often independent of other demographic, physical, or behavioral factors (Jousilahti et al., 2003; Lubbock et al., 2005; Obinwa et al., 2016; Owen et al., 2003). The operationalization of SES varies, with different studies utilizing occupation, income, and neighborhood conditions (or a combination of these factors) to define SES (Shavers, 2007). Level of education is also often used as a proxy measure for SES (Shavers, 2007) and is also inversely correlated with CRP levels (Kershaw et al., 2010; Panagiotakos et al., 2004). Smoking, drinking, physical activity, and obesity may act as mediating factors in the relationship between SES and CRP (Alley et al., 2006; Gimeno et al., 2007; Kershaw et al., 2010). Notably, the association between SES and CRP is very robust at the highest levels of CRP (Alley et al., 2006) and remains stable over time (Gimeno et al., 2007). Childhood poverty may even predict CRP levels in offspring (Schreier and Chen, 2010).
Lower SES is also linked to depressive disorders; a meta-analysis found that lower educational status and income were specifically associated with higher likelihood of MDD (Lorant et al., 2003). Causal factors linking low SES to MDD include adverse childhood experiences, neighborhood and poverty-related stress, sexual and racial discrimination, and lower levels of social support (Belle and Doucet, 2003; Blair et al., 2014; Santiago et al., 2011; Williams, 1999).
5.2. Race and ethnicity
Although race has been found to be an important factor in overall levels of CRP (for review, see O’Connor et al, 2009), less research has focused on race as a moderating factor in the association between CRP and depression. Despite recommendations (O’Connor et al., 2009), many studies do not control for these variables or stratify results by racial or ethnic group; often it is difficult to obtain clear information about if this information was collected. Therefore, although there are indications that race and ethnicity may influence inflammatory mechanisms of depression, this meta-analysis will not examine it as an explicit factor as too few of the studies examined reliably reported these statistics. Instead, we devote a section of the discussion to elaborating on the importance of collecting these important demographic variables in future research.
6. Summary
In summary, several factors are related to both CRP and depression. However, studies vary widely in accounting for these variables when examining the relationship between CRP and depression. As such, we cannot reject the possibility that some findings regarding the association between CRP and depression may be epiphenomenal – an apparent association that is not inherent to these two variables, but that is actually causally reliant on a third, unmeasured variable. Indeed, evidence suggests that controlling for specific factors, such as BMI or antidepressant use, may independently attenuate the association between CRP and depression (e.g., Shelton et al., 2015; White et al., 2017). Further, some confounders (e.g., obesity) may mediate the relationship between depression and CRP, while others are less likely to be causally linked to depression or CRP and instead are best conceptualized as proxies for an unmeasured mechanism (e.g., race and ethnicity may be a proxy for discrimination). However, no review to date has synthesized how the statistical control of multiple covariates affects the strength of the relationship between CRP and depression. Equally important, other key methodological inconsistencies exist in the literature, including variation in sample collection procedures, handling of outliers, and data transformation decisions.
7. The present study
Without a comprehensive synthesis of data that has adhered to the most rigorous and appropriate methodological techniques (and a comparison of results across techniques), it will be difficult for this area of research to be reproduced and therefore replicated, allowing our scientific inferences to be stronger. In order to address these issues, the current study has three aims: 1) to conduct a meta-analysis on specific studies utilizing the most rigorous and theoretically justifiable methodology, and to compare results to what is commonly reported in the field; 2) to systematically identify and evaluate methodological inconsistencies that may hinder replication and reproducibility efforts; and 3) to establish a set of empirically grounded guidelines for best practice methodology in CRP research to help inform future replication and reproducibility efforts. To address the aims, first a systematic review of all studies investigating the association between CRP and depression (either diagnosed MDD or depressive symptoms) in otherwise healthy individuals was conducted, in order to synthesize the status of the current field regarding the statistical adjustments for key covariates as well as methodological issues, including the assaying, handling, cleaning, and testing of data. Secondly, a focused meta-analysis was conducted to specifically investigate the role of crucial confounding factors: age, sex, BMI/adiposity, and chronic medical conditions, substance-related factors (e.g., nicotine, alcohol, and caffeine), medication-related factors (e.g., antidepressant, NSAID, and statin/antihypertensive use), and psychosocial factors (e.g., SES and education) as well as proper and transparent data handling techniques.
The meta-analysis was conducted in stages both to examine how an increase in methodological integrity affects the strength of the relationship between CRP and depression, and how separate groups of potential confounders may differentially impact this association. Additionally, this approach will address how the level of heterogeneity in studies examining the association between CRP and depression differs across stages. The final meta-analysis, which reflects the highest standard of methodological rigor with respect to these issues, will be the first meta-analysis on this topic to examine only studies that adhere to stringent quality standards. This study was pre-registered on 06/15/2017 with Open Science Framework (link: osf.io/x5wug) and the data analytic plan and code was uploaded on 09/20/2017.
8. Method
8.1. Systematic review
8.1.1. Identification and selection of studies
A systematic review was conducted of the PubMed and Google Scholar electronic databases for English language studies to identify studies that have conducted statistical analyses of the association between CRP and unipolar depression. Searches were conducted for the following key terms: “CRP”, “C-reactive protein”, “depressive,” and “depression” to capture a broad range of potential articles. Further, the reference lists of all relevant publications and prior meta-analyses were scrutinized for additional articles.
Eligibility for inclusion was independently determined by two of the authors (SH and ML). Studies reporting cross-sectional or longitudinal analyses for unipolar depression and CRP in either clinical or community adult populations were included. Depression could be assessed by a clinician-based interview (e.g., Structured Clinical Interview for DSM-5; (First et al., 2016), with symptom-based psychometric instruments (e.g., Beck Depression Inventory (BDI) (Beck et al., 1996), or via medical records. Unstimulated measures of CRP via venous blood samples, blood spots, or saliva were included. Given the wide range of physical health disorders that impact immunological functioning, studies in which physical conditions were the primary focus (e.g., a study of the association between a physical health condition and CRP), such as metabolic syndrome or CVD, were excluded. However, studies that included participants with stable medical conditions (e.g., HTN, DM) were included. Further, clinical studies in which depression was not the primary mental health disorder (e.g., studies investigating CRP and anxiety disorders) were excluded as there were not properly defined a priori hypotheses focused on depression.
8.1.2. Study selection and data extraction
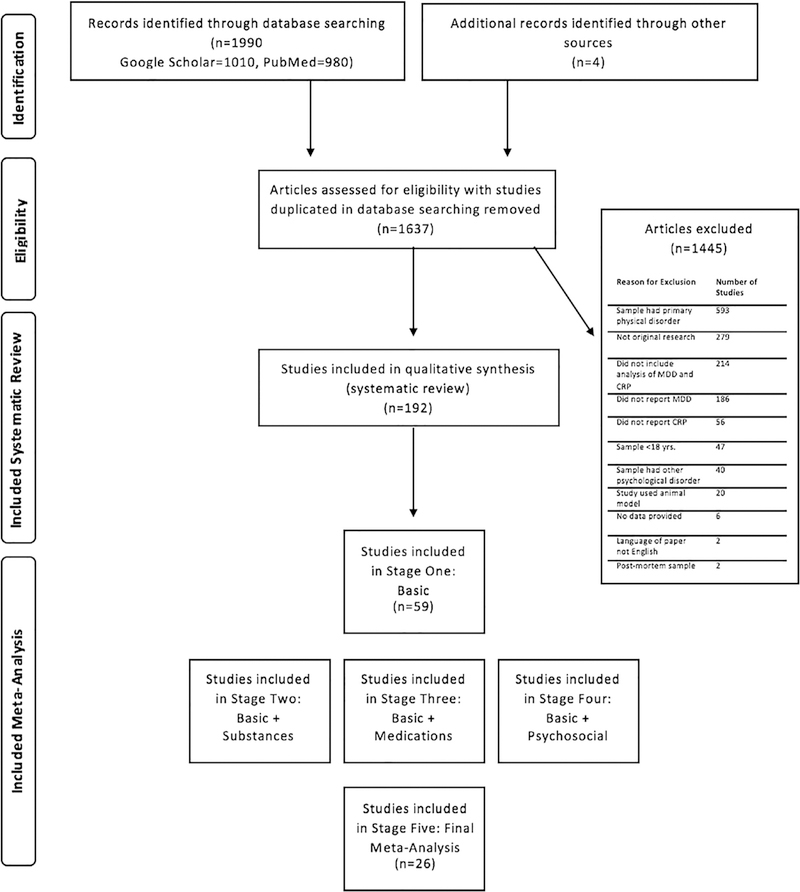
A consort chart of the literature search is shown in Fig. 1. Studies that provided sufficient information about the relationship between depression and CRP (e.g., a statistical analysis) were included in the systematic review (a total of 192 original studies).
Fig. 1.
Consort chart for systematic review and meta-analysis.
A standardized data coding system was developed to extract the following information from each study: Study characteristics included: 1) authors and citation; 2) sample size and description of the sample (e.g., mean age, sex, percent with depression, clinical or population-based), 3) main aim of the study, 4) study design (e.g., cross-sectional, longitudinal, randomized controlled trial-RCT), 5) measure(s) of depression; sample collection procedures included: 6) mode of specimen collected for CRP analysis (serum, plasma, blood spot, saliva, or unspecified venous blood), 7) fasting status, 8) time of day of sample collection, 9) freezer temperature/details, 10) additional details of collection (e.g., type of tubes used, supine/resting position, time resting before blood draws); assay technology included: 11) assay type (name, brand), 12) intra-inter assay coefficients provided (yes or no with value), 13) if samples were measured in duplicate; data transformation and handling included: 14) normality statistics reported (yes or no), 15) type of transformation applied (e.g., log-transformation, square root transformation), 16) type of statistical test utilized (parametric or non-parametric), 17) handling of high value outliers (e.g., number excluded, statistical handling), 18) handling of “nondetect” values, 19) additional exclusion criteria. In addition, confounders coded for included: 20) demographic confounders (age, sex, race and/or ethnicity, SES, education), 21) health variables (BMI, waist circumference, waist-to-hip ratio, cholesterol levels, triglyceride levels, glucose levels, blood pressure, exercise/physical activity, acute sleep deprivation, body temperature, acute illness/infection (e.g., fever), and chronic medical conditions (e.g., CVD, DM, HTN, cancer, asthma), 22) substance variables (smoking/nicotine use, alcohol use, caffeine use), 23) medication variables (birth control/estrogen, hormone replacement therapies, NSAID use, antibiotics, stimulants, antidepressants, antipsychotics, anxiolytics, sleep medications/sedatives, antiarrhythmic drugs, and statin/antihypertensive use). For confounders, studies were coded for if the variable was statistically controlled for, found to be non-significantly associated with both CRP and depression, matched between groups, excluded, or not accounted for in the statistical model.
Data were searched and extracted independently by three authors (SH, ML, BN) and a team of trained research assistants. Each article was coded by one author and checked by a separate author. All articles were verified with a quality check by the first author. Disagreements were resolved through group discussion.
8.2. Meta-analysis
8.2.1. Identification and selection of studies
For the meta-analysis, we developed a set of quality indicators that reflect established recommendations (e.g., O’Connor et al, 2009), and theoretically justifiable methodological practices. The meta-analysis was conducted in five stages to explore how methodological quality may influence the strength of the relationship between CRP and depression. In order to maintain as much homogeneity as possible across studies, and because the strength of correlation of CRP values across modes of collection has yet to be determined (Brindle et al., 2010; Ouellet-Morin et al., 2011), the few studies that measured CRP via saliva (n = 1) or blood spots (n = 3) were not included in the metaanalysis. The present meta-analysis focused on the strength of concurrent associations between CRP and depression, therefore, all studies included in the meta-analysis were of cross-sectional design; in cases of longitudinal studies or RCTs, if a cross-sectional analysis was conducted and reported, the study was considered for inclusion. Stage One of the meta-analysis reflected studies that met the basic baseline level of methodological integrity, described below. Stages Two - Four included studies that met criteria for Stage One and also controlled for particular confounding variables, described below. Stage Five included studies that met criteria for Stages One-Four. Eligibility for inclusion in all stages of meta-analysis was independently determined by three of the authors (SH, ML, and BN). Two authors (SH, MB) independently extracted outcome data (e.g., effect size) for the association between depression and CRP and relevant variables (e.g., sample size, level of significance, type of effect size). Disagreements were resolved through group discussion until a consensus was reached. Fewer than 8% of all studies required discussion.
8.2.2. Stages of meta-analysis criteria
For inclusion in Stage One of the meta-analysis, the study must have used a valid measure of MDD or depressive symptoms, such as the SCID or BDI-II. To investigate the overall strength of the association between CRP and depression as a whole disorder or the full range of depressive symptomatology, studies that only measured a subset of clinical depressive symptoms (e.g., cognitive symptoms of depression) or used non-validated measures of depression (e.g., a single question regarding depression status or the use of antidepressants) were excluded. Further, given the skewed nature of CRP values (Woloshin and Schwartz, 2005), studies must have utilized and reported clear and consistent data transformation and handling techniques in line with recommendations for non-normal data (Bishara and Hittner, 2012). Specifically, if the distribution of CRP was non-normal and values were left raw, studies using non-parametric testing were included. Studies that employed parametric tests on raw CRP data were also included if the researchers provided kurtosis and skewness statistics to justify treating the data as normally distributed. If the CRP values were transformed, the transformation must have been clear (e.g., type of transformation) and appropriate corresponding parametric tests must have been utilized. In addition, studies included in Stage One must have also controlled or accounted for age, sex, BMI/waist circumference/waist-to-hip ratio, and chronic medical conditions. We chose these variables because, as outlined in the introduction, they have the strongest and most consistent empirical associations with both CRP and depression. Studies were included if they conducted separate sex analyses (e.g., male versus female), controlled for the variable, matched groups based on the variable, or excluded relevant cases (e.g., excluding chronic medical conditions). A total of n = 59 articles was included in Stage One.
Studies included in Stage Two of the meta-analysis must have met for all criteria in Stage One and accounted for at least one of the following substance-related covariates: nicotine use, alcohol use, and/or caffeine use (n = 57). Studies included in Stage Three of the metaanalysis must have met for all criteria in Stage One and accounted for at least one of the following medication-related covariates: antidepressant use, NSAID use, and/or statin/anti-hypertensive use (n = 43). Stage Four of the meta-analysis included studies that met criteria for Stage One and accounted for at least one psychosocial factor: SES and/or education level (n = 35).
The final and most rigorous meta-analysis stage included studies that met for criteria from all the above stages (n = 26). These studies fulfilled Stage One criteria and accounted for at least one covariate from each category: substance- related confounders (nicotine, alcohol, caffeine), medication-related confounders (antidepressants, NSAID, statin/antihypertensive use), and psychosocial-related confounders (SES, education).
8.2.3. Quality score
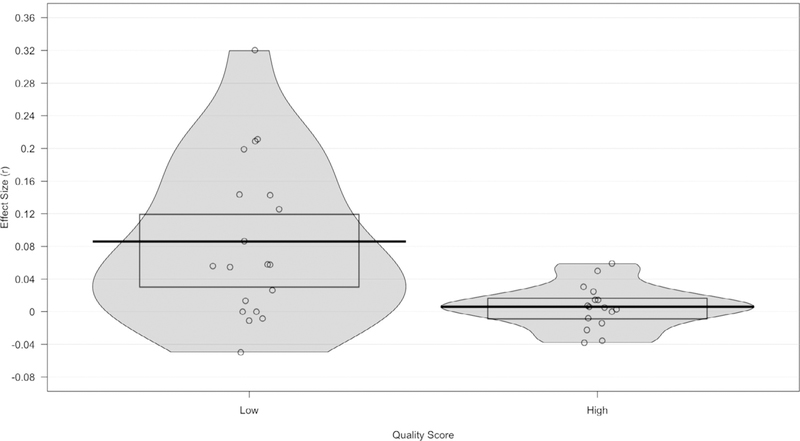
In addition to the meta-analysis stages, an a priori quality composite score was calculated that considered other notable methodological considerations. The quality score included the covariates from Stages Two- Four (smoking, alcohol, caffeine, antidepressant use, NSAID use, statin/antihypertensive use, SES, and/or education). Other key covariates that were highlighted in O’Connor’s review, such as physical exercise/activity, sleep deprivation, and acute illness, were included. The quality score also accounted for whether the study statistically adjusted the model for covariates or demonstrated that the variable was non-significantly associated with depression and CRP (higher score) or simply matched groups on covariates (lower score), as matching the groups by depression status may only account for the variability in depression rather than in CRP. Additionally, the quality score considered recommended sample collection procedures, such as if the participants were resting before the sample was collected, if the study reported proper handling of “nondetect” values and high-value outliers, if the sample was measured in duplicate, and if the study reported an effect size. The highest possible quality score was 16. All design factors contributed equally to the quality score. All quality score calculations were double checked by the first author. The quality score was considered as a potential continuous moderator for Stage 5 of the metaanalysis if considerable heterogeneity was still observed.
8.2.4. Calculation and aggregation of study effect sizes
The metafor and userfriendlyscience packages on RStudio 1.0.136 were used to compute and aggregate effect sizes (Team, 2015). All code for this meta-analysis is in Supplementary material and at the Open Science Framework pre-registration link. Random-effects models are the most appropriate approach for the purposes of this paper and were used in all analyses. Random-effects models assume a distribution of population effect sizes across studies and account for within- and between-study variation. Lastly, random-effects models produce wider confidence intervals (CI) and are considered a more conservative analytic strategy compared to the fixed-model approach (Egger et al., 1997; Hedges and Vevea, 1998). Effect sizes were calculated as r-values, with positive r-values representing higher levels of CRP in depression or a positive association between CRP and levels of depressive symptoms. R-values were selected as the effect size index as this meta-analysis includes continuous and categorical predictors (range of depressive symptoms and diagnosis of depression versus no depression). R values are comparable across the studies with different types of predictors and readily computable from the information reported in the articles. Finally, r values are interpretable and the primary effect size recommended for meta-analyses of correlational data, particularly for meta-analyses including studies conducted with one group (Borenstein et al., 2009).
In the event that a statistical test was reported as non-significant with no additional information provided, the effect size was set to r = 0.00 and weighted according to sample size. This approach yields the most conservative effect size estimate, and has been utilized in past meta-analytic approaches (Howren et al., 2009). If results were reported for both a continuous measure of depression and a categorical diagnosis of depression versus no depression, effect sizes from the continuous measure of depression were included as continuous variables contain more variability. Similarly, in papers that report both continuous and dichotomized or binned CRP (e.g., CRP “low versus high” or CRP in quartiles), effect sizes derived from the continuous measure of CRP were included. Lastly, if studies provided separate results by sex or type of depression (e.g., atypical versus melancholic), the results were treated as separate analyses from the same parent study.
Heterogeneity among effect sizes was calculated and assessed with the Q statistic, which is distributed as χ2, and indicates if the variability among study outcomes is sufficiently large to reject the null hypothesis that they are drawn from a common population. An I2 value was also calculated which describes the percentage of variation across studies due to heterogeneity. A separate meta-analysis was conducted for each stage, with studies utilizing both continuous and categorical predictors combined. A post-hoc analysis at each stage was run separating the two types of samples (e.g., continuous predictor studies and categorical predictor studies). Lastly, the quality score was entered as a continuous moderator for the Stage 5 analysis.
Forest plots displaying the effect sizes for each study with associated CIs are included for each stage (Fig. 3a–e). Funnel plots that show the distribution of effect sizes in the analysis were created to illustrate any potential publication bias (Supplemental Fig. 1a–e). In the funnel plot, an asymmetrical distribution indicates that there is an overrepresentation of positive results in the published literature.
Fig. 3a.

Studies in Stage One Meta-Analysis. Sample size included to the left of the effect size. Fisher’s z transformed correlation coefficient and 95% Confidence Interval.
Fig. 3e.
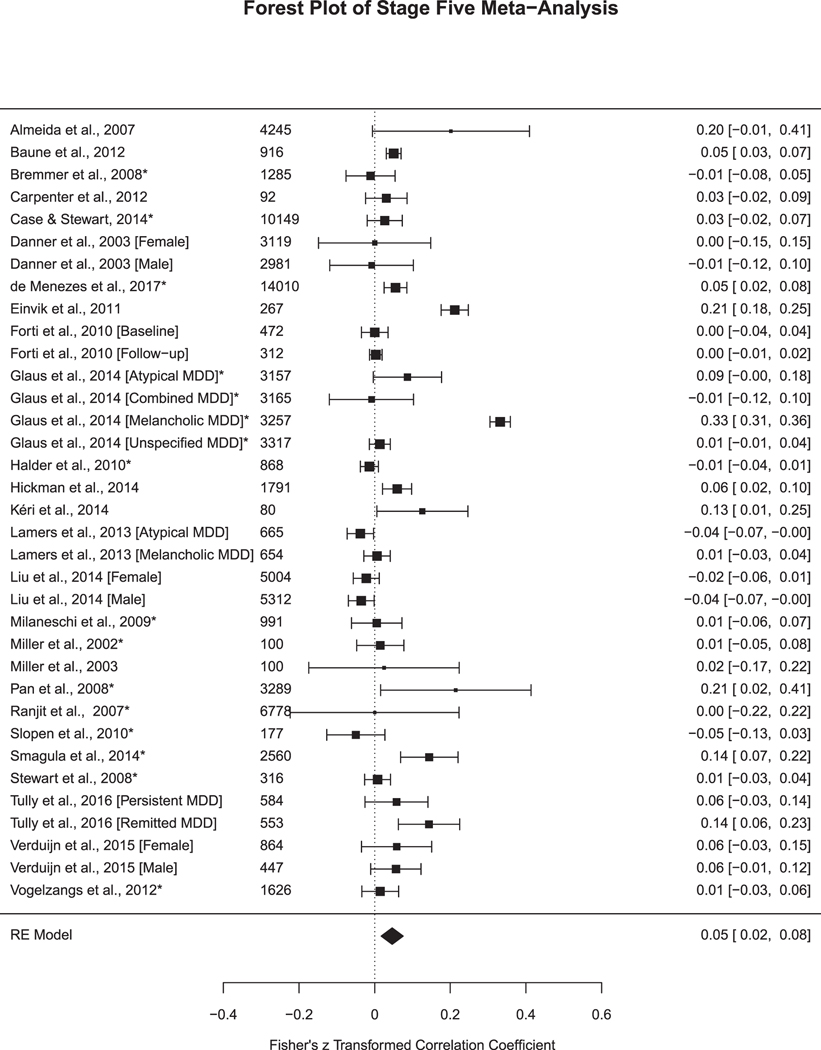
Studies in Stage Five Meta-Analysis. Sample size included to the left of the effect size. Fisher’s z transformed correlation coefficient and 95% Confidence Interval. * =high-quality study for moderator analysis.
9. Results
9.1. Systematic review
Basic defining features of all studies are summarized in Supplemental Table 1.
9.2. Methodological practices
9.2.1. Sample collection and assaying protocols
Nearly 97% of studies measured CRP in blood samples (n = 104 in serum, n = 52 in plasma, and n = 30 in blood sample that was not specified). Four studies measured CRP in blood spots and 2 studies measured CRP in saliva. Sixty-eight studies (35.4%) reported assaying their samples in duplicate, either by including an intra-assay coefficient or statement that samples were analyzed in duplicate. In terms of studies reporting assay detection sensitivity values, 108 (56.3%) did not and 84 (43.6%) did report assay sensitivity values. Across studies, the lower assay detection limit ranged from 0.008 mg/L to 0.16 mg/L.
Sixteen studies (8.3%) reported that participants were in a supine, resting position prior to the specimen collection. The time spent resting prior to the blood draw ranged from 5 min to 45 min across studies. Fifty-one studies (26.7%) accounted for individuals with acute illness either via exclusion criteria or statistical control.
9.2.2. Data handling and transformation
Fourteen studies (7.3%) explicitly reported the handling of non-detect values with four studies assigning nondetect values to a random number, two studies assigning nondetect values to half of the detection limit, and one study assigning nondetect values equal to 0. Four studies excluded nondetect CRP values. Forty studies included dichotomized or binned categorical analyses of CRP; however, only three studies explicitly stated that nondetect values were included in the lowest CRP category. Ten of those studies also included continuous analyses of CRP and depression without specifying the handling of nondetect values. Fifty-two studies (27%) reported handling of high outliers. The most reported cut-off utilized was 10 mg/L (n = 34 studies) with the remaining studies using a range of cut-offs from 5 mg/L to 20 mg/L. Seven studies explicitly stated that they included high values in their analysis.
Half of the studies (50.5%, n = 97) transformed the CRP data and ran parametric tests, while only 8 (4.2%) reported that the CRP data was normal and then used parametric tests. Thirty-four studies (17.7%) left the CRP data raw and ran non-parametric tests and 20 studies (10.4%) indicated that they ran both parametric and non-parametric tests. The remaining 33 studies (17.2%) ran parametric tests without providing normality statistics or provided insufficient information about the type of statistical test employed.
9.2.3. Confounding variables
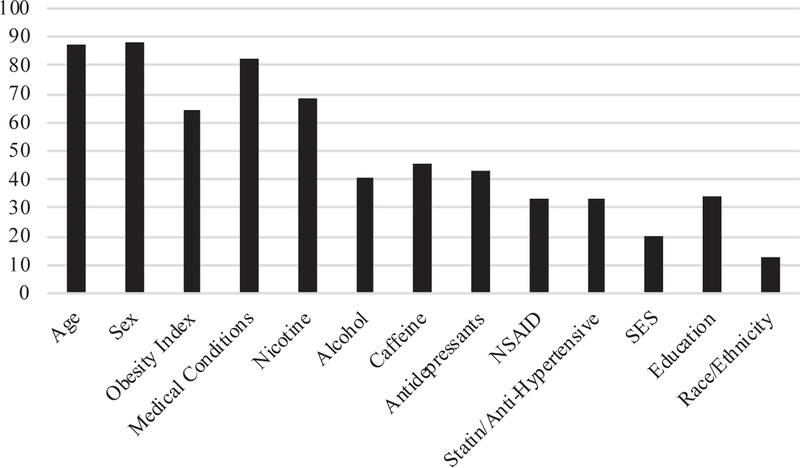
Fig. 2 illustrates the percentage of studies that accounted for the following confounding variables.
Fig. 2.
Percent of studies in systematic review controlling for confounding variables.
9.2.4. Age, sex, BMI/waist circumference/waist-to-hip ratio, and chronic medical conditions
Most studies accounted for age (n = 168, 87.5%). Only 22 studies (11.5%) did not account for sex differences; 141 (73.4%) studies either examined the sexes separately or statistically controlled for sex, while 29 (15.1%) studies were conducted in all-male or all-female populations. A total of 123 articles (64.1%) statistically accounted for the role of BMI, waist circumference, and/or waist-to-hip ratio. The majority of studies accounted for chronic medical conditions (n = 159 studies, 82.8%) with roughly equivalent numbers of studies excluding medical conditions (43%) and controlling for them (40%). There was widespread inconsistency in the reporting of which medical conditions were accounted for; the two most common medical conditions controlled for or excluded were CVD-related disorders (n = 107; 55.7%) and DM (n = 80, 41.7%). Several studies provided only general statements, such as exclusion of “any condition known to affect the immune system” or “chronic health morbidities,” which prevented examination of which conditions specifically were being accounted for. Overall, studies ranged from reporting 1–39 physical health conditions accounted for, with 40% of studies not providing sufficient information to know how many conditions were accounted for in the statistical analysis of CRP and depression.
9.2.5. Substance-related variables: nicotine, alcohol, and caffeine
120 studies (62.5%) controlled for nicotine use and 12 (6.3%) studies excluded acute or chronic nicotine use prior to the sample collection. The most common method of measuring nicotine use was via self-report (n = 91 studies). Out of the studies controlling for nicotine use, n = 48 controlled for both current and former nicotine use while n = 50 reported controlling for only current nicotine use. For alcohol use, 69 (35.9%) studies controlled for alcohol use while 9 studies (4.7%) excluded individuals with a diagnosis of alcohol-use disorder or heavy drinking patterns. Studies accounted for alcohol with varying methods; 29% of studies used a self-report regarding frequency of drinking, 24.7% of studies specifically controlled for the number of drinks in the prior week or month, 21.2% calculated and controlled for the number of grams of alcohol consumed in the prior week or month, and 10.6% prohibited alcohol consumption prior to the sample collection. Eighty-seven (45.3%) studies controlled for caffeine intake; 88.5% of the studies accounting for caffeine intake instructed participants to fast overnight prior to the sample collection. The remaining 11.5% of studies specifically reported asking subjects to abstain from caffeine intake or controlled for caffeine intake.
9.2.6. Medication-related variables: NSAID use, antidepressant use, and statin/anti-hypertensive medication
Sixty-four studies (33.3%) accounted for NSAID use with 17% of those studies excluding NSAID use and 16% statistically controlling for subject’s NSAID use. Eighty-three studies (43.2%) accounted for antidepressant use with 22% excluding individuals taking antidepressants and 21% statistically controlling for antidepressant use. Lastly, 27 (14%) studies statistically controlled for anti-hypertensive and/or statin use and 37 (19.2%) studies excluded individuals on these medications.
9.2.7. Psychosocial variables: SES, education, and race/ethnicity
Only 20% of studies (n = 39) controlled for SES and the mode of measuring SES varied across studies, with income being the most widely used measure of SES (n = 18), followed by a combination of different factors (e.g., income, occupation, neighborhood; n = 8), employment status or occupation type (n = 7), a validated scale (e.g., Hollingshead Four-Factor Index of SES; (Hollingshead, 1975); n = 3), neighborhood/zip code (n = 2), or unspecified (n = 1). In comparison, 34.4% of studies (n = 66) accounted for education level.
Race and ethnicity variables were typically presented together. A total of 24 studies (12.5%) explicitly controlled for race and ethnicity and one study excluded African-American participants. The majority of studies were conducted in the United States (n = 58) followed by the United Kingdom (n = 20), the Netherlands (n = 18), and Germany (n = 14). Most studies were conducted in Western European countries with predominantly Caucasian populations (e.g., Germany, Finland; 55.4%). Roughly 8% of studies were conducted in Asian countries (e.g., Thailand, Japan, China) and 7% of studies were conducted in Eastern European, Middle Eastern, and/or Mediterranean-based countries (e.g., Israel, Greece, Croatia). A total of 143 studies either did not report their race and ethnicity break-down or only reported the country in which the study was conducted.
9.3. Meta-analysis results
Studies included in the meta-analysis with basic defining features are summarized in Table 1. The meta-analysis results are presented by stage and type of predictor (Table 2).
Table 1.
Studies included in meta-analysis.
| Author and Year | Country of Study |
Sample |
Mode of Specimen collection |
Depression Measure |
Medical Condition exclusion criteria | Confounders Accounted** For |
Included Stages of Meta- Analysis |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean Age or Age Range |
% Male | Name | Categorical or Continuous |
Substances | Medication | Psychosocial | |||||
| Almeida et al., 2007 | Australia | 4245 | 70 + | 100.0% | Serum | GDS-15 | Categorical | CVD conditions | Nicotine Caffeine | Antidepressant | SES Education | 5 |
| Almeida et al., 2009 | Australia | 3700 | 70–85 + | 100.0% | Serum | GDS-15 | Categorical | Physical disorder | Nicotine Caffeine | None | None | 2 |
| Baune et al., 2012 | Australia | 916 | 78.8 | 44.8%. | Serum | GDS-15 | Continuous | Neurological illness, MS, malignancy | Nicotine Caffeine | NSAID Antidepressant Statin | Education | 5 |
| Bjerkeset et al., 2011 | Norway | 8994 | 55.6 | 46.0%. | Serum | HADS-D | Categorical | CVD conditions, DM, asthma, cancer | Nicotine Alcohol Caffeine | None | SES Education | 2,4 |
| Bremmer et al., 2008 | Netherlands | 1285 | 70.0 | 48.9%. | Serum | CES-D 20 | Continuous | CVD conditions, HTN, DM, lung disease | Nicotine Alcohol | NSAID Antidepressant Statin | Education | 5 |
| Carpenter et al., 2012 | USA | 92 | 30.5 | 48.9%. | Plasma | IDS-R | Continuous | All medical and psychiatric disorders | Nicotine | NSAID Antidepressant Statin | SES | 5 |
| Case & Stewart, 2014 | USA | 10,149 | 44.3 | 50.8%. | Serum | PHQ-9 | Continuous | CVD conditions, bronchitis, emphysema, arthritis, HIV, liver disease, kidney disease | Nicotine Alcohol Caffeine | NSAID Statin | Education | 5 |
| Danner et al., 2003 (Female) | USA | 3119 | 17–39 | 0.0%. | Serum | DIS-III | Categorical | Acute illness, chronic inflammatory illness, CVD conditions | Nicotine | Antidepressant Statin | Education | 5 |
| Danner et al., 2003 (Male) | USA | 2981 | 17–39 | 100.0%. | Serum | DIS-III | Categorical | Acute illness, chronic inflammatory illness, CVD conditions | Nicotine | Antidepressant Statin | Education | 5 |
| de Menezes et al., 2017 | Brazil | 14,010 | 51.0 | 45.7% | Serum | CIS-R | Categorical | CVD conditions, DM | Nicotine Alcohol | NSAID Antidepressant | Education | 5 |
| Douglas et al., 2004 (Female) | USA | 125 | 44.0 | 18.0%. | Serum | PHQ-9 | Continuous | CVD conditions | Nicotine Alcohol Caffeine | Statin | None | 2,3 |
| Douglas et al., 2004 (Male) | USA | 571 | 44.0 | 82.0%. | Serum | PHQ-9 | Continuous | CVD conditions | Nicotine Alcohol Caffeine | Statin | None | 2,3 |
| Einvik et al., 2011 | Norway | 267 | 48.0 | 56.0%. | Serum | SCID-IV | Categorical | CVD conditions, DM | Nicotine Caffeine | Statin | Education | 5 |
| Einvik et al., 2013 | Norway | 520 | 48.0 | 54.6%. | Serum | BDI-I | Continuous | CVD conditions, DM | Nicotine Caffeine | None | None | 2 |
| Elovainio et al., 2006 | Finland | 1201 | 31.5 | 40.6%. | Serum | BDI-I | Continuous | CVD conditions, DM, Rheumatoid disease | Nicotine Alcohol | None | SES Education | 2,4 |
| Elovainio et al., 2009 (Female) | Finland | 3257 | 53.0 | 0.0%. | Serum | BDI-I | Continuous | Chronic illness, metabolic syndrome | Nicotine Alcohol | None | Education | 2,4 |
| Elovainio et al., 2009 (Male) | Finland | 2748 | 51.5 | 100.0%. | Serum | BDI-I | Continuous | Chronic illness, metabolic syndrome | Nicotine Alcohol | None | Education | 2,4 |
| Eurelings et al., 2015 (Baseline) | Netherlands | 2047 | 74.2 | 39.3%.* | Serum | GDS-15 | Categorical | CVD conditions | Nicotine | None | Education | 2, 4 |
| Eurelings et al., 2015 (Year 2) | Netherlands | 899 | 74.2 | 39.3%.* | Serum | GDS-15 | Categorical | CVD conditions | Nicotine | None | Education | 2,4 |
| Eurelings et al., 2015 (Year 4) | Netherlands | 566 | 74.2 | 39.3%.* | Serum | GDS-15 | Categorical | CVD conditions | Nicotine | None | Education | 2,4 |
| Forti et al., 2010 (baseline) | Italy | 472 | 74.5 | 45.0%. | Serum | GDS-30 | Categorical | CVD conditions, DM, HTN, cancer, COPD | Caffeine | NSAID | Education | 5 |
| Forti et al., 2010 (Follow-up) | Italy | 312 | 74.5 | 45.0%.* | Serum | GDS-30 | Categorical | CVD conditions, DM, HTN, cancer, COPD | Caffeine | NSAID | Education | 5 |
| Frodi et al., 2012 | Ireland | 83 | 39.1 | 41.0%. | Plasma | HRSD | Categorical | Severe medical illness, head injury | Alcohol | None | None | 2 |
| Gambi et al., 2005 | Italy | 37 | 41.3 | 45.9%. | Serum | BDI-I | Continuous | CVD conditions, DM, Endocrine disorders | Alcohol Caffeine | NSAID Statin Antidepressant Statin | None | 2,3 |
| Glaus et al., 2014 (Atypical depression) | Switzerland | 3157 | 50.9 | 47.0%.* | Plasma | DIGS | Categorical | DM | Nicotine Alcohol Caffeine | SES | 5 | |
| Glaus et al., 2014 (Combination depression) | Switzerland | 3165 | 50.9 | 47.0%* | Plasma | DIGS | Categorical | DM | Nicotine Alcohol Caffeine | Antidepressant Statin | SES | 5 |
| Glaus et al., 2014 (Melancholic depression) | Switzerland | 3257 | 50.9 | 47.0%* | Plasma | DIGS | Categorical | DM | Nicotine Alcohol Caffeine | Antidepressant Statin | SES | 5 |
| Glaus et al., 2014 (unspecified depression) | Switzerland | 3317 | 50.9 | 47.0%* | Plasma | DIGS | Categorical | DM | Nicotine Alcohol Caffeine | Antidepressant Statin | SES | 5 |
| Häfner et al., 2011 (Female) | Germany | 700 | 50.4 | 0.0% | Serum | DEEX | Categorical | Chronic medical conditions, CVD conditions, DM, cancer | Nicotine Alcohol | None | None | 2 |
| Häfner et al., 2011 (Male) | Germany | 847 | 50.3 | 100% | Serum | DEEX | Categorical | Chronic medical conditions, CVD conditions, DM, cancer | Nicotine Alcohol | None | None | 2 |
| Haider et al., 2010 | USA | 868 | 44.8 | 50.2% | Plasma | CES-D | Continuous | CVD conditions, kidney disease, liver disease, cancer, DM | Nicotine Alcohol Caffeine | Antidepressant Statin | Education | 5 |
| Hickman et al., 2014 | USA | 1791 | 29.1 | 30.1% | Serum | CIDI | Categorical | CVD conditions, Kidney disease, emphysema, Rheumatoid arthritis, Liver condition, DM | Nicotine | Antidepressant | Education | 5 |
| Hughes et al., 2012 | Ireland | 78 | 39.5 | 42.3% | Plasma | HAM-D | Continuous | Severe medical illness, head injury | Nicotine Alcohol | Antidepressant | None | 2, 3 |
| Kéri et al., 2014 | Hungary | 80 | 23.2 | 35.0% | Blood (not specified) | SCID-IV, HAM-D |
Categorical | Conditions requiring medication | Nicotine Alcohol Caffeine | Antidepressant Statin |
SES Education | 5 |
| Lamers et al., 2013 (Melancholic Depression) | Netherlands | 654 | 40.2 | 34.2% | Plasma | CIDI | Categorical | CVD, DM | Nicotine Caffeine | Antidepressant Statin |
Education | 5 |
| Lamers et al., 2013 (Atypical Depression) | Netherlands | 665 | 39.6 | 21.5% | Plasma | CIDI | Categorical | CVD, DM | Nicotine Caffeine | Antidepressant Statin |
Education | 5 |
| Lehto et al., 2010 | Finland | 122 | 53.8 | 31.1% | Serum | SCID-IV, HAM-D | Categorical | CVD conditions, rheumatoid arthritis | Alcohol Caffeine | NSAID | None | 2, 3 |
| Liu et al., 2014 (Female) | USA | 5004 | 46.7 | 0.0% | Blood (not specified) | PHQ-9 | Categorical | Chronic illness, CVD conditions, cancer | N ic otine Alcohol | NSAID | SES Education | 5 |
| Liu et al., 2014 (Male) | USA | 5312 | 46.7 | 100.0% | Blood (not specified) | PHQ-9 | Categorical | Chronic illness, CVD conditions, cancer | Nicotine Alcohol | NSAID | SES Education | 5 |
| Meier et al., 2016 | USA | 110 | 32.8 | 32.0% | Serum | SCID | Categorical | CVD conditions, respiratory illness, endocrine disorders, neurological diseases, autoimmune diseases | Alcohol Caffeine | Antidepressant | None | 2, 3 |
| Milaneschi et al., 2009 | Italy | 991 | 75.0 | 44.1% | Serum | CES-D | Continuous | CVD conditions, HTN, DM, COPD, arthritis | Nicotine Alcohol Caffeine | NSAID Antidepressant | Education | 5 |
| Miller et al., 2002 | USA | 100 | 30.0 | 32.0% | Serum | DISH | Categorical | All medical conditions and acute infections | Nicotine | NSAID Antidepressant Statin | Education | 5 |
| Miller et al., 2003 | USA | 100 | 30.3 | 32.0% | Serum | HAM-D | Categorical | Acute illness, Chronic medical conditions | Nicotine | NSAID Antidepressant Statin | Education | 5 |
| Morris et al., 2011 (Caucasian Female) | USA | 166 | 52 | 0.0% | Plasma | BDI-II | Continuous | DM | Nicotine | None | Education | 2, 4 |
| Morris et al., 2011 (Black Female) | USA | 147 | 50 | 0.0% | Plasma | BDI-II | Continuous | DM | Nicotine | None | Education | 2, 4 |
| Morris et al., 2011 (Caucasian Male) | USA | 104 | 52 | 100.0% | Plasma | BDI-II | Continuous | DM | Nicotine | None | Education | 2,4 |
| Morris et al., 2011 (Black Male) | USA | 95 | 48 | 0.0% | Plasma | BDI-II | Continuous | DM | Nicotine | None | Education | 2,4 |
| Mwendwa et al., 2013 | USA | 198 | 45.6 | 48.0% | Serum | BDI-II | Continuous | Physical illness | None | None | Education | 4 |
| Naghashpour et al., 2011 | Iran | 98 | 37.0 | 0.0% | Serum | BDI-I | Categorical | CVD conditions, DM, HTN, allergies, asthma, cancer, polycystic ovarian syndrome | Caffeine | NSAID Antidepressant Statin | None | 2, 3 |
| Pan et al., 2008 | China | 3289 | 58.6 | 44.3% | Plasma | CES-D | Categorical | CVD conditions, Self-care disabilities, cancer, neurological disorders, AIDS | Nicotine Alcohol Caffeine | NSAID | SES Education | 5 |
| Panagiotakos et al., 2004 (Female) | Greece | 400 | 44 | 0.0% | Serum | ZDRS | Continuous | Hypercholesterolemia, DM | Nicotine Alcohol Caffeine | None | SES Education | 2,4 |
| Panagiotakos et al., 2004 (Male) | Greece | 453 | 45 | 100.0% | Serum | ZDRS | Continuous | Hypercholesterolemia, DM | Nicotine Alcohol Caffeine | None | SES Education | 2,4 |
| Penninx et al., 2003 | USA | 3024 | 73.6 | 48.5% | Serum | CES-D | Categorical | CVD conditions, DM, osteoarthritis, lung disease | Nicotine Alcohol | NSAID | None | 2, 3 |
| Piletz et al., 2009 | USA | 39 | 39.5 | 15.4% | Plasma | SCID-IV, HAM-D | Categorical | CVD conditions, DM, seizures, HTN, Acute infection | Nicotine Alcohol Caffeine | NSAID Antidepressant Statin | None | 2, 3 |
| Pizzi et al., 2008 | Italy | 415 | 57.6 | 51.7% | Blood (not specified) | BDI-II | Continuous | CVD conditions, DM, kidney failure, liver failure, neurological conditions, inflammatory disesae | Nicotine Caffeine | Antidepressant Statin | None | 2, 3 |
| Politi et al., 2008 | Italy | 50 | 53.4 | 47.0% | Blood (not specified) | Clinical Interview (DSM-IV) |
Categorical | CVD conditions, physical disorders, abnormal hematological, renal, liver function tests | Nicotine | None | None | 2 |
| Prohan et al., 2014 | Iran | 60 | 21.0 | 100.0% | Serum | BDI-II | Categorical | All medical disorders | Nicotine Caffeine | None | None | 2 |
| Ranjit et al., 2007 | USA | 6778 | 62.2 | 38.5% | Serum | CES-D | Categorical | DM | Nicotine Alcohol Caffeine | NSAID Statin | SES Education | 5 |
| Sarandol et al., 2006 | Turkey | 122 | 39.5 | 27.9% | Serum | HDRS | Categorical | Chronic illness via lab tests, acute infection | Nicotine Caffeine | NSAID Statin | None | 2, 3 |
| Savitz et al., 2015 | USA | 107 | 33.5 | 35.0% | Serum | SCID IV | Categorical | CVD conditions, respiratory illness, endocrine disorders, neurological diseases | Caffeine | NSAID Antidepressant | None | 2, 3 |
| Slopen et al., 2010 | USA | 177 | 57.9 | 44.6% | Serum | CES-D | Continuous | CVD conditions, DM, prior cancer | Nicotine Caffeine | Antidepressant Statin | Education | 5 |
| Smagula et al., 2014 | USA | 2560 | 76.4 | 100.0% | Serum | GDS-15 | Categorical | CVD conditions, DM, HTN, COPD, Parkinson’s disease, arthritis | Nicotine Alcohol Caffeine | NSAID Antidepressant | Education | 5 |
| Song et al., 2015 (Female) | Korea | 341 | 71.6 | 0.0% | Serum | CES-D | Continuous | CVD conditions, DM, HTN, metabolic syndrome, dyslipidemia, asthma, cancer, osteoporosis, hepatitis B, glaucoma, asthma, pulmonary tuberculosis | Nicotine Alcohol Caffeine | None | Education | 2,4 |
| Song et al., 2015 (Male) | Korea | 223 | 72.8 | 100.0% | Serum | CES-D | Continuous | CVD conditions, DM, HTN, metabolic syndrome, dyslipidemia, asthma, cancer, osteoporosis, hepatitis B, glaucoma, asthma, pulmonary tuberculosis | Nicotine Alcohol Caffeine | None | Education | 2,4 |
| Stewart et al., 2008 | USA | 316 | 60.6 | 50.9% | Serum | BDI-II | Continuous | Chronic medical disorders, high blood pressure | Nicotine Alcohol Caffeine | Statin | Education | 5 |
| Su et al., 2009 | USA | 188 | 55.0 | 100.0% | Plasma | BDI-II | Continuous | CVD conditions, HTN | Nicotine Caffeine | None | Education | 2, 4 |
| Suarez, 2004 | USA | 127 | 27.6 | 55.1% | Blood (not specified) | BDI-I | Continuous | CVD conditions, acute infection, DM, HTN, rheumatoid arthritis Asthma, Allergies, Chronic pain, Cancer | Nicotine Alcohol Caffeine | NSAID Antidepressant Statin | None | 2, 3 |
| Toker et al., 2005 (Female) | Israel | 630 | 45.2 | 0.0% | Serum | PHQ-9 | Continuous | CVD conditions, Inflammatory illness, rheumatic diseases, peripheral blood diseases, cancer | Nicotine Caffeine | NSAID Antidepressant Statin | None | 2, 3 |
| Toker et al., 2005 (Male) | Israel | 933 | 45.2 | 100.0% | Serum | PHQ-9 | Continuous | CVD conditions, Inflammatory illness, rheumatic diseases, peripheral blood diseases, cancer | Nicotine Caffeine | NSAID Antidepressant Statin | None | 2, 3 |
| Tully et al., 2016 (Persistent MDD) | Australia | 584 | 51.2 | 100.0% | Blood (not specified) | BDI-I | Categorical | CVD conditions, DM, osteoarthritis, OS A, rheumatoid arthritis | Caffeine | Statin | SES | 5 |
| Tully et al., 2016 (Remitted Depression+) | Australia | 553 | 51.7 | 100.0% | Blood (not specified) | BDI-I | Categorical | CVD conditions, DM, osteoarthritis, OS A, rheumatoid arthritis | Caffeine | Statin | SES | 5 |
| Ventorp et al., 2015 | Sweden | 38 | 36.8 | 45.6% | Plasma | Clinical Interview (DSM-IV) |
Categorical | CVD conditions, Somatic conditions | None | Antidepressant | None | 3 |
| Verduijn et al., 2015 (Female) | Netherlands | 864 | 41.8 | 0.0% | Plasma | IDS | Categorical | CVD conditions, DM, lung diseases, rheumatic diseases, cancer, ulcer, intestinal problems, liver disease, epilepsy, thyroid problems | Nicotine Alcohol Caffeine | NSAID Antidepressant |
Education | 5 |
| Verduijn et al., 2015 (Male) | Netherlands | 447 | 41.8 | 100.0% | Plasma | CIDI | Categorical | CVD conditions, DM, lung diseases, arthritis, cancer, ulcer, intestinal problems, liver disease, epilepsy, thyroid gland disease | Nicotine Alcohol Caffeine | NSAID Antidepressant |
Education | 5 |
| Vogelzangs et al., 2012 | Netherlands | 1626 | 41.8 | 33.0% | Plasma | CIDI | Categorical | CVD conditions, DM, lung diseases, arthritis, cancer, ulcer, intestinal problems, liver disease, epilepsy, thyroid gland disease | Nicotine Alcohol | NSAID Antidepressant Statin |
Education | 5 |
| Zeman et al., 2009 | Czech Republic |
76 | 58.5 | 0.0% | Blood (not specified) | HAM-D | Categorical | CVD conditions, DM, renal disease, hypothyroidism, malignancies, macroalbuminuria | Nicotine Alcohol Caffeine | None | SES Education | 2 |
| Zoga et al., 2014 | Greece | 80 | 51.7 | 0.0% | Serum | SCID, HDRS | Categorical | Chronic immune illness, acute infection, allergic reactions, neurological disorders | Caffeine | NSAID Statin | None | 2, 3 |
A total of 58 studies, with k = 78 analyses, were included in the meta-analysis.
% male given is for N of entire study rather than the subgroup. If the mean age was not provided for stratified results (e.g., sex), the mean age of the entire sample is provided.
Confounders were considered accounted for if they were part of exclusion criteria, controlled for statistically, or in the case of substances and medications, were controlled through abstinence. Statin use also includes control for additional anti-hypertensive medications.
in the case of Tully et al. (2016), the analysis included was a cross-sectional of individuals with depression which later remitted. The analysis included represents the association between GRP and depression prior to remission.
All studies in this table were included in Stage One meta-analysis. Inclusion in Stages Two-Four was a prerequisite for inclusion in Stage Five.
Depression Measure Acronyms Self-Report: GDS: Geriatric Depression Scale; HADS: Hospital Anxiety and Depression Scale; CESD-R: Center for Epidemiologic Studies Depression Scale-Revised; IDS-R: Inventory for Depressive Symptomatology-Revised; PHQ-9: Patient Health Questionnaire; BDI: Beck Depression Inventory; DEEX: DEpression and Exhaustion subscale; ZDRS: Zung Depression Rating Scale.
Depression Measure Acronyms Clinician Scales: DIS: Diagnostic Interview Schedule; CIS-R: Clinical Interview Schedule-Revised; SGID: Structured Clinical Interview Diagnostic; HRSD/HAM-D: Hamilton Rating Scale for Depression; DIGS: Diagnostic Interview for Genetic Studies; CIDI: Composite International Diagnostic Interview; DISH: Depression Interview and Structured Hamilton.
Physical Condition Acronyms: CVD: Cardiovascular Disease, DM: Diabetes Mellitus, MS: Multiple Sclerosis, HTN: Hypertension, HIV: human immunodeficiency virus, AIDS: acquired immunodeficiency syndrome. COPD; Chronic obstructive pulmonary disease, OSA: obstructive sleep apnea.
Table 2.
Meta-analysis key statistics.
| Stage | Combined—All Studies | Continuous Predictors | Categorical Predictors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | Q | r | SE | 95% CI | k | Q | r | SE | 95% CI | k | Q | r | SE | 95% CI | |
| 1 | 78 | 976.56*** | 0.07*** | 0.01 | 0.04–0.09 | 30 | 212.23*** | 0.09** | 0.03 | 0.04–0.14 | 48 | 760.88*** | 0.05*** | 0.01 | 0.02–0.08 |
| 2 | 76 | 975.29*** | 0.07*** | 0.01 | 0.04–0.10 | 29 | 210.76*** | 0.10** | 0.03 | 0.04–0.15 | 47 | 760.74*** | 0.05*** | 0.01 | 0.02–0.08 |
| 3 | 53 | 825.07*** | 0.07*** | 0.01 | 0.04–0.10 | 16 | 119.62*** | 0.10** | 0.04 | 0.02–0.18 | 37 | 705.13*** | 0.06*** | 0.02 | 0.03–0.09 |
| 4 | 52 | 795.46*** | 0.05*** | 0.01 | 0.02–0.08 | 21 | 95.11*** | 0.06* | 0.02 | 0.01–0.11 | 31 | 699.53*** | 0.04* | 0.02 | 0.01–0.07 |
| 5 | 35 | 684.17*** | 0.05** | 0.01 | 0.02–0.08 | 8 | 8.50 | 0.03 | 0.01 | 0–0.05 | 27 | 675.47*** | 0.05** | 0.02 | 0.02–0.09 |
···k represents the number of unique analyses per stage.
p < .05.
p < .01.
p < .001.
9.3.1. Stage one (Appropriate data transformation and statistical adjustment for age, sex, BMI/waist circumference/waist-to-hip ratio, and chronic medical conditions)
A total of n = 58 articles, with 78 independent analyses, were included in Stage One (Fig. 3a; Table 2). Seventy-five percent of the analyses (k = 78) included in the Stage One analysis reported a positive effect size between CRP and depression (Fig. 2a). Out of the 78 analyses, 27% reported a statistically significant relationship between depression and CRP. As indicated in Table 2, the aggregated correlation coefficient was small, yet highly significant (r = 0.07, SE = 0.01, 95% CI = 0.04–0.09, p < .001) and demonstrated high heterogeneity (Q (df = 77) = 976.65, p < .001, I2 = 93.98%). The funnel plot was asymmetric and suggests considerable publication bias with an overrepresentation of positive effect sizes reported (Supplemental Fig. 1a).
9.3.2. Stage two (Stage one articles and control for at least one of the following: nicotine, alcohol, or caffeine)
Stage Two included 57 articles (Fig. 3b), with k = 76 analyses, and the effect size was identical (r = 0.07, SE = 0.01; Table 2). Further, the heterogeneity was comparable (Q(df = 76) = 975.29, p < .001, I2 = 94.19%). Out of the 76 analyses, 25% reported a statistically significant relationship between depression and CRP.
Fig. 3b.

Studies in Stage Two Meta-Analysis. Sample size included to the left of the effect size. Fisher’s z transformed correlation coefficient and 95% Confidence Interval.
9.3.3. Stage three (Stage one articles and control for at least one of the following: antidepressant, NSAID, and statin/anti-hypertensive)
Stage Three included 43 articles with k = 53 analyses (Fig. 3c). The aggregated effect size estimate was r = 0.07, SE = 0.01 (Table 2). The heterogeneity decreased, yet remained highly significant (Q (df = 53) = 825.07, p < .001, I2 = 94.36%). Out of the 53 analyses, 26% reported a statistically significant relationship between depression and CRP. Additional results comparing effect sizes between studies that controlled for antidepressant medication versus excluding for antidepressant medication at Stage 5 can be found in Supplemental Material.
Fig. 3c.
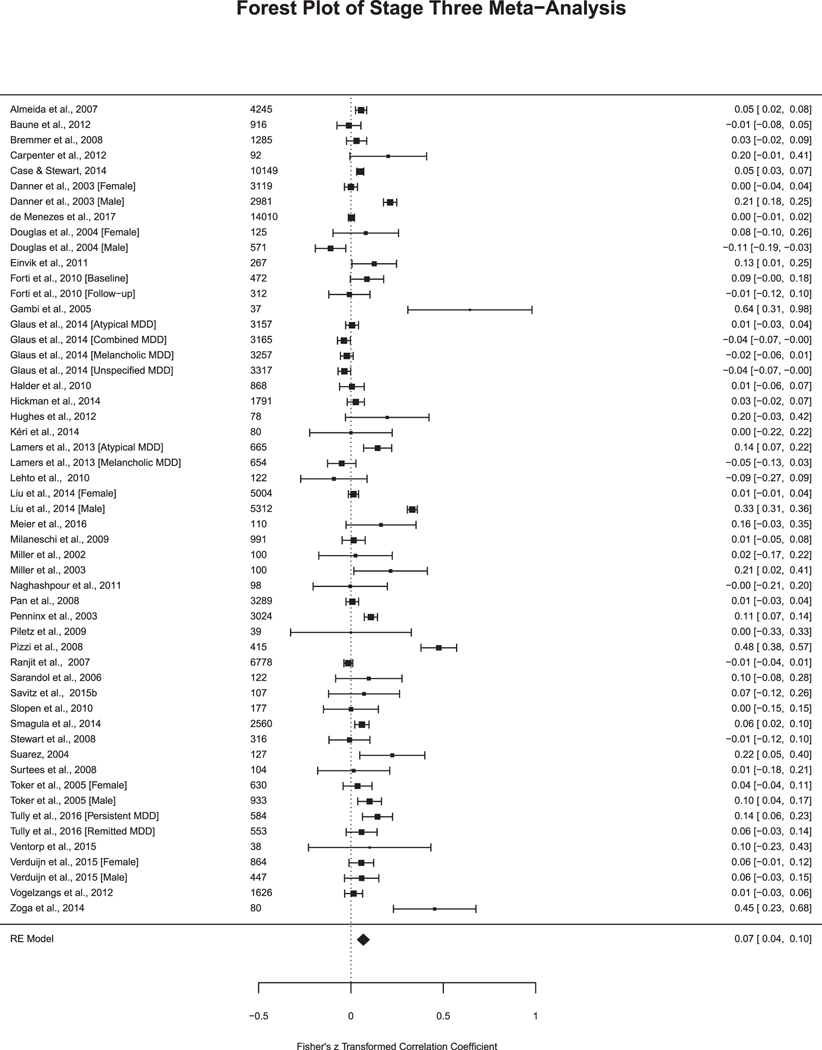
Studies in Stage Three Meta-Analysis. Sample size included to the left of the effect size. Fisher’s z transformed correlation coefficient and 95% Confidence Interval.
9.3.4. Stage four (Stage one articles and control for at least one of the following: SES or education)
Notably, in Stage Four (n = 35 articles, k = 52 analyses; Fig. 3d), the effect size reduced, yet remained significant (r = 0.05, SE = 0.01, 95% = 0.02–0.08, p < .001) as did the heterogeneity (Q (df = 51) = 795.46, p < .001, I2 = 93.69%) (Table 2). Out of the 52 analyses, 21% reported a statistically significant relationship between depression and CRP.
Fig. 3d.
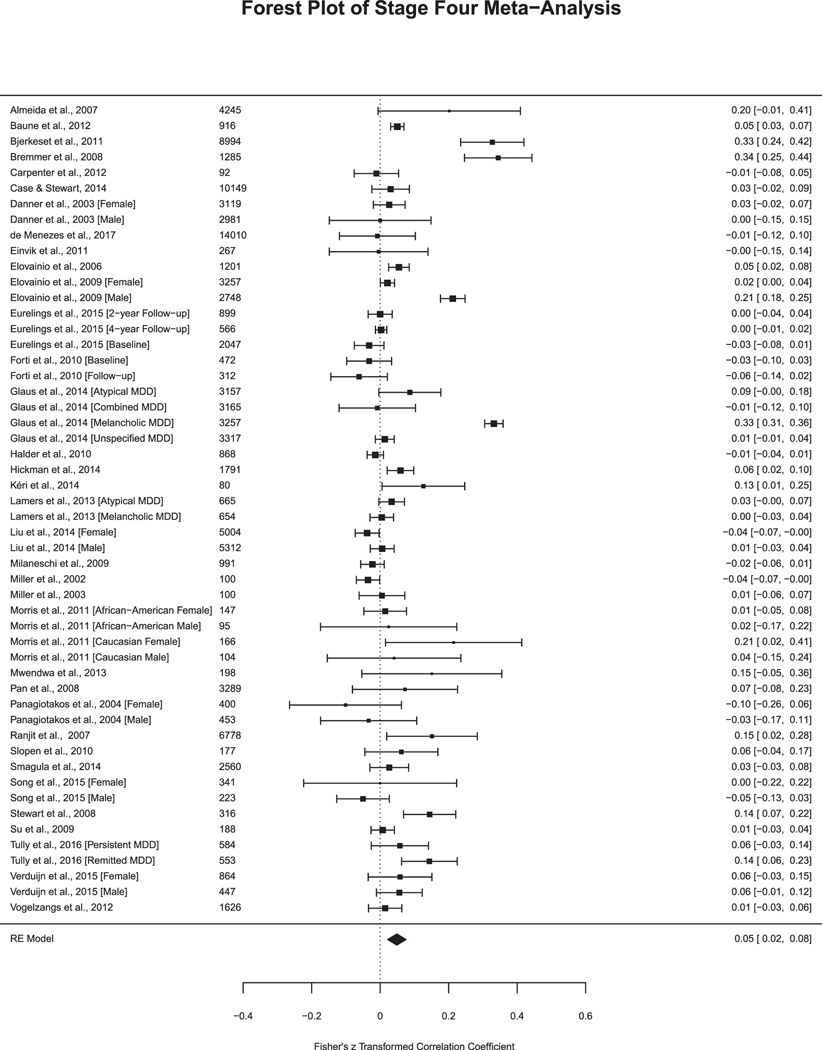
Studies in Stage Four Meta-Analysis. Sample size included to the left of the effect size. Fisher’s z transformed correlation coefficient and 95% Confidence Interval.
9.3.5. Stage five (Met for all of the above stages)
In Stage Five (n = 26 articles, k = 35 analyses; Fig. 3e), the effect size remained significant at r = 0.05, SE = 0.01, 95% CI = 0.02–0.08, p < .01. The heterogeneity reduced, but remained high (Q (df = 34) = 684.17, p < .001, I2 = 93.32%) (Table 2). Out of the 35 analyses, 23% of the studies reported a statistically significant relationship between depression and CRP. Additional results comparing effect sizes between studies that controlled for antidepressant medication versus excluding for antidepressant medication at Stage 5 can be found in Supplemental Material.
9.3.6. Comparison of studies employing continuous versus categorical predictors
Within the 78 separate analyses, 61.5% used depression as a categorical predictor (k = 48). Out of the analyses using a categorical definition of depression, 26 analyses (54%) used a clinical interview to diagnose depression while the remaining 22 analyses used a scale with a cut-off score for probable depression (19 self-report, 3 clinician-administered scale). For studies utilizing a categorical predictor of depression, the effect size was significant and ranged from r = 0.04–0.06 depending on the stage (Table 2). The effect size was relatively consistent across the stages with the lowest effect size observed at Stage 4. The heterogeneity was highly significant across the stages, but lowest at Stage 5 (Table 2). Within studies employing categorical predictors, the type of depression assessment (i.e., self-report versus clinician diagnosis) was not a significant moderator at any stage (p > .3).
In comparison, the remaining 30 analyses included statistical models with a continuous predictor of depression. The effect size ranged from r = 0.03–0.10 depending on the stage. In Stages 1–3, the effect size was highest (Stage 1: r = 0.09, SE = 0.03; Stage 2: r = 0.10, SE = 0.03, Stage 3: r = 0.10, SE = 0.04). The effect size dropped to r = 0.06, SE = 0.02 at Stage Four and further dropped to r = 0.03, SE = 0.01 at Stage 5. The effect size was significant at all stages, except Stage 5. Notably, at Stage 5, there were only k = 8 analyses included. Considerable heterogeneity was observed at all stages, except Stage 5 (Table 2).
9.3.7. Quality score
Given the significant heterogeneity observed at Stage 5, a quality score was entered as a continuous moderator. The quality score ranged continuously from 4 to 11 and accounted for the number of confounding variables accounted for from Stages 2–4 and additional significant methodological considerations. Fig. 4 contains a violin plot to illustrate the distribution of effect sizes separated by quality score for Stage Five analyses. Results of the moderation analysis indicate that part of the heterogeneity in the true effects is related to the moderator (QM(df = 1) = 8.17, p < .01) with approximately 20% of the variance in CRP due to the interaction between depression and the quality score (r = −0.02, p < .01). For illustrative purposes (Fig. 4), in a post-hoc analysis, the Stage 5 analyses were separated into “higher quality scores” (above the median, scores 9–11, k =16 analyses) and “lower quality scores” (equal to/below the median, scores 4–8, k = 19 analyses) to examine how low versus high quality influenced the results. In the analyses with lower quality scores, considerable heterogeneity remained (Q(df = 18) = 446.5, p < .001, I2 = 95.97%) and the aggregated effect size was small, yet significant (r = 0.08, SE = 0.02, 95% CI: 0.04–0.13, p < .001). In comparison, in analyses with a higher quality score, the heterogeneity was notably reduced (Q (df = 15) = 45.27, p < .001, I2 = 66.87%) and the effect size diminished greatly and became non-significant (r = 0.005, SE = 0.009, 95% CI: −0.01–0.02, p = .55). To note, the quality score was a significant or trending moderator at all stages, except Stage 4 (Stage 1: QM (df = 1) = 2.76, p = .09); Stage 2: QM(df = 1) = 3.29, p = .07; Stage 3: QM(df =1) = 6.83, p = .01); Stage 4: QM(df =1) = 0.30; p = .58).
Fig. 4.
Stage Five effect sizes by quality score.
10. Discussion
To date, this is the first systematic review and meta-analysis focused specifically on the methodological processes utilized and their influence on the relationship between depression and CRP. Results from the systematic review and meta-analysis illustrate the current state of the field, identifying both positive strides made in the past three decades as well as inconsistencies that may hinder the validity of replication and reproducibility efforts. First, we will review the methodological issues (i.e., sample collection and data transformation) identified. Second, we will discuss results pertaining to confounding variables in the systematic review and the corresponding meta-analysis stages. Lastly, we will present recommendations for future studies examining CRP and depression that will promote valid replicability.
10.1. Methodological issues
10.1.1. Sample collection procedures
The lack of consistent protocols around the collection and assaying of samples for CRP is an issue whose importance cannot be understated. The systematic review revealed that there is significant heterogeneity in the sample collection and assaying procedures. Specifically, most studies did not specify any procedure, while others highlighted that patients were in the supine position or resting for 5–45 min prior to the collection. Further, while roughly 40% of the studies reported that participants were fasting, protocols ranged in time from 5 to 12 h fasting with other protocols specifying “overnight” fasting, while still others allowed some food groups. Lastly, reporting of coefficient of variation ranges, assay detection sensitivity, and reporting assay duplicate varied highly across studies with most not providing sufficient information, limiting our ability to compare these protocols across studies. Greater sample collection and assay heterogeneity inherently leads to more noise when comparing CRP findings across studies and impedes progress in the field.
10.1.2. Data cleaning and statistical methods
Another related issue was the variability in the handling of data and statistical transformations. The lack of transparency around the handling of non-detectable values of CRP makes it unclear what proportion of data are missing from the final dataset. Even in those studies that did report handling of non-detectable values, the techniques varied. Some replaced those values with random numbers, zeroes, or half the detection limit. Most others excluded non-detectable values listwise, which has implications for introducing bias (if data is not missing at random) or reducing power. Equally variable was the treatment of high-value outliers. Nearly ¾ of the studies in the systematic review did not provide critical details on how high-value outliers were handled. Out of the studies that did report these procedures, the most common approach was to exclude CRP values over 10 mg/L, as this may be indicative of an acute infection (Pearson et al., 2003). However, the exclusion of values over 10 mg/L may reduce important variability in the sample as other factors (e.g., BMI, SES, alcohol use) can also result in elevated CRP levels. However, only 4% of the studies in the systematic review ran their analyses with and without outliers to examine if the relationship remained similar. Obviously, these inconsistencies will contribute to lack of replicability.
Furthermore, most studies either transformed non-normal data or reported that data was normal (and corresponding normality statistics) and used parametric statistics, or they left non-normal data raw and conducted non-parametric statistics. These were also required in order to reach Stage One of the meta-analysis. However, 17.6% of studies in the systematic review conducted parametric tests without providing normality statistics or provided insufficient information about the type of statistical test employed. Correct reporting and handling of nonnormal data is another recommendation that will allow for more consistency and therefore vertical progress in the field.
10.2. Confounding variables
10.2.1. Stage one confounders
The importance of proper assessment and control of relevant confounding variables is paramount. A wide range of variables that affect both CRP and depression has been identified in the literature; however, the adoption of those guidelines is mixed. The confounding variables included as criteria in Stage One of the meta-analysis (age, sex, BMI/waist circumference/waist-to-hip ratio, and medical conditions) have the most support in the research (Hamer et al., 2011; Harris et al., 1999; Khera et al., 2005, 2009; Smith et al., 2014). Encouragingly, these variables were among the most consistent variables statistically accounted for in the systematic review. Specifically, the majority of studies accounted for age, sex, and medical health conditions (83–87%), while BMI, waist circumference, or waist-to-hip ratio was statistically controlled for in 65% of the studies. However, significant inconsistencies were observed that introduce substantial heterogeneity in the studies to date and could deter replication efforts in the future. For example, while most studies accounted for chronic medical conditions, the number and type of conditions controlled or excluded varied widely, from 1 condition up to 39 health conditions or more. Further, 40% of the studies did not provide explicit information or details on which conditions were accounted for in their models. For example, some studies excluded or adjusted for “any significant medical disorders” or “disorders known to affect inflammatory functioning.” The participants in studies exploring CRP and depression therefore could be very inconsistent with respect to the types of medical disorders that are excluded (and therefore included), limiting generalizability. Clear inclusion and exclusion criteria are critical in future replication efforts.
Obesity is increasingly identified as among the most robust confounders affecting depression and CRP (Hamer et al., 2011, 2009; O’Connor et al., 2009; Shelton et al., 2015); yet, one-third of studies did not properly assess or control for BMI, waist circumference, or waist-to-hip ratio. Notably, nearly half of the studies that did not account for these obesity indices were published in 2011 or later, two years following Howren et al.’s (2009) meta-analysis and O’Connor et al.’s (2009) recommended guidelines. Given the strength of the role of obesity, it is a potentially significant problem that so many published studies did not include BMI, waist circumference, or waist-to-hip ratio in their analysis.
In the current meta-analysis, even when controlling for these well-established covariates, the association between CRP and depression remained significant. However, the aggregated effect size of r = 0.07 was quite small. Results suggest that while age, sex, obesity indices, and medical conditions may attenuate the relationship between depression and CRP, they do not fully account for the association. Very high rates of heterogeneity were observed at this stage, as well as considerable publication bias, suggesting caution regarding this finding (see Table 2; Supplemental Fig. 1a).
10.2.2. Stage two confounders
It has been recommended that researchers assess and adjust accordingly for nicotine, alcohol, and caffeine (O’Connor et al., 2009). Nicotine use was controlled for in 68% of the studies, comparable to the proportion of studies adjusting for BMI, waist circumference, or waist-to-hip ratio. However, over half of the studies that adjusted for nicotine use only accounted for current nicotine use. This is troubling given that nicotine use in both current and former smokers has been linked to heightened CRP levels (Bazzano et al., 2003; Hastie et al., 2008). In contrast, alcohol and caffeine use were assessed less frequently, with over half of the studies in the systematic review not adjusting for these substances. As both substances have been linked to depression (Grant et al., 2004; Lucas et al., 2011) and CRP (Bell et al., 2017; Swirski and Nahrendorf, 2017), it is discouraging that so few studies included them in their analyses.
In the meta-analysis, only one article was dropped from Stage One to Stage Two. This suggests that in studies adjusting for age, sex, obesity, and medical conditions, substances are also generally evaluated and controlled for accordingly. Not surprisingly, the heterogeneity from Stage One to Stage Two reduced only minimally and the effect size remained constant (r = 0.07), suggesting that statistical adjustment for substances does not appear to account for the association between depression and CRP or significantly reduce the heterogenenity.
10.2.3. Stage three confounders
Assessment and control for medication usage was also inconsistent. Antidepressants, commonly prescribed for both depressive disorders and other health conditions (Pratt et al., 2011; Simon et al., 2014) may modulate inflammatory functioning (Lanquillon et al., 2000; O’Brien et al., 2006; Tuglu et al., 2003; Uher et al., 2014). Yet, over half of the studies in the systematic review (57%) did not adjust for antidepressant use. Interestingly, near equal number of studies excluded or controlled for antidepressant use. Excluding individuals who use antidepressant medication may eliminate more severely symptomatic patients and limit the generalizability of the findings. NSAIDs and statins/antihypertensives, were each only controlled for in one-third of the studies. Both medications are prescribed at even higher rates in individuals with depression and have been linked to lowered CRP levels in individuals with a physical health condition (Di Napoli and Papa, 2003; Ikonomidis et al., 1999; Joynt et al., 2004; Palmas et al., 2007; Solheim et al., 2003).
Comparable to Howren, control for medication usage did not appear to significantly alter the effect size (Howren et al., 2009). In the metaanalysis, Stage Three saw a significant drop of the number of studies from n = 58 in Stage One to n = 43 in Stage Three, yet, the level of heterogeneity was still relatively high. Only 14 studies with continuous predictors controlled for one of the three medications, highlighting that this recommendation is rarely considered in community-based studies or studies examining a range of depressive symptoms. It is possible that studies that did not use a formal diagnosis of depressed versus nondepressed may have considered the prevalence of medication use to be neglible. However, antidepressants, NSAIDs, and statin/antihypertensive medications are frequently prescribed for other conditions. Future research that properly assesses and accounts for medication use in community-based samples will further elucidate the potential role of medication use on the relationship between CRP and depression.
10.2.4. Stage four confounders
Only 20% of studies assessed and adjusted for SES in their models, though 34% of studies adjusted for education. Given the stable association between low SES/education and higher levels of CRP and rates of depression (Belle and Doucet, 2003; Obinwa et al., 2016), it is alarming that so few studies controlled for SES or education. Results from Stage Four of the meta-analysis revealed a significant drop in the number of studies that properly adjusted for SES or education. Interestingly, the effect size also decreased at this Stage, both across studies and within categorical and continuous predictors (Table 2). In studies utilizing a continuous predictor, the effect size only changed significantly at Stage Four, when it dropped from r = 0.09 at Stage One to r = 0.06 at Stage Four (Table 2). The change in effect size was less drastic in studies with a categorical predictor (Stage One, r= 0.05, Stage Four, r = 0.04). While preliminary, these findings highlight that psychosocial factors, particularly SES/education, may have a stronger influence on the relationship between depression and CRP and merit additional attention. To extend these findings, a future direction in the field could utilize structural equation modeling approaches to create psychosocial profiles to combine predictors that are the highest risk factors for depression and dysregulated immune functioning.
The current systematic review elucidates that race and ethnicity have received far too little attention in the study of the association between CRP and depressive symptoms, which is particularly troublesome given that cultural variables have historically been considered nuisance variables (Hall et al., 2016; Sue, 1999) that receive passing or even hostile attention by researchers (Neville and Carter, 2005). Notably, 75% of the studies in the systematic review did not report basic descriptive participant data, such as demographic and ethnicity/race data, and for those that did, minority populations made up such a small aspect of their sample size that there is likely not enough power in order to detect robust, race/ethnicity specific, and generalizable results. It has been established that race and ethnicity influence outcomes in psychological science, specifically due to their relevance for scientific reproducibility (Collaboration, 2012; Van Bavel et al., 2016), making their omission from ¾ of studies all the more worrisome. One study indicated that they collected variables on race, but then removed all African-Americans from their sample to reduce participant heterogeneity in CRP (Halder et al., 2010), which indicates that race differences may moderate the association between inflammation and depression. Prior research has demonstrated that African-American race is associated with elevated CRP levels (Kelley-Hedgepeth et al., 2008; Matthews et al., 2005; McDade et al., 2006; Morimoto et al., 2014). Indeed, out of the 24 studies that controlled for race, 8 studies or one-third found race to be a significant covariate influencing CRP levels.
10.2.5. Stage five meta-analysis
In Stage Five of the meta-analysis, which included studies that had controlled for age, sex, BMI/waist circumference/waist-to-hip ratio, medical conditions and at least one substance, medication, and psychosocial factor, the effect size was further attenuated from r = 0.06 to r = 0.05, though it remained significant. Of note, while the heterogeneity decreased across the stages, it remained highly significant in Stage Five, indicating that even with proper control of these variables, other factors are still likely influencing the association between depression and CRP. These findings suggest that even when studies adhered to higher degree of methodological integrity, results should be interpreted carefully as additional factors are likely at play in evaluating the association between CRP and depression.
A quality score was entered as a continuous moderator at Stage Five, to explore if other important factors, including the methodological issues discussed previously, may reduce heterogeneity, thus getting closer to understanding the strength of the direct association between CRP and depression. The quality score was conceived a priori and weighted studies that accounted for more of the variables from Stages 2–4 and also met other recommended guidelines (e.g., proper handling of outliers and nondetect values, assessment of acute illness). Results indicated that the quality score was a significant moderator in that within studies that had a quality score above the median, (> 8) the effect size diminished (r = 0.005), becoming non-significant (see Fig. 4). In comparison, the studies with quality scores at or below the median (≤8), had a significant effect size (r = 0.08) with higher rates of heterogeneity. Fig. 4 illustrates that studies in the “high quality” group were normally distributed, with significantly lower levels of heterogeneity (Q(df = 15) = 45.27,I2 = 67%), and centered around 0. In comparison, the “low quality” studies were skewed, with significant heterogenenity (Q(df = 18) = 446.5, I2 = 96%) (Fig. 4). Of note, the quality score was significant or trending across all stages, except Stage 4. These compelling results indicate that in studies that adhered to the highest number of recommendations in the field, the association between depression and CRP attenuated to near zero and was not statistically significant. However, given the small number of analyses included in Stage Five, and the smaller group with a quality score above 8 (n = 13 studies, k = 16 analyses), the results should be interpreted with caution.
It is important to note that the inclusion of multiple individual confounders, even when each contributes a low percent of variability, will cumulatively reduce the effect size, and significance, of the CRP-depression relationship. However, at all stages, even when adjusting for several covariates, the association between CRP and depression remained significant, albeit small. It was only when a quality score that also accounted for data practices and methodological rigor was incorporated into Stage 5 that the effect size diminished to non-significance. Additionally, results from this meta-analysis further illustrate that CRP, while a useful and readily available general marker of systemic inflammation, may be affected by other factors and confounders that contribute to systemic inflammatory process. For example, there are other specific immune markers that directly act on the brain and thus affect behavior and depression (as reviewed in, Dantzer et al., 2008). It should be noted that such markers, or studies that calculate composite scores of several inflammatory markers, may be less sensitive to certain confounders. It will be important for future research to examine and report on these confounders with other immune markers or composite scores to help elucidate such associations. Thus, we may not anticipate that CRP would robustly correlate with behavior once con-founders and other methodological factors are accounted for. More studies need to be conducted that follow this level of methodological rigor in order to elucidate the strength and accuracy of the association between CRP and depression that is currently accepted in the field.
10.2.6. Effect size across stages
The effect size between depression and CRP remained stable across Stages One-Three, attenuating further at Stages Four and Five (Table 2). However, the effect size, particularly in Stage Five, is quite small, yet, highly significant across the stages until the quality score was introduced. These findings indicate that the effect size between CRP and depression may be inflated in studies that do not adhere to higher methodological standards, or even that depression may not be the most salient predictor of inflammation over and above the other confounding factors. Results do not suggest that any specific group of confounding variables is uniquely moderating the relationship between CRP and depression, but rather, that the control variables, when combined, may weaken the association.
Interesting patterns in the effect sizes across the Stages emerged between continuous and categorical predictors. In studies utilizing categorical predictors, the effect size remained relatively stable across the Stages, ranging from r = 0.04–0.06. Of the studies that categorized depression, 45% used a validated self-report measure with a cut-off indicative of probable depression while 55% of studies compared groups with diagnosed depression versus healthy controls free of any psychological disorder. Wide variability was noted in how the control groups were defined for studies that used a cut-off score for probable depression. Several studies dichotomized the sample with the depression group meeting the “depression” cut-off score and any participants with scores lower than that score were defined as “no or mild depression” (e.g., Almeida et al., 2007; Eurelings et al., 2015; Forti et al., 2010; Naghashpour et al., 2011; Ranjit et al., 2007), while others designated a score for probable depression and a score for no evidence of depression (Liu et al., 2014; Prohan et al., 2014). Other studies did not explicitly define their comparison group, labeling them as “no depression” without providing a mean depression score or any indication of how that group was defined (Almeida et al., 2009; Pan et al., 2008; Penninx et al., 2003; Smagula et al., 2014). While self-report continuous measures (e.g., the BDI) often have acceptable validity and reliability, they are not intended to be measures of a depression diagnosis. Rather, they are helpful tools for screening for possible depressive disorders and measures of symptom levels, particularly in large, population-based studies. It must be noted that symptom scales do not take into account key elements required for valid diagnosis, such as duration of illness, level of impairment, or comorbidity. However, given the limitations of dichotomizing a continuous scale, these studies are better conceptualized as investigations of distinct constructs that may reflect distinct psychobiological phenomena. Future research should report results from analysis of the continuous result.
Studies that compared individuals with a depression diagnosis versus healthy controls have typically yielded higher effect sizes (Haapakoski et al., 2015), however, this meta-analysis did not explicitly compare effect sizes in clinical versus community samples that were categorized. The studies with categorical predictors had lower and more consistent effect sizes across the stages (Table 2). This lower effect size may have resulted from some of these studies including individuals with subsyndromal symptoms of depression in their control group, thereby increasing the within group variances in their non-depressed samples. As for the greater consistency of effect sizes across the stages, it is not immediately obvious why this would particularly be the case with the studies that defined depression categorically, although it may be that those studies that used matched case-control designs also indirectly matched their samples more closely on variables that were unmeasured and/or not explicitly controlled, meaning that the introduction of explicit controls had less impact than it might in correlational designs.
In the studies with continuous predictors, the effect size was larger in Stages One-Three compared to categorical predictors (Table 2), diminishing significantly at Stages Four and Five. In Stage Five for the continuous predictors, the heterogeneity lessened greatly and became non-significant and the effect size attenuated to r = 0.03 and was also no longer significant (Table 2). However, only 8 studies qualified for Stage Five with continuous predictors, demonstrating that very few studies using depression as a continuous predictor are following recommended guidelines from the field. Results do indicate that the strength of the relationship between depression and CRP may be significantly smaller, and potentially non-significant, in these cases but given the small number of studies, this result is preliminary. Future research is required to build upon these early findings and elucidate how continuous measures of depression may relate to CRP, particularly in population and community based samples.
10.2.7. Defining depression
As defined by the DSM, MDD is a highly heterogenous disorder. Using DSM-5 criteria, there are over 200 unique symptom profiles for MDD (Fried and Randolph, 2015). More research is now being conducted to identify subtypes of depression, which will enhance precision in diagnosis and treatment. It is possible that there is an “inflammatory depression” subtype. In fact, several ongoing clinical trials of immunomodulators for depression use indicators of low-grade inflammation (e.g., CRP) as an inclusion criterion (e.g., clinical trial NCT02363738).
However, very little research thus far has examined how discreet depressive symptoms, or subtypes of depression, are related to inflammation, with notable exceptions (e,g., Duivis et al., 2013; Glaus et al., 2014; Jokela et al., 2016; Lamers et al., 2013; White et al., 2017). In this meta-analysis, only two studies met criteria and stratified results by subtype of depression (Glaus et al., 2014; Lamers et al., 2013). Lamers and colleagues found a higher and significant association only between atypical depression and CRP (Lamers et al., 2013) while Glaus and colleagues reported that “unspecified depression” (i.e., neither melancholic nor atypical) was significantly associated with CRP, while neither atypical nor melancholic presentations alone were significantly correlated to CRP (Glaus et al., 2014). Jokela and colleagues compiled data from three large cross-sectional studies and found that depressive symptoms mimicking sickness behaviors, including fatigue, reduced appetite, withdrawal, and inhibited motivation, were most related to CRP (Jokela et al., 2016). While this study did not adjust for several important confounders (e.g., BMI), it is an important first step to investigating if there is an “inflammatory subtype” of depression, which may explain the great deal of heterogeneity and inconsistency observed in the field thus far.
As bipolar disorder also includes depressive episodes, further research is also needed to explore differences in immunological systems between unipolar and bipolar depressive disorders. A meta-analysis of 30 studies of bipolar depression has provided preliminary evidence for significant elevation of inflammatory cytokines in bipolar disorder (Modabbernia et al., 2013). However, fewer studies have explicitly contrasted inflammatory dysregulations between unipolar and bipolar depressive disorders. Bai and colleagues documented higher levels of CRP, and other inflammatory cytokines, in bipolar disorder compared to unipolar depression (Bai et al., 2015), highlighting that this is an important area of research.
Results from the present study contribute to a shared consensus on methodological practices that will promote replicability and rigor and complement these preliminary investigations to challenge the prevailing view in the field that CRP is strongly and independently associated with case level MDD as defined by the DSM. In addition to depression symptom profiles, other potential moderators, such as disease course and chronicity (e.g., number of episodes), severity of illness, and treatment outcome will be very useful in not only understanding the pathophysiology of depression but in setting up future clinical trials. Future research should include information about discreet depressive symptoms and make hypotheses based on symptom profiles (such as those based on sickness behaviors as in the study by Jokela and colleagues) and treatment outcomes.
Overall, results from the meta-analysis stages and systematic review illustrate widespread inconsistency in the adoption of recommended guidelines for control variables (O’Connor et al., 2009), calling into doubt the nature and strength of reported associations between depression and CRP. The control variables have unique and complex relationships both with depressive pathology and inflammatory systems. However, a very small proportion of studies considered variables from each domain discussed (demographic, substance, medication, and psychosocial). While over 85% of studies may have included age and sex as control covariates, far fewer considered the many other variables known to affect depression and CRP. Only 31% of studies met the criteria for Stage One of the meta-analysis, indicating that they utilized proper statistical methods and adjusted appropriately for age, sex, BMI/waist circumference/waist-to-hip ratio, and chronic medical conditions. Further, only 13.5% of studies from the systematic review met for Stage Five, highlighting that only a small proportion of the work completed in the field so far has followed recommended guidelines.
10.3. Recommendations
Table 3 includes a summary of recommendations for the field, described in detail below.
Table 3.
Summary recommendations for future replication efforts.
|
Sample Collection Procedures Sample collected after resting period of 20–45 min with participant in supine position Sample collected following overnight fast Rule out acute infection via collection of body temperature and vital signs* |
|
Assaying Procedures Run all assays in duplicate with coefficients of variation under 10% Report inter- and intra-assay coefficients Report assay detection and sensitivity limits |
|
Data Cleaning and Statistical Methods Report proportion of non-detectable values and patterns of missingness, and number of high out-of-range CRP values, and include all available data (no listwise deletion) Visualize CRP values to determine outliers Winsorization for non-detectable and high out-of-range values (three standard deviations) Correct and transparent reporting of handling of non-normal data, including reporting of kurtosis and skewness statistics Report results from continuous analyses |
|
Control variables to assess and statistically adjust (as necessary) Demographic Variables Age Sex Race/ethnicity Health Variables Body mass index, waist circumference, or waist-to-hip ratio Chronic medical conditions or health markers (e.g., cholesterol, triglycerides)* Substance Variables Nicotine use (current and former)* Alcohol use (frequency and amount)* Caffeine use* Medication Variables Antidepressant use (frequency, type & dose) NSAID use (frequency and dose)* Statin/anti-hypertensive use (frequency and dose) Psychosocial Variables SES and/or education Additional variables to consider (included in quality score) Physical activity/exercise Sleep deprivation |
Indicates that it is appropriate for the study to exclude based on this variable or to exclude based on acute use.
10.3.1. Sample collection and assaying procedures
We propose three guidelines for sample collection procedures. First, we recommend that samples are collected after a resting period of 20–45 min with participants in a supine position. Acute psychological distress associated with a blood draw may lead to specious results. A supine position is recommended as the references established for CRP levels, including cut-offs for elevated CRP (e.g., 3 mg/L), were derived from studies in which participants were in a supine position (Erlandsen and Randers, 2000). Second, due to the effects of caffeine and substances on circulating biomarkers and depression, it is recommended that participants fast overnight prior to the blood draw. Third, researchers should rule out any evidence of acute infection via objective methods such as collection of body temperature and vital signs, which will ensure a valid collection and reduce the observation of high value outliers (Fortmann et al., 2004).
10.3.2. Assaying procedures
We recommend that future studies use standardization of assays by referring to reference materials (Kimberly et al., 2009, 2003); run all assays in duplicate, ideally within an upper limit of coefficients of variation of 5% (National Heart, Lung, and Blood Institute, 2017), but realistically no greater than 10%; report analytical, within subject, and between subject assay variance (Fraser and Harris, 1989); and report CRP detection sensitivity.
10.3.3. Data cleaning and statistical methods
We recommend clearly reporting in the methods section what proportion of CRP values were non-detectable and the patterns of missingness (missing completely at random, missing at random, or missing not at random) and include all available data. We do not recommend deleting any outliers listwise due to loss of power and the likelihood of introducing bias to the sample, especially if data are not missing at random. Furthermore, the substantial time and effort given to the studies from participants ethically requires researchers to include data if it is valid. For both non-detectable and high out of range values, we recommend winsorization to retain the ordinal value of the data without excluding it. Although it is common to replace any outliers via this process, there are no guidelines for exactly what the threshold should be. This is because the definition of an outlier depends on the sample itself and the norms of the population (Ghosh and Vogt, 2012). Therefore, we recommend that researchers first visualize the distribution of their CRP data using boxplots and/or histograms to see which values are clearly outliers, and as a very general guideline, replace any values at three standard deviations and above (and report this threshold in the manuscript), similar to other studies of CRP (Riis et al., 2015). We also recommend reporting the number of outliers that were win-sorized. Lastly, we recommend transparent reporting of handling of non-normal data, including calculation of kurtosis and skewness statistics to justify the use of parametric testing.
Further, we recommend that researchers who have collected symptoms or biological data on a continuous scale, always present continuous results (e.g., non-categorical continuous depression measures, CRP levels) even if a clinical cut-off for the continuous scale, for either depression or CRP, is utilized to divide people into groups (e.g., probable depression versus no depression). Studies that have made a clinical diagnosis of MDD should also collect data on discreet depressive symptoms (either by interview or self-report) and present those continuous results.
10.3.4. Confounding variables
We recommend that each of the following variables be properly assessed and controlled for accordingly. Specifically, to avoid extraneous co-variates that reduce power and may introduce unnecessary heterogeneity and issues of multicollinearity, we recommend that researchers first run and report a series of zero-order bivariate correlations, exploring the association between the control co-variates with CRP and depression separately. Then, researchers should only control for variables that are significantly associated with CRP or depression. This approach is preferable to matching depressed and control on these variables, which fails to account for the potential influence of the variable on CRP. Further, reporting associations between depression and CRP with these confounding variables also promotes transparency in the field and helps accrue knowledge on how these factors relate independently to depression and CRP. Lastly, to promote standardization and reproducibility, we note that all following recommendations are best conceptualized as “default” criteria in the absence of specific design-related considerations.
10.3.5. Descriptives and health
Age, sex, race/ethnicity, BMI/waist circumference/waist-to-hip ratio, and medical conditions should all be assessed. Medical conditions can also be exclusion criteria, though explicit reporting of which and how many conditions were exclusionary is necessary. Cholesterol, triglycerides, blood pressure, and fasting glucose levels may be substituted as control variables and can also mitigate limitations related to self-report.
10.3.6. Substance use
Lifetime and current nicotine use should be assessed, including the amount, type, and frequency as well as the date of abstinence for former smokers. Smoking and other nicotine use can be assessed reliably via self-report or measurement of serum cotinine levels (Welsh et al., 2008). Acute nicotine use can be excluded; however, given the higher prevalence of nicotine use in MDD, this may lead to a less representative sample. Therefore, we instead recommend that participants should be instructed to avoid nicotine for 24 h prior to the collection and to provide detailed information on amount, type, and frequency, including last time they smoked. Proper control of alcohol use should include assessment and consideration of both the amount consumed and frequency of use. Alcohol dependence should be excluded as a heavy levels of alcohol are known to impact CRP levels. Further, to reduce variability, participants should be instructed to avoid alcohol for 24 h prior to their collection. For both nicotine and alcohol use, withdrawal effects after withholding such agents should be monitored. Since valid assessment of caffeine consumption is difficult given the nuanced influences of types of caffeine and methods of preparation (Grosso et al., 2016; Zampelas et al., 2004), it is recommended that studies investigating stress-related biomarkers instruct subjects to abstain from caffeine use prior to the specimen collection, preferably via an overnight fast.
10.3.7. Medications
We recommend that antidepressant type, use, and dosage should be carefully assessed and adjusted for accordingly, even in participants without depressive disorders or symptoms. Given the many types and classes of antidepressant medication, it is imperative that researchers document type, use, and dosage and examine and report if these variables significantly influence CRP or depression, before deciding if they should be included in the statistical model. We also recommend that researchers carefully document the type, dosage, and frequency, and dates of use of NSAID, antihypertensive, and statin use and control the medications in subsequent analyses if necessary. In the case of NSAID use, which is often used “as needed,” participants should be instructed to not take any NSAID or aspirin on the day of the collection, unless as part of a routine medication regimen.
10.3.8. Psychosocial variables
Socioeconomic status must be considered in analyses of depression and CRP. It is preferable that a valid measure of SES is utilized (e.g., Hollingshead Four-Factor Index of SES), which considers marital status, educational attainment, employment status, and occupation; (Hollingshead, 1975). However, if a single parameter of SES is to be utilized, education is preferred as it has the most robut and consistent correlation with cardiovascular risk factors (Winkleby et al., 1992) and validated scales of SES (Cirino et al., 2002).
10.3.9. Additional variables
Additional variables to consider that were included in the quality score include physical activity/exercise habits and sleep deprivation. Greater levels of cardiorespiratory fitness have been linked to lower levels of circulating CRP in both healthy and clinical populations (Plaisance and Grandjean, 2006). Physical activity is also a protective factor against the development of depressive disorders (Strawbridge et al., 2002). Sleep deprivation has been increasingly linked to elevated CRP levels (Meier-Ewert et al., 2004) as has poor quality of sleep (Huang et al., 2017). Moreover, disruptions in sleep have also been linked to more severe presentations of depression (Tsuno et al., 2005).
10.4. Limitations of the current study
The coding for the systematic review presented several challenges, as studies often did not include critical information. Therefore, it is possible that certain studies did adhere to a methodological standard that was not noted in the publication, possibly due to word limit restrictions. However, the estimates provided in this review reflect the knowledge available. In a push towards Open Science and transparency, it is imperative to report all steps taken in the study protocol, data analysis, and results, even if published in Supplemental Material. The Open Science Framework fosters reproducibility efforts and enables researchers to have an additional space to document their protocols (Collaboration, 2012).
A significant limitation is that we did not examine the differential impacts of excluding versus controlling for certain confounders, such as chronic illness or medication use. Results may also vary by class of antidepressant. Given the high levels of heterogeneity across the field in the treatment of these variables, the present paper is an important step into gathering ongoing evidence for the role of these confounders and for developing a shared set of guidelines that help orient the field and set the stage for future progress. For example, an important area of research will be to delineate how different classes of medication impact inflammatory functioning in the context of depression. Additionally, investigating how excluding medications compares to statistically controlling for them will provide useful information on how to best accommodate such variables in the design and analysis of studies examining inflammation and depression. However, such goals can only be achieved by adhering to a shared set of guidelines that initially include the collection, assessment, and proper reporting of the role of such variables. Additional research may shed light on important distinctions that will enable the field to re-evaluate, and if necessary, update these guidelines.
While all studies included in the meta-analysis (Stages 1–5) did account for sex, we were unable to specifically examine sex differences in the association between CRP and depression. At Stage 5, only three studies stratified results by sex. In addition to presenting results from the overall model, future studies should consider presenting results stratified by sex to advance knowledge of sex differences in the field.
Another notable limitation is that certain control variables that may influence depression and CRP were not included in the meta-analysis. Most notable amongst these is race/ethnicity, but physical activity and sleep deprivation are also potentially important confounders. The reporting of descriptive data on race and ethnicity was limited and often hard to extract (e.g., only reporting the country of the study). While research has also identified physical activity and sleep deprivation as potential control variables to consider (O’Connor et al., 2009), the knowledge base is more limited and far fewer studies accounted for these variables. Therefore, they were entered in the quality score and we recommend that researchers assess them in future studies.
Finally, in the meta-analysis, even at the highest Stage, there was marked heterogeneity across studies. Random-effects models were utilized to mitigate this concern. Further, at Stage Five with the quality score moderator, the heterogeneity was significantly diminished. We cannot deduce the individual role of any single confounding variable. Prior efforts have already reported on the singular role of age, sex, and BMI. The purpose of the current study was to see how the classes of confounding variables together affected the heterogenenity and effect size.
10.5. Conclusions
In recent years, there has been a push for Open Science, a framework that aims to promote transparency and foster reproducibility efforts (Collaboration, 2012). Ideally, scientific findings can be reproduced independently, but this will rely on clear, transparent descriptions of the original methods. The last three decades have witnessed a surge of research exploring depression and CRP. However, there is marked variability in the methods utilized across the field, limiting reproducibility efforts and calling into question the heretofore accepted findings in the literature. While there may be an independent association between depression and CRP, the validity and robustness of that relationship is still undetermined, given that it was altered or became non-significant depending when confounding variables and quality measures were controlled.
While prior meta-analyses have included quality scores or explored moderators, this study is the first focused meta-analysis that specifically excluded studies that did not meet a certain threshold for methodological rigor. Despite the increasing methodological standards with each Stage, significant heterogeneity between studies persisted. Results from the moderation analysis at Stage Five are particularly compelling, demonstrating that when studies met the highest bar of methodological quality as suggested by the field, the aggregated effect size was attenuated to become non-significant, the heterogeneity diminished significantly, and the studies’ reported outcomes were normally distributed. Notably, only 6.7% from the systematic review, or 13 studies (with 16 analyses), met this standard. In order to make progress in the field, and accurately delinate the relationship between CRP and depression, more studies must adhere to these recommendations (Table 3). Future research should be dedicated to following the guidelines as closely as possible to reduce heterogeneity in methods and outcomes and pave the way for valid replication efforts.
It is an exciting time for the field as advanced research methods continue to explore and elucidate the neurobiological underpinnings of depression. Innovative designs have paved the way for exciting developments, such as the investigation of new treatments for MDD targeting inflammatory functioning (Köhler et al., 2014; Na et al., 2014), utilizing CRP as diagnostic biomarkers for MDD and treatment response to antidepressants and psychotherapy (Harley et al., 2010; Jha et al., 2017; Uher et al., 2014), and employing dimensional approaches to elucidate if CRP is distinctly related to specific depressive symptoms (Jokela et al., 2016; Lamers et al., 2017; White et al., 2017). As we further explore the complex association between depression and CRP, and apply these findings in novel directions that will optimize treatment options for afflicted individuals, we must agree on a common set of guidelines to follow that will set the stage for successful reproducibility efforts (Table 3). Moreover, most of these clinical applications of the putative link between CRP (or inflammatory processes more generally) and depression rely on the association between these variables being causal and mechanistic, not epiphenomenal (i.e., based on a third variable). In order to ensure that these translational activities are likely to really improve the health and wellbeing of those affected by depressive conditions, our basic research on the links between inflammatory and depressive phenomena must now meet the most stringent standards – ones where our research designs put our hypotheses at increasing risk of disconfirmation. Only when we find effects that shine through in such rigorous tests, will we truly start to unravel the puzzle of depression in a way that can reduce the heavy burden of suffering associated with these conditions.
Supplementary Material
Acknowledgements
The authors thank Abigail Collins, Sarah Rosenthal, and Leticia Hayes for their dedicated work on coding articles for the systematic review.
Research support was provided by the following Grants
Sarah R. Horn received support from National Science Foundation Graduate Research Fellowship Program, Fellow ID: 2017244647. Philip A. Fisher received support from NIH Grants R01 HD075716 and P50 DA035763. Michelle L. Byrne is supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K01MH111951. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016Xj.bbi.2018.06.016.
References
- Abbing-Karahagopian V, Huerta C, Souverein P, De Abajo F, Leufkens H, Slattery J, Alvarez Y, Miret M, Gil M, Oliva B, 2014. Antidepressant prescribing in five European countries: application of common definitions to assess the prevalence, clinical observations, and methodological implications. Eur. J. Clin. Pharmacol 70, 849–857. [DOI] [PubMed] [Google Scholar]
- Albert MA, Glynn RJ, Ridker PM, 2004. Effect of physical activity on serum C-reactive protein. Am. J. Cardiol 93, 221–225. [DOI] [PubMed] [Google Scholar]
- Alley DE, Seeman TE, Kim JK, Karlamangla A, Hu P, Crimmins EM, 2006. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain Behav. Immun 20, 498–504. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Norman P, Hankey GJ, Jamrozik K, Flicker L, 2007. The association between C-reactive protein concentration and depression in later life is due to poor physical health: results from the Health in Men Study (HIMS). Psychol. Med 37, 1775–1786. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Norman PE, Allcock R, Bockxmeer FV, Hankey GJ, Jamrozik K, Flicker L, 2009. Polymorphisms of the CRP gene inhibit inflammatory response and increase susceptibility to depression: the Health in Men Study. Int. J. Epidemiol 38, 1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ, 2001. The prevalence of comorbid depression in adults with diabetes. Diabetes Care 24, 1069–1078. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM, 1998. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol. Med 28, 51–61. [DOI] [PubMed] [Google Scholar]
- Angold A, Rutter M, 1992. Effects of age and pubertal status on depression in a large clinical sample. Dev. Psychopathol 4, 5–28. [Google Scholar]
- Azar RR, Klayme S, Germanos M, Kassab R, Tawm S, Aboujaoude S, Naman R, 2003. Effects of aspirin (325 mg/day) on serum high-sensitivity C-reactive protein, cytokines, and adhesion molecules in healthy volunteers. Am. J. Cardiol 92, 236–239. [DOI] [PubMed] [Google Scholar]
- Aziz N, Fahey JL, Detels R, Butch AW, 2003. Analytical performance of a highly sensitive C-reactive protein-based immunoassay and the effects of laboratory variables on levels of protein in blood. Clin. Diagn. Lab. Immunol 10, 652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai YM, Su TP, Li CT, Tsai SJ, Chen MH, Tu PC, Chiou WF, 2015. Comparison of pro-inflammatory cytokines among patients with bipolar disorder and unipolar disorder and normal controls. Bipolar Disord 17, 269–277. [DOI] [PubMed] [Google Scholar]
- Baune BT, Smith E, Reppermund S, Air T, Samaras K, Lux O, Brodaty H, Sachdev P, Trollor JN, 2012. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology 37, 1521–1530. [DOI] [PubMed] [Google Scholar]
- Bautista LE, Vera-Cala LM, Colombo C, Smith P, 2012. Symptoms of depression and anxiety and adherence to antihypertensive medication. Am. J. Hypertens 25, 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzano LA, He J, Muntner P, Vupputuri S, Whelton PK, 2003. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann. Intern. Med 138, 891–897. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Beck Depression Inventory-II (BDI-II). Psychological Corporation, San Antonio, TX. [Google Scholar]
- Bell S, Mehta G, Moore K, Britton A, 2017. Ten-year alcohol consumption typologies and trajectories of C-reactive protein, interleukin-6 and interleukin-1 receptor antagonist over the following 12 years: a prospective cohort study. J. Intern. Med 281, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle D, Doucet J, 2003. Poverty, inequality, and discrimination as sources of depression among US women. Psychol. Women Q. 27, 101–113. [Google Scholar]
- Benros ME, Waltoft BL, Nordentoft M, 0stergaard SD, Eaton WW, Krogh J, Mortensen PB, 2013. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry 70, 812–820. [DOI] [PubMed] [Google Scholar]
- Bishara AJ, Hittner JB, 2012. Testing the significance of a correlation with nonnormal data: comparison of Pearson, Spearman, transformation, and resampling approaches. Psychol. Methods 17, 399–417. [DOI] [PubMed] [Google Scholar]
- Bjerkeset O, Romild U, Smith GD, Hveem K, 2011. The associations of high levels of C-reactive protein with depression and myocardial infarction in 9258 women and men from the HUNT population study. Psychol. Med 41, 345–352. [DOI] [PubMed] [Google Scholar]
- Blair A, Ross NA, Gariepy G, Schmitz N, 2014. How do neighborhoods affect depression outcomes? A realist review and a call for the examination of causal pathways. Social Psychiatry Psychiat. Epidemiol 49, 873–887. [DOI] [PubMed] [Google Scholar]
- Bo S, Gentile L, Ciccone G, Baldi C, Benini L, Dusio F, Lucia C, Forastiere G, Nuti C, Cassader M, 2005. The metabolic syndrome and high C-reactive protein: prevalence and differences by sex in a southern-European population-based cohort. Diabetes Metab. Res. Rev 21, 515–524. [DOI] [PubMed] [Google Scholar]
- Bonita JS, Mandarano M, Shuta D, Vinson J, 2007. Coffee and cardiovascular disease: in vitro, cellular, animal, and human studies. Pharmacol. Res 55, 187–198. [DOI] [PubMed] [Google Scholar]
- Borenstein, M., Hedges, L.V., Higgins, J., Rothstein, H.R., 2009. References. Wiley Online Library.
- Bremmer MA, Beekman AT, Deeg DJ, Penninx BW, Dik MG, Hack CE, Hoogendijk WJ, 2008. Inflammatory markers in late-life depression: results from a population-based study. J. Affect. Disord 106, 249–255. [DOI] [PubMed] [Google Scholar]
- Brindle E, Fujita M, Shofer J, O’Connor KA, 2010. Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. J. Immunol. Methods 362, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GC, Blaha MJ, Blumenthal RS, 2010. Relation of C-reactive protein to abdominal adiposity. Am. J. Cardiol 106, 56–61. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA, 2006. Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychol. Aging 21, 140–151. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Price LH, 2012. C-reactive protein, early life stress, and wellbeing in healthy adults. Acta. Psychiatr. Scand 126, 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case SM, Stewart JC, 2014. Race/ethnicity moderates the relationship between depressive symptom severity and C-reactive protein: 2005–2010 NHANES data. Brain Behav. Immun 41, 101–108. [DOI] [PubMed] [Google Scholar]
- Chang HH, Lee IH, Gean PW, Lee S-Y, Chi MH, Yang YK, Lu R-B, Chen PS, 2012. Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain Behav. Immun 26, 90–95. [DOI] [PubMed] [Google Scholar]
- Chen H, Quandt SA, Grzywacz JG, Arcury TA, 2013. A Bayesian multiple imputation method for handling longitudinal pesticide data with values below the limit of detection. Environmetrics 24, 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Joseph L, Pilote L, 2013. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes. Rev 14, 232–244. [DOI] [PubMed] [Google Scholar]
- Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD, 2002. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment 9, 145–155. [DOI] [PubMed] [Google Scholar]
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, Dickens C, Ferrier I, Geddes J, Gilbody S, 2015. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J. Psychopharmacol 29, 459–525. [DOI] [PubMed] [Google Scholar]
- Collaboration OS, 2012. An open, large-scale, collaborative effort to estimate the reproducibility of psychological science. Perspect. Psychol. Sci [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ, 2012. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol. Psychiatry 71, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner M, Kasl SV, Abramson JL, Vaccarino V, 2003. Association between depression and elevated C-reactive protein. Psychosom. Med 65, 347–356. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Capuron L (Eds.), 2017. Inflammation-Associated Depression: Evidence, Mechanisms, and Implications. 31 Springer, New York. [Google Scholar]
- Dantzer R, Kelley KW, 2007. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun 21, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From in-flammaiton to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood T, Lambert EA, Barton DA, Laude D, Elghozi J-L, Esler MD, Haikerwal D, Kaye DM, Hotchkin EJ, Lambert GW, 2007. Specific serotonin reuptake inhibition in major depressive disorder adversely affects novel markers of cardiac risk. Hypertens. Res 30, 285–293. [DOI] [PubMed] [Google Scholar]
- de Menezes ST, de Figueiredo RC, Goulart AC, Nunes MA, Bensenor IM, Viana MC, Barreto SM, 2017. Lack of association between depression and C-reactive protein level in the baseline of Longitudinal Study of Adult Health (ELSA-Brasil). J. Affect. Disord 208, 448–454. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Papa F, 2003. Angiotensin-converting enzyme inhibitor use is associated with reduced plasma concentration of C-reactive protein in patients with first-ever ischemic stroke. Stroke 34, 2922–2929. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Avenevoli S, Stolar M, Merikangas K, 2002. Smoking and depression: an examination of mechanisms of comorbidity. Am. J. Psychiatry 159, 947–953. [DOI] [PubMed] [Google Scholar]
- Douglas KM, Taylor AJ, O’Malley PG, 2004. Relationship between depression and C-reactive protein in a screening population. Psychosom. Med 66, 679–683. [DOI] [PubMed] [Google Scholar]
- Du Clos TW, 2000. Function of C-reactive protein. Ann. Med 32, 274–278. [DOI] [PubMed] [Google Scholar]
- Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW, 2013. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology 38, 1573–1585. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Phillips AN, 1997. Meta-analysis: principles and procedures. BMJ Br. Med. J 315, 1610–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einvik G, Hrubos-Strøm H, Randby A, Nordhus IH, Somers VK, Omland T, Dammen T, 2011. Major depressive disorder, anxiety disorders, and cardiac biomarkers in subjects at high risk of obstructive sleep apnea. Psychosom. Med 73, 378–384. [DOI] [PubMed] [Google Scholar]
- Einvik G, Flyvbjerg A, Hrubos-Strøm H, Randby A, Frystyk J, Bjerre M, Namtvedt SK, Kristiansen HA, Nordhus IH, Somers VK, Dammen T, Omland T, 2013. Novel cardiovascular risk markers in depression: no association between depressive symptoms and osteoprotegerin adiponectin in persons at high risk for sleep apnea. J. Affect. Disord 145, 400–404. [DOI] [PubMed] [Google Scholar]
- Elderon L, Whooley MA, 2013. Depression and cardiovascular disease. Prog. Cardiovasc. Dis 55, 511–523. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Keltijangas-Jarvinen L, Pulkki-Raback L, Kivimaki M, Puttonen S, Viikari L, Rasanen L, Mansikkaniemi K, Viikari J, Raitakari OT, 2006. Depressive symptoms and C-reactive protein: the Cardiovascular Risk in Young Finns Study. Psychol. Med 36, 797–805. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Aalto A-M, Kivimäki M, Pirkola S, Sundvall J, Lönnqvist J, Reunanen A, 2009. Depression and C-reactive protein: population-based Health 2000 Study. Psychosom. Med 71, 423–430. [DOI] [PubMed] [Google Scholar]
- EPA U, 2000. Guidance for data quality assessment Practical methods for data analysis. Office of Environmental Information; EPA QA/G-9, QA00 Version Washington, DC. [Google Scholar]
- Erlandsen E, Randers E, 2000. Reference interval for serum C-reactive protein in healthy blood donors using the Dade Behring N Latex CRP mono assay. Scand. J. Clin. Lab. Invest 60, 37–43. [DOI] [PubMed] [Google Scholar]
- Eurelings LS, Richard E, Eikelenboom P, van Gool WA, van Charante EPM, 2015. Low-grade inflammation differentiates between symptoms of apathy and depression in community-dwelling older individuals. Int. Psychogeriatr 27, 639–647. [DOI] [PubMed] [Google Scholar]
- Feldman M, Jialal I, Devaraj S, Cryer B, 2001. Effects of low-dose aspirin on serum C-reactive protein and thromboxane B 2 concentrations: a placebo-controlled study using a highly sensitive C-reactive protein assay. J. Am. Coll. Cardiol 37, 2036–2041. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Somerville A, Baxter A, Norman R, Patten S, Vos T, Whiteford H, 2013. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol. Med 43, 471–481. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JB, Karg RS, Spitzer RL, 2016. User’s Guide for the SCID-5-CV: Structured Clinical Interview for DSM-5 Disorders, Clinician Version. American Psychiatric Association. [Google Scholar]
- Fiske A, Gatz M, Pedersen NL, 2003. Depressive symptoms and aging: the effects of illness and non-health-related events. J. Gerontol. B Psychol. Sci. Soc. Sci 58, P320–P328. [DOI] [PubMed] [Google Scholar]
- Ford DE, Erlinger TP, 2004. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med 164, 1010–1014. [DOI] [PubMed] [Google Scholar]
- Ford ES, Loucks EB, Berkman LF, 2006. Social integration and concentrations of C-reactive protein among US adults. Ann. Epidemiol 16, 78–84. [DOI] [PubMed] [Google Scholar]
- Forouhi N, Sattar N, McKeigue P, 2001. Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. Int. J. Obesity 25, 1327–1331. [DOI] [PubMed] [Google Scholar]
- Forti P, Rietti E, Pisacane N, Olivelli V, Mariani E, Chiappelli M, Licastro F, Ravaglia G, 2010. Blood inflammatory proteins and risk of incident depression in the elderly. Dement. Geriatr. Cogn. Disord 29, 11–20. [DOI] [PubMed] [Google Scholar]
- Fortmann SP, Ford E, Criqui MH, Folsom AR, Harris TB, Hong Y, Pearson TA, Siscovick D, Vinicor F, Wilson PF, 2004. CDC/AHA workshop on markers of inflammation and cardiovascular disease. Circulation 110, e554–e559. [DOI] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J, 2010. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 303, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GG, Harris EK, 1989. Generation and application of data on biological variation in clinical chemistry. Crit. Rev. Clin. Lab. Sci 27, 409–437. [DOI] [PubMed] [Google Scholar]
- Fried EI, Randolph MN, 2015. Depression is not a consistent syndrome: an investigation of unique symptom patterns in the STAR*D study. J. Affect. Disord 172, 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Carballedo A, Hughes MM, Saleh K, Fagan A, Skokauskas N, McLoughlin DM, Meaney J, O’Keane V, Connor TJ, 2012. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl. Psychiatry 13, e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich M, Sund M, Löwel H, Imhof A, Hoffmeister A, Koenig W, 2003. Independent association of various smoking characteristics with markers of systemic inflammation in men: Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95). Eur. Heart J 24, 1365–1372. [DOI] [PubMed] [Google Scholar]
- Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B, Ganio EA, Fragiadakis GK, Spitzer MH, Douchet I, 2017. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med 23, 174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambi F, De Barardis D, Campanella D, Carano A, Sepede G, Salini G, Scorrano B, Spinella S, Conti CM, La Rovere R, Valchera A, 2005. A retrospective evaluation of the inflammatory marker C-reactive protein (CRP), cholesterol and high-density lipoproteins in patients with major depression: preliminary findings. Eur. J. Inflamm 3, 127–134. [Google Scholar]
- Ghosh D, Vogt A, 2012. Outliers: An Evaluation of Methodologies, Joint Statistical Meetings. American Statistical Association; San Diego, CA, pp. 3455–3460. [Google Scholar]
- Gimeno D, Brunner EJ, Lowe GD, Rumley A, Marmot MG, Ferrie JE, 2007. Adult socioeconomic position, C-reactive protein and interleukin-6 in the Whitehall II prospective study. Eur. J. Epidemiol 22, 675–683. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, Lowe GD, Rumley A, Marmot MG, 2009. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med 39, 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass TA, De Leon CFM, Bassuk SS, Berkman LF, 2006. Social engagement and depressive symptoms in late life: longitudinal findings. J. Aging Health 18, 604–628. [DOI] [PubMed] [Google Scholar]
- Glaus J, Vandeleur CL, von Kanel R, Lasserre AM, Strippoli MPF, Gholam-Rezaee M, Castelao E, Marques-Vidal P, Bovet P, Merikangas K, Mooser V, 2014. Associations between mood anxiety, or substance use disorders and inflammatory markers after adjustment for multiple covariates in a population-based study. J. Psychiatr. Res 58, 36–45. [DOI] [PubMed] [Google Scholar]
- Gonsalves R, Coletta R, Silverio K, Benevides L, Casati M, Da Silva J, Nociti F, 2011. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm. Res 60, 409–424. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K, 2004. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry 61, 807–816. [DOI] [PubMed] [Google Scholar]
- Greenfield JR, Samaras K, Jenkins AB, Kelly PJ, Spector TD, Gallimore JR, Pepys MB, Campbell LV, 2004. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation 109, 3022–3028. [DOI] [PubMed] [Google Scholar]
- Grosso G, Micek A, Castellano S, Pajak A, Galvano F, 2016. Coffee, tea, caffeine and risk of depression: a systematic review and dose–response meta-analysis of observational studies. Mol. Nutr. Food Res 60, 223–234. [DOI] [PubMed] [Google Scholar]
- Gu Q, Paulose-Ram R, Dillon C, Burt V, 2006. Antihypertensive medication use among US adults with hypertension. Circulation 113, 213–221. [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M, 2015. Cumulative meta-analysis of interleukins 6 and 1ß, tumour necrosis factor a and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun 49, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner S, Emeny R, Lacruz M, Baumert J, Herder C, Koenig W, Thorand B, Ladwig K, Investigators KS, 2011. Association between social isolation and inflammatory markers in depressed and non-depressed individuals: results from the MONICA/KORA study. Brain Behav. Immun 25, 1701–1707. [DOI] [PubMed] [Google Scholar]
- Halder I, Marsland AL, Cheong J, Muldoon MF, Ferrell RE, Manuck SB, 2010. Polymorphisms in the CRP gene moderate an association between depressive symptoms and circulating levels of C-reactive protein. Brain Behav. Immun 24, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GCN, Yip T, Zárate MA, 2016. On becoming multicultural in a monocultural research world: a conceptual approach to studying ethnocultural diversity. Am. Psychol 71, 40–51. [DOI] [PubMed] [Google Scholar]
- Hamer M, Molloy GJ, de Oliveira C, Demakakos P, 2009. Persistent depressive symptomatology and inflammation: to what extent do health behaviours and weight control mediate this relationship? Brain Behav. Immun 23, 413–418. [DOI] [PubMed] [Google Scholar]
- Hamer M, Batty GD, Marmot MG, Singh-Manoux A, Kivimäki M, 2011. Anti-depressant medication use and C-reactive protein: results from two population-based studies. Brain Behav. Immun 25, 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley J, Luty S, Carter J, Mulder R, Joyce P, 2010. Elevated C-reactive protein in depression: a predictor of good long-term outcome with antidepressants and poor outcome with psychotherapy. J. Psychopharmacol 24, 625–626. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Heimovitz H, Cohen HJ, Wallace R, 1999. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med 106, 506–512. [DOI] [PubMed] [Google Scholar]
- Hastie CE, Haw S, Pell JP, 2008. Impact of smoking cessation and lifetime exposure on C-reactive protein. Nicotine Tob. Res 10, 637–642. [DOI] [PubMed] [Google Scholar]
- Heckman MA, Weil J, Mejia D, Gonzalez E, 2010. Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci 75, R77–R87. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Vevea JL, 1998. Fixed-and random-effects models in meta-analysis. Psychol. Methods 3, 486–504. [Google Scholar]
- Heffner KL, Waring ME, Roberts MB, Eaton CB, Gramling R, 2011. Social isolation, C-reactive protein, and coronary heart disease mortality among community-dwelling adults. Soc. Sci. Med 72, 1482–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman RJ, Khambaty T, Stewart JC, 2014. C-reactive protein is elevated in atypical but not nonatypical depression: data from the National Health and Nutrition Examination survey (NHANES) 1999–2004. J. Behav. Med 37, 621–629. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, 1975. Four Factor Index of Social Status. Yale University, New Haven, Connecticut. [Google Scholar]
- Hollon SD, Thase ME, Markowitz JC, 2002. Treatment and prevention of depression. Psychol. Sci. Public Interest 3, 39–77. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J, 2009. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med 71, 171–186. [DOI] [PubMed] [Google Scholar]
- Huang WY, Huang CC, Chang CC, Kor CT, Chen TY, Wu HM, 2017. Associations of self-reported sleep quality with circulating interferon gamma-inducible protein 10, interleukin 6, and high-sensitivity C-reactive protein in healthy menopausal women. PLoS One 12, e0169216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MM, Carballedo A, McLoughlin DM, Amico F, Harkin A, Frodl T, Connor TJ, 2012. Tryptophan depletion in depressed patients occurs independent of ky-nurenine pathway activation. Brain Behav. Immun 26, 979–987. [DOI] [PubMed] [Google Scholar]
- Ikonomidis I, Andreotti F, Economou E, Stefanadis C, Toutouzas P, Nihoyannopoulos P, 1999. Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation 100, 793–798. [DOI] [PubMed] [Google Scholar]
- Imhof A, Woodward M, Doering A, Helbecque N, Loewel H, Amouyel P, Lowe G, Koenig W, 2004. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: results from three MONICA samples (Augsburg, Glasgow, Lille). Eur. Heart J 25, 2092–2100. [DOI] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, Mayes TL, Rush AJ, Trivedi MH, 2017. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 78, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M, Virtanen M, Batty GD, Kivimäki M, 2016. Inflammation and specific symptoms of depression. JAMA Psychiatry 73, 87–88. [DOI] [PubMed] [Google Scholar]
- Jousilahti P, Salomaa V, Rasi V, Vahtera E, Palosuo T, 2003. Association of markers of systemic inflammation, C reactive protein, serum amyloid A, and fibrinogen, with socioeconomic status. J. Epidemiol. Community Health 57, 730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joynt KE, Gattis WA, Hasselblad V, Fuzaylov SY, Serebruany VL, Gurbel PA, Gaulden LH, Felker GM, Whellan DJ, O’Connor CM, 2004. Effect of angiotensin-converting enzyme inhibitors, beta blockers, statins, and aspirin on C-reactive protein levels in outpatients with heart failure. Am. J. Cardiol 93, 783–785. [DOI] [PubMed] [Google Scholar]
- Kasapis C, Thompson PD, 2005. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J. Am. Coll. Cardiol 45, 1563–1569. [DOI] [PubMed] [Google Scholar]
- Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, Matthews KA, Johnston J, Sowers MR, Sternfeld B, Pasternak RC, Chae CU, 2008. Ethnic differences in C-reactive protein concentrations. Clin. Chem 54, 1027–1037. [DOI] [PubMed] [Google Scholar]
- Kéri S, Szabo C, Kelemen O, 2014. Expression of Toll-like Receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain Behav. Immun 40, 235–243. [DOI] [PubMed] [Google Scholar]
- Kershaw KN, Mezuk B, Abdou CM, Rafferty JA, Jackson JS, 2010. Socioeconomic position, health behaviors, and C-reactive protein: a moderated-mediation analysis. Health Psychol 29, 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Foster C, Webster PS, House JS, 1992. The relationship between age and depressive symptoms in two national surveys. Psychol. Aging 7, 119–126. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, 2003. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289, 3095–3105. [DOI] [PubMed] [Google Scholar]
- Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Grundy SM, de Lemos JA, 2005. Race and gender differences in C-reactive protein levels. J. Am. Coll. Cardiol 46, 464–469. [DOI] [PubMed] [Google Scholar]
- Khera A, Vega GL, Das SR, Ayers C, McGuire DK, Grundy SM, de Lemos JA, 2009. Sex differences in the relationship between C-reactive protein and body fat. J. Clin. Endocrinol. Metab 94, 3251–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, 2002. Depression and immune function: central pathways to morbidity and mortality. J. Psychosom. Res 53, 873–876. [DOI] [PubMed] [Google Scholar]
- Kim M, Dunkle R, Lehning A, Shen H, Feld S, Perone A, 2017. Caregiver stressors and depressive symptoms among older husbands and wives in the United States: the role of religiosity as a proective factor of suicide attempts in elderly Brazilians. Innovation Aging 1, 861. [DOI] [PubMed] [Google Scholar]
- Kimberly MM, Vesper HW, Caudill SP, Cooper GR, Rifai N, Dati F, Myers GL, 2003. Standardization of immunoassays for measurement of high-sensitivity C-reactive protein. Phase I: evaluation of secondary reference materials. Clin. Chem 49, 611–616. [DOI] [PubMed] [Google Scholar]
- Kimberly MM, Caudill SP, Vesper HW, Monsell EA, Miller WG, Rej R, Rifai N, Dati F, Myers GL, 2009. Standardization of high-sensitivity immunoassays for measurement of C-reactive protein; II: two approaches for assessing commutability of a reference material. Clin. Chem 55, 342–350. [DOI] [PubMed] [Google Scholar]
- Kling MA, Alesci S, Csako G, Costello R, Luckenbaugh DA, Bonne O, Duncko R, Drevets WC, Manji HK, Charney DS, 2007. Sustained low-grade pro-inflammatory state in unmedicated, remitted women with major depressive disorder as evidenced by elevated serum levels of the acute phase proteins C-reactive protein and serum amyloid A. Biol. Psychiatry 62, 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM, 2002. β-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 288, 351–357. [DOI] [PubMed] [Google Scholar]
- Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J, 2014. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 71, 1381–1391. [DOI] [PubMed] [Google Scholar]
- Krousel-Wood M, Islam T, Muntner P, Holt E, Joyce C, Morisky DE, Webber LS, Frohlich ED, 2010. Association of depression with antihypertensive medication adherence in older adults: cross-sectional and longitudinal findings from CoSMO. Ann. Behav. Med 40, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D’agostino RB, Herrington DM, 2006. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am. Heart J 152, 593–598. [DOI] [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW, 2013. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry. 18, 692–699. [DOI] [PubMed] [Google Scholar]
- Lamers F, Milaneschi Y, de Jonge P, Giltay E, Penninx B, 2017. Metabolic and inflammatory markers: associations with individual depressive symptoms. Psychol. Med 1–11. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg J, Bening-Abu-Shach U, Vedder H, 2000. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 22, 370–379. [DOI] [PubMed] [Google Scholar]
- Lee J, Taneja V, Vassallo R, 2012. Cigarette smoking and inflammation: cellular and molecular mechanisms. J. Dent. Res 91, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto SM, Niskanen L, Herzig KH, Tolmunen T, Huotari A, Vinamaki H, Koivumaa-Honkanen H, Honkalampi K, Ruotsalainen H, Hintikka J, 2010. Serum chemokine levels in major depressive disorder. Psychoneuroendocrinology 35, 226–232. [DOI] [PubMed] [Google Scholar]
- Little R, Rubin D, 1987. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons, New York. [Google Scholar]
- Liu Y, Al-Sayegh H, Jabrah R, Wang W, Yan F, Zhang J, 2014. Association between C-reactive protein and depression: modulated by gender and mediated by body weight. Psychiatry Res 219, 103–108. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia E, van Dam RM, Qi L, Hu FB, 2006. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am. J. Clin. Nutr 84, 888–893. [DOI] [PubMed] [Google Scholar]
- Lorant V, Deliège D, Eaton W, Robert A, Philippot P, Ansseau M, 2003. Socioeconomic inequalities in depression: a meta-analysis. Am. J. Epidemiol 157, 98–112. [DOI] [PubMed] [Google Scholar]
- Lowe G, 2005. Circulating inflammatory markers and risks of cardiovascular and non-cardiovascular disease. J. Thromb. Haemost 3, 1618–1627. [DOI] [PubMed] [Google Scholar]
- Lubbock LA, Goh A, Ali S, Ritchie J, Whooley MA, 2005. Relation of low socioeconomic status to C-reactive protein in patients with coronary heart disease (from the heart and soul study). Am. J. Cardiol 96, 1506–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Lumley MA, Flack JM, Wegner R, Pierce J, Goetz S, 2016. A preliminary experimental examination of worldview verification, perceived racism, and stress reactivity in African Americans. Health Psychol 35, 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Mirzaei F, Pan A, Okereke OI, Willett WC, O’Reilly ÉJ, Koenen K, Ascherio A, 2011. Coffee, caffeine, and risk of depression among women. Arch. Intern. Med 171, 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG, 2010. Overweight, obesity, and depression: a systematic review and metaanalysis of longitudinal studies. Arch. Gen. Psychiatry 67, 220–229. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR, 1998. Cytokines for psychologists: implications ofbidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol. Rev 105, 83–107. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Sowers MF, Derby CA, Stein E, Miracle-McMahill H, Crawford SL, Pasternak RC, 2005. Ethnic differences in cardiovascular risk factor burden among middle-aged women: study of Women’s Health Across the Nation (SWAN). Am. Heart J 149, 1066–1073. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, Cacioppo JT, 2006. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom. Med 68, 376–381. [DOI] [PubMed] [Google Scholar]
- Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, Bodurka J, Teague TK, Dantzer R, Savitz J, 2016. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav. Immun 53, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM, 2004. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J. Am. Coll. Cardiol 43, 678–683. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Corsi AM, Penninx BW, Bandinelli S, Guralnik JM, Ferucci L, 2009. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: the InCHIANTI study. Biol. Psychiatry 65, 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, 1998. Neuroendocrine and immune system interactions in stress and depression. Psychiatr. Clin. North Am 21, 443–463. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL, 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA, 2002. Clinical depression and inflammatory risk markers for coronary heart disease. Am. J. Cardiol 15, 1279–1283. [DOI] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA, 2003. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav. Immun 17, 276–285. [DOI] [PubMed] [Google Scholar]
- Mitchell DC, Knight CA, Hockenberry J, Teplansky R, Hartman TJ, 2014Beverage caffeine intakes in the US. Food Chem. Toxicol 63, 136–142. [DOI] [PubMed] [Google Scholar]
- Modabbernia A, Taslimi S, Brietzke E, Ashrafi M, 2013. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol. Psychiatry 74, 15–25. [DOI] [PubMed] [Google Scholar]
- Moreira RO, Marca K, Appolinario J, Coutinho W, 2007. Increased waist circumference is associated with an increased prevalence of mood disorders and depressive symptoms in obese women. Eat. Weight Disord. 12, 35–40. [DOI] [PubMed] [Google Scholar]
- Morimoto Y, Conroy SM, Ollberding NJ, Kim Y, Lim U, Cooney RV, Franke AA, Wilkens LR, Hernandez BY, Goodman MT, 2014. Ethnic differences in serum adipokine and C-reactive protein levels: the multiethnic cohort. Int. J. Obesity 38, 1416–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AA, Zhao L, Ahmed Y, Stoyanova N, De Staercke C, Hooper WC, Gibbons G, Din-Dzietham R, Quyyumi A, Vaccarino V, 2011. Association between depression and inflammation-differences by race and sex: the META-Health study. Psychosom. Med 73, 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwendwa DT, Ali MK, Sims RC, Cole AP, Lipscomb MW, Levy SA, Callender CO, Campbell AL, 2013. Dispositional depresion and hostility are associated with inflammatory markers of cardiovascular disease in African Americans. Brain Behav. Immun 28, 72–82. [DOI] [PubMed] [Google Scholar]
- Na KS, Lee KJ, Lee JS, Cho YS, Jung HY, 2014. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Progr. Neuro-psychopharmacol. Biol. Psychiatry 48, 79–85. [DOI] [PubMed] [Google Scholar]
- Naghashpour M, Amani R, Nutr R, Nematpour S, Haghighizadeh MH, 2011Riboflavin status and its association with serum hs-CRP levels among clinical nurses with depression. J. Am. Coll. Nutr 30, 340–347. [DOI] [PubMed] [Google Scholar]
- Nanri A, Moore MA, Kono S, 2007. Impact of C-reactive protein on disease risk and its relation to dietary factors: literature review. Asian Pac. J. Cancer Prev 8, 167–177. [PubMed] [Google Scholar]
- Nazmi A, Oliveira I, Victora CG, 2008. Correlates of C-reactive protein levels in young adults: a population-based cohort study of 3827 subjects in Brazil. Braz. J. Med. Biol. Res 41, 357–367. [DOI] [PubMed] [Google Scholar]
- Neville HA, Carter RT, 2005. Race and racism in counseling psychology research, training, and practice: a critical review, current trends, and future directions. Counseling Psychol 33, 413–418. [Google Scholar]
- Nwankwo T, Yoon SS, Burt V, Gu Q, 2013. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 1–8. [PubMed] [Google Scholar]
- O’Brien SM, Scott LV, Dinan TG, 2006. Antidepressant therapy and C-reactive protein levels. Br. J. Psychiatry 188, 449–452. [DOI] [PubMed] [Google Scholar]
- O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, 2009. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun 23, 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obinwa UC, Morrissey K, Li F, Mandapati SR, Aryal S, Robledo M, 2016. Exploring the Relationship between Socioeconomic status and C-Reactive protein levels in the US population using NHANES Survey Data. [Google Scholar]
- Ouellet-Morin I, Danese A, Williams B, Arseneault L, 2011. Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain Behav. Immun 25, 640–646. [DOI] [PubMed] [Google Scholar]
- Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A, 2003. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behav. Immun 17, 286–295. [DOI] [PubMed] [Google Scholar]
- Pai JK, Hankinson SE, Thadhani R, Rifai N, Pischon T, Rimm EB, 2006Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis 186, 113–120. [DOI] [PubMed] [Google Scholar]
- Palmas W, Ma S, Psaty B, Goff DC, Darwin C, Barr RG, 2007. Antihypertensive medications and C-reactive protein in the multi-ethnic study of atherosclerosis. Am. J. Hypertens 20, 233–241. [DOI] [PubMed] [Google Scholar]
- Pan A, Ye X, Franco OH, Li H, Yu Z, Wang J, Qi Q, Gu W, Pang X, Liu H, 2008. The association of depressive symptoms with inflammatory factors and adipokines in middle-aged and older Chinese. PLoS One 3, e1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos CE, Chrysohoou CA, Skoumas J, Toutouza M, Belegrinos D, Toutouzas PK, Stefanadis C, 2004. The association between educational status and risk factors related to cardiovascular disease in healthy individuals: the ATTICA study. Ann. Epidemiol 14, 188–194. [DOI] [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C, 2005. The implication of obesity and central fat on markers of chronic inflammation: the ATTICA study. Atherosclerosis 183, 308–315. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, 2003. Markers of inflammation and cardiovascular disease. Circulation 107, 499–511. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M, 2003. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol. Psychiatry 54, 566–572. [DOI] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G, 2000. Gender differences in depression. Br. J. Psychiatry 177, 486–492. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, Sinacore J, Devane CL, 2009. Pro-inflammatory biomarkers in depression: treatment with venlafaxine. World J. Biol. Psychiatry 10, 313–323. [DOI] [PubMed] [Google Scholar]
- Pizzi C, Manzoli L, Mancini S, Costa GM, 2008. Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function. Eur. Heart J 29, 1110–1117. [DOI] [PubMed] [Google Scholar]
- Plaisance EP, Grandjean PW, 2006. Physical activity and high-sensitivity C-reactive protein. Sports Med 36, 443–458. [DOI] [PubMed] [Google Scholar]
- Politi P, Brondino N, Emanuele E, 2008. Increased proapoptotic serum activity in patients with chronic mood disorders. Arch. Med. Res 39, 242–245. [DOI] [PubMed] [Google Scholar]
- Prasad K, 2006. C-reactive protein (CRP)-lowering agents. Cardiovasc. Ther 24, 33–50. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, Gu Q, 2011. Antidepressant use in persons aged 12 and over: United States, 2005–2008. [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS, 2000. Sex-specific genetic influences on the comorbidity of alcoholism and major depression in a population-based sample of US twins. Arch. Gen. Psychiatry 57, 803–811. [DOI] [PubMed] [Google Scholar]
- Procopio DO, Saba LM, Walter H, Lesch O, Skala K, Schlaff G, Vanderlinden L, Clapp P, Hoffman PL, Tabakoff B, 2013. Genetic markers of comorbid depression and alcoholism in women. Alcohol. Clin. Exp. Res 37, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohan M, Amani R, Nematpour S, Jomehzadeh N, Haghighizadeh MH, 2014. Total antioxidant capacity of diet and serum, dietary antioxidant vitamins intake, and serum hs-CRP levels in relation to depression scales in university male students. Redox Rep. 19, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzianowska-Kuźnicka M, Owczarz M, Wieczorowska-Tobis K, Nadrowski P, Chudek J, Slusarczyk P, Skalska A, Jonas M, Franek E, Mossakowska M, 2016. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun. Ageing 13, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH, 2006. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, Ni H, 2007. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch. Intern. Med 167, 174–181. [DOI] [PubMed] [Google Scholar]
- Raum E, Gebhardt K, Buchner M, Schiltenwolf M, Brenner H, 2007. Long-term and short-term alcohol consumption and levels of C-reactive protein. Int. J. Cardiol 121, 224–226. [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM, 2003. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann. Epidemiol 13, 674–682. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM Jr., 2001. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N. Engl. J. Med 344, 1959–1965. [DOI] [PubMed] [Google Scholar]
- Riis JL, Granger DA, DiPietro JA, Bandeen-Roche K, Johnson SB, 2015. Salivary cytokines as a minimally-invasive measure of immune functioning in young children: correlates of individual differences and sensitivity to laboratory stress. Dev. Psychobiol 57, 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmond R, Lapidus L, Mårin P, Björntorp P, 1996. Mental distress, obesity and body fat distribution in middle-aged men. Obesity 4, 245–252. [DOI] [PubMed] [Google Scholar]
- Saijo Y, Kiyota N, Kawasaki Y, Miyazaki Y, Kashimura J, Fukuda M, Kishi R, 2004. Relationship between C-reactive protein and visceral adipose tissue in healthy Japanese subjects. Diabetes Obes. Metab 6, 249–258. [DOI] [PubMed] [Google Scholar]
- Santiago CD, Wadsworth ME, Stump J, 2011. Socioeconomic status, neighborhood disadvantage, and poverty-related stress: prospective effects on psychological syndromes among diverse low-income families. J. Econ. Psychol 32, 218–230. [Google Scholar]
- Sarandol A, Sarandol E, Eker SS, Karaagac EU, Hizli BZ, Dirican M, Kirli S, 2006. Oxidation of apolipoproetin B-containing lipoproteins and serum paraoxonase/ arylesterase activities in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 1103–1108. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PS, Victor TA, Bodurka J, Teague TK, Dantzer R, 2015. Reduction of kynurenic acid to quinolinic acid ration in both the depressed and remitted phases of major depressive disorder. Brain Behav. Immun 46, 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier HM, Chen E, 2010. Socioeconomic status in one’s childhood predicts offspring cardiovascular risk. Brain Behav. Immun 24, 1324–1331. [DOI] [PubMed] [Google Scholar]
- Shavers VL, 2007. Measurement of socioeconomic status in health disparities research. J. Natl. Med. Assoc 99, 1013–1023. [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Falola M, Li L, Zajecka J, Fava M, Papakostas GI, 2015. The pro-inflammatory profile of depressed patients is (partly) related to obesity. J. Psychiatr. Res 70, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Stewart C, Beck A, Ahmedani BK, Coleman KJ, Whitebird RR, Lynch F, Owen-Smith AA, Waitzfelder BE, Soumerai SB, 2014. National prevalence of receipt of antidepressant prescriptions by persons without a psychiatric diagnosis. Psychiatric Serv 65, 944–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin NL, Kumar AD, Gehi AK, Whooley MA, 2016. Direction of association between depressive symptoms and lifestyle behaviors in patients with coronary heart disease: the Heart and Soul Study. Ann. Behav. Med 50, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, Williams DR, 2010. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosom. Med 72, 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagula SF, Ancoli-Israel S, Barrett-Connor E, Lane NE, Redline S, Stone KL, Cauley JA, Group OFIMR, 2014. Inflammation, sleep disturbances, and depressed mood among community-dwelling older men. J. Psychosom. Res 76, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, 1991. The macrophage theory of depression. Med. Hypotheses 35, 298–306. [DOI] [PubMed] [Google Scholar]
- Smith DJ, McLean G, Martin D, Langan MJ, Guthrie B, Gunn J, Mercer SW, 2014. Depression and multimorbidity: a cross-sectional study of1,751,841 patients in primary care. J. Clin. Psychiatry 75, 1202–1208. [DOI] [PubMed] [Google Scholar]
- Solheim S, Arnesen H, Eikvar L, Hurlen M, Seljeflot I, 2003. Influence of aspirin on inflammatory markers in patients after acute myocardial infarction. Am. J. Cardiol 92, 843–845. [DOI] [PubMed] [Google Scholar]
- Song BM, Lee J-M, Choi W, Youm Y, Chu SH, Park Y-R, Kim HC, 2015. Association between C reactive protein level and depressive symptoms in an elderly Korean population: Korean Social Life, Health and Aging Project. BMJ Open 5, e006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht S, Owen N, 2003. Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol. Med 33, 667–674. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Janicki-Deverts D, Muldoon MF, Kamarck TW, 2008. Depressive symptoms moderate the influence of hostility on serum interleukin-6 and C-reactice protein. Psychosom. Med 70, 197–204. [DOI] [PubMed] [Google Scholar]
- Strawbridge WJ, Deleger S, Roberts RE, Kaplan GA, 2002. Physical activity reduces the risk of subsequent depression for older adults. Am. J. Epidemiol 156, 328–334. [DOI] [PubMed] [Google Scholar]
- Su S, Miller AH, Snieder H, Bremner JD, Ritchie J, Maisano C, Jones L, Murrah NV, Goldberg J, Vaccarino V, 2009. Common genetic contributions to depressive symptoms and inflammatory markers in middle-aged men: the Twins Heart Study. Psychosom. Med 71, 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC, 2004. C-reactive protein is associated with psychological risk factors of cardiovascular disease in apparently healthy adult. Psychosom. Med 66, 684–691. [DOI] [PubMed] [Google Scholar]
- Sue S, 1999. Science, ethnicity, and bias: where have we gone wrong? Am. Psychol 54, 1070–1077. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR, 2000. The comorbidity of depression and substance use disorders. Clin. Psychol. Rev 20, 173–189. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, 2017. Inflammation: old, caffeinated, and healthy. Nat. Rev. Cardiol 14, 194–196. [DOI] [PubMed] [Google Scholar]
- Team R, 2015. RStudio: Integrated Development for R RStudio, Inc, Boston, MA. [Google Scholar]
- Timpson NJ, Nordestgaard BG, Harbord RM, Zacho J, Frayling TM, Tybjærg-Hansen A, Smith GD, 2011. C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int. J. Obesity 2005 (35), 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker S, Shirom A, Shapira I, Berliner S, Melamed S, 2005. The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. J. Occup. Health Psychol 10, 344–362. [DOI] [PubMed] [Google Scholar]
- Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, Cornell ES, Kuller LH, 1997. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler. Thromb. Vasc. Biol 17, 2167–2176. [DOI] [PubMed] [Google Scholar]
- Tsuno N, Besset A, Ritchie K, 2005. Sleep and depression. J. Clin. Psychiatry 66, 1254–1269. [DOI] [PubMed] [Google Scholar]
- Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E, 2003. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology 170, 429–433. [DOI] [PubMed] [Google Scholar]
- Tully PJ, Baumeister H, Martin S, Atlantis E, Jenkins A, Januszewski A, O’Loughlin P, Taylor A, Wittert GA, Florey Adelaide Male Ageing Study, 2016. Elucidating the biological mechanisms linking depressive symptoms with Type 2 diabetes in men: the longitudinal effects of inflammation, microvascular dysfunction, and testosterone. Psychosom. Med 78, 221–232. [DOI] [PubMed] [Google Scholar]
- Uchida N, Chong MY, Tan CH, Nagai H, Tanaka M, LEE MS, Fujii S, Yang SY, Si T, Sim K, 2007. International study on antidepressant prescription pattern at 20 teaching hospitals and major psychiatric institutions in East Asia: analysis of 1898 cases from China, Japan, Korea, Singapore and Taiwan. Psychiatry Clin. Neurosci 61, 522–528. [DOI] [PubMed] [Google Scholar]
- Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A, 2014. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am. J. Psychiatry 171, 1278–1286. [DOI] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL, 2013. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord 150, 736–744. [DOI] [PubMed] [Google Scholar]
- Van Bavel JJ, Mende-Siedlecki P, Brady WJ, Reinero DA, 2016. Contextual sensitivity in scientific reproducibility. Proc. Natl. Acad. Sci 113, 6454–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek P, Radder A, Hermans J, 1990. The significance of body temperature, sedimentation, C-reactive protein, leukocyte count and differential for the diagnosis of infections in an internal medicine emergency department. Ned. Tijdschr. Geneeskd. 134, 2536–2540. [PubMed] [Google Scholar]
- van Reedt Dortland AK, Giltay EJ, van Veen T, Zitman FG, Penninx BW, 2013. Longitudinal relationship of depressive and anxiety symptoms with dyslipidemia and abdominal obesity. Psychosom. Med 75, 83–89. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Correll CU, Wampers M, Sienaert P, Mitchell A, De Herdt A, Probst M, Scheewe TW, De Hert M, 2014. Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalences and moderating variables. Psychol. Med 44, 2017–2028. [DOI] [PubMed] [Google Scholar]
- Vashist SK, Venkatesh A, Schneider EM, Beaudoin C, Luppa PB, Luong JH, 2016. Bioanalytical advances in assays for C-reactive protein. Biotechnol. Adv 34, 272–290. [DOI] [PubMed] [Google Scholar]
- Vaucher J, Marques-Vidal P, Waeber G, Vollenweider P, 2014. Cytokines and hs-CRP levels in individuals treated with low-dose aspirin for cardiovascular prevention: a population-based study (CoLaus Study). Cytokine 66, 95–100. [DOI] [PubMed] [Google Scholar]
- Ventorp F, Gustafsson A, Träskman-Bendz L, Westrin Å, Ljunggren L, 2015Increased soluble urokinase-type plasminogen activator receptor (suPAR) levels in plasma of suicide attempters. PLoS One 10, e0140052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verduijn J, Milaneschi Y, Schoevers RA, van Hemert AM, Beekman AT, Penninx BW, 2015. Pathophysiology of major depressive disorder: mechanisms involved in etiology are not associated with clinical progression. Transl. Psychiatry 29, e649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Kritchevsky SB, Beekman AT, Newman AB, Satterfield S, Simonsick EM, Yaffe K, Harris TB, Penninx BW, 2008. Depressive symptoms and change in abdominal obesity in older persons. Arch. Gen. Psychiatry 65, 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Duivis HE, Beekman AT, Kluft C, Neuteboom J, Hoogendijk W, Smit JH, de Jonge P, Penninx BW, 2012. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl. Psychiatry 21, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JJ, Tung T-H, Yin W-H, Huang CM, Jen HL, Wei J, Young M-S, 2008. Effects of moderate alcohol consumption on inflammatory biomarkers. Acta Cardiol 63, 65–72. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU, 1993. Sex differences in rates of depression: cross-national perspectives. J. Affect. Disord 29, 77–84. [DOI] [PubMed] [Google Scholar]
- Welsh P, Woodward M, Rumley A, Lowe G, 2008. Associations of plasma pro-inflammatory cytokines, fibrinogen, viscosity and C-reactive protein with cardiovascular risk factors and social deprivation: the fourth Glasgow MONICA study. Br. J. Haematol 141, 852–861. [DOI] [PubMed] [Google Scholar]
- Whicher J, Chambers R, Higginson J, Nashef L, Higgins P, 1985. Acute phase response of serum amyloid A protein and C reactive protein to the common cold and influenza. J. Clin. Pathol 38, 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Kivimäki M, Jokela M, Batty GD, 2017. Association of inflammation with specific symptoms of depression in a general population of older people: the English Longitudinal Study of Ageing. Brain Behav. Immun 61, 27–30. [DOI] [PubMed] [Google Scholar]
- Wierzbicki A, 2006. Statins and hypertension. J. Hum. Hypertens 20, 554–556. [DOI] [PubMed] [Google Scholar]
- Williams DR, 1999. Race, socioeconomic status, and health the added effects of racism and discrimination. Ann. N. Y. Acad. Sci 896, 173–188. [DOI] [PubMed] [Google Scholar]
- Williams CJ, Fargnoli JL, Hwang JJ, Van Dam RM, Blackburn GL, Hu FB, Mantzoros CS, 2008. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes. Diabetes Care 31, 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkleby MA, Jatulis DE, Frank E, Fortmann SP, 1992. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am. J. Public Health 82, 816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshin S, Schwartz LM, 2005. Distribution of C-reactive protein values in the United States. N. Engl. J. Med 352, 1611–1613. [DOI] [PubMed] [Google Scholar]
- Xu SJ, Jiang CQ, Zhang WS, Cheng KK, Schooling CM, Xu L, Liu B, Jin YL, Lam KBH, Lam TH, 2016. Alcohol sensitivity, alcohol use and high-sensitivity C-reactive protein in older Chinese men: the Guangzhou Biobank Cohort Study. Alcohol 57, 41–48. [DOI] [PubMed] [Google Scholar]
- Yamada S, Gotoh T, Nakashima Y, Kayaba K, Ishikawa S, Nago N, Nakamura Y, Itoh Y, Kajii E, 2001. Distribution of serum C-reactive protein and its association with atherosclerotic risk factors in a Japanese population: Jichi Medical School Cohort Study. Am. J. Epidemiol 153, 1183–1190. [DOI] [PubMed] [Google Scholar]
- Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF, 2007. Systemic effects of smoking. Chest 131, 1557–1566. [DOI] [PubMed] [Google Scholar]
- Zampelas A, Panagiotakos DB, Pitsavos C, Chrysohoou C, Stefanadis C, 2004. Associations between coffee consumption and inflammatory markers in healthy persons: the ATTICA study. Am. J. Clin. Nutr 80, 862–867. [DOI] [PubMed] [Google Scholar]
- Zeman M, Jirak R, Jachymova M, Vecka M, Tvrzicka E, Zak A, 2009. Leptin, adiponectin, leptin to adiponection ratio and insulin resistance in depressive women. Neuro. Endocrinol. Lett 30, 387–395. [PubMed] [Google Scholar]
- Zhao G, Ford ES, Li C, Tsai J, Dhingra S, Balluz LS, 2011. Waist circumference, abdominal obesity, and depression among overweight and obese US adults: National Health and Nutrition Examination Survey 2005–2006. BMC Psychiatry 11, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Kremers HM, Yawn BP, Bobo WV, Sauver JLS, Ebbert JO, Rutten LJF, Jacobson DJ, Brue SM, Rocca WA, 2014. Time trends of antidepressant drug prescriptions in men versus women in a geographically defined US population. Archiv. Women’s Mental Health 17, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Boudreau DM, Freedman AN, 2014. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general US population. Pharmacoepidemiol. Drug Saf 23, 43–50. [DOI] [PubMed] [Google Scholar]
- Zoga M, Oulis P, Chatzipanagiotou S, Masdrakis VG, Pilatsika P, Boufidou F, Foteli S, Soldatos CR, Nikolaou C, Papageorgiou C, 2014. Indoleamine 2, 3-dioxygenase and immune changes under antidepressive treatment in major depression in females. In Vivo 28, 633–638. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.