SYNOPSIS
Objectives:
To compare the effectiveness and tolerability of micafungin versus posaconazole during chemotherapy-induced neutropenia in acute leukemia (AL) and myelodysplastic syndrome (MDS).
Methods:
Patients with AL or MDS undergoing chemotherapy were randomized to open-label micafungin 100 mg intravenously daily or posaconazole suspension 400 mg orally twice daily until neutrophil recovery, up to 28 days. Patients were followed for 12 weeks. The primary endpoint was prophylaxis failure (premature discontinuation due to infection, intolerance, adverse event, or death). Time to failure and survival were calculated by Kaplan-Meier analysis.
Results:
From March 2011 to May 2016, 113 patients who received at least 2 doses of prophylaxis were analyzed (58 patients randomized to micafungin and 55 to posaconazole). Prophylaxis failure occurred in 34.5% and 52.7% of patients on micafungin and posaconazole, respectively (P = 0.0118). The median number of days on prophylaxis was 16 [interquartile range (IQR) 12-20] for micafungin and 13 [IQR 6-16] for posaconazole (P = 0.01). Micafungin failures were largely due to antifungal treatment; posaconazole failures were mostly due to gastrointestinal intolerance or adverse effects. IFI incidence and survival were similar between study arms.
Conclusions:
Our data support micafungin as alternative antifungal prophylaxis in patients with AL and MDS.
Keywords: immunocompromised hosts, neutropenia, invasive fungal infections, prophylaxis, posaconazole, micafungin, leukemia, myelodysplastic syndrome
1. INTRODUCTION
Invasive fungal infections (IFIs) are common in patients receiving intensive chemotherapy for acute leukemia (AL) and myelodysplastic syndrome (MDS) and are associated with significant morbidity and mortality.1, 2 For this reason, antifungal prophylaxis is recommended for these high-risk patient groups who have prolonged neutropenia due to intensive chemotherapy.3, 4 Prophylaxis with posaconazole oral suspension has been shown to effectively prevent IFIs and improve survival compared with fluconazole or itraconazole prophylaxis in patients undergoing intensive chemotherapy for AL or MDS.5 Oral posaconazole suspension and delayed-release tablets are currently approved by the United States Food and Drug Administration (FDA) for this indication in patients 13 years and older and is recommended by the Infectious Diseases Society of America (IDSA) and the National Comprehensive Cancer Network (NCCN) as the preferred prophylaxis for these specific high-risk patient groups.3, 4, 6
The administration of posaconazole prophylaxis can be challenging due to pharmacokinetic variability, drug interactions, and mucositis or colitis precluding administration of oral medications or nutritional supplements required for adequate posaconazole absorption.7 Therapeutic drug monitoring is sometimes used due to an association between low drug levels and breakthrough IFIs during prophylaxis although this monitoring increases provider burden and treatment cost.8 Delayed-release posaconazole tablets, approved by the FDA in 2013, have improved bioavailability compared with the suspension, but administration with food is still recommended for optimal absorption, and the tablets cannot be crushed or split for patients with swallowing impairment.6 However, this newer formulation of posaconazole, as well as the parenteral form, do not remedy other potential disadvantages of the drug. Posaconazole has substantial interactions with commonly used medications; it also may potentiate vincristine-associated neurotoxicity and interacts with vincristine and tyrosine kinase inhibitors and other treatments for AL.6, 9 Furthermore, posaconazole has been linked with QT-interval prolongation and hepatotoxicity, which sometimes requires drug discontinuation.6
Micafungin is an attractive alternative to posaconazole as antifungal prophylaxis because of its activity against yeasts and Aspergillus species, availability as an intravenous (IV) formulation, favorable safety profile, and minimal drug interactions. Potential concerns include lack of activity against Cryptococcus and other organisms and lower tissue levels than achieved by some azoles.10 Micafungin prophylaxis has been shown to be effective in patients with hematopoietic cell transplantation (HCTs) in randomized trials and cohort studies and is approved by the FDA for this indication.11-18 However, limited data are available on the use of micafungin prophylaxis in patients with AL or MDS. Two randomized trials have shown similar effectiveness of caspofungin compared with other agents, mostly itraconazole and fluconazole.19, 20 Cohort studies comparing echinocandins with voriconazole and posaconazole have shown variable results.21, 22 Though these cohort studies suggest the potential effectiveness of echinocandin prophylaxis in these patients, micafungin has not been compared to posaconazole in a prospective, randomized trial.
Since 2008, posaconazole has been the preferred antifungal prophylaxis in patients with neutropenia due to intensive chemotherapy for AL and MDS at our institution. However, approximately one-third of patients have historically been switched to alternative antifungal prophylactic agents due to inability to tolerate or absorb oral medications or due to interactions of posaconazole with concomitant medications. We conducted a randomized open-label study to compare micafungin with posaconazole for antifungal prophylaxis in patients with AL or MDS undergoing intensive chemotherapy at our institution.
2. PATIENTS AND METHODS
2.1. Study Design
The study was reviewed and approved by the Memorial Sloan Kettering (MSK) Institutional Review Board. Written informed consent was obtained from each participant.
This was a single-center, randomized, parallel-group, open-label study of micafungin versus posaconazole as antifungal prophylaxis (Clinicaltrials.gov: ). Patients were assigned in a 1:1 ratio to receive posaconazole oral suspension or micafungin starting 24 to 48 hours after completion of intensive chemotherapy. Patients were followed through 12 weeks after the first study drug dose for occurrence of IFI and survival.
2.2. Patients
Starting on March 1, 2011, patients aged 18 and older admitted to receive intensive chemotherapy for newly diagnosed or first relapsed acute myeloid leukemia (AML), acute lymphocytic leukemia, or MDS with an anticipated duration of neutropenia (absolute neutrophil count [ANC] less than 500/μL) of at least 7 days and able to take oral medications at the time of randomization were eligible to participate in the study. Exclusion criteria included diagnosis of IFI less than 30 days prior to randomization or significant hepatic or renal disease. Other exclusion criteria can be found in the Supplemental Material.
2.3. Administration of the Study Drug
Antifungal prophylaxis started 24 to 48 hours after completion of intensive chemotherapy. Posaconazole oral suspension (400 mg orally twice daily) was administered until neutrophil recovery or up to 28 days. Posaconazole delayed release tablets became available in January 2014, but because however, enrollment had reached 70% accrual by that point, it was decided to complete the study with oral suspension per protocol. Twice-daily dosing of posaconazole is approved for treatment of fungal infections and has been shown to produce similar serum drug levels to the standard dosing regimen (posaconazole suspension 200 mg three times daily) recommended for prophylaxis.23-25 Micafungin (100 mg) was administered intravenously once daily, consistent with guidelines that recommend a dose range of 50 to 100 mg per day for prophylaxis.26 Patients who were unable to tolerate the oral study drug could receive intravenous micafungin prophylaxis at the discretion of the treating physician not to exceed a total of 3 days. Patients in either group were permitted to receive liposomal amphotericin B (L-AmB) or another systemic agent as empirical antifungal therapy for a suspected IFI.
2.4. Monitoring and Treatment of Enrolled Patients
All patients underwent comprehensive evaluation per MSK institutional standards of care for IFI. Blood cultures were obtained for workup of fever and neutropenia, and empiric antibiotics were started per IDSA and NCCN guidelines.4, 8 Patients with signs or symptoms of infection, including fever, underwent a complete evaluation, including microbiologic testing and computerized tomography of the chest, abdomen, pelvis, and sinuses, as clinically indicated. Bronchoscopy with bronchoalveolar lavage or other sampling was obtained if clinically feasible for patients with pulmonary infiltrates. Surveillance blood specimens were collected at baseline and twice weekly during prophylaxis for the Aspergillus galactomannan assay and (1→3)-ß-D-glucan (BDG) assay.
For patients with persistent fever after 3 days of broad-spectrum antibiotics but without an apparent infectious source, a switch to empiric antifungal treatment using L-AmB (3 mg/kg IV daily) was acceptable. If there was a suspected IFI, voriconazole (6 mg/kg IV every 12 hours for 2 doses, then 4 mg/kg IV every 12 hours) or L-AmB (5 mg/kg IV daily) was used at the discretion of the treating physician. Alternative doses and other treatment approaches were sometimes employed by the treating physician based on clinical judgment.
2.5. Definition of Endpoints
2.5.1. Primary Endpoint
The primary endpoint was time to prophylaxis failure. Failure was defined as any of the following: occurrence of a proven or probable IFI during prophylaxis; receipt of IV prophylaxis for more than 3 consecutive days due to concerns about inability to tolerate or absorb oral posaconazole (as determined by the treating clinicians due to severe nausea, emesis, mucositis, or colitis; serum posaconazole levels were generally not available to clinicians at the time of discontinuing posaconazole); receipt of any other systemic antifungal agent for more than 3 days for persistent fever or suspected IFI; occurrence of a treatment-emergent adverse event (TEAE), regardless of causality, resulting in discontinuation of prophylaxis; withdrawal from the study with no additional follow-up; or death.
2.5.2. Secondary Endpoints
Secondary endpoints included reasons for prophylaxis failure, the incidence and types of IFI during the prophylaxis phase and follow-up phase, and overall survival at end of study. Time to IFI was also assessed. Posaconazole serum levels were collected and analyzed as secondary explorative endpoints.
Proven, probable, and possible IFI were scored by standard European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC) criteria.27 Suspected IFIs included these proven, probable, and possible IFIs, along with manifestations not meeting these criteria (such as persistent fever in an appropriate clinical context) assessed by the treating clinician to potentially represent an IFI for which empiric treatment was indicated. Prophylaxis phase was defined as the duration of the prophylaxis study drug plus 7 days after the study drug was discontinued. Follow-up phase was defined as the period from 7 days after discontinuation of the prophylaxis study drug through the end of study (12 weeks from study drug initiation).
Statistical Analysis
Baseline demographic and clinical characteristics were compared between the two treatment arms. The Wilcoxon rank-sum test (Mann-Whitney U test) was used for continuous variables; the χ2 or Fischer’s exact tests were used for categorical variables.
2.5.3. Primary Outcomes
The primary efficacy analysis was based on a modified intention-to-treat (mITT) approach, with the use of data from patients who underwent randomization and received 2 or more doses of prophylaxis. The trial was designed with power to detect absolute differences of approximately 25% of prophylaxis failure in the two groups with approximately 80% power and a significance level of 5% using a two-tail test.
The time to failure of prophylaxis was calculated from the Kaplan-Meier method. The log-rank test was used to compare time to failure between micafungin and posaconazole. Patients who discontinued prophylaxis prematurely and did not meet criteria for failure were censored at the time of drug discontinuation.
2.5.4. Secondary Outcomes
The time to first probable or proven IFI was evaluated with the Kaplan-Meier method and the two arms were compared with the log-rank test. Death was a competing risk for IFI. The Kaplan-Meier survival estimates were used to calculate overall survival at 12 weeks and the log-rank test was used for comparison between the two arms. Unless otherwise specified, statistical significance was defined as a P value of less than 0.05. Similarly, two-tailed tests assuming independent data were used unless otherwise stated. SAS statistical software version 9.4 (SAS Institute, Cary, NC) was used.
We measured the steady-state plasma concentrations of posaconazole and calculated the arithmetic means using liquid chromatography with a mass-spectrometric detection method.28 Prior to May 2014, serum posaconazole levels were determined at Focus Technologies (Cypress, CA); after May 2014, levels were assayed at the clinical microbiology laboratory at Memorial Sloan Kettering.
3. RESULTS
3.1. Patient Characteristics
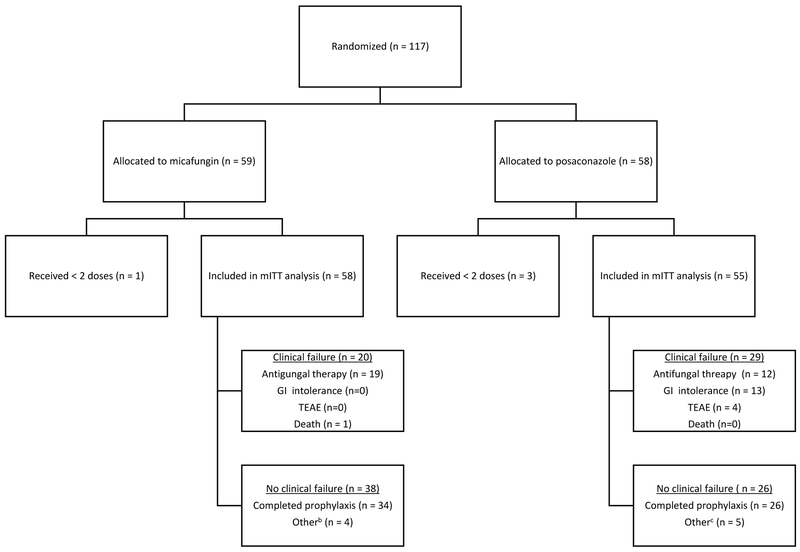
Between March 1, 2011 and May 31, 2016, a total of 117 patients were randomized. One hundred and thirteen patients who received 2 or more doses of prophylaxis were included in the mITT analysis. Demographic and clinical characteristics were similar between the two arms (Table 1).
Table 1.
Baseline characteristics of patients included in the mITT analysis1
| Characteristic | Micafungin (N=58) |
Posaconazole (N=55) |
P value |
|
|---|---|---|---|---|
| Age, years | Median (range) |
Median (range) |
||
| 61 (32-75) | 59 (26-74) | 0.10 | ||
| N (%) | N (%) | |||
| Race | 0.14 | |||
| White | 50 (86.2) | 43 (78.2) | ||
| Black | 6 (10.3) | 3 (5.5) | ||
| Asian | 1 (1.7) | 5 (9.1) | ||
| Other/Refused | 1 (1.7) | 4 (7.3) | ||
| Male sex | 33 (56.9) | 29 (52.7) | 0.66 | |
| Treatment history | Treatment naïve | 42 (72.4) | 40 (72.7) | 0.28 |
| Prior chemotherapy without alloHCT | 13 (22.4) | 8 (14.5) | ||
| Prior allogeneic HCT | 3 (5.2) | 7 (12.7) | ||
| Underlying disease | 0.57 | |||
| AML | 51 (87.9) | 45 (81.8) | ||
| MDS | 4 (6.9) | 7 (12.7) | ||
| ALL | 1 (1.7) | 2 (3.6) | ||
| Other2 | 2 (3.4) | 1 (1.8) | ||
| Chemotherapy type | 0.49 | |||
| 7 + 33 | 42 (72.4) | 40 (72.7) | ||
| MEC4 | 8 (13.8) | 4 (7.3) | ||
| Purine-analog-based | 2 (3.4) | 5 (9.1) | ||
| Other5 | 6 (10.3) | 6 (10.9) | ||
| Prior hypomethylating agents | 12 (20.7) | 18 (32.7) | 0.15 | |
| Median (IQR) | Median (IQR) | |||
| Days of neutropenia6 | 18 (15-26) | 21 (16-28) | 0.07 |
Abbreviations: mITT = modified intention-to-treat; alloHCT = allogeneic hematopoietic cell transplant; AML = acute myeloid leukemia; MDS = myelodysplastic syndrome; ALL = acute lymphocytic leukemia.
Includes adult T-cell leukemia/lymphoma; chronic myeloid leukemia with accelerated phase versus blast crisis; and acute leukemia, multilineage.
Includes either daunorubicin or idarubicin when combined with 7 days of cytarabine.
Mitoxantrone, etoposide and intermediate-dose cytarabine.
Includes HiDAC (high-dose cytarabine), elacytarabine, CALGB (Cancer and Leukemia Group B) 10403 regimen, cytarabine + etoposide, cytarabine + mitoxantrone, etoposide + mitoxantrone, and 5 + 3 with idarubicin.
Capped at 28 days from first dose of study drug.
Baseline fungal biomarkers were available for 91% of patients randomized to micafungin and 100% of patients randomized to posaconazole. Baseline serum galactomannan was negative for all tested patients. Baseline BDG was negative for all patients except for 1 patient randomized to posaconazole who had a transient and mild elevation at baseline and a negative result on repeat testing after 3 days of posaconazole.
3.2. Primary Outcome
Prophylaxis failure occurred in 20 of 58 patients (34.5%) in the micafungin arm and 29 of 55 patients (52.7%) in the posaconazole arm (P = 0.0118) (Figure 2).
Figure 2.
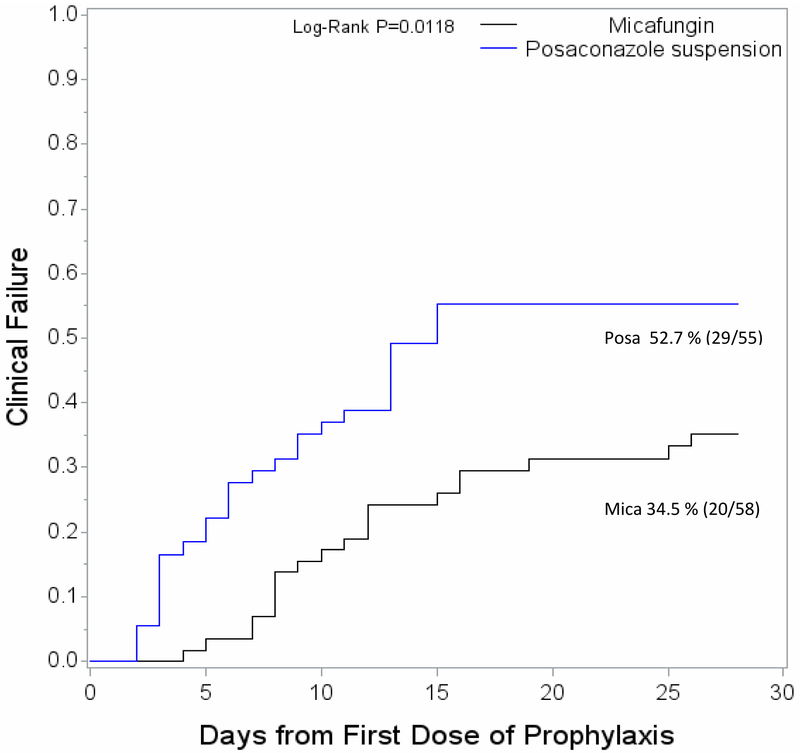
Time to clinical failure of prophylaxis
The median duration of prophylaxis on the study drug was 16 days (interquartile range [IQR]) 12-20) for micafungin versus 13 days (IQR 6-16) for posaconazole (P = 0.01).
Table 2 shows patient disposition for the 2 arms. The reasons for prophylaxis failure differed substantially between the 2 arms (P < 0.001). Initiation of systemic antifungal therapy due to suspected IFI (including possible, probable, and proven IFIs and suspected IFI not meeting EORTC criteria) accounted for the majority (95%) of all discontinuations in the micafungin group. In contrast, clinician concerns regarding ability to tolerate or reliably absorb oral medication accounted for nearly half (44.8%) of all discontinuations in the posaconazole arm, followed by initiation of systemic antifungal therapy (41.4%) and TEAE (13.8%). The time-to-discontinuation of posaconazole due to clinician concerns over intolerance or poor absorption was a median of 5 days (IQR 3-6) compared to discontinuation for suspected IFI or persistent fever at a median of 12 days (IQR 9-14).
Table 2.
Disposition of patients included in the mITT analysis7
| Disposition | Micafungin (N=58) |
Posaconazole (N=55) |
||
|---|---|---|---|---|
| N (%) | N (%) | |||
| Did not complete prophylaxis | 24 (41.4) | 21 (38.2) | ||
| Clinical failure | 20 (34.5) | 29 (52.7) | ||
| Other8 | 4 (6.9) | 5 (9.1) | ||
| Did not complete study9 | 7 (12.1) | 2 (3.6) | ||
| Median (IQR) |
Median (IQR) | |||
| Days on prophylaxis | 16 (12-20) | 13 (6-16) | ||
| N (%) | N (%) | |||
| Reasons for failure | ||||
| Antifungal therapy | 19 (95%) | 12 (41.4) | ||
| Possible IFI | 6 | 3 | ||
| Probable IFI | 1 | 0 | ||
| Persistent fever | 3 | 3 | ||
| Abnormal CT chest10 | 8 | 6 | ||
| Other11 | 1 | 0 | ||
| Gastrointestinal intolerance | 0 | 13 (44.8) | ||
| TEAE | 0 | 4 (13.8) | ||
| Death | 1 (5%) | 0 (0) |
Abbreviations: mITT = modified intention-to-treat; IFI = invasive fungal infection; CT = computed tomography; TEAE = treatment-emergent adverse event.
These did not complete prophylaxis but did not meet criteria for clinical failure.
12 weeks from first dose of antifungal prophylaxis.
New findings on chest CT not meeting EORTC (European Organization for Research and Treatment of Cancer) criteria for IFI.
Throat fungal culture with Aspergillus spp., not meeting EORTC criteria for IFI.
Among patients who discontinued prophylaxis for suspected IFI, the time-to-discontinuation was similar between the 2 arms: median of 11 days (IQR 8-16) in the micafungin arm versus 12 days (IQR 9-14) in the posaconazole arm.
3.3. Secondary Outcomes
3.3.1. IFI Incidence
The incidence of probable or proven IFI during the study was low and similar between the two study arms (P = NS) (Figure 3). Overall, a total of 8 IFIs occurred during the study. Of the 3 IFIs occurring during prophylaxis, 2 occurred in the micafungin arm and 1 in the posaconazole arm. Of the 5 IFIs that occurred during the follow-up period, 3 occurred in the micafungin arm and 2 in the posaconazole arm. Table 3 shows the types of IFIs by study arm. Due to the small number of cases, no formal comparison was performed with regards to the IFI types between the two study arms.
Figure 3.
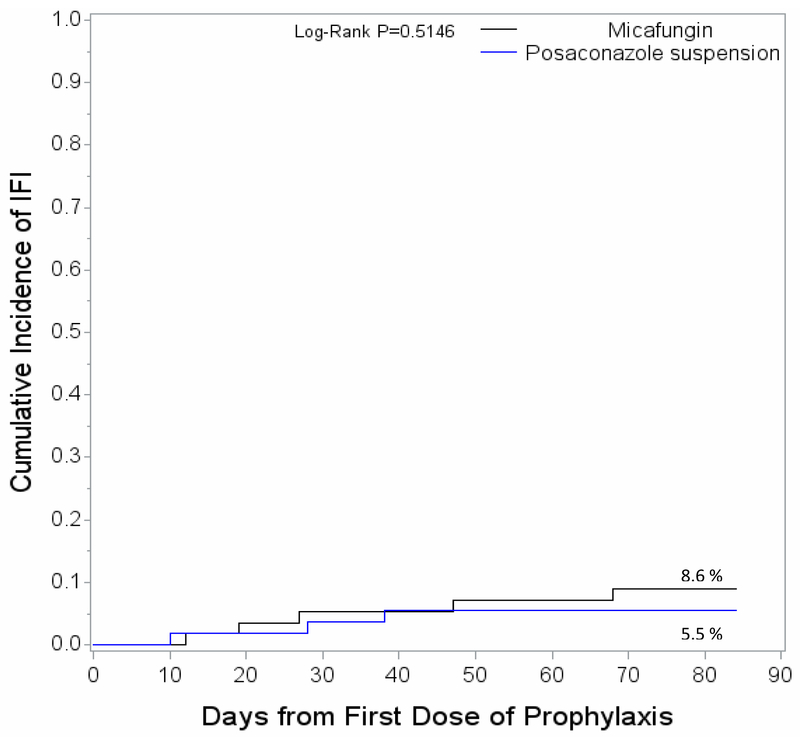
Cumulative incidence of invasive fungal infections during the study
Table 3.
Probable or proven IFIs occurring during study in the mITT population12
| IFI | Micafungin (N=58) |
Posaconazole (N=55) |
|
|---|---|---|---|
| N (%) | N (%) | ||
| Prophylaxis period: During and for up to 7 days following study drug administration | 2 (3.4) | 1 (1.7) | |
| Aspergillus spp.13 | 0 | 1 | |
| Candida albicans | 1 | 0 | |
| Cryptococcus spp.14 | 1 | 0 | |
| Follow-up period: From 7 days after drug discontinuation through end of study (12 weeks or death) | 3 (5.5) | 2 (3.6) | |
| Aspergillus spp. | 2 | 0 | |
| Candida spp.15 | 0 | 1 | |
| Candida tropicalis | 1 | 0 | |
| Trichosporon asahii16 | 0 | 1 |
Abbreviations: IFI = invasive fungal infection; mITT = modified intention-to-treat.
Aspergillus spp. was diagnosed by the serum galactomannan assay.
Cryptococcus spp. was diagnosed by fungal polymerase chain reaction (PCR).
Candida spp. was diagnosed by presence of fungal elements consistent with Candida in biopsy specimen.
Trichosporon asahii was identified by PCR of liver tissue.
3.3.2. Overall Survival
Overall, 9 patients died during the study, including 7 patients in the micafungin arm and 2 in the posaconazole arm. The Kaplan-Meier estimate for overall survival was 87.9% and 96.3% for micafungin and posaconazole, respectively (P = 0.1036).
Of the 7 deaths in the micafungin arm, 2 deaths were related to IFIs, and both occurred in the follow-up period. One patient died of disseminated Candida tropicalis infection during consolidation therapy, and another patient with refractory AML died of disseminated aspergillosis. The remaining deaths in the micafungin arm were not attributable to IFI. Causes of death included bacterial sepsis (due to mucosal barrier injury laboratory-confirmed bloodstream infection) during prophylaxis (n = 1), relapse of underlying malignancy (n = 3), and transplant-related mortality (n = 1). Of the 2 deaths in the posaconazole arm, both occurred during the follow-up period and were due to relapsed disease.
3.3.3. Posaconazole levels
Twenty-four patients had serum posaconazole levels assessed during prophylaxis; patients with drug discontinuation earlier than 7 days did not have posaconazole levels drawn. Thirteen patients (54%) had serum levels of at least 0.7 pg/mL and 11 (46%) had levels less than 0.7 pg/mL. Because the time to discontinuation of posaconazole due to intolerance or concern for poor absorption was a median of 5 days (IQR 3-6), determination of posaconazole level was not performed for many patients.
3.3.4. Other
Data on fungal colonization were collected and are described in the Supplemental Material.
4. DISCUSSION
We conducted a randomized, parallel-group, open-label study at a major cancer center in the United States to compare the effectiveness and tolerability of micafungin versus posaconazole during neutropenia from intensive chemotherapy for AL and MDS. Approximately one-quarter of the enrolled patients received re-induction chemotherapy, including 10 (8.8%) with prior HCT, with a median duration of significant neutropenia (defined as ANC less than 100/μL) of greater than 2 weeks. These patient characteristics have been associated with an increased risk for IFI, for which mold-active antifungal prophylaxis is recommended.3
During the study, posaconazole suspension was administered at 400 mg twice daily for tolerability and convenience. Twice-daily dosing of posaconazole is approved for treatment of fungal infections and has been shown in multiple studies to produce similar serum drug levels to the standard dosing regimen (posaconazole suspension 200 mg three times daily) recommended for prophylaxis.23-25 The primary endpoint of the study was time to failure of prophylaxis. This composite endpoint encompassed the most common reasons for discontinuing or switching to alternative antifungal prophylaxis in clinical practice. Thus, discontinuation of oral posaconazole due to intolerance of oral medications or physician concerns over absorption constituted failure of prophylaxis. Similarly, discontinuation of the allocated prophylaxis due to a TEAE regardless of severity was at the discretion of the treating physician and constituted failure of prophylaxis.
Patients stayed on micafungin prophylaxis significantly longer than posaconazole suspension. Fifty- nine percent of patients in the micafungin arm completed prophylaxis successfully compared to 38% of patients in the posaconazole arm. A major driver of clinical failure in the posaconazole arm, accounting for nearly half of failures, was intolerance or concern for poor absorption of oral medication in patients with severe mucositis, colitis, diarrhea, nausea, or emesis. One-third of patients in the posaconazole arm discontinued the assigned prophylaxis by day 7 prior to achieving posaconazole steady state. Of patients with clinical failure on posaconazole, 13.8% had the drug discontinued due to a TEAE. While the most common TEAE was mild and reversible biochemical hepatotoxicity, discontinuation reflects common current clinical practice, where, in the setting of abnormal liver function tests, all potentially hepatotoxic medications are discontinued if feasible. Of note, discontinuations in the posaconazole arm due to intolerance, or clinician concern for absorption and TEAE, occurred much earlier than discontinuations for suspected IFI.
In the micafungin arm, 95% of prophylaxis failures were due to initiation of systemic antifungal therapy for suspected IFIs. The time-to-discontinuation due to suspected IFI was similar between the micafungin and posaconazole arms.
The number of observed IFIs during the study was low and similar to rates reported in recent studies of posaconazole prophylaxis. Notably, one of the 3 IFIs occurring during the prophylaxis phase was a case of cryptococcosis in the micafungin arm. Micafungin does not have activity against Cryptococcus species. Overall survival was similar in both arms, and the majority of deaths were due to disease relapse.
Our study has several limitations. First, the open-label design may have introduced a bias favoring micafungin. When clinicians know of an alternative IV medication and patients experience even mild diarrhea or nausea, providers may opt to change to the IV medication even if the oral medication would have been effective if continued. For this reason, it is plausible that clinicians may have discontinued posaconazole earlier than they would have done in a blinded study. Second, the choice of alternative antifungal drug for prophylaxis or therapy after discontinuation of the study drug was at the clinician’s discretion. This heterogeneity in antifungal drugs and variable duration of prophylaxis between the two arms may have introduced differences in rates and types of IFI observed during follow-up. Since the rates of IFI during the study were exceedingly low in both arms, we can only conclude that the current standard of care is associated with low IFI rates. Finally, many centers now use posaconazole tablets rather than suspension for prophylaxis. While posaconazole tablets have higher bioavailability, issues with toxicity and drug interactions remain. Since posaconazole suspension has established benefit in preventing IFIs in this patient population, and since posaconazole tablets have not been proven to be more effective than suspension for this indication, posaconazole suspension remains a valid reference standard against which other agents can be compared.
While acknowledging these limitations, our study has several strengths. It is the first randomized study comparing micafungin to posaconazole as antifungal prophylaxis in patients with AL and MDS treated at a major cancer center. The composite endpoint of failure reflects the multitude of challenges in clinical practice and real-world management decisions in this complex patient population.
Our data confirm the safety and tolerability of echinocandins in patients with hematologic malignancies, concordant with prior studies. 19, 20, 22 The rates of IFIs in both arms were low and also consistent with prior studies of antifungal prophylaxis with posaconazole and echinocandins.22, 23 Notably, micafungin is only available in parenteral form, complicating its use in patients discharged while still neutropenic and requiring antifungal prophylaxis.
In summary, our data support the use of micafungin as an alternative to posaconazole suspension for antifungal prophylaxis in patients with AL and MDS undergoing intensive chemotherapy. Our findings are particularly relevant in view of novel anti-leukemia therapies that have significant interactions with azoles.
Supplementary Material
Figure 1.
CONSORT flow charta
a Abbreviations: mITT = modified intention-to-treat; TEAE = treatment-emergent adverse event; GI intolerance includes inability to tolerate and/or absorb oral medication or food intake
b 4 patients discontinued micafungin prophylaxis prematurely because they were discharged prior to neutrophil recovery
c 5 patients discontinued posaconazole prophylaxis prematurely due to concern for drug-drug interactions with new medications or for administrative reasons
Figure 4.
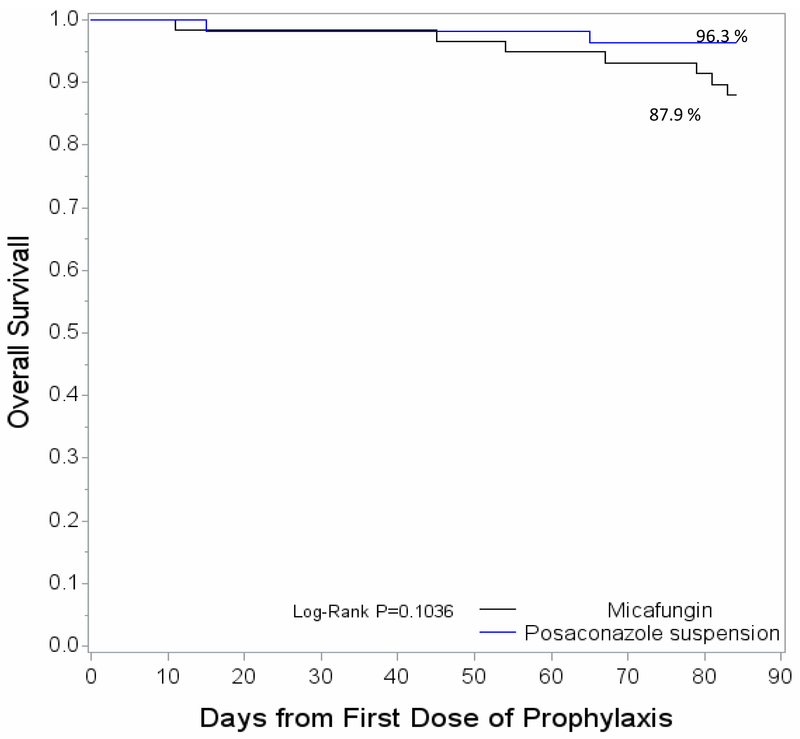
Overall survival at 12 weeks from first dose of antifungal prophylaxis
5. ACKNOWLEDGEMENTS
We acknowledge Elizabeth Halton NP, Gaelle Senatus NP, Jamilla Brutus NP, Michel Turner PA, and all advanced care practitioners and nursing staff of the MSK Leukemia Service. We acknowledge Leslie Cheteyan, clinical research coordinator, and Amanda Walker, research study assistant.
6. FUNDING
This work was supported by an investigator-initiated study grant from Astellas Pharma Global Development, Inc. Micafungin for this study was provided by Astellas Pharma Global Development, Inc.
This work was also in part supported by the National Cancer Institute at the National Institutes of Health [P30 CA008748].
Footnotes
TRANSPARENCY DECLARATIONS
GAP and MGF have received research funding and/or consulting fees from Astellas Pharma and Merck & Co.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pagano L, Caira M, Candoni A et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 2006; 91: 1068–75. [PubMed] [Google Scholar]
- 2.Tang JL, Kung HC, Lei WC et al. High Incidences of Invasive Fungal Infections in Acute Myeloid Leukemia Patients Receiving Induction Chemotherapy without Systemic Antifungal Prophylaxis: A Prospective Observational Study in Taiwan. PLoS One 2015; 10: e0128410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, Swaminathan S, Angarone M et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016; 14: 882–913. [DOI] [PubMed] [Google Scholar]
- 4.Freifeld AG, Bow EJ, Sepkowitz KA et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52: 427–31. [DOI] [PubMed] [Google Scholar]
- 5.Cornely OA, Maertens J, Winston DJ et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356: 348–59. [DOI] [PubMed] [Google Scholar]
- 6.Posaconazole [package insert]. Whitehouse Station, NJ: Merck & Co., Inc, 2014. [Google Scholar]
- 7.Dolton MJ, Bruggemann RJ, Burger DM et al. Understanding variability in posaconazole exposure using an integrated population pharmacokinetic analysis. Antimicrob Agents Chemother 2014; 58: 6879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattaneo C, Panzali A, Passi A et al. Serum posaconazole levels during acute myeloid leukaemia induction therapy: correlations with breakthrough invasive fungal infections. Mycoses 2015; 58: 362–7. [DOI] [PubMed] [Google Scholar]
- 9.Moriyama B, Henning SA, Leung J et al. Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses 2012; 55: 290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev 2014; 27: 68–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiramatsu Y, Maeda Y, Fujii N et al. Use of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantation. Int J Hematol 2008; 88: 588–95. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Chen H, Han M et al. Multicenter, randomized, open-label study comparing the efficacy and safety of micafungin versus itraconazole for prophylaxis of invasive fungal infections in patients undergoing hematopoietic stem cell transplant. Biol Blood Marrow Transplant 2012; 18: 1509–16. [DOI] [PubMed] [Google Scholar]
- 13.van Burik JA, Ratanatharathorn V, Stepan DE et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 2004; 39: 1407–16. [DOI] [PubMed] [Google Scholar]
- 14.El-Cheikh J, Venton G, Crocchiolo R et al. Efficacy and safety of micafungin for prophylaxis of invasive fungal infections in patients undergoing haplo-identical hematopoietic SCT. Bone Marrow Transplant 2013; 48: 1472–7. [DOI] [PubMed] [Google Scholar]
- 15.Heimann SM, Vehreschild MJ, Meintker L et al. Different doses of micafungin for prophylaxis of invasive fungal diseases in hemato-oncological high-risk patients: a web-based non-interventional trial in four large university hospitals in Germany. Transpl Infect Dis 2014; 16: 968–74. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi C, Hanadate T, Niwa T et al. Safety and efficacy of micafungin for prophylaxis against invasive fungal infections in Japanese patients undergoing hematopoietic stem cell transplantation: Results of a post-marketing surveillance study. J Infect Chemother 2015; 21: 438–43. [DOI] [PubMed] [Google Scholar]
- 17.Hashino S, Morita L, Takahata M et al. Administration of micafungin as prophylactic antifungal therapy in patients undergoing allogeneic stem cell transplantation. Int J Hematol 2008; 87: 91–7. [DOI] [PubMed] [Google Scholar]
- 18.Langebrake C, Rohde H, Lellek H et al. Micafungin as antifungal prophylaxis in recipients of allogeneic hematopoietic stem cell transplantation: results of different dosage levels in clinical practice. Clin Transplant 2014; 28: 286–91. [DOI] [PubMed] [Google Scholar]
- 19.Mattiuzzi GN, Alvarado G, Giles FJ et al. Open-label, randomized comparison of itraconazole versus caspofungin for prophylaxis in patients with hematologic malignancies. Antimicrob Agents Chemother 2006; 50: 143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cattaneo C, Monte S, Algarotti A et al. A randomized comparison of caspofungin versus antifungal prophylaxis according to investigator policy in acute leukaemia patients undergoing induction chemotherapy (PROFIL-C study). J Antimicrob Chemother 2011; 66: 2140–5. [DOI] [PubMed] [Google Scholar]
- 21.Gomes MZ, Jiang Y, Mulanovich VE et al. Effectiveness of primary anti-Aspergillus prophylaxis during remission induction chemotherapy of acute myeloid leukemia. Antimicrob Agents Chemother 2014; 58: 2775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nachbaur D, Angelova O, Orth-Holler D et al. Primary antifungal prophylaxis with micafungin in patients with haematological malignancies: real-life data from a retrospective single-centre observational study. Eur J Haematol 2015; 94: 258–64. [DOI] [PubMed] [Google Scholar]
- 23.Cornely OA, Helfgott D, Langston A et al. Pharmacokinetics of different dosing strategies of oral posaconazole in patients with compromised gastrointestinal function and who are at high risk for invasive fungal infection. Antimicrob Agents Chemother 2012; 56: 2652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebeaux D, Lanternier F, Elie C et al. Therapeutic drug monitoring of posaconazole: a monocentric study with 54 adults. Antimicrob Agents Chemother 2009; 53: 5224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross AL, Slain D, Cumpston A et al. Evaluation of an alternative posaconazole prophylaxis regimen in haematological malignancy patients receiving concomitant stress ulcer prophylaxis. Int J Antimicrob Agents 2012; 40: 557–61. [DOI] [PubMed] [Google Scholar]
- 26.Patterson TF, Thompson GR, Denning DW et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63: e1–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Pauw B, Walsh TJ, Donnelly JP et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogeser M, Schiel X, Spohrer U. Quantification of voriconazole in plasma by liquid chromatography-tandem mass spectrometry. Clin Chem Lab Med 2005; 43: 730–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.