Abstract
SLC1A2 is a trimeric transporter essential for clearing glutamate from neuronal synapses. Recurrent de novo SLC1A2 missense variants cause a severe, early-onset developmental and epileptic encephalopathy via an unclear mechanism. We demonstrate that all three variants implicated in this condition localize to the trimerization domain of SLC1A2, and that the Leu85Pro variant acts via a dominant negative mechanism to reduce, but not eliminate, wild-type SLC1A2 protein localization and function. Finally, we demonstrate that treatment of a 20-month-old SLC1A2-related epilepsy patient with the SLC1A2 modulating agent ceftriaxone did not result in a significant change in daily spasm count.
Introduction
Glutamate is the predominant central nervous system excitatory neurotransmitter, and its synaptic concentration is kept under tight control to prevent neuronal damage from excessive activation of glutamate receptors1,2. Of the five glutamate transporters, the trimeric transporter SLC1A2 (also known at GLT-1 and EAAT2) and SLC1A3 mediate the bulk of glutamate clearance from the synaptic cleft via their expression in astrocytes. Pan-knockout or astrocyte-specific knockout of Slc1a2 in mice results in neuronal excitotoxicity, epilepsy and premature death3,4. Despite the severe epilepsy phenotype of homozygous Slc1a2 knockout mice, mice heterozygote for the Slc1a2 deletion demonstrate no apparent clinical phenotype3. Furthermore, heterozygous deletion of SLC1A2 in humans (as is frequently seen in the WAGR deletion syndrome) is only rarely associated with epilepsy5. In contrast to individuals with heterozygous SLC1A2 deletions, three heterozygous SLC1A2 missense variants have recently been implicated in severe early-onset epilepsy (OMIM: #617105)6–8. The mechanism by which these SLC1A2 missense variants cause epilepsy remains unclear, significantly hindering the ability to apply glutamate and SLC1A2-modulating therapies to treat these patients.
Methods
Identification of SLC1A2 variant
Singleton whole exome sequencing (Prevention Genetics, Marshfield, WI) revealed a pathogenic heterozygous c.254T>C (p.Leu85Pro) variant in the SLC1A2 gene (NM_004171.3), which was classified as de novo using parental testing.
Cell culture, transfection, plasma membrane isolation and immunoblotting
HEK293 cells were transfected using Lipofectamine 2000 (Invitrogen) following manufacturer’s protocol. To isolate plasma membrane bound proteins, cells were treated with 1.5mg/ml sulfo-NHS-SS-biotin for 1 hour at 4°C, then washed with PBS containing 1mM MgCl2, 0.1mM CaCl2 and 100mM glycine, rinsed with PBS, then lysed with 150mM NaCl, 5mM EDTA, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, and 50mM Tris-HCl pH7.4 containing protease inhibitors. Insoluble debris was cleared by centrifugation and protein equivalent amounts of cleared lysates were incubated with streptavidin-agarose beads overnight at 4°C, followed by three washes with 100mM NaCl, 5mM EDTA and 40mM Tris-HCl pH7.4, two washes with 500mM NaCl and 50mM Tris-HCl pH7.4, and one wash with 50mM Tris-HCl pH7.4. Beads were resuspended in 2X Laemmli buffer and heated to 95°C. Elute was resolved on SDS-polyacrylamide gels and transferred onto PVDF membrane prior to immunoblotting. Antibodies for immunoblots include: α-SLC1A2 (PA5–17099; Thermo Scientific); rabbit polyclonal anti-actin antibody (Santa Cruz Biotechnology); and HRP-conjugated goat anti-rabbit IgG (W4011; Promega). Optical densitometry determination was made using ImageJ.
Cycloheximide treatment
Transfected HEK293 cells were treated with DMEM media containing 20µg/mL Cycloheximide for different amounts of time, then harvested with RIPA buffer and assayed by immunoblotting as above.
L-[3H]-glutamate uptake
HEK293 cells were transiently transfected in triplicate, and after 24 hours each well was washed three times with uptake buffer (140mM NaCl, 2.5mM KCl, 1mM CaCl2, 1mM MgCl2, 1.2mM K2HPO4, 10mM glucose, 10mM HEPES pH7.4), then incubated for 10 minutes at room temperature with 100µL of uptake buffer containing 100µM L-glutamate and 0.05µCi of L-[3H]-glutamate. Cells were then washed three times with ice-cold uptake buffer, then treated with 100µL of MicroScint-20 (PerkinElmer) for 1 hour at room temperature. L-[3H]-glutamate uptake was determined using TopCount microplate scintillation and luminescence counter (PerkinElmer) and cpm values were transformed into flux rates and averaged and normalized as follows:
Ceftriaxone trial
An innovative therapy protocol was approved by the Boston Children’s Hospital Institutional Review Board (IRB). After observing baseline epileptic spasm frequency via both caregiver observed spasm count and continuous video-EEG for 24 hours, IV ceftriaxone was initiated at 80mg/kg/day. The patient was observed for two days after ceftriaxone initiation with continuous video-EEG, then discharged after placement of a peripherally inserted central catheter for home ceftriaxone infusion. On days 7 and 14 of ceftriaxone therapy she was admitted for planned monitoring of spasm counts using continuous video-EEG. Per the IRB protocol, treatment was stopped after 14 days of ceftriaxone therapy. Daily spasm counts were collected by the caregiver during treatment and for 12 days after ceftriaxone discontinuation.
Results
Clinical spectrum of SLC1A2-related epilepsy
We present an expanded clinical phenotype of SLC1A2-related epilepsy based on seven independent cases, including: one new de novo dominant case; five previously reported de novo dominant cases6–8; and one previously reported recessive case10. All six de novo dominant cases developed early onset epilepsy with symptom onset between 2 days and 6 weeks of life (Supplemental Table 1). Seizures typically presented as focal motor events (often tonic or myoclonic), and progressed to multiple seizure types including epileptic spasms, myoclonic seizures, focal and generalized tonic seizures, and tonic-clonic seizures. Seizures were systematically refractory to multiple medications. Brain MRIs were all normal for the first several months of life, but around 5 months of life began to show evidence of delayed myelination, thinning of the corpus callosum, and cerebral cortical atrophy. EEG patterns demonstrated multifocal and generalized epileptiform discharges with abnormal background activity, including modified and classic hypsarrhythmia. Common clinical features included severe global developmental delay (6/6 cases), cortical visual impairment (3/6 cases), axial hypotonia (6/6 cases), spasticity and/or joint contractures (5/6 cases), and kyphoscoliosis (2/6 cases). Of note, the case of recessive SLC1A2-related epilepsy appears milder, with seizure onset at 2 years of age that was controlled with a single medication and mild developmental delay.
Recurrent de novo SLC1A2-related epilepsy variants localize to the trimerization domain of SLC1A2
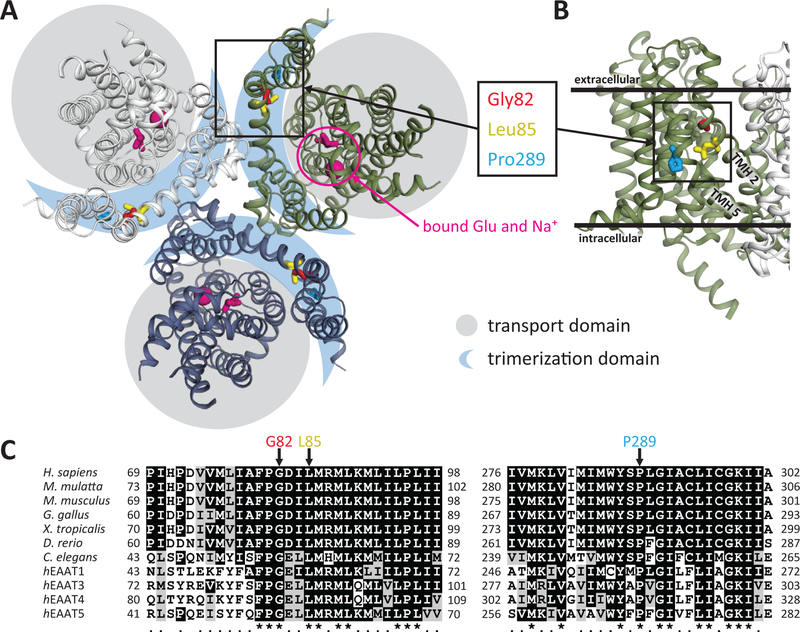
Five of the six de novo dominant cases result from recurrent alterations at SLC1A2 Gly82 (Gly82Arg) and Leu85 (Leu85Pro), whereas Pro289Arg has only been observed in one case. All three of these amino acids are highly conserved and colocalize within the same region of the SLC1A2 trimerization domain, far from the glutamate binding site (Fig 1). Both Gly82 and Leu85 are located in a close helix-helix contact between transmembrane helix (TMH) 2 and 5, whereas Pro289 is required to create a kink in TMH5. All three of these variants are predicted to disrupt TMH2 or TMH5.
Figure 1. Structural relationship and evolutionary conservation of de novo SLC1A2 variants associated with epileptic encephalopathy.
(A) Crystal structure of thermostable SLC1A3 (PDB ID: 5LLU) marked using conserved residues homologous to SLC1A2 Gly82, Leu85 and Pro289 positions, and the glutamate substrate binding pocket.
(B) Close-up view of TMH2 and 5 in reference to the inner and outer layers of the cytoplasmic membrane as well as the trimerization domain.
(C) ClustalW 2.1 sequence alignment of SLC1A2 orthologs and different EAAT proteins for the SLC1A2 protein regions containing G82, L85 and P289.
The Leu85Pro variant significantly reduced SLC1A2 activity
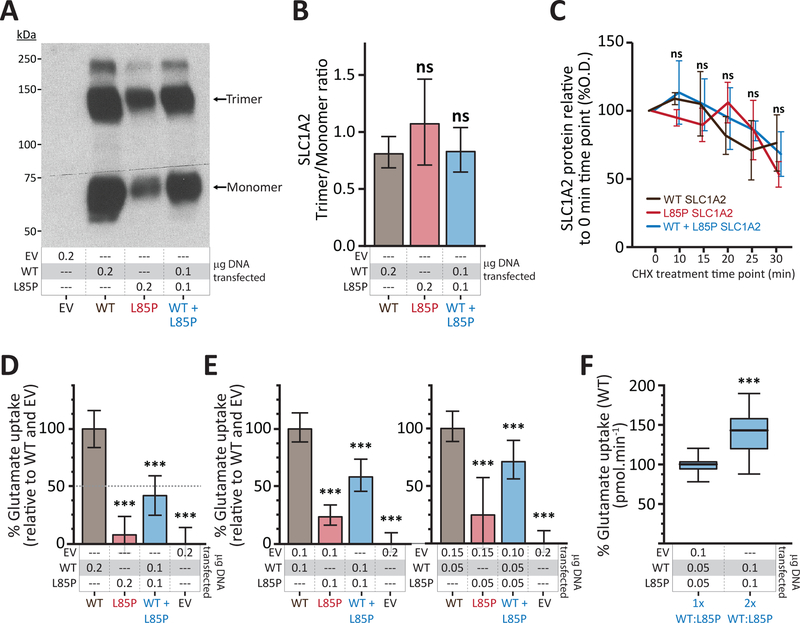
To evaluate the effects of these de novo dominant variants on SLC1A2 function, we studied the Leu85Pro variant in a well-established in vitro model of transiently expressed SLC1A2 in HEK cells9. Both wild-type SLC1A2 (SLC1A2wt) and Leu85Pro SLC1A2 (SLC1A2L85P) proteins were readily expressed in HEK cells and were able to form trimeric plasma membrane-bound proteins (Fig 2A). However, compared to SLC1A2wt, SLC1A2L85P formed fewer monomeric (37±9.4% of WT) and trimeric (47±8.9% of WT) plasma membrane-bound SLC1A2 protein (Fig 2A), yet maintained a preserved trimer-to-monomer ratio (Fig 2B). The protein half-life did not differ between SLC1A2wt and SLC1A2L85P (Fig 2C), demonstrating that the Leu85Pro variant is associated with decreased plasma membrane-bound SLC1A2 protein, but not an overt defect in trimerization or increased protein degradation. Furthermore, whereas cells transfected with SLC1A2wt demonstrated robust glutamate transport activity, cells transfected with SLC1A2L85P had significantly reduced glutamate uptake (Fig 2D), indicating that SLC1A2L85P has significantly reduced functional activity despite its ability to form stable trimeric plasma-membrane bound protein. However, when both SLC1A2wt and SLC1A2L85P were transfected together the glutamate transport activity was reduced below 50% suggesting a dominant negative effect of the mutant allele over the SLC1A2wt
Figure 2. SLC1A2 Leu85Pro is a dominant negative variant that interferes with wild type SLC1A2 function.
(A) Plasma membrane expression of SLC1A2wt and SLC1A2L85P in transiently transfected HEK cells. EV = empty vector.
(B) Trimer/Monomer ratio of plasma membrane SLC1A2 from cells transfected with SLC1A2wt, SLC1A2L85P or both SLC1A2wt and SLC1A2L85P. Mean±SD of 3 independent experiments is represented. ns = not significant (p>0.05).
(C) SLC1A2 protein expression levels in total lysates from cells transfected with SLC1A2wt, SLC1A2L85P or both SLC1A2wt and SLC1A2L85P after incremental treatment with cycloheximide. Values obtained in triplicate and corrected against actin loading and normalized to the value of the untreated condition (sample 0). Values represent mean±SD. ns = non-significant differences in the values obtained at each time point (p>0.05)
(D–E) Uptake of L-[3H] glutamate by HEK cells transiently transfected with the indicated plasmids. Data from 4 independent experiments (N=20–65) expressed as percentage of average SLC1A2wt uptake (pmol.min−1) controlled for EV uptake, and are represented as the mean ± SD. *** significant difference (p<0.001) in level of SLC1A2wt versus the indicated sample.
(F) Uptake of L-[3H] glutamate by HEK cells transiently transfected with equal amounts of SLC1A2wt and SLC1A2L85P plasmids, as well as twice as much of each plasmid. Data from 3 independent experiments expressed as percentage of average uptake (pmol.min−1) of the leftward experiment are represented as the mean ± SD. *** significant difference (p<0.001) calculated using unpaired Student’s test.
SLC1A2L85P acts via a dominant negative mechanism to reduce SLC1A2wt activity
To directly evaluate whether SLC1A2L85P acts via a loss-of-function or dominant negative mechanism we compared the activity of SLC1A2 between cells expressing only SLC1A2wt or both SLC1A2wt and SLC1A2L85P, using different DNA ratios. Identical amounts of SLC1A2wt DNA was used in both transfections, enabling us to quantify the effect of SLC1A2L85P on SLC1A2wt protein function. Notably, glutamate transport activity in cells expressing both SLC1A2wt and SLC1A2L85P was reduced by 33% compared to cells expressing only SLC1A2wt (67±10.8% of WT) (Fig 2E), demonstrating that SLC1A2L85P acts in a dominant negative manner to reduce, but not eliminate, SLC1A2wt glutamate transporter function. The dominant negative effect of SLC1A2L85P on SLC1A2wt function is likely mediated via an effect on SLC1A2 folding, trafficking or functional disruption of mixed trimeric proteins given the ability of SLC1A2L85P to form stable trimeric protein.
Effect of ceftriaxone on seizure frequency in a patient with SLC1A2-related epilepsy
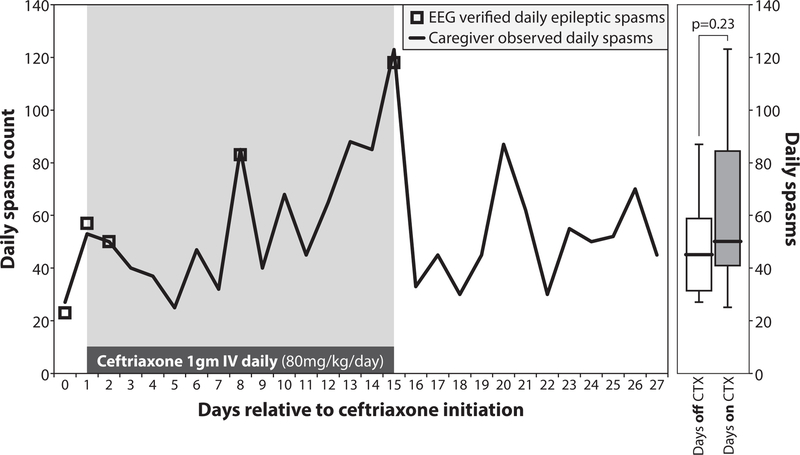
Given our observation in vitro that transfection with increasing amount of both SLC1A2wt and SLC1A2L85P resulted in increased SLC1A2 function (Fig 2F), we hypothesized that increasing both SLC1A2L85P and SLC1A2wt expression in vivo may help partially alleviate the glutamate transporter defect caused by SLC1A2L85P. To test this, we evaluated the SLC1A2-modulating agent ceftriaxone on a 20-month-old with de novo dominant Leu85Pro SLC1A2-related epilepsy. Ceftriaxone is an FDA-approved antibiotic that crosses the blood-brain barrier and increases SLC1A2 expression and function within several days in neural tissue11–15. After IRB approval, the patient was initiated on IV ceftriaxone at 80mg/kg/day for 14 days. Caregiver assessed daily spasms counts were recorded for 28 days and demonstrated good agreement with continuous video-electroencephalography (EEG) monitoring (Fig 3). Ceftriaxone therapy did not result in a significant change in the daily spasm count (median 50 with versus 45 without ceftriaxone; p=0.23, two-tailed Welch’s t-test) (Fig 3), although subjectively the caregiver thought that the patient’s overall level of alertness improved during ceftriaxone therapy. EEG background was unchanged, and treatment was not associated with any major adverse events. Overall, these findings indicate that the SLC1A2-modulating agent ceftriaxone did not improve daily epileptic spasm frequency over the short-term in a 20-month-old with Leu85Pro SLC1A2-related epilepsy.
Figure 3. The SLC1A2 modulating agent ceftriaxone does not significantly alter daily spasm count in a 20-month-old heterozygous for Leu85Pro SLC1A2.
(Left) Daily spasm counts before, during and after a 14 day trial of IV ceftriaxone in a 20-month-old girl with a heterozygous Leu85Pro SLC1A2 variant causing SLC1A2-related epilepsy. EEG verified daily epileptic spasms are indicated in green. (Right) Box-and-whisker plots showing the median, quartile and minimum/maximum daily spasm counts across the 13 days without ceftriaxone treatment and the 15 days with ceftriaxone treatment. P-value calculated using the two-tailed Welch’s t-test.
Discussion
We demonstrate that the recurrent de novo Leu85Pro SLC1A2 variant causes a severe, early-onset developmental and epileptic encephalopathy via a dominant negative mechanism that reduces, but does not eliminate, wild-type SLC1A2 protein localization and function. Based on these findings, as well as the lack of seizures in humans and mice with heterozygous SLC1A2 loss-of-function variants, it appears that there is a critical dosage of functional SLC1A2 protein (somewhere between 0 and 50% of wild-type) under which seizures develop. It is possible that the differences in seizure severity between the dominant and recessive forms of this condition (Supplemental Table 1) result from differences in the amount of remaining functional SLC1A2 protein, with the recessive variants resulting in a higher amount of remaining functional SLC1A2 protein. Finally, although ceftriaxone is one of the best studied SLC1A2-modulating agents across mice and humans, our report represents the first trial of ceftriaxone in a human with a condition resulting from a direct alteration in SLC1A2. Although we observed no benefit of a two week trial of ceftriaxone in this patient, additional studies are warranted. It is likely, based on progressive neuroimaging abnormalities in this disorder, that there is cumulative neuronal damage over time from excessive activation of glutamate receptors. As such, earlier initiation of SLC1A2-modulating agents may be efficacious to overcome the cumulative dominant negative effects of the variant SLC1A2 allele.
Supplementary Material
Acknowledgements
We thanks Tamara Lochner and Yvonne Amrein for their dedication and technical contribution as well as all the patients and families described in this case series. M.A.H was supported by Swiss National Science Foundation grant #31003A_156376. J.P. and G.G. were supported by Marie Curie Actions International Fellowship Program TransCure. A.B.S was supported by NIH T32 grant GM007748 and P.A.R. by NIH grants NS066019 and MH104318.
Footnotes
Conflicts of Interest
No significant conflicts of interest.
References
- 1.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988;1(8):623–34. [DOI] [PubMed] [Google Scholar]
- 2.Epstein FH, Lipton SA, Rosenberg PA. Excitatory Amino Acids as a Final Common Pathway for Neurologic Disorders. N. Engl. J. Med 1994;330(9):613–622. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka K, Watase K, Manabe T, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 1997;276(5319):1699–702. [DOI] [PubMed] [Google Scholar]
- 4.Petr GT, Sun Y, Frederick NM, et al. Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes. J. Neurosci 2015;35(13):5187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu S, Han JC, Morales A, et al. Characterization of 11p14-p12 deletion in WAGR syndrome by array CGH for identifying genes contributing to mental retardation and autism. Cytogenet. Genome Res 2008;122(2):181–7. [DOI] [PubMed] [Google Scholar]
- 6.Allen AS, Berkovic SF, Cossette P, et al. De novo mutations in epileptic encephalopathies. Nature 2013;501(7466):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epi4K Consortium CT, McMahon JM, Schneider AL, et al. De Novo Mutations in SLC1A2 and CACNA1A Are Important Causes of Epileptic Encephalopathies. Am. J. Hum. Genet 2016;99(2):287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guella I, McKenzie MB, Evans DM, et al. De Novo Mutations in YWHAG Cause Early-Onset Epilepsy. Am. J. Hum. Genet 2017;101(2):300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonin A, Montalbetti N, Gyimesi G, et al. The Hydroxyl Side Chain of a Highly Conserved Serine Residue Is Required for Cation Selectivity and Substrate Transport in the Glial Glutamate Transporter GLT-1/SLC1A2. J. Biol. Chem 2015;290(51):30464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner M, Gusic M, Günthner R, et al. Biallelic Mutations in SLC1A2; an Additional Mode of Inheritance for SLC1A2-Related Epilepsy. Neuropediatrics 2017 [DOI] [PubMed]
- 11.Zschocke J, Bayatti N, Clement AM, et al. Differential Promotion of Glutamate Transporter Expression and Function by Glucocorticoids in Astrocytes from Various Brain Regions. J. Biol. Chem 2005;280(41):34924–34932. [DOI] [PubMed] [Google Scholar]
- 12.Carbone M, Duty S, Rattray M. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem. Int 2012;60(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zink M, Rapp S, Donev R, et al. Fluoxetine treatment induces EAAT2 expression in rat brain. J. Neural Transm 2011;118(6):849–855. [DOI] [PubMed] [Google Scholar]
- 14.Karki P, Webb A, Smith K, et al. cAMP response element-binding protein (CREB) and nuclear factor κB mediate the tamoxifen-induced up-regulation of glutamate transporter 1 (GLT-1) in rat astrocytes. J. Biol. Chem 2013;288(40):28975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothstein JD, Patel S, Regan MR, et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 2005;433(7021):73–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.