INTRODUCTION
The most important component of proper mammalian embryo culture is high-quality rat serum. Some commercial sources exist, but control over anesthetization methods and serum extractions means that preparations consistently give superior results. To obtain ~100 mL of rat serum, 30 adult male Sprague Dawley rats must be used. Depending on the number of cultures performed per week, this amount of rat serum can last for several months. This protocol requires two people to collect blood from the rat.
RELATED INFORMATION
For additional information, see Live Imaging of Mouse Embryos (Garcia et al. 2011a). Protocols for Preparation of Postimplantation Mouse Embryos for Imaging (Garcia et al. 2011b) and Time-Lapse Imaging of Postimplantation Mouse Embryos (Garcia et al. 2011c) are also available.
METHOD
Anesthetize an adult male rat with ether inside of a fume hood.
Once the rat is anesthetized and unresponsive, but breathing, spray the abdomen with 70% ethanol and make a V-shaped incision into the lower abdominal cavity.
-
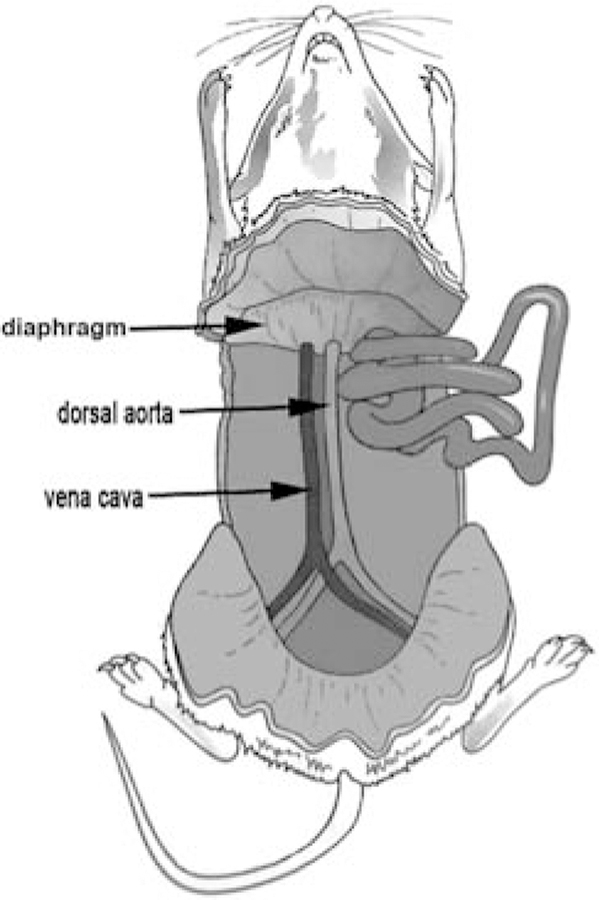
Push aside internal organs and expose the dorsal aorta, which is located next to the larger vena cava. It is the smaller (~1-mm-diameter) pulsing blood vessel (Fig. 1).
Excess fascia may be removed for better visualization of the dorsal aorta by gently rubbing a tissue along the region where the dorsal aorta and vena cava are located.
(Person 1) Puncture the aorta using a beveled butterfly needle from the blood collection set, with the beveled edge facing down.
-
Once the dorsal aorta has been punctured with the butterfly needle, (Person 2) press the outlet needle into a blood collection (Vacutainer) tube. This will create a mild suction that allows blood to collect in the tube.
Collect blood under low suction. At higher suction, the red blood cells can lyse, reducing the quality of the serum. It is possible to use a syringe for blood collection, rather than Vacutainer tubes; however, too much force will cause hemolysis, making the serum unusable.
(Person 2) Invert the Vacutainer tube a few times during collection to allow the anticlotting reagents inside the tube to mix with the blood.
-
Once the rat has been fully exsanguinated, place the collected blood on ice.
One rat should yield 7–10 mL of blood.
- Kill the rats using one of the following methods:
- Use a guillotine. Allow the ether to evaporate from the carcasses in the fume hood before discarding the rats.
- Place the rats in an explosion-proof freezer. Allow the ether to evaporate before discarding the rats.
After blood has been collected from all rats, centrifuge the blood at 1300g for 20 min.
-
Remove and pool the supernatants, and discard the pellets.
Each tube should yield ~2–3 mL of serum.
Centrifuge the serum again at 1300g for 10 min to remove any remaining cells and debris. Collect the supernatant.
-
Heat-inactivate the serum in a 56°C water bath for 30 min with the tube lid partially unscrewed to allow residual ether to evaporate.
The use of ether is essential to the procedure. Other anesthetics remain in the blood and can affect embryonic growth. Ether, however, will easily evaporate from the serum during heat inactivation.
Working in a sterile tissue culture hood, filter the serum through a 0.45-μm filter. Place the sterile serum in microcentrifuge tubes in 1-mL aliquots.
Freeze the aliquots and store them at −80°C for up to 1 yr.
FIGURE 1.
Location of the rat dorsal aorta.
MATERIALS.
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
Reagents
Ethanol (70%)
Ether
Rats, Sprague Dawley adult male (~12 wk old or 300–325 g)
Equipment
Blood collection set (BD Vacutainer 367283; BD Biosciences)
Blood collection tubes (BD Vacutainer 367985; BD Biosciences)
Centrifuge, benchtop
Dissection instruments
Fume hood
Guillotine or explosion-proof freezer (see Step 8)
Ice
Laboratory tissue (optional; see Step 3)
Syringe filter (0.45-μm)
Tissue culture hood
Tubes, microcentrifuge (1.5-mL)
Water bath preset to 56°C
REFERENCES
- Garcia MD, Udan RS, Hadjantonakis A-K, Dickinson ME. 2011a. Live imaging of mouse embryos. Cold Spring Harb Protoc doi: 10.1101/pdb.top104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MD, Udan RS, Hadjantonakis A-K, Dickinson ME. 2011b. Preparation of postimplantation mouse embryos for imaging. Cold Spring Harb Protoc doi: 10.1101/pdb.prot5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MD, Udan RS, Hadjantonakis A-K, Dickinson ME. 2011c. Timelapse imaging of postimplantation mouse embryos. Cold Spring Harb Protoc doi: 10.1101/pdb.prot5595. [DOI] [PMC free article] [PubMed] [Google Scholar]