Abstract
Background:
Paroxysmal atrial fibrillation (PAF) is broadly defined despite high variability in the occurrence and duration of PAF episodes.
Objective:
We aimed to identify rhythm patterns in a large cohort of individuals with PAF who wore an ambulatory single-lead ECG patch sensor as part of standard clinical care.
Methods:
We performed a retrospective analysis of longitudinal rhythm data obtained from 13,293 individuals with PAF.
Results:
In this study, 7,934 men and 5,359 women with PAF wore an ambulatory single-lead ECG patch sensor for 11.4 days on average, experiencing 1,041,504 PAF episodes. The median daily rate of PAF was 1.21 episodes per day (IQR: 0.31, 4.99), and the median maximum duration per individual was 7.5 hours (IQR: 2.4, 18.6 hours). There was an inverse relationship between the duration of PAF episodes and the frequency in which they occurred, which became pronounced at moderate and high overall burden of AF. This produced a spectrum of PAF flanked by two distinct subtypes of the disease: the staccato subtype characterized by many, short AF episodes, and the legato subtype characterized by fewer, longer episodes. Longer but less frequent episodes became more common with increasing age. Only 49.4% of individuals experienced an episode in the first 24 hours of monitoring, increasing to 89.7% after 1 week of monitoring.
Conclusions:
We identified subtypes of the disease that we labeled staccato and legato. Although further study is required, these subtypes may result from differing elements of pathophysiology and disease progression, and may confer differing stroke risks.
Keywords: paroxysmal atrial fibrillation, single-lead ECG patch sensor, atrial fibrillation burden, mobile health, digital medicine
Subject terms: atrial fibrillation, electrocardiology (ECG)
INTRODUCTION
Atrial fibrillation (AF) is the most common pathologic heart rhythm disturbance affecting over 5 million people in the United States alone 1. Lifetime risks of AF currently exceed 30% with this figure expected to rise in the coming years 2,3 AF is a major, independent contributor to incidence of ischemic stroke and death 4,5 with approximately one-fifth of all strokes attributable to AF 6. While there is substantial heterogeneity in the behavior of the disease, AF is broadly defined by its pattern and duration of occurrence: paroxysmal, persistent, or permanent. Paroxysmal AF (PAF) is typically defined as self-terminating episodes of AF lasting shorter than 7 days 7 Compared to more sustained AF phenotypes, PAF has been associated with a lower rate of stroke, whether treated with anticoagulation or not 8,9 By virtue of its paroxysmal nature there is substantial variability in both the frequency and duration of PAF episodes. Recent efforts have sought to identify factors within PAF that confer differing risks for stroke 10,11.
Early detection and treatment of PAF is critical to reducing the substantial morbidity and mortality attached to such a prevalent condition 12 Estimates suggest that 20% to 30% of individuals with AF have PAF 13, and a substantial percentage of these often go undiagnosed and therefore untreated 14,15. High-definition, longitudinal rhythm data on individuals with PAF has largely remained unexplored due to routine practices of short-term, 24-hour monitoring. Now, the ability to unobtrusively monitor every heartbeat for extended periods of time with a wearable electrocardiogram (ECG) patch offers a broad, temporal window for PAF diagnosis and monitoring in a person’s daily life.
We obtained up to two weeks of continuous ECG data on 13,293 individuals with PAF, including over 1 million discrete PAF episodes of 30 seconds or longer. Here we evaluated the frequency, duration, and timing of these events in the largest ever data set of individuals with PAF during extended single-lead ECG patch sensor monitoring. Quantifying the variation in rhythm patterns of PAF is the first step in developing individualized risk assessment and treatment approaches, potentially through the identification of disease subtypes.
METHODS
A retrospective analysis was performed on 13,293 individuals who wore a single-lead patch ECG monitor (Zio Patch, iRhythm Inc.) for up to two weeks, and were determined to have PAF based on the Zio service proprietary algorithm (iRhythm Inc.) with confirmation by Certified Cardiographic Technicians. These represent individuals in the iRhythm Inc. database with PAF identified over a 22 month period from November 2014 through September 2016. All subjects were referred for extended cardiac rhythm evaluation on the basis of a clinical indication as determined by their managing care provider. This study was approved by an institutional review board.
Longitudinal data showing each individual’s rhythm pattern over the wear time of the ECG patch was obtained. Artifacts, as determined by the manufacturer’s specifications, were removed, and PAF episodes were re-identified. From this processed data, individuals with no PAF episodes (n=35) or appeared to demonstrate persistent AF (>99% of wear time in AF; n=53) were excluded prior to evaluation. Only age and sex information was provided beyond the de-identified rhythm reports. No clinical data was available. PAF episodes that were shorter than 30 seconds were omitted in the primary analysis. A selection of cross-sectional metrics were calculated on each individual, including the total number of PAF events, median and maximum duration of PAF events, the total percentage of wear time in AF (burden of AF), and the time until first detected PAF event. Longitudinal analysis focused on the duration of PAF events. The presence of ventricular tachycardia (VT) and supraventricular tachycardia (SVT) was defined based on 3 or more seconds of the arrhythmia, with sustained VT lasting 30 seconds or more, according to the manufacturer’s algorithm. All analyses were performed in R version 3.2.3 and figures were generated using the ggplot2 package.
RESULTS
Extended cardiac rhythm monitoring was obtained from an ambulatory single-lead patch ECG worn by 13,293 individuals with PAF, including 7,934 (59.7%) men and 5,359 (40.3%) women. The average age was 69.4 (SD=11.1) years, with women (71.9) older on average than men (67.7; p<2.2 × 10−16) (Supplemental Figure S1). The average wear time among all individuals was 11.4 days with 11.1 days of analyzable data. The occurrence of additional arrhythmia events and primary indication for ordering rhythm monitoring is presented in Supplemental Table S1.
Frequency, duration, and burden of AF
Across all individuals, 1,041,504 PAF episodes exceeding 30 seconds were identified. The median daily rate of PAF events was 1.21 (interquartile range (IQR): 0.31, 4.99) (Figure 1). While the majority of individuals experienced only a few episodes of PAF per day, some individuals had numerous events per day: 13.0% of the study population averaged one PAF event every two hours, and 6.5% averaged at least one event each hour. This contrasts with the 1,791 (13.5%) individuals that only experienced a single event throughout their full monitoring period. The frequency of PAF was higher in men (median 1.38 events per day) than women (1.00 events per day; p<2.2 × 10−16). Younger individuals tended to have more frequent PAF episodes than older individuals. For those individuals younger than 75 years the median daily event rate was 1.36 compared to 0.97 for those 75 or older (p=1.09 × 10−14).
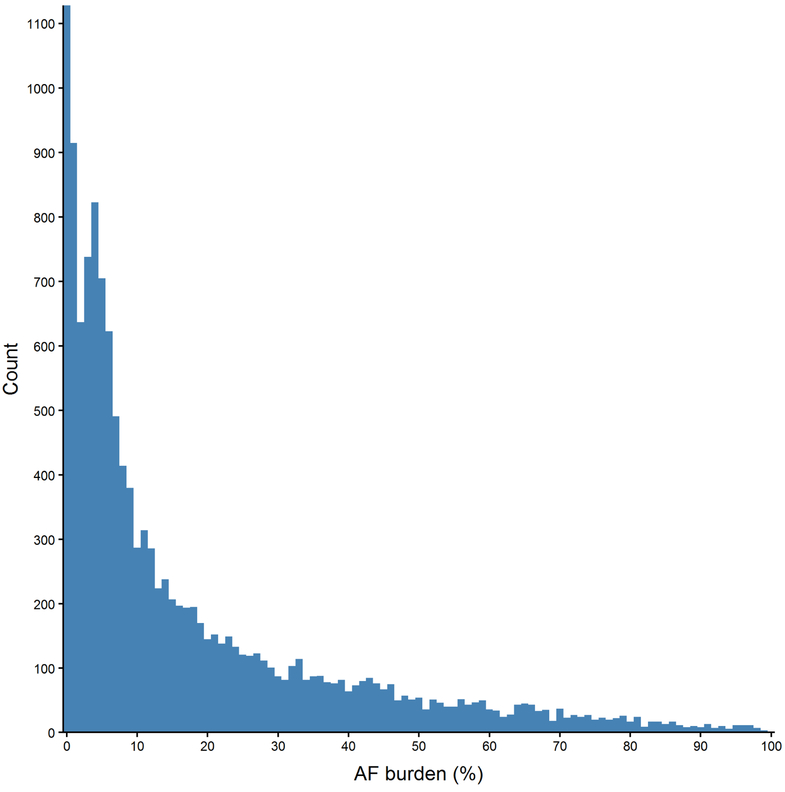
Figure 1.
Cumulative distribution of the daily PAF event rate, defined as the number of distinct PAF events an individual experienced divided by the wear time of the device.
Across all PAF episodes, the average duration was 16.6 minutes, with a median of 2.0 minutes (IQR: 54 seconds, 6.7 minutes). On an individual basis, the median duration of an individual’s longest episode of PAF was 7.5 hours (IQR: 2.4, 18.6 hours). There were 502 (3.8%) individuals without an episode longer than 5 minutes, with 5,894 (44.3%) having no episodes longer than 6 hours. No difference in duration was noted between men or women (p=0.14), but older individuals tended to have a longer duration episodes. The median duration of PAF for individuals younger than 75 years was 11.3 minutes, and 22.8 minutes for individuals 75 years or older (p<2.2 × 10−16). Accordingly, the median burden of AF (% of time in AF relative to entire period of monitoring) was 8.9% (IQR: 3.4%, 25.2%) (Figure 2). The burden of AF was higher in men (9.9%) than women (7.7%; p=4.51 × 10−14), and positively correlated with age among women (p=3.48 × 10−3), but not men (p=0.21). Trends across age and gender are presented in Table 1.
Figure 2.
Histogram of AF burden, defined as the proportion of wear time spent in AF.
Table 1.
Median AF event rate, maximum duration, and burden of PAF cases by age and sex. Cases with unspecified age omitted (n=30).
| N | AF events/day | Maximum Duration (hrs) | Burden (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | M | F | All | M | F | All | M | F | All | M | F | All |
| < 40 | 109 | 33 | 142 | 2.58 | 0.93 | 1.59 | 5.94 | 2.13 | 4.16 | 9.25 | 5.62 | 8.37 |
| 40-69 | 4,307 | 2,032 | 6,339 | 1.51 | 1.32 | 1.45 | 7.56 | 6.07 | 6.95 | 10.04 | 7.49 | 9.04 |
| 70+ | 3,488 | 3,294 | 6,782 | 1.21 | 0.86 | 1.01 | 8.21 | 7.64 | 7.94 | 9.74 | 7.99 | 8.81 |
| All | 7,904 | 5,359 | 13,263 | 1.38 | 1.00 | 1.21 | 7.80 | 6.84 | 7.49 | 9.84 | 7.73 | 8.92 |
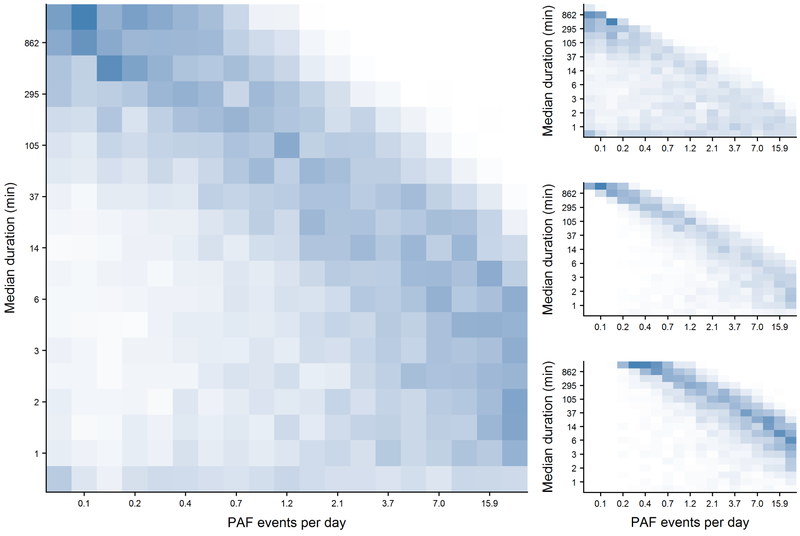
AF burden was positively correlated with both the PAF event rate (p<2.2 × 10−16) and the median duration of PAF episodes (p<2.2 × 10−16). Yet, there was an inverse relationship between the frequency and duration. This trend was less distinct at a lower burden of PAF, but became more pronounced at moderate and higher burden levels (Figure 3). The resulting frequency-duration bivariate distribution of individuals with PAF demonstrates a continuum of rhythm patterns bookended by a staccato rhythm, or many short PAF events, on one end, and a legato rhythm, or few long events, on the other. Higher frequency and shorter duration events, a characterization of the staccato phenotype, were associated with the presence of VT, with individuals with sustained VT demonstrating even more frequent, shorter events. Alternatively, SVT was more commonly observed in individuals displaying the legato phenotype with lower frequency and longer duration events (all p<2.2 × 10−16).
Figure 3.
Heatmaps demonstrating the frequencies of PAF cases based on their daily PAF event rate (x-axis) and median event duration (y-axis). Left panel: all PAF cases; Right panels broken down by AF burden tertiles, Top: low AF burden (less than 4.7%); Middle: moderate AF burden (4.7% to 17.8%); Bottom: high AF burden (greater than 17.8%). Boxes represent every 5 percentile on the corresponding distribution. Individuals in the upper left of each figure display the legato subtype while individuals in the bottom right are staccato.
Time to PAF Detection
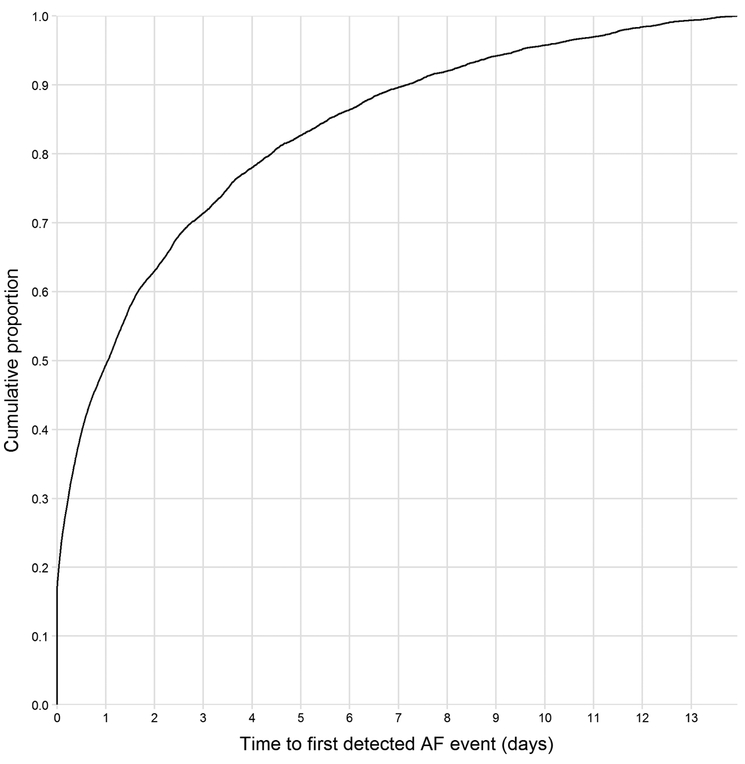
Overall, 15.4% of individuals were in AF at the time of patch application (Figure 4). The median time to the first detected PAF event was 24.9 hours (IQR: 2.7, 83.9 hours). After 24 hours of monitoring, only 49.4% of individuals with PAF had experienced a PAF event, with this metric increasing to 63.1% after 48 hours of monitoring. Meanwhile, extending monitoring to 7 days increased the detection rate to 89.7%. This suggests that extending monitoring from one day to one week may reduce the rate of undetected PAF by up to 79.6%.
Figure 4.
Cumulative distribution of the time to first PAF episode detected.
DISCUSSION
Currently, the AF phenotype is broadly classified based solely on the duration and permanence of AF episodes. Specific treatment strategies remain general, and it is unknown if targeting therapies or preventative strategies to specific taxonomies of AF based on a more detailed rhythm assessment is clinically effective. Here, we mined the largest ECG database of PAF to date, with extended rhythm monitoring, in an effort to assess and define discrete subtypes of PAF. Our primary finding demonstrates an inverse relationship between the number of PAF events and the average and maximum duration of the PAF events. Accordingly, individuals can largely be classified along a spectrum of PAF rhythm patterns from staccato to legato. Comparatively, there were few people with only a small number of short events and almost no individuals with numerous long events. This finding raises the question if staccato and legato subtypes share the same underlying pathophysiology, just on different ends of natural variation; or are they distinct sub-phenotypes of PAF, among potential numerous, that may have distinct etiology, clinical trajectories, and outcomes. Our discovery that the staccato phenotype was enriched for VT while the legato phenotype was enriched for SVT suggests the latter may be true. We also observed that the frequency of PAF events decreased with age, while the duration of events increased. One explanation could be that PAF progresses over time from the staccato to the legato phenotype, where these shorter events grow longer and begin to link together with one another to generate more sustained bouts. Longer-term follow-up studies are needed to examine how the PAF rhythm pattern in an individual may evolve over time.
Importantly, we demonstrated that over 50% of individuals with PAF did not display a PAF episode within the first day of AF monitoring. While traditional 24-hour Holter monitoring may be warranted in certain clinical settings, it would miss the PAF diagnosis in over half the individuals we assessed. As longer term clinical rhythm monitoring has become more common, cost effective, and of greater comfort for patients, it would be reasonable to utilize these devices when PAF is in question. Extending monitoring to one week would reduce the number of PAF cases missed by nearly 80%. More widespread use of sensors that capture AF and PAF, such as through a smartwatch, have been approved by the FDA 16,17 Ultimately, such technologies may help alleviate the issue of undiagnosed PAF. However, our understanding of clinically significant PAF for which anticoagulation is warranted to reduce the risk of stroke remains limited 18.
Although evidence suggests the risk of stroke and systemic embolism among patients with PAF is roughly half that of patients with persistent or permanent AF 19, these risks remain higher than the general population – stroke/systemic embolism incidence was 1.7 per 100 person-years among PAF patients receiving oral anticoagulation. Furthermore, the wide variation we observed in the frequency, duration, and burden of AF among PAF cases may contribute to variation in risk, with particular concern regarding the appropriate management of subclinical AF when detected by a consumer-facing or implantable cardiac rhythm device. A recent study found that individuals with the highest tertile of AF burden (11.4% or higher in this study) had slightly over a 3-fold increased risk of thromboembolism 20. It is clear that AF burden, and not simply the presence AF, is an important characteristic when assessing outcome risks 21,22 that is inadequately quantified from intermittent, short-term monitoring via the Holter. Meanwhile, we observed marked variation in frequency and duration of AF events even among individuals with the highest AF burden. As such, the interplay between these factors may be an important consideration regarding the relationship of PAF events and stroke risk, along with the progression of PAF to persistent and permanent AF. High-definition, longitudinal inspection of rhythm patterns to better quantify PAF, including other derived metrics such as AF density which evaluates non-uniformity of the occurrence of PAF episodes 23, may enable these discoveries.
There are several limitations to this study. Chiefly is our inability to relate the subtypes of PAF we identified to meaningful clinical risk factors and outcomes. We were neither able to correlate specific rhythm patterns to recognized risk factors for AF, nor determine how different medical procedures or antiarrhythmic drugs may influence our observations. Accordingly, the lack of clinical data and subsequent follow-up did not allow us to assess differences in stroke occurrence. As such, clinical validation tied to AF-related outcomes is needed. Orthogonal biological data may also yield novel insights into the etiology of PAF. Lastly, it is important to note that the PAF episodes we observed are derived from the manufacturer’s proprietary algorithm. Though the data we examined is the same provided to clinicians and can thus be assumed to be reasonably correct following technologist review, efforts are underway to improve the diagnostic accuracy of these sensors 24
Our findings from this large cohort of patients diagnosed with PAF and with more than a week of monitoring demonstrate broad variability in the frequency, duration, and timing of PAF episodes. We observed a spectrum of PAF subtypes displaying characteristics that range from staccato to legato rhythm patterns. It is our suggestion that future efforts aimed at studying this disease would consider these rhythm patterns as opposed to broadly labeling all non-sustained AF cases as PAF. Doing so may provide explanations into disease etiology, reduce the rate of undetected or inappropriately anticoagulated AF cases, and ultimately lead to more individualized care.
Supplementary Material
ACKNOWLEDGEMENTS
Data was generated and provided to the authors by iRhythm Inc.
SOURCE OF FUNDING
This work was supported, in part, by the NIH/NCATS Clinical and Translation Science Award (CTSA) UL1 TR001114-05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. : Heart Disease and Stroke Statistics’ 2017 Update: A Report from the American Heart Association. Circulation. 2017, pp. e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng L-C, Preis SR, Hulme OL, et al. : Genetic Predisposition, Clinical Risk Factor Burden, and Lifetime Risk of Atrial Fibrillation. Circulation United States, 2017; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnussen C, Niiranen TJ, Ojeda FM, et al. : Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts: Results From the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 2017; 136:1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf PA, Abbott RD, Kannel WB: Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988. [DOI] [PubMed] [Google Scholar]
- 5.Glotzer T V, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, Cook J, Paraschos A, Love J, Radoslovich G, Lee KL, Lamas GA: Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: Report of the atrial diagnostics ancillary study of the MOde Selection Trial (MOST). Circulation 2003; 107:1614–1619. [DOI] [PubMed] [Google Scholar]
- 6.Marini C, De Santis F, Sacco S, Russo T, Olivieri L, Totaro R, Carolei A: Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke 2005; 36:1115–1119. [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P, Benussi S, Kotecha D, et al. : 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016,. [DOI] [PubMed] [Google Scholar]
- 8.Vanassche T, Lauw MN, Eikelboom JW, et al. : Risk of ischaemic stroke according to pattern of atrial fibrillation: Analysis of 6563 aspirin-treated patients in active-a and averroes. Eur Heart J 2015; 36:281–287. [DOI] [PubMed] [Google Scholar]
- 9.Link MS, Giugliano RP, Ruff CT, Scirica BM, Huikuri H, Oto A, Crompton AE, Murphy SA, Lanz H, Mercuri MF, Antman EM, Braunwald E: Stroke and Mortality Risk in Patients with Various Patterns of Atrial Fibrillation: Results from the ENGAGE AF-TIMI 48 Trial (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Circ Arrhythmia Electrophysiol 2017; 10. [DOI] [PubMed] [Google Scholar]
- 10.Van Gelder IC, Healey JS, Crijns HJGM, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau CP, Morillo CA, Hobbelt AH, Rienstra M, Connolly SJ: Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017; 38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 11.Inohara T, Shrader P, Pieper K, et al. : Association of of Atrial Fibrillation Clinical Phenotypes With Treatment Patterns and Outcomes: A Multicenter Registry Study. JAMA Cardiol United States, 2018; 3:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You JJ, Singer DE, Howard PA, et al. : Antithrombotic therapy for atrial fibrillation: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest; 2012,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S: Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014; 6:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowres N, Neubeck L, Redfern J, Ben Freedman S: Screening to identify unknown atrial fibrillation: A systematic review. Thromb Haemost 2013; 110:213–222. [DOI] [PubMed] [Google Scholar]
- 15.Kishore A, Vail A, Majid A, Dawson J, Lees KR, Tyrrell PJ, Smith CJ: Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: A systematic review and meta-analysis. Stroke. 2014, pp. 520–526. [DOI] [PubMed] [Google Scholar]
- 16.Time: Fitbit May Have a New Way To Detect an Irregular Heartbeat [Internet]. Available from: http://time.com/4907284/fitbit-detect-atrial-fibrillation/
- 17.Apple is working with Stanford and American Well to test whether its watch can detect heart problems [Internet]. Available from: https://www.cnbc.com/2017/09/11/apple-watch-caridac-arrhythmia-tests-stanford-american-well.html [Google Scholar]
- 18.Gold MR: Treatment of Subclinical Atrial Fibrillation: Does One Plus One Always Equal Two? Circulation United States, 2018; 137:217–218. [DOI] [PubMed] [Google Scholar]
- 19.Takabayashi K, Hamatani Y, Yamashita Y, et al. : Incidence of stroke or systemic embolism in paroxysmal versus sustained atrial fibrillation: The fushimi atrial fibrillation registry. Stroke 2015; 46:3354–3361. [DOI] [PubMed] [Google Scholar]
- 20.AS G, K R, J Y, et al. : Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: The kp-rhythm study. JAMA Cardiol 2018; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, Keung EK, Singer DE: Atrial Fibrillation Burden and Short-Term Risk of Stroke: Case-Crossover Analysis of Continuously Recorded Heart Rhythm from Cardiac Electronic Implanted Devices. Circ Arrhythmia Electrophysiol 2015; . [DOI] [PubMed] [Google Scholar]
- 22.Wechselberger S, Kronborg M, Huo Y, et al. : Continuous monitoring after atrial fibrillation ablation: the LINQ AF study. EP Eur 2018; :euy038–euy038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charitos EI, Ziegler PD, Stierle U, Sievers HH, Paarmann H, Hanke T: Atrial fibrillation density: A novel measure of atrial fibrillation temporal aggregation for the characterization of atrial fibrillation recurrence pattern. Appl Cardiopulm Pathophysiol 2013;. [Google Scholar]
- 24.Rajpurkar P, Hannun AY, Haghpanahi M, Bourn C, Ng AY: Cardiologist-Level Arrhythmia Detection with Convolutional Neural Networks. arXiv 2017; arXiv:1707.01836v1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.