Graphical Abstract

The viable use of photodynamic therapy (PDT) in cancer therapy has never been fully realized because of its undesirable effects on healthy tissues. Herein we summarize some physicochemical factors that can make PDT a more viable and effective option to provide future oncological patients with better-quality treatment options. These physicochemical factors include light sources, photosensitizer (PS) carriers, microwaves, electric fields, magnetic fields, and ultrasound. This Review is meant to provide current information pertaining to PDT use, including a discussion of in vitro and in vivo studies. Emphasis is placed on the physicochemical factors and their potential benefits in overcoming the difficulty in transitioning PDT into the medical field. Many advanced techniques, such as employing X-rays as a light source, using nanoparticle-loaded stem cells and bacteriophage bio-nanowires as a photosensitizer carrier, as well as integration with immunotherapy, are among the future directions.
Keywords: cancer therapy, medical chemistry, microwaves, photodynamictherapy(PDT), ultrasound
1. Introduction
Photodynamic therapy (PDT) is a cancer therapy method with minimal invasiveness and is different from other light-triggered cancer therapies such as photothermal therapy.[1] This form of treatment requires the presence of three components to be considered effective, these are oxygen, light, and a photosensitizer (PS).[2] The light activates the photosensitizer to produce lethal reactive oxygen species (ROS), such as singlet oxygen (1O2), by transferring energy to ground-state oxygen present in the system via triplet state energy transfer.[3] PDT is a viable cancer therapy option because of its low impact on the living system, its few side effects and low invasiveness; overall the “Quality of Life” of patients can be enhanced.[4] PDT has been successfully employed in the last two decades to treat various types of cancer, including breast, oral cavity, head and neck, skin, esophageal, and bladder cancer.[5] Cancer treatments are not the only field in which PDT has proven useful. Other successful applications have been in ophthalmology and urology and in antimicrobial processes.[6]
There are many reasons to use PDT over traditional cancer therapies, these reasons include low levels of systemic toxicity while maintaining a high tumor selectivity, the possibility of repetitive treatment cycles, fewer secondary effects, and the ability to combine PDT with other treatment options, such as radiotherapy.[7] Like all therapies, PDT has limitations, such as light penetration depths because PDT is effective only when light can hit the target area. As a result of these limitations, PDT is confined to treating cancers that have not spread to multiple locations, primarily treating regions of skin or immediately below the organ linings that are possible to reach with a light source. The difficulty in systemic administration of this form of therapy is that photosensitizers are usually hydrophobic, limiting the clinical efficacy of PDT. Because of these challenges, extensive research is being carried out to help in the optimization and identification of viable photosensitizer systems, which include using functional dyes[8] and carriers.[9] Furthermore, the effects of various light sources on PDT are also being studied.[10]
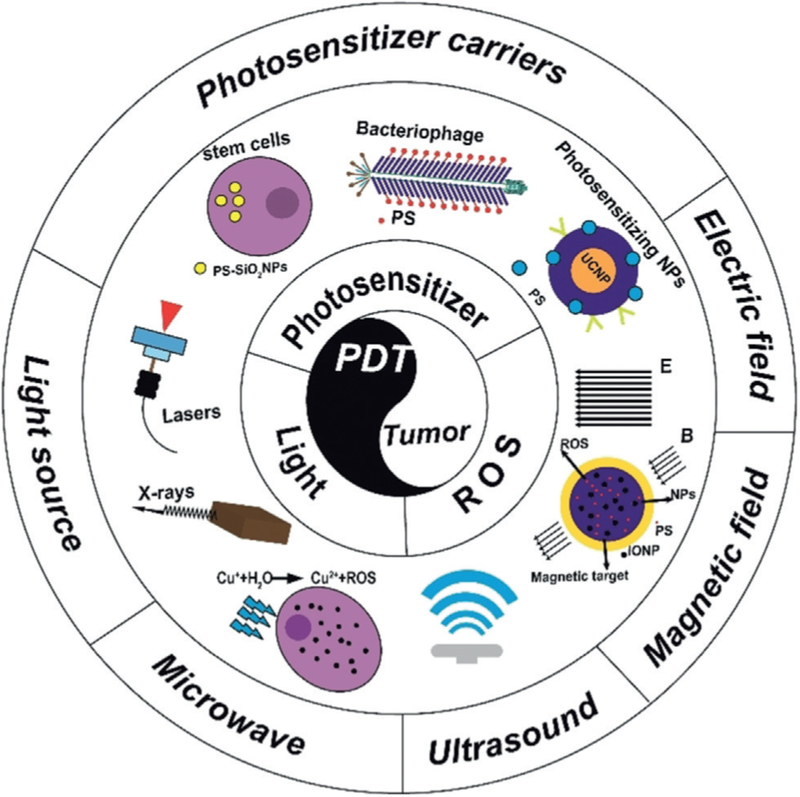
The focus of this Review is to address the physicochemical factors available for the enhancement of PDT. Therefore, the goal of this Review is to critically assess research on the physicochemical factors used in PDT, such as light sources (e.g., laser and radioactive rays), magnetic fields, electric fields, microwaves, and ultrasound (Figure 1). In particular, how these factors can enhance PDT use in cancer therapy is systematically summarized and critically analyzed.
Figure 1.
Schematic illustration showing how PDT can be enhanced by various physicochemical factors.
2. Choice of Light Source
Just as important as in light-triggered drug release and photothermal therapy,[10d,11] the choice of light sources has an important role in PDT. Also, the correct light dose, sufficient photosensitizer concentration, and oxygen are needed within the tumor tissue.[12] Since the parameters must be so closely regulated, the light source must be delivered to the affected area in a homogeneous manner to ensure the treatment benefits reach the tissue. To date, several laser and non-laser light sources have been tested in PDT.[13] Recent research will be discussed regarding advances in PDT light delivery systems being used.
2.1. Laser light sources
Presently, the use of a multi-laser system is common when treating different diseases with PDT. The mono-chromaticity of lasers allows for irradiation with a precise wavelength at which a photosensitizer reaches its maximum absorption capabilities. These factors provide what is necessary to drive light to the targeted area of the body via optic fibers.[14] There are three types of laser light sources: argon-pumped, metal-vapor-pumped, and solid-state lasers.
2.1.1. Argon/Dye Laser
The argon/dye laser is currently the primary light source for PDT employed in a medical setting. The high-powered argon/dye laser system can deliver light of continuous wave (CW) with a power of 1 to 7 W at a wavelength of 630 nm. However, argon/dye lasers have a “large frame” and are often immobile and need to be mounted to a structure, such as an optical bench.[13c] For use in PDT procedures, the unit should be less cumbersome and require little optical adjustment to maintain sufficient power.
2.1.2. Metal-Vapor Lasers
Metal-vapor lasers are considered versatile; they have been found to be portable and lack the requirement for special electrical and cooling equipment. For example, gold-vapor lasers can generate a wavelength of 628 nm,[15] but they require charges of gold to maintain their power output; this makes them an expensive option. A copper-vapor laser, conversely, has less expensive maintenance, but still requires the use of a dye-laser module. These lasers characteristically must be allowed to warm-up and then cool down, making them less desirable for use in the medical field, where meeting these demands can prove difficult.
2.1.3. Solid-State Lasers
Solid-state lasers take advantage of solid-state gain media. These lasers utilize arc or flash lamps as optical pumps,[13a] such as Nd:YAG lasers, Ho:YAG lasers, and KTP:YAG/dye lasers. These all have applications in PDT, much like argon and metal vapor lasers. Among the advances newly developed laser processes is the use of biocompatible silica optical-fiber delivery systems; this has driven development in treatment procedures. The Ho:YAG laser is a multipurpose laser used in urology, this is due to the laser’s ability to penetrate multiple soft- and hard-tissues. This system provides advantages in PDT including low cost, durability, portability, tenability, and ease of use.[15]
2.1.4. Diode Lasers
Diode lasers are based on semiconductors. There is a lot of potential for incorporating these lasers into clinical use because they are portable and easy to use. They are considered ideal for endoscopic PDT and are generally coupled with optical fibers.[13c] Moslemi et al. reported that PDT using a diode laser could suppress the growth of Aggregatibacter actinomycetemcomitans, an aggressive micro-organism causing oral diseases.[16] At present, diode lasers have limited applicability to PDT because of their single wavelength output. However, these systems are under development to adapt the laser for use with multiple wavelengths. An advantage of using a system of lasers as a way of delivering light for PDT is the easy incorporation of the same light into fiber optics, allowing for the activation of photons to happen directly at the target site.
2.2. Non-Laser Light Sources
Photonics technology is constantly under development. New light sources are being designed and improved for use with PDT on a large scale in the near future.
2.2.1. Lamp Light Sources
Lamp light sources are being explored as an alternative to laser lights with highly successful results; these novel light sources include the use of halogen, xenon, and metal-halide lamps.[17] When comparing lamps to lasers, the lamps provide a wider spectral output. They are advantageous in that they are portable, easy to use, have the ability to cover a large area and can be used cooperatively with cross-section light guides. This allows the therapy to be incorporated into the treatment of large superficial lesions. The ongoing struggle is the challenge of coupling small optical fibers with lamps without diminishing their power output, which otherwise would minimize the use of this method to treating skin lesions. Limitations have kept this light source from being used for endoscopic PDT.[13a] Kimura et al.[17] studied the differences in using flash wave (FW) and CW lights. They found, specifically at a low irradiation frequency, PDT efficacy could be enhanced when FW was used in comparison with CW light, in spite of both light sources having equivalent irradiation fluence. The primary cause for the resulting efficacy of FW over CW light is the exceptional ability at which FW generates 1O2 at this frequency. Despite the advantages, there may be undesirable side effects when using PDT with lamps, such as a significant thermal effect.[13a,b]
2.2.2. Light-Emitting Diodes (LEDs)
LED utilization in PDT is a developing concept.[13d,18] An LED light source has the capability to emit high-energy light at a wavelength that can be specified, while having customizable shapes and configurations. LED provides diverse treatment options, allowing customization for PDT that can treat brain tumors and fit into balloon catheters intraoperatively, while also being useful for treating minimally invasive procedures such as the implantation of a flexible LED catheter into a tumor percutaneously.[19] Comparing a 660 nm LED desk lamp to the LD670–05 diode and the metal-halide lamps, it was found that the LED desk lamp was not only more potent but needed half as much irradiation time to produce the same effects.[10b] In this way, a large LED arrangement has the potential to be a viable option for superficial lesions that cover a wide area.[20] LEDs have advantages over other PDT light sources, such as low cost and low hazard.[21] LED lights have numerous advantages, they are compact, lightweight, and require low amounts of energy when producing desired wavelengths. Also, according to Mang et al. LED lights can be produced at varying wavelengths such as 630, 670, and 690 nm, useful for PDT methods requiring flat surface illumination.[13c]
2.2.3. Daylight
Daylight studies have been published illustrating the effectiveness of daylight PDT procedures. Direct and reflected outdoor sunlight can be combined to trigger day-light-based PDT (DL-PDT).[22] DL-PDT has been evaluated as an alternative use to the laser-based PDT. It can effectively treat non-melanoma skin cancers. It is shown that DL-PDT has an ability comparable to that of other PDT approaches and is easier to tolerate than standard PDT in treating mild to moderate actinic keratosis.[23] DL-PDT has made PDT more widespread, cheaper, and less painful.[24] Nevertheless, important disadvantages include difficulty in scheduling due to the dependence of daylight on weather and times as well as the inconvenience in controlling daylight exposure.[23c]
2.2.4. X-rays
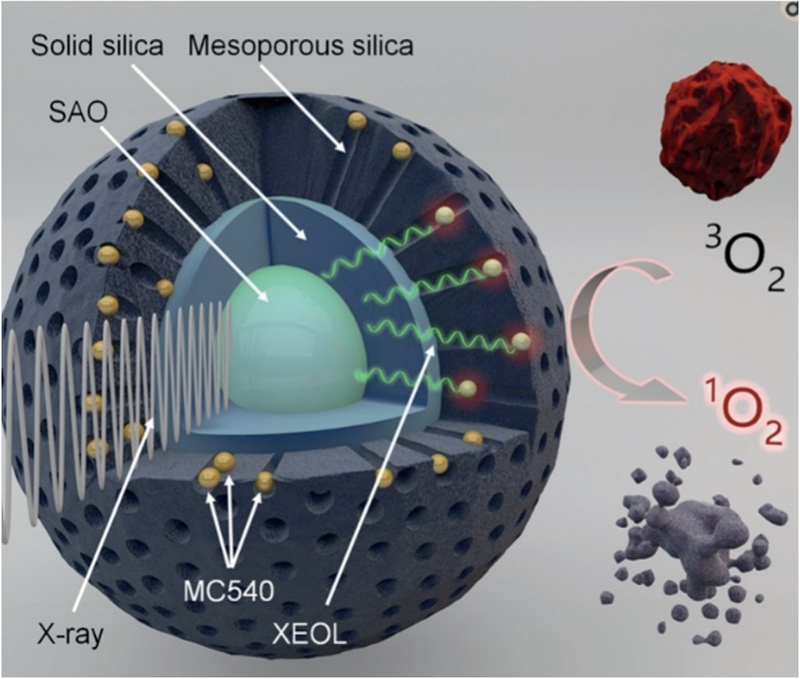
X-ray-based radiotherapy holds promise in cancer therapy because X-rays have excellent tissue penetration capability compared to other light sources. When X-rays hit normal tissue, side effects will arise. Thus, design of novel X-ray responsive material is very important for successful radiotherapy. Using materials containing heavy elements may lead to more sensitive x-ray absorption, resulting in the development of materials that present increased photoelectric cross-sections compared to the tissues. Recently, PDT and X-ray based radiotherapy have been merged to establish a new mode of PDT, the X-ray induced PDT (X-PDT).[25] X-PDT requires the use of nanoparticles that can absorb X-ray to emit light capable of triggering a photosensitizer through energy transfer. Because X-rays have good tissue penetration capability and can reach a tumor that cannot be reached by other light source, X-PDT can treat tumors deep in the tissue, making it a frontier in PDT research.[25f,26] For example, nanoparticles with a composition of (n-Bu4N)2[Mo6I8-(OCOCF3)6] that contained a heavy element molybdenum were found to be able to absorb X-ray to become excited triplet states, which led to the formation of 1O2 capable of killing cancer cells.[26] In another example, nanoparticles made of both X-ray sensitive SrAl2O4 :Eu2+ (SAO) and photosensitizer (merocyanine 540, MC540) were used to absorb X-rays to emit visible light that activated PDT, converting 3O2 into 1O2 for killing cancer cells and shrinking tumors (Figure 2).[25g]
Figure 2.
An example of X-PDT. The SrAl2O4:Eu2+ (SAO) core was decorated by two silica layers (bearing MC540 photosensitizer). When the nanoparticle was irradiated with X-rays, the SAO absorbed X-ray to emit visible light (XEOL). The emitted visible light triggered the PDT by converting 3O2 into 1O2 for killing cancer cells and shrinking tumors.[25g]
For widespread use of PDT, light source availability and complexity, as well as cost and delivery systems all need to be considered. The most prominent barrier to be considered is the absence of suitable light delivery technology.[13c]
3. Photosensitizers (PS)
The photosensitizer used in PDT is pivotal to the treatment results. Ideally, a photosensitizer suitable for PDT needs to be an amphiphile that is tunable in biological environments. It should show high molar absorptivity in response to long light wavelengths and be non-toxic when not under light irradiation. It also needs to have long triplet lifetime (micro-seconds) and present an increased quantum yield of 1O2 generation (over 50%). In addition, an ideal photosensitizer should be able to target tumor tissue where it has a long retention and then be quickly removed from the body once PDT is completed.[27] Recently, many studies have reported the development of efficient photosensitizers with tunable solubility, high 1O2 quantum yields, and tissue targeting.
3.1. Photosensitizer-Delivering Nanosystems
Using nanosized carriers for photosensitizer delivery is a technique that is turning out desirable results, improving the capability of photodynamic activity while overcoming many side effects.[28] Using nanosized carriers has been found beneficial in many ways. First, the photosensitizer itself usually does not have tumor-targeting capability, but it can always be loaded in a nanocarrier that is modified by tumor-homing motifs, such as tumor-homing peptides. Namely, the tumor-targeting nanocarriers have the ability to home into tumor tissues and assist in concentrating the photosensitizer there; this helps minimize the harmful effects of treatment to healthy tissues, making PDT a more desirable option. Second, unlike many other nanoformulated chemotherapeutics, payload bioavailability and efficacy difficulties are avoided by containing the photosensitizer within nanoparticles, such as mesoporous silica nanoparticles (MSNs) and activating them via an external source. Thus it is reasonable to suggest that specifically delivering photosensitizer by way of nanocarriers could improve PDT.[1a,29] In recent years, studies have been performed to show the varied roles which nanostructured platforms can play in PDT.
3.1.1. Silica Nanoparticles (SiO2NPs): Alone or Loaded in Stem Cells
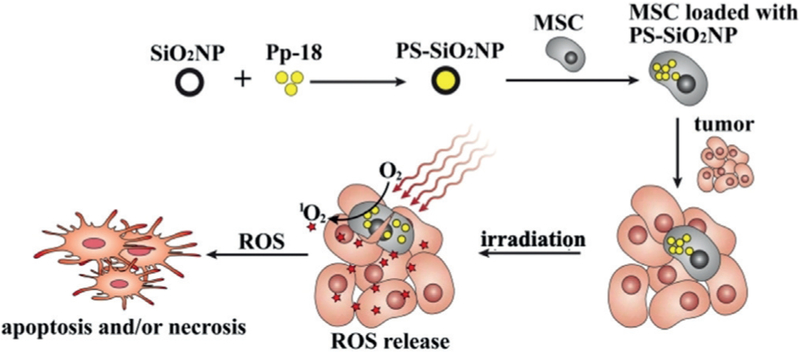
Silica Nanoparticles (SiO2NPs) are spheres of a uniform shape (e.g., ca. 50 nm in diameter). They are being researched for use as a carrier for PDT drug.[5b] When comparing organic polymeric systems to inorganic silica (SiO2), SiO2 has several advantages; SiO2NPs are inert and stable, while being nontoxic in nature and potentially non-reactive toward microbes and enzymes.[30] Therefore, SiO2NPs are used for loading various photosensitizers, such as purpurin-18,[5e] hematoporphyrin (HP),[19,20] hypocrellin A,[31] chlorin,[32] methylene blue,[33] PHPP and ZnPc.[34] Especially, our group[5e] chose mesenchymal stem cells (MSCs), which are naturally tumor-homing, to transport photosensitizer-loaded silica NPs to tumors for breast cancer therapy (Figure 3). The photosensitizer-loaded MSCs suppressed the tumor growth under PDT treatment. The silica NPs could load many photosensitizers and did not significantly impact the tumor-homing capability of MSCs. This combination of MSCs and silica NPs holds promise for developing new strategies of targeted cancer therapy.
Figure 3.
Scheme of using silica nanoparticle (SiO2NP)-loaded mesenchymal stem cells (MSCs) for tumor-targeted PDT. The photosensitizer used is purpurin-18 (Pp-18).[5e]
3.1.2. Liposomes and Micelles
Liposomes and micelles are artificially developed vessels for drug and nutrient transport; they have been incorporated as a way to deliver photosensitizer drugs in PDT.[35] There are many reasons for this, including their ability to target and release the drugs at specific locations while maintaining protection of the drugs and their biocompatibility.[36] The encapsulated photosensitizer has a higher uptake ability because its endocytosis is optimized and eventually it is localized in the organelles.[37] Traditionally, liposomes are categorized by their short plasma half-life. Unfortunately this short time span is not long enough for a tumor cell to uptake the drug. The reason being the quick expulsion by the reticuloendothelial system and breakdown, which result in an exchange of lipids with liposome components.[38] Recently, liposomes have undergone modifications to lengthen their circulation time through the bloodstream and improve their structural stability. PEGylated liposomal nanocarriers have been refined so that they can be used to deliver the photosensitizer, m-THPC, to specific target tissues by improving their circulation times and bioavailablilty.[39] Moreover, the result is a decrease in damage to the healthy tissue in the surrounding area, with a lower risk for functional damage and scar-tissue development.
Micelles can be pictured as a nanocage formed from a single surfactant layer with a polar surface and hydrophobic core, or vice versa.[40] Micelles can be modified to become responsive to the pH value. For example, pH-responsive micelles, with protoporphyrin IX (PpIX) encapsulated, were developed to achieve demicellization at tumoral acidic pH, enabling the photosensitizer-loaded micelles to be used for in vivo tumor diagnosis (by fluorescence imaging) and enhanced PDT (by generating singlet oxygen).[41] So far, micelles loaded with a PDT drugs have been shown to be an ideal candidate for treating a variety of tumors, such as human hepatocellular carcinoma by PDT.[42] Furthermore, polymer-based micelles can be developed for delivering photosensitizer to enhance PDT. For instance, it was found that pH-sensitive graft-polymer-based micelles could be employed to effectively deliver photosensitizer into cells to achieve PDT with improved efficiency.[40]
3.1.3. Carbon Nanotubes
Carbon nanotubes provide an alternative method for photosensitizer delivery to required tissues. These single-, double-, or multi-walled structures can be pictured as materials formed by making tubes out of rolled sheets of carbon. Single walled carbon nanotubes (SWCNTs) can absorb near-infra-red (NIR) light which can cause cell death due to extreme localized heating. They also possess a hollow interior that can encapsulate drug molecules.[43] Nyokong’s group reported that SWCNTs had the ability to increase the effect of zinc monocarboxylphenylphthalocyanine in PDT.[44]
3.1.4. Peptide-Based Nanoparticles
Peptide-based nanoparticles, as a unique type of drug carrier, have received more attention because of their stability, low cost, long-term storage, functional disposition, low immunogenicity, and easy handling.[45] Ross and coworkers[46] prepared F3-targeted polymeric nanoparticles containing iron oxide or a fluorescent probe and a Photofrin photosensitizer. The studies provide evidence that, when comparing animals receiving peptide-based nanoparticles to those dosed with non-targeted nanoparticles or systemic Photofrin, there was a notable increase in survival rate of those treated with peptide-based nanoparticles after PDT. Additionally, van Hell et al.[47] produced peptide vesicles containing phthalocyanine and demonstrated that cell death was linked to the active photodynamic response of these vesicles against cells under illumination. Conversely, the peptide-based nanoparticle vesicles, which had already released the drug, were not cytotoxic. These studies have demonstrated the pivotal role that the photosensitizer delivery system plays in the overall efficacy of PDT treatments.
Recently, trifunctionalized MSNs were designed to contain optical-tracking NIR fluorescent beacons, photosensitizer, and moieties specific to certain cells for targeting purposes in order to decrease collateral damage to healthy tissues and increase MSN absorption.[48] The PDT efficiency for MSNs loaded with photosensitizer was improved by using the tumor-targeting cRGDyK peptides.[48] Certainly, peptides also present some drawbacks such as their metabolic instability. One approach to increasing metabolic stability and pharmacokinetics is by altering peptide sequences, for example using d-amino acids or multivalent sequences.[44a]
3.1.5. Bacteriophage Nanowires
Filamentous bacteriophages (also called phages) are biological nanowires that can be genetically or chemically modified to bear peptides or other molecules, such as a photosensitizer. Phage display techniques have recently been widely used in biomedical materials research.[49] Phages are shown to be useful in the delivery of vaccines, genes, or drugs because they are flexible, cost-effective, stable and biocompatible.[50] Our group has also made some exploration in this area in the context of using phages to develop PDT.[2,5e,f,51] Phage display is powerful in discovering cell- or tissue-targeting peptides from a phage-displayed random peptide library.[50b,52] Such peptides or peptide-displaying phages can be utilized to guide the transport of photosensitizer to cancer cells and tumor tissues. We successfully developed the phage–photosensitizer complex by chemically conjugating phages (displaying a discovered SKBR-3 breast cancer cell-homing peptide with a sequence of VSSTQDFP) and photosensitizer (pyropheophorbide a, PPa; Figure 4). The results clearly indicated that the conjugated PPa-phage complex could selectively destroy the cancer cells by PDT through the cancer-targeting peptides displayed on the phages. By effectively incorporating phage-display technology, a variety of phage–photosensitizer complexes can be developed for treating various cancers by PDT.
Figure 4.
Illustration of PPa-conjugated phage nanowires for targeted PDT. A) Nanowire-like phages with all the copies of major coat protein (pVIII) conjugated with PPa, there is no singlet oxygen production because of the close packing of PPa and thus excitonic coupling. B) Nanowire-like phages with some copies of pVIII conjugated with PPa, resulting in singlet oxygen production. C) Live/dead assay showing the use of PPa-conjugated phage nanowires for killing SKBR-3 cells by PDT triggered by 658 nm laser. The SKBR-3 cell-targeting peptide (pink line) is displayed on pVIII (green).[51]
3.1.6. Graphene
Graphene has distinct physical and chemical properties, which give it the potential for various uses in biomedicine. Graphene oxide (GO), is a derivative that is characteristically water soluble, is important in drug delivery[53] and enzyme immobilization[13c,54] because it has a large, potentially specific surface area with an abundance of functional groups. Graphene also has a large optical absorption within the NIR widow. PEGylated graphene oxide (GO-PEG) has recently been used in animal experiments to test photothermal ablation and has been found efficient.[55] Tian et al. observed that GO-PEG injected at a dose of 20 mgkg−1 was nontoxic in mice. Moreover, they found that the water-soluble GO-PEG-Ce6 complex could produce cytotoxic 1O2 and offer note-worthy improvements in regard to killing cancer cells compared to free Ce6.[55b] The PDT efficacy against cancer cells can be enhanced by the photothermal properties of graphene; it can be employed for the purpose of delivering Ce6 through exposing the target to a low power density NIR laser.
Some research groups[56] developed folic acid-conjugated (FA-conjugated) GO as a drug delivery system for PDT. They demonstrated that the photosensitizers, Ce6, and hypocrellin A, can be loaded into the FA-conjugated-GO-based delivery system; this led to an extraordinary photodynamic effect on MGC803 cells. The PEG-decorated GO-Ce6 complex showed a combination of PTT and PDT under the excitation of 660 nm and 808 nm, respectively. These findings indicated the possible use of FA-conjugated-GO in targeted PDT. However, the improved outcome required the use of multiple laser treatments, suggesting that this approach is expensive and has a prolonged therapeutic time.
3.2. Photosensitizing Nanosystems
3.2.1. Upconversion Nanoparticles (UCNPs)
UCNPs are a group of emerging luminescent materials, which absorb light of longer wavelength (e.g., NIR) to produce light of shorter wavelength (e.g., green light).[57] UCNPs have been found to be chemically stable and unlike organic fluorescent dyes, UCNPs avoid traditional photobleaching complications. Recently UCNPs have been utilized as nanocarriers for photosensitizer in PDT.[58] UCNPs have been integrated with photosensitizers including merocyanine,[59] docetaxel,[60] zinc phthalocyanine,[61] chlorin e6,[56b] doxorubicin (Figure 5),[62] and pyropheophorbide a[34] to develop PDT. The resultant conjugates exhibit efficient singlet oxygen generation after NIR light exposure giving an improved therapeutic effect, both in vitro and in vivo. Furthermore, some groups[56b] prepared nanorattles made of a hydrophilic rare-earth doped NaYF4 shell and magnetic nanoparticle cores. This material showed excellent water dispersion capability, a high drug-loading capacity, and low cytotoxicity; it was also shown to be useful for cell imaging.
Figure 5.
The use of the NaGdF4:Yb/Er@NaGdF4-based NPs in cancer theranostics. The UCNPs (as imaging probes) were loaded with two therapeutic drugs, including doxorubicin (DOX) and camptothecin (CPT).[62]
The use of UCNPs can alleviate the challenge of other biomolecules being auto-fluorescent and causing interference, thus, the signal-to-noise ratio is higher. In particular, when UCNPs possess cancer biomarkers and are then excited by NIR light (wavelength range 700–1,000 nm), theoretically their penetration capability could be improved without damaging tissues through re-absorption and scattering.[63] All of these studies show that UCNPs are promising platforms for developing strategies for cancer theranostics.
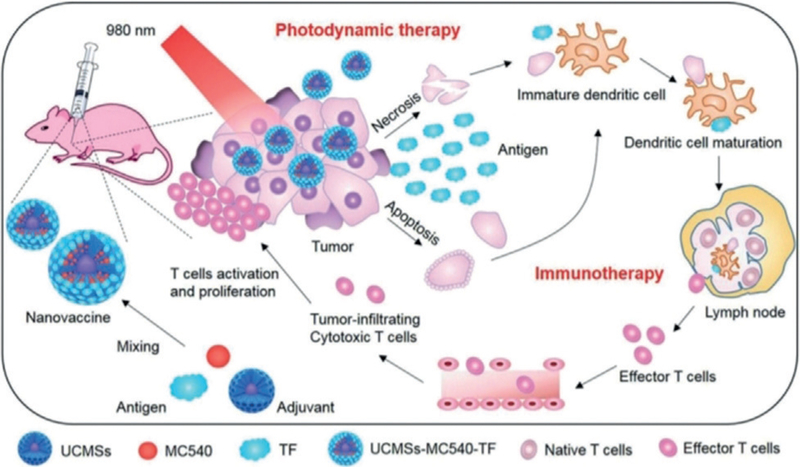
Recently, UCNPs encapsulated by mesoporous silica (UCMSs) were used as an immunoadjuvant to integrate immunotherapy and PDT (Figure 6).[64] Photosensitizer (merocyanine 540, MC540), chicken ovalbumin (OVA), and tumor cell fragment (TF), were loaded into the UCMSs. When a 980 nm NIR light was applied to the resultant UCMSs-MC540-OVA, PDT was triggered followed by immunotherapy. The immunotherapy was verified by Th1 and Th2 immune responses and effector-memory T cells. Moreover, the integrated PDT and immunotherapy was found to be more effective in inhibiting colon tumor growth and increasing cancer survival than PDT or immunotherapy alone. These studies suggest that a combination of PDT and immunotherapy is a new promising direction in cancer therapy.
Figure 6.
Use of UCMSs-MC540-TF nanovaccines to enable the integration of PDT and immunotherapy. UCNPs (β-NaYF4:20%Yb,2%Er) encapsulated by porous silica were mixed with merocyanine 540 (MC540) and antigens (tumor cell fragment). The resultant UCMSs-MC540-TF were given to a mice model. Upon irradiation of 980 nm NIR light, PDT was triggered to form tumor-associated antigens, which activated the maturation of dendritic cells. As a result, effector T cells were released to trigger immunotherapy for more effective cancer therapy.[64]
3.2.2. X-ray Luminescent Nanoparticles (XLNPs)
XLNPs are considered a novel method to improve PDT.[25a,26,65] Because X-rays have an improved tissue penetration capability, the use of X-ray responsive nanoparticles as a photosensitizer in PDT could solve the problems of low penetration depth met in traditional PDT.[25b,66] photosensitizer-conjugated XLNPs, such as LaF3:Ce3+, LaF3:Tb3+ and Y2O3 nanoparticles, have been studied in a new approach for PDT.[65c,67] Combining a photosensitizer (e.g., Photofrin) and radiation therapy was found to achieve effective cancer therapy.[68] Photosensitizer-XLNPs complexes can be triggered at the tumor sites by X-rays through the effective energy transfer from the excited XLNPs to photosensitizer. More importantly, because X-rays can penetrate deep tissue, this method has the advantage of being able to treat deep tumors. Therefore, by linking radiotherapy with PDT, allows the treatment of deep tumors and increases the therapeutic effect.
3.2.3. Quantum Dots
Quantum dots (QDs) are semiconductor nanocrystals and exhibit a quantum confinement effect when the radius of the nanoparticle is less than the Bohr radius.[69] Narrow emission bands, size-dependent tunable photoluminescence[70] and high two-photon absorption cross sections[71] are characteristic of QD. Photosensitizers can be covalently linked to the exterior of QDs to form QD-photosensitizer conjugates, which are then utilized in PDT. The schematic representation of the cytotoxic effect mechanisms by QD-photosensitizer is shown in Figure 7.[72] CdSe and CdTe QDs have been heavily researched so far because their quantum confinement region spans the complete visible spectrum.[73] Even though CdSe QD-photosensitizers show promising capabilities for a high efficiency of generating singlet oxygen, there is a concern with the high toxicity of this QD-photosensitizer that will stifle their potential use in the medical field. As a less toxic option, InP/ZnS QDs complexed with Ce6, were employed for use with PDT.[74] When InP/ZnS-Ce6 was incubated with breast cancer cells (MDA-MB-231) and irradiated with UV light, the results showed a decrease in cell viability as a result of PDT. Recently, it was found that defect engineering of QDs could enhance ROS generation,[69b] further suggesting the potential use of QDs in enhancing PDT.
Figure 7.
Two modes of generating free radicals in QD-based PDT, including electron transfer in Path I and ROS generation in Path II.[72a]
4. Microwaves
Local hyperthermia is recognized as one of the most efficient methods in combined oncotherapy, since it results in tumor sensitization to chemotherapeutic agents and to ionizing radiation.[75] To induce clinical hyperthermia, microwaves, radiowaves, laser, or ultrasound can be employed.[76] For example, a microwave-induced PDT was reported using copper cysteamine (Cu-Cy) nanoparticles.[77] Microwaves could activate the Cu-Cy nanoparticles, generating singlet oxygen. It was shown that bone tumor cells could be effectively killed by the microwave-induced PDT. Further studies indicated that the microwave irradiation could induce heating and Cu ion release, which in turn promoted the ROS generation.[77] Research from Dooley et al. provides information regarding preferential heating and damage to breast carcinoma, a result of the high water/ion content of these tumors, when microwave energy was employed; conversely the heating and damage to low water and ion-containing adipose and glandular tissues was less significant.[78] Further, PDT can also be made much more effective if the blood supply to the tumor is increased through heating. As the tissue is heated, the blood vessels dilate, thereby increasing blood supply. Hemoglobin in blood contains oxygen, so heating increases the presence of oxygen, thereby increasing the effectiveness of the reaction.[79a] Some NPs may make tumor more leaky,[79b] potentially further enhancing the PDT. Therefore, using microwave-responsive NPs could enhance PDT for more effective cancer therapy (Figure 8).
Figure 8.
Microwave-induced nanoparticle activation to enhance PDT for more effective cancer therapy.
As early as the 1980s, microwave thermotherapy was being used in medicine for cancer treatment and also used in combination with PDT.[80] The photorejuvenation combination therapy gives excellent clinical results through the PDT radiofrequency-induced hyperthermia interactions, resulting in dysplastic, photodamaged cells that have a higher mortality rate. Many studies have also demonstrated that microwave hyperthermia could enhance the effect of PDT in vivo.[81] The presence of oxygen was found to be a critical factor in determining the effectiveness of PDT. Oxygen availability during PDT could be increased by promoting a higher oxygen supply to tissues that have been illuminated via the increase of blood flow which can be achieved by localized tissue heating.
These techniques can be harmful to healthy tissues through overheating of healthy tissues when targeting deep tumor tissue, causing concern regarding this method of treatment. Over the last two decades, several minimally invasive microwave antenna designs have become available for hyperthermia induction.[82] The antenna would need to be transparent enough to let through as much of the light emitted by the laser as possible and must also effectively deliver microwaves to induce heating of the cancerous area. Rosen et al.[82c] proposed a novel microwave applicator antenna, which was designed to cut the power off after a certain time frame. This antenna was for use on balloon catheters and applied in different areas. According to Rosen et al. this design provided large optical power, which was able to activate the photosensitizers utilized in this method of PDT. When fixed onto balloon catheters, the light delivery system and microwave antenna together were able to provide sufficient heat to the esophageal lumen, increasing the amount of O2 in the tissue.[82c] This method provides a means to control the heating and the depth at which the microwave can penetrate allowing it to be effective in esophageal wall tissue.
Recently, Gu[83] put forward a new idea on microwave applications. He used a microwave frequency-sensitive light-emitting polymer as a light source to excite an administered photosensitizer to exert cytotoxicity at a site containing undesired or diseased tissue. The selection of a photosensitizer will depend at least in part on the radio-microwave frequency, which determined the wavelength of the light-emitting agent. The wavelength, once determined, may then be paired with one or more suitable photosensitizers that display desirable tissue distribution and/or uptake. Some suitable photosensitizers included, for example, foscan (652 nm), porfimer sodium (Photofrin, 630 nm), photosens (675 nm), visudyne (693 nm), and silicon phthalocyanine (Pc, 600–700 nm). The microwave-absorbing agent was the light-emitting polymer diazoluminomelanin (DALM), a biosynthesized polymer that has been shown to be chemiluminescent. When DALM was activated by microwave frequency to emit light, any light going into the surrounding normal, or non-targeting tissue was harmlessly absorbed. However, the light going into the photosensitizer caused the generation of 1O2, which will then cause cell death in tissue containing the photosensitizer.
5. Electrical Fields (EF)
Electrical fields (EF) have recently been used in PDT. In the past decade, studies have been performed on antimitotic, frequency-tuned EF therapy for use as a new confined oncology therapy.[84] An intense EF can affect the cell membrane by the mechanism of electroporation (EP).[85] EP has been utilized to deliver both genes[86] and drugs.[87] One deciding feature of PDT was that the photosensitizer has minimal ability to access tumor cells. Because of the nature of the electroporation effect on the cell membrane, it is possible to exploit this effect to enhance drug delivery to cells, which in turn can increase the antitumor effectiveness of the drug. Further, numerous studies have developed the electroporation effect to help the photosensitizer pass the cell membrane, thus promoting the PDT.[88] The results demonstrated that electric pulses used in combination with PDT enhance the photodynamic effectiveness. Additionally, Ward et al,[89] observed that the degree of increased photosensitized erythrocyte lysis under EF and laser irradiation sources was more than that of electric pulses when no laser light was present. Cell lysis was also promoted with increasing hematoporphyrin derivative (HpD) concentration and higher EF strength. Studies found in vitro tumor cells had increased cell death when EF-induced activation was used with HpD-treated tumor cells; the viability of HpD-treated HeLa was found to be reliant on both EF strength and HpD concentration. Many researchers are engaged in studying charged photosensitizers and their solubility in aqueous media.[90] Aggregation of photosensitizers in an aqueous solution is problematic. One method to minimize this problem is through the electrostatic repulsion of molecules of the same charge to reduce aggregation. Makarov et al.[91] demonstrated that the phthalocyanine became less aggregated in the aqueous solutions when the number of pyridiniomethyl or cholinyl substituents increased. This has led to increased fluorescence quantum efficiency and 1O2 generation. Moreover, Donnelly’s group[92] investigated the possibility of employing iontophoresis (ITP) for quick photosensitizer delivery and photodynamic chemotherapy (Figure 9). They found that PEG-crosslinked hydro-gels loaded with methylene blue and placed in between an anode and a cathode provided the highest chance of quick delivery of photosensitizer to the targeted tissue under the action of electric field as those hydrogels were the most flexible, least adhesive and released the greatest amount of photosensitizer. Thus, these results indicated that iontophoteric delivery of photosensitizers by employing PEG-crosslinked hydrogels is a feasible choice in the efficient PDT for cancer therapy.
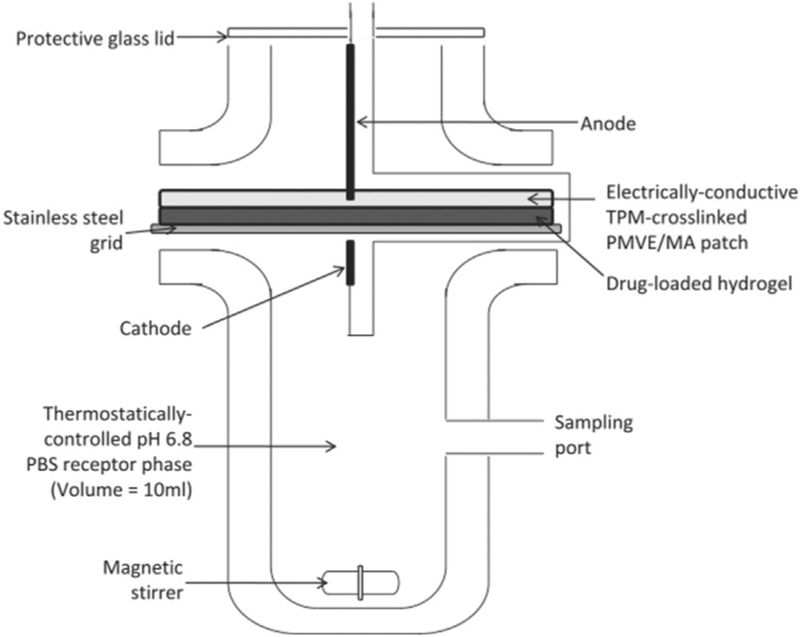
Figure 9.
Illustration of using adapted Franz cell to investigate the promoted release of drugs (photosensitizers) from hydrogels by means of iontophoresis.[92]
6. Magnetic fields (MF)
Recently, work has been started to use magnetic nanoparticles as a way to concentrate the heat deposition at the tumorous region in the body. To induce localized hyperthermia in biological tissues, magnetic nanoparticles capable of absorbing NIR light are used. The NIR light can penetrate into the tissues when exposed to an alternating current(for generating a magnetic field) than when not exposed to an alternating current. The resultant deeper penetration shows an effective cellular uptake and obvious production of 1O2 in vitro.[82b] Tedesco and co-workers found an increase in diffusional flux for Foscan when coupled with a magnetic nanoemulsion, which improved in vivo drug penetration in the skin layers. They found that a higher penetration of photosensitizers in skin layer due to the presence of MF resulted in accumulation of the drug (Foscan) in the skin tumor.[94] On a similar note, there is a promising possibility of a magnetically targeted drug delivery system with tissue targeting controlled through an external MF. PEGylated fullerene/iron oxide nanocomposites,[93f] magnetic liposomes (with magnetic nanoparticles in the lipid membrane or inside the interior cavity),[95] micro-emulsions (containing purpurin-18 silica-coated magnetic particles)[96] and magnetic fluids[97] have all been developed and all have shown good results (Figure 10). Oliveira et al.[98] identified a magnetic liposome containing the zinc phthalocyanine photosensitizer for improved PDT. Overall, a combination of the photosensitizer and magnetic nanoparticles in liposome leads to enhancement of PDT efficacy.[99]
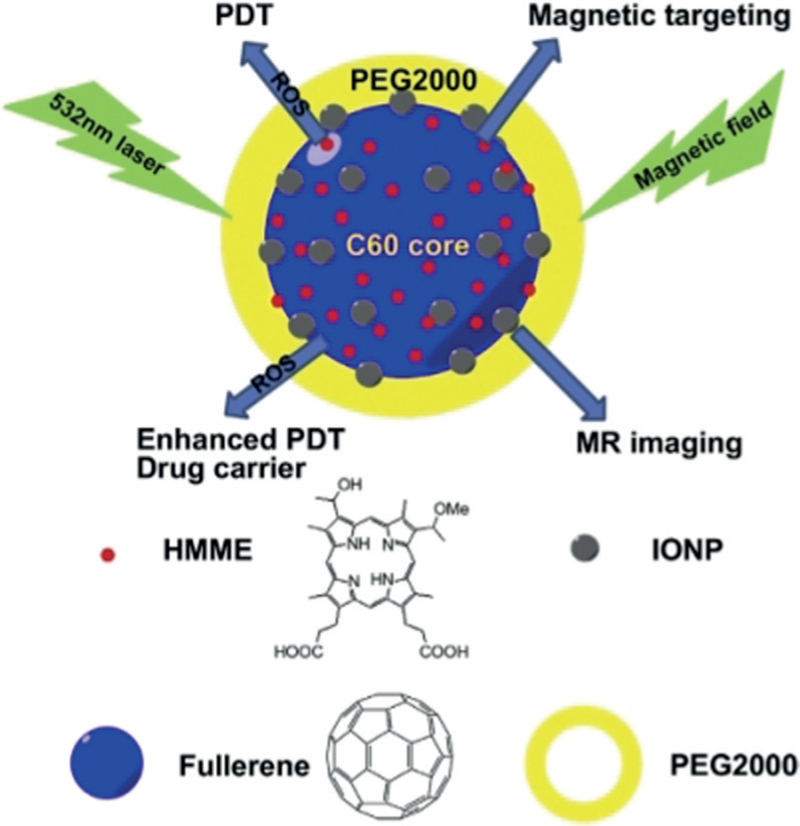
Figure 10.
Illustrations of the applications of C60-IONP-PEG/HMME. IONP and PEG are responsible for magnetic targeting and making the complex stable at physiological condition. HMME is a photosensitizer for PDT. PEG=polyethylene glycol; IONP=iron oxide nanoparticles; HMME=hematoporphyrin monomethyl ether.[93f]
The electromagnetic fields (EMF) can be viewed as the combination of an EF and MF. A major advantage of EMF hyperthermia is the ability to control the heating process by using a single antenna applicator. Recently, studies of electromagnetism have been conducted to look deeper into chemical reaction models and cell biological studies to aid in clinical theories.[93b] Pang et al.[100] found that the pulsating electromagnetic field (PEMF) as a treatment method had the ability to increase the photodynamic efficacy over 40% when compared to the used control value over 12 min. Additionally, they also treated yeast cells and noticed successful coupling of PEMF with amplified PDT post treatment. The reason may be that PEMF is a non-invasive route to promote dye penetration by affecting cell membrane composition and protein channels.[101] In addition, Radeva et al.[102] also reported the necrosis and apoptosis in cancer cells by the combination of EMF and photosensitizer quinoids. Due to the possibilities of inducing apoptosis and necrosis of cancer cells, EMF gained interest as a possible supplementary therapy. The coupling of EMF and PDT activity was considered as a new way to promote the efficacy of cancer therapy. Afterwards, they observed that pulsating EMF (50 Hz) could induce necrosis, which was further enhanced when quinoid anthracyclines and actinomycin-C were present. So the EMF assisted PDT, as a possible non-invasive treatment, will be advantageous in the development of possible supplemental therapies.
7. Ultrasound
Ultrasound is defined as frequencies that are too high for humans to hear. It has become an increasingly important tool in medical science. For example, when targeting a small tumorous region in an attempt to activate photosensitizer for the triggering of tissue or tumor cell destruction, ultrasound can be useful for penetrating deep tissue focused on a small area. This novel approach has been dubbed sonodynamic therapy (SDT) compared with PDT. Over a decade ago, evidence that photosensitizers responded to ultrasound in a way that caused a cytotoxic effect was found, it has since been shown that the cytotoxic effects were mediated due to the production of cytotoxic ROS. Due to the ability of ultrasound to penetrate tissue, it is theorized that this method could provide activation of photosensitizers in a clinical setting where light activation is unavailable.[103] McHale et al. found the generation of ROS could possibly be promoted if the photosensitizers, rose bengal, methylene blue, or indocyanine-green, were irradiated with coupled ultrasound and light, which led to enhanced cytotoxicity in vitro.[104] Many studies also have reported that combining PDT and SDT can cause increased tumor suppression compared to SDT or PDT alone, for example, using the photosensitizers, such as chlorin e6 (Ce6), ClAlPcS2, TiO2, mTHPC, and Sonoflora.[105] It is still unknown what the best time to apply ultrasound is (before or after PDT), though there are studies underway to identify which is more beneficial.
Liu et al.[105b] indicated that applying ultrasound either before or after PDT, termed SPDT or PSDT, respectively, was more effective in killing MDA-MB-231 cells than PDT or SDT alone, with SPDT being more powerful than PSDT. Further, SDT alone was found not to increase ROS formation while PDT alone could promote ROS formation inside MDA-MB-231 cells. However, SPDT perhaps increased the uptake of photosensitizers by changing cell membrane permeability. These observations suggest the ultrasound exposure before initiating PDT could promote the PDT effect. Xu et al.[106] found that the antitumor efficacy was markedly improved by Ce6 mediated SPDT (Ce6-SPDT) compared with PDT or SDT alone. Particularly, Ce6-SPDT also significantly inhibited tumor metastasis in breast cancer 4T1 xenograft model.
Photoacoustic technology was also applied to the monitoring, guidance, and evaluation of PDT. The photoacoustic effect is the use of a short laser pulse to irradiate an object, causing it to emit ultrasonic waves. Absorption of the diffusely propagating light in tissue showed a centralized rise in temperature before the thermoelastic expansion and emission of an ultrasonic pulse. Therefore, photoacoustic technology[107] (Figure 11) is utilized to define the distribution of optical energy throughout tissues because of the intrinsic optical absorption, and the optical absorption by the photosensitizer. With this information, it is possible to identify spatial distribution, and dynamic change of the photosensitizer in tissues that have been targeted as well as additional biological structures and functional hemodynamic properties.
Figure 11.
Photoacoustic and ultrasound bimodal imaging. a) administration of CCl4 to generate liver fibrosis in mice. Healthy mice were given olive oil and served as controls. b) A handheld device with photoacoustic and ultrasound imaging capability. c) Working principle of the handheld device. d) Imaging of the mice by the device.[107]
8. Conclusions and outlook
PDT is a developing strategy for use as a treatment for cancer. Nevertheless, there is evidence that PDT has advantages over traditional cancer treatments, which helps to further drive research in this field. Many issues need to be solved for successful PDT. For instance, it is important to choose the right photosensitizer and concentration, photosensitizer administration mode, light source type, and light radiation conditions (including, wavelength, energy, exposure time, pulse frequencies).[14] In this Review, we described some physicochemical factors that can be considered to give better treatment options when utilizing PDT for oncological patients, including light source, magnetic fields, microwave, ultrasound, and photosensitizer carriers. Additionally, employing X-rays as a light source, multiple advanced drug carriers (such as nanoparticle-loaded stem cells and bacteriophage bio-nanowires), as well as integration with immunotherapy, are promising factors that can significantly improve PDT efficacy in cancer therapy.
Acknowledgements
We would like to acknowledge the financial support from National Institutes of Health (EB021339 and GM116116).
Biographies

Mingying Yang graduated from Tokyo University of Agriculture and Technology in 2015. After postdoctoral training at this University, she became an associate professor at Zhejiang University and was promoted to full professor in 2014. Her main research interests include biomolecular materials for cancer therapy and regenenerative medicine.

Tao Yang received his BS from the University of Science and Technology Beijing in 2016. He is now a PhD student at the School of Materials Science and Engineering of Zhejiang University. His research is focused on cancer therapy and regenerative medicine.

Chuanbin Mao received graduate training at Northeastern University in China and post-doctoral training at Tsinghua University and the University of Texas at Austin. He is a recipient of NSF Career award and a fellow of the Royal Society of Chemistry (RSC) and American Institute for Medical and Biological Engineering (AIMBE). His research is focused on phage display, cancer therapy, regenerative medicine and nanomedicine.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
The ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/anie.201814098.
Contributor Information
Mingying Yang, College of Animal Science, Zhejiang University, Hangzhou, Zhejiang, 310058 (China).
Tao Yang, School of Materials Science and Engineering, Zhejiang University, Hangzhou, Zhejiang, 310027 (China).
Chuanbin Mao, School of Materials Science and Engineering, Zhejiang University, Hangzhou, Zhejiang, 310027 (China); Department of Chemistry & Biochemistry, Stephenson Life Science Research Center, Institute for Biomedical Engineering, Science and Technology, University of Oklahoma, 101 Stephenson Parkway, Norman, OK 73019 (USA).
References
- [1].a) Qiu P, Yang M, Qu X, Huai Y, Zhu Y, Mao C, Biomaterials 2016, 104, 138–144; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wan SS, Zeng JY, Cheng H, Zhang XZ, Biomaterials 2018, 185, 51–62; [DOI] [PubMed] [Google Scholar]; c) Zhang D, Feng F, Li QL, Wang XY, Yao L, Biomaterials 2018, 173, 22–33; [DOI] [PubMed] [Google Scholar]; d) Shaughnessy MJO, Murray KS, La Rosa SP, Budhu S, Merghoub T, Somma A, Monette S, Kim K, Corradi RB, Scherz A, Coleman JA, Clin. Cancer Res 2018, 24, 592–599; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Ryu TK, Baek SW, Kang RH, Jeong KY, Jun DR, Choi SW, J. Controlled Release 2018, 270, 237–245; [DOI] [PubMed] [Google Scholar]; f) Baran TM, Lasers Surg. Med 2018, 50, 476–482; [DOI] [PubMed] [Google Scholar]; g) Sun XH, Zebibula A, Dong XB, Li GH, Zhang GX, Zhang DQ, Qian J, He SL, Nano Res 2018, 11, 2756–2770. [Google Scholar]
- [2].a) Ngweniform P, Abbineni G, Cao B, Mao C, Small 2009, 5, 1963–1969; [DOI] [PubMed] [Google Scholar]; b) Ngweniform P, Li D, Mao C, Soft Matter 2009, 5, 954–956. [Google Scholar]
- [3].a) Xia L, Kong X, Liu X, Tu L, Zhang Y, Chang Y, Liu K, Shen D, Zhao H, Zhang H, Biomaterials 2014, 35, 4146–4156; [DOI] [PubMed] [Google Scholar]; b) Staicu A, Pascu A, Nuta A, Sorescu A, Raditoiu V, Pascu ML, Rom. Rep. Phys 2013, 65, 1032–1051. [Google Scholar]
- [4].Krummenauer F, Braun M, Dick HB, Eur. J. Ophthalmol 2005, 15, 74–80. [DOI] [PubMed] [Google Scholar]
- [5].a) Wang H, Agarwal P, Zhao S, Yu J, Lu X, He X, Biomaterials 2016, 97, 62–73; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Master A, Livingston M, Gupta AS, J. Controlled Release 2013, 168, 88–102; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Khurshid A, Firdous S, Ahmat L, Ferraria J, Vollet-Filho JD, Kurachi C, Bagneto VS, Nawaz M, Ikram M, Ahmad M, Laser Phys 2012, 22, 317–321; [Google Scholar]; d) Succo G, Rosso S, Fadda GL, Fantini M, Crosetti E, Photodiagn. Photodyn. Ther 2014, 11, 63–70; [DOI] [PubMed] [Google Scholar]; e) Cao B, Yang M, Zhu Y, Qu X, Mao C, Adv. Mater 2014, 26, 4627–4631; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Sreeram KJ, Narayan S, Abbineni G, Hayhurst A, Mao C, Mol. Cancer Ther 2010, 9, 2524–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Bozzini G, Colin P, Betrouni N, Nevoux P, Ouzzane A, Puech P, Villers A, Mordon S, Photodiagn. Photodyn. Ther 2012, 9, 261–273; [DOI] [PubMed] [Google Scholar]; b) Al Amri MD, Kellesarian SV, Ahmed A, Al-Kheraif AA, Romanos GE, Javed F, Photodiagn. Photodyn. Ther 2016, 14, 166–169; [DOI] [PubMed] [Google Scholar]; c) Hanakova A, Bogdanova K, Tomankova K, Pizova K, Malohlava J, Binder S, Bajgar R, Langova K, Kolar M, Mosinger J, Kolarova H, Microbiol. Res 2014, 169, 163–170. [DOI] [PubMed] [Google Scholar]
- [7].Sanabria LM, Rodriguez ME, Cogno IS, Vittar NBR, Pansa MF, Lamberti MJ, Rivarola VA, Biochim. Biophys. Acta Rev. Cancer 2013, 1835, 36–45. [DOI] [PubMed] [Google Scholar]
- [8].a) Ormond AB, Freeman HS, Materials 2013, 6, 817–840; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K, Chem. Soc. Rev 2013, 42, 77–88; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) James NS, Joshi P, Ohulchanskyy TY, Chen Y, Tabaczynski W, Durrani F, Shibata M, Pandey RK, Eur. J. Med. Chem 2016, 122, 770–785; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Bandera Y, Burdette MK, Shetzline JA, Jenkins R, Creager SE, Foulger SH, Dyes Pigm 2016, 125, 72–79. [Google Scholar]
- [9].a) Lv R, Yang P, He F, Gai S, Yang G, Dai Y, Hou Z, Lin J, Biomaterials 2015, 63, 115–127; [DOI] [PubMed] [Google Scholar]; b) Bhaumik J, Mittal AK, Banerjee A, Chisti Y, Banerjee UC, Nano Res 2015, 8, 1373–1394; [Google Scholar]; c) Park KE, Noh YW, Kim A, Yong TL, Carbohydr. Polym 2017, 157, 476–483; [DOI] [PubMed] [Google Scholar]; d) Wang H, Zhu X, Han R, Wang X, Yang L, Wang Y, Microporous Mesoporous Mater 2017, 239, 78–85. [Google Scholar]
- [10].a) Lau JTF, Lo P-C, Jiang X-J, Wang Q, Ng DKP, J. Med. Chem 2014, 57, 4088–4097; [DOI] [PubMed] [Google Scholar]; b) Takahashi H, Nakajima S, Ogasawara K, Asano R, Nakae Y, Sakata I, Iizuka H, J. Dermatol 2014, 41, 729–731; [DOI] [PubMed] [Google Scholar]; c) Chen T-C, Huang L, Liu C-C, Chao P-J, Lin F-H, Proc. Biochem 2012, 47, 1903–1908; [Google Scholar]; d) Li X, Zhang Q, Ahmad Z, Huang J, Ren Z, Weng W, Han G, Mao C, J. Mater. Chem. B 2015, 3, 7449–7456; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Liu H, Fu Y, Li Y, Ren Z, Li X, Han G, Mao C, Langmuir 2016, 32, 9083–9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Fu Y, Li X, Sun C, Ren Z, Weng W, Mao C, Han G, ACS Appl. Mater. Interfaces 2015, 7, 25514–25521; [DOI] [PubMed] [Google Scholar]; b) Zhang Q, Xiang L, Ren Z, Han G, Mao C, Eur. J. Inorg. Chem 2015, 4532–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) Huang RB, Mocherla S, Heslinga MJ, Charoenphol P, Eniola-Adefeso O, Mol Membr. Biol 2010, 27, 312–327; [DOI] [PubMed] [Google Scholar]; b) Kruijt B, van der Snoek EM, Sterenborg HJCM, Amelink A, Robinson DJ, Photodiagn. Photodyn. Ther 2010, 7, 3–9. [DOI] [PubMed] [Google Scholar]
- [13].a) Jeon YM, Lee HS, Jeong D, Oh HK, Ra KH, Lee MY, Life Sci 2015, 124, 56–63; [DOI] [PubMed] [Google Scholar]; b) Lerche C, Heerfordt I, Heydenreich J, Wulf H, Int. J. Mol. Sci 2016, 17, 309; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Etcheverry ME, Pasquale MA, Garavaglia M, J. Photochem. Photobiol. B 2016, 160, 271–277; [DOI] [PubMed] [Google Scholar]; d) Weijer R, Broekgaarden M, Kos M, van Vught R, Rauws EAJ, Breukink E, van Gulik TM, Storm G, Heger M, J. Photochem. Photobiol. C 2015, 23, 103–131; [Google Scholar]; e) Munck C, Mordon S, Betrouni N, Photodiagn. Photodyn. Ther 2016, 16, 23–26. [DOI] [PubMed] [Google Scholar]
- [14].Calin MA, Diaconeasa A, Savastru D, Tautan M, Arch. Dermatol. Res 2011, 303, 145–151. [DOI] [PubMed] [Google Scholar]
- [15].Panjehpour OB, Haydek M, Gastrointest. Endosc. Clin. N. Am 2000, 10, 513–532. [PubMed] [Google Scholar]
- [16].Moslemi N, Soleiman-zadeh Azar P, Bahador A, Rouzmeh N, Chiniforush N, Paknejad M, Fekrazad R, Lasers Med. Sci 2015, 30, 89–94. [DOI] [PubMed] [Google Scholar]
- [17].Kimura M, Kashikura K, Yokoi S, Koiwa Y, Tokuoka Y, Kawashima N, Opt. Rev 2005, 12, 207–210. [Google Scholar]
- [18].a) Hino H, Murayama Y, Nakanishi M, Inoue K, Nakajima M, Otsuji E, J. Surg. Res 2013, 185, 119–126; [DOI] [PubMed] [Google Scholar]; b) Reddy MLP, Bejoymohandas KS, J. Photochem. Photobiol. C 2016, 29, 29–47. [Google Scholar]
- [19].Lustig RA, Vogl TJ, Fromm D, Cuenca R, Hsi RA, D'Cruz AK, Krajina Z, Turic M, Singhal A, Chen JC, Cancer 2003, 98, 1767–1771. [DOI] [PubMed] [Google Scholar]
- [20].Chen J, Keltner L, Christopherson J, Zheng F, Krouse M, Singhal A, Wang SS, Cancer J 2002, 8, 154–163. [DOI] [PubMed] [Google Scholar]
- [21].a) Neupane J, Ghimire S, Shakya S, Chaudhary L, Shrivastava VP, Photodiagn. Photodyn. Ther 2010, 7, 44–49; [DOI] [PubMed] [Google Scholar]; b) Trindade FZ, Pavarina AC, Ribeiro APD, Bagnato VS, Vergani CE, de Souza Costa CA, Lasers Med. Sci 2012, 27, 403–411. [DOI] [PubMed] [Google Scholar]
- [22].Wiegell SR, Wulf HC, Szeimies RM, Basset-Seguin N, Bissonnette R, Gerritsen MJP, Gilaberte Y, Calzavara-Pinton P, Morton CA, Sidoroff A, Braathen LR, J. Eur. Acad. Dermatol. Venereol 2012, 26, 673–679. [DOI] [PubMed] [Google Scholar]
- [23].a) Wiegell SR, Fabricius S, Gniadecka M, Stender IM, Berne B, Kroon S, Andersen BL, Mork C, Sandberg C, Ibler KS, Jemec GBE, Brocks KM, Philipsen PA, Heydenreich J, Haedersdal M, Wulf HC, Br. J. Dermatol 2012, 166, 1327–1332; [DOI] [PubMed] [Google Scholar]; b) Wiegell SR, Skodt V, Wulf HC, J. Eur. Acad. Dermatol. Venereol 2014, 28, 169–175; [DOI] [PubMed] [Google Scholar]; c) Cantisani C, Paolino G, Faina V, Frascani F, Cantoresi F, Bianchini D, Fazia G, Calvieri S, Int. J. Photoenergy 2014, 2014, 304862; [Google Scholar]; d) Togsverd-Bo K, Lerche CM, Philipsen PA, Haedersdal M, Wulf HC, Photochem. Photobiol. Sci 2013, 12, 2130–2136; [DOI] [PubMed] [Google Scholar]; e) Turan IS, Yildiz D, Turksoy A, Gunaydin G, Akkaya EU, Angew. Chem. Int. Ed 2016, 55, 2875–2878; Angew. Chem. 2016,128, 2925–2928. [DOI] [PubMed] [Google Scholar]
- [24].Wiegel SR, Haedersdal M, Philipsen PA, Eriksen P, Enk CD, Wulf HC, Br. J. Dermatol 2008, 158, 740–746. [DOI] [PubMed] [Google Scholar]
- [25].a) Wang H, Lv B, Tang ZM, Zhang M, Ge WQ, Liu YY, He XH, Zhao KL, Zheng XP, He MY, Bu WB, Nano Lett 2018, 18, 5768–5774; [DOI] [PubMed] [Google Scholar]; b) Larue L, Mihoub AB, Youssef Z, Colombeau L, Acherar S, Andre JC, Arnoux P, Baros F, Vermandel M, Frochot C, Photochem. Photobiol. Sci 2018, 17, 1612–1650; [DOI] [PubMed] [Google Scholar]; c) Hsu CC, Lin SL, Chang CA, Acs Appl. Mater Interfaces 2018, 10, 7859–7870; [DOI] [PubMed] [Google Scholar]; d) Ren XD, Hao XY, Li HC, Ke MR, Zheng BY, Huang JD, Drug Discovery Today 2018, 23, 1791–1800; [DOI] [PubMed] [Google Scholar]; e) Song L, Li PP, Yang W, Lin XH, Liang H, Chen XF, Liu G, Li J, Yang HH, Adv. Funct. Mater 2018, 28, 1707496; [Google Scholar]; f) Clement S, Chen WJ, Deng W, Goldys EM, Int. J. Nanomed 2018, 13, 3553–3570; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Chen HM, Wang GD, Chuang YJ, Zhen ZP, Chen XY, Biddinger P, Hao ZL, Liu F, Shen BZ, Pan ZW, Xie J, Nano Lett 2015, 15, 2249–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kirakci K, Zelenka J, Rumlova M, Martincik J, Nikl M, Ruml T, Lang K, J. Mater Chem. B 2018, 6, 4301–4307. [DOI] [PubMed] [Google Scholar]
- [27].Stacey OJ, Pope SJA, RSC Adv 2013, 3, 25550–25564. [Google Scholar]
- [28].a) Zhao HJ, Li L, Zheng CX, Hao YW, Niu MY, Hu YJ, Chang JB, Zhang ZZ, Wang L, Colloids Surf. B 2018, 167, 299–309; [DOI] [PubMed] [Google Scholar]; b) Callaghan S, Senge MO, Photochem. Photobiol. Sci 2018, 17, 1490–1514. [DOI] [PubMed] [Google Scholar]
- [29].Chen WH, Luo GF, Qiu WX, Lei Q, Liu LH, Wang SB, Zhang XZ, Biomaterials 2017, 117, 54. [DOI] [PubMed] [Google Scholar]
- [30].Allison RR, Bagnato VS, Sibata CH, Future Oncol 2010, 6, 929–940. [DOI] [PubMed] [Google Scholar]
- [31].Paszko E, Ehrhardt C, Senge MO, Kelleher DP, V Reynolds J, Photodiagn. Photodyn. Ther 2011, 8, 14–29. [DOI] [PubMed] [Google Scholar]
- [32].Bechet D, Auger F, Couleaud P, Marty E, Ravasi L, Durieux N, Bonnet C, Plénat F, Frochot C, Mordon S, Tillement O, Vanderesse R, Lux F, Perriat P, Guillemin F, Barberi-Heyob M, Nanomedicine 2015, 11, 657–670. [DOI] [PubMed] [Google Scholar]
- [33].Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, Khurshid A, Hasan T, Adv. Drug Delivery Rev 2010, 62, 1094–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y, Nat. Med 2012, 18, 1580–U190. [DOI] [PubMed] [Google Scholar]
- [35].a) Lee CH, Lai PS, Lu YP, Chen HY, Chai CY, Tsai RK, Fang KT, Tsai MH, Hsu CY, Hung CC, Wu DC, Yu HS, Chang CH, Tsai DP, J. Dermatol. Sci 2015, 80, 124–132; [DOI] [PubMed] [Google Scholar]; b) Broekgaarden M, Weijer R, Krekorian M, van den IJssel B, Kos M, Alles LK, van Wijk AC, Bikadi Z, Hazai E, van Gulik TM, Heger M, Nano Res. 2016, 9, 1639–1662; [Google Scholar]; c) Mahmoud G, Jedelska J, Strehlow B, Bakowsky U, Eur. J. Pharm. Biopharm 2015, 95, 88–98; [DOI] [PubMed] [Google Scholar]; d) L JJ, Meng X, Deng J, Lu D, Zhang X, Chen YR, Zhu JD, Fan AP, Ding D, Kong DL, Wang Z, Zhao YJ, Acs Appl. Mater. Interfaces 2018, 10, 17117–17128. [DOI] [PubMed] [Google Scholar]
- [36].a) Perez AP, Casasco A, Schilrreff P, Tesoriero MVD, Duempelmann L, Altube MJ, Higa L, Morilla MJ, Petray P, Romero EL, Int. J. Nanomed 2014, 9, 3335–3345; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li W, Peng J, Tan L, Wu J, Shi K, Qu Y, Wei X, Qian Z, Biomaterials 2016, 106, 119–133. [DOI] [PubMed] [Google Scholar]
- [37].Guelluy P-H, P Fontaine-Aupart M, Grammenos A, Lecart S, Piette J, Hoebeke M, Photochem. Photobiol. Sci 2010, 9, 1252–1260. [DOI] [PubMed] [Google Scholar]
- [38].Lim C-K, Heo J, Shin S, Jeong K, Seo YH, Jang W-D, Park CR, Park SY, Kim S, Kwon IC, Cancer Lett 2013, 334, 176–187. [DOI] [PubMed] [Google Scholar]
- [39].Bovis MJ, Woodhams JH, Loizidou M, Scheglmann D, Bown SG, MacRobert AJ, J. Controlled Release 2012, 157, 196–205. [DOI] [PubMed] [Google Scholar]
- [40].Shibu ES, Hamada M, Murase N, Biju V, J. Photochem. Photobiol. C 2013, 15, 53–72. [Google Scholar]
- [41].Koo H, Lee H, Lee S, Min KH, Kim MS, Lee DS, Choi Y, Kwon IC, Kim K, Jeong SY, Chem. Commun 2010, 46, 5668–5670. [DOI] [PubMed] [Google Scholar]
- [42].Wu D-Q, Li Z-Y, Li C, Fan J-J, Lu B, Chang C, Cheng S-X, Zhang X-Z, Zhuo R-X, Pharm. Res 2010, 27, 187–199. [DOI] [PubMed] [Google Scholar]
- [43].Sah U, Sharma K, Chaudhri N, Sankar M, Gopinath P, Colloids Surf B 2018, 162, 108–117. [DOI] [PubMed] [Google Scholar]
- [44].a) Ogbodu RO, Limson JL, Prinsloo E, Nyokong T, Synth. Met 2015, 204, 122–132; [Google Scholar]; b) Ogbodu RO, Nyokong T, Spectrochim. Acta Part A 2015, 151, 174–183. [DOI] [PubMed] [Google Scholar]
- [45].Benachour H, Seve A, Bastogne T, Frochot C, Vanderesse R, Jasniewski J, Miladi I, Billotey C, Tillement O, Lux F, Barberi-Heyob M, Theranostics 2012, 2, 889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo Y-EL, Woolliscroft MJ, Sugai JV, Johnson TD, Philbert MA, Kopelman R, Rehemtulla A, Ross BD, Clin. Cancer Res 2006, 12, 6677–6686. [DOI] [PubMed] [Google Scholar]
- [47].van Hell AJ, Fretz MM, Crommelin DJA, Hennink WE, Mastrobattista E, J. Controlled Release 2010, 141, 347–353. [DOI] [PubMed] [Google Scholar]
- [48].Cheng S-H, Lee C-H, Chen M-C, Souris JS, Tseng F-G, Yang C-S, Mou C-Y, Chen C-T, Lo L-W, J. Mater. Chem 2010, 20, 6149–6157. [Google Scholar]
- [49].a) Merzlyak A, Indrakanti S, Lee S-W, Nano Lett. 2009, 9, 846–852; [DOI] [PubMed] [Google Scholar]; b) Cao B, Mao C, Biomacromolecules 2009, 10, 555–564; [DOI] [PubMed] [Google Scholar]; c) Cao B, Yang M, Mao C, Acc. Chem. Res 2016, 49, 1111–1120; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Huai Y, Dong S, Zhu Y, Li X, Cao B, Gao X, Yang M, Wang L, Mao C, Adv. Healthcare Mater 2016, 5, 786–794; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Kalarical Janardhanan S, Narayan S, Abbineni G, Hayhurst A, Mao C, Mol Cancer Ther 2010, 9, 2524–2535; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Sunderland K, Yang M, Mao C, Angew. Chem. Int. Ed 2017, 56, 1964–1992; Angew. Chem. 2017, 129, 1992–2022; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Zhou X, Cao P, Zhu Y, Lu W, Gu N, Mao C, Nat. Mater 2015, 14, 1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].a) Lankes HA, Zanghi CN, Santos K, Capella C, Duke CMP, Dewhurst S, J. Appl Microbiol 2007, 102, 1337–1349; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Abbineni G, Modali S, Safiejko-Mroczka B, Pet-renko VA, Mao CB, Mol. Pharm 2010, 7, 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].a) Gandra N, Abbineni G, Qu X, Huai Y, Wang L, Mao C, Small 2013, 9, 215–221; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yang M, Li Y, Huai Y, Wang C, Yi W, Mao C, Chem. Commun 2018, 54, 1631–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].a) Ma K, Wang DD, Lin Y, Wang J, Petrenko V, Mao C, Adv. Funct. Mater 2013, 23, 1172–1181; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gandra N, Wang DD, Zhu Y, Mao C, Angew. Chem. Int. Ed 2013, 52, 11278–11281; Angew. Chem. 2013, 125, 11488–11491; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang DD, Yang M, Zhu Y, Mao C, Biomacromolecules 2015, 16, 3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].a) Yan X, Niu G, Lin J, Jin AJ, Hu H, Tang Y, Zhang Y, Wu A, Lu J, Zhang S, Huang P, Shen B, Chen X, Biomaterials 2015, 42, 94–102; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wu Q, Chu M, Shao Y, Wo F, Shi D, Carbon 2016, 108, 21–37. [Google Scholar]
- [54].Mang TS, Photodiagn. Photodyn. Ther 2004, 1, 43–48. [DOI] [PubMed] [Google Scholar]
- [55].a) Yang K, Zhang S, Zhang G, Sun X, Lee S-T, Liu Z, Nano Lett 2010, 10, 3318–3323; [DOI] [PubMed] [Google Scholar]; b) Tian B, Wang C, Zhang S, Feng L, Liu Z, Acs Nano 2011, 5, 7000–7009; [DOI] [PubMed] [Google Scholar]; c) Dong H, Zhao Z, Wen H, Li Y, Guo F, Shen A, Frank P, Lin C, Shi D, Sci. China Chem 2010, 53, 2265–2271; [Google Scholar]; d) Yang K, Wan J, Zhang S, Zhang Y, Lee S-T, Liu Z, Acs Nano 2011, 5, 516–522; [DOI] [PubMed] [Google Scholar]; e) Meng HM, Zhao D, Li N, Chang JB, Analyst 2018, 143, 4967–4973. [DOI] [PubMed] [Google Scholar]
- [56].a) Huang P, Xu C, Lin J, Wang C, Wang X, Zhang C, Zhou X, Guo S, Cui D, Theranostics 2011, 1, 240–250; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhou L, Wang W, Tang J, Zhou J-H, Jiang H-J, Shen J, Chem. Eur. J 2011, 17, 12084–12091. [DOI] [PubMed] [Google Scholar]
- [57].a) Haase M, Schaefer H, Angew. Chem. Int. Ed 2011, 50, 5808–5829; Angew. Chem. 2011, 123, 5928–5950; [DOI] [PubMed] [Google Scholar]; b) Wang M, Nano Res. 2015, 8, 1800–1810; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang M, Li M, Yu A, Wu J, Mao C, ACS Appl. Mater. Interfaces 2015, 7, 28110–28115; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wang M, Mi CC, Wang WX, Liu CH, Wu YF, Xu ZR, Mao CB, Xu SK, ACS Nano 2009, 3, 1580–1586; [DOI] [PubMed] [Google Scholar]; e) Wang M, Zhu Y, Mao C, Langmuir 2015, 31, 7084–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li FY, Du Y, Liu JA, Sun H, Wang J, Li RQ, Kim D, Hyeon T, Ling DS, Adv. Mater 2018, 30, 1802808. [DOI] [PubMed] [Google Scholar]
- [59].Zhang P, Steelant W, Kumar M, Scholfield M, J. Am. Chem. Soc 2007, 129, 4526–4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Du B, Han S, Zhao F, Lim KH, Xi H, Su X, Yao H, Jie Z, Nanomedicine 2016, 12, 2071–2080. [DOI] [PubMed] [Google Scholar]
- [61].a) Lim ME, Lee Y.-l., Zhang Y, Chu JJH, Biomaterials 2012, 33, 1912–1920; [DOI] [PubMed] [Google Scholar]; b) Cui S, Chen H, Zhu H, Tian J, Chi X, Qian Z, Achilefu S, Gu Y, J. Mater. Chem 2012, 22, 4861–4873. [Google Scholar]
- [62].Tian G, Yin W, Jin J, Zhang X, Xing G, Li S, Gu Z, Zhao Y, J. Mater. Chem. B 2014, 2, 1379–1389. [DOI] [PubMed] [Google Scholar]
- [63].Cui S, Yin D, Chen Y, Di Y, Chen H, Ma Y, Achilefu S, Gu Y, Acs Nano 2013, 7, 676–688. [DOI] [PubMed] [Google Scholar]
- [64].Ding B, Shao S, Yu C, Teng B, Wang M, Cheng Z, Wong KL, Ma P, Lin J, Adv. Mater 2018, 30, 1802479. [DOI] [PubMed] [Google Scholar]
- [65].a) Chatterjee DK, Yong Z, Nanomedicine 2008, 3, 73–82; [DOI] [PubMed] [Google Scholar]; b) Zou X, Yao M, Ma L, Hossu M, Han X, Juzenas P, Chen W, Nanomedicine 2014, 9, 2339–2351; [DOI] [PubMed] [Google Scholar]; c) Liu Y, Chen W, Wang S, Joly AG, Appl. Phys. Lett 2008, 92, 043901–043903; [Google Scholar]; d) Ma L, Zou X, Bui B, Chen W, Song K, Solberg T, Appl. Phys. Lett 2014, 105, 013702–013705. [Google Scholar]
- [66].Morgan NY, Kramer-Marek G, Smith PD, Camphausen K, Capala J, Radiat. Res 2009, 171, 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].a) Chen W, Zhang J, J. Nanosci. Nanotechnol 2006, 6, 1159–1166; [DOI] [PubMed] [Google Scholar]; b) Scaffidi JP, Gregas MK, Lauly B, Zhang Y, Vo-Dinh T, Acs Nano 2011, 5, 4679–4687. [DOI] [PubMed] [Google Scholar]
- [68].a) Schaffer M, Ertl-Wagner B, Schaffer PM, Kulka U, Jori G, Duhmke E, Hofstetter A, Current Med. Chem 2005, 12, 1209–1215; [DOI] [PubMed] [Google Scholar]; b) Kulka U, Schaffer M, Siefert A, Schaffer PM, Olsner A, Kasseb K, Hofstetter A, Duhmke E, Jori G, Biochem. Biophys. Res. Commun 2003, 311, 98–103. [DOI] [PubMed] [Google Scholar]
- [69].a) Drbohlavova J, Adam V, Kizek R, Hubalek J, Int. J. Mol. Sci 2009, 10, 656–673; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ding X, Peng F, Zhou J, Gong W, Slaven G, Loh KP, Lim CT, Leong DT, Nat. Commun 2019, 10, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].a) Medintz IL, Uyeda HT, Goldman ER, Mattoussi H, Nat. Mater 2005, 4, 435–446; [DOI] [PubMed] [Google Scholar]; b) Biju V, Itoh T, Anas A, Sujith A, Ishikawa M, Anal. Bioanal. Chem 2008, 391, 2469–2495. [DOI] [PubMed] [Google Scholar]
- [71].a) Biju V, Mundayoor S, Omkumar RV, Anas A, Ishikawa M, Biotechnol. Adv 2010, 28, 199–213; [DOI] [PubMed] [Google Scholar]; b) Biju V, Itoh T, Ishikawa M, Chem. Soc. Rev 2010, 39, 3031–3056; [DOI] [PubMed] [Google Scholar]; c) Yaghini E, Seifalian AM, MacRobert AJ, Nanomedicine 2009, 4, 353–363. [DOI] [PubMed] [Google Scholar]
- [72].a) Geszke-Moritz M, Moritz M, Mater. Sci. Eng. C 2013, 33, 1008–1021; [DOI] [PubMed] [Google Scholar]; b) Tshangana C, Nyokong T, J. Fluoresc 2015, 25, 199–210; [DOI] [PubMed] [Google Scholar]; c) Bekasova OD, Revina AA, Kornienko ES, Kurganov BI, Appl. Biochem. Biotechnol 2015, 176, 1141–1150; [DOI] [PubMed] [Google Scholar]; d) Diaz-Diestra D, Beltran-Huarac J, Bracho-Rincon DP, González-Feliciano JA, González CI, Weiner BR, Morell G, J. Nanopart. Res 2015, 17, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].a) Li L, Zhao J-F, Won N, Jin H, Kim S, Chen J-Y, Nanoscale Res. Lett 2012, 7, 386–393; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Juzenas P, Chen W, Sun Y-P, Coelho MAN, Generalov R, Generalova N, Christensen IL, Adv. Drug Delivery Rev 2008, 60, 1600–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Charron G, Stuchinskaya T, Edwards DR, Russell DA, Nann T, J. Phys. Chem. C 2012, 116, 9334–9342. [Google Scholar]
- [75].a) Pandita TK, Pandita S, Bhaumik SR, Crit. Rev. Eukaryotic Gene Expression 2009, 19, 235–251; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Horsman MR, Overgaard J, Clin. Oncol 2007, 19, 418–426. [DOI] [PubMed] [Google Scholar]
- [76].Manthe RL, Foy SP, Krishnamurthy N, Sharma B, Labhasetwar V, Mol. Pharm 2010, 7, 1880–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yao M, Ma L, Li L, Zhang J, Lim R, Chen W, Zhang Y, J. Biomed. Nanotechnol 2016, 12, 1835–1851. [DOI] [PubMed] [Google Scholar]
- [78].a) Dooley WC, Vargas HI, Fenn AJ, Tomaselli MB, Harness JK, Ann. Surg. Oncol 2010, 17, 1076–1093; [DOI] [PubMed] [Google Scholar]; b) Vargas HI, Dooley WC, Gardner RA, Gonzalez KD, Venegas R, Heywang-Kobrunner SH, Fenn AJ, Ann. Surg. Oncol 2004, 11, 139–146. [DOI] [PubMed] [Google Scholar]
- [79].a) Vorst AV, Rosen A, Kotsuka Y, RF/Microwave Interaction with Biological Tissues, Wiley, New York, 2006; [Google Scholar]; b) Setyawati MI, Tay CY, Bay BH, Leong DT, ACS Nano 2017, 11, 5020–5030. [DOI] [PubMed] [Google Scholar]
- [80].a) Waldow SM, Henderson BW, Dougherty TJ, Laser Surg. Med 1987, 7, 12–22; [DOI] [PubMed] [Google Scholar]; b) Waldow SM, Henderson BW, Dougherty TJ, Lasers Surg. Med 1984, 4, 79–85; [DOI] [PubMed] [Google Scholar]; c) Matsumoto N, Miyoshi N, Saito H, Hisazumi H, Sawada M, Fukuda M, J. Jpn. Soc. Laser Surg. Med 1989, 10, 233–236; [Google Scholar]; d) Waldow SM, Henderson BW, Dougherty TJ, Lasers Surg. Med 1985, 5, 83–94; [DOI] [PubMed] [Google Scholar]; e) Henderson BW, Waldow SM, Potter WR, Dougherty TJ, Cancer Res 1985, 45, 6071–6077. [PubMed] [Google Scholar]
- [81].a) Foster KR, Lozano-Nieto A, Riu PJ, Bioelectromagnetics 1998, 19, 420–428; [DOI] [PubMed] [Google Scholar]; b) Seegenschmiedt MH, Brady LW, Sauer R, Am. J. Clin. Oncol 1990, 13, 352–363; [PubMed] [Google Scholar]; c) Sato M, Watanabe Y, Ueda S, Iseki S, Abe Y, Sato N, Kimura S, Okubo K, Onji M, Gastroenterology 1996, 110, 1507–1514. [DOI] [PubMed] [Google Scholar]
- [82].a) Charamisinau I, Happawana G, Evans G, Rosen A, Hsi RA, Bour D, Appl. Opt 2005, 44, 5055–5068; [DOI] [PubMed] [Google Scholar]; b) Park S, Hwang J, Kwon Y, Cheon C, Electron. Lett 2012, 48, 1179–U123; [Google Scholar]; c) Premasiri HG, Evans G, Rosen A, ASME 2006 Frontiers in Biomedical Devices Conference, Nanotechnology Institute, Irvine, California, USA 2006, S. 29–30; [Google Scholar]; d) Li L, Che W, Chang Y, Microwave Opt. Technol. Lett 2012, 54, 405–409. [Google Scholar]
- [83].Gu Y, Microwave induced photothermal therapy, US20130012754A1, USA patent, 2013.
- [84].Davies AM, Weinberg U, Palti Y, Ann. N. Y. Acad. Sci 2013, 1291, 86–95. [DOI] [PubMed] [Google Scholar]
- [85].Saczko J, Nowak M, Skolucka N, Kulbacka J, Kotulska M, Bioelectrochemistry 2010, 79, 90–94. [DOI] [PubMed] [Google Scholar]
- [86].Covello G, Siva K, Adami V, Denti MA, Cytotechnology 2014, 66, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Charoo NA, Rahman Z, Repka MA, Murthy SN, Current Drug Delivery 2010, 7, 125–136. [DOI] [PubMed] [Google Scholar]
- [88].a) Lambreva M, Gluck B, Radeva M, Berg H, Bioelectro-chemistry 2004, 62, 95–98; [DOI] [PubMed] [Google Scholar]; b) Labanauskiene J, Gehl J, Didziapetriene J, Bioelectrochemistry 2007, 70, 78–82; [DOI] [PubMed] [Google Scholar]; c) Traitcheva N, Berg H, Bioelectrochemistry 2010, 79, 257–260; [DOI] [PubMed] [Google Scholar]; d) Kulbacka J, Kotulska M, Rembialkowska N, Choromanska A, Kaminska I, Garbiec A, Rossowska J, Daczewska M, Jachimska B, Saczko J, Cell Stress Chaperones 2013, 18, 719–731; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Wezgowiec J, Kotulska M, Saczko J, Derylo MB, Teissie J, Rols M-P, Orio J, Garbiec A, Kulback J, Photodiagn. Photodyn. Ther 2013, 10, 490–502. [DOI] [PubMed] [Google Scholar]
- [89].Ward T, The effect of electric fields on photodynamic activation, Ulster University, 1998. [Google Scholar]
- [90].a) Makarov DA, Yuzhakova OA, Slivka LK, Kuznetsova NA, Negrimovsky VM, Kaliya OL, Lukyanets EA, J. Porphyrins Phthalocyanines 2007, 11, 586–595; [Google Scholar]; b) Balaz M, Collins HA, Dahlstedt E, Anderson HL, Org. Biomol. Chem 2009, 7, 874–888. [DOI] [PubMed] [Google Scholar]
- [91].Makarov DA, Kuznetsova NA, Yuzhakova OA, Savvina LP, Kaliya OL, Lukyanets EA, Negrimovskii VM, Strakhovskaya MG, Russian J Phys. Chem. A 2009, 83, 1044–1050. [Google Scholar]
- [92].Fallows SJ, Garland MJ, Cassidy CM, Tunney MM, Singh TRR, Donnelly RF, J. Photochem. Photobiol. B 2012, 114, 61–72. [DOI] [PubMed] [Google Scholar]
- [93].a) Fan Z, Shelton M, Singh AK, Senapati D, Khan SA, Ray PC, Acs Nano 2012, 6, 1065–1073; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Miyoshi N, Idehara T, Khutoryan E, Fukunaga Y, Bibin AB, Ito S, Sabchevski SP, J. Infrared Millimeter Terahertz, Waves 2016, 37, 805–814; [Google Scholar]; c) Tada DB, Vono LLR, Duarte EL, Itri R, Kiyohara PK, Baptista MS, Rossi LM, Langmuir 2007, 23, 8194–8199; [DOI] [PubMed] [Google Scholar]; d) Lai C-W, Wang Y-H, Lai C-H, Yang M-J, Chen C-Y, Chou P-T, Chan C-S, Chi Y, Chen Y-C, Hsiao J-K, Small 2008, 4, 218–224; [DOI] [PubMed] [Google Scholar]; e) Penon O, Marin MJ, Amabilino DB, Russell DA, Perez-Garcia L, J. Colloid Interface Sci 2016, 462, 154–165; [DOI] [PubMed] [Google Scholar]; f) Shi J, Yu X, Wang L, Liu Y, Gao J, Zhang J, Ma R, Liu R, Zhang Z, Biomaterials 2013, 34, 9666–9677; [DOI] [PubMed] [Google Scholar]; g) Chen HP, Tung FI, Chen MH, Liu TY, J. Controlled Release 2016, 226, 182–192; [DOI] [PubMed] [Google Scholar]; h) Shah SA, Khan MUA, Arshad M, Awan SU, Hashmi MU, Ahmad N, Colloids Surf. B 2016, 148, 157–164. [DOI] [PubMed] [Google Scholar]
- [94].Primo FL, Michieleto L, Rodrigues MAM, Macaroff PP, Morais PC, Lacava ZGM, Bentley MVLB, Tedesco AC, J. Magn. Magn. Mater 2007, 311, 354–357. [Google Scholar]
- [95].a) Basoglu H, Bilgin MD, Demir MM, Photodiagn. Photodyn. Ther 2016, 13, 81–90; [DOI] [PubMed] [Google Scholar]; b) Bolfarini GC, Siqueira-Moura MP, Demets GJF, Morais PC, Tedesco AC, J. Photochem. Photobiol. B 2012, 115, 1–4; [DOI] [PubMed] [Google Scholar]; c) Curry T, Kopelman R, Shilo M, Popovtzer R, Contrast Media Mol Imaging 2014, 9, 53–61; [DOI] [PubMed] [Google Scholar]; d) Corato R, Bealle G, Kolosnjaj-Tabi J, Espinosa A, Clement O, Silva AK, Menager C, Wilhelm C, ACS Nano 2015, 9, 2904–2916; [DOI] [PubMed] [Google Scholar]; e) Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Doeblinger M, Banerjee R, Bahadur D, Plank C, J. Controlled Release 2010, 142, 108–121. [DOI] [PubMed] [Google Scholar]
- [96].Liu F, Zhou X, Chen Z, Huang P, Wang X, Zhou Y, Mater. Lett 2008, 62, 2844–2847. [Google Scholar]
- [97].Mbakidi J-P, Brégier F, Ouk T-S, Granet R, Alves S, Rivière E, Chevreux S, Lemercier G, Sol V, ChemPlusChem 2015, 80, 1416–1426. [DOI] [PubMed] [Google Scholar]
- [98].Oliveira DM, Lacava ZGM, Lima ECD, Morais PC, Tedesco AC, J. Nanosci. Nanotechnol 2006, 6, 2432–2437. [DOI] [PubMed] [Google Scholar]
- [99].Muehlmann LA, Joanitti GA, Silva JR, Longo JPF, Azevedo RB, Brazilian J Med. Biol Res 2011, 44, 729–737. [DOI] [PubMed] [Google Scholar]
- [100].Pang LJ, Baciu C, Traitcheva N, Berg H, J. Photochem. Photobiol. B 2001, 64, 21–26. [DOI] [PubMed] [Google Scholar]
- [101].Shopova M, Mantareva V, Krastev K, Hadjiolov D, Milev A, Spirov K, Jori G, Ricchelli F, J. Photochem. Photobiol. B 1992, 16, 83–89. [DOI] [PubMed] [Google Scholar]
- [102].Radeva M, Berg A, Berg H, Electromagn. Biol Med 2004, 23, 185–200. [Google Scholar]
- [103].a) Rosenthal I, Sostaric JZ, Riesz P, Ultrason. Sonochem 2004, 11, 349–363; [DOI] [PubMed] [Google Scholar]; b) Hiraoka W, Honda H, Feril LB, Kudo N, Kondo T, Ultrason. Sonochem 2006, 13, 535–542. [DOI] [PubMed] [Google Scholar]
- [104].a) McCaughan B, Rouanet C, Fowley C, Nomikou N, McHale AP, McCarron PA, Callan JF, Bioorg. Med. Chem. Lett 2011, 21, 5750–5752; [DOI] [PubMed] [Google Scholar]; b) Nomikou N, Sterrett C, Arthur C, McCaughan B, Callan JF, McHale AP, ChemMedChem 2012, 7, 1465–1471. [DOI] [PubMed] [Google Scholar]
- [105].a) Tomankova K, Kolarova H, Vachutka J, Zapletalova J, Hanakova A, Kaplova E, Indian J. Biochem. Biophys 2014, 51, 19–28; [PubMed] [Google Scholar]; b) Wang H, Wang X, Wang P, Zhang K, Yang S, Liu Q, Ultrasound Med. Biol 2013, 39, 1713–1724; [DOI] [PubMed] [Google Scholar]; c) Tserkovsky DA, Alexandrova EN, Chalau VN, Istomin YP, Exp. Oncol 2012, 34, 332–335; [PubMed] [Google Scholar]; d) Wang P, Li C, Wang X, Xiong W, Feng X, Liu Q, Leung AW, Xu C, Ultrason. Sonochem 2015, 23, 116–127; [DOI] [PubMed] [Google Scholar]; e) Li J-H, Chen Z-Q, Huang Z, Zhan Q, Ren F-B, Liu J-Y, Yue W, Wang Z, Oncol. Lett 2013, 5, 702–706; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Li Q, Liu Q, Wang P, Feng X, Wang H, Wang X, Ultrasonics 2014, 54, 981–989; [DOI] [PubMed] [Google Scholar]; g) Gao S, Wang G, Qin Z, Wang X, Zhao G, Ma Q, Zhu L, Biomaterials 2017, 112, 324; [DOI] [PubMed] [Google Scholar]; h) Liu Y, Wang P, Liu Q, Wang X, Ultrason. Sonochem 2016, 31, 437; [DOI] [PubMed] [Google Scholar]; i) Tennert C, Drews AM, Walther V, Altenburger MJ, Karygianni L, Wrbas KT, Hellwig E, Alahmad A, Photodiagn. Photodyn. Ther 2015, 12, 244–251. [DOI] [PubMed] [Google Scholar]
- [106].Wang P, Li C, Wang X, Xiong W, Feng X, Liu Q, Leung AW, Xu C, Ultrason. Sonochem 2015, 23, 116–127. [DOI] [PubMed] [Google Scholar]
- [107].van den Berg PJ, Bansal R, Daoudi K, Steenbergen W, Prakash J, Biomed. Opt. Express 2016, 7, 5081–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]