Abstract
In this review, an effort is made to discuss the most recent progress and future trend in the two-way traffic of the interactions between plants and nanoparticles (NPs). One way is the use of plants to synthesize NPs in an environmentally benign manner with a focus on the mechanism and optimization of the synthesis. Another way is the effects of synthetic NPs on plant fate with a focus on the transport mechanisms of NPs within plants as well as NP-mediated seed germination and plant development. When NPs are in soil, they can be adsorbed at the root surface, followed by their uptake and inter/intra-cellular movement in the plant tissues. NPs may also be taken up by foliage under aerial deposition, largely through stomata, trichomes, and cuticles, but the exact mode of NP entry into plants is not well documented. The NP-plant interactions may lead to inhibitory or stimulatory effects on seed germination and plant development, depending on NP compositions, concentrations, and plant species. In numerous cases, radiation-absorbing efficiency, CO2 assimilation capacity, and delay of chloroplast aging have been reported in the plant response to NP treatments, although the mechanisms involved in these processes remain to be studied.
Keywords: nanoparticles, plants, mechanism, plant response
Graphical Abstract
Critical review is made to discuss the two-way traffic of interactions between plants and nanoparticles (NPs). One way is the plant-mediated green synthesis of NPs, in particular, its mechanism and the commercial potentials of NP phytosynthesis. Another way is the NP-mediated fate of plant cells and tissues, in particular, the transport mechanisms of NPs within plants as well as NP-mediated seed germination and plant development.
1. Introduction
Nanotechnology concerns the synthesis of metal, semi-metal and nonmetal nanoparticles (NPs), offering promising solutions for a wide range of disciplines including agriculture.[1] NPs can be considered as colloidal particles with one or more dimensions of 1–100 nm.[2] They bear unique properties such as an extremely high ratio of surface-to-volume and specific surface area, which determine excellent chemical, optical, mechanical and electronical properties.[3] From the points of view of structure and chemical composition, nanomaterials are heterogeneous group of compounds, include carbonaceous materials,[4] semiconductors, metal oxides,[5] lipids,[6] zero-valent metals,[7] quantum dots, nanopolymers,[8] and dendrimers[3] with different morphologies such as rods, fibers, wires and sheets.[9]
Nanotechnology has developed into a high-tech industry with stupendous market growth; hundreds of nanomaterials produced by physical or chemical methods[10] are commercially available. Currently, the most popular and important methods to synthesize NPs are ultraviolet (UV) irradiation,[11] microwave irradiation,[12] chemical reduction,[13] photochemical methods,[14] electron irradiation,[15] and sono electrochemical methods.[16] Due to few decades of intensive and extensive research and development in this field, these methods give repetitive and demanded size particles, but they are also costly and hazardous.[17] Physical methods are time-consuming and need to use expensive equipment.[18] Chemical methods involve greater risks of contamination of NPs with precursor chemicals, the toxic solvents, and the formation of hazardous byproducts.[19] In view of the environmental risks associated with chemical methods, demand for green technologies such as biological synthesis of NPs via microorganisms and plant extracts[20] has been increasing. Among microorganisms, numerous yeasts,[21] algae and cynobacteria,[22] fungi,[23] bacteria,[24] flagella,[24a] pili,[25] and viruses[26] are potential candidates to facilitate controlled NP synthesis.[27] To meet the increasing demand for NPs and expand their use in various fields, a switch to green synthesis methods,[28] especially phytosynthesis, is a rational approach.
Although microorganisms especially algae, cyanobacteria, fungi and bacteria[29] constitute important candidates for NP green synthesis,[30] none of the reported reviews on these aspects described the two-way traffic of plant-NP interactions. Hence we aim our attention at the two-way traffic of such interactions. We first describe the use of plants to interact with NPs and then summarized the effects of NPs on plant development. Specifically, we critically assess the principles and mechanisms of the plant-mediated synthesis of NPs as well as the fundamental rules governing the NP fate in plant tissues/ cells and the way by which NPs affect plant development.
2. Current status of plant-mediated synthesis of nanoparticles
NP synthesis via plant extracts has been known for more than a century, but this process has only been explored and exploited in the last twenty years.[31] Reviews on the synthesis of NPs utilizing green methods have been published in recent years.[20d, 20e, 32] However, almost no reports have systematically presented the mechanism of plant-mediated NP synthesis, factors affecting this synthesis, the NP effects on the plants themselves, and the mechanism of the effects. The present review offers detailed and critical information on the aforementioned aspects of NP synthesis, which are, by and large, undescribed. Critical efforts have been made to streamline the mechanism(s) involved in NP synthesis and to identify efficient plant species for commercial exploitation. The effects of NPs on phytosystems have been examined for crop production and phytotoxicity; however, the possible route of metabolism of NPs in the plant system remains difficult to explain.
In general, NPs are formed by two pathways, depending on the form and size of the metal in the solvent. For example, if the metal particles are smaller than nanosized, i.e., atoms, ions or molecules, the “bottom-up” pathway operates. In this pathway, NP synthesis occurs through aggregation of atoms, molecules and/or clusters through the action of reducing agents of chemical or biological origin.[19] The second pathway is “top-down” and operates when particles are larger than nanosized. In this pathway, particle size is gradually reduced.[33]
Phytosynthesis is a technique in which extract of parts from plants are used to synthesize NPs.[34] This technique may prove to be highly effective, quick, economical, and eco-friendly and free of hazardous byproducts. Researchers have used different preparations of plant extracts such as whole plant biomass,[35] aerial parts,[36] leaves[37] and seeds.[38] These plant parts were macerated in different solvents, including ethanol,[39] distilled water[35] or deionized water,[40] followed by filtration through a filter paper to separate plant tissue and other debris. The plant extract (containing multiple molecules) is applied to the metal salt solution to mediate synthesis of nanoparticles of the respective metal. This general procedure is reported to yield stable NPs with good quality as well as lesser risk of contamination. In phytosynthesis, the whole plant as well as soft tissues such as foliage, and more specifically the leaves and buds[41] are generally used. The NP synthesis can also be accomplished with dry leaf powder.[11] Extracts from plant tissue act as reducing and anti-agglomerating agents.[42] Leaf extract or biomass contains triterpenes, flavonoids, phenols flavonoids, carbohydrates, anthraquinones etc., which reduce metal ions into NPs and stabilize the resultant NPs.[43] Other researchers have reported that plant extracts contain alkaloids, terpenoids, and proteins. Among these, flavonoids and proteins have been hypothesized to be pivotal in bringing out the reduction of NP precursors and stabilization of NPs.[44] Some researchers have shown that Au NPs can be synthesized by plants rich in tannic acid. Such plants can also efficiently catalyse to synthesize Ag NPs.[27a, 45]
3. Mechanism of NP phytosynthesis
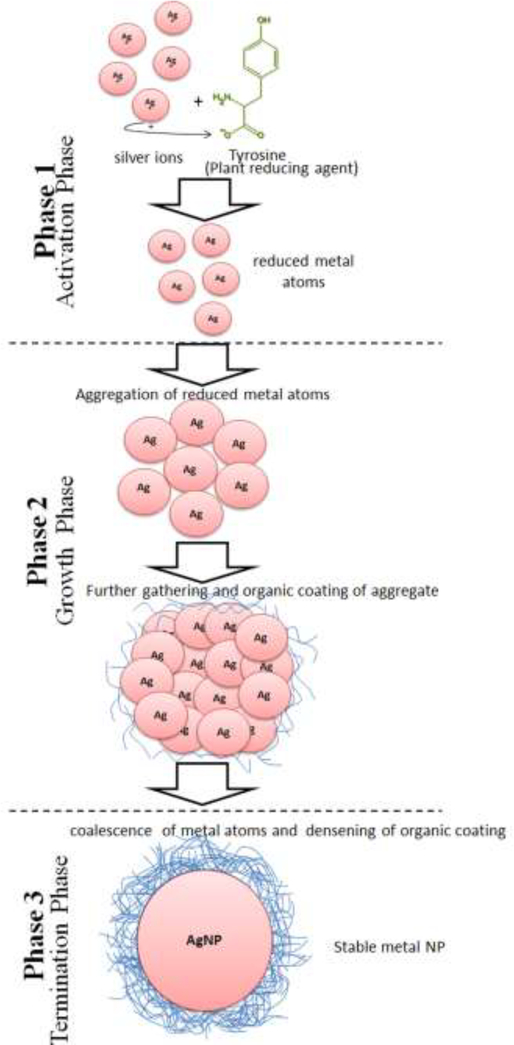
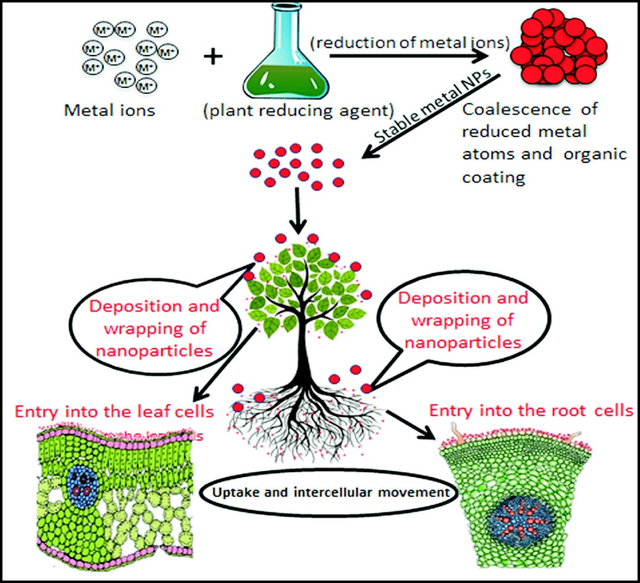
The bottom-up pathway is widely used to synthesize metallic NPs. NPs formed through this pathway have fewer surface structural defects and greater homogeneity with regard to size and chemical composition than those formed through the top-down pathway.[19] It is believed that this pathway is initiated with the reduction of metal ions by phytomolecules. A range of phytomolecules existing in plant extracts, are believed to be responsible for reducing metal ions in aqueous salt solutions, resulting in the solution-phase formation of NPs through the bottom-up pathway (Figure 1). Ion reduction does not appear to occur directly; instead, intermediate biosynthetic products or reduced cofactors are formed during the reduction of the respective ions to neutral particles. The reduction of ions is followed by the growth and coalescence of small particles. Coalescence occurs through a coarsening process. Finally, particles with stable shape and size are formed by a continuous process of coalescence and growth. Aggregated particles are stabilized by the formation of a surrounding organic coating.[46] In general, the mechanism of phytosynthesis of metal NPs operates in three phases:
Figure 1.
Schematic diagram showing important steps involved in the phytosynthesis of metallic NPs such as Ag NPs.
Phase 1 - activation phase
During this phase metal ions are reduced before the atoms nucleate. When plant extract is introduced to the metal salt solution, the metal ions react with the reducing phytomolecules such as polyphenols, vitamins, sterols, triterpenes, alkaloids, sugars, proteins, polysaccharides, saponins, β-phenylethylamines, phenols, amino acids, antioxidant metabolites, flavonoids and stabilizing agents.[47] As a result, the ions are reduced to metal atoms (Figure 1). The resulting complex of metal atoms, ions, and phytomolecules further interact and react to form small metal NPs. The reducing agents vary by plant species. In the case of the leaf extract of neem, metal ion reduction possibly occurs through reducing sugars present in the leaves.[48] The leaf extract of Capsicum annum, however, contains amine-bearing proteins that may reduce the Ag+ ions in the solution, as evidenced by the change in the secondary structure of the proteins following their interaction with Ag+ ions.[49] The leaf extract of Ficus benghalensis showed a higher concentration of antioxidants and polyphenols (flavonoids).[50] These phytomolecules hunt active oxygen molecular species. The antioxidant action of flavonoid donation of electrons or hydrogen atoms may change keto groups to the enol form of phenols and other chemicals, thereby enabling the reduction of Ag+ ions and imparting high stability to NPs.[50]
Zheng and colleagues developed a detailed study to understand the bioreductive mechanism of NP phytosynthesis.[51] They principally examined the relative role of saccharides, reducing sugars, flavonoids, and proteins in reducing the metal ions.[51] The concentrations of flavonoids and proteins were observed to be much greater than those of reducing sugars and saccharides. After the reaction was completed, a notably lower concentration of reducing sugars and flavonoids was recorded, whereas the saccharide (non-reducing sugars) and protein contents remained significantly unchanged. These results indicated that the reducing flavonoids and sugars participated in NP synthesis.[52] The major phytochemicals involved in metal ion reduction also vary by plant groups. Mesophytes contain terpenoids, flavones, ketones, aldehydes, amines, flavones, organic acids, and quinones, immediately leading to ion reduction,[53] whereas xerophytes contain the anthraquinone emodin, which undergoes tautomerization, leading to silver ion reduction.[53] In general, cyperoquinone, dietchequinone and remirin basically benzoquinones, were present in mesophytes, which played a direct role in ionic reduction and Ag NP formation.[54]
Phase 2 - growth phase
In this phase, the smaller particles formed in phase-1 undergo spontaneous coalescence. The coalescence mainly occurs between adjacent small particles, leading to the formation of larger particles. Additionally, heterogeneous nucleation, coupled with the growth of existing nuclei and further reduction of metal ions, occurs with larger particles, leading to the formation of NPs in a process called Ostwald ripening.[55] As the duration of the growth phase increases, NP aggregates of different shapes such as nanotubes, nanoprisms, nanohexahedra, and other irregular shapes are formed. Little identification of biomolecules that cause aggregation and coalescence of ions has been reported. Egorova and Revina reported the involvement of quercetin for the quick and stable synthesis of Ag and Cu NPs.[55] They demonstrated that when a synthetic quercetin solution (0.15 mol octane or heptane/L) was added to aqueous AgNO3 or Cu(NO3)2 solutions, Ag and Cu NPs were formed, respectively.
Phase 3 - termination phase
The final phase of NP synthesis involves the final shaping of NPs. In this process, NPs require energy to become stable. Gibbs free energy (GFE) is liberated during a reaction to form a stable product.[47] The energy input requirements vary with NP shape.[47] For example, nanotriangles have very high surface energy, which makes them less stable; if the stability of NPs is not supported by counter equivalent GFE in a given plant extract, the nanotriangles shift to a more stable shape, such as that of a truncated triangle. The shape transformation occurs because of the lesser GFE available in the plant extract; thus, the reaction proceeds toward the formation of particles with more stable shapes. Therefore, the type of plant extract having different levels of GEE governs the stabilization of NPs, and growth and coalescence are terminated after the particles are stabilized. Stability refers to a stage where further growth of particle has ceased and the particle has assumed a permanent shape. The particle stability occurs when the surface energy and GFE are equal. Thereafter, the particle is coated by an organic material and attains a specific size (Figure 1). To prevent the dispersal of aggregated particles, the aggregate is encased or covered by organic materials such as proteins and polysaccharides. These materials provide additional stability to the particles, and after this process further growth in the particles is ceased or the particles are capped. Li and colleagues identified 3-nm protein moieties in C. annum extracts that served as Ag NP capping agents.[49] Ag+ ion reduction was induced by the amino groups of proteins present in the pepper extract. Shanker and colleagues identified citronellol and geraniol in the decocted Geranium leaves for Au NP synthesis and capping.[27a] Similarly, proteins and other biomolecules present in chickpea leaf extract bring about the stabilizing Au NPs.[56] The neem leaf extract contains terpenoids that could serve as a surface active molecule to stabilize NPs.[48]
The above information has revealed that the plant extracts containing different molecules such as proteins, polysaccharides, phenols, flavenoids reducing sugers etc. are involved in the reduction of metal ions to metal nanoparticles.[57] Most of these molecules also participate in the final stabilization and capping of NPs.[58] It shows that a plant molecule may perform different functions during NP synthesis. On the contrary, different molecules may be involved in a specific process and may bring about reduction, capping or stabilization of NPs.[59] Since, plant extract contains large number of chemical compounds along with sugars, proteins and other residual materials, it is hard to define exact role of each and every constituents at different phases of NP synthesis.[60] However, concerted research efforts are needed to pin point the role of individual phytomolecules in the synthesis.
Chemical modification
Nanoparticles synthesized by the green methods are less stable and low in viability in comparison with NPs produced by conventional synthesis.[61] The plant extracts are high in ionic strengths which usually induce NP aggregation, and in other molecules such as proteins, lipids, sugars, and nucleic acids., Especially, proteins molecules affect NP stability and its viability.[62]
Two methods are typically used for the colloidal stability of green synthesized NPs: electrostatic repulsion and steric stabilization. The stabilization through electrostatic repulsion is the simplest and widely used method. In this method, at low ionic strengths, the diffuse double layer (DDL) extends far from the particle surface, facilitating particle–particle repulsions. However, at high ionic strengths, the NP DDL is compressed and neutralized with the subsequent aggregation due to van der Waals forces.[63] Thus, electrostatic stabilization largely fails to provide sufficient colloidal stability in biological media.[64] The other method is based on the generation of a physical barrier at the NP surface like steric stabilization. Attachment of polymers on the nanoparticle surface such as polyethylene glycol (PEG) or polyvinyl pyrrolidone (PVP), are used to increase NPs stability in suspensions.[65] The PEG and PVP are hydrophilic in nature, inducing extra stabilization through the short range repulsive hydration forces.[66]
4. Factors affecting biosynthesis of NPs
Only a few deliberate efforts have been made to identify the factors that affect NP yield during the phytosynthesis of NPs. The reported pace and yield of phytosynthesis has not been consistent. Some researchers have shown a good NP yield, whereas others have reported a low NP yield using the same metal salt and plant species.[67] This indicates that other factors, namely, temperatures, reaction time, and pH values, may also influence the yield, size or shape of the resulting NPs. Although this generalization is broad because of a lack of information concerning the effect of different factors on NP yield, the available data is sufficient to support the argument that these factors require standardization for commercial exploitation of phytosynthesis, which is described below.[67]
4.1. Plant species
A critical analysis of the published information reveals that plants such as Aloe vera, Avena sativa, Azadiracta indica, Chenopodium album, Jatropha and Pelargonium graveolens have been extensively used to produce NPs and have been found to mediate NP synthesis more efficiently than other plant species. The concentrations of plant extract may also affect the NP synthesis and particle morphology.[68] The concentrations of lemongrass leaf extract were found to control the shapes of the NPs,[20a] which could be somewhat connected with chemical composition and synergic effect of unique chemicals found in this plant species. The average count of hexagonal and triangular shapes decreased and the number of spherical NPs (vs. non-spherical NPs) increased when the leaf extract concentration was increased from 0.2 to 1.6 mL.[20a] The leaf extract of A. vera, A. sativa, A. indica, C. album and Jatropha with a concentration lower than that of other plant species led to the effective synthesis of NPs; hence, these plant species can be considered “NP efficient plants”. Chemical analyses of leaf extracts have indicated that these plants contain greater amounts of primary, secondary and tertiary compounds such as flavonoids, phenols, polyphenols, and antioxidants.[69] The phenols and antioxidants[69] are strong reducing and stabilizing agents; hence, their concentrations in the extract appears to actually govern the NP synthesis and particle stability.
Research has also indicated that the specific plant species and extract concentration may also control the particle shape and size. The addition of Geranium leaf extract at 1 mmol/L to an HAuCl4 aqueous solution bring about rapid formation of rod-, flat-, and triangular-shaped Au NPs, whereas lower or higher concentrations yielded NPs of either shape.[70] Leaf extracts of lemongrass (Cymbopogon flexuosus) and alfalfa are notably efficient plant species with regard to NP synthesis.[20a] Lemongrass extract in HAuCl4 solution induced rapid formation of triangular and hexagonal Au NPs,[20a] whereas the alfalfa extract synthesized icosahedral NPs.[71] Above information has revealed that plant extracts prepared by macerating the plant parts in a solvent and filtering through a simple filter paper were used to convert metal ions into metal NPs. Surprisingly, none of the researchers concerned for impurities present in the extract which might influence synthesis or quality of NPs. Probably for rapid synthesis of NPs induced by plant extracts, researchers did not consider undertaking additional purification steps to separate the impurities. However, we presume that these impurities may pose some hindrance in the NP production at commercial scale. A further purification of plant extract may resolve this issue but it may increase the process cost.
4.2. pH of reaction solvents
Solvent pH may regulate the pace of synthesis and morphology of NPs. Studies indicated that smaller NPs are formed at pH 3–4 and larger NPs are synthesized at pH 2,[31a] suggesting that higher acidity may cause greater aggregation of neutral particles, resulting in larger particles. In another study, chemically modified hop biomass (Sargassum wightii) led to 70, 80 and 84% yields of Au NPs from esterified, native, and hydrolyzed hop biomass, respectively. Chemical treatments of the hop biomass in different forms resulted in different NP sizes, i.e., 0.92 nm (esterified), 1.73 nm (native), and 2.5 nm (hydrolyzed).[72] Some researchers have used plant biomass for NP synthesis and have demonstrated the are governed by the pH of reaction size and shape of NPs mixture.[31a] Ground wheat biomass (Triticum aestivum) was added to KAuCl4 solutions (0.3 mmol/L) with various pH values, i.e., pH 2.0–6.0, and incubated for 3.5 h. The process led to formation of 10–30 nm Au NPs with tetrahedral, hexagonal, irregular, decahedral and other shapes. The maximal synthesis of Au NPs was achieved at pH 2.0–3.0 with a rod-like shape. In similar studies, oat biomass (A. sativa) mediated the synthesis of Au NPs at pH 2–6 in 1 h. Maximum NP synthesis was achieved at pH 3.0.[31a] On the basis of the aforementioned information, an acidic pH range of 2.0–3.0 appears to be optimal for the phytosynthesis of NPs.
4.3. Reaction time
The reaction duration may also influence NP synthesis. The leaf extract of Geranium (P. graveolens) in HAuCl4 solution led to the synthesis of rods, flat sheets, and triangle-shaped Au NPs within 2 h.[70] The reduction of Au3+ ions was almost completed in 60 min. In the experiments with a fungus (Fusarium oxysporum[73]) and an actinomycete (Thermomonospora sp.[55]) under nearly identical reaction conditions, Au NP synthesis was finished after 48 and 120 h, respectively. Geranium leaf extract induced the formation of crystalline, highly stable Ag NPs (from AgNO3 solution) in very high yield.[74] Rapid reduction of the silver ions occurred, achieving 90% reduction within 9 h of reaction. Li and colleagues reported that the size of Ag particles (16–40 nm) varied with the reaction time.[49] An increase in the reaction time led first to polycrystalline and then to single-crystalline particles, and NP size also increased to 40 nm. Spherical and polycrystalline particles of 10 ± 2 nm were formed in 5 h. A further increase to 9 and 13 h in the reaction time resulted in 25 ± 3 and 40 ± 5 nm particles, respectively.71
5. NP effects on plant growth and mechanism of action
NPs enter plant systems mainly through roots and leaves. After entry, NPs interact with plants at the cellular and subcellular levels, facilitating changes to morphological and physiological states,[75] which may be suppressive or stimulatory depending on the NP properties. NP effects on the plant systems may be determined by the chemical nature, size, reactivity, and specifically, the amount of NPs present in or on the plant.[76] To understand the NP-plant interaction and to assess the agriculture risks/benefits, we discuss the inhibitory (negative) and promoting (positive) effects of NPs on the growth and development of plants separately. Table 1 and 2 summarize the inhibitory and promoting effects of NPs the seed priming, plant growth and development, respectively. Researchers have used different methods of NP application viz., soil application, foliar spray or seed treatment while examining the influence of nanoparticles on seed germination or plant growth. However, seed treatment has been used in majority of the studies, apparently due to its handiness.
Table 1.
Inhibitory effects of nanoparticles on the seed priming, plant growth and development.
| Nanoparticles | Plant species | Effect on plant | References | |
|---|---|---|---|---|
| Type and size (nm) | Concentration | |||
| CdSe | Rice | Inhibited the germination | [78] | |
| C, Al, Zn | Corn, cucumber, lettuce, radish rape canola, and ryegrass. | Adversely affected seed germination | [79–80] | |
| Metal and Metal oxides [Al (18), ZnO (20), Zn (35), Al2O3 (60)] | 2,000 mg/L | Raphanus raphanistrum, Brassica napus, Lolium perenne, Lactuca sativa, Zea mays, Cucumis sativus | Phytotoxic effect | [80] |
| Al2O3 | 100, 500, 1,000 mg/L | Nicotiana tabacum | Dose-dependent decrease in the average root length, average biomass, and leaf count of the seedlings | [82a] |
| NiO (23.34) | 25, 50, 100, 250, 500, 1,000, 2,000 mg/L | Solanum lycopersicum | Induced apoptosis in tomato root cells. | [90] |
| NiO (< 100) | 87.8, 131.7, 197.5, 296.5, 444.4, 66.7, 1,000 mg/Kg | Hordeum vulgare | Decrease in leaf surface area, chlorophyll and carotenoids. | [91] |
| ZnO (20 ±5) | 10, 20, 50, 100, 200, 1,000 mg/L | Lolium perenne | Dose-dependent inhibition of root elongation. Above 20 mg/L decreased the seedling biomass. |
[92] |
| ZnO | 30–40 mg/L | Peanut, onion, bean, wheat | Negative effect on germination | [81] |
| ZnO (85) | 200, 400, 800 mg/L | Allium cepa | Increased cytotoxicity in root cells. | [93] |
| ZnO (15.37) | 100, 200μM | Triticum aestivum | Reduced photosynthetic efficiency | [85b] |
| CuNPs (20) | 200, 400, 600, 800, 1,000 mg/L |
Phaseolus radiates Triticum aestivum |
No adverse effect on shoot growth in P. radiates till 800 mg/L whereas T. aestivum shoot growth adversely affected from 200 mg/L concentration. Roots were more sensitive than the shoot. |
[94] |
| CuO (<50) | 0, 5, 15, 30, 45, 60, 100, 200, 400, 600, 800, 1,000, 1,500, 2,000 mg/L |
Glycine max Cicer arietinum |
Glycine max: Decline in root and shoot growth from 100 mg/L concentration. Cicer arietinum: Decline in root and shoot growth from 45 mg/L concentration. |
[95] |
| CuO (30–40) | 680 ± 60, 1004 ± 120, 2008 ± 340, 4051 ± 950 mg/L | Lemna gibba | Dose-dependent decrease in plant growth, and PSII activity and PSII reaction centers inactivated. | [96] |
| CuO (<50) | 0.5, 1, 1.5mM | Barley | Dose dependent reduction in shoot and root growth. | [97] |
| CuO (30) | 0.5, 1, 2, 5, 10, 20, 50, 100 mg/L | Arabidopsis thaliana | Dose dependent reduction in fresh weight, root length, and total chlorophyll. | [98] |
| CuO (43 ± 9) | 100, 200, 500, 1,000 mg/L | Elsholtzia splendens | Dose-dependent decrease in root length and leaf chlorophylls. | [99] |
| CuO (30–50) | 10 mg/L | Elodea nuttallii | Phytotoxic effect | [100] |
| CuO (<50) | 2.5, 10, 50, 100, 1,000 mg/L | Oryza sativa | Dose-dependent decrease in thylakoid number per grana, Photosynthetic rate, transpiration rate, stomatal conductance, maximal quantum yield of PSII photochemistry, and photosynthetic pigment contents. | [101] |
| CuO (40) | 10, 50, 100, 150, 200 mg/L | Lemna minor | Inhibition of plant growth. | [87] |
| CuO (<50) | 3, 10, 30, 300 mg/Kg | Triticum aestivum | Inhibition of root elongation (>10 mg/kg). Exposure resulted in root hair proliferation and shortening of the zones of division and elongation. |
[102] |
| CuO (30 ± 10) | 10, 200, 1,000 mg/L | Transgenic cotton (Bt-29317) Conventional cotton (Jihe321) |
Decrease in growth, development, nutrient content, indole-3-acetic acid and abscisic acid concentrations. | [103] |
| CuO (20–40) | 20, 50 mg/L | Arabidopsis thaliana | Inhibition in seedling growth | [104] |
| CuO (<100) | 10, 100, 50, 1,000 mg/L |
Raphanus sativus Lolium perenne Lolium rigidum |
DNA damaged was found to be increased with an increase in NP concentration. | [105] |
| AgNPs (2) | 0, 125, 250, 500 mg/L | Raphanus sativus | Concentration-dependent reduction in seedling elongation and water content. | [79] |
| Ag NPs | 40 mg/L | Carex, Juncus, Lobelia, Panicum, Phytolaca and Scirpus, | Inhibited seed germination | [12a] |
| AgNPs (25) | 1,000 mg/L | Oryza sativa | Phytotoxic effect on plant | [106] |
| AgNPs (11 ± 0.7) | 0.05, 0.1, 1, 18.3, 36.7, 73.4 mg/L | Zea mays, Brassica oleracea | Structural change in maize primary root cells. Phytotoxic effect on root development. |
[107] |
| AgNPs (18.34) | 0.30–60 mg/L | Oryza sativa | 60 mg/L damaged the root cell structure. Up to 30 mg/L accelerated root growth whereas 60 μg/mL restricted the root growth. 60 mg/L decreased Chl b concentration and increased shoot carotenoid content |
[108] |
| AgNPs (10) | 0.2, 0.5, 3 mg/L | Arabidopsis thaliana | Root growth inhibition. Decrease in chlorophyll a, chlorophyll b and total chlorophyll. |
[109] |
| AgNPs (10) | 10 0.5, 1.5, 2.5, 3.5, 5 mg/kg | Triticum aestivum | Reduced the length of shoots and roots. | [110] |
| AgNPs (60) | 12.5, 25, 50, 100 mg/L | Vicia faba | Genotoxic effect | [111] |
| AgNPs (2) | 0, 125, 250, 500 mg/L | Raphanus sativus | Less Ca, Mg, B, Cu, Mn, and Zn in seedlings at 50 mg/L | [79] |
| AgNPs (20) | 5, 10, 20 mg/L | Allium cepa | Various chromosomal aberrations were induced in both mitotic and meiotic cells even at lower concentrations | [112] |
| AgNPs (5.6 ± 2.1) | 25, 50, 75, 100μM | Allium cepa | Inhibition in root growth | [85a] |
| AgNPs (20) | 1000, 3000μM | Pisum sativum | Declined growth parameters, photosynthetic pigments and chlorophyll fluorescence. | [85b] |
| AgNPs (12.9 ± 9.1) | 0.01, 0.05, 0.1, 0.5, 1 mg/L | Capsicum annuum | Dose dependent decrease in plant growth and increase in cytokinin Concentration | [86] |
| AgNPs (<100) | 250, 750 mg/L | Cucurbita pepo | Reduction in plant biomass and transpiration | [113] |
| Al2O3 NPs | 10%, 100%, or 432% | Corn, cucumber, soybean, cabbage, and carrot | Suppressed root growth | [88] |
| CeO2 | 2000 mg/L | Lettuce | Suppressed elongation of roots | [76] |
| CeO2 (8) | 500 mg/Kg | Oryza sativa | NP could compromise the quality of rice grain. | [114] |
| CeO2 (10 ± 3.2) | 100, 500 mg/L | Transgenic cotton (Bt-29317) Conventional cotton (Jihe321) |
Decrease in indole-3-acetic acid and abscisic acid in the roots and destruction of vascular bundles. Conventional cotton was more sensitive than transgenic cotton. | [115] |
| CeO2 (8) | 400 mg/Kg | Triticum aestivum | Decrease in leaf chlorophylls and increase in catalase and superoxide dismutase activities. | [116] |
| TiO2 (25) | 300 mg/L | Zea mays | Inhibition in leaf growth and transpiration due to physical effects on root water transport | [117] |
| TiO2 (<100) | 2,000, 10,000, 20,000, 40,000 mg/L | Vicia narbonensis, Zea mays | Decrease in root elongation. | [118] |
| TiO2 (14, 25, 140) | 100 mg/L |
Brassica napus Triticum aestivum |
Moderate or no effect on plant growth. | [119] |
| TiO2 (15) | 100 mg/L | Linum usitatissimum | Reduction in seed germination, root biomass, and root length. | [120] |
| TiO2 (21) | 10, 100, 1,000 mg/L | Allium cepa | Dose dependent increase in genotoxicity. | [121] |
| TiO2 (90–98) | 12.5, 25, 50, 100 mg/L | Allium cepa | Dose dependent increase in genotoxicity. | [122] |
| TiO2 (11.93–18.67) | 80, 100 mg/Kg | Triticum aestivum | Decrease in root and shoot length | [123] |
| TiO2 and Al2O3 | Tobacco | Inhibition in seed sprouting, plant growth and development | [82] | |
| La2O3, Gd2O3 and Yb2O3 | 2000 mg/L | Cabbage, lettuce, radish, rape, cucumber, tomato, wheat | Inhibition in the plant elongation | [76] |
| TiO2 | 0.1% | Tobacco | Inhibited root and shoot development and decreased plant biomass | [82b] |
Table 2.
Promoting effects of nanoparticles on the seed priming, plant growth and development
| Nanoparticles | Plant species | Effect on plant | References | |
|---|---|---|---|---|
| Type and size (nm) | Concentration | |||
| SiO2 NPs | 2–8 g/L | Tomato | Promoted seed germination | [127] |
| SiO2 | Maize | Promoted seed sprouting | [128] | |
| SiO2 | Tomato and squash | Enhanced sprouting and antioxidant activity | [131], [127] | |
| SiO2 | 10 mL/L | Improved chlorophyll content, leaf dry weight, and proline accumulation | [132a, 159] | |
| Si NPs | Rice, Equisetum arvense, wheat, cucumber | Promoted plant growth and yield and provided resistance to abiotic | [135] | |
| and biotic stresses | ||||
| Si NPs | Rice | Act as a physical barrier | ||
| Si NPs | Soybean | Enhanced seed germination and seedling growth | [157] | |
| ZnO (25) | 400, 1,000, 2,000 mg/L | Arachis hypogaea | Promoted seed germination and growth vigor up to 1,000 mg/L | [160] |
| ZnO | 10–20 μg/mL | Peanut, onion, soybean, and wheat | Promoted seed germination | [81a, 81b] |
| ZnO | 30 and 40 μg/mL | Peanut, onion, soybean, wheat | Inhibited seed germination | [81a, 81b] |
| ZnO | Cucumber, alfalfa, tomato | Enhanced the plant growth, seed germination | [136] | |
| ZnO | Soybean, onion, peanut, wheat | Promoted plant growth | [81a] [81b] [137] [138] | |
| ZnO | Cluster bean | Enhanced plant development, biomass, leaf pigments, and protein content | [139] | |
| ZnO | Green gram, black gram | Increased length and biomass of root and shoot were increased. | [140] | |
| AgNPs (10) | 2.5 mg/kg | Triticum aestivum | Increased root branching | [110] |
| AgNPs (20) | 40 gha–1 Field, | Zea mays | Improved foliage yield | [161] |
| AgNPs (6 and 20) | 0.5, 5, 10 mg/L | Spirodela polyrhiza | Dose dependent increase in levels of ROS, superoxide dismutase, peroxidase, and glutathione activity | [162] |
| AgNPs | 1 mg/L | Trigonella foenum-graecum | Enhanced in plant growth | [163] |
| AgNPs (35–40) | 50, 75 mg/L |
Triticum aestivum, Vigna sinensis Brassica juncea |
Wheat: relatively unaffected Vigna: optimum plant growth and root nodulation at 50 ppm treatment (cowpea). Brassica: improved shoot growth |
[164] |
| at 75 ppm | ||||
| Ag NPs | Bacopa monnieci | Increased seed germination and proteins, carbohydrates synthesis | [148a] | |
| Ag NPs | 4 μg/mL | Boswellia ovaliofoliolata | Facilitated the sprouting and seedling growth | [149] |
| Ag NPs | 20–60 ppm | Mustards, beans, corns | Promoted root, shoot and leaf growth carbohydrate, chlorophyll, proteins | [142, 150] |
| Ag NPs | 1–10 mg/L | Barley, lettuce | Barley: increased root length Lettuce: decreased root growth |
|
| Ag NPs | 40 mg/L | Eupatorium fistulosum | Germination rate increased | [12a] |
| CuO (30) | 0.025, 0.25, 0.5, 1, 5 mg/L | Elodea densa | Stimulated photosynthesis up to 0.25mg/L but suppressed at 1–5 mg/L | [165] |
| CuO (30 ± 10) | 10, 200, 1,000 mg/L | Transgenic cotton (Bt-29317) | Enhance the expression of Bt-toxin protein in leaves and roots. | [103] |
| TiO2 (5) | 300 mg/L | Spinacia oleraces | More than 60% increase in plant fresh and dry weight. | [166] |
| TiO2 (11.93–18.67) | 0, 20, 40, 60 mg/Kg | Triticum aestivum | Increased root and shoot | [123] |
| TiO2 (25 ± 0.64) | 0, 100, 250, 500, 750, 1,000 mg/Kg | Solanum lycopersicum | Promoted plant height, root length, and biomass Up to a 250 mg/Kg and chlorophyll up to 750 mg/Kg | [167] |
| TiO2 (< 25) | 0.01, 0.1, 1, 10 mg/L | Hydrilla verticillata | Catalase and glutathione reductase activities increased | [168] |
| TiO2 | 2000 mg/L | Canola seeds | Promoted seed germination | [152] |
| TiO2 | 0.02% | Wheat under water stress | Enhanced plant growth and yield | [153] |
| TiO2 | 2.5% | Spinach, wheat | Enhanced germination of aged seeds | [154] |
| TiO2 | 0.25% | Spinach | Promoted seed germination, vigor indices, promoted photosynthesis and nitrogen metabolism | [130] |
| TiO2 (30–40) | 0.25–4.0% | Spinach | Increased plant dry weight, photosynthesis and chlorophyll formation | [154a] |
| CeO2 (8) | 62.5, 125, 250, 500 mg/Kg | Solanum lycopersicum | NP increased total chlorophyll, chl-a, and chl-b increased at 250 mg/kg and stem length at 500 mg/kg | [169] |
| CeO2 (8) | 0–500 mg/Kg | Phaseolus vulgaris | Increased antioxidant enzyme activities in the aerial tissues. | [170] |
| Fe3O4 (10) | 5, 10, 15, 20 mg/L | Triticum aestivum | No effect on seed germination, plant growth and chlorophyll content. | [171] |
| Fe3O4 (17.7 ± 3.9) | 20, 50, 100 mg/L | Zea mays | Germination index was increased at 20 and 50 mg/L, but decreased at 100 mg/L | [172] |
| CdO (7–60) | 2.03 ± 0.45 × 105 particles cm−3 | Hordeum vulgare | No change in total chlorophyll concentration, with minor change in Fv/Fm. Increase in total amino acids in all three cases. |
[173] |
| Au NPs | 62 μg/mL | Lactuca sativa, Cucumis sativus | Improved seed germination | [141] |
| Au NPs | 10 ppm | Brassica juncea | Improved seed germination | [142] |
| Au NPs | 1000 μM | Gloriosa superba | Improved seed germination | [143] |
5.1. Inhibitory effects of NPs
The negative effects of metallic NPs are very evident because of phytotoxicity.[75, 77] The degree of toxicity varies by the type of NPs and their concentrations. Seed treatment with cadmium selenide (CdSe) quantum dots inhibited the germination of rice seeds.[78] Lin and Xing reported that seed treatment with NPs of carbon, aluminum and zinc adversely affected seed germination in corn, cucumber, lettuce, radish,[79] rape canola, and ryegrass.[80] They found that high concentration of 2000 mg/L of 20 nm and 35 nm ZnO NPs negatively affected the sprouting of seeds of corn and ryegrass, respectively. Conversely, NPs of neither size affected seed germination at 200 mg/L but did suppress root growth in ryegrass and corn. Adverse effects of ZnO NPs on peanut, onion, bean and wheat were observed, though not at low concentrations (i.e., 30–40 mg/L as seed treatment).[81] Ma et al. examined the effects of seed treatment with four nano-oxides, viz., gadolinium(III) oxide (Gd2O3), cerium(IV) oxide (CeO2), ytterbium oxide (Yb2O3) and lanthanum(III) oxide (La2O3) at a much higher concentration, rarely used in practice on the growth and development of cabbage, lettuce, radish, rape, cucumber, tomato, and wheat.[76] Their results show that CeO2 suppressed the elongation of the root at 2000 mg/L in lettuce, whereas the La2O3, Gd2O3 and Yb2O3 at 2000 mg/L severely inhibited the elongation in all seven plant species. Similarly the seed treatment with titanium dioxide (TiO2) and aluminum oxide (Al2O3) inhibited seed sprouting, growth and development of tobacco plants.[82] This effect may be regulated by small regulatory microRNAs (miRNAs)—a vast RNA classification that is key in almost all biological and metabolite processes[83] and plays a central role in gene regulation.[84] The treatment of Ag NPs of the size lower than 30 nm inhibited the root shoot growth in plants.[85] Treatment of Capsicum annum seedling with 12.9 nm with 1 mg/L decreased the plant growth.[86] Enhancement in peroxidase, catalase, superoxide, dismutase activity and inhibition in plant growth was recorded in Lemna minor with treatment of 40 nm CuO (200 mg/L).[87]
Above information has shown that the lowest observed effects concentration (LOEC) of nanoparticles depends largely on metal species, its concentration and plant tolerance on a given metal kind as well. For example, Ag NPs at 40 mg/L inhibited seed germination of ten wetland plants species from the genera Carex, Juncus, Lobelia, Panicum, Phytolaca and Scirpus.[12a] However, Al2O3 NPs caused phytotoxic effects and suppressed the root growth of corn, cucumber, soybean, cabbage, and carrot root at 10%, 100%, or 432% concentration (w/v).[88] Other studies have also shown that minimum phytotoxic concentration of NPs varied with the metal species such as carbon nanotubes,[89], ZnO NPs (30–40 mg/L) [81] and CuO NPs (200 mg/L).[87]
5.2. Mechanism of inhibitory effects of NPs on plant growth
Negative influences of NPs on the plant growth mainly occur because of an overdose of NPs. The suppressive abilities of NPs on seed sprouting or plant growth occur because of inhibition of root development and AQP function. AQPs are the primary proteins involved in various molecules moving across the cells. A higher concentration of NPs may cause structural damage to cells and interfere in vital metabolic processes. Lin and Xing observed that ZnO NPs applied to soil significantly reduced the biomass of ryegrass.[124] The anatomical study of roots showed shrinkage of the root tip. The epidermal cells and parenchymatous cells in the root were collapsed or highly vacuolated. NPs accumulated on the root hairs and surface of feeder roots; as a result, absorption of water and minerals was adversely affected. One study found that NPs reached the apoplast and protoplast in the endodermal and stelar cells of the root, but the particles were profusely stuck at the root surface.[124] Rico et al. cultivated three cultivars of rice with various amylose character (low, medium, and high) in the soil applied with 500 mg CeO2 NPs/kg soil.[114] The grains from CeO2 NP treatment contained less starch, iron, sulphur, glutelin, prolamin, lauric and valeric acids.. The NP treatment decreased antioxidant levels, except for that of flavonoids, in grains. The accumulation of Ce in the grains was greater in the low- and medium-amylose cultivars than the high-amylose cultivar.
5.3. Stimulatory effects of NPs on plants
Available information has revealed that various NPs at concentrations below certain limits may promote the plant growth, development[125] and seed germination.[126] Among these studies, most have been conducted under artificial treatment conditions such as growth medium on plate, hydrophobic or pot conditions. To better understand plant growth enhanced by NPs, the information is organized here according to the type of NPs.
5.3.1. Silicon dioxide
The ability of SiO2 NPs to affect the germination of seeds is reported to be concentration-dependent. The seed treatment with 2–8 g/L SiO2 NPs promoted the germination of tomato seeds.[127] Suriyaprabha et al. have reported that SiO2 promoted seed sprouting by making nutrients more available to maize seeds.[128] NP inoculation may modify the conductivity and pH values of the growing environment suitable for germination. The exogenous application of nano-SiO2 as root-dip treatment induced significant improvement in length and other growth parameters of root and shoot.[129] Under abiotic stress, SiO2 NPs enhanced seed germination. Treatment with nano-SiO2 promoted seed germination and enhanced the production of antioxidants. Treatments with nanosized Au, Cu, Pd, and Si significantly improved lettuce germination. SiO2 NPs and TiO2 NPs enhanced soybean sprouting by promoting the activity of nitrate reductase. The NP seed treatments improved water uptake and utilization, and enhanced nutrient availability for seeds from soil.[130] In tomato[131] and squash,[127] seed treatment with nano-SiO2 when plants encountered NaCl stress enhanced sprouting and stimulated antioxidant activities. Soil application of SiO2 NPs at 10 mL/L improved chlorophyll content, leaf dry weight, and proline accumulation.[132] Exogenous application of silica (6 g/L) significantly enhanced the growth of root and shoot in rice.[133] It was observed that treatment with SiO2 NPs promoted physiological processes, viz., transpiration, stomatal conductance, electron transport rate and photochemical efficiency. [127, 134] Si NPs treatments have been found to promote plant growth and yield in addition to the resistance to abiotic and biotic stress in many plant species, including rice, Equisetum arvense, wheat,[135] and cucumber. Si NPs may act as a physical barrier by accumulating on epidermal layer and stelar tissues of stem and leaf sheath of rice plants. The seedling root-dip with Si NPs at 500 μL/L showed key regulating activity in physiological processes.[129]
5.3.2. Zinc oxide
Treatment with ZnO NPs of a low concentration (10–20 μg/mL) stimulated the seed germination of peanut, onion, soybean, and wheat; however, ZnO NPs of high concentrations (30 and 40 μg/mL) inhibited seed germination.[81a, 81b] It was also found that seed treatment with ZnO NPs affected the growth of cucumber, alfalfa, and tomato.[136] Among these three crops, cucumber exhibited enhanced seed germination. The addition of ZnO NPs promoted plant growth in soybean,[81a] onion,[81b] peanut[137] and wheat.[138] Raliya and Tarafdar provided evidence that exogenous inoculation of ZnO NPs on seeds markedly promoted plant development, biomass generation, leaf pigments, and protein content in cluster bean, Cyamopsis tetragonoloba.[139] The soil application of ZnO NPs increased microbial populations and enhanced the activity of phytase and acid and alkaline phosphatase182 in the rhizosphere of cluster bean (C. tetragonoloba). Mahajan et al. reported that root system of seedling of green gram and black gram absorbed ZnO NPs and that as a result, the length and biomass of root and shoot were increased.[140]
5.3.3. Gold NPs
Understanding of the effect of Au NPs on the plants is still limited. Seed or seedling treatment with Au NPs (62 μg/mL) improved seed germination in Lactuca sativa and Cucumis sativus,[141] Brassica juncea (10 ppm),[142] and Gloriosa superba (1000 μM).[143] Au NP treatments also enhanced plant growth and yield, including the number, area, and chlorophyll and sugar content of leaves,[142–143] and 10 and 80 μg/mL Au NPs promoted antioxidant production and seed germination in Arabidopsis thaliana.[144] These positive effects, however, may be connected with stimulation of biochemical pathways as the response on the low doses of xenobiotics. Xenobiotics is a foreign substance or exogenous chemical which the plant or animal body does not recognize, such as drugs, pollutants, some food additives and cosmetics. Besides, stimulating the production of antioxidants, xenobiotics etc. AuNPs may also act as workhorse of biolistic gene delivery in plants.[145] The extended matrices of AuNPs may offer high efficiency for transformation of plasmids, requiring exceedingly a small amount of gold.[146]
5.3.4. Silver NPs
Numerous investigations have tested the effect of Ag NPs on microbial and animal cells,[147] but little information is available on the growth and development of the plants.[148] The germination of the seeds and the synthesis of the proteins and carbohydrates increased in Bacopa monnieci after seed treatment with Ag NPs, whereas the phenol content, catalase activity, and peroxidase activity decreased.[148a] Seed treatment with 4 μg/mL silver NPs facilitated the sprouting and seedling growth in a tree species, Boswellia ovaliofoliolata.[149] The treatment of seedlings or seeds with Ag NPs (20–60 ppm) positively impacted root and shoot length as well as the leaf area of mustards, beans, and corns.[142, 150] The NP treatment also significantly increased carbohydrate, chlorophyll, and proteins (e.g., antioxidant enzymes) in these crops. Both negative and positive influences of Ag NPs (1–10 mg/L) were found on the barley and lettuce root growth, in which root length was increased for barley whereas that for lettuce decreased due to seed treatments. Similarly, among eleven wetland plant species, the germination rate increased only in Eupatorium fistulosum after seed treatment with 40 mg/L Ag NPs.[12a] The impact of different shapes of Ag NPs (spherical, 2 and 10 μm, and decahedral, 100 μm applied as root-dip) was studied to understand Arabidopsis physiology and molecular response.[151] It was found that Ag NPs of decahedral shape induced the greatest enhancement in root growth, followed by polyhedral particles. The spherical particles caused maximum accumulation of anthocyanin without affecting the root growth of Arabidopsis seedlings. The seedling treatment with decahedral and spherical Ag NPs resulted in enhanced activity of Cu superoxide dismutase, but inhibited the expression of Zn superoxide dismutase.
5.3.5. Titanium dioxide NPs
The seed treatment with TiO2 NPs at 2000 mg/L promoted the germination of canola seeds.[152] The emergence of radicle (root initial) and growth of plumule (shoot initial) were also promoted. Application of 0.02% (w/v) TiO2 NPs enhanced plant growth and yield in wheat under water stress.[153] The 2.5% (w/v) nano-TiO2 seed treatment enhanced germination of aged seeds in spinach[154] and wheat. However, Frazier et al. did not observe this promotion in tobacco after TiO2 NP seed treatment, although they observed a slight increase in the seed germination rate after 0.1% (w/v) TiO2 NP treatment.[82b] TiO2 NP treatment strongly inhibited root and shoot development and further decreased plant biomass (fresh and dry weight).[82b]
TiO2 NPs treatments were found to improve plant growth by regulating the synthesis of enzymes, glutamine synthase, glutamate dehydrogenase, glutamic-pyruvic transaminase and nitrate reductase.[155] These enzymes help plants absorb and convert nitrate into organic nitrogen. A study showed that TiO2 NPs may catalyze the oxidation-reduction reaction.[156] It was discovered that seed treatment with TiO2 NPs promoted seedling vigor and biosynthesis of chlorophylls; TiO2 NP treatment stimulated the activity of an enzyme, ribulose 1,5-bisphosphate carboxylase, promoting photosynthesis and plant growth.[155a] Additionally, NP treatment increased the absorbance and conversion of light energy and prevented the aging of chloroplasts. Hong et al. investigated the impact of seed treatment with TiO2 and nano- TiO2 at 0.25% (w/v) on seed sprouting and plant growth in spinach (Spinacia oleracea).[130] The seed germination and vigor indices increased as much as 4% after treatment compared to those treated with non-nano TiO2. The TiO2 NPs treatment also promoted photosynthesis and nitrogen metabolism in spinach.[130] Zheng et al. showed that seed treatment with nano-TiO2 (0.25–4.0% w/v) promoted plant dry weight (73%), photosynthesis (three-fold), and chlorophyll formation (45%) over those of the control group for 30 days after germination of spinach seeds. Seed germination varied according to NP size, and the spinach rate of growth was negatively correlated to the size of TiO2 (30–40 nm). Overall, a higher seed germination rate was observed when seeds were treated with nano-TiO2 than with non-nano-TiO2 particles.[154a] The NPs promoted photosterilization and the photogeneration of active oxygen ions such as superoxide and hydroxide. Active oxygen ions contribute significantly in enhancing the ability of seeds to resist stress and promote the intake of water and oxygen, which are vital for proper seed germination. Smaller TiO2 NPs were observed to promote inorganic nutrient uptake and organic material breakdown.[154a] Similarly, Si NP treatment enhanced the germination and seedling growth of soybeans and protected them from rotting.[157] The NP treatment also promoted the activity of nitrate reductase, superoxide dismutase, and catalase enzymes, resulting in a marked improvement in stress resistance in soybean plants.[157] TiO2 NPs treatments were found to accelerate the Hill reaction and the overall photochemical reaction of chloroplasts in spinach.[130, 158] The non-cyclic photophosphorylation activity was enhanced compared with the cyclic photophosphorylation activity. The entry of TiO2 NPs into chloroplasts and induction of oxidation-reduction reactions resulted in accelerated electron transport and oxygen evolution.[130, 158]
5.4. Mechanism of stimulatory effects of NPs on plants
5.4.1. Mechanisms involved in NP-enhanced seed germination
The mechanism by which NP treatments improve seed germination rates is not fully understood. It is possibly attributable to the NP treatments enhancing seed uptake and retention of water. To better understand this mechanism, Khodakovskaya et al. conducted experiments with CNTs and tomato seeds that had been treated for their water level.[174] They placed dry seeds in media inoculated with CNTs and in media inoculated with Murashige and Skoog medium; after 2 d of incubation, the moisture content of seeds was measured. The seeds treated with CNTs contained 19% more water than the untreated seeds, which suggests that CNT treatments enabled the seeds to take up and retain water. The mechanisms by which NPs improve water entry and retention inside the seeds is not well understood. CNTs may create micropores [175] and channels for water permeation into the seed coat.[174] CNTs have been assumed to regulate the gates of aquaporins (AQPs) in the seed coat.[174] AQPs are membrane proteins, and act as channels for water transfer across the cell membrane.[176] In addition, small solutes or molecules including NPs may enter seeds as well as cell-to-cell contact through AQPs, endocytosis, plasmodesmata and cell wall pores (Figure 2). Little information is available on plant cell water channel gating; however, the available information suggests that CNTs play a role in accelerating water intake through AQPs.
Figure 2.
Schematic of the movement of NPs through cell wall pores in plants.
5.4.2. Mechanism of plant growth promotion
Several studies have shown that NP treatment enhanced plant growth and development, possibly through protein-coding and miRNA gene expression regulation. Rezani et al. recorded that Ag NPs treatment enhanced growth of saffron roots (Crocus sativus) by blocking ethylene signaling in these plants.[177] The Ag NPs (100 μm/L) promoted indole acetic acid protein 8 gene expression but suppressed the ACC oxidase 2 expression as well as ACC synthase 7 synthesis. These data indicate that Ag NPs may interfere in the biosynthesis of ethylene by inhibiting ethylene perception.[151] Both TiO2 and Al2O3 affected the expression of ascorbate peroxidase and alcohol dehydrogenase in tobacco, which regulate reactive oxygen generation and various biotic and abiotic stresses.[82] In addition, miRNAs may be involved in NP-regulated plant growth and development; miRNAs are evolutionarily conserved small regulatory molecules that exist in all plant species and are key to all biological and metabolic processes.[178] Kurklew and colleagues reported that miR395, miR397, miR398, and miR399 were highly expressed in tobacco when 1% (w/v) Al2O3 NPs were applied compared with their expression levels with different NP treatments and the control. Frazier and colleagues also reported that nano-TiO2 impacted miRNA expression significantly.[82b] Treatment with TiO2 NPs at 0.1 and 1% induced drastic expression of miRNAs in tobacco seedlings.[82b]
CNTs promote the growth of plant cells by accelerating cell division through regulation of gene expression.[179] Notably improved elongation of root cells and greater activity of dehydrogenase was reported in wheat treated with oxidized-MWCNTs (Multi walled CNTs). MWCNT uptake followed by accumulation in roots resulted in the promotion of dehydrogenase activity in Onobrychis arenaria.[180] Two separate studies consistently show that the absorption and accumulation of MWCNTs may result in greater synthesis of photosynthetic enzymes.[179] Soluble CNTs inside the plants were attributed to enhancements in the growth of roots and shoots in wheat.[181] MWCNTs have also been shown to enhance water retention capacity, plant growth and fruit production in tomato and alkaloid synthesis in medicinal plants, viz., Morinda citri, Cinnomomun camphora and Pangamia pinnata.[127]
5.4.3. Mechanism of acceleration in photosynthesis
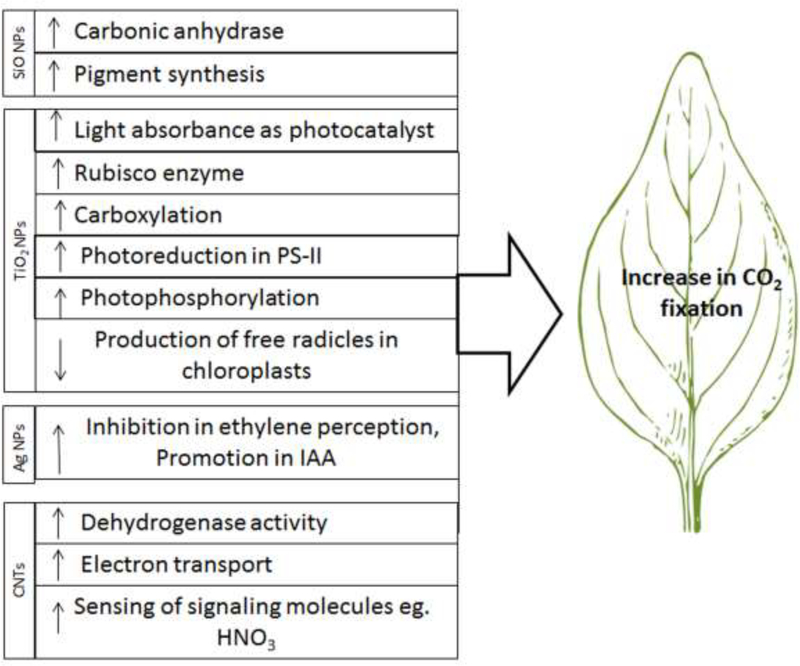
Recently, concerted efforts have been dedicated to improving the CO2 fixation efficiency of C3 plants.[182] NP application may enhance the photosynthesis efficiency in plants via different pathways (Figure 3). An in vitro study showed a three-fold increase in photosynthetic activity and a greatly accelerated rate of electron transport in an isolated chloroplast embedded with single walled carbon nanotubes abbreviated as SWCNTs.[183] SWCNTs also allowed the transmission of signal molecules such as nitric oxide by plants. [184] Noji et al. reported that photosynthetic oxygen-evolving reaction was achieved with a stable activity when a nanomesoporous silica compound was bound to photosystem II (PSII).[185] The application of nanosilica enhanced the synthesis of chlorophylls and the activity of carbonic anhydrase.[127, 186] Nano-anatase TiO2, acting as a photocatalyst, may improve the absorbance of light and the conversion of light energy into chemical energy, thereby leading to greater CO2 assimilation.[187] Gao and colleagues reported that the application of nano-anatase TiO2 promoted the carboxylation by activating RuBisCO enzymes.[155a] Ma et al. showed that nano-anatase may act at the gene level by inducing the expression of the marker gene for RuBisCO activase (rca) mRNA,[124] which may result in greater synthesis of proteins, thereby enhancing RuBisCO carboxylation.[124]
Figure 3.
Schematic of the stimulatory effect of NPs on different steps/enzymes of photosynthesis that lead to increased CO2 fixation in green plants. Downward and upward arrows indicate a decrease and an increase, respectively.
Treatment with TiO2 NPs improved the transpiration and the rate of photosynthesis.[188] Nano-anatase strongly increased electron transport, photoreduction, photophosphorylation and chlorophyll formation under a visible or ultraviolet light. The accumulation of free radicals is a key mechanism of organism aging, and chloroplast aging is related to the same accumulation.[158] Chloroplasts generate free radicals of reactive oxygen, such as O2.–, H2O2 and singlet oxygen (1O2), under light that causes chloroplast aging; consequently, photosynthetic capacity and plant growth decrease. TiO2 NP treatment may protect chloroplasts from aging for longer illumination life spans.[130, 155a] When TiO2 NP treatment was applied to chloroplasts, the production rate of free radicals was lower than in the case of untreated chloroplasts.[158] A dramatic difference in the production rate of free radicals was found between TiO2 NP-treated and untreated groups with 40-min illumination.[158]
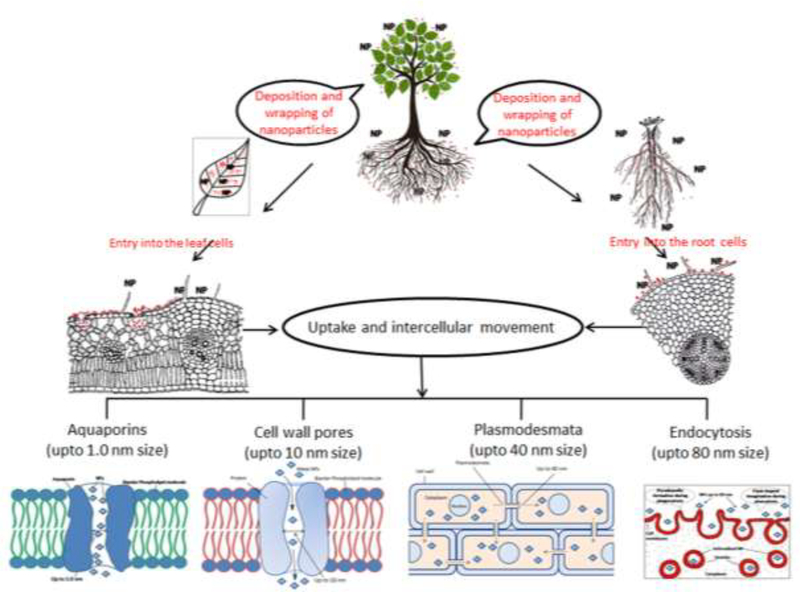
6. Uptake of NPs by plants
The mode of NPs uptake by plants depends on the way of exposure (Figure 4). When NPs are applied to foliage, they directly deposit onto the aerial parts of plants. However, when applied to soil, the NPs gradually move in soil pores through various forces, such as water movement and physical pressure. As a result, NPs enter the vicinity of the roots. After they are in contact with a root or leaf surface, NPs adhere to the plant surfaces via forces such as electrostatic, hydrophobic, and van der Waals forces.[84] This process is followed by wrapping or docking with the surface and subsequent entry into cells near the surface.[189] The uptake of NPs through underground parts occurs mainly through physiologically active lateral roots and root hairs, whereas in the leaf, NPs enter largely via stomata but also through trichomes.[10] The plants may also block the uptake of NPs through the exudation of mucilage, which bonds the NPs on the root surface.[190] After entering roots and leaves, NPs spread from one cell to another and move upward and downward through xylem or phloem tissues, respectively.[191] Investigations of NP uptake in animal and plant cells have indicated that NPs may enter plant cells and move intercellularly through different modes and mechanisms, such as cell wall pores,[192] endocytosis,[193] plasmodesmata,[194] AQP,[195] and ion transporters[196] (Figure 4). The mechanisms that control the NP entry and movement into the plant cells are largely dependent on the NP size.[191]
Figure 4.
Schematic depicting events potentially involved in the uptake of NPs by plants and their intercellular movement.
6.1. Cell wall pores
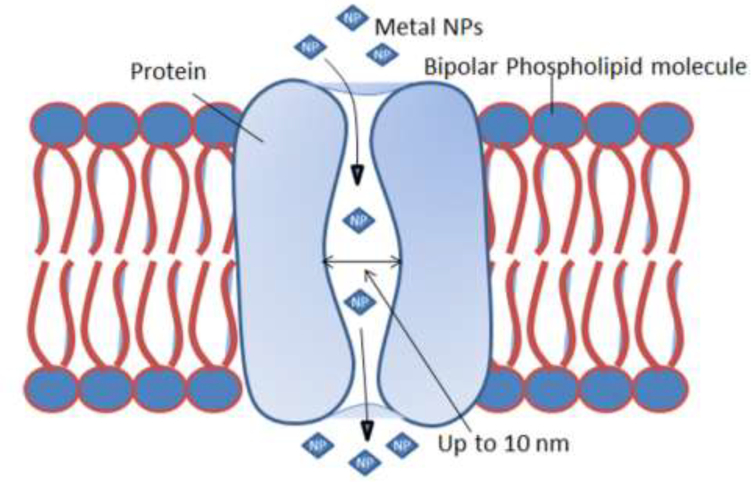
After application of NPs to plants, the epidermis is the first barrier for NP entry into the epidermal and cortical cells of roots. Cellulose microfibrils provide the main structural components of cell wall. The cell wall pores are believed to be cellulose chains. Numerous pores are formed in the cell wall with a size of 5–10 nm and a length of micrometers[192] (Figure 2). The cell wall pores allow entry/diffusion for movement of NPs as large as 10 nm, small proteins and other molecules. Hence, a limited range of NPs (up to 10 nm size) may also enter and pass through these pores. The cell wall acts prevents entry of foreign particles larger than the cell wall pores.[197]
6.2. Endocytosis
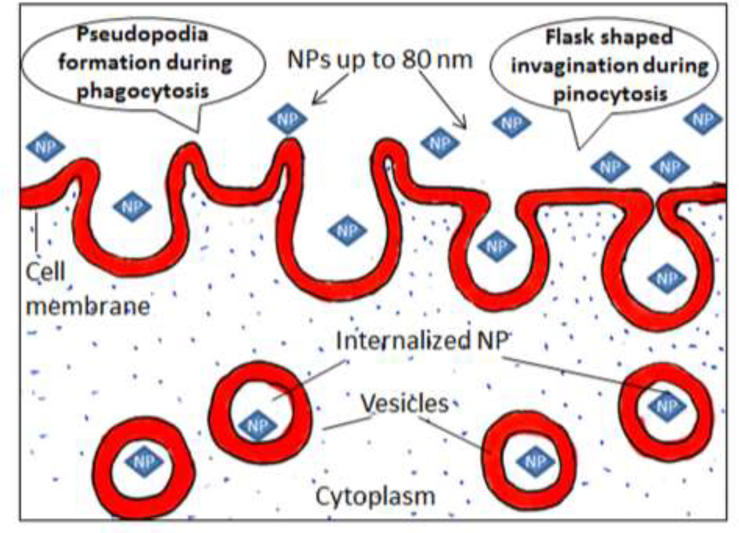
Endocytosis is a process by which the cell membrane engulfs an object for cellular uptake. Endocytosis is thought to be a more common process which operates uptake of NPs by plants (Figure 5). When the particles come in close contact with the surface of root or leaf cells, they become wrapped/docked on the cell surface.[198] Wrapping of NPs requires a minute curving of the cell surface. This process provides adhesion, which is accomplished through hydrophobic interactions, electrostatic interactions, van der Waals forces, and/ or through ligand receptor binding.[199] These forces also initiate endocytosis, where intracellular vesicles are formed followed by invagination of the plasma membrane [200] (Figure 5).
Figure 5.
Schematic of the endocytosis of NPs through the plant cell membrane.
On the basis of vesicle formation, endocytosis may occur in various ways, including phagocytosis, pinocytosis, and macropenocytosis.[201] The NPs may be taken up by cells through endocytosis via various endocytic pathways, depending on particle size. Clathrin- and caveolin-dependent endocytosis are mainly involved in the uptake of NPs of up to 80 nm in diameter.[202] During caveolin-dependent endocytosis, hairpin-like caveolin pores and cytosolic sites of plasma membranes are formed (Figure 5). As a result, flask-shaped caveolin of 50–80 nm width is formed.[203] Large NPs up to a 1000 nm in size may enter cells through phagocytosis[204] and endocytosis.[205] In the former, the cell membrane protrudes to form a cup-shaped vesicle that gradually surrounds a particle till it is fully enclosed, followed by engulfing of the particle by the cell[198] (Figure 5). Although, nanoparticles of varying size can move through endocytosis, the fastest movement has been recorded for NPs of 20–50 nm in size.[206]
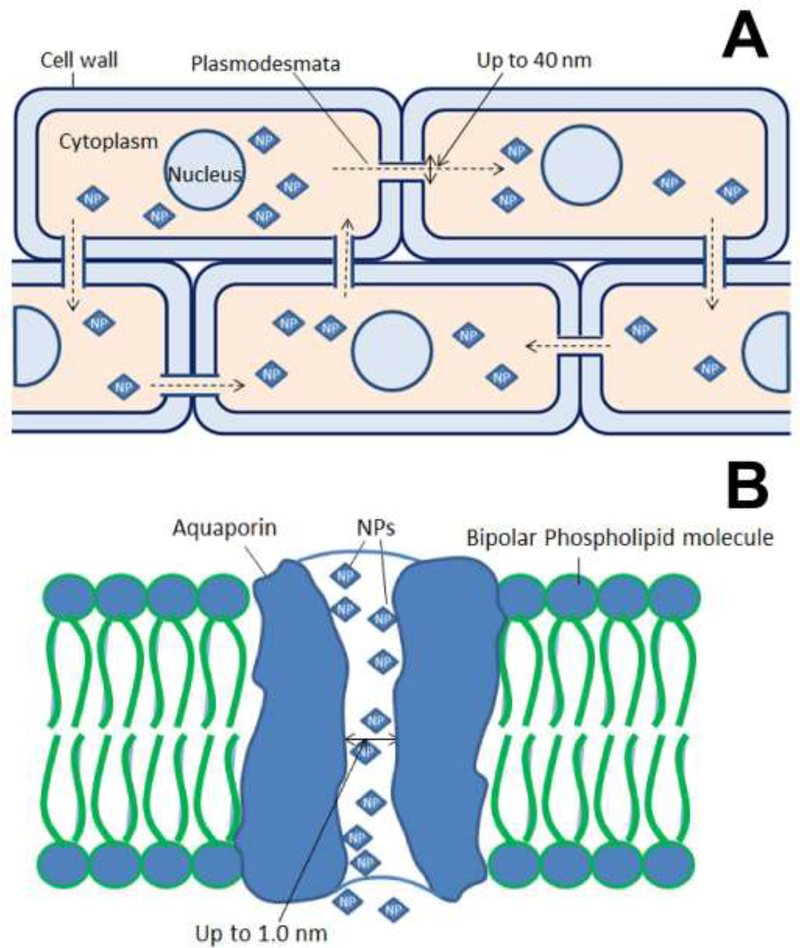
6.3. Plasmodesmata
Plasmodesmata provide a complex intracellular path of cytoplasmic bridges that connect cells (Figure 5A). The endoplasmic reticulum (ER) forms a tube that passes through the plasmodesmata, connecting the ER of neighboring cells and thereby providing endomembrane continuity among cells. The plasmodesmata are microchannels for the transport of various molecules. Structurally, they are cylindrical and approximately 40 nm wide.[207]
The plasmodesmata may serve as an alternate passage for NPs larger than 10 nm[92] that cannot pass through cell wall pores.[208] Hence, 40-nm particles can be transported through plasmodesmata. Larger particles may also move through the plasmodesmata.[209] However, the exact mechanism of the regulation of transport for larger particles is not understood. Some molecules have been observed to temporarily widen plasmodesmata channels to transport larger particles.[209] The outward opening of plasmodesmata may become wider to allow passage of larger particles and molecules[210] (Figure 5A). For example, plant virus particles move through plasmodesmata by manipulating width of the passage.[210] In the same way, the ingress of larger NPs may also occur through the plasmodesmata, by modifying its width through physical pressure induced by a concentration gradient.[211] However, the movement of larger particles is believed to be much slower.
6.4. Aquaporins and ion channels
Aquaporins (AQPs) are membrane water channels up to 1.0 nm in diameter; they are known for their key contribution to water concentration control in the cells.[212] In addition to water, other ultrasmall molecules are also transported through AQPs.[213] Generally, AQPs operate via the principle of permeation; however, AQPs also act as “always open channels”.[214] Ultrasmall NPs with sizes below 1.0 nm may move through AQPs (Figure 5B). Structurally, AQPs are similar to cell wall pores. The transport carrier proteins and ion transporters may also participate in the movement of NPs through the cell wall and plasma membrane. [215] Tani and Barrington reported significant role of ion transporters in the movement of NPs in the root cells.[216] However, more information on NP transport through ion transporters is not available. In a very recent study regarding the gene expression encoding the activity of AQPs under NPs treatment and water-stress conditions, it was observed that the downregulation of AQPs genes due to NPs treatment resulted in the reduction of water uptake.[217]
6.5. The upward and downward movement of NPs
The intercellular movement of particles may finally lead to NP arrival into stellar tissues, through which the NPs may move to different plant parts at a higher rate. NPs, when sprayed on aerial parts, enter stomata, trichomes and cuticle,[218] finally reach the phloem, and are transported downward.[191] Similarly, when applied to the soil, they are absorbed by roots.[219] The intercellular movement of NPs through different modes may also lead to their arrival in the xylem tissue. Through the xylem, NPs are transported upward to different plant parts.[219]
7. Conclusion and future perspectives
Demand for nanomaterials, including NPs, has drastically increased in recent decades. With respect to other production processes, green and eco-friendly technologies are required for risk-free nanomaterial manufacturing. At present, plants and microbes are the only sources for green synthesis of NPs. In this process microbial cultures function as the reducing and stabilizing/capping agents. Phytosynthesis process is a simple and inexpensive with relatively lesser environmental risks. Leaf extracts have been largely used to synthesize metal and carbonaceous NPs. The pH, temperature, and, to some extent, reaction time control the formation and size of NPs. Depending on the concentration, NPs may suppress or improve seed sprouting and plant growth. Many studies have shown that the application of NPs at low dose promotes seed germination, plant growth and development. In many cases, radiation-absorbing efficiency, CO2 assimilation capacity, and aging of chloroplasts have been improved in response to NP treatments. However, numerous studies have also indicated negative effects of NPs, especially at the higher concentrations. When NPs are applied to the soil, particles accumulate at the root surface, followed by entry through cell wall pores (up to 10 nm), endocytosis (up to 80 nm), plasmodesmata (up to 40 nm), AQPs (up to 1.0 nm), or induced pores (bigger NPs). From aerial parts, NPs are taken up largely through stomata and through the trichomes and cuticle. The further course of NP movement in shoots resembles that in the root, except that NP dispersal occurs through the phloem. Critical examination of the relevant information has revealed that basic information on the mode and mechanism of synthesis of NPs and their entry and action on plant synthesis is not available in detail. To date, the phytosynthesis of NPs has been carried out only under laboratory conditions, and the advantage of biosynthesis over conventional methods of NPs production needs to be proved at the large scale. Further, there is limited practical evidence that plant extracts containing phyto-impurities or byproducts formed during the process may not affect the yield and stability of NPs at large scale production. Hence, holistic approach and concerted research efforts are needed to understand the mechanism of phytosynthesis including factors (impurities/byproducts) affecting the synthesis. These studies will be helpful to standardize reaction conditions and to develop an industrial protocol for the production of NPs of desired shape and size. To address biosafety concerns, research efforts are required to determine the influences of NPs at the molecular, physiological, and biochemical levels in much detail. Moreover, NPs developed by phytosynthesis may also be used as probes for human disease diagnosis[220] or as a therapeutic agent for human disease treatment[221] because they may be more human-safe than their chemically synthesized counterparts. Phytosynthesized NPs therefore also hold great promise as nanomedicines in human healthcare.
Figure 6.
Illustration showing movement of NPs (up to 40 nm) through plasmodesmata (A) and Schematic showing the movement of fine NPs (up to 1.0 nm size) through AQPs (B).
Acknowledgements
Y.Z. and C.B.M. would like to thank the financial support from National Institutes of Health (EB021339).
Biography

Chuanbin Mao graduated with a PhD from Northeastern University in China in 1997. He then worked at Tsinghua University, first as a postdoctoral fellow and then as a faculty member until late 1999. He was a visiting scholar at the University of Tennessee at Knoxville in early 2000, followed by postdoctoral studies at the University of Texas at Austin. He became an assitant professor at the University of Oklahoma in 2005, and promoted to associate professor in 2010 and to full professor in in 2011. His research is centered at the interface between chemistry, biology, materials science, bioengineering and medicine.

Mujeebur Rahman Khan completed M.Sc. in 1984 and Ph.D. in 1988 in Plant Pathology and Nematology from Aligarh Muslim University, India. Worked as Post-Doc at North Carolina State University, USA and California Department of Food and Agriculture, USA. Received advanced training in nematode identification at the Institute of Parasitology, Commonwealth Agricultural Bureau, UK. Joined as a faculty in 1993 in the Institute of Agriculture, and presently serving as Professor and Chairman in the Department of Plant Protection, Aligarh Muslim University, Aligarh, India. Research areas include plant-microbe interaction, microbial control of pathogenic fungi and nematodes, and plant diseases under climate change.
References
- [1].Talapin DV, Shevchenko EV, Chem. Rev 2016, 116, 10343–10345. [DOI] [PubMed] [Google Scholar]
- [2].Oleszczuk P, Crit. Rev. Environ. Sci. Technol 2013, 43, 2581–2616. [Google Scholar]
- [3].Astruc D, Nat. Chem 2012, 4, 255. [DOI] [PubMed] [Google Scholar]
- [4].Baughman RH, Zakhidov AA, De Heer WA, Science 2002, 297, 787–792. [DOI] [PubMed] [Google Scholar]
- [5].a Lang X, Hirata A, Fujita T, Chen M, Nat. Nanotechnol 2011, 6, 232–236; [DOI] [PubMed] [Google Scholar]; b Rizzello L, Pompa PP, Chem. Soc. Rev 2014, 43, 1501–1518. [DOI] [PubMed] [Google Scholar]
- [6].Yang K, Ma Y-Q, Nat. Nanotechnol. 2010, 5, 579–583. [DOI] [PubMed] [Google Scholar]
- [7].Diao M, Yao M, Water Res. 2009, 43, 5243–5251. [DOI] [PubMed] [Google Scholar]
- [8].Ljubimova JY, Holler E, Nanomed. 2012, 7, 1467–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eltarahony M, Zaki S, Abd-El-Haleem D, J Nanomater 2018, 2018, 1–14. [Google Scholar]
- [10].Khan MR, Rizvi TF, Plant Pathol. J 2014, 13, 214–231. [Google Scholar]
- [11].Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N, Nanotechnology 2007, 18, 105104. [Google Scholar]
- [12].a Yin L, Colman BP, McGill BM, Wright JP, Bernhardt ES, PLoS ONE 2012, 7, e47674; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Nadagouda MN, Speth TF, Varma RS, Acc. Chem. Res 2011, 44, 469–478; [DOI] [PubMed] [Google Scholar]; c Ali K, Ahmed B, Dwivedi S, Saquib Q, Al-Khedhairy AA, Musarrat J, PLoS ONE 2015, 10, e0131178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Golubeva OY, Shamova O, Orlov D, Pazina TY, Boldina A, Kokryakov V, Glass Phys. Chem 2010, 36, 628–634. [Google Scholar]
- [14].Harada M, Kawasaki C, Saijo K, Demizu M, Kimura Y, J. Colloid Interface Sci 2010, 343, 537–545. [DOI] [PubMed] [Google Scholar]
- [15].Li K, Zhang F-S, J. Nanopart. Res 2010, 12, 1423–1428. [Google Scholar]
- [16].Zhu J, Liu S, Palchik O, Koltypin Y, Gedanken A, Langmuir 2000, 16, 6396–6399. [Google Scholar]
- [17].Khan I, Saeed K, Khan I, Arab J Chem 2017. [Google Scholar]
- [18].Agarwal H, Venkat Kumar S, Rajeshkumar S, Resource-Efficient Technol. 2017, 3, 406–413. [Google Scholar]
- [19].Thakkar KN, Mhatre SS, Parikh RY, Nanomed-Nanotechnol 2010, 6, 257–262. [DOI] [PubMed] [Google Scholar]
- [20].a Shankar SS, Rai A, Ahmad A, Sastry M, Chem. Mater 2005, 17, 566–572; [Google Scholar]; b Khan MR, Rizvi TF, in Nanoscience and Plant–Soil Systems, Springer, 2017, pp. 405–427; [Google Scholar]; c Joe MH, Jeong HT, Lee HM, Park HJ, Kim DH, Park DH, Bai S, J Nanomater 2017, 2017, 8734758; [Google Scholar]; d Baranwal A, Mahato K, Srivastava A, Maurya PK, Chandra P, Rsc Adv 2016, 6, 105996–106010; [Google Scholar]; e Kumar CS, Mahesh A, Antoniraj MG, Vaidevi S, Ruckmani K, Rsc Adv 2016, 6, 26874–26882; [Google Scholar]; f Parveen M, Ahmad F, Malla AM, Azaz S, Appl Nanosci 2016, 6, 267–276. [Google Scholar]
- [21].Mourato A, Gadanho M, Lino AR, Tenreiro R, Bioinorg. Chem. Appl 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Konishi Y, Ohno K, Saitoh N, Nomura T, Nagamine S, Hishida H, Takahashi Y, Uruga T, J. Biotechnol 2007, 128, 648–653. [DOI] [PubMed] [Google Scholar]
- [23].a Vahabi K, Mansoori GA, Karimi S, Insciences J. 2011, 1, 65–79; [Google Scholar]; b Neethu S, Midhun SJ, Radhakrishnan EK, Jyothis M, Microb. Pathog. 2018, 116, 263–272. [DOI] [PubMed] [Google Scholar]
- [24].a Wei X, Luo M, Li W, Yang L, Liang X, Xu L, Kong P, Liu H, Bioresour. Technol 2012, 103, 273–278; [DOI] [PubMed] [Google Scholar]; b Singh H, Du J, Yi TH, Artif Cell Nanomed B 2017, 45, 584–590; [DOI] [PubMed] [Google Scholar]; c Saravanan M, Barik SK, MubarakAli D, Prakash P, Pugazhendhi A, Microb. Pathog 2018, 116, 221–226. [DOI] [PubMed] [Google Scholar]
- [25].Cao BR, Xu H, Mao CB, Angew Chem Int Edit 2011, 50, 6264–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang F, Nimmo SL, Cao B, Mao C, Chem Sci 2012, 3, 2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].a Shankar SS, Ahmad A, Pasricha R, Sastry M, J. Mater. Chem 2003, 13, 1822–1826; [Google Scholar]; b Lengke MF, Fleet ME, Southam G, Langmuir 2006, 22, 2780–2787. [DOI] [PubMed] [Google Scholar]
- [28].a Li K, Ma CY, Jian TC, Sun HS, Wang L, Xu H, Li WH, Su HY, Cheng XH, J Food Sci Tech Mys 2017, 54, 3569–3576; [DOI] [PMC free article] [PubMed] [Google Scholar]; b de Barros CHN, Cruz GCF, Mayrink W, Tasic L, Nanotechnol Sci Appl 2018, 11, 1–14; [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ma YH, Liu CY, Qu D, Chen Y, Huang MM, Liu YP, Biomed. Pharmacother 2017, 89, 351–357. [DOI] [PubMed] [Google Scholar]
- [29].a Prasannaraj G, Venkatachalam P, J. Cluster Sci 2017, 28, 645–664; [Google Scholar]; b Meva FE, Ebongue CO, Fannang SV, Segnou ML, Ntoumba AA, Kedi PBE, Loudang REN, Wanlao AY, Mang ER, Mpondo EAM, J Nanomater 2017, 2017, 6834726. [Google Scholar]
- [30].a Das B, Dash SK, Mandal D, Ghosh T, Chattopadhyay S, Tripathy S, Das S, Dey SK, Das D, Roy S, Arab J Chem 2017, 10, 862–876; [Google Scholar]; b Ahmad A, Wei Y, Syed F, Tahir K, Rehman AU, Khan A, Ullah S, Yuan QP, Microb. Pathog 2017, 102, 133–142; [DOI] [PubMed] [Google Scholar]; c Qayyum S, Oves M, Khan AU, PLoS ONE 2017, 12; [DOI] [PMC free article] [PubMed] [Google Scholar]; d Siddiqi KS, Husen A, Rao RAK, J. Nanobiotechnol 2018, 16, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].a Armendariz V, Herrera I, Jose-yacaman M, Troiani H, Santiago P, Gardea-Torresdey JL, J. Nanopart. Res 2004, 6, 377–382; [Google Scholar]; b Kandasamy K, Alikunhi NM, Manickaswami G, Nabikhan A, Ayyavu G, Appl Nanosci 2013, 3, 65–73; [Google Scholar]; c Mishra S, Singh BR, Naqvi AH, Singh HB, Sci. Rep 2017, 7, 45154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].a Kumar V, Yadav SK, J. Chem. Technol. Biotechnol 2009, 84, 151–157; [Google Scholar]; b Mittal AK, Bhaumik J, Kumar S, Banerjee UC, J. Colloid Interface Sci 2014, 415, 39–47; [DOI] [PubMed] [Google Scholar]; c Dauthal P, Mukhopadhyay M, Ind. Eng. Chem. Res 2016, 55, 9557–9577; [Google Scholar]; d Jadhav MS, Kulkarni S, Raikar P, Barretto DA, Vootla SK, Raikar US, New J. Chem 2018, 42, 204–213. [Google Scholar]
- [33].Meyers MA, Mishra A, Benson DJ, Prog Mater Sci 2006, 51, 427–556. [Google Scholar]
- [34].Ahmad A, Syed F, Imran M, Khan AU, Tahir K, Khan ZU, Yuan QP, J. Food Biochem 2016, 40, 420–427. [Google Scholar]
- [35].Pirtarighat S, Ghannadnia M, Baghshahi S, J. Nanostruct. Chem. 2018, 9, 1–9. [Google Scholar]
- [36].Shaik M, Khan M, Kuniyil M, Al-Warthan A, Alkhathlan H, Siddiqui M, Shaik J, Ahamed A, Mahmood A, Khan M, Adil S, Sustainability 2018, 10, 913. [Google Scholar]
- [37].Aljabali AAA, Akkam Y, Al Zoubi MS, Al-Batayneh KM, Al-Trad B, Abo Alrob O, Alkilany AM, Benamara M, Evans DJ, Nanomaterials (Basel) 2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jain S, Mehata MS, Sci. Rep 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vélez E, Campillo G, Morales G, Hincapié C, Osorio J, Arnache O, J Nanomater 2018, 2018, 1–7. [Google Scholar]
- [40].Tippayawat P, Phromviyo N, Boueroy P, Chompoosor A, PeerJ 2016, 4, e2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A, Colloids Surf. A 2009, 339, 134–139. [Google Scholar]
- [42].a Sharma NC, Sahi SV, Nath S, Parsons JG, Gardea-Torresde JL, Pal T, Environ. Sci. Technol 2007, 41, 5137–5142; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Iravani S, Green Chem. 2011, 13, 2638–2650. [Google Scholar]
- [43].Gomathi M, Rajkumar P, Prakasam A, Ravichandran K, Resource-Efficient Technol. 2017, 3, 280–284. [Google Scholar]
- [44].a Chandrasekharan DK, Khanna PK, Kagiya TV, Nair CKK, Cancer Biother. Radiopharm 2011, 26, 249–257; [DOI] [PubMed] [Google Scholar]; b Qin Y, Ji X, Jing J, Liu H, Wu H, Yang W, Colloids Surf. A 2010, 372, 172–176. [Google Scholar]
- [45].Jadhav K, Deore S, Dhamecha D, Rajeshwari HR, Jagwani S, Jalalpure S, Bohara R, Acs Biomater Sci Eng 2018, 4, 892–899. [DOI] [PubMed] [Google Scholar]
- [46].Thanh NTK, Maclean N, Mahiddine S, Chem. Rev 2014, 114, 7610–7630. [DOI] [PubMed] [Google Scholar]
- [47].Makarov V, Love A, Sinitsyna O, Makarova S, Yaminsky I, Taliansky M, Kalinina N, Acta Naturae 2014, 6, 35–44. [PMC free article] [PubMed] [Google Scholar]
- [48].Shankar SS, Rai A, Ahmad A, Sastry M, J. Colloid Interface Sci 2004, 275, 496–502. [DOI] [PubMed] [Google Scholar]
- [49].Li S, Shen Y, Xie A, Yu X, Qiu L, Zhang L, Zhang Q, Green Chem. 2007, 9, 852–858. [Google Scholar]
- [50].a Saxena A, Tripathi R, Zafar F, Singh P, Mater. Lett 2012, 67, 91–94; [Google Scholar]; b Tripathi RM, Ranac D, Shrivastav A, Singh RP, Shrivastav BR, Nano Biomed. Eng 2013, 5. [Google Scholar]
- [51].Zheng B, Kong T, Jing X, Odoom-Wubah T, Li X, Sun D, Lu F, Zheng Y, Huang J, Li Q, J. Colloid Interface Sci 2013, 396, 138–145. [DOI] [PubMed] [Google Scholar]
- [52].Zhou Y, Lin W, Huang J, Wang W, Gao Y, Lin L, Li Q, Lin L, Du M, Nanoscale Res. Lett 2010, 5, 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Prabhu S, Poulose EK, Intl. Nano Lett 2012, 2, 32. [Google Scholar]
- [54].Jha AK, Prasad K, Prasad K, Kulkarni A, Colloids Surf. B 2009, 73, 219–223. [DOI] [PubMed] [Google Scholar]
- [55].Egorova E, Revina A, Colloids Surf. A 2000, 168, 87–96. [Google Scholar]
- [56].Ghule K, Ghule AV, Liu J-Y, Ling Y-C, J. Nanosci. Nanotechnol. 2006, 6, 3746–3751. [DOI] [PubMed] [Google Scholar]
- [57].Kuppusamy P, Yusoff MM, Maniam GP, Govindan N, Saudi Pharm J 2016, 24, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mohammed AE, Al-Qahtani A, Al-Mutairi A, Al-Shamri B, Aabed KF, Nanomaterials (Basel) 2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Das RK, Pachapur VL, Lonappan L, Naghdi M, Pulicharla R, Maiti S, Cledon M, Dalila LMA, Sarma SJ, Brar SK, Nanotechnology for Environmental Engineering 2017, 2. [Google Scholar]
- [60].Geraldes AN, da Silva AA, Leal J, Estrada-Villegas GM, Lincopan N, Katti KV, Lug AB atildeo, Advances in Nanoparticles 2016, 05, 176–185. [Google Scholar]
- [61].Henriksen-Lacey M, Carregal-Romero S, Liz-Marzan LM, Bioconjug. Chem. 2017, 28, 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].a Ariga K, Minami K, Ebara M, Nakanishi J, Polym. J. (Tokyo, Jpn.) 2016, 48, 371–389; [Google Scholar]; b Jessl S, Tebbe M, Guerrini L, Fery A, Alvarez-Puebla RA, Pazos-Perez N, Small 2018, 14, e1703879. [DOI] [PubMed] [Google Scholar]
- [63].Bostrom M, Williams DR, Ninham BW, Phys. Rev. Lett. 2001, 87, 168103. [DOI] [PubMed] [Google Scholar]
- [64].Edwards SA, Williams DR, Phys. Rev. Lett. 2004, 92, 248303. [DOI] [PubMed] [Google Scholar]
- [65].a Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Muller R, Colloids Surf. B 2000, 18, 301–313; [DOI] [PubMed] [Google Scholar]; b Morla-Folch J, Guerrini L, Pazos-Perez N, Arenal R, Alvarez-Puebla RA, ACS Photonics 2014, 1, 1237–1244. [Google Scholar]
- [66].Moore TL, Rodriguez-Lorenzo L, Hirsch V, Balog S, Urban D, Jud C, Rothen-Rutishauser B, Lattuada M, Petri-Fink A, Chem. Soc. Rev. 2015, 44, 6287–6305. [DOI] [PubMed] [Google Scholar]
- [67].Patra JK, Baek K-H, J Nanomater 2014, 2014, 417305. [Google Scholar]
- [68].Vadlapudi V, Kaladhar D, Behara M, Sujatha B, Naidu GK, Orient. J. Chem. 2014, 29, 1589–1595. [Google Scholar]
- [69].Özyürek M, Güngör N, Baki S, Gü K, Apak R. a., Anal. Chem. 2012, 84, 8052–8059. [DOI] [PubMed] [Google Scholar]
- [70].Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M, Nat. Mater 2004, 3, 482–488. [DOI] [PubMed] [Google Scholar]
- [71].Gardea-Torresdey J, Parsons J, Gomez E, Peralta-Videa J, Troiani H, Santiago P, Yacaman MJ, Nano Lett. 2002, 2, 397–401. [Google Scholar]
- [72].Singaravelu G, Arockiamary J, Kumar VG, Govindaraju K, Colloids Surf. B 2007, 57, 97–101. [DOI] [PubMed] [Google Scholar]
- [73].Durán N, Teixeira MF, De Conti R, Esposito E, Crit. Rev. Food Sci. Nutr. 2002, 42, 53–66. [DOI] [PubMed] [Google Scholar]
- [74].Shankar SS, Ahmad A, Sastry M, Biotechnol. Prog. 2003, 19, 1627–1631. [DOI] [PubMed] [Google Scholar]
- [75].Sheikh MM, Sakthi KD, in Plant Nanotechnology (Eds.: Kole C, Kumar D, Khodakovskaya M), Springer, Cham, 2016. [Google Scholar]
- [76].Ma Y, Kuang L, He X, Bai W, Ding Y, Zhang Z, Zhao Y, Chai Z, Chemosphere 2010, 78, 273–279. [DOI] [PubMed] [Google Scholar]
- [77].Yang J, Cao WD, Rui YK, J Plant Interact 2017, 12, 158–169. [Google Scholar]
- [78].Nair R, Poulose AC, Nagaoka Y, Yoshida Y, Maekawa T, Kumar DS, J. Fluoresc 2011, 21, 2057. [DOI] [PubMed] [Google Scholar]
- [79].Zuverza-Mena N, Armendariz R, Peralta-Videa JR, Gardea-Torresdey JL, Front. Plant Sci. 2016, 7, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lin D, Xing B, Environ. Pollut. 2007, 150, 243–250. [DOI] [PubMed] [Google Scholar]
- [81].a Sedghi M, Hadi M, Toluie SG, Annals of West University of Timişoara, Ser. Biology 2013, 16, 73–78; [Google Scholar]; b Raskar S, Laware S, Int. J. Current Microbiol. Appl. Sci. 2014, 3, 467–473; [Google Scholar]; c Ahmed B, Dwivedi S, Abdin MZ, Azam A, Al-Shaeri M, Khan MS, Saquib Q, Al-Khedhairy AA, J. Musarrat, Sci. Rep 2017, 7, 40685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].a Burklew CE, Ashlock J, Winfrey WB, Zhang B, PLoS ONE 2012, 7, e34783; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Frazier TP, Burklew CE, Zhang B, Funct. Integr. Genomics 2014, 14, 75–83. [DOI] [PubMed] [Google Scholar]
- [83].a Li C, Zhang B, J. Cell. Physiol. 2016, 231, 303–313; [DOI] [PubMed] [Google Scholar]; b Zhang B, Wang Q, J. Cell. Physiol 2015, 230, 1–15. [DOI] [PubMed] [Google Scholar]
- [84].Zhang B, J. Exp. Bot. 2015, 66, 1749–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].a Cvjetko P, Milosic A, Domijan AM, Vinkovic Vrcek I, Tolic S, Peharec Stefanic P, Letofsky-Papst I, Tkalec M, Balen B, Ecotoxicol. Environ. Saf 2017, 137, 18–28; [DOI] [PubMed] [Google Scholar]; b Tripathi DK, Singh S, Singh S, Srivastava PK, Singh VP, Singh S, Prasad SM, Singh PK, Dubey NK, Pandey AC, Chauhan DK, Plant Physiol. Biochem 2017, 110, 167–177. [DOI] [PubMed] [Google Scholar]
- [86].Vinkovic T, Novak O, Strnad M, Goessler W, Jurasin DD, Paradikovic N, Vrcek IV, Environ. Res. 2017, 156, 10–18. [DOI] [PubMed] [Google Scholar]
- [87].Song G, Hou W, Gao Y, Wang Y, Lin L, Zhang Z, Niu Q, Ma R, Mu L, Wang H, Bot Stud 2016, 57, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yang L, Watts DJ, Toxicol. Lett. 2005, 158, 122–132. [DOI] [PubMed] [Google Scholar]
- [89].a Begum P, Fugetsu B, J. Hazard. Mater 2012, 243, 212–222; [DOI] [PubMed] [Google Scholar]; b Tiwari D, Dasgupta-Schubert N, Cendejas LV, Villegas J, Montoya LC, Garcia SB, Appl Nanosci 2014, 4, 577–591. [Google Scholar]
- [90].Faisal M, Saquib Q, Alatar AA, Al-Khedhairy AA, Hegazy AK, Musarrat J, J. Hazard. Mater 2013, 250–251, 318–332. [DOI] [PubMed] [Google Scholar]
- [91].Soares C, Branco-Neves S, de Sousa A, Pereira R, Fidalgo F, Chemosphere 2016, 165, 442–452. [DOI] [PubMed] [Google Scholar]
- [92].Lin D, Xing B, Environ. Sci. Technol 2008, 42, 5580–5585. [DOI] [PubMed] [Google Scholar]
- [93].Ghosh M, Jana A, Sinha S, Jothiramajayam M, Nag A, Chakraborty A, Mukherjee A, Mukherjee A, Mutat. Res., Genet. Toxicol. Environ. Mutagen 2016, 807, 25–32. [DOI] [PubMed] [Google Scholar]
- [94].Lee W-M, An Y-J, Yoon H, Kweon H-S, Toxico Enviro Chem 2008, 27, 1915–1921. [DOI] [PubMed] [Google Scholar]
- [95].Adhikari T, Kundu S, Biswas AK, Tarafdar JC, Rao AS, J Agr Sci Tech-Iran 2012, 2, 815–823. [Google Scholar]
- [96].Perreault F, Samadani M, Dewez D, Nanotoxicology 2014, 8, 374–382. [DOI] [PubMed] [Google Scholar]
- [97].Shaw AK, Ghosh S, Kalaji HM, Bosa K, Brestic M, Zivcak M, Hossain Z, Environ. Exp. Bot. 2014, 102, 37–47. [Google Scholar]
- [98].Nair PMG, Chung IM, Environ Sci Pollut R 2014, 21, 12709–12722. [DOI] [PubMed] [Google Scholar]
- [99].Shi J, Peng C, Yang Y, Yang J, Zhang H, Yuan X, Chen Y, Hu T, Nanotoxicology 2014, 8, 179–188. [DOI] [PubMed] [Google Scholar]
- [100].Regier N, Cosio C, von Moos N, Slaveykova VI, Chemosphere 2015, 128, 56–61. [DOI] [PubMed] [Google Scholar]
- [101].Da Costa MVJ, Sharma PK, Photosynthetica 2015, 54, 110–119. [Google Scholar]
- [102].Adams J, Wright M, Wagner H, Valiente J, Britt D, Anderson A, Plant Physiol. Biochem. 2017, 110, 108–117. [DOI] [PubMed] [Google Scholar]
- [103].Van NL, Ma C, Shang J, Rui Y, Liu S, Xing B, Chemosphere 2016, 144, 661–670. [DOI] [PubMed] [Google Scholar]
- [104].Wang Z, Xu L, Zhao J, Wang X, White JC, Xing B, Environ. Sci. Technol. 2016, 50, 6008–6016. [DOI] [PubMed] [Google Scholar]
- [105].Atha DH, Wang H, Petersen EJ, Cleveland D, Holbrook RD, Jaruga P, Dizdaroglu M, Xing B, Nelson BC, Environ. Sci. Technol. 2012, 46, 1819–1827. [DOI] [PubMed] [Google Scholar]
- [106].Mazumdar H, Ahmed GU, Int.J. ChemTech Res 2011, 3, 1494–1500. [Google Scholar]
- [107].Pokhrel LR, Dubey B, Sci. Total Environ. 2013, 452–453, 321–332. [DOI] [PubMed] [Google Scholar]
- [108].Mirzajani F, Askari H, Hamzelou S, Farzaneh M, Ghassempour A, Ecotoxicol. Environ. Saf. 2013, 88, 48–54. [DOI] [PubMed] [Google Scholar]
- [109].Qian H, Peng X, Han X, Ren J, Sun L, Fu Z, J Environ Sci-China 2013, 25, 1947–1956. [DOI] [PubMed] [Google Scholar]
- [110].Dimkpa CO, McLean JE, Martineau N, Britt DW, Haverkamp R, Anderson AJ, Environ. Sci. Technol. 2013, 47, 1082–1090. [DOI] [PubMed] [Google Scholar]
- [111].Patlolla AK, Berry A, May L, Tchounwou PB, Int. J. Environ. Res. Public. Health 2012, 9, 1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Saha N, Dutta Gupta S, J. Hazard. Mater. 2017, 330, 18–28. [DOI] [PubMed] [Google Scholar]
- [113].Hawthorne J, Musante C, Sinha SK, White JC, Int J Phytoremediation 2012, 14, 429–442. [DOI] [PubMed] [Google Scholar]
- [114].Rico CM, Morales MI, Barrios AC, McCreary R, Hong J, Lee W-Y, Nunez J, Peralta-Videa JR, Gardea-Torresdey JL, J. Agric. Food Chem 2013, 61, 11278–11285. [DOI] [PubMed] [Google Scholar]
- [115].Van Nhan L, Ma C, Rui Y, Cao W, Deng Y, Liu L, Xing B, Front Plant Sci 2015, 6, 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Du W, Gardea-Torresdey JL, Ji R, Yin Y, Zhu J, Peralta-Videa JR, Guo H, Environ. Sci. Technol. 2015, 49, 11884–11893. [DOI] [PubMed] [Google Scholar]
- [117].Asli S, Neumann PM, Plant Cell Environ. 2009, 32, 577–584. [DOI] [PubMed] [Google Scholar]
- [118].Ruffini Castiglione M, Giorgetti L, Geri C, Cremonini R, J. Nanopart. Res. 2010, 13, 2443–2449. [Google Scholar]
- [119].Larue C, Laurette J, Herlin-Boime N, Khodja H, Fayard B, Flank AM, Brisset F, Carriere M, Sci. Total Environ. 2012, 431, 197–208. [DOI] [PubMed] [Google Scholar]
- [120].Clement L, Hurel C, Marmier N, Chemosphere 2013, 90, 1083–1090. [DOI] [PubMed] [Google Scholar]
- [121].DemİR E, Kaya N, Kaya B, Turk. J. Biol. 2014, 38, 31–39. [Google Scholar]
- [122].Pakrashi S, Jain N, Dalai S, Jayakumar J, Chandrasekaran PT, Raichur AM, Chandrasekaran N, Mukherjee A, PLoS ONE 2014, 9, e87789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Rafique R, Arshad M, Khokhar MF, Qazi IA, Hamza A, Virk N, NUST Journal of Engineering Sciences 2014, 7, 42–46. [Google Scholar]
- [124].Linglan M, Chao L, Chunxiang Q, Sitao Y, Jie L, Fengqing G, Fashui H, Biol. Trace Elem. Res. 2008, 122, 168–178. [DOI] [PubMed] [Google Scholar]
- [125].a Aslani F, Bagheri S, Muhd Julkapli N, Juraimi AS, Hashemi FSG, Baghdadi A, Sci. World J 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pallavi, Mehta CM, Srivastava R, Arora S, Sharma AK, 3 Biotech 2016, 6; [DOI] [PMC free article] [PubMed] [Google Scholar]; c Nair R, in Plant Nanotechnology (Eds.: Kole C, Kumar D, Khodakovskaya M), Springer, Cham, 2016. [Google Scholar]
- [126].a Hatami M, in Nanoscience and Plant-soil systems, Vol. 48 (Eds.: Ghorbanpour M, Manika K, Varma A), Springer, Cham, 2017; [Google Scholar]; b Klanjscek T, Muller EB, Holden PA, Nisbet RM, Environ. Sci. Technol 2017, 51, 4944–4950. [DOI] [PubMed] [Google Scholar]
- [127].Siddiqui MH, Al-Whaibi MH, Saudi J Biol. Sci. 2014, 21, 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Suriyaprabha R, Karunakaran G, Yuvakkumar R, Rajendran V, Kannan N, Curr Nanosci 2012, 8, 902–908. [Google Scholar]
- [129].Bao-shan L, Chun-hui L, Li-jun F, Shu-chun Q, Min Y, J. Forestry Res 2004, 15, 138–140. [Google Scholar]
- [130].Hong F, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P, Biol. Trace Elem. Res. 2005, 105, 269–279. [DOI] [PubMed] [Google Scholar]
- [131].Haghighi M, Afifipour Z, Mozafarian M, J. Biol. Environ. Sci. 2012, 6, 87–90. [Google Scholar]
- [132].a Kalteh M, Alipour ZT, Ashraf S, Aliabadi MM, Nosratabadi AF, J. Chem. Health Risks 2014, 4; [Google Scholar]; b LI B, TAO G, XIE Y, CAI X, J. Nanjing Forestry Univ. (Natural Sciences Edition) 2012, 4, 038. [Google Scholar]
- [133].Wang L, Guo Z, Li T, Li M, Prog. Chem. 1999, 11, 119–128. [Google Scholar]
- [134].Xie X, Hu L, Pasta M, Wells GF, Kong D, Criddle CS, Cui Y, Nano Lett. 2010, 11, 291–296. [DOI] [PubMed] [Google Scholar]
- [135].a Ma JF, Yamaji N, Trends Plant Sci. 2006, 11, 392–397; [DOI] [PubMed] [Google Scholar]; b Pilon-Smits EA, Quinn CF, Tapken W, Malagoli M, Schiavon M, Curr. Opin. Plant Biol 2009, 12, 267–274; [DOI] [PubMed] [Google Scholar]; c Saqib M, Zörb C, Schubert S, Funct. Plant Biol 2008, 35, 633–639; [DOI] [PubMed] [Google Scholar]; d Pei Z, Ming D, Liu D, Wan G, Geng X, Gong H, Zhou W, J. Plant Growth Regul 2010, 29, 106–115; [Google Scholar]; e Ma Y, Zhang J, Zhang G, He H, J. Am. Chem. Soc 2004, 126, 7097–7101. [DOI] [PubMed] [Google Scholar]
- [136].de la Rosa G, López-Moreno ML, de Haro D, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL, Pure Appl. Chem. 2013, 85, 2161–2174. [Google Scholar]
- [137].Prasad T, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Sreeprasad T, Sajanlal P, Pradeep T, J. Plant Nutr 2012, 35, 905–927. [Google Scholar]
- [138].Ramesh M, Palanisamy K, Babu K, Sharma NK, J Glob Biosci 2014, 3, 415–422. [Google Scholar]
- [139].Raliya R, Tarafdar JC, Agric. Res. 2013, 2, 48–57. [Google Scholar]
- [140].Mahajan P, Dhoke S, Khanna A, J. Nanotechnol 2011, 2011, 696535. [Google Scholar]
- [141].Barrena R, Casals E, Colón J, Font X, Sánchez A, Puntes V, Chemosphere 2009, 75, 850–857. [DOI] [PubMed] [Google Scholar]
- [142].Arora S, Sharma P, Kumar S, Nayan R, Khanna P, Zaidi M, Plant Growth Regul. 2012, 66, 303–310. [Google Scholar]
- [143].Gopinath K, Gowri S, Karthika V, Arumugam A, J. Nanostruct. Chem. 2014, 4, 115. [Google Scholar]
- [144].Kumar V, Guleria P, Kumar V, Yadav SK, Sci. Total Environ. 2013, 461, 462–468. [DOI] [PubMed] [Google Scholar]
- [145].Demirer GS, Landry MP, Chem. Eng. Prog. 2017, 113, 40–45. [Google Scholar]
- [146].Kumari M, Pandey S, Mishra A, Nautiyal CS, FEMS Microbiol. Lett. 2017, 364. [DOI] [PubMed] [Google Scholar]
- [147].a Arul D, Balasubramani G, Balasubramanian V, Natarajan T, Perumal P, Pathog Glob Health 2017, 111, 367–382; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kasithevar M, Periakaruppan P, Muthupandian S, Mohan M, Microb. Pathog 2017, 107, 327–334; [DOI] [PubMed] [Google Scholar]; c Zheng KY, Setyawati MI, Leong DT, Xie JP, Coord. Chem. Rev 2018, 357, 1–17; [Google Scholar]; d Acharya D, Singha KM, Pandey P, Mohanta B, Rajkumari J, Singha LP, Sci. Rep 2018, 8, 201; [DOI] [PMC free article] [PubMed] [Google Scholar]; e Pareek V, Gupta R, Panwar J, Mat Sci Eng C-Mater 2018, 90, 739–749; [DOI] [PubMed] [Google Scholar]; f Ertem E, Gutt B, Zuber F, Allegri S, Le Ouay B, Mefti S, Formentin K, Stellacci F, Ren Q, ACS Appl. Mater. Interfaces 2017, 9, 34762–34772; [DOI] [PubMed] [Google Scholar]; g Panacek A, Kvitek L, Smekalova M, Vecerova R, Kolar M, Roderova M, Dycka F, Sebela M, Prucek R, Tomanec O, Zboril R, Nat. Nanotechnol 2018, 13, 65–+. [DOI] [PubMed] [Google Scholar]
- [148].a Krishnaraj C, Jagan E, Ramachandran R, Abirami S, Mohan N, Kalaichelvan P, Process Biochem. 2012, 47, 651–658; [Google Scholar]; b Monica RC, Cremonini R, Caryologia 2009, 62, 161–165. [Google Scholar]
- [149].Savithramma N, Ankanna S, Bhumi G, Nano Vision 2012, 2, 2. [Google Scholar]
- [150].Salama HM, Int. Res. J. Biotech 2012, 3, 190–197. [Google Scholar]
- [151].Syu Y.-y., Hung J-H, Chen J-C, Chuang H.-w., Plant Physiol. Biochem 2014, 83, 57–64. [DOI] [PubMed] [Google Scholar]
- [152].Mahmoodzadeh H, Nabavi M, Kashefi H, J Ornamental Hortic Plants 2013, 3, 25–32. [Google Scholar]
- [153].Jaberzadeh A, Moaveni P, MOGHADAM HRT, Zahedi H, Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2013, 41, 201. [Google Scholar]
- [154].a Zheng L, Hong F, Lu S, Liu C, Biol. Trace Elem. Res 2005, 104, 83–91; [DOI] [PubMed] [Google Scholar]; b Feizi H, Moghaddam PR, Shahtahmassebi N, Fotovat A, Biol. Trace Elem. Res 2012, 146, 101–106. [DOI] [PubMed] [Google Scholar]
- [155].a Yang F, Hong F, You W, Liu C, Gao F, Wu C, Yang P, Biol. Trace Elem. Res 2006, 110, 179–190; [DOI] [PubMed] [Google Scholar]; b Mishra V, Mishra RK, Dikshit A, Pandey AC, in Emerging Technologies and Management of Crop Stress Tolerance, Vol. 1, Elsevier, 2014, pp. 159–180. [Google Scholar]
- [156].Crabtree RH, Science 1998, 282, 2000–2001. [Google Scholar]
- [157].Harrison CC, Phytochemistry 1996, 41, 37–42. [DOI] [PubMed] [Google Scholar]
- [158].Hong F, Yang F, Liu C, Gao Q, Wan Z, Gu F, Wu C, Ma Z, Zhou J, Yang P, Biol. Trace Elem. Res. 2005, 104, 249–260. [DOI] [PubMed] [Google Scholar]
- [159].LI B, TAO G, XIE Y, CAI X, Journal of Nanjing Forestry University (Natural Sciences Edition) 2012, 4, 038. [Google Scholar]
- [160].Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Sreeprasad TS, Sajanlal PR, Pradeep T, J. Plant Nutr 2012, 35, 905–927. [Google Scholar]
- [161].Berahmand AA, Ghafariyan Panahi A, Sahabi H, Feizi H, Rezvani Moghaddam P, Shahtahmassebi N, Fotovat A, Karimpour H, Gallehgir O, Biol. Trace Elem. Res. 2012, 149, 419–424. [DOI] [PubMed] [Google Scholar]
- [162].Jiang HS, Qiu XN, Li GB, Li W, Yin LY, Environ. Toxicol. Chem. 2014, 33, 1398–1405. [DOI] [PubMed] [Google Scholar]
- [163].Jasim B, Thomas R, Mathew J, Radhakrishnan EK, Saudi Pharm J 2017, 25, 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Pallavi CM Mehta R. Srivastava, Arora S, Sharma AK, 3 Biotech 2016, 6, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Nekrasova GF, Ushakova OS, Ermakov AE, Uimin MA, Byzov IV, Russ. J. Ecol. 2011, 42, 458–463. [Google Scholar]
- [166].Gao F, Liu C, Qu C, Zheng L, Yang F, Su M, Hong F, Biometals 2008, 21, 211–217. [DOI] [PubMed] [Google Scholar]
- [167].Raliya R, Nair R, Chavalmane S, Wang W-N, Biswas P, Metallomics 2015, 7, 1584–1594. [DOI] [PubMed] [Google Scholar]
- [168].Okupnik A, Pflugmacher S, Environ. Toxicol. Chem 2016, 35, 2859–2866. [DOI] [PubMed] [Google Scholar]
- [169].Barrios AC, Rico CM, Trujillo-Reyes J, Medina-Velo IA, Peralta-Videa JR, Gardea-Torresdey JL, Sci. Total Environ 2016, 563–564, 956–964. [DOI] [PubMed] [Google Scholar]
- [170].Majumdar S, Peralta-Videa JR, Trujillo-Reyes J, Sun Y, Barrios AC, Niu G, Margez JPF, Gardea-Torresdey JL, Sci. Total Environ. 2016, 569–570, 201–211. [DOI] [PubMed] [Google Scholar]
- [171].Iannone MF, Groppa MD, de Sousa ME, Fernández van Raap MB, Benavides MP, Environ. Exp. Bot 2016, 131, 77–88. [Google Scholar]
- [172].Li J, Hu J, Ma C, Wang Y, Wu C, Huang J, Xing B, Chemosphere 2016, 159, 326–334. [DOI] [PubMed] [Google Scholar]
- [173].Vecerova K, Vecera Z, Docekal B, Oravec M, Pompeiano A, Triska J, Urban O, Environ. Pollut. 2016, 218, 207–218. [DOI] [PubMed] [Google Scholar]
- [174].Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris AS, ACS Nano 2009, 3, 3221–3227. [DOI] [PubMed] [Google Scholar]
- [175].Sanborn JR, Chen X, Yao YC, Hammons JA, Tunuguntla RH, Zhang Y, Newcomb CC, Soltis JA, De Yoreo JJ, Van Buuren A, Parikh AN, Noy A, Adv. Mater 2018, 30, e1803355. [DOI] [PubMed] [Google Scholar]
- [176].Yu S, Chen R, Zhang G, Cheng J, Meng Z, Appl. Phys. Lett. 2006, 89, 212906. [Google Scholar]
- [177].Rezvani N, Sorooshzadeh A, Farhadi N, World Acad. Sci. Eng. Technol 2012, 6, 517–522. [Google Scholar]
- [178].Zhang B, Pan X, Cannon CH, Cobb GP, Anderson TA, Plant J 2006, 46, 243–259. [DOI] [PubMed] [Google Scholar]
- [179].Wang X, Han H, Liu X, Gu X, Chen K, Lu D, J. Nanopart. Res 2012, 14, 841. [Google Scholar]
- [180].Smirnova E, Gusev A, Zaytseva O, Sheina O, Tkachev A, Kuznetsova E, Lazareva E, Onishchenko G, Feofanov A, Kirpichnikov M, Front. Chem. Sci. Eng 2012, 6, 132–138. [Google Scholar]
- [181].Tripathi S, Sarkar S, Appl Nanosci 2015, 5, 609–616. [Google Scholar]
- [182].Kirschbaum MU, Plant Physiol. 2011, 155, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Giraldo JP, Landry MP, Faltermeier SM, McNicholas TP, Iverson NM, Boghossian AA, Reuel NF, Hilmer AJ, Sen F, Brew JA, Nat. Mater 2014, 13, 400–408. [DOI] [PubMed] [Google Scholar]
- [184].Kwak SY, Lew TTS, Sweeney CJ, Koman VB, Wong MH, Bohmert-Tatarev K, Snell KD, Seo JS, Chua NH, Strano MS, Nat. Nanotechnol 2019, 14, 447–455. [DOI] [PubMed] [Google Scholar]
- [185].a Noji T, Kamidaki C, Kawakami K, Shen J-R, Kajino T, Fukushima Y, Sekitoh T, Itoh S, Langmuir 2010, 27, 705–713; [DOI] [PubMed] [Google Scholar]; b Torney F, Trewyn BG, Lin VS, Wang K, Nat. Nanotechnol 2007, 2, 295–300. [DOI] [PubMed] [Google Scholar]
- [186].Xie Y, Bo LI, Tao G, Zhang Q, Zhang C, J. Nanjing Forestry Univ. 2012, 36, 59–63. [Google Scholar]
- [187].Yang F, Liu C, Gao F, Su M, Wu X, Zheng L, Hong F, Yang P, Biol. Trace Elem. Res 2007, 119, 77–88. [DOI] [PubMed] [Google Scholar]
- [188].Qi M, Liu Y, Li T, Biol. Trace Elem. Res 2013, 156, 323–328. [DOI] [PubMed] [Google Scholar]
- [189].a Taylor GJ, Foy CD, Can. J. Bot 1985, 63, 1271–1275; [Google Scholar]; b Hartley W, Lepp NW, Sci. Total Environ 2008, 390, 35–44. [DOI] [PubMed] [Google Scholar]
- [190].Zhang Z, He X, Zhang H, Ma Y, Zhang P, Ding Y, Zhao Y, Metallomics 2011, 3, 816–822. [DOI] [PubMed] [Google Scholar]
- [191].Hauck TS, Ghazani AA, Chan WC, Small 2008, 4, 153–159. [DOI] [PubMed] [Google Scholar]
- [192].Davison BH, Parks J, Davis MF, Donohoe BS, in Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals (Ed.: Wyman CE), John Wiley & Sons, Ltd, 2013, pp. 23–38. [Google Scholar]
- [193].Kurepa J, Paunesku T, Vogt S, Arora H, Rabatic BM, Lu J, Wanzer MB, Woloschak GE, Smalle JA, Nano Lett. 2010, 10, 2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Kendall SK, The Charleston Advisor 2012, 13, 19–21. [Google Scholar]
- [195].Jensen MØ, Tajkhorshid E, Schulten K, Structure 2001, 9, 1083–1093. [DOI] [PubMed] [Google Scholar]
- [196].Taran N, Batsmanova L, Konotop Y, Okanenko A, Nanoscale Res. Lett 2014, 9, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].a Lin S, Reppert J, Hu Q, Hudson JS, Reid ML, Ratnikova TA, Rao AM, Luo H, Ke PC, Small 2009, 5, 1128–1132; [DOI] [PubMed] [Google Scholar]; b Zhang L, Feng C, Chen Z, Liu L, Jiang K, Li Q, Fan S, Nano Lett. 2008, 8, 2564–2569. [DOI] [PubMed] [Google Scholar]
- [198].Zhang S, Gao H, Bao G, ACS Nano 2015, 9, 8655–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Decuzzi P, Ferrari M, Biomaterials 2007, 28, 2915–2922. [DOI] [PubMed] [Google Scholar]
- [200].a Sandvig K, Torgersen ML, Raa HA, Van Deurs B, Histochem. Cell Biol. 2008, 129, 267–276; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Doherty GJ, McMahon HT, Annu. Rev. Biochem 2009, 78, 857–902. [DOI] [PubMed] [Google Scholar]
- [201].Howes MT, Mayor S, Parton RG, Curr. Opin. Cell Biol. 2010, 22, 519–527. [DOI] [PubMed] [Google Scholar]
- [202].Iversen T-G, Skotland T, Sandvig K, Nano Today 2011, 6, 176–185. [Google Scholar]
- [203].Palade GE, J. Appl. Phys. 1953, 24, 1424–1424. [Google Scholar]
- [204].a Swanson JA, Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pérez-de-Luque A, Frontiers in Environmental Science 2017, 5. [Google Scholar]
- [205].McNeil PL, Tissue Cell 1984, 16, 519–533. [DOI] [PubMed] [Google Scholar]
- [206].Chithrani BD, Chan WC, Nano Lett. 2007, 7, 1542–1550. [DOI] [PubMed] [Google Scholar]
- [207].Tilney LG, Cooke TJ, Connelly PS, Tilney MS, J. Cell Biol 1991, 112, 739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Milewska-Hendel A, Gawecki R, Zubko M, Stróż D, Kurczyńska E, Acta Agrobot. 2016, 69, 1694. [Google Scholar]
- [209].Erwee M, Goodwin P, Planta 1985, 163, 9–19. [DOI] [PubMed] [Google Scholar]
- [210].Faulkner CR, Blackman LM, Collings DA, Cordwell SJ, Overall RL, Eur. J. Cell Biol 2009, 88, 357–369. [DOI] [PubMed] [Google Scholar]
- [211].Nair R, Mohamed MS, Gao W, Maekawa T, Yoshida Y, Ajayan PM, Kumar DS, J. Nanosci. Nanotechnol 2012, 12, 2212–2220. [DOI] [PubMed] [Google Scholar]
- [212].Hill AE, J. Membr. Biol 1994, 137, 197–203. [DOI] [PubMed] [Google Scholar]
- [213].Tyerman S, Niemietz C, Bramley H, Plant, Cell Environ. 2002, 25, 173–194. [DOI] [PubMed] [Google Scholar]
- [214].Tornroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P, Nature 2006, 439, 688–694. [DOI] [PubMed] [Google Scholar]
- [215].Demirer GS, Zhang H, Matos JL, Goh NS, Cunningham FJ, Sung Y, Chang R, Aditham AJ, Chio L, Cho MJ, Staskawicz B, Landry MP, Nat. Nanotechnol. 2019, 14, 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Tani F, Barrington S, Environ. Pollut. 2005, 138, 538–547. [DOI] [PubMed] [Google Scholar]
- [217].Yue L, Ma C, Zhan X, White JC, Xing B, Environ. Sci. Nano 2017, 4, 843–855. [Google Scholar]
- [218].Sharif F, Westerhoff P, Herckes P, Environ. Sci.: Processes & Impacts 2013, 15, 267–274. [DOI] [PubMed] [Google Scholar]
- [219].Cifuentes Z, Custardoy L, de la Fuente JM, Marquina C, Ibarra MR, Rubiales D, Pérez-de-Luque A, J. Nanobiotechnol 2010, 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Wang Y, Ju Z, Cao B, Gao X, Zhu Y, Qiu P, Xu H, Pan P, Bao H, Wang L, Mao C, ACS Nano 2015, 9, 4475–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Sunderland KS, Yang M, Mao C, Angew Chem Int Edit 2017, 56, 1964–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]