Abstract
PURPOSE
Immune checkpoint inhibitor (ICI) therapy often is suspended because of immune-mediated diarrhea and colitis (IMDC). We examined the rate of and risk factors for IMDC recurrence after ICI resumption.
METHODS
This retrospective multicenter study examined patients who resumed ICI therapy after improvement of IMDC between January 2010 and November 2018. Univariable and multivariable logistic regression analyses assessed the association of clinical covariates and IMDC recurrence.
RESULTS
Of the 167 patients in our analysis, 32 resumed an anti–cytotoxic T-cell lymphocyte-4 (CTLA-4) agent, and 135 an anti–programmed cell death 1 or ligand 1 (PD-1/L1) agent. The median age was 60 years (interquartile range [IQR], 50-69 years). The median duration from IMDC to restart of ICI treatment was 49 days (IQR, 23-136 days). IMDC recurred in 57 patients (34%) overall (44% of those receiving an anti–CTLA-4 and 32% of those receiving an anti–PD-1/L1); 47 of these patients (82%) required immunosuppressive therapy for recurrent IMDC, and all required permanent discontinuation of ICI therapy. The median duration from ICI resumption to IMDC recurrence was 53 days (IQR, 22-138 days). On multivariable logistic regression, patients who received anti–PD-1/L1 therapy at initial IMDC had a higher risk of IMDC recurrence (odds ratio [OR], 3.45; 95% CI, 1.59 to 7.69; P = .002). Risk of IMDC recurrence was higher for patients who required immunosuppression for initial IMDC (OR, 3.22; 95% CI, 1.08 to 9.62; P = .019) or had a longer duration of IMDC symptoms in the initial episode (OR, 1.01; 95% CI, 1.00 to 1.03; P = .031). Risk of IMDC recurrence was lower after resumption of anti–PD-1/L1 therapy than after resumption of anti–CTLA-4 therapy (OR, 0.30; 95% CI, 0.11 to 0.81; P = .019).
CONCLUSION
One third of patients who resumed ICI treatment after IMDC experienced recurrent IMDC. Recurrence of IMDC was less frequent after resumption of anti–PD-1/L1 than after resumption of anti–CTLA-4.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy and have been the focus of intensive clinical and basic research in recent years. ICIs augment antitumor immune response by blocking cytotoxic T-cell lymphocyte-4 (CTLA-4) or programmed cell death 1 or ligand-1 (PD-1/L1) or both, resulting in significant response rates in a subset of malignancies.1 Currently, studies are assessing ICI safety and efficacy in an increasing array of solid tumors as well as hematologic malignancies.2
The toxicity profile of ICIs is distinct from those of other cancer therapies and falls under the umbrella term immune-related adverse events (irAEs) because of their autoimmune nature.1 In theory, irAEs can affect any organ system and frequently manifest as dermatitis, diarrhea, colitis, pneumonitis, hepatitis, and pancreatitis.3 Development of irAEs is indicative of an augmented immune response, which is associated with prolonged overall survival, and should be considered a surrogate marker for positive response to ICI therapy.4-6 We previously reported longer overall survival among patients who develop immune-mediated diarrhea and colitis (IMDC).5,6 However, considerable controversy exists with regard to the impact of irAEs on survival because some investigators have not found a similar impact.7,8
IMDC can be severe enough to cause colon perforation and death if not treated appropriately.9 Therefore, timely and precise management of IMDC is critical for favorable outcomes.10-12 Current treatment guidelines recommend holding ICI therapy in patients with grade 2 or higher IMDC and initiating corticosteroid therapy.13-15 A subset of these patients may resume ICI therapy, particularly anti–PD-1/L1, when IMDC symptoms subside to grade 1. Furthermore, it may be possible to hold ICI therapy temporarily (in cases of cancer progression) or indefinitely (in cases of cancer remission) because the optimum number of doses of anti–PD-1/L1 remains unknown. However, a paucity of evidence exists on the safety profile of ICI resumption in patients who stopped treatment because of the development of an irAE.
One large-scale study that assessed the safety of resuming anti–PD-1 therapy included 80 patients previously treated with a combination anti–CTLA-4 and anti–PD-1 ICI regimen who developed a treatment-limiting irAE.16 Although the findings were encouraging and have added to the current body of evidence, they did not address patients who were previously treated with anti–PD-1/L1 or anti–CTLA-4 therapy as a single agent and then resumed the same or a different ICI agent. Because IMDC frequently requires interruption of ICI treatment, the current study aimed to identify the incidence and characteristics of and risk factors for recurrent IMDC after resumption of ICI therapy in patients in whom ICI treatment was withheld because of IMDC.
METHODS
Patient Cohort
This retrospective multicenter study was approved by the institutional review boards of the participating institutions (Appendix Table A1, online only). Included patients were 18 years of age or older who received an ICI and developed IMDC between January 2010 and November 2018 and then resumed ICI therapy after it was suspended because of IMDC onset. Patients were identified from pharmacy and institutional databases.
Clinical Data
Demographic information, medical and oncologic history, and data related to ICI therapy and IMDC were extracted from the medical record of each identified patient. Comorbid conditions included in the Charlson comorbidity index score were recorded.17 Cancer stage was determined according to the seventh edition of the American Joint Committee on Cancer Staging System but was not recorded for patients with hematologic malignancies because of the intricate staging systems used.
ICI and IMDC Information
The recorded information that pertained to ICI included type, number of infusions, and duration of treatment. In addition, we documented the reason for resuming ICI therapy after IMDC as cancer progression, resolution of irAE, or maintenance therapy. Cancer progression was assessed as reported in the medical chart by the treating oncologist and radiologist according to the immune-modified Response Evaluation Criteria in Solid Tumors (imRECIST) and immune RECIST (iRECIST).18,19 The Common Terminology Criteria of Adverse Events (version 5.0) was used to grade IMDC at its peak severity.20 The duration of IMDC symptoms was measured as the cumulative number of days from IMDC onset until symptom resolution.
Clinical Outcomes
The primary end point of this study was recurrence of IMDC after resuming ICI therapy. Time from IMDC occurrence to ICI restart, type of ICI restarted, reason to restart ICI, time from ICI restart to IMDC recurrence (if any), peak grade of the recurrent IMDC, and treatment required for the recurrent IMDC were recorded.
Statistical Analyses
The distributions of continuous variables were summarized using medians and interquartile ranges (IQRs). The distributions of categorical variables were summarized using frequencies and percentages. Categorical variables were compared between groups by Fisher’s exact and χ2 tests. Continuous variables were compared by Wilcoxon rank sum test. To assess the risk factors for IMDC recurrence, we performed a univariable followed by a multivariable logistic regression. Clinical characteristics that were significant in the univariable analysis or were relevant to clinical practice (ie, type of ICI resumed) were included in the multivariable model. Requirement for infliximab or vedolizumab was not added to the multivariable analysis because its effect is included as part of immunosuppression. Hosmer-Lemeshow test was used to measure the goodness of fit of the included variables in the multivariable logistic regression model and revealed P = .22, which indicates a good fit of the variables in the multivariable logistic model. In addition, collinearity was evaluated using the variance inflation factor, which assesses how much the variance of an estimated regression coefficient increases if the predictors are correlated. The highest variance inflation factor for our model was 1.66, which indicates that there was no multicollinearity. All statistical tests were two-sided. P ≤ .05 was considered statistically significant. Statistical analyses were carried out using SAS 9.4 (SAS Institute, Cary, NC) and SPSS version 24.0 (IBM Corporation, Chicago, IL) software.
RESULTS
Patient Selection
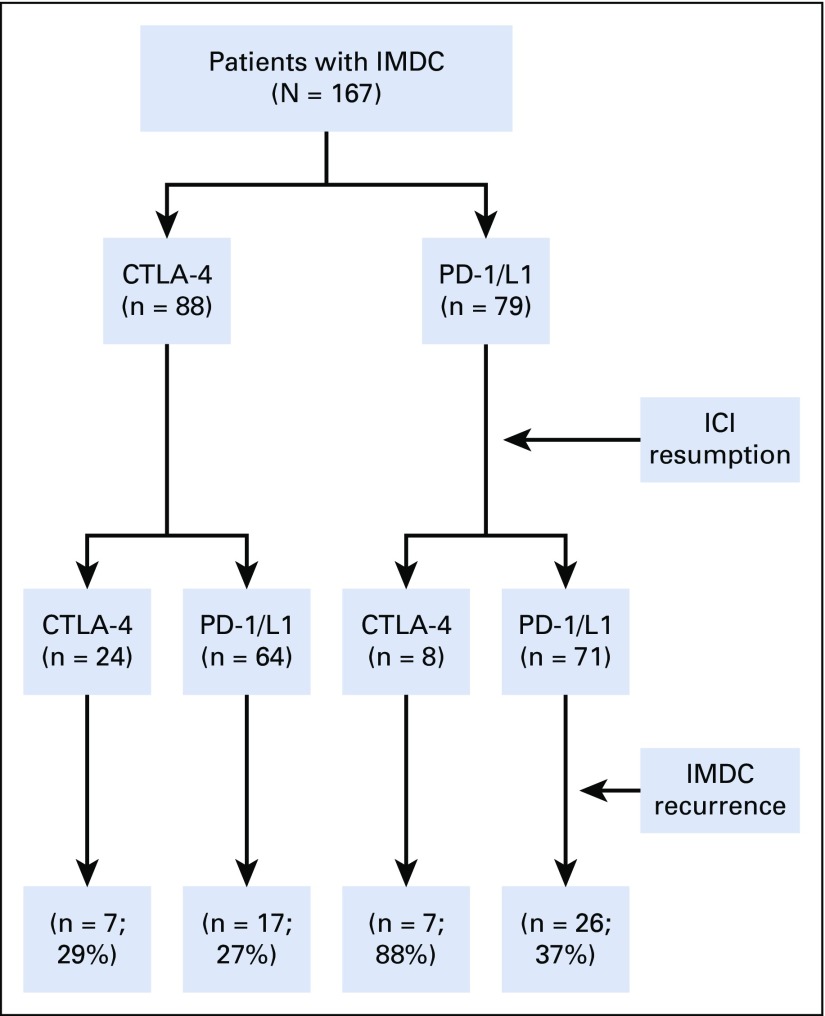
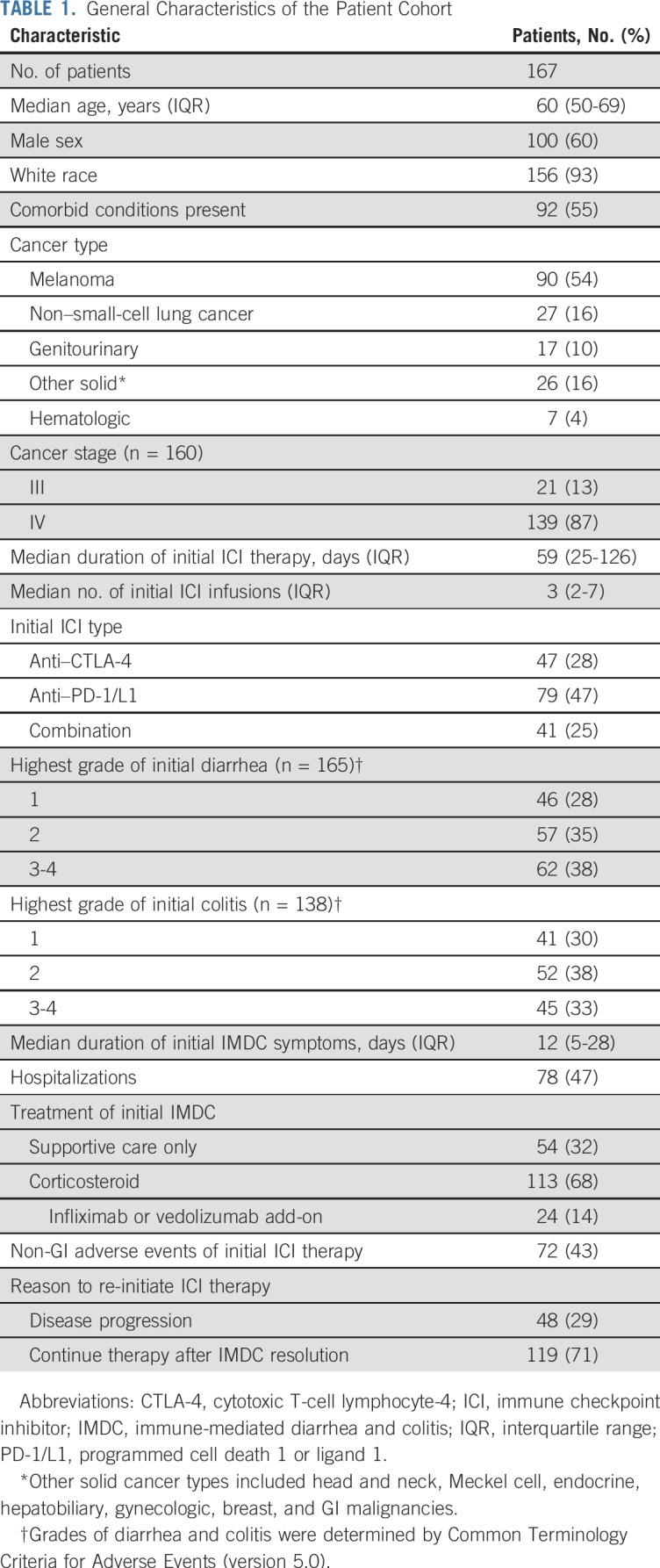
Among 550 patients who had IMDC, we identified 167 (30%) who received ICI treatment after the onset of IMDC; patient demographic and clinical characteristics are listed in Table 1. The median age was 60 years (IQR, 50-69 years). Initial ICI therapy was a PD-1/L1 inhibitor in 79 patients (47%), a CTLA-4 inhibitor in 47 (28%), and a combination in 41 (25%). Melanoma was the most common malignancy type in this cohort (54%) followed by non–small-cell lung cancer (16%) and genitourinary cancer (10%).
TABLE 1.
General Characteristics of the Patient Cohort
Initial IMDC and Non-GI irAEs
The median duration of initial IMDC symptoms was 12 days (IQR, 5-28 days). Seventy-five patients had an endoscopic evaluation. A majority of the patients who developed IMDC required immunosuppression with a corticosteroid (113; 68%); among them, 24 patients (14%) required treatment escalation to addition of infliximab or vedolizumab. Non-GI irAEs were reported in 72 patients (43%) and involved skin (n = 22), liver (n = 11), endocrine organs (n = 29), lung (n = 9), or other sites (n = 19).
ICI Resumption and IMDC Recurrence
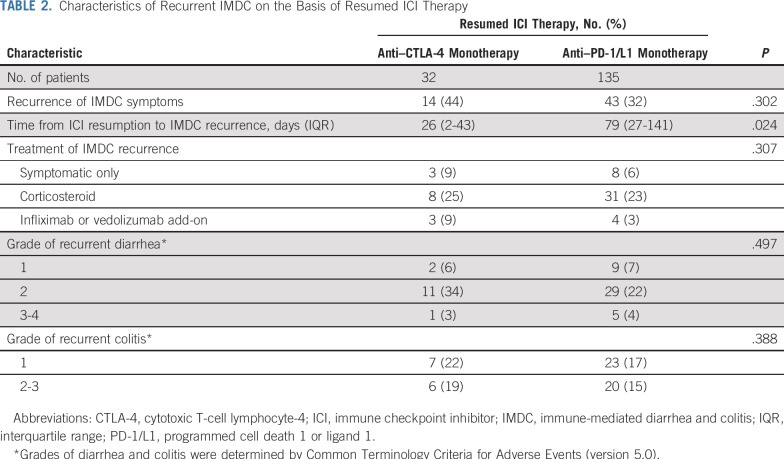
ICI therapy was re-initiated because of cancer progression or relapse in 48 patients (29%), whereas 119 (71%) either continued therapy as maintenance therapy with good response to ICI or resumed therapy after irAE resolution. The median duration from initial IMDC to ICI resumption was 49 days (IQR, 23-136 days). On resuming ICI therapy, a majority of patients received an anti–PD-1/L1 agent (135; 81%), whereas 32 (19%) received an anti–CTLA-4 agent (Table 2). Overall, IMDC recurred in 57 patients (34%) after a median of 53 days (IQR, 22-138 days) after ICI resumption. Of the 57 patients who experienced IMDC recurrence, the majority experienced grade 2 diarrhea (40; 70%) and grade 1 colitis (30; 54%). Most IMDC recurrences required corticosteroid therapy (46; 81%). Seven patients (12%) required treatment escalation to addition of infliximab or vedolizumab for the recurrent IMDC.
TABLE 2.
Characteristics of Recurrent IMDC on the Basis of Resumed ICI Therapy
Of the 43 patients (32%) who experienced IMDC recurrence after resuming anti–PD-1/L1 therapy, 26 (37%) initially received an anti–PD-1/L1 agent, and 17 (27%) initially received an anti–CTLA-4 agent. Of the 14 patients (44%) who experienced IMDC recurrence after resuming anti–CTLA-4 therapy, seven (88%) initially received an anti–PD-1/L1 agent, and seven (29%) initially received an anti–CTLA-4 agent (Fig 1). Monthly nivolumab was the re-initiated ICI type in 44 patients; among them, 10 (23%) had IMDC recurrence. Of the 113 patients who received immunosuppressive therapy for initial IMDC, 47 (42%) had recurrent IMDC. Of the seven patients who had IMDC grade 4 initially and resumed ICI therapy (anti–CTLA-4 in two patients and anti–PD-1/L1 in five), four (57%) had IMDC recurrence.
FIG 1.
Recurrence of immune-mediated diarrhea and colitis (IMDC) after immune checkpoint inhibitor (ICI) resumption. CTLA-4, cytotoxic T-cell lymphocyte-4; PD-1/L1, programmed cell death 1 or ligand 1.
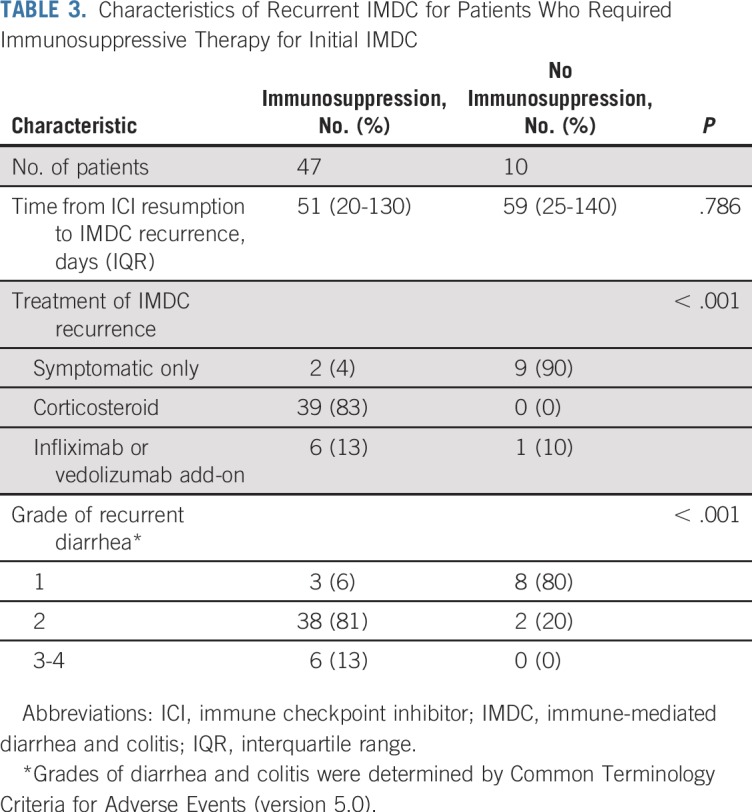
In patients who resumed anti–CTLA-4 therapy, IMDC recurrence was reported after a median of 26 days (IQR, 2-43 days) and occurred significantly earlier than recurrence after anti–PD-1/L1 resumption (median, 79 days; IQR, 27-141 days; P = .024; Table 2). No differences were observed in the severity of IMDC recurrence between the two groups. The recurrence of IMDC was more severe (P < .001) and required more intensive immunosuppressive therapy (P < .001) in patients who received immunosuppressive therapy for the initial event than in those who did not (Table 3).
TABLE 3.
Characteristics of Recurrent IMDC for Patients Who Required Immunosuppressive Therapy for Initial IMDC
Risk Factors for IMDC Recurrence
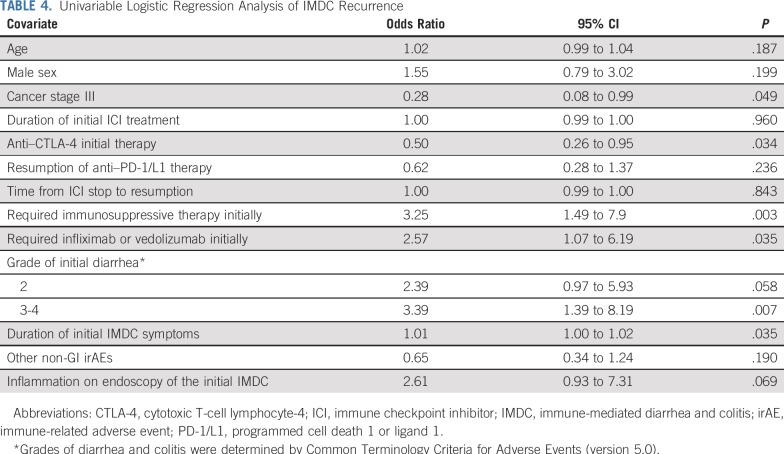
On univariable analysis (Table 4), initial use of anti–PD-1/L1 therapy (P = .034), cancer stage III (P = .049), requirement for immunosuppressive therapy at initial IMDC (P = .003), higher grades of IMDC (P = .007), requirement for infliximab or vedolizumab at initial IMDC (P = .035), and longer duration of initial IMDC symptoms (P = .035) were associated with an increased likelihood of IMDC recurrence after ICI resumption. Inflammation identified by endoscopy in the initial IMDC episode tended to increase the risk of IMDC recurrence (P = .069).
TABLE 4.
Univariable Logistic Regression Analysis of IMDC Recurrence
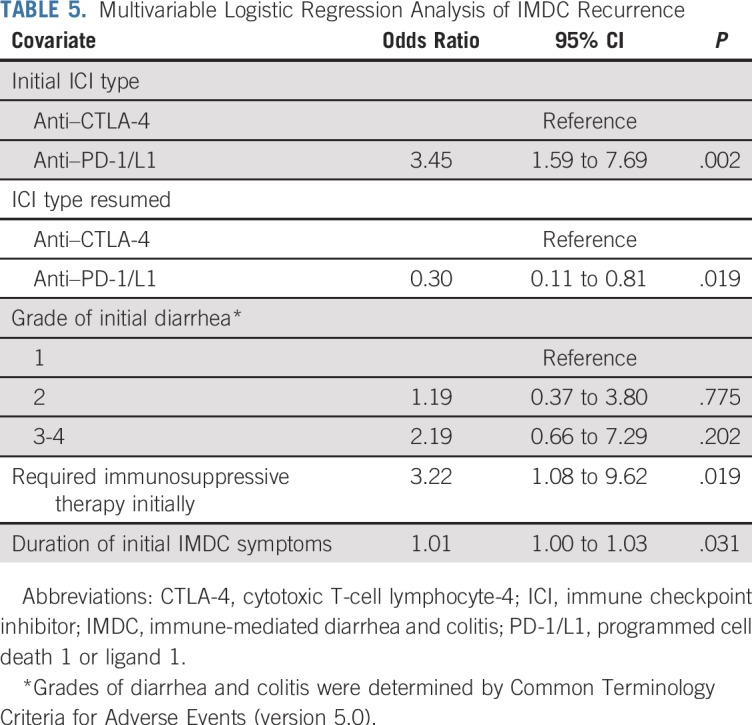
Multivariable logistic regression analysis (Table 5) revealed that the initial use of anti–CTLA-4 was associated with a lower risk of IMDC recurrence upon ICI resumption (P = .002). Regardless of initial therapy, however, the resumption of anti–PD-1/L1 therapy, as opposed to anti–CTLA-4 therapy, was associated with a lower risk of IMDC recurrence (P = .019). In addition, patients who required immunosuppressive therapy for initial IMDC were more likely to experience IMDC recurrence after the resumption of ICI therapy (P = .019). Likewise, a long duration of IMDC symptoms in the initial episode was associated with a higher risk of IMDC recurrence (P = .031).
TABLE 5.
Multivariable Logistic Regression Analysis of IMDC Recurrence
DISCUSSION
This multicenter study aimed to evaluate and characterize the recurrence of IMDC after the resumption of ICI therapy after a first occurrence of IMDC. Our approach of studying recurrent ICI toxicity differs from previously published studies in that our cohort comprised patients with different types of cancer who received different types of ICI therapy. Although such an approach limits in-depth analysis of oncologic outcomes, it allows for generalizable data about the toxicity profile of ICIs irrespective of cancer type. We found the incidence of recurrent IMDC to be lower in patients who resumed an anti–PD-1/L1 agent than in those who resumed an anti–CTLA-4 agent.
We found that IMDC after ICI resumption was mostly grade 1 to 2 in severity, irrespective of the ICI type resumed. This finding agrees with the limited body of evidence about the resumption of ICIs. In the study by Pollack et al,16 which comprised 80 patients with melanoma previously treated with a combination ICI regimen who developed a clinically significant irAE and were rechallenged with anti–PD-1 therapy, the recurrence of grade 3/4 colitis was 3%. In another study of eight patients with melanoma who were previously treated with anti–PD-1 therapy and rechallenged with an anti–PD-1 agent, recurrent grade 1 IMDC was reported in one.21 In addition to melanoma, the resumption of ICI therapy in patients with non–small-cell lung cancer has been reported (two case series and one case report).22-24 None of these three studies reported a recurrence of grade 3/4 IMDC. The discrepancy between these reports and our findings could be the result of the low rate of high-grade initial IMDC in those reports because we found that more severe IMDC is a predictor of recurrence. In addition, because ICI use has become more widespread and practitioners have become more comfortable with irAE management, ICI rechallenge has broadened.
Recurrent IMDC in our cohort was generally manageable by established treatment approaches. No serious AEs that led to mortality were identified in this IMDC cohort. By contrast, Pollack et al16 reported progression of a grade 2 rash as the initial event to a grade 3 rash with anti–PD-1 resumption, which evolved into fatal Stevens-Johnson syndrome. Hence, although the severity of IMDC after resumption of ICI therapy seems to be mild, caution should be practiced. Close monitoring for progression in severity of irAEs should prompt timely management efforts, including adequate immunosuppressive therapy as well as ICI discontinuation if deemed appropriate. In the setting of IMDC, colon biopsy of mucosal ulcerations and nonulcerative inflammation, as well as seemingly normal mucosal areas, on colonoscopy can help to identify active inflammation and guide the duration of immunosuppressive therapy.11,12,25
Our findings on multivariable analysis that anti–CTLA-4 therapy resumption was associated with an increased risk of IMDC recurrence, whereas prior anti–CTLA-4 use was a predictor of low risk of IMDC recurrence, hint at the relative significance of prior and resumed ICI therapy and their impact on toxicity recurrence. The clinical implication of this observation should be taken into consideration when planning ICI resumption in patients in whom previous ICI therapy failed or had to be terminated altogether because of AEs. Similarly, Pollack et al16 concluded that patients with colitis and hypophysitis can safely resume anti–PD-1 ICI therapy. In real-life settings, however, treatment decisions are made on a per-patient basis, depending on comorbid conditions and cancer therapy options.
Timely recognition and treatment of IMDC are critical to sustain ICI therapy.31 To achieve this goal, the use of potent nonsteroidal immunosuppressive therapy, such as infliximab or vedolizumab, may be considered, although the long-term effect of such drugs on cancer outcomes is not yet known.10,26 A new IMDC treatment strategy with great promise and no known immunosuppressive effect is fecal microbiota transplantation, which was found by our group to be a novel treatment of immunosuppression-refractory IMDC.27
Our findings reveal that even upon resumption of anti–CTLA-4 therapy, the risk of severe IMDC seems to be acceptable. Although resumption of anti–PD-1/L1 therapy is more desirable when limitation of the toxicity profile is among the major goals of care, the presumed toxicity risk associated with anti–CTLA-4 therapy should not prevent clinicians from considering resumption of these drugs when this may be the only resort for prolonging survival. In addition, patients who develop irAEs tend to have high rates of response to ICI therapy, which are sustained and may obviate ICI resumption.1,28-30 Hence, the decision to resume ICI therapy in patients with an ongoing durable response can be difficult, and more studies are needed to outline the subset of patients who will benefit from ICI resumption. Careful delineation of goals of care should be discussed with patients before treatment planning because this will help in making decisions that are in line with each patient’s wishes and optimize the subsequent quality of life of already debilitated patients with cancer.1
In addition to the inherent fallacies of a retrospective design, the patient cohort was heterogeneous because our aim was to characterize the toxicity profile of ICI resumption. One limitation of such an approach is the inability to perform cancer-specific survival analyses. Second, no predetermined criteria were used to decide which patients could resume ICI therapy, and decisions relied on the clinical judgment of the individual treating physician. Third, because our cohort only consisted of patients who resumed ICI therapy, we did not account for patients who were deemed not to be candidates for ICI resumption for any reason, which reflects an inherent selection bias and limits the generalizability of our findings. Fourth, because the focus of our study was IMDC, determination of the characteristics and recurrence of non-GI irAEs was not attempted. Fifth, practice standards could have been different at the various institutions of this study and could have had an impact on our data. Finally, because of the limited sample size, multiple subgroup analyses may have been underpowered.
In conclusion, one third of patients who resumed ICI therapy after IMDC experienced recurrent IMDC that was mostly mild and could be managed adequately with immunosuppressive therapy. The lower risk of recurrent IMDC upon resumption of ICI is intriguing and suggests that ICI resumption in many patients is at least safe, especially anti–PD-1/L1 therapy. Caution should be practiced before resuming ICI therapy, especially in patients with severe initial IMDC. Future prospective studies with larger patient cohorts and predetermined criteria for resumption should validate the safety and efficacy of ICI resumption in the setting of IMDC.
ACKNOWLEDGMENT
Medical editing of this article was provided by the Department of Scientific Publications at MD Anderson Cancer Center. The biostatistical analysis was performed by Dr. Wei Qiao in the Department of Biostatistics at MD Anderson Cancer Center.
Appendix
TABLE A1.
Included Patients From Each Institution
Footnotes
Presented at the Society for Immunotherapy of Cancer 2018, Washington, DC, November 7-11, 2018.
Listen to the podcast by Dr Oh at http://ascopubs.org/jco/podcasts
See accompanying Oncology Grand Rounds on page 2714
AUTHOR CONTRIBUTIONS
Conception and design: Hamzah Abu-Sbeih, Yinghong Wang
Provision of study material or patients: Abdul Rafeh Naqash, Sandipkumar Patel, Gregory A. Otterson, Kari Kendra, Rita Chiari, Jarushka Naidoo, Yinghong Wang
Collection and assembly of data: Hamzah Abu-Sbeih, Abdul Rafeh Naqash, Dwight H. Owen, Sandipkumar Patel, Gregory A. Otterson, Kari Kendra, Biagio Ricciuti, Rita Chiari, Andrea De Giglio, Joseph Sleiman, Pauline Funchain, Beatriz Wills, Jiajia Zhang, Jarushka Naidoo
Data analysis and interpretation: Hamzah Abu-Sbeih, Faisal S. Ali, Sandipkumar Patel, Kari Kendra, Joseph Sleiman, Jiajia Zhang, Jarushka Naidoo, Jessica Philpott, Jianjun Gao, Sumit K. Subudhi, Yinghong Wang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Dwight H. Owen
Consulting or Advisory Role: L&M Policy Research, AstraZeneca
Research Funding: Bristol-Myers Squibb (Inst), Palobiofarma (Inst), Merck Sharp & Dohme (Inst), Genentech (Inst), Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Gregory A. Otterson
Consulting or Advisory Role: Novartis, Takeda Pharmaceuticals, Novocure
Research Funding: Genentech (Inst), Roche (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), Merck (Inst), AstraZeneca (Inst), Celgene (Inst)
Kari Kendra
Research Funding: Novartis (Inst), Bristol-Myers Squibb (Inst), GlaxoSmithKline (Inst), Karyopharm Therapeutics (Inst), Merck (Inst)
Rita Chiari
Speakers’ Bureau: Takeda Pharmaceuticals
Pauline Funchain
Consulting or Advisory Role: Eisai
Jarushka Naidoo
Honoraria: Bristol-Myers Squibb, AstraZeneca, MedImmune
Consulting or Advisory Role: Bristol-Myers Squibb, AstraZeneca, MedImmune, Roche, Genentech
Research Funding: Merck (Inst), Calithera Biosciences (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, AstraZeneca, MedImmune
Jianjun Gao
Consulting or Advisory Role: AstraZeneca, ARMO BioSciences, CRISPR Therapeutics, Jounce Therapeutics, Nektar, Polaris, Pfizer
Travel, Accommodations, Expenses: AstraZeneca
Sumit K. Subudhi
Stock and Other Ownership Interests: Apricity Health
Honoraria: Compugen, Apricity Health, Janssen Pharmaceuticals, Dendreon
Consulting or Advisory Role: Valeant, Dendreon, Apricity Health, Janssen Pharmaceuticals, Polaris
Travel, Accommodations, Expenses: Janssen Pharmaceuticals, Compugen, Dendreon
No other potential conflicts of interest were reported.
REFERENCES
- 1.Toi Y, Sugawara S, Kawashima Y, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncologist. 2018;23:1358–1365. doi: 10.1634/theoncologist.2017-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Sbeih H, Ali FS, Qiao W, et al. Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol Immunother. 2019;68:553–561. doi: 10.1007/s00262-019-02303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Sbeih H, Tang T, Ali FS, et al. The impact of immune checkpoint inhibitor-related adverse events and their immunosuppressive treatment on patients’ outcomes. J Immunother Precis Oncol. 2018;1:7–18. [Google Scholar]
- 6.Wang Y, Abu-Sbeih H, Mao E, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: Retrospective review at MD Anderson. J Immunother Cancer. 2018;6:37. doi: 10.1186/s40425-018-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen DH, Wei L, Bertino EM, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. 2018;19:e893–e900. doi: 10.1016/j.cllc.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian Y, Abu-Sbeih H, Wang Y. Immune checkpoint inhibitors-induced colitis. Adv Exp Med Biol. 2018;995:151–157. doi: 10.1007/978-3-030-02505-2_7. [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1186/s40425-018-0412-0. Johnson DH, Zobniw CM, Trinh VA, et al: Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer 6:103, 2018 [Erratum: J Immunother Cancer 7:107, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Sbeih H, Ali FS, Luo W, et al. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2018;6:95. doi: 10.1186/s40425-018-0411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Abu-Sbeih H, Mao E, et al. Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis. 2018;24:1695–1705. doi: 10.1093/ibd/izy104. [DOI] [PubMed] [Google Scholar]
- 13.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson JA. New NCCN guidelines: Recognition and management of immunotherapy-related toxicity. J Natl Compr Canc Netw. 2018;16:594–596. doi: 10.6004/jnccn.2018.0047. [DOI] [PubMed] [Google Scholar]
- 15.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29:250–255. doi: 10.1093/annonc/mdx642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Hodi FS, Ballinger M, Lyons B, et al. Immune-modified Response Evaluation Criteria in Solid Tumors (imRECIST): Refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018;36:850–858. doi: 10.1200/JCO.2017.75.1644. [DOI] [PubMed] [Google Scholar]
- 19.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, 2018. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
- 21.Blasig H, Bender C, Hassel JC, et al. Reinduction of PD1-inhibitor therapy: First experience in eight patients with metastatic melanoma. Melanoma Res. 2017;27:321–325. doi: 10.1097/CMR.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 22.Niki M, Nakaya A, Kurata T, et al. Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget. 2018;9:32298–32304. doi: 10.18632/oncotarget.25949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita K, Uchida N, Kanai O, et al. Retreatment with pembrolizumab in advanced non-small cell lung cancer patients previously treated with nivolumab: Emerging reports of 12 cases. Cancer Chemother Pharmacol. 2018;81:1105–1109. doi: 10.1007/s00280-018-3585-9. [DOI] [PubMed] [Google Scholar]
- 24.Hakozaki T, Okuma Y, Kashima J. Re-challenging immune checkpoint inhibitor in a patient with advanced non-small cell lung cancer: A case report. BMC Cancer. 2018;18:302. doi: 10.1186/s12885-018-4212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. doi: 10.1093/ibd/izy240. Choi K, Abu-Sbeih H, Samdani R, et al: Can immune checkpoint inhibitors induce microscopic colitis or a brand new entity? Inflamm Bowel Dis, 25:385-393, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Sbeih H, Ali FS, Alsaadi D, et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: A multi-center study. J Immunother Cancer. 2018;6:142. doi: 10.1186/s40425-018-0461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. doi: 10.1038/s41591-018-0238-9. Wang Y, Wiesnoski DH, Helmink BA, et al: Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med 24:1804-1808, 2018 [Erratum: Nat Med 25:188, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martini DJ, Hamieh L, McKay RR, et al. Durable clinical benefit in metastatic renal cell carcinoma patients who discontinue PD-1/PD-L1 therapy for immune-related adverse events. Cancer Immunol Res. 2018;6:402–408. doi: 10.1158/2326-6066.CIR-17-0220. [DOI] [PubMed] [Google Scholar]
- 29.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 30.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abu-Sbeih H, Ali FS, Wang X, et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor–induced colitis. J Immunother Cancer. 2019;7(1):93. doi: 10.1186/s40425-019-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]