Visual Abstract
Keywords: bipolar disorder, copy number variations, GABAergic neurons, glutamatergic neurons, induced pluripotent stem cells, schizophrenia
Abstract
Bipolar disorder (BP) and schizophrenia (SCZ) are major psychiatric disorders, but the molecular mechanisms underlying the complicated pathologies of these disorders remain unclear. It is difficult to establish adequate in vitro models for pathological analysis because of the heterogeneity of these disorders. In the present study, to recapitulate the pathologies of these disorders in vitro, we established in vitro models by differentiating mature neurons from human induced pluripotent stem cells (hiPSCs) derived from BP and SCZ patient with contributive copy number variations, as follows: two BP patients with PCDH15 deletion and one SCZ patient with RELN deletion. Glutamatergic neurons and GABAergic neurons were induced from hiPSCs under optimized conditions. Both types of induced neurons from both hiPSCs exhibited similar phenotypes of MAP2 (microtubule-associated protein 2)-positive dendrite shortening and decreasing synapse numbers. Additionally, we analyzed isogenic PCDH15- or RELN-deleted cells. The dendrite and synapse phenotypes of isogenic neurons were partially similar to those of patient-derived neurons. These results suggest that the observed phenotypes are general phenotypes of psychiatric disorders, and our in vitro models using hiPSC-based technology may be suitable for analysis of the pathologies of psychiatric disorders.
Significance Statement
Useful in vitro models of psychiatric disorders such as schizophrenia and bipolar disorder are urgently required for pathological analysis and drug discovery. In this study, mature excitatory and inhibitory neurons were induced from patient-derived induced pluripotent stem cells. The patient-derived induced neurons exhibited abnormalities in dendrite and synapse formation in vitro, which are similar to the previously reported findings observed in postmortem brains. Our in vitro model may reflect general phenotypes of psychiatric disorders and can be used to further examine therapeutic targets.
Introduction
Both bipolar disorder (BP) and schizophrenia (SCZ) are chronic and debilitating psychiatric disorders that affect ∼1% of the worldwide population (McGrath et al., 2008; Grande et al., 2016). Although these disorders are highly heritable (Craddock and Sklar, 2013; Millan et al., 2016), the molecular mechanisms underlying the complex pathology of these disorders remain to be elucidated.
There are limitations to the recapitulation of clinical characteristics in animal models and postmortem brain studies because of genetic heterogeneity (O'Shea and McInnis, 2016; Prytkova and Brennand, 2017). Therefore, reliable models that functionally mimic live human brains are sought after. Induced pluripotent stem cells (iPSCs) are expected to become a promising tool for recapitulating disease-specific phenotypes in vitro (Okano and Yamanaka, 2014; O'Shea and McInnis, 2016; Watmuff et al., 2016; Prytkova and Brennand, 2017; Tobe et al., 2017). Although recent studies established iPSCs from BP and SCZ patients and induced neurons to analyze phenotypes (O'Shea and McInnis, 2016; Prytkova and Brennand, 2017; Wen, 2017), the maturity and subtype specificity of induced neurons remain to be considered. Thus, analysis of mature and subtype-specific neurons is required for further elucidation of the pathologies. It has been suggested that the collapse of the excitation–inhibition (E/I) balance plays key roles in BP and SCZ (Gao and Penzes, 2015; Lee et al., 2018). Therefore, it is important to focus on certain neurons that are the main players in the E/I balance, such as glutamatergic neurons and GABAergic neurons. Recent studies have shown that transcription factor overexpression enabled iPSCs to be differentiated into specific neurons, including glutamatergic neurons (Zhang et al., 2013) and GABAergic neurons (Colasante et al., 2015; Yang et al., 2017).
Many genetic mutations are associated with these disorders, especially copy number variations (CNVs), which are important contributive factors that affect the onset and treatment resistance of BP and SCZ (Georgieva et al., 2014; Green et al., 2016; Kushima et al., 2017). Thus, to analyze the pathologies, we used iPSC lines derived from patients who carried certain CNVs: two BP patients with PCDH15 exonic deletions and an SCZ patient who carried an RELN exonic deletion. Protocadherin 15 (PCDH15), encoded by PCDH15, is a member of the cadherin superfamily. PCDH15 mutations cause Usher syndrome, which results in hearing vision loss (Ahmed et al., 2001; Alagramam et al., 2001; Kim et al., 2011). A recent genome-wide association study suggested that PCDH15 is associated with psychiatric disorders (Lo et al., 2017). In addition, de novo or rare exonic deletions in PCDH15 were identified in BP patients (Georgieva et al., 2014; Noor et al., 2014). These studies suggested that PCDH15 is a risk gene for psychiatric disorders. Reelin, which is encoded by RELN, is an extracellular matrix protein that regulates brain developmental processes, such as neuronal migration and dendrite formation, and modulates synaptic functions in adult brains (Ishii et al., 2016; Lee and D'Arcangelo, 2016). Reelin is associated with various psychiatric disorders such as SCZ, autism spectrum disorders, and BP (Folsom and Fatemi, 2013; Ishii et al., 2016). Two SCZ patients with exonic deletions in RELN have been reported in previous studies (Costain et al., 2013; Kushima et al., 2017).
In this study, to recapitulate the pathologies in BP and SCZ in vitro, we differentiated patient-derived iPSCs into neurons and identified a set of similar phenotypes among induced neurons derived from patients with different genetic lesions, which potentially represents a cell biological basis for shared clinical features between these two different disorders.
Materials and Methods
Subjects
A human subject was recruited at Keio University, and another from Kyushu University. Written informed consent for the present study was obtained from this patient. All the experimental procedures for biopsy, genetic analysis, and iPSC production were approved by the Keio University School of Medicine Ethics Committee (approval #20080016), the Ethics Committee of Nagoya University (approval #2010-1033-3 and #2012-0184-14), and the Ethics Committee of Kyushu University (approval #IRB-30-537).
We recruited two patients with BP. The first patient (BP1) was a 43-year-old Japanese female with a family history of alcohol use disorder. Her developmental history was unremarkable. In her early thirties, she had depressive moods and suicidal thoughts. Although she was treated with antidepressant medications, a significant treatment effect was not observed. After 3 years, she received a diagnosis of bipolar I disorder because she had her first manic episode, which included an elevated mood, increased activity and talkativeness, and decreased need for sleep. In her late thirties, she became depressed again after she received a diagnosis of a malignant tumor. Her depressive symptoms were chronic and refractory to pharmacological treatments, requiring multiple hospitalizations. At the time of the study evaluation, she was receiving treatment with lithium and quetiapine.
The second patient (BP2) was a 57-year-old Japanese female with no remarkable developmental history and no family history of psychiatric disorders. She had been committed to various social activities and was sometimes hyperactive until her middle thirties (hypomanic episodes). Since her late thirties, she had experienced insomnia, depressive moods, and severe suicidal thoughts two or three times a year, requiring multiple hospitalizations. Despite treatment with antidepressant medications, a considerable treatment effect was not observed. After she had received a diagnosis of bipolar I disorder based on her life history and hyperactivity between depressive episodes, lithium was prescribed. However, the response was still poor. At age 50 years, lithium was completely replaced with valproate and lamotrigine. After that, her mood stabilized and no subsequent hospitalizations were necessary. At the time of the study evaluation, she was still receiving valproate and lamotrigine. Although she had once received a diagnosis of bipolar I disorder, the diagnosis was revised to bipolar II disorder because of the lack of severe manic episodes.
iPSC generation and culture
The 1210B2 (Nakagawa et al., 2014), 201B7 (Takahashi et al., 2007), WD39 (Imaizumi et al., 2012), 414C2 (Okita et al., 2011), eKA3 (Matsumoto et al., 2016), eTKA4 (Matsumoto et al., 2016), and NC1032-1-2 cell lines were used as the healthy control human iPSC lines. The NC1032-1-2 line was established from cells obtained from a healthy 30-year-old Japanese woman using episomal vectors as previously described (Okita et al., 2013), which was then used for establishment of the isogenic PCDH15-deleted iPSC line. iPSC lines derived from an SCZ patient with heterozygous RELN deletion (SCZ1-1, SCZ1-2) were established in a previous study (Arioka et al., 2018). BP patient-derived iPSCs (BP-iPSCs) were generated by a previously reported method (Okita et al., 2013; Hosoya et al., 2017). Briefly, episomal plasmids encoding six factors (OCT4, SOX2, KLF4, LIN28, L-MYC, EBNA1, and dominant-negative p53) were transduced into human peripheral blood mononuclear cells obtained from the patient. Established iPSCs were evaluated based on the expression of pluripotent markers, episomal transgene elimination, and the absence of clinically significant CNVs.
iPSCs were cultured with or without feeder cells. For on-feeder culture, iPSCs were grown on mitomycin-C-treated SNL murine fibroblast feeder cells in standard human pluripotent stem cell medium (DMEM/F12 medium, FUJIFILM Wako Pure Chemical) containing 20% KnockOut Serum Replacement (KSR; Thermo Fisher Scientific), 0.1 mm nonessential amino acids (NEAAs; Merck), 0.1 mm 2-mercaptoethanol (Merck), and 4 ng/ml fibroblast growth factor 2 (PeproTech) at 37°C in an atmosphere containing 3% CO2. For the feeder-free culture, iPSCs were grown on tissue culture dishes coated with 1 μg/ml iMatrix-511 (Laminin-511 E8, Nippi). iPSCs were cultured in StemFit AK02N (Ajinomoto) at 37°C in an atmosphere containing 5% CO2.
Identification and validation of an exonic deletion of PCDH15
Genomic DNA was extracted from blood or iPSC lines. To identify an exonic deletion of PCDH15, we performed array comparative genomic hybridization (aCGH) using an SurePrint G3 Human CGH Microarray, 2 × 400K (Agilent). The experiment was performed following the manufacturer instructions. CNV calls were made with Nexus Copy Number software version 9.0 (BioDiscovery) using the Fast Adaptive States Segmentation Technique 2 algorithm, which is a hidden Markov model-based approach. The log2 ratio thresholds for copy number loss and copy number gain were set at −0.7 and 0.45, respectively. The significance threshold p value was set at 1 × 10−6, and at least four contiguous probes were required for CNV calls. To validate the exonic deletion of PCDH15, quantitative real-time PCR was performed with TaqMan copy number assay (Hs03733966_cn for BP1 and Hs00117569_cn for BP2; Applied Biosystems). All genomic locations are given in NCBI36/hg18.
In vitro three-germ differentiation via embryoid body formation
To check the pluripotency of iPSCs, iPSCs treated with TrypLE Select (Thermo Fisher Scientific) were dissociated into single cells and plated in low-cell adhesion 96-well plates with V-bottomed conical wells. The cells were cultured in embryoid body (EB) medium (DMEM/F-12 containing 5% KSR, 2 mm l-glutamine, 1% NEAAs, and 0.1 mm 2-ME). On day 7, EBs were plated on culture plates coated with 0.1% gelatin (Merck) and 10 μg/ml fibronectin (Merck). The plated EBs were cultured for 3 weeks at 37°C in an atmosphere containing 5% CO2.
qRT-PCR
RNA was extracted from the cells using the RNeasy Kit (Qiagen). One hundred nanograms of RNA was used to prepare cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). qRT-PCR was performed with SYBR Premix Ex TaqII (TaKaRa Bio) on the ViiA 7 real-time PCR system (Thermo Fisher Scientific). Values were normalized to GAPDH levels. The comparative (ΔΔCt) method was used to analyze the data. Relative expression levels are presented as geometric means ± geometric SDs. The primers used for qRT-PCR were as follows: GAPDH: forward, AATCCCATCACCATCTTCCA; reverse, TGGACTCCACGACGTACTCA; PCDH15 (set1): forward, GAGGCAGCCTTGGCAAGAAA; reverse, CTGTCGAAACATCTTCTGTCAAAGT; PCDH15 (set2): forward, GGGACCATGGTTGGTGTAAT; reverse, CACCTGTGATGTTATTAATTCCAAA; and RELN: forward, AGAAGGACAAGACTCACAATGCT; reverse, GCTTCACAACCCACCACAAT.
Neuron differentiation via dual SMAD inhibitor-treated EB formation
For dual SMAD inhibitor-treated EB (DSi-EB) formation, the cells were cultured in medium hormone mix (MHM; Shimazaki et al., 2001; Okada et al., 2004) containing B27 supplement (Thermo Fisher Scientific) with 5 μm SB431542 (Tocris Bioscience), 150 nm LDN193189 (StemRD), and 2.5 μm IWP-2 (Merck; Imaizumi et al., 2015). On day 7, DSi-EBs were plated on culture plates coated with Matrigel (Corning). The medium was replaced every 3 d.
Plasmids construction
The NEUROG2 expression vector (PB-TET-PH-lox66FRT-NEUROG2; Matsushita et al., 2017) was a gift from Dr. Minoru S.H. Ko (Keio Uniersity,Tokyo, Japan). ASCL1 and DLX2 expression vectors [PB-P(tetO)-hAscl1-pA-PGK-PuroTK-pA and PB-P(tetO)-hDLX2-pA-floxPGKneo-pA] were established by Gateway cloning (Thermo Fisher Scientific). First, three plasmids with att-cloning sites (pProF-PHtetO2, pMK-pA-PGKpacTK-pA1, pMK-pA-floxPGKneo-pA) were constructed according to the manufacturer protocol for the MultiSite Gateway Three-Fragment Vector Construction Kit (Thermo Fisher Scientific) from pDONR-P4P1r and pDONR-P2rP3. Primer sequences and templates are listed below, as follows:
pProF-PHtetO2 (template: PB-TET-PH; Matsushita et al., 2017), primers: ggggACAACTTTGTATagaaaaGTTGttaattaagtcgacATTAAGTTGGGTAACGCCAGGG, ggggACTGCTTTTTTGTACAAACTTgcgatcgcGATGGCCGCCACCGCGGAGGC); pMK-pA-PGKpacTK-pA1 (template: pUC19PGKpacDeltaTKpA), primers: ggggACAGCTTTCTTGTACAAAGTGGTTAATTAAGGATCGGCAATAAAAAGACAGAATAAAACGCACGGGTGTTGGGTCGT, gggACAACTTTGTATAATAAAGTTGCCCGGGTGCATGCCTGCAGGTCGACTCTAGA);pMK-pA-floxPGKneo-pA (template: pL452), primers: ggggACAGCTTTCTTGtacaaaGTGGggatcggcaataaaaagacagaataaaacgcacgggtgttgggtcgtttgttcGTCGACCTGCAGCCAAGCTATCGAATTCC, ggggACAACtttgtaTAATAAAGTTGgcggccgcTCTAGAACTAGTGGATCCCC).
Then, the entry vector pENTR-L4-P(tetO)-R1 was established from pProF-PHtetO2 by restriction enzyme digestion with PacI (New England Biolabs Japan) and DraI (TaKaRa Bio). The destination vector PB-DEST-R4R3 was established from the PB-TET plasmid (Addgene). PB-TET was digested with SalI (TaKaRa) and XbaI (TaKaRa) to be ligated with a SpeI-XhoI-digested ccdB fragment. Then, the two expression vectors were constructed by three-fragment LR cloning using the LR Clonase II enzyme (Thermo Fisher Scientific). PB-P(tetO)-hAscl1-pA-PGK-PuroTK-pA was constructed from four plasmids, as follows: pENTR-L4-P(tetO)-R1, pENTR221-hASCL1 (DNAFORM), pMK-pA-PGKpacTK-pA1, and PB-DEST-R4R3. PB-P(tetO)-hDLX2-pA-floxPGKneo-pA was also constructed from the following four plasmids: pENTR-L4-P(tetO)-R1, pENTR/D-hDLX2 (RIKEN BRC), pMK-pA-floxPGKneopA, and PB-DEST-R4R3.
Neuronal differentiation by transcription factor overexpression
Based on a previous study (Matsushita et al., 2017), NEUROG2-inducible iPSCs were established using the following vectors: PB-TET-PH-lox66FRT-NEUROG2, pCMV-HyPBase-PGK-Puro (a gift from Dr. Kosuke Yusa, Wellcome Sanger Institute, Hinxton, U.K.), and PB-CAGrtTA3G-IH. These vectors were cotransfected into iPSCs using Gene Juice Transfection Reagent (Merck). The transfectants were cultured in StemFit AK02N containing 450 μg/ml hygromycin (FUJIFILM Wako Pure Chemical) and 0.1-1.0 μg/ml puromycin (Merck). To establish ASCL1- and DLX2-inducible iPSCs, we used the following vectors: PB-P(tetO)-hAscl1-pA-PGK-PuroTK-pA, PB-P(tetO)-hDLX2-pA-floxPGKneo-pA, pCMV-HyPBase-PGK-Puro, and PB-CAGrtTA3G-IH. These vectors were cotransfected into iPSCs using Gene Juice Transfection Reagent (Merck). The transfectants were cultured in StemFit AK02N containing 450 μg/ml hygromycin (FUJIFILM Wako Pure Chemical), 0.1-1.0 μg/ml puromycin (Merck), and 100 μg/ml G418 (nacalai tesque).
Feeder-free cultured iPSCs were used for the induction of neuronal differentiation. The cells were dissociated and seeded on culture dishes coated with poly-l-lysine, iMatrix-511 (Laminin-511 E8), and Laminin (R&D Systems). To induce glutamatergic neurons, NEUROG2-transduced cells were cultured in induction medium [MHM+B27 containing Y27632, FUJIFILM Wako Pure Chemical; 10 μm DAPT, Merck; 2.5 μm IWP-2; and 2 μg/ml doxycycline (Dox), FUJIFILM Wako Pure Chemical] on day 0. For GABAergic neuron induction, ASCL1- and DLX2-transfected cells were cultured in induction medium with 80 ng/ml recombinant human sonic hedgehog (R&D Systems). On day 5, the medium was replaced with neuron culture medium [MHM+B27 containing 20 ng/ml brain-derived neurotrophic factor (BDNF), R&D Systems; 10 ng/ml glial cell line-derived neurotrophic factor (GDNF), Alomone Labs; 200 μm l-ascorbic acid, Merck; and 100 μm dibutyryl cyclic adenosine monophosphate, Merck] or function assay medium (BrainPhys Neuronal Medium and N2-A/SM1, STEMCELL Technologies; containing 20 ng/ml BDNF, 20 ng/ml GDNF, 200 μm l-ascorbic acid, and 1 mm dibutyryl cyclic adenosine monophosphate) . Treatment with 2 μm PD0332991 isethionate (Merck) was also performed for both types of neuron induction from day 5 to day 21. The medium was replaced every 4 d after day 5.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 20 min at room temperature and then incubated with blocking buffer (PBS containing 2% normal fetal bovine serum, 2% normal goat serum, 2% bovine serum albumin, and 0.2% Triton X-100) for 1 h at room temperature. Then, the cells were incubated overnight at 4°C with primary antibodies diluted with blocking buffer without Triton X-100. The cells were washed three times with PBS and then incubated with secondary antibodies with Alexa Fluor 488, Alexa Fluor 555, or Alexa Fluor 647 (1:1000; Thermo Fisher Scientific) and Hoechst 33342 (Merck) for 1–2 h at room temperature. After washing with PBS, the cells were examined with a BZ-9000 microscope (Keyence) and an IN Cell Analyzer 6000 (GE Healthcare). The following antibodies were used as the primary antibodies in this study: Oct4 (1:500; mouse, catalog #sc-5279, Santa Cruz Biotechnology), Nanog (1:500; rabbit, RCAB0004PF, REPLPCELL), SSEA4 (1:500; mouse, catalog #ab16287, Abcam), Tra1-60 (1:500; mouse, MAB4360, Millipore), βIII tubulin (1:500; mouse, T8660, Sigma-Aldrich), α-fetoprotein (AFP; 1:250; mouse, MAB1368, R&D Systems), α-smooth muscle actin (αSMA; 1:300; mouse, A2547, Sigma-Aldrich), microtubule-associated protein 2 (MAP2; 1:1000; rabbit, M4403, Merck), MAP2 (1:1000; mouse, AB5622, Millipore), vesicular glutamate transporter 2 (VGluT2; 1:500; mouse, ab79157, Abcam), GABA (1:500; rabbit, A2052, Sigma-Aldrich), Synapsin I (1:2000; rabbit, S193, Sigma-Aldrich), Homer I (1:500; mouse, 2a8, Synaptic Systems), and Gephyrin (1:500; mouse, 147111, Synaptic Systems).
High-content image analysis
For morphologic analysis, 96-well plates with V-bottomed conical wells containing 1 EB in each well were imaged on an IN Cell Analyzer 6000 using the 2× objective. EBs were identified based on the contrast with the background; from the EB images obtained, the area and form factor were calculated using IN Cell Developer Toolbox version 1.9 (GE Healthcare).
For fluorescence intensity analysis and neurite length analysis of the EBs, stained plates were imaged on an IN Cell Analyzer 6000 high-content cellular analysis system; only the field containing the EB was selectively collected from each well using the 2× objective, resulting in 1 EB being scored per well. For cell population assays and dendrite length analysis, stained plates were imaged with an IN Cell Analyzer 6000; a set of 5 × 5 fields was collected from each well using the 20× objective. For quantitative analysis of the synaptic puncta, stained plates were imaged with an IN Cell Analyzer 6000; a set of 6 × 6 fields was collected from each well using the 60× objective.
Analysis using IN Cell Developer Toolbox version 1.9 (GE Healthcare) began by identifying intact nuclei stained by the Hoechst dye, which were defined as traced nuclei that were >50 μm2 in surface area and with intensity levels that were typical and lower than the threshold brightness of pyknotic cells. Each traced nuclear region was then expanded by 50% and cross-referenced with an endodermal marker (AFP), a mesodermal marker (αSMA), and an ectodermal marker (βIII tubulin) for identification; from these images, the fluorescence intensity of each marker was calculated. Using the expanded nuclear region images, neuron markers (βIII tubulin and MAP2), a glutamatergic neuron marker (VGluT2), and a GABAergic neuron marker (GABA) were identified; from these images, the percentage of each marker was calculated (for neural subtype markers stained as granules, the number of βIII tubulin + cells containing one or more of the markers was quantified). Using the above-described traced neuronal images of each cell, neurite length, dendrite length, and the number of synaptic puncta (Homer I+, Gephyrin+, and/or Synapsin I+ puncta) were also analyzed.
Microarray analysis
RNA was extracted with the RNeasy Kit (QIAGEN), and the RNA quality and quantity were assessed using a bioanalyzer (model 2100, Agilent Technologies). Microarray analysis was performed using the Clariom S Assay for humans (Thermo Fisher Scientific). The accession number was referred later. Data were analyzed by Transcriptome Analysis Console 4.0 (Thermo Fisher Scientific). Gene ontology (GO) and pathway analyses were performed using DAVID 6.8 Bioinformatics Resources (https://david.ncifcrf.gov). The data shown in this publication have been deposited in the NCBI Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/). The accession number of our microarray analysis data was GSE116820.
Genetic engineering of PCDH15-mutant iPSCs
PCDH15-deleted iPSCs were established using the CRISPR/Cas9 system, as previously described (Arioka et al., 2018). In brief, single-guide RNA (sgRNA) expression vectors were constructed using pHL-H1-ccdB-mEF1α-RiH vector (catalog #60601, Addgene) with two oligos containing the sgRNA target site and a universal reverse primer (Table 1). The T7 endonuclease I (T7E1) assay was performed using genomic DNA from HEK293FT cells co-transfected with the sgRNA expression vector and Cas9 expression vector (Addgene ID: 60599) to assess sgRNA activity. The target region of sgRNA was amplified from the genomic DNA using a specific primer set (Table 1). The PCR products were digested using T7E1 (New England Biolabs) after denaturing and reannealing. To establish the isogenic PCDH15-deleted iPSC line, sgRNA and Cas9 expression vectors were cotransfected into healthy control iPSCs, NC1032-1-2 (Control 1) and cells were selected with puromycin.
Table 1.
Used primers for generating isogenic PCDH15 deletion iPSCs
| Targets | Primers | Sequence (5´ → 3´) |
|---|---|---|
| sgRNAs construction | sgRNA#1-Fw | GAGACCACTTGGATCCGGACGGCAATCACGAGTGTTGTTTTAGAGCTAGAAATAGCA |
| sgRNA#2-Fw | GAGACCACTTGGATCCGTCGCCTCTCATTCAGATTTGTTTTAGAGCTAGAAATAGCA | |
| sgRNA#3-Fw | GAGACCACTTGGATCCGTGGCAGCTTGATAAGTGAGGTTTTAGAGCTAGAAATAGCA | |
| sgRNA#4-Fw | GAGACCACTTGGATCCGCGCCTCTCATTCAGATTTTGTTTTAGAGCTAGAAATAGCA | |
| sgRNA#5-Fw | GAGACCACTTGGATCCGCTCATTCAGATTTTGGGCAGTTTTAGAGCTAGAAATAGCA | |
| sgRNA#universal-Rv | GCCCGGGTTTGAATTCAAAAAAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAA | |
| T7E1 assay | Fw | CTCAGTTTACATCCTGACTCAACCAC |
| Rv | CCTTCAAACGGCCAAACATAATCTCC |
Sequence information of the primers for sgRNAs construction and T7E1 assay. Fw, Forward primer; Rv, reverse primer. Also see Materials and Methods.
Microelectrode array analysis
The microelectrode array (MEA) was assayed using the Maestro system (Axion Biosystems). Neuronal inductions from iPSCs were performed in 48-well MEA plates coated with poly-l-lysine, iMatrix-511, and laminin. For neuronal differentiation, iPSCs were cultured in induction medium for the first 5 d and then in function assay medium from day 5 until day 28 or day 42. Data were acquired using a sampling rate of 12.5 kHz and filtered using a 200–3000 Hz Butterworth bandpass filter. The detection threshold was set to +6.0 × SD of the baseline electrode noise. Five minutes of activity was subsequently recorded at 37°C. Given the considerable variation in spike count between wells and plates, we focused on active electrodes (electrodes with an average of >5 spikes/min; Isoda et al., 2016), which were certain to make contact with neurons. The number of active electrodes and the mean spike rate per active electrode (spikes per second) was calculated using the spike count file generated by the Axion Integrated Studio program (Axion Biosystems). The spike raster plot was generated by Neural Metric Tool (Axion Biosystems). To determine the responses to the agonist or antagonists of glutamate or the GABA receptor, 50 µm CNQX (Tocris Bioscience), 50 µm AP-5 (Alomone Labs), or 10 µm GABA (nacalai tesque) was applied as treatment, and the activity was recorded.
Calcium imaging
Neurons were induced from iPSCs by culture in induction medium for 5 d followed by function assay medium from day 5. Calcium imaging analyses were performed on days 28–30. Cells were loaded with 1 μg/ml fluo-8 AM (AAT Bioquest) in recording medium (20 mm HEPES, 115 mm NaCl, 5.4 mm KCl, 0.8 mm MgCl2, 1.8 mm CaCl2, and 13.8 mm glucose; Dojindo) containing 0.02% Cremophor EL (Dojindo) and incubated for 20 min at 37°C and 5% CO2. After washing with PBS, the medium was changed to the recording medium. Changes in fluorescence intensity were measured using an IX83 inverted microscope (Olympus) equipped with an Electron Multiplying CCD Camera (Hamamatsu Photonics) and LED illumination system pE-4000 (CoolLED). We recorded 3500 frames (1 frame: 31–32 ms) per well using the stream acquisition mode. MetaMorph Image Analysis Software (Molecular Devices) was used to analyze the live cell calcium traces. Regions of interest (ROIs) were drawn on cells based on time projection images of the recordings. ROI traces of the time course of changes in fluorescence intensity were generated and used as substrates for subsequent analyses. To adjust for photobleaching, the difference in intensity between the first frame and last frame was calculated and subtracted from the raw intensity. The change in fluorescence intensity over time was normalized as ΔF/F = (F − F0)/F0, where F0 is fluorescence at the starting point of exposure (time 0). ΔFmax was defined as the difference of the largest change of ΔF/F associated with each calcium spike.
Statistical analysis
Statistical analyses were performed using a Dunnett’s or Tukey’s test for multiple comparisons. For comparison between two groups, an unpaired Student’s t test or a paired t test was used. All statistical analyses were performed using SAS 9.2 (SAS Institute). Probability values (p value) <0.05 were considered to be statistically significant. Statistical test results are included in Table 2.
Table 2.
p Values of the indicated statistical comparisons
| Figures | Measurement | Type of test | comparison | p Value |
|---|---|---|---|---|
| Figure 2C | EB size (EB) | Dunnett’s test | Control vs BP1 | 0.4406 |
| Control vs SCZ1 | 0.5982 | |||
| EB size (DSi-EB) | Dunnett’s test | Control vs BP1 | 0.5619 | |
| Control vs SCZ1 | 0.9784 | |||
| Figure 2D | EB form factor (EB) | Dunnett’s test | Control vs BP1 | 0.5015 |
| Control vs SCZ1 | 0.7476 | |||
| EB form factor (DSi-EB) | Dunnett’s test | Control vs BP1 | 0.0662 | |
| Control vs SCZ1 | 0.0562 | |||
| Figure 2E | PCDH15 gene expression | Dunnett’s test | Control vs BP1 | 0.3861 |
| Control vs SCZ1 | 0.3897 | |||
| Reln gene expression | Control vs BP1 | 0.7288 | ||
| Control vs SCZ1 | 0.5178 | |||
| Figure 2G | Intensity/area (βIII tubulin) | Dunnett’s test | Control vs BP1 | 0.9826 |
| Control vs SCZ1 | 0.2543 | |||
| Intensity/area (αSMA) | Dunnett’s test | Control vs BP1 | 0.9778 | |
| Control vs SCZ1 | 0.9238 | |||
| Intensity/area (AFP) | Dunnett’s test | Control vs BP1 | 0.7731 | |
| Control vs SCZ1 | 0.9345 | |||
| Figure 2J | Neurite length (day 10) | Dunnett’s test | Control vs BP1 | 0.7746 |
| Control vs SCZ1 | 0.8824 | |||
| Neurite length (day13) | Dunnett’s test | Control vs BP1 | 0.0293 | |
| Control vs SCZ1 | 0.0324 | |||
| Neurite length (day16) | Dunnett’s test | Control vs BP1 | 0.0192 | |
| Control vs SCZ1 | 0.01 | |||
| Neurite length (day22) | Dunnett’s test | Control vs BP1 | 0.0036 | |
| Control vs SCZ1 | 0.0025 | |||
| Figure 3C | No. of cells (βIII tubulin+/HO+) | Tukey’s test | 1210B2 vs 201B7 | 0.9993 |
| 1210B2 vs BP1-1 | 1 | |||
| 1210B2 vs BP1-2 | 1 | |||
| 1210B2 vs BP2-1 | 0.0218 | |||
| 1210B2 vs SCZ1-1 | 0.5216 | |||
| 1210B2 vs SCZ1-2 | 0.875 | |||
| 201B7 vs BP1-1 | 0.9975 | |||
| 201B7 vs BP1-2 | 0.9989 | |||
| 201B7 vs BP2-1 | 0.0036 | |||
| 201B7 vs SCZ1-1 | 0.2079 | |||
| 201B7 vs SCZ1-2 | 0.5352 | |||
| BP1-1 vs BP1-2 | 1 | |||
| BP1-1 vs BP2-1 | 0.0077 | |||
| BP1-1 vs SCZ1-1 | 0.4016 | |||
| BP1-1 vs SCZ1-2 | 0.8143 | |||
| BP1-2 vs BP2-1 | 0.0042 | |||
| BP1-2 vs SCZ1-1 | 0.3116 | |||
| BP1-2 vs SCZ1-2 | 0.7283 | |||
| BP2-1 vs SCZ1-1 | 0.5238 | |||
| BP2-1 vs SCZ1-2 | 0.1306 | |||
| SCZ1-1 vs SCZ1-2 | 0.9847 | |||
| No. of cells (MAP2+/βIII tubulin+) | Tukey’s test | 1210B2 vs 201B7 | 1 | |
| 1210B2 vs BP1-1 | 0.6317 | |||
| 1210B2 vs BP1-2 | 0.9984 | |||
| 1210B2 vs BP2-1 | 0.6973 | |||
| 1210B2 vs SCZ1-1 | 0.9988 | |||
| 1210B2 vs SCZ1-2 | 0.9994 | |||
| 201B7 vs BP1-1 | 0.7153 | |||
| 201B7 vs BP1-2 | 1 | |||
| 201B7 vs BP2-1 | 0.7806 | |||
| 201B7 vs SCZ1-1 | 1 | |||
| 201B7 vs SCZ1-2 | 1 | |||
| BP1-1 vs BP1-2 | 0.7807 | |||
| BP1-1 vs BP2-1 | 1 | |||
| BP1-1 vs SCZ1-1 | 0.8539 | |||
| BP1-1 vs SCZ1-2 | 0.7703 | |||
| BP1-2 vs BP2-1 | 0.8448 | |||
| BP1-2 vs SCZ1-1 | 1 | |||
| BP1-2 vs SCZ1-2 | 1 | |||
| BP2-1 vs SCZ1-1 | 0.8955 | |||
| BP2-1 vs SCZ1-2 | 0.8325 | |||
| SCZ1-1 vs SCZ1-2 | 1 | |||
| No. of cells (VGluT2+βIII tubulin+/βIII tubulin+) | Tukey’s test | 1210B2 vs 201B7 | 0.9629 | |
| 1210B2 vs BP1-1 | 0.2892 | |||
| 1210B2 vs BP1-2 | 0.9885 | |||
| 1210B2 vs BP2-1 | 0.8416 | |||
| 1210B2 vs SCZ1-1 | 0.6553 | |||
| 1210B2 vs SCZ1-2 | 0.9756 | |||
| 201B7 vs BP1-1 | 0.7818 | |||
| 201B7 vs BP1-2 | 0.9999 | |||
| 201B7 vs BP2-1 | 0.9996 | |||
| 201B7 vs SCZ1-1 | 0.9859 | |||
| 201B7 vs SCZ1-2 | 1 | |||
| BP1-1 vs BP1-2 | 0.5003 | |||
| BP1-1 vs BP2-1 | 0.9484 | |||
| BP1-1 vs SCZ1-1 | 0.9956 | |||
| BP1-1 vs SCZ1-2 | 0.6512 | |||
| BP1-2 vs BP2-1 | 0.9885 | |||
| BP1-2 vs SCZ1-1 | 0.9093 | |||
| BP1-2 vs SCZ1-2 | 1 | |||
| BP2-1 vs SCZ1-1 | 0.9998 | |||
| BP2-1 vs SCZ1-2 | 0.9975 | |||
| SCZ1-1 vs SCZ1-2 | 0.9616 | |||
| Figure 3D | PCDH15 gene expression (iPS) | Dunnett’s test | Control vs BP | 0.9527 |
| Control vs SCZ | 0.9354 | |||
| PCDH15 gene expression (neuron) | Dunnett’s test | Control vs BP | 0.2862 | |
| Control vs SCZ | 0.4102 | |||
| RELN gene expression (iPS) | Dunnett’s test | Control vs BP | 0.1826 | |
| Control vs SCZ | 0.3746 | |||
| RELN gene expression (neuron) | Dunnett’s test | Control vs BP | 0.6918 | |
| Control vs SCZ | 0.9941 | |||
| Figure 3G | No. of cells (βIII tubulin+/HO+) | Tukey’s test | 1210B2 vs 201B7 | 0.98 |
| 1210B2 vs BP1-1 | 0.9914 | |||
| 1210B2 vs BP1-2 | 0.9863 | |||
| 1210B2 vs BP2-1 | 0.4556 | |||
| 1210B2 vs SCZ1-1 | 1 | |||
| 1210B2 vs SCZ1-2 | 0.9992 | |||
| 201B7 vs BP1-1 | 0.7241 | |||
| 201B7 vs BP1-2 | 1 | |||
| 201B7 vs BP2-1 | 0.1395 | |||
| 201B7 vs SCZ1-1 | 0.9894 | |||
| 201B7 vs SCZ1-2 | 0.9993 | |||
| BP1-1 vs BP1-2 | 0.7192 | |||
| BP1-1 vs BP2-1 | 0.7873 | |||
| BP1-1 vs SCZ1-1 | 0.9536 | |||
| BP1-1 vs SCZ1-2 | 0.8677 | |||
| BP1-2 vs BP2-1 | 0.1102 | |||
| BP1-2 vs SCZ1-1 | 0.9937 | |||
| BP1-2 vs SCZ1-2 | 0.9998 | |||
| BP2-1 vs SCZ1-1 | 0.2366 | |||
| BP2-1 vs SCZ1-2 | 0.1646 | |||
| SCZ1-1 vs SCZ1-2 | 0.9999 | |||
| No. of cells (MAP2+/βIII tubulin+) | Tukey’s test | 1210B2 vs 201B7 | 0.9997 | |
| 1210B2 vs BP1-1 | 0.9897 | |||
| 1210B2 vs BP1-2 | 0.9829 | |||
| 1210B2 vs BP2-1 | 0.5806 | |||
| 1210B2 vs SCZ1-1 | 0.9812 | |||
| 1210B2 vs SCZ1-2 | 0.9842 | |||
| 201B7 vs BP1-1 | 1 | |||
| 201B7 vs BP1-2 | 0.9998 | |||
| 201B7 vs BP2-1 | 0.8884 | |||
| 201B7 vs SCZ1-1 | 0.9037 | |||
| 201B7 vs SCZ1-2 | 0.9149 | |||
| BP1-1 vs BP1-2 | 1 | |||
| BP1-1 vs BP2-1 | 0.902 | |||
| BP1-1 vs SCZ1-1 | 0.6069 | |||
| BP1-1 vs SCZ1-2 | 0.6541 | |||
| BP1-2 vs BP2-1 | 0.9712 | |||
| BP1-2 vs SCZ1-1 | 0.6265 | |||
| BP1-2 vs SCZ1-2 | 0.6636 | |||
| BP2-1 vs SCZ1-1 | 0.0941 | |||
| BP2-1 vs SCZ1-2 | 01225 | |||
| SCZ1-1 vs SCZ1-2 | 1 | |||
| No. of cells (GABA+βIII tubulin+/βIII tubulin+) | Tukey’s test | 1210B2 vs 201B7 | 0.9415 | |
| 1210B2 vs BP1-1 | 0.2961 | |||
| 1210B2 vs BP1-2 | 0.6938 | |||
| 1210B2 vs BP2-1 | 0.2707 | |||
| 1210B2 vs SCZ1-1 | 0.343 | |||
| 1210B2 vs SCZ1-2 | 0.8786 | |||
| 201B7 vs BP1-1 | 0.9599 | |||
| 201B7 vs BP1-2 | 0.9994 | |||
| 201B7 vs BP2-1 | 0.949 | |||
| 201B7 vs SCZ1-1 | 0.9747 | |||
| 201B7 vs SCZ1-2 | 1 | |||
| BP1-1 vs BP1-2 | 0.9981 | |||
| BP1-1 vs BP2-1 | 1 | |||
| BP1-1 vs SCZ1-1 | 1 | |||
| BP1-1 vs SCZ1-2 | 0.9345 | |||
| BP1-2 vs BP2-1 | 0.9967 | |||
| BP1-2 vs SCZ1-1 | 0.9994 | |||
| BP1-2 vs SCZ1-2 | 0.9993 | |||
| BP2-1 vs SCZ1-1 | 1 | |||
| BP2-1 vs SCZ1-2 | 0.9169 | |||
| SCZ1-1 vs SCZ1-2 | 0.9587 | |||
| Figure 3H | PCDH15 gene expression (iPS) | Dunnett’s test | Control vs BP | 0.2334 |
| Control vs SCZ1 | 0.8567 | |||
| PCDH15 gene expression (neuron) | Dunnett’s test | Control vs BP | 0.3458 | |
| Control vs SCZ | 0.5146 | |||
| RELN gene expression (iPS) | Dunnett’s test | Control vs BP | 0.999 | |
| Control vs SCZ | 0.9874 | |||
| RELN gene expression (neuron) | Dunnett’s test | Control vs BP | 0.081 | |
| Control vs SCZ | 0.0663 | |||
| Figure 5D | Dendrite length | Dunnett’s test | Control vs BP | 0.0001 |
| Control vs SCZ | 0.0002 | |||
| Figure 5E | Homer I puncta No. | Dunnett’s test | Control vs BP | 0.0093 |
| Control vs SCZ | 0.009 | |||
| Synapsin I puncta No. | Dunnett’s test | Control vs BP | 0.0067 | |
| Control vs SCZ | 0.0014 | |||
| Homer I-Synapsin I puncta No. | Dunnett’s test | Control vs BP | 0.0182 | |
| Control vs SCZ | 0.0117 | |||
| Figure 5H | Dendrite length | Dunnett’s test | Control vs BP | 0.004 |
| Control vs SCZ | 0.0016 | |||
| Figure 5I | Gephyrin puncta No. | Dunnett’s test | Control vs BP | 0.0065 |
| Control vs SCZ | 0.0048 | |||
| Synapsin I puncta No. | Dunnett’s test | Control vs BP | 0.0173 | |
| Control vs SCZ | 0.0099 | |||
| Gephyrin-Synapsin I puncta No. | Dunnett’s test | Control vs BP | 0.0044 | |
| Control vs SCZ | 0.0042 | |||
| Figure 6E | Gene expression of PCDH15 | Student’s t test | Control 1 vs PCDH15del (glutamatergic neurons) | 0.1757 |
| Control 1 vs PCDH15del (GABAergic neurons) | 0.01 | |||
| Figure 6G | Gene expression of RELN | Student’s t test | Control 2 vs RELNdel (glutamatergic neurons) | <0.0001 |
| Control 2 vs RELNdel (GABAergic neurons) | 0.0014 | |||
| Figure 7B | No. of cells (βIII tubulin+/HO+) | Student’s t test | Control 1 vs PCDH15del | 0.1781 |
| Control 1 vs RELNdel | 0.2969 | |||
| No. of cells (MAP2+/βIII tubulin+) | Student’s t test | Control 1 vs PCDH15del | 0.0003 | |
| Control 1 vs RELNdel | 0.2568 | |||
| No. of cells (VGluT2+βIII tubulin+/βIII tubulin+) | Student’s t test | Control 1 vs PCDH15del | 0.0098 | |
| Control 1 vs RELNdel | 0.2754 | |||
| Figure 7C | Dendrite length | Student’s t test | Control 1 vs PCDH15del | 0.0418 |
| Control 1 vs RELNdel | 0.063 | |||
| Figure 7D | Homer I puncta No. | Student’s t test | Control 1 vs PCDH15del | 0.0282 |
| Control 1 vs RELNdel | 0.0209 | |||
| Synapsin I puncta No. | Student’s t test | Control 1 vs PCDH15del | 0.2101 | |
| Control 1 vs RELNdel | 0.3278 | |||
| Homer I-Synapsin I puncta No. | Student’s t test | Control 1 vs PCDH15del | 0.982 | |
| Control 1 vs RELNdel | 0.7786 | |||
| Figure 7F | No. of cells (βIII tubulin+/HO+) | Control 1 vs PCDH15del | 0.1441 | |
| Control 1 vs RELNdel | 0.1954 | |||
| No. of cells (MAP2+/βIII tubulin+) | Control 1 vs PCDH15del | 0.0281 | ||
| Control 1 vs RELNdel | 0.4007 | |||
| No. of cells (GABA+βIII tubulin+/βIII tubulin+) | Control 1 vs PCDH15del | 0.1269 | ||
| Control 1 vs RELNdel | 0.66 | |||
| Figure 7G | Dendrite length | Student’s t test | Control 1 vs PCDH15del | 0.0055 |
| Control 1 vs RELNdel | 0.9907 | |||
| Figure 7H | Gephyrin puncta No. | Student’s t test | Control 1 vs PCDH15del | 0.553 |
| Control 1 vs RELNdel | 0.0452 | |||
| Synapsin I puncta No. | Student’s t test | Control 1 vs PCDH15del | 0.2637 | |
| Control 1 vs RELNdel | 0.0431 | |||
| Gephyrin-Synapsin I puncta No. | Student’s t test | Control 1 vs PCDH15del | 0.5136 | |
| Control 1 vs RELNdel | 0.3123 | |||
| Figure 8B | Dendrite length (Glutamatergic neurons) | Dunnett’s test | Control vs BP | 0.0001 |
| Control vs SCZ | 0.0002 | |||
| Homer I puncta No. | Dunnett’s test | Control vs BP | 0.0003 | |
| Control vs SCZ | 0.001 | |||
| Synapsin I puncta No. | Dunnett’s test | Control vs BP | 0.0011 | |
| Control vs SCZ | 0.0012 | |||
| Homer I-Synapsin I puncta No. | Dunnett’s test | Control vs BP | <0.0001 | |
| Control vs SCZ | <0.0001 | |||
| Dendrite length (GABAergic neurons) | Dunnett’s test | Control vs BP | 0.0021 | |
| Control vs SCZ | 0.0027 | |||
| Gephyrin puncta No. | Dunnett’s test | Control vs BP | 0.0002 | |
| Control vs SCZ | 0.0011 | |||
| Synapsin I puncta No. | Dunnett’s test | Control vs BP | 0.1728 | |
| Control vs SCZ | 0.1023 | |||
| Gephyrin-Synapsin I puncta No. | Dunnett’s test | Control vs BP | <0.0001 | |
| Control vs SCZ | <0.0001 | |||
| Figure 8E | Spike frequency | Dunnett’s test | Control vs BP (day28) | 0.8839 |
| Control vs SCZ (day28) | 0.924 | |||
| Control vs BP (day42) | 0.7486 | |||
| Control vs SCZ (day42) | 0.9812 | |||
| Paired t test | day28 vs day42 | 0.0104 | ||
| Figure 8F | Spike frequency | Dunnett’s test | Control 1 vs PCDH15del (day28) | 0.6129 |
| Control 2 vs RELNdel (day28) | 0.7575 | |||
| Control 1 vs PCDH15del (day42) | 0.2678 | |||
| Control 2 vs RELNdel (day42) | 0.9187 | |||
| Paired t test | day28 vs day42 | 0.0483 | ||
| Figure 8G | Spike number ratio (CNQX) | Dunnett’s test | Control vs BP | 0.0351 |
| Control vs SCZ | 0.0479 | |||
| Spike number ratio (AP-5) | Control vs BP | 0.1922 | ||
| Control vs SCZ | 0.1165 | |||
| Spike number ratio (GABA) | Control vs BP | 0.1204 | ||
| Control vs SCZ | 0.0071 | |||
| Figure 8I | ΔFmax | Dunnett’s test | 1210B2 vs BP1-1 | 0.9215 |
| 1210B2 vs BP1-2 | 0.9233 | |||
| 1210B2 vs BP2-1 | 1 | |||
| 1210B2 vs SCZ1-1 | 0.9999 | |||
| 1210B2 vs SCZ1-2 | 0.9997 | |||
| Spike frequency | Dunnett’s test | 1210B2 vs BP1-1 | 0.2334 | |
| 1210B2 vs BP1-2 | 0.7435 | |||
| 1210B2 vs BP2-1 | 0.9984 | |||
| 1210B2 vs SCZ1-1 | 0.9985 | |||
| 1210B2 vs SCZ1-2 | 1 | |||
| Figure 8J | ΔFmax | Student’s t test | Control 1 vs PCDH15del | 0.7346 |
| Control 1 vs RELNdel | 0.5067 | |||
| Spike frequency | Student’s t test | Control 1 vs PCDH15del | 0.0795 | |
| Control 1 vs RELNdel | 0.3771 | |||
p Values <0.05 were considered to be statistically significant in this study.
Results
Generation of iPSCs from BP patients
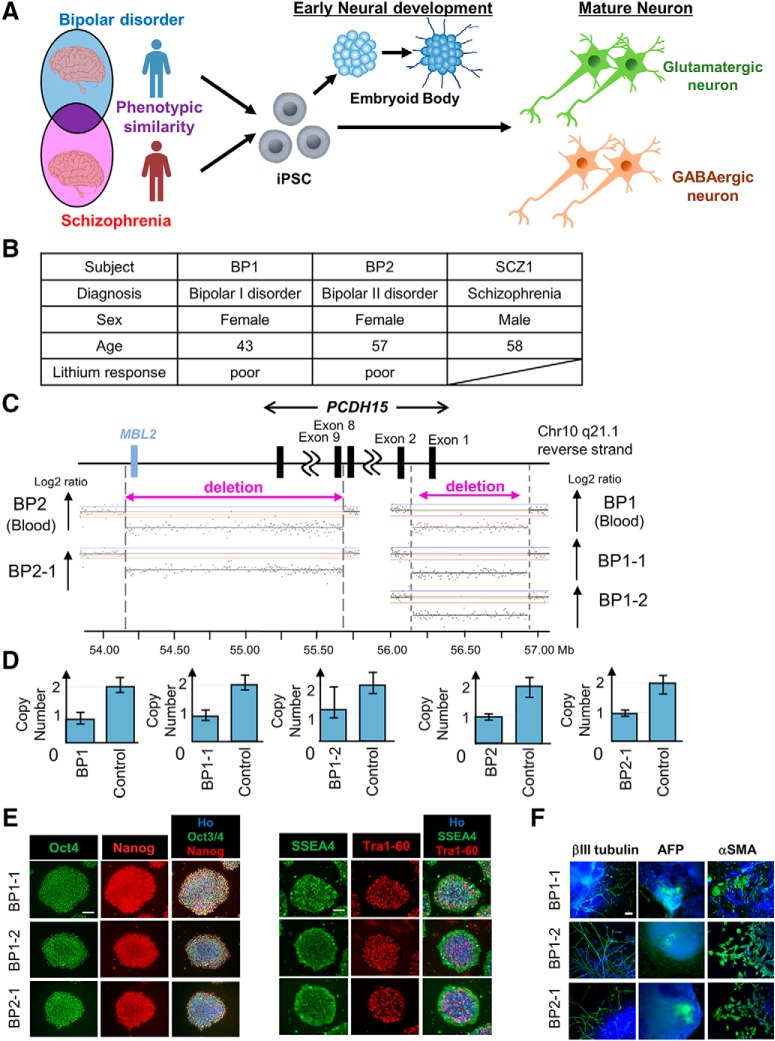
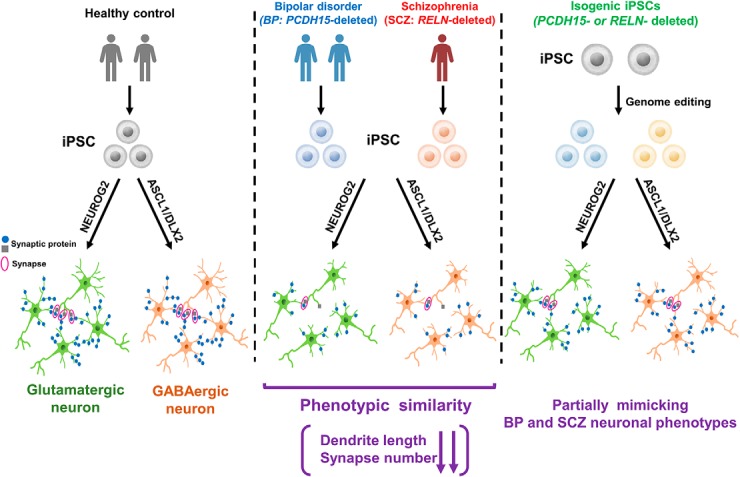
BP and SCZ are distinct neuropsychiatric diseases but share a subset of similar pathologies and symptoms (Maggioni et al., 2017). To recapitulate the shared BP and SCZ pathologies in vitro, we prepared iPSCs derived from BP and SCZ patients to induce neurons (Fig. 1A). We focused on two BP patients (BP1 and BP2), who were evaluated in a high-resolution CNV analysis study (our unpublished observation). The patient characteristics are listed in Figure 1B (see also Materials and Methods). Using aCGH, we identified an exonic deletion of PCDH15 in each patient with BP (Fig. 1C). The coordinate of this deletion of BP1 was chr10:56,149,683-56,924,932, and the deletion of BP2 was chr10:54,162,953-55,694,398. The deletion of BP1 included exon 1 of most of the PCDH15 transcripts, and the deletion of BP2 included PCDH15 exon 9 to the last exon and MBL2 (mannose binding lectin 2). These PCDH15 deletions were validated by the TaqMan copy number assay (Fig. 1D). We generated two iPSC clones (BP1-1, BP1-2) from BP1 and one iPSC clone (BP2-1) using episomal plasmids (see Materials and Methods), and we detected the same exonic deletions of PCDH15 as those detected in blood cells (Fig. 1C,D). Immunocytochemical analysis demonstrated that our newly established BP-iPSCs expressed the pluripotent markers Oct4, Nanog, SSEA4, and Tra1-60 (Fig. 1E). In vitro three-germ layer differentiation analysis via EBs also showed the differentiation potentials of the iPSCs into three germ layers (Fig. 1F). These results suggest that the BP-iPSCs conserved the patient’s genetic background and had adequate pluripotency.
Figure 1.
Generation and characterization of iPSCs derived from a BP patient. A, Schematic diagram of the strategy to explore the phenotypes of BP and SCZ in vitro. B, Subjects list containing basic information. C, CNVs in chromosome 10 were detected in blood and iPSCs derived from two BP patients by aCGH. D, The exonic deletions of PCDH15 identified in this study were validated by the TaqMan copy number assays. Bars indicate copy numbers predicted by the TaqMan copy number assays. Capped bars indicated the minimum and maximum copy number calculated for the sample replicate group (n = 4). Controls carried no aCGH-detected CNVs of PCDH15 (copy number = 2) and were used to calibrate the assays. E, The generated iPSCs expressed the pluripotent markers Oct4, Nanog, SSEA4, and Tra1-60. Scale bar, 100 μm. F, Representative images of immunocytochemical analysis for in vitro three-germ layer differentiation. βIII-Tubulin, αSMA, and AFP are pluripotent markers of the ectoderm, mesoderm, and endoderm, respectively. Blue indicates Ho, and green indicates the pluripotent marker. Scale bar, 100 μm.
We also used two previously established iPSC clones (SCZ1-1 and SCZ1-2) derived from an SCZ patient with RELN deletion (SCZ1) (Fig. 1B; Arioka et al., 2018; Sobue et al., 2018) as SCZ patient-derived iPSCs (SCZ-iPSCs) in subsequent experiments.
Ectoderm-enriched embryoid body and neuron formation by chemical treatment
There are two major methods for neural differentiation from iPSCs or embryonic stem cells (ESCs). One is the chemical induction method, in which iPSCs are treated with several pharmacological agents or bioactive proteins for differentiation into neurons via EB or neurosphere formation (Han et al., 2011; Espuny-Camacho et al., 2013; Matsumoto et al., 2016). The other is the direct conversion method, involving the overexpression of specific transcription factors (Zhang et al., 2013; Colasante et al., 2015; Sun et al., 2016; Yang et al., 2017). To select the best method for recapitulating the disease-specific mature neuron phenotypes, we used both methods to differentiate iPSCs into neurons (Fig. 1A).
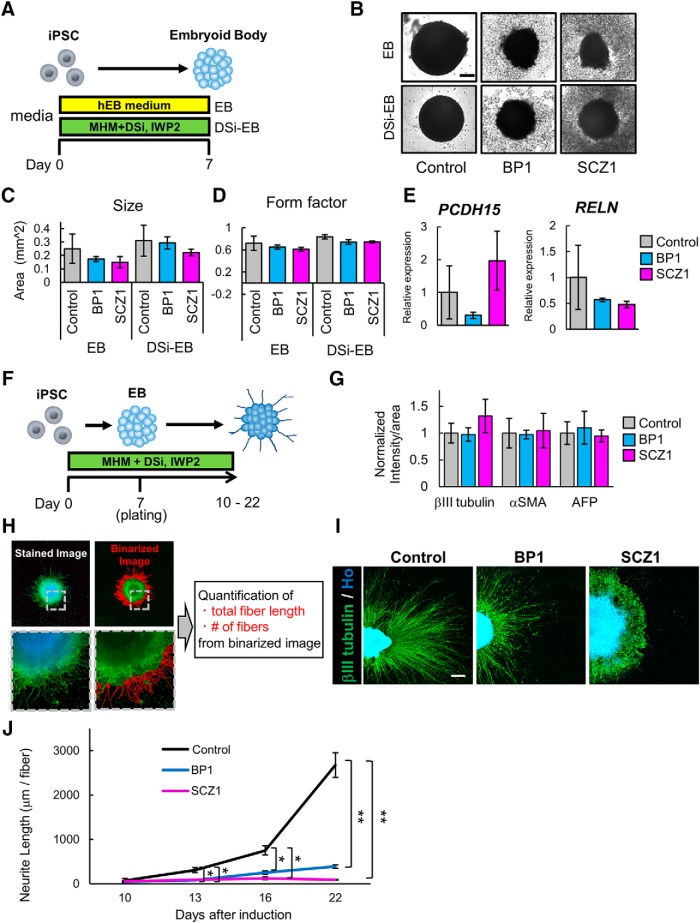
First, we performed chemical treatment. In previous studies, SCZ or BP patient-derived iPSCs exhibited abnormal phenotypes of early-stage neural differentiation when this type of method was used (Madison et al., 2015; Toyoshima et al., 2016). We used BP-iPSCs (BP1-1, BP1-2) and SCZ-iPSCs (SCZ1-1, SCZ1-2) as patient-derived iPSCs. To confirm whether such phenotypes occurred in our iPSC lines, neurons were induced via EB formation. First, we checked the potential of the two types of EB formation. Normal EBs were formed in EB medium (Fig. 2A). Another type of EB is induced by dual SMAD inhibition to facilitate neural differentiation (Chambers et al., 2009). DSi-EBs were induced in MHM with two DSis, namely, SB431542 and LDN193189 (Imaizumi et al., 2015; Matsumoto et al., 2016). The Wnt inhibitor IWP-2 was also used for DSi-EB formation to induce anterior regionality of the brain (Fig. 2A; Imaizumi et al., 2015; Nehme et al., 2018). Six clones of iPSCs (1210B2, 201B7, eKA3, eTKA4, WD39, and 414C2) were used as healthy control iPSCs. Each group of iPSCs formed EBs by both methods (Fig. 2B). To assess EB development, we examined morphometric parameters including size and form factor. The patient-derived iPSCs tended to form smaller and more distorted EBs than the control iPSCs, although there were no significant differences in size and form factor among EBs derived from each group of iPSC lines (Fig. 2C,D). In addition, the PCDH15 and RELN gene expression levels of DSi-EBs were analyzed by qRT-PCR. The expression of both genes did not change significantly between the control and BP or SCZ, although PCDH15 tended to exhibit low expression in BP samples and RELN exhibited a similar trend in SCZ samples (Fig. 2E).
Figure 2.
Neuron differentiation via EB formation. A, Overview of the protocol for EB formation. DSi represents SB431542 and LDN193189. B, Representative images of EBs on day 7. Scale bar, 200 μm. C, Quantification of EB sizes (n = 3 independent experiments; mean ± SD; Dunnett’s test, no significant differences were observed). D, The form factor of the EBs was calculated as an indicator of roundness. E, Gene expression of iPSCs and DSi-EBs. E, Relative gene expression levels of PCDH15 and RELN in DSi-EBs derived from six control lines, two BP lines (BP1-1 and BP1-2), and two SCZ lines (SCZ1-1 and SCZ1-2; n = 3 independent experiments; mean ± SD; Dunnett’s test). Values were normalized to that of the control, which was considered to be 1.0. One sample of 1210B2-derived DSi-EBs, in which PCDH15 expression was under the detection limit, was removed from the analysis. F, Overview of the protocol for neuron differentiation via EB formation. DSi represents SB431542 and LDN193189. G, Intensity levels of three germ layer markers, namely, βIII-tubulin, αSMA, and AFP. Intensity levels were normalized to that of the control, which was considered to be 1.0 (n = 3 independent experiments; mean ± SD; Dunnett’s test among each group, no significant differences were observed). H, Schematic diagram of the analysis protocol. Fiber length and number of βIII tubulin+ cells were quantified from binarized image data obtained from stained images. I, Representative images of βIII-tubulin+ neurons. Scale bar, 300 μm. J, Time-dependent changes of βIII-tubulin+ mean neurite length per neurite fiber (n = 3 independent experiments; mean ± SD; *p < 0.05, **p < 0.01; Dunnett’s test among each group). Mean neurite length is shown as the mean length of βIII-tubulin+ neurite fiber.
To investigate whether neurons from the patient-derived iPSCs exhibited aberrant phenotypes, we induced neural differentiation via DSi-EB formation. DSi-EBs were plated on culture plates on day 7. Plated DSi-EBs were cultured for up to an additional 15 d in MHM with DSi and IWP-2 (Fig. 2F). We used 1210B2 and 201B7 as control iPSCs, and BP-iPSCs (BP1-1, BP1-2) and SCZ-iPSCs (SCZ1-1, SCZ1-2) were used as patient-derived iPSCs. Immunocytochemical analysis showed that the expression levels of markers of the three germ layers, namely, βIII tubulin (ectoderm), αSMA (mesoderm), and AFP (endoderm), in the patient-derived cells did not differ significantly from those in the control cells (Fig. 2G). Thus, the capacity of differentiation into the three germ layers among the control and both patient-derived iPSCs was likely to be the same. We then measured the length of neurite extension from the cell clusters. To visualize the neurites, cells were immunostained for βIII tubulin. Binarized images were formed from stained images to quantify the total fiber length of each cell cluster (a) and fiber numbers of each cell cluster (b) of the βIII tubulin+ cells (Fig. 2H). Average neurite length was defined as (a)/(b). The neurite lengths of patient-derived cells were significantly shortened from day 13 onward (Fig. 2I,J). We found that this method was not suitable for the analysis of mature neurons because of early-stage neuron abnormalities, although these phenotypes may reflect developmental abnormalities of the pathologies of BP and SCZ. Thus, we decided not to perform further analysis using this method.
Neuronal differentiation of iPSCs by overexpression of transcription factors
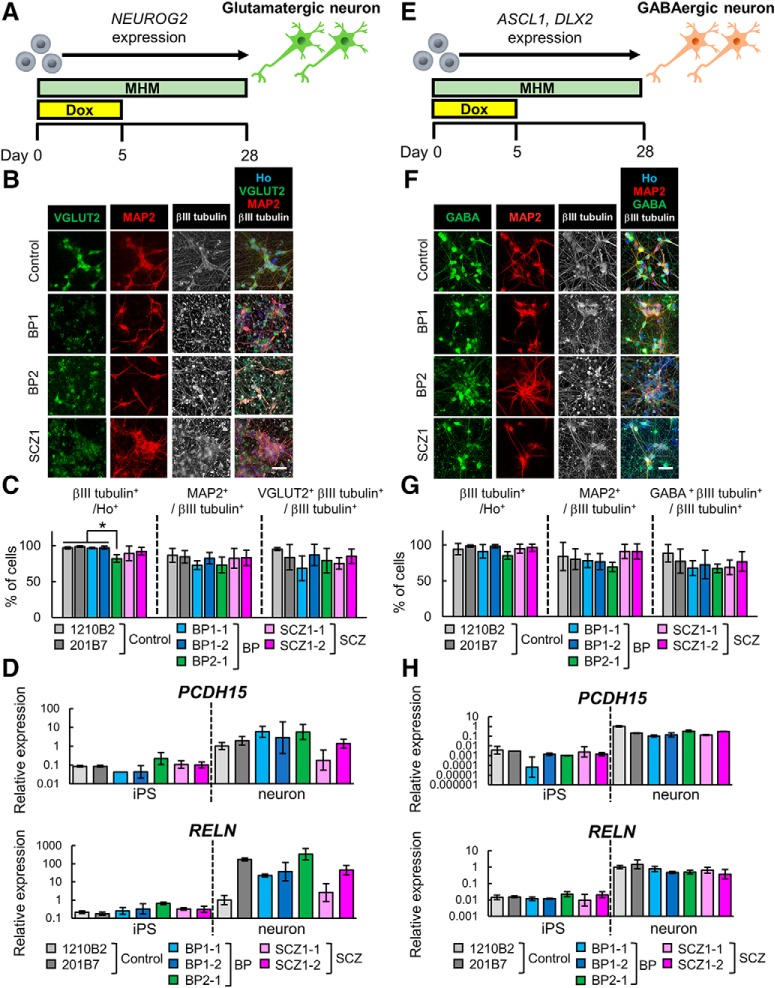
We next tested the direct method for neuronal conversion of iPSCs by transcription factor overexpression. This method can induce specific neuron subtypes. Previous studies have shown that Neurog2 overexpression induced glutamatergic neuron differentiation (Zhang et al., 2013), and co-overexpression of ASCL1 and DLX2 induced GABAergic neuron differentiation (Yang et al., 2017). Although these studies used lentivirus as a tool for introducing transcription factors, use of the PiggyBac transposon vector system enabled the establishment of transgenic cells that can be easily stored and used for experiments. One report using the PiggyBac system for the introduction of transcription factors into human ESCs resulted in highly efficient neural differentiation (Matsushita et al., 2017). Therefore, we established transgenic iPSC lines in which transcription factors can be induced by Dox. We used 1210B2 and 201B7 as healthy control iPSCs, while we used BP-iPSCs (BP1-1, BP1-2, and BP2-1) and SCZ-iPSCs (SCZ1-1, SCZ1-2) as patient-derived iPSCs. NEUROG2 expression vector-transduced iPSCs were established for glutamatergic neuronal induction, and Ascl1- and DLX2-transduced iPSCs were established for GABAergic neuronal induction (see Materials and Methods). The iPSCs were treated with Dox for 5 d and cultured in MHM for 28 d (Fig. 3A,E). Immunocytochemical analysis showed that the neuronal marker βIII tubulin was expressed in most of the cells induced from control iPSCs (control neurons), BP-iPSCs (BP neurons), and SCZ-iPSCs (SCZ neurons; Fig. 3B,C,F,G). The conversion efficiency of iPSCs into βIII tubulin+ neurons was close to 100% in all lines, except line BP2-1 which still showed >80% efficiency (Fig. 3C). βIII tubulin+ neurons derived from NEUROG2-transduced iPSCs expressed the mature neuronal marker MAP2 and the glutamatergic neuronal marker VGLUT2 at similar ratios in each line (Fig. 3B,C). Next, the PCDH15 and RELN gene expression levels of NEUROG2-transduced cells (iPSCs and induced glutamatergic neurons) were analyzed by qRT-PCR. We used the primer sets targeting the deletion of PCDH15 in BP1 or RELN in SCZ1. Both PCDH15 and RELN were substantially upregulated on neuronal induction but were not differentially expressed between control, BP, SCZ iPSCs, or neurons (Fig. 3D). Induced neurons derived from ASCL1- and DLX2-transduced iPSCs expressed MAP2 and the GABAergic neuronal marker GABA at similar ratios in each line (Fig. 3F,G). The PCDH15 and RELN gene expression levels of ASCL1- and DLX2-cotransduced cells (iPSCs and induced GABAergic neurons) are shown in Figure 3H. The expression of both genes was upregulated in GABAergic neurons compared with undifferentiated iPSCs. Neither PCDH15 nor RELN expression changed significantly in patient-derived cells. These results showed that this method enabled both healthy and patient-derived iPSCs to efficiently differentiate into subtype-specific and mature neurons. Thus, we chose this method for further analyses.
Figure 3.
Neuron differentiation by transcription factor overexpression. A, Overview of the protocol for differentiation into glutamatergic neurons. B, Immunocytochemical analysis of neuronal markers (VGluT2, MAP2, and βIII tubulin). Scale bar, 40 μm. C, Ratio of positive cells for each marker; βIII tubulin+ cells/all cells (βIII tubulin+/Ho+), MAP2+ cells/βIII tubulin+ cells (MAP2+/ βIII-tubulin+), and VGluT2 and βIII tubulin double+ cells/βIII tubulin+ cells (VGLUT2+βIII tubulin+/βIII tubulin+). (n = 3-6 independent experiments; mean ± SD; *p < 0.05; Tukey’s test, the population of βIII tubulin+ cells in BP2-1-derived neurons was significantly lower than those in 1210B2-, 201B7-, BP1-1-, and BP1-2-derived neurons). D, Relative gene expression levels of PCDH15 (primer set1) and RELN in NEUROG2-transduced iPSCs and induced neurons (n = 3 independent experiments; mean ± SD; Dunnett’s test among iPSCs and among neurons, no significant differences were observed). Values were normalized to that for 1210B2-derived neurons, which was considered to be 1.0. Some iPSC samples, in which PCDH15 expression was under the detection limit, were removed from the analysis. Specifically, two samples of BP1-1, and one sample of 1210B2, 201B7, BP2-1, and SCZ1-1 were excluded from the analysis. E, Overview of the protocol for differentiation into GABAergic neurons. F, Immunocytochemical analysis of neuronal markers (MAP2, GABA, and βIII tubulin). Scale bar, 40 μm. G, Ratio of positive cells for each marker; βIII tubulin+ cells/all cells (βIII tubulin+/Ho+), MAP2+ cells/βIII tubulin+ cells (MAP2+/βIII tubulin+), and GABA and βIII-tubulin double-positive cells/βIII tubulin+ cells (GABA+βIII tubulin+/βIII tubulin+). (n = 3-6 independent experiments; mean ± SD; Tukey’s test; no significant differences were observed). H, Relative gene expression levels of PCDH15 and RELN in ASCL1- and DLX2-transduced iPSCs and induced neurons. (n = 3 independent experiments; mean ± SD; Dunnett’s test among iPSCs and among neurons; no significant differences were observed). Values were normalized to that for 1210B2-derived neurons, which was considered to be 1.0. Some iPSC samples, in which PCDH15 expression was under the detection limit, were removed from the analysis. Specifically, each one sample of 1210B2 and BP2-1 was excluded from the analysis.
Characterization of gene expression in neurons induced from iPSCs
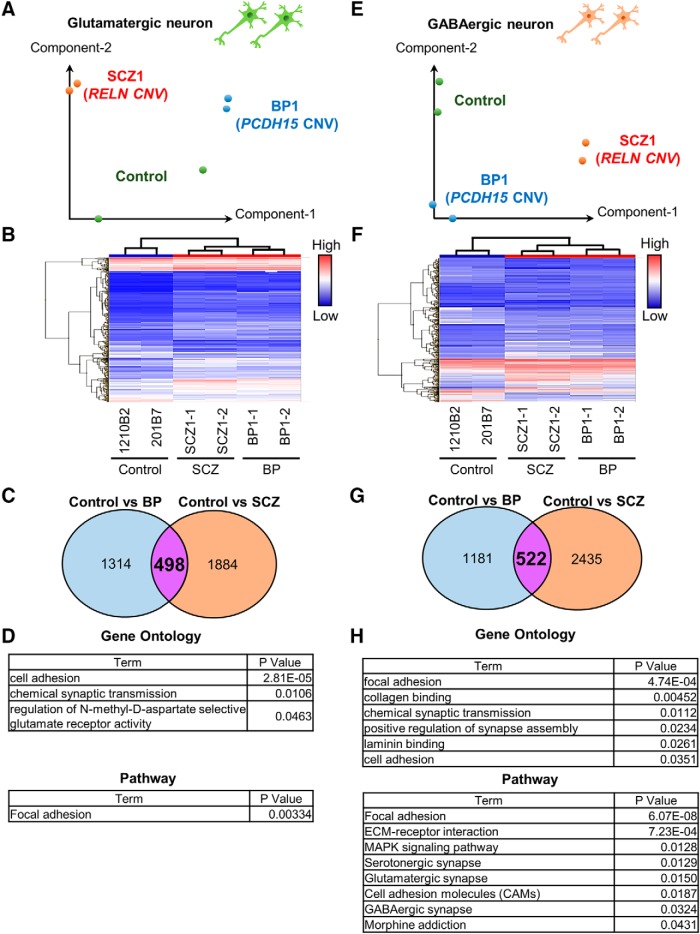
To identify the characteristics of neurons induced from patient-derived iPSCs, the global gene expression profiles of neurons induced from control-iPSCs (1210B2 and 201B7) and patient-derived iPSCs (BP1-1, BP1-2, SCZ1-1, and SCZ1-2) were examined by a microarray analysis. Principal component analysis (PCA; Fig. 4A,E) and hierarchical analysis (Fig. 4B,F) showed that the control, BP, and SCZ neurons were placed in different groups. In particular, the profiles of the BP and SCZ neurons were closer to each other than to that of the control neurons (Fig. 4B,F). We next identified the differentially expressed genes (DEGs) in neurons induced from patient-derived iPSCs compared with control neurons (fold change, >2.0). We identified 1812 genes of BP glutamatergic neurons and 2382 genes of SCZ glutamatergic neurons that were upregulated or downregulated compared with the control neurons (Fig. 4C). For the GABAergic neurons, the expression levels of 1703 genes of BP neurons and 2357 genes of SCZ neurons were changed (Fig. 4G). To explore the cellular functions associated with the DEGs in the BP and SCZ neurons, GO analysis and pathway analysis were performed. Featured GO terms and pathways of common DEGs between BP and SCZ are shown in Figure 4, D and H. In both glutamatergic and GABAergic neurons, multiple GO terms and pathways related to cell adhesion and neural function were enriched compared with control neurons (Fig. 4D,H).
Figure 4.
Comparison of the global gene expression profiles. A, PCA of the gene expression data of glutamatergic neurons. B, Hierarchical clustering analysis of DEGs in glutamatergic neurons. C, Venn diagram of DEGs of BP or SCZ glutamatergic neurons compared with control neurons (fold change, >2.0). A total of 498 genes were common between the two groups compared. D, Featured GO and pathway terms for the 498 common DEGs among the control vs BP and control vs SCZ. Adhesion- and neuron-associated terms were extracted. E, PCA plot of the gene expression data of GABAergic neurons. Green, control (1210B2, 201B7); blue, BP; red, SCZ. F, Hierarchical clustering analysis of DEGs in GABAergic neurons. G, Venn diagram of DEGs of BP or SCZ GABAergic neurons in comparison with control neurons (fold change, >2.0). A total of 522 genes were common among the two comparisons. H, Featured GO and pathway terms for 522 common DEGs among the control vs BP and control vs SCZ. Adhesion- and neuron-associated terms were extracted.
Neurons induced from patient-derived iPSCs exhibited shorter dendrites and reduced formation of excitatory and inhibitory synapses
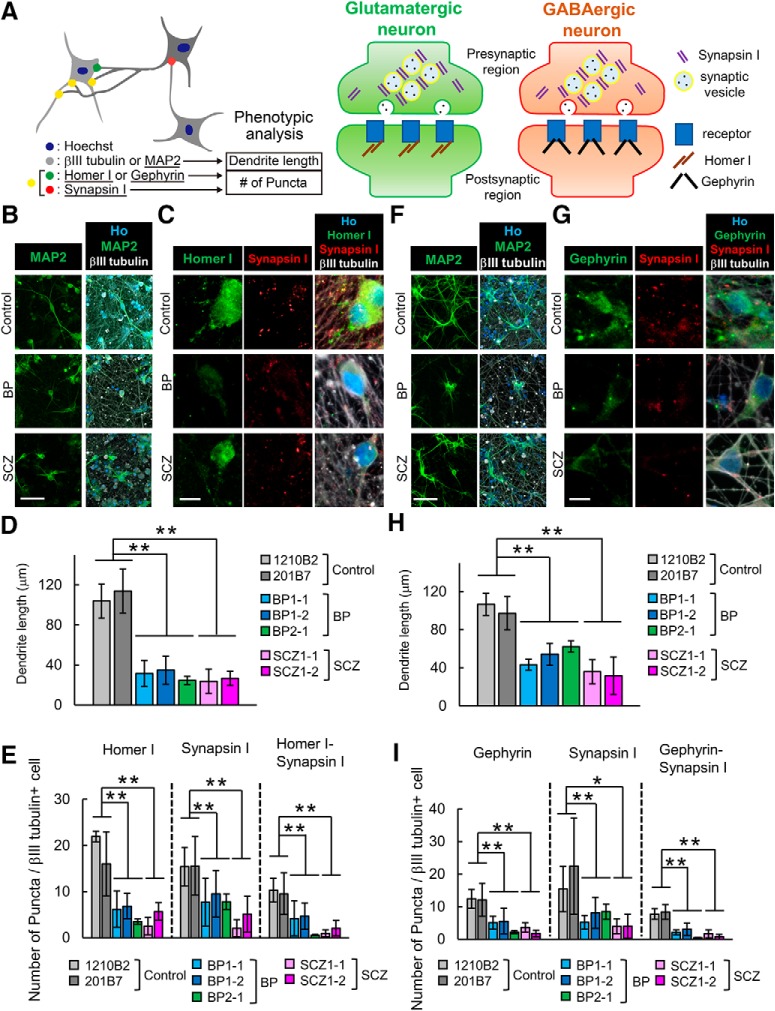
Microarray and GO analyses suggested that genes involved in cell adhesion may contribute to pathogenetic mechanisms (Fig. 4). Cell adhesion is a key event for neurite extension and synapse formation (Kiryushko et al., 2004; Missler et al., 2012). In addition, previous studies using postmortem human brains showed reduced spine density and dendrite length in the brains of BP and SCZ patients (Glantz and Lewis, 2000; Konopaske et al., 2014). To investigate whether BP and SCZ neurons have similar phenotypes, we determined the dendrite lengths and synaptic densities of induced neurons by immunocytochemical analysis with an IN Cell Analyzer 6000. Dendrite length was measured as the length of the region immunostained with the dendrite marker MAP2 (Fig. 5A). Then, to detect the number of morphological synapses in glutamatergic neurons, we counted the puncta of Synapsin I and Homer I as presynaptic or postsynaptic markers. For GABAergic neurons, we determined the number of puncta positive for Synapsin I and Gephyrin, which is a postsynaptic scaffolding protein found in GABAergic synapses (Fig. 5A). MAP2+ dendrites of BP and SCZ neurons were significantly shorter than those of control neurons in both glutamatergic and GABAergic neurons (Fig. 5B,D,F,H). In addition, fewer puncta of Homer I and Synapsin I on glutamatergic neurons were detected in BP and SCZ neurons than in control neurons (Fig. 5C,E). The number of puncta of GABAergic Synapsin I and Gephyrin were also reduced in BP and SCZ neurons compared with control neurons (Fig. 5G,I). Moreover, the number of colocalized puncta between presynaptic and postsynaptic markers was also significantly decreased in BP and SCZ neurons (Fig. 5E,I). Thus, the number of synapses decreased in both BP and SCZ neurons. These results suggest that neurons induced from patient-derived iPSCs exhibit synapse- and dendrite-related phenotypes that are consistent with the phenotypes often observed in patients with psychiatric disorders and can thus serve as disease models of psychiatric disorders in vitro.
Figure 5.
Neurons induced from patient-derived iPSCs exhibit abnormal phenotypes of dendrite length and synapse formation. A, Schematic diagram of the protocol for phenotypic analysis of dendrite lengths and number of synaptic markers of the neurons. B, C, Representative images of immunocytochemical analysis of a dendrite marker (MAP2; scale bar, 60 μm; B) and synaptic markers (Homer I, Synapsin I; scale bar, 10 μm; C) in glutamatergic neurons. D, Quantitative analysis of dendrite length in glutamatergic neurons (n = 3-6 independent experiments; mean ± SD; **p < 0.01; Dunnett’s test). E, Quantitative analysis of the number of synaptic marker puncta in glutamatergic neurons (n = 3-6 independent experiments; mean ± SD; *p < 0.05, **p < 0.01; Dunnett’s test). Homer I puncta, Synapsin I puncta and Homer-Synapsin I colocalized puncta on βIII-tubulin+ cells were counted. F, G, Representative images of immunocytochemical analysis of a dendrite marker (MAP2; scale bar, 60 μm; F) and synaptic markers (Gephyrin, Synapsin I; scale bar, 10 μm; G) in GABAergic neurons. H, Quantitative analysis of dendrite length in GABAergic neurons (n = 3-6 independent experiments; mean ± SD; **p < 0.01, Dunnett’s test). I, Quantitative analysis of the number of synaptic marker puncta in glutamatergic neurons (n = 3-6 independent experiments; mean ± SD; *p < 0.05, **p < 0.01, Dunnett’s test). Gephyrin puncta, Synapsin I puncta, and Gephyrin-Synapsin I colocalized puncta on βIII tubulin+ cells were counted.
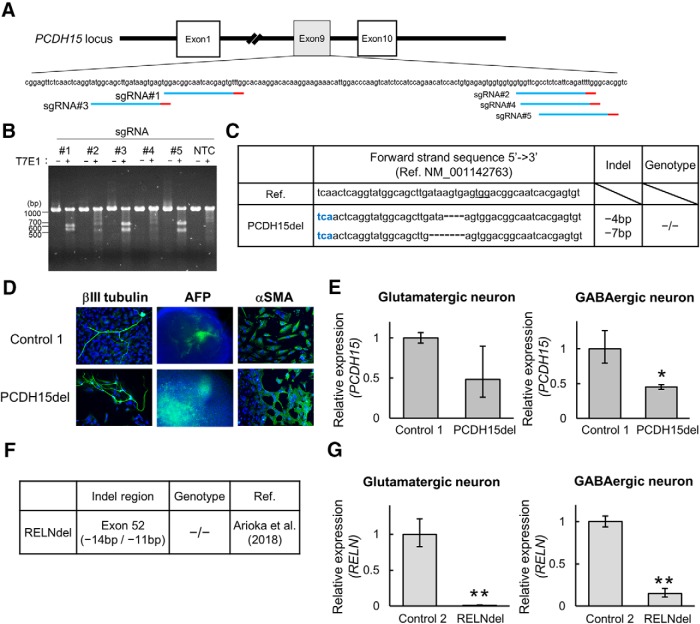
Establishment of isogenic PCDH15-deleted iPSCs
It is difficult to perform large-scale analyses because of the infrequency of patients carrying particular CNVs. Moreover, line-to-line variability caused by different genetic backgrounds complicate genotype–phenotype correlation. Thus, we sought to establish mutant and control iPSCs by targeted genome editing using the CRISPR/Cas9 system. To generate PCDH15-deleted iPSCs, sgRNAs were constructed in exon 9 (Fig. 6A). According to the results of the T7E1 assay (see Materials and Methods), sgRNA#3 showed the strongest cleavage activity among the five sgRNA candidates (Fig. 6B). We obtained an isogenic line with the homozygous PCDH15 deletion (PCDH15del) from healthy control NC1032-1-2 (Control 1; Fig. 6C). PCDH15del mutant iPSCs showed the capacity to differentiate into three germ layers as well as Control 1 (Fig. 6D). Then, we analyzed the expression level of PCDH15 by qRT-PCR using the primer set targeting the deleted region. The mRNA level of PCDH15 was decreased by about 50% in PCDH15del-induced neurons (PCDH15del neurons) when normalized to Control 1-induced neurons (Fig. 6E). We also prepared an isogenic line with the homozygous RELN deletion (RELNdel) that was established from 201B7 (Control 2) in a previous study (Arioka et al., 2018; Fig. 6F). Remarkably, the expression level of RELN in RELNdel-induced neurons (RELNdel neurons) was much lower than that in Control 2-induced neurons (Fig. 6G).
Figure 6.
Generation of isogenic PCDH15 deletion iPSCs by targeted genome editing. A, The target sites of CRISPR-sgRNAs in exon 9 of the PCDH15 gene (NM_001142763). Red bars represent PAM (protospacer adjacent motif) sequences. B, Analysis of CRISPR-sgRNA activity by the T7E1 assay using HEK293FT. NTC, No transfection. Among the constructed sgRNAs, sgRNA#3 showed the strongest cleavage activity. C, Indel pattern of the isogenic line using CRISPR-sgRNA#3. Blue letters represent stop codon. D, Representative images of immunocytochemical analysis for in vitro three-germ layer differentiation. Blue indicates Ho and green indicates the pluripotent marker (βIII-tubulin, αSMA, and AFP). Scale bar, 100 μm. E, Relative gene expression levels of PCDH15 (primer set2) in glutamatergic or GABAergic neurons on day 28 (n = 3 independent experiments; mean ± SD; *p < 0.05, Student’s t test). F, Information of isogenic RELN-deleted iPSCs generated in the previous study (Arioka et al., 2018). G, Relative gene expression levels of RELN in induced glutamatergic or GABAergic neurons on day 28 (n = 3 independent experiments; mean ± SD; **p < 0.01, Student’s t test).
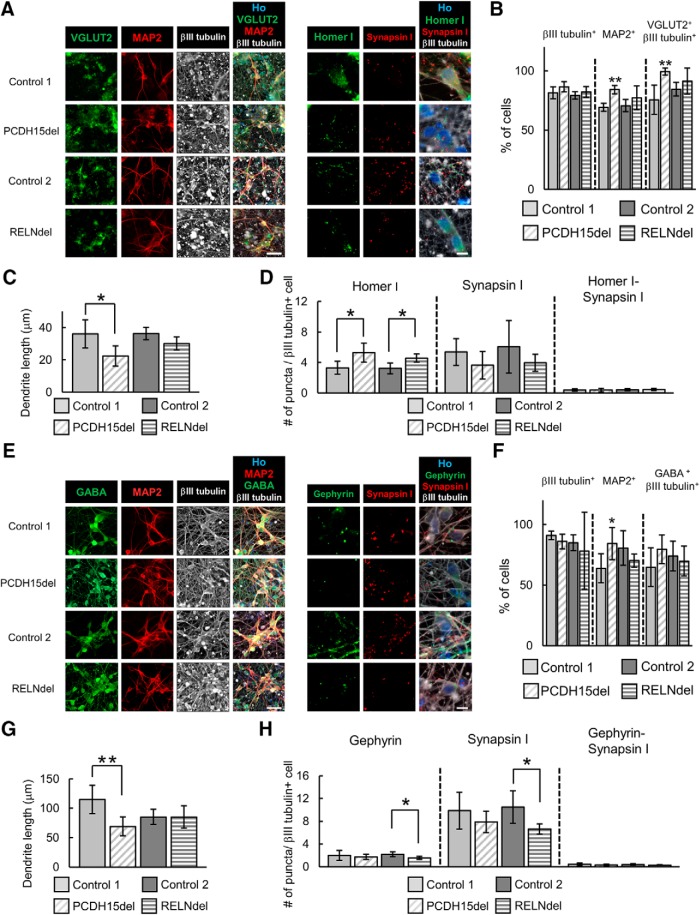
Dendritic and synaptic characterization of isogenic PCDH15- or RELN-deleted neurons
To investigate whether our isogenic gene-edited iPSC-derived neurons recapitulate patient iPSC-derived neurons, we performed phenotypic analyses focusing on their dendrite length and synapse formation using the same method as performed in Figure 5. Both glutamatergic and GABAergic neurons were efficiently induced from PCDH15del and RELNdel (Fig. 7A,B,E,F). While some differentiation characteristics were confirmed, >60% of cell populations constantly differentiated into our target neurons in all cell lines we tested (Fig. 7B,F).
Figure 7.
Isogenic PCDH15 or RELN deleted neurons showed partial phenotypes of dendrite and synapse formation. A, Representative images of immunocytochemical analysis of neuronal markers (scale bar, 40 μm) and synaptic markers (scale bar, 10 μm) in glutamatergic neurons. B, Ratio of positive cells for each marker; βIII tubulin+ cells/all cells (βIII tubulin+), MAP2+ cells/βIII tubulin+ cells (MAP2+), and VGLUT2 and βIII tubulin double-positive cells/βIII tubulin+ cells (VGluT2+βIII tubulin+). (n = 4–6 independent experiments; mean ± SD; **p < 0.01, Student’s t test between each pair of control and isogenic lines). C, Quantitative analysis of dendrite length in glutamatergic neurons (n = 4–6 independent experiments; mean ± SD; *p < 0.05, Student’s t test). D, Quantitative analysis of the number of synaptic marker puncta in glutamatergic neurons (n = 3–6 independent experiments; mean ± SD; *p < 0.05, Student’s t test). E, Representative images of immunocytochemical analysis of neuronal markers (scale bar, 40 μm) and synaptic markers (scale bar, 10 μm) in GABAergic neurons. F, Ratio of positive cells for each marker; βIII tubulin+, MAP2+, and GABA and βIII tubulin double-positive cells/βIII tubulin+ neuronal cells (GABA+βIII tubulin+). (n = 4–6 independent experiments; mean ± SD; *p < 0.05, Student’s t test between Control 1 and PCDH15del or between Control 2 and RELNdel). G, Quantitative analysis of dendrite length in GABAergic neurons (n = 4–6 independent experiments; mean ± SD; **p < 0.01, Student’s t test). H, Quantitative analysis of the number of synaptic marker puncta in GABAergic neurons (n = 4–6 independent experiments; mean ± SD; *p < 0.05, Student’s t test).
Regarding glutamatergic neurons, MAP2+ dendrites of PCDH15del neurons were significantly shorter than those of Control 1 neurons, while RELNdel neurons tended to form shorter dendrites than Control 2 neurons, although the difference was not statistically significant (p = 0.063; Fig. 7C). Unlike patient-derived neurons, the number of Homer I puncta was increased in both PCDH15del neurons and RELNdel neurons compared with Control 1 or 2 neurons (Fig. 7D). The number of Synapsin I puncta and colocalized puncta of Homer I and Synapsin I tended to be lower in isogenic lines, although the changes were not significant (Fig. 7D). Regarding GABAergic neurons, MAP2+ dendrite shortening was observed only in PCDH15del neurons (Fig. 7G), while both Gephyrin and Synapsin I puncta were significantly decreased only in RELNdel neurons (Fig. 7H). The number of colocalized puncta was not significantly different in either isogenic line (Fig. 7H). Based on these findings, isogenic gene-edited lines partially recapitulated the structural phenotypes observed in patient-derived lines.
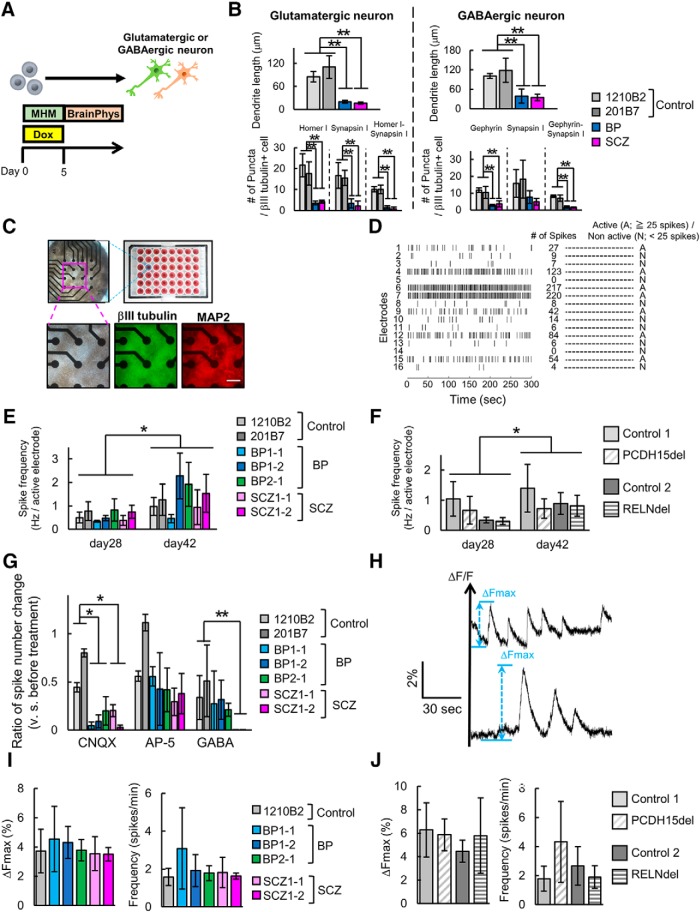
Assessment of neuronal function of patient-derived and isogenic iPSCs-derived neurons
We showed that patient-derived neurons or isogenic gene-edited neurons displayed structural phenotypes. However, it remains unknown whether these neurons show differences in functional activity. Thus, we investigated their neuronal function by assaying spontaneous neuronal activity. To clearly assess neuronal activity, we evaluated the effect of BrainPhys medium, which was developed to support neurophysiological activity (Bardy et al., 2015) on neuronal phenotypes we detected (Fig. 8A). Even by culturing with BrainPhys, BP and SCZ neurons showed the recapitulated phenotypes of dendrite shortening and decreased synapse number and for both glutamatergic and GABAergic neurons (Fig. 8B). Based on these results, we used BrainPhys as the neuron culture media for functional analysis.
Figure 8.
Spontaneous activity of neurons induced from patient-derived or isogenic iPSCs. A, Overview of the protocol for neuronal differentiation for functional analysis. B, Quantitative analysis of dendrite length and synaptic markers puncta in neurons cultured in BrainPhys for differentiation on day 28 (n = 3–4 independent experiments; mean ± SD; **p < 0.01, Dunnett’s test among control, BP, and SCZ neurons; BP: BP1-2, SCZ: SCZ1-2). C, Overview of MEA plate and representative images of neurons induced from iPSCs on the 48-well MEA plates. Bright-field image and immunocytochemical images of neuron markers. Scale bar, 200 μm. D, Representative image of raster plot and definition of active electrodes. E, Spike frequency of control or patient-derived glutamatergic neurons on day 28 and day 42 (n = 4–6 independent experiments; mean ± SD; Dunnett’s test among Control, BP, and SCZ neurons, no significant differences were observed; paired t test between day 28 and day 42, *p < 0.05). F, Spike frequency of isogenic iPSC-derived glutamatergic neurons (n = 3–4 independent experiments; mean ± SD; Dunnett’s test among Control, BP, and SCZ neurons, no significant differences were observed; paired t test between day 28 and day 42, *p < 0.05). G, Relative change in the total number of spikes after drug treatment in glutamatergic neurons on day 42 (n = 3 independent experiments; mean ± SD; *p < 0.05, **p < 0.01, Dunnett’s test). H, Representative image of calcium spikes and display of parameters (ΔFmax and calcium spike numbers). I, ΔFmax and calcium spike frequency in control or patient-derived GABAergic neurons (n = 3–6 independent experiments; mean ± SD; Dunnett’s test among each line, no significant differences were observed). J, ΔFmax and calcium spike frequency in control or patient-derived GABAergic neurons (n = 4–6 independent experiments; mean ± SD; Student’s t test, no significant differences were observed).
For MEA analysis, we induced neurons from iPSCs directly on the MEA plates at a high density (Fig. 8C). Spontaneous activity was detected by 16 electrodes per well in the MEA plate, and the activities of neurons were recorded at each electrode (Fig. 8D). In glutamatergic neurons, active electrodes were stably detected on day 28 after induction, while few active electrodes appeared in GABAergic neurons on day 28 (data not shown). In spike frequency of glutamatergic neurons on day 28 and day 42, there were no significant differences in spike frequency among control, BP, and SCZ groups, although the time-dependent increase in spike frequencies of each group (Fig. 8E). Isogenic PCDH15-deleted or RELN-deleted neurons also showed no significant differences compared with Control 1 or Control 2 both on day 28 and day 42, except for the time-dependent increase in spike frequencies (Fig. 8F). These results indicate that spontaneous neuronal activity was maintained at a comparable level among glutamatergic neurons whether they were control neurons, patient-derived neurons, or isogenic neurons.
Next, we investigated receptor reactivity by treatment with a glutamate receptor antagonist (AMPA receptor antagonist CNQX and NMDA receptor antagonist AP-5) or GABA receptor agonist (GABA). By using induced glutamatergic neurons, we measured changes in the total number of spikes per well before and after treatment. We found that the spike number was significantly decreased both in BP and SCZ neurons after CNQX treatment, but not after AP-5 treatment compared with Control neurons (Fig. 8G). Similarly, GABA treatment resulted in a significant decrease in spike number in SCZ neurons compared with Control neurons (Fig. 8G). These results suggest that BP and SCZ neurons have higher sensitivities in AMPA receptor and GABA receptor stimulation, which might lead to the maintenance of spontaneous activity of neurons.
In GABAergic neurons, 6 weeks of differentiation enabled neurons to have active spikes in most patient-derived and isogenic lines. However, some cell lines did not show sufficient neuronal activity to be analyzed (data not shown). Then, we used a calcium imaging system to detect the GABAergic neuron activity with higher sensitivity. We used 1210B2 as a healthy control for comparison with patient-derived lines. We recorded spontaneous calcium spikes from GABAergic neurons on day 28 and focused on the following parameters: ΔFmax and spike number (Fig. 8H; see also Materials and Methods). There were no significant differences both in ΔFmax and spike frequency among control- and patient-derived lines (Fig. 8I). Isogenic neurons also showed no significant differences in these parameters compared with controls (Fig. 8J). These results of calcium imaging thus suggest that the spontaneous activity of GABAergic neurons is comparable among control, patient-derived, and isogenic neurons, indicating some compensatory mechanism for structural abnormalities.
Discussion
It is challenging to elucidate the molecular mechanisms and general pathologies of BP and SCZ for the following two reasons: the genetic background complexity of these disorders and the difficulty of pathological recapitulation. However, neurons induced from patient-derived iPSCs can overcome these problems. In particular, the use of iPSCs derived from patients with disease-associated CNVs is a promising strategy (Flaherty et al., 2017; Rutkowski et al., 2017). In the present study, we focused on the following two CNVs: the heterozygous deletion of PCDH15 and of RELN. We used newly generated iPSCs derived from BP patients with PCDH15 deletion and differentiated the BP-iPSCs (PCDH15-deleted) and SCZ-iPSCs (RELN-deleted) into neurons efficiently. We identified several neuronal abnormalities in neurons induced from patient-derived iPSCs.
In this study, we used the following two types of methods for neural differentiation: induction by chemical treatment or overexpression of transcription factors. Neurons induced from patient-derived iPSCs using the chemical treatment method exhibited abnormal phenotypes of neurite extension in the early differentiation stage. Although these phenotypes may support the neurodevelopmental hypothesis for BP and SCZ (O'Shea and McInnis, 2016; Owen and O'Donovan, 2017), this differentiation method is not suited for the analysis of adult and specific pathologies for medical treatment. On the other hand, we showed that transcription factor overexpression is suitable for the preparation of specific types of mature neurons and analysis of the phenotypes of clinical pathologies. NEUROG2 overexpression induced glutamatergic neurons, and ASCL1 and DLX2 co-overexpression induced GABAergic neurons, from iPSCs efficiently, which enabled analysis of the phenotype of each type of neuron. In addition, this type of method is expected to be applicable for electrophysiological analysis of specific neurons or functional analysis of neural networks by coculturing different types of neurons (Sun et al., 2016). However, the induction of specific subtypes of GABAergic neurons is also needed for further understanding of the pathologies. It was reported that ASCL1 and DLX2 overexpression induced various subtypes of GABAergic neurons, such as calretinin+, somatostatin+, and parvalbumin+ neurons (Yang et al., 2017). Although parvalbumin+ neurons have important roles in BP and SCZ (Wang et al., 2011; Marin, 2012), the existing method can induce parvalbumin+ neurons at only low levels (Sun et al., 2016; Wen, 2017; Yang et al., 2017). Therefore, an efficient method to induce parvalbumin+ neurons is required for further investigation of the roles of GABAergic neurons in BP and SCZ.
Interestingly, we identified similar phenotypes of decreased MAP2+ dendrite length and synapse number between BP and SCZ neurons and between glutamatergic and GABAergic neurons. These phenotypes seem to be associated with the common and general pathologies in BP and SCZ in terms of neurite extension and synapse formation, similar to the phenotypes observed in the brains of patients. Our microarray analysis suggested that these common phenotypes were associated with cell adhesion. PCDH15 participates in cell–cell adhesion of hair cells at the inner ear by forming tip-link filaments (Jaiganesh et al., 2018), while there are no reports on the precise role of PCDH15 in neural adhesion. Reelin is also involved in cell adhesion. Reelin-dependent signaling pathways regulate neural adhesion (Sekine et al., 2012; Hirota and Nakajima, 2017). Thus, PCDH15 and Reelin are expected to play key roles in neural adhesion to regulate dendrite or synapse formation. Indeed, it is well known that cell adhesion plays important roles in synapse formation and neurite extension (Missler et al., 2012). Previous studies have suggested that cell adhesion molecules are associated with BP and SCZ (O'Dushlaine et al., 2011; Sakurai, 2017). In addition, if such a basic system of neural development is associated with these phenotypes, the phenotypes may be associated with a broader range of psychiatric disorders. Although, based on these observations, cell adhesion is expected to play important roles in the pathologies of BP and SCZ, it remains unknown whether these phenotypes reflect common pathologies in general patients or are specific to certain genetic backgrounds because of two limitations. First, the number of donors was limited in this study, and further studies are warranted to confirm our findings. Second, the PCDH15 or RELN mRNA levels did not decrease significantly in neurons induced from patient-derived iPSCs according to our qRT-PCR data in contrast to isogenic homozygous PCDH15-deleted or RELN-deleted neurons. A previous report using NEUROG2 overexpression-induced neurons from iPSCs of SCZ patients with heterozygous gene deletion obtained a similar result (Flaherty et al., 2017). Therefore, heterozygous gene deletion does not always indicate the downregulation of gene expression in neurons. Considering that these genes tended to be downregulated in patient-derived DSi-EBs, although these changes were not significant, it is possible that gene expression in patient-derived cells was different from that in the control cells during differentiation.
For further insights into the relationship between CNVs and phenotypes, we established and analyzed isogenic PCDH15-deleted or RELN-deleted iPSCs using genome-editing technology. Although the isogenic lines showed dendritic or synaptic phenotypes partially correlated with those of patient iPSC-derived neurons, these phenotypes of isogenic neurons were weaker. These results suggest that CNVs as well as other factors contribute to these phenotypes. We also confirmed that other clinically important CNVs were not present in the patients, although it is still possible that an unknown genetic background affected the phenotypes or clinical pathologies because BP and SCZ are highly polygenic disorders. If large-scale genome-editing technologies that allow for the rescue of CNVs become available in the future, the contributions of CNVs can be clearly and directly proved. Under these conditions, patient-derived iPSCs may be a better tool than isogenic iPSCs for modeling BP and SCZ.
In addition, we performed functional analysis by measuring spontaneous neural activity to investigate whether the observed structural phenotypes led to functional abnormalities. However, we did not identify any functional differences in spontaneous activity in patient or isogenic neurons. Although our neurons seemed to be immature compared with in vivo neurons, which might make it difficult to detect functional differences, our data suggest that the electrophysiological functions of patient-derived glutamatergic neurons were compensated by the enhancement of sensitivity in their receptors. Although structural abnormalities do not always cause functional defects because a compensatory mechanism might contribute to functional maintenance, detailed electrophysiological analyses are warranted in future studies. We also showed that our neurons were electrically active to some extent. Therefore, our in vitro model is suitable for further electrophysiological analyses to elucidate the functional significance of CNVs. Also, coculture of both types of our neurons (glutamatergic and GABAergic) or coculture with glial cells, which is expected to promote further functional maturation and neural network formation, may support this approach in the future studies.
For various diseases, potential therapeutic drugs were identified from phenotypic screening using induced tissues from patient-derived iPSCs (Okano and Yamanaka, 2014; Wainger et al., 2014; Hino et al., 2017; Hosoya et al., 2017; Imamura et al., 2017; Kondo et al., 2017; Fujimori et al., 2018; Tabata et al., 2018). Thus, phenotype-recapitulated in vitro model using iPSC technology is available for exploring new therapeutic agents. Our model showing disorder-related phenotypes can be a useful tool for identifying novel drug candidates.
Overall, our approach for the induction of specific neurons from patient-derived iPSCs with disease-related genetic polymorphisms enabled us to analyze pathology-associated phenotypes. Our in vitro model, which may show general phenotypes of psychiatric disorders, is expected to be applicable for further elucidation of molecular mechanisms or drug discovery.
Acknowledgments
Acknowledgments: We thank the Collaborative Research Resources, School of Medicine, Keio University for technical assistance with microarray analysis. We also thank Dr. Minoru S.H. Ko (Keio University) and Dr. K. Yusa (Sanger Institute) for the provision of plasmids; Dr. T. Sone (Keio University) for the preparation of plasmids; Dr. N. Kuzumaki (Hoshi University) for assistance in experimental design and supervision of the study; and A. Ohnishi (Hoshi University) for technical assistance in the generation of iPSCs.
Synthesis
Reviewing Editor: Julie Andersen, Buck Institute
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below.
This revised manuscript studying the possible effect CNV's in the genes RELN and PCDH15 have in psychiatric patients deserves applaud. The authors sincerely addressed the reviewer comments to enhance their study, and created a more complete story by adding advanced and technical experiments. We believe after making the following minor revisions (none being experiments), the manuscript will be ready for publication.
1. In line 14, the authors describe their observations as “general phenotypes for BP and SCZ”, and maintain that perspective throughout their study. Instead, we suggest framing their observations as “general phenotypes of psychiatric disorders” as both RELN and PCDH15 are not known to be specific to either disease.
2. Starting at line 88, the authors describe the 2nd BP patient iPSC line. In the description, they detail that the subject had hypomanic episodes (but no mention of full manic episodes) as well as other symptomology that may indicate the subject may have a lesser version of bipolar disorder, such as bipolar 2 disorder (even though she was diagnosed as BP1). We suggest the authors include a remark addressing this possible incongruency.
3. After line 660, “We did not find any functional differences in patient or isogenic neurons”, can the authors please add a possible explanation of their results being due to their neurons being immature compared to their in vivo counterparts. And that as differentiation protocols become better at generating more mature neural cultures, these cell lines may display functional differences
4. In figure 1D, the authors should list if there are statistical tests for each histogram and if there is significance.
5. For Figure 2B-J, the authors must explain why they only performed these tests with one iPSC line for both BP and SCZ, as it negates the value of the having the additional lines if they are not being utilized. This figure would be much more impactful if its findings were consistent across all the psychiatric lines, and specifically with the isogenic lines. We will not ask the author's to perform these additional experiments, but the authors must address this choice in the manuscript.
6. Figure 2 I&J do not appear consistent with one another. The plot of neurite length implies the psychiatric lines generate neurites that are over 10 times shorter than healthy controls, but the images in panel I do not show that. We ask the authors either re-examine the validity of their neurite length calculations or provide an explanation for this discrepancy.
7. Figure 4A-B, can the authors label the genes in the PCA diagrams?
8. Figure 6E-G. The histograms' y-axis should be labeled with their respective genes of interest. Also in E, looking at histograms and error bars in the GLUT/PCDH15, it appears there should be statistical significance, especially compared to some of the other graphs (ie Fig. 7E).
9. The statistical significance found in Figure 7C,D,F,G,H are hard to believe based of their mean values and error bars. We ask the authors review their statistical analyses to verify the appropriate tests were performed.
10. Adding to point 3, the MEA data in figure 8D seems to indicate that the cultures had yet to reach an in vivo level of maturity based off their limited spiking activity, and could explain why no functional differences were found.
11. Figure 8G, can the authors clarify the y-axis title, is the “spike number change” in Hz, percentage, frequency, etc...?
12. THIS POINT IS OF HIGHEST IMPORTANCE IN OUR REVIEW. In table 2, the authors list all of their statistical tests. Many of the tests appear to be inappropriate for the experiment performed, or there are unexplained variations in the chosen tests between similar experiments.
a. Specifically, in multiple comparison tests, the authors oscillate between Dunnett and Tukey tests. We ask the authors be consistent between the analyses to maintain an ad hoc versus post hoc appearance.
b. For Figures 7F-H, 8B, & 8J it is inappropriate for the authors to use T-tests, and instead should use a test that considers either multiple samples and/or comparisons.
c. The authors oscillate between SD and SEM error bars, we ask the authors pick a consistent error bar to not confuse the future readers of this study.
Author Response
Point-by-Point Responses to editor and reviewers' comments
We appreciate the editor and reviewers for evaluating our manuscript (#eN-NWR-0403-18) entitled “In vitro modeling of the bipolar disorder and schizophrenia using patient-derived induced pluripotent stem cells with copy number variations of PCDH15 and RELN.” We found the insightful comments made by the reviewers to be very helpful, and we have addressed the issues raised.
We hope that you will find revised manuscript suitable for publication in eNeuro.
Response to the comments by Reviewers:
(Comment 1) In line 14, the authors describe their observations as “general phenotypes for BP and SCZ”, and maintain that perspective throughout their study. Instead, we suggest framing their observations as “general phenotypes of psychiatric disorders” as both RELN and PCDH15 are not known to be specific to either disease.
(Response) We appreciate the reviewer's insightful suggestion. Although we mainly focused on BP and SCZ in this study using iPSCs derived from the patients with these disorders, RELN and PCDH15 can be associated with other disorders as the reviewer pointed out. In fact, both RELN and PCDH15 were also said to be related to ASD. Thus, according to the comment, we added the sentences that the observed phenotypes could be general in psychiatric disorders.
(Comment 2) Starting at line 88, the authors describe the 2nd BP patient iPSC line. In the description, they detail that the subject had hypomanic episodes (but no mention of full manic episodes) as well as other symptomology that may indicate the subject may have a lesser version of bipolar disorder, such as bipolar 2 disorder (even though she was diagnosed as BP1). We suggest the authors include a remark addressing this possible incongruency.
(Response) Thank you for your kind suggestion. According to your comment, we have considerably checked her previous clinical record, and we could not find her severe manic episodes which are necessary for the diagnosis of Bipolar I disorder. Thus, we have revised the diagnosis from Bipolar I disorder to Bipolar II disorder. We corrected the patient information in the manuscript and in Figure 1B.
(Comment 3) After line 660, “We did not find any functional differences in patient or isogenic neurons”, can the authors please add a possible explanation of their results being due to their neurons being immature compared to their in vivo counterparts. And that as differentiation protocols become better at generating more mature neural cultures, these cell lines may display functional differences.
(Comment 10) Adding to point 3, the MEA data in figure 8D seems to indicate that the cultures had yet to reach an in vivo level of maturity based off their limited spiking activity, and could explain why no functional differences were found.
(Response to above two comments) We appreciate the reviewer's important comments. As the reviewer pointed out, our in vitro neurons seemed to be functionally immature compared to in vivo neurons, and more matured neurons may show functional differences. We think co-culture of both glutamatergic neurons and GABAergic neurons or co-culture with glial cells may support further functional maturation or neural network formation. On the other hand, some compensatory mechanisms might also contribute to functional maintenance in patient neurons as suggested in Figure 8G. These issues should be considered in our future studies. We discussed this point and added explanation.
(Comment 4) In figure 1D, the authors should list if there are statistical tests for each histogram and if there is significance.
(Response) Thank you for the thoughtful comment. We analyzed the data using CopyCaller Software (Applied Biosystems). In this software, at least 7 subjects of the same copy number (in this case, PCDH15- or RELN-deleted patients) were required to calculate confidence value, which show an estimate of the confidence that the assigned copy number was the true copy number. Since the subject number was limited, we did not perform the statistical analysis. However, the purpose of this assay is to confirm the CNVs which we identified using array CGH (Figure 1C). And the results of taqman copy number assays (Figure 1D) clearly supported the results of array CGH. Therefore, we believe that the validity of CNVs were assessed in Figure 1C, D.
(Comment 5) For Figure 2B-J, the authors must explain why they only performed these tests with one iPSC line for both BP and SCZ, as it negates the value of the having the additional lines if they are not being utilized. This figure would be much more impactful if its findings were consistent across all the psychiatric lines, and specifically with the isogenic lines. We will not ask the author's to perform these additional experiments, but the authors must address this choice in the manuscript.
(Response) We appreciate the thoughtful and kind comment. Basically, our purpose in Figure 2 was to determine whether the method of neuron induction via EB formation by chemical treatment was suitable for analyzing mature neuron phenotypes. We picked out one donor with BP or SCZ from which two iPSC clones were established, which allowed for considering the variation between clones. In other words, we used two iPSC clones for each psychiatric group.
As a result, the observed phenotypes suggested that this method was not suitable for analysis of mature neurons even though the phenotypes reflected neurodevelopmental pathologies of the disorders. Thus, we did not perform further analysis using isogenic lines. We added the sentences that mentioned this point in the manuscript.
(Comment 6) Figure 2 I&J do not appear consistent with one another. The plot of neurite length implies the psychiatric lines generate neurites that are over 10 times shorter than healthy controls, but the images in panel I do not show that. We ask the authors either re-examine the validity of their neurite length calculations or provide an explanation for this discrepancy.
(Response) We appreciate for the instructive comment. We detected neurites and quantified the neurite length from stained images as shown in Figure 2H. We reconfirmed that the calculations were correctly performed. In Figure 2I, the neurites of the psychiatric lines, especially BP1, might look longer in comparison with their measured values. However, their neurites included many short neurites, and resulted in showing the low averaged values. In addition, we excluded NPC-like migrated cells and cell-crowded regions expressing both βIII-tubulin and Ho for high-accuracy analysis of neurites (Figure 2H). Therefore, we succeeded in accurately visualizing/quantifying their pathologies using our neurite-specific analysis system.
(Comment 7) Figure 4A-B, can the authors label the genes in the PCA diagrams?
(Response) According to the comment, we added the genetic characteristics (PCDH15 CNV or RELN CNV) in the PCA diagrams in Figure 4A, E to clarify the samples information.
(Comment 8) Figure 6E-G. The histograms' y-axis should be labeled with their respective genes of interest. Also in E, looking at histograms and error bars in the GLUT/PCDH15, it appears there should be statistical significance, especially compared to some of the other graphs (ie Fig. 7E).
(Response) Thank you for the kind suggestion. We added the labels of gene names according to the comment. Regarding Figure 6E, we showed the error bars representing SEM (and SD error bars were used in Figure 7). Due to this, it might look there was significant difference compared to Figure 7E. We unified error bars in SD error bars in all figures (please also refer the response to Comment 12C).
(Comment 9) The statistical significance found in Figure 7C,D,F,G,H are hard to believe based of their mean values and error bars. We ask the authors review their statistical analyses to verify the appropriate tests were performed.
(Response) We apologize for any confusion. We used SD error bars, which could overlap with each other if there is significant difference between two groups, while SEM error bars cannot overlap each other when there is significant difference. We used both SD and SEM error bars in the manuscript, which might confuse the reviewers. We reconfirmed that the results of statistical analysis were correct. Then, we unified error bars in SD error bars in all figures (please also refer the response to comment 12C).
(Comment 11) Figure 8G, can the authors clarify the y-axis title, is the “spike number change” in Hz, percentage, frequency, etc...?
(Response) Thank you for the comment. The graph in Figure 8G showed relative spike number changes before and after the treatment. We altered the y-axis title as follows: “Ratio of spike number change (v. s. before treatment)” to clarify it.
(Comment 12) THIS POINT IS OF HIGHEST IMPORTANCE IN OUR REVIEW. In table 2, the authors list all of their statistical tests. Many of the tests appear to be inappropriate for the experiment performed, or there are unexplained variations in the chosen tests between similar experiments.
a. Specifically, in multiple comparison tests, the authors oscillate between Dunnett and Tukey tests. We ask the authors be consistent between the analyses to maintain an ad hoc versus post hoc appearance.
b. For Figures 7F-H, 8B, & 8J it is inappropriate for the authors to use T-tests, and instead should use a test that considers either multiple samples and/or comparisons.
c. The authors oscillate between SD and SEM error bars, we ask the authors pick a consistent error bar to not confuse the future readers of this study.
(Response) We appreciate the reviewer's important comments. We addressed the concerns as follows.
(a) As the reviewer pointed out, it is better to use consistent tests as much as possible. In this study, we used Dunnett's or Tukey's test for multiple comparisons depending on the purpose. Dunnett's test was mainly utilized when we wanted to assess whether or not there were significant changes in each patient group compared with the control group in most figures. On the other hand, we used Tukey's test when we evaluated the ratios of marker-positive cells (Figure 3C, 3G) to compare each clone with each other in these figures because we wanted to assess not the differences from control but the variation in induction efficiency among each clone. Regarding Figure 2G, although we originally used Tukey's test, Dunnett's test seems to be more suitable. Thus, we reanalyzed using Dunnett's test in Figure 2G.
(b) As the reviewer pointed out, we performed Dunnett's test in Figure 8B. Regarding Figure 7F-H and 8J, we made comparisons between Control 1 and PCDH15del or between Control 2 and RELNdel. Because these two pairs (Control 1-PCDH15del and Control 2-RELNdel) or two comparisons were independent of each other, we think that T-tests are appropriate for each comparison.
(c) As the reviewer suggested, using both types of error bar may confuse the future readers. We unified error bars in SD error bars in all figures (SEM error bars were originally used in Figure 2E, 3D, 3H, 6E, 6G).
References
- Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, Griffith AJ, Morell RJ, Friedman TB, Riazuddin S, Wilcox ER (2001) Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am J Hum Genet 69:25–34. 10.1086/321277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, Srisailpathy CR, Lowry RB, Knaus R, Van Laer L, Bernier FP, Schwartz S, Lee C, Morton CC, Mullins RF, Ramesh A, Van Camp G, Hageman GS, Woychik RP, Smith RJ (2001) Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum Mol Genet 10:10. [DOI] [PubMed] [Google Scholar]
- Arioka Y, Shishido E, Kubo H, Kushima I, Yoshimi A, Kimura H, Ishizuka K, Aleksic B, Maeda T, Ishikawa M, Kuzumaki N, Okano H, Mori D, Ozaki N (2018) Single-cell trajectory analysis of human homogenous neurons carrying a rare RELN variant. Transl Psychiatry 8:129. 10.1038/s41398-018-0177-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardy C, Van den Hurk M, Eames T, Marchand C, Hernandez RV, Kellogg M, Gorris M, Galet B, Palomares V, Brown J, Bang AG, Mentens J, Bohnke L, Boyer L, Simon S, Gage FH (2015) Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc Natl Acad Sci U S A 112:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27:275–280. 10.1038/nbt.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasante G, Lignani G, Rubio A, Medrihan L, Yekhlef L, Sessa A, Massimino L, Giannelli SG, Sacchetti S, Caiazzo M, Leo D, Alexopoulou D, Dell'Anno MT, Ciabatti E, Orlando M, Studer M, Dahl A, Gainetdinov RR, Taverna S, Benfenati F, Broccoli V (2015) Rapid conversion of fibroblasts into functional forebrain GABAergic interneurons by direct genetic reprogramming. Cell Stem Cell 17:719–734. 10.1016/j.stem.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Costain G, Lionel AC, Merico D, Forsythe P, Russell K, Lowther C, Yuen T, Husted J, Stavropoulos DJ, Speevak M, Chow EW, Marshall CR, Scherer SW, Bassett AS (2013) Pathogenic rare copy number variants in community-based schizophrenia suggest a potential role for clinical microarrays. Hum Mol Genet 22:4485–4501. 10.1093/hmg/ddt297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Sklar P (2013) Genetics of bipolar disorder. Lancet 381:1654–1662. 10.1016/S0140-6736(13)60855-7 [DOI] [PubMed] [Google Scholar]
- Espuny-Camacho I, Michelsen KA, Gall D, Linaro D, Hasche A, Bonnefont J, Bali C, Orduz D, Bilheu A, Herpoel A, Lambert N, Gaspard N, Péron S, Schiffmann SN, Giugliano M, Gaillard A, Vanderhaeghen P (2013) Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron 77:440–456. 10.1016/j.neuron.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Flaherty E, Deranieh RM, Artimovich E, Lee IS, Siegel AJ, Levy DL, Nestor MW, Brennand KJ (2017) Patient-derived hiPSC neurons with heterozygous CNTNAP2 deletions display altered neuronal gene expression and network activity. NPJ Schizophr 3:35 10.1038/s41537-017-0033-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom TD, Fatemi SH (2013) The involvement of Reelin in neurodevelopmental disorders. Neuropharmacology 68:122–135. 10.1016/j.neuropharm.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori K, Ishikawa M, Otomo A, Atsuta N, Nakamura R, Akiyama T, Hadano S, Aoki M, Saya H, Sobue G, Okano H (2018) Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat Med 24:1579–1589. 10.1038/s41591-018-0140-5 [DOI] [PubMed] [Google Scholar]
- Gao R, Penzes P (2015) Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med 15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva L, Rees E, Moran JL, Chambert KD, Milanova V, Craddock N, Purcell S, Sklar P, McCarroll S, Holmans P, O'Donovan MC, Owen MJ, Kirov G (2014) De novo CNVs in bipolar affective disorder and schizophrenia. Hum Mol Genet 23:6677–6683. 10.1093/hmg/ddu379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA (2000) Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 57:65–73. 10.1001/archpsyc.57.1.65 [DOI] [PubMed] [Google Scholar]
- Grande I, Berk M, Birmaher B, Vieta E (2016) Bipolar disorder. Lancet 387:1561–1572. 10.1016/S0140-6736(15)00241-X [DOI] [PubMed] [Google Scholar]
- Green EK, Rees E, Walters JT, Smith KG, Forty L, Grozeva D, Moran JL, Sklar P, Ripke S, Chambert KD, Genovese G, McCarroll SA, Jones I, Jones L, Owen MJ, O'Donovan MC, Craddock N, Kirov G (2016) Copy number variation in bipolar disorder. Mol Psychiatry 21:89–93. 10.1038/mp.2014.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SS, Williams LA, Eggan KC (2011) Constructing and deconstructing stem cell models of neurological disease. Neuron 70:626–644. 10.1016/j.neuron.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Hino K, Horigome K, Nishio M, Komura S, Nagata S, Zhao C, Jin Y, Kawakami K, Yamada Y, Ohta A, Toguchida J, Ikeya M (2017) Activin-A enhances mTOR signaling to promote aberrant chondrogenesis in fibrodysplasia ossificans progressiva. J Clin Invest 127:3339–3352. 10.1172/JCI93521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Nakajima K (2017) Control of neuronal migration and aggregation by reelin signaling in the developing cerebral cortex. Front Cell Dev Biol 5:40. 10.3389/fcell.2017.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya M, Fujioka M, Sone T, Okamoto S, Akamatsu W, Ukai H, Ueda HR, Ogawa K, Matsunaga T, Okano H (2017) Cochlear cell modeling using disease-specific iPSCs unveils a degenerative phenotype and suggests treatments for congenital progressive hearing loss. Cell Rep 18:68–81. 10.1016/j.celrep.2016.12.020 [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Sone T, Ibata K, Fujimori K, Yuzaki M, Akamatsu W, Okano H (2015) Controlling the regional identity of hpsc-derived neurons to uncover neuronal subtype specificity of neurological disease phenotypes. Stem Cell Reports 5:1010–1022. 10.1016/j.stemcr.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y, Okada Y, Akamatsu W, Koike M, Kuzumaki N, Hayakawa H, Nihira T, Kobayashi T, Ohyama M, Sato S, Takanashi M, Funayama M, Hirayama A, Soga T, Hishiki T, Suematsu M, Yagi T, Ito D, Kosakai A, Hayashi K, et al. (2012) Mitochondrial dysfunction associated with increased oxidative stress and α-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol Brain 5:35 10.1186/1756-6606-5-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Izumi Y, Watanabe A, Tsukita K, Woltjen K, Yamamoto T, Hotta A, Kondo T, Kitaoka S, Ohta A, Tanaka A, Watanabe D, Morita M, Takuma H, Tamaoka A, Kunath T, Wray S, Furuya H, Era T, Makioka K, et al. (2017) The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Transl Med 9:eaaf3962. [DOI] [PubMed] [Google Scholar]
- Ishii K, Kubo KI, Nakajima K (2016) Reelin and neuropsychiatric disorders. Front Cell Neurosci 10:229. 10.3389/fncel.2016.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M, Kohyama J, Iwanami A, Sanosaka T, Sugai K, Yamaguchi R, Matsumoto T, Nakamura M, Okano H (2016) Robust production of human neural cells by establishing neuroepithelial-like stem cells from peripheral blood mononuclear cell-derived feeder-free iPSCs under xeno-free conditions. Neurosci Res 110:18–28. 10.1016/j.neures.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Jaiganesh A, Narui Y, Araya-Secchi R, Sotomayor M (2018) Beyond cell-cell adhesion: sensational cadherins for hearing and balance. Cold Spring Harb Perspect Biol 10:a029280 10.1101/cshperspect.a029280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-Y, Yasuda S, Tanaka H, Yamagata K, Kim H (2011) Non-clustered protocadherin. Cell Adh Migr 5:97–105. 10.4161/cam.5.2.14374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryushko D, Berezin V, Bock E (2004) Regulators of neurite outgrowth: role of cell adhesion molecules. Ann N Y Acad Sci 1014:140–154. 10.1196/annals.1294.015 [DOI] [PubMed] [Google Scholar]
- Kondo T, Imamura K, Funayama M, Tsukita K, Miyake M, Ohta A, Woltjen K, Nakagawa M, Asada T, Arai T, Kawakatsu S, Izumi Y, Kaji R, Iwata N, Inoue H (2017) iPSC-based compound screening and in vitro trials identify a synergistic anti-amyloid β combination for Alzheimer’s disease. Cell Rep 21:2304–2312. 10.1016/j.celrep.2017.10.109 [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Lange N, Coyle JT, Benes FM (2014) Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry 71:1323–1331. 10.1001/jamapsychiatry.2014.1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushima I, Aleksic B, Nakatochi M, Shimamura T, Shiino T, Yoshimi A, Kimura H, Takasaki Y, Wang C, Xing J, Ishizuka K, Oya-Ito T, Nakamura Y, Arioka Y, Maeda T, Yamamoto M, Yoshida M, Noma H, Hamada S, Morikawa M, et al. (2017) High-resolution copy number variation analysis of schizophrenia in Japan. Mol Psychiatry 22:430–440. 10.1038/mp.2016.88 [DOI] [PubMed] [Google Scholar]
- Lee GH, D'Arcangelo G (2016) New insights into reelin-mediated signaling pathways. Front Cell Neurosci 10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Zhang Y, Kim S, Han K (2018) Excitatory and inhibitory synaptic dysfunction in mania: an emerging hypothesis from animal model studies. Exp Mol Med 50:12. 10.1038/s12276-018-0028-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MT, Hinds DA, Tung JY, Franz C, Fan CC, Wang Y, Smeland OB, Schork A, Holland D, Kauppi K, Sanyal N, Escott-Price V, Smith DJ, O'Donovan M, Stefansson H, Bjornsdottir G, Thorgeirsson TE, Stefansson K, McEvoy LK, Dale AM, et al. (2017) Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nat Genet 49:152–156. 10.1038/ng.3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison JM, Zhou F, Nigam A, Hussain A, Barker DD, Nehme R, van der Ven K, Hsu J, Wolf P, Fleishman M, O'Dushlaine C, Rose S, Chambert K, Lau FH, Ahfeldt T, Rueckert EH, Sheridan SD, Fass DM, Nemesh J, Mullen TE, et al. (2015) Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol Psychiatry 20:703–717. 10.1038/mp.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggioni E, Crespo-Facorro B, Nenadic I, Benedetti F, Gaser C, Sauer H, Roiz-Santiañez R, Poletti S, Marinelli V, Bellani M, Perlini C, Ruggeri M, Altamura AC, Diwadkar VA, Brambilla P (2017) Common and distinct structural features of schizophrenia and bipolar disorder: the European Network on Psychosis, Affective disorders and Cognitive Trajectory (ENPACT) study. PLoS One 12:e0188000. 10.1371/journal.pone.0188000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O (2012) Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 13:107–120. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Fujimori K, Andoh-Noda T, Ando T, Kuzumaki N, Toyoshima M, Tada H, Imaizumi K, Ishikawa M, Yamaguchi R, Isoda M, Zhou Z, Sato S, Kobayashi T, Ohtaka M, Nishimura K, Kurosawa H, Yoshikawa T, Takahashi T, Nakanishi M, et al. (2016) Functional neurons generated from T cell-derived induced pluripotent stem cells for neurological disease modeling. Stem Cell Reports 6:422–435. 10.1016/j.stemcr.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Nakatake Y, Arai I, Ibata K, Kohda K, Goparaju SK, Murakami M, Sakota M, Chikazawa-Nohtomi N, Ko SBH, Kanai T, Yuzaki M, Ko MSH (2017) Neural differentiation of human embryonic stem cells induced by the transgene-mediated overexpression of single transcription factors. Biochem Biophys Res Commun 490:296–301. 10.1016/j.bbrc.2017.06.039 [DOI] [PubMed] [Google Scholar]
- McGrath J, Saha S, Chant D, Welham J (2008) Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 30:67–76. 10.1093/epirev/mxn001 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, Gallinat J, Giedd J, Grayson DR, Heinrichs M, Kahn R, Krebs MO, Leboyer M, Lewis D, Marin O, Marin P, Meyer-Lindenberg A, McGorry P, McGuire P, Owen MJ, et al. (2016) Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov 15:485–515. 10.1038/nrd.2016.28 [DOI] [PubMed] [Google Scholar]
- Missler M, Südhof TC, Biederer T (2012) Synaptic cell adhesion. Cold Spring Harb Perspect Biol 4:a005694. 10.1101/cshperspect.a005694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, Asano K, Morizane A, Doi D, Takahashi J, Nishizawa M, Yoshida Y, Toyoda T, Osafune K, Sekiguchi K, Yamanaka S (2014) A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep 4:3594. 10.1038/srep03594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme R, Zuccaro E, Ghosh SD, Li C, Sherwood JL, Pietilainen O, Barrett LE, Limone F, Worringer KA, Kommineni S, Zang Y, Cacchiarelli D, Meissner A, Adolfsson R, Haggarty S, Madison J, Muller M, Arlotta P, Fu Z, Feng G, et al. (2018) Combining NGN2 programming with developmental patterning generates human excitatory neurons with NMDAR-mediated synaptic transmission. Cell Rep 23:2509–2523. 10.1016/j.celrep.2018.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor A, Lionel AC, Cohen-Woods S, Moghimi N, Rucker J, Fennell A, Thiruvahindrapuram B, Kaufman L, Degagne B, Wei J, Parikh SV, Muglia P, Forte J, Scherer SW, Kennedy JL, Xu W, McGuffin P, Farmer A, Strauss J, Vincent JB (2014) Copy number variant study of bipolar disorder in Canadian and UK populations implicates synaptic genes. Am J Med Genet B Neuropsychiatr Genet 165B:303–313. 10.1002/ajmg.b.32232 [DOI] [PubMed] [Google Scholar]
- O'Dushlaine C, Kenny E, Heron E, Donohoe G, Gill M, Morris D, Corvin A (2011) Molecular pathways involved in neuronal cell adhesion and membrane scaffolding contribute to schizophrenia and bipolar disorder susceptibility. Mol Psychiatry 16:286–292. 10.1038/mp.2010.7 [DOI] [PubMed] [Google Scholar]
- Okada Y, Shimazaki T, Sobue G, Okano H (2004) Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev Biol 275:124–142. 10.1016/j.ydbio.2004.07.038 [DOI] [PubMed] [Google Scholar]
- Okano H, Yamanaka S (2014) iPS cell technologies: significance and applications to CNS regeneration and disease. Mol Brain 7:22. 10.1186/1756-6606-7-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S (2011) A more efficient method to generate integration-free human iPS cells. Nat Methods 8:409–412. 10.1038/nmeth.1591 [DOI] [PubMed] [Google Scholar]
- Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, Goshima N, Yamanaka S (2013) An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells 31:458–466. 10.1002/stem.1293 [DOI] [PubMed] [Google Scholar]
- O'Shea KS, McInnis MG (2016) Neurodevelopmental origins of bipolar disorder: iPSC models. Mol Cell Neurosci 73:63–83. 10.1016/j.mcn.2015.11.006 [DOI] [PubMed] [Google Scholar]
- Owen MJ, O'Donovan MC (2017) Schizophrenia and the neurodevelopmental continuum: evidence from genomics. World Psychiatry 16:227–235. 10.1002/wps.20440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prytkova I, Brennand KJ (2017) Prospects for modeling abnormal neuronal function in schizophrenia using human induced pluripotent stem cells. Front Cell Neurosci 11:360. 10.3389/fncel.2017.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski TP, Schroeder JP, Gafford GM, Warren ST, Weinshenker D, Caspary T, Mulle JG (2017) Unraveling the genetic architecture of copy number variants associated with schizophrenia and other neuropsychiatric disorders. J Neurosci Res 95:1144–1160. 10.1002/jnr.23970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T (2017) The role of cell adhesion molecules in brain wiring and neuropsychiatric disorders. Mol Cell Neurosci 81:4–11. 10.1016/j.mcn.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Sekine K, Kawauchi T, Kubo K, Honda T, Herz J, Hattori M, Kinashi T, Nakajima K (2012) Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin α5β1. Neuron 76:353–369. 10.1016/j.neuron.2012.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki T, Shingo T, Weiss S (2001) The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci 21:7642–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue A, Kushima I, Nagai T, Shan W, Kohno T, Aleksic B, Aoyama Y, Mori D, Arioka Y, Kawano N, Yamamoto M, Hattori M, Nabeshima T, Yamada K, Ozaki N (2018) Genetic and animal model analyses reveal the pathogenic role of a novel deletion of RELN in schizophrenia. Sci Rep 8:13046 10.1038/s41598-018-31390-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AX, Yuan Q, Tan S, Xiao Y, Wang D, Khoo AT, Sani L, Tran HD, Kim P, Chiew YS, Lee KJ, Yen YC, Ng HH, Lim B, Je HS (2016) Direct induction and functional maturation of forebrain GABAergic neurons from human pluripotent stem cells. Cell Rep 16:1942–1953. 10.1016/j.celrep.2016.07.035 [DOI] [PubMed] [Google Scholar]
- Tabata Y, Imaizumi Y, Sugawara M, Ando-Noda T, Banno S, Chai M, Sone T, Yamazaki K, Ito M, Tsukahara K, Saya H, Hattori N, Kohyama J, Okano H (2018) T-type calcium channels determine the vulnerability of dopaminergic neurons to mitochondrial stress in familial Parkinson’s disease. Stem Cell Reports 11:1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Tobe BTD, Crain AM, Winquist AM, Calabrese B, Snyder EY (2017) Probing the lithium-response pathway in hiPSCs implicates the phosphoregulatory set-point for a cytoskeletal modulator in bipolar pathogenesis. Proc Natl Acad Sci U S A 114:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima M, Akamatsu W, Okada Y, Ohnishi T, Balan S, Hisano Y, Iwayama Y, Toyota T, Matsumoto T, Itasaka N, Sugiyama S, Tanaka M, Yano M, Dean B, Okano H, Yoshikawa T (2016) Analysis of induced pluripotent stem cells carrying 22q11.2 deletion. Transl Psychiatry 6:e934. 10.1038/tp.2016.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainger BJ, Kiskinis E, Mellin C, Wiskow O, Han SS, Sandoe J, Perez NP, Williams LA, Lee S, Boulting G, Berry JD, Brown RH Jr, Cudkowicz ME, Bean BP, Eggan K, Woolf CJ (2014) Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep 7:1–11. 10.1016/j.celrep.2014.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AY, Lohmann KM, Yang CK, Zimmerman EI, Pantazopoulos H, Herring N, Berretta S, Heckers S, Konradi C (2011) Bipolar disorder type 1 and schizophrenia are accompanied by decreased density of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region. Acta Neuropathol 122:615–626. 10.1007/s00401-011-0881-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watmuff B, Berkovitch SS, Huang JH, Iaconelli J, Toffel S, Karmacharya R (2016) Disease signatures for schizophrenia and bipolar disorder using patient-derived induced pluripotent stem cells. Mol Cell Neurosci 73:96–103. 10.1016/j.mcn.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z (2017) Modeling neurodevelopmental and psychiatric diseases with human iPSCs. J Neurosci Res 95:1097–1109. 10.1002/jnr.24031 [DOI] [PubMed] [Google Scholar]
- Yang N, Chanda S, Marro S, Ng YH, Janas JA, Haag D, Ang CE, Tang Y, Flores Q, Mall M, Wapinski O, Li M, Ahlenius H, Rubenstein JL, Chang HY, Buylla AA, Südhof TC, Wernig M (2017) Generation of pure GABAergic neurons by transcription factor programming. Nat Methods 14:621–628. 10.1038/nmeth.4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, Xu W, Yang N, Danko T, Chen L, Wernig M, Südhof TC (2013) Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78:785–798. 10.1016/j.neuron.2013.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]