Significance
We found that orders and initial doses of treatment in the hospital were strongly influenced by time of day, regardless of drug type, diagnosis, or care unit. As the first large-scale account of 24-h rhythms in hospital medicine, this study identifies a potential operational barrier to best clinical care. Clinical decisions should be made around the clock; pain, infection, hypertensive crisis, and other conditions do not occur selectively in the morning. Systemic bias in the timing of medicine may also conflict with circadian biology, which can influence when certain treatments are most effective or safe. Our findings suggest that time of day in hospital operations deserves further consideration.
Keywords: circadian medicine, hospital systems operation, biological rhythms, shift work
Abstract
Hospitals operate 24 h a day, and it is assumed that important clinical decisions occur continuously around the clock. However, many aspects of hospital operation occur at specific times of day, including medical team rounding and shift changes. It is unclear whether this impacts patient care, as no studies have addressed this. We analyzed the daily distribution of ∼500,000 doses of 12 separate drugs in 1,546 inpatients at a major children’s hospital in the United States from 2010 to 2017. We tracked both order time (when a care provider places an electronic request for a drug) and dosing time (when the patient receives the drug). Order times were time-of-day−dependent, marked by distinct morning-time surges and overnight lulls. Nearly one-third of all 103,847 orders for treatment were placed between 8:00 AM and 12:00 PM. First doses from each order were also rhythmic but shifted by 2 h. These 24-h rhythms in orders and first doses were remarkably consistent across drugs, diagnosis, and hospital units. This rhythm in hospital medicine coincided with medical team rounding time, not necessarily immediate medical need. Lastly, we show that the clinical response to hydralazine, an acute antihypertensive, is dosing time-dependent and greatest at night, when the fewest doses were administered. The prevailing dogma is that hospital treatment is administered as needed regardless of time of day. Our findings challenge this notion and reveal a potential operational barrier to best clinical care.
Hospitals operate 24 h a day, yearlong. Caretakers are available around the clock, and it is assumed that important clinical decisions occur continuously regardless of time of day. However, many universal aspects of hospital operation occur at particular times of day, including medical team rounding and staff shift changes (1, 2). It is unclear whether this impacts patient care, as an empirical account of 24-h treatment patterns is lacking.
We conducted an observational cohort study to investigate whether the ordering or administration of hospital treatment is influenced by time of day. Blood pressure (BP) is a continuous phenotype universally tracked in an intensive care unit (ICU) setting. We analyzed the 24-h distribution of order and administration times in 1,546 patients who received at least one dose of hydralazine, a commonly used antihypertensive in the ICU, at a tertiary pediatric hospital from 2010 to 2017. Although hydralazine treatment was the basis for patient inclusion, our analysis also included 11 other drugs commonly administered to these patients.
We found that treatment was strongly time-of-day−dependent. Leveraging this large dataset of ∼500,000 doses, we examined the 24-h distribution of order and administration times of multiple different drug classes, across different care units. Lastly, we explored the impact of dosing time on clinical outcomes.
Results
Hydralazine is a vasodilator used to treat essential hypertension. We analyzed its daily use in 1,546 patients who received at least one dose of the drug (Table 1). In total, 5,485 orders for hydralazine were placed, each stipulating a dosing frequency of “once,” “as needed,” or “scheduled” (Table 2). We tracked both order time (when a care provider places an electronic request for a drug) and dosing time (when the patient receives the drug) for each dose. Initial analyses considered only the first dose administered after each order.
Table 1.
Patient admissions and treatment summary
| Age, y | ||||||
| Patient attributes | 1–5 | 5–7 | 7–10 | 10–15 | 15–20 | >20 |
| Sex (%) | ||||||
| Female | 232 (40) | 239 (42) | 594 (39) | 685 (40) | 514 (42) | 1,088 (52) |
| Male | 349 (60) | 332 (58) | 936 (61) | 1,018 (60) | 699 (58) | 1,000 (48) |
| Diagnosis % | ||||||
| Fever | 7 | 10 | 11 | 12 | 6 | 5 |
| Respiratory distress | 3 | 2 | 3 | 3 | 2 | 3 |
| Hemophagocytic syndromes | 2 | 2 | 2 | 1 | 1 | 1 |
| Leukemia | 2 | 3 | 2 | 5 | 4 | 3 |
| Dehydration | 1 | 1 | 2 | 1 | 1 | 1 |
| Neutropenia | 1 | 2 | 2 | 2 | 2 | 1 |
| Renal failure/injury | 1 | 1 | 3 | 3 | 1 | 3 |
| Pneumonia | 1 | 1 | 2 | 2 | 2 | 2 |
| Cystic fibrosis | <1 | <1 | <1 | <1 | <1 | 3 |
| Other | 31 | 32 | 38 | 37 | 40 | 48 |
| Not reported | 51 | 44 | 34 | 35 | 40 | 29 |
Total admissions (n = 7,686) for 1,546 patients from 2010 to 2017.
Table 2.
Treatment summary
| Drug | Orders | % order frequency | Doses | % treatment unit | |||||
| Once | Sched. | As needed | Total | % first | ICU | Floor | Other/NA | ||
| Hydralazine | 5,485 | 41 | 5 | 53 | 16,662 | 33 | 43 | 39 | 18 |
| Acetaminophen | 22,174 | 30 | 13 | 57 | 85,599 | 26 | 21 | 54 | 24 |
| Furosemide | 16,199 | 71 | 29 | 0 | 40,815 | 40 | 41 | 44 | 15 |
| Morphine | 13,084 | 28 | 7 | 64 | 65,463 | 20 | 46 | 33 | 21 |
| Lorazepam | 9,510 | 30 | 33 | 37 | 72,879 | 13 | 43 | 37 | 21 |
| Ondansetron | 8,005 | 20 | 37 | 44 | 62,061 | 13 | 14 | 61 | 25 |
| Fentanyl | 6,882 | 40 | 2 | 58 | 25,668 | 27 | 67 | 8 | 25 |
| Hydrocortisone | 6,623 | 26 | 67 | 7 | 45,996 | 14 | 24 | 58 | 18 |
| Diphenhydramine | 7,616 | 38 | 10 | 51 | 26,761 | 28 | 12 | 65 | 24 |
| Methylprednisolone | 3,818 | 32 | 67 | 1 | 20,118 | 19 | 35 | 44 | 21 |
| Labetalol | 2,441 | 33 | 38 | 29 | 17,021 | 14 | 41 | 37 | 22 |
| Vancomycin | 2,010 | 18 | 82 | 0 | 15,070 | 13 | 30 | 33 | 36 |
Treatment orders (n = 103, 847) and doses (n = 494,113). Sched., scheduled (e.g., take every 4 hours); NA, not reported in medical record.
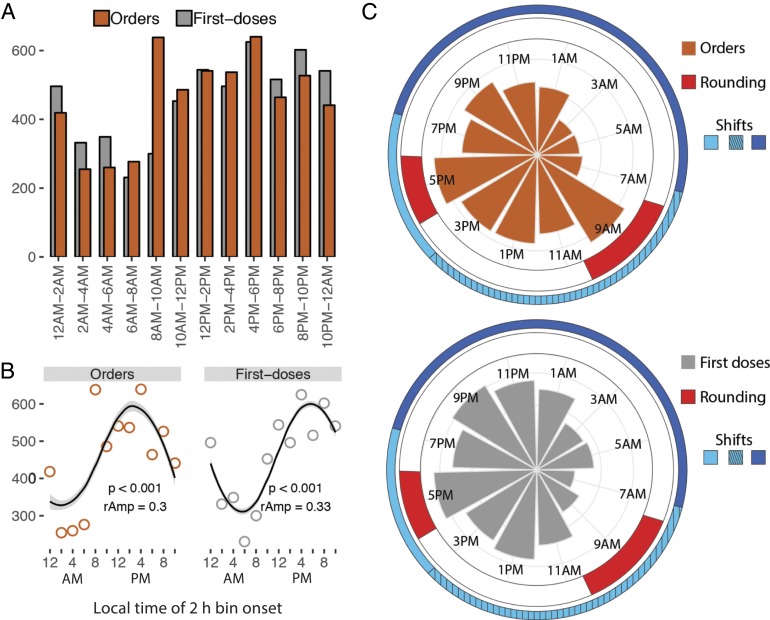
Hydralazine order and first-dosing times were nonuniformly distributed over 24 h [Kuiper’s test (3), P < 0.01], marked by distinct morning-time surges and overnight lulls (Fig. 1). Nearly twice as many treatments were ordered between 8:00 AM and 6:00 PM (2,842) compared to 10:00 PM and 8:00 AM (1,652). The profiles were described by 24-h rhythms using 3 separate detection methods [cosinor analysis (4), Jonckheere-Terpstra-Kendall (JTK)_CYCLE (5, 6), and Rhythmicity Analysis Incorporating Nonparametric (RAIN) (7), P < 0.05] (Fig. 1B and Dataset S1). The morning surge in hydralazine order times coincides with team rounding and a medical staff shift change (Fig. 1C). Caretaker shifts at our institution include 7:00 AM to 7:00 PM, 7:00 AM to 3:00 PM, and 7:00 PM to 7:00 AM shifts. Team rounds typically occur from ∼7:30 AM to 10:30 AM and 4:00 PM to 6:00 PM. These schedules are common across the United States.
Fig. 1.
Daily rhythms in the timing of hydralazine treatment. (A) Nearly twice as many treatments were ordered from 10:00 AM to 6:00 PM (2,842) compared to 10:00 PM to 8:00 AM (1,652). (B) The number of orders and first doses in 2-h bins were modeled by a cosinor waveform with a 24-h period. P value (p) and relative amplitude (rAmp) are indicated. (C) Polar histograms indicate the times of hydralazine orders (Top) and first doses (Bottom). Team rounds occur from 7:30 AM to 10:30 AM and 4:00 PM to 6:00 PM. Clinical caretakers at this study site work either a 7:00 AM to 7:00 PM (light blue), 7:00 AM to 3:00 PM (light blue hashed), or 7:00 PM to 7:00 AM shift (dark blue). This schedule is common throughout hospitals in the United States.
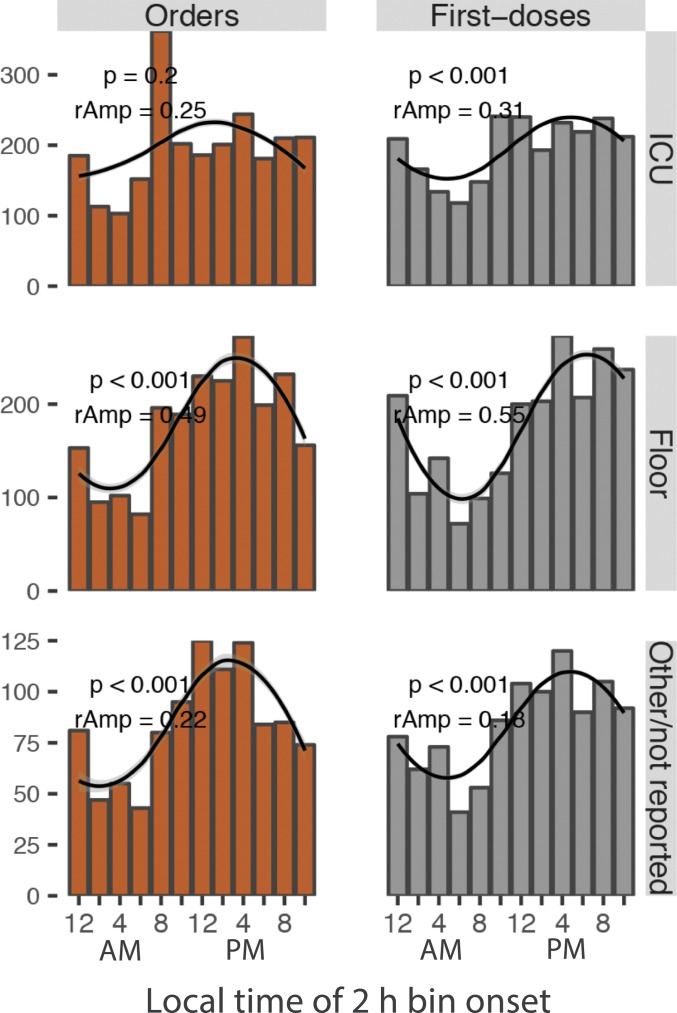
Rhythms in hydralazine orders and first doses were consistent across patient age groups and treatment units––ICU or general floor (Fig. 2 and SI Appendix, Fig. S1A). To determine whether the 24-h rhythm in dosing of hydralazine reflected immediate medical need for treatment, we analyzed BPs immediately prior to drug dosing (d-BP). For systolic d-BP, there were no statistically discernible differences between any 2-h time bins, in any age group (one-way ANOVA, P > 0.05) (SI Appendix, Fig. S1A). For diastolic d-BP, although multivariate analysis detected marginally significant time-of-day differences in 2 of the 6 age groups (one-way ANOVA, P < 0.05), post hoc analyses found only one significant difference (Tukey’s test, adjusted P < 0.05) across all 2-h time bin comparisons (SI Appendix, Fig. S1B and Dataset S2). This suggested that 24-h rhythms in treatment were not solely driven by immediate medical need. Why was the distribution of dosing BPs discordant with dosing time?
Fig. 2.
Rhythms in hydralazine orders and first doses across hospital units. The number of orders and first doses in 2-h bins were modeled by a cosinor waveform with a 24-h period. P value (p) and relative amplitude (rAmp) are indicated.
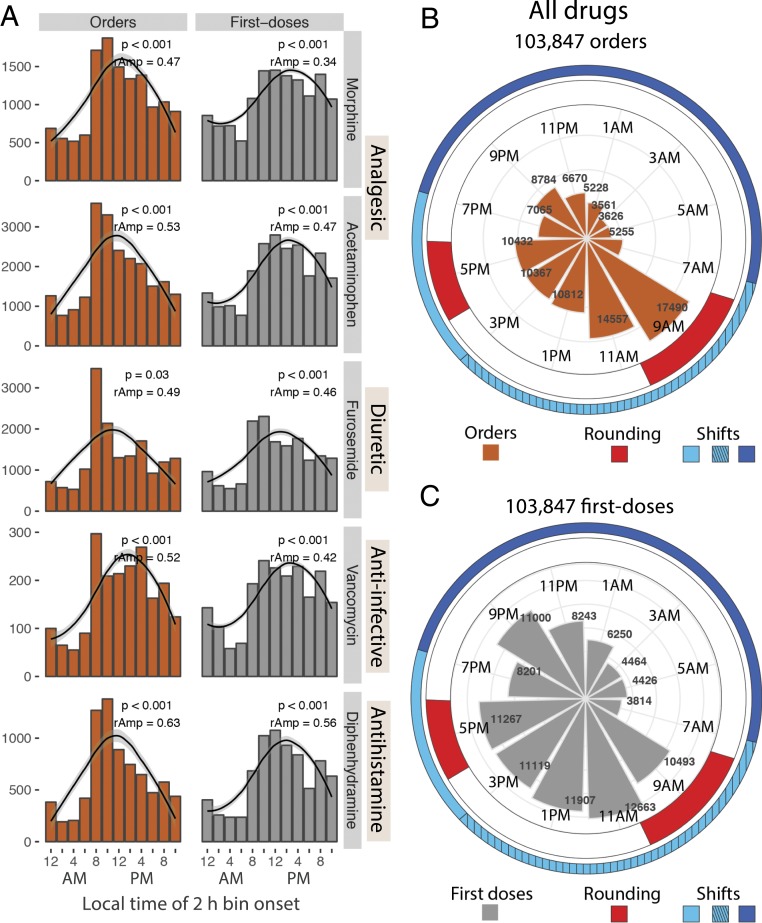
We next tested whether the 24-h patterns in hydralazine use generalized to other therapies, including analgesics, antiinfectives, antihistamines, diuretics, and other drug classes commonly used in the hospital. For each of the additional 11 drugs analyzed, order and first dose times were nonuniformly distributed (Kuiper’s test, P < 0.01), with characteristic morning surges and overnight lulls (Fig. 3A and SI Appendix, Fig. S2A). The majority (9 out of 11) were described by 24-h rhythms (cosinor, P < 0.05; RAIN or JTK_CYCLE, P < 0.05), regardless of treatment unit (Dataset S1 and SI Appendix, Fig. S2B). No single model provided the best fit for all. Whereas cosinor methods fit well to profiles with smoother rises and falls, RAIN (an umbrella function) better fit several of the spiky (sawtooth) profiles. Overall, nearly one-third of the total 103,847 drug orders were placed during the 4-h time window from 8:00 AM to 12:00 PM. This morning surge in ordering and first doses of all drugs coincides with team rounding (Fig. 3 B and C).
Fig. 3.
Rhythms in treatment across drug classes coincide with hospital-wide operational activity. (A) Ordering and first dose administration of several different drug classes all showed 24-h rhythms marked by morning time surges and nighttime lulls. (B and C) The polar histogram indicates the times of (B) all drug orders and (C) first doses administered. The onset of peak ordering time coincides with morning team rounding (indicated in red). Clinical caretakers at this study site work either a 7:00 AM to 7:00 PM (light blue), 7:00 AM to 3:00 PM (light blue hashed), or 7:00 PM to 7:00 AM shift (dark blue). Team rounds occur from 7:30 AM to 10:30 AM and 4:00 PM to 6:00 PM.
The distribution of first doses resembled orders, but was shifted by ∼1 h to 2 h (Fig. 3 and Dataset S1). At our institution, orders are entered, filled by the central pharmacy, sent to the ordering site, and then administered by staff. This creates a time lag from order to first dose, which, interestingly, was also influenced by time of day (SI Appendix, Fig. S3A). Lags were longest for orders placed from 8:00 AM to 12:00 PM (one-way ANOVA, P < 0.05, 9 out of 12 drugs). In sum, we think first dose times are primarily determined by order time plus an operational lag.
Previous analyses included all order types (“once,” “scheduled,” or “as needed”) but considered only the first doses from each order, which comprised 21% of all doses. Next, we compared first doses with “all other doses,” focusing on hydralazine as an example (SI Appendix, Fig. S3B). As expected, first dose times were rhythmically distributed regardless of order type. Conversely, “all other doses” from scheduled orders had no discernible 24-h rhythm. This was not surprising, as timings were stipulated (e.g., “take every 8 h”). Interestingly, “all other doses” from “as needed” orders (e.g., “take every 8 h as needed”) showed a weaker but still discernible 24-h rhythm. Thus, although first doses showed the strongest 24-h patterns, decisions to administer subsequent doses may also be influenced by time of day.
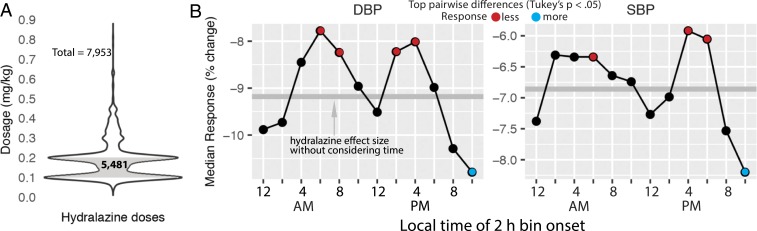
Rhythms in hospital medicine may conflict with circadian biology, as dosing time influences responses to many types of treatment (8). For example, in the outpatient setting, short-acting antihypertensives are most effective at lowering BP if taken before bedtime (9). To test whether this applies to acute antihypertensive therapy in the hospital, we analyzed inpatient responses to 7,953 doses of intravenous (IV) hydralazine as a function of dosing time (Fig. 4 and SI Appendix, SI Methods). Response was computed as the percent change between dosing BP (just before dose) and mean BP over the 3 h following each dose. To control for the impact of dosage, we stratified doses by concentration (milligrams per kilogram body weight) (Fig. 4A). The response to hydralazine varied over 24 h (analysis of covariance [ANCOVA], diastolic P = 2e-13; systolic P = 6e-4) in the most common dosage group (0.1 ≥ mg/kg ≤ 0.2; n = 5,481 of 7,953 doses) (Fig. 4B and SI Appendix, Fig. S4). BP was more responsive to nighttime dosing (10:00 PM to 12:00 AM) compared to morning (6:00 AM to 10:00 AM) and late afternoon (2:00 PM to 6:00 PM) dosing (Fig. 4B, Tukey’s test, p-adjusted < 0.05; see Dataset S2 for difference and confidence levels for all pairwise comparisons). Both diastolic and systolic responses followed this pattern, although differences in diastolic BP were stronger. In sum, nighttime hydralazine was associated with an ∼3 to 4% greater reduction in diastolic BP, on average, than morning dosing (Tukey’s test, difference = −3.7, 95% CI upper level = −5.7, lower level = −1.1, adjusted P = 1e-07). This effect was also independent of patient sex (Dataset S2).
Fig. 4.
Clinical response to hydralazine varies by time of administration. (A) The 5,481 doses of IV hydralazine ranging from 0.1 to 0.2 mg/kg body weight. (B) Median response to hydralazine in diastolic BP (DBP) and systolic BP (SBP) as a function of dosing time. Response was computed as the percent change between dosing BP (just before dose) and mean BP over the 3 h following each dose (Methods). Tukey’s post hoc pairwise tests between all 2-h time bins detected strongest differences between 10:00 PM and 12:00 AM versus 6:00 AM to 8:00 AM, 8:00 AM to 10:00 AM, 2:00 PM to 4:00 PM, and 4:00 PM to 6:00 PM (Dataset S2).
We did not detect robust 24-h variation in response for any of the other 3 dosage groups, which included far fewer doses (SI Appendix, Fig. S4). Given the many potential sources of variation in response in this cohort, a larger sample may be required to detect 24-h patterns. Nevertheless, for the majority of doses, the clinical response to hydralazine varied over 24 h.
Discussion
Order and first dosing times of antihypertensives, analgesics, antiinfectives, and other drug therapies all followed a 24-h rhythm characterized by a morning surge and an overnight lull. There are no practice guidelines or institutional policies specifying time-of-day recommendations for any of the drugs in this study. The surge in orders (8:00 AM to 10:00 AM) coincides with rounding—when the medical care team visits each patient and collaboratively develops a treatment plan. Orders for diagnostics, therapies, and referrals to specialty services are commonly placed during rounds or immediately thereafter, once final consensus is reached among the team.
Systemic bias toward treatment at a particular time of day is problematic. Antiinfectives should be administered when an infection is identified, whereas analgesics should be administered when there is pain. Neither of these happens selectively in the morning. In fact, evidence shows that pain is more severe in the evening (10). Circadian clocks coordinate physiologic functions (11) and affect therapeutic responses according to the time of day (8). For example, patterns in BP are closely tied to both endogenous circadian rhythms and the sleep−wake cycle (12). BP characteristically decreases during sleep, often referred to as “dipping” (13). Cardiovascular disease is more common in people that demonstrate nondipping BPs while asleep (13). Circadian variability is now being exploited to improve treatments for various disorders, including cardiovascular disease (9, 14–16). Short-acting antihypertensives taken by millions of adults, for example, are most effective before bedtime (17). Here, we show that this is also true in the pediatric hospital setting. The clinical response to an acute antihypertensive, hydralazine, varied by 3 to 4% over 24 h. Paradoxically, patients were more responsive to therapy during the window of time (nighttime) when relatively few orders were placed and first doses administered.
Conversely, other treatments have been shown to be more effective if administered in the morning [vaccines (18), antipsychotics (19)], or midday [corticosteroids (20), radiotherapy (21), cardiac surgery (22)]. There is no single optimal dosing time for all drugs and patients. Given the broad impact of circadian rhythms on physiology, exploring the optimal time of day for drug administration may improve medical practice.
Hospital medical staff are available to provide treatment around the clock, and prevailing dogma is that treatment is given as needed regardless of time of day. Our findings challenge this notion and reveal a potential barrier to best clinical care. Interestingly, studies show that more-frequent rounding by nurses can improve measures of patient care (23). Would medical rounding designed to be more evenly distributed over 24 h improve clinical outcomes? Indeed, there are other long-standing aspects of hospital operation that have been challenged. For example, recent evidence shows that traditional continuous dim lighting in ICUs (24) lacks a medical rationale, disrupts patients, and may impede recovery (25–27).
As with all retrospective clinical studies, there were a number of potential sources of variability whose specific contribution could not be evaluated. For example, patient genotype [single nucleotide polymorphisms (SNPs) can affect hydralazine metabolism (28)], drug–drug interactions, or special clinical circumstances could each impact the clinical response to hydralazine. This is a population-level study and not necessarily applicable for individual patients. We hope that future multicenter, prospective trials will further elucidate the importance of drug timing on individual patient outcome measures.
In sum, time of day in hospital operations deserves further consideration.
Methods
We performed an observational cohort study of all patients receiving hydralazine while admitted to our institution from January 2010 to December 2017. Demographic data, medical history, BP, and order and administration times of 12 different drug therapies were recorded. We analyzed the 24-h distribution of orders, doses, and clinical responses (SI Appendix, SI Methods). Prior to initiation of this project, approval was obtained from the Cincinnati Children's Hospital Medical Center institutional review board (Study IRB2017-3080). Consent was not required for our retrospective analysis of electronic medical records. Anonymized data and code for reproducing the analyses in this study are available on GitHub (https://github.com/MarcDRuben/Hospital-Medicine).
Supplementary Material
Acknowledgments
This work is supported by Cincinnati Children’s Hospital Medical Center (D.F.S. and J.B.H.). J.B.H. is supported by the National Institute of Neurological Disorders and Stroke (Grant 2R01NS054794), National Heart, Lung, and Blood Institute (Grant R01HL138551), and National Human Genome Research Institute (2R01HG005220).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Anonymized data and code for reproducing the analyses in this study have been deposited in GitHub (https://github.com/MarcDRuben/Hospital-Medicine).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909557116/-/DCSupplemental.
References
- 1.Kleiber C., Davenport T., Freyenberger B., Open bedside rounds for families with children in pediatric intensive care units. Am. J. Crit. Care 15, 492–496 (2006). [PubMed] [Google Scholar]
- 2.Aronson P. L., Yau J., Helfaer M. A., Morrison W., Impact of family presence during pediatric intensive care unit rounds on the family and medical team. Pediatrics 124, 1119–1125 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Kuiper N. H., Tests concerning random points on a circle Nederl. Akad. Wetensch. Proc. Ser. A 63, 38–47 (1960). [Google Scholar]
- 4.Cornelissen G., Cosinor-based rhythmometry. Theor. Biol. Med. Model. 11, 16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes M. E., Hogenesch J. B., Kornacker K., JTK_CYCLE: An efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J. Biol. Rhythms 25, 372–380 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu G., Anafi R. C., Hughes M. E., Kornacker K., Hogenesch J. B., MetaCycle: An integrated R package to evaluate periodicity in large scale data. Bioinformatics 32, 3351–3353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaben P. F., Westermark P. O., Detecting rhythms in time series with RAIN. J. Biol. Rhythms 29, 391–400 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruben M. D., Smith D. F., FitzGerald G. A., Hogenesch J. B., Dosing time matters. Science 365, 547–549 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smolensky M. H., Hermida R. C., Ayala D. E., Tiseo R., Portaluppi F., Administration-time-dependent effects of blood pressure-lowering medications: Basis for the chronotherapy of hypertension. Blood Press. Monit. 15, 173–180 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Glynn C. J., Lloyd J. W., The diurnal variation in perception of pain. Proc. R. Soc. Med. 69, 369–372 (1976). [PMC free article] [PubMed] [Google Scholar]
- 11.Skarke C., et al. , A pilot characterization of the human chronobiome. Sci. Rep. 7, 17141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smolensky M. H., Hermida R. C., Castriotta R. J., Portaluppi F., Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med. 8, 668–680 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Okamoto L. E., et al. , Nocturnal blood pressure dipping in the hypertension of autonomic failure. Hypertension 53, 363–369 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemmer B., The importance of biological rhythms in drug treatment of hypertension and sex-dependent modifications. ChronoPhysiol. Ther. 2, 9–18 (2012). [Google Scholar]
- 15.Hermida R. C., et al. , Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am. J. Hypertens. 24, 383–391 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Smolensky M. H., Hermida R. C., Ayala D. E., Portaluppi F., Bedtime hypertension chronotherapy: Concepts and patient outcomes. Curr. Pharm. Des. 21, 773–790 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Carter B. L., et al. , Efficacy and safety of nighttime dosing of antihypertensives: Review of the literature and design of a pragmatic clinical trial. J. Clin. Hypertens. (Greenwich) 16, 115–121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long J. E., et al. , Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 34, 2679–2685 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chipchura D. A., Freyberg Z., Edwards C., Leckband S. G., McCarthy M. J., Does the time of drug administration alter the metabolic risk of aripiprazole? Front. Psychiatry 9, 494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beam W. R., Weiner D. E., Martin R. J., Timing of prednisone and alterations of airways inflammation in nocturnal asthma. Am. Rev. Respir. Dis. 146, 1524–1530 (1992). [DOI] [PubMed] [Google Scholar]
- 21.Squire T., et al. , Does chronomodulated radiotherapy improve pathological response in locally advanced rectal cancer? Chronobiol. Int. 34, 492–503 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Montaigne D., et al. , Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by rev-erbα antagonism: A single-centre propensity-matched cohort study and a randomised study. Lancet 391, 59–69 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Blakley D., Kroth M., Gregson J., The impact of nurse rounding on patient satisfaction in a medical-surgical hospital unit. Medsurg Nurs. 20, 327–332 (2011). [PubMed] [Google Scholar]
- 24.Rivkees S. A., Mayes L., Jacobs H., Gross I., Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics 113, 833–839 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Beauchemin K. M., Hays P., Dying in the dark: Sunshine, gender and outcomes in myocardial infarction. J. R. Soc. Med. 91, 352–354 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vásquez-Ruiz S., et al. , A light/dark cycle in the NICU accelerates body weight gain and shortens time to discharge in preterm infants. Early Hum. Dev. 90, 535–540 (2014). [DOI] [PubMed] [Google Scholar]
- 27.McKenna H., van der Horst G. T. J., Reiss I., Martin D., Clinical chronobiology: A timely consideration in critical care medicine. Crit. Care 22, 124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sim E., Abuhammad A., Ryan A., Arylamine N-acetyltransferases: From drug metabolism and pharmacogenetics to drug discovery. Br. J. Pharmacol. 171, 2705–2725 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.