Significance
We have previously demonstrated that induction of pathogenic eomesodermin-positive CD4+ T cells (Eomes+ T helper [Th] cells) is associated with transition from an acute stage to a later stage of experimental autoimmune encephalomyelitis (EAE). In the late phase of EAE, B cells and non-B cell antigen-presenting cells (APCs) recruited to the central nervous system strikingly up-regulate prolactin (PRL). The PRL-producing APCs have the potential to promote generation of Eomes+ Th cells from naïve T cells in an MHC class II-restricted manner, and therapies inhibitory for PRL production suppress the induction of Eomes+ Th cells and ameliorate clinical signs of EAE. Our study highlights the unexpected role of extrapituitary PRL in the development of persistent neuroinflammation.
Keywords: multiple sclerosis, experimental autoimmune encephalomyelitis, prolactin, antigen-presenting cell
Abstract
Induction of eomesodermin-positive CD4+ T cells (Eomes+ T helper [Th] cells) has recently been correlated with the transition from an acute stage to a later stage of experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis. Moreover, these cells’ pathogenic role has been experimentally proven in EAE. While exploring how the pathogenic Eomes+ Th cells are generated during the course of EAE, we unexpectedly found that B cells and MHC class II+ myeloid cells isolated from the late EAE lesions strikingly up-regulated the expression of prolactin (PRL). We demonstrate that such PRL-producing cells have a unique potential to induce Eomes+ Th cells from naïve T cells ex vivo, and that anti-MHC class II antibody could block this process. Furthermore, PRL levels in the cerebrospinal fluid were significantly increased in the late phase of EAE, and blocking the production of PRL by bromocriptine or Zbtb20-specific siRNA significantly reduced the numbers of Eomes+ Th cells in the central nervous system (CNS) and ameliorated clinical signs in the later phase of EAE. The PRL dependency of Eomes+ Th cells was confirmed in a series of in vitro and ex vivo experiments. Collectively, these results indicate that extrapituitary PRL plays a crucial role in the CNS inflammation mediated by pathogenic Eomes+ Th cells. Cellular interactions involving PRL-producing immune cells could be considered as a therapeutic target for the prevention of chronic neuroinflammation.
Experimental autoimmune encephalomyelitis (EAE) has greatly contributed to our understanding of the mechanism for autoimmune inflammation in the central nervous system (CNS), a vital factor in the pathogenesis of multiple sclerosis (MS). Although T helper (Th) 17 cells play an essential role in the early phase of EAE induced by acute inflammation, it has recently been demonstrated that the late phase of EAE is maintained independently of Th17 cells. Actually, a lack of NR4A2 gene expression in T cells abolishes the functions of Th17 cells and prevents the early phase of EAE; however, it does not influence on the late phase. We have identified cytotoxic Th cells expressing the T-box transcription factor eomesodermin (Eomes) as pathogenic lymphocytes involved in the late/chronic phase of EAE (1–3). Collective data indicate that the transition from the acute stage to the later stage of EAE corresponds to a switch from Th17 cell-dependent to Eomes+ Th cell-dependent pathology in vivo.
Strikingly, Eomes+ Th cells are also increased in the peripheral blood and cerebrospinal fluid (CSF) obtained from patients with secondary progressive multiple sclerosis (SPMS) (3). Moreover, we have recently confirmed that Eomes+ Th cells are enriched in lymphocytes isolated from brain samples of patients with SPMS. On the other hand, Eomes+ Th cells are not increased in peripheral lymphoid tissue samples in mice EAE (3) or human SPMS. Because previous studies showed that similar CD4+ T cells expressing Eomes are generated under chronic inflammatory conditions (4, 5), we have been curious to know whether Eomes+ Th cells involved in EAE and SPMS are generated in the inflammatory CNS tissue. Knowing that the T cell receptor (TCR) use of Eomes+ Th cells is significantly biased in the CNS, we suspected that the cells might be stimulated with TCR ligands presented by CNS antigen-presenting cells (APCs). Thus, we analyzed the properties of CNS infiltrating APCs in EAE that might contribute to the induction of Eomes+ Th cells in such an inflammatory milieu.
Here we report that B cells and MHC class II+ myeloid cells infiltrating the late EAE lesions produce a large quantity of prolactin (PRL), and that the extrapituitary PRL and PRL-producing cells have the ability to induce Eomes+ Th cells from naïve T cells. PRL levels are actually increased in the CSF obtained from EAE mice at the late phase of EAE, despite reductions in pituitary PRL production and serum PRL levels. PRL blockade therapies, including bromocriptine (BRC), can reduce the number of Eomes+ Th cells in the CNS and suppress the signs of chronic EAE.
Results
Infiltrating B Cells and Myeloid APCs Have the Potential to Induce Eomes+ Th Cells.
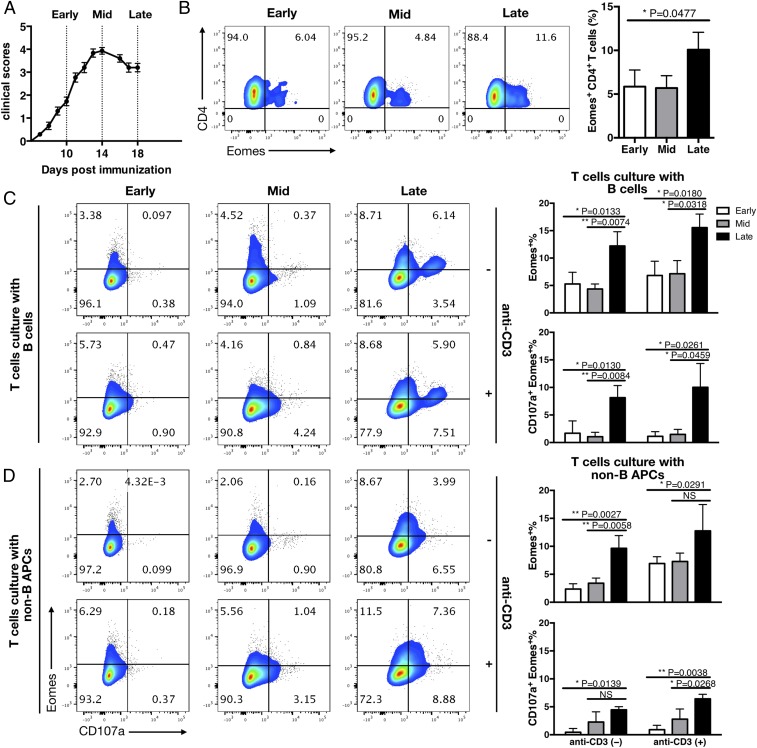
As we have demonstrated previously (3), the number of Eomes+ Th cells was increased in the CNS during the late phase of EAE (Fig. 1 A and B), although we did not see such a change in other tissues or lymphoid organs. Notably, TCR use by Eomes+ Th cells was significantly biased for Vβ5.1/5.2 and Vβ8.3 in the CNS compared with spleen CD4+ T cells or Eomes−CD4+ T cells in the CNS. Previous studies have shown that myeloid cells recruited to the CNS from the periphery can serve as APCs for priming pathogenic T cells with an alternative antigen epitope (6, 7). Eomes+CD4+ T cells found in the CNS were reactive to an epitope other than MOG35–55 (SI Appendix, Fig. S1).
Fig. 1.
B cells and non-B cell APCs from late EAE lesions have the potential to induce Eomes expression in Th cells. (A) Clinical scores for EAE in WT B6 mice sensitized for MOG35–55 peptide emulsified in CFA. Pertussis toxin (PT) was injected i.p. on days 0 and 2. Error bars represent SEM values, and dashed lines indicate the early (before peak), middle (at peak), and late (after peak) time points of EAE. Eomes+CD4+ T cells were absent or scarce at the early and middle time points but increased at the late time point. (B) Eomes expression by CNS-derived CD4+ T cells. The cells were isolated following enzymatic digestion of pooled CNS tissues at each time point (early, middle, and late) after EAE induction. (Left) FACS plots representative of at least 3 independent experiments. (Right) Summary of data for the frequency of Eomes+ CD4+ T cells. (C and D) Induction of Eomes and CD107a on CD4+ T cells after culture with CNS APCs. CD19+ B cells and CD19−CD45hi MHC II+ APCs (non-B APC) were purified from the CNS cellular infiltrates via FACS sorting at the early, middle, and late time points and then cultured with FACS-sorted splenic CD226+CD4+ T cells derived from congenic naïve mice in the presence of fluorescein isothiocyanate (FITC)-conjugated anti-CD107a antibodies. Anti-CD3 antibody was added into one-half of the culture wells. After 8 h, cells were stained to analyze the intracellular expression of Eomes. FACS plots show the expression of Eomes and surface CD107a in the CD4+ T cells that were cultured with B cells (Top) or non-B cell APCs (Bottom). Data are representative of 3 independent experiments. Columns shown at the right summarize the percentage values for Eomes+ and Eomes+CD107a+ cells among CD4+ T cells after culture with B cells (Top) or non-B cell APCs (Bottom). Error bars represent mean ± SD values. n = 5–7 mice in each group. P values were calculated using 1-way ANOVA with Dunnett’s multiple comparisons test. NS, not significant; *P < 0.05; **P < 0.01.
The foregoing observations led us to speculate that Eomes+ Th cells might be generated in the inflamed CNS tissue in response to antigen(s) presented by the CNS-recruited APCs. To explore this possibility, we isolated CD19+ B cells and CD19− MHC class II-positive cells from the CNS of mice with EAE (SI Appendix, Fig. S2B) and cultured these cells with splenic CD4+ T cells isolated from naïve congenic mice or CD226+CD4+ T cells as responders to assess the potentials of inducing Eomes+ Th cells (SI Appendix, Fig. S2 A and C). The proportions of CD19+ B cells and CD19− MHC class II-positive cells in the CNS increased greatly during the subacute phase (or at a middle time point) of EAE, followed by some reductions in the late phase (SI Appendix, Fig. S2B). CD226+CD4+ T cells were used, because they are relatively enriched for Eomes+ cells (SI Appendix, Fig. S2A). We found that both B cells and non-B cell APCs derived from late EAE preferentially induced the expression of intracellular Eomes in the CD4+ T cells compared with those derived at earlier time points (Fig. 1 C and D). In addition to Eomes, cell surface CD107a, an index of continuing lysosomal degranulation and cytotoxicity, was induced in the majority of the cells (Fig. 1D and SI Appendix, Fig. S2D). Consequently, CD107a+Eomes+ cells (CD107a+Eomes+%) were most significantly induced when the responder T cells were cultured with the B cells and non-B cell APCs derived from late EAE. Notably, anti-MHC class II antibody significantly inhibited the induction of CD107a on responder T cells, as well as induction of CD107a+Eomes+ cells after culture with the B cells or non-B cells (SI Appendix, Fig. S3). By contrast, the addition of anti-CD3 antibody significantly promoted CD107a expression and induction of the CD107a+Eomes+ cells in the presence of B cells but not in the presence of non-B cells. In the absence of the B cells or non-B cell APCs, anti-CD3, anti-CD28, or a combination of the 2 antibodies did not show any significant effects. Collectively, these results indicate the possible involvement of MHC II-associated ligand in the induction of cytotoxic Eomes+ Th cells ex vivo.
PRL Is Produced by APCs Capable of Inducing Eomes+ Th Cells.
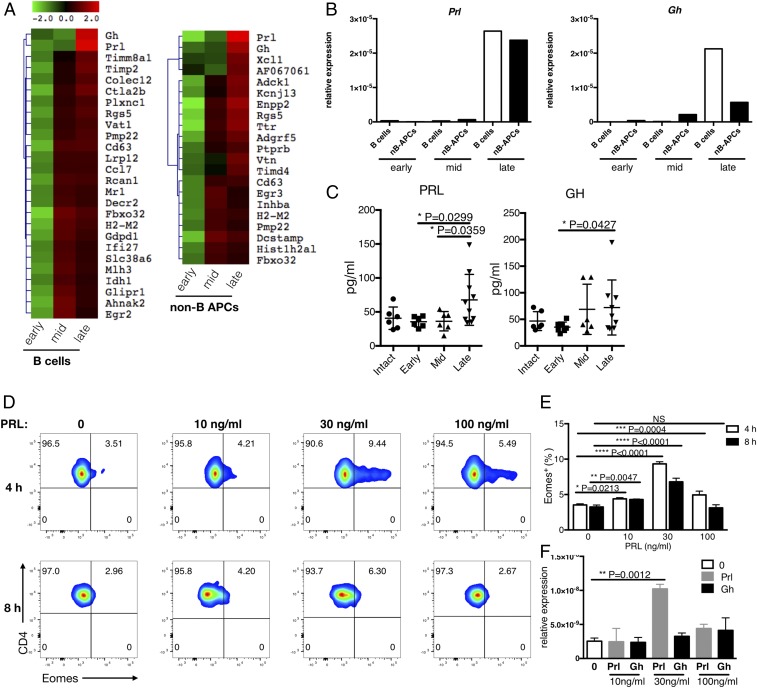
To understand the reason why APCs from late EAE lesions are superior to others in their ability to induce CD107a+Eomes+ Th cells, we analyzed the gene expression profiles of the APCs obtained from early, middle, and late time points during EAE (Fig. 1A). Analysis of gene sets that were highly up-regulated in late EAE revealed the enrichment of biological processes associated with immune-related Gene Ontology terms (SI Appendix, Table S1), indicating a drastic change in the properties of APCs after they enter the CNS. Unexpectedly, we found that PRL and growth hormone (GH), the hormonal sibs produced predominantly by pituitary glands, were highly up-regulated in both B cells and non-B cell APCs from late EAE (Fig. 2A). Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) analysis confirmed an outstanding up-regulation of PRL in the APCs recruited to the late lesions (Fig. 2B). GH expression was also up-regulated in those samples, although this was less significant in non-B cell APCs compared with B cells. We further observed that concentrations of PRL and GH in the CSF were significantly increased at the late time point during EAE compared with the early or middle time point (Fig. 2C). In contrast, PRL transcripts in the pituitary glands were reduced in mice showing signs of EAE, as reported previously (8). More conspicuously, serum PRL levels were greatly reduced in EAE mice, although GH levels were not changed significantly (SI Appendix, Fig. S4 A and B). These results indicate that the production of PRL by CNS APCs is not a trivial phenomenon but could play a pathogenic role in the late stage of EAE.
Fig. 2.
PRL-positive APCs play a role in the induction of Eomes+ Th cells. (A) Gene expression in B cells and non-B cell APCs. At different time points (early, middle, and late), B cells and MHC class II+ non-B cell APCs were isolated from CNS lesions for expression microarray analysis. The relative expression of selected genes is summarized in the heat maps. (B) Expression levels of PRL and GH were determined by qRT-PCR. (C) Protein levels of PRL and GH in the CSF of EAE mice were determined using the Luminex system (Methods). (D) Eomes induction in CD226+CD4+ T cells in the presence of PRL. CD226+CD4+ T cells from the spleens of naïve WT B6 mice were cultured with the indicated concentrations of PRL (0 to 100 ng/mL) for 4 or 8 h. Data shown are representative of 3 independent experiments. (E) Frequency of Eomes+ cells among cells after culture. Eomes+ (%) represents the frequency of Eomes+ cells determined in the experiments in D. Error bars represent mean ± SD values. P values were calculated using 1-way ANOVA with Dunnett’s multiple comparisons test. (F) Expression of Eomes. NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
As immunostimulatory functions of PRL have been described previously (9–13), we next examined the ability of PRL and GH to induce Eomes+ Th cells in vitro. Eomes expression was significantly up-regulated in naïve CD226+CD4+ T cells from the spleen after they were cultured with exogenous PRL for 4 h (Fig. 2 D and E). In contrast, GH showed only a marginal effect on the induction of Eomes (Fig. 2F). A very narrow range of PRL concentrations are effective for the induction of Eomes, as has been described for the effect of PRL on T-bet expression in T cells (14). Since the ability of CNS APCs to induce Eomes+ Th cells is correlated with the potential to produce PRL, we speculated that the ectopic secretion of PRL by the APCs might facilitate the generation of Eomes+ Th cells in the inflamed CNS tissue.
Zbtb20 Is Necessary for PRL Expression in CNS APCs.
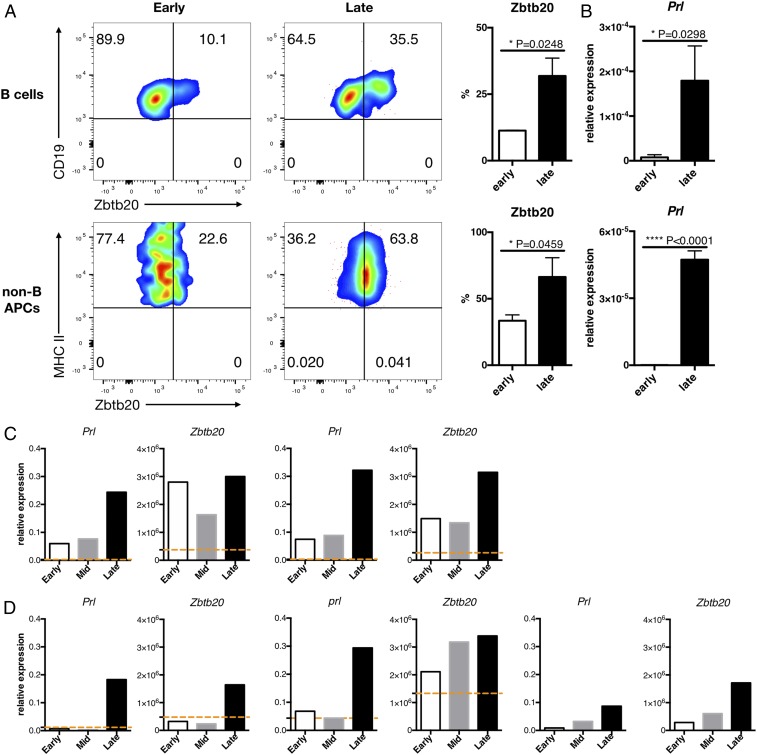
The transcription factor Zbtb20 is essential for the development of lactotrophs and is responsible for the production of PRL in the anterior pituitary gland (15, 16). Since Zbtb20, originally cloned from human dendritic cells (DCs) (17), is expressed in the B cell lineage (18, 19), we examined the expression of Zbtb20 in the CNS-recruited B cells and non-B cell APCs in the late phase of EAE. Flow cytometry assessments showed that the proportion of Zbtb20-expressing cells in these myeloid cells were increased in late EAE compared with early EAE (Fig. 3A). Moreover, the in vivo injection of Zbtb20-specific siRNA inhibited the expression of PRL in the APCs from late EAE (Fig. 3B), suggesting that Zbtb20 plays a critical role in the production of extrapituitary PRL.
Fig. 3.
Zbtb20 is involved in PRL production and is highly expressed in CNS APCs during the late phase of EAE. Mixed B cells and non-B cell APC populations were isolated from the CNS of WT B6 mice at the early or late time point. (A) Proportions of Zbtb20+ cells in CNS B cells and non-B cell APCs were evaluated by intracellular flow cytometry. FACS staining plots are representative of at least 3 independent EAE experiments. (B) The expression level of PRL was determined by qRT-PCR. Error bars represent mean ± SD values. P values were calculated using Student’s t test. (C and D) Expression levels of Prl and Zbtb20 in CNS B cell subsets (C) and non-B cell APCs subsets (D) were determined by qRT-PCR. The orange dashed lines in each bar graph (except CD11c−) represent the values of counterparts of intact splenocytes. Error bars represent mean ± SD values. P values were calculated using Student’s t test. *P < 0.05; ****P < 0.0001.
Based on the flow cytometry results, we next analyzed the expression of PRL and Zbtb20 in subsets of B cells and non-B cells: CD5+ B cells, CD5− B cells, conventional DCs (cDCs), plasmacytoid DCs (pDCs), and CD11c−PDCA-1− (CD11c−) cells (SI Appendix, Fig. S4 C and D). In all of these subsets, PRL expression was selectively up-regulated in the cells obtained from late EAE (Fig. 3 C and D). Expression of Zbtb20 in CD5+ B cells and pDCs was found in early stages of EAE, whereas late EAE-specific expression of Zbtb20 was confirmed in CD5− B cells, cDCs, and CD11c− cells (Fig. 3 C and D). Consistent with a previous report (18), CD5+ B cells (Fr. 2 and Fr. 3 in SI Appendix, Fig. S4C) contained a higher proportion of Zbtb20-expressing cells compared with conventional CD5− B cells (Fr. 1 and Fr. 4). Interestingly, in all subsets except pDCs, B cells and non-B cell APCs isolated from the spleens of naïve mice expressed only marginal levels of Zbtb20 or PRL (orange dashed lines in Fig. 3 C and D). These results imply that a wide variety of B cells and non-B cell APCs acquire an ability to express PRL after entering the CNS of EAE mice.
Inhibition of PRL and Zbtb20 Attenuates the Development of Late EAE.
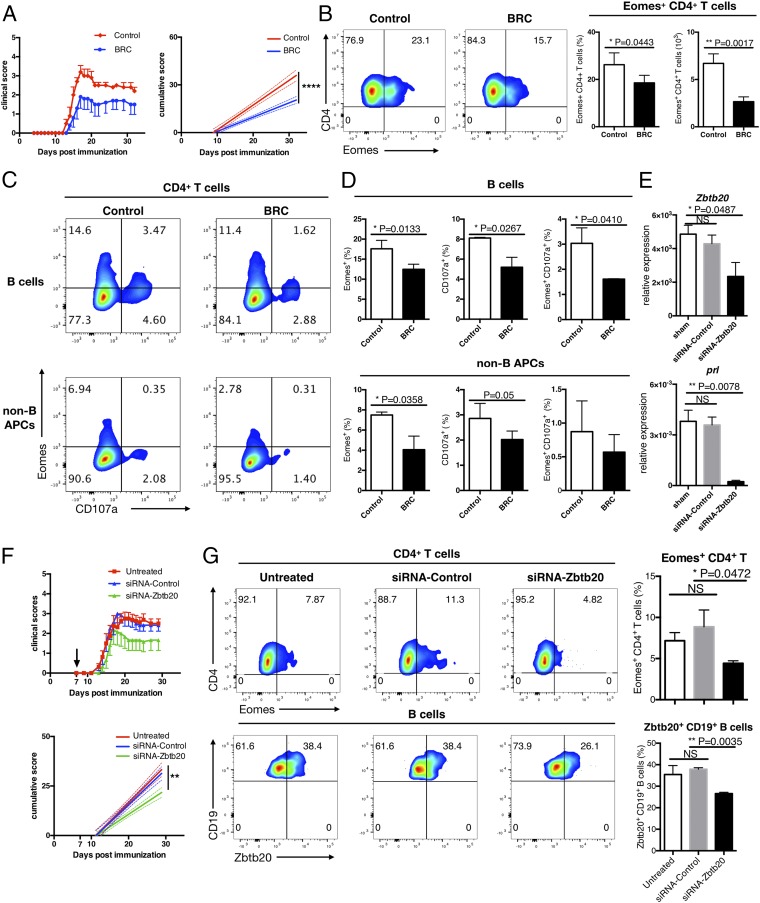
Although hyperprolactinemia can be associated with autoimmune diseases, such as systemic lupus erythematosus and MS (10, 20), the role of PRL in the pathogenesis of EAE/MS remains controversial. In fact, a previous report has suggested that the development of acute EAE might not depend on PRL (8). We evaluated the effect of the PRL inhibitor BRC in a model of EAE induced in NR4A2cKO mice. Because NR4A2cKO mice are deficient for Th17 cells but maintain Eomes+ Th cells, the chronic EAE induced in these mice is suitable for evaluating the pathogenicity of Eomes+ Th cells (2, 3). Treatment with BRC significantly reduced the clinical symptoms of EAE in this model (Fig. 4A), which is not attributable to the effect of BRC on Th17 cells. Moreover, we observed a reduced infiltration of Eomes+ CD4+ T cells in the CNS lesions of the mice treated with BRC (Fig. 4B). The CNS APCs isolated from BRC-treated mice showed a reduced capability to induce Eomes+ T cells from naïve Th cells ex vivo (Fig. 4 C and D). These results are consistent with our postulation that PRL produced by CNS APCs plays a critical role in the induction of Eomes+ Th cells.
Fig. 4.
Inhibition of PRL and Zbtb20 attenuates the transition to late EAE. (A) NR4A2cKO mice were immunized with MOG35–55 peptide emulsified in CFA and were given i.p. injections of pertussis toxin on days 0 and 2. The mice were then given i.p. injections of BRC or DMSO/PBS every other day starting on day 4 postimmunization. Clinical EAE scores are shown, with error bars representing SEM values. On the right, solid lines represent cumulative disease scores and dashed lines indicate the 95% confidence intervals. ****P < 0.0001, linear regression analysis. (B) Freshly isolated CNS CD4+ T cells were stained intracellularly to detect the presence of Eomes. (Left) Flow cytometry plots showing representative data from 3 independent experiments. (Right) Collective data indicating the frequency and exact number of CNS Eomes+CD4+ T cells. Error bars represent mean ± SD values. P values were calculated using Student’s t test. (C and D) CNS CD19+ B cells and CNS CD19−CD45hi MHC II+ non-B APCs were purified by FACS sorting, then cocultured with FACS sorted splenic CD4+ T cells derived from congenic naïve mice in the presence of FITC-conjugated anti-CD107a antibodies. After 8 h of culture, cells were then stained intracellularly for expression of Eomes. (C) FACS plots showing the expression of Eomes and CD107a surface trapping in CD4+ T cells cocultured with CNS B cells (Top) or CNS non-B APCs (Bottom) from control (Left) or BRC-treated (Right) mice. (D) Summary of expression of Eomes+, CD107a, and Eomes+CD107a+ in CD4+ T cells after coculturing with CNS B cells or CNS non-B APCs from control or BRC-treated mice. Error bars show mean ± SD. *P < 0.05, Student’s test. Data are representative of 3 independent experiments. (E) CNS APCs were isolated from late EAE mice. CNS CD19−CD45hi MHC II+ non-B cell APCs were purified from the pooled APCs by FACS sorting, then transfected with Zbtb20-specific or control scrambled siRNA and cultured in the presence of LPS for 24 h. The expression levels of Zbtb20 and Prl were determined by qRT-PCR. (F) Zbtb20-specific (green triangle) or control scrambled (blue triangle) siRNA was stabilized in the atelocollagen substrate and administered i.v. into WT B6 mice that had been sensitized for MOG35–55 peptide 7 d earlier. Clinical EAE scores are shown, with error bars representing SEM values. On the right, solid lines represent cumulative disease scores, and dashed lines indicate the 95% confidence intervals. P = 0.0012, linear regression analysis. (G) CNS CD4+ T cells and B cells were freshly isolated from untreated, control siRNA-treated, or Zbtb20-specific siRNA-treated EAE mice and then stained intracellularly for Eomes or Zbtb20. Flow cytometry plots show representative data from 2 independent experiments. Summarized data are shown in the bar graphs. Error bars represent mean ± SD values. P values were calculated using 1-way ANOVA test with Dunnett’s multiple comparisons test. NS, not significant; *P < 0.05; **P < 0.01.
Because BRC is a dopamine D2 receptor agonist, we further examined the effect of dopamine and its precursor l-DOPA in late EAE. We observed that in vitro treatment with dopamine markedly reduced the expression levels of PRL and Zbtb20 in APCs isolated from late EAE (SI Appendix, Fig. S5A). Clinical manifestations of late EAE were significantly ameliorated by the administration of l-DOPA in vivo (SI Appendix, Fig. S5B), whereas the numbers of Eomes+ Th cells and Zbtb20+ CNS APCs were reduced. PRL expression was significantly suppressed in the CNS APCs derived from l-DOPA–treated mice (SI Appendix, Fig. S5C). Furthermore, late/chronic EAE induced in NR4A2cKO mice was significantly ameliorated by the systemic administration of Zbtb20-specific siRNA (Fig. 4F). In parallel, the proportions of Eomes+ Th cells and Zbtb20+ B cells in the CNS were significantly reduced in mice treated with this siRNA (Fig. 4G). These results indicate that the up-regulation of Zbtb20 in the CNS APCs is essential for the ectopic production of PRL, generation of Eomes+ Th cells, and progression of late EAE.
Inflammatory Cytokines Induce the Ectopic Expression of Zbtb20 and PRL in the CNS APCs.
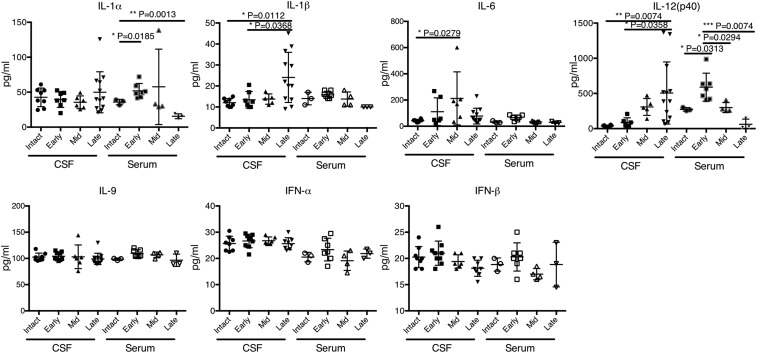
Since the persistent inflammatory milieu appears to be a prerequisite for the expression of Zbtb20/PRL in the APCs recruited to the CNS lesions, we next evaluated the ability of inflammatory cytokines to induce the production of Zbtb20/PRL in splenic B cells. Among a panel of cytokines used, IL-1β, IL-6, IL-9, IL-12, IL-33, type I IFNs (IFN-α and -β), and IFN-γ were able to significantly increase the proportion of Zbtb20+ cells in splenic B cells (SI Appendix, Fig. S6 A and B). Many of the cytokines and chemokines in the CSF were differentially expressed during the course of EAE (Fig. 5 and SI Appendix, Fig. S7). Although the kinetics suggest that IL-6 and IL-12 (increased from mid to late stages of EAE) might be involved in the induction of Zbtb20 and PRL in the CNS APCs, other cytokines may also participate in the induction of Zbtb20/PRL. This is because cytokines produced in the CNS parenchyma might not always be identified by CSF analysis. We actually observed that type I IFNs are produced by CNS pDCs (SI Appendix, Fig. S8); therefore, further study is needed to identify cytokines that make the greatest contribution to ectopic PRL expression.
Fig. 5.
Protein levels of cytokines in mouse CSF and serum were determined using the Luminex system. Shown are the results of cytokines that can significantly up-regulate Zbtb20 expression in splenic B cells in vitro (SI Appendix, Fig. S6). Error bars represent mean ± SD values. P values were calculated using 1-way ANOVA with Dunnett’s multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001.
Amelioration of the Late Phase of EAE by B Cell Depletion Accompanies a Reduction of Eomes+ Th Cells.
Efficacy of B cell depletion by the anti-CD20 mAb has been reported in the treatment of autoimmune diseases, such as MS (21, 22). We hypothesized that the efficacy of B cell depletion therapy might be achieved in part through the depletion of PRL-producing B cells and subsequent inhibition of the induction of Eomes+ Th cells. To explore this possibility, we treated EAE mice with B cell-depleting anti-CD20 mAb and evaluated its effects on clinical EAE symptoms and Eomes+ Th cell numbers in the CNS. The depletion of B cells before immunization with MOG35–55 EAE (day −7) modestly increased disease severity, probably due to a depletion of B regulatory cells, as reported previously (23) (SI Appendix, Fig. S9A). In contrast, B cell depletion shortly after the onset of disease (day 13) reduced EAE severity at the peak phase and at later phases. The suppression of EAE symptoms was accompanied by reduced numbers of Zbtb20+ B cells and Eomes+ Th cells in the CNS (SI Appendix, Fig. S9 B–D). These results suggest that the efficacy of the anti-CD20 mAb in MS might be related to its effect on ectopic PRL production in the CNS.
Discussion
An increasing body of evidence suggests that bidirectional interactions between the neuroendocrine and immune systems play critical roles in the maintenance of homeostasis in the body. Consequently, disturbing the mutual communication between these systems might trigger or promote the development of a wide variety of diseases (24). Among the endocrine hormones known to influence the immune system, those produced by the specialized endocrine organs have been widely studied (25). However, hormones of ectopic origin have not been a major area of research with regard to the pathogenesis of human disorders. Although PRL is a pituitary hormone known to be involved in lactation, luteal functioning, and reproduction (26), we here disclose the unexpected role of extrapituitary PRL in the pathogenesis of persistent neuroinflammation mediated by Eomes+ Th cells, and report that cytotoxic Eomes+ Th cells are under the control of extrapituitary PRL.
After noting the biased TCR use by Eomes+ Th cells in the CNS (SI Appendix, Fig. S1), we examined the properties of potential APCs that may interact with precursors of Eomes+ Th cells. Subsequently, we came across the up-regulation of PRL in B cells and non-B cells recruited to the chronic EAE lesions, implying that CNS-recruited myeloid cells acquire the ability to secrete PRL several days after CNS entry. Further analysis indicated that such PRL-producing APCs are able to induce potentially cytotoxic CD107a+Eomes+ Th cells from the splenic naïve T cells in vitro, which is blocked in the presence of anti-MHC class II antibody (SI Appendix, Fig. S3). The link between extrapituitary PRL and Eomes+ Th cells has been explored in a series of experiments using anti-PRL drugs and siRNA, which showed that blocking PRL production by BRC, Zbtb20-specific siRNA, or l-DOPA all led to reduced induction of Eomes+ Th cells in vivo.
Although the efficacy of BRC for EAE has been reported previously (20, 27), another study has shown that the development of EAE does not necessarily require PRL (8). In the present study, we have demonstrated a role of PRL in maintaining the late phase of EAE that is dependent on Eomes+ Th cells, by introducing NR4A2 cKO mice in which Th17 cells are dysfunctional (3). These results from in vitro and ex vivo analyses allow us to propose a model in which B cells and non-B cells recruited to the CNS acquire the ability to secrete PRL and induce Eomes+ Th cells via the presentation of MHC class II-associated antigen in the presence of PRL (SI Appendix, Fig. S10). In support of this, costimulatory roles of PRL in the in vitro activation of T cells and B cells have been reported previously (28, 29). Analysis with MOG35–55–loaded class II tetramer revealed that MOG35–55 is not the epitope recognized by Eomes+ Th cells (SI Appendix, Fig. S1A). Although the TCR ligands remain to be identified, autoantigens from the CNS tissue, superantigens (30, 31), or gut-derived antigens can be considered candidates.
There is a critical incubation period for the CNS-recruited APCs to acquire an ability of promoting the induction of Eomes+ Th cells, although such APCs are most abundant at the middle phase of EAE. We have indicated that expression of Zbtb20 in the APCs is probably induced by inflammatory cytokines; however, we have not specified which cytokine is most critical. Our next goal is to determine this Zbtb20/PRL-inducing cytokine in vivo and identify its cellular source. We also need to learn more about the cellular interactions leading to the generation of Eomes+ Th cells on the molecular terms, as such efforts may provide a better understanding of the pathogenesis of CNS inflammatory diseases and lead to the design of novel therapeutic strategies for preventing persistent neuroinflammation.
Methods
Mice.
All mice used were 6 to 8 wk old and maintained in specific pathogen-free conditions in accordance with institutional guidelines. C57BL/6 (WT) mice were purchased from CLEA Japan. CD4-Cre NR4A2 mice were generated in house as described previously (3). All animal experiments were approved by the National Center of Neurology and Psychiatry’s Committee for Small Animal Research and Animal Welfare. All efforts were made to minimize animal suffering in clinical disease experiments.
EAE Induction and Scoring.
Mice were injected s.c. with 50 mg of MOG35–55 peptide (synthesized at the Toray Research Center) and 1 mg of heat-killed Mycobacterium tuberculosis H37RA emulsified in complete Freund’s adjuvant (CFA; Difco). Then 100 ng of pertussis toxin (List Biological Laboratories was injected i.p. on days 0 and 2 after immunization). Neurologic deficits were evaluated on a scale of 0 to 5 (0, no clinical signs; 0.5, tail weakness; 1, partial tail paralysis; 1.5, severe tail paralysis; 2, flaccid tail; 2.5, flaccid tail and hind limb weakness; 3, partial hind limb paralysis; 3.5, severe hind limb paralysis; 4, total hind limb paralysis; 4.5, hind and fore leg paralysis; 5, dead).
BRC Treatment.
BRC (Wako) was dissolved in DMSO and diluted by PBS, and a dose of 2.5 mg/kg was administered i.p. starting on day 4 postimmunization and continuing every other day up to day 32.
l-DOPA Treatment.
l-DOPA (Sigma-Aldrich) was dissolved in DMSO and diluted with PBS, and a dose of 50 mg/kg was administered i.p. starting on day 4 postimmunization and continuing every other day up to day 30.
Anti-CD20 Treatment.
Here 250 µg of anti-mouse CD20 or isotype control (all from BioLegend) was administered i.v. on day -7 or day 13 related to MOG35–55 peptide immunization.
Systemic siRNA Treatment.
Mice received i.v. 20 mM Zbtb20-specific siRNA (sequence 50-ggaaacuacuaaaguauaauu-30; synthesized by Koken) or negative control siRNA stabilized with an AteloGene collagen systemic kit (Koken) on day 7 after MOG35–55 peptide immunization.
Cell Isolation.
Single-cell suspensions of splenocytes were generated by mechanical disruption of tissues. To obtain CNS cell suspensions, the spinal cord was flushed out with PBS, and the brain was removed from the skull. The brain and spinal cord were cut into small pieces, followed by digestion with 1.4 mg/mL collagenase H and 100 µg/mL DNase I (Roche) in RPMI medium for 40 min at 37 °C. The cell suspension was filtered through a 70-μm cell strainer and enriched by a discontinuous 37%/70% Percoll gradient centrifugation (GE Healthcare Life Sciences).
Flow Cytometry.
Cells were treated with anti-mouse CD16/CD32 (BioLegend) to block Fc receptors before staining. Staining was performed in PBS solution containing 5% FCS. For all experiments, dead cells were excluded using an Aqua Live/Dead fixable staining reagent (Invitrogen). mAbs CD1d (1B1), CD4 (GK1.5), CD5 (53-7.3), CD8 (53-6.7), CD11b (M1/70), CD11c (N418), CD19 (6D5), CD28(37.51), CD45 (30F11), CD107a (1D4B), CD138 (281-2), CD226 (TX42.1), CD317 (129C1), Eomes (Dan11Mag), IL-9 (RM9A4), IL-10 (JES5-16E3), TCR-β (H57-597), MHC class II I-A/I-E (M5/114.15.2), and Zbtb20 (4A3) were obtained from BD Biosciences, BioLegend, or eBioscience. For surface staining, cells were incubated with mAbs for 30 min on ice. For intracellular cytokine staining, cells were incubated for 6 h at 37 °C in 10% FCS in RPMI medium with PMA (50 ng/mL; Sigma-Aldrich) and ionomycin (500 ng/mL; Sigma-Aldrich) in the presence of GolgiPlug (BD Bioscience; 1:1,000 dilution).
For Eomes and Zbtb20 intracellular staining for CNS cells, fresh isolated cells were used without restimulation. After surface staining, a Foxp3 staining kit (eBioscience) was used according to the manufacturer’s instructions, and then cells were incubated with the respective antibodies for 30 min at room temperature. Flow cytometry analysis was performed with a FACSCanto II flow cytometer (BD Biosciences) with FACSDiva software, and data were analyzed using FlowJo software (Tree Star).
Cell Sorting.
From splenocytes, T cells and B cells were isolated using a CD4 T cell or CD19 B cell MACS isolation kit, respectively, with an AutoMACS separator (Miltenyi Biotech) according to the manufacturer’s instructions. CD226+CD4+ T cells were further purified using a FACSAria IIu cell sorter (BD Biosciences). For CNS cells, CD45hiCD19+ MHC II+ B cells, CD45hiCD19−MHC II+CD11c+CD317hiB220+ pDCs, CD45hi TCR-β+CD4+ T cells, and CD45intCD11b+ microglia cells were sorted using a FACSAria IIu cell sorter (BD Biosciences).
Mouse Vβ TCR Analysis.
Groups of NR4A2 cKO and control mice were immunized with MOG35–55 to induce EAE. During the late phase of EAE (day 26), single-cell suspensions were prepared from spleen and CNS tissue. Cells were stained with antibodies against TCR-β and CD4 combined with the Mouse Vβ TCR screen panel kit (BD Biosciences), followed by fixation and permeabilization with an intracellular transcription factor staining reagent kit (eBioscience). Subsequently, intracellular staining with antibodies against Eomes was conducted for flow cytometer analysis.
Cell Culture.
The culture medium was RPMI supplemented with 10% FCS, 100 U/mL penicillin–streptomycin, and 50 mM 2-mercaptoethanol (all from Invitrogen).
T Cell-APC Coculture.
Flow cytometry-sorted CNS B cells or non-B APCs from EAE mice (ly5.2) were cultured with flow cytometry-sorted splenic CD226+ CD4+ T cells from naïve congenic mice (ly5.1) in the presence of an anti-CD107a-fluorochrome-conjugated antibody with or without anti-CD3 (17A2; BioLegend) stimulation for 8 h at 37 °C. In the indicated experiments, anti-mouse class II I-A/I-E (M5/114.15.2) was added. Cells were subjected to FACS analysis as described above.
Tetramer Staining.
CNS-derived lymphocytes were treated with neuraminidase (Sigma-Aldrich), followed by staining with MOG35–55/MHC class II tetramer-BV421 (NIH Tetramer Core Facility) at room temperature. Human-CLIP/MHC class II tetramer-BV421 served as a staining control. Cells were costained with antibodies against surface markers, followed by fixation and intracellular staining for Eomes.
Induction of Zbtb20.
AutoMACS-isolated splenic B cells from naïve mice were cultured in the presence of IL-1β (5 ng/mL; BioLegend), IL-6 (40 ng/mL; BioLegend) IL-9 (12.5 ng/mL; BioLegend), IL-12 (5 ng/mL; R&D Systems), IL-33 (40 ng/mL; Peprotech), IFN-α (1000 U/mL; BioLegend), IFN-β (500 U/mL; BioLegend), or IFN-γ (200 ng/mL; BioLegend) with lipopolysaccharide (LPS; 10 ng/mL) for 24 h at 37 °C. Cells were subjected to Zbtb20 intracellular staining or lysed for qRT-PCR assays.
Block of PRL Signaling.
Flow cytometry-sorted CNS B cells or non-B APCs from late EAE mice were cultured in the presence of 50 µmol/L dopamine (Sigma-Aldrich) with 10 ng/mL LPS for 24 h, 48 h, and 96 h at 37 °C. Cells were then lysed for qRT-PCR assays.
qRT-PCR.
Total RNA was extracted from cell populations using the RNeasy Mini Kit (Qiagen), then transcribed into cDNA using the First-Strand cDNA Kit (Takara) according to the manufacturer’s instructions. Gene expression was measured by real-time qRT-PCR using a LightCycler instrument (Roche) with SYBR Green Master Mix (Roche). All primers were purchased from Qiagen. Expression levels were normalized to Rplp0 or β2m.
Microarray Analysis.
Expression microarrays were carried out on FACS-sorted CNS B cells and non-B APCs using GeneChip Mouse Gene 2.0 ST Arrays prepared using the RNeasy Mini Kit (Qiagen) and a GeneChip Hybridization, Wash, and Stain Kit (Affymetrix), according to the manufacturers’ instructions. Arrays were washed in a GeneChip Fluidics Station 450 and scanned using a GeneChip Scanner 3000 7G (Qiagen). Array data were compiled using Affymetrix GCOS software. Differential gene expression analysis was performed using MeV software (32), and Gene Ontology analysis was performed using PANTHER (33).
CSF Collection and Multiplex Immunoassay.
Mouse CSF was collected as described previously (34). In brief, mice were anesthetized i.p. using a mixture of 3 anesthetic agents (medetomidine, midazolam, and butorphanol). Skin was sagittally incised posterior to the occipital crest. The subcutaneous tissues and muscles were carefully separated to expose the glistening surface of the dura covering the cisterna magna. The dura matter was poked by a glass capillary tube, after which the CSF spontaneously flowed into the capillary tube. CSF was stored in a –80 °C freezer. An array of cytokines, chemokines and hormones—IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL- 9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, eotaxin, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), IFN-α, IFN-β, IFN-γ, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1 (MIP-1) α, MIP-1β, normal T cell expressed and secreted (RANTES), TNF-α, PRL, and GH—were examined in CSF samples using a Bio-Plex Pro Mouse Cytokine 23-Plex Assay (Bio-Rad), Mouse Pituitary Magnetic Bead Panel (EMD Millipore), and ProcartaPlex Mouse IFN-α/IFN-β Assays (Invitrogen) and then read on a Bio-Rad Bio-Plex 200 system according to the manufacturer’s instructions.
Statistical Analysis.
Statistical analysis was performed with GraphPad Prism 6 using the unpaired Student’s t test, 1-way or 2-way ANOVA as specified, with Tukey’s or Dunnett’s multiple comparison test. A P value < 0.05 was considered to indicate statistical significance.
Supplementary Material
Acknowledgments
We thank W. Li, A. Takeo, and C. Koto for excellent illustration design, technical assistance and animal care. We also thank the NIH Tetramer Core Facility for providing the MOG35–55– and CLIP-specific MHC class II tetramers. This work was supported by Health and Labor Sciences Research Grant for research on rare and intractable diseases from the Japanese Ministry of Health, Labor, and Welfare; Japan Society for the Promotion of Science KAKENHI Grant 18H04045; Japan Agency for Medical Research and Development Practical Research Project for Rare/Intractable Diseases (17ek0109097 and 17ek0109155); and National Institute of Neuroscience Intramural Research Grant 30-5 for Neurological and Psychiatric Disorders.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1906438116/-/DCSupplemental.
References
- 1.Doi Y., et al. , Orphan nuclear receptor NR4A2 expressed in T cells from multiple sclerosis mediates production of inflammatory cytokines. Proc. Natl. Acad. Sci. U.S.A. 105, 8381–8386 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raveney B. J., Oki S., Yamamura T., Nuclear receptor NR4A2 orchestrates Th17 cell-mediated autoimmune inflammation via IL-21 signalling. PLoS One 8, e56595 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raveney B. J., et al. , Eomesodermin-expressing T-helper cells are essential for chronic neuroinflammation. Nat. Commun. 6, 8437 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall N. B., Swain S. L., Cytotoxic CD4 T cells in antiviral immunity. J. Biomed. Biotechnol. 2011, 954602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curran M. A., et al. , Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J. Exp. Med. 210, 743–755 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey S. L., Schreiner B., McMahon E. J., Miller S. D., CNS myeloid DCs presenting endogenous myelin peptides “preferentially” polarize CD4+ T(H)-17 cells in relapsing EAE. Nat. Immunol. 8, 172–180 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Serafini B., Rosicarelli B., Magliozzi R., Stigliano E., Aloisi F., Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 14, 164–174 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costanza M., Musio S., Abou-Hamdan M., Binart N., Pedotti R., Prolactin is not required for the development of severe chronic experimental autoimmune encephalomyelitis. J. Immunol. 191, 2082–2088 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Bauernhofer T., et al. , Role of prolactin receptor and CD25 in protection of circulating T lymphocytes from apoptosis in patients with breast cancer. Br. J. Cancer 88, 1301–1309 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correale J., Farez M. F., Ysrraelit M. C., Role of prolactin in B cell regulation in multiple sclerosis. J. Neuroimmunol. 269, 76–86 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Flores-Fernández R., et al. , Prolactin rescues immature B-cells from apoptosis induced by B-cell receptor cross-linking. J. Immunol. Res. 2016, 3219017 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochendoerfer S. K., Krishnan N., Buckley D. J., Buckley A. R., Prolactin regulation of Bcl-2 family members: Increased expression of bcl-xL but not mcl-1 or bad in Nb2-T cells. J. Endocrinol. 178, 265–273 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Matera L., Mori M., Galetto A., Effect of prolactin on the antigen presenting function of monocyte-derived dendritic cells. Lupus 10, 728–734 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Tomio A., et al. , Prolactin can modulate CD4+ T-cell response through receptor-mediated alterations in the expression of T-bet. Immunol. Cell Biol. 86, 616–621 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Dong Q., Chen X. Y., Li G. M., Effect of transcription factor ZBTB20 on mouse pituitary development. Genet. Mol. Res. 14, 17622–17629 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Cao D., et al. , ZBTB20 is required for anterior pituitary development and lactotrope specification. Nat. Commun. 7, 11121 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W., et al. , Identification and characterization of DPZF, a novel human BTB/POZ zinc finger protein sharing homology to BCL-6. Biochem. Biophys. Res. Commun. 282, 1067–1073 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Chevrier S., et al. , The BTB-ZF transcription factor Zbtb20 is driven by Irf4 to promote plasma cell differentiation and longevity. J. Exp. Med. 211, 827–840 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Bhattacharya D., Adjuvant-specific regulation of long-term antibody responses by ZBTB20. J. Exp. Med. 211, 841–856 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orbach H., Shoenfeld Y., Hyperprolactinemia and autoimmune diseases. Autoimmun. Rev. 6, 537–542 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Hauser S. L., et al. ; HERMES Trial Group , B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 358, 676–688 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Montalban X., et al. ; ORATORIO Clinical Investigators , Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 376, 209–220 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Matsushita T., Yanaba K., Bouaziz J. D., Fujimoto M., Tedder T. F., Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 118, 3420–3430 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coronel-Restrepo N., Posso-Osorio I., Naranjo-Escobar J., Tobón G. J., Autoimmune diseases and their relation with immunological, neurological and endocrinological axes. Autoimmun. Rev. 16, 684–692 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Verburg-van Kemenade B. M. L., Cohen N., Chadzinska M., Neuroendocrine-immune interaction: Evolutionarily conserved mechanisms that maintain allostasis in an ever-changing environment. Dev. Comp. Immunol. 66, 2–23 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Freeman M. E., Kanyicska B., Lerant A., Nagy G., Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 80, 1523–1631 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Dijkstra C. D., et al. , Therapeutic effect of the D2-dopamine agonist bromocriptine on acute and relapsing experimental allergic encephalomyelitis. Psychoneuroendocrinology 19, 135–142 (1994). [DOI] [PubMed] [Google Scholar]
- 28.Chavez-Rueda K., et al. , Identification of prolactin as a novel immunomodulator on the expression of co-stimulatory molecules and cytokine secretions on T and B human lymphocytes. Clin. Immunol. 116, 182–191 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Ignacak A., et al. , Prolactin—not only lactotrophin. A “new” view of the “old” hormone. J. Physiol. Pharmacol. 63, 435–443 (2012). [PubMed] [Google Scholar]
- 30.Woodland D. L., et al. , Endogenous superantigen expression is controlled by mouse mammary tumor proviral loci. J. Exp. Med. 174, 1255–1258 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morandi E., Tarlinton R. E., Gran B., Multiple sclerosis between genetics and infections: Human endogenous retroviruses in monocytes and macrophages. Front. Immunol. 6, 647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeed A. I., et al. , TM4: A free, open-source system for microarray data management and analysis. Biotechniques 34, 374–378 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Mi H., et al. , PANTHER version 11: Expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 45, D183–D189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L., Duff K., A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J. Vis. Exp., 21 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.