Significance
Photosystem II (PSII) catalyzes light-induced water oxidation in photosynthesis. The PSII core is associated with light-harvesting complex II (LHCII) subunits, forming a PSII–LHCII supercomplex. To adapt to various light environments, photosynthetic organisms have developed different antenna systems during evolution. So far the structure of a C2S2M2-type PSII–LHCII from higher plants has been reported. Here we report a near–atomic-resolution structure of a complete, C2S2M2N2-type PSII–LHCII supercomplex from the green alga Chlamydomonas reinhardtii by cryoelectron microscopy. Our results reveal the detailed supramolecular organization and arrangement of a huge number of protein subunits and pigments of the PSII–LHCII supercomplex, enabling us to examine the possible energy transfer pathways in great detail in the green algal PSII–LHCII.
Keywords: photosynthesis, cryoelectron microscopy, photosystem II, light-harvesting complex, structural analysis
Abstract
Photosystem II (PSII) in the thylakoid membranes of plants, algae, and cyanobacteria catalyzes light-induced oxidation of water by which light energy is converted to chemical energy and molecular oxygen is produced. In higher plants and most eukaryotic algae, the PSII core is surrounded by variable numbers of light-harvesting antenna complex II (LHCII), forming a PSII–LHCII supercomplex. In order to harvest energy efficiently at low–light-intensity conditions under water, a complete PSII–LHCII supercomplex (C2S2M2N2) of the green alga Chlamydomonas reinhardtii (Cr) contains more antenna subunits and pigments than the dominant PSII–LHCII supercomplex (C2S2M2) of plants. The detailed structure and energy transfer pathway of the Cr-PSII–LHCII remain unknown. Here we report a cryoelectron microscopy structure of a complete, C2S2M2N2-type PSII–LHCII supercomplex from C. reinhardtii at 3.37-Å resolution. The results show that the Cr-C2S2M2N2 supercomplex is organized as a dimer, with 3 LHCII trimers, 1 CP26, and 1 CP29 peripheral antenna subunits surrounding each PSII core. The N-LHCII trimer partially occupies the position of CP24, which is present in the higher-plant PSII–LHCII but absent in the green alga. The M trimer is rotated relative to the corresponding M trimer in plant PSII–LHCII. In addition, some unique features were found in the green algal PSII core. The arrangement of a huge number of pigments allowed us to deduce possible energy transfer pathways from the peripheral antennae to the PSII core.
Light-driven photochemical reactions in oxygenic photosynthesis take place in photosystem I (PSI) and photosystem II (PSII), 2 pigment–protein complexes embedded in the thylakoid membranes of higher plants, algae, and cyanobacteria (1). PSII is a multisubunit pigment–protein complex that catalyzes the light-driven oxidation of water into molecular oxygen and the reduction of plastoquinone, thereby transforming sunlight into chemical energy (2, 3). PSII is composed of 2 moieties: the core complex and the peripheral antenna system. Except for some luminal extrinsic subunits, the PSII core complex is relatively conserved in all photosynthetic organisms and contains all of the subunits and cofactors involved in charge separation, electron transport, and water-splitting reactions (1–3). The atomic structure of the PSII core complex from thermophilic cyanobacteria has been solved by X-ray crystallography up to 1.9-Å resolution (4–9), which revealed the detailed organization of the PSII core including the catalytic center for water oxidation.
The peripheral antenna system bound to the PSII core enhances the capacity of light capture and exhibits a large variety in different photosynthetic organisms (10). In cyanobacteria and red algae, a water-soluble protein complex of phycobilisome (PBS) attaches to the stromal side of the PSII core and serves as the antenna to harvest light energy. The complete structure of PBS from the red alga Griffithsia pacifica has been solved at 3.5-Å resolution by cryoelectron microscopy (cryo-EM) (11), which reveals detailed architecture and energy transfer pathways within PBS. In higher plants and green algae, the peripheral antenna system is composed of membrane-spanning Lhcb proteins encoded by the light-harvesting complex (Lhc) multigenic family (12). In higher plants, there are mainly 6 types of Lhcb proteins (Lhcb1 to 6) encoded by Lhcb1 to 6 genes. Lhcb1 to 3 organize as homo- or heterotrimers to form the major light-harvesting complex II (LHCII) (13, 14). The other chlorophyll (Chl) a/b-binding proteins, Lhcb4, Lhcb5, and Lhcb6, also called CP29, CP26, and CP24, exist as minor monomeric antennae (15–18). In green algae, a larger number of LHC subunits are found to associate with the PSII core (19, 20). The major LHCII trimer in Chlamydomonas reinhardtii, a unicellular green alga used as a model organism for photosynthesis studies, is encoded by 9 genes (lhcbm1 to 9) and has been divided into 4 groups based on their sequence homology: type I (LHCBM3, LHCBM4, LHCBM6, LHCBM8, and LHCBM9), type II (LHCBM5), type III (LHCBM2 and LHCBM7), and type IV (LHCBM1) (20). On the other hand, only 2 minor antenna subunits, CP26 and CP29, are present and the gene encoding CP24 is not found in the genome of this alga (21, 22). Each PSII core was found to be surrounded by 3 LHC trimers and 2 minor antenna subunits (23, 24).
LHCII trimers associated with the PSII core are divided into 3 types, namely S (strongly associated), M (moderately associated), and L (loosely bound), based on their position in the PSII–LHCII supercomplex and affinities with the core (23, 25). In the PSII–LHCII supercomplex of C. reinhardtii, the L-type trimer was also named the N type (N, naked) for the reason that this trimer was directly associated with the PSII core without involvement of any monomeric antenna (24). In the past few years, the organization of PSII–LHCII has been studied intensively in higher plants and green algae (23–30). The structures of a C2S2-type PSII–LHCII from spinach and a C2S2M2-type PSII–LHCII from pea were analyzed by single-particle cryo-EM at 2.7- to 3.2-Å resolution (18, 31). PSII–LHCII complexes containing 1 L trimer have been observed in spinach from projection maps of negatively stained specimens (25), and 2D projection maps of the supercomplex with 6 LHCII trimers per dimeric core from C. reinhardtii have been obtained at 13- to 16.8-Å resolution by single-particle electron microscopy analysis (23, 24). However, the high-resolution structure of a complete C2S2M2L2(N2)-type PSII–LHCII supercomplex has not been analyzed so far, in part due to the weak association of the L trimer with the PSII core. To understand the molecular details of this large PSII supercomplex, we purified the complete, C2S2M2N2-type PSII supercomplex from C. reinhardtii and analyzed its structure using single-particle cryo-EM at an overall resolution of 3.37 Å. The detailed structure and pigment arrangement of PSII–LHCII from the green alga are revealed, enabling us to examine the detailed energy transfer pathways among the peripheral antennae and from the peripheral antennae to the core.
Results
Overall Structure.
The PSII–LHCII supercomplex was isolated from a green alga, C. reinhardtii CC-137 (Materials and Methods and SI Appendix, Fig. S1A), and characterized by electrophoresis, size-exclusion chromatography, absorption and fluorescence spectroscopy, and pigment quantification (SI Appendix, Fig. S1 B–F). The results obtained showed that the sample (hereafter designated Cr-PSII–LHCII) is composed of major components of the PSII core and a large number of LHCII subunits, and is homogeneous enough to be suitable for cryo-EM analysis. We collected 4,436 cryo-EM micrographs, and picked 208,263 particles for subsequent data processing (SI Appendix, Figs. S2 and S3). After 2D and 3D classifications (SI Appendix, Figs. S2 and S3 and Table S1), particles with a major population were selected and processed, which yielded a cryo-EM map with an overall resolution of 3.37 Å for the whole PSII–LHCII supercomplex. The local resolutions for the PSII core and peripheral antennae were determined to be 3.35 and 3.73 Å, respectively (SI Appendix, Fig. S3).
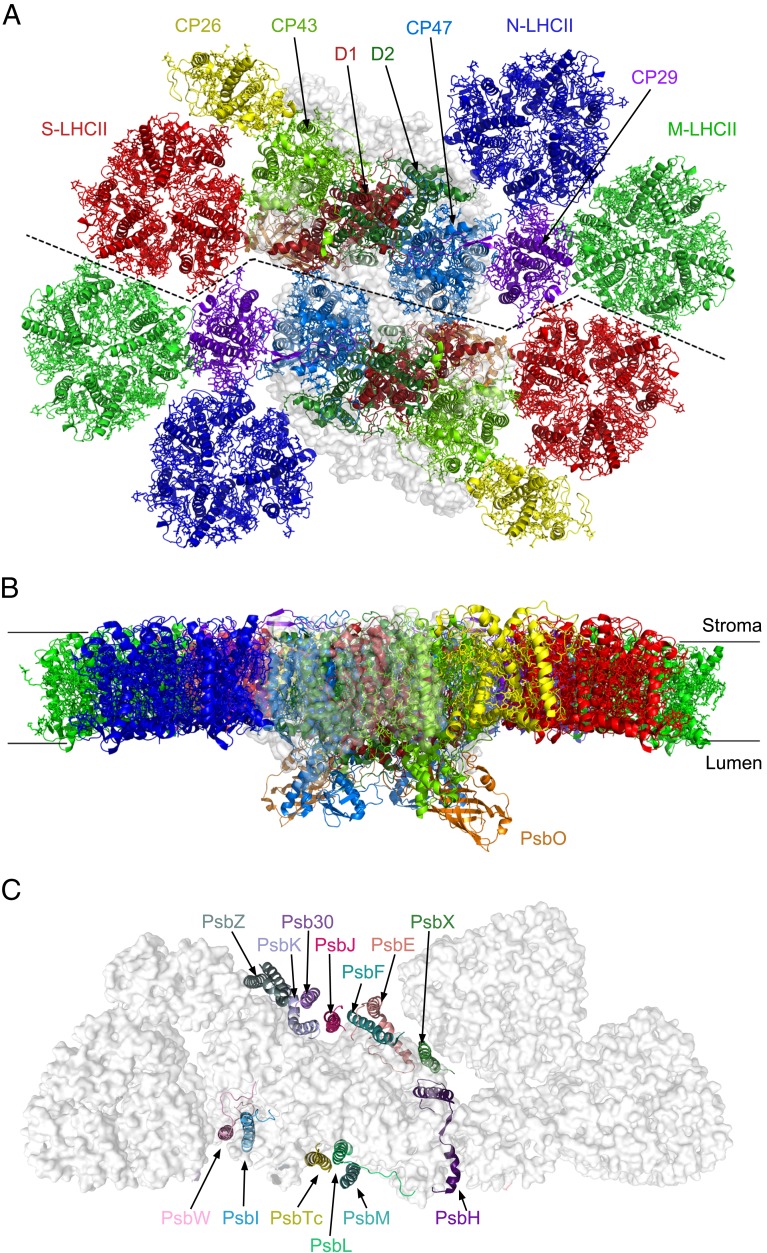
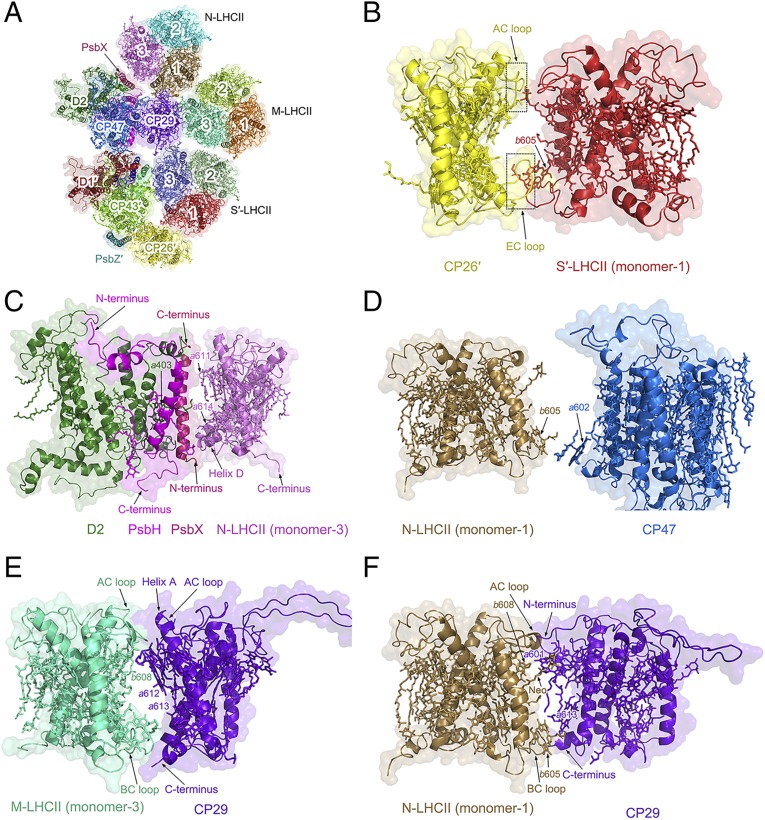
The overall structure of this Cr-PSII–LHCII is a homodimer with a 2-fold symmetry, and consists of 2 PSII core complexes (C2), 2 strongly bound LHCII trimers (S-LHCIIs), 2 moderately bound LHCII trimers (M-LHCIIs), and 2 naked LHCII trimers (N-LHCIIs), forming a C2S2M2N2-type supercomplex (Fig. 1). In addition, 1 CP26 subunit and 1 CP29 subunit are associated with each PSII core.
Fig. 1.
Overall architecture of the C2S2M2N2-type PSII–LHCII supercomplex from C. reinhardtii. (A) Cartoon representation of the overall structure with a top view from the stromal side. Four core subunits (D1, D2, CP43, and CP47), 1 extrinsic subunit PsbO, and the peripheral antennae are colored differently, whereas the 13 small intrinsic subunits are shown as a gray transparent surface. (B) Side view along the membrane plane. (C) The arrangement of 13 small membrane-intrinsic proteins in each monomer of the C2S2M2N2-type PSII–LHCII supercomplex viewed from the stromal side.
Similar to the plant PSII–LHCII, S-LHCII and CP26 attach to the core directly at the CP43 side, whereas the M-LHCII trimer attaches to the core at the CP47 side through CP29. The N-LHCII trimer takes the position occupied by CP24 in plant PSII–LHCII (31), and attaches to the core at the CP47 side partially directly and partially through interactions with CP29 (Fig. 1A). In addition to the protein subunits, we identified 189 Chls, 53 carotenoids, 2 pheophytins, 1 heme, and numerous other cofactors and lipids in each PSII–LHCII monomer (SI Appendix, Fig. S4 and Table S2). Due to the limited resolution, it was not possible to distinguish between Chl a and Chl b, as well as between different types of carotenoids unambiguously. Thus, we modeled these pigments according to the structure of plant LHCII (13, 18, 31). Loroxanthin, a carotenoid found in the PSII–LHCII of C. reinhardtii, was found in our purified sample by high-performance liquid chromatography (HPLC) (SI Appendix, Fig. S1F); however, its location cannot be distinguished from other carotenoids, again due to the limited resolution.
The Structure of the PSII Core.
The structure of the PSII core is similar to that of cyanobacterial and higher-plant PSII. In each monomer of the PSII core, 4 large intrinsic transmembrane subunits (D1, D2, CP43, and CP47) (SI Appendix, Fig. S5), 13 low–molecular-mass intrinsic transmembrane subunits (PsbE, PsbF, PsbH, PsbI, PsbJ, PsbK, PsbL, PsbM, PsbTc, PsbW, PsbX, PsbZ, and Psb30), and 1 extrinsic subunit (PsbO) attached at the luminal surface are observed (Fig. 1 and SI Appendix, Fig. S5). The rmsd values between the green algal PSII core (not including PsbW and Psb30) and those from a cyanobacterium (8) and higher plant (31) are 1.869 and 2.272, respectively.
The extrinsic subunits PsbP, PsbQ, and PsbTn present in higher-plant PSII (18, 31) are absent in the cryo-EM map of the green algal PSII, indicating that they associate with the PSII core weakly and have been lost during purification of the sample. PsbR was also not found in the cryo-EM structure despite it being detected by SDS/PAGE (SI Appendix, Fig. S1B), suggesting that it has also been lost during sample purification owing to its loose association with the PSII core.
Among the low–molecular-mass subunits, PsbW is observed in the structure of Cr-PSII. This subunit is absent in the cyanobacterial PSII (8, 9) but found in the PSII core of higher plants (18, 31). A transmembrane helix corresponding to the position of PsbW was also found in a red algal PSII (32). Psb30 is another unique subunit found in the current Cr-PSII structure which is present in the structures of cyanobacterial (8, 9) and red algal PSII (32) but absent in higher-plant PSII (18, 31). These features suggest that the green algal PSII core retains some features of cyanobacterial, red algal, and higher-plant PSII, reflecting its intermediate feature between cyanobacteria and higher plants during evolution.
Structure of the Peripheral Antennae.
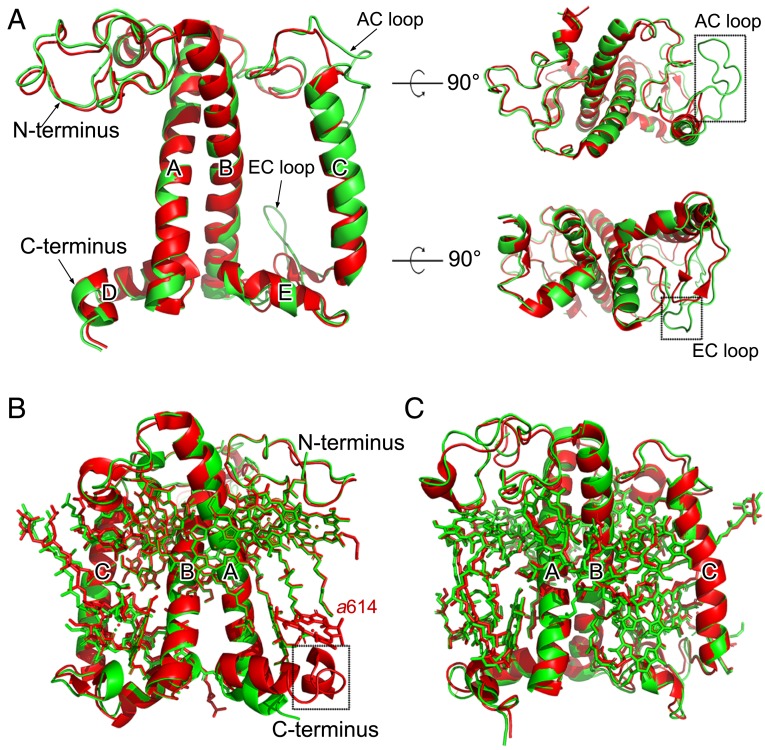
The light-harvesting antenna subunits of C. reinhardtii share common structural features as those of green algal and plant LHCI (33–37) and the major and minor antenna proteins of plant PSII (13, 15, 18, 31) in that all of them have 3 transmembrane helices (A, B, and C) and 2 short amphipathic helices (D and E) parallel to the luminal membrane. However, there are some distinct differences in the detailed structures and pigment bindings among the different LHC subunits. The cryo-EM density of the 3 LHCII trimers and CP26 and CP29 of C. reinhardtii obtained in the present study is shown in SI Appendix, Fig. S6, and the numbers of Chls and carotenoids bound to these subunits are summarized in SI Appendix, Table S2. The chromophore assignment was based on the cryo-EM density map (SI Appendix, Fig. S4) as well as previous high-resolution crystal and cryo-EM structures of LHCs and their complexes with the photosystem cores from plants (13, 18, 31). In Cr-CP26, 13 Chl binding sites and 3 carotenoid binding sites are found, which are the same as those found in plant CP26. However, the AC loop and EC loop of Cr-CP26 are longer than that of plant CP26 (Fig. 2A and SI Appendix, Fig. S7). These 2 extra regions are able to interact with monomer 1 of the S-LHCII trimer, forming unique interactions with S-LHCII. This may contribute to the energy transfer between CP26 and monomer 1 of the S-LHCII trimer. Cr-CP29 binds 13 Chls and 3 carotenoids but lacks a pigment molecule at Chl b614 found in plant CP29 (Fig. 2B). The Cr-CP29 possesses a shorter C terminus than that of plant CP29, whose C-terminal helix interacts with the BC loop of CP24 and strengthens the binding of Chl b614. The shorter C terminus of Cr-CP29 may be due to the fact that, in Cr-PSII–LHCII, there is no CP24 and its position is occupied by the larger N-LHCII trimer, leading to a smaller space between N-LHCII and CP29, and hence only a shorter C-terminal region of CP29 can be accommodated.
Fig. 2.
Structural comparison of peripheral antennae between C. reinhardtii and pea. (A) Superposition of the cryo-EM structure of the CP26 apo-protein from C. reinhardtii with that from pea (PDB ID code 5XNL). The 2 extra loop regions in Cr-CP26 are highlighted with black boxes. (B) Superposition of the cryo-EM structure of CP29 (including pigments) from C. reinhardtii with that from pea (PDB ID code 5XNL). The missing helix in Cr-CP29 is highlighted with a black box. Chl a614 existing in pea CP29 is absent in Cr-CP29. (C) Superposition of the cryo-EM structure of an LHCII subunit from the S-LHCII trimer from C. reinhardtii with that from pea (PDB ID code 5XNL). Green, C. reinhardtii; red, pea.
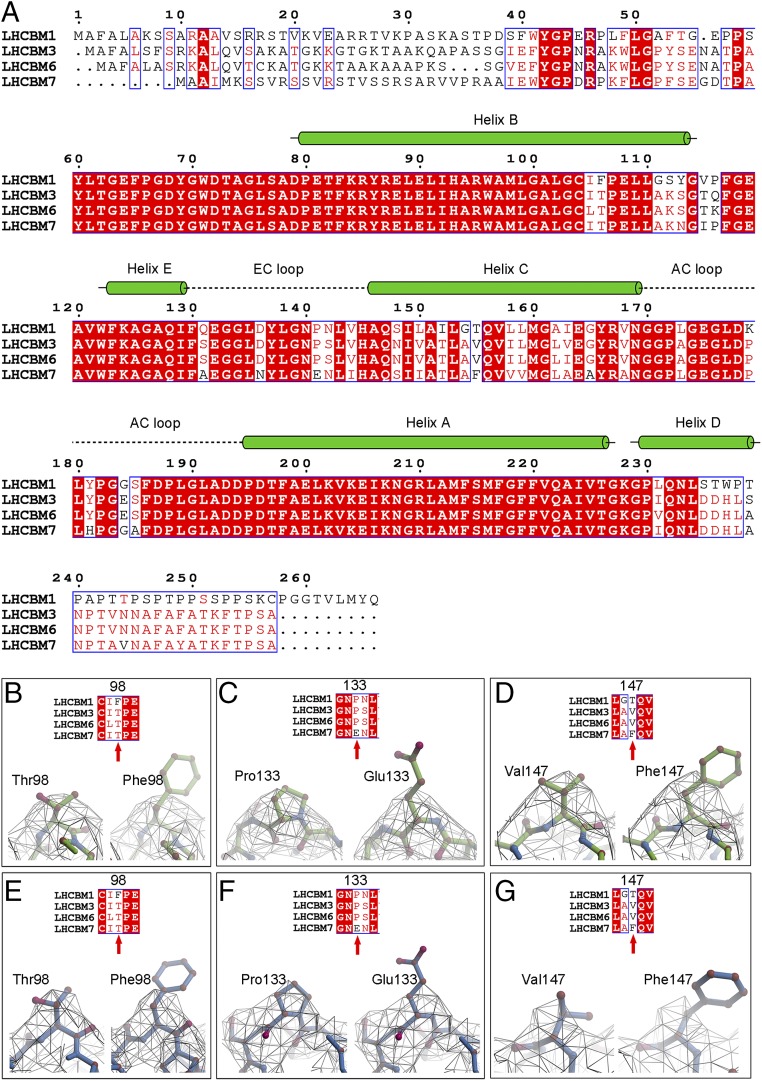
In C. reinhardtii, the major light-harvesting complexes of PSII (S-LHCII, M-LHCII, and N-LHCII) are encoded mainly by lhcbm1 to 9 genes. The protein bands corresponding to the LHCII antennae from SDS/PAGE were analyzed by mass spectrometry and the results showed that 4 subunits, namely LHCBM1, LHCBM3, LHCBM6, and LHCBM7, are abundantly present in the purified PSII–LHCII sample used for the cryo-EM studies (SI Appendix, Fig. S1B). Since the sequences of LHCBM1, LHCBM3, LHCBM6, and LHCBM7 (Fig. 3A) show a high degree of homology, the assignment of the LHC subunits constituting the 3 trimers, in particular for M-LHCII and N-LHCII, is difficult from the current cryo-EM density map (SI Appendix, Fig. S6). However, monomer 1 and monomer 3 of S-LHCII showed structural features of some special amino acid residues, allowing us to deduce their corresponding lhcbm genes. By comparing the structural features of residues Thr98 and Phe98, Pro133 and Glu133, as well as Val147 and Phe147 among the different LHCBM subunits (Fig. 3 B–G), LHCBM1 and LHCBM7 can be excluded for monomers 1 and 3 of S-LHCII. Thus, either LHCBM3 or LHCBM6 may be assigned to monomer 1 or 3 of S-LHCII. Previously, Drop et al. also analyzed the protein composition of Chlamydomonas PSII–LHCII supercomplexes by mass spectrometry, and suggested that LHCBM2/7, LHCBM1, and LHCBM3 are the most abundant LHCBM proteins in the supercomplex (24). These results are very similar to our results on the protein composition analysis. Taken together, we tentatively assign LHCBM3 as the protein subunit for monomers 1 and 3 of S-LHCII.
Fig. 3.
Sequence alignment of LHCBM subunits and the assignment of protein subunits in the cryo-EM structure from C. reinhardtii. (A) Sequence alignment of LHCBM1, LHCBM3, LHCBM6, and LHCBM7, which were detected by mass spectrometry from the SDS/PAGE bands of the Cr-PSII–LHCII supercomplex. Information on secondary structures shown above the sequences is taken from the cryo-EM structure of LHCII of the Cr-PSII–LHCII supercomplex obtained here. Helices are represented by green cylinders. Loops are denoted by dashed lines. (B–D) Assignment of the monomer 1 subunit of S-LHCII by comparison of the structural features between amino acids Thr98 and Phe98 (B), Pro133 and Glu133 (C), and Val147 and Phe147 (D). The possibilities of LHCBM1 and LHCBM7 as S-LHCII (monomer 1) in the cryo-EM structure of Cr-PSII–LHCII are excluded on the basis of B–D. (E–G) Assignment of subunit S-LHCII (monomer 3) by comparison of the structural features between amino acids Thr98 and Phe98 (E), Pro133 and Glu133 (F), and Val147 and Phe147 (G). The possibilities of LHCBM1 and LHCBM7 as S-LHCII (monomer 3) in the cryo-EM structure of Cr-PSII–LHCII are excluded on the basis of E, F and G. Thus, either LHCBM3 or LHCBM6 can be assigned to S-LHCII (monomer 1) or S-LHCII (monomer 3).
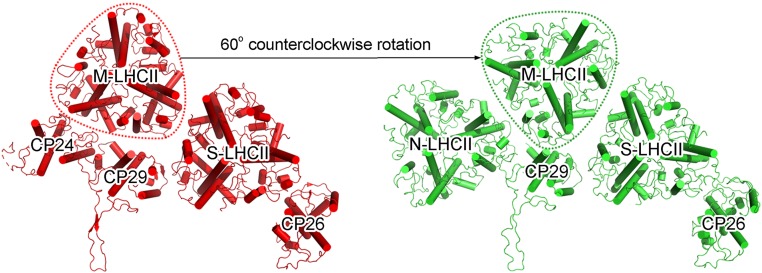
The Cr-LHCII trimers exhibited similar overall structures to that of higher plants in terms of the number and location of pigments as well as the protein secondary structure. All of the Cr-LHCII trimers consist of 8 Chls a, 6 Chls b, and 4 carotenoids in each subunit (Fig. 2C). One distinct feature of the Cr-LHCII trimers is that its M-LHCII trimer is rotated counterclockwise by 60° relative to the position and orientation of M-LHCII in higher plants (Fig. 4), possibly due to its interactions with nearby LHCII subunits (see below).
Fig. 4.
Comparison of the orientations of M-LHCII in PSII–LHCII from green algae (C. reinhardtii; green; Right) and a higher plant (pea; red; Left). For simplicity, only the peripheral antenna complexes are shown. M-LHCII is encircled with dashed lines.
Antenna–Core and Antenna–Antenna Interactions.
The LHCII subunits in Cr-PSII–LHCII (Fig. 5A) interact with other subunits in a similar way to those of the C2S2M2-type PSII–LHCII supercomplex in higher plants, except for the presence of the N trimer and some structural differences between Cr-LHCII and higher-plant LHCIIs. CP26 interacts with CP43 and PsbZ in the PSII core (SI Appendix, Fig. S8A) at both the stromal and luminal sides. At the stromal side, the N-terminal region of CP26 connects with the loop between the 2 helices of PsbZ and Chl a513 of CP43, whereas at the luminal side, the extra helix F in the C terminus of CP26 connects with CP43 through a lipid molecule (monogalactosyldiacylglycerol; MGDG), and also interacts with the C-terminal helix of PsbZ. CP29 interacts with CP47 of the PSII core extensively and also with the stromal loop-helix-loop region of PsbH (SI Appendix, Fig. S8B). At the stromal side, the long loop region in the N terminus of CP29 extends to the stromal side of CP47, and interacts with the loop region between the second and third transmembrane helices of CP47. At the luminal side, Chl b607 of CP29 interacts with the third and fourth transmembrane helices of CP47 through another MGDG molecule.
Fig. 5.
Antenna–antenna and antenna–core interactions in the C2S2M2N2-type PSII–LHCII supercomplex of C. reinhardtii. (A) Surface and cartoon representations of the local structure including LHCII (S-LHCII, M-LHCII, and N-LHCII), CP26, CP29, D1, D2, CP43, CP47, PsbH, PsbI, PsbX, PsbW, and PsbZ, viewed from the stromal side. (B) Interactions between S′-LHCII (monomer 1) and CP26′. The closed interactions between S′-LHCII (monomer 1) and the extra stromal as well as luminal loops of CP26′ are highlighted with solid boxes. (C) Interactions between N-LHCII (monomer 3) and D2 mediated by PsbX. (D) Interactions between N-LHCII (monomer 1) and CP47. (E) Interactions between M-LHCII (monomer 3) and CP29. (F) Interactions between N-LHCII (monomer 1) and CP29.
S-LHCII associates with the PSII core through interactions between monomer 3 of S-LHCII and CP43/PsbW (SI Appendix, Fig. S8C). Monomer 3 of S-LHCII connects with CP43 directly and also interacts with PsbW extensively, and the latter interactions appear to be more pivotal for the association of S-LHCII with the PSII core. At the stromal side, Chl a612 of monomer 3 interacts with the transmembrane helix of PsbW, and the C terminus of PsbW inserts into the stromal side of CP43 and interacts with the loop region between the fourth and fifth transmembrane helices of CP43. The N terminus of PsbW is connected to D1 directly at the luminal side, and a part of PsbW is also connected with D1 through PsbI.
The association of N-LHCII with the PSII core is less extensive and is mediated by interactions between monomer 3 of N-LHCII and PsbX as well as CP47 (Fig. 5 C and D). At the stromal side, Chl a611 of N-LHCII monomer 3 interacts with the C-terminal helix of PsbX, whereas at the luminal side, Chl a614 of monomer 3 and its helix D interact with the N-terminal helix of PsbX, which is in turn connected to D1 and PsbH of the PSII core.
Within the peripheral antenna complexes, CP26 connects with monomer 1 of S-LHCII through interactions between Pro194 and Lys198 at the AC loop of CP26 and Asp179 and Gly182 at the AC loop of monomer 1, as well as interactions between the EC loop of CP26 and the EC loop and helix B of monomer 1 (Fig. 5B). The interaction patterns between M-LHCII and S′-LHCII from the adjacent PSII–LHCII monomer in Cr-PSII–LHCII are different from that of higher plants because of the rotation of M-LHCII. In C. reinhardtii, monomer 2 of S′-LHCII interacts with 2 monomers (monomer 1 and monomer 3) of M-LHCII simultaneously (SI Appendix, Fig. S8 D and E), whereas only 1 monomer from M-LHCII is involved in the interaction with S′-LHCII in higher plants. Multiple interactions are observed at the luminal side between the 2 helices D of S′-LHCII (monomer 2) and M-LHCII (monomer 3), and between Chl a614 of S′-LHCII (monomer 2) and M-LHCII (monomer 3). In addition, 1 monomer (monomer 1) of N-LHCII may also interact with the 2 monomers (monomer 3 and monomer 2) of M-LHCII (SI Appendix, Fig. S8 F and G). At the stromal side, the AC loop of N-LHCII (monomer 1) interacts with the neoxanthin (Neo) of M-LHCII (monomer 3), whereas interactions between N-LHCII (monomer 1) and M-LHCII (monomer 2) are less extensive.
CP29 associates with 3 major LHCII trimers (M-LHCII, N-LHCII, and S′-LHCII) tightly, and therefore mediates the interactions between these LHCII trimers as well as their interactions with the core. At the stromal side, Neo623 of CP29 interacts with Pro180 at the AC loop of S′-LHCII (monomer 3), and Leu182 at the AC loop of CP29 interacts with Neo623 of S′-LHCII (monomer 3) (SI Appendix, Fig. S8H). At the luminal side, close connections between the Leu139-to-Pro142 region at the EC loop of CP29 and the Ser134-to-Leu135 region at the EC loop and Chl b605 of S′-LHCII (monomer 3) are observed.
Strong interactions between CP29 and monomer 3 of M-LHCII are found at both the stromal and luminal sides (Fig. 5E). At the stromal side, helix A, the AC loop, and the Chl a611–Chl a612 pair of CP29 interact with Chl b608, Neo623, and the AC loop of M-LHCII (monomer 3). At the luminal side, Chl a613 and the C-terminal helix of CP29 interact with Chl b605 and the BC loop of M-LHCII (monomer 3). The interactions between CP29 and M-LHCII are enhanced by the rotation of M-LHCII, resulting in their tight association. In contrast, the interactions between M-LHCII and S′-LHCII are formed mostly by pigments laying in their interface and hence may be weaker (SI Appendix, Fig. S8 D and E).
The interactions between CP29 and N-LHCII are unique to Cr-PSII–LHCII (Fig. 5F). They include interactions between Chl a601 at the N terminus of CP29 and Chl b608, Neo623, and the AC loop of monomer 1 of N-LHCII at the stromal side, and between Chl a613, the C-terminal helix of CP29, and Chl b605 and the BC loop from monomer 1 of N-LHCII at the luminal side. These extensive interactions between CP29 and M-LHCII, and between CP29 and N-LHCII, on both the stromal and luminal sides lead to a close association of the peripheral antenna complexes with the PSII core.
Possible Energy Transfer Pathways.
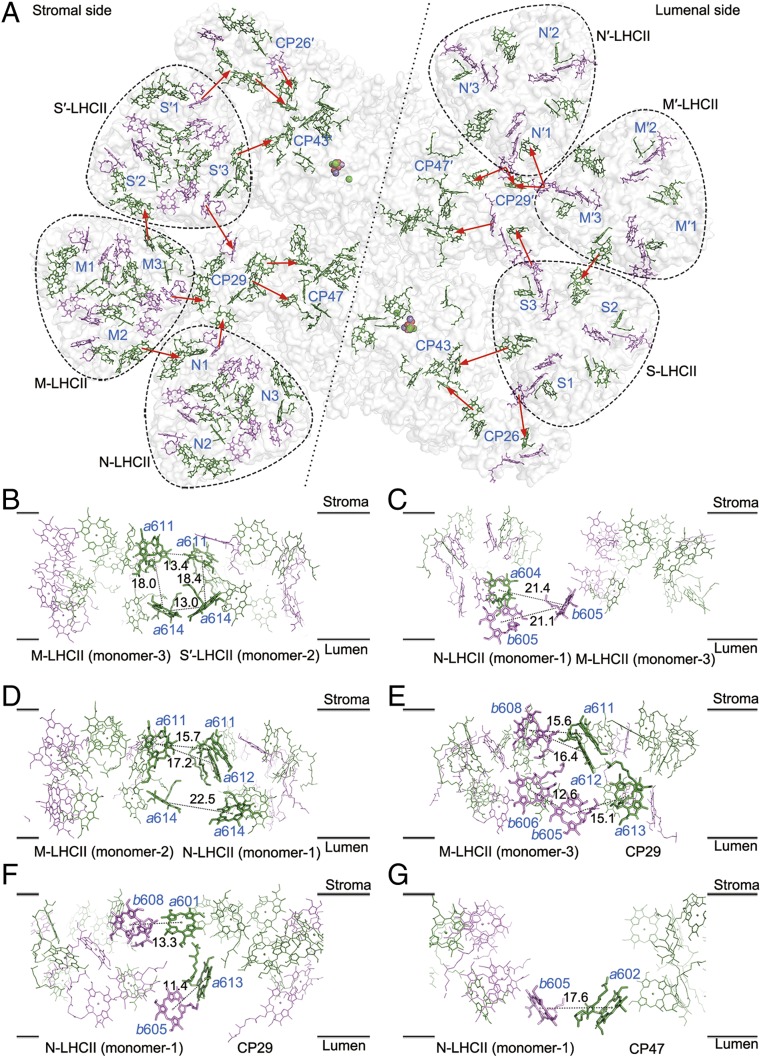
Based on the arrangement of pigments revealed in the present study, we propose possible excitation energy transfer (EET) pathways in the Cr-PSII–LHCII supercomplex (Fig. 6A). The pathways of EET from S-LHCII to CP26, and from these 2 LHCII units to the PSII core in C. reinhardtii, are similar to those in higher plants (SI Appendix, Fig. S9A). Although the association of CP26 with S-LHCII is enhanced by the presence of 2 extra loops in CP26, the distance between pigments in their interface is similar to those found in the pea supercomplex (31).
Fig. 6.
Pigment arrangement and possible excitation energy transfer pathways from peripheral antenna complexes to the reaction center in the C2S2M2N2-type PSII–LHCII supercomplex of C. reinhardtii. (A) Chlorophyll distribution and possible energy transfer pathways within the whole PSII–LHCII supercomplex. Chlorophylls distributed in the stromal side layer are shown (Left), as are those distributed in the luminal side layer (Right). Chl a and Chl b are colored in green and violet, respectively. Possible energy transfer pathways from the peripheral antennae to the PSII core and among the antennae are indicated by red arrows. (B–G) The interfacial chlorophylls between M-LHCII (monomer 3) and S′-LHCII (monomer 2) (B), N-LHCII (monomer 1) and M-LHCII (monomer 3) (C), N-LHCII (monomer 1) and M-LHCII (monomer 2) (D), M-LHCII (monomer 3) and CP29 (E), N-LHCII (monomer 1) and CP29 (F), and N-LHCII (monomer 1) and CP47 (G). These panels are viewed from the side of the membrane, with the upper layer representing the stromal layer and the lower layer representing the luminal layer. Chlorophylls involved in the possible energy transfer are highlighted as sticks and labeled with blue. The Mg-to-Mg distances (Å) between 2 adjacent interfacial chlorophylls are indicated in black.
The EET pathways involving M-LHCII differ from those of higher plants due to the 60° rotation of the M-LHCII trimer in Cr-PSII–LHCII. Although the interactions between M-LHCII and S′-LHCII are less extensive, pigments in M-LHCII (monomer 3) and S′-LHCII (monomer 2) are very close in space, which results in different EET pathways (Fig. 6B). At the stromal side, the Chl a611 pair between M-LHCII (monomer 3) and S′-LHCII (monomer 2) has an Mg-to-Mg distance of 13.4 Å. An Mg-to-Mg distance of 13.0 Å is observed for the Chl a614 pair between M-LHCII (monomer 3) and S′-LHCII (monomer 2) at the luminal side. The edge-to-edge distances between Chl a611M-LHCII (monomer 3) and Chl a611S′-LHCII (monomer 2), and between Chl a614M-LHCII (monomer 3) and Chl a614S′-LHCII (monomer 2), are both 6.1 Å. The Mg-to-Mg distances between Chl a611M-LHCII (monomer 3) and a614M-LHCII (monomer 3), and between Chl a611S′-LHCII (monomer 2) and Chl a614S′-LHCII (monomer 2), are 18.0 and 18.4 Å, respectively. Thus, these 4 Chls form a pigment cluster at the interface between M-LHCII and S′-LHCII, enabling efficient energy transfer among them possible. This facilitates efficient EET between 2 PSII–LHCII monomers, illustrating the importance of the formation of a PSII–LHCII dimer unit.
The EET between M-LHCII and N-LHCII is found between M-LHCII (monomer 3) and N-LHCII (monomer 1) at the luminal side, and between M-LHCII (monomer 2) and N-LHCII (monomer 1) at both the luminal and stromal sides. At the interface between M-LHCII (monomer 3) and N-LHCII (monomer 1) (Fig. 6C), the Mg-to-Mg distance from Chl b605M-LHCII (monomer 3) to Chl a604N-LHCII (monomer 1) and Chl b605N-LHCII (monomer 1) are 21.4 and 21.1 Å, respectively. At the interface between M-LHCII (monomer 2) and N-LHCII (monomer 1) (Fig. 6D), the Mg-to-Mg distance between Chl a614M-LHCII (monomer 2) and Chl a614N-LHCII (monomer 1) at the luminal side is 22.5 Å. At the stromal side, the Mg-to-Mg distances of the Chl a611–Chl a611 and Chl a611–Chl a612 pairs between M-LHCII (monomer 2) and N-LHCII (monomer 1) are 15.7 and 17.2 Å, respectively, which are shorter than the Mg-to-Mg distances observed at the interface between M-LHCII (monomer 3) and N-LHCII (monomer 1). Therefore, the Chl a611–a612 and Chl a611 pairs between M-LHCII (monomer 2) and N-LHCII (monomer 1) are likely the dominant pathway for EET between M-LHCII and N-LHCII.
The minor antenna CP29 plays a vital role in facilitating EET from the outermost antennae to the PSII core. EET between CP29 and S′-LHCII (monomer 3) in C. reinhardtii is similar to that observed in higher plants (SI Appendix, Fig. S9B). For the EET between M-LHCII and CP29 (Fig. 6E), the Mg-to-Mg distances from Chl b608M-LHCII (monomer 3) to Chl a611CP29 or Chl a612CP29 are 15.6 and 16.4 Å, respectively, at the stromal side. At the luminal side, Chl b605M-LHCII (monomer 3) locates at a position between Chl b606M-LHCII (monomer 3) and Chl a613CP29, with Mg-to-Mg distances of 12.6 Å for the Chl b605–Chl b606 pair and 15.1 Å for the Chl b605–Chl b613 pair. Thus, Chl b605M-LHCII (monomer 3) likely mediates EET from M-LHCII to CP29. For the energy transfer between N-LHCII and CP29 (Fig. 6F), Chl b608N-LHCII (monomer 1) and Chl a601CP29 form an intersubunit pair with an Mg-to-Mg distance of 13.3 Å at the stromal side, and Chl b605N-LHCII (monomer 1) and Chl a613CP29 form a close pair with an Mg-to-Mg distance of 11.4 Å at the luminal side. Since Chl b possesses a higher energy level than Chl a, EET usually takes place from Chl b to Chl a (18). Therefore, EET from N-LHCII to CP29 may occur from Chl b608N-LHCII (monomer 1) and Chl b605N-LHCII (monomer 1) to Chl a601CP29 and Chl a613CP29.
The energy absorbed by peripheral antenna complexes is first transferred to the inner antenna complexes CP43 and CP47 before arriving at the reaction center to trigger the photochemical reaction at the core. CP43 is able to collect the excitation energy from CP26 owing to their close interactions (SI Appendix, Fig. S9C). At the stromal side, the Mg-to-Mg distances between Chl b601CP26 and Chl a513CP43, and between Chl a611CP26 and Chl a512CP43, are 12.3 and 18.6 Å, respectively. At the luminal side, the Mg-to-Mg distance between Chl a614CP26 and Chl a503CP43 is 16.6 Å. Thus, energy from CP26 will be transferred to the Chl a513–a512 pair at the stromal side and Chl a503 at the luminal side of CP43. Meanwhile, CP43 may receive the energy from S-LHCII directly (SI Appendix, Fig. S9D). At the stromal side, energy from Chl a611S-LHCII (monomer 3) may be transferred to Chl a506CP43 at an Mg-to-Mg distance of 17.5 Å, and energy from Chl a614S-LHCII (monomer 3) may be transferred to Chl a501CP43 at a relatively far Mg-to-Mg distance of 25.0 Å.
CP47 accepts the excitation energy from CP29 and N-LHCII. In the EET pathways from CP29 to CP47 (SI Appendix, Fig. S9E), energy from Chl a616 of CP29 may be transferred to Chl a616 of CP47 at an Mg-to-Mg distance of 14.8 Å, and Chl a603 of CP29 may transfer excitation energy to Chl a610 of CP47 at an Mg-to-Mg distance of 19.4 Å, at the stromal side. At the luminal side, Chl b607 of CP29 may transfer energy to Chl a607 of CP47 at an Mg-to-Mg distance of 19.4 Å. In addition, energy absorbed by N-LHCII may be transferred to CP47 directly in addition to being partially transferred to CP47 through CP29 (Fig. 6G). This may occur mainly at the luminal side between Chl b605 of N-LHCII (monomer 1) and Chl a602 of CP47 with an Mg-to-Mg distance of 17.6 Å. Moreover, it was found that at the luminal side, Chl b605N-LHCII (monomer 1), Chl a602CP47, Chl a613CP29, and Chl b605M-LHCII (monomer 3) form a pigment cluster (SI Appendix, Fig. S9F), which enhances the efficient EET within this antenna network involving N-LHCII (monomer 1), CP47, CP29, and M-LHCII (monomer 3).
Discussion
The C2S2M2N2-type structure of PSII–LHCII solved in this study provides a complete, near–atomic-resolution structure of the PSII–LHCII supercomplex throughout green algae and higher plants. Among the PSII core components, most are conserved from cyanobacteria to higher plants but the Cr-PSII core shows the presence of some special subunits. PsbW is absent in cyanobacteria but present in both green algae and higher plants. Previous studies have shown that it is essential for formation of the PSII–LHCII supercomplex in higher plants (18, 31). The present Cr-PSII–LHCII structure shows that PsbW mediates the connection of LHCII with the PSII core, in agreement with its importance for the formation of the PSII–LHCII supercomplex, and also suggests its role in mediating energy transfer from LHCII to the PSII core. Interestingly, a similar transmembrane helix at the position of PsbW was found in a red algal PSII (32), although no transmembrane LHCIIs were found and the role of PsbW in the red algal PSII needs to be clarified. In addition, the current structure shows that PsbX also plays a vital role in mediating the binding of the peripheral antennae, although previous studies have shown that PsbX may be involved in quinone turnover at the QB binding site (38, 39).
Another PSII core subunit, Psb30, found in the present structure is present in the cyanobacterial PSII but not in higher-plant PSII. Previous mutation studies have shown that Psb30 is necessary for the optimal functioning of PSII in cyanobacteria and C. reinhardtii under high-light conditions (40, 41), and deletion of this subunit led to the increase of photosensitivity in C. reinhardtii (41). It is thus likely that Psb30 is involved in an adaptation mechanism used by cyanobacteria and green algae to cope with the highly fluctuating light conditions under water, which has been lost in land plants possibly due to the different light environment.
The green algal PSII–LHCII solved in the present study bears the largest number of LHCII trimers among the PSII–LHCII structures solved so far. Among them, the positions of S-LHCII and M-LHCII are similar to those in higher-plant PSII (31). However, the orientation of the M-LHCII trimer is rotated counterclockwise by 60° compared with that of the M-LHCII in higher-plant PSII (Fig. 4 and SI Appendix, Fig. S10), which may have been caused by its interactions with the nearby subunits different from that of higher-plant PSII–LHCII. One such nearby subunit is the N-LHCII (or L-LHCII) trimer located at the position occupied by CP24 in higher-plant PSII. This trimer is associated with the PSII core as well as CP29 and M-LHCII. This is apparently different from what has been suggested in higher-plant PSII (25), where L-LHCII is located at a position between CP24 and CP26 but not directly connected with M-LHCII. Multiple interactions between N-LHCII and the core, CP29, and M-LHCII suggests its rather tight binding, which may contribute to a larger antenna size or cross-section in the green alga that is likely required for harvesting enough energy under low-light conditions under water.
In summary, the complete Cr-PSII–LHCII structure demonstrates a highly efficient network of energy transfer. Compared with the C2S2M2-type supercomplex of higher-plant PSII, the rotation of M-LHCII enhances the interactions and connections between M-LHCII and CP29, which may increase the efficiency of EET from M-LHCII to CP29. Moreover, the interactions between N-LHCII and CP29 may mediate the binding and energy transfer from N-LHCII to the PSII core. In addition, due to the interaction between N-LHCII and M-LHCII, energy harvested by M-LHCII may be transferred to CP29 by N-LHCII. Taken together, these features ensure a more efficient EET, which may be needed for green algae to adapt to the low-light intensities available in aquatic environments.
Materials and Methods
Purification of the C2S2M2N2-Type PSII–LHCII.
Cells of C. reinhardtii wild type (strain CC-137) were cultured in a Tris-acetate-phosphate medium bubbled with 3% (vol/vol) CO2-enriched air at room temperature (25 °C) under a continuous light intensity of 30 to 50 μmol photons⋅m−2⋅s−1. For purification of the C2S2M2N2-type PSII–LHCII supercomplex, the thylakoid membranes were prepared under dim green light at 4 °C as described previously (42).
PSII–LHCII supercomplexes were isolated as described in ref. 28 with modifications described in ref. 24 at 4 °C. Briefly, thylakoid membranes were washed with 10 mM Hepes-KOH (pH 7.5), 5 mM EDTA and then centrifuged at 40,000 × g for 10 min. Subsequently, the pellets were suspended in a buffer containing 20 mM Hepes-KOH (pH 7.5), 10 mM MgCl2, 5 mM CaCl2 at a Chl concentration of 1.2 mg⋅mL−1. The washed membranes were solubilized by adding an equal volume of 1.0% (wt/vol) n-dodecyl-α-d-maltoside (α-DDM) for 5 min on ice in the dark. The solubilized membranes were centrifuged at 15,000 × g for 10 min to remove unsolubilized materials. The supernatant was loaded onto a continuous sucrose density gradient (prepared by freeze–thawing of a 0.7 M sucrose solution containing 10 mM Hepes-KOH, pH 7.5, 5 mM CaCl2, and 0.01% [wt/vol] α-DDM) and centrifuged at 38,000 rpm for 20 h at 4 °C using an SW41 rotor with a Beckman Coulter Optima XPN-100 centrifuge. The green band at the lowest position on the sucrose gradient (SI Appendix, Fig. S1A) was collected carefully using a syringe. For electron microscopy studies, the bottom band with a Chl a/b ratio of around 2.2 was collected and concentrated to 1 mg Chl⋅mL−1 by using a 100-kDa cutoff membrane concentrator. Then, the sample was subjected to size-exclusion chromatography (GE; Superose 6 Increase 10/300 GL) in a buffer containing 10 mM Hepes-KOH (pH 7.5), 50 mM NaCl, 5 mM CaCl2, and 0.01% α-DDM (SI Appendix, Fig. S1C). The peak fractions were collected for cryo-EM analysis. The concentration of Chls was determined according to ref. 43.
Characterization of the C2S2M2N2-Type PSII–LHCII.
The protein compositions of thylakoid membranes and purified PSII–LHCII supercomplexes were analyzed by electrophoresis using a gel containing 16% polyacrylamide and 7.5 M urea (44). For mass spectrometry, Coomassie brilliant blue-stained bands were cut out from the gel and digested using sequencing-grade modified trypsin, and the resultant peptides were extracted. The peptides were separated by a 60-min gradient elution at a flow rate of 0.30 mL/min with an EASY-nLC 1000 System, which was directly interfaced with a Thermo Orbitrap Fusion mass spectrometer. The analytical column used was a homemade fused silica capillary column (75-μm inner diameter, 150-mm length; Upchurch) packed with C18 resin (300 Å, 5 μm; Varian). The MS/MS spectra from each LC-MS/MS run were searched against the selected database using the Proteome Discovery searching algorithm (version 1.4).
UV absorption spectra were measured by a UV-vis spectrophotometer (UV-2700; Shimadzu) at room temperature. Fluorescence emission spectra were measured with a fluorescence spectrometer (F-4500; Hitachi) equipped with a xenon lamp source and a 1.0-cm quartz cell, and the spectra were recorded at a wavelength range from 600 to 800 nm with the excitation wavelengths of 436, 473, and 500 nm at room temperature. The slit widths of both excitation and emission were set at 5.0 nm.
Pigment composition was analyzed by high-performance liquid chromatography as described previously (45). The sample after size-exclusion chromatography was mixed with 90% (vol/vol) cold acetone to extract pigments, and the extract was centrifuged at 10,000 × g for 10 min under dim light. The supernatant was injected onto a C18 reversed-phase column (Alltima C18 5μ) in a Waters e2695 separation module equipped with a Waters 2998 photodiode array detector. Pigments were identified based on their absorption spectra and elution times. The HPLC results indicated that the C2S2M2N2-type PSII–LHCII supercomplex contained Chl a, Chl b, neoxanthin, loroxanthin, violaxanthin, lutein, and β-carotene.
Cryo-EM Sample Preparation and Data Collection.
An aliquot of 2.5 μL purified C2S2M2N2-type PSII–LHCII sample was applied to graphene-oxide–covered holey carbon grids (Quantifoil R2/2, Au, 300 mesh), blotted for 6.5 s at a humidity of 100% and 22 °C, and plunge-frozen in liquid ethane using a Vitrobot (FEI). The microscope was carefully aligned before data acquisition, including the coma-free alignment to minimize beam tilt. Cryo-EM images of PSII–LHCII were acquired manually with an FEI Titan Krios electron microscope operated at 300-kV accelerating voltage at a nominal magnification of 22,500×, and the sample stage was tilted at 30° to overcome the problem of preferred orientations of the particles. The dose rate of the electron beam was set to ∼8 e−/s per physical pixel, and cryoimage stacks were recorded on a Gatan K2 summit camera at 4 frames per s for 10 s using the mode of superresolution. Defocus values on the specimen were set from −2.0 to −3.0 μm. A total of 4,436 image stacks were collected, and the drift correction and dose weighting of all 40 frames in each image stack were performed with MotionCor2 (46) to generate 2× binned images with a pixel size of 1.307 Å per pixel. The total dose of each merged image is ∼47 e−/Å2.
Cryo-EM Image Processing.
A total of 208,263 particles were automatically selected with Gautomatch (https://www.mrc-lmb.cam.ac.uk/kzhang/Gautomatch/), and the local defocus and astigmatism values of individual particles were determined using Gctf (47). Image processing was accomplished with cryoSPARC (48). After 2D classification, 20 out of 50 classes were selected, resulting in a total of 135,829 particles (SI Appendix, Fig. S3). Subsequently, 3D classification was performed, which yielded 4 classes as follows: 1) The first class (Left side) does not correspond to any of the PSII-LHCII particles and thus was discarded. 2) The remaining 3 classes with 124,456 particles were processed using both C1 and C2 symmetries, resulting in structures with a resolution of 3.52 and 3.37 Å, respectively (SI Appendix, Fig. S3). 3) Among these 3 classes, the 2 classes on the Left side have 89,018 particles and were processed using a C1 symmetry, resulting in a structure with a resolution of 3.77 Å, and focused refinements for the antenna and the core regions resulted in 3.73- and 3.35-Å resolution, respectively (SI Appendix, Fig. S3).
Model Building and Refinement.
The atomic structure was built with Coot (49) using the structure of the stacked C2S2M2-type PSII–LHCII supercomplex from Pisum sativum (Protein Data Bank [PDB] ID code 5XNL) as the starting reference. The structures of each subunit were then refined in real space using Phenix (50) and displayed with UCSF Chimera (51) and PyMOL (Molecular Graphics System).
Data Availability.
The cryo-EM density map and atomic models have been deposited in the Electron Microscopy Data Bank and Protein Data Bank for the PSII–LHCII supercomplex structure at 3.37 Å (EMD ID code 9957 and PDB ID code 6KAF). The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Supplementary Material
Acknowledgments
We thank the Center of Cryo Electron Microscopy, Zhejiang University for their technical assistance with cryo-EM data collection; C. Ma from the Protein Facility, School of Medicine, Zhejiang University for providing this platform for sample purification; X. Meng in the Center of Biomedical Analysis, Tsinghua University, for protein MS analysis; and W. Tang and Y. Yin from the Institute of Botany, Chinese Academy of Sciences (CAS) for instrumental support in sample preparation, fluorescence measurement, and high-performance liquid chromatography analysis. The project was funded by the National Key R&D Program of China (2017YFA0503700, 2017YFA0504803, 2018YFA0507700), the Chinese Academy of Sciences Key Research Program of Frontier Sciences (QYZDY-SSW-SMC003), a Strategic Priority Research Program of CAS (XDB17000000), a National Basic Research Program of China (2015CB150100), and the Fundamental Research Funds for the Central Universities (2018XZZX001-13).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The cryo-EM density map and atomic models for the PSII–LHCII supercomplex structure at 3.37 Å reported in this paper have been deposited in the Electron Microscopy Data Bank and the Protein Data Bank, respectively (EMD ID code 9957 and PDB ID code 6KAF).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912462116/-/DCSupplemental.
References
- 1.Nelson N., Yocum C. F., Structure and function of photosystems I and II. Annu. Rev. Plant Biol. 57, 521–565 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Barber J., Photosystem II: The engine of life. Q. Rev. Biophys. 36, 71–89 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Shen J.-R., The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu. Rev. Plant Biol. 66, 23–48 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Kamiya N., Shen J.-R., Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7-Å resolution. Proc. Natl. Acad. Sci. U.S.A. 100, 98–103 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira K. N., Iverson T. M., Maghlaoui K., Barber J., Iwata S., Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Loll B., Kern J., Saenger W., Zouni A., Biesiadka J., Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 438, 1040–1044 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Guskov A., et al. , Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nat. Struct. Mol. Biol. 16, 334–342 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Umena Y., Kawakami K., Shen J.-R., Kamiya N., Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–60 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Suga M., et al. , Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 517, 99–103 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Croce R., van Amerongen H., Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 10, 492–501 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., et al. , Structure of phycobilisome from the red alga Griffithsia pacifica. Nature 551, 57–63 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Jansson S., A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 4, 236–240 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Liu Z., et al. , Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428, 287–292 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Standfuss J., Terwisscha van Scheltinga A. C., Lamborghini M., Kühlbrandt W., Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J. 24, 919–928 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan X., et al. , Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat. Struct. Mol. Biol. 18, 309–315 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Croce R., Canino G., Ros F., Bassi R., Chromophore organization in the higher-plant photosystem II antenna protein CP26. Biochemistry 41, 7334–7343 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Passarini F., Wientjes E., Hienerwadel R., Croce R., Molecular basis of light harvesting and photoprotection in CP24: Unique features of the most recent antenna complex. J. Biol. Chem. 284, 29536–29546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei X., et al. , Structure of spinach photosystem II-LHCII supercomplex at 3.2 Å resolution. Nature 534, 69–74 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Minagawa J., Takahashi Y., Structure, function and assembly of photosystem II and its light-harvesting proteins. Photosynth. Res. 82, 241–263 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Natali A., Croce R., Characterization of the major light-harvesting complexes (LHCBM) of the green alga Chlamydomonas reinhardtii. PLoS One 10, e0119211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elrad D., Grossman A. R., A genome’s-eye view of the light-harvesting polypeptides of Chlamydomonas reinhardtii. Curr. Genet. 45, 61–75 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Merchant S. S., et al. , The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245–250 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokutsu R., Kato N., Bui K. H., Ishikawa T., Minagawa J., Revisiting the supramolecular organization of photosystem II in Chlamydomonas reinhardtii. J. Biol. Chem. 287, 31574–31581 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drop B., et al. , Light-harvesting complex II (LHCII) and its supramolecular organization in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1837, 63–72 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Boekema E. J., Van Roon H., Van Breemen J. F. L., Dekker J. P., Supramolecular organization of photosystem II and its light-harvesting antenna in partially solubilized photosystem II membranes. Eur. J. Biochem. 266, 444–452 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Yakushevska A. E., et al. , Supermolecular organization of photosystem II and its associated light-harvesting antenna in Arabidopsis thaliana. Eur. J. Biochem. 268, 6020–6028 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Nield J., Barber J., Refinement of the structural model for the photosystem II supercomplex of higher plants. Biochim. Biophys. Acta 1757, 353–361 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Caffarri S., Kouřil R., Kereïche S., Boekema E. J., Croce R., Functional architecture of higher plant photosystem II supercomplexes. EMBO J. 28, 3052–3063 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagliano C., et al. , Proteomic characterization and three-dimensional electron microscopy study of PSII-LHCII supercomplexes from higher plants. Biochim. Biophys. Acta 1837, 1454–1462 (2014). [DOI] [PubMed] [Google Scholar]
- 30.van Bezouwen L. S., et al. , Subunit and chlorophyll organization of the plant photosystem II supercomplex. Nat. Plants 3, 17080–17091 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Su X., et al. , Structure and assembly mechanism of plant C2S2M2-type PSII-LHCII supercomplex. Science 357, 815–820 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Ago H., et al. , Novel features of eukaryotic photosystem II revealed by its crystal structure analysis from a red alga. J. Biol. Chem. 291, 5676–5687 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin X., Suga M., Kuang T., Shen J.-R., Photosynthesis. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 348, 989–995 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Mazor Y., Borovikova A., Caspy I., Nelson N., Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nat. Plants 3, 17014–17023 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Qin X., et al. , Structure of a green algal photosystem I in complex with a large number of light-harvesting complex I subunits. Nat. Plants 5, 263–272 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Su X., et al. , Antenna arrangement and energy transfer pathways of a green algal photosystem-I-LHCI supercomplex. Nat. Plants 5, 273–281 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Suga M., et al. , Structure of the green algal photosystem I supercomplex with a decameric light-harvesting complex I. Nat. Plants 5, 626–636 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Funk C., Functional analysis of the PsbX protein by deletion of the corresponding gene in Synechocystis sp. PCC 6803. Plant Mol. Biol. 44, 815–827 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Katoh H., Ikeuchi M., Targeted disruption of psbX and biochemical characterization of photosystem II complex in the thermophilic cyanobacterium Synechococcus elongatus. Plant Cell Physiol. 42, 179–188 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Inoue-Kashino N., et al. , Evidence for a stable association of Psb30 (Ycf12) with photosystem II core complex in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth. Res. 98, 323–335 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Inoue-Kashino N., Kashino Y., Takahashi Y., Psb30 is a photosystem II reaction center subunit and is required for optimal growth in high light in Chlamydomonas reinhardtii. J. Photochem. Photobiol. B 104, 220–228 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Chua N. H., Bennoun P., Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: Wild-type and mutant strains deficient in photosystem II reaction center. Proc. Natl. Acad. Sci. U.S.A. 72, 2175–2179 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porra R. J., Thompson W. A., Kriedemann P. E., Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975, 384–394 (1989). [Google Scholar]
- 44.Ikeuchi M., Inoue Y., A new 4.8-kDa polypeptide intrinsic to the PS II reaction center, as revealed by modified SDS-PAGE with improved resolution of low-molecular-weight proteins. Plant Cell Physiol. 29, 1233–1239 (1988). [Google Scholar]
- 45.Angeler D. G., Schagerl M., Distribution of the xanthophyll loroxanthin in selected members of the Chlamydomondales and Volvocales (Chlorophyta). Phyton Ann. Rei Bot. A 37, 119–132 (1997). [Google Scholar]
- 46.Zheng S. Q., et al. , MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang K., Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Punjani A., Rubinstein J. L., Fleet D. J., Brubaker M. A., cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams P. D., et al. , PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pettersen E. F., et al. , UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The cryo-EM density map and atomic models have been deposited in the Electron Microscopy Data Bank and Protein Data Bank for the PSII–LHCII supercomplex structure at 3.37 Å (EMD ID code 9957 and PDB ID code 6KAF). The data that support the findings of this study are available from the corresponding authors upon reasonable request.