Significance
The cochlea is essential for sound detection. It consists of highly differentiated hair cells and nonsensory supporting cells radially patterned into lateral and medial compartments. However, mechanisms regulating radial patterning and specification of hair cell and supporting cell subtypes, each of which serves unique functions, are unclear. Here we show that β-catenin is required for radial patterning and differentiation of sensory and nonsensory cell subtypes in the developing cochlea. β-Catenin-deleted cochleae displayed disrupted compartment borders containing ectopic hair cell and supporting cell subtypes, whereas inhibiting transcriptional and preserving cell adhesion-mediated activities of β-catenin maintained radial patterning and cell identity. This study thus characterizes a role for β-catenin-mediated cell adhesion in the developing cochlea.
Keywords: Wnt signaling, hair cell, cochlea, development, cell adhesion
Abstract
Development of multicellular organs requires the coordination of cell differentiation and patterning. Critical for sound detection, the mammalian organ of Corti contains functional units arranged tonotopically along the cochlear turns. Each unit consists of sensory hair cells intercalated by nonsensory supporting cells, both specified and radially patterned with exquisite precision during embryonic development. However, how cell identity and radial patterning are jointly controlled is poorly understood. Here we show that β-catenin is required for specification of hair cell and supporting cell subtypes and radial patterning of the cochlea in vivo. In 2 mouse models of conditional β-catenin deletion, early specification of Myosin7-expressing hair cells and Prox1-positive supporting cells was preserved. While β-catenin-deficient cochleae expressed FGF8 and FGFR3, both of which are essential for pillar cell specification, the radial patterning of organ of Corti was disrupted, revealed by aberrant expression of cadherins and the pillar cell markers P75 and Lgr6. Moreover, β-catenin ablation caused duplication of FGF8-positive inner hair cells and reduction of outer hair cells without affecting the overall hair cell density. In contrast, in another transgenic model with suppressed transcriptional activity of β-catenin but preserved cell adhesion function, both specification and radial patterning of the organ of Corti were intact. Our study reveals specific functions of β-catenin in governing cell identity and patterning mediated through cell adhesion in the developing cochlea.
The mammalian organ of Corti is a highly organized sensory epithelium crucial for auditory function. As mechanoreceptors for detecting sound vibration, hair cells (HCs) are interlaced with supporting cells, forming a checkerboard arrangement replicated along the length of the cochlea. Location along the cochlea constitutes a tonotopic map in which high frequencies are encoded in the base and low frequencies in the apex. HC progenitors exit mitosis in an apical-to-basal direction, starting around embryonic day 12 (E12), and HC differentiation begins in the opposing direction, starting in the midbasal region and extending apically around E13.5 (1). Inner HCs (IHCs) in the medial compartment (Fig. 1A) at the midbasal region are the first sensory cells specified in the developing cochlea (2). The wave of differentiation extends radially into the lateral compartment, where 3 rows of outer HCs (OHCs) develop (1). Around E16, IHCs in the midbasal turn begin expressing FGF8, which directs the formation of the pillar cells, a supporting cell subtype (3). Pillar cells will later form the tunnel of Corti, which separates IHCs and OHCs, and thus represent the border between medial and lateral compartments. In the mature mammalian cochlea, IHCs are the primary mechanoreceptors, whereas the OHCs serve as amplifiers to augment sound perception and frequency selectivity (4). Supporting cell subtypes in the medial and lateral compartments are distinct in their morphology and gene expression (5, 6). Hence, precise radial patterning and cell fate determination are fundamental for proper cochlear function.
Fig. 1.
β-Catenin deletion does not impair hair cell formation. (A) The Sox2 domain spans the lateral GER and the organ of Corti, including IHCs and OHCs, inner border cells (Bo), inner phalangeal cells (IPh), inner pillar cells (IP), outer pillar cells (OP), Deiters’ cells (DC), and Hensen cells (HC). The Fgf20 domain additionally encompasses portions of the LER and medial parts of the GER. (B) Schematic of the experimental setup. Fgf20-Cre starts to be expressed at E10.5, and tissues were examined at E18.5. Sox2-Cre mediated recombination was initiated with tamoxifen injections at E13.5 and E14.5, and tissues were examined at E18.5/E20.5. (C) Immunostaining of Myosin7a and Prox1 shows disorganized HCs and supporting cells in the basal turn of Sox2CreERT2/+; Ctnnb1fl/fl cochlea. Controls had 3 orderly rows of OHCs and 1 row of IHCs, separated by the apical processes of the pillar cells (D) Myosin7a+ HCs counts in Sox2CreERT2/+; Ctnnb1fl/fl (n = 6 to 8) and control (n = 5) cochleae. (E) Immunostaining of Myosin6 shows disorganized HCs in the basal turn of Fgf20Cre/+; Ctnnb1fl/fl cochlea and normal organization in control tissues. (F) In situ hybridization of basal turns of control and Sox2CreERT2/+; Ctnnb1fl/fl cochleae, showing a loss of β-catenin mRNA transcripts containing exons 2 to 6 in the cKO tissues. (G) Myosin7a+ HCs counts in Fgf20Cre/+; Ctnnb1fl/fl (n = 5) and control (n = 3) cochleae. Data are shown with mean ± SD. ns, not statistically significant (P ≥ 0.05).
Three major pathways have been implicated in establishing cochlear radial patterning. Before sensory cell specification (E11.5 to E13.5), a gradient of BMP is present in the cochlear epithelium, with the highest level located in the lesser epithelial ridge (LER) (7). A reduction in BMP signaling eliminates both the prosensory domain and the nonsensory LER, suggesting that BMP signaling dictates radial patterning before HC specification. As HCs become specified, Notch signaling mediates cell fate decisions and directs the establishment of the mosaic pattern of HCs and supporting cells via the process of lateral inhibition. A severe reduction in Notch signaling results in excessive HC formation in both the medial and lateral compartments at the expense of supporting cells (8, 9). However, a partial reduction in Notch signaling results in duplication of IHCs and their adjacent supporting cells, and thus expansion of only the medial compartment (10, 11), suggesting cell identity may be differentially regulated in the 2 compartments. In support of this notion, FGF20 signaling via FGFR1 is required for the specification of both HCs and supporting cells in the lateral, but not the medial, compartment (12, 13). In addition to BMP and Notch signaling, in vitro administration of glycogen synthase kinase 3β (GSK3β) inhibitors prevents the formation of OHCs and supporting cells in the lateral compartment while increasing HCs and supporting cells in the medial compartment (14). At present, we lack insights into the mechanisms dictating the precise separation of these 2 compartments and differential specification of their corresponding sensory and nonsensory cell types, including the determination of an IHC versus an OHC identity.
Wnt/β-catenin signaling is implicated in regulating HC formation both in vitro and in vivo (14–16). Here we show that β-catenin deletion does not prevent HC formation, but instead disrupts the boundaries between the lateral and medial compartments, resulting in ectopic pillar cell and IHC formation at the expense of OHCs. In contrast, in a mouse model with presumably diminished transcriptional activity of β-catenin and preserved adhesion properties, cell specification and radial patterning were relatively intact. These results indicate that while β-catenin is dispensable for HC establishment, it is essential for radial patterning and cell identity in the developing cochlea, primarily via its role as a mediator of cell adhesion.
Results
Hair Cells and Supporting Cells Are Disorganized in β-Catenin-Deficient Cochleae.
Sox2 and Fgf20 both mark prosensory cells in the developing cochlea (17, 18) (Fig. 1A). To determine whether β-catenin is required for HC specification, we generated embryos carrying the Sox2CreERT2/+ or Fgf20Cre/+ alleles, each combined with Ctnnb1ex2-6-floxed alleles (Sox2-β-cat-cKO and Fgf20-β-cat-cKO; Fig. 1B) (19, 20). For the Sox2-β-cat-cKO mice, we administered tamoxifen to pregnant dams at E13.5 to E14.5 to delete β-catenin in postmitotic prosensory cells. Due to tamoxifen-related dystocia, we collected and examined cochleae at E18.5 and E20.5 (Fig. 1B). In parallel, we employed the Fgf20Cre/+ mouse line to delete β-catenin from E10.5 onward (Fig. 1B) (18).
Sox2-β-cat-cKO cochleae contained 4 rows of Myosin7a+ HCs (Fig. 1C and SI Appendix, Fig. S1A). However, HCs were misaligned with no clear separation of IHCs and OHCs throughout the cochlea. Immunostaining for Prox1, a marker of Deiters’ and pillar cells, showed that supporting cells were also disorganized in all 3 turns of β-catenin-deleted cochleae (Fig. 1C and SI Appendix, Fig. S1B). Similar to Sox2-β-cat-cKO cochleae, Fgf20-β-cat-cKO cochleae displayed a disorganized arrangement of Myosin6+ HCs and Prox1+ supporting cells (Fig. 1E and SI Appendix, Fig. S1 C and D). The efficacy of Cre-mediated excision was confirmed by in situ hybridization specific for the floxed exons (2–6) in Sox2-β-cat-cKO cochleae and immunostaining in the Fgf20-β-cat-cKO cochleae (Fig. 1F and SI Appendix, Fig. S2 A–E). In both conditional knockout models, we found no differences in HC density between β-catenin-deleted and control tissues (Fig. 1 D and G). Neither did we detect differences in supporting cell density nor cochlear length in Sox2-β-cat-cKO cochleae compared with controls (SI Appendix, Fig. S3 A and B). A small increase in supporting cell density and decrease in cochlear length were noted in the Fgf20-β-cat-cKO cochleae (SI Appendix, Fig. S3 C and D), possibly due to the earlier deletion of β-catenin. Last, HCs in both the Sox2-β-cat-cKO and Fgf20-β-cat-cKO models exhibited stereocilia (Fig. 2A and SI Appendix, Fig. S4), a hallmark of HC maturation. Taken together, these data suggest that β-catenin is required for proper cell organization in the organ of Corti, but that it is dispensable for the initial establishment of HC and supporting cell identity.
Fig. 2.
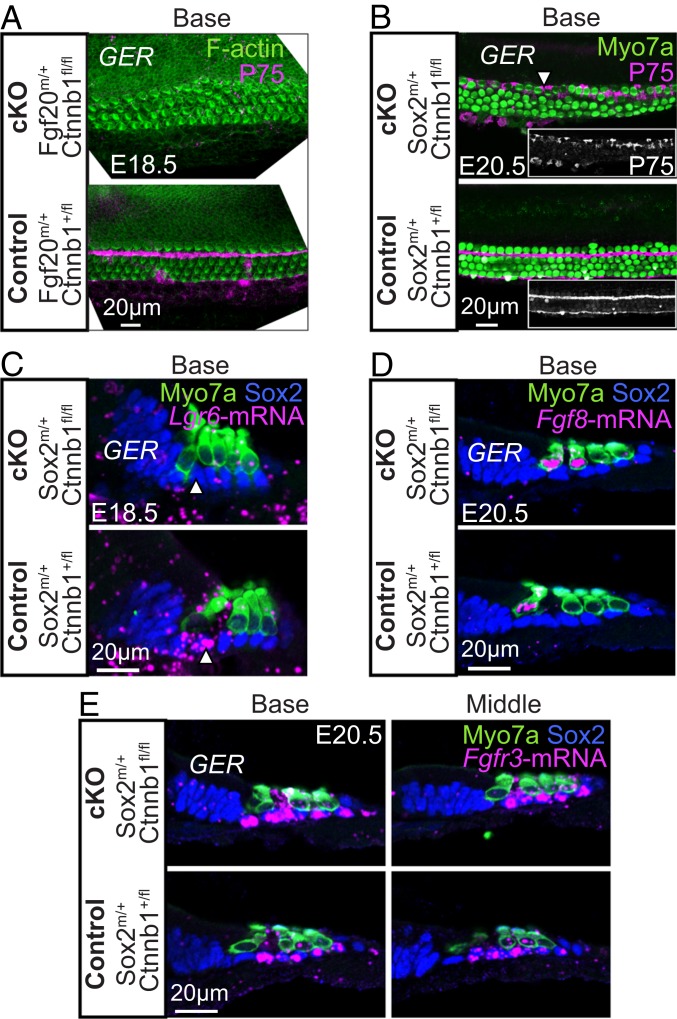
Pillar cell differentiation is compromised in β-catenin-deleted cochleae. (A) The pillar cell marker P75 was absent in E18.5 Fgf20Cre/+; Ctnnb1fl/fl cochleae. F-actin-rich HCs were stained with phalloidin (B) Sox2CreERT2/+; Ctnnb1fl/fl mice were examined at E20.5. Ectopic expression of P75 was seen around disorganized IHCs (arrowheads). Control tissues showed P75 expression in the apical process of the pillar cells. (Insets) P75 in grayscale. (C) Sox2CreERT2/+; Ctnnb1fl/fl tissues were probed for Lgr6 mRNA expression, using in situ hybridization, and then immunostained for Myosin7a and Sox2. In control tissues, Lgr6 mRNA was present in the inner pillar cells (arrowhead). Lgr6 was undetectable in the inner pillar cell region in cKO tissues. (D) In situ hybridization demonstrated the presence of Fgf8 in both Sox2CreERT2/+; Ctnnb1fl/fl and control cochleae. (E) Similarly, Fgfr3 transcripts were detected in supporting cells in β-catenin-deleted tissues.
Misexpressed Supporting Cell Markers in β-Catenin-Deficient Cochleae.
Formation of the tunnel of Corti by pillar cells is a cardinal feature of the mammalian cochlea. Because the separation between IHCs and OHCs was ambiguous after β-catenin ablation, we postulated that pillar cell differentiation was perturbed. In control embryonic cochleae, pillar cells robustly expressed P75 and formed a linear separation between IHCs and OHCs (Fig. 2 A and B and SI Appendix, Fig. S5 A and B). In Sox2-β-cat-cKO cochleae, we noted either absent or disorganized P75 expression in all cochlear turns (Fig. 2B). Furthermore, P75 was ectopically expressed in the supporting cells surrounding IHCs (Fig. 2B and SI Appendix, Fig. S5 A and B). In Fgf20-β-cat-cKO cochleae, P75 expression was primarily missing from all 3 turns (Fig. 2A). We attribute this difference in P75 expression phenotype to the earlier ablation in the Fgf20Cre model, which displayed diminished β-catenin protein levels at E14.5. In contrast, β-catenin protein levels appeared unchanged in the E14.5 Sox2-β-cat-cKO cochleae (SI Appendix, Fig. S2 B and C).
Lgr6 is expressed in developing inner pillar cells from E16.5 to postnatal (P) day 0 (21) (SI Appendix, Fig. S6 A–D). Combining immunostaining for Myosin7a+ HCs and Sox2+ supporting cells and in situ hybridization for Lgr6, we detected abundant Lgr6 mRNA expression in inner pillar cells in control tissues and a notable absence of Lgr6 transcripts in the same regions in β-catenin-deleted cochleae (Fig. 2C and SI Appendix, Fig. S6E). Together, our data indicate that nonsensory cells in β-catenin-deleted cochlea expressed early supporting cell markers such as Sox2 (Fig. 2C), Prox1 (Fig. 1C), and Jagged1 (SI Appendix, Fig. S6F), but that the expression of markers for more mature supporting cell subtypes, such as P75 and Lgr6, was disrupted. To elucidate the mechanisms underlying misexpressed pillar cell markers in both conditional knockout models, we next examined components of the Fgf signaling pathway, which dictates pillar cell specification.
Developing IHCs secrete FGF8 to neighboring FGFR3+ supporting cells to direct pillar cell differentiation (3, 22). Mice lacking either Fgfr3 or Fgf8 are missing pillar cells (23, 24). To determine whether the FGF8-FGFR3 axis was abnormal, we hybridized tissue sections from β-catenin-ablated cochleae with probes against Fgf8 and Fgfr3 and found expression of both (Fig. 2 D and E and SI Appendix, Fig. S5 C–E). Fgf8 was present in IHCs bordering the greater epithelial ridge (GER), and Fgfr3 mRNA was expressed in supporting cells, including both pillar cells and Deiters’ cells. These data indicate that FGF8-FGFR3 signaling is preserved in the β-catenin-ablated cochleae.
β-Catenin Deletion Results in Ectopic IHCs.
Based on the observed lack of a distinct separation between IHCs and OHCs in β-catenin-ablated cochleae, we postulate that the border between the medial and lateral compartments was disrupted. This spurred the question as to whether IHCs and OHCs were properly specified.
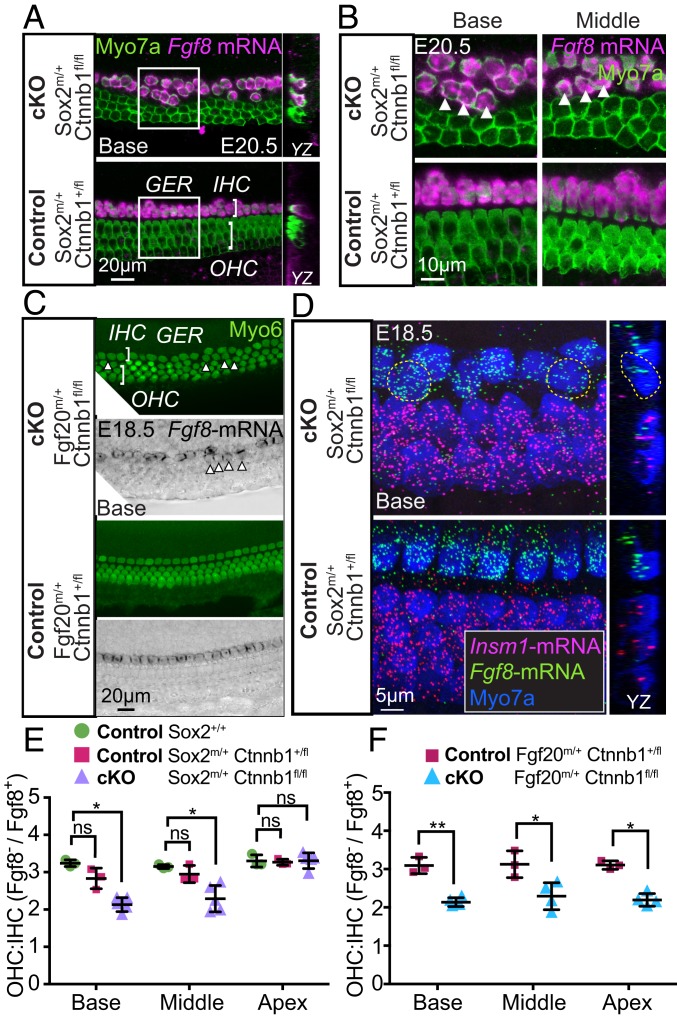
To distinguish IHCs from OHCs, we combined in situ hybridization of Fgf8 with immunostaining for Myosin7a on whole mount Sox2-β-cat-cKO cochleae (Fig. 3A). Sox2CreERT2/+; Ctnnb1fl/+ control tissues exhibited rare extranumerary IHCs positioned medially in the inner phalangeal region, and otherwise displayed a normal cochlear organization consisting of a single row of IHCs and 3 rows of OHCs (Fig. 3A). Ectopic IHCs can be attributed to Sox2 haploinsufficiency in the knock-in control mice, as observed in Sox2 hypomorphs (17). We noted, however, that while β-catenin-deleted cochleae had a comparable number of Myosin7a+ HCs to that of control cochleae (Fig. 1D), there were regions with 2 rows of Fgf8+, Myosin7a+ cells (presumably IHCs) positioned medial to 2 rows of Fgf8−, Myosin7a+ cells (presumably OHCs; Fig. 3 A–C and SI Appendix, Fig. S7 A–C). Last, we performed multiplex in situ hybridization and found that ectopic Fgf8+ IHCs lacked expression of Insm1 (Fig. 3D), which is required for driving an OHC identity (25). Together, these results suggest ectopic formation of IHCs at the expense of OHCs in the lateral compartment before E18.5.
Fig. 3.
β-Catenin deletion results in ectopic inner hair cells. (A) Whole mount preparation of Sox2CreERT2/+; Ctnnb1fl/fl cochleae processed for fluorescent in situ hybridization with probes directed against Fgf8 and immunostained for Myosin7a. Ectopic IHCs were found in all turns of the cochlea, frequently in regions with 2 rows of OHCs (boxed areas). (B) Higher magnification of boxed areas in A showing areas of ectopic IHCs (arrowheads) (C) Fgf20Cre/+; Ctnnb1fl/fl and control (Fgf20Cre/+; Ctnnb1fl/+) tissues were hybridized with probes against Fgf8 mRNA and then immunostained for Myosin6. Arrowheads mark the presence of ectopic IHCs. (D) Whole mount preparation of Sox2CreERT2/+; Ctnnb1fl/fl cochleae processed for fluorescent in situ hybridization with probes directed against Insm1 and Fgf8 with immunostaining for Myosin7a. In regions with 2 rows of OHCs (dashed circles), supernumerary IHCs were found to be Fgf8 positive and Insm1 low/negative. (E) Ratios of Myosin7a-positive, Fgf8-negative OHCs to Myosin7a-positive, Fgf8-positive IHCs in each turn on Sox2CreERT2/+; Ctnnb1fl/fl (n = 5) and control (n = 3) cochleae. The apical tip was excluded from this analysis. (F) Ratios of Fgf8-negative OHCs to Fgf8-positive IHCs in each turn of the Fgf20Cre/+; Ctnnb1fl/fl (n = 4) and control (n = 3) cochlea. The apical tip was excluded from this analysis. Data are shown with mean ± SD. *P < 0.05; **P < 0.01; ns, not statistically significant (P ≥ 0.05).
Although the apical turn of the cochlea also displayed areas with misexpression of OHCs and IHCs, we found some areas with 4 to 5 rows of FGF8−, Myosin7a+ HCs in the apical tip (SI Appendix, Fig. S7D). Overall, the average OHC:IHC ratios along the entire cochlea (excluding the apical tip) in control cochleae were 3.23 (Sox2+/+) and 3.02 (Sox2CreERT2/+; Ctnnb1fl/+). In contrast, this ratio was significantly decreased to 2.58 in Sox2-β-cat-cKO cochleae with significant differences between wild-type control and cKO cochlea in the middle and basal turns and a downward trend between heterozygous and cKO tissues (Fig. 3E). Similar to the Sox2-β-cat-cKO cochleae, the Fgf20-β-cat-cKO cochleae also contained many laterally positioned, ectopic IHCs with a corresponding decrease in the OHC number (Fig. 3C). Quantitative analyses found that the OHC:IHC ratios were also significantly decreased in each cochlear turn of the Fgf20-β-cat-cKO model in comparison with controls (2.21 vs. 3.11, respectively; Fig. 3F).
In summary, these results indicate that β-catenin deletion in the developing cochlea resulted in more HCs exhibiting an IHC phenotype in the lateral compartment at the expense of OHCs. Considering the aberrant expression of pillar cell markers (P75 and Lgr6), our data imply that β-catenin is required for the establishment of the border separating the medial and lateral compartments of the organ of Corti, which in turn is requisite for the proper specification of HCs and supporting cells subtypes.
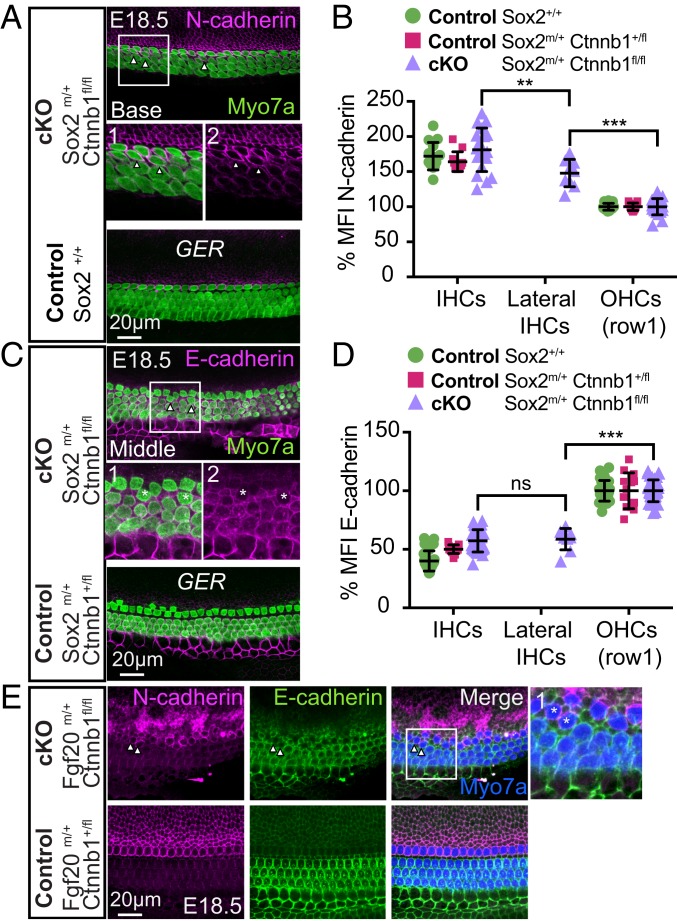
The medial–lateral boundary in the organ of Corti can be marked by the opposing expression of N-cadherin and E-cadherin (26, 27). We found that N-cadherin, which is normally restricted to the medial compartment of the organ of Corti, including the IHCs, was expressed in ectopic IHCs in the lateral compartment in both Sox2-β-cat-cKO and Fgf20-β-cat-cKO cochleae (Fig. 4 A, B, and E and SI Appendix, Fig. S8A). Conversely, E-cadherin, normally restricted to the lateral compartment including OHCs, was expressed at significantly lower levels in ectopic IHCs compared with OHCs in cKO tissues (Fig. 4 C–E and SI Appendix, Fig. S8B). Moreover, E-cadherin expression was comparable between ectopic IHCs in cKO tissues and those in controls. These data suggest that β-catenin is required for the radial patterning of cadherins in the developing cochlea.
Fig. 4.
Cadherin expression marks disrupted the medial–lateral boundary in β-catenin-deleted cochleae. (A) N-cadherin expression is restricted to the medial compartment in controls, including the IHCs. In Sox2CreERT2/+; Ctnnb1fl/fl cochlea, areas with presumed duplicated IHCs displayed ectopic N-cadherin (arrowheads, boxed area). Boxed area with ectopic expression of N-cadherin is magnified in subpanels A1 (Myosin7a and N-cadherin) and A2 (N-cadherin). (B) Relative mean fluorescence intensity (MFI) of membrane N-cadherin. (C) Sox2CreERT2/+; Ctnnb1fl/fl and littermate control tissues showing E-cadherin expression restricted to the lateral compartment, including OHCs. In β-catenin-deleted cochlea, presumed ectopic IHCs displayed loss of E-cadherin (arrowheads, boxed area). Boxed area with E-cadherin expression is magnified in subpanels C1 (Myosin7a and E-cadherin) and C2 (E-cadherin). (D) MFI of membrane E-cadherin. (E) Fgf20Cre/+; Ctnnb1fl/fl and control tissues were stained with Myosin7a, N-cadherin, and E-cadherin. In β-catenin-deleted cochlea, presumed ectopic IHCs expressed N-cadherin (boxed area). Boxed area with ectopic expression of N/E-cadherin is magnified in subpanels E1. **P < 0.01, ***P < 0.001; ns, not statistically significant (P ≥ 0.05).
Medial to Lateral Border Formation Is Dependent on β-Catenin Cell Adhesion.
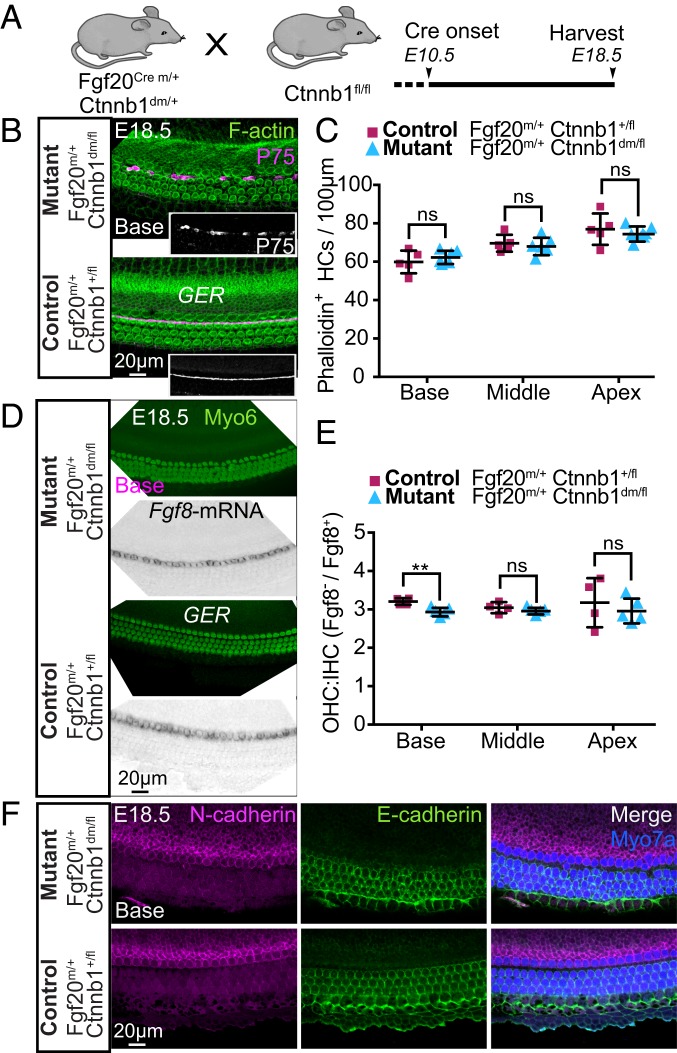
In addition to its function as a transcriptional activator for Wnt signaling, β-catenin is an essential component of cadherin-mediated cell adhesion (28). To demonstrate that the β-catenin adhesion function is required for radial patterning, we examined a β-catenin double mutant (dm) mouse strain in which the transcriptional nuclear function of β-catenin at the nucleus level is disrupted (constitutive and not inducible) while preserving the cell adhesion function (Fig. 5A and SI Appendix, Fig. S9A) (29). Because a single wild-type allele of β-catenin is sufficient for normal cochlear development (Fig. 1 C–G) and homozygous Ctnnb1dm/dm mice exhibit early embryonic lethality, we used Fgf20Cre mice to delete 1 β-catenin allele, while the remaining allele expressed the dm version of β-catenin (Fgf20Cre/+; Ctnnb1dm/fl; Fig. 5A). Fgf20Cre/+; Ctnnb1+/fl mice were used as controls.
Fig. 5.
Inhibiting β-catenin transcriptional activity does not severely impair radial patterning of the organ of Corti. (A) Schematic of the experimental setup. A dm β-catenin allele with preserved cell–cell adhesion function (Fgf20Cre/+; Ctnnb1dm/fl) was used. Tissues were harvested at E18.5. (B) Mutant cochleae displayed a clear separation between IHC and OHC and patchy loss of P75 staining along the length of the cochlea. (C). Quantification of hair cells in Fgf20Cre/+; Ctnnb1dm/fl (n = 6) and control (n = 5) cochleae. (D) Fgf8 mRNA and Myosin6 expression in mutant tissues. (E) The ratio of Fgf8− OHCs to Fgf8+ IHCs in mutant (n = 5) and control (n = 4) cochleae. (F) Mutant and control tissues both showed segregated expression of N-cadherin (magenta) and E-cadherin (green). Data are shown with mean ± SD. *P < 0.05; **P < 0.01; ns, not statistically significant, P ≥ 0.05.
Fgf20Cre/+; Ctnnb1dm/fl mice at E18.5 had a largely normal organ of Corti displaying the typical separation of the IHCs and OHCs by P75+ pillar cells (Fig. 5B and SI Appendix, Fig. S9B). In contrast to both β-cat-cKO models, organization of HCs and supporting cells in the Fgf20Cre/+; Ctnnb1dm/fl cochleae appeared preserved and comparable to controls, containing 4 rows of Myosin6+ HCs and 5 rows of Prox1+/Fgfr3+ supporting cells with densities of both similar to control tissues (Fig. 5 B and C and SI Appendix, Fig. S9 C–E). Immunostaining revealed that the Fgf20Cre/+; Ctnnb1dm/fl cochlea had areas with preserved P75 expression and only occasionally showed Fgf8+, Myosin6+ cells in the lateral compartment (Fig. 5 B and E). However, we detected no corresponding decrease in OHCs close to these ectopic IHCs (Fig. 5D and SI Appendix, Fig. S9F). Ectopic expression of N-cadherin and E-cadherin was rarely observed in the Fgf20Cre/+; Ctnnb1dm/fl cochleae (Fig. 5F). Overall, there was no significant difference in the ratio of OHC:IHC in the Fgf20Cre/+; Ctnnb1dm/fl cochleae compared with controls (Fig. 5E). In contrast to the Fgf20-β-cat-cKO model, the phenotypes observed in the Fgf20Cre/+; Ctnnb1dm/fl cochleae were remarkably milder and more closely resembled control tissues (Fgf20Cre/+; Ctnnb1fl/+). Therefore, we conclude that cochlear radial patterning and cell identify are primarily dependent on the cell adhesion function of β-catenin.
Discussion
β-Catenin Is Dispensable for the Initial Establishment of Cochlear HCs.
Deletion of Wnt ligands and receptors during inner ear development causes a wide range of morphological and differentiation defects (30). Canonical Wnt/β-catenin signaling is necessary for the specification of the otic placode from the surrounding epidermis (31). At later developmental stages, studies suggest that Wnt/β-catenin might be necessary for HC formation in the auditory and vestibular sensory epithelia (15, 16). Here we show that HCs are specified despite β-catenin deletion. Rather, β-catenin-deficient cochleae displayed disorganized sensory HCs and nonsensory supporting cells with anomalous cell identity. Thus, our study has implicated a role for β-catenin in dictating radial patterning and specifying cell identity in the cochlea.
β-Catenin Is Required for Cochlear Radial Patterning.
After the initial HC specification, nonsensory cells in the β-catenin-deficient cochleae (Deiters’ and pillar cells) express Prox1, Sox2, and Jagged1, suggesting they are specified supporting cells. However, the subsequent steps of supporting cell differentiation are impaired, as illustrated by the disrupted expression of pillar cell markers (P75 and Lgr6).
Pillar cell specification requires FGF8 secreted from IHCs and FGFR3 expression in the developing supporting cells (3); however, our data suggest that such a directive signal by the FGF8–FGFR3 axis is likely preserved in the β-catenin-deficient cochlea. As such, we propose a FGF8–FGFR3-independent mechanism in which the border separating the medial and lateral compartments of the organ of Corti is disrupted (SI Appendix, Fig. S10 A–C), causing radial mispatterning of IHCs, OHCs, and pillar cells. We propose that the establishment of such a border is dependent on β-catenin, and loss of this demarcation leads to aberrant cell identity.
A combination of morphogen signaling across the prosensory zone is involved in establishing the GER, the organ of Corti and the LER. Before HC specification, loss of BMP4 results in a prosensory region that takes on the phenotype of the GER (7). During HC specification, FGF20 deletion prevents formation of OHCs, but not IHCs, further suggesting that cell specification signals differ between the medial and lateral compartments (12). Our work establishes that the demarcation between the 2 compartments is dependent on β-catenin’s cell adhesion function, thus adding β-catenin to the repertoire of signals dictating radial patterning and cell identity.
β-Catenin-Mediated Cell Adhesion Is Required for Cell Identity.
Cell adhesion to substrates in the microenvironment and to neighboring cells is crucial for diverse cellular processes such as cell shape, migration, proliferation, apoptosis, and differentiation, which together dictate tissue patterning (32). The cadherin superfamily works parallel to cell-extracellular matrix pathways to establish intercellular adhesion. Cadherins recruit α- and β-catenin to form cell–cell contacts known as adherens junctions (33). N-cadherin and E-cadherin have distinct opposing expression patterns in the developing cochlea: E-cadherin is expressed mainly in the lateral compartment OHCs and supporting cells, and N-cadherin is expressed in the developing GER and IHCs (26, 27). Since we found an up-regulation of N-cadherin and down-regulation of E-cadherin in the lateral compartment of β-catenin-deleted cochleae, future studies to better understand the interaction between cadherins and β-catenin in the different compartments of the organ of Corti would be of interest.
β-Catenin is abundantly expressed in the cellular membrane of cochlear ductal cells before and after HC specification (27). By comparing the β-catenin knockout models (loss of both β-catenin-mediated cell adhesion and transcription; SI Appendix, Fig. S10B) with the dm model (SI Appendix, Fig. S10C), we conclude that the cell adhesion function of β-catenin contributes to forming radial patterning. Along the same vein, the cell adhesion function of β-catenin played a larger role than the transcriptional function in dictating cell fate and tissue patterning in the developing germ layers and forebrain (34, 35). However, our results do not rule out the potential contribution of transcriptional activity of β-catenin to cochlear radial patterning. Instead, an alternative interpretation may be that this developmental process is more sensitive to the relative changes of cell adhesion than transcriptional activities of β-catenin.
A Model System to Study Inner versus Outer HC Fate.
Mechanisms that specify IHCs and OHCs in the cochlea have recently been revealed (25). However, there is a dearth of model systems favoring 1 HC fate at the expense of the other. Deletion of NeuroD1 results in such a switch in cell fate, although it is limited to the apical turn of the cochlea (36). The β-catenin-deficient cochlea can serve as a model system to study this important topic. Recent data demonstrated that the transcription factor Insm1 is required for consolidating OHC fate (25). However, Insm1 was shown to be dispensable for the initiation of OHC differentiation. Since Insm1 is absent in ectopic IHCs, it is possible that β-catenin acts upstream of Insm1 in governing hair cell identity.
Finally, a better understanding of how radial patterning is governed and how the identity of individual HCs is established may have crucial implications for future efforts to restore hearing via regeneration.
Methods
Mice.
Sox2CreERT2 mice (JAX #017593), Fgf20Cre mice (18), Ctnnb1-flox mice (JAX #004152) (20), and Ctnnb1dm/+ mice (gift from K. Basler, University of Zurich) (29) were used. The day of vaginal plug detection was termed E0.5. A tamoxifen/progesterone mixture in corn oil (Sigma Aldrich, 250 and 125 µg/g, respectively) was injected IP once daily on 2 consecutive days. Animals were examined at the stated time point, and E20.5 was equated with P0, as tamoxifen-induced dystocia was common in the model. All protocols were approved by the Animal Care and Use Committees at Stanford University, Washington University, and University of Nebraska (IACUC numbers 18606, 20130201, and 16-005-02-EP, respectively).
Imaging.
Confocal images were acquired on a Zeiss LSM500/LSM700 microscope (Carl Zeiss AG,) using Zen software. Transmitted light images were captured on an Evos Xl digital microscope (Thermo Fisher Scientific) and Zeiss Apotome.2 (Carl Zeiss AG). Cell counts and measurements were performed using ImageJ software (NIH).
Supplementary Material
Acknowledgments
We thank S. Heller and our laboratory members for discussion, W. Dong for technical support, and K. Basler for sharing the β-catenin dm mice. This work was supported by the Swedish Research Council (L.J.); NIH R21DC017042 (to D.M.O.); NIH K99/R00DC012825, GM110768, Edna Ittner Trust, and DHHS 44388-Y3 (to S.-H.H.); and NIH K08DC011043, R01DC01910, R01DC016919, Department of Defense MR130316, and the Yu and Oberndorf families (to A.G.C.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. M.W.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910223116/-/DCSupplemental.
References
- 1.Groves A. K., Zhang K. D., Fekete D. M., The genetics of hair cell development and regeneration. Annu. Rev. Neurosci. 36, 361–381 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobin A., Anniko M., Early development of cochlear hair cell stereociliary surface morphology. Arch. Otorhinolaryngol. 241, 55–64 (1984). [DOI] [PubMed] [Google Scholar]
- 3.Jacques B. E., Montcouquiol M. E., Layman E. M., Lewandoski M., Kelley M. W., Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development 134, 3021–3029 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Corwin J. T., Warchol M. E., Auditory hair cells: Structure, function, development, and regeneration. Annu. Rev. Neurosci. 14, 301–333 (1991). [DOI] [PubMed] [Google Scholar]
- 5.Burns J. C., Kelly M. C., Hoa M., Morell R. J., Kelley M. W., Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat. Commun. 6, 8557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waldhaus J., Durruthy-Durruthy R., Heller S., Quantitative high-resolution cellular map of the organ of corti. Cell Rep. 11, 1385–1399 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohyama T., et al. , BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J. Neurosci. 30, 15044–15051 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiernan A. E., Cordes R., Kopan R., Gossler A., Gridley T., The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development 132, 4353–4362 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Brooker R., Hozumi K., Lewis J., Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development 133, 1277–1286 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Lanford P. J., et al. , Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat. Genet. 21, 289–292 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Basch M. L., et al. , Fine-tuning of Notch signaling sets the boundary of the organ of corti and establishes sensory cell fates. eLife 5, e19921 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huh S. H., Jones J., Warchol M. E., Ornitz D. M., Differentiation of the lateral compartment of the cochlea requires a temporally restricted FGF20 signal. PLoS Biol. 10, e1001231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi T., Ray C. A., Bermingham-McDonogh O., Fgf20 is required for sensory epithelial specification in the developing cochlea. J. Neurosci. 28, 5991–5999 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munnamalai V., Fekete D. M., Notch-Wnt-Bmp crosstalk regulates radial patterning in the mouse cochlea in a spatiotemporal manner. Development 143, 4003–4015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacques B. E., et al. , A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development 139, 4395–4404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi F., et al. , β-catenin is required for hair-cell differentiation in the cochlea. J. Neurosci. 34, 6470–6479 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiernan A. E., et al. , Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434, 1031–1035 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Huh S. H., Warchol M. E., Ornitz D. M., Cochlear progenitor number is controlled through mesenchymal FGF receptor signaling. eLife 4, e05921 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold K., et al. , Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9, 317–329 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brault V., et al. , Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., et al. , Dynamic expression of Lgr6 in the developing and mature mouse cochlea. Front. Cell. Neurosci. 9, 165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller K. L., Jacques B. E., Kelley M. W., Fibroblast growth factor signaling regulates pillar cell development in the organ of corti. J. Neurosci. 22, 9368–9377 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colvin J. S., Bohne B. A., Harding G. W., McEwen D. G., Ornitz D. M., Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat. Genet. 12, 390–397 (1996). [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T., Cunningham D., Bermingham-McDonogh O., Loss of Fgfr3 leads to excess hair cell development in the mouse organ of corti. Dev. Dyn. 236, 525–533 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Wiwatpanit T., et al. , Trans-differentiation of outer hair cells into inner hair cells in the absence of INSM1. Nature 563, 691–695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chacon-Heszele M. F., Ren D., Reynolds A. B., Chi F., Chen P., Regulation of cochlear convergent extension by the vertebrate planar cell polarity pathway is dependent on p120-catenin. Development 139, 968–978 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonneau L., Gallego M., Pujol R., Comparative expression patterns of T-, N-, E-cadherins, beta-catenin, and polysialic acid neural cell adhesion molecule in rat cochlea during development: Implications for the nature of Kölliker’s organ. J. Comp. Neurol. 459, 113–126 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Nelson W. J., Nusse R., Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303, 1483–1487 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valenta T., et al. , Probing transcription-specific outputs of β-catenin in vivo. Genes Dev. 25, 2631–2643 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansson L., Kim G. S., Cheng A. G., Making sense of Wnt signaling-linking hair cell regeneration to development. Front. Cell. Neurosci. 9, 66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohyama T., Mohamed O. A., Taketo M. M., Dufort D., Groves A. K., Wnt signals mediate a fate decision between otic placode and epidermis. Development 133, 865–875 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Thiery J. P., Cell adhesion in development: A complex signaling network. Curr. Opin. Genet. Dev. 13, 365–371 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Jamora C., Fuchs E., Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell Biol. 4, E101–E108 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Junghans D., Hack I., Frotscher M., Taylor V., Kemler R., Beta-catenin-mediated cell-adhesion is vital for embryonic forebrain development. Dev. Dyn. 233, 528–539 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Lyashenko N., et al. , Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat. Cell Biol. 13, 753–761 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jahan I., Pan N., Kersigo J., Fritzsch B., Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One 5, e11661 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.