Significance
[FeFe]-hydrogenases catalyze H2 evolution at extraordinary rates at low overpotential using base metal (not platinum). In the field of renewable energy, the biosynthesis of these enzymes’ active sites, the H-cluster, is of intense interest. Among its many cofactors, the azadithiolate is unique, and its biosynthesis remains enigmatic. Three Fe–S proteins, HydE, HydF, and HydG, are essential for the H-cluster bioassembly. This paper describes an in vitro assembly approach where a synthetic [Fe(cysteine)(CO)2(CN)] complex, “syn-B,” allows HydG-free biosynthesis of the active enzyme. Using isotopic and S/Se labeling, syn-B is shown to contribute Fe(CO)2(CN)S to the biosynthesis. This work sets the stage for further investigation of the H-cluster bioassembly.
Keywords: iron carbonyl cyanides, cysteine, H-cluster biosynthesis
Abstract
The enzyme [FeFe]-hydrogenase (HydA1) contains a unique 6-iron cofactor, the H-cluster, that has unusual ligands to an Fe–Fe binuclear subcluster: CN−, CO, and an azadithiolate (adt) ligand that provides 2 S bridges between the 2 Fe atoms. In cells, the H-cluster is assembled by a collection of 3 maturases: HydE and HydF, whose roles aren’t fully understood, and HydG, which has been shown to construct a [Fe(Cys)(CO)2(CN)] organometallic precursor to the binuclear cluster. Here, we report the in vitro assembly of the H-cluster in the absence of HydG, which is functionally replaced by adding a synthetic [Fe(Cys)(CO)2(CN)] carrier in the maturation reaction. The synthetic carrier and the HydG-generated analog exhibit similar infrared spectra. The carrier allows HydG-free maturation to HydA1, whose activity matches that of the native enzyme. Maturation with 13CN-containing carrier affords 13CN-labeled enzyme as verified by electron paramagnetic resonance (EPR)/electron nuclear double-resonance spectra. This synthetic surrogate approach complements existing biochemical strategies and greatly facilitates the understanding of pathways involved in the assembly of the H-cluster. As an immediate demonstration, we clarify that Cys is not the source of the carbon and nitrogen atoms in the adt ligand using pulse EPR to target the magnetic couplings introduced via a 13C3,15N-Cys–labeled synthetic carrier. Parallel mass-spectrometry experiments show that the Cys backbone is converted to pyruvate, consistent with a cysteine role in donating S in forming the adt bridge. This mechanistic scenario is confirmed via maturation with a seleno-Cys carrier to form HydA1–Se, where the incorporation of Se was characterized by extended X-ray absorption fine structure spectroscopy.
Hydrogenases are nature’s machines for the metabolism of H2, both its production and its oxidation. [FeFe]-hydrogenases are hyperefficient, operating at rates up to ∼104 per second (1, 2). Investigations into [FeFe]-hydrogenases are motivated by their potential applications related to renewable energy and the design of fuel cells (3). It is therefore mandatory that we understand how nature assembles the elaborate catalytic centers at the heart of these remarkable machines.
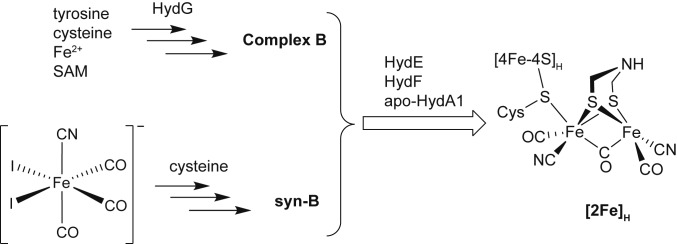
The active site of [FeFe]-hydrogenase, the H-cluster, is a 6-Fe ensemble consisting of a canonical [4Fe–4S]H subcluster linked through a bridging Cys residue to a [2Fe]H subcluster, in which the 2 Fe ions are coordinated by distinctive cofactors: 3 CO ligands, 2 CN− ligands, and an unprecedented azadithiolate [adt; HN(CH2S−)2] ligand. The [2Fe]H subcluster is proposed to be the site of substrate (H+, H2) binding and activation (4–7). The unique structural features and catalytic activity of the H-cluster have attracted considerable interest regarding its biosynthesis, particularly of this [2Fe]H subcluster (8–14), which poses one of the greatest active-site biosynthesis challenges known for biology with multiple-component, step-by-step assembly involving toxic ligands, O2 sensitivity, and an unusual organic bridging ligand that has little inherent stability. From low-molecular-mass precursors, the [2Fe]H subcluster is orchestrated by 3 maturases: HydE, HydF, and the multifunctional enzyme HydG. While understanding of HydE and HydF remains limited (9, 15–19), recent work has demonstrated that HydG is responsible for the biosynthesis of CN− and CO, which are produced by degradation of tyrosine, catalyzed by a radical S-adenosyl-l-methionine (SAM) site (20–24). These diatomic ligands combine with a Fe(Cys) center that is bound to the auxiliary [4Fe–4S] cluster within HydG. Once assembled, this [Fe(Cys)(CO)2(CN)] module, termed “Complex B,” is quickly consumed in the biosynthesis of the [2Fe]H module (the active site) of HydA1 (25–29). Due to its lability—decomposition and release of free CO occurs on the order of minutes (25)—isolated Complex B has not been tested in vitro as a precursor to the H-cluster, which, in principle, should be possible in the absence of HydG. In this paper, we report an analog of Complex B, which we call syn-B, which allows us to probe the biosynthesis of HydA1, leading to several important insights. We show that syn-B functionally replaces the proposed [Fe(Cys)(CO)2(CN)] product of HydG to generate fully active [FeFe] hydrogenase with identical spectroscopic signatures. As a robust functional surrogate of Complex B, syn-B allows us to carry out H-cluster assembly with isotopically labeled cysteine and with selenocysteine. A combination of mass spectrometry (MS) and extended X-ray absorption fine structure (EXAFS) spectroscopy reveal that cysteine/selenocysteine donates the S/Se atoms of the adt bridge, linking the 2 Fe atoms of the binuclear cluster. Finally, experiments reveal that, while Cys serves as a source of the S in the adt, it is not the source of its central HN(CH2)2.
Results
Efforts were initially directed toward generation of Complex B by combining cysteinate salts and sources of “Fe(CO)2(CN)+.” As a precursor to “Fe(CO)2(CN)+,” we focused on [FeI2(CN)(CO)3]− (30), which we generated both by cyanation of FeI2(CO)4 or, preferably, the iodination of [Fe(CN)(CO)4]− (31, 32). Provided that it is protected from light, the resulting [FeI2(CN)(CO)3]− is stable for several minutes at room temperature as a solution in methanol (see SI Appendix, Fig. S1 for electrospray ionization [ESI]-MS). It reacts rapidly with dipotassium cysteinate to afford a homogeneous orange solution, which upon evaporation yields a light-orange solid of syn-B (Scheme 1).
Scheme 1.
Synthetic scheme showing maturation of [FeFe] hydrogenase H-cluster under normal conditions (Upper) and through our modular surrogate strategy (Lower).
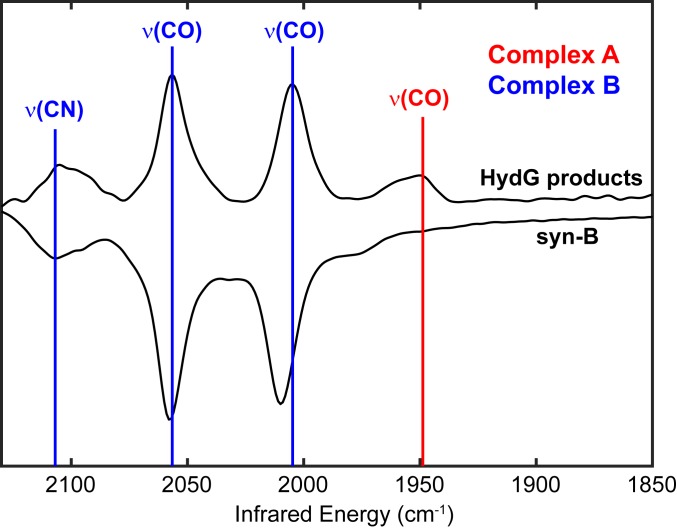
The Fourier transform infrared (FT-IR) spectrum of aqueous solution of syn-B is nearly identical to the spectrum observed for Complex B (Fig. 1), with bands at 2,107; 2,058; and 2,010 cm−1 [compared to 2,105; 2,057; and 2,006 cm−1 for natural isotopic abundance Complex B reported (25); see SI Appendix, Table S1 for the summary of IR bands]. The 3-band pattern is diagnostic of a facial Fe(CO)2(CN) center (33). Although they are very similar by FT-IR, syn-B and Complex B exhibit very different stabilities: Complex B decomposes on the order of minutes at room temperature (25), whereas syn-B is stable in water for hours. Additionally, syn-B is stable as a solid, showing no signs of decomposition after months.
Fig. 1.
FT-IR comparison of HydG reaction products observed in enzymatic reactions using Tyr as substrate (upper trace; adapted from ref. 26) and syn-B dissolved in H2O (lower trace).
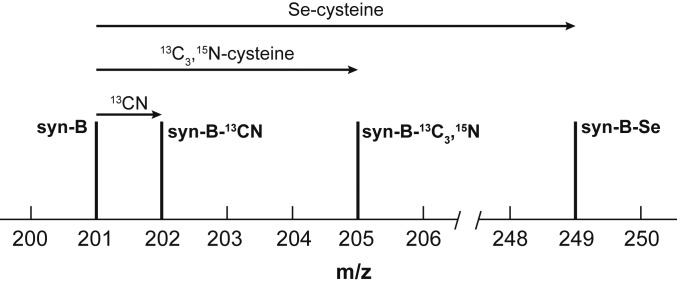
ESI-MS for syn-B (Fig. 2 and SI Appendix, Fig. S2) revealed a strong peak in the negative mode at m/z = 201.0 corresponding to [Fe(Cys)(CN)]− (carboxylate deprotonated). The nonobservations of [Fe(Cys)(CN)(CO)2]− is consistent with the lability of FeII-CO bonds. The assignment of the m/z 201.0 signal to [Fe(Cys)(CN)]− ion was confirmed by the preparation of several isotopologues and selenocysteine analog (vide infra; SI Appendix, Figs. S2–S5).
Fig. 2.
Schematic representation of the effect of labeling on the parent [Fe(Cys)(CN)]− peak in the ESI-MS of syn-B and its analogs. Incorporation of 13CN causes a shift of 1 m/z, the isotopologue containing 13C3,15N-cysteinate results in a shift of 4 m/z, and incorporation of selenocysteine (80Se) causes a shift of 48 m/z.
Although its formulation cannot be described precisely in the absence of X-ray crystallographic analysis, syn-B is a multiiron ensemble consisting of Fe(CO)2(CN)(Cys) centers bound to a high-spin ferrous iodide site. Elemental analyses (percent C, H, N, and I) are consistent with the formula (Et4N)4{FeI2[Fe(Cys)(CN)(CO)2(H2O)]4}, i.e., a cluster composed of 4 [Fe(Cys)(CN)(CO)2(H2O)] centers bound to FeI2. The ensemble exhibits a highly reproducible magnetic moment of 4.6 μB (calculated by Evan’s method; SI Appendix, Fig. S6) and the presence of Et4N+ (observable by NMR spectroscopy). The paramagnetic ferrous center is assumed to derive from [FeI2(CN)(CO)2]−, which is prone to decomposition. This high-spin ferrous Lewis acid provides crucial stabilization of the Fe(Cys)(CO)2(CN) fragments, analogous to the interaction of the dangler Fe and the [4Fe–4S] cluster in HydG (26, 34). Mixed-spin ferrous thiolate complexes are well precedented (35, 36).
The essential and remarkable feature of syn-B is that it serves as a kinetically well-behaved source of Fe(CO)2(CN)(Cys), directly applicable to the in vitro maturation of HydA1. These experiments are enumerated below.
syn-B Facilitates the Maturation of HydA1 in the Absence of HydG.
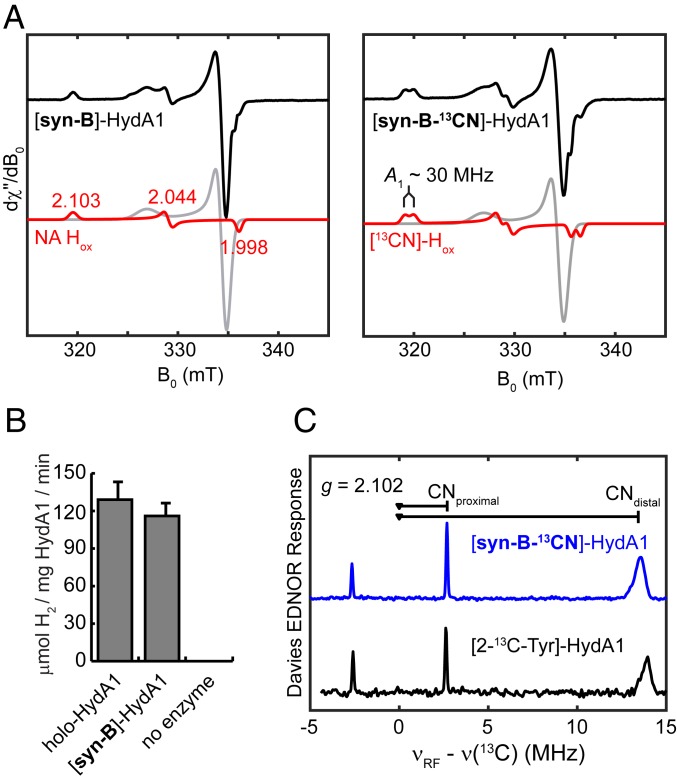
The maturation of apo-CrHydA1 (that harbors the [4Fe–4S]H subcluster) requires 3 maturases—HydE, HydF, and HydG—as well as various low-molecular-mass components necessary for the enzymatic reactions (37, 38). Using syn-B, HydG (along with its substrate, Tyr) can be omitted from this canonical recipe, leaving everything else unchanged. The electron paramagnetic resonance (EPR) spectrum of the resulting [syn-B]-CrHydA1 poised in the thionine-oxidized state exhibits the lineshape and g values of [2.103, 2.044, 1.998] that are characteristic of CrHydA1 Hox (Fig. 3A, red traces) (1). Also present is the CO-inhibited form of the H-cluster, Hox–CO, commonly seen in [FeFe]-hydrogenase samples (Fig. 3A, gray traces) (1).
Fig. 3.
Characterization of CrHydA1 maturated with syn-B. (A) X-band CW EPR spectra of [syn-B]-CrHydA1 (Left) and [syn-B-13CN]-CrHydA1 (Right). Both spectra were simulated with 2 components: Hox, shown in red traces, and Hox-CO, in gray traces. Conditions were as follows: temperature, 30 K; microwave power, 0.05 mW. (B) H2 evolution activity of [syn-B]-CrHydA1 and a holo-CrHydA1 standard measured at 20 °C. (C) Q-band Davies ENDOR spectrum of [syn-B-13CN]-CrHydA1 Hox and [2-13C-Tyr]-CrHydA1 Hox recorded at g = 2.102 (g1 of Hox). Conditions were as follows: frequency = 34.1 GHz; temperature = 15 K; inversion pulse = 80 ns; τ = 300 ns; radiofrequency pulse = 20 µs.
Most importantly, [syn-B]-CrHydA1 shows similar H2 production activity to the standard holo-CrHydA1 sample under parallel conditions (Fig. 3B and SI Appendix, Fig. S7). These results clearly indicate that syn-B can be used as a surrogate of the HydG reaction product to build the H-cluster. Stated more generally, syn-B is a fully competent precursor to the H-cluster, without the need for HydG. This result confirms the role of HydG in building [Fe(Cys)(CO)2(CN)] as an intermediate in the maturation of the hydrogenase. Furthermore, HydG-free maturation shows that direct interactions between the HydG protein and other maturation partners are not necessary for the bioassembly of the H-cluster in the cell-free system.
Maturation of apo-HydA1 with syn-B-13CN Affords HydA1-13CN (also in the Absence of HydG).
Having established the efficacy of syn-B for HydG-free maturation, additional experiments were undertaken to verify the function of syn-B. These experiments also provided further support for its formulation. Starting from [Fe(13CN)I2(CO)3]−, we prepared the 13CN-isotopologue of syn-B, i.e., syn-B-13CN. The FT-IR spectrum of syn-B-13CN and syn-B differ only slightly in the νCO region, showing a shift of ∼11 cm−1 for one of the CO bands. Moreover, the νCN region was perturbed by ∼33 cm−1 to lower energy for syn-B-13CN, as expected (SI Appendix, Table S1). The ESI-MS of syn-B-13CN was analogous to that for syn-B, except that the peak of interest assigned to [Fe(Cys)(CN)]− was shifted to 1-Da higher mass, consistent with the fragment [Fe(Cys)(13CN)]− (Fig. 2 and SI Appendix, Fig. S3). Maturation using syn-B-13CN generated [syn-B-13CN]-CrHydA1, the EPR spectrum of which clearly shows hyperfine splitting that has been observed in the 13CN-labeled H-cluster with the 13CN ligands sourced from HydG-cleaved 2-13C-Tyr (39). The hyperfine interactions in [syn-B-13CN]-CrHydA1 were further probed by Q-band Davies on electron nuclear double resonance (ENDOR) spectroscopy, revealing 2 13C hyperfine values of 29.2 and 5.34 MHz at g1 of Hox, identical to those found in the 13CN-labeled CrHydA1 (Fig. 3C) (39). This result unambiguously shows that the cyanide in syn-B is indeed delivered into HydA1 and validates that syn-B is fully competent precursor to the H-cluster.
An Intact (Cys)Fe(CO)2(CN) Ensemble Is Required for Maturation.
The cysteamine (cysamH = H2NCH2CH2SH) analog of syn-B, syn-B-cysam, was prepared by the reaction of Kcysam with [FeI2(CN)(CO)3]−. The FT-IR spectrum of syn-B-cysam closely matched that of syn-B, except that the νCO bands were shifted to lower energies (ΔνCO ≤ 9 cm−1; SI Appendix, Table S1), consistent with the better electron-donation ability of cysteamine versus cysteine. Its stability is also similar to syn-B. The many similarities between syn-B-cysam and syn-B imply that the carboxylate remains uncoordinated in syn-B and probably also in Complex B.
Interestingly, replacement of syn-B with syn-B-cysam in our standard HydG-free maturation protocol did not produce holo-HydA1. This result is striking because the maturation medium contains ∼1 mM Cys; thus, the mere presence of Cys was insufficient for maturation, even in the presence of a source of “Fe(CN)(CO)2.” These results imply that the presence of Cys attached to the Fe(CO)2(CN) moiety is essential for maturation.
The conversion of Complex B into [2Fe]H entails many steps: loss of CO, formation of a [2Fe–2SR] center, and, most remarkable of all, the generation of the adt cofactor. It is well known that adtH2 and adt2− are unstable (40), which implies that the cofactor is produced on an Fe2 template. The 2Fe–2S clusters are well known to mediate the biosynthesis of other organosulfur cofactors (biotin and lipoic acid) (19, 41). Since l-cysteine is required for the biosynthesis of the [2Fe-2SR] center, it is thus appealing to suggest that the cysteine in Complex B, and here syn-B, could provide all of the ingredients (CH2, NH, and S) required to assemble the adt cofactor. This Cys → adt hypothesis has not been unambiguously tested by using the reported maturation procedure, because Cys is already present in the auxiliary 5Fe–4S cluster of HydG (34), which makes labeling of Cys by adding labeled Cys exogenously inefficient. This issue can be clearly avoided by using syn-B as a surrogate for HydG.
Maturation of apo-HydA1 with syn-B-13C3,15N Affords Unlabeled HydA1, i.e., Cys Is Not the Source of adt Backbone.
Starting with HS13CH213CH(15NH2)13CO2H, we prepared an isotopologue of syn-B, which we call syn-B-13C3,15N. The FT-IR spectra for syn-B-13C3,15N and syn-B are virtually identical, except that the carboxylate absorbance is shifted from 1,615 to 1,575 cm−1, reflecting the presence of 13CO2− substituent in the heavy isotopologue. The ESI-MS of syn-B-13C3,15N was also analogous to that for syn-B, except that the peak for [Fe(Cys)(CN)]− was shifted by 4 Da to higher mass, consistent with the fragment [Fe(13C3,15N-Cys)(CN)]− (Fig. 2 and SI Appendix, Fig. S4). These systematic and predictable shifts in the m/z values give us a high level of confidence regarding the identity of this fragment.
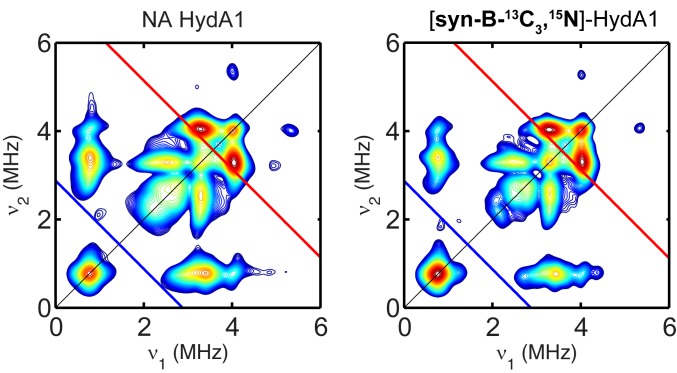
As expected, HydG-free maturation with syn-B-13C3,15N proceeded normally to afford active HydA1. We then employed pulse EPR spectroscopy to test whether the adt ligand is labeled. The hyperfine coupling to the 15N in adt has been investigated by hyperfine sublevel correlation (HYSCORE) spectroscopy, which revealed a hyperfine tensor of A 15N = [1.9, 1.6, 1,6] MHz (42). The hyperfine values of the 2 13C carbons, while not previously reported, may be estimated to be comparable to or larger than the 15N hyperfine values, because 13C has larger nuclear gn value, and they are closer to the spin center. Alternatively, they may be comparable to the 13C hyperfine value of the C9 C in the paramagnetic intermediate found in the C–S bond-forming biotin synthase BioB (aiso = 2.7 MHz), in which the C is located at a similar relative geometry to the diiron cluster as the H-cluster (43). In any case, both 13C and 15N hyperfine couplings should be readily resolved in X-band HYSCORE spectrum. Clearly, however, the HYSCORE spectrum of HydA1 maturated from syn-B-13C3,15N exhibited exactly the same patterns as natural abundance HydA1 (Fig. 4) and can be simulated (SI Appendix, Fig. S8) by using the 14N hyperfine tensors from the adt and the distal CN ligand, as reported (42). This result indicates that while syn-B-13C3,15N results in maturation as expected, the C2N portion of adt is not labeled, and therefore is not derived from Cys activated in HydG. Additionally, we also added the 13C3,15N-Cys into the maturation reaction directly and didn’t find labeled adt ligand either (SI Appendix, Fig. S9), indicating that the C and N in adt are indeed not sourced from Cys.
Fig. 4.
Comparison of X-band HYSCORE spectra of a CrHydA1 and CrHydA1 maturated from syn-B-13C3,15N collected at g = 2.102 (g1 of Hox). The Larmor frequencies of 13C and 15N are indicated in both spectra by red and blue lines, respectively. Conditions were as follows: frequency = 9.8 GHz; temperature = 15 K; τ = 140 ns; π/2 pulse = 12 ns.
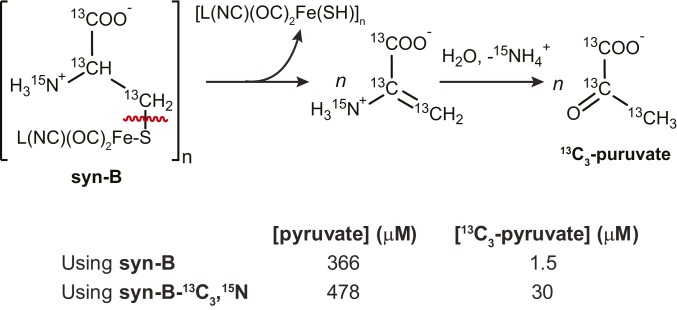
Preparation of HydA1 with syn-B-13C3,15N also allows the determination of the fate of the cysteine backbone. Reaction mixtures of maturations using syn-B and syn-B-13C3,15N were each analyzed for pyruvate and 13C3-pyruvate [13CH313C(O)13CO2−] by gas chromatography (GC)-MS (following derivatization; see SI Appendix for details). Inspection of the results shows a large background level of pyruvate for both maturations (Scheme 2); however, for the syn-B-13C3,15N maturation, 13C3-pyruvate was readily distinguished from the unlabeled pyruvate not sourced from syn-B-13C3,15N. The maturation reaction using syn-B-13C3,15N was found to contain 30 μM 13C3-pyruvate, corresponding to approximately twice the concentration of apo-HydA1 used for the maturation. The fact that Cys was converted into pyruvate reinforces our hypothesis that Cys serves only as a source of S for the construction of adt.
Scheme 2.
Proposed origin of 13C3-pyruvate from maturation using syn-B-13C3,15N (Upper) along with quantification of pyruvate in syn-B and syn-B-13C3,15N maturation reactions (Lower). L, unspecified labile ligand.
Maturation of Apo-HydA1 with Syn-B-Secys Gives the Seleno-adt Derivative.
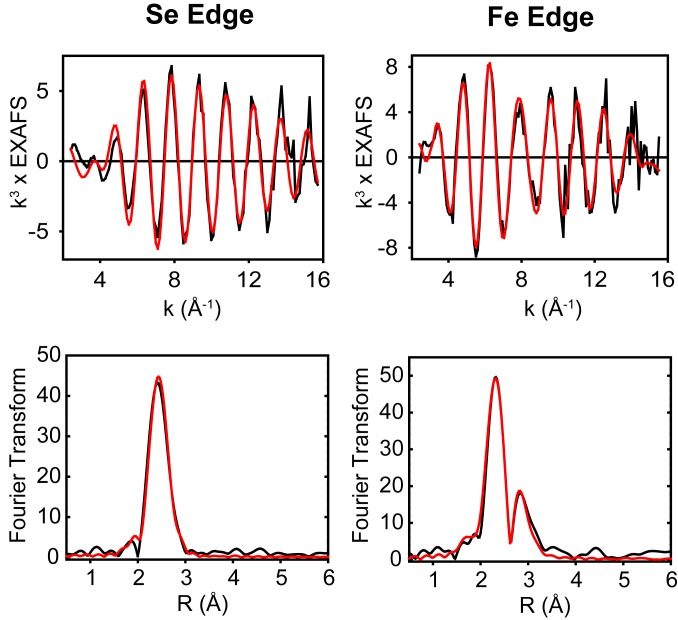
To further establish that Cys serves as a S donor, experiments were conducted to demonstrate that selenocysteine serves as a Se donor. The required carrier syn-B-Secys was prepared completely analogously to the preparation of syn-B by reaction of selenocysteine and [FeI2(CN)(CO)3]−. In terms of its ESI-MS and FT-IR properties, syn-B-Secys and syn-B are very similar (SI Appendix, Fig. S5 and Table S1). Maturation with syn-B-Secys gave CrHydA1-Se, whose EPR spectrum was virtually indistinguishable from native CrHydA1, except some broadening at g1 (SI Appendix, Fig. S10). Incorporation of Se into HydA1-Se was definitively characterized with EXAFS, both at the Se and Fe edges (44). The Se K EXAFS and FT are shown in Fig. 5, with simulated metrical parameters listed in SI Appendix, Table S2. The data simulated well to 2 Se–Fe interactions with Se–Fe = 2.43 Å and a Debye–Waller (DW) term of 0.0065 Å2. The value of the DW factor is similar to that obtained for a SeCys ligand bridging between 2 Cu(I) centers in SeCys-labeled Cu(I)-CCS (45) and is consistent with the Se atom bridging between 2 Fe atoms of the subcluster. The Se data do not permit the number of Se bridges to be determined, but if the Se is incorporated into the adt ligand, the expectation is that 2 such bridges will be present. To gain insight, we also analyzed the Fe K EXAFS of the S- and Se-containing HydA1 systems, which are shown for comparison in SI Appendix, Figs. S11 and S12. Although these spectra are similar, there are clear differences which can be accounted for by inclusion of Fe–Se scattering in the SeCys derivative. Importantly, the best fits to the Fe data were obtained when 2 Fe–Se scatterers per Fe atom of the [2Fe]H subcluster were included with identical Fe–Se distances and DW factors as determined at the Se edge. Thus, we conclude that our data are supportive of an HydA1 subcluster containing 2 bridging Se-containing ligands, as expected for an Se–adt interaction. To summarize, these results reveal the fate of Cys during the H-cluster bioassembly as donating the S atom to the adt ligand with its backbone converted into pyruvate, as depicted in Scheme 3.
Fig. 5.
Se K-edge (Left) and Fe K-edge (Right) EXAFS (Upper) and the corresponding Fourier transforms (Lower) of the Se-labeled CrHydA1. Black traces are experimental data and red traces are simulations using the parameters listed in SI Appendix, Table S2.
Scheme 3.
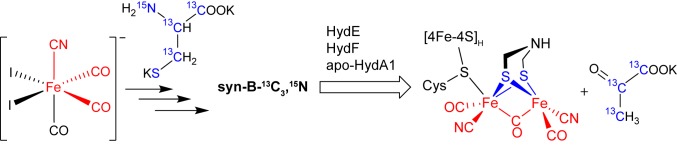
Summary and implications of maturation with syn-B-13C3,15N: HydG produces the precursor to the Fe2S2(CN)2(CO)3 portion of HydA1.
Discussion
A combination of synthetic organometallic chemistry, biochemical techniques, and biophysical analyses provides unique insights into the biosynthesis of the catalytic center of the [FeFe]hydrogenase. The synthetic surrogate syn-B is a fully functional analog of Complex B, the proposed product of the radical SAM maturase enzyme HydG. Because it is far more robust than Complex B, syn-B has allowed us to eliminate HydG in the in vitro maturation protocol. While the exact composition of syn-B in not fully understood, FT-IR, ESI-MS, and elemental analyses confirmed that it contains the targeted [Fe(Cys)(CN)(CO)2] fragment, which has been postulated to be the essential component of Complex B.
Regardless of the precise structure of syn-B, this surrogate allows us to gain major insights into the biosynthesis of the H-cluster of [FeFe] hydrogenase. With the added control associated with syn-B, we were able to selectively test the effects on maturation by sequentially replacing cysteine with cysteamine (lacking the carboxylate). The inability of syn-B-cysam to form maturated HydA1 indicates that cysteine is necessary for maturation. Furthermore, the finding that mixtures of syn-B-cysam and Cys do not maturate implies that syn-B, and logically, Complex B, is a source, not only of the diatomic ligands but of Fe(CN)(CO)2S. These findings imply that the Fe(CN)(CO)2(Cys) is a cohesive module. Such behavior is consistent with its being a low-spin d6 center, which confers kinetic robustness to this module.
This strategy also allows for isotopic labeling of the cysteine in the maturation, which has not been possible with typical maturation using HydG because protocols do not exist for replacing auxiliary Fe–S cluster-ligated natural abundance cysteine with labeled cysteine or selenocysteine. Maturation with syn-B-13C3,15N was successful; however, no 13C or 15N appeared to incorporate into the adt of the final HydA1 produced. This result is surprising: It shows that Complex B is not the source of C or N for adt formation. Instead, the Cys backbone is found to convert into pyruvate. Consistent with these results, maturation with syn-B-Se results in the incorporation of Se into the H-cluster. These results clearly indicate that Cys only donates its S atom to the H-cluster. Given that in HydG, the cysteine ligand stabilizes the dangler Fe relative to its highly labile form without cysteine (26, 34), it is interesting to observe that the Fe–S bond remains intact in the formation of the Fe2S2 core of the binuclear subcluster.
This work sets the stage for other studies involving the preparation of modified and isotopically labeled versions of Complex B. We have shown that this synthetic surrogate strategy is a fruitful venture that powerfully complements biochemical strategies aimed at understanding pathways involved in the maturation of [FeFe]-hydrogenase. Future work will focus on synthesizing more well-defined surrogates for Complex B, while also developing new complexes incorporating other ligands to identify the source of C and N for adt formation. The abstraction of S from cysteine is reminiscent of the Fe–S cluster biogenesis system (the isc operon) employed for the biosynthesis of ferredoxins (46), except that S transfer affords pyruvate, not alanine. This indicates interesting C–S bond cleavage and formation reaction(s) mediated probably by HydE or HydF. In the future, as prospective products of HydE and HydF are hypothesized, this surrogate strategy will likely be useful in investigating the molecular mechanism of these enzymes and identifying intermediates at later stages in the maturation pathway.
Materials and Methods
Synthetic organometallic chemistry leading to syn-B, syn-B-13CN, syn-B-13C3,15N, syn-B-cysam, and syn-B-Secys were conducted by using glovebox and Schlenkline techniques. Maturation experiments followed recently published protocols (37, 47). Additional details together with spectra and simulation details can be found in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants 1R35GM126961-01 (to R.D.B.), GM61153 (to T.B.R.), and R01GM123725 (to N.J.B.). Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences Contract DE-AC02-76SF00515. The Stanford Synchrotron Radiation Lightsource Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences (including Grant P41 GM103393). We thank Prof. Frank E. Osterloh and Dr. Zeqiong Zhao (University of California, Davis) for the help with GC detection of H2; and Dr. Lucas Li and Dr. Alexander Ulanov at the Roy J. Carver Biotechnology Center (University of Illinois Urbana–Champaign) for help with pyruvate detection and quantification.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913324116/-/DCSupplemental.
References
- 1.Lubitz W., Ogata H., Rüdiger O., Reijerse E., Hydrogenases. Chem. Rev. 114, 4081–4148 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Pandey K., Islam S. T., Happe T., Armstrong F. A., Frequency and potential dependence of reversible electrocatalytic hydrogen interconversion by [FeFe]-hydrogenases. Proc. Natl. Acad. Sci. U.S.A. 114, 3843–3848 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans R. M., et al. , The value of enzymes in solar fuels research—Efficient electrocatalysts through evolution. Chem. Soc. Rev. 48, 2039–2052 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Mulder D. W., Guo Y., Ratzloff M. W., King P. W., Identification of a catalytic iron-hydride at the H-cluster of [FeFe]-hydrogenase. J. Am. Chem. Soc. 139, 83–86 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Pelmenschikov V., et al. , Reaction coordinate leading to H2 production in [FeFe]-hydrogenase identified by nuclear resonance vibrational spectroscopy and density functional theory. J. Am. Chem. Soc. 139, 16894–16902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reijerse E. J., et al. , Direct observation of an iron-bound terminal hydride in [FeFe]-hydrogenase by nuclear resonance vibrational spectroscopy. J. Am. Chem. Soc. 139, 4306–4309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer C., et al. , Proton coupled electronic rearrangement within the H-cluster as an essential step in the catalytic cycle of [FeFe] hydrogenases. J. Am. Chem. Soc. 139, 1440–1443 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Shepard E. M., et al. , [FeFe]-hydrogenase maturation. Biochemistry 53, 4090–4104 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Betz J. N., et al. , [FeFe]-hydrogenase maturation: Insights into the role HydE plays in dithiomethylamine biosynthesis. Biochemistry 54, 1807–1818 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broderick J. B., et al. , H-cluster assembly during maturation of the [FeFe]-hydrogenase. J. Biol. Inorg. Chem. 19, 747–757 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Mulder D. W., et al. , Insights into [FeFe]-hydrogenase structure, mechanism, and maturation. Structure 19, 1038–1052 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Peters J. W., et al. , [FeFe]- and [NiFe]-hydrogenase diversity, mechanism, and maturation. Biochim. Biophys. Acta 1853, 1350–1369 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Berggren G., et al. , Biomimetic assembly and activation of [FeFe]-hydrogenases. Nature 499, 66–69 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulder D. W., et al. , Stepwise [FeFe]-hydrogenase H-cluster assembly revealed in the structure of HydAΔEFG. Nature 465, 248–251 (2010). [DOI] [PubMed] [Google Scholar]
- 15.McGlynn S. E., et al. , HydF as a scaffold protein in [FeFe] hydrogenase H-cluster biosynthesis. FEBS Lett. 582, 2183–2187 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Shepard E. M., et al. , Synthesis of the 2Fe subcluster of the [FeFe]-hydrogenase H cluster on the HydF scaffold. Proc. Natl. Acad. Sci. U.S.A. 107, 10448–10453 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berto P., et al. , The [4Fe-4S]-cluster coordination of [FeFe]-hydrogenase maturation protein HydF as revealed by EPR and HYSCORE spectroscopies. Biochim. Biophys. Acta 1817, 2149–2157 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Caserta G., et al. , Structural and functional characterization of the hydrogenase-maturation HydF protein. Nat. Chem. Biol. 13, 779–784 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Rohac R., et al. , Carbon-sulfur bond-forming reaction catalysed by the radical SAM enzyme HydE. Nat. Chem. 8, 491–500 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Kuchenreuther J. M., et al. , A radical intermediate in tyrosine scission to the CO and CN- ligands of FeFe hydrogenase. Science 342, 472–475 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Pilet E., et al. , The role of the maturase HydG in [FeFe]-hydrogenase active site synthesis and assembly. FEBS Lett. 583, 506–511 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Shepard E. M., et al. , [FeFe]-hydrogenase maturation: HydG-catalyzed synthesis of carbon monoxide. J. Am. Chem. Soc. 132, 9247–9249 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Driesener R. C., et al. , Biochemical and kinetic characterization of radical S-adenosyl-L-methionine enzyme HydG. Biochemistry 52, 8696–8707 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Pagnier A., Martin L., Zeppieri L., Nicolet Y., Fontecilla-Camps J. C., CO and CN- syntheses by [FeFe]-hydrogenase maturase HydG are catalytically differentiated events. Proc. Natl. Acad. Sci. U.S.A. 113, 104–109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchenreuther J. M., et al. , The HydG enzyme generates an Fe(CO)2(CN) synthon in assembly of the FeFe hydrogenase H-cluster. Science 343, 424–427 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinis P., et al. , X-ray crystallographic and EPR spectroscopic analysis of HydG, a maturase in [FeFe]-hydrogenase H-cluster assembly. Proc. Natl. Acad. Sci. U.S.A. 112, 1362–1367 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suess D. L., Kuchenreuther J. M., De La Paz L., Swartz J. R., Britt R. D., Biosynthesis of the [FeFe] hydrogenase H cluster: A central role for the radical SAM enzyme HydG. Inorg. Chem. 55, 478–487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suess D. L., et al. , The radical SAM enzyme HydG requires cysteine and a dangler iron for generating an organometallic precursor to the [FeFe]-hydrogenase H-cluster. J. Am. Chem. Soc. 138, 1146–1149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao G., Tao L., Suess D. L. M., Britt R. D., A [4Fe-4S]-Fe(CO)(CN)-L-cysteine intermediate is the first organometallic precursor in [FeFe] hydrogenase H-cluster bioassembly. Nat. Chem. 10, 555–560 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stock A., et al. , Synthesis of fac-[FeII(CN)(CO)3I2]− and chemistry of the fac-[FeII(CN)x(CO)3I(3−x)]− series (x=1–3). Inorg. Chem. Commun. 18, 105–109 (2012). [Google Scholar]
- 31.Liaw W. F., et al. , Dinuclear iron(II)-cyanocarbonyl complexes linked by two/three bridging ethylthiolates: Relevance to the active site of [Fe] hydrogenases. Inorg. Chem. 42, 2783–2788 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Chen C.-H., et al. , Preparative and structural studies on iron(II)-thiolate cyanocarbonyls: Relevance to the [NiFe]/[Fe]-hydrogenases. Dalton Trans., 137–143 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Lai C. H., et al. , Responses of the Fe(CN)2(CO) unit to electronic changes as related to its role in [NiFe]hydrogenase. J. Am. Chem. Soc. 120, 10103–10114 (1998). [Google Scholar]
- 34.Suess D. L., et al. , Cysteine as a ligand platform in the biosynthesis of the FeFe hydrogenase H cluster. Proc. Natl. Acad. Sci. U.S.A. 112, 11455–11460 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaasjager V. E., et al. , A structural model for [Fe]-only hydrogenases. Angew. Chem. Int. Ed. Engl. 37, 1668–1670 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Volkers P. I., et al. , Precursors to [FeFe]-hydrogenase models: Syntheses of Fe2(SR)2(CO)6 from CO-free iron sources. Inorg. Chem. 47, 7002–7008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao G., Britt R. D., Electronic structure of two catalytic states of the [FeFe] hydrogenase H-cluster as probed by pulse electron paramagnetic resonance spectroscopy. Inorg. Chem. 57, 10935–10944 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuchenreuther J. M., George S. J., Grady-Smith C. S., Cramer S. P., Swartz J. R., Cell-free H-cluster synthesis and [FeFe] hydrogenase activation: All five CO and CN− ligands derive from tyrosine. PLoS One 6, e20346 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers W. K., et al. , The cyanide ligands of [FeFe] hydrogenase: Pulse EPR studies of 13C and 15N-labeled H-cluster. J. Am. Chem. Soc. 136, 12237–12240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angamuthu R., et al. , N-substituted derivatives of the azadithiolate cofactor from the [FeFe] hydrogenases: Stability and complexation. Inorg. Chem. 54, 5717–5724 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cronan J. E., Advances in synthesis of biotin and assembly of lipoic acid. Curr. Opin. Chem. Biol. 47, 60–66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adamska-Venkatesh A., et al. , Spectroscopic characterization of the bridging amine in the active site of [FeFe] hydrogenase using isotopologues of the H-cluster. J. Am. Chem. Soc. 137, 12744–12747 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Tao L., Stich T. A., Fugate C. J., Jarrett J. T., Britt R. D., EPR-derived structure of a paramagnetic intermediate generated by biotin synthase BioB. J. Am. Chem. Soc. 140, 12947–12963 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kertess L., et al. , Chalcogenide substitution in the [2Fe] cluster of [FeFe]-hydrogenases conserves high enzymatic activity. Dalton Trans. 46, 16947–16958 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Barry A. N., Blackburn N. J., A selenocysteine variant of the human copper chaperone for superoxide dismutase. A Se-XAS probe of cluster composition at the domain 3-domain 3 dimer interface. Biochemistry 47, 4916–4928 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Lill R., Function and biogenesis of iron-sulphur proteins. Nature 460, 831–838 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Kuchenreuther J. M., Shiigi S. A., Swartz J. R., Cell-free synthesis of the H-cluster: A model for the in vitro assembly of metalloprotein metal centers. Methods Mol. Biol. 1122, 49–72 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.