Significance
Interleukin-2 (IL-2), a cytokine produced by activated T cells, acting in a paracrine/autocrine fashion, is essential for T cell proliferation. In adult T cell leukemia (ATL) and in autoimmune/graft-vs.-host diseases, anti–IL-2Rα antibody therapies were established to inhibit proliferation, with only limited success. We demonstrated that IL-2/15 receptors start to assemble in the endoplasmic reticulum/Golgi and demonstrated signaling by Jak1/Jak3 in the Golgi. Thus, in T cells also producing IL-2 (e.g., ATL cells), signaling can already take place before receptors reach cell membranes. This scenario explains resistance to antibody therapies targeting receptors at cell surfaces. Our study sheds light on an autocrine signaling mechanism, in which an intracellularly synthesized ligand activates its receptor en route toward the cell surface.
Keywords: adult T cell leukemia/lymphoma, antiproliferative antibody therapy, autocrine signaling, IL-2/15 receptor preassembly, Förster resonance energy transfer
Abstract
Interleukin-2 (IL-2) and IL-15 play pivotal roles in T cell activation, apoptosis, and survival, and are implicated in leukemias and autoimmune diseases. Their heterotrimeric receptors share their β- and γc-chains, but have distinct α-chains. Anti–IL-2Rα (daclizumab) therapy targeting cell surface-expressed receptor subunits to inhibit T cell proliferation has only brought limited success in adult T cell leukemia/lymphoma (ATL) and in multiple sclerosis. We asked whether IL-2R subunits could already preassemble and signal efficiently in the endoplasmic reticulum (ER) and the Golgi. A combination of daclizumab and anti–IL-2 efficiently blocked IL-2–induced proliferation of IL-2–dependent wild-type (WT) ATL cells but not cells transfected with IL-2, suggesting that in IL-2–producing cells signaling may already take place before receptors reach the cell surface. In the Golgi fraction isolated from IL-2–producing ATL cells, we detected by Western blot phosphorylated Jak1, Jak3, and a phosphotyrosine signal attributed to the γc-chain, which occurred at much lower levels in the Golgi of WT ATL cells. We expressed EGFP- and mCherry-tagged receptor chains in HeLa cells to study their assembly along the secretory pathway. Confocal microscopy, Förster resonance energy transfer, and imaging fluorescence cross-correlation spectroscopy analysis revealed partial colocalization and molecular association of IL-2 (and IL-15) receptor chains in the ER/Golgi, which became more complete in the plasma membrane, further confirming our hypothesis. Our results define a paradigm of intracellular autocrine signaling and may explain resistance to antagonistic antibody therapies targeting receptors at the cell surface.
Interleukin-2 (IL-2) has been applied for many years therapeutically as an immune adjuvant in certain types of lymphoproliferative diseases and cancers [metastatic renal cell carcinoma, metastatic melanoma (1, 2)]. IL-2R antagonist [daclizumab, a humanized form of the anti–IL-2Rα monoclonal antibody (3)] has been used to inhibit proliferation of adult T cell leukemia/lymphoma (ATL) cells (4), to prevent organ transplant rejection (5), and for the treatment of multiple sclerosis (6). However, the efficacy of daclizumab therapies was limited; no responses were observed in 18 patients with acute or lymphoma ATL and 6 partial responses were observed in 16 chronic and smoldering ATL (4). The molecular background of resistance to antiproliferative antibody therapies is unclear. The mechanism of action of IL-2 is paracrine or autocrine, i.e., IL-2 is secreted by neighboring T cells or the target cell itself. Activated T cells produce IL-2 transiently, whereas smoldering/chronic ATL cells due to their constitutive expression of HTLV-1 Tax express IL-2 and IL-2Rα constitutively creating an autocrine loop (7). We hypothesize that, in cells expressing IL-2 and its receptor, signaling might take place in the cell before receptor subunits reach the plasma membrane, which could explain the limited efficiency of daclizumab therapies.
IL-2 and -15 are responsible for many processes in the immune system by controlling the life and death of immune cells (8). They are expressed by activated T cells, natural killer (NK) cells, and some B cells (IL-2) or by dendritic cells and monocytes (IL-15). Their heterotrimeric receptors have 2 subunits, the β (CD122) and γc (CD132), in common (9, 10). These shared subunits are necessary and sufficient for effective signaling (11, 12), whereas the cytokine-specific α subunits are responsible for the specific, high-affinity binding of ligand. IL-15Rα expressed by dendritic cells or monocytes also expressing IL-15 can present the ligand in trans to T or NK cells expressing the β- and γc-chains (13). The common γc subunit is also a component of the receptors for IL-4, IL-7, IL-9, and IL-21 (14–18). Due to the shared signaling receptor chains, both cytokines activate similar signal transduction pathways (Jak/STAT, PI-3K/Akt, Ras/Raf/MAPK) and stimulate the proliferation of T and NK cells, and induce the generation of cytotoxic T lymphocytes. At the same time, they also play distinct and often contrasting roles: IL-2 has a pivotal role in activation-induced cell death and is crucial for the maintenance of peripheral Treg cells; in contrast, IL-15 has an antiapoptotic effect and stimulates long-term survival of memory CD8+ T cells (19–23).
We and others have already characterized associations of the receptor chains at the surface of T cells. In addition to the high-affinity receptor heterotrimers, the subunits can form dimers with different ligand binding affinities (reviewed in ref. 24). The existence of preassembled heterocomplexes of the receptor subunits in the plasma membrane, which could be modulated by ligand binding, was first reported in a Förster resonance energy transfer (FRET)-based study (25). The homoassociation of IL-2Rα was also observed on the IL-2–independent Kit225 IG3 T lymphoma cell line, while no significant homoassociation occurred on the IL-2–dependent Kit225 K6 and the Hut102 cells (26). The β–β homodimer as a new form of functional IL-2 receptor was also reported to assemble spontaneously in the absence of γc subunit at the cell surface (27). γc ectodomains may exist as stable homotrimers in the membrane of transfected insect cells (28). Coexpression of IL-2Rβ significantly reduces the level of homomeric γc in BOSC23 cells (29). The presence of the IL-2Rα subunit does not affect the oligomerization of the β- and γc-chains (29). It was described that the extracellular domains of IL-2Rβ and γc could interact at the cell surface in the absence of cytokine, whereas the cytoplasmic and transmembrane domains did not significantly contribute to heterodimerization. Binding of IL-2 brought the transmembrane domains of the β- and γc-chains closer together (30). We found that the 4 subunits of IL-2/15R (IL-2Rα, IL-15Rα, β, and γc) could form heterotetrameric complexes in the absence of cytokine in the plasma membrane of T lymphoma cells (31), which were rearranged upon the addition of relevant ligands.
The life cycle of membrane receptors starts with their synthesis in the rough endoplasmic reticulum (ER), followed by chaperone-assisted folding, posttranslational modifications and quality control in the ER, then further posttranslational modifications in the Golgi apparatus, from where they travel in targeted transport vesicles toward the plasma membrane. The general view is that membrane receptors can signal efficiently while they are in the plasma membrane, the subunits being in an already assembled form or brought together by their ligand. After ligand binding—or spontaneously—receptors are then internalized and degraded in endosomes (such as IL-2R/15Rβ and γc) or recycled to the membrane (like IL-2Rα or IL-15Rα) (32). Signaling through IL-4R was found to be promoted by receptor enrichment in endosomes following their actin-dependent internalization (33). It is an intriguing question whether the newly synthesized constituents of multicomponent membrane receptors find each other only in the plasma membrane, or they arrive there in a preassembled form.
Therefore, we aimed to investigate the preassembly of IL-2 and IL-15 receptors inside the cell using fluorescence microscopy techniques. Here, we demonstrate that in living HeLa cells: 1) the β subunit can assemble partially with IL-2Rα, IL15Rα, as well as with γc subunits prior to reaching the cell surface, in the ER and the Golgi, but the extent of the association between the β and α subunits is more extensive in the plasma membrane; 2) IL-2Rα and IL-15Rα can also partially assemble in the ER and Golgi, and to an even larger extent in the plasma membrane; and 3) γc subunits can form homodimers diffusing stably together in the ER and the Golgi. 4) We also show that the proliferation of the ED40515(+) wild-type (WT) IL-2–dependent ATL line can be blocked by a combination of daclizumab (anti–IL-2Rα) and anti–IL-2 antibodies, whereas cells transfected with the gene of IL-2 [ED40515(+)/IL-2] so as to produce their own ligand evade this block and keep proliferating. 5) Finally, we detect phosphorylated Jak1/Jak3 and a phosphotyrosine signal attributed to γc-chains in the Golgi fraction of ED40515(+)/IL-2, which occur at much lower levels in the Golgi of WT cells.
Our results may explain resistance to antagonistic antibody therapies targeting receptor subunits at the cell surface and define an intracellular autocrine signaling mechanism.
Results
Inhibition of Proliferation by Anti–IL-2Rα and Anti–IL-2 Antibodies Is Abolished in IL-2–Producing ATL Cells.
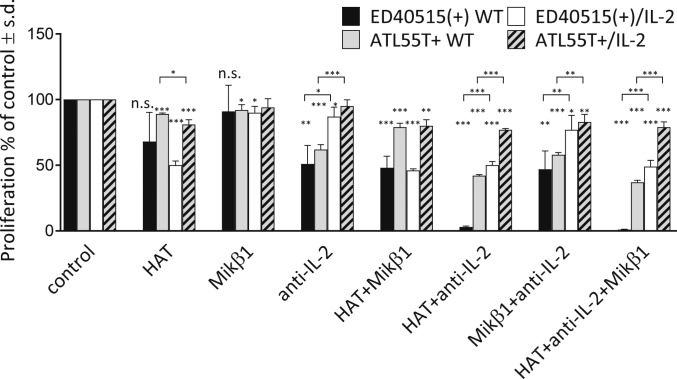
Activated T cells (and e.g., ATL cells) produce IL-2 and also express its receptor. The question arises whether newly synthesized receptor subunits could assemble in the ER, bind intracellularly produced IL-2, and signal before reaching the cell surface. To test this possibility, we measured the proliferation rates of ATL cell lines as assessed by thymidine uptake in response to externally administered IL-2, and their change in the presence of antibodies inhibiting interaction between IL-2R subunits and IL-2. We used the IL-2–dependent ED40515(+) WT cell line and its virally transfected, IL-2–producing derivative, ED40515(+)/IL-2, as well as ATL55T+ and its IL-2–transfected version ATL55T+/IL-2 (Fig. 1). Since ATL55T+ can survive for 5 to 7 d without IL-2, this cell line and its IL-2–producing version were used as controls in proliferation inhibition experiments. Daclizumab, the humanized version of the anti-Tac antibody targeting IL-2Rα (denoted HAT in the figure), has been tested to treat ATL and to prevent autoimmune responses or graft-vs.-host diseases via inhibiting binding of IL-2 to IL-2Rα on T cells (3, 4). As shown in Fig. 1, HAT treatment reduced proliferation rates of ED40515(+) and ED40515(+)/IL-2 cells by ∼30 to 50% but hardly affected ATL55T+ and ATL55T+/IL-2 cells. Mikβ1, an antibody targeting the IL-2/15Rβ chain (34), had hardly any effect on either cell line but increased the effect of HAT when applied to ED40515(+) or ED40515(+)/IL-2 cells. Anti–IL-2 treatment caused 40 to 50% reduction in ED40515(+) and ATL55T+ WT cells but had hardly any effect on their IL-2–producing counterparts. Importantly, HAT and anti–IL-2 applied together reduced proliferation of the IL-2–dependent ED40515(+) cells dramatically, by 97%. However, this treatment caused only ∼50% reduction in the proliferation of IL-2–producing ED40515(+)/IL-2 cells and had an even weaker effect on ATL55T+ WT and ATL55T+/IL-2 cells. Evasion of the block in IL-2–producing cells suggests that, in a situation when binding of IL-2 to its receptors at the cell surface is prevented, those receptors binding endogenously produced IL-2 can still signal efficiently.
Fig. 1.
Antibody block of IL-2–induced proliferation of ATL cell lines is lifted by expression of own IL-2. The effect of antibodies against IL-2Rα (HAT), IL-2/15Rβ (Mikβ1), or IL-2 or their combination was assayed on the proliferation of the cytokine-dependent ATL cell lines ED40515(+) WT and ATL55T+ or these cells transfected with IL-2 [ED40515(+)/IL-2 and ATL55T+/IL-2]. Cells were plated in medium containing IL-2 (100 U/mL) for the nontransfected cells and without IL-2 for the IL-2–transfected cells. Cells were then incubated for 3 d with the indicated antibodies directed against IL-2 or a receptor chain. Cells were pulsed with 1 μCi of 3H-thymidine during the last 6 h of the cultures, harvested, and counted in a microplate counter. The value of percent proliferation was calculated as follows: (cpm of proliferation with antibody)/(cpm of proliferation without antibody) × 100%. HAT + anti–IL-2 effectively inhibited the proliferation of ED40515(+) WT cells mediated by IL-2. ED40515(+)/IL-2 cells producing their own IL-2 evaded this block. The experiments were performed in triplicate, and the data are expressed as mean ± SD. Significant differences are indicated by the following: *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test).
Confocal Microscopy Shows That Cotransfected Receptor Chains Are Partially Colocalized in the ER and the Golgi.
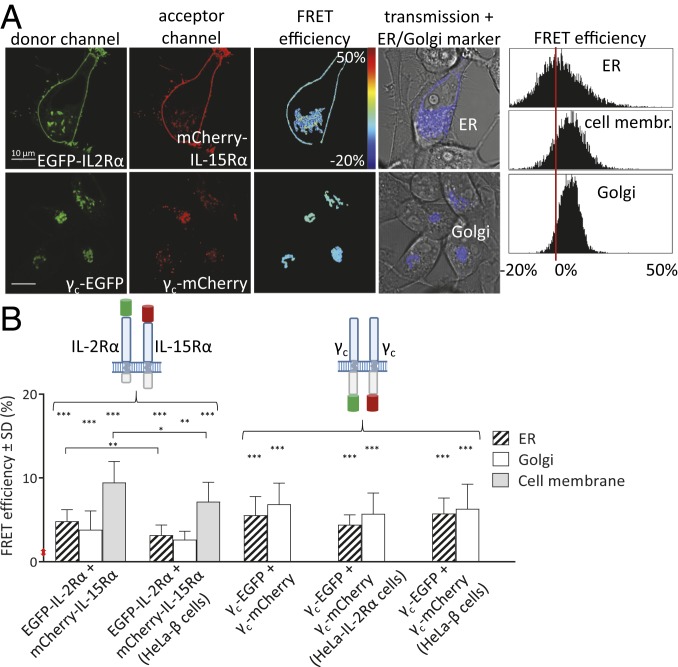
A plausible explanation for the evasion of IL-2–producing cells from antagonistic antibody block targeting cell surface receptors (and exogenous IL-2) would be that IL-2 receptors could already preassemble and signal before reaching the cell surface using endogenous IL-2. Therefore, we set out to investigate the colocalization and molecular vicinity of receptor subunits along the secretory pathway, in the ER and the Golgi. A schematic drawing of the controls and the receptor subunits (tagged with enhanced green fluorescent protein [EGFP] or mCherry at the extracellular N or cytoplasmic C termini) is shown in Fig. 2. We gained a first insight into the extent of colocalization between coexpressed receptor chains at ∼200- to 300-nm resolution by confocal microscopy in different compartments of living HeLa cells. Codistributions of different receptor chains tagged with EGFP or mCherry at various compartments are presented in a large format in SI Appendix, Fig. S1. The extent of colocalization was assessed by the Pearson’s correlation coefficient, C, between the green and red pixel intensities in the 2 images (Table 1). The maximal value of this parameter is 1 for perfectly overlapping distributions; it is 0 for independent ones and negative for mutually exclusive ones. As a positive control, we used the EGFP-mCherry fusion protein, for which the signals in the green and red channels were proportional, resulting a homogenous yellow merged image and a high average C value of 0.93 (SI Appendix, Fig. S1A). As a negative control, the pixels of confocal images were randomized, yielding an average C value of zero. We used TagBFP-labeled ER/Golgi-resident proteins as markers to visualize these organelles and study colocalization of receptor chains in an organelle-specific manner. The colocalization of the γc-EGFP and IL-2/15Rβ-mCherry chains was only partial in the ER and the Golgi; besides areas where both chains were present at similar concentrations, there were also regions where one or the other protein dominated (SI Appendix, Fig. S1B). The partial colocalization is reflected by the lower values of C (0.55 and 0.5 in the ER/Golgi) compared to the positive control. For IL-2Rα-EGFP and β-C7-mCherry, the C-terminally truncated version of IL-2/15Rβ, the correlation was high in the ER and the Golgi (C ∼ 0.7), but even higher in the plasma membrane (C = 0.83; SI Appendix, Fig. S1C). For IL-15Rα-EGFP and β-C7-mCherry, the tendency was similar: partial colocalization in the ER and the Golgi (C = 0.62 and 0.67) and more extensive in the plasma membrane (C = 0.83; SI Appendix, Fig. S1D). The distinct EGFP-IL-2Rα and mCherry-IL-15Rα subunits had intermediate C values in the ER/Golgi (0.54, 0.48) and a very high value in the plasma membrane (0.89; SI Appendix, Fig. S1E). The colocalization of cotransfected γc-EGFP and γc-mCherry in the ER/Golgi was also partial (C = 0.5 and 0.68; SI Appendix, Fig. S1F).
Fig. 2.
Schematic drawing of IL-2 and -15 receptor subunits and FRET controls. IL-2Rα and IL-15Rα subunits, full-length and C-terminally truncated IL-2/15Rβ subunits (279 aa shorter than the full-length version), and γc are shown. In FRET experiments, the subunits were tagged with EGFP (donor) or mCherry (acceptor) fluorescent proteins N- or C-terminally. As a positive control, an EGFP-mCherry fusion protein was used containing a 5-aa linker between the 2 dyes. As a negative control, cells were cotransfected with either EGFP and mCherry empty vectors or with N- or C-terminally tagged IL-2Rα (carrying the label at the extracellular or cytoplasmic tail) and mCherry-GPI, which carries the dye at the extracellular side.
Table 1.
Pearson’s correlation coefficients and FRET efficiencies between donor-acceptor–tagged protein pairs
| Interaction partners | ER | Golgi | Cell membrane | Whole cell |
| Correlation coefficient C, mean ± SD (n) | ||||
| pos ctrl (EGFP-mCh) | 0.93 ± 0.01 (13) | |||
| neg ctrl (randomized images) | 0.00 ± 0.00 (13) | |||
| β + γc (C) | 0.55 ± 0.22 (13) | 0.50 ± 0.16 (15) | ||
| IL-2Rα + β-C7 (C) | 0.70 ± 0.14 (16) | 0.69 ± 0.21 (18) | 0.83 ± 0.06 (27) | |
| IL-15Rα + β-C7 (C) | 0.62 ± 0.07 (10) | 0.67 ± 0.19 (10) | 0.83 ± 0.06 (18) | |
| IL-2Rα + IL-15Rα (N) | 0.54 ± 0.21 (12) | 0.48 ± 0.23 (10) | 0.89 ± 0.05 (15) | |
| γc + γc (C) | 0.50 ± 0.33 (10) | 0.68 ± 0.32 (16) | ||
| FRET efficiency E (%), mean ± SD (n) | ||||
| pos ctrl (EGFP-mCh) | 24.5 ± 2.3 (277) | |||
| neg ctrl1 (EGFP + mCh) | 0.9 ± 1.7 (180) | |||
| neg ctrl2 (EGFP-IL2Rα + mCh-GPI) | 0.4 ± 1.1 (13) | 1.5 ± 0.9 (11) | ||
| neg ctrl3 (IL2Rα-EGFP + mCh-GPI) | 1.1* ± 0.6 (12) | 0.4 ± 0.9 (22) | ||
| β + γc (C) | 5.5 ± 2.4 (11) | 3.7 ± 2.2 ( 21) | ||
| γc + β (C) | 5.5 ± 2.7 (46) | 4.6 ± 2.5 (27) | ||
| γc + β-C7 (C) | 4.4 ± 1.5 (11) | 4.1 ± 2.0 (22) | ||
| β + IL-2Rα (N) | 5.5 ± 1.3 (11) | 5.0 ± 1.7 (10) | ||
| β + IL-15Rα (N) | 6.9 ± 2.4 (16) | 6.2 ± 2.3 (25) | ||
| IL-2Rα + β (C) | 4.3 ± 1.1 (12) | 2.3n.s. ± 0.9 (13) | ||
| IL-2Rα + β-C7 (C) | 5.0 ± 1.7 (16) | 4.7 ± 2.0 (17) | 21.6 ± 5.4 (23) | |
| IL-15Rα + β-C7 (C) | 4.9 ± 1.6 (10) | 4.5 ± 1.3 (11) | 21.6 ± 5.1 (21) | |
| IL-15Rα + β-C7 (HeLa-γc) (C) | 7.1 ± 2.4 (10) | 4.6 ± 2.7 (17) | 21.1 ± 4.8 (26) | |
| IL-2Rα + IL-15Rα (N) | 4.8 ± 1.4 (16) | 3.8 ± 2.2 (18) | 9.5 ± 2.5 (27) | |
| IL-2Rα + IL-15Rα (HeLa-β) (N) | 3.2 ± 1.2 (12) | 2.6 ± 1.0 (10) | 7.2 ± 2.3 (15) | |
| γc + γc (C) | 5.6 ± 2.2 (53) | 6.8 ± 2.5 (62) | ||
| γc + γc (HeLa-IL-2Rα) (C) | 4.4 ± 1.2 (10) | 5.7 ± 2.5 (17) | ||
| γc + γc (HeLa-β) (C) | 5.7 ± 1.9 (32) | 6.3 ± 3.0 (39) |
Average Pearson’s correlation coefficients between the pixelwise fluorescence intensities of coexpressed protein pairs determined from confocal microscopic images indicate the extent of colocalization at the few-hundred-nanometers level. The first receptor chain is tagged with EGFP, the second one with mCherry. The position of the tags (N or C terminal, first column) and the numbers of measured cells (after SD values) are shown in parentheses. Average FRET efficiency values between receptor chains were all significantly different (P < 0.01) from the negative control (EGFP + mCherry), except for IL-2Rα-EGFP+IL-2/15Rβ-mCherry in the Golgi. Each FRET result was calculated based on at least 3 independent measurements. Ctrl, control; neg, negative; pos, positive; n.s., not significant.
These data were measured in the cytoplasm (no ER/Golgi markers were expressed).
Control Samples for Confocal Microscopic FRET Measurements.
We used confocal microscopic FRET measurements to assess the intracellular preassembly of IL-2/15 receptor subunits at the molecular level in an organelle-specific manner. FRET results are summarized in Table 1. The positive control, the EGFP-mCherry fusion protein, showed an average FRET efficiency of E = 24.5 ± 2.3% (mean ± SD) (Fig. 3A, first row). We applied 3 kinds of negative controls. First, we used cotransfected EGFP and mCherry, which were distributed evenly in the whole cell, yielding E = 0.9 ± 1.7% (Fig. 3A, second row). Second, we coexpressed N-terminally tagged EGFP-IL-2Rα with mCherry-GPI, a glycosylphosphatidylinositol-anchored protein, in which case the donor and the acceptor are both at the extracellular side of the plasma membrane (Fig. 3A, third row), resulting in E = 0.4 ± 1.1% in the ER and 1.5 ± 0.9% in the plasma membrane. This control allowed us to assess the extent of random FRET that may occur between 2 unrelated membrane components expressed in lipid rafts at similar concentrations as those of the receptor chains in our experiments. The low E values indicate that random FRET is not a concern at the expression levels used. Third, we applied C-terminally labeled IL-2Rα-EGFP coexpressed with mCherry-GPI (Fig. 3A, fourth row), resulting in E = 1.1 ± 0.6% in the cytosol (no ER/Golgi marker was used) and 0.4 ± 0.9% in the plasma membrane. In this case, IL-2Rα is tagged at the cytoplasmic, whereas GPI at the extracellular side, the donor and the acceptor being separated by a distance at least as large as the thickness of the lipid bilayer (5 to 10 nm). This control showed that, for a sample where the donor–acceptor distance is outside the range of FRET, the resultant FRET efficiency is practically zero, thus proving that spectral cross talk factors (SI Appendix) are determined accurately, making our FRET calculations accurate. Importantly, mean FRET efficiencies were ≤1.5% for each negative control.
Fig. 3.
FRET control measurements. (A) Confocal microscopic FRET results from representative cells of the control samples are shown. HeLa cells were transfected with the plasmid encoding for the EGFP-mCherry fusion protein as a positive control (first row). The first column is the donor signal from EGFP (green); the second is the acceptor signal from mCherry (red); the third column displays the map of FRET efficiencies calculated on a pixel-by-pixel basis. The fourth column shows the TagBFP signal (blue) from fragments of Sac1 (ER marker) or giantin (Golgi marker) overlaid on the transmission image. The fifth column is the FRET histogram from the pixels of the indicated cellular localizations. As a negative control, cells were cotransfected with EGFP and mCherry empty vectors (second row); their gene products were distributed evenly in the whole cell. As membrane localized negative controls, cells were cotransfected with 2 membrane proteins: N- or C-terminally tagged IL-2Rα carrying the label at the extracellular or the cytoplasmic tail, and mCherry-GPI tagged at the extracellular side (third and fourth rows). (Scale bars: 10 µm.) (B) FRET efficiencies (±SD) averaged for all measured cells are shown in different cellular locations. The schematic drawings represent the donor- and acceptor-tagged proteins in the given sample. FRET efficiencies and the numbers of cells measured for each sample are also summarized in Table 1.
The IL-2/15Rβ Chain Assembles with γc in the ER and the Golgi.
After determining the FRET efficiencies of the control samples, we studied the assembly of the intermediate affinity IL-2/15Rβ–γc heterodimer (Table 1). FRET measurements were carried out first with cells cotransfected with γc-EGFP and IL-2/15Rβ-mCherry. FRET efficiencies calculated in pixels where the ER or Golgi marker was present resulted in 5.5 ± 2.7% and 4.6 ± 2.5% (average ± SD), respectively (Fig. 4A, first row; average FRET efficiency shown in Fig. 4B). Using donor and acceptor labeling in reverse order (IL-2/15Rβ-EGFP and γc-mCherry) resulted in similar FRET efficiencies, 5.5 ± 2.4% (ER) and 3.7 ± 2.2% (Golgi). All of these values are significantly higher than that of the negative control (EGFP + mCherry) indicating that the IL-2/15Rβ and γc-chains are at least partially preassembled in these organelles. We plotted E as a function of the acceptor-to-donor molecular ratio, which showed an increasing tendency with increasing NA/ND and reached higher values (SI Appendix, Fig. S2A). At a higher NA/ND ratio, the probability that a donor associates with an acceptor and transfers its excitation energy to it is greater according to the law of mass action. The fluorescence intensity from coexpressed γc-EGFP and IL-2/15Rβ-mCherry subunits at the cell surface was hardly discernible from the autofluorescence intensity; therefore, we did not measure FRET in the plasma membrane. Among the IL-2/15R subunits, IL-2/15Rβ has the longest cytoplasmic tail, 200 amino acids (aa) longer than the γc-chain. To bring the C termini of the 2 receptor chains closer together, we also used a C-terminally truncated version of the β-chain (β-C7) (Fig. 2), having a cytoplasmic tail of only 7 aa. Contrary to our expectations, this modification did not influence the FRET efficiency to a significant extent; its value was E = 4.4 ± 1.5% in the ER and 4.1 ± 2.0% in the Golgi (Fig. 4A, second row).
Fig. 4.
Interaction of γc with the IL-2/15Rβ subunit demonstrated by confocal FRET microscopy. (A) FRET results measured between γc and IL-2/15Rβ subunits C-terminally tagged with EGFP (first column, green) and mCherry (second column, red) are shown for representative cells. Merged images are also presented (third column). TagBFP-Sac1 (ER marker) or giantin (Golgi marker) were also expressed (fifth column, blue). The fourth column displays the map of FRET efficiencies calculated only in pixels where the ER or Golgi marker was present. The first row shows data for the full-length IL-2/15Rβ (ER), whereas the second row displays data on the β-C7, a C-terminally truncated version of the β subunit having only 7 aa in its cytoplasmic tail (Golgi). FRET histograms are calculated for the selected cells. (B) FRET efficiencies (±SD) averaged for all measurements are shown for the ER/Golgi. The red arrow on the ordinate axis marks the range of the 3 negative control samples, E = 0.4 to 1.5%. Significant differences compared to negative control 1 (EGFP + mCherry) are indicated by the following: ***P < 0.001 (Student’s t test). FRET efficiencies and the numbers of cells measured for each sample are also summarized in Table 1.
IL-2/15Rβ Subunit Assembles Partially with IL-2Rα or IL15Rα along the Secretory Pathway before Full Assembly at the Cell Surface.
Next, we assessed the association of the IL-2/15Rβ chain with the cytokine-specific IL-2Rα and IL-15Rα chains. We compared N- or C-terminally labeled subunit pairs and examined the effects of shortening the β subunit as well as the presence of unlabeled γc-chains. FRET efficiencies measured between N-terminally tagged subunits, EGFP-IL-2/15Rβ and mCherry-IL-2Rα (5.5 ± 1.3% in the ER; 5.0 ± 1.7% in the Golgi) or mCherry-IL-15Rα (6.9 ± 2.4% in the ER; 6.2 ± 2.3% in the Golgi) (Fig. 5A, first and second rows; average FRET efficiencies, Fig. 5B), were significantly higher than those for the negative control. These results suggest that each α-chain is at least partially preassembled with the β-chain prior to reaching the cell surface. We received smaller FRET efficiencies between C-terminally tagged IL-2Rα-EGFP and IL-2/15Rβ-mCherry (4.3% ± 1.1% in the ER; 2.3% ± 0.9% in the Golgi; Fig. 5A, third row, and Fig. 5B) than between the N-terminally labeled counterparts, which is probably due to a larger distance between the C termini of these subunits (Fig. 1). Therefore, we also measured FRET (Fig. 5A, fourth and fifth rows) between the C-terminally truncated β-C7-mCherry and the IL-2Rα-EGFP (5.0 ± 1.7% in the ER; 4.7 ± 2.0% in the Golgi) or IL-15Rα-EGFP (4.9 ± 1.6% in the ER; 4.5 ± 1.3% in the Golgi), yielding higher FRET efficiencies than achieved with the full-length β-chains (Fig. 5B). E vs. NA/ND plots (SI Appendix, Fig. S2B) display an increasing tendency with increasing NA/ND ratio in all cellular compartments as expected, reaching higher values. When β-C7-mCherry was coexpressed with IL-2Rα-EGFP or IL-15Rα-EGFP, all receptor subunits were highly expressed in the plasma membrane, where they resulted in much higher FRET efficiencies (21.6 ± 5.4% for IL-2Rα and 21.6 ± 5.1% for IL-15Rα) than in the ER or the Golgi. The high FRET efficiencies measured in the plasma membrane imply that full assembly of these receptor chains ensues only at their final destination, i.e., preassembly along the secretory pathway is partial. In order to examine whether γc, the third element of the high-affinity αβγc receptor heterotrimers, influences the assembly of the α- and β-chains, we also used a cell line stably expressing nontagged, dark γc. As shown in Fig. 5B (columns for “HeLa-γc cells”), the presence of γc did not significantly influence the interaction between the IL-15Rα and β-C7 subunits.
Fig. 5.
Interaction of IL-2Rα and IL-15Rα with full-length or truncated IL-2/15Rβ revealed by FRET microscopy. (A) FRET efficiency was measured by confocal microscopy between the IL-2/15Rβ chain N-terminally tagged with EGFP (donor) and the IL-2Rα or IL-15Rα subunits tagged with mCherry (acceptor) (first and second rows), and C-terminally tagged IL-2Rα-EGFP coexpressed with the full-length IL-2/15Rβ-mCherry (third row). Interaction of C-terminally truncated and tagged β-C7 subunit (having only 7 aa in its cytoplasmic tail) and IL-2Rα or IL-15Rα subunits (fourth and fifth rows) are also presented. To allow identification of organelles, TagBFP-tagged fragments of Sac1 (ER marker) or giantin (Golgi marker) were also expressed (fourth column, blue). FRET efficiencies were calculated on a pixel-by-pixel basis in an organelle-specific manner (third column). The fifth column shows the FRET histograms at the different cellular localizations. Note that FRET efficiencies determined from the plasma membrane are higher than in the ER/Golgi. (B) Average FRET efficiencies are shown (±SD). The interaction between IL-15Rα and β-C7 was not influenced by the presence of nontagged, dark γc (see last group of columns for the HeLa-γc cell line). The red arrow on the ordinate axis marks the range of the 3 negative control samples, E = 0.4 to 1.5%. Significant differences compared to negative control 1 (EGFP + mCherry) are indicated by the following: ***P < 0.001 (Student’s t test). FRET efficiencies and the numbers of cells measured for each sample are also summarized in Table 1.
IL-2Rα and IL-15Rα Form a Common Cluster.
Earlier, we have shown that IL-2Rα and IL-15Rα were coexpressed in a common complex on T cells, which probably plays a role in efficient sharing of the signaling β and γc subunits. We were interested whether the 2 α-chains are also preassembled along the secretory pathway. The distinct α-chains, N-terminally tagged with EGFP or mCherry, displayed a positive FRET efficiency, suggesting that they could form heterodimers in the ER and the Golgi (ER, E = 4.8 ± 1.4%; Golgi, 3.8 ± 2.2%; Fig. 6A, top row). In the plasma membrane, a higher FRET efficiency, E = 9.5 ± 2.5%, was measured; thus, the intracellular assembly is probably partial. To test whether the presence of the IL-2/15Rβ subunit influences the interaction of the 2 distinct α subunits, we used a HeLa cell line stably transfected with nontagged IL-2/15Rβ. There was a slight decrease of FRET efficiency at each cellular organelle (Fig. 6B, “HeLa-β cells”), suggesting that there is a competition between the interaction of the 2 α-chains and their heteroassociation with IL-2/15Rβ.
Fig. 6.
FRET microscopy measurements demonstrating the heteroassociation of IL-2Rα and IL-15Rα and the homoassociation of γc subunits. (A) Confocal microscopic FRET results measured between IL-2Rα and IL-15Rα subunits N-terminally tagged with EGFP (donor) and mCherry (acceptor) are shown for representative cells (A, first row). Measurements were carried out in HeLa cells as well as in HeLa-β cells expressing the nontagged IL-2/15Rβ subunit (shown in B). Association of C-terminally tagged γc-chains was measured in HeLa (A, second row) as well as in HeLa-IL2Rα and HeLa-β cell lines (B). (B) Average FRET efficiencies (±SD) are shown. The red arrow on the ordinate axis marks the range of the 3 negative control samples, E = 0.4 to 1.5%. Significant differences compared to negative control 1 (or between HeLa and HeLa-β cells) are indicated by the following: *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test). FRET efficiencies and the numbers of cells measured for each sample are also summarized in Table 1.
Homotypic Complexes of γc in the ER and the Golgi.
It was found in vitro by gel filtration that purified γc subunits could form stable homotrimers, which dissociated when incubated with IL-2Rα, IL-2/15Rβ, and IL-2 (28). We were interested whether this self-association could also occur in live cells. The γc-chains C-terminally tagged with EGFP or mCherry were transiently coexpressed to test their possible complex formation. The γc subunit localized to the ER and the Golgi; in the plasma membrane, its signal was not discernible from autofluorescence (Fig. 6A, second row). The FRET efficiencies measured between γc-chains (ER, 5.6 ± 2.2%; Golgi, 6.8 ± 2.5%) were significantly higher than those of the negative controls (Fig. 6B). We also carried out the experiment in cells stably transfected with untagged, dark IL-2Rα or IL-2/15Rβ (referred to as “HeLa-IL-2Rα cells, HeLa-β cells”). The presence of neither IL-2Rα nor IL-2/15Rβ influenced the homoassociation of the γc-chains significantly (Fig. 6B).
Single-Plane Illumination Microscopy-Based Fluorescence Cross-Correlation Spectroscopy Measurements Evidence Stable Codiffusion of Homotypic γc Complexes.
Fluorescence cross-correlation spectroscopy (FCCS) is a dynamic technique capable of demonstrating the joint motion of associated molecules that form a stable complex for at least the dwell time of the molecules diffusing across the femtoliter-sized sensitive volume (typically a few tens or hundreds of milliseconds for membrane proteins). Analysis of the temporal autocorrelation and cross-correlation functions (ACFs, CCFs) of the fluorescence intensities reveals the mobilities of the diffusing molecular species and the fraction of molecules forming a complex. Earlier, we and others have demonstrated the stable codiffusion of the IL-2Rα + IL-15Rα chains (31) and the IL-2/15Rβ + γc-chains (30) in the membrane of T cells by using confocal FCCS. To confirm the FRET-detected intracellular homoassociation of γc-chains, we investigated the comobility of these molecules using single-plane illumination microscopy-based FCCS (SPIM-FCCS). Whereas confocal FCCS detects comobility only at a single point, SPIM-FCCS creates a 2D map of diffusion properties over a whole thin (∼1.3-μm) section of a cell.
Experiments were carried out on cells transfected with the EGFP-mCherry fusion protein as a positive control, cells cotransfected with EGFP + mCherry as a negative control, and cells cotransfected with γc-EGFP + γc-mCherry to detect homooligomerization. In the SPIM setup, it was not possible to detect TagBFP fluorescence; thus, the ER and the Golgi could not be discerned via the fluorescent markers used in FRET microscopy. We carried out measurements in the cytoplasm, which was clearly discernible from the plasma membrane. Fig. 7A shows the ACFs and CCFs for selected cells from the 3 samples. The ACFs and CCFs of the γc–γc sample decay at longer lag times, clearly showing the slower diffusion of the receptor chains compared to the soluble controls. A nonzero CCF amplitude proves the joint diffusion of proteins tagged with green and red labels. To approximate the extent of dimerization, we calculated the model independent ratio RCCFg (SI Appendix, Eq. S5) of the amplitudes of the CCF and the ACF of the green channel by forming the ratio of the averaged values at the first 5 lag times of the correlation functions. Fig. 7B displays the transmission images and 2D maps of the fluorescence intensities (first row) and the maps of RCCFg in the ROI (second row) from representative cells. The soluble proteins of the positive and negative controls are distributed evenly in the whole cytoplasm, whereas the γc-chains are restricted in smaller areas. In Fig. 7C, pixel-by-pixel distributions of RCCFg are shown for n = 11 to 13 cells. With this evaluation, we obtained an average RCCFg value of 0.26 ± 0.11 (SD) for the positive and 0.06 ± 0.07 for the negative control, and 0.15 ± 0.1 for the γc-EGFP + γc-mCherry sample (Fig. 7D). The value for the γc-EGFP + γc-mCherry sample was lower than that of the positive control but significantly higher than for the negative control. These results indicate that at least a fraction of the intracellular γc subunits exists in a dimeric (or oligomeric) form. Fitting the correlation curves (SI Appendix, Eq. S4) also yielded the concentrations of the individual molecular populations. Another estimate of the green–red dimer fraction, cgr/(cg-only+cr-only+cgr), can be obtained from the fitted ACF and CCF curves (Fig. 7E). The negative control had the lowest “dimer” fraction, 0.05 ± 0.04; the positive control gave the largest fraction, 0.19 ± 0.1; and the γc-EGFP + γc-mCherry sample gave an intermediate but, in comparison to the negative control, significantly larger dimer fraction, 0.14 ± 0.14. The green–red dimer fraction is less than unity even for the positive control, which may be due to partial photobleaching of the fluorescent proteins and imperfect overlap of the green and red microscopic observation volumes.
Fig. 7.
SPIM-FCCS data analysis shows stable codiffusion of γc subunits in homomeric complexes in the cytoplasm. (A) Autocorrelation and cross-correlation functions (ACFs and CCFs) from SPIM-FCCS measurements. Green, EGFP ACF; red, mCherry ACF; blue, CCF. Solid lines indicate experimental data; dashed lines are fits assuming 2 diffusing components for the ACFs and CCFs of the positive and negative controls and the ACFs of the γc-EGFP + γc-mCherry sample, and 1 diffusing component for the CCF of γc-EGFP + γc-mCherry. As a positive control, the EGFP-mCherry fusion protein, whereas as a negative control coexpressed EGFP and mCherry were used. (B, Top row) Fluorescence intensity and transmission images with the region of interest (ROI) of the observed area of the cell. (B, Bottom row) Map of relative CCF amplitudes RCCFg characterizing the green–red dimer fraction (fraction of red molecules associated with green molecules). (C) Histograms show the distributions of RCCFg from pixels within the ROI. (D) Average RCCFg values. (E) Average fractions of green–red dimers obtained from global fits to the correlation curves. (F) Average diffusion coefficients derived from global fits of ACFs and CCFs. Significant differences compared to the negative control are denoted by the following: ***P < 0.001; *P < 0.05 (t test). (G) Average diffusion coefficients of the fast (D1) and slow populations (D2), and fractions of the slow component (ρ2) are shown (mean ± SD). For C–G, parameters for all measured cells (n = 11 to 13) were averaged.
Finally, we determined the mobility of the particles from the ACF and CCF functions (Fig. 7 F and G and SI Appendix, Fig. S3). The ACFs of all samples and the CCFs of the controls were fitted with a model having 2 diffusing components. The average values of the fast and slow diffusion coefficients derived from the ACFs are similar for the controls and the γc-EGFP + γc-mCherry sample (Fig. 7G). However, the fraction of the slow component for the γc–γc dimer (ρ2 = 0.58 and 0.85 for the green and red ACFs) is much larger than for the controls (0.28 to 0.37), reflecting the slower diffusion of the γc subunits in the cytoplasm. For fitting the CCF of the γc–γc sample, we used a single-component model since there was no detectable fast component, suggesting that all γc dimers diffused slowly. The D value of the CCF, 0.12 ± 0.08 µm2/s, was similar to the slow component of the ACFs, D2 = 0.13 ± 0.03 and 0.11 ± 0.04 µm2/s (Fig. 7G). The CCF of the positive control could be fitted with 2 components, where the slow component had a faster D2 value, 0.20 ± 0.05 µm2/s, than that of the γc dimer, and its fraction was only 0.52 ± 0.26. SI Appendix, Fig. S3 shows 2D maps of the diffusion coefficients and fractions of the slow populations for selected cells where the average tendencies are exemplified. Our SPIM-FCCS measurements confirmed the existence of γc dimers or oligomers in the cytoplasm and demonstrated their slow codiffusion there.
Phosphorylation of IL-2 Signaling Elements in the Golgi/ER of ATL Cells.
To assess the signaling competence of preassembled IL-2R in the ER/Golgi, we isolated Golgi from our ATL cell lines and investigated phosphorylation of signaling elements of the IL-2 pathway by Western blot (Fig. 8). Jak1 binds and phosphorylates the β-chain, whereas Jak3, binding constitutively, phosphorylates the γc-chain. pJak1, pJak3, and phosphotyrosine bands around the expected molecular mass of γc (65 to 70 kDa) chains were detected from the isolated Golgi fraction of IL-2–expressing ED40515(+)/IL-2 cells; the signals from all 3 phosphorylated components were weaker for IL-2–nonexpressing, IL-2–starved ED40515(+) WT cells. The isolated Golgi fractions were GM130 positive (Golgi marker), but also contained calreticulin, characteristic for the ER. No lysosome/late endosome marker (LAMP1), recycling endosome marker (Rab11), or plasma membrane marker [MDR1 having a half-life of >100 h (35)] were found in the isolates. Thus, the Golgi fraction does not contain a significant fraction of plasma membrane contamination.
Fig. 8.
Detection of (phospho)proteins by Western blot from isolated Golgi of ATL cells. Representative Western blot results from total cell lysates and 2 fractions, called Golgi+ (containing the GM130 marker) and a Golgi−, from ultracentrifugal isolation. Both fractions contained calreticulin (ER marker) as well but were free of Rab11 (recycling endosome marker), Lamp1 (lysosome/late endosome marker), and MDR1 (plasma membrane marker). In the Golgi+ fraction, phospho-Jak1, phospho-Jak3, and the phosphotyrosine (pY20) bands at ∼65 to 70 kDa have stronger signals for IL-2–expressing ED40515(+)/IL-2 cells than for IL-2–dependent, IL-2-starved ED40515(+) WT cells. pY20 at 65 to 70 kDa may represent phosphorylated γc as suggested by the TUGh4 bands. Based on 3 independent experiments (n = 3).
Discussion
IL-2 and -15 receptor subunits can form various homomeric and heteromeric complexes in the plasma membrane having different ligand binding affinities. Of these, the βγc intermediate-affinity heterodimers (Kd ∼ 10−9 M ligand binding affinity) and the high-affinity αβγc heterotrimers (Kd ∼ 10−11 M) are capable of efficient signaling (11, 12). Earlier, we have shown that IL-2 and -15 receptors are preassembled in the plasma membrane even in the absence of ligand, and their conformation changes upon ligand binding (25, 31, 36, 37). We also demonstrated that the 2 receptor kinds form a common complex including the shared β- and γc-chains and the cytokine-specific IL-2Rα and IL-15Rα chains (31). It is an intriguing question whether the newly synthesized constituents of multicomponent membrane receptors find each other only in the plasma membrane, or they arrive there in a preassembled form. If the receptor subunits can already associate along the secretory pathway, they may potentially signal even before reaching the plasma membrane. This would define a paradigm of intracellular autocrine signaling in cells that produce the receptors and their ligands as well.
We tested this possibility by monitoring the IL-2–induced proliferation of ATL cell lines. Anti–IL-2 alone was not sufficient to block proliferation in IL-2–dependent ED40515(+) cells, but it efficiently blocked proliferation when applied in combination with HAT (anti–IL-2Rα). The very high-affinity binding of IL-2 to the heterotrimeric receptor with a Kd of 10−11 M is reduced to 10−9 M (the affinity of the βγc heterodimer) when HAT blocks binding of IL-2 to IL-2Rα. In particular, the antibody binding to IL-2 added may not be in sufficient quantity and affinity to be effective in fully blocking binding to the high-affinity receptor; however, it sufficiently blocked when the affinity of the receptor was reduced. This block was lifted in ED40515(+)/IL-2 cells transfected with the IL-2 gene; cells proliferated even in the presence of anti–IL-2, HAT, and Mikβ1 (anti–IL-2/15Rβ). We also demonstrated the presence of pJak1 and pJak3 as well as a phosphotyrosine signal around the expected molecular mass of the γc-chain (which is phosphorylated by Jak3) on Western blots from the isolated Golgi fraction of ED40515(+)/IL-2 cells. These findings can be explained by formation of efficient ligand–receptor signaling complexes in the intracellular space before receptors reached the membrane.
HTLV-1–associated ATL and the neurological disorder human T-cell lymphotropic virus-1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) represent disorders characterized by an IL-2/IL-2R autocrine loop. In the early phases of ATL and in HAM/TSP expression of HTLV-1, a viral encoded Tax gene induces T-cell proliferation through the induction of a number of cytokine–cytokine receptor autocrine stimulation loops including IL-2/IL-2Rα–induced proliferation (7). However, only 6 partial responses were observed in 16 chronic and smoldering ATL patients treated with daclizumab (4). Similarly, following therapy of patients with HAM/TSP with daclizumab, there was only partial down-regulation of activated CD4+ lymphocytes (38). Daclizumab has also been administered to multiple sclerosis patients, causing a reduction of T cell responses reducing the relapse rate (by 45%) and the number of new lesions (by 54%) (6). It was also used together with cyclosporine and corticosteroids to prevent acute rejection of kidney transplants until being discontinued because of side effects (5). Production and intracellular binding of the IL-2 ligand to its receptor in T cells involved in these immune reactions may reduce the efficiency of antibody therapies targeting receptors expressed in the plasma membrane only but having no access to them in intracellular organelles.
To test the hypothesis that receptors could preassemble before reaching the plasma membrane, thereby creating the possibility of intracellular signaling, we studied the interaction of the IL-2 and -15 receptor chains in the ER and the Golgi. FRET microscopy is a well-established method to report on molecular interactions (39–41). Earlier, we used this technique to study the interaction between IL-2/9/15 receptor subunits (31, 36, 37, 42, 43) or c-Fos and c-Jun transcription factors (44–46). Here, we used fluorescent ER and Golgi markers, allowing us to study protein–protein interactions in living cells in an organelle-specific manner. It must be mentioned that the lateral and axial resolutions of the confocal microscope are ∼200 and ∼500 nm, respectively; therefore, an area identified as positive, e.g., for the ER marker contains not only the membrane and lumen of the ER but also the cytoplasmic space between neighboring cisternae. Our FRET data gained from HeLa cells expressing fluorescent protein-tagged IL-2/15 receptor chains revealed the pairwise association of the β + γc (Fig. 4), β + IL-2Rα, as well as β + IL-15Rα subunits (Fig. 5) in the ER and the Golgi. We also found that the distinct α-chains of IL-2R and IL-15R were associated with each other in these organelles (Fig. 6), in line with our previous findings in T cell plasma membranes. These observations imply that, following their synthesis, the subunits of IL-2R and also IL-15R start their assembly along the secretory pathway, which may be a general phenomenon for different receptors. The very low FRET efficiency detected between EGFP-IL-2Rα and mCherry-GPI used as a negative control proved that accidental colocalization of unrelated proteins expressed at similar levels as those of the labeled receptor chains could not result in a significant random FRET (47) in the membrane or the ER.
The crystal structure of the cytoplasmic tails of the receptor chains has not been solved; thus, their exact conformation is unknown. FRET efficiency depends on the negative sixth power of the donor–acceptor distance, which facilitates its use as a molecular ruler. Even though our FRET data are not sufficient to get a detailed insight into the structure of the receptor chains, we may use them to restrain the range of possible conformations. The Förster radius, the distance at which E = 0.5, is R0 ∼ 5.4 nm for the EGFP-mCherry pair (48). E values measured for the different pairs of receptor chains ranged between 0.03 and 0.22. If assuming an orientation factor of κ2 = 2/3 [in case of random orientation of the transition dipoles of the dyes (49)] and assuming that each donor has an acceptor in its vicinity, these E values translate to a D–A distance range of 9.6 to 6.7 nm. We can assess the extended lengths of the cytoplasmic tails of the receptor chains based on the length of a peptide bond (Cα–Cα distance, ∼0.38 nm): IL-2Rα: 13 aa, 4.9 nm; IL-15Rα: 37 aa, 14.1 nm; IL-2/15Rβ: 286 aa, 108.7 nm; truncated β-C7: 7 aa, 2.7 nm; γc: 86 aa, 32.7 nm. The difference between the stretched lengths of the cytoplasmic tails of γc and IL-2/15Rβ would be ∼76 nm, which is well beyond the range (∼10 nm) of FRET. Thus, from the observed FRET efficiencies, it can be concluded that the IL-2/15Rβ chain is in a strongly folded form, in which its C terminus is closer to the C termini of the other (γc as well as IL-2Rα and IL-15Rα) receptor chains. Similarly, we also measured a significant FRET efficiency between the cytoplasmic tails of the β-C7 and the 30-nm longer γc-chains, suggesting that the γc-chain is probably also in a folded form.
The FRET efficiencies characterizing the interactions between the β- and α-chains or the IL-2Rα and IL-15Rα subunits were lower in the ER/Golgi than in the plasma membrane. Such a discrepancy may result from the following scenarios. On the one hand, the conformation of the receptor chains at different cellular localizations may differ, leading to different donor–acceptor distances and thus different FRET efficiencies within a single D–A pair. On the other hand, in the ER/Golgi, there may be a mixture of assembled receptor complexes and monomers, while this equilibrium is shifted toward a higher ratio of assembled complexes in the plasma membrane. Overlay images of the fluorescence intensity distributions of coexpressed receptor chains showed that their relative concentration ratios were different at various regions of the ER or the Golgi. After their synthesis by distinct ribosomes at different parts of the ER, receptor chains start to mix in the endomembrane systems by diffusion; however, their mixing is obviously incomplete. Besides areas where both receptor chains are present at similar concentrations, there are also regions where 1 of the 2 receptor chains is dominantly present (green and red areas). In contrast, in the plasma membrane, the mixing of the receptor chains at the few-hundred-nanometer scale is more perfect as seen from the more uniform yellow/orange color of the merged images. The values of the Pearson’s correlation coefficient, a measure of colocalization at this distance scale, were higher in the plasma membrane than at the ER/Golgi for all studied pairs of receptor chains.
We may speculate about further possible reasons for this discrepancy. The IL-2Rα chain contains 2 N-linked and several O-linked glycosylation sites (50, 51), whereas IL-15Rα has 1 N-linked and several O-linked sites (52). N- and O-linked glycosylation occur in the ER and the Golgi, respectively. Partially glycosylated proteins might have a different affinity to each other than in a fully glycosylated state, which may result in a different degree of association at different cellular localizations. Another possibility is that the lipid environment in the ER/Golgi may differ from that in the plasma membrane, which may also affect interactions between the receptor chains. We have shown earlier that the cholesterol content of the plasma membrane, probably due to its contribution to the integrity of lipid rafts, plays an important role in the distribution and function of IL-2R in Kit225 T lymphoma cells. Cholesterol depletion significantly reduced the efficiency of IL-2 signaling and made the originally patchy, clustered distribution of IL-2R more diffuse in the membrane (36, 53). It also reduced interactions between IL-2R subunits and MHC I and II glycoproteins coexpressed in lipid rafts. In the ER, the correct folding of membrane proteins undergoes quality control (54); binding of chaperones and cochaperones to the receptor chains may also interfere with receptor preassembly.
In addition to heteromeric complexes, different homomeric complexes of the IL-2/15R subunits have also been reported: IL-2Rα oligomers (26) and IL-2/15Rβ dimers (27) were found in the membrane with as-yet-undefined functions, and γc was shown to form trimers in vitro (28). Our FRET analysis indicated that γc dimers/oligomers were also present in the ER and the Golgi. Our SPIM-FCCS data corroborated the stable association of γc complexes and their joint diffusion in the cytoplasm; in these latter experiments, the ER and the Golgi were not discerned. Our SPIM-FCCS measurements also proved that γc subunits were mobile, similar to Golgi-resident proteins according to a fluorescence recovery after photobleaching study (55) and an FCS study showing their anomalous subdiffusion (56). Our analysis demonstrated that γc-chains had a fast and a slow diffusing subpopulation, but the dimeric/oligomeric form belonged fully to the slow species. The diffusion coefficient of this complex (∼0.1 μm2/s) was similar to that measured in the plasma membrane for IL-2Rα and IL-15Rα subunits (37, 57), suggesting diffusion in an intracellular membrane. The diffusion coefficient of the fast fraction of the monomeric γc was 2 orders of magnitude higher, similar to that of the soluble controls: EGFP, mCherry, and the EGFP-mCherry fusion (Fig. 7G). We may speculate that a fraction of the γc-chains could be in a non–membrane-bound form; e.g., some misfolded receptor chains could be bound to a chaperone complex diffusing in the cytoplasm between the cisternae toward proteasomal degradation. This could also contribute to the lower values of FRET efficiency measured between receptor subunits in the cytoplasm relative to the plasma membrane.
Based on our FRET data, combined with results gained about the evasion from antibody-mediated block of IL-2–induced proliferation in cells synthesizing their own IL-2, we put forward the possibility of an intracellular autocrine signaling mechanism that utilizes intracellular IL-2 binding to preassembled receptors along the secretory pathway. This mechanism has clinical implications regarding antibody therapies targeting receptors in the plasma membrane.
Materials and Methods
Plasmid Construction.
Plasmids coding for IL-2/15R subunits tagged at the N or C termini with EGFP or mCherry, for control samples (EGFP-mCherry fusion, EGFP and mCherry, mCherry-GPI), and for ER/Golgi markers (target sequences of Sac1 and giantin tagged with TagBFP) are described in SI Appendix, Supplementary Materials.
Cell Culture, Transient and Stable Transfection.
Human cervix carcinoma (HeLa) cells, which do not express any of the IL-2/15R chains, were cultured in RPMI 1640 (Sigma-Aldrich) supplemented with 10% (vol/vol) FBS, l-glutamine, and gentamycin. ED40515(+) and ATL55T+ cells are human, IL-2–dependent ATL cell lines, which were maintained in RPMI medium 1640 containing 10% heat-inactivated FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, and 100 U/mL recombinant human IL-2. The cytokine-independent IL-2–transfected cells, ED40515(+)/IL-2 and ATL55T+/IL-2, were maintained in the same medium as above in the absence of IL-2. All cells were grown in 5% CO2 humidified atmosphere. Transient transfections of HeLa cells with fluorescent protein-tagged receptor subunits and ER/Golgi markers, generation of HeLa cells stably expressing IL-2Rα, IL-2/15Rβ, or γc-chains (referred to as HeLa-IL-2Rα, HeLa-β, and HeLa-γc) and of ED40515(+)/IL-2 and ATL55T+/IL-2 cells producing IL-2 are described in SI Appendix.
Cell Proliferation Assay.
The effect of antibodies to IL-2Rα (HAT), to IL-2/15Rβ (Mikβ1), or to IL-2 or their combination was assayed on the proliferation of the cytokine-dependent ATL cell lines ED40515(+) and ATL55+ or these cells transfected with IL-2 [ED40515(+)/IL-2 and ATL55T+/IL-2]. Aliquots (1 × 104) of these cells were seeded in 96-well culture plates in medium containing IL-2 (100 U/mL) for the cytokine-dependent nontransfected cells and without IL-2 for the cytokine-dependent IL-2–transfected cells. The cells were then incubated for 3 d with the indicated antibody doses of 10 μg/mL for a single antibody directed against directed against IL-2 or a receptor chain or 5 μg/mL each when used in combination. Cells were pulsed with 1 µCi (0.037 MBq) of 3H-thymidine (PerkinElmer) during the last 6 h of the cultures. Cells were then harvested and counted in a MicroBeta2 Microplate Counter (PerkinElmer). The value of percent proliferation was calculated as (cpm of proliferation with antibody) divided by (cpm of proliferation without antibody) × 100%. These assays were performed in triplicate.
Immunofluorescent Labeling.
Stably transfected HeLa cells were labeled with mouse monoclonal antibodies conjugated with Alexa Fluor 546 (Thermo Fisher Scientific) to detect the plasma membrane expression of untagged receptor subunits (SI Appendix, Fig. S4). For details, see SI Appendix.
Determination of FRET Efficiency and Colocalization by Confocal Microscopy.
Molecular associations of the receptor chains at the 2- to 10-nm level were assessed by FRET microscopy on a pixel-by-pixel basis (44, 46) using LSM 880 (Carl Zeiss) or TCS SP5 II (Leica Microsystems) confocal microscopes. The average FRET efficiency in a pixel or a cell depends on the relative number of donor (EGFP)- and acceptor (mCherry)-tagged receptor subunits. The positive control (EGFP-mCherry fusion) expresses the donor and the acceptor in equal quantities, allowing us to determine the acceptor-to-donor molecular ratios in other samples also as described (46). In our analyses, we used cells where this ratio was between 1 and 3.
We used target sequences of Sac1 (ER marker) or giantin (Golgi marker) tagged with TagBFP (excited at 405 nm) to identify these cellular organelles. Thus, by selecting pixels where TagBFP fluorescence was above a threshold, we could evaluate FRET data in an organelle-specific manner. The advantage of using TagBFP, a blue-emitting fluorescent protein, as a third marker is that it does not interfere with the FRET measurement between the green and red donor–acceptor pair. Data analysis was carried out using the RiFRET plugin (41) written for the FiJi software.
Colocalization of receptor chains in different organelles with a few-hundred-nanometer resolution was assessed by calculating the Pearson’s correlation coefficients between the pixel intensities of the green (EGFP) and red (mCherry) detection channels of confocal images as described in ref. 53. As a positive control, the EGFP-mCherry fusion protein was used. As a negative control, the pixels of the images of all samples were randomized 1,000 times, and the correlation coefficients of these randomized images were calculated (58). Further details of FRET and colocalization analyses are described in SI Appendix.
Determination of the Comobility of γ-Chains by SPIM-FCCS.
The comobility of receptor subunits was assessed by SPIM-FCCS measurements, also called imaging FCCS. This method is capable of demonstrating the stable codiffusion of fluorescent particles within the observation time window (the diffusion time of the particles across the detection volume element). For studying the homodimerization of γc subunits in HeLa cells, we used γc-EGFP + γc-mCherry cotransfection. Measurements were performed with a SPIM setup built in-house, described in ref. 59; for details, see SI Appendix. Briefly, selected cells mounted on a coverslip were illuminated by 2 overlapping, 1.3-μm-thick light sheets from 491- and 561-nm lasers to excite EGFP and mCherry. Fluorescence was collected perpendicular to the light sheet by a water-immersion objective, split to a green and a red component, and projected side by side onto a 128 × 128-pixel EMCCD camera. For each cell, we acquired 100,000 frames at a rate of 2,000 frames/s. From the time series of fluorescence images, the ACF of each channel as well as their CCF were calculated for every pixel using the QuickFit 3 software (60). We made a global fit for the ACFs and CCFs within every pixel as described in ref. 61. For the EGFP-mCherry fusion and EGFP + mCherry samples, we used the equation for 2 normally diffusing components for both the ACFs and the CCF. The ACFs of the γc–γc sample were also fitted with the 2-component model, whereas its CCF, with a single diffusing component. The fit yielded the following parameters: the concentration c of each species χ, ρχ, its fractional amplitude, Dχ, its diffusion coefficient, and the green–red dimer fractions. Cells that moved during the measurement were excluded from analysis. During the evaluation, first we masked the fluorescence images with intensity thresholding to exclude pixels outside the cells and without fluorophore presence.
As a model-independent approximation of the fraction of molecules in a green–red complex, i.e., the fraction of interacting proteins, we also calculated the relative amplitude of the CCF to the ACF, denoted RCCF, for the selected pixels. The dynamic range of the RCCF ratio is defined by values derived for the negative control (coexpressed EGFP and mCherry) and the positive control (EGFP-mCherry fusion protein). We only considered pixels with a value of −1 < RCCF < 1.
Western Blot from Isolated Golgi.
Golgi compartment was isolated from ATL cells [ED40515(+) WT cells IL-2–starved for 48 h and from ED40515(+)/IL-2 cells] by ultracentrifugation, and phosphorylation of IL-2 signaling elements (Jak kinases, γc subunit) was detected by Western blot. For details, see SI Appendix.
Statistical Analysis.
Student’s t tests with Holm–Sidak correction for multiple comparisons were performed with GraphPad Prism software (GraphPad). Data indicate mean ± SD in all bar graphs. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Drs. F. Bestvater and D. Krunic for their help at the Light Microscopy Core Facility of the German Cancer Research Center (Heidelberg, Germany); Dr. B. Rehó, L. Lau, and F. Taheri for help in SPIM-FCCS data acquisition and analysis; A. Fekete for useful discussions; and E. Nagy for excellent technical assistance. This work was funded by Short-Term Fellowship from the European Federation of Immunological Societies and Immunology Letters (to J.V.); the Intramural Research Program of the National Cancer Institute, NIH (to T.A.W.); GINOP-2.3.2-15-2016-00026, GINOP-2.3.3-15-2016-00003, GINOP-2.3.3-15-2016-00030, and NN129371 from the National Research, Development and Innovation Office, Hungary (to G.V.); EFOP-3.6.3-VEKOP-16-2017-00009 co-financed by the European Union and the European Social Fund (to A.B.); and the German Academic Exchange Service and the Tempus Public Foundation (273478) (to G.V. and K.T.). All experiments have been conducted according to the principles expressed in the Declaration of Helsinki.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901382116/-/DCSupplemental.
References
- 1.Rosenberg S. A., Raising the bar: The curative potential of human cancer immunotherapy. Sci. Transl. Med. 4, 127ps8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soiffer R. J., et al. , Administration of R24 monoclonal antibody and low-dose interleukin 2 for malignant melanoma. Clin. Cancer Res. 3, 17–24 (1997). [PubMed] [Google Scholar]
- 3.Waldmann T. A., Anti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: A 25-year personal odyssey. J. Clin. Immunol. 27, 1–18 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz J. L., et al. , Safety, efficacy, and pharmacokinetics/pharmacodynamics of daclizumab (anti-CD25) in patients with adult T-cell leukemia/lymphoma. Clin. Immunol. 155, 176–187 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nashan B., Light S., Hardie I. R., Lin A., Johnson J. R.; Daclizumab Double Therapy Study Group , Reduction of acute renal allograft rejection by daclizumab. Transplantation 67, 110–115 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Lycke J., Monoclonal antibody therapies for the treatment of relapsing-remitting multiple sclerosis: Differentiating mechanisms and clinical outcomes. Ther. Adv. Neurol. Disorder. 8, 274–293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tendler C. L., et al. , Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: Pathogenic implications and a rationale for immunotherapy. Proc. Natl. Acad. Sci. U.S.A. 87, 5218–5222 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldmann T. A., Dubois S., Tagaya Y., Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: Implications for immunotherapy. Immunity 14, 105–110 (2001). [PubMed] [Google Scholar]
- 9.Giri J. G., et al. , Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 13, 2822–2830 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X., Lupardus P., Laporte S. L., Garcia K. C., Structural biology of shared cytokine receptors. Annu. Rev. Immunol. 27, 29–60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson B. H., Lord J. D., Greenberg P. D., Cytoplasmic domains of the interleukin-2 receptor beta and gamma chains mediate the signal for T-cell proliferation. Nature 369, 333–336 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y., et al. , Heterodimerization of the IL-2 receptor beta- and gamma-chain cytoplasmic domains is required for signalling. Nature 369, 330–333 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Dubois S., Mariner J., Waldmann T. A., Tagaya Y., IL-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity 17, 537–547 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Kondo M., et al. , Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science 262, 1874–1877 (1993). [DOI] [PubMed] [Google Scholar]
- 15.Noguchi M., et al. , Interleukin-2 receptor gamma chain: A functional component of the interleukin-7 receptor. Science 262, 1877–1880 (1993). [DOI] [PubMed] [Google Scholar]
- 16.Russell S. M., et al. , Interleukin-2 receptor gamma chain: A functional component of the interleukin-4 receptor. Science 262, 1880–1883 (1993). [DOI] [PubMed] [Google Scholar]
- 17.Kimura Y., et al. , Sharing of the IL-2 receptor gamma chain with the functional IL-9 receptor complex. Int. Immunol. 7, 115–120 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Asao H., et al. , Cutting edge: The common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 167, 1–5 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Becker T. C., et al. , Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195, 1541–1548 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontenot J. D., Rasmussen J. P., Gavin M. A., Rudensky A. Y., A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Marks-Konczalik J., et al. , IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 97, 11445–11450 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldmann T. A., The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Zhang X., Sun S., Hwang I., Tough D. F., Sprent J., Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Bodnár A., et al. , A biophysical approach to IL-2 and IL-15 receptor function: Localization, conformation and interactions. Immunol. Lett. 116, 117–125 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Damjanovich S., et al. , Preassembly of interleukin 2 (IL-2) receptor subunits on resting Kit 225 K6 T cells and their modulation by IL-2, IL-7, and IL-15: A fluorescence resonance energy transfer study. Proc. Natl. Acad. Sci. U.S.A. 94, 13134–13139 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eicher D. M., Damjanovich S., Waldmann T. A., Oligomerization of IL-2Ralpha. Cytokine 17, 82–90 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Pillet A. H., et al. , Human IL-Rbeta chains form IL-2 binding homodimers. Eur. Cytokine Netw. 19, 49–59 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Stauber D. J., Debler E. W., Horton P. A., Smith K. A., Wilson I. A., Crystal structure of the IL-2 signaling complex: Paradigm for a heterotrimeric cytokine receptor. Proc. Natl. Acad. Sci. U.S.A. 103, 2788–2793 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malka Y., et al. , Ligand-independent homomeric and heteromeric complexes between interleukin-2 or -9 receptor subunits and the gamma chain. J. Biol. Chem. 283, 33569–33577 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pillet A. H., et al. , IL-2 induces conformational changes in its preassembled receptor core, which then migrates in lipid raft and binds to the cytoskeleton meshwork. J. Mol. Biol. 403, 671–692 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Vámosi G., et al. , IL-2 and IL-15 receptor alpha-subunits are coexpressed in a supramolecular receptor cluster in lipid rafts of T cells. Proc. Natl. Acad. Sci. U.S.A. 101, 11082–11087 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hémar A., et al. , Endocytosis of interleukin 2 receptors in human T lymphocytes: Distinct intracellular localization and fate of the receptor alpha, beta, and gamma chains. J. Cell Biol. 129, 55–64 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurgonaite K., et al. , Essential role of endocytosis for interleukin-4-receptor-mediated JAK/STAT signalling. J. Cell Sci. 128, 3781–3795 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Hakimi J., et al. , Humanized Mik beta 1, a humanized antibody to the IL-2 receptor beta-chain that acts synergistically with humanized anti-TAC. J. Immunol. 151, 1075–1085 (1993). [PubMed] [Google Scholar]
- 35.Sandoval P. C., et al. , Proteome-wide measurement of protein half-lives and translation rates in vasopressin-sensitive collecting duct cells. J. Am. Soc. Nephrol. 24, 1793–1805 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matkó J., et al. , GPI-microdomains (membrane rafts) and signaling of the multi-chain interleukin-2 receptor in human lymphoma/leukemia T cell lines. Eur. J. Biochem. 269, 1199–1208 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Mocsár G., et al. , MHC I expression regulates co-clustering and mobility of interleukin-2 and -15 receptors in T cells. Biophys. J. 111, 100–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehky T. J., et al. , Reduction in HTLV-I proviral load and spontaneous lymphoproliferation in HTLV-I-associated myelopathy/tropical spastic paraparesis patients treated with humanized anti-Tac. Ann. Neurol. 44, 942–947 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Brameshuber M., et al. , Monomeric TCRs drive T cell antigen recognition. Nat. Immunol. 19, 487–496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renz M., Daniels B. R., Vámosi G., Arias I. M., Lippincott-Schwartz J., Plasticity of the asialoglycoprotein receptor deciphered by ensemble FRET imaging and single-molecule counting PALM imaging. Proc. Natl. Acad. Sci. U.S.A. 109, E2989–E2997 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roszik J., Lisboa D., Szöllosi J., Vereb G., Evaluation of intensity-based ratiometric FRET in image cytometry—approaches and a software solution. Cytometry A 75, 761–767 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Nizsalóczki E., et al. , Distinct spatial relationship of the interleukin-9 receptor with interleukin-2 receptor and major histocompatibility complex glycoproteins in human T lymphoma cells. Chemphyschem 15, 3969–3978 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nizsalóczki E., et al. , Minimum degree of overlap between IL-9R and IL-2R on human T lymphoma cells: A quantitative CLSM and FRET analysis. Cytometry A 93, 1106–1117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szalóki N., et al. , High throughput FRET analysis of protein-protein interactions by slide-based imaging laser scanning cytometry. Cytometry A 83, 818–829 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Szalóki N., Krieger J. W., Komáromi I., Tóth K., Vámosi G., Evidence for homodimerization of the c-Fos transcription factor in live cells revealed by fluorescence microscopy and computer modeling. Mol. Cell. Biol. 35, 3785–3798 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vámosi G., et al. , Conformation of the c-Fos/c-Jun complex in vivo: A combined FRET, FCCS, and MD-modeling study. Biophys. J. 94, 2859–2868 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kenworthy A. K., Edidin M., Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 Å using imaging fluorescence resonance energy transfer. J. Cell Biol. 142, 69–84 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam A. J., et al. , Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 9, 1005–1012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dale R. E., Eisinger J., Blumberg W. E., The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys. J. 26, 161–193 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greene W. C., Depper J. M., Krönke M., Leonard W. J., The human interleukin-2 receptor. J. Cell Sci. Suppl. 3, 97–106 (1985). [DOI] [PubMed] [Google Scholar]
- 51.Leonard W. J., et al. , Structure of the human interleukin-2 receptor gene. Science 230, 633–639 (1985). [DOI] [PubMed] [Google Scholar]
- 52.Dubois S., et al. , Natural splicing of exon 2 of human interleukin-15 receptor alpha-chain mRNA results in a shortened form with a distinct pattern of expression. J. Biol. Chem. 274, 26978–26984 (1999). [DOI] [PubMed] [Google Scholar]
- 53.Vereb G., et al. , Cholesterol-dependent clustering of IL-2Ralpha and its colocalization with HLA and CD48 on T lymphoma cells suggest their functional association with lipid rafts. Proc. Natl. Acad. Sci. U.S.A. 97, 6013–6018 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Araki K., Nagata K., Protein folding and quality control in the ER. Cold Spring Harb. Perspect. Biol. 3, a007526 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole N. B., et al. , Diffusional mobility of Golgi proteins in membranes of living cells. Science 273, 797–801 (1996). [DOI] [PubMed] [Google Scholar]
- 56.Weiss M., Hashimoto H., Nilsson T., Anomalous protein diffusion in living cells as seen by fluorescence correlation spectroscopy. Biophys. J. 84, 4043–4052 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagy É., et al. , Membrane potential distinctly modulates mobility and signaling of IL-2 and IL-15 receptors in T cells. Biophys. J. 114, 2473–2482 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costes S. V., et al. , Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys. J. 86, 3993–4003 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krieger J. W., et al. , Imaging fluorescence (cross-) correlation spectroscopy in live cells and organisms. Nat. Protoc. 10, 1948–1974 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Krieger J., Langowski J., QuickFit 3.0. https://www.dkfz.de/Macromol/quickfit/. Accessed 29 October 2015.
- 61.Krieger J. W., Singh A. P., Garbe C. S., Wohland T., Langowski J., Dual-color fluorescence cross-correlation spectroscopy on a single plane illumination microscope (SPIM-FCCS). Opt. Express 22, 2358–2375 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.