Abstract
Aim: Several members of secreted frizzled-related protein (SFRP) are involved in the process of myocardial ischemia-reperfusion injury. However, little is known about the role of SFRP5 in patients with acute ST-segment elevation myocardial infarction (STEMI).
Methods: In this cross-sectional study, 85 patients with first-time anterior STEMI who underwent timely primary percutaneous coronary intervention (PCI) and 35 patients without coronary artery disease (CAD) were enrolled. Serum SFRP5 levels were measured using an enzyme-linked immunosorbent assay kit. Patients with STEMI were divided into low-SFRP5 and high-SFRP5 groups according to their median baseline serum SFRP5 levels. To evaluate cardiac function and structure after infarction, the left ventricular ejection fraction (LVEF) and left ventricular end-diastolic volume (LVEDV) were measured using echocardiography. The associations between changes in LVEF and reduced LVEF (≤ 50%) and clinical variables were determined by univariate and multivariate analyses.
Results: Baseline serum SFRP5 levels were significantly higher in patients with STEMI than in those without CAD (23.3 ng/mL vs 19.8 ng/mL, P = 0.008), although they decreased over time. Also, baseline serum SFRP5 levels were inversely correlated with peak hypersensitive cardiac troponin I (hs-cTnI) levels (r = −0.234, P = 0.025) and peak hypersensitive C-reactive protein (hs-CRP) levels (r = −0.262, P = 0.015). A multivariate linear regression model showed that changes in LVEF were positively correlated with serum SFRP5 levels at baseline (β = 0.249, 95% confidence interval (CI) 0.018–0.245, P = 0.024) and 24 h after admission (β = 0.220, 95% CI 0.003–0.264, P = 0.045). At 3 months, LVEF in patients with high SFRP5 levels was significantly improved over baseline [(60.8 ± 7.1) % vs (56.1 ± 7.5) %, P = 0.001]. LVEF was also significantly higher in patients with high SFRP5 levels than in those with low at the 3-month follow-up [(60.8 ± 7.1) % vs (56.8 ± 8.9) %, P = 0.028]. Consequently, high serum SFRP5 levels at baseline were associated with a decreased risk of reduced LVEF at 3 months, independent of peak hs-cTnI and baseline cardiac function (hazard ratio 0.190, 95% CI 0.036–0.996; P = 0.049).
Conclusions: High serum SFRP5 levels measured during the acute phase of STEMI were significantly associated with promoting myocardial recovery at an early phase following primary PCI, suggesting that SFRP5 is a potential therapeutic target in acute STEMI.
Keywords: Secreted frizzled-related protein 5, Adipokine, ST-segment elevation myocardial infarction, Left ventricular ejection fraction, Inflammation
Aim
Adipocytokine is recognized as a bridge between obesity-related metabolic disorders and cardiovascular disease. The unbalanced production of pro-inflammatory and anti-inflammatory adipocytokines by dysfunctional adipose tissue in obese persons contributes to a chronic low-grade inflammatory state and thus affects the initiation and progression of coronary artery disease (CAD)1–3). Acute ST-segment elevation myocardial infarction (STEMI) is more complicated as the secretion of adipocytokines is markedly perturbed, a condition that has a complex role in myocardial ischemia/reperfusion (I/R) injury, postinfarction remodeling, and subsequently heart failure (HF)4–6). Therefore, it is necessary to elucidate the links between adipocytokines and the adaptive and maladaptive myocardial healing processes because of the substantial mortality and morbidity associated with acute STEMI7, 8).
Secreted frizzled-related protein (SFRP), a Wnt antagonist, consists of five members (SFRP1–SFRP5)9). It has been reported that the SFRP–Wnt modulatory axis is involved in the process of myocardial I/R injury. Genetic overexpression of SFRP1, a modulator of the Wnt/Fzd pathway, has been shown to reduce scar size and improve cardiac hemodynamics in a myocardial infarction (MI) model10). Also, SFRP2 has been shown to promote myocardial healing following MI via inhibition of Wnt and bone morphogenetic protein signaling11).
SFRP5, an adipocyte-specific secretory protein, is highly expressed in white adipose tissue. It exerts an anti-inflammatory effect on lipid metabolism, inflammation, and type 2 diabetes mellitus (T2DM) via inhibition of the Wnt signaling pathway12–14). Recently, the large-scale KORA F4 study confirmed the metaboloprotective role of SFRP5, which showed significantly inverse correlations between serum SFRP5 levels and multiple metabolic risk factors after dedicated statistical adjustments15). The cardioprotective role of SFPR5 in the setting of myocardial I/R injury, however, has never been determined. Therefore, in this study, we investigated serum SFRP5 levels in patients with acute STEMI who underwent successful primary percutaneous coronary intervention (PCI) and determined its association with early-stage improvement of cardiac function following MI.
Methods
Subjects
Between June 2017 and May 2018, patients presenting with first-time, acute, anterior STEMI were consecutively screened for the following inclusion criteria: age > 18 years; symptom duration ≤ 6 h; electrocardiography showed consistent anterior ST elevation; emergent coronary angiography showed that the infarct-related artery (IRA) was only the left anterior descending artery; and primary PCI had been performed successfully. We also consecutively enrolled a control group of individuals with suspected CAD, whose elective coronary angiography showed no obvious coronary artery stenosis. Exclusion criteria were patients aged > 80 years, prior history of MI, prior coronary artery revascularization, thrombolytic therapy prior to primary PCI, cardiogenic shock, liver or renal failure, chronic inflammation disease, pharmacological glucocorticoid or immunosuppressive therapy, and/or refusal to give written informed consent. Finally, 85 eligible patients were enrolled in the STEMI group. Baseline demographics and clinical data were obtained from the hospital case records. Body mass index (BMI) was calculated as the patient's weight divided by the height squared (kg/m2).
The study protocol adhered to the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Anzhen Hospital of Capital Medical University. Written informed consent was obtained from each patient before enrollment.
Blood Sample Measurement
For patients in the STEMI group, blood samples were obtained at hospital admission and 24 h and 72 h after admission. For patients in the control group, blood samples were drawn after overnight fasting. Venous blood samples were collected in sodium heparin Vacutainers (Becton-Dickinson, Franklin Lakes, BJ, USA), and serum samples were stored at −80°C after centrifugation. Routine blood, urine, and biochemical tests, as well as markers of myocardial injury, were measured in the central laboratory of Beijng Anzhen Hospital of Capital Medical University. Peak hypersensitive cardiac Troponin I (hs-cTnI) and peak hypersensitive C-reactive protein (hs-CRP) levels were defined as the maximum value measured during hospitalization.
Serum SFRP5 concentrations were detected by a commercially available enzyme-linked immunosorbent assay kit (Shanghai BlueGene Biotech Co., Ltd., Shanghai, China) following the manufacturer's instructions. Both intra-assay and inter-assay coefficients of variation were < 5%. All samples were measured in duplicate.
Echocardiography
Left ventricular ejection fraction (LVEF) and left ventricular end-diastolic volume (LVEDV) within the first 24 h after admission and 3 months later were measured by the modified Simpson's equation with a GE ViVid E7 ultrasonography machine (GE Healthcare, Piscataway, NJ, USA). Reduced LVEF was defined as LVEF ≤ 50%.
Procedure and Periprocedural Management
Diagnostic coronary angiography, primary PCI, and periprocedural management were applied according to the current guidelines16). All participants underwent coronary angiography emergently or electively. CAD was defined as the presence of stenosis of > 50% of the luminal diameter of at least one of the major epicardial coronary arteries. For patients in the STEMI group, judgment about the IRA was based on electrocardiography, echocardiography, and coronary angiography results. In this study, only IRA was treated emergently, with the other diseased vessels managed with elective PCI after 3 months if indicated. All implanted stents were second-generation, drug-eluting stents. The use of thrombus aspiration or glycoprotein IIb/IIIa inhibitor was left to the operator's discretion. Total ischemia time was defined as the period from the onset of chest pain to IRA reopening.
Statistical Analysis
Continuous data were expressed as the mean ± standard deviation or the median (lower quartile, upper quartile) where indicated. Mean and median values were compared using Student's t-test and the Mann–Whitney U test, respectively. Categorical data were expressed as percentages and analyzed using a χ2 test. Spearman's correlation testing was performed between serum SFRP5 levels and clinical parameters. The associations between changes in LVEF and other variables were assessed using both univariate and multivariate linear regression analyzes. In addition, the associations between reduced LVEF and variables were determined by univariate and logistic regression analysis. All statistical analyzes were performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). A value of P < 0.05 was considered to indicate statistical significance.
Results
Baseline and Procedural Characteristics of the Study Population
During the study period, a total of 190 patients with STEMI were consecutively screened, and 85 (44.7%) eligible patients with first-time anterior STEMI who underwent timely primary PCI were enrolled in this study. We also consecutively enrolled 35 individuals without CAD as a control group. The mean age of the patients at the first presentation of acute anterior STEMI was 56 years, of which 76% were men, and the proportion of self-reported CAD was 21% (Table 1). Specifically, compared with the controls, patients with STEMI were more likely to be male and smokers and have hypertension and low LVEF. Other baseline characteristics and medications were similar between the two groups. As shown in Tables 2 and 3, preinfarction angina accounted for 30% of STEMI patients, 94% of STEMI patients presented with Killip classes I–II acute HF, and half of the STEMI patients had multivessel disease, which was higher than the prevalence of self-reported CAD. Intracoronary imaging was not routinely used; second-generation, drug-eluting stents were implanted in 99% of patients; 92% of patients had a thrombolysis in MI (TIMI) flow of ≥ 2 after the procedure; and the overall median ischemia time was 4.6 h, which indicated a timely and successful primary PCI.
Table 1. Baseline characteristics of participants in the Control and STEMI groups.
| Variables | Control group (N = 35) | STEMI group (N = 85) | P value |
|---|---|---|---|
| Age (years) | 52.3 ± 9.9 | 55.7 ± 11.8 | 0.133 |
| Male | 21 (60.0) | 65 (76.5) | 0.069 |
| BMI (kg/m2) | 24.9 ± 3.5 | 26.0 ± 3.4 | 0.137 |
| Hypertension | 6 (17.1) | 45 (52.9) | < 0.001 |
| Hypercholesteromia | 5 (14.3) | 24 (28.2) | 0.105 |
| T2DM | 3 (8.6) | 17 (20.0) | 0.127 |
| Smoking | 7 (20.0) | 59 (69.4) | < 0.001 |
| CAD | 0 | 18 (21.2) | - |
| PAD or stroke | 4 (11.4) | 19 (22.4) | 0.167 |
| LVEF (%) | 61.5 ± 5.2 | 56.4 ± 7.0 | 0.001 |
| SFRP5 (ng/ml) | 19.8 (16.8, 21.8) | 23.3 (17.8, t36.8) | 0.008 |
| Medications (admission) | |||
| Aspirin | 4 (11.4) | 19 (22.4) | 0.167 |
| P2Y12 inhibitor | 0 | 2 (2.4) | - |
| Beta-blocker | 2 (5.7) | 8 (9.4) | 0.722 |
| Statin | 3 (8.6) | 14 (16.5) | 0.389 |
| ACEI/ARB | 3 (8.6) | 19 (22.4) | 0.076 |
STEMI, ST-segment elevation myocardial infarction; BMI, body mass index; T2DM, type 2 diabetes mellitus; CAD, coronary artery disease; PAD, peripheral arterial disease; LVEF, left ventricular ejection fraction; SFRP5, serum secreted frizzled-related protein 5; ACEI, angiotensin-converting enzyme inhibitor; ARB, Angiotensin II receptor blocker.
Table 2. Baseline characteristics of STEMI patients according to median baseline serum SFRP5 level.
| Variables | Total (N = 85) | Low-SFRP5 Group (N = 42) | High-SFRP5 Group (N = 43) | P value |
|---|---|---|---|---|
| Age (years) | 55.7 ± 11.8 | 57.8 ± 10.2 | 53.6 ± 13.0 | 0.099 |
| Male | 65 (76.5) | 30 (71.4) | 35 (81.4) | 0.279 |
| BMI (kg/m2) | 26.0 ± 3.4 | 25.3 ± 3.3 | 26.7 ± 3.5 | 0.065 |
| Hypertension | 45 (52.9) | 23 (54.8) | 22 (51.2) | 0.740 |
| Hypercholesteromia | 24 (28.2) | 13 (31.0) | 11 (25.6) | 0.582 |
| T2DM | 17 (20.0) | 9 (21.4) | 8 (18.6) | 0.745 |
| Smoking | 59 (69.4) | 26 (61.9) | 33 (76.7) | 0.138 |
| CAD | 18 (21.2) | 8 (19.0) | 10 (23.3) | 0.635 |
| Pre-infarction angina | 26 (30.6) | 12 (28.6) | 14 (32.6) | 0.815 |
| PAD or stroke | 19 (22.4) | 12 (28.6) | 7 (16.3) | 0.174 |
| LVEF (%) | 56.4 ± 7.0 | 56.7 ± 6.5 | 56.1 ± 7.5 | 0.942 |
| LVEDV (ml) | 45.6 ± 3.7 | 45.5 ± 4.3 | 45.7 ± 3.0 | 0.740 |
| Acute heart failure (Killip classification) | 0.598 | |||
| Class I | 45 (52.9) | 24 (57.1) | 21 (48.8) | |
| Class II | 35 (41.2) | 15 (35.7) | 20 (46.5) | |
| Class III | 5 (5.9) | 3 (7.1) | 2 (4.7) | |
| Class IV | 0 | - | - | |
| SFRP5 (ng/ml) | 23.3 (17.8, 36.8) | 17.8 (13.6, 20.7) | 36.8 (27.0, 48.6) | < 0.01 |
| Peak hs-cTnI (pg/ml) | 34.5 (23.6, 46.6) | 39.1 (30.0, 52.1) | 29.1 (22.8, 42.4) | 0.024 |
| Peak hs-CRP (mg/L) | 23.0 (17.5, 29.5) | 27.3 (18.6, 32.1) | 21.0 (15.4, 27.6) | 0.017 |
| Peak CK-MB (U/L) | 222.0 (120.0, 330.5) | 249.5 (151.3, 358.0) | 189.0 (98.0, 300.0) | 0.041 |
| Medications (Discharge) | ||||
| Aspirin | 85 (100.0) | 42 (100.0) | 43 (100.0) | - |
| P2Y12 inhibitor | 85 (100.0) | 42 (100.0) | 43 (100.0) | - |
| Beta-blocker | 75 (88.2) | 39 (92.9) | 36 (83.7) | 0.313 |
| Statin | 85 (100.0) | 42 (100.0) | 43 (100.0) | - |
| ACEI/ARB | 60 (70.6) | 29 (69.0) | 31 (72.1) | 0.758 |
STEMI, ST-segment elevation myocardial infarction; SFRP5, serum secreted frizzled-related protein 5; BMI, body mass index; T2DM, type 2 diabetes mellitus; CAD, coronary artery disease; PAD, peripheral arterial disease; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; hs-cTnI, hypersensitive cardiac Troponin I; hs-CRP, hypersensitive C reactive protein; CK-MB, creatine kinase MB; ACEI, angiotensin-converting enzyme inhibitor; ARB, Angiotensin II receptor blocker.
Table 3. Procedural characteristics of STEMI patients according to median baseline serum SFRP5 level.
| Variables | Total (N = 85) | Low-SFRP5 Group (N = 42) | High-SFRP5 Group (N = 43) | P value |
|---|---|---|---|---|
| Angiography | ||||
| No. of diseased vessels | 0.443 | |||
| 1-vessel disease | 45 (52.9) | 22 (52.4) | 23 (53.5) | |
| 2-vessel disease | 22 (25.9) | 9 (21.4) | 13 (30.2) | |
| 3-vessel disease | 18 (21.2) | 11 (26.2) | 7 (16.3) | |
| Location of culprit lesion | 0.952 | |||
| Proximal LAD | 32 (37.6) | 16 (38.1) | 16 (37.2) | |
| Middle LAD | 44 (51.8) | 21 (50.5) | 23 (53.5) | |
| Distal LAD | 9 (10.6) | 5 (11.9) | 4 (9.3) | |
| Pre-PCI TIMI flow | 0.346 | |||
| Grade 0 | 72 (84.7) | 33 (78.6) | 39 (90.7) | |
| Grade 1 | 10 (11.8) | 7 (16.7) | 3 (7.0) | |
| Grade 2 | 3 (3.5) | 2 (4.8) | 1 (2.3) | |
| Grade 3 | 0 | - | - | |
| Intervention therapy | ||||
| Total ischemic time (min) | 278 (194, 413) | 322 (200, 553) | 271 (190, 408) | 0.399 |
| Stent implantation | 84 (98.8) | 42 (100) | 42 (97.7) | 1.000 |
| 2nd-generation DES | 84 (98.8) | 42 (100) | 42 (97.7) | 1.000 |
| No. of stents per patient | 1.11 ± 0.31 | 1.10 ± 0.30 | 1.12 ± 0.33 | 1.000 |
| No. of stent diameter ≤ 2.5 mm | 5 (5.9) | 3 (7.1) | 2 (4.7) | 0.676 |
| Length of stent | 30 (24, 36) | 29 (25, 36) | 30 (24, 33) | 0.780 |
| Thrombus aspiration | 9 (10.6) | 4 (9.5) | 5 (11.6) | 1.000 |
| GP IIb/IIIa inhibitor | 54 (63.5) | 28 (66.7) | 26 (60.5) | 0.654 |
| Post-PCI TIMI flow ≥ 2 | 78 (91.8) | 38 (90.5) | 40 (93.0) | 0.713 |
STEMI, ST-segment elevation myocardial infarction; SFRP5, serum secreted frizzled-related protein 5; LAD, left anterior descending; PCI, percutaneous coronary intervention; TIMI: thrombolysis in myocardial infarction; DES, drug eluting stent; GP IIb/IIIa: Glycoprotein IIb/IIIa.
Serum SFRP5 Levels in STEMI Patients
We investigated serum SFRP5 levels in patients who presented with acute STEMI on admission and their changes over time. Compared with controls, STEMI patients had significantly higher baseline serum SFRP5 levels (23.3 ng/mL vs 19.8 ng/mL, P = 0.008) (Fig. 1A). Furthermore, serum SFRP5 levels in STEMI patients were consistently higher than those in the controls during the first 72 h after admission, but they declined gradually over time (Fig. 1B). We also found that baseline serum SFRP5 levels were negatively associated with BMI (r = −0.367, P = 0.030) and T2DM (r = −0.384, P = 0.023) in the control group, whereas these associations were not observed in the STEMI group.
Fig. 1.
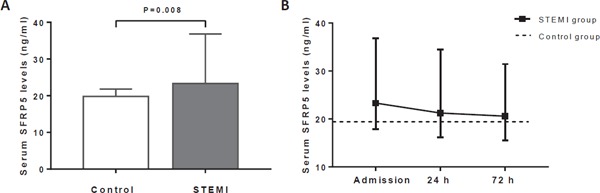
Serum SFRP5 levels in the STEMI and control groups at admission (A) and their changes over time (B). SFRP5, serum secreted frizzled-related protein 5; STEMI, ST-segment elevation myocardial infarction.
Serum SFRP5 Levels and Myocardial Injury and Inflammation during the I/R Process
To evaluate myocardial injury and systematic inflammation after MI, we used the indices of the peak serum hs-cTnI and peak serum hs-CRP levels, respectively. Patients in the STEMI group were divided into low-SFRP5 and high-SFRP5 groups according to their median baseline serum SFRP5 concentration (23.3 ng/ml). As shown in Tables 2 and 3, baseline and procedural characteristics were not significantly different between the two groups, whereas patients in the high-SFRP5 group had significantly lower peak hs-cTnI and peak hs-CRP levels than the low-SFRP5 group (peak hs-cTnI 29.1 pg/mL vs 39.1 pg/mL, respectively, P = 0.024; peak hs-CRP 21.0 mg/L vs 27.3 mg/L, respectively, P = 0.017). Furthermore, baseline serum SFRP5 levels were inversely correlated with the peak hs-cTnI levels (r = −0.234, P = 0.025) and the peak hs-CRP levels (r = −0.262, P = 0.015) (Fig. 2A, B).
Fig. 2.
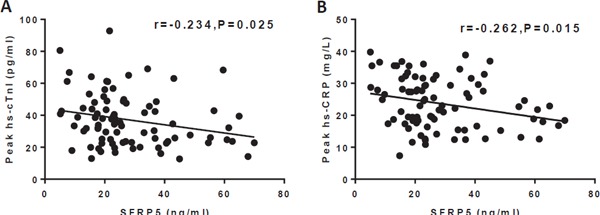
Associations of baseline serum SFRP5 levels with peak hs-cTnI levels (A) and peak hs-CRP levels (B). SFRP5, secreted frizzled-related protein 5; hs-cTnI, hypersensitive cardiac troponin I; hs-CRP, hypersensitive C-reactive protein.
Serum SFRP5 Levels and Left Ventricular Function and Structure after Infarction
At the 3-month follow-up, all participants were still alive and underwent cardiac echocardiography. To study the cardiac function alterations (Table 4), we found that changes in LVEF were positively correlated with the serum SFRP5 levels at baseline (β = 0.276, P = 0.011) and 24 h after admission (β = 0.216, P = 0.048), but they were not significantly correlated with serum SFRP5 levels 72 h after admission (β = 0.147, P = 0.180). The former two correlations were further confirmed in a multivariate linear regression model involving age, sex, T2DM, number of diseased vessels, TIMI flow (pre-PCI), and total ischemia time [admission: β = 0.249, 95% confidence interval (CI) 0.018–0.245, P = 0.024; 24 h after admission: β = 0.220, 95% CI 0.003–0.264, P = 0.045].
Table 4. Association between Changes in LVEF and variables using univariate and multivariate analysis.
| Variables | Univariate Analysis | Multivariate Analysis 1 | Multivariate Analysis 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age | −0.074 | −0.203, 0.100 | 0.501 | 0.015 | −0.142, 0.163 | 0.891 | −0.003 | −0.154, 0.149 | 0.975 |
| Female | −0.162 | −7.272, 1.026 | 0.138 | −0.044 | −5.376, 3.699 | 0.714 | −0.060 | −5.442, 3.191 | 0.605 |
| T2DM | −0.171 | −7.893, 0.893 | 0.117 | −0.111 | −6.891, 2.354 | 0.331 | −0.137 | −7.418, 1.802 | 0.229 |
| No. of diseased vessels | −0.140 | −3.631, 0.783 | 0.203 | −0.143 | −3.678, 0.758 | 0.194 | −0.150 | −3.764, 0.694 | 0.174 |
| TIMI flow (pre-PCI) | −0.199 | −7.132, 0.264 | 0.068 | −0.141 | −6.188, 1.300 | 0.197 | −0.140 | −6.213, 1.375 | 0.208 |
| Total ischemia time | −0.096 | −0.013, 0.005 | 0.382 | −0.146 | −0.015, 0.003 | 0.183 | −0.150 | −0.015, 0.003 | 0.175 |
| SFRP5 (admission) | 0.276 | 0.035, 0.256 | 0.011 | 0.249 | 0.018, 0.245 | 0.024 | |||
| SFRP5 (24 h after admission) | 0.216 | 0.001, 0.260 | 0.048 | 0.220 | 0.003, 0.264 | 0.045 | |||
| SFRP5 (72 h after admission) | 0.147 | −0.042, 0.219 | 0.180 | ||||||
LVEF, left ventricular ejection fraction; HR, hazard ratio; CI, confidence interval; T2DM, type 2 diabetes mellitus; TIMI: thrombolysis in myocardial infarction; PCI, percutaneous coronary intervention; SFRP5, serum secreted frizzled-related protein 5.
Consequently, at 3 months, the LVEF of STEMI patients in the high-SFRP5 group had significantly improved over baseline [(60.8 ± 7.1) % vs (56.1 ± 7.5) %, P = 0.001], whereas the LVEF of STEMI patients in the low-SFRP5 group was unchanged over time [(56.8 ± 8.9) % vs (56.7 ± 6.5) %, P = 0.555] (Fig. 3A). In addition, LVEF was significantly higher in STEMI patients in the high-SFRP5 group than in the low-SFRP5 group at the 3-month follow-up [(60.8 ± 7.1) % vs (56.8 ± 8.9) %, P = 0.028], although they were similar at baseline [(56.1 ± 7.5) % vs (56.7 ± 6.5) %, P = 0.942)]. In contrast, the improvement of LVEDV was not significant in STEMI patients with high SFRP5 levels at 3 months [high-SFRP5 group: baseline vs 3 months, (45.7 ± 3.0) mL vs (46.3 ± 4.1) mL, P = 0.474; 3-month follow-up: high-SFRP5 group vs low-SFRP5 group, (46.3 ± 4.1) mL vs (47.1 ± 4.3) mL, P = 0.416] (Fig. 3B).
Fig. 3.
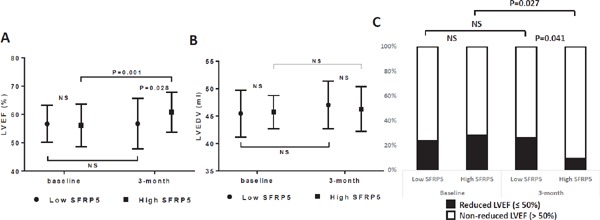
Cardiac function (LVEF) (A), cardiac structure (LVEDV) (B), and reduced LVEF (C) in STEMI patients in the low-SFRP5 and high- SFRP5 groups at baseline and at the 3-month follow-up. LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; NS, no significant difference; SFRP5, serum secreted frizzled-related protein 5; STEMI, ST-segment elevation myocardial infarction.
Finally, the proportion of reduced LVEF (≤ 50%) was significantly lower in STEMI patients in the high-SFRP5 group than in the low-SFRP5 group at 3 months (9.3% vs 26.2%, P = 0.041). It was also lower in STEMI patients with higher SFPR5 levels at 3 months than at baseline (9.3% vs 27.9%, P = 0.027) (Fig. 3C). In line with these findings, as shown in Table 5, high serum SFRP5 levels at baseline were associated with a decreased risk of having reduced LVEF at 3 months [hazard ratio (HR) 0.289, 95% CI 0.084–0.997; P = 0.049]. This association persisted after adjusting for age, sex, reduced LVEF at baseline, and peak hs-cTnI (HR 0.190, 95% CI 0.036–0.996; P = 0.049).
Table 5. Association between reduced LVEF (≤ 50%) at 3-month and variables using univariate and multivariate analysis.
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.044 | 0.992, 1.099 | 0.099 | 1.042 | 0.972, 1.116 | 0.245 |
| Female | 2.667 | 0.813, 8.743 | 0.105 | 2.982 | 0.660, 13.477 | 0.156 |
| Reduced LVEF (≤ 50%) at baseline | 9.667 | 2.796, 33.420 | < 0.001 | 18.753 | 3.805, 92.428 | < 0.001 |
| Peak hs-cTnI | 1.027 | 0.995, 1.061 | 0.102 | 1.024 | 0.984, 1.066 | 0.241 |
| High-SFRP5 group | 0.289 | 0.084, 0.997 | 0.049 | 0.190 | 0.036, 0.996 | 0.049 |
LVEF, left ventricular ejection fraction; HR, hazard ratio; CI, confidence interval; hs-cTnI, hypersensitive cardiac Troponin I; SFRP5, serum secreted frizzled-related protein 5.
Discussion
As far as we have been able to determine, this was the first study showing that serum SFRP5 levels were higher in patients with first-time, acute, anterior STEMI on admission than in those without CAD and that it decreased over time after timely reperfusion therapy. Additionally, elevated baseline serum SFRP5 levels were correlated with decreased myocardial injury and suppressed systematic inflammation, evidenced by lower peak hs-cTnI and hs-CRP levels, respectively. Furthermore, we showed that serum SFRP5 levels at baseline or 24 h after admission were positively and independently correlated with LVEF changes at the 3-month follow-up. Consequently, we concluded that the higher is the baseline serum SFRP5 levels, the higher is LVEF and the lower is the risk of developing HF at the 3-month follow-up, independent of peak cTnI levels and cardiac function at baseline, after successful primary PCI following optimal medications. These findings suggested that the elevated SFRP5 levels in circulation were an active reaction to alleviate myocardial I/R injury and promote myocardial recovery after MI.
Accumulating evidence shows that circulating SFRP5 levels are decreased in overweight/obese individuals and those with impaired glucose intolerance or T2DM and metabolic syndrome14, 17, 18). Similarly, Miyoshi et al. found lower serum SFRP5 levels in patients with stable CAD19). Conversely, in other studies, higher circulating SFRP5 levels were observed in those with T2DM20, 21). Although these inconsistent results are difficult to explain, the anti-inflammatory role of SFRP5 has been widely recognized in cardiometablic disease. As shown in the in vivo experiment of Ouchi et al., transient administration of SFRP5 improved metabolic function and reduced adipose inflammation in obese and diabetic mice13). In the present study, we found markedly higher serum SFRP5 levels during the early phase of acute STEMI. Similar findings were observed in patients with chronic kidney disease associated with acute STEMI22). Interestingly, the responses of serum SFRP5 to chronic and acute myocardial ischemia were markedly divergent, which might indicate an emergent compensatory mechanism of SFRP5 in the context of acute MI.
The negative correlations of baseline serum SFRP5 levels with BMI and T2DM in this study were present only in individuals with multiple cardiometabolic risk factors in the control group, but not in the STEMI group. This difference was partially due to the drastic disturbance of the cardiometabolic system during acute STEMI. To explore the time course of SFRP5, we showed that the elevated serum SFRP5 declined continuously and was close to the control levels within 3 days after the infarction. This reduction in anti-inflammatory SFRP5 might result from the acute enhancement of oxidant stress and inflammation during the myocardial I/R process23). This pattern was in line with a previous report by Ouchi et al. regarding the early stages of murine obesity13). Adiponectin, another well-established anti-inflammatory adipokine, was reported at its highest level in the circulation in patients with STEMI at the time of admission, but reached its low point 24 h after reperfusion24, 25).
Inflammation plays a vital role in the process of myocardial I/R, and hs-CRP was a prognostic marker of long-term mortality after infarction26). It was also reported that late gadolinium enhancement with cardiac magnetic resonance imaging following STEMI showed that peak cTnI strongly correlated with infarct size27). In the present study, we found that serum SFRP5 levels on admission were negatively correlated with peak serum hs-CRP and hs-cTnI levels, which revealed an adaptive response of SFPR5 to counteract elevated systematic inflammation and acute myocardial necrosis following acute STEMI. Similar to our findings, Nakamura et al. showed that genetic knockout of SFRP5 in mice led to severe cardiomyocyte apoptosis, elevated inflammation infiltration in the infarct zone, and greater MI size23). Kikuchi et al. also found that SFRP5 deficiency resulted in impaired reperfusion and the capillary density of ischemic limbs28).
Subsequently, we investigated the improvement of cardiac function and structure after infarction. We found that serum SFRP5 levels upon admission or 24 h after admission were associated with LVEF improvement at the 3-month follow-up. Moreover, these correlations were independent of potential confounders. This cardioprotective role of decreased SFRP5 at 72 h, however, was not significant. We also showed that patients with acute STEMI and high serum SFRP5 levels at admission were more likely to have better recovery of cardiac function and were less likely to suffer left ventricular systolic dysfunction during the early postinfarction phase after successful primary PCI following optimal medications. More importantly, this correlation was independent of peak cTnI levels and baseline cardiac functions. However, such obvious early recovery of left ventricular function was not accompanied by significant improvement of left ventricular geometry.
As already reported, the anti-inflammatory effect of SFRP5 is mediated via suppression of the Wnt5a/JNK signaling pathway in metabolic disorders13), although the specific mechanism of the SFRP5–Wnt5a axis in cardiac I/R injury is less well understood. SFRP5 inhibits the proliferation and activation of cardiac fibroblasts and so alleviates the postinfarction process of myocardial fibrosis29). Wnt5a promotes human myocardial inflammation and fibrosis through tissue inhibition of metalloproteinases 1 and extracellular signal-regulated kinase 1 and 2, thereby contributing to HF progression30). Blocking of Wnt signaling by Wnt3a and Wnt5a homologs inhibited the occurrence of HF following MI31). Nakamura et al. also showed that the activation of cytokines by Wnt5a was inhibited by recombinant SFRP5, possibly through non-canonical Wnt5a/JNK signaling23). In addition, Wnt5a stimulated hypertrophy in cultured myocytes, thereby contributing to worsened postinfarction remodeling32).
In this proof-of-concept study, we confirmed the cardioprotective role of SFPR5 in the setting of acute STEMI. Importantly, we provide a new insight into the conceptual framework of “obesity-inflammation-atherothrombosis,” that is, residual inflammation might contribute to poor prognosis of patients with atherothrombosis though managed with contemporary and comprehensive therapies. Adipocytokine, such as SFRP5, is a promising therapeutic target focus on distinct inflammatory pathway to alleviate I/R injury and improve cardiac remodeling postinfarction. The study has several limitations. First, because of the cross-sectional design and relatively small sample size, a definitively causal relation cannot be established between serum SFRP5 levels and improved cardiac function. Second, the most accurate imaging modalities for evaluating scar size and cardiac remodeling after infarction were not used. Third, the relatively strict inclusion criteria that were used to reduce the heterogenicity of the participants precluded the application of our results to the general population. Fourth, we did not enroll patients with stable CAD and non-ST-segment elevation acute coronary syndrome and did not have long-term follow-up of STEMI patients on adverse clinical outcomes. Fifth, intracoronary imaging was not assessed during primary PCI, which would have considerably strengthened our findings. Finally, the serum concentration of Wnt5a, a target antagonistic to SFRP5, was not measured.
Conclusions
We showed, for the first time, that serum SFRP5 levels were sharply increased during the early phase of acute STEMI. The elevated serum SFRP5 level was significantly associated with low myocardial injury and systematic inflammation during the I/R process. It was also associated with early improvement of cardiac function. Therefore, SFRP5 is a promising target for enhancing infarct healing and limiting cardiac remodeling after MI.
Declarations Authors' Contributions
YD and YZ interpreted the data and wrote the manuscript. YXZ and YJZ designed the study protocol and supervised the project. CPH measured serum SFRP5 levels using ELISA kits. DZ and ST performed echocardiography and analyzed echocardiographic data. JWZ and QWJ enrolled the participants. WL, HYH, YHG, XLL, ZJW, and LXY managed the participants. All authors read and approved the final manuscript.
Acknowledgments
Not applicable.
Abbreviations
- BMI
body mass index
- CAD
coronary artery disease
- CI
confidence interval
- CRP
C-reactive protein
- HF
heart failure
- HR
hazard ratio
- hs-CRP
hypersensitive C-reactive protein
- hs-cTnI
hypersensitive cardiac troponin I
- I/R
ischemia/reperfusion
- IRA
infarct-related artery
- LVEDV
left ventricular end-diastolic volume
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- SFRP
secreted frizzled-related protein
- STEMI
ST-segment elevation myocardial infarction
- T2DM
type 2 diabetes mellitus
Competing Interests
The authors declare that they have no competing interests.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Consent for Publication
Not applicable.
Ethics Approval and Consent to Participate
The study protocol adhered to the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Anzhen Hospital of Capital Medical University. Written informed consent was obtained from each patient before enrollment.
Funding
This work was supported by the grant from Beijing Municipal Science and Technology Commission (Z171100000417042), National Key Research and Development Program of China (2017YFC0908800), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201303), the National Key Clinical Specialty Construction Project (2013–2014), and the “Beijing Municipal Administration of Hospitals” Ascent Plan (DFL20150601).
References
- 1). Nakamura K, Fuster JJ, Walsh K: Adipokines: a link between obesity and cardiovascular disease. J Cardiol, 2014; 63: 250-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Antoniades C: ‘Dysfunctional’ adipose tissue in cardiovascular disease: a reprogrammable target or an innocent bystander? Cardiovasc Res, 2017; 113: 997-998 [DOI] [PubMed] [Google Scholar]
- 3). Vilahur G, Ben-Aicha S, Badimon L: New insights into the role of adipose tissue in thrombosis. Cardiovasc Res, 2017; 113: 1046-1054 [DOI] [PubMed] [Google Scholar]
- 4). Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, Sam F, Ouchi N, Walsh K: Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol, 2007; 42: 1065-1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Kataoka Y, Shibata R, Ohashi K, Kambara T, Enomoto T, Uemura Y, Ogura Y, Yuasa D, Matsuo K, Nagata T, Oba T, Yasukawa H, Numaguchi Y, Sone T, Murohara T, Ouchi N: Omentin prevents myocardial ischemic injury through AMP-activated protein kinase- and Akt-dependent mechanisms. J Am Coll Cardiol, 2014; 63: 2722-2733 [DOI] [PubMed] [Google Scholar]
- 6). Montecucco F, Carbone F, Schindler TH: Pathophysiology of ST-segment elevation myocardial infarction: novel mechanisms and treatments. Eur Heart J, 2016; 37: 1268-1283 [DOI] [PubMed] [Google Scholar]
- 7). Retraction and republication--ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet (London, England: ), 2015; 385: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Bogaty P, L'Allier PL, Segal E, Rinfret S, Racine N, Harvey R, Ross D, Maire S, Kouz S, Carroll C, Boothroyd LJ, Kezouh A, Azzi L, Brown KA, Nasmith J, Lambert LJ: Clinical Profiles Related to Timing of Death, Including In-Hospital Deaths Before Admission, in Patients With ST-Elevation Myocardial Infarction. Am J Cardiol, 2016; 117: 347-352 [DOI] [PubMed] [Google Scholar]
- 9). Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J: Beyond Wnt inhibition: new functions of secreted Frizzled- related proteins in development and disease. J Cell Sci, 2008; 121: 737-746 [DOI] [PubMed] [Google Scholar]
- 10). Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, Leroux L, Moreau C, Dare D, Duplaa C: Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation, 2003; 108: 2282-2289 [DOI] [PubMed] [Google Scholar]
- 11). Alfaro MP, Vincent A, Saraswati S, Thorne CA, Hong CC, Lee E, Young PP: sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. J Biol Chem, 2010; 285: 35645-35653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Jura M, Jaroslawska J, Chu DT, Kozak LP: Mest and Sfrp5 are biomarkers for healthy adipose tissue. Biochimie, 2016; 124: 124-133 [DOI] [PubMed] [Google Scholar]
- 13). Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K: Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science (New York, NY), 2010; 329: 454-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Liu LB, Chen XD, Zhou XY, Zhu Q: The Wnt antagonist and secreted frizzled-related protein 5: implications on lipid metabolism, inflammation, and type 2 diabetes mellitus. Biosci Rep, 2018; 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Carstensen-Kirberg M, Kannenberg JM, Huth C, Meisinger C, Koenig W, Heier M, Peters A, Rathmann W, Roden M, Herder C: Inverse associations between serum levels of secreted frizzled-related protein-5 (SFRP5) and multiple cardiometabolic risk factors: KORA F4 study. Cardiovasc Diabetol, 2017; 16: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P. Widi: 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J, 2018; 39: 119-177 [DOI] [PubMed] [Google Scholar]
- 17). Hu W, Li L, Yang M, Luo X, Ran W, Liu D, Xiong Z, Liu H, Yang G: Circulating Sfrp5 is a signature of obesity- related metabolic disorders and is regulated by glu and liraglutide in humans. J Clin Endocrinol Metab, 2013; 98: 290-298 [DOI] [PubMed] [Google Scholar]
- 18). Xu Q, Wang H, Li Y, Wang J, Lai Y, Gao L, Lei L, Yang G, Liao X, Fang X, Liu H, Li L: Plasma Sfrp5 levels correlate with determinants of the metabolic syndrome in Chinese adults. Diabetes Metab Res Rev, 2017; 33. [DOI] [PubMed] [Google Scholar]
- 19). Miyoshi T, Doi M, Usui S, Iwamoto M, Kajiya M, Takeda K, Nosaka K, Nakayama R, Okawa K, Takagi W, Nakamura K, Hirohata S, Ito H: Low serum level of secreted frizzled-related protein 5, an anti-inflammatory adipokine, is associated with coronary artery disease. Atherosclerosis, 2014; 233: 454-459 [DOI] [PubMed] [Google Scholar]
- 20). Lu YC, Wang CP, Hsu CC, Chiu CA, Yu TH, Hung WC, Lu LF, Chung FM, Tsai IT, Lin HC, Lee YJ: Circulating secreted frizzled-related protein 5 (Sfrp5) and wingless-type MMTV integration site family member 5a (Wnt5a) levels in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev, 2013; 29: 551-556 [DOI] [PubMed] [Google Scholar]
- 21). Canivell S, Rebuffat S, Ruano EG, Kostov B, Siso-Almirall A, Novials A, Ceriello A, Gomis R: Circulating SFRP5 levels are elevated in drug-naive recently diagnosed type 2 diabetic patients as compared with prediabetic subjects and controls. Diabetes Metab Res Rev, 2015; 31: 212-219 [DOI] [PubMed] [Google Scholar]
- 22). Wang CP, Yu TH, Wu CC, Hung WC, Hsu CC, Tsai IT, Tang WH, Chung FM, Houng JY, Lee YJ, Lu YC: Circulating secreted frizzled-related protein 5 and chronic kidney disease in patients with acute ST-segment elevation myocardial infarction. Cytokine, 2018; 110: 367-373 [DOI] [PubMed] [Google Scholar]
- 23). Nakamura K, Sano S, Fuster JJ, Kikuchi R, Shimizu I, Ohshima K, Katanasaka Y, Ouchi N, Walsh K: Secreted Frizzled-related Protein 5 Diminishes Cardiac Inflammation and Protects the Heart from Ischemia/Reperfusion Injury. J Biol Chem, 2016; 291: 2566-2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Trifunovic D, Stankovic S, Marinkovic J, Beleslin B, Banovic M, Djukanovic N, Orlic D, Tesic M, Vujisic-Tesic B, Petrovic M, Nedeljkovic I, Stepanovic J, Djordjevic-Dikic A, Giga V, Ostojic M: Time-dependent changes of plasma adiponectin concentration in relation to coronary microcirculatory function in patients with acute myocardial infarction treated by primary percutaneous coronary intervention. J Cardiol, 2015; 65: 208-215 [DOI] [PubMed] [Google Scholar]
- 25). Natsukawa T, Maeda N, Fukuda S, Yamaoka M, Fujishima Y, Nagao H, Sato F, Nishizawa H, Sawano H, Hayashi Y, Funahashi T, Kai T, Shimomura I: Significant Association of Serum Adiponectin and Creatine Kinase-MB Levels in ST-Segment Elevation Myocardial Infarction. J Atheroscler Thromb, 2017; 24: 793-803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Suleiman M, Khatib R, Agmon Y, Mahamid R, Boulos M, Kapeliovich M, Levy Y, Beyar R, Markiewicz W, Hammerman H, Aronson D: Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C-reactive protein. J Am Coll Cardiol, 2006; 47: 962-968 [DOI] [PubMed] [Google Scholar]
- 27). Costello BT, Stub D, Hare J, Ellims AH, Wang X, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, Cameron P, Barger B, Meredith IT, Kaye DM, Iles L, Taylor AJ, AVOID Investigators : Comparison of Magnetic Resonance Analysis of Myocardial Scarring With Biomarker Release Following S-T Elevation Myocardial Infarction. Heart Lung Circ, 2018 [DOI] [PubMed] [Google Scholar]
- 28). Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT, Shimizu I, Fuster JJ: An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med, 2014; 20: 1464-1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Bie ZD, Sun LY, Geng CL, Meng QG, Lin XJ, Wang YF, Wang XB, Yang J: MiR-125b regulates SFRP5 expression to promote growth and activation of cardiac fibroblasts. Cell biology international, 2016; 40: 1224-1234 [DOI] [PubMed] [Google Scholar]
- 30). Abraityte A, Vinge LE, Askevold ET, Lekva T, Michelsen AE, Ranheim T, Alfsnes K, Fiane A, Aakhus S, Lunde IG, Dahl CP, Aukrust P, Christensen G, Gullestad L, Yndestad A, Ueland T: Wnt5a is elevated in heart failure and affects cardiac fibroblast function. J Mol Med (Berl), 2017; 95: 767-777 [DOI] [PubMed] [Google Scholar]
- 31). Laeremans H, Hackeng TM, van Zandvoort MA, Thijssen VL, Janssen BJ, Ottenheijm HC, Smits JF, Blankesteijn WM: Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation, 2011; 124: 1626-1635 [DOI] [PubMed] [Google Scholar]
- 32). Hagenmueller M, Riffel JH, Bernhold E, Fan J, Katus HA, Hardt SE: Dapper-1 is essential for Wnt5a induced cardiomyocyte hypertrophy by regulating the Wnt/PCP pathway. FEBS letters, 2014; 588: 2230-2237 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


