Abstract
Background
Parkinson’s disease is a disorder growing in prevalence, disability, and deaths. Healthcare databases provide a ‘real-world’ perspective for millions of individuals. We envisioned helping accelerate drug discovery by using these databases.
Objectives
The objectives of this study were to assess the association of marketed medications with the risk of parkinsonism in four US claims databases and to evaluate the consistency of the association of β-adrenoreceptor modulation with parkinsonism.
Methods
The study was conducted using a self-controlled cohort design in which subjects served as their own control. The time from treatment initiation until discontinuation or end of observation was the exposed period and a similar time preceding medication was the unexposed period. Medications were studied at ingredient and class level. The incidence rate ratio (IRR) and combined IRR were calculated.
Results
We assessed 2181 drugs and 117,015,066 people. Diphenhydramine, isradipine, methylphenidate, armodafinil, and modafinil were associated with reduced risk for parkinsonism in at least two databases. Armodafinil, modafinil, methylphenidate, and the β-agonist albuterol were associated with a 56%, 54%, 39%, and 17% reduction in the risk of having parkinsonism, respectively. Isradipine results were heterogeneous and no significant association was found. Propranolol was associated with a 32% increased risk, the only β-adrenoceptor antagonist (β-blocker) associated with an increased risk.
Conclusions
Armodafinil, modafinil, and methylphenidate were associated with a decreased risk of parkinsonism, as were β-agonists. Of the β-blockers, only propranolol was associated with increased risk. Healthcare database analyses that incorporate scientific rigor provide insight and direction for drug discovery efforts. These findings show association not causality; however, they offer considerable support to the association between β-adrenergic receptor modulation and risk of Parkinson’s disease.
Electronic supplementary material
The online version of this article (10.1007/s40261-019-00830-4) contains supplementary material, which is available to authorized users.
Key Points
| An analysis of healthcare databases can help provide insight and direction for drug discovery efforts. |
| Diphenhydramine, isradipine, methylphenidate, armodafinil, modafinil, and β-agonists were associated with a reduced risk of parkinsonism, opening the door for potential new drug targets. |
Introduction
Neurological disorders are now the leading source of disability globally, and Parkinson’s disease is one of the fastest growing in prevalence, disability, and deaths. In fact, since the 1990s the global burden of Parkinson’s disease has more than doubled as a result of increasing numbers of older people, with potential contributions from longer disease duration and environmental factors [1].
To date, carbidopa and levodopa remain the first line of treatment for Parkinson’s disease and disease modification therapies remain elusive. Various approaches to identifying such therapies have been proposed, including the use of large datasets such as those produced by genome-wide association studies and other molecular research, prompting calls for ever more data sharing and collaboration [2, 3]. In addition to such molecular data, there are also significant opportunities to derive information from observational healthcare data to inform drug development, especially as ‘real-world’ evidence from healthcare databases, which includes medication use and health outcomes, is becoming increasingly available.
Administrative healthcare databases have traditionally been used to monitor the safety of medications and for comparative effectiveness research [4, 5]. These databases frequently have healthcare information for many millions of individuals and provide a ‘real-world’ perspective, as opposed to data gathered from highly controlled clinical trials. Increasingly, the role of such healthcare databases has been expanded to help in early drug development [6] and even drug repurposing [7]. For example, Mittal et al. [6] recently proposed that, based on pre-clinical model studies, β-adrenoreceptor agonism would be protective for Parkinson’s disease, and went on to show some human validation for this concept using large population data from Scandinavia. However, the analysis of such observational healthcare data is complicated by multiple confounders along with bias and potential for conflicting results from multiple studies due to the use of different methods. We set out to replicate and then extend the Mittal et al. [6] findings by using multiple databases, which included very large numbers of individuals, using methods that enable integration of such data with a common analytical approach, and an analysis agnostic to mechanism of action or type of drug interaction with the target disease—in this case, Parkinson’s disease and parkinsonism.
We conducted a study using several administrative healthcare databases to assess the association of marketed medications with risk of parkinsonism and evaluate the consistency of the association of β-adrenoreceptor modulation with parkinsonism.
Methods
Study Design
We used a self-controlled cohort design to assess the association between initiation of medications and the incidence of Parkinson’s disease. In this design, the subjects served as their own controls [8]. This study design has been shown to be superior to other more commonly used designs, such as case–control studies, in terms of less biased estimates and higher predictive accuracy [9–11]. The advantage of the self-controlled cohort design is that the control of confounding is improved as subjects are their own controls.
The first use of a medication was considered the index date and the time from treatment initiation until discontinuation of the therapy or end of observation was considered the exposed period. We allowed for a maximum of the medication supply plus a 30-day gap between consecutive dispensings in defining the exposed period. A similar duration of time, directly preceding the index date, was considered the unexposed period. We then captured the first diagnosis of parkinsonism for each patient. We use the term parkinsonism from this point forward because the validated algorithm used to identify Parkinson’s disease includes both Parkinson’s disease and parkinsonism [12]. This algorithm requires at least two encounters with a Parkinson’s or parkinsonism diagnosis within 12 months, and the earliest diagnosis date was considered the date of incident parkinsonism.
For the group of patients exposed to the medication of interest we calculated an incidence rate (IR) of parkinsonism during the exposed period as the number of patients with parkinsonism first diagnosed with the disease during the exposed period divided by the total exposed person-time observed in the population. The unexposed IRs were calculated in an identical fashion and an IR ratio (IRR) was calculated as the exposed IR divided by the unexposed IR. Rate ratios, standard errors, and 95% confidence intervals (CIs) were obtained using Poisson regression. An IRR lower than 1 indicated that the occurrence of parkinsonism was observed less often while exposed to the drug—a ‘potential benefit’. Patients were identified from June 2000 to April 2018.
Only medications requiring a prescription were included because claims databases do not include over-the-counter medications. Medications were studied at the ingredient and class level. The SNOMED concepts that we used were parkinsonism, Parkinson’s disease, and secondary parkinsonism. The International Classification of Diseases, 9th Revision (ICD-9) and 10th Revision (ICD-10) codes used are listed in Electronic Supplementary Material 1.
Data Sources
The following four US claims databases were used:
IBM MarketScan® Commercial Claims and Encounters (CCAE) database. This is a large US claims database that includes data from 142 million individuals enrolled in employer-sponsored insurance health plans. Data include adjudicated health insurance claims (e.g., inpatient, outpatient, and outpatient pharmacy) as well as enrollment data from large employers and health plans who provide private healthcare coverage to employees, their spouses, and dependents. Data elements are outpatient pharmacy dispensing claims (coded with National Drug Codes [NDCs]) and inpatient and outpatient medical claims that provide diagnosis codes (ICD-9 Clinical Modification [ICD-9-CM] or ICD-10 Clinical Modification [ICD-10-CM]).
IBM MarketScan® Multi-State Medicaid Database (MDCD). MDCD is an adjudicated health insurance claims database for 26 million Medicaid enrollees from multiple states and includes hospital discharge diagnoses, outpatient diagnoses, and outpatient pharmacy claims. Data elements are outpatient pharmacy dispensing claims (coded with NDCs), inpatient and outpatient medical claims, and diagnosis codes (coded in ICD-9-CM or ICD-10-CM).
IBM MarketScan® Medicare Supplemental Database (MDCR). The MDCR represents the health services of 9 million retirees with primary or Medicare supplemental coverage through privately insured fee-for-service, point-of-service, or capitated health plans. These data include adjudicated health insurance claims (e.g., inpatient, outpatient, and outpatient pharmacy dispensing claims). Data elements are outpatient pharmacy dispensing claims (coded with NDCs) and inpatient and outpatient medical claims that provide diagnosis codes (coded in ICD-9-CM or ICD-10-CM).
Optum® De-Identified Clinformatics® Data Mart Database. Optum® includes de-identified data from 84 million members with private health insurance, who are fully insured in commercial plans or in administrative services only and Medicare Advantage (Medicare Advantage Prescription Drug coverage. The population is representative of US commercial claims patients (0–65 years old) with some Medicare (65+ years old). Data elements are outpatient pharmacy dispensing claims (coded with NDCs) and inpatient and outpatient medical claims that provide procedure codes (coded in Current Procedural Terminology, 4th Edition [CPT-4], Healthcare Common Procedure Coding System [HCPCS], ICD-9-CM or ICD-10-PCS) and diagnosis codes (coded in ICD-9-CM or ICD-10-CM).
Analytical Methods
A caveat to interpretation of study results using a self-controlled cohort design is that the association of the medications used for the treatment of the disease with Parkinson’s disease will be confounded. The direction of the bias will depend on when these medications are given in relation to the first occurrence of the diagnosis of parkinsonism in the database. For example, drugs such as carbidopa/levodopa will appear as protective under the design we implemented, i.e., associated with an apparently decreased risk, because the first diagnosis of parkinsonism often occurs before receiving the treatment. Therefore, medications used for the treatment of Parkinson’s disease such as carbidopa/levodopa and antipsychotics were excluded.
To avoid spurious associations for the examination between all medications and parkinsonism due to the multiple comparisons being made, we limited our attention to medications associated with a statistically significant reduction of at least 30% in the risk for parkinsonism in at least two of the four databases.
To assess the association with β-adrenoreceptor modulation, we report individual β-adrenoreceptor agonists and antagonists that have been evaluated in recent literature. The β-blockers (β-adrenoceptor antagonists) included were propranolol, carvedilol, and metoprolol. The β-agonist included was albuterol. In addition, we assessed the association of β-adrenoreceptor modulation as a therapeutic class. For this categorization, we used level 4 of the Anatomical Therapeutic Chemical (ATC) classification system. Electronic Supplementary Material 2 lists the β-adrenoreceptor modulation medications by ATC category.
Individual and Combined Relative Risks
The relative IRR of each medication in each database with its 95% CI is presented separately. We also combined the estimates using meta-analytic techniques. We used I2 to measure heterogeneity of the results [13] across the four databases. I2 describes the percentage of total variation that is due to heterogeneity among databases, relative to the average within-database variability. A value of < 50% was defined as indicating low heterogeneity, 50–74% indicates moderate heterogeneity, and ≥ 75% indicates serious heterogeneity. We combined the relative risks using the DerSimonian–Laird random effects model that allows for differences in the treatment effect from database to database [14].
Results
To examine the relation between prescribed medications and the risk of Parkinson’s disease, we examined four databases including 117,015,066 people, of whom 429,530 were diagnosed with Parkinson’s disease, and 2181medications. Five of these drugs were associated with at least a 30% decreased risk in at least two of the databases.
Overall, 95.4% of the subjects were identified through codes reflecting Parkinson’s disease and 4.6% of subjects were identified through codes reflecting secondary parkinsonism. In the CCAE database, 94.6% of the subjects were identified through codes reflecting Parkinson’s disease; in the MDCD, 91.6%; in the MDCR, 96.6%; and in the Optum® database, 95.6%. The number of subjects exposed to each medication, the number of parkinsonism cases observed during the exposed and unexposed time, and the duration of exposure are presented in Table 1. We found that diphenhydramine, isradipine, methylphenidate, and two related drugs (armodafinil and modafinil; armodafinil is the R-enantiomer of modafinil) were associated with a reduced risk for parkinsonism in at least two databases (Table 1).
Table 1.
Number of subjects exposed and number of Parkinson’s cases observed during the unexposed and exposed period and duration of exposure for medications found to be associated with the risk of having parkinsonism in at least two databases and for β-adrenoreceptor modulators at the ingredient level and therapeutic class level
| Medication | Database | Number of persons exposed | Mean ± SD treatment time (days) | Number of Parkinson’s cases while exposed | Number of Parkinson’s cases while unexposed | IRR | 95% CI |
|---|---|---|---|---|---|---|---|
| α- and β-blocking agents | Optum® | 409,704 | 585 ± 531 | 602 | 592 | 0.92 | 0.82–1.03 |
| CCAE | 416,992 | 552 ± 367 | 80 | 68 | 1.08 | 0.77–1.52 | |
| MDCR | 257,087 | 594 ± 497 | 599 | 603 | 0.89 | 0.79–1.0 | |
| MDCD | 101,623 | 464 ± 431 | 65 | 98 | 0.61 | 0.44–0.84 | |
| β-Blocking agents, non-selective | Optum® | 522,487 | 420 ± 455 | 883 | 635 | 1.28 | 1.15–1.41 |
| CCAE | 690,041 | 322 ± 344 | 386 | 226 | 1.59 | 1.35–1.89 | |
| MDCR | 226,444 | 392 ± 396 | 675 | 514 | 1.18 | 1.05–1.32 | |
| MDCD | 106,493 | 361 ± 380 | 85 | 95 | 0.85 | 0.63–1.15 | |
| β-Blocking agents, selective | Optum® | 1,536,628 | 570 ± 511 | 2108 | 1734 | 1.05 | 0.98–1.11 |
| CCAE | 1,765,729 | 501 ± 480 | 416 | 311 | 1.17 | 1.01–1.36 | |
| MDCR | 736,044 | 492 ± 433 | 1897 | 1690 | 0.95 | 0.88–1.01 | |
| MDCD | 205,896 | 356 ± 359 | 180 | 203 | 0.8 | 0.65–0.98 | |
| Selective β2-adrenoreceptor agonists | Optum® | 4,370,912 | 133 ± 198 | 371 | 432 | 0.84 | 0.73–0.97 |
| CCAE | 7,458,835 | 138 ± 232 | 68 | 87 | 0.78 | 0.56–1.08 | |
| MDCR | 925,988 | 161 ± 257 | 339 | 362 | 0.9 | 0.78–1.05 | |
| MDCD | 1,618,746 | 181 ± 265 | 75 | 112 | 0.66 | 0.49–0.89 | |
| Albuterol | Optum® | 4,269,322 | 96 ± 133 | 326 | 388 | 0.83 | 0.72–0.97 |
| CCAE | 7,328,721 | 114 ± 206 | 60 | 79 | 0.76 | 0.53–1.07 | |
| MDCR | 914,514 | 133 ± 239 | 280 | 309 | 0.89 | 0.75–1.05 | |
| MDCD | 1,608,646 | 182 ± 292 | 68 | 97 | 0.69 | 0.5–0.96 | |
| Armodafinil | Optum® | 25,805 | 323 ± 289 | 15 | 19 | 0.75 | 0.36–1.57 |
| CCAE | 56,039 | 529 ± 359 | 4 | 21 | 0.18 | 0.05–0.55 | |
| MDCR | 3809 | 591 ± 421 | 9 | 24 | 0.36 | 0.15–0.8 | |
| MDCD | 2283 | 55 ± 36 | 2 | 3 | 0.64 | 0.05–5.61 | |
| Carvedilol | Optum® | 342,191 | 588 ± 532 | 562 | 556 | 0.92 | 0.81–1.03 |
| CCAE | 300,541 | 601 ± 436 | 72 | 64 | 1.03 | 0.73–1.47 | |
| MDCR | 239,282 | 609 ± 504 | 557 | 563 | 0.89 | 0.79–1 | |
| MDCD | 77,413 | 470 ± 436 | 61 | 97 | 0.57 | 0.41–0.79 | |
| Diphenhydramine | Optum® | 420,560 | 167 ± 361 | 21 | 42 | 0.5 | 0.28–0.86 |
| CCAE | 1,174,920 | 117 ± 122 | 13 | 8 | 1.62 | 0.62–4.51 | |
| MDCR | 206,491 | 142 ± 173 | 24 | 44 | 0.54 | 0.32–0.91 | |
| MDCD | 793,022 | 255 ± 322 | 49 | 54 | 0.89 | 0.59–1.33 | |
| Isradipine | Optum® | 7840 | 557 ± 631 | 2 | 14 | 0.13 | 0.01–0.58 |
| CCAE | 11,030 | 545 ± (NA) | 1 | 21 | 0.04 | 0–0.28 | |
| MDCR | 9195 | 487 ± 445 | 18 | 13 | 1.29 | 0.6–2.86 | |
| Methylphenidate | Optum® | 276,941 | 297 ± 394 | 60 | 95 | 0.6 | 0.43–0.83 |
| CCAE | 578,187 | 234 ± 259 | 22 | 26 | 0.79 | 0.43–1.46 | |
| MDCR | 16,569 | 215 ± 269 | 53 | 90 | 0.56 | 0.39–0.79 | |
| MDCD | 265,809 | 226 ± 156 | 9 | 9 | 0.92 | 0.32–2.62 | |
| Metoprolol | Optum® | 1,105,532 | 549 ± 492 | 1647 | 1512 | 0.97 | 0.9–1.04 |
| CCAE | 1,213,182 | 517 ± 489 | 293 | 255 | 1.03 | 0.87–1.22 | |
| MDCR | 625,495 | 515 ± 442 | 1732 | 1609 | 0.92 | 0.86–0.99 | |
| MDCD | 163,187 | 362 ± 380 | 144 | 167 | 0.78 | 0.62–0.98 | |
| Modafinil | Optum® | 51,484 | 251 ± 259 | 43 | 69 | 0.6 | 0.4–0.88 |
| CCAE | 115,888 | 455 ± 438 | 16 | 62 | 0.25 | 0.13–0.43 | |
| MDCR | 16,948 | 374 ± 285 | 65 | 123 | 0.51 | 0.37–0.69 | |
| MDCD | 5094 | 181 ± 215 | 3 | 3 | 0.95 | 0.13–7.06 | |
| Propranolol | Optum® | 268,507 | 418 ± 456 | 704 | 481 | 1.32 | 1.18–1.49 |
| CCAE | 479,976 | 314 ± 338 | 372 | 202 | 1.72 | 1.44–2.05 | |
| MDCR | 49,163 | 380 ± 366 | 499 | 303 | 1.37 | 1.19–1.59 | |
| MDCD | 79,254 | 375 ± 386 | 77 | 88 | 0.83 | 0.6–1.14 |
CCAE IBM MarketScan® Commercial Claims and Encounters Database, CI confidence interval, IRR incidence rate ratio, MDCD IBM MarketScan® Multi-State Medicaid Database, MDCR IBM MarketScan® Medicare Supplemental Database, NA not applicable, Optum® Optum® De-Identified Clinformatics® Data Mart Database, SD standard deviation
The association of armodafinil and methylphenidate with parkinsonism was similar across the four databases. The use of armodafinil and modafinil was associated with a 56% and 54% reduction in the risk of having Parkinson’s disease, respectively (Table 2), using the combined relative risk estimate.
Table 2.
Incidence rate ratio in each database and combined estimate of medications found to be associated with the risk of having parkinsonism in at least two databases
| Medication | Database | IRR (95% CI) |
|---|---|---|
| Armodafinil | Optum® | 0.75 (0.36–1.57) |
| CCAE | 0.18 (0.05–0.55) | |
| MDCR | 0.36 (0.15–0.8) | |
| MDCD | 0.64 (0.05–5.61) | |
| Combined | I2 = 28.7% | 0.44 (0.23–0.82) |
| Diphenhydramine | Optum® | 0.5 (0.28–0.86) |
| CCAE | 1.62 (0.62–4.51) | |
| MDCR | 0.54 (0.32–0.91) | |
| MDCD | 0.89 (0.59–1.33) | |
| Combined | I2 = 51.8% | 0.72 (0.47–1.09) |
| Isradipine | Optum® | 0.13 (0.01–0.58) |
| CCAE | 0.04 (0–0.28) | |
| MDCR | 1.29 (0.6–2.86) | |
| MDCD | 0 | |
| Combined | I2 = 78.2% | 0.26 (0.03–2.15) |
| Methylphenidate | Optum® | 0.6 (0.43–0.83) |
| CCAE | 0.79 (0.43–1.46) | |
| MDCR | 0.56 (0.39–0.79) | |
| MDCD | 0.92 (0.32–2.62) | |
| Combined | I2 = 0 | 0.61 (0.49–0.77) |
| Modafinil | Optum® | 0.6 (0.4–0.88) |
| CCAE | 0.25 (0.13–0.43) | |
| MDCR | 0.51 (0.37–0.69) | |
| MDCD | 0.95 (0.13–7.06) | |
| Combined | I2 = 55.6% | 0.459 (0.31–0.68) |
CCAE IBM MarketScan® Commercial Claims and Encounters Database, CI confidence interval, IRR incidence rate ratio, MDCD IBM MarketScan® Multi-State Medicaid Database, MDCR IBM MarketScan® Medicare Supplemental Database, Optum® Optum® De-Identified Clinformatics® Data Mart Database
Methylphenidate use was associated with a 39% reduced risk of being diagnosed with parkinsonism (Table 2). Isradipine was associated with a decreased risk, but the results were heterogeneous and the sample sizes were small. When this heterogeneity was incorporated, the combined estimate showed no statistically significant association between the use of isradipine and parkinsonism (Table 2).
β-Adrenergic Receptor Modulation
Given the previous findings in relation to β-adrenergic receptor medication and risk of Parkinson’s disease [6], we examined the relationship of the disease with individual drugs and by ATC class. Exposure to the β-adrenergic receptor agonist albuterol was associated with a reduced risk of being subsequently diagnosed with parkinsonism across all four databases, ranging from an 11% to a 31% reduction in risk. The overall estimate for the association of albuterol was of modest magnitude, with the 95% CI ranging from an 8% to a 25% reduction in risk (Table 3). In contrast, the non-selective β-adrenergic receptor antagonist propranolol was associated with a 32% increased risk in three of the databases; it was the only β-blocker associated with an increased risk (Table 3).
Table 3.
Incidence rate ratio and combined estimate for β-adrenoreceptor modulators and parkinsonism in each database
| Medication | Database | IRR (95% CI) |
|---|---|---|
| Albuterol | Optum® | 0.83 (0.72–0.97) |
| CCAE | 0.76 (0.53–1.07) | |
| MDCR | 0.89 (0.75–1.05) | |
| MDCD | 0.69 (0.5–0.96) | |
| Combined | I2 = 0 | 0.83 (0.75–0.92) |
| Carvedilol | Optum® | 0.92 (0.81–1.03) |
| CCAE | 1.03 (0.73–1.47) | |
| MDCR | 0.89 (0.79–1.0) | |
| MDCD | 0.57 (0.41–0.79) | |
| Combined | I2 = 60.7% | 0.86 (0.74–1.0) |
| Metoprolol | Optum® | 0.97 (0.9–1.04) |
| CCAE | 1.03 (0.87–1.22) | |
| MDCR | 0.92 (0.86–1.0) | |
| MDCD | 0.78 (0.62–0.98) | |
| Combined | I2 = 22.7 | 0.94 (0.89–0.99) |
| Propranolol | Optum® | 1.32 (1.18–1.49) |
| CCAE | 1.72 (1.44–2.05) | |
| MDCR | 1.37 (1.19–1.59) | |
| Truven MDCD | 0.83 (0.6–1.14) | |
| Combined | I2 = 81.4% | 1.32 (1.09–1.60) |
CCAE IBM MarketScan® Commercial Claims and Encounters Database, CI confidence interval, IRR incidence rate ratio, MDCD IBM MarketScan® Multi-State Medicaid Database, MDCR IBM MarketScan® Medicare Supplemental Database, Optum® Optum® De-Identified Clinformatics® Data Mart Database
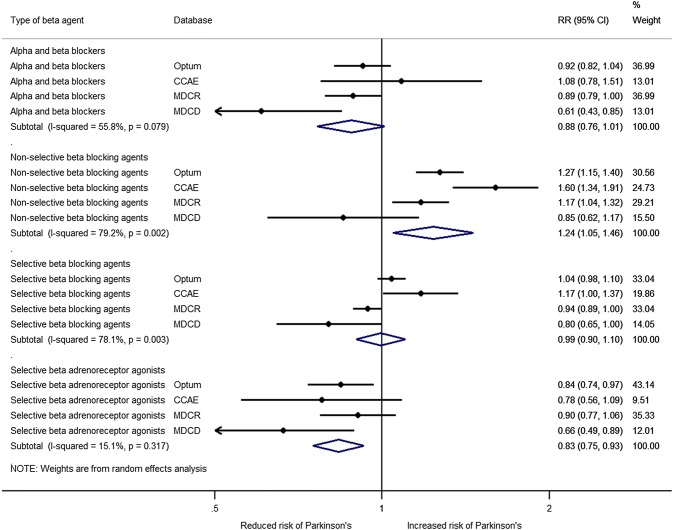
We then performed an analysis by ATC class and found that exposure to selective β2-adrenoreceptor agonists (e.g., albuterol) was associated with a reduction in the risk of having parkinsonism in all of the databases and the results were homogeneous (Fig. 1). Exposure to α- and β-blockers (e.g., carvedilol) showed some heterogeneity and no significant association with parkinsonism was found. Analysis of the exposure to non-selective β-blocking agents (e.g., propranolol) also had substantial heterogeneity. An increased risk was present in three of the databases and the combined estimated showed a 23% increase in the risk of having Parkinson’s disease. Exposure to selective β-blocking agents (e.g., metoprolol) also showed substantial heterogeneity and no significant association was found.
Fig. 1.
Forest plot of the association of parkinsonism with β-adrenoreceptor modulators at the therapeutic class level. CCAE IBM MarketScan® Commercial Claims and Encounters Database, CI confidence interval, MDCD IBM MarketScan® Multi-State Medicaid Database, MDCR IBM MarketScan® Medicare Supplemental Database, Optum® Optum® De-Identified Clinformatics® Data Mart Database, RR relative risk
Discussion
Previous research has shown that the results of observational studies can vary dramatically when different healthcare databases are used [15]. For example, albuterol, a β-adrenoreceptor agonist, has been found to be associated with both a decreased and increased risk of Parkinson’s disease [6, 16]. Such findings attest to the importance of assessing different databases to capture such heterogeneity [15]. However, results from single databases are often used, in part because differences in design, organization, and terminologies make integrated analysis challenging.
To systematically include more than one database requires transforming the databases so that they all have a common format (data model) as well as a common representation of terminologies [5, 17]. In addition to the implementation of a common data model across healthcare databases, improvements in large-scale data management and the use of study designs that address lack of randomization have allowed us to expand the scalability of studies.
Using this innovative approach, we found that treatment with armodafinil, modafinil, and methylphenidate was associated with a decreased risk of having parkinsonism. In terms of the effect of β-adrenoreceptor modulators, we found that albuterol and other β-agonists were associated with a decreased risk and that propranolol was associated with an increased risk of having parkinsonism.
It has been proposed that the observed protective effect of albuterol in comparative cohort studies may be due to confounding, as albuterol is more often prescribed to smokers, and smoking could be protective against Parkinson’s disease [18, 19]. However, our use of a self-controlled cohort design controls for smoking and all other subject-specific confounders that often do not change over time. The increased risk of parkinsonism associated with propranolol could be due to the drug being prescribed for the treatment of tremors [16]. In our study design, this could confound our results if the diagnosis and treatment of tremors precedes the diagnosis of Parkinson’s disease. Since propranolol was the only β-blocker associated with an increased risk, and results showed substantial variability, the effect of β-blockers on the incidence of Parkinson’s disease is unclear.
The substantial variability observed could be attributed to differences in receptor pharmacology and selectivity of the various β-adrenergic ligands. For example, the binding affinity of metoprolol for the β2-adrenoreceptors is extremely different from the affinity of propanol or carvedilol [20, 21]. In contrast, the association between β-agonists and the decreased risk of parkinsonism is consistent across databases and therapeutic classes. This, in conjunction with the body of work on how β-adrenoreceptor modulation affects protein accumulation in neurons, gives the findings a biological plausibility; however, the association is of small magnitude.
Methylphenidate inhibition of the reuptake of dopamine and norepinephrine may have potential neuroprotective effects as the abnormal accumulation of dopamine inside dopaminergic neurons is attenuated [22]. There are suggestions that therapies targeting the catecholaminergic system may attenuate functional deficits after traumatic brain injury [23]. Similarly, the mechanism of action of armodafinil and modafinil, which is dependent on the dopaminergic and adrenergic signaling mechanism of action, could also explain the observed protective effect [24]. Therefore, the protective effect observed with armodafinil, modafinil, and methylphenidate could be due to their catecholaminergic effect on neurons.
On the other hand, the apparent ‘protective’ effect of armodafinil, modafinil, and methylphenidate could be explained by the study design. Subjects with Parkinson’s disease often complain of sleepiness or fatigue [22] and could receive these medications for symptom relief. Because the condition would occur more often before the exposure, the comparisons of rates before and after exposure would lead to an IRR lower than 1.
Isradipine was found to have a substantial reduction in the risk of Parkinson’s disease in two databases; however, in Medicare subjects that ‘protective effect’ was not present and when we accounted for that variability in the analysis, the association was not statistically significant. Isradipine is a calcium channel antagonist indicated for the treatment of hypertension. Animal studies have shown isradipine to have a protective neuronal effect [25]. A recent randomized controlled trial conducted to study it as a disease-modifying agent in patients with early parkinsonism [26] did not show any benefit [27].
There are limitations to our study. The definition we used to identify Parkinson’s disease will also include some subjects with parkinsonism, not just Parkinson’s disease. Also, since Parkinson’s disease is an age-dependent neurodegenerative disease, some prodromal symptoms may appear long before the clinical diagnosis; thus, medications used for the treatment of such symptoms may appear to increase the risk.
A self-controlled cohort study design has the advantage of controlling for time-invariant confounders (e.g., genetic attributes) because subjects serve as their own control. However, it underestimates variability, with CIs that tend to be too narrow resulting in higher false-positive rates than in traditional cohort studies. In the present study, we required that, in addition to being statistically significant, the magnitude of the estimated association of the drug with parkinsonism be at least a 30% risk reduction and consistent in a least two of the four databases. This strategy reduced the possibility of false-positive results. On the other hand, we evaluated thousands of medications and conducted many statistical tests and our strategy could be criticized for having the potential for false-positive results due to multiple comparisons. However, despite this risk, we found that only a handful of medications were associated with a reduced risk of Parkinson’s disease. It is important to emphasize that the aim of this study was to identify potential pathways that may have been overlooked to help guide future drug discovery research. So, unlike randomized controlled studies used for regulatory approval, where false-positive results are completely undesired, in this instance the risk of erring on the side of over-inclusion due to false-positive results was mitigated by comparing data across multiple databases.
Symptoms of Parkinson’s disease start gradually, and formal diagnosis of the condition may occur years after the actual onset. This slow progression can lead to a temporal misclassification of the outcome, as the parkinsonism diagnosis would be captured more often after drug exposure even though the condition was present before drug exposure. This bias would lead to medications erroneously appearing to be associated with an increased risk. Similarly, the risk of Parkinson’s disease increases with age. In this study we compared the rate of events before versus after drug exposure and so subjects are always older when exposed to a medication. Therefore, in theory, more parkinsonism diagnoses may appear following drug exposure and medications could be associated with an apparent increased risk due to age. We found that armodafinil, modafinil, and methylphenidate were associated with a decreased risk of having parkinsonism, and thus these considerations do not explain why these medications are associated with a reduced risk.
The inconsistency of effects with medications that have similar mechanisms of action may in part be explained by differences in drug absorption and/or brain penetration as well as potential differences in receptor reserve and/or in intrinsic ligand activities. For many β-adrenergic receptor ligands, direct head-to-head pharmacological comparisons have not been conducted. This makes the interpretation of data involving partial agonists in cells or tissues with different receptor reserves problematic. Thus, a comprehensive understanding of the pharmacological properties and target selectivity of the medications is essential to help interpret their clinical effects. While our analysis comparing before and after the index date of diagnosis in the same individuals controlled for some confounders, the possibility of persistent bias remains. Finally, as in all observational studies, this analysis speaks only to an association, not causality. Despite this, the robust findings derived from over 117 million lives in four independent US datasets offers considerable support to the association between β-adrenergic receptor modulation and the risk of Parkinson’s disease.
The data sources of this study represent a variety of populations, including commercially insured and government funded populations, but the sources are US databases. Medications not marketed in the USA were not assessed and treatment patterns for Parkinson’s disease may be different outside the USA. Thus, the results may not be generalizable to other countries.
Conclusion
We employed a self-controlled cohort design to assess the association between initiation of medications and the incidence of Parkinson’s disease and studied the association of all marketed drugs on the risk of having parkinsonism in four large US administrative healthcare databases at the same time. We looked for unknown associations that would allow researchers to identify potential novel therapeutic targets that could lead to the development of medications for the prevention or treatment of Parkinson’s disease, a paradigm where old drugs help the discovery of new drugs. We found that armodafinil, modafinil, and methylphenidate were associated with a decreased risk of having parkinsonism, as were β-agonists. Of the β-blockers, only propranolol was associated with an increased risk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with Ethical Standards
Funding
Janssen Research & Development, LLC funded the study.
Conflict of Interest
M. Soledad Cepeda, David M. Kern, Guy R. Seabrook, and Simon Lovestone are employees of Janssen Research & Development, LLC. Janssen Research & Development, LLC has an interest in Parkinson’s disease.
Ethical Approval
The New England Institutional Review Board (NEIRB) determined that studies conducted with the Optum® database (NEIRB #12-286) or the MarketScan databases (NEIRB #12-284) do not qualify as human subjects research.
References
- 1.Dorsey ER. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephenson D, Hu MT, Romero K, Breen K, Burn D, Ben-Shlomo Y, et al. precompetitive data sharing as a catalyst to address unmet needs in Parkinson’s disease. J Parkinsons Dis. 2015;5(3):581–594. doi: 10.3233/JPD-150570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sardi SP, Cedarbaum JM, Brundin P. Targeted therapies for Parkinson’s disease: from genetics to the clinic. Mov Dis. 2018;33(5):684–696. doi: 10.1002/mds.27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stang PE, Ryan PB, Racoosin JA, Overhage JM, Hartzema AG, Reich C, et al. Advancing the science for active surveillance: rationale and design for the observational medical outcomes partnership. Ann Intern Med. 2010;153(9):600–606. doi: 10.7326/0003-4819-153-9-201011020-00010. [DOI] [PubMed] [Google Scholar]
- 5.Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, et al. Observational health data sciences and informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574–578. [PMC free article] [PubMed] [Google Scholar]
- 6.Mittal S, Bjornevik K, Im DS, Flierl A, Dong X, Locascio JJ, et al. β2-Adrenoreceptor is a regulator of the alpha-synuclein gene driving risk of Parkinson’s disease. Science. 2017;357(6354):891–898. doi: 10.1126/science.aaf3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng F, Desai RJ, Handy DE, Wang R, Schneeweiss S, Barabasi AL, et al. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat Commun. 2018;9(1):2691. doi: 10.1038/s41467-018-05116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan PB, Schuemie MJ, Madigan D. Empirical performance of a self-controlled cohort method: lessons for developing a risk identification and analysis system. Drug Saf. 2013;36(Suppl 1):S95–S106. doi: 10.1007/s40264-013-0101-3. [DOI] [PubMed] [Google Scholar]
- 9.Ryan PB, Stang PE, Overhage JM, Suchard MA, Hartzema AG, DuMouchel W, et al. A comparison of the empirical performance of methods for a risk identification system. Drug Saf. 2013;36(Suppl 1):S143–S158. doi: 10.1007/s40264-013-0108-9. [DOI] [PubMed] [Google Scholar]
- 10.Ryan PB, Schuemie MJ. Evaluating performance of risk identification methods through a large-scale simulation of observational data. Drug Saf. 2013;36(Suppl 1):S171–S180. doi: 10.1007/s40264-013-0110-2. [DOI] [PubMed] [Google Scholar]
- 11.Madigan D, Schuemie MJ, Ryan PB. Empirical performance of the case-control method: lessons for developing a risk identification and analysis system. Drug Saf. 2013;36(Suppl 1):S73–S82. doi: 10.1007/s40264-013-0105-z. [DOI] [PubMed] [Google Scholar]
- 12.Butt DA, Tu K, Young J, Green D, Wang M, Ivers N, et al. A validation study of administrative data algorithms to identify patients with Parkinsonism with prevalence and incidence trends. Neuroepidemiology. 2014;43(1):28–37. doi: 10.1159/000365590. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 15.Madigan D, Ryan PB, Schuemie M, Stang PE, Overhage JM, Hartzema AG, et al. Evaluating the impact of database heterogeneity on observational study results. Am J Epidemiol. 2013;178(4):645–651. doi: 10.1093/aje/kwt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Searles Nielsen S, Gross A, Camacho-Soto A, Willis AW, Racette BA. β2-adrenoreceptor medications and risk of Parkinson disease. Ann Neurol. 2018;84(5):683–693. doi: 10.1002/ana.25341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voss EA, Makadia R, Matcho A, Ma Q, Knoll C, Schuemie M, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc. 2015;22(3):553–564. doi: 10.1093/jamia/ocu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma C, Liu Y, Neumann S, Gao X. Nicotine from cigarette smoking and diet and Parkinson disease: a review. Transl Neurodegener. 2017;6:18. doi: 10.1186/s40035-017-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalia L, Yo R. β2-Adrenoreceptor agonists and antagonists and Parkinson’s disease. 2019. https://www.movementdisorders.org/MDS/Scientific-Issues-Committee-Blog/B2-Adrenoceptor-agonists.htm. Accessed 01 Feb 2019.
- 20.Baker JG. The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol. 2010;160(5):1048–1061. doi: 10.1111/j.1476-5381.2010.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. Comparative pharmacology of human beta-adrenergic receptor subtypes–characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(2):151–159. doi: 10.1007/s00210-003-0860-y. [DOI] [PubMed] [Google Scholar]
- 22.Sheng P, Hou L, Wang X, Wang X, Huang C, Yu M, et al. Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: a systematic review and meta-analysis. PLoS One. 2013;8(12):e81802. doi: 10.1371/journal.pone.0081802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osier ND, Dixon CE. Catecholaminergic based therapies for functional recovery after TBI. Brain Res. 2016;1640(Pt A):15–35. doi: 10.1016/j.brainres.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wisor J. Modafinil as a catecholaminergic agent: empirical evidence and unanswered questions. Front Neurol. 2013;7(4):139. doi: 10.3389/fneur.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang QM, Xu YY, Liu S, Ma ZG. Isradipine attenuates MPTP-induced dopamine neuron degeneration by inhibiting up-regulation of L-type calcium channels and iron accumulation in the substantia nigra of mice. Oncotarget. 2017;8(29):47284–47295. doi: 10.18632/oncotarget.17618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simuni T, Holloway R, Oakes D, Biglan K, Lungu C. A Phase 3 study of Isradipine as a disease modifying agent in patients with early Parkinson’s disease (STEADY-PD III): baseline characteristics and study update [abstract no. P2.039] Neurology. 2018;90(15 Suppl):P2.39. [Google Scholar]
- 27.Bowser AD. Isradipine for Parkinson’s disease fails in phase 3 study. AHS 2019 conference news. 2019. https://www.mdedge.com/neurology/article/199907/parkinsons-disease/isradipine-parkinsons-disease-fails-phase-3-study. Accessed 07 Jun 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.