Significance
Development in multicellular organisms consists of a series of cell divisions followed by cell expansions, which are tightly controlled both spatially and temporally. Disorganized cell division causes serious growth defects in both animals and plants. Auxin has been repeatedly implicated in regulating cell division patterns during organogenesis in plants; however, the underlying mechanisms remain to be further investigated. Here, we demonstrate that auxin signaling controls cell division pattern by activating Mitogen-Activated Protein Kinase (MAPK)-signaling cascade during lateral root development. Our work reveals how signal transduction pathways regulate cell division pattern and also provides a connection between auxin signaling and MAPK signaling in plants.
Keywords: auxin, TMKs, MKK4/5, MPK3/6, cell division pattern
Abstract
In both plants and animals, multiple cellular processes must be orchestrated to ensure proper organogenesis. The cell division patterns control the shape of growing organs, yet how they are precisely determined and coordinated is poorly understood. In plants, the distribution of the phytohormone auxin is tightly linked to organogenesis, including lateral root (LR) development. Nevertheless, how auxin regulates cell division pattern during lateral root development remains elusive. Here, we report that auxin activates Mitogen-Activated Protein Kinase (MAPK) signaling via transmembrane kinases (TMKs) to control cell division pattern during lateral root development. Both TMK1/4 and MKK4/5-MPK3/6 pathways are required to properly orient cell divisions, which ultimately determine lateral root development in response to auxin. We show that TMKs directly and specifically interact with and phosphorylate MKK4/5, which is required for auxin to activate MKK4/5-MPK3/6 signaling. Our data suggest that TMK-mediated noncanonical auxin signaling is required to regulate cell division pattern and connect auxin signaling to MAPK signaling, which are both essential for plant development.
Coordinated cell division and cell expansion are essential for proper organ development in both animals and plants (1–3). Cell division orientation determines cell fate, and formative cell division drives organ morphogenesis and tissue differentiation. Extensive research has focused on the regulation of the polarized direction of cell expansion, such as the interdigitated pavement cell expansion (4, 5), the tip growth of pollen tubes (6), and root hair cells (7), but little is known about how the direction of cell division is determined.
The phytohormone auxin plays a pivotal role in regulating plant growth and development in a concentration-dependent manner. Disruption of the auxin concentration gradient causes severe defects in cell division patterns and aberrant organ development (8–13). Lateral root (LR) initiation and development, a tightly coordinated process involving the strictly controlled cell division pattern (14), provides a model system to study the underlying mechanism of auxin regulation in cell division patterns during organogenesis (15, 16).
Previous work demonstrated that abundant key transcription factors, regulated by the TIR1/AFB-based canonical auxin pathway, were essential for organogenesis, including LR development (17–20). Recently, TMK-mediated auxin signaling was identified to regulate the ROP signaling pathways in the pavement cells morphogenesis (21) and the noncanonical Aux/IAAs in the apical hook development (22). This discovery provides a new perspective for understanding the action of auxin in many developmental processes.
Mitogen-Activated Protein Kinase (MAPK) cascades are highly conserved signaling modules found in all eukaryotic cells. Plant MAPK pathways, which convert signals into cellular responses, regulate various aspects of plant growth and development (23–25). Accumulating evidences illustrate that plant MAPKs are involved in regulating cell division during organogenesis. The NPK1-NQK1-NRK1 cascade regulates cytokinesis in Nicotiana tabacum (26, 27), and the activated NPK1, a MAPKKK, acts at the late M-phase to mediate the cell plate formation (28). MKK4/MKK5 together with MPK3/MPK6 regulate stomatal patterning by controlling asymmetric cell division (29) and inflorescence architecture by promoting localized cell proliferation in Arabidopsis (30). Recently, MPK6 was proved to affect cell division orientation in both primary and lateral roots (31, 32), and the MKK4/MKK5-MPK3/MPK6 module was essential for LR emergence (33).
Notably, mutual regulation between plant MAPK signal transduction modules and auxin signaling has been reported (34, 35). Exogenous auxin application can specifically activate MPK3/MPK6 in root (33, 36). However, it is still unknown how MPK3/MPK6 are activated by auxin. In this study, we demonstrate that the local auxin maximum at LR initiation sites triggers TMK1/TMK4-mediated phosphorylation and activation of MKK4/MKK5, followed by the activation of MPK3/MPK6 to regulate cell division pattern during LR development.
Results
TMK1 and TMK4 Are Required for Auxin-Regulated Cell Division Pattern during LR Development.
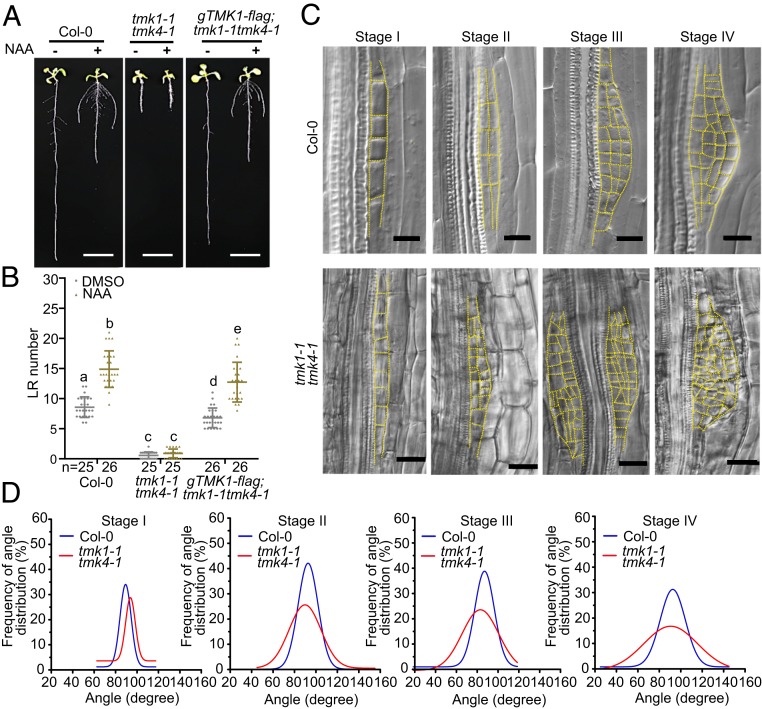
To investigate potential nontranscriptional mechanisms underlying auxin-mediated regulation of LR development in Arabidopsis, we examined a transmembrane kinases (TMKs) family that was recently found to participate in auxin signaling (22). Of the 4 TMK members, tmk1-1 and tmk4-1 mutants showed slightly fewer LRs and lower LR density than wild type (SI Appendix, Fig. S1 A–C), and LRs were mostly abolished in the tmk1-1tmk4-1 double-mutant (Fig. 1 A and B and SI Appendix, Fig. S1 A and B), suggesting that TMK1 and TMK4 play an essential but redundant role in regulating LR development. Notably, GUS reporter analyses driven by the native promoters of both TMK1 and TMK4 suggested that TMK1 and TMK4 were highly expressed at the different stages of LR development (SI Appendix, Fig. S2), which were confirmed by pTMK1-TMK1-GFP;tmk1-1 and pTMK4-TMK4-GFP;tmk4-1 transgenic lines (SI Appendix, Fig. S3A). These were in agreement with the strong LR phenotype in the tmk1-1tmk4-1 mutant. Exogenous auxin treatment with 150 nM NAA (1-Naphthaleneacetic acid) strongly promoted LR development including LR number and lateral root primordia (LRP) in wild type but not in the tmk1-1tmk4-1 mutant (Fig. 1 A and B and SI Appendix, Fig. S4). However, the tmk1-1tmk4-1 mutant had a bit higher LRP density compared to wild type (SI Appendix, Fig. S4B), which may be caused by the severe growth defect, such as the much shorter primary root. Therefore, we generated an estradiol-inducible TMK4 RNAi in the tmk1 mutant background to further investigate the role of TMK1 and TMK4 in LR development. The estradiol treatment in the tmk1−/−tmk4RNAi-est mutant resulted in the reduced expression and protein level of TMK4 (SI Appendix, Fig. S5 D and F). This mutant showed similar phenotype as in the tmk1-1tmk4-1 double-mutant (SI Appendix, Fig. S5 A–C). To avoid the effect of primary root defect in the tmk1-1tmk4-1 mutant, we transferred the 5-d-old noninduced mutant to the 1/2MS medium containing 50 μM estradiol for another 5 d and found that the lateral roots were partially abolished in the newly generated primary roots of the estradiol treated tmk1−/−tmk4RNAi-est mutant (SI Appendix, Fig. S5E). We further noticed that the cell division patterns were disorganized at the different stages of LR development in the tmk1-1tmk4-1 mutant, leading to the LR growth defects in the mutant compared to the wild type (Fig. 1 C and D and SI Appendix, Fig. S3B). Together, these data suggest that TMK1 and TMK4 are required to regulate cell division pattern and LR development in response to auxin.
Fig. 1.
TMK1 and TMK4 regulate auxin-mediated cell division pattern during lateral root development. (A) Lateral root from 9-d-old seedlings in Col-0, tmk1-1tmk4-1 and complementing gTMK1-flag;tmk1-1tmk4-1 lines. Seedlings grown on medium with dimethyl sulfoxide (DMSO) or 150 nM NAA. (Scale bar, 1 cm.) (B) Number of LRs per seedling of 9-d-old Col-0, tmk1-1tmk4-1 mutant and gTMK1-flag in tmk1-1tmk4-1 transgenic line seedlings with or without NAA treatment. Values indicate mean ± SD from 3 individual biological repeats. n denotes number of independent seedlings, and different letters denote significant differences from 1-way ANOVA with Tukey multiple comparisons test. (C) Cell division pattern in lateral root primordia at stage I to IV in Col-0, tmk1-1tmk4-1. (Scale bar, 20 μm.) (D) Frequency of angle distribution in LRP cells (From stage I to IV) in Col-0, tmk1-1tmk4-1. Ten primordia of each stage from 10 individual seedlings showed similar results.
TMK1 and TMK4 Interact with MKK4 and MKK5.
To determine how TMK1/TMK4 regulate cell division orientation and LR development, we identified proteins that interact with TMK1 in Arabidopsis. Briefly, we used GFP-trap agarose beads to immunoprecipitate TMK1-GFP and interacting proteins from pTMK1-TMK1-GFP transgenic plants, followed by mass spectrometry. Among the TMK1-interaction candidates, we were particularly interested in MAP-kinase kinase 4 (MAPKK4 or MKK4), a key component of the MAPK-signaling pathway that was previously reported to regulate both cell division and LR development in plant (30, 33).
Arabidopsis contains 10 MKK family members (37). To comprehensively investigate these MKKs, we used the unbiased yeast 2-hybrid assay to screen all MKK proteins for interactions with the C terminus of the 4 TMK family members. We found that the C terminus of TMK1 and TMK4 specifically interacted with MKK4 and MKK5 (Fig. 2A and SI Appendix, Fig. S6A), which belong to the same subfamily (SI Appendix, Fig. S6C). The TMK2C terminus interacted with MKK5 (SI Appendix, Fig. S6A); however, TMK2 was not expressed in the LR (38). Transiently expressed MYC-tagged MKK4 and MKK5 coimmunoprecipitated with HA-tagged TMK1 from protoplasts (Fig. 2B). Consistently, HA-tagged MKK4 and MKK5 coimmunoprecipitated with MYC-tagged TMK1 (SI Appendix, Fig. S6B). These interactions were further confirmed on plasma membrane by the FLIM-FRET (Fluorescence Lifetime Imaging Microscopy–Fluorescence Resonance Energy Transfer) assay in protoplasts (Fig. 2 C and D). Similar to TMK1 and TMK4, both MKK4 and MKK5 were also highly expressed at the different stages of LR development, assessed in pMKK4-MKK4-RFP and pMKK5-MKK5-RFP transgenic lines (SI Appendix, Fig. S7). Based on these data, we hypothesized that MKK4/5 participate with TMK1/4 to regulate LR development.
Fig. 2.
TMK1 interacts with MKK4 and MKK5. (A) Yeast 2-hybrid assay of TMK1C-terminus (as bait) and MKK4 and MKK5 protein (as prey). Three independent biological repeats. AD, activation domain; BD, DNA-binding domain; -TL, synthetic dextrose minimal medium without leucine and tryptophan; -TLHA, synthetic dextrose minimal medium without leucine, tryptophan, adenine, and histidine. (B) α-MYC coimmunoprecipitated TMK1-HA with MKK4-MYC and MKK5-MYC in protoplasts. The 35S-TMK1-HA was transiently coexpressed with 35S-MKK4-MYC or 35S-MKK5-MYC in Arabidopsis protoplasts. Three independent repeats showed similar results. (C) Images of FLIM-FRET (Fluorescence Lifetime Imaging Microscopy–Fluorescence Resonance Energy Transfer) assay between TMK1 and MKK4 or MKK5. The 35S-TMK1-GFP was transiently coexpressed with 35S-MKK4-RFP or 35S-MKK5-RFP in Arabidopsis protoplasts, and the protoplasts were harvested after 6 h for image analysis. (Scale bar, 10 μm.) (D) Quantification of lifetimes of GFP signals (C). n denotes number of independent protoplasts. Asterisks indicate a statistically significant difference (****P value < 0.0001), according to a Student’s t test.
MKK4 and MKK5 Regulate Cell Division Pattern during LR Development in Response to Auxin.
The mkk4mkk5 double-mutant is embryonic lethal; therefore, we generated an estradiol-inducible MKK4 and MKK5 RNAi mutant to further investigate the role of MKK4 and MKK5 in LR development. Estradiol treatment of the mkk4,5RNAi-est mutant resulted in the reduced expression of MKK4 and MKK5, but not other MKKs (SI Appendix, Fig. S8 A and C). Similar to the tmk1-1tmk4-1 mutant, the estradiol-treated mkk4,5RNAi-est mutant showed strong defects in LR development which were also insensitive to auxin treatment (Fig. 3 A and B). The LR density in the estradiol-treated mkk4,5RNAi-est was insensitive to auxin, but LRP density in the mutant was a bit higher than in the wild type (SI Appendix, Fig. S9 A and B). To avoid differences in primary root growth, we grew wild-type and mkk4,5RNAi-est seedlings in 1/2 MS medium for 5 d and then transferred them to the medium containing 50 μM estradiol together with 150 nM NAA for another 5 d (SI Appendix, Fig. S10A). Again, we observed defective LR development in the estradiol-treated mkk4,5RNAi-est seedlings that was not rescued by auxin treatment (Fig. 3 A and B). These defects in the estradiol-treated mkk4,5RNAi-est mutant were also associated with the disorganized cell division patterns at different stages of LR development (Fig. 3 E and F). Thus, MKK4 and MKK5 also regulate LR development in response to auxin.
Fig. 3.
MKK4/5-MPK3/6 regulates auxin-dependent cell division pattern during lateral root development. (A) Lateral root phenotype of 9-d-old seedlings in Col-0 and estradiol-inducible mutant mkk4,5RNAi-est. Seedlings grown on 1/2MS medium with dimethyl sulfoxide (DMSO) or 50 µM estradiol or 50 μM estradiol plus 150 nM NAA. (Scale bar, 1 cm.) (B) Number of LRs per seedling of Col-0 and estradiol-inducible mutant mkk4,5RNAi-est (A). Error bars indicate SD of 3 individual biological repeats. n denotes number of independent seedlings. (C) Lateral root phenotype of 9-d-old seedlings in Col-0 and estradiol-inducible mutant mpk6−/−mpk3RNAi-est. Seedlings grown on 1/2MS medium with DMSO or 20 nM estradiol or 20 nM estradiol plus 150 nM NAA. (Scale bar, 1 cm.) (D) Number of LRs per seedling of 9-d-old Col-0 and estradiol-inducible mutant mpk6−/−mpk3RNAi-est (C). Error bars indicate SD of 3 individual biological repeats. n denotes number of independent seedlings, and different letters denote significant differences from 1-way ANOVA with Tukey multiple comparisons test in B and D. (E) Cell division pattern in LRP at stage I to IV in Col-0, estradiol-inducible mkk4,5RNAi-est, and mpk6−/−mpk3RNAi-est mutant. (Scale bar, 20 µm.) (F) Frequency of angles distribution in LRP cells (from stage I to IV) in Col-0, estradiol-inducible mkk4,5RNAi-est, and mpk6−/−mpk3RNAi-est mutant. Ten primordia of each stage from 10 individual seedlings showed similar results.
Auxin Induces TMK1/4-Dependent Phosphorylation of MKK4/5.
The TMK-mediated signaling pathway has been implicated in auxin signal transduction (22, 38), which led us to hypothesize that auxin triggers the activation of MKK4 and MKK5 via TMKs. To test this hypothesis, we treated Arabidopsis protoplasts transiently expressing MKK4 or MKK5 with 100 nM NAA for 1 h. Auxin treatment led to the mobility shift of MKK4 and MKK5 in Phos-tag polyacrylamide gels that was abolished by Lambda Protein Phosphatase (λ-PPase) treatment (Fig. 4A), suggesting that auxin induces MKK4/5 phosphorylation. Using the same approach, we found that coexpression of TMK1, but not a kinase inactive TMK1(K616E), induced the phosphorylation of MKK4/5 (Fig. 4B). These data suggest that TMK1 also can phosphorylate MKK4/5. Indeed, an in vitro phosphorylation assay confirmed that both MKK4 and MKK5 were specifically and directly phosphorylated by a recombinant TMK1C terminal fragment (Fig. 4C). These observations collectively suggest that TMK-based auxin signaling may participate in regulating the phosphorylation of MKK4/5, which is consistent with the similar LR defects in both tmk1-1tmk4-1 and mkk4,5RNAi-est mutants.
Fig. 4.
TMKs mediate the phosphorylation of MKK4/5-MPK3/6 by auxin. (A) Phos-tag gel shift assay shows that auxin triggers phosphorylation of MKK4 and MKK5. The 35S-MKK4-MYC or 35S-MKK5-MYC (5 h) were transiently expressed in Arabidopsis protoplasts and then treated with 100 nM NAA for 1 h; dimethyl sulfoxide (DMSO) was used as a control. MKK4/5 proteins were detected with an anti-MYC antibody. Similar results were obtained with 3 biological repeats. (B) Phos-tag gel shift assay shows that TMK1, but not kinase inactive TMK1(K616E), triggers phosphorylation of MKK4 and MKK5. Arabidopsis protoplasts transiently expressed 35S-MKK4-MYC or 35S-MKK5-MYC with 35S-TMK1-HA or 35S-TMK1(K616E)-HA for 6 h. The 35S-HA transformed with 35S-MKK4-MYC or 35S-MKK5-MYC were used as control. Two independent repeats showed similar results. (C) In vitro kinase assay of TMK1 kinase domain with MKK4(PD) and MKK5(PD) protein. Phosphorylation is detected by radioactive P32. MKK4(PD), kinase inactive MKK4(K108M); MKK5(PD), kinase inactive MKK5(K99M). Protein loading was detected by Western blotting. (D) Auxin levels correlated with levels of phosphorylated MPK6 and MPK3 in seedlings root. The 6-d-old seedlings were used for 100 µM l-Kyn treatment for 1 h, then added back 10 µM NAA; the seedlings roots were harvested for protein extraction. Three biological repeats showed similar results. (E) Quantification of relative MPK6 and MPK3 kinase activity in auxin treatment shown in D. n = 3 biological repeats; data are mean ± SD. (F) Auxin induces MPK6 and MPK3 phosphorylation partially via TMK1 and TMK4. The 6-d-old tmk1−/−tmk4RNAi-est seedlings grown on 1/2MS were transferred to medium containing 10 μM estradiol for another 4 d, the seedlings were harvested for 100 μM l-Kyn treatment for 1 h, then added back 10 μM NAA, and the seedling roots were harvested for protein extraction. Three biological repeats. (G) Quantification of relative MPK6 and MPK3 kinase activity in tmk1−/−tmk4RNAi-est seedlings’ treatment with auxin shown in F. n = 3 biological repeats; data are mean ± SD. Asterisks indicate a statistically significant difference (*P value < 0.05; **P value < 0.01; ***P value < 0.001; ****P < 0.0001), according to a Student’s t test; n.s., not significant.
Auxin Activates MPK3 and MPK6 through TMK1/4 to Regulate Cell Division Pattern in LR.
MPK3/6 have been reported to function downstream of MKK4/5 in multiple developmental processes (29, 30, 39, 40). Similar to TMK1/4 and MKK4/5, both MPK3 and MPK6 were also highly accumulated at different stages of LR development (SI Appendix, Fig. S7). To determine whether MPK3/6 play a role in LR development, we generated an estradiol-inducible MPK3 RNAi in the mpk6 mutant, since the mpk3mpk6 double-mutant was also embryonic lethal. Estradiol treatment of the mpk6−/−mpk3RNAi-est mutant resulted in the reduced expression of MPK3 in the mpk6 mutant background (SI Appendix, Fig. S8B). Similar to the tmk1-1tmk4-1 and estradiol-inducible mkk4,5RNAi-est mutants, estradiol treatment of mpk6−/−mpk3RNAi-est also led to LR developmental defects (SI Appendix, Figs. S9 C and D and S10B) and the disorganized cell division pattern, which was insensitive to auxin (Fig. 3 C–F).
To further verify whether auxin activates MPK3/6 to regulate LR development, we examined yuc1-D mutant, which produces excessive auxin (41), and wei8-3tar2-1 mutant, which produces reduced auxin (42). The levels of phosphorylated MPK3/6 correlated with auxin levels in the respective mutants (SI Appendix, Fig. S11 A and B). In addition, we treated wild-type seedlings with l-kynurenine (l-Kyn) (43) to inhibit auxin biosynthesis, followed by the exogenous auxin (NAA or IAA) treatment. We found that l-Kyn treatment led to slightly reduced levels of phosphorylated MPK3/6, which was restored by auxin treatment. Auxin treatment also restored MPK3/6 phosphorylation in the auxin-deficient wei8-3tar2-1 mutant (Fig. 4 D and E and SI Appendix, Fig. S11 C and D). It has been reported that MKK4/5 mediate the activation of MPK3/6 by auxin (33). More importantly, we found that the auxin-induced phosphorylation of MPK3/6 was partially abolished in the tmk1−/−tmk4RNAi-est mutant, but not in the tir1afb2afb3 mutant (Fig. 4 F and G and SI Appendix, Fig. S11 E and F). Taken together, our observations collectively suggest that auxin activates the MKK4/5-MPK3/6-signaling cascade via TMK1/4 to regulate cell division pattern during LR development.
Discussion
The molecular mechanism underlying the strict control of cell division pattern, by which multicellular organisms produce new and specific tissues and organs, is a basic and central theme in developmental biology. The formative cell divisions are required for multiple developmental processes both in plants and animals. Unlike animals, most plant organs, including lateral roots, are primarily postembryonically initiated from continuous de novo development, implying that these mechanisms establish tight spatiotemporal coordination of cell division and patterning (16).
In this study, we show that auxin activates MKK4/5-MPK3/6 cascade through TMK1/4 to regulate cell division pattern during LR morphogenesis. TMKs, as the key components of auxin signaling on plasma membrane, mediate noncanonical auxin-signaling transduction to control versatile developmental processes (21, 22), which is distinct from TIR1-based canonical transcriptional auxin signaling. This finding provides a mechanism to regulate cell division pattern during organ morphogenesis and a perspective of auxin action at the cellular level. Previous work demonstrated that TIR1-based auxin signaling regulates lateral root development, especially initiation, through the transcriptional regulation of the key components involved in this developmental process. That TMK1/TMK4 mediate auxin activation of the MKK4/5-MPK3/6 cascade provides a posttranscriptional regulation mechanism, which coordinates with the TIR1 pathway to control the LR development precisely (SI Appendix, Fig. S12). This also fills a missing link in our understanding of both auxin and MAPK-signaling events, whose modes of action are critical for plant growth and development. Given the pivotal roles of auxin in cell division patterning, our findings shed light on how hormonal signaling leads to changes in cell division orientation, which is important for plant cells (44), but distinct from animal cells.
The new lateral roots grow from LR initiation sites where auxin accumulates (SI Appendix, Fig. S2) (9), the same place as the protein abundance of TMK1/TMK4, MKK4/MKK5 and MPK3/MPK6 (SI Appendix, Figs. S2, S3A, and S7). Both auxin and MKK4/5-MPK3/6 activities are required for LR development (9, 33, 45). In the meanwhile, MKK4 and MKK5, as well as MPK3 and MPK6, have redundant functions in diverse biological processes, including stomatal differentiation (29), inflorescence localized cell division (30), and zygote division (46). Although our work here focuses on the roles of this auxin-signaling mechanism in LR cells, it is likely that similar auxin-TMK-MAPK modules operate in other plant cells and tissues.
Plant cell division orientation relies on the PPB (preprophase band), a specialized MT (microtubule) apparatus composed of MTs and F-actins, to organize the cell division plane (47). Recent evidence indicates that MPK6 is associated with MT dynamics (48) and involved in the control of cell division plane formation (32), which provides some hints for further exploring the detailed mechanism of how the auxin–TMK–MAPK pathway elaborately regulates cell division orientation. Moreover, MPK3 and MPK6 are protein kinases and likely function with downstream substrates or transcription factors, whose identification will be crucial to understand how this signaling executes and exports outputs.
The MKK4/5-MPK3/6 cascade has been reported as downstream of several RLKs [e.g., ER (30), FLS2 (49), HAE/HSL2 (50), CLV1/CLV2 (51)] and senses various upstream signals (e.g., various stress stimuli and development signals). For example, IDA-HAE/HLS2 ligand–receptor mediates auxin-facilitated LR formation through MKK4/5-MPK3/6. Auxin also activates MPK3 and MPK6 in an IDA-HAE/HSL2-independent manner, suggesting the existence of unknown signaling pathways (33). The finding of the TMK-MKK4/5-MPK3/6 cascade may fill the gap between the auxin and MAPK cascade. Due to the overlapped downstream components, the auxin–TMK-signaling pathway may reciprocally crosstalk with IDA-HAE/HLS2 signaling, which means phytohormone and plant small peptides coordinate to regulate LR development. Meanwhile, it remains unclear how plants apply the same signaling pathway in distinct biological processes. One possibility is that the MKK4/5-MPK3/6 cascade functions as a molecular switch over the course of evolution, which links the various tissue- or cell-specific activation of upstream inputs and the spatiotemporal control of downstream substrates, i.e., TMK1/TMK4, are highly expressed at LR primordia where the upstream input auxin accumulates (SI Appendix, Fig. S2). Thus, it is worthwhile to understand how these multiple signaling pathways conjugated to regulate cellular events in response to the complex developmental cues.
Materials and Methods
Plant growth conditions and materials, phenotype analyses, plasmid constructs, GUS staining and imaging, RT-qPCR analyses, recombinant protein purification, Western blots, Coimmunoprecipitation, FLIM-FRET assay, in vitro kinase assay, MAPK activity assay, and primers (SI Appendix, Table S1) are provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by the National Key R&D Program of China (2016YFA0503200), the National Natural Science Foundation of China (Grants 31870256 and 31422008, to T.X.), and by National Natural Science Foundation of China (Grant 31470359, to J.H.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910916116/-/DCSupplemental.
References
- 1.Rasmussen C. G., Humphries J. A., Smith L. G., Determination of symmetric and asymmetric division planes in plant cells. Annu. Rev. Plant Biol. 62, 387–409 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Gillies T. E., Cabernard C.; Cell Division Orientation in Animals , Cell division orientation in animals. Curr. Biol. 21, R599–R609 (2011). [DOI] [PubMed] [Google Scholar]
- 3.De Smet I., Beeckman T., Asymmetric cell division in land plants and algae: The driving force for differentiation. Nat. Rev. Mol. Cell Biol. 12, 177–188 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Fu Y., Gu Y., Zheng Z., Wasteneys G., Yang Z., Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120, 687–700 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Xu T., et al. , Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143, 99–110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung A. Y., Wu H. M., Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu. Rev. Plant Biol. 59, 547–572 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Fischer U., et al. , Vectorial information for Arabidopsis planar polarity is mediated by combined AUX1, EIN2, and GNOM activity. Curr. Biol. 16, 2143–2149 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Mattsson J., Ckurshumova W., Berleth T., Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol. 131, 1327–1339 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benková E., et al. , Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi N., et al. , A molecular framework for auxin-mediated initiation of flower primordia. Dev. Cell 24, 271–282 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y., Dai X., Zhao Y., Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19, 2430–2439 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinhardt D., et al. , Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Rademacher E. H., et al. , Different auxin response machineries control distinct cell fates in the early plant embryo. Dev. Cell 22, 211–222 (2012). [DOI] [PubMed] [Google Scholar]
- 14.De Smet I., et al. , Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 322, 594–597 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Marhavý P., et al. , Auxin reflux between the endodermis and pericycle promotes lateral root initiation. EMBO J. 32, 149–158 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marhavý P., et al. , Targeted cell elimination reveals an auxin-guided biphasic mode of lateral root initiation. Genes Dev. 30, 471–483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okushima Y., Fukaki H., Onoda M., Theologis A., Tasaka M., ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19, 118–130 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., et al. , WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 26, 1081–1093 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H. W., Kim N. Y., Lee D. J., Kim J., LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol. 151, 1377–1389 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian H., Jia Y., Niu T., Yu Q., Ding Z., The key players of the primary root growth and development also function in lateral roots in Arabidopsis. Plant Cell Rep. 33, 745–753 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Xu T., et al. , Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343, 1025–1028 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao M., et al. , TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 568, 240–243 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Xu J., Zhang S., Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 20, 56–64 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez M. C., Petersen M., Mundy J., Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 61, 621–649 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Tena G., Boudsocq M., Sheen J., Protein kinase signaling networks in plant innate immunity. Curr. Opin. Plant Biol. 14, 519–529 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishihama R., et al. , The NPK1 mitogen-activated protein kinase kinase kinase is a regulator of cell-plate formation in plant cytokinesis. Genes Dev. 15, 352–363 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soyano T., Nishihama R., Morikiyo K., Ishikawa M., Machida Y., NQK1/NtMEK1 is a MAPKK that acts in the NPK1 MAPKKK-mediated MAPK cascade and is required for plant cytokinesis. Genes Dev. 17, 1055–1067 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishihama R., et al. , Expansion of the cell plate in plant cytokinesis requires a kinesin-like protein/MAPKKK complex. Cell 109, 87–99 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Wang H., Ngwenyama N., Liu Y., Walker J. C., Zhang S., Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19, 63–73 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng X., et al. , A MAPK cascade downstream of ERECTA receptor-like protein kinase regulates Arabidopsis inflorescence architecture by promoting localized cell proliferation. Plant Cell 24, 4948–4960 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smékalová V., et al. , Involvement of YODA and mitogen activated protein kinase 6 in Arabidopsis post-embryogenic root development through auxin up-regulation and cell division plane orientation. New Phytol. 203, 1175–1193 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller J., et al. , Arabidopsis MPK6 is involved in cell division plane control during early root development, and localizes to the pre-prophase band, phragmoplast, trans-Golgi network and plasma membrane. Plant J. 61, 234–248 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Zhu Q., et al. , A MAPK cascade downstream of IDA-HAE/HSL2 ligand-receptor pair in lateral root emergence. Nat. Plants 5, 414–423 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Kovtun Y., Chiu W. L., Zeng W., Sheen J., Suppression of auxin signal transduction by a MAPK cascade in higher plants. Nature 395, 716–720 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Jia W., et al. , Mitogen-activated protein kinase cascade MKK7-MPK6 plays important roles in plant development and regulates shoot branching by phosphorylating PIN1 in Arabidopsis. PLoS Biol. 14, e1002550 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mockaitis K., Howell S. H., Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J. 24, 785–796 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Group M.; MAPK Group , Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 7, 301–308 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Dai N., Wang W., Patterson S. E., Bleecker A. B., The TMK subfamily of receptor-like kinases in Arabidopsis display an essential role in growth and a reduced sensitivity to auxin. PLoS One 8, e60990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao C., et al. , EDR1 physically interacts with MKK4/MKK5 and negatively regulates a MAP kinase cascade to modulate plant innate immunity. PLoS Genet. 10, e1004389 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim T. W., Michniewicz M., Bergmann D. C., Wang Z. Y., Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 482, 419–422 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y., et al. , A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291, 306–309 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Stepanova A. N., et al. , TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191 (2008). [DOI] [PubMed] [Google Scholar]
- 43.He W., et al. , A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23, 3944–3960 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao W., Dong J., Polarity in plant asymmetric cell division: Division orientation and cell fate differentiation. Dev. Biol. 419, 121–131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Himanen K., et al. , Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14, 2339–2351 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lukowitz W., Roeder A., Parmenter D., Somerville C., A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell 116, 109–119 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Müller S., Wright A. J., Smith L. G., Division plane control in plants: New players in the band. Trends Cell Biol. 19, 180–188 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Kohoutová L., et al. , The Arabidopsis mitogen-activated protein kinase 6 is associated with γ-tubulin on microtubules, phosphorylates EB1c and maintains spindle orientation under nitrosative stress. New Phytol. 207, 1061–1074 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Asai T., et al. , MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415, 977–983 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Cho S. K., et al. , Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 105, 15629–15634 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Betsuyaku S., et al. , Mitogen-activated protein kinase regulated by the CLAVATA receptors contributes to shoot apical meristem homeostasis. Plant Cell Physiol. 52, 14–29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.