Abstract
Multi-angle light scattering coupled with size-exclusion chromatography (SEC-MALS) is a standard approach for protein characterization. Recently MALS detection has been coupled with ion-exchange chromatography (IEX) which demonstrated the feasibility and high value of MALS in combination with non-sized-based fractionation methods. In this study we coupled reverse-phase ultra-high pressure liquid chromatography (RP-UPLC) with a low-dispersion MALS detector for the characterization of intact monoclonal antibody (mAbs) and their fragments. We confirmed a constant refractive index increment value for mAbs in RP gradients, in good agreement with the values in literature for other classes of proteins. We showed that the impurities eluting from a RP column can often be related to aggregated species and we confirmed that in most cases those oligomers are present also in SEC-MALS. Yet, in few cases small aggregates fractions in RP-UPLC are an artifact. In fact, proteins presenting thermal and physical stability not suitable for the harsh condition applied during the RP separation of mAbs (i.e. organic solvents at high temperature) can aggregate. Further, we applied RP-UPLC-MALS during a long term stability studies. The different principle of separation used in RP-UPLC- MALS provides an additional critical level of protein characterization compared to SEC-MALS and IEX-MALS.
Subject terms: Proteins, Analytical chemistry
Introduction
Light scattering is one of the widely-used techniques for the characterization of macromolecules and particles in solution in biological and biopharmaceutical sciences1. By far the most common application of light scattering in this field is the determination of mass and size of proteins by means of multi-angle light scattering coupled to size-exclusion chromatography (SEC-MALS)2 or field flow fractionation (FFF-MALS)3. Other important applications include the characterization of protein conformational and colloidal stability and the characterization of both specific and non-specific protein-protein interaction1. The use of MALS with fractionated samples yields a calculation of the absolute molecular weight (Mw) at each point of the chromatogram. As the Mw estimated by the retention time is often inaccurate4,5, SEC-MALS provides a useful tool for determination of accurate monomer and fragment Mw, oligomeric state and hydrodynamic radius (Rh)1,2,6. Recently the advantages of coupling MALS with ion exchange chromatography (IEX) have been demonstrated7. IEX separates proteins according to surface charge based on differences in ionic interaction with the support matrix8. The different principle used in the separation of IEX-MALS provides additional critical information and can resolve SEC-MALS shortcomings7. In this study, we coupled MALS with another type of liquid chromatography, reversed-phase (RPLC). RPLC is a highly promising technique to study chemical changes9–11 and to quantify12,13 peptides and proteins, including monoclonal antibodies (mAbs). Historically, the use of RP to monitor intact mAb was limited because the complex hydrophobic and hydrophilic nature of these large proteins caused poor recovery and limited resolution. More recently, the use of columns with large pores (300 ) at high temperatures (60–75 °C) in combination with non-traditional solvent system containing ion pairing agents has been consolidated as standard procedure for the analysis of mAbs, overcoming previous difficulties14,15. Small chemical differences cannot be separated by standard RP-HPLC16, as they are often insufficient to yield significant changes in polarity17. Here, we took advantage of ultra-high pressure LC (UPLC) instrumentation to further refine the separation of mAb species and their derivatives. We investigated RP-UPLP-MALS for mAb characterization, focusing on two common applications: (i) analysis and characterization of mAb fragments, which are typically studied by mass spectrometry, (ii) analysis of mAbs after long term storage. The former is a real-time stability testing which permits the establishment of recommended storage condition and shelf life of the bio-therapeutic products. The addition of MALS allows the Mw assignment for each individual peak in the chromatogram enabling differentiation between chemical variants of the monomeric form and other impurities or degradation products as aggregates and fragments.
Results and Discussion
RP-HPLC-MALS technique
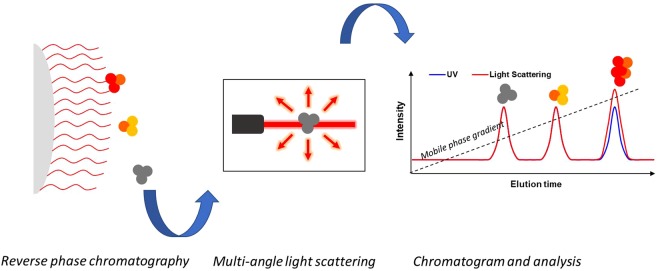
The principle of RP-HPLC-MALS is the combination of RP chromatography with an online MALS detector. As shown in Fig. 1, multiple hydrophobic areas of protein molecules interact with the alkyl silane-derivated surface of the stationary phase. The separation is achieved by decreasing the water concentration in the mobile phase increasing the organic solvent fraction (e.g. acetonitrile). This in turn weakens the hydrophobic attraction of the protein to the column. During elution from the column the molecules are then introduced into a concentration detector (i.e. UV) and subsequently in a MALS detector. Using these detectors to measure the Mw of eluting molecules is especially important as no column calibration procedure, analogous to that of analytical SEC, can be applied to relate the size of a molecule to its hydrophobic interaction with a column matrix.
Figure 1.
Schematic illustration of the RP-UPLC-MALS method. A protein sample is injected in the RP chromatography column in-line with a MALS detector. The protein interacts with the hydrophobic matrix.
Development of RP-UPLC-MALS
Good RP-HPLC conditions for intact protein analysis are typically achieved with a UPLC, a stationary phase with short alkyl chain length and large pore size, a strong ion-pairing agent and an adequate gradient decreasing the water content of the mobile phase at high temperature9. We coupled a low-volume, low-dispersion MALS detector to our UPLC system allowing for small peak width and high resolution. Six different IgG1s (PPI01, PPI02, PPI03, PPI04, PPI10, PPI13), one IgG2 (PPI17), one bispecific (PPI08), and one protein-drug conjugate (PPI18) were used to develop and assess our RP-UPLC-MALS method. IFNα2a served as a reference, as RPLC is a well-established technique to detect its chemically-changed species18–21. During the development of the RP-UPLC-MALS method column type, temperature, flow rate, injection volume, mobile phase and gradient were evaluated14,15. Some proteins presented better resolution with the BEH-300 C4 column compared with the Zorbax 300SB-C8 column. However, we noticed a fast decrease of efficiency with the BEH-300 C4 after just 400 injections, while the Zorbax 300SB-C8 showed good robustness. This is possibly due to the fact that the C4 phase chemistry is less resistant to hydrolysis in acidic media than the C8 phase chemistry. As screen of proteins in multiple formulation and across many time points involve thousands of injections, we selected the Zorbax 300SB-C8 as workhorse.
In order to determine Mw correctly, it is necessary to know the refractive index increment of solute in solution value dn/dc and the concentration for each slice of a peak. It has been shown that MALS is compatible with RP elution gradients22. Different classes of proteins have been investigated in literature with various mobile phase compositions containing aqueous buffer and acetonitrile yielding a dn/dc values close to 0.175 ml/g22–25. It has been shown that assuming a constant dn/dc in the narrow interval of an eluting peak only induces an error at most 3–4%22. This is due to the fact that the solvent refractive index changes only very slightly within the time frame of peak elution26. We first calculated the protein Mw using the dn/dc of proteins in water at 660 nm of 0.185 mL/g27. The obtained Mw was approx. 25% below the Mw calculated based on the primary sequence. Consequently, we fixed the Mw of the monomer as calculated from the primary sequence and confirmed by SEC-MALS to obtain a dn/dc in the RP-MALS eluent. This yielded a dn/dc value of 0.1742 +/− 0.0017 mL/g for the proteins, which is in very good agreement with the literature22–25, and was used for calculating the Mw of the investigated proteins.
Analysis of intact monoclonal antibodies using RP-UPLC-MALS
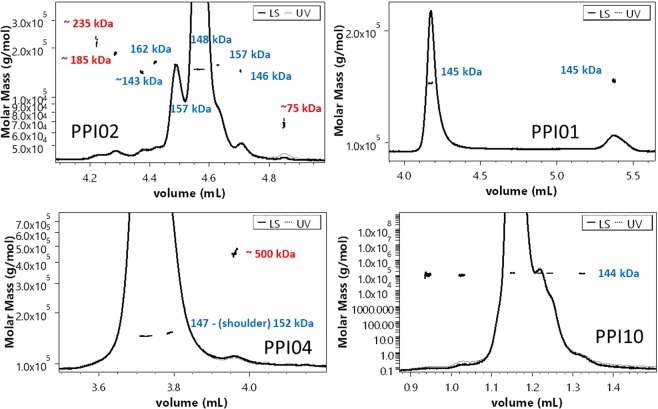
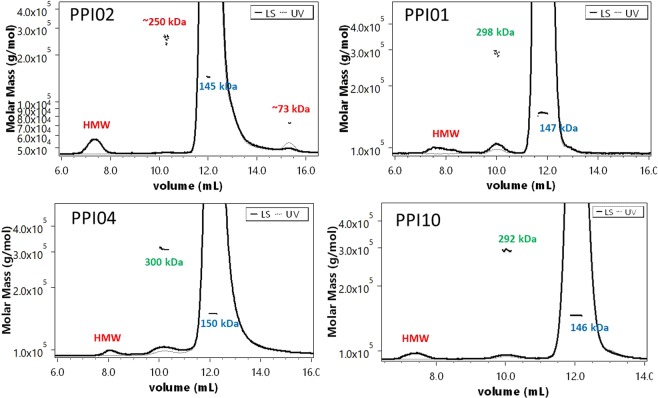
Proteins with similar size cannot be separated by SEC, but if they have a different hydrophobicity they can be separated by RP-UPLC. In our study we encountered three cases: (i) The Mw of all the peaks reflects monomeric variants (e.g. PPI01 and PPI10), (ii) The main peak represents a monomeric form while other impurity peaks are identified as aggregates (e.g. PPI04), (iii) The main peak represents a monomeric form while other impurities peaks are either identified as aggregates, fragments or close to, but not equal within the experimental error, to the monomer Mw (e.g. PPI02) (Fig. 2). Dimers detected in SEC-MALS (Fig. 3) were not found in RP-UPLC-MALS (Fig. 2). As the RP-UPLC recovery was often close or exactly 100% (Table 1) we hypothesize that (i) the monomer-dimer equilibrium is shifted completely towards the monomeric form in the RPLC eluent, (ii) the dimers are prompted to further aggregation, (iii) the dimers are lost over the column. Both RP-UPLC-MALS and SEC-MALS confirmed the absence of oligomers beyond the dimers visible in SEC for PPI01 and PPI10 (Fig. 2). Similar conclusions were reached for PPI13, PPI08 and PPI17 (Supplementary information (SI) 1). Differently, PPI04 (Fig. 2) and PPI18 (SI 2) showed a very small fraction of oligomers by RP-UPLC-MALS, which were not detected in SEC-MALS (Fig. 3).These oligomers may have been induced by the high temperature of 75 °C applied during the RP separation. The first temperature of unfolding (Tm1), the temperature of aggregation (Tagg), and the diffusion interaction parameter (kD) for PPI01, PPI02, PPI03, PP10 and PP17 are 66, 61 °C and 5.6 mg/L (data averaged from 24 formulation conditions, Gentiluomo L, et al.)28 as compared to 54 °C, 47 °C and 4.7 mg/L resp. for PPI18 and 64 °C, 55 °C and −1.9 mg/L for PPI04. This lower thermal and/or colloidal stability of PPI18 and PP4 could explain their susceptibility to aggregation under the RP conditions. Finally, PPI02 showed aggregates and fragments (highlighted in red in Fig. 2) that were also detected in SEC-MALS (Fig. 3). The averaged Mw of the PPI02 aggregates from SEC-MALS and RP-UPLC-MALS are respectively of 250 kDa and 235 kDa. This difference is probably due to the high error in the Mw calculations, which is in turn due to the small concentration of such aggregates. Further, the 235 kDa aggregate in RP-UPLC-MALS is not baseline separated. PPI02 further presented a series of peaks and shoulders with 5 to 15 kDa difference to the monomer Mw, which were not visible by SEC-MALS. The Mw difference may be possibly due to post-translational modifications of the IgG. These typically include methionine oxidation, asparagine and glutamine deamidation, N-terminal acetylation or cyclization, glycation of lysine and variable glycosylation29. Physically, the refractive index increment is insensitive to the long-range structure of macromolecules27 and is nearly independent of its amino acid composition30. However, carbohydrate moieties do affect the refractive index value31. This would suggest that PPI02 comes with a high degree of variation in glycosylation.
Figure 2.
RP-UPLC-MALS of mAbs. Typical chromatograms showing the UV and the MALS signals of PPI02, PPI01, PPI04 and PPI10 analyzed by RP-UPLC-MALS. The MW of the monomer, aggregates/fragments, and dimers are highlighted in blue and respectively. (*) denotes aggregates.
Figure 3.
SEC-MALS of mAbs. Typical chromatograms of the proteins investigated by SEC-MALS showing UV and LS signals along superimposed with calculated molar mass. The MW of the monomer, aggregates/fragments, and dimers are highlighted in blue, red and green, respectively. HMW stands for high-molecular weight species, which are usually not separated, and in all our investigated cases presented no UV detectable signal. (*) denotes aggregates; (**) denotes dimers.
Table 1.
Information on the investigated protein. The theoretical Mw is calculated from the primary sequence. Mass recovery is calculated over all the visible UV peaks as described in material and method.
| Type | Provider | Ɛ at 280 nm (mg/ml/cm) | Theoretical MW (kDa) | MALS Mw (kDa) | Mass recovery | pI | Notes | ID |
|---|---|---|---|---|---|---|---|---|
| IgG1λ | AstraZeneca | 1.56 | 144.8 | 144.1 ± 0.2% | 99.9% | 7.96 | — | PPI01 |
| Human IgG1κ | AstraZeneca | 1.47 | 148.2 | 148.1 ± 0.1% | 100% | 8.53 | — | PPI02 |
| Human IgG1κ | AstraZeneca | 1.435 | 144.8 | 144.6 ± 0.2% | 100% | 8.44 | WT IgG | PPI03 |
| IgG1λ YTE | AstraZeneca | 1.755 | 146.2 | 146.5 ± 0.1% | 97.2% | 8.99 | — | PPI04 |
| IgG1κ + scFv | AstraZeneca | 1.57 | 204.4 | 204.4 ± 0.1% | 98% | 9.2 | Bispecific | PPI08 |
| Human IgG1 | AstraZeneca | 1.533 | 144.2 | 144.6 ± 0.2% | 96.5% | 8.95 | — | PPI10 |
| Human IgG1κ | AstraZeneca | 1.66 | 148.9 | 148.7 ± 0.2% | 100% | 9.04 | — | PPI13 |
| IgG2κ | AstraZeneca | 1.31 | 145.1 | 145.6 ± 0.3% | 99.9% | 7.78 | PPI17 | |
| HSA-NEP | AstraZeneca | 1.04 | 146.7 | 146.3 ± 0.1% | 100% | 5.8 | Conjugate | PPI18 |
| Intα-2A | Roche | 0.972 | 19.2 | 20.1 ± 7.5% | 100% | 5.97 | — | PPI30 |
Characterization of Fab and Fc fragments
Complete proteolytic digestion of mAb (peptide mapping) followed by RP-UPLC coupled with mass spectrometry (MS) is a well-established method for the identification and quantification of chemical modification of mAbs32,33. Alternatively, the analysis by MALS of large fragments, such as Fab and Fc, requires little sample preparation and can provide a high-throughput alternative. The preparation and purification of the fragments was performed as described in material and methods. Subsequently, we investigated the Fab and Fc fragments of PPI01 by RP-UPLC-MALS. The Fc fragment eluted before the intact mAb which in turn eluted before the Fab fragment (Fig. 4). The latter exhibited two shoulders on the left and right of the 47 kDa monomer with a Mw close to that of a Fab dimer (~90 kDa). The Fc fragment elutes with a series of peaks after the main peak of ~110, ~700, ~170 kDa with longer elution time. SEC-MALS measurements on the purified fragments confirmed the presence of Fab dimer and of Fc dimer and trimer (fragments showed in SI 3, intact mAb showed in Fig. 3). However, the 700 kDa Fc aggregate was not detected in SEC-MALS. As mentioned previously, the formation of small fraction of high molecular-weight oligomers due to the RP conditions can affect proteins with insufficient thermal and/or colloidal stability. PP01 shows averaged Tm1, typically reflecting unfolding of the CH2 domain and Tm2, typically reflecting unfolding of the CH3 and Fab fragment, of 64 and 77 °C34–36. This would explain the higher susceptibility of the Fc fragment to unfolding and aggregation. Thus, it could be useful to couple MALS with RP-UPLC-MS to differentiate between monomer and aggregates peak before analyzing the MS spectra.
Figure 4.
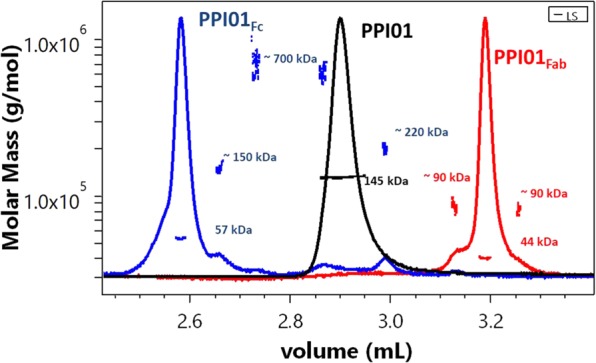
RP-UPLC-MALS of PPI01 and its fragments. PPI01 Fc fragment, PPI01 (whole mAb) and PPI01 Fab fragment are plotted in blue, black and red lines, respectively.
Long term stability studies
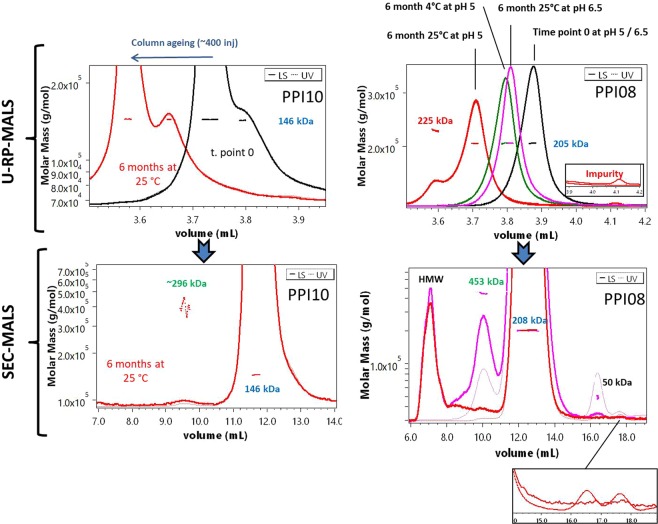
Finally, we performed a long term stability study and analyzed samples with the RP-UPLC-MALS method developed herein to learn whether we can gain additional insights from the MALS information on the chemical stability of our proteins. PP02, PP03, PP04, PP08, PP10, PP13 were tested in 8 different formulations for six month at 4 °C and 25 °C (see SI 4 for the formulations list). We observed an overall high chemical stability. Significant changes upon storage stress occurred only in a few conditions. PP10, formulated in 10 mM His at pH 6.5 stored at 25 °C, exhibited an increased hydrophobicity of the shoulder, presenting the same Mw of the monomer (Fig. 5). Chemical changes can perturb the local conformation backbone of proteins, such in the case of deamidation, the most common hydrolytic reaction for protein, and Asp isomerization. Conformational variants of proteins often present increased hydrophobicity and are more prone to aggregate37. Other chemical reactions, such Met oxidation, could on the other side decrease the hydrophobicity of proteins38. However, RP-UPLC-MALS cannot provide mechanistic insight behind an increased hydrophobicity after isothermal stress. For such purpose mass spectroscopy, which could be coupled with RP-UPLC-MALS, could provide quantitation of degradation products, such in the case of deamidation products39. PPI08 stored at 25 °C in 10 mM Histidine at pH 5 showed a new peak with an Mw of 225 kDa, which was not observed in any other formulation and was not noticeable in SEC-MALS (Fig. 5). This aggregate is probably formed by a mixture of fragments formed during the stress e.g. Fab, Fc, Heavy chain or by a complex formed by monomer and light chain. Comparison with SEC-MALS, confirmed the presence of fragments (Fig. 5). As baseline separation was not obtained between the monomer and the dimer, we could not tell whether the small complex is present in the formulation or formed during the RP separation. Regardless, MALS provided the exact Mw of the peaks eluting upon RP-UPLC, which allowed differentiation between chemical variants of the monomer (i.e. in cases of PPI10) and aggregates (i.e. in case of PPI08) formed during long term storage.
Figure 5.
RP-UPLC-MALS and SEC-MALS of mAbs for long term stability studies. Typical chromatograms of the long-term-storage study, showing the regions of eluting sample. Top: RP-UPLC-MALS chromatograms; bottom: SEC-MALS chromatograms. Left: PPI10; right: PPI08. The Mw of the monomer, aggregates/fragments, and dimers are highlighted in blue, red and green, respectively. HMW stands for high-molecular-weight species, which are usually not separated, and in all our investigated cases presented no detectable UV signal. A magnified section shows the impurities for PP08. The shifting of the chromatograms at different time points is due to column ageing. PPI10 is shown in one formulation only (His 10 mM at pH 6.5), where the chromatograms before and after 6 months at 25 °C are depicted in black and red, respectively. PPI08 is shown formulated at pH 6.5 (His 10 mM) before stress, in black, and after 6 months at 25 °C, in magenta, and formulated at pH 5 (His 10 mM) before, in black, and after 6 months at 4 °C, in green, and 6 months at 25 °C, in red. PPI08 fragments are zoomed.
Conclusions
We successfully coupled RP-UPLC with MALS to calculate the Mw of each eluting peak of intact mAbs and of Fc and Fab fragments. The different principle of separation used in RP-UPLC-MALS provides an additional critical level of protein characterization compared to SEC-MALS and IEX-MALS. RP is one of the most promising analytical techniques to analyze proteins11,12,40. Yet, peaks eluting from the column can often be related to aggregated species. Thanks to MALS, it is possible to tell whether an impurity is indeed a chemical variant of the monomer, an aggregate or a fragment. Furthermore, we highlight that the organic solvent and the temperature applied during the RP separation of mAbs could artificially induce aggregates which may lead to false interpretation of protein purity. Nonetheless, MALS could not be enough to describe detailed mechanisms and further coupling with MS (i.e. RP-UPLC-MALS-MS) could prove in the future natural development to characterize RP chromatograms.
Material and Methods
Sample preparation
Five antibodies IgG1s (PPI02, PPI03, PPI04, PPI10, PPI13), one bispecific antibody (PPI08), one IgG2 (PPI17), and one HSA-fusion protein (PPI18) were provided by AstraZeneca (Cambridge, UK). Interferon alpha-2a (PPI30) was provided from Roche Diagnostics GmbH. A summary of the protein’s physical properties is listed in Table 1. The proteins were dialyzed overnight using Slide-A-Lyzer™ cassettes (Thermo Fisher Scientific, Waltham, USA) with suitable membrane cut-off against excess of 10 mM of histidine HCl buffer with pH 5.0, 5.5, 6.0, 6.5, 7.0, 7.5. The excipient (e.g. NaCl) stock solutions were prepared in the respective buffers. Protein concentration was measured on a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, USA) using the protein extinction coefficient calculated from the primary sequence. All conditions were prepared in 1.5 mL non-coated PP Eppendorf tubes. Finally, the formulations were sterile-filtered with 0.22 μm cellulose acetate filters from VWR International (Darmstadt, Germany). The purity of the proteins was studied by SEC and cEIF (SI 5).
Ultra-high-pressure reverse-phase chromatography combined with multi-angle light scattering (RP-UPLC-MALS)
RP-UPLC-MALS was conducted on an ACQUITY UPLC H-Class system (Waters, UK) equipped with a quaternary pump, an autosampler, UV detector and a μDAWN detector (Wyatt Technology, USA). The separation was performed with both an Acquity BEH-300 C4 (Waters, UK) and a Zorbax 300SB-C8 column (Agilent Technologies, USA). The samples were diluted to 1 mg/mL before injection. For monoclonal antibodies a pilot gradient of 20 to 40% of eluent B in A over 20 minutes was used. Eluent A consisted of 10% w/v acetonitrile and 0.1% w/v trifluoracetic acid in ultrapure water. Eluent B consisted of 0.1% w/v trifluoracetic acid in acetonitrile. The flow rate was 0.2 mL/min. The column oven temperature was set at 75 °C. A preheater was included before the column. Subsequently, depending on the protein and the column used the gradient was fine-tuned. All methods were based on a gradient from 20–25 to 40%. On-column adsorption of the mAbs was evaluated systematically and almost complete mass recovery was reached for all the protein (Table 1). All the calculations were performed with ASTRA V7.1 software (Wyatt Technology, USA). Mass recovery is calculated from the injected mass versus the calculated mass from the concentration detector (i.e. UV). Therefore, to achieve an accurate determination of the mass recovery the sample concentration needs to be measured accurately. Thus, the concentration was measured again before injection in real triplicates by a Nanodrop One (Thermo Fisher Scientific, Waltham, USA). The theoretical extinction coefficients were double-checked re-calculating the values from the RI monomeric peaks during the SEC-MALS experiments. PPI30 (int-2alpha) was used as a standard. Finally, to achieve a flat baseline, we collected and subtracted the blanks by the algorithm included in the ASTRA V7.1 software.
Size-exclusion chromatography combined with multi angle light scattering (SEC–MALS)
SEC-MALS was conducted on Agilent 1260 Bio-Inert system with a variable wavelength UV detector operated at 280 nm (Thermo Fischer Scientific, USA), followed by a TREOS II detector (Wyatt Technology, USA) and an Optilab T-rEX (Wyatt Technology, USA). The temperature controlled-autosampler was kept at 4 °C. Separation was performed with a Superdex 200 increased 10/30 GL column. Data were collected and processed using the ASTRA® software V7.2 (Wyatt Technology, USA). The aqueous mobile phase consisted of 38 mM NaH2PO4, 12 mM Na2HPO4, 150 mM NaCl and 200 ppm NaN3 at pH 7.4 dissolved in HPLC-grade water, filtered through Durapore VVPP 0.1 m membrane filters (Millipore Corporation, USA). The samples were centrifuged and injected in duplicates of 25 µl.
Stress assay
0.2 mL of each protein solution was aliquoted at a concentration of 1 mg/mL and filtered in 0.5 mL sterile non-coated PP Eppendorf tubes. The samples were incubated at 4 °C and 25 °C, for 6 months. After storage, the samples were quenched in an ice bath, left at 4 °C and measured within two weeks. Sample concentration was measured after the stress in real triplicates by a Nanodrop One (Thermo Fisher Scientific, Waltham, USA). Similarly, the pH was measured after the stress showing no changes within the experimental error (i.e. ±0.1).
Preparation and purification of Fab and Fc Fragments
Immobilized Papain (Thermo Fisher Scientific, Waltham, USA) was used to digest PPI01 into its Fab and Fc fragments. PPI01 at 20 mg/mL was pipetted into 15 mL glass vial, the vial capped with the resin separator provided with the kit to remove all the air-liquid interface. The vial was gently rotated by a Sunlab rotator SU1100 for 5 h at 37 °C. An ÄKTA purifier 10 (GE Healthcare, Uppsala, Sweden) equipped with a Pierce Protein A chromatography cartridge (Thermo Fisher Scientific, Waltham, USA) (column volume, CV = 5 ml) was used to separate Fc (and undigested mAb) from the Fab fragments. The binding buffer was composed of 100 mM sodium phosphate with 150 mM NaCl at pH 7.2. The column was equilibrated with 2 CV of binding buffer with a flow of 2 ml/min. Fractions were collected in 15-ml PP tubes using a Frac 920 fraction collector (GE Healthcare, Uppsala, Sweden) capturing any unbound species e.g. Fab. The elution buffer (100 mM sodium phospate at pH 3) was kept at 100% over 7 CV. The eluting protein was collected in 15-ml PP tubes using the fraction collector, and was immediately neutralized with a 1 M sodium phosphate buffer at pH 8.5. Ultrafiltration was performed using Vivaspin® tubes with a 10 kDa MWCO PES membrane (Sartorius Stedim Biotech, Göttingen, Germany). Success of the purification was monitored by HP-SEC (see 3.4).
Supplementary information
Acknowledgements
This study was funded by a project part of the EU Horizon 2020 Research and Innovation program under the Marie Skłodowska-Curie grant agreement No 675074. The first author would like to thank the whole PIPPI consortium (http://www.pippi.kemi.dtu.dk) for the continuous support offered. The first author thanks Wyatt Technology staff members for their many contributions, with a special mention to Dan Some for the for the passionate discussion related to this work and for reviewing the manuscript. The first author would like to thanks Hristo Svinelov, Andreas Tosstorff, Marcello Morales and Maria Laura Greco for formulating some of the sample presented in this work.
Author contributions
L.G., D.R. and W.F. conceived and designed the study. L.G. supervised the study. V.S. conducted the experiments and analyzed the data. L.G. and W.F. contributed to the writing of the manuscript.
Data availability
The raw data presented in this work will be made available via a specially-designed publicly-available database (https://pippi-data.kemi.dtu.dk/), currently in phase alpha.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-51233-6.
References
- 1.Minton AP. Recent applications of light scattering measurement in the biological and biopharmaceutical sciences. Anal. Biochem. 2016;501:4–22. doi: 10.1016/j.ab.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyatt PJ. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta. 1993;272:1–40. doi: 10.1016/0003-2670(93)80373-S. [DOI] [Google Scholar]
- 3.Wittgren B, Wahlund KG. Fast molecular mass and size characterization of polysaccharides using asymmetrical flow field-flow fractionation-multiangle light scattering. J. Chromatogr. A. 1997;760:205–218. doi: 10.1016/S0021-9673(96)00777-7. [DOI] [Google Scholar]
- 4.Sahin E, Roberts CJ. Size-exclusion chromatography with multi-angle light scattering for elucidating protein aggregation mechanisms. Methods Mol. Biol. 2012;899:403–423. doi: 10.1007/978-1-61779-921-1_25. [DOI] [PubMed] [Google Scholar]
- 5.Goyon A, et al. Evaluation of size exclusion chromatography columns packed with sub-3 μm particles for the analysis of biopharmaceutical proteins. J. Chromatogr. A. 2017;1498:80–89. doi: 10.1016/j.chroma.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 6.Nettleship JE, Brown J, Groves MR, Geerlof A. Methods for Protein Characterization by Mass Spectrometry, Thermal Shift (ThermoFluor) Assay, and Multiangle or Static Light Scattering. Methods Mol. Biol. 2008;426:299–318. doi: 10.1007/978-1-60327-058-8_19. [DOI] [PubMed] [Google Scholar]
- 7.Amartely H, Avraham O, Friedler A, Livnah O, Lebendiker M. Coupling Multi Angle Light Scattering to Ion Exchange chromatography (IEX-MALS) for protein characterization. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-25246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fekete S, Beck A, Veuthey JL, Guillarme D. Ion-exchange chromatography for the characterization of biopharmaceuticals. J. Pharm. Biomed. Anal. 2015;113:43–55. doi: 10.1016/j.jpba.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 9.Staub A, Guillarme D, Schappler J, Veuthey JL, Rudaz S. Intact protein analysis in the biopharmaceutical field. J. Pharm. Biomed. Anal. 2011;55:810–822. doi: 10.1016/j.jpba.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Grotefend S, et al. Protein quantitation using various modes of high performance liquid chromatography. J. Pharm. Biomed. Anal. 2012;71:127–138. doi: 10.1016/j.jpba.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Fekete S, Rudaz S, Fekete J, Guillarme D. Analysis of recombinant monoclonal antibodies by RPLC: Toward a generic method development approach. J. Pharm. Biomed. Anal. 2012;70:158–168. doi: 10.1016/j.jpba.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Molnár I, Horváth C. Separation of amino acids and peptides on non-polar stationary phases by high-performance liquid chromatography. J. Chromatogr. A. 1977;142:623–640. doi: 10.1016/S0021-9673(01)92073-4. [DOI] [PubMed] [Google Scholar]
- 13.Navas N, et al. Quantification of an intact monoclonal antibody, rituximab, by (RP)HPLC/DAD in compliance with ICH guidelines. Anal. Bioanal. Chem. 2013;405:9351–9363. doi: 10.1007/s00216-013-7368-1. [DOI] [PubMed] [Google Scholar]
- 14.Dillon TM, Bondarenko PV, Speed Ricci M. Development of an analytical reversed-phase high-performance liquid chromatography-electrospray ionization mass spectrometry method for characterization of recombinant antibodies. J. Chromatogr. A. 2004;1053:299–305. doi: 10.1016/S0021-9673(04)01410-4. [DOI] [PubMed] [Google Scholar]
- 15.Dillon TM, et al. Optimization of a reversed-phase high-performance liquid chromatography/mass spectrometry method for characterizing recombinant antibody heterogeneity and stability. J. Chromatogr. A. 2006;1120:112–120. doi: 10.1016/j.chroma.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Wang S, Liu J, Raghani A. Determination of tryptophan oxidation of monoclonal antibody by reversed phase high performance liquid chromatography. J. Chromatogr. A. 2007;1156:174–182. doi: 10.1016/j.chroma.2007.01.140. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Ortega A, et al. Study and ICH validation of a reverse-phase liquid chromatographic method for the quantification of the intact monoclonal antibody cetuximab. J. Pharm. Anal. 2016;6:117–124. doi: 10.1016/j.jpha.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svilenov H, Winter G. Rapid sample-saving biophysical characterisation and long-term storage stability of liquid interferon alpha2a formulations: Is there a correlation? Int. J. Pharm. 2019;562:42–50. doi: 10.1016/j.ijpharm.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Sharma, V. K. & Kalonia, D. S. Polyethylene glycol-induced precipitation of interferon alpha-2a followed by vacuum drying: Development of a novel process for obtaining a dry, stable powder. AAPS PharmSci6 (2004). [DOI] [PMC free article] [PubMed]
- 20.Mohl S, Winter G. Continuous release of rh-interferon α-2a from triglyceride implants: Storage stability of the dosage forms. Pharm. Dev. Technol. 2006;11:103–110. doi: 10.1080/10837450500464230. [DOI] [PubMed] [Google Scholar]
- 21.Hermeling S, et al. Structural characterization and immunogenicity in wild-type and immune tolerant mice of degraded recombinant human interferon alpha2b. Pharm. Res. 2005;22:1997–2002. doi: 10.1007/s11095-005-8177-9. [DOI] [PubMed] [Google Scholar]
- 22.Astafieva IV, Eberlein GA, Wang YJ. Absolute on-line molecular mass analysis of basic fibroblast growth factor and its multimers by reversed-phase liquid chromatography with multi-angle laser Light scattering detection. J. Chromatogr. A. 1996;740:215–229. doi: 10.1016/0021-9673(96)00134-3. [DOI] [PubMed] [Google Scholar]
- 23.Mhatre R, Krull IS, Stuting HH. Determination of biopolymer (protein) molecular weights by gradient elution, reversed-phase high-performance liquid chromatography with low-angle laser light scattering detection. J. Chromatogr. A. 1990;502:21–46. doi: 10.1016/S0021-9673(01)89561-3. [DOI] [PubMed] [Google Scholar]
- 24.Mhatre R, Krull IS. Determination of On-Line Differential Refractive Index and Molecular Weight via Gradient HPLC Interfaced with Low-Angle Laser Light Scattering, Ultraviolet, and Refractive Index Detection. Anal. Chem. 1993;65:283–286. doi: 10.1021/ac00051a016. [DOI] [PubMed] [Google Scholar]
- 25.Stuting HH, Krull IS. Determination of pituitary and recombinant human growth hormone molecular weights by modern high-performance liquid chromatography with low angle laser light scattering detection. J. Chromatogr. A. 1991;539:91–109. doi: 10.1016/S0021-9673(01)95363-4. [DOI] [PubMed] [Google Scholar]
- 26.An N, Zhuang B, Li M, Lu Y, Wang ZG. Combined Theoretical and Experimental Study of Refractive Indices of Water-Acetonitrile-Salt Systems. J. Phys. Chem. B. 2015;119:10701–10709. doi: 10.1021/acs.jpcb.5b05433. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Brown PH, Schuck P. On the distribution of protein refractive index increments. Biophys. J. 2011;100:2309–2317. doi: 10.1016/j.bpj.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gentiluomo L, et al. Application of interpretable artificial neural networks to early monoclonal antibodies development. Eur. J. Pharm. Biopharm. 2019;141:81–89. doi: 10.1016/j.ejpb.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Walsh CT, Garneau-Tsodikova S, Gatto GJ. Protein posttranslational modifications: The chemistry of proteome diversifications. Angew. Chem. Int. Ed. Engl. 2005;44:7342–7372. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]
- 30.Wen J, Arakawa T, Philo JS. Size-exclusion chromatography with on-line light-scattering, absorbance, and refractive index detectors for studying proteins and their interactions. Anal. Biochem. 1996;240:155–166. doi: 10.1006/abio.1996.0345. [DOI] [PubMed] [Google Scholar]
- 31.Arakawa T, Wen J. Determination of carbohydrate contents from excess light scattering. Anal. Biochem. 2001;299:158–161. doi: 10.1006/abio.2001.5432. [DOI] [PubMed] [Google Scholar]
- 32.Lundell N, Schreitmüller T. Sample preparation for peptide mapping - A pharmaceutical quality- control perspective. Anal. Biochem. 1999;266:31–47. doi: 10.1006/abio.1998.2919. [DOI] [PubMed] [Google Scholar]
- 33.Williams KR, Stone KL. Identifying Sites of Posttranslational Modifications in Proteins Via HPLC Peptide Mapping. Methods Mol Biol. 1995;40:157–175. doi: 10.1385/0-89603-301-5:157. [DOI] [PubMed] [Google Scholar]
- 34.Menzen T, Friess W. High-throughput melting-temperature analysis of a monoclonal antibody by differential scanning fluorimetry in the presence of surfactants. J. Pharm. Sci. 2013;102:415–428. doi: 10.1002/jps.23405. [DOI] [PubMed] [Google Scholar]
- 35.Ionescu RM, Vlasak J, Price C, Kirchmeier M. Contribution of variable domains to the stability of humanized IgG1 monoclonal antibodies. J. Pharm. Sci. 2008;97:1414–1426. doi: 10.1002/jps.21104. [DOI] [PubMed] [Google Scholar]
- 36.Garber E, Demarest SJ. A broad range of Fab stabilities within a host of therapeutic IgGs. Biochem. Biophys. Res. Commun. 2007;355:751–757. doi: 10.1016/j.bbrc.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 37.Wang, W. & Roberts, C. J. Aggregation of therapeutic protein. John Wiley & Sons (2010).
- 38.Uversky VN, Yamin G, Souillac PO, Goers J, Glaser CB. Methionine oxidation inhibits fibrillation of human alpha-synuclein in vitro. FEBS Lett. 2002;517:239–44. doi: 10.1016/S0014-5793(02)02638-8. [DOI] [PubMed] [Google Scholar]
- 39.Barnes, C. A., Lim, A. Applications of mass spectrometry for the structural characterization of recombinant protein pharmaceuticals. Mass Spectrom Rev. 26, 370–388 [DOI] [PubMed]
- 40.Molnar I, Boysen RI, Erdmann VA. High-performance-liquid chromatography of Thermus aquaticus 50S and 30S ribosomal proteins. Chromatographia. 1989;28:39–44. doi: 10.1007/BF02290381. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data presented in this work will be made available via a specially-designed publicly-available database (https://pippi-data.kemi.dtu.dk/), currently in phase alpha.