Abstract
Background
The increasing emission of flue gas from industrial plants contributes to environmental pollution, global warming, and climate change. Microalgae have been considered excellent biological materials for flue gas removal, particularly CO2 mitigation. However, tolerance to high temperatures is also critical for outdoor microalgal mass cultivation. Therefore, flue gas- and thermo-tolerant mutants of Chlorella vulgaris ESP-31 were generated and characterized for their ability to grow under various conditions.
Results
In this study, we obtained two CO2- and thermo-tolerant mutants of Chlorella vulgaris ESP-31, namely, 283 and 359, with enhanced CO2 tolerance and thermo-tolerance by using N-methyl-N-nitro-N-nitrosoguanidine (NTG) mutagenesis followed by screening at high temperature and under high CO2 conditions with the w-zipper pouch selection method. The two mutants exhibited higher photosynthetic activity and biomass productivity than that of the ESP-31 wild type. More importantly, the mutants were able to grow at high temperature (40 °C) and a high concentration of simulated flue gas (25% CO2, 80–90 ppm SO2, 90–100 ppm NO) and showed higher carbohydrate and lipid contents than did the ESP-31 wild type.
Conclusions
The two thermo- and flue gas-tolerant mutants of Chlorella vulgaris ESP-31 were useful for CO2 mitigation from flue gas under heated conditions and for the production of carbohydrates and biodiesel directly using CO2 from flue gas.
Keywords: Flue gas, Microalgae, Chlorella sp., Thermo-tolerance
Background
The threat of global warming caused by the increasing levels of CO2 in the atmosphere due to the combustion of fossil fuels is of great concern [1]. Flue gases emitted from industrial plants containing high concentrations of CO2, NOX and SOX are mostly responsible for global CO2 emissions [2, 3]. The use of microalgae for biological CO2 mitigation and flue gas cleaning (removal) has attracted global attention because (1) microalgae do not compete directly with food crops for land or water; (2) they can fix CO2 via photosynthesis at much higher rates than terrestrial higher plants can [4–7]; and (3) the microalgal biomass obtained by the consumption of CO2 is a very useful feedstock for the production of biofuels and many valuable chemicals [8, 9].
In general, typical flue gas emitted from combustion sources contains 10–15% CO2, and coupling microalgae cultivation with CO2 generated by industrial plants has the potential to reduce the cost of flue gas pretreatment of industrial plants and industrial-scale microalgae production [10, 11]. However, the presence of high concentrations of CO2, NOX, SOX, and other impurities of industrial flue gases inhibits the growth of most microalgae [12, 13]. Therefore, the generation and selection of fast-growing microalgal strains or mutants with high CO2 fixation efficiency and the ability to tolerate high concentrations of CO2, NOX, and SOX in flue gases will improve the efficiency and cost-effectiveness of microalgal flue gas CO2 mitigation processes [12, 14, 15].
The tolerance to high temperatures is also critical for outdoor microalgal mass cultivation. The optimal growth temperature for most algae is approximately 20–30 °C [12, 16], and the high temperatures during outdoor cultivation suppress the growth of most microalgae.
In subtropical regions such as Taiwan, the temperature of microalgal culture medium in photobioreactors (PBRs) frequently surpasses 40 °C due to sunlight irradiation during the daytime [17]. Thus, using microalgae strains that can tolerate high temperatures would significantly reduce the cooling costs for outdoor cultivation. Although many thermo-tolerant microalgal strains have been isolated from hot springs [18–20], they may not be able to tolerate the high levels of CO2, SOX, and NOX in flue gases.
A lipid-rich green microalga, Chlorella vulgaris ESP-31, exhibits 30–31 mg/L/day lipid productivity and 35–44% lipid content, respectively, when grown under phototrophic and photoheterotrophic conditions using CO2 and acetic acid as the carbon source, respectively [21]. However, when grown outdoors, the biomass productivity obtained from phototrophic and photoheterotrophic conditions was only 0.07 and 0.11 g/L/day, respectively, compared to 0.26 and 0.20 g/L/day obtained under indoor cultivation conditions [21].
In this study, C. vulgaris ESP-31 was subjected to N-methyl-N′-nitro-N-nitrosoguanidine (NTG) mutagenesis [22] and then screened for mutants tolerant to high temperature and high CO2 concentration. Two mutants of C. vulgaris ESP-31, mutants 283 and 359, with the ability to tolerate heat and high levels of CO2, NO, and SO2 were isolated and characterized. These mutants showed the ability to grow at high temperature (40 °C) in simulated flue gas containing 25% CO2/air, 80–90 ppm SO2, and 90–100 ppm NO. The mutants also showed higher carbohydrate and lipid contents than those of the C. vulgaris ESP-31 wild type, and they may be useful for CO2 mitigation and the production of carbohydrates and biodiesel by directly using CO2 from flue gas.
Results
Isolation of Chlorella vulgaris ESP-31 mutants under the conditions of high temperature and high CO2 concentration
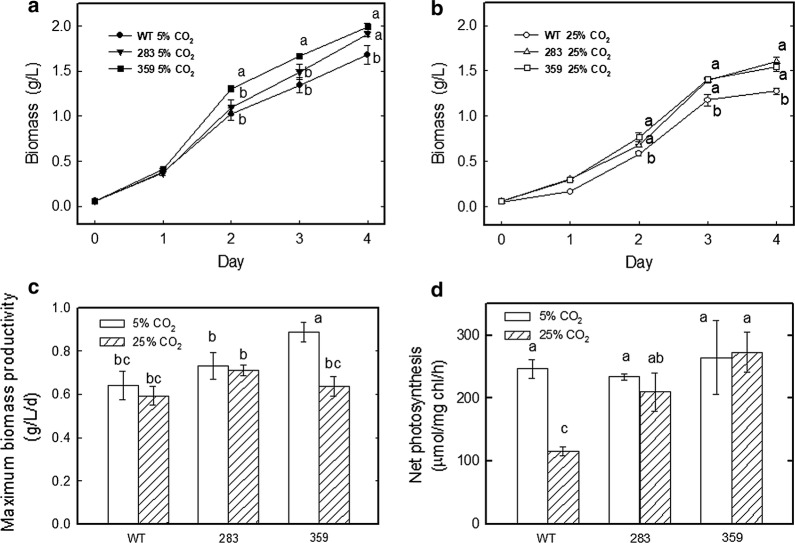
Over 500 potential mutants of C. vulgaris ESP-31 were obtained after NTG mutagenesis, all of which were screened for the tolerance of high temperature (40 °C) and high CO2 concentration (15% CO2/air) using the w-zipper pouch selection method. Of them, two mutants (namely, mutant 283 and mutant 359) showed better growth than the wild type under 40 °C and 15% CO2/air. The two mutants and wild type were grown at 28 °C in BG-11 medium under two different CO2 concentrations (5 and 25% CO2/air) and 150 μmol m−2 s−1 for 4 days, followed by detection and quantification of the biomass, maximum biomass productivity and net photosynthetic rates. The results showed that the biomass and net photosynthetic O2 evolution rates of mutants 283 and 359 were increased when compared with those of the wild type.
When aerated with 5% CO2/air, compared to the wild type, mutants 283 and 359 exhibited biomass increases of 14% and 18%. The biomass values were measured as 1.68, 1.91, and 1.99 g/L for the wild type and mutants 283 and 359, respectively (Fig. 1a). As a consequence, the maximum biomass productivity of strains 283 and 359 was increased by 14% and 39%, respectively, when compared to that of the wild type, with a maximum biomass productivity of 0.64, 0.73 and 0.89 g/L/day for the wild type and mutants 283 and 359, respectively, after 4 days (Fig. 1c).
Fig. 1.
Effects of different CO2 concentrations on the growth and photosynthesis rates of the wild type and mutants 283 and 359 of Chlorella vulgaris ESP-31. a Growth curves under 5% CO2. b Growth curves under 25% CO2. c Biomass productivity. d Net photosynthesis rates. A 50-mL culture of each strain aerated with different CO2 (0.1 vvm) was grown in BG-11 at 28 °C under 12 h light–dark (light intensity 150 μmol m−2 s−1) conditions for 4 days. Data shown are the means (along with the standard deviations) of at least two independent experiments
When grown under 25% CO2/air, compared with the wild type, mutants 283 and 359 exhibited biomass increases of 25% and 20%, respectively. The biomass was measured as 1.28, 1.61, and 1.54 g/L for the wild type and mutants 283 and 359, respectively (Fig. 1b). Consistently, the maximum biomass productivity of mutants 283 and 359 was increased by 20% and 8%, respectively, when compared to that of the wild type, with a maximum biomass productivity of 0.59, 0.71 and 0.64 g/L/day for the wild-type, 283 and 359 strains, respectively, after 4 days (Fig. 1c).
The net photosynthetic O2 evolution rate was determined in mutants 283 and 359 in response to different CO2 concentrations (5 and 25% CO2/air). As shown in Fig. 1d, the net photosynthetic O2 evolution rates of mutants 283 and 359 were similar to those of the wild type with 5% CO2/air. However, when grown in medium aerated with 25% CO2/air, the wild type presented inhibited net photosynthetic O2 evolution rates compared with those under 5% CO2/air. In contrast, the values for mutants 283 and 359 remained almost the same as those with 5% CO2/air aeration. Therefore, under 25% CO2/air, the net photosynthetic O2 evolution rates were increased by 1.8- to 2.4-fold in the mutants in comparison with the wild type. The net photosynthetic O2 evolution rates were measured as 115.7, 210.4, and 272.9 μmol/mg chl/h for the wild type and mutants 283 and 359, respectively (Fig. 1d).
Taken together, when grown at 28 °C in 5% CO2/air under 150 μmol m−2 s−1 light intensity, the growth and photosynthetic efficiency of mutants 283 and 359 were significantly higher than those of the wild type. As the CO2 level increased to 25%, both the growth and photosynthetic efficiency of these two mutants and the wild type were decreased. However, under 25% CO2 conditions, both the growth and photosynthetic efficiency of the two mutants were much higher than those of the wild type. This result indicated that mutants 283 and 359 can tolerate higher levels of CO2 than can the wild type.
Effects of high temperature on the cell growth and photosynthesis rates of Chlorella vulgaris ESP-31 mutants
High temperature impacts the growth and photosynthesis of algae in outdoor cultivation systems [23, 24]. The temperatures in the closed PBRs usually surpass 40–45 °C without temperature control in tropical and subtropical regions. Therefore, high temperatures have become an issue for outdoor cultivation. In the present study, to examine the combined effects of high CO2 concentration and high temperature on cell growth, the two mutants (mutants 283 and 359) and the wild type were grown at 40 °C in BG-11 medium under two different CO2 concentrations (5 and 25% CO2/air) and 150 μmol m−2 s−1 for 4 days, separately, followed by detection of the biomass, maximum biomass productivity, net photosynthetic rates and maximum PSII activity. The biomass and net photosynthetic O2 evolution rates of mutants 283 and 359 were increased in comparison with those of the wild type (Fig. 2).
Fig. 2.
Effects of high temperature and different CO2 concentrations on the growth and photosynthetic rates of the wild type and mutants 283 and 359 of Chlorella vulgaris ESP-31. a The growth performance under different CO2 concentrations (5 and 25% CO2/air) at 40 °C. b Biomass productivity. c Net photosynthesis rates. (D) Maximum PSII activity. A 50-mL culture of each strain aerated with different CO2 (0.1 vvm) was grown in BG-11 at 40 °C under 12 h light–dark (light intensity 150 μmol m−2 s−1) conditions for 4 days. Data shown are the means (along with the standard deviations) of at least two independent experiments
When aerated with 5% CO2/air, compared with the wild type, mutants 283 and 359 exhibited biomass increases of 1.7- and 2.1-fold. The values of biomass were measured as 0.50, 0.84, and 1.07 g/L for the wild type and mutants 283 and 359, respectively (Fig. 2a). Meanwhile, the maximum biomass productivity of mutants 283 and 359 was increased by 1.5- and twofold, respectively, when compared to that of the wild type, with a biomass productivity of 0.21, 0.31 and 0.42 g/L/day for the wild type and mutants 283 and 359, respectively, after 4 days (Fig. 2b).
When grown at 40 °C in the medium aerated with 5% CO2/air, compared with the wild type, mutants 283 and 359 exhibited net photosynthetic O2 evolution rate increases of 20.3% and 30.2%, respectively. The photosynthetic O2 evolution rates were measured as 145.1, 174.5, and 188.9 μmol/mg chl/h for the wild type and mutants 283 and 359, respectively (Fig. 2c).
When aerated with 25% CO2/air, mutants 283 and 359 exhibited biomass increases of 5.4- and 3.8-fold, respectively. The biomass was measured as 0.20, 1.08 and 0.75 g/L for the wild type and mutants 283 and 359, respectively (Fig. 2a). The maximum biomass productivity of mutants 283 and 359 increased by 3.5-fold and 1.8-fold, respectively, when compared to that of the wild type, with a biomass productivity of 0.12, 0.42 and 0.22 g/L/day for the wild type and mutants 283 and 359, respectively, after 4 days (Fig. 2b). Although the mutant strain 359 showed a lower biomass under the 25% CO2 condition than under the 5% CO2 condition, strain 283 showed a higher biomass under the 25% CO2 condition than under the 5% CO2 condition. When grown at 40 °C in the medium aerated with 25% CO2/air, compared with the wild type, mutants 283 and 359 exhibited photosynthetic O2 evolution rate increases of 30% and 25.4%. The photosynthetic O2 evolution rates were measured as 144.8, 188.9, and 181.5 μmol/mg chl/h for the wild type and mutants 283 and 359, respectively (Fig. 2c).
The PSII activities of mutants 283 and 359 were determined in response to 40 °C under different CO2 concentrations (5 and 25% CO2/air). As shown in Fig. 2d, the growth of mutants 283 and 359 was similar to that of the wild type with 5% CO2/air. However, when mutants 283 and 359 were grown in the medium aerated with 25% CO2/air, the maximum PSII activity of remained stable. The maximum PSII activity was measured as 0.095, 0.615 and 0.49 for the wild type and mutants 283 and 359, respectively (Fig. 2d).
Effects of simulated flue gas and high temperature on the cell growth, photosynthesis rates, and carbohydrate and lipid levels of Chlorella vulgaris ESP-31 mutants under indoor conditions
To investigate the effects of simulated flue gas (25% CO2, 80–90 ppm SO2, 90–100 ppm NO) and high temperature on cell growth, the wild type and mutants 283 and 359 were grown at 40 °C in ¼ N BG11 medium (0.375 g/L NaNO3) aerated with the simulated flue gas (0.1 vvm) under 150 μmol m−2 s−1 light intensity, followed by measurement of the biomass, biomass productivity, net photosynthetic rates, maximum PSII activity and nitrate concentration.
When aerated with simulated flue gas (25% CO2, 80–90 ppm SO2, 90–100 ppm NO) and grown at 40 °C, mutants 283 and 359 showed a better survival rate than the wild type; the values of biomass were measured as 0.08, 0.72 and 0.58 g/L for the wild type and mutants 283 and 359, respectively, after 9 days of aeration with simulated flue gas and grown at 40 °C (Fig. 3a). The maximum biomass productivity of mutants 283 and 359 increased by 4.5- and 3.4-fold, respectively, when compared to that of the wild type, with a biomass productivity of 0.028, 0.126 and 0.095 g/L/day for the wild type and mutants 283 and 359, respectively (Fig. 3b). The net photosynthetic O2 evolution rates of mutants 283 and 359 increased by 55.2% and 24.5% after 2 days of cultivation. The photosynthetic O2 evolution rates were measured as 99.4, 154.3 and 123.8 μmol/mg chl/h for the wild type and mutants 283 and 359, respectively (Fig. 3c). As shown in Fig. 3d, when mutants 283 and 359 were grown at 40 °C, the maximum PSII activity remained stable in comparison with that of the wild type (Fig. 3d).
Fig. 3.
Effects of simulated flue gas and high temperature on the growth and photosynthetic rates of the wild type and mutants 283 and 359 of Chlorella vulgaris ESP-31. a Growth curves. b Biomass productivity. c Net photosynthesis rates. d Maximum PSII activity. e Nitrate concentration. f Nile red staining. Bright field: A, C, E, G, I, K. Fluorescence: B, D, F, H, J, L. An 800-mL culture of each strain aerated with simulated flue gas (0.1 vvm) was grown in ¼ N BG-11 medium (containing 0.375 g/L NaNO3) at 40 °C with a speed at 150 rpm under a light cycle consisting of 12 h of light with 150 μmol m−2 s−1 and then aeration with air (0.1 vvm) at 28 °C with a speed at 150 rpm of 12 h of dark conditions for 9 days. Next, the cells were cultivated by aeration with air (0.1 vvm) at 28 °C with a speed at 150 rpm and a light/dark cycle of 12 h/12 h at a light intensity of 150 μmol m−2 s−1 until 28 days. Data shown are the means (along with the standard deviations) of at least two independent experiments
After 9 days of cultivation, mutants 283 and 359 showed that the nitrogen source concentration in the medium was depleted (Fig. 3e). Figure 3f shows images of cells of the wild type and mutants 283 and 359 after 9 days in simulated flue gas. The use of fluorescent Nile red for cellular lipid measurement showed that Nile red fluorescence cannot be found during simulated flue gas injection (9 days) while algal cells were stained with Nile red fluorescence after air aeration for 28 days. The simulated flue gases containing NO might influence the lipid accumulation of cells. Therefore, to promote the lipid accumulation, cultures were switched to air aeration after 9 days of growth in simulated flue gas until 28 days.
After 28 days of culture, the carbohydrate content in mutants 283 and 359 was 29.98 and 24.02%, respectively, and the lipid content in the wild type and mutants 283 and 359 was 3.72, 17.84 and 17.49%, respectively (Table 1). The carbohydrate production of mutants 283 and 359 was 234.53 and 152.71 mg/L, respectively, and the lipid production of the wild type and mutants 283 and 359 was 5.57, 139.31 and 111.03 mg/L, respectively (Table 1).
Table 1.
Carbohydrate and lipid production by Chlorella vulgaris ESP-31 mutants grown in simulated flue gas and high temperature
| Strain | Carbohydrate content (%)a | Carbohydrate production (mg/L)b | Lipid content (%)c | Lipid production (mg/L)d |
|---|---|---|---|---|
| Wild type | – | – | 3.72 ± 0.002 | 5.57 ± 0.70 |
| 283 | 29.98 ± 1.71 | 234.53 ± 18.39 | 17.84 ± 0.016** | 139.31 ± 9.69** |
| 359 | 24.02 ± 1.04 | 152.71 ± 4.23 | 17.49 ± 0.030* | 111.03 ± 17.27* |
Each datum indicates the mean ± SD from two experiments
* P < 0.05 and **P < 0.01 were statistically significant as compared with Chlorella vulgaris ESP-31 wild type
–, no data because the cells were dead
aCarbohydrate content (%) = (carbohydrate dry weight/biomass dry weight) × 100%
bCarbohydrate production (mg/L) = (Max. biomass production × carbohydrate content)/100 × 1000
cLipid content (%) = (lipid dry weight/biomass dry weight) × 100%
dLipid production (mg/L) = (Max. biomass production × lipid content)/100 × 1000
Cell growth, photosynthesis, and carbohydrate and lipid production performance of Chlorella vulgaris ESP-31 mutants grown in simulated flue gas under outdoor culture conditions
The growth performance of C. vulgaris ESP-31 mutant 283 and 359 strains in the field was examined in a 1800-mL outdoor PBR from September 6-October 6, 2017, in the School of Environment and Life Sciences, Fooyin University, Kaohsiung, Taiwan (22°60′15.65″N, 120°38′89.70″E). The wild-type, 283 and 359 strains were grown in 1800 mL ¼ BG-11 medium under the conditions of intermittent simulated flue gas aeration (25% CO2, 90–100 ppm NO, 80–90 ppm SO2) (0.1 vvm) at daytime and then aeration with air (0.1 vvm) at night for 6 days. Next, cells were cultivated by aeration with air (0.1 vvm) until 30 days, followed by comparison of the biomass, nitrate concentration and net photosynthetic rates.
When grown in simulated flue gas under outdoor culture conditions, compared with the wild type, strains 283 and 359 exhibited maximum biomass increases of 1.8-fold and 1.7-fold, respectively. The maximum biomass was measured as 0.53, 0.94 and 0.89 g/L for the wild-type, 283 and 359 strains, respectively (Fig. 4a). As a consequence, the nitrate concentration in the medium was depleted after 4 days (Fig. 4b).
Fig. 4.
Growth and photosynthesis rates of the wild type and mutants 283 and 359 of Chlorella vulgaris ESP-31 in a closed photobioreactor aerated with simulated flue gas under outdoor cultivation. a Growth curves, b Nitrate concentration, c net photosynthesis rate, d Nile red staining. Bright field: A, C, E, G, I, K. Fluorescence: B, D, F, H, J, L. A 1800-mL culture of each strain aerated with simulated flue gas (0.1 vvm) was grown in ¼ N BG-11 medium (containing 0.375 g/L NaNO3) at daytime and then aerated with air (0.1 vvm) at night for 6 days. Next, cells were cultivated by aeration with air (0.1 vvm) until 30 days. Data shown are the means (along with the standard deviations) of at least two independent experiments
The net photosynthetic O2 evolution rates of strains 283 and 359 were increased by 1.3- and 1.3-fold in comparison with that of the wild type after 2 days of culture. The photosynthetic O2 evolution rates were measured as 122.5, 163.0 and 163.3 μmol/mg chl/h for the wild-type, 283 and 359 strains, respectively (Fig. 4c).
The carbohydrate and lipid contents of C. vulgaris ESP-31 wild type, 283 and 359 strain cultures that were aerated with simulated flue gas (25% CO2, 90–100 ppm NO, 80–90 ppm SO2) are shown in Table 2. After 30 days of cultivation, the carbohydrate and lipid contents were similar among the wild-type, 283 and 359 strains. The carbohydrate content in the wild type and mutants 283 and 359 was 28.98%, 27.28% and 29.78%, respectively, and the lipid content was 14.08%, 12.24% and 13.78% for the wild-type, 283 and 359 strains, respectively. Figure 4e also shows that the algal cells of the wild-type, 283 and 359 strains were stained by Nile red fluorescence at 6 day before the change to air aeration, and also at 30 day. The carbohydrate production of the wild-type, 283 and 359 strains was 153.36, 255.83 and 266.60 mg/L, respectively, and the lipid production of the wild-type, 283 and 359 strains was 75.10, 115.81 and 123.61 mg/L, respectively (Table 2).
Table 2.
Carbohydrate and lipid production by Chlorella vulgaris ESP-31 mutants grown in closed outdoor PBR aerated with simulated flue gas
| Strain | Carbohydrate content (%)a | Carbohydrate production (mg/L)b | Lipid content (%)c | Lipid production (mg/L)d |
|---|---|---|---|---|
| Wild type | 28.98 ± 1.60 | 153.36 ± 30.65 | 14.08 ± 2.32 | 75.1 ± 23.01 |
| 283 | 27.28 ± 0.70 | 255.83 ± 21.78 | 12.24 ± 1.76 | 115.81 ± 29.27 |
| 359 | 29.78 ± 0.25 | 266.60 ± 30.02 | 13.78 ± 0.73 | 123.61 ± 19.36 |
Each datum indicates the mean ± SD from two experiments
aCarbohydrate content (%) = (carbohydrate dry weight/biomass dry weight) × 100%
bCarbohydrate production (mg/L) = (Max. biomass production × carbohydrate content)/100 × 1000
cLipid content (%) = (lipid dry weight/biomass dry weight) × 100%
dLipid production (mg/L) = (Max. biomass production × lipid content)/100 × 1000
Comparison between indoor and outdoor conditions
The results in Table 3 show the maximum biomass, maximum biomass productivity, CO2 biofixation rate and specific growth rate of C. vulgaris ESP-31 mutants grown under indoor and outdoor conditions with simulated flue gas. In indoor cultivations, the maximum biomass of the wild-type, 283, and 359 strains was 0.15, 0.78 and 0.64 g/L, respectively, indicating a fivefold and fourfold increase for the 283 and 359 strains over the wild-type strain, respectively (Table 3). In addition to higher maximum biomass, when compared with C. vulgaris ESP-31 wild type, mutants 283 and 359 have relatively higher maximum biomass productivity (125.76 and 94.79 mg/L/day), CO2 biofixation rate (236.42 and 178.20 mg/L/day) and specific growth rate (0.52 and 0.35 day−1).
Table 3.
Maximum biomass, maximum biomass productivity, CO2 biofixation rate and specific growth rate of Chlorella vulgaris ESP-31 wild type and mutants under indoor and outdoor condition with simulated flue gas
| Cultivation system | Strain | Maximum biomass (g/L) | Maximum biomass productivity (mg/L/day) | CO2 biofixation rate (mg/L/day) | specific growth rate (day−1) |
|---|---|---|---|---|---|
| Indoor with simulated flue gas | Wild type | 0.15 ± 0.01 | 28.38 ± 1.83 | 53.35 ± 3.44 | 0.27 ± 0.02 |
| 283 | 0.78 ± 0.02*** | 144.72 ± 0.17*** | 272.06 ± 0.31*** | 0.52 ± 0.07* | |
| 359 | 0.64 ± 0.01*** | 103.21 ± 4.58** | 194.03 ± 8.61** | 0.35 ± 0.03 | |
| Outdoor with simulated flue gas | Wild type | 0.53 ± 0.08 | 112.18 ± 10.66 | 210.89 ± 20.04 | 0.40 ± 0.11 |
| 283 | 0.94 ± 0.10 | 243.15 ± 70.77 | 457.13 ± 133.05 | 0.55 ± 0.01 | |
| 359 | 0.89 ± 0.09 | 225.96 ± 27.48* | 424.81 ± 51.66** | 0.66 ± 0.23 |
Each datum indicates the mean ± SD from two experiments
* P < 0.05, **P < 0.01 and ***P < 0.001 were statistically significant as compared with Chlorella vulgaris ESP-31 wild type
When grown in simulated flue gas under outdoor culture conditions, the wild-type, 283, and 359 strains presented a maximum biomass of 0.53, 0.94 and 0.89 g/L, respectively, indicating a 1.8-fold and 1.7-fold increase for the 283 and 359 strains over the wild-type strain, respectively (Table 3). In addition to higher maximum biomass, compared with C. vulgaris ESP-31 wild type, mutants 283 and 359 have relatively higher maximum biomass productivity (243.15 and 225.96 mg/L/day), CO2 biofixation rate (457.13 and 424.81 mg/L/day) and specific growth rate (0.55 and 0.66 day−1).
These results clearly indicated that C. vulgaris ESP-31 mutants enhance both simulated flue gas and heat tolerances to the extent that they could successfully grow in closed outdoor PBR with simulated flue gas under severe high temperature and high illumination conditions.
Discussion
Microalgae have been recognized as organisms for capturing CO2 in flue gases [25]. The cultivation of microalgae usually faces adverse effects of high temperature under outdoor culture conditions [26]. Therefore, C. vulgaris ESP-31 was subjected to NTG mutagenesis to obtain mutants tolerant to flue gas and high temperature. Two mutants exhibited the ability to tolerate simulated flue gases at 40 °C under indoor conditions, as evidenced by higher biomass, biomass productivity, and photosynthetic efficiency than that of the wild type.
The flue gases of thermal power plants in Taiwan have shown high CO2, NOx, and SOx emissions [27]. The current study showed that the high concentrations of CO2, NO and SO2 present in the flue gases had negative effects on the growth and CO2 sequestration processes of C. vulgaris ESP-31 wild type (net photosynthetic O2 evolution rate). The C. vulgaris ESP-31 mutants 283 and 359 could tolerate high concentrations of CO2, NO and SO2 present in the flue gases. These strains might be useful resources for CO2 sequestration and utilization of flue gases. Microalgae are considered to be organisms that can remove CO2, NOx, and SOx in flue gases [28]. Our present study showed that the mutants can grow well in simulated flue gas. This finding supported that these mutants could possibly use CO2, NO and SO2 for growth. Although we did not detect the concentrations of CO2, NO and SO2, we found that compared to the wild type, these mutants showed significant growth and photosynthetic efficiency under high CO2, NO, and SO2 concentrations. Our findings indicated that these mutants can utilize CO2, NO and SO2.
CO2 is a greenhouse gas that contributes to global warming [29]; it is mainly produced by burning fossil fuels and biomaterials and is emitted into the atmosphere as a global warming gas [30]. Algae production is well recognized as a solution for CO2 sequestration [31]. Chlorella spp., belonging to Chlorophyta, are freshwater and single-cell algae and represent one of the common algal species used for CO2 capture. The present study found that biomass production could reach 1.68, 1.91, and 1.99 g/L for the wild type and mutants 283 and 359, respectively, under 5% CO2 conditions (Fig. 1a), while comparatively, under 25% CO2 conditions, biomass production decreased, with values of 1.28, 1.61, and 1.54 g/L (Fig. 1b). A study examining the growth of the wastewater alga C. vulgaris ARC 1 in response to different CO2 concentrations ranging from 0.036 to 20% showed the highest biomass production of 0.21 g/L at 6% CO2 under a light intensity of 47 μmol m−2 s−1 [32]. The authors also found that CO2 concentration > 16% decreased the biomass production. This result was similar to our present results. A decrease in biomass was also found in Chlorella sp. KR-1 in response to high CO2 concentrations [33]. However, our data showed that even though the biomass decreased as the CO2 concentration increased in both mutants and the wild type, the mutants still retained a higher biomass than the wild type did. This result clearly demonstrated that these two mutants exhibited higher CO2 utilization ability.
Chlorella vulgaris ESP-31 wild type showed the highest biomass productivity of 0.029 g/L/day when 1200 mg/L NaHCO3 was used as the carbon source [34]. This value was lower than that from the present study using CO2 gas as the carbon source. This finding suggested that the growth of C. vulgaris ESP-31 wild type favoured CO2 as a carbon source compared to NaHCO3. In another previous study [21] using 2% CO2/air aeration, C. vulgaris ESP-31 wild type showed 0.26 g/L/day biomass productivity under indoor conditions. This value was also lower than that for C. vulgaris ESP-31 wild type cultured in the present study.
Chlorella vulgaris ESP-31 mutants 283 and 359 showed enhanced thermo-tolerance and 25% CO2 tolerance (Fig. 2). To further investigate the feasibility of using C. vulgaris ESP-31 mutants 283 and 359 for CO2 sequestration from industrial flue gas, C. vulgaris ESP-31 wild type and mutants 283 and 359 were grown at high temperature in medium aerated with simulated flue gas (25% CO2, 80–90 ppm SO2, 90–100 ppm NO) under both indoor (40 °C) and outdoor (~45 °C) conditions.
The maximum biomass, maximum biomass productivity, specific growth, CO2 biofixation rate, and specific growth rate of the mutants under the outdoor conditions were higher than those under the indoor conditions. It is obvious that the outdoor conditions were more suitable for algal cell growth. For example, the irradiance was higher in outdoor conditions and thus promoted algal cell growth.
The growth of microalgae under flue gas conditions not only varied between species but also was dependent on different flue gas compositions. In the present study, the maximum biomass of the C. vulgaris ESP-31 mutants 283 and 359 grown under outdoor conditions was 0.94 and 0.89 g/L, respectively, while the productivity was 243.15 and 225.96 mg/L/day. A Chlorella sp. cultured for 7 days with flue gas from burning coal containing 10% (v/v) CO2, 61 ppm NOx, and 0.3% (v/v) SOx showed maximum biomass and productivity values of 1.4 g/L and 191 mg/L/day, respectively [35]. Radmann et al. found that the cultivation of Chlorella vulgaris isolated from the coal-burning President Medici thermoelectric plant (UTPM, Candiota town, RS state, Brazil) using 12% (v/v) CO2, 60 ppm SO2 and 100 ppm NO in a serial tubular PBR showed maximum biomass and productivity values of 0.8 g/L and 80 mg/L/day, respectively [36].
In indoor cultivations, the wild type did not grow while the maximum biomass of the mutants 283 and 359 was 0.78 and 0.64 g/L, respectively, the maximum biomass productivity was 125.76 and 94.79 mg/L/day, the specific growth rates were 0.52 and 0.35 day−1, the CO2 biofixation rates were 236.42 and 178.20 mg/L/day, and the specific growth rates were 0.52 and 0.35 day−1. In contrast to the indoor condition, the wild type can grow under outdoor culture condition although the growth was smaller than mutants (Fig. 4a). Probably, high temperature of 40 °C maintained over the daytime in indoor condition while in outdoor condition, temperature remained low (< 40 °C) at the start of the daytime and then gradually increased to around 45 °C for approximately 2–3 h at noon, followed by a decrease in temperatures. Evidently, the exposure to high temperature in outdoor condition was shorter than the indoor condition. Under outdoor culture conditions the maximum biomass of the 283 and 359 mutants was 0.94 and 0.89 g/L, respectively, and the maximum biomass productivity was 243.15 and 225.96 mg/L/day. The CO2 biofixation rates were 457.13 and 424.81 mg/L/day, respectively, and the specific growth rates were 0.55 and 0.66 day−1.
The current study applied NTG mutagenesis (leading to transition mutations between GC and AT) together with high temperature and CO2 conditions to successfully obtain a mutant that can grow in simulated flue gas. In fact, microalgal species tolerant to flue gas can be obtained through the adaptive evolution method. The use of real flue gas injection into the microalgal community for a long time can also successfully select the species that can grow under flue gas conditions [37]. A recent study by Cheng et al. revealed that a new strain, Chlorella sp. Cv, can be obtained through adaptive evolution (46 cycles) against simulated flue gas (10% CO2, 200 ppm NOx and 100 ppm SOx) with a maximum CO2 fixation rate of 1200 mg/L/day [38]. This value was higher than that of our outdoor results, in which the maximum CO2 biofixation rates of C. vulgaris ESP-31 mutants 283 and 359 were 457.13 and 424.81 mg/L/day, respectively. It is evident that the CO2 utilization ability of microalgae was higher for the species selected by adaptive selection.
Microalgal growth is known to fix CO2 from flue gas and can also use NOx and SOx as nutrients for their growth [9]. According to our results that were obtained by using NO and SO2, we did not know whether the mutants obtained in the present study can use or tolerate NOx and SOx. A study similar to our present work was also using simulated flue gas containing CO2, SO2, NO and ash for adaptive evolution selection of the algal species that can grow in simulated flue gas [39]. However, further study is needed to identify the tolerance of the C. vulgaris ESP-31 mutant to real flue gas containing CO2, NOx, SOx and ash.
SO2 was much more dangerous than NO. The presence of SO2 at high concentrations can inhibit the growth of microalgae [40] due to a decrease in pH [41]. Our findings showed that the injection of simulated flue gas containing 80–100 ppm SO2 promoted the growth of the C. vulgaris ESP-31 mutants. Furthermore, microalgal growth was not affected by 50 ppm SO2 but was significantly affected by 400 ppm SO2 due to a decrease in pH [41]. Evidently, the 80–90 ppm SO2 used in the present study was not high enough to inhibit algal growth. Several studies have also shown Chlorella spp. can tolerate high SO2 gas levels. Duarte et al. found that Chlorella fusca LEB 111 showed no inhibition of cell growth with 400 ppm SO2 gas present in the flue gas from a coal power plant [39]. Using the injection of flue gas every 2 h of the light period (between 7 and 19 h) for 10 min through a porous curtain sparger fixed on the PBR base, Chlorella fusca LEB 111 can even grow in the flue gas containing SOx (5000 ppm) as well as CO2 (10–12%, v/v), NOx (400 ppm), and ash (650 ppm) [14].
The impact of SO2 on microalgal growth may be due to a drop in the pH caused by SOx injection in the waters [42]. Although our present study did not determine the medium pH, it would be expected that the medium pH under simulated flue gas conditions did not drop during the culture of the mutant, possibly due to the utilization of CO2 by the mutants with normal photosynthetic ability. However, for the control cell, the photosynthetic ability was significantly decreased, and as a result, the pH was decreased by SO2 injection; hence, cell growth was inhibited. Further study is needed to prove this hypothesis in the future.
SO2 may exert different effects in different temperature condition. It is because the solubility of SO2 was lower under 40 °C than that under 28 °C. Therefore, under indoor condition, the concentration of SO2 in night time was higher than the daytime period As a result, SO2 in night time can be much more utilized than that in the daytime. This can be also found in the outdoor conditions.
In addition to the increase in growth rate, a significant increase in the production of carbohydrates and lipids in mutants 283 and 359 grown under flue gas and high-temperature conditions was observed in both indoor and outdoor cultivations. Carbohydrates can be utilized for fermentation into ethanol as fuel energy. However, the lipid content of the wild type and 283 and 359 mutants was lower than that in a previous study using C. vulgaris ESP-31 as a material [21]. Besides, the present data from lipid content and Nile red stain showed that the wild type cultured did not accumulate lipid under indoor condition, but can accumulate under outdoor condition. It was possible because of the death of algal cells under indoor condition but the maintenance of growth under outdoor condition. These results indicated that flue gases and/or high temperature exhibited a negative effect on lipid accumulation in the lipid-rich green microalga, C. vulgaris ESP-31.
Obviously, the photosynthetic activity (photosynthetic O2 evolution rate and PSII activity) was increased after mutation for enhanced CO2 utilization in C. vulgaris ESP-31 in CO2-enriched flue gas. Photosystem II biogenesis is important in cyanobacteria [43]. The results suggest that the efficiency of the photosystem is enhanced in the mutants and therefore improves the light energy input used for CO2 fixation. Thus, the biomass is increased under increased CO2 supplementation.
High temperature (HT) stress inhibits photosynthetic machinery, and the primary sites of targets of high temperature stress are photosystem II (PSII) by increasing the fluidity of thylakoid membranes at high temperature, which causes dislodging of PSII light-harvesting complexes on the thylakoid membrane and affects electron dynamics depending on PSII integrity [44]. High temperature also impacts ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) kinetics and induces photorespiration [44]. A higher PSII activity and photosynthetic O2 evolution rate in the mutants demonstrated that photosynthesis in C. vulgaris ESP-31 wild type decreased upon exposure to high temperature, while the mutants could overcome the inhibitory effect of high temperature. It is obvious that enhanced CO2 sequestration was achieved in these mutant strains. In addition, although we did not exactly study the tolerance mechanism in these mutants, it has been suggested that major tolerance mechanisms, including ion transporters, increased production of late embryogenesis abundant (LEA) proteins, accumulation of heat shock proteins (HSPs) and osmoprotectants, and involvement of the antioxidant defence system are essential to cope with high temperature stress [44].
Conclusions
The present study reported the isolation and characterization of two thermo-tolerant and high CO2-tolerant mutants of C. vulgaris ESP-31, namely, mutants 283 and 359. These two mutants gained the ability to directly and efficiently grow in simulated flue gas (25% CO2, 80–90 ppm SO2, 90–100 ppm NO) by using outdoor PBR. The results also revealed that the photosynthetic activity (photosynthetic O2 evolution rate and PSII activity) of mutants 283 and 359 increased after mutation for enhanced CO2 utilization in C. vulgaris ESP-31. These strains represent promising microalgae for sequestration and utilization of CO2 from flue gases using outdoor PBR.
Methods
Microalgae cultivation
Chlorella vulgaris ESP-31 was obtained from Prof. Jo-Shu Chang, Department of Chemical Engineering, National Cheng-Kung University, Tainan, Taiwan [34]. Chlorella vulgaris ESP-31 cells were cultured in 50 mL BG-11 medium, pH 7.4 [45]. The cultures were normally incubated at 28 °C under continuous light conditions (approximately 50 μmol m−2 s−1, illuminated by a TL5 lamp (Philips, Singapore). The light intensity was measured by a Li-250A Light Meter with a Li-190 quantum sensor (Li-COR Inc., Lincoln, Nebraska, USA).
NTG mutagenesis
To isolate heat-tolerant mutants of C. vulgaris ESP-31, the wild-type strain was mutagenized with N-methyl-N′-nitro-N-nitrosoguanidine (NTG) [46]. First, 30 mL log-phase cultures of strain C. vulgaris ESP-31 were harvested and resuspended in 10 mL 10-mM citrate buffer, pH 6.0. These cells were then treated with NTG (500 μg/mL) for 30 min at room temperature in the light. The treated cells were washed three times with 10 mL BG-11 medium and then harvested by centrifugation. The survivors were plated onto BG-11 plates at 40 °C and grown under continuous light. The colonies that grew better on plates at 40 °C were picked and then restreaked onto fresh BG-11 plates.
Screening of Chlorella vulgaris ESP-31 mutants with the ability to tolerate heat and high levels of CO2
To screen the thermo-tolerant and high CO2 concentration-tolerant mutants of C. vulgaris ESP-31, a 48-well microtitre plate (without lid) containing multiple mutant strains was placed inside a w-zipper standard pouch (C-43, Mitsubishi Gas Chemical Company, Inc., Japan), and the bag was aerated with 15% CO2/air. The sealed bags were incubated at 40 °C and illuminated with 50 μmol m−2 s−1. Growth was monitored by measuring the OD680 every 24 h using a Synergy HT Multi-Detection Microplate Reader (BioTek Instruments, Inc., USA). After the reading, the culture plates were placed back into the bags and re-charged with 15% CO2/air (the process is shown in Fig. 5).
Fig. 5.
Schematic representation of NTG mutagenesis and screening of Chlorella vulgaris ESP-31 mutants for high temperature tolerance and high CO2 concentration tolerance
Growth of cells under various CO2 concentrations and high temperature stress conditions
Chlorella vulgaris ESP-31 wild type and mutant cells were collected and diluted to an OD680 of 0.2 with 50 mL freshly prepared BG-11 medium (containing 1.5 g/L NaNO3). Cells were cultivated at 28 °C with a speed of rocking at 150 rpm and a light/dark cycle of 12 h/12 h at a light intensity of 150 μmol m−2 s−1. For growth experiments in different CO2 concentrations, cells were cultivated by aeration with 5% and 25% CO2 (0.1 vvm), separately. For growth experiments under high temperature stress, cells were cultivated in an incubator at 12 h light (40 °C)/12 h dark (28 °C) by aeration with 25% CO2 (0.1 vvm). Growth was determined by measuring the OD at 680 nm using a spectrophotometer (CT-5600, ChromTech, USA) every 24 h.
Growth of cells under indoor simulated flue gas conditions
Chlorella vulgaris ESP-31 wild type and mutant cell pre-cultures were normally incubated at 28 °C under continuous light conditions (approximately 50 μmol m−2 s−1, illuminated by a TL5 lamp). Then, the cultures were collected and diluted to an OD680 of 0.4 with 800 mL freshly prepared ¼ N BG-11 medium (containing 0.375 g/L NaNO3) in a 1 L PBR (length × i.d. = 22 × 8 cm). In the intermittent flue gas aeration, culture aeration was controlled by a gas switch, and a gas-switching cycle was performed with simulated flue gas containing 25% CO2/air, 80–90 ppm SO2, 90–100 ppm NO at a rate of 0.1 vvm. The temperature was controlled at 40 °C with a rocking speed of 150 rpm under a light cycle consisting of 12 h of light with 150 μmol m−2 s−1 on single sides of the bottle and then aeration with air (0.1 vvm) at 28 °C with a rocking speed of 150 rpm for 12 h of dark for 9 days. Then, the cells were cultivated by aeration with air (0.1 vvm) at 28 °C with a rocking speed of 150 rpm and a light/dark cycle of 12 h/12 h at a light intensity of 150 μmol m−2 s−1 until 28 days. Growth was monitored by measuring the OD680 every 24 h.
Growth of cells in a closed PBR with simulated flue gas under outdoor culture conditions
Chlorella vulgaris ESP-31 wild type and mutant cells were collected and diluted to an OD680 of 0.4 with 1.8 L freshly prepared ¼ N BG-11 medium (containing 0.375 g/L NaNO3) in a 2 L PBR (length × i.d. = 25 × 12 cm). In the intermittent flue gas aeration, culture aeration was controlled by a gas switch, and a gas-switching cycle was performed with simulated flue gas containing 25% CO2/air, 80–90 ppm SO2, 90–100 ppm NO (0.1 vvm) at daytime and then aeration with air (0.1 vvm) at night for 6 days. Then, cells were cultivated by aeration with air (0.1 vvm) until 30 days. The outdoor PBR cultivations lasted from September 6–October 6, 2017. Growth was monitored by measuring the OD680 every 24 h.
Determination of chlorophyll content
To determine the chlorophyll content, 1 mL algal cell culture was centrifuged at 12,000×g at 4 °C for 5 min, and the pellet was ground in liquid nitrogen and extracted with 1 mL chilled 95% ethanol at 4 °C for 10 min. After centrifugation at 12,000×g at 4 °C for 5 min, the supernatant was collected, and the volume was adjusted to 1 mL with 95% ethanol. The absorbance of the supernatant was detected at 645 nm and 663 nm for the estimation of the chlorophyll content. The contents of chlorophyll, chlorophyll a, and chlorophyll b were determined by the following equations [47]:
| 1 |
| 2 |
| 3 |
Measurements of photosynthetic oxygen evolution rate
Photosynthesis was determined by detecting the amount of O2 evolution and consumption by algal cells using a Clark-type oxygen electrode fitted with a DW3 chamber (Hansatech, King’s Lynn, Norfolk, England) thermostat at 28 °C. The cell culture was centrifuged at 12,000×g for 5 min at room temperature. The cells were resuspended in 3 mL 50-mM HEPES buffer (pH 7.4) containing 5 mM NaHCO3. The O2 evolution of net photosynthesis was determined in 300 μmol m−2 s−1 for 8 min. The net photosynthesis rate was expressed as the change in O2 concentration per hour, as previously described [48]. Two replicates per treatment were measured.
Chlorophyll a fluorescence determination of maximum PSII activity using pulse amplitude modulation (PAM) fluorometry
Chlorophyll a fluorescence parameters were employed to determine the activity of photosystem II (PSII) using an AP-C 100 system (AquaPen, Brno, Czech Republic). An aliquot of the algal culture was diluted with BG-11 medium to an OD680 = 0.2. A 2-mL aliquot of diluted algal cells was then transferred to an AquaPen cuvette and subjected to a pulse of saturating light of 4000 μmol m−2 s−1 PAR to obtain the light-adapted minimal fluorescence (Ft) and the light-adapted maximal fluorescence (Fm’). To determine the maximum PSII activity, Fv/Fm (=Fm − Fo/Fm), 2 mL diluted algal cell suspension in an AquaPen cuvette was incubated in the dark for 30 min and then flushed with saturated light (4000 μmol m−2 s−1) to obtain the dark-adapted minimal fluorescence (Fo) and dark-adapted maximal fluorescence (Fm). The maximum PSII activity, Fv/Fm = Fm − Fo/Fm, was then calculated.
Nile red staining and microscopy
To detect lipid droplets, Nile red was used to stain the wild type and mutants 283 and 359 of C. vulgaris ESP-31. An aqueous solution of Nile red of 0.1 μg/mL was incubated for approximately 10 min in the dark at room temperature [49, 50]. After staining, Nile red fluorescence and phase contrast micrographs were taken on a LEICA microscope (LEICA DM2500) with 100× objective using specific emission filter sets (bypass excitation (475 nm) and longpass emission (510 nm) filters).
Determination of carbohydrate content of Chlorella vulgaris ESP-31
The carbohydrate content in the C. vulgaris ESP-31 wild type and mutants was determined using the modified quantitative saccharification (QS) method reported by the Nation Renewable Energy Laboratory (NREL), USA [51]. A small amount of dry cell powder was added to 1 mL 72% (v/v) sulfuric acid and incubated for 60 min at 30 °C for primary hydrolysis. The hydrolysate was then diluted to 4% (v/v) with sulfuric acid and incubated at 121 °C (sterilization) for 20 min as the secondary hydrolysis. The supernatant was neutralized and analysed by high-performance liquid chromatography for sugar assays [52].
Determination of lipid content
The biomass of Chlorella vulgaris ESP-31 wild type and mutants was harvested by centrifugation (6000 rpm for 10 min) and then washed twice with deionized water and lyophilized. The lipid content of cells was determined as fatty acid methyl esters (FAMEs) through the direct transesterification method. The samples were analysed by gas chromatography (GC-2014, Shimadzu, Kyoto, Japan) equipped with a flame ionization detector (FID) [34, 53, 54].
Determination of growth biomass productivity and specific growth rate
The biomass productivity (P, mg/L/day) was calculated from the change in biomass concentration (X, mg/L) within a culture time (t, d) according to the equation P = (X1 − X0)/(t1 − t0). The specific growth rate μ (day−1) was calculated from the equation μ = ln (X1 − X0)/(t1 − t0), where X1 and X0 represented the biomass concentration (mg L−1) on days t1 and t0, respectively [28].
Determination of CO2 biofixation rate
The fixation rate of CO2 (mg/L/day) was calculated from the equation CO2 fixation rate = 1.88 × P, where P is the biomass productivity (mg/L/day) [57].
Statistical analysis
Experimental data were calculated and expressed as the mean ± SE. Duncan’s new multiple range was used for the test of the data among the wild type and two mutants. A difference was considered to be statistically significant when P < 0.05.
Acknowledgements
We would like to express our thanks to National Energy Program-Phase II (NEP-II) granted by Taiwan’s Ministry of Science and Technology for financial support in this research.
Abbreviations
- PBR
photobioreactor
- HT
high temperature
- FAMEs
fatty acid methyl esters
- FID
flame ionization detector
- Fm
maximal fluorescence
- Fo
minimal fluorescence
- HSPs
heat shock proteins
- LEA
late embryogenesis abundant
- NTG
N-methyl-N′-nitro-N-nitrosoguanidine
- PAM
pulse amplitude modulation
- PSII
photosystem II
- Rubisco
ribulose-1,5-bisphosphate carboxylase/oxygenase
- WT
wild type
Authors’ contributions
HHC, HYS, and XDS performed the microalgal cultivation experiments and the MNNG mutagenesis tests. CLC performed the lactic acid fermentation experiments. CYC and JSC provided the Chlorella vulgaris ESP-31 strain. TJC, TML, and JSC critically reviewed the manuscript draft. TJC, TML and JSC designed the experiments and prepared/polished the manuscript. All authors read and approved the final manuscript.
Authors’ information
TML is currently a Professor at National Sun Yat-sen University (NSYSU), Taiwan. HHC is currently a Ph.D. student at NSYSU (supervised by TML and TJC). HYS is a postdoctoral fellow at Dongguan University of Technology. XDS is a graduate student at Fooying University (supervised by TJC). TJC is currently an Associate Professor of Fooying University. CYC is currently an Associate Research Professor at National Cheng Kung University, Taiwan. JSC is currently a Professor at National Cheng Kung University, Taiwan.
Funding
This work was supported by Grants NSC103-2623-E-242-001-ET, MOST105-2221-E-242-002, MOST103-3113-E-006-006, MOST104-3113-E-006-003 and MOST 105-3113-E-006-003 from Taiwan’s Ministry of Science and Technology.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consented to the publication of this work.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hsiang-Hui Chou, Email: d042010007@student.nsysu.edu.tw.
Hsiang-Yen Su, Email: SHY16100@outlook.com.
Xiang-Di Song, Email: tw255023@gmail.com.
Te-Jin Chow, Email: dr008@fy.edu.tw.
Chun-Yen Chen, Email: ccy.ncku@gmail.com.
Jo-Shu Chang, Email: changjs@mail.ncku.edu.tw.
Tse-Min Lee, Email: tmlee@faculty.nsysu.edu.tw, Email: tmlee@mail.nsysu.edu.tw.
References
- 1.Favre E, Bounaceur R, Roizard D. Biogas, membranes and carbon dioxide capture. J Memb Sci. 2009;238:11–14. doi: 10.1016/j.memsci.2008.12.017. [DOI] [Google Scholar]
- 2.Gielen D. CO2 removal in the iron and steel industry. Energ Convers Manage. 2003;44:1027–1037. doi: 10.1016/S0196-8904(02)00111-5. [DOI] [Google Scholar]
- 3.Kadam KL. Environmental implications of power generation via coal- microalgae cofiring. Energy. 2002;27:905–922. doi: 10.1016/S0360-5442(02)00025-7. [DOI] [Google Scholar]
- 4.Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev. 2010;14:217–232. doi: 10.1016/j.rser.2009.07.020. [DOI] [Google Scholar]
- 5.Hannon M, Gimpel J, Tran M, Rasala B, Mayfield S. Biofuels from algae: challenges and potential. Biofuels. 2010;1:763–784. doi: 10.4155/bfs.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho SH, Lu WB, Chang JS. Photobioreactor strategies for improving the CO2 fixation efficiency of indigenous Scenedesmus obliquus CNW-N: statistical optimization of CO2 feeding, illumination, and operation mode. Bioresour Technol. 2012;105:106–113. doi: 10.1016/j.biortech.2011.11.091. [DOI] [PubMed] [Google Scholar]
- 7.Garzon-Sanabria AJ, Davis RT, Nikolov ZL. Harvesting Nannochloris oculata by inorganic electrolyte flocculation: effect of initial cell density, ionic strength, coagulant dosage, and media pH. Bioresour Technol. 2012;118:418–424. doi: 10.1016/j.biortech.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 8.Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol. 2011;102:71–81. doi: 10.1016/j.biortech.2010.06.159. [DOI] [PubMed] [Google Scholar]
- 9.Yen HW, Hu IC, Chen CY, Ho SH, Lee DJ, Chang JS. Microalgae-based biorefinery—from biofuels to natural products. Bioresour Technol. 2013;135:166–174. doi: 10.1016/j.biortech.2012.10.099. [DOI] [PubMed] [Google Scholar]
- 10.de Morais MG, Costa JAV. Biofixation of carbon dioxide by Spirulina sp and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechnol. 2007;129(3):439–445. doi: 10.1016/j.jbiotec.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Hughes E, Benemann JR. Biological fossil CO2 mitigation. Energy Convers Manag. 1997;38:S467–S473. doi: 10.1016/S0196-8904(96)00312-3. [DOI] [Google Scholar]
- 12.Kumar K, Dasgupta CN, Nayak B, Lindblad P, Das D. Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour Technol. 2011;102:4945–4953. doi: 10.1016/j.biortech.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Lee JP. Review of advances in biological CO2 mitigation technology. Biotechnol Bioprocess Eng. 2003;8:354–359. doi: 10.1007/BF02949279. [DOI] [Google Scholar]
- 14.Jiang Y, Zhang W, Wang J, Chen Y, Shen S, Liu T. Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour Technol. 2013;128:359–364. doi: 10.1016/j.biortech.2012.10.119. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Li Y, Wu N, Lan CQ. CO2 bio-mitigation using microalgae. Appl Microbiol Biotechnol. 2008;79:707–718. doi: 10.1007/s00253-008-1518-y. [DOI] [PubMed] [Google Scholar]
- 16.Ono E, Cuello JL. Design parameters of solar concentrating systems for CO2-mitigating algal photobioreactors. Energy. 2004;29:1651–1657. doi: 10.1016/j.energy.2004.03.067. [DOI] [Google Scholar]
- 17.Chiu PH, Soong K, Chen CN. Cultivation of two thermotolerant microalgae under tropical conditions: influences of carbon sources and light duration on biomass and lutein productivity in four seasons. Bioresour Technol. 2016;212:190–198. doi: 10.1016/j.biortech.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 18.Hsueh HT, Chu H, Chang CC. Identification and characteristics of a cyanobacterium isolated from a hot spring with dissolved inorganic carbon. Environ Sci Technol. 2007;41:1909–1914. doi: 10.1021/es0620639. [DOI] [PubMed] [Google Scholar]
- 19.de Bashan LE, Trejo A, Huss VAR, Hernandez JP, Bashan Y. Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Bioresour Technol. 2008;99:4980–4989. doi: 10.1016/j.biortech.2007.09.065. [DOI] [PubMed] [Google Scholar]
- 20.Ong SC, Kao CY, Chiu SY, Tsai MT, Lin CS. Characterization of the thermal-tolerant mutants of Chlorella sp. with high growth rate and application in outdoor photobioreactor cultivation. Bioresour Technol. 2010;101:2880–2883. doi: 10.1016/j.biortech.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Chen CY, Yeh KL, Chang HY, Chang JS. Strategies to improve oil/lipid production of microalgae in outdoor cultivation using vertical tubular-type photobioreactors. Energy Procedia. 2014;61:2755–2758. doi: 10.1016/j.egypro.2014.12.298. [DOI] [Google Scholar]
- 22.Cordero BF, Obraztsova I, Couso I, Leon R, Vargas MA, Rodriguez H. Enhancement of lutein production in Chlorella sorokiniana (Chlorophyta) by improvement of culture conditions and random mutagenesis. Mar Drugs. 2011;9:1607–1624. doi: 10.3390/md9091607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borowitzka MA. Algal physiology and large-scale outdoor cultures of microalgae. In: Borowitzka MA, Beardall J, Raven JA, editors. The physiology of microalgae. London: Springer; 2015. pp. 601–652. [Google Scholar]
- 24.Béchet Q, Laviale M, Arsapin N, Bonnefond H, Bernard O. Modeling the impact of high temperatures on microalgal viability and photosynthetic activity. Biotechnol Biofuels. 2017;10:136–147. doi: 10.1186/s13068-017-0823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aslam A, Mughal TA. A review on microalgae to achieve maximal carbon dioxide (CO2) mitigation from industrial flue gases. Int J Res Advent Technol. 2016;4:12–29. [Google Scholar]
- 26.Ras M, Steyer JP, Bernard O. Temperature effect on microalgae: a crucial factor for outdoor production. Rev Environ Sci Bio. 2013;12:153–164. doi: 10.1007/s11157-013-9310-6. [DOI] [Google Scholar]
- 27.Liu CH, Lin SJ, Lewis C. Evaluation of NOx, Sox and CO2 emissions of Taiwan’s thermal power plants by data envelopment analysis. Aerosol Air Qual Res. 2013;13:1815–1823. doi: 10.4209/aaqr.2013.03.0070. [DOI] [Google Scholar]
- 28.Yen HW, Ho SH, Chen CY, Chang JS. CO2, Nox and Sox removal from flue gas via microalgae cultivation: a critical review. Biotechnol J. 2015;10:829–839. doi: 10.1002/biot.201400707. [DOI] [PubMed] [Google Scholar]
- 29.Anderson TR, Hawkins E, Jones PD. CO2, the greenhouse effect and global warming: from the pioneering work of Arrhenius and Callendar to today’s Earth System Models. Endeavor. 2016;40:178–187. doi: 10.1016/j.endeavour.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Kumar M, Sundaram S, Gnansounou E, Larroche C, Thakur IS. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: a review. Bioresour Technol. 2018;247:1059–1068. doi: 10.1016/j.biortech.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 31.Singh SP, Singh P. Effect of CO2 concentration on algal growth: a review. Renew Sust Energ Rev. 2014;38:172–179. doi: 10.1016/j.rser.2014.05.043. [DOI] [Google Scholar]
- 32.Chinnasamy S, Ramakrishnan B, Bhatnagar A, Das K. Biomass production potential of a wastewater alga Chlorella vulgaris ARC1 under elevated levels of CO2 and temperature. Int J Mol Sci. 2009;10:518–532. doi: 10.3390/ijms10020518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung KD, Lee JS, Shin CS, Park SC. Isolation of a new highly CO2 tolerant fresh water microalga Chlorella sp. KR-1. Renew Energ. 1999;16:1019–1022. doi: 10.1016/S0960-1481(98)00362-0. [DOI] [Google Scholar]
- 34.Yeh KL, Chang JS, Chen WM. Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP-31. Eng Life Sci. 2010;10:201–208. doi: 10.1002/elsc.200900116. [DOI] [Google Scholar]
- 35.Yadav G, Karemore A, Dash SK, Sen R. Performance evaluation of a green process for microalgal CO2 sequestration in closed photobioreactor using flue gas generated in-situ. Bioresour Technol. 2015;191:399–406. doi: 10.1016/j.biortech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 36.Radmann EM, Camerini FV, Santos TD, Costa JAV. Isolation and application of Sox and Nox resistant microalgae in biofixation of CO2 from thermoelectricity plants. Energ Convers Manage. 2011;52:3132–3136. doi: 10.1016/j.enconman.2011.04.021. [DOI] [Google Scholar]
- 37.Aslam A, Thomas-Hall SR, Mughal TA, Schenk PM. Selection and adaptation of microalgae to growth in 100% unfiltered coal-fired flue gas. Bioresour Technol. 2017;233:271–283. doi: 10.1016/j.biortech.2017.02.111. [DOI] [PubMed] [Google Scholar]
- 38.Cheng D, Li X, Yuan Y, Yang C, Tang T, Zhao Q, Sun Y. Adaptive evolution and carbon dioxide fixation of Chlorella sp in simulated flue gas. Sci Total Environ. 2019;650:2931–2938. doi: 10.1016/j.scitotenv.2018.10.070. [DOI] [PubMed] [Google Scholar]
- 39.Duarte JH, de Morais EG, Radmann EM, Costa JAV. Biological CO2 mitigation from coal power plant by Chlorella fusca and Spirulina sp. Bioresour Technol. 2017;234:472–475. doi: 10.1016/j.biortech.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 40.Costa JAV, Moraess L, Moreirra JB, daRosa GM, Henrard ASA, deMorais MG. Microalga-based biorefineries as a promising approach to biofuel production. In: Tripathi BN, Kumar D, editors. Prospects and challenges in algal biotechnology. New York: Springer; 2017. pp. 113–140. [Google Scholar]
- 41.Negoro M, Shioji N, Miyamoto K, Miura Y. Growth of microalgae in high CO2 gas and effects of SOX and NOX. Appl Biochem Biotechnol. 1991;28–29:877–886. doi: 10.1007/BF02922657. [DOI] [PubMed] [Google Scholar]
- 42.Heinz S, Liauw P, Nickelsen J, Nowaczyk M. Analysis of photosystem II biogenesis in cyanobacteria. Biochim Biophys Acta. 2016;1857:274–287. doi: 10.1016/j.bbabio.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Mathur S, Agrawal D, Jajoo A. Photosynthesis: response to high temperature stress. J Photochem Photobiol, B. 2014;137:116–126. doi: 10.1016/j.jphotobiol.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Rippka R, Deruelles J, Waterbury JB. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 45.Golden SS. Mutagenesis of cyanobacteria by classical and gene-transfer-based methods. In: Meth Enzymol. 1988; 167, p. 714–27. [DOI] [PubMed]
- 46.Su S, Zhou Y, Qin JG, Yao W, Ma Z. Optimization of the method for Chlorophyll extraction in aquatic plants. J Freshw Ecol. 2010;25(4):531–538. doi: 10.1080/02705060.2010.9664402. [DOI] [Google Scholar]
- 47.Su HY, Chou HH, Chow TJ, Lee TM, Chang JS, Huang WL, Chen HJ. Improvement of outdoor culture efficiency of cyanobacteria by over-expression of stress tolerance genes and its implication as bio-refinery feedstock. Bioresour Technol. 2017;244:1294–1303. doi: 10.1016/j.biortech.2017.04.074. [DOI] [PubMed] [Google Scholar]
- 48.Greenspan M, Mayer EP, Fowler SD. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen W, Zhang C, Song L, Sommerfeld M, Hu Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods. 2009;77:41–47. doi: 10.1016/j.mimet.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Moxley G, Zhang YHP. More accurate determination of acid-labile carbohydrates in lignocellulose by modified quantitative saccharification. Energy Fuels. 2007;21:3684–3688. doi: 10.1021/ef7003893. [DOI] [Google Scholar]
- 51.Chow TJ, Su HY, Tsai TY, Chou HH, Lee TM, Chang JS. Using recombinant cyanobacterium (Synechococcus elongatus) with increased carbohydrate productivity as feedstock for bioethanol production via separate hydrolysis and fermentation process. Bioresour Technol. 2014;184:33–41. doi: 10.1016/j.biortech.2014.10.065. [DOI] [PubMed] [Google Scholar]
- 52.Chen CY, Yeh KL, Su HM, Lo YC, Chen WM, Chang JS. Strategies to enhance cell growth and achieve high-level oil production of a Chlorella vulgaris isolate. Biotechnol Progr. 2010;26:679–686. doi: 10.1002/btpr.381. [DOI] [PubMed] [Google Scholar]
- 53.Su CH, Giridhar R, Chen CW, Wu WT. A novel approach for medium formulation for growth of a microalga using motile intensity. Bioresour Technol. 2007;98:3012–3016. doi: 10.1016/j.biortech.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 54.Tang D, Han W, Li PL, Miao X, Zhong JJ. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol. 2011;102:3071–3076. doi: 10.1016/j.biortech.2010.10.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.