Abstract
Introduction
Adolescent and young adult periods are characterized by increased risk-taking, impulsive behavior, and nonadherence issues, which makes it equally challenging for patients and their health care professionals. Health information technology (IT) has the potential to empower patients.
Objective
Determine the effects and features of IT-based interventions for self-management of adolescents and young adults in kidney transplant recipients.
Materials and Methods
A comprehensive survey was done on Medline and Scopus in September 2018. Eligible studies included randomized controlled trials (RCTs) and quasi-experimental studies focused on automated IT-based interventions. Studies contained information about adolescent and young adult kidney transplant recipients aged under 25, all published in English. The articles were combined with each other based on the classification of outcomes, the type of interventions, and their impact. The studies were categorized based on the impact of interventions as positive and statistically significant, with no effect, or a combined effect (both positive significance and without effect).
Results
In this review, of a total of 2,242 retrieved articles, collected from Scopus and PubMed databases, 5 studies met the full-text inclusion criteria. Interventions were performed using computerized systems (3 studies), smartphone application/personal digital ass (PDA) (1 study), and multiple components (1 study). These studies evaluated 15 outcomes, including 7 care process and 8 clinical outcomes. In 6 of 15 outcomes (40%), interventions had a statistically significant positive effect.
Conclusion
IT-based interventions such as mobile health/personal digital assistant(PDA), computer systems and multi-component have the potential to improve self-management in adolescents and young adult kidney transplant recipients (care process outcomes). It is recommended to conduct complementary research to examine the effect of IT-based self-management interventions on clinical outcomes in kidney transplant recipients.
Keywords: adolescent, young adult, information technology, self-management
Introduction
The puberty and emerging adulthood period in humans coincides with physical and morphologic changes, as well as brain development.1–3 As such, adolescents and young adult patients require special medical attention. Adolescents suffering from end-stage renal disease reach developmental milestones less or later than their healthy peers,4 while enjoying a lower mental quality of life.5
The survival rate of allograft in child and young adult kidney transplant recipients is low as compared to the elderly population, in whom up to 30% of all allografts fail in the first 5 years.6 The risk of graft rejection increases significantly during the transition from childhood to adolescence and reaches its peaks at the age of 19 so that during this period, it is almost three times more likely than younger children and the elderly.7 These results have profound negative impacts on patients’ health and quality of life, and more broadly, on the whole health care system. Increased risk-taking, impulsive behavior, and nonadherence issues are among the features characterized by this period of life, making it equally challenging for patients and health care professionals.1 Also, adolescents and young adults are typically less adherent to immunosuppressive medication than elderly transplant recipients,8,9 thus increasing the risk of graft rejection or loss.2,8 Based on previous research, the highest allograft rejection rate has been observed in 16- to 21-year-old patients.2,10 Also, the worst clinical outcomes of kidney transplantation are observed in adolescent and young adult transplant recipients as compared to other age groups.11 Graft rejection leads to the return of dialysis, which in turn reduces the quality of life and life expectancy, and increases morbidity rates and health care costs.12,13 However, in order for a transplantation to be successful, transplant recipients should strictly follow complex and dynamic therapeutic regimes such as frequent visits by physician, carrying out medical laboratory tests, and changing lifestyle and nutritional diets.14
It is assumed that self-management could increase adherence to medical standards, primary physical changes, and patients’ autonomy.15,16 Self-management is the interaction between health behavior and related processes, in which patients and families are engaged to manage a chronic condition.17 The primary focus of self-care and self-management interventions is to encourage patients to change their behaviors in the face of a chronic illness or condition, which requires knowledge sharing, education, and condition understanding.18
Health information technology (IT) and electronic tools have provided new opportunities for patients to empower themselves in order to be able to actively participate in their health monitoring process, to be their health care providers’ assistant19 and to be aware and involved in the decision-making processes, as opposed to being a passive care service recipient. IT has also provided novel opportunities for patients’ health care and training.20
The use of IT has been investigated in a large number of interventional studies conducted on patient training and self-management monitoring programs designed for patients with chronic diseases, with both effective and ineffective reported outcomes. These seemingly heterogeneous, paradoxical results made it necessary to carry out a literature review in which the main findings of previous studies were systematically reiterated and categorized. There are several systematic reviews conducted on self-management in patients with chronic kidney disease.21–24
An IT-based intervention, by itself, has the ability to reduce error, promote self-management, and improve patient’s awareness and self-care. To the best of our knowledge, none of the IT-based systematic reviews were focused on adolescent and young adult transplant recipients, while these groups of patients have a fundamental need for self-management before and after transplantation. Therefore, this study aimed to combine the results of IT-based interventions conducted on self-management outcomes in adolescent and young adult transplant recipients. To achieve this objective, the present study will focus on the following questions: What interventional studies have been conducted in this area? What are the main features of these studies? How have these interventions affected self-management in adolescent and young adult kidney transplant recipients?
Materials and methods
The current systematic review was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) items created and reported by Moher et al (2009).25
Data source and research strategy
A comprehensive search was done on Medline (through PubMed) and Scopus databases, for articles published in the 1980–2018 period. The search was conducted from August to September 2018. Comprehensive research was done using a combination of keywords and MeSH terms associated with participation, empowerment, and self-management as well as kidney transplant. Table 1 shows a combination of keywords and MeSH terms used in our search. See Appendix A for the full search strategy.
Table 1.
Keyword and MeSH terms in the search strategy
| Kidney transplantation | Keywords | Kidney transplantation, renal transplantation, kidney grafting, kidney transplant |
|---|---|---|
| MeSH terms | Kidney transplantation | |
| Self-management | Keywords | Self-Care, Self-Management,Disease Management, Decision Aids, Patient Participation, Patient Involvement, Medication Alert System, Reminder Systems, Patient Education Patient Empowerment, Patient Activation, Patient Engagement, Patient Participation patient education, Reminder Systems |
| MeSH terms | Self-Care, Disease Management, Patient Participation, patient education, Decision Support Systems, Clinical, Reminder Systems, Decision Support Techniques |
Eligibility criteria
The inclusion criteria were determined based on the population, intervention, comparator, outcomes, and study design (PICOS). An IT-based system is a system with an automatic function and without direct human involvement. The inclusion criteria for studies to be included in our survey were as follows: 1) IT-based interventions with automatic functioning, 2) interventions involving an IT tool to support self-management and self-care, including smartphones, tablets, and computers, 3) studies published in scientific journals, 4) studies published during 1980–2018, 5) studies published in English, 6) studies designed as a randomized clinical trial or quasi-experimental (before–after intervention and interrupted time series), and 7) studies conducted on adolescents and young adult kidney transplant patients.
Exclusion criteria were as follows: 1) interventions with direct human involvement, such as nonautomatic phone calls, nonautomatic SMS systems, and nonautomatic video systems, 2) studies focusing on applicability or description of IT-based tools, 3) descriptive studies without a comparative group, case study, study protocol, 4) conference papers, and 5) studies in languages other than English.
Data extraction
The first selection was based on the article’s title and abstract to identify the articles that fit into the research question domain. Second, selection was based on the inclusion criteria, focusing on studies that use IT-based interventions for self-care and self-management of kidney transplant patients. After these two steps, the full text of the remaining articles was studied for further investigation, and these studies were classified based on the type of IT-based tools.
A special spreadsheet was designed to systematically extract data from the studies. Data extracted from the studies were about participants, type of intervention, study performance, description of the study, duration of intervention, outcomes, and findings. Data were examined by the second author (FK) and revised and confirmed by the second reviewer (first author, RG) in terms of accuracy of data extraction.
Risk of bias
Cochrane Collaboration’s evaluation tool was used to assess the quality of clinical trials.26 This tool evaluates6 areas including random sequence generation, allocation concealment, blinding participants and personnel, blinding outcome assessor, incomplete outcomes data, and selective outcome reporting. The first and second items are to prevent selection bias, while others are designed to prevent performance bias, detection bias, attrition bias, and reporting bias, respectively.
Quasi-experimental studies were assessed by using the Quality Assessment Tool for Pre- and Post-Intervention Designs by Brown et al, which is adopted from Estabrooks et al.27,28 This tool evaluates6 areas including sampling, design, control of confounders, data collection and outcome measurement, statistical analysis, and conclusions and dropouts. Each study was evaluated independently by the two reviewers (RG, MRMH), and a consensus was reached on the disagreements by holding a bilateral discussion.
Synthesis and analysis
The synthesis of articles was based on the classification of outcomes, type of intervention, and its impact. The outcomes were categorized into two groups: clinical and process outcomes. Clinical outcomes are biomarkers that indicate the severity of the disease, such as blood pressure. Care process outcomes are described as outcomes that affect the patient care by improving the quality of care and provider–patient interaction.29
The interventions based on the studies were classified as follows:
Smartphones and digital assistant tools (software and SMS).
Coverage tools: Wearable tools that record physiological changes, such as blood pressure monitoring.
Computer systems: Systems in which data are recorded by the patient and delivered through the internet.
Multicomponent systems: Interventions involving more than one of the above tools.30
The function of IT-based systems was classified according to the technology performance framework. This categorization was based on whether the system could:
Inform: Provide information in a variety of formats (text, photo, video)
Instruct: Provide instructions to the user
Record: Capture user-entered data
Display: display user-entered data/output user-entered data
Guide: Provide guidance based on user-entered information
Remind/Alert: Provide reminders to the user
Communicate: Provide communication with health care provider (HCP) patients and/or provide links.31,32
The impact of the interventions was classified according to the following: a positive and statistically significant effect, no effect (not statistically significant), or a combined effect (both positive significance and without effect).
Results
Study selection
As shown in Figure 1, 1,081 records from PubMed database and 1,161 records from Scopus database were collected. After eliminating duplicate records, 1,809 records were retrieved. After evaluating the title and abstract of the studies, and taking into account our inclusion criteria, 31 papers were selected for full-text evaluation, and 1,778 papers did not meet our inclusion criteria. In the full-text assessment stage, 26 papers were eliminated based on the exclusion criteria and 5 studies remained for evaluation.
Figure 1.
Flow diagram of the literature search and publication selection.
General characteristics of the included studies
Table 2 presents the general characteristics of the five selected studies. The oldest study was published in 2010, and the most recent study was published in 2018. Two of the studies were designed as quasi-experimental interventions,33,34 while three other studies were randomized controlled trials (RCTs).35–37
Table 2.
General characteristics of included studies
| Source, author, year, country | Participants (age range) | Type of studies | Sample size (n) | Intervention | Duration | Outcomes | Results | Effect | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Christina Freier et al, 2010, Germany | Adolescents following transplantation (15–19 years) | Randomized controlled trial | IGR (26) CGr (24) | Educational programme using the OTIS system | 24 months |
|
|
|
The results presented demonstrate that this medium holds the potential to improve perceived IRK and behavior. Moreover, this medium can support the challenging transition period from pediatric to adult care |
| Kullgren KA et al 2015, USA | Pediatric kidney transplant recipients (7– 19 years) | Randomized controlled trial | IGR (16) CGr (16) | Interactive water bottle | 1 month |
|
|
|
While an interactive water bottle providing real-time feedback may be a promising intervention to help pediatric kidney transplant patients meet fluid goals, it did not appear to impact kidney function. |
| Bethany J. Foster et al, 2018, Canada and the United States | Kidney transplant recipients (11–24 years) | Randomized controlled trial | IGR (81) CGr (88) | TAKE-IT intervention which includes electronic adherence monitoring, receive text message, e-mail, and/or visual cue dose reminders | 12 months |
|
|
|
The multicomponent TAKE-IT intervention resulted in significantly better medication adherence than the control condition. Better medication adherence may result in improved graft outcomes, but this will need to be demonstrated in larger studies. |
| David K. Hooper et al, 2012, USA | Kidney transplant recipients (3–26) | Interrupted times series | 62 |
|
2 years | 1. Process outcome (the proportion of patients due for cholesterol testing who had it performed within 1 week of their clinic visit) 2. Process outcome (the proportion of patients who achieved low-density lipoprotein (LDL) and cholesterol control) |
|
|
Using quality improvement and health information technology, we achieved sustained, reliable, and efficient personalized monitoring of cholesterol and 11 other tests. This approach enabled substantial improvement in LDL cholesterol control. Structured methods of system redesign that leverage information technology systems hold promise for rapidly achieving reliable individualized care in other settings. |
| Malone et al, 2016, USA | Kidney transplant recipients >2 years | Before–after | 122 | EHR-generated reminders for providers to give the vaccines to eligible patients. | 12 months | Process outcome (increase pneumococcal vaccine rates) | Increase the percentage of transplant patients receiving the PCV13 and PPSV23 from 6% to 52%. | Positive effect | Utilizing an age-based algorithm and the electronic medical record, vaccine champions can track both missed visit opportunities and the number of vaccinated patients to improve pneumococcal immunization coverage for these high-risk patients. |
Abbreviations: OTIS, organ transplantation information system; IRK, illness-related knowledge; IRB, illness-related behavior; GFR, glomerular filtration rate; eGFR, estimated glomerular filtration rate; Na, sodium; BUN, blood urea nitrogen; TAKE-IT, Teen Adherence in Kidney Transplant Effectiveness of Intervention Trial; EHR, electronic health record; LDL, low-density lipoprotein; PCV13, pneumococcal conjugate vaccine; PPSV23, pneumococcal polysaccharide vaccine.
Four studies were conducted in the United States,33–35,37 and the remaining one study was conducted in Germany.35 In all studies, participants were kidney transplant recipients. The median number of participants in the studies was 62 (32–169), and the median duration of the studies was 12 months (1–24 months). Most of the studies (4/5, 80%) examined more than one outcome.
Risk of bias assessment
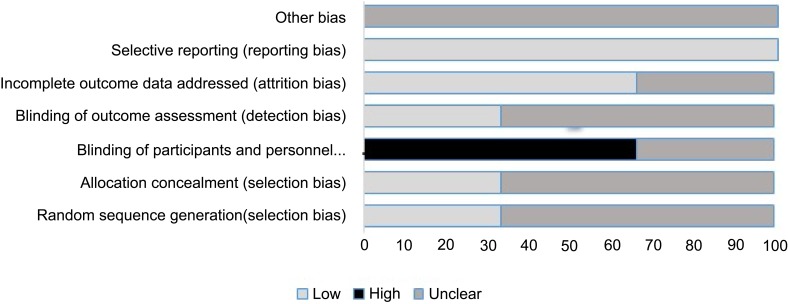
The risk of bias assessment for the three RCT studies is shown in Figure 2. One of the studies (33%) had specifically identified random sequence generation data and allocation concealment. Moreover, one of the studies (33%) reported incomplete data in relation to the method of blinding participants and staff, and one of the studies (33%) provided complete data regarding the blinding of outcome assessors. The bias was also low in 66% of the studies in assessing the cause of participants’ loss and exclusion. As acknowledged in all three studies, the outcomes were reviewed based on the prespecified and previously reported outcomes. This bias was low in all of the studies. There was no reported form of other bias in all of the studies. Of the three RCTs, one study had adequate random sequence generation; computer-generated systems were used. One of the studies had a low risk of bias for allocation assessment, mainly because of the use of numbered sealed envelopes. Other aspects that were rated for risk of bias were 1) blinding of outcome assessment and 2) incomplete outcome data. These items were often not reported, and therefore, scored as an unclear risk of bias according to the Cochrane handbook.19
Figure 2.
Risk of bias assessment of the included RCT studies.
Two quasi-experimental studies were assessed, one of which had moderate quality 33 and the other one with weak quality.34 Quality appraisal results for included papers are shown in Appendix B. Ratings displayed in each table are the final ratings agreed on by both reviewers.
The impact of interventions on the outcome
Table 3 shows a summary of the impact of interventions on the outcomes. In total, 15 outcomes were evaluated in the studies, including 7 care process and 8 clinical outcomes. The studies showed that in 6 (40%) of 15 outcomes, interventions had a statistically significant positive effect. In 9 outcomes (60%), no significant difference was observed between groups. In total, 12 outcomes were assessed by RCT studies and 3 outcomes were evaluated by quasi-experimental studies.
Table 3.
Summary of measured effects of IT-based interventions
| Outcome category | Outcome | Total | Effect | Effective interventions | Ineffective interventions | Mix effective interventions | ||
|---|---|---|---|---|---|---|---|---|
| Positive effect N (%) | No effect N (%) | Mix effect N (%) | ||||||
| Clinical outcome (n=8) | GFR | 2 | - | 2 (100) | - | - | Computerized systems Multiple components |
- |
| Graft failure | 1 | - | 1 (100) | - | - | Multiple components | - | |
| Concentrations of tacrolimus | 1 | - | 1 (100) | - | - | Multiple components | - | |
| Na | 1 | 1 (100) | - | - | Smartphones or PDA | - | ||
| BUN | 1 | 1 (100) | - | Smartphones or PDA | - | |||
| Creatinine | 1 | 1 (100) | - | Smartphones or PDA | - | |||
| Acute rejection | 1 | - | 1 (100) | - | - | Multiple components | - | |
| Process of care (n=7) | IRB and IRK | 1 | 1 (100) | - | - | Computerized systems | - | - |
| Fluid intake | 1 | 1 (100) | Smartphones or PDA | - | - | |||
| Electronically measured “taking” adherence | 1 | 1 (100) | - | - | Multiple components | - | - | |
| Electronically measured “timing” adherence | 1 | 1 (100) | - | - | Multiple components | - | - | |
| Proportion of patients who performed cholesterol testing within 1 week of their clinic visit | 1 | - | 1 (100) | - | - | Computerized systems | - | |
| The proportion of patients who achieved low-density lipoprotein (LDL) and cholesterol control | 1 | 1 (100) | Computerized systems | - | - | |||
| Increase pneumococcal vaccine rates | 1 | 1 (100) | - | - | Computerized systems | - | - | |
| Total | - | 15 | 6 (40) | 9 (60) | - | Computerized systems (3) Multiple components (2) Smartphones or PDA (1) |
Computerized systems (2) Multiple components (4) Smartphones or PDA (3) |
- |
Abbreviations: GFR, glomerular filtration rate; Na, sodium; BUN, blood urea nitrogen; IRK, illness-related knowledge; IRB, illness-related behavior; LDL, low-density lipoprotein.
Clinical outcomes
The clinical outcomes evaluated in these studies were glomerular filtration rate (GFR) changes (2 studies), graft failure (1 study), tacrolimus concentrations (1 study), Na serum level (1 study), blood urea nitrogen (BUN) serum level (1 study), creatinine serum level (1 study), and acute rejection (1 study). Totally, the impact of IT-based interventions on clinical outcomes was not statistically significant on clinical outcome. Two of the retrieved studies evaluated IT-based interventions on GFR, which was the most frequent outcome compared to the other clinical outcomes. In one study, the impact of computer-based patient education intervention on GFR changes was evaluated, with no reported significant effect.36 Another multicomponent intervention, involving text messages, e-mail, and/or visual cue dose reminders, found no significant effect on GFR changes.35 In one study, the impact of available interactive water bottle (HydraCoach water bottle) on serum chemistries (Na, BUN, and creatinine) was evaluated, having no significant effect.37 As well, the effect of multicomponent interventions (receive text message, mail, and/or visual cue dose reminders) on graft failure, acute rejection, and concentrations of tacrolimus was evaluated, with no reported significant effect.35
Care process outcomes
The care process outcomes evaluated in these studies consisted of illness-related knowledge (IRK) and illness-related behavior (IRB) (one study), electronically measured “taking” adherence (one study), electronically measured “timing” adherence (one study), fluid intake (one study), proportion of patients who performed cholesterol testing within 1 week of their clinic visit (one study), proportion of patients who achieved low-density lipoprotein (LDL) and cholesterol control (one study), and increased pneumococcal vaccine rates (1 study). Overall, the effect of IT-based interventions on care process outcomes was reported as statistically significant in 6 of 7 outcomes (85%) and not statistically significant as reported in one outcome (15%).
One of the included studies reported the effect of IT-based interventions on “taking” and “timing” of medication adherence as statistically significant.35 In this study, it was shown that use of an electronic monitoring system in medication adherence showed a statistically significant positive effect on taking and timing of medication. Three studies used computerized systems for measuring the process outcomes. The interventions involving computerized systems evaluated process care outcomes such as IRB and IRK, the proportion of patients who achieved LDL and cholesterol control, and increased pneumococcal vaccine rates. Computer systems were shown to have a statistically significant positive effect on all the above three process outcomes. Also, one of the studies examined the effect of computer systems on proportion of patients who performed cholesterol testing within 1week of their clinic visit and found no statistically significant effect. Regarding the other process care outcomes, one study showed that the daily fluid intake was significantly increased in the intervention group who used the interactive water bottle.
Intervention classification based on the type of technology and characteristics
Table 4 shows a summary of interventions classification based on technology. One study evaluated the effect of smartphone or (PDA) interventions using the interactive water bottle. The functions of the smartphones consisted of reminders, recording, and displaying. Based on the outcomes assessment results, the impact of smartphones was positive on one out of four outcomes, while no significant effect was observed on three outcomes. Three studies evaluated the impact of computerized system interventions using a computer-based educational program on IRK and IRB, GFR, the proportion of patients who achieved LDL and cholesterol control, increased pneumococcal vaccine rates, and proportion of patients who performed cholesterol testing within 1 week of their clinic visit. The technological functionalities of the computerized systems include reminding, informing, instructing, guiding, and displaying. These studies concluded that interventions had a positive impact on 3 of 5 evaluated outcomes, while they were found to be ineffective for the other two outcomes. Also, one study evaluated the impact of multicomponents, including text message, e-mail, and/or visual cue dose reminders. The impact of using multicomponent interventions was evaluated as positive on two outcomes, while there was no observed effect on four outcomes.
Table 4.
Classification of interventions based on technology type and features
| Reference | Classification of consumer health informatics | Technology platform | Technology functionality | Technology description |
|---|---|---|---|---|
| Freier C et al 36 | Computerized system | Computer-based educational programme | Inform Instruct |
The content of the programme is distributed in six main modules including one module on the time on the waiting list, the perioperative period, immunosuppressant and other medications, prevention of infections and rejection sand long-term issues. Knowledge transfer is provided in written format, short video clips with interviews of other transplanted adolescents, comics, and chart pictures. A quiz or interactive practice is at the end of each submodule. The computer program provides active and neutral feedback during the quiz by informing the patient of both a correct or incorrect answer. Module “‘your transplantation medication’” was used for the intervention and provides information about the following pharmaceutical drug groups: immunosuppressants, antihypertensives, antibiotics, antiviral drugs, and gastric protective medications. For each pharmaceutical drug, a detailed description of the generic and brand name, major effects, and important side effects is provided. |
| Kullgren KA et al 37 | Smartphones or PDA | interactive water bottle | Record Display Remind/Alert |
The HydraCoach water bottle is an interactive water bottle that calculates personal hydration needs, tracks real-time fluid consumption, and monitors fluid intake pacing through the day. The HydraCoach is the small removable computer on the bottle. A person merely enters their weight and the bottle automatically calculates a target fluid intake goal for that person. The HydraCoach also prompts the user to drink by continuously visually displaying the percentage consumed in either liters or ounces. It displays total amount consumed in a 24-hr period and can be easily reset every 12–24 hrs by the push of a few buttons. The display flashes after the 24-hr period to remind the user to reset it. |
| Foster BJ et al 35 | Multiple component | Text message, e-mail, and/or visual cue dose reminders |
Instruct Remind/Alert Record |
At enrollment, all participants were given an electronic multidose pillbox in which all medications were stored. During the first 4–6 months of recruitment, participants received a Medminder or Simplemed pillbox, with the same types of adherence tracking and reminder functions. Both devices connected using cellular telephone technology. Prescribed dosing times were recorded in each participant’s web-based pillbox record. The date and time of each pillbox compartment opening were registered in the patient’s electronic pillbox record. Intervention-arm participants could also choose to receive text message, e-mail, or visual cue dose reminders throughout the study. |
| Hooper DK et al 33 | Computerized system | EMR-automated reminders | Inform Display Remind/Alert Guide |
First intervention, simplified laboratory monitoring schedules for 12 selected tests, including fasting lipid profile, for personalized monitoring and developed 18 discrete individualized schedules based on evidence and published guidelines. second intervention was to develop a decision-support report automatically generated from our EMR to 1) identify all KTRs coming to clinic in the upcoming week, 2) assign 1 of the new 18 unique testing schedules to each patient according to dyslipidemia risk, and 3) report the most recent test results, whether additional testing was due, and the next due date for each test. The EMR was configured to automatically forward laboratory results to the ordering physician after the patient’s visit. |
| Malone K et al 34 | Computerized system | EHR-generated reminder | Remind/Alert Inform guide |
EHR-generated reminders to identify eligible patients before their clinic visit (ie pre-visit planning). This system created monthly report to track vaccination rates. As a general reminder to providers seeing transplant patients and progress note templates with built-in documentation reminders. Also they developed best practice advisory (BPA) alert that was added to EHR. |
| SUM | Computerized system (3 studies) Multiple component (1 study) Smartphones or PDA (1study) |
|||
Abbreviations: EMR, electronic medical record; KTR, kidney transplant recipient; BPA, best practice advisory; EHR, electronic health record; PDA, personal digital assistant.
Discussions
There is a limited body of literature that covers the value of technology-based interventions on self-management in the population of young adults who are recipients of kidney transplants. In the present review, we reviewed the best available publications to date. All articles and abstracts were assessed by two independent investigators, and both the inclusion of the studies and the data extraction were performed by reaching a consensus.
This systematic review focused on clinical trials and quasi-experimental studies, which analyzed the effect of IT-based interventions on the self-management outcomes in adolescents and young adult kidney transplant recipients.
Our search of the literature leads us to 5 studies with a total of 435 participants and showed that IT-based interventions have the potential to improve the self-management outcomes in their respective target population. The effectiveness of interventions on self-management outcomes was statistically significant in 3 of 5 studies.
In particular, a 40% rate of success was observed in the effectiveness of interventions on process outcomes, while there were no statistically significant reported effects of interventions on clinical outcomes. The studies which reported a positive impact of technology-based interventions utilized tools such as PDA and computer systems, as well as multicomponent interventions. However, there were no studies that evaluated the effect of using wearable devices on process and clinical outcomes. An interesting finding of the present review is the positive effect of IT-based interventions on process outcomes in transplant recipients, which is in contrast with some other systematic reviews based on IT-based interventions.31,32–34 Therefore, we may conclude that IT-based tools are the ideal form of intervention to improve process outcomes in adolescents and young adults after kidney transplantation.
The process outcomes such as medication adherence are not directly related to clinical outcomes. Nevertheless, the facilitated improvement in process outcomes can lead to an improvement in the patient’s clinical conditions.38 Findings of the present survey showed that IT-based interventions can lead to improved adherence to medication in adolescents and young adult kidney transplant recipients. This is similar to the findings of a systematic review which had been done for population of patients with chronic disease,39 in which they reiterated that automated IT-based interventions can potentially improve adherence to medication by sending electronic reminders. Given the importance of timely medication use in chronic diseases,40–42 IT-based tools are the recommended solution to improve adherence to medication in this population of patients.
There are many studies that focus on the use of IT for patient education and disease management. However, knowledge is categorized as one of the care process outcomes. Increased knowledge has the capability to empower patients by increasing their awareness.35 In this study, the impact of IT interventions on increasing kidney transplant knowledge was found to be important, in line with results of other studies.36,37–39 One of the studies in our review investigated the effect of a tool that used an electronical interactive water bottle on clinical outcomes and reported no significant effect. However, the duration of intervention in this study was short, signifying the fact that the clinical relevance of the findings of this study is debatable.
None of the studies in our review was free from the risk of bias. Some form of bias is inherent to the type of intervention, since the blinding of either the participants or professionals was not possible. The methodological quality of the five selected studies in this review varied. Only one of the 3 RCTs was rated as having a good methodological quality. Limited information about the blinding process as well as insufficient description of the randomization and concealment method were the two most important factors that contributed to the low scores on the risk of bias. The associated bias may have influenced the results of these studies. This is because previous evidence shows that poor methodological approaches in controlled trials, particularly those representing weak allocation concealment, are associated with bias.43
HIT approaches have the potential to address the challenges faced in adherence to pharmacological therapy or overcome the complications in behavioral interventions. In this perspective, HITs may be advantageous in these therapeutic approaches. With its advantages of being pragmatic, highly engaging, cost-effective, and scalable,44 these technologies are capable of providing an interactive communication between the patients and their health care providers, providing timely reminders for medication or cues for behavioral change, enhancing treatment or intervention effects, and ultimately, assisting patients to achieve self-management.
One of the strength points of this review was the application of a comprehensive search strategy that extracted a large number of publications, thus reducing the chances of missing relevant studies. The present review only included experimental studies (randomized clinical trials and quasi-experimental studies), and other types of studies were excluded. As well, the quality of all studies was thoroughly investigated. This review mainly comprised of long-term interventions, with the exception of one study, which involved an intervention of 1month. The literature suggests that long-term changes in behavior can only be proven by studies with long-term follow-up periods.45 However, no guidelines exist regarding the optimal duration of interventions. Combining multiple IT-based interventions and proposing a comprehensive solution for obtaining better results in various clinical findings can lead to better self-management and may be the suggested direction for future research.
One of the limitations of this study was the exclusion of conference articles, which was due to lack of accessibility. Furthermore, this review comprised of two short-term interventions. Only three studies incorporated interventions of 6 months or longer. Another limitation of this study was the presence of heterogeneity across outcomes of the five studies, which implied that no meta-analysis of the outcomes was feasible.
Future experiments should consider studies with a larger sample size, thus improving the generalization ability of the subsequent outcomes. The duration of interventions should be consistent with the expected goals and outcomes of the studies. Based on the quality evaluation of the studies, there is a need for improved reporting.
Conclusion
IT-based interventions such as mHealth, computer systems, and multicomponent systems can improve process outcomes for self-management in adolescents and young adult kidney transplant recipients. Moreover, IT-based interventions may potentially improve clinical outcomes. Future studies should consider larger sample sizes and longer follow-up periods.
Acknowledgments
This study was supported by a grant from Mashhad University of Medical Sciences (951645) Research Council and National Institute for Medical Research Development (963562).
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Chaturvedi S, Jones CL, Walker RG, Sawyer SM. The transition of kidney transplant recipients: a work in progress. Pediatric nephrology (Berlin, Germany) 2009;24(5):1055–1060. doi: 10.1007/s00467-009-1124-y [DOI] [PubMed] [Google Scholar]
- 2.Foster BJ. Heightened graft failure risk during emerging adulthood and transition to adult care. Pediatr Nephrol. 2015;30(4):567–576. doi: 10.1007/s00467-014-2859-7 [DOI] [PubMed] [Google Scholar]
- 3.Watson AR, Harden PN, Ferris ME, Kerr PG, Mahan JD, Ramzy MF. Transition from pediatric to adult renal services: a consensus statement by the International Society of Nephrology (ISN) and the International Pediatric Nephrology Association (IPNA). Kidney Int. 2011;80(7):704–707. doi: 10.1038/ki.2011.209 [DOI] [PubMed] [Google Scholar]
- 4.Stam H, Hartman EE, Deurloo JA, Groothoff J, Grootenhuis MA. Young adult patients with a history of pediatric disease: impact on course of life and transition into adulthood. J Adolesc Health. 2006;39(1):4–13. doi: 10.1016/j.jadohealth.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 5.Grootenhuis MA, Stam H, Last BF, Groothoff JW. The impact of delayed development on the quality of life of adults with end-stage renal disease since childhood. Pediatr Nephrol. 2006;21(4):538–544. doi: 10.1007/s00467-006-0030-9 [DOI] [PubMed] [Google Scholar]
- 6.Varnell CD Jr, Rich KL, Nichols M, et al. Assessing barriers to adherence in routine clinical care for pediatric kidney transplant patients. Pediatr Transplant. 2017;21(7). doi: 10.1111/petr.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA. Association between age and graft failure rates in young kidney transplant recipients. Transplantation. 2011;92(11):1237–1243. doi: 10.1097/TP.0b013e31823411d7 [DOI] [PubMed] [Google Scholar]
- 8.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant. 2010;14(5):603–613. doi: 10.1111/j.1399-3046.2010.01299.x [DOI] [PubMed] [Google Scholar]
- 9.Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9(11):2597–2606. doi: 10.1111/j.1600-6143.2009.02798.x [DOI] [PubMed] [Google Scholar]
- 10.Steinberg EA, Moss M, Buchanan CL, Goebel J. Adherence in pediatric kidney transplant recipients: solutions for the system. Pediatr Nephrol. 2018;33(3):361–372. doi: 10.1007/s00467-017-3637-0 [DOI] [PubMed] [Google Scholar]
- 11.Francis A, Johnson DW, Craig JC, Wong G. Moving on: transitioning young people with chronic kidney disease to adult care. Pediatr Nephrol. 2018;33(6):973–983. doi: 10.1007/s00467-017-3728-y [DOI] [PubMed] [Google Scholar]
- 12.Ingerski L, Perrazo L, Goebel J, Pai AL. Family strategies for achieving medication adherence in pediatric kidney transplantation. Nurs Res. 2011;60(3):190–196. doi: 10.1097/NNR.0b013e318215fdfe [DOI] [PubMed] [Google Scholar]
- 13.Kreuzer M, Prüfe J, Tönshoff B, Pape L. Survey on management of transition and transfer from pediatric- to adult-based care in pediatric kidney transplant recipients in Europe. Transplant Direct. 2018;4(7):e361–e. doi: 10.1097/TXD.0000000000000798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patzer RE, Serper M, Reese PP, et al. Medication understanding, non-adherence, and clinical outcomes among adult kidney transplant recipients. Clin Transplant. 2016;30(10):1294–1305. doi: 10.1111/ctr.12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagger MS, Orbell S. A meta-analytic review of the common-sense model of illness representations. Psychol Health. 2003;18(2):141–184. doi: 10.1080/088704403100081321 [DOI] [Google Scholar]
- 16.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01 [DOI] [PubMed] [Google Scholar]
- 17.Modi AC, Pai AL, Hommel KA, et al. Pediatric self-management: a framework for research, practice, and policy. Pediatrics. 2012;129(2):e473. doi: 10.1542/peds.2011-1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wantland DJ, Portillo CJ, Holzemer WL, Slaughter R, McGhee EM. The effectiveness of web-based vs. non-web-based interventions: a meta-analysis of behavioral change outcomes. J Med Internet Res. 2004;6(4):e40. doi: 10.2196/jmir.6.4.e40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kay M, Santos J, Takane M. mHealth: new horizons for health through mobile technologies. World Health Organ. 2011;64(7):66–71. [Google Scholar]
- 20.Välimäki M, Hätönen H, Lahti M, Kuosmanen L, Adams CE. Information and communication technology in patient education and support for people with schizophrenia. Cochrane Database of Systematic Reviews 2012;10: CD007198. doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeddi FR, Nabovati E, Amirazodi S. Features and effects of information technology-based interventions to improve self-management in chronic kidney disease patients: a systematic review of the literature. J Med Syst. 2017;41(11):170. doi: 10.1007/s10916-017-0820-6 [DOI] [PubMed] [Google Scholar]
- 22.McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. The Lancet. 2010;376(9736):163–172. doi: 10.1016/S0140-6736(10)60964-6 [DOI] [PubMed] [Google Scholar]
- 23.Strand H, Parker D. Effects of multidisciplinary models of care for adult pre‐dialysis patients with chronic kidney disease: a systematic review. Int J Evid Based Healthc. 2012;10(1):53–59. doi: 10.1111/j.1744-1609.2012.00253.x [DOI] [PubMed] [Google Scholar]
- 24.Taylor S, Pinnock H, Epiphaniou E, Pearce G, Parke H, Schwappach A, et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: PRISMS - Practical systematic Review of Self-Management Support for long-term conditions. Southampton (UK); NIHR Journals Library 2014:1–580. [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 26.Higgins Julian P T, Altman Douglas G, GøtzschePeter C, Jüni Peter, Moher David, Oxman Andrew Det al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estabrooks C, Goel V, Thiel E, Pinfold P, Sawka C, Williams I. Decision aids: are they worth it? A systematic review. J Health Serv Res Policy. 2001;6(3):170–182. doi: 10.1258/1355819011927431 [DOI] [PubMed] [Google Scholar]
- 28.Estabrooks CA, Cummings GG, Olivo SA, Squires JE, Giblin C, Simpson N. Effects of shift length on quality of patient care and health provider outcomes: systematic review. Qual Saf Health Care. 2009;18(3):181–188. doi: 10.1136/qshc.2007.024232 [DOI] [PubMed] [Google Scholar]
- 29.Beratarrechea A, Lee AG, Willner JM, Jahangir E, Ciapponi A, Rubinstein A. The impact of mobile health interventions on chronic disease outcomes in developing countries: a systematic review. Telemed J E Health. 2014;20(1):75–82. doi: 10.1089/tmj.2012.0328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vegesna A, Tran M, Angelaccio M, Arcona S. Remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed J E Health. 2017;23(1):3–17. doi: 10.1089/tmj.2016.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aitken M, Gauntlett C. Patient Apps for Improved Healthcare: From Novelty to Mainstream. Parsippany, NJ: IMS Institute for Healthcare Informatics; 2013. [Google Scholar]
- 32.Chaet AV, Morshedi B, Wells KJ, Barnes LE, Valdez R. Spanish-language consumer health information technology interventions: a systematic review. J Med Internet Res. 2016;18:8. doi: 10.2196/jmir.5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malone K, Clark S, Palmer JA, et al. A quality improvement initiative to increase pneumococcal vaccination coverage among children after kidney transplant. Pediatr Transplant. 2016;20(6):783–789. doi: 10.1111/petr.12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster BJ, Pai AL, Zelikovsky N, et al. A randomized trial of a multicomponent intervention to promote medication adherence: the teen adherence in kidney transplant effectiveness of intervention trial (TAKE-IT). Am J Kidney Dis. 2018. doi: 10.1053/j.ajkd.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freier C, Oldhafer M, Offner G, Dorfman S, Kugler C. Impact of computer‐based patient education on illness‐specific knowledge and renal function in adolescents after renal transplantation. Pediatr Transplant. 2010;14(5):596–602. doi: 10.1111/j.1399-3046.2010.01297.x [DOI] [PubMed] [Google Scholar]
- 37.Kullgren KA, Scholl P, Kidwell KM, Hmiel SP. Using an interactive water bottle to target fluid adherence in pediatric kidney transplant recipients: a pilot study. Pediatr Transplant. 2015;19(1):35–41. doi: 10.1111/petr.12385 [DOI] [PubMed] [Google Scholar]
- 38.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163(12):1409–1416. doi: 10.1001/archinte.163.12.1409 [DOI] [PubMed] [Google Scholar]
- 39.Vervloet M, Linn AJ, van Weert JC, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc. 2012;19(5):696–704. doi: 10.1136/amiajnl-2011-000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid H, Hartmann B, Schiffl H. Adherence to prescribed oral medication in adult patients undergoing chronic hemodialysis: a critical review of the literature. Eur J Med Res. 2009;14(5):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva AN, Moratelli L, Tavares PL, et al. Self-efficacy beliefs, locus of control, religiosity and non-adherence to immunosuppressive medications in kidney transplant patients. Nephrology. 2016;21(11):938–943. doi: 10.1111/nep.12695 [DOI] [PubMed] [Google Scholar]
- 42.Weng FL, Chandwani S, Kurtyka KM, Zacker C, Chisholm-Burns MA, Demissie K. Prevalence and correlates of medication non-adherence among kidney transplant recipients more than 6 months post-transplant: a cross-sectional study. BMC Nephrol. 2013;14:261. doi: 10.1186/1471-2369-14-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Jama. 1995;273(5):408–412. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida Y, Boren SA, Soares J, Popescu M, Nielson SD, Simoes EJ. Effect of health information technologies on glycemic control among patients with type 2 diabetes. Curr Diab Rep. 2018;18(12):130. doi: 10.1007/s11892-018-1107-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simons-Morton DG, Calfas KJ, Oldenburg B, Burton NW. Effects of interventions in health care settings on physical activity or cardiorespiratory fitness. Am J Prev Med. 1998;15(4):413–430. [DOI] [PubMed] [Google Scholar]