Abstract
Background
Chronic kidney disease (CKD) is a global public health problem associated with progressive decline in kidney function and adverse cardiovascular outcome. Anemia of CKD has substantial adverse outcomes in CKD patients. There is paucity of published data on prevalence of anemia and its associated factors among CKD patients in Northwest Ethiopia.
Objective
This study aimed to determine the prevalence of anemia and its associated factors among CKD patients at the University of Gondar hospital, Northwest Ethiopia.
Methods
A hospital-based cross-sectional study was conducted from May 1, to September 30, 2018. Consecutive sampling was used to recruit 251 study subjects. Patients were interviewed to obtain demographic data, and the patients’ medical records were reviewed to obtain information on relevant medical history and laboratory parameters. Data was analyzed using SPSS version 20. Bivariate and multivariate logistic regression analyses were used to identify independently associated factors of anemia among CKD patients. P-value <0.05 was used to declare association.
Results
The overall prevalence of anemia in CKD patients was high (64.5%), and the magnitude worsened as kidney function declined. Hypertension (45%), chronic glomerulonephritis (24%) and diabetes (20%) were common causes of CKD. Multivariate logistic regression analysis revealed rural residence (AOR= 2.75, 95%CI: 1.34–5.65, P=0.006), BMI <18.5 kg/m2 (AOR=6.78, 95%CI: 1.32–34.73, P=0.022) and BMI of 18.5–24.9 kg/m2 (AOR=5.04, 95%CI: 1.26–20.10, P=0.022), and having hemodialysis history (AOR=3.59, 95%CI: 1.24–10.38, P=0.018) were independently associated with anemia among CKD patients.
Conclusion
Periodic screening and intervention programs for anemia of CKD should be practiced to change the existing situation in the setting.
Keywords: chronic kidney disease, anemia, Northwest Ethiopia
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Chronic kidney disease (CKD) is progressive, irreversible damage to the kidneys, which leads to inability of the kidneys to perform homeostatic, synthetic and excretory functions. Estimated epidemiological prevalence of CKD in global and sub-Saharan Africa was reported to be 15% and 10% respectively.1–7 The evident reasons for escalating burden of kidney failure in Africa were due to growing urbanization, environmental pollutant exposure, high burden of infectious diseases and increasing rate of noncommunicable diseases such as diabetes and hypertension.5–7
Common etiologies of CKD in Africa were hypertension, chronic glomerulonephritis and diabetes. Ten percent of HIV-positive patients were documented to have renal dysfunction. Complications of CKD included anemia, metabolic bone disease, metabolic acidosis, fluid and electrolyte imbalance and uremia, which imposed considerable burden on health care resources.5–7,11–14
Anemia is a common and significant complication in CKD patients. Study-based estimated prevalence of anemia was 15% in US CKD patients, 45–55% in Asian CKD patients, and 50–90% in African CKD patients.3,4,11–13
The etiopathogenesis of anemia in CKD is multifactorial and includes erythropoietin deficiency from reduced renal mass, uremic environment-induced inhibitors of erythropoiesis, shortened red cell survival, and disordered iron homeostasis.8,9
Anemia in CKD patients is significantly associated with worsening CKD, and development of heart failure and stroke. It contributes to impaired physical activity, neurocognitive dysfunction and poor quality of life.9–13,15,16,22–24
Documented predictors of anemia in CKD include, but are not restricted to, female gender, advanced stage of CKD, diabetic nephropathy as etiology, nonsmoking status, nonobese body habitus, low serum albumin, abnormal bone minerals level (high phosphorus and low calcium levels), abnormal iron markers (transferrin saturation <20%), and low leukocyte count.4,13,17
However, prevalence of anemia of CKD in Northwest Ethiopia has not been fully investigated. Therefore, we aimed to disclose prevalence of anemia and its associated factors among CKD patients in the setting.
Methods
Study Settings And Design
A hospital-based cross sectional study was conducted from May 1, 2018 to September 30, 2018 at the University of Gondar hospital. The hospital is located in Northwest Ethiopia, which is 750 km away from the capital, Addis Ababa. The hospital has a catchment population of five million people.
Study Subjects And Variables
Source population was all CKD patients visiting University of Gondar hospital. Patients 18 years and above with an established diagnosis of CKD as per kidney disease/improving global outcomes (KDIGO) criteria regardless of its primary cause were considered as study population. Those study subjects who provided informed consent were included in the study. Patients with known cause of anemia other than kidney disease, pregnant women, and those unwilling to participate were excluded.
Sample Size And Sampling Procedure
The sample size was calculated using Fisher’s formula18 at a prevalence of 80% with a confidence interval of 95% and degree of precision of 5%. Consecutive sampling was used to recruit 251 study subjects.
Data Collection
Data were collected through an investigator administered pretested questionnaire. Patients were interviewed to obtain demographic data, and the patients’ medical records were reviewed to obtain information on relevant medical history and laboratory parameters. The determination of the primary cause of kidney disease was based on clinical history, physical examination, and laboratory investigations including complete blood count, urinalysis, blood chemistry, ultrasound and HIV serology.
Blood and urine samples were collected for laboratory tests. Complete blood counts were obtained after processing the blood sample using AR-6300 (ARI Medical Equipment Co. Ltd, China) hematology analyzer. Serum glucose and creatinine were determined by enzymatic glucose oxidase and kinetic alkaline picrate method respectively using Mindray BS-480 (Shenzhen Mindray Bio-Medical Electronics Co., Ltd, China) clinical chemistry analyzer. Urine albumin was measured semiquantitatively by dip stick urinalysis (COMBINA 11S, Human) using fresh, midstream urine samples.
The hemoglobin level was used to define anemia according to WHO criteria.19 The serum creatinine was used to estimate GFR using chronic kidney disease epidemiology collaboration (CKD-EPI) equation.20
Dependent variable: anemia was the dependent variable, as defined by WHO criteria.
Independent variables: sociodemographic factors including age, sex, place of residence, educational status and marital status; behavioral factors including smoking and alcohol drinking; and clinical parameters such as cause, duration and stage of CKD, presence of comorbidity, dialysis status, body mass index (BMI), blood pressure level, presence/absence of albuminuria and treatment status for anemia.
Data Analysis
Data was entered into EPI Info version 4.4.1 and transported to SPSS version 20 for analysis. Descriptive statistics, such as median and IQRs were used to compute continuous variables, and counts with percentage for categorical variables. Both bivariate and multivariate logistic regression analyses were used to identify independently associated factors of anemia in CKD patients. Those variables with a P-value <0.2 in the bivariate analysis were exported to multivariate analysis to control the possible effect of confounders. Adjusted odds ratio (AOR) with 95%CI and P-value <0.05 were used to select variables associated with anemia in CKD patients.
Definition Of Terms
Chronic kidney disease (CKD) was defined as abnormalities of kidney structure or function present for more than three months, with implications for health. CKD is classified based on cause and GFR category (G1–G5). GFR was determined by using CKD-EPI equation for eGFR, stage 1, albuminuria with eGFR >90 mL/min/1.73m2; stage 2, albuminuria with eGFR 60–89 mL/min/1.73m2; stage 3a, eGFR 45–59ml/min/1.73m2; stage 3b, eGFR 30–44 mL/min/1.73m2; stage 4, eGFR 15–29 mL/min/1.73m2; and stage 5, eGFR<15 mL/min/1.73m2.
Anemia was defined as hemoglobin level <12g/dL in females and <13g/dL in males in patients aged 18 years of age and above. Severity of anemia was classified as mild (11–11.9 g/dL (females), 11–12.9 g/dL (males)), moderate (8–11 g/dL), and severe (<8 g/dL).
Albuminuria was defined as presence of albumin in the urine (from trace to 4+). Massive proteinuria is defined as albumin in urine of 2+ in two of three determinations over three months.
Hypertension was defined as the presence of persistently elevated systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg in patients aged 18 years of age and above, history of hypertension, or the use of antihypertensive drug(s).
Hypertensive kidney disease was defined as presence of documented history of hypertension predated kidney disease (reduced eGFR).
Diabetes was defined as fasting serum glucose ≥126 mg/dL, history of diabetes, or use medications for diabetes.
Diabetic nephropathy (DN) was defined as long-standing known diabetes, and urinalysis evidence of significant proteinuria.
Chronic glomerulonephritis was defined as presence of proteinuria, hematuria, and/or ultrasound-proven reduced kidney size (≤8 cm) or loss of cortico-medullary differentiation.
Ethical Considerations
Ethical clearance was obtained from the Research Ethical Review Board of College of Medicine and Health Sciences, University of Gondar. Formal letter of permission was obtained from University of Gondar hospital administrative body. Written informed consent was obtained from participants, and confidentiality of information obtained was maintained by coding and restricting access to the questionnaire. During the data collection process, those patients who were found to have anemia were taken care of as per the recommendations of CKD guidelines, and advice on preventive measures was given to all participants.
Result
Sociodemographic Characteristics Of Study Participants
Among a total of 251 CKD patients, 161 (64.1%) were males and 90 (35.9%) were females. The median age of study subjects was 60 years (IQR=44–69). The majority of patients (61%) were aged from 45 to 75 years. Most of them were nonsmokers (95%), nonalcoholic (76%), married (77%), and Orthodox Christian by religion (84%). The majority 142 (57%) were urban dwellers and 118 (47%) joined formal education (Table 1).
Table 1.
Sociodemographic Characteristics Of CKD Patients (N=251)
| Variables | Frequency (%) | Anemia | P-value | |
|---|---|---|---|---|
| Present | Absent | |||
| Sex | ||||
| Male | 161 (64.1) | 102 | 59 | 0.599 |
| Female | 90 (35.9) | 60 | 30 | |
| Age (years) | ||||
| 19–30 | 30 (12.0) | 24 | 6 | 0.003 |
| 31–45 | 40 (15.9) | 33 | 7 | |
| 46–60 | 78 (31.1) | 40 | 38 | |
| 61–75 | 75 (29.9) | 50 | 25 | |
| 76–85 | 28 (11.1) | 15 | 13 | |
| Residence | ||||
| Urban | 142 (56.6) | 76 | 66 | <0.001 |
| Rural | 109 (43.4) | 86 | 23 | |
| Occupation | ||||
| Civil servant | 43 (17.1) | 21 | 22 | <0.001 |
| Merchant | 37 (14.7) | 18 | 19 | |
| Farmer | 61 (24.3) | 52 | 9 | |
| Housewife | 60 (23.9) | 43 | 17 | |
| Self-employed | 13 (5.2) | 9 | 4 | |
| Daily laborer | 11 (4.4) | 9 | 2 | |
| Student | 5 (2.0) | 3 | 2 | |
| Othersa | 21 (8.4) | 7 | 14 | |
| Religion | ||||
| Orthodox Christian | 210 (83.7) | 143 | 67 | 0.018 |
| Protestant Christian | 4 (1.6) | 2 | 2 | |
| Muslim | 37 (14.7) | 17 | 20 | |
| Marital status | ||||
| Single | 22 (8.8) | 15 | 7 | 0.767 |
| Married | 193 (76.9) | 124 | 69 | |
| Divorced | 21 (8.3) | 12 | 9 | |
| Widowed | 15 (6.0) | 11 | 4 | |
| Educational status | ||||
| Unable to read and write | 101 (40.2) | 78 | 23 | 0.007 |
| Able to read and write | 32 (12.7) | 20 | 12 | |
| Primary education | 41 (16.3) | 25 | 16 | |
| Secondary education | 37 (14.7) | 20 | 17 | |
| College and above | 40 (15.9) | 19 | 21 | |
Note: a= retired or no job.
Clinical and Laboratory Profile Of CKD Patients
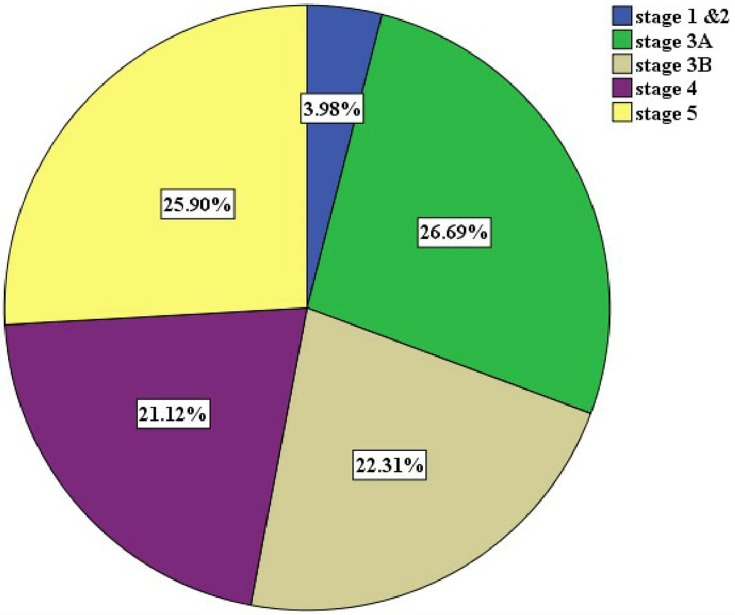
Hypertension accounted for 45% as a cause of CKD, followed by chronic glomerulonephritis (24%) and diabetes (20%). HIV/AIDS was ascertained as etiology of CKD in 2% of cases (Table 2). Majority of the patients were in KDIGO stage 3 (49%), followed by KDIGO stage 5 (26%) and stage 4 (21%) (Figure 1). Two-thirds of patients 180 (71.7%) were positive for albuminuria using urinalysis dipstick. One hundred four (53.3%) had massive proteinuria (≥2+ albuminuria). One-fifth 51 (20.3%) had ultrasound-proven bilateral shrunken kidneys (≤8 cm) (Table 3).
Table 2.
Clinical Characteristics Of CKD Patients (N=251)
| Variables | Frequency (%) | Anemia | P-value | |
|---|---|---|---|---|
| Present | Absent | |||
| Treatment status for anemia | ||||
| Yes | 42 (16.7) | 42 | 0 | <0.001 |
| No | 209 (83.3) | 120 | 89 | |
| Type of treatment for treated cases | ||||
| Blood transfusion (BT) | 19 | 19 | 0 | ? |
| Hematinics only | 14 | 14 | 0 | |
| BT and hematinics | 3 | 3 | 0 | |
| Hematinics and ESA | 5 | 5 | 0 | |
| BT, hematinics, and ESA | 1 | 1 | 0 | |
| Smoking | ||||
| Yes | 13 (5.2) | 7 | 6 | 0.408 |
| No | 238 (94.8) | 155 | 83 | |
| Alcohol drinking | ||||
| Yes | 61 (24.3) | 39 | 22 | 0.909 |
| No | 190 (75.7) | 123 | 67 | |
| Presence of comorbidity | ||||
| Yes | 88 (35.1) | 58 | 60 | 0.014 |
| No | 163 (64.9) | 104 | 59 | |
| Taking other medicationsa | ||||
| Yes | 212 (84.1) | 132 | 79 | 0.132 |
| No | 40 (15.9) | 30 | 10 | |
| Family history of kidney disease | ||||
| Yes | 10 (4.0) | 9 | 1 | 0.085 |
| No | 241 (96.0) | 153 | 88 | |
| Underlying cause of CKD | ||||
| Chronic GN | 60 (23.9) | 54 | 6 | <0.001 |
| Hypertension | 115 (45.4) | 66 | 48 | |
| Diabetes | 51 (20.9) | 23 | 28 | |
| Others | 26 (9.8) | 20 | 6 | |
| Hemodialysis history | ||||
| Yes | 32 (12.7) | 26 | 6 | 0.034 |
| No | 219 (87.3) | 136 | 83 | |
| SBP category (mmHg) | ||||
| ≥160 | 38 (15.1) | 22 | 16 | 0.151 |
| 150–159 | 68 (27.1) | 41 | 27 | |
| 120–139 | 85 (33.9) | 53 | 32 | |
| 90–119 | 60 (23.9) | 46 | 14 | |
| DBP category (mmHg) | ||||
| ≥100 | 21 (8.4) | 16 | 5 | 0.033 |
| 90–99 | 52 (20.7) | 31 | 21 | |
| 80–89 | 70 (27.9) | 37 | 33 | |
| 60–79 | 108 (43.0) | 78 | 30 | |
| BMI (kg/m2) | ||||
| <18.5 | 43 (17.1) | 35 | 8 | <0.001 |
| 18.5–24.9 | 160 (63.7) | 109 | 51 | |
| 25–29.9 | 31 (12.4) | 14 | 17 | |
| ≥30 | 17 (6.8) | 4 | 13 | |
Note: aACE inhibitors, calcium channel blockers, diuretics, insulin, oral antidiabetic drugs (metformin, glibenclamide), ASA and statins.
Figure 1.
Stages of CKD by eGFR using CKD-EPI equation.
Table 3.
Laboratory And Imaging Profile Of CKD Patients (N=251)
| Variables | Frequency (%) | Anemia | P-value | |
|---|---|---|---|---|
| Present | Absent | |||
| Albuminuria on urinalysis dipstick | ||||
| Present | 180 (71.7) | 120 | 60 | 0.264 |
| Absent | 71 (28.3) | 42 | 29 | |
| Degree of albuminuria | ||||
| Negative | 71 (28.3) | 42 | 29 | 0.025 |
| 1+ | 46 (18.3) | 23 | 23 | |
| 2+ | 101 (40.2) | 75 | 26 | |
| 3+ | 33 (13.2) | 22 | 11 | |
| eGFR (mL/min/1.73m2) | ||||
| Early stage (stage 1/2) | 10 (4.0) | 2 | 8 | <0.001 |
| Stage 3A (45–59.99) | 67 (26.7) | 30 | 37 | |
| Stage 3B (30–44.99) | 56 (22.3) | 26 | 30 | |
| Stage 4 (15–29.99) | 53 (21.1) | 43 | 10 | |
| Stage 5 (≤14.99) | 65 (25.9) | 61 | 4 | |
| Renal ultrasound findings | ||||
| Normal sized kidneys | 123 (49.0) | 71 | 52 | 0.264 |
| Shrunken kidneys | 51 (20.3) | 35 | 16 | |
| Large kidneys | 4 (1.6) | 3 | 1 | |
| Congenital anomalies | 9 (3.6) | 5 | 4 | |
| Obstructive | 15 (6.0) | 11 | 4 | |
| Others | 49 (19.5) | 37 | 12 | |
| HIV status | ||||
| Reactive | 12 (4.8) | 10 | 2 | 0.002 |
| Nonreactive | 99 (39.4) | 75 | 24 | |
| Unknown | 140 (55.8) | 77 | 63 | |
Prevalence Of Anemia Among CKD Patients
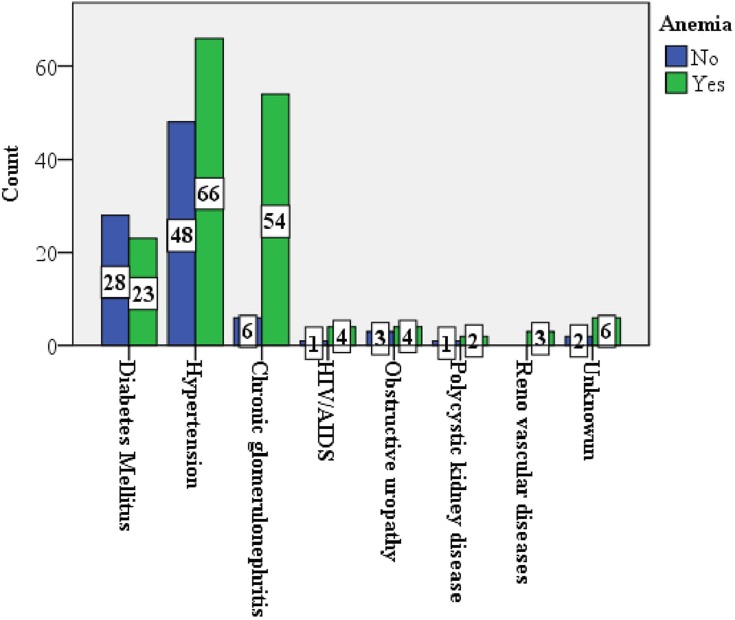
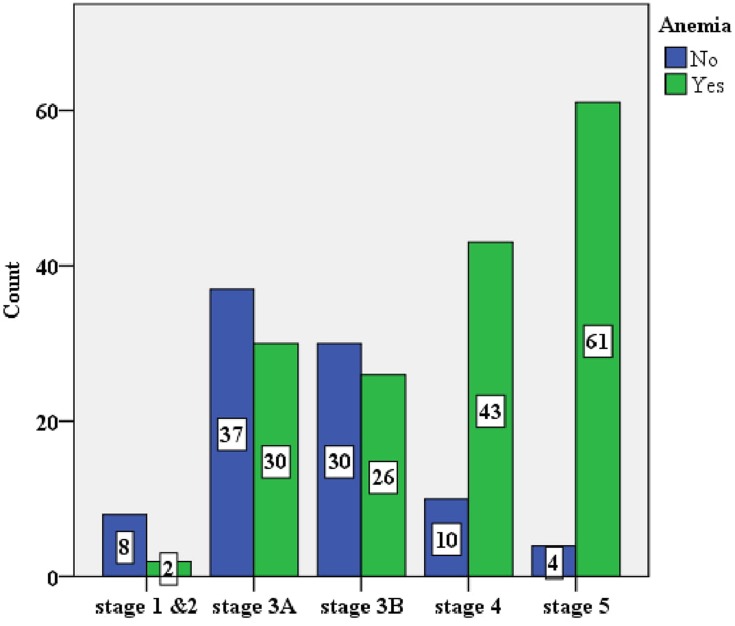
The overall prevalence of anemia in this study was 64.5% (58.6 to 70.5%). Anemia was significantly prevalent in glomerular disease among other underlying causes of CKD (P-value <0.001) (Figure 2). The prevalence of anemia increased with worsening kidney function: stage 1 and 2, 3A, 3B, 4 and 5 CKD were 20%, 44.8%, 46.4%, 81.1%, 93.8% respectively (Figure 3). Severe anemia (Hgb <8g/dL) was documented in 49/251 (19.5%) of CKD patients. One quarter (42/162) of patients received therapy among those who had documented anemia. Options of therapy among treated cases were packed blood transfusions 23/42 (55%), hematinics 23/42 (55%), and erythropoiesis stimulating agent (ESA) 6/42 (14.3%) (Table 2).
Figure 2.
The prevalence of anemia across the underlying causes of CKD.
Figure 3.
Prevalence of anemia across stages of CKD.
Factors Associated With Anemia Among CKD Patients
Multivariate logistic regression analysis was used to identify independently associated factors of anemia among CKD patients. Accordingly, rural residence (AOR=2.75, 95%CI: 1.34–5.65, P=0.006), BMI <18.5 kg/m2 (AOR=6.78, 95%CI: 1.32–34.73, P=0.022) and BMI of 18.5–24.9 kg/m2 (AOR=5.04, 95%CI: 1.26–20.10, P=0.022) and, having hemodialysis history (AOR=3.59, 95%CI: 1.24–10.38, P=0.018) were found to be associated with anemia among CKD patients. Those with diastolic blood pressure (DBP) between 80 and 89 mmHg (AOR=0.37, 95%CI: 0.16–0.92, P=0.025) were at lower risk for anemia of CKD (Table 4).
Table 4.
Bivariate And Multivariate Logistic Regression Analysis Of Factors Associated With Anemia Among CKD Patients At University Of Gondar Hospital, Northwest Ethiopia, May 1, To September 30, 2018 (N=251)
| Variable | Anemia | COR (95%CI) | AOR (95%CI) | P–value | |
|---|---|---|---|---|---|
| Present | Absent | ||||
| Sex | |||||
| Male | 102 | 59 | 1 | ||
| Female | 60 | 30 | 1.16 (0.67–1.99) | ||
| Age (years) | |||||
| 19–30 | 24 | 6 | 1 | 1 | |
| 31–45 | 33 | 7 | 1.18 (0.35–3.96) | 1.30 (0.34–5.07) | |
| 46–60 | 40 | 38 | 0.26 (0.01–0.71) | 0.53 (0.16–1.71) | |
| 61–75 | 50 | 25 | 0.50 (0.18–1.38) | 1.00 (0.29–3.42) | |
| 76–85 | 15 | 13 | 0.29 (0.09–0.92) | 0.69 (0.16–2.94) | |
| Residence | |||||
| Urban | 76 | 66 | 1 | 1 | |
| Rural | 86 | 23 | 3.25 (1.84–5.72) | 2.75 (1.34–5.65) | 0.006 |
| Educational status | |||||
| Unable to read &write | 78 | 23 | 3.75 (1.73–8.14) | 1.65 (0.58–4.64) | |
| Able to read and write | 20 | 12 | 1.84 (0.71–4.75) | 1.46 (0.46–4.66) | |
| Primary education (1–8) | 25 | 16 | 1.73 (0.72–4.17) | 1.14 (0.38–3.37) | |
| Secondary education (9–10) | 12 | 6 | 2.21 (0.69–7.05) | 1.31 (0.33–5.17) | |
| Preparatory (11–12) | 8 | 11 | 0.80 (0.27–2.42) | 0.71 (0.17–2.88) | |
| College and above | 19 | 21 | 1 | 1 | |
| Marital status | |||||
| Married | 124 | 69 | 1 | ||
| Single | 15 | 7 | 1.19 (0.46–3.07) | ||
| Divorced | 12 | 9 | 0.74 (0.30–1.85) | ||
| Widowed | 11 | 4 | 1.53 (0.47–4.99) | ||
| Alcohol drinking | |||||
| Yes | 39 | 22 | 0.97 (0.53–1.76) | ||
| No | 123 | 67 | 1 | ||
| Presence of comorbidity | |||||
| Yes | 58 | 30 | 1.01 (0.64–1.89) | ||
| No | 104 | 59 | 1 | ||
| Taking other medications | |||||
| Yes | 132 | 79 | 0.56 (0.26–1.20) | 0.79 (0.30–2.08) | |
| No | 30 | 10 | 1 | 1 | |
| SBP category (mmHg) | |||||
| 90–119 | 46 | 14 | 1 | 1 | |
| 120–139 | 53 | 32 | 0.50 (0.24–1.06) | 1.38 (0.52–3.65) | |
| 140–159 | 41 | 27 | 0.46 (0.21–1.00) | 1.03 (0.34–3.14) | |
| 160+ | 22 | 16 | 0.42 (0.17–1.00) | 0.96 (0.25–3.59) | |
| DBP category(mmHg) | |||||
| 60–79 | 78 | 30 | 1 | 1 | |
| 80–89 | 37 | 33 | 0.43 (0.23–0.81) | 0.38 (0.16–0.92) | 0.025 |
| 90–99 | 31 | 21 | 0.57 (0.28–1.14) | 0.62 (0.23–1.71) | |
| 100+ | 16 | 5 | 1.23 (0.41–3.67) | 1.69 (0.35–8.09) | |
| Hemodialysis status | |||||
| Yes | 26 | 6 | 2.24 (0.93–5.39) | 3.59 (1.24–10.38) | 0.018 |
| No | 136 | 83 | 1 | 1 | |
| BMI (kg/m2) | |||||
| 10–18.49 | 35 | 8 | 14.22 (3.66–55.32) | 6.78 (1.32–34.73) | 0.022 |
| 18.5–24.9 | 109 | 51 | 6.95 (2.16–22.36) | 5.04 (1.26–20.10) | 0.022 |
| 25–29.9 | 14 | 17 | 2.68 (0.71–10.07) | 3.14 (0.63–15.77) | |
| 30–50 | 4 | 13 | 1 | 1 | |
| Proteinuria on urinalysis dipstick | |||||
| Absent | 42 | 29 | 1 | ||
| Present | 120 | 60 | 1.38 (0.78–2.43) | ||
| Degree of proteinuria | |||||
| None | 42 | 29 | 1 | 1 | |
| 1+ | 23 | 23 | 0.69 (0.33–1.46) | 0.58 (0.23–1.47) | |
| 2+ | 75 | 26 | 1.99 (1.04–3.82) | 1.82 (0.83–4.00) | |
| 3+ | 22 | 11 | 1.38 (0.58–3.28) | 1.66 (0.57–4.80) | |
| WBC count | Median=6 (IQR=5–8) | 1.09 (0.98–1.22) | 1.07 (0.94–1.21) | ||
| Neutrophil (%) | Median=70 (IQR=61–76) | 1.01 (0.99–1.03) | 1.02 (0.97–1.07) | ||
| Lymphocyte (%) | Median=23 (IQR=14.7–30) | 0.98 (0.96–0.99) | 0.99 (0.95–1.05) | ||
| Platelet count | Mean=247.91 (SD=102.7) | 0.99 (0.99–1.00) | |||
Abbreviations: COR, crude odds ratio; AOR, adjusted odds ratio.
Discussion
The overall prevalence of anemia among CKD stages 1–5 was 64.5%, with in the range in African studies report (51–87%).8,11–13 Recent NHANES report in the United States revealed the prevalence of anemia was twice (15.4%) in CKD patients compared to the nonCKD population (7.5%).2,3
The study demonstrated prevalence of anemia increased with stage of CKD. A similar finding was reported from NHANES survey in the United States with prevalence of anemia from 8.4% at stage 1 to 53.4% at stage 5. Comparable trend was also observed from recent Korean cohort with prevalence of 10% at stage 1 to 96.5% at stage 5.2,4
The main etiologies of CKD were hypertension, chronic glomerulonephritis and diabetes, congruent with studies from low and middle-income countries.5,7,9–14 Chronic glomerulonephritis remained an important cause of CKD, possibly related to high prevalence of viral, bacterial, protozoan and helminthic infections/infestations in sub-Saharan Africa.9–13
As in other developing countries, most patients (96%) in this study presented in advanced stage of CKD (stage 3–5), unlike the situation in developed countries. Presumed reasons for patients’ late presentation for renal medical care might be due to low detection and treatment rate of CKD risk factors such as hypertension and diabetes, lack of regular CKD screening program and insufficient follow-up care, and preference to use alternative treatment.4,6,7,10,12,13
This study demonstrated anemia was significantly prevalent in CKD of chronic glomerulonephritis compared to other underlying causes of CKD (P-value <0.001). Studies from the Western world documented diabetic nephropathy were strongly correlated with anemia of CKD among other underlying causes. Putative reasons for high prevalence of anemia in diabetic nephropathy might be hyperglycemia-induced renal interstitial fibrosis, decrease in hypoxia-inducible transcription factor, and increase in inflammatory cytokines.4,12,17
This study showed that anemia was significantly prevalent in rural residents. The odds of being anemic were threefold higher in rural residents (AOR=2.75, 95%CI: 1.34–5.65, P=0.006) as compared to urban dwellers. Rural resident patients might visit health facility late due to lack of access to or remote from health care service. Concomitant presence of nutritional deficiencies, gastrointestinal blood loss or helminthic infestations might contribute to anemia in rural residents.10,13
Patients with nonobese body habitus had five to sevenfold increased risk for anemia compared to patients with obese body habitus. A cohort study in Korea showed lower BMI was associated with elevated risk of anemia, while lower odds for anemia was reported for higher BMI. Malnutrition defined by protein-energy wasting is indicator of advanced renal disease.4
Patients with hemodialysis history had fourfold higher odds for anemia (AOR=3.59, 95%CI: 1.24–10.38, P=0.018) compared to patients without hemodialysis history. Various studies revealed hemodialysis requiring patients were those with advanced renal disease, in which presence and severity of anemia was prevalent. Intractable anemic state in later stages of CKD is mainly due to inflammation, protein-energy wasting and bone marrow fibrosis.9,11,12
There was a 63% less likely chance of developing anemia among patients with DBP (80–89 mmHg) (AOR=0.37, 95%CI: 0.16–0.92, P=0.025). Normal or controlled blood pressure in CKD patients might indicate early stage renal disease or attenuated renal disease progression respectively.21
Literatures revealed anemia of CKD caused maladaptive cardiovascular mechanisms leading to left ventricular hypertrophy, heart failure, and myocardial ischemia and infarction. Anemia with CKD was also associated with a substantial higher risk of stroke. As well, it increased risk of cardiovascular related morbidity and mortality.9,15,16,22–25
Suboptimal treatment of anemia with ESA and hematinics were noticed in this study, possibly explained by unavailability or high cost of drugs. Clinical studies have shown that correction of anemia using ESA and hematinics improved quality of life, physical activity and cardiac function, and might decrease kidney disease progression and morbidity.11–13,16,20,25
Limitation Of The Study
Data were cross-sectional, not longitudinal, preventing assessment of whether identified associated factors caused or resulted from CKD. Type and cause of anemia in CKD patients were not determined due to logistic reasons. Albuminuria was evaluated using a semiquantitative urine dipstick test due to unavailability of quantitative albuminuria test.
Conclusion
Hypertension (45%), chronic glomerulonephritis (24%) and diabetes (20%) were common causes of CKD. Anemia was prevalent across stages of chronic kidney disease, and worsened with progressive decline in kidney function. Rural residence, nonobese body habitus and having hemodialysis history were independently associated with anemia in CKD patients.
Recommendation
The authors recommend longitudinal studies to determine actual predictors of anemia in CKD patients. Periodic screening and intervention programs for anemia of CKD should be practiced to change the existing situation in the setting.
Acknowledgments
We are grateful to the College of Medicine and Health Sciences, University of Gondar for providing financial assistance for the study. We also acknowledge the study participants and their health personnel.
Funding Statement
Funding for research was obtained from College of Medicine and Health Sciences, University of Gondar.
Abbreviations
CKD, chronic kidney disease; CGN, chronic glomerulonephritis; AOR, adjusted odds ratio; COR, crude odds ratio; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; BMI, body mass index, KDIGO, kidney disease/improving global outcomes; CKD-EPI, chronic kidney disease epidemiology collaboration; ESA, erythropoiesis stimulating agents.
Ethics Approval And Consent To Participate
Ethical approval was obtained from College of Medicine and Health Sciences, University of Gondar. Informed consent was obtained from all study participants in written format.
Declaration Of Helsinki
The research was conducted in accordance with the “Declaration of Helsinki”.
Availability Of Data And Materials
All data generated and analyzed are included in this research article.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Hill NR, Stf J, Oke L, et al. Global prevalence of chronic kidney disease-A systematic review and meta-analysis. PLoS One. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McFarlane SI, Chen S-C, Whaley-Connell AT, et al. Prevalence and associations of anemia of CKD: Kidney Early Evaluation Program(KEEP)and National Health and Nutrition Examination Survey (NHANES)1999-2004. Am J Kidney Dis. 2008;51(4):S46–S55. doi: 10.1053/j.ajkd.2007.12.019 [DOI] [PubMed] [Google Scholar]
- 3.Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1):e84943. doi: 10.1371/journal.pone.0084943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryu SR, Park SK, Jung JY, et al. The prevalence and management of anemia in chronic kidney disease patients: result from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD). J Korean Med Sci. 2017;32(2):249–256. doi: 10.3346/jkms.2017.32.2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanifer JW, Muiru A, Jafat TH, Patel UD. Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant. 2016;31:868–874. doi: 10.1093/ndt/gfv466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaze AD, Ilori T, Jaar BG, Echouffo-Tcheugui JB. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 2018;19:125–136. doi: 10.1186/s12882-018-0930-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ElHafeez SA, Bolignano D, D’Arrigo G, Dounousi E, Tripepi G, Zocccali C. prevalence and burden of chronic kidney disease among the general population and high-risk groups in Africa: asystematic review. BMJ Open. 2018;8:e015069. doi: 10.1136/bmjopen-2016-015069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–1634. doi: 10.1681/ASN.2011111078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivera RF, Lullo LD, Pascalis AD, et al. Anemia in patients with chronic kidney disease: current screening and management approaches. Nephrol Renal Dis. 2016;1(1):1–9. [Google Scholar]
- 10.Stanifer JW, Jing B, Tolan S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e174–e181. doi: 10.1016/S2214-109X(14)70002-6 [DOI] [PubMed] [Google Scholar]
- 11.Maina CK, Karimi PN, Mariita K, Nyamu DG, Mugendi GA, Opanga SA. Correlates and management of anemia of chronic kidney disease in a Kenyan Tertiary hospita. East Afri Med J. 2016;93(10):489–499. [Google Scholar]
- 12.Amoako YA, Laryea DO, Beddu-Addo G, Andoh H, Awuku YA. Clinical and demographic characteristics of chronic kidney disease patients in a tertiary facility in Ghana. Pan Afric Med J. 2014;18:274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ijoma C, Ulasi I, Ijoma U, Ifebunandu N. High prevalence of anemia in predialysis patients in Egunu, Nigeria. Nephrol Rev. 2010;2:61–65. doi: 10.4081/nr.2010.e14 [DOI] [Google Scholar]
- 14.Fesiha T, Kassim M, Yemane T. Prevalence of chronic kidney disease and associated risk factors among diabetic patients in Southern Ethiopia. Am J Health Res. 2014;2(4):216–221. doi: 10.11648/j.ajhr.20140204.28 [DOI] [Google Scholar]
- 15.He J, Shlipak M, Anderson A, et al. Risk Factors for Heart Failure in Patients With Chronic Kidney Disease: the CRIC (Chronic Renal Insufficiency Cohort) Study. J Am Heart Assoc. 2017;6(5). doi: 10.1161/JAHA.116.005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt R, Dalton CL. Treating anemia of chronic kidney disease in the primary care settings: cardiovascular outcomes and management recommendations. Osteopath Med Prim Care. 2007;1(14):1–11. doi: 10.1186/1750-4732-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loutradis C, Skodra A, Georgianos P, et al. Diabetes mellitus increases the prevalence of anemia in patients with chronic kidney disease: A nested case-control study. World J Nephrol. 2016;5(4):358–366. doi: 10.5527/wjn.v5.i4.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wayne WD, Cross CL. Biostatistics: A Foundation for Analysis in Health Sciences. 10 ed. New Jersey: John Wiley and Sons Ltd; 2013. [Google Scholar]
- 19.World Health Organization: Nutritional Anemia. Report of a WHO Scientific Group. Vol. 41 Geneva, Switzerland: WHO; 1968:6–8. [Google Scholar]
- 20.Eknoyan G, Lameire N, Eckardt K, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):5–14. [DOI] [PubMed] [Google Scholar]
- 21.Wright JT Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and anti-hypertensive drug class on progression of hypertensive kidney disease: results from AASK trial. Jama. 2002;288(19):2421–2431. doi: 10.1001/jama.288.19.2421 [DOI] [PubMed] [Google Scholar]
- 22.Daniel Eriksson DG, Teitsson S, Jackson J, van Nooten. F. Cross-sectional survey in CKD patients across Europe describing the association between quality of life and anaemia. BMC Nephrol. 2016;17:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abramson JL, Jurkovitz CT, Vaccarino V, William S, Weintraub WM. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: theARIC study. Kidney Int. 2003;64:610–615. doi: 10.1046/j.1523-1755.2003.00109.x [DOI] [PubMed] [Google Scholar]
- 24.Del Fabbro P, Luthi J-C, Carrera E, Michel P, Burnier M, Burnand B. Anemia and chronic kidney disease are potential risk factors for mortality in stroke patients: a historic cohort study. BMC Nephrol. 2010;11(27):1–10. doi: 10.1186/1471-2369-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iimori S, Naito S, Noda Y, et al. Anaemia management and mortality risk in newly visiting patients with chronic kidney disease in Japan: the CKD-ROUTE study. Nephrology. 2015;20(9):601–608. doi: 10.1111/nep.12493 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed are included in this research article.