Abstract
Background
Despite substantial improvements in the success of treatments through assisted reproduction technologies (ART), live birth rates remain constantly low, and practitioners are seeking aetiologic treatments to improve the outcomes.
Local inflammatory response is believed to contribute to implantation failure, where prostaglandins may increase uterine contractions and decrease uterine receptivity, decreasing the possibility of an IVF cycle leading to successful embryo transfer. In this context, nonsteroidal anti‐inflammatory drugs (NSAIDs) have been employed to inhibit the negative prostaglandin effect. They are often offered in clinical practice to improve ART outcomes, but current robust evidence on their efficacy is lacking.
Objectives
To evaluate the effectiveness and safety of nonsteroidal anti‐inflammatory drugs as co‐treatments in infertile women undergoing assisted reproduction, in terms of improving live birth and miscarriage rates.
Search methods
We designed the search using standard Cochrane methods and performed it on databases from their inception to 20 February 2019.
We searched the Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials, CENTRAL via the Cochrane Central Register of Studies Online, MEDLINE, Embase, CINAHL, and the trial registers for ongoing and registered trials, grey literature and treatment guidelines. We handsearched reference lists of relevant systematic reviews and RCTs, and PubMed and Google for any recent trials. There were no restrictions by language or country of origin.
Selection criteria
All RCTs on the use of NSAIDs as co‐treatment during an ART cycle compared with no use or the use of placebo or any other similar drug, along with the comparison of any NSAID to another.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. Our primary outcomes were live birth/ongoing pregnancy and miscarriage. We performed statistical analysis using Review Manager 5. We assessed evidence quality using GRADE methods.
Main results
We found 11 RCTs (1884 women) suitable for inclusion in the review. Most studies were at unclear or high risk of bias. The main limitations in the overall quality of the evidence were high risk of bias, unexplained heterogeneity and serious imprecision and indirectness.
There were no data on our primary outcome — live birth per woman randomised — in any review comparisons.
NSAIDs vs. placebo/no treatment
We are uncertain of an effect on ongoing pregnancy when NSAIDs were compared to placebo/no treatment (risk ratio (RR) 1.06, 95% confidence interval (CI) 0.71 to 1.59; 4 studies, 1159 participants; I² = 53%; very low quality evidence). Results suggest that if the chance of ongoing pregnancy following placebo or no treatment is assumed to be 15%, the chance following the use of NSAIDs is estimated to be between 12% and 24%. Subgroup analysis according to the type of NSAID yielded similar results.
We are also uncertain of an effect on miscarriage rates when NSAIDs were compared to placebo/no treatment (RR 0.62, 95% CI 0.33 to 1.16; 4 studies, 525 participants; I² = 43%; very low quality evidence). Results suggest that if the chance of miscarriage following placebo or no treatment is assumed to be 21%, the chance following the use of NSAIDs is estimated to be between 7% and 27%. The results were similar when two studies were excluded due to high risk of bias.
Concerning the secondary outcomes, we are uncertain of an effect on clinical pregnancy rates (RR 1.23, 95% CI 1.00 to 1.52; 6 studies, 1570 participants; I² = 49%; low‐quality evidence); on ectopic pregnancy (RR 0.56, 95% CI 0.05 to 5.89; 1 study, 72 participants); on multiple pregnancy (RR 2.00, 95% CI 0.18 to 21.67; 1 study, 180 participants); and on side effects (RR 1.39, 95% CI 0.02 to 119.35; 3 studies, 418 participants; I² = 79%). The evidence suggests that if the chance of clinical pregnancy following placebo or no treatment is assumed to be 30%, the chance following the use of NSAIDs is estimated to be between 31% and 45%. If the chance of ectopic pregnancy following placebo or no treatment is assumed to be 5%, the chance following the use of NSAIDs is estimated to be between 0.3% and 31%. If the chance of multiple pregnancy following placebo or no treatment is assumed to be 1%, the chance following the use of NSAIDs is estimated to be between 0.2 % and 24%.
There were no cases of congenital anomalies during antenatal ultrasound screening of the women in one study.
NSAID vs. another NSAID
Only one study compared piroxicam with indomethacin: we are uncertain of an effect on ongoing pregnancy (RR 1.12, 95% CI 0.63 to 2.00; 1 study, 170 participants; very low quality evidence); and on miscarriage (RR 1.00, 95% CI 0.44 to 2.28; 1 study, 170 participants; very low quality evidence). The evidence suggests that if the chance of ongoing pregnancy following indomethacin is assumed to be 20%, the chance following the use of piroxicam is estimated to be between 13% and 40%; while for miscarriage, the evidence suggests that if the chance following indomethacin is assumed to be 12%, the chance following the use of piroxicam is estimated to be between 5% and 27%.
Similar results were reported for clinical pregnancy (RR 1.07, 95% CI 0.71 to 1.63; 1 study, 170 participants; very low quality evidence).
There were no data for the other outcomes specified in this review.
NSAID vs. aspirin
No study reported this comparison.
Authors' conclusions
Currently we are uncertain of an effect of the routine use of NSAIDs as co‐treatments in infertile women undergoing assisted reproduction in order to improve ongoing pregnancy and miscarriage rates. This is based on available data from RCTs, where very low quality evidence showed that there is no single outcome measure demonstrating a benefit with their use. Further large, well‐designed randomised placebo‐controlled trials reporting on live births are required to clarify the exact role of NSAIDs.
Plain language summary
Nonsteroidal anti‐inflammatory drugs for assisted reproductive technology
Review question Researchers reviewed the evidence about the effect of nonsteroidal anti‐inflammatory drugs (NSAIDs) as co‐treatments when applied in infertile women undergoing assisted reproduction.
Background Assisted reproductive technologies (ART) include techniques used for treating subfertility, and in vitro fertilization (IVF) is the most common. Despite both clinical and laboratory efforts and improvements in the success of these treatments, pregnancy rates remain low. Local inflammatory response is believed to cause implantation difficulties for the embryo, through the action of prostaglandins. These substances mainly cause a differentiated local inflammatory response and uterine contractions during embryo transfer, inhibiting the embryo from implanting successfully. For this reason, clinicians usually use anti‐prostaglandin agents to block this action: NSAIDs are such agents. In clinical practice, they are often offered to improve ART outcomes, but evidence is based on various types of studies. Thus because there is lack of clear evidence, their efficacy and safety still remain controversial. In this Cochrane Review we have summarised the available evidence on the use of NSAIDs in infertile women undergoing IVF, in an attempt to identify gaps and limitations in our current understanding.
Study characteristics We performed a comprehensive literature search of the standard medical databases (from database inception to February 2019) in consultation with the Cochrane Gynaecology and Fertility Group Information Specialist, for all randomised controlled trials (studies in which participants are assigned to a treatment group using a random method) investigating the efficiency of NSAIDs compared to the use of placebo or no treatment or compared to each other in infertile women undergoing IVF. We searched for and included studies irrespective of language and country of origin. Two review authors independently selected and evaluated studies, extracted data, and attempted to contact the authors of studies for which data were missing. We found 11 studies (2384 women); data were not available in one study, so we analysed data on 1884 patients; two studies were published as abstracts in international conference reports; and we found one ongoing trial that met our inclusion requirements.
Key results We are uncertain of an effect on ongoing pregnancy and miscarriage when NSAIDs were compared to placebo/no treatment. Results suggest that if the chance of ongoing pregnancy and miscarriage following placebo or no treatment is assumed to be 15% and 21%, respectively, the chance following the use of NSAIDs is estimated to be between 12% and 24%, and 7% and 27%, respectively. Only one study compared piroxicam with indomethacin: we are uncertain of an effect on ongoing pregnancy and on miscarriage. The evidence suggests that if the chance of ongoing pregnancy following indomethacin is assumed to be 20%, the chance following the use of piroxicam is estimated to be between 13% and 40%; while for miscarriage, the evidence suggests that if the chance following indomethacin is assumed to be 12%, the chance following the use of piroxicam is estimated to be between 5% and 27%.
Concerning the secondary outcomes, we are equally uncertain of any effect.
Currently we are uncertain of an effect of the routine use of NSAIDs as co‐treatments in infertile women undergoing assisted reproduction in order to improve pregnancy rates. This is based on available data from randomised controlled trials, where no single outcome reported in the studies demonstrated a benefit with their use.
Quality of the evidence The quality of the evidence was very low for all outcomes. This is because of several limitations including poorly reported study methods, imprecision, small study numbers and low numbers of events reported.
Summary of findings
Background
Description of the condition
An IVF cycle involves ovulation stimulation, oocyte retrieval, fertilisation and embryo transfer (Speroff 2005). With the exception of fertilisation, each of these steps may lead to pain, with a localized inflammatory response and, sometimes, uterine contractions. These factors, including those at the time of embryo transfer, may contribute to implantation failure (Al‐Ghamdi 2008; Fanchin 1998; Speroff 2005). The anti‐prostaglandin effects of nonsteroidal anti‐inflammatory drugs (NSAIDs) are commonly used to reduce or eliminate both the inflammatory response and the uterine/myometrial contractility (Hawkey 2003).
Despite substantial improvements in the success of these treatments since the inception of IVF, pregnancy rates remained unchanged during the last decade, reaching 30% to 45% for cases undergoing intracytoplasmic sperm injection (ICSI) (Kuczynski 2001; Motteram 2015). Practitioners are constantly seeking adjunct treatments to improve the outcomes, either in the form of medical (Akhtar 2013; Siristatidis 2016) or non‐medical (Cheong 2013) co‐therapies. This Cochrane Review focuses on the adjunct use of NSAIDs in terms of safety and efficacy in assisted reproduction. Studies involving the use of aspirin in ART have not been considered, since a previous review showed clear evidence of no effect of aspirin for this purpose (Siristatidis 2016).
Description of the intervention
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are sometimes referred to as nonsteroidal anti‐inflammatory agents/analgesics (NSAIAs) or nonsteroidal anti‐inflammatory medicines (NSAIMs) (Brown 2017). They have analgesic, antipyretic and, in higher doses, anti‐inflammatory effects. The term 'nonsteroidal' is used to distinguish these drugs from steroids which (among a broad range of other effects) have a similar eicosanoids‐depressing, anti‐inflammatory action. NSAIDs are non‐narcotic.
Their action lies in their inhibitory effect on the cyclooxygenase enzymes (COX‐1 or COX‐2, or both). In cells, these enzymes are involved in the synthesis of prostaglandins. Prostaglandins are a group of chemicals produced by various cells in the body. They promote inflammation, pain and fever.
The widespread use of NSAIDs has led to their increasingly prevalent adverse effects. The two main adverse drug reactions linked with NSAIDs are gastrointestinal and renal effects, and are associated with the different roles and tissue localisations of each COX isoenzyme (Baigent 2003; Green 2001; Hawkey 2003).
How the intervention might work
Each step of an ART cycle, with the exception of fertilisation, may lead to pain due to the inflammatory reaction. This reaction leads to the production of inflammatory cytokines, which in excess may be detrimental (Chaouat 2002). Similarly, it is known that an inflammatory response produces prostaglandins locally, which may increase uterine contractions and also decrease uterine receptivity. It has been reported that uterine/myometrial contractility has to be considered as an important factor in determining endometrial receptivity (Fanchin 2001; Moon 2004). In addition to any exogenous manipulation, introducing extrauterine particles (cervical mucus, bacteria, and detritus) could trigger a ‘pro‐inflammatory status’. These factors could potentially contribute to implantation failure (Al‐Ghamdi 2008; Speroff 2005).
The anti‐prostaglandin effects of NSAIDs act by reducing or eliminating the local inflammatory response and uterine contractility by inhibiting prostaglandin release through inhibition of cyclo‐oxygenase activity (Bernabeu 2006; Hawkey 2003; Speroff 2005). Piroxicam and indomethacin — two drugs that are widely used in clinical practice and have well‐known anti‐prostaglandin effects that reduce uterine/myometrial contractility — have been evaluated by Moon 2004 and Dal Prato 2009 (piroxicam), and Bernabeu 2006 (indomethacin), looking for a possible improvement in pregnancy outcome after IVF–ET.
Why it is important to do this review
The use of NSAIDs in ART is increasing, as they may improve the chances of conception in subfertile women. These products are inexpensive and simple to use, yet remain controversial because of a lack of robust evidence for their efficacy and safety. In this Cochrane Review we have summarised the available evidence on the use of NSAIDs in subfertile women who are undergoing ART and tried to identify any gaps or limitations in our current understanding. Our aim is to provide a clear view on the effectiveness of this pharmacological intervention in order to encourage or disprove its clinical application. Moreover, this meta‐analysis can be used to identify gaps in research that can be used in designing future trials.
Objectives
To evaluate the effectiveness and safety of nonsteroidal anti‐inflammatory drugs as co‐treatments in infertile women undergoing assisted reproduction, in terms of improving live birth and miscarriage rates.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials, published or unpublished, comparing the relative effectiveness or safety of one of the interventions compared to the other treatment. We did not include quasi‐randomised trials. Cross‐over trials were not eligible for inclusion unless pre‐cross‐over data were available.
Types of participants
Participants were women, subfertile for any cause, who were undergoing IVF/ICSI.
We excluded trials of ovum recipients, peri‐ or post‐menopausal women, and others in which assisted hatching was used, as an issue of embryo quality impairment might be introduced.
Types of interventions
We considered all trials where one of these interventions was compared.
NSAIDs versus placebo/no treatment
One NSAID versus another NSAID
We did not include the comparison of aspirin versus placebo, as this was the topic of another Cochrane Review (Siristatidis 2016).
Types of outcome measures
Primary outcomes
Live birth or ongoing pregnancy rate per woman randomised: we defined live birth as delivery of a live fetus after 20 completed weeks of gestation; we defined ongoing pregnancy as a pregnancy beyond 12 weeks of gestation.
Miscarriage rate per woman randomised, defined as the number of pregnancies lost before 20 weeks of gestation.
Secondary outcomes
Clinical pregnancy rate per woman randomised, defined as evidence of a gestational sac on ultrasound.
Adverse effects to the woman (per woman randomised: ectopic pregnancy, multiple birth, antenatal and perinatal complications).
Adverse fetal effects including fetal anomalies (chromosomal, congenital and anatomical abnormalities, preterm labour, growth restriction).
Quality of life (considering both mother and newborn).
Relief of pain, using validated pain scale (VPS) or subjective report.
Search methods for identification of studies
We developed a comprehensive literature search strategy in consultation with the Information Specialist of the Cochrane Gynaecology and Fertility Group (CGF).
Two review authors (DV and IM) independently conducted a systematic search of the published and unpublished literature. There were no restrictions on language or publication status.
We developed the key search terms in accordance with the structured question. We identified synonyms and related terms for each of the PICO elements and added them to the strategy. We identified the relevant subject indexing terms used within individual databases and added them to the strategy as appropriate. We used database facilities, such as truncation, explosion and proximity searching, when they were available. We selected search filters from the ISSG search filter web site, for other databases and to identify systematic reviews (www.york.ac.uk/inst/crd/intertasc). We sought relevant publications in all languages (including full papers and abstracts).
Electronic searches
We searched the following electronic databases, trial registers and websites from inception to February 2019: the CGF Specialised Register of Controlled Trials, ProCite platform, searched 02 February 2019 (Appendix 1); CENTRAL via the Cochrane Register of Studies Online (CRSO); web platform, searched 02 February 2019 (Appendix 2); MEDLINE; OVID platform, searched from 1946 to 02 February 2019 (Appendix 3); Embase; OVID platform, searched from 1980 to 02 February 2019 (Appendix 4); PsycINFO; OVID platform, searched from 1806 to 02 February 2019 (Appendix 5); and CINAHL; EBSCO platform, searched from 1961 to 02 February 2019 (Appendix 6).
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials, which appears in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The Embase search was combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (https://www.sign.ac.uk/search‐filters.html).
We also searched the following electronic sources for trials.
Trial registers for ongoing and registered trials: Current Controlled Trials (www.controlled‐trials.com); ClinicalTrials.gov, a service of the US National Institutes of Health (ClinicalTrials.gov/ct2/home); and the World Health Organization International Clinical Trials Registry Platform search portal (WHO ICTRP) (www.who.int/trialsearch/Default.aspx) (Appendix 7).
Citation indexes (scientific.thomson.com/products/sci).
Conference abstracts in the Web of Science (wokinfo.com).
LILACS database (a source of Portuguese‐ and Spanish language trials). (regional.bvsalud.org/php/index.php?lang=en).
PubMed (www.ncbi.nlm.nih.gov/pubmed), using the random control filter for PubMed from the searching chapter of the Cochrane Handbook for Systematic Reviews of Interventions.
OpenSIGLE database (opensigle.inist.fr) and Google Scholar for grey literature (Appendix 8).
Professional societies, organisations and individuals (e.g. RCOG, ACOG, BFS, ESHRE, ASRM), guidelines (NICE, the (US) National Guidelines Clearinghouse and other collections) were contacted.
We asked external referees who are experts in this field to check the completeness of the search strategy, and to identify any additional, ongoing and planned trials.
Searching other resources
We handsearched the reference lists from all searched published articles for additional studies. We also contacted experts in the subject area for further references. Similarly, we handsearched the conference proceedings and abstracts not covered in the CGF Specialized Register of Controlled Trials for relevant unpublished reports, theses and any other sources of potentially relevant references or studies, in liaison with the CGF Trials Search Coordinator.
Data collection and analysis
Selection of studies
We developed a data screening, assessment and standardized extraction form for this Cochrane Review, and pilot‐tested the form on a randomly selected sample of apparently applicable studies. This form included details and criteria of all relevant trial characteristics (Appendix 9, Appendix 10).
Two review authors (AN and DV) reviewed titles and abstracts of references for compliance with the inclusion criteria for the review and excluded those considered irrelevant at this screening stage. The same review authors assessed full‐text articles for eligibility and resolved disagreements by discussion with the third review author (CS). We sought further information from the study authors when papers contained insufficient information to make a decision about eligibility.
We provide a PRISMA flow diagram to show the results of the search and the number of included and excluded trials (Figure 1). We documented the reasons for excluding any studies identified by the search in the 'Characteristics of excluded studies' table.
1.

Study flow diagram.
Data extraction and management
Two review authors (AN and DV) independently extracted data from the included studies using the standardized data extraction form (Appendix 10). We extracted data on trial characteristics including patient characteristics (mean and SD for age, median duration of subfertility), study settings, methods, the types of interventions (used dose, type of preparation, regimens, co‐interventions), and the outcomes. We extracted the number randomised and the number analysed in each treatment group for each outcome and reported the loss to follow‐up in each group. Where there were insufficient data to enable us to make a decision on inclusion or exclusion, we included the trial provisionally and contacted the authors of the trial report for further data on methods or results, or both.
Assessment of risk of bias in included studies
AN and CS independently assessed the risk of bias for each trial using Cochrane's 'Risk of bias' assessment tool (Higgins 2011). We resolved differences of opinion through discussion. We assessed whether adequate steps were taken to reduce the risk of bias across six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other sources of bias.
For sequence generation and allocation concealment, we reported the methods used. For blinding, we described who was blinded and the blinding method. For incomplete outcome data, we reported the percentage and proportion lost to follow‐up. For selective outcome reporting, we stated any discrepancies between the methods used and the results, in terms of the outcomes measured or the outcomes reported. For other biases, we described any other trial features that we thought could affect the trial result (e.g. funding).
We categorized our judgements as 'low risk of bias', 'high risk of bias', or 'unclear risk of bias', and this information was used to guide our interpretation of the presented data table, which was incorporated into the interpretations of review findings by means of sensitivity analyses. Where our judgement was unclear, we contacted the trial authors for clarification and resolved any differences of opinion through discussion.
The 'Risk of bias' tables describe all judgements and present our conclusions; for summaries see Figure 2 and Figure 3
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
For dichotomous data we used the number of events in the control and intervention groups of each study to calculate the risk ratio (RR). We have provided 95% confidence intervals (CIs) for all outcomes. There were no continuous data.
Unit of analysis issues
All analyses were per woman randomised. We planned to summarise in a separate table those data that did not allow valid analysis (such as data reported 'per cycle' and not 'per woman', where results might include the same woman at more than one time point or cycle) and exclude it from meta‐analyses. We would only have included first‐phase data from cross‐over trials.
As we were considering pain as an outcome we anticipated that we would have to convert different pain scales; this, however, was not the case. If outcomes were reported per cycle, we contacted authors of the trials to get data per woman/couple randomised.
Dealing with missing data
As far as possible, we analysed data on an intention‐to‐treat (ITT) analysis. We asked trial authors via e‐mail to provide further details where reported data were insufficient or missing. If we had seen dropouts and if follow‐up had not been complete then we would have assumed that no pregnancy had occurred.
Assessment of heterogeneity
We assessed the characteristics of the included studies to decide whether there were sufficient similarities — in study populations, methodologies used, comparisons applied (vs. placebo or no treatment or another NSAID), length of treatments and outcomes — for meta‐analysis to be appropriate.
We examined heterogeneity between results of different studies by visually inspecting the overlap of the CIs of the forest plots, considering P values, Chi² statistics relative to the degree of freedom and interpreting the I² statistic. We took an I² statistic value greater than 50% to indicate substantial heterogeneity (Higgins 2011). If this had been found, we had planned to explore this by means of sensitivity analysis, as described below.
Assessment of reporting biases
We aimed to minimise publication and other biases and their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. Our review of each trial report included an evaluation of unreported or insufficiently reported outcomes; where we suspected this within‐study reporting bias, we obtained the protocols where possible and compared the prespecified outcomes with those reported in the published study results. Our intention was to use a comparison‐adjusted funnel plot to explore the possibility of small‐study effects if there were 10 or more studies in an analysis (Chaimani 2013).
Data synthesis
We gave included trials an identity code, comprising the first author and the year published, and listed them in chronological order in forest plots.
We planned to combine results from primary studies using meta‐analysis with Review Manager 5 (Review Manager 2014), using fixed‐effect models in comparisons as follows.
All NSAIDs versus placebo or no treatment.
One NSAID versus another active intervention (NSAID or not).
In the meta‐analyses we have graphically displayed an increase in the odds of a particular outcome (whether beneficial (e.g. live birth) or detrimental (e.g. miscarriage)) to the right of the centre line and a decrease in the odds of an outcome to the left of the centre line. We pooled dichotomous data to calculate pooled RRs with 95% CIs.
Subgroup analysis and investigation of heterogeneity
The review authors planned the following steps if, after confirming data, they detected substantial heterogeneity.
Perform a random‐effects meta‐analysis
Consider if a meta‐analysis was warranted
Consider completing a meta‐regression analysis
Consider completing a subgroup analysis, according to meaningful factors, such as number of embryos transferred, previous failed cycles, maternal age, duration of treatment; and type of COX inhibitors.
Consider ignoring the heterogeneity
If we detected substantial heterogeneity, we explored possible explanations in subgroup analyses (e.g. differing populations) and/or sensitivity analyses (e.g. differing risk of bias). We took any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect.
Sensitivity analysis
We planned sensitivity analyses for the primary outcomes and clinical pregnancy rates to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis. These analyses would have included consideration of whether the review conclusions would have differed if:
eligibility had been restricted to studies judged to have low risk of bias (concerning all six domains);
the type of publication had been different (full text or abstract);
alternative imputation strategies had been implemented;
the summary effect measure was odds ratio rather than risk ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using the browser‐based version of GRADEpro (GRADEpro GDT) and Cochrane methods. This table presents the overall quality of the body of evidence for the main review outcomes (live birth, ongoing pregnancy, miscarriage, clinical pregnancy, multiple pregnancy, adverse effects) for each comparison. We assessed the quality of the studies using five GRADE criteria: study limitations, consistency of effect, imprecision, indirectness, and publication bias (Higgins 2011). Two authors independently assessed the quality of the evidence for each outcome. We justified, documented, and incorporated judgements about the quality of the evidence (high, moderate, low or very low) when reporting the results for each outcome.
Results
Description of studies
Results of the search
From the database search we located a total of 275 titles and abstracts that we thought might provide data addressing our question of interest. Eleven proved to be duplicates and 235 were not relevant: we excluded them based on the abstract. Of the remaining titles, we assessed 29 full‐text papers for eligibility: we excluded a total of 17, with reasons (Characteristics of excluded studies); and we deemed 11 eligible for inclusion (Characteristics of included studies; Figure 1). There was one ongoing study (NCT02642601).
Eleven RCTs met the inclusion criteria for this review (Asgharnia 2007; Dal Prato 2009; Fekih 2013; Firouzabadi 2007; Kailasam 2008; Kumbasar 2017; Moon 2004; Rijken‐Zijlstra 2013; Sohrabvand 2009; Sohrabvand 2014; Zhao 2017). Asgharnia 2007 was published as an abstract at a conference; data were not available, so we did not include it in the final meta‐analysis.
We sent emails to authors of all included and ongoing studies (and the appropriate reminders).
Included studies
Study design and setting
Of the 11 included studies, two were published as abstracts at conferences (Asgharnia 2007; Fekih 2013) (see Characteristics of included studies).
A published study by Rijken‐Zijlstra 2013 included the data presented as an abstract during ESHRE conference by Hammer and colleagues in 2008. Ten were parallel‐design trials and one was a three‐arm study with two intervention groups (Kumbasar 2017). They were conducted in IVF units in Iran (four trials), Italy, Korea, the Netherlands, Tunisia, UK, Turkey and China.
Participants
The included studies involved 2384 subfertile women undergoing ART; after subtracting the 500 participants studied in Asgharnia 2007, whose data were not available, we analysed data on 1884 women. All couples had similar baseline characteristics: the aetiology of infertility was male factor, tubal factor, ovulatory factor and unexplained; the mean age of female partners was around 35 years; exclusion criteria involved endometrial pathology (space‐occupying lesions, submucous myomas or polyps), congenital or acquired cavity disorders, contraindications for the use of NSAIDs (e.g. asthma or hypersensitivity), systemic diseases and endometriosis.
Interventions
All IVF cycles were fresh, except from one study, which reported on fresh and frozen embryos (Moon 2004). Most ovarian stimulation protocols were long GnRH agonist protocols: in one study, modified natural‐cycle IVF cycles were analysed (Rijken‐Zijlstra 2013); in two studies, further protocols were implemented as well (e.g. GnRH antagonists) (Zhao 2017; Kumbasar 2017); in one study additional co‐interventions included the use of dexamethasone and norethisterone (Kailasam 2008); and in one study, the IVF protocol was not stated (Fekih 2013).
NSAID vs. placebo/no treatment
Six studies were performed on piroxicam administered at a dosage of 10 mg once at 1 to 2 hours before embryo transfer (Asgharnia 2007; Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017; Moon 2004; Sohrabvand 2014).
Three studies were performed on indomethacin (Kumbasar 2017; Rijken‐Zijlstra 2013; Sohrabvand 2009), whereas in one study indomethacin was administered at a dosage of 50 mg capsules (three times a day from the day of hCG trigger to the morning of oocyte retrieval) (Rijken‐Zijlstra 2013), and in the other studies indomethacin was administered at a dosage of 10 mg as rectal suppositories before embryo transfer (Kumbasar 2017; Sohrabvand 2009).
In one study patients received an oral dose of 200 mg of ibuprofen 90 minutes before embryo transfer (Fekih 2013); in a second, flurbiprofen axetil as a single dose 30 minutes before oocyte retrieval (Zhao 2017); and in a third, sodium dilclofenac suppository was administered at a dosage of 100 mg at the end of oocyte retrieval (Kailasam 2008).
NSAID vs. another NSAID
Piroxicam was compared with indomethacin, and the exact interventions have been described above (Kumbasar 2017).
Outcomes
None of the studies reported on live birth per woman randomised; four studies reported on ongoing pregnancy (Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017; Rijken‐Zijlstra 2013); and four on miscarriage rates (Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017; Fekih 2013).
Of the secondary outcomes, clinical pregnancy rates were reported by seven studies (Kailasam 2008; Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017; Moon 2004; Fekih 2013; Zhao 2017); and adverse effects by five studies (Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017; Rijken‐Zijlstra 2013; Sohrabvand 2009).
In the comparison between piroxicam versus indomethacin, Kumbasar 2017 reported ongoing pregnancy, miscarriage and clinical pregnancy.
Excluded studies
Of the 264 records identified after removal of duplicates, we excluded 236 studies initially on the basis of the abstract (Figure 1).
Of the remaining 28 papers, we retrieved 17 but subsequently excluded them on various grounds: as non‐RCTs; as a trial on oocyte recipients; or as not answering the precise review question or not addressing the outcomes of interest (see Characteristics of excluded studies).
Risk of bias in included studies
We describe the 'Risk of bias' assessments in detail in the 'Risk of bias' tables in Characteristics of included studies, and in Figure 2 and Figure 3. We made the decisions after sending emails to the study authors in an attempt to retrieve further data.
Allocation
Random sequence generation
Random sequence generation was at unclear risk of bias in three studies (Fekih 2013; Kumbasar 2017; Sohrabvand 2009); at high risk of bias in two (Asgharnia 2007; Sohrabvand 2014); and at low risk in the rest of the included studies.
Allocation concealment
Allocation concealment was at high risk of bias in five studies (Asgharnia 2007; Fekih 2013; Firouzabadi 2007; Kumbasar 2017; Moon 2004); and unclear in two (Rijken‐Zijlstra 2013; Sohrabvand 2014).
Blinding
Performance bias
Blinding of participants was at high risk of bias as it was not performed in one study (Dal Prato 2009), where authors stated that it was not possible to provide a placebo, and not described in an abstract (Asgharnia 2007), and two full text articles (Kumbasar 2017; Sohrabvand 2014).
Detection bias
Blinding of outcome assessment was at low risk only in one study (Zhao 2017), while most of the studies were at high risk.
Incomplete outcome data
None of the studies reported loss to follow‐up. Four of them addressed the primary outcomes of this review (Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017; Rijken‐Zijlstra 2013), although none reported on live birth.
Selective reporting
Reporting bias was low in four studies, and high in the two which were published as abstracts (Asgharnia 2007; Fekih 2013).
Other potential sources of bias
No other sources of bias were identified in four studies (Dal Prato 2009; Firouzabadi 2007; Kailasam 2008; Zhao 2017). In one study, funding was provided by a pharmaceutical company which also provided the drug, where the role of the company was not adequately described (Rijken‐Zijlstra 2013).
Effects of interventions
Summary of findings for the main comparison. Nonsteroidal anti‐inflammatory drugs compared to placebo/ no treatment for assisted reproductive technology.
| Nonsteroidal anti‐inflammatory drugs compared to placebo/ no treatment for assisted reproductive technology | ||||||
| Patient or population: assisted reproductive technology Setting: Intervention: nonsteroidal anti‐inflammatory drugs Comparison: placebo/ no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo/ no treatment | Risk with nonsteroidal anti‐inflammatory drugs | |||||
| Ongoing pregnancy | 151 per 1000 | 167 per 1000 (116 to 244) | RR 1.06 (0.71 to 1.59) | 1159 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | We are uncertain about the effect of nonsteroidal anti‐inflammatory drugs on ongoing pregnancy |
| Miscarriage | 205 per 1000 | 127 per 1000 (68 to 273) | RR 0.62 (0.33 to 1.16) | 525 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 5 | |
| Clinical pregnancy | 300 per 1000 | 372 per 1000 (305 to 454) | RR 1.23 (1.00 to 1.52) | 1570 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | |
| Ectopic Pregnancy | 53 per 1000 | 29 per 1000 (3 to 310) | RR 0.56 (0.05 to 5.89) | 72 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 6 7 | |
| Multiple pregnancy | 11 per 1000 | 22 per 1000 (2 to 241) | RR 2.00 (0.18 to 21.67) | 180 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 6 7 | |
| Side effects | 18 per 1000 | 25 per 1000 (0 to 1000) | RR 1.39 (0.02 to 119.35) | 418 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 4 6 7 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 1 level for serious risk of bias including poor reporting of methods, attrition bias, selective reporting, and other biases.
2 Downgraded 1 level for serious imprecision: wide confidence interval was compatible with benefit in either arm, or no difference between the groups
3 Downgraded 1 level for serious inconsistency / moderate heterogeneity across trials
4 Downgraded 2 levels for very serious indirectness
5 Downgraded 1 level for serious indirectness
6 Downgraded 2 levels for very serious imprecision
7 Downgraded 2 levels for very large effect
Summary of findings 2. Piroxicam compared to indomethacin for assisted reproductive technology.
| Piroxicam compared to Indomethacin for assisted reproductive technology | ||||||
| Patient or population: assisted reproductive technology Setting: Clinic Intervention: Piroxicam Comparison: Indomethacin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Indomethacin | Risk with Piroxicam | |||||
| Live birth | Not reported in any study | |||||
| Ongoing pregnancy | 200 per 1000 | 224 per 1000 (126 to 400) | RR 1.12 (0.63 to 2.00) | 170 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | |
| Miscarriage | 118 per 1000 | 118 per 1000 (52 to 268) | RR 1.00 (0.44 to 2.28) | 170 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | |
| Clinical pregnancy | 329 per 1000 | 352 per 1000 (234 to 537) | RR 1.07 (0.71 to 1.63) | 170 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| Ectopic pregnancy | Not reported in any study | |||||
| Multiple pregnancy | Not reported in any study | |||||
| Side effects | Not reported in any study | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 2 levels for very serious risk of bias including poor reporting of methods, attrition bias, selective reporting, and other biases.
2 Downgraded 2 levels for very serious indirectness
3 Downgraded 2 levels for very serious imprecision
4 Downgraded 1 level for large effect
1 NSAIDs vs placebo/no treatment
Primary outcomes
1.1 Live birth or ongoing pregnancy
None of the included studies reported live birth.
There were four studies reporting ongoing pregnancy. Three studies involved the use of piroxicam (Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017); and two used indomethacin (Kumbasar 2017; Rijken‐Zijlstra 2013).
Owing to heterogeneity we conducted all analyses in this comparison using a random‐effects model.
Overall results indicated that we are uncertain of an effect on ongoing pregnancy when NSAIDs were compared to placebo/no treatment (RR 1.06, 95% CI 0.71 to 1.59; 4 studies, 1159 participants; I² = 53%; very low quality evidence). The evidence suggests that if the chance of ongoing pregnancy following placebo or no treatment is assumed to be 15%, the chance following the use of NSAIDs is estimated to be between 12% and 24%.
Similarly, none of the subgroups analysed according to the type of NSAID showed certainty of an effect of NSAIDs versus placebo/no treatment in ongoing pregnancy — piroxicam: (RR 1.30, 95% CI 0.70 to 2.39; 3 studies, 508 participants; I² = 68%); indomethacin: (RR 0.77, 95% CI 0.48 to 1.25; participants = 651; studies = 2; I2 = 0%) (Analysis 1.1; Figure 4).
1.1. Analysis.

Comparison 1 NSAID versus placebo/no treatment, Outcome 1 Ongoing pregnancy.
4.

Forest plot of comparison: 1 NSAID versus Placebo/No treatment, outcome: 1.1 Ongoing pregnancy.
The comparators were no treatment in Dal Prato 2009, Kumbasar 2017 and Placebo in Firouzabadi 2007, Rijken‐Zijlstra 2013.
There were too few studies to conduct other planned subgroup or sensitivity analyses.
1.2 Miscarriage
Four studies reported this outcome: three of them involved the use of piroxicam (Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017); one involved the use of indomethacin (Kumbasar 2017); and one involved the use of ibuprofen (Fekih 2013).
Overall results indicated that we are uncertain of an effect on miscarriage rates when NSAIDs were compared to placebo/no treatment (RR 0.62, 95% CI 0.33 to 1.16; 4 studies, 525 participants; I² = 43%, very low quality evidence) (Analysis 1.2, Figure 5). The evidence suggests that if the chance of miscarriage following placebo or no treatment is assumed to be 21%, the chance following the use of NSAIDs is estimated to be between 7% and 27%.
1.2. Analysis.

Comparison 1 NSAID versus placebo/no treatment, Outcome 2 Miscarriage.
5.
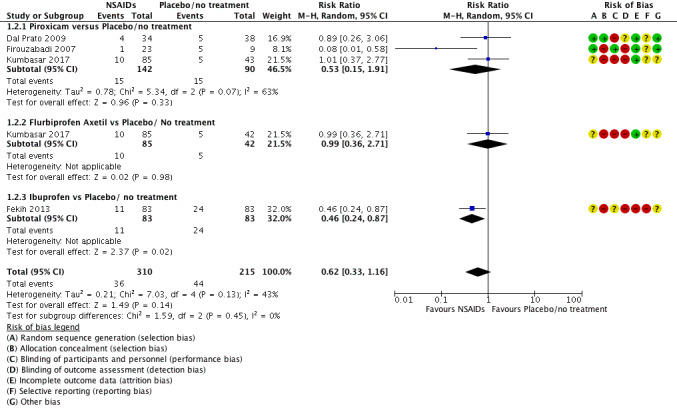
Forest plot of comparison: 1 NSAID versus Placebo/No treatment, outcome: 1.2 Miscarriage.
The comparators were no treatment in Dal Prato 2009, Kumbasar 2017 and Placebo in Fekih 2013, Firouzabadi 2007
Concerning the use of piroxicam, there was no certainty of an effect compared to placebo/no treatment on miscarriage (RR 0.53, 95% CI 0.15 to 1.91; 3 studies, 232 participants; I² = 63%) (Analysis 1.2, Figure 5).
There were too few studies to conduct a subgroup analysis in the other predefined parameters.
Overall results of sensitivity analysis — excluding the study of Fekih 2013 (conference abstract only) — indicated that we are uncertain of an effect on miscarriage rates when NSAIDs were compared to placebo/no treatment (RR 0.69, 95% CI 0.30 to 1.61; 3 RCTs, 359 participants; I² = 47%).
When two studies were excluded due to high risk of bias (Fekih 2013; Kumbasar 2017), overall results indicated that there was no certainty of an effect in miscarriage rates when NSAIDs were compared to placebo/no treatment (RR 0.30, 95% CI 0.03 to 3.30; 2 RCTs, 104 participants; I² = 76%).
There were no studies to conduct a sensitivity analysis in the other predefined parameters.
Secondary outcomes
1.3 Clinical pregnancy
A total of seven studies reported on clinical pregnancy. One study used diclofenac (Kailasam 2008); four used piroxicam (Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017; Moon 2004); one study used indomethacin (Kumbasar 2017); one study used flurbiprofen axetil (Zhao 2017); and one used ibuprofen (Fekih 2013).
There was no certainty of an effect in clinical pregnancy rates when NSAIDs were compared to placebo/no treatment (RR 1.23, 95% CI 1.00 to 1.52; 7 studies, 1570 participants; I² = 49%, low quality of evidence) (Analysis 1.3, Figure 6). The evidence suggests that if the chance of clinical pregnancy following placebo or no treatment is assumed to be 30%, the chance following the use of NSAIDs is estimated to be between 1% and 45%.
1.3. Analysis.

Comparison 1 NSAID versus placebo/no treatment, Outcome 3 Clinical pregnancy.
6.

Forest plot of comparison: 1 NSAID versus Placebo/No treatment, outcome: 1.3 Clinical pregnancy.
The comparators were no treatment in Dal Prato 2009, Kailasam 2008, Kumbasar 2017, and Placebo in Firouzabadi 2007, Moon 2004, Zhao 2017
Similarly, none of the individual subgroups analysed showed certainty of an effect of NSAID versus placebo/no treatment on clinical pregnancy (diclofenac ‒ RR 1.20, 95% CI 0.92 to 1.58; 1 study, 381 participants; piroxicam ‒ RR 1.36, 95% CI 0.89 to 2.08; 4 studies, 696 participants; I² = 69%; indomethacin ‒ RR 0.95, 95% CI 0.56 to 1.62; 1 study, 127 participants; flurbiprofen axetil ‒ RR 1.00, 95% Cl 0.73 to 1.37; 1 study, 200 participants; ibuprofen ‒ RR 1.71, 95% Cl 1.02 to 2.86; 1 study, 166 participants).
There were too few studies to conduct a subgroup analysis in the other predefined parameters.
Overall results of sensitivity analysis (excluding the study of Fekih 2013 (abstract only form)), indicated that there was no certainty of an effect in clinical pregnancy rates when NSAIDs were compared to placebo/no treatment (RR 1.19, 95% CI 0.96 to 1.47; 6 studies, 1404 participants; I² = 49%).
When two studies were excluded due to high risk of bias (Fekih 2013; Kumbasar 2017), overall results indicated that there was no certainty of an effect in clinical pregnancy rates when NSAIDs were compared to placebo/no treatment (RR 1.26, 95% CI 0.95 to 1.66; 5 studies, 1149 participants; I² = 64%).
There were no studies to conduct a sensitivity analysis in the other predefined parameters.
1.4 Adverse effects to the woman
There were five studies reporting adverse effects in pregnancy. One study reported ectopic pregnancy (Dal Prato 2009); one reported multiple pregnancy (Firouzabadi 2007); and three reported side effects (Kumbasar 2017; Rijken‐Zijlstra 2013; Sohrabvand 2009).
1.4.1 Ectopic pregnancy
Only one study involving piroxicam reported this outcome (Dal Prato 2009). There was no certainty of an effect in ectopic pregnancy when piroxicam was compared to placebo (RR 0.56, 95% CI 0.05 to 5.89; 1 RCT, 72 participants; very low quality evidence). The evidence suggests that if the chance of ectopic pregnancy following placebo or no treatment is assumed to be 5%, the chance following the use of NSAIDs is estimated to be between 0.3% and 31%.
There were no studies to conduct further analyses in the other predefined parameters.
1.4.2 Multiple pregnancy
Only one study involving piroxicam reported this outcome (Firouzabadi 2007). There was no certainty of an effect in multiple pregnancy rate when piroxicam was compared to placebo (RR 2.00, 95% CI 0.18 to 21.67; 1 RCT, 180 participants; I² = 0%; very low quality evidence). The evidence suggests that if the chance of multiple pregnancy following placebo or no treatment is assumed to be 1%, the chance following the use of NSAIDs is estimated to be between 0.2 % and 24%
There were no studies to conduct further analyses in the other predefined parameters.
1.4.3 Side effects
Three studies reported on side effects (Kumbasar 2017; Rijken‐Zijlstra 2013; Sohrabvand 2009). In the first study, side effects related to indomethacin included headache, gastrointestinal complaints, nausea and dizziness; in the second, although authors reported the presence of muscle cramps in the control group, there were no events observed in the indomethacin group; in the third study, authors reported no side effects in either compared groups.
Overall results indicated that there was no certainty of an effect concerning side effects when NSAIDs were compared to placebo/no treatment (RR 1.39, 95% CI 0.02 to 119.3; 3 studies, 418 participants; I² = 79%; very low quality evidence). Apart from the fact that side effects were minimal and different among studies, we are unable to account further for this heterogeneity (Analysis 1.4; Figure 7).
1.4. Analysis.

Comparison 1 NSAID versus placebo/no treatment, Outcome 4 Adverse effects.
7.

Forest plot of comparison: 1 NSAID versus placebo/no treatment, outcome: 1.4 Adverse effects.
The comparators were no treatment in Dal Prato 2009, Kumbasar 2017, Sohrabvand 2009 and Placebo in Firouzabadi 2007, Rijken‐Zijlstra 2013.
There were not enough studies to conduct further analyses in the other predefined parameters.
1.5 Adverse fetal effects
There were no congenital anomalies reported during antenatal ultrasound screening in both groups (Kumbasar 2017).
1.6 Quality of life
No studies reported this outcome.
1.7 Relief of pain
Four studies reported relief of pain (Kailasam 2008; Sohrabvand 2009; Sohrabvand 2014; Zhao 2017); one study reported scores of post‐operative and discharge pain, with significant difference between the treatment and the control group in the second comparison (Kailasam 2008).
2 One NSAID vs another NSAID
One study compared piroxicam with indomethacin (Kumbasar 2017).
Primary outcomes
2.1 Live birth or ongoing pregnancy
There were no data on live birth.
There was no certainty of an effect in ongoing pregnancy when piroxicam was compared with indomethacin (RR 1.12, 95% CI 0.63 to 2.00; 1 study, 170 participants; very low quality evidence) (Analysis 2.1, Figure 8). The evidence suggests that if the chance of ongoing pregnancy following indomethacin is assumed to be 20%, the chance following the use of piroxicam is estimated to be between 13% and 40%.
2.1. Analysis.

Comparison 2 One NSAID vs another NSAID, Outcome 1 Ongoing pregnancy.
8.

Forest plot of comparison: 2 One NSAID vs another NSAID, outcome: 2.1 Ongoing pregnancy.
There were insufficient data to conduct further analyses in the other predefined parameters.
2.2 Miscarriage
There was no certainty of an effect in miscarriage when piroxicam was compared with indomethacin (RR 1.00, 95% CI 0.44 to 2.28; 1 study, 170 participants; very low quality evidence) (Analysis 2.2, Figure 9). The evidence suggests for miscarriage that if the chance following indomethacin is assumed to be 12%, the chance following the use of piroxicam would be between 5% and 27%.
2.2. Analysis.

Comparison 2 One NSAID vs another NSAID, Outcome 2 Miscarriage.
9.

Forest plot of comparison: 2 One NSAID vs another NSAID, outcome: 2.2 Miscarriage.
There were insufficient data to conduct further analyses in the other predefined parameters.
Secondary outcomes
2.3 Clinical Pregnancy
There was no certainty of an effect in clinical pregnancy when piroxicam was compared with indomethacin (RR 1.07, 95% CI 0.71 to1.63; 1 study, 170 participants; very low quality evidence) (Analysis 2.3, Figure 10). The evidence suggests that if the chance of clinical pregnancy following indomethacin is assumed to be 33%, the chance following the use of piroxicam is estimated to be between 23% and 54%.
2.3. Analysis.

Comparison 2 One NSAID vs another NSAID, Outcome 3 Clinical pregnancy.
10.

Forest plot of comparison: 2 One NSAID vs another NSAID, outcome: 2.3 Clinical pregnancy.
There were insufficient data to conduct further analyses in the other predefined parameters.
2.4 Adverse effects to the woman
2.4.1 Ectopic pregnancy
No data available.
2.4.2 Multiple pregnancy rate
No data available.
2.4.3 Antenatal and perinatal complications
No data available.
2.4.4 Adverse events
There were no adverse events in either group.
2.5 Adverse fetal effects
There were no congenital anomalies during antenatal ultrasound screening in either group.
2.6 Quality of life
No data available.
2.7 Relief of pain
No data available.
NSAID vs. aspirin
No study reported this comparison.
Discussion
Summary of main results
This Cochrane review evaluated the effectiveness and safety of nonsteroidal anti‐inflammatory drugs (NSAIDs) as co‐treatments in infertile women undergoing in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI). We identified and included 11 randomised controlled trials (RCTs) and one ongoing study; data were available for 10 of them, including a total of 1884 women, which investigated piroxicam, indomethacin, ibuprofen and diclofenac versus placebo/no treatment, while one study compared piroxicam with indomethacin.
Four RCTs with very low quality evidence compared NSAIDs to placebo/no treatment (Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017; Rijken‐Zijlstra 2013); three studies involved the use of piroxicam (Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017); and two used indomethacin (Kumbasar 2017; Rijken‐Zijlstra 2013). After analysis of the data, we are uncertain of the effect of NSAIDs on ongoing pregnancy, as the studies were at high risk of bias and pooled estimates were imprecise.
Four RCTs with very low quality evidence compared NSAIDs to placebo/no treatment, addressing miscarriage (Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017; Fekih 2013). Three of them involved the use of piroxicam (Dal Prato 2009; Firouzabadi 2007; Kumbasar 2017); one involved the use of indomethacin (Kumbasar 2017); and one involved the use of ibuprofen (Fekih 2013). After analysis of the data, we are uncertain of the effect of NSAIDs on miscarriage, as the studies were at high risk of bias and pooled estimates were imprecise.
We did not find different results when we removed data from RCTs with high risk of bias in our sensitivity analysis for miscarriage and when we performed subgroup analysis of ongoing pregnancy according to the type of NSAID used.
We found low‐quality evidence from seven and five RCTs of no certainty of an effect in clinical pregnancy rates and in adverse effects to the woman, respectively, when NSAIDs were compared to placebo/no treatment. There were sparse data for adverse fetal effects, quality of life and relief of pain in this comparison.
In the second comparison of this review, we found one study comparing piroxicam with indomethacin (Kumbasar 2017): we found very low quality evidence of no certainty of an effect in ongoing pregnancy, miscarriage and clinical pregnancy rates. Data on other secondary outcomes set for this review were not available, apart from the adverse fetal effects that were reported as null in both groups.
We found no studies comparing NSAIDs with aspirin.
Overall completeness and applicability of evidence
We included 11 studies with data relevant to the review question and a total of 1884 women in this Cochrane Review. Women eligible for randomisation had an average age of less than 35 years; they were defined as infertile women with an expected good prognosis in terms of ovarian response after stimulation. These studies reported on outcomes which included ongoing pregnancy and miscarriage amongst primary outcomes and clinical pregnancy, adverse effects to the woman (including ectopic and multiple pregnancy, drugs side effects) and fetus (chromosomal abnormalities) and relief of pain amongst secondary outcomes. Of note: none of them reported on live births.
We are uncertain that our findings support the use of NSAIDs to improve pregnancy outcomes in infertile woman undergoing ART. This conclusion came out through the comparison of the most usually used drugs to placebo/no treatment and through the comparison between piroxicam and indomethacin that was reported by one included study.
All studies were conducted in an urban setting in IVF units in countries around the world. Participants suffered from infertility from the conventional causes and seemed to be good responders. There were no data on high or poor responders, or in specific categories of infertile patients, such as those with recurrent implantation failures.
In addition to the published data collected, we retrieved only a small amount of extra details on the trials after communication with authors: a lot of information is therefore missing in many cases. We had no data on the quality of life in the first comparison and in most of the secondary outcomes in the second.
Quality of the evidence
We found 28 potentially eligible studies. Of them, 11 studies were eligible for inclusion and 10 for further analysis. The overall quality ranged from very low for the primary outcomes in both comparisons and from very low to low for the secondary outcomes (Table 1; Table 2) In addition to the published data collected, we retrieved some extra details by communicating authors of the original studies; specified information remained missing in many cases. Of note: one study was published only as a conference abstract.
Concerning the first comparison (NSAIDs vs. placebo/no treatment), we found very low quality evidence for the primary outcomes of ongoing pregnancy and miscarriage, because we assessed very serious risk of bias including poor reporting of methods, attrition, selective reporting, other biases, serious inconsistency/moderate heterogeneity across trials, very serious to serious indirectness and serious imprecision. And this concerned the comparison of both NSAIDs and no treatment/placebo and between piroxicam and indomethacin.
We found low‐quality evidence for the secondary outcome of clinical pregnancy, due to serious risk of bias including poor reporting of methods, attrition bias, selective reporting, and other biases and serious inconsistency/moderate heterogeneity across trials.
We found very low quality evidence for the secondary outcome of adverse effects, due to serious risk of bias including poor reporting of methods, attrition bias, selective reporting, and other biases, very serious indirectness, very serious imprecision and wide confidence interval.
For fetal (congenital) abnormalities, we found one very low quality trial at high risk of attrition and reporting biases that investigated both review comparisons. It reported no events in either comparison.
Concerning the second comparison (NSAID vs. another NSAID), we found very low quality of evidence for the primary outcome of ongoing pregnancy and miscarriage, because we assessed very serious risk of bias including poor reporting of methods, attrition, selective reporting, other biases, serious inconsistency/moderate heterogeneity across trials, very serious to serious indirectness and serious imprecision. We found very low quality evidence for the secondary outcome of clinical pregnancy due to serious risk of bias including poor reporting of methods, attrition bias, selective reporting, and other biases, serious inconsistency/moderate heterogeneity across trials and serious imprecision.
Potential biases in the review process
We made every effort to identify all eligible studies, following standard Cochrane procedures. We did not identify any potential source of bias, except from the fact that very few trial authors responded to our requests for additional information, and that, understandably, the data could not be retrieved for the ongoing trial.
Agreements and disagreements with other studies or reviews
This current review aimed to establish whether NSAIDs may play a beneficial role in improving pregnancy outcomes in infertile women undergoing ART. Our review showed that there is no certainty of a benefit of the use of NSAIDs for ART treatment. There are no similar reviews to date addressing this comparison. These drugs have been linked in the past with "reversible female infertility" (Stone 2002), and are currently used in medical conditions (pain due to endometriosis) related to infertility (Brown 2017), primary dysmenorrhoea (Marjoribanks 2015), and in the management of uterine adenomyosis (Vannuccini 2018), amongst others.
Authors' conclusions
Implications for practice.
We found little evidence from properly conducted randomised controlled trials (RCTs) regarding the use of NSAIDs in infertile women undergoing assisted reproduction, in spite of some promising data in the literature, through their physiology and potential action on localized inflammatory response, delaying or preventing ovulation and uterine contractions during an IVF cycle.
We have no data on live births; this might be explained by the difficulty in follow‐up together with the reporting bias that is quite frequent in such trials. Individual studies have concluded that there was a reduction in miscarriage rates using ibuprofen and an improvement in clinical pregnancy rates using piroxicam (when compared to placebo), but the synthesis of all included studies showed no evidence to support the use of NSAIDs. This could be due to the small number of eligible studies and variations in dose administration and time of treatment commencement across studies.
The quality of the evidence is very low for the primary outcomes of ongoing pregnancy and miscarriage when NSAIDs were compared to placebo/no treatment and when piroxicam was compared to indomethacin. In secondary outcomes for both comparisons, including clinical pregnancy, adverse effects to the woman and fetus, the quality varied from low to very low.
We are awaiting the results of one ongoing trial, but it is unlikely that these could change the results of our review.
Implications for research.
We aimed to provide a clear overview of the effectiveness and safety of NSAIDs as co‐treatments in infertile women undergoing assisted reproduction, in order to facilitate a decision over their use. We identified 11 studies: two were published in the form of abstracts and there was one ongoing study; data were available for 10 of them, including a total of 1884 women. After the synthesis of data, we arrived at no robust conclusions concerning their use.
Proper RCTs with sufficient power and appropriate endpoints (live‐birth and miscarriage rates must be the primary outcomes) that compare the use of NSAIDs with that of a placebo in assisted reproductive technology, that have sufficient power through sample size calculation based on current data and estimated differences in outcomes and that are performed according to CONSORT guidelines are urgently needed in women with infertility.
Notably, participants so far have been infertile women, relatively young and identified as normal responders to ovarian stimulation. Other subgroups, such as high or poor responders (although in the latter there might be a restriction because of the low number of embryos anticipated), or even women with recurrent implantation failures, could be included in the trials. Accurate documentation of the randomisation, allocation concealment, and blinding methods is highly desirable, so that risks of bias could be eliminated and the quality of the conclusions could be at high levels. In addition to the primary outcomes of live birth and miscarriage, study protocols should include the reporting of other adverse effects, and of crucial secondary outcomes.
Moreover, further studies on piroxicam and ibuprofen should be conducted with a longer follow‐up until live birth with adequate power.
Finally, studies on frozen‐thawed cycles should also be performed, as such strategies (e.g. freeze‐all policy) have begun to be the preferred choice for most of the infertile population undergoing assisted reproduction.
History
Protocol first published: Issue 1, 2009 Review first published: Issue 10, 2019
| Date | Event | Description |
|---|---|---|
| 12 May 2008 | Amended | Converted to new review format. |
| 19 June 2006 | New citation required and major changes | Substantive amendment |
Notes
None.
Acknowledgements
We thank Helen Nagels (Managing Editor), Marian Showell (Information Specialist), and the editorial board of the Cochrane Gynaecology and Fertility Group for their invaluable assistance in developing this review. In addition, we thank the authors of all included studies for supplying further information in response to our queries. We also thank Irene Moridi, Mina Mahdian and Miss Babalwa Zani for their contributions to the protocol and to early drafts of this review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Specialised Register of Controlled Trials
Searched 20 February 2019
Procite platform
Keywords CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or "intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "assisted reproduction" or "assisted reproduction techniques" or "IUI" or "Intrauterine Insemination" or "subfertility" or "infertility" or Title CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or "intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "assisted reproduction" or "assisted reproduction techniques" or "IUI" or "Intrauterine Insemination" or "subfertility" or "infertility"
AND
Keywords CONTAINS "non steroidal" or "NSAID" or "mefenamic acid" or "naproxen" or "ibuprofen" or "Flurbiprofen" or "Meclofenamic Acid" or "Meclofenamate" or "diclofenac" or "indomethacin" or "indometacin" or "Ketoprofen" or "Piroxicam" or "Flufenamic Acid" or "nimesulide" or "COX‐2 inhibitors" or "valdecoxib" or "etoricoxib" or "lumiracoxib" or "rofecoxib" or "parecoxib sodium" or "cyclooxygenase" or "Aspirin" or "acetly salicylic acid" or "nonsteroidal" or "cyclooxygenase" or "anti‐inflammatory effect" or "NSAIDs" or Title CONTAINS "non steroidal" or "NSAID" or "mefenamic acid" or "naproxen" or "ibuprofen" or "Flurbiprofen" or "Meclofenamic Acid" or "Meclofenamate" or "diclofenac" or "indomethacin" or "indometacin" or "Ketoprofen" or "Piroxicam" or "Flufenamic Acid" or "nimesulide" or "COX‐2 inhibitors" or "valdecoxib" or "etoricoxib" or "lumiracoxib" or "rofecoxib" or "parecoxib sodium" or "cyclooxygenase" or "Aspirin" or "nonsteroidal" or "cyclooxygenase" or "anti‐inflammatory effect" or "NSAIDs" (120 hits)
Appendix 2. CENTRAL search strategy
Via Cochrane Central Register of Studies Online (CRSO)
Searched 20 February 2019
Web platform
#1 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES 1029
#2 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES 1959
#3 MESH DESCRIPTOR Sperm Injections, Intracytoplasmic EXPLODE ALL TREES 510
#4 (embryo* adj2 transfer*):TI,AB,KY 2894
#5 (vitro fertili?ation):TI,AB,KY 2568
#6 ivf:TI,AB,KY 3838
#7 icsi:TI,AB,KY 1714
#8 (intracytoplasmic sperm injection*):TI,AB,KY 1500
#9 (blastocyst* adj2 transfer*):TI,AB,KY 247
#10 MESH DESCRIPTOR Reproductive Techniques, Assisted EXPLODE ALL TREES 2996
#11 (assisted reproduct*):TI,AB,KY 915
#12 (artificial insemination):TI,AB,KY 184
#13 MESH DESCRIPTOR Insemination, Artificial EXPLODE ALL TREES 355
#14 IUI:TI,AB,KY 555
#15 (intrauterine insemination*):TI,AB,KY 790
#16 (ovulation induc*):TI,AB,KY 2133
#17 (ovar* adj2 stimulat*):TI,AB,KY 1497
#18 superovulat*:TI,AB,KY 183
#19 (ovarian hyperstimulation):TI,AB,KY 1008
#20 COH:TI,AB,KY 276
#21 infertil*:TI,AB,KY 5739
#22 subfertil*:TI,AB,KY 576
#23 (ovar* adj2 induction):TI,AB,KY 189
#24 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 10977
#25 MESH DESCRIPTOR Anti‐Inflammatory Agents, Non‐Steroidal EXPLODE ALL TREES 18653
#26 MESH DESCRIPTOR diclofenac EXPLODE ALL TREES 1768
#27 MESH DESCRIPTOR Ibuprofen EXPLODE ALL TREES 1700
#28 MESH DESCRIPTOR Mefenamic Acid EXPLODE ALL TREES 121
#29 MESH DESCRIPTOR Naproxen EXPLODE ALL TREES 1060
#30 MESH DESCRIPTOR Piroxicam EXPLODE ALL TREES 626
#31 MESH DESCRIPTOR Cyclooxygenase Inhibitors EXPLODE ALL TREES 14524
#32 MESH DESCRIPTOR Cyclooxygenase 2 Inhibitors EXPLODE ALL TREES 1291
#33 nsaid*:TI,AB,KY 3474
#34 (cyclooxygenase inhibitor*):TI,AB,KY 1099
#35 (non‐steroidal anti‐inflammator*):TI,AB,KY 1869
#36 cox‐2:TI,AB,KY 1015
#37 (etoricoxib* or lumiracoxib* or parecoxib*):TI,AB,KY 710
#38 (rofecoxib* or valdecoxib*):TI,AB,KY 551
#39 sulphonanilide*:TI,AB,KY 0
#40 (diclofenac or voltaren):TI,AB,KY 3813
#41 ibuprofen:TI,AB,KY 3408
#42 (mefenamic acid or naproxen):TI,AB,KY 2169
#43 piroxicam:TI,AB,KY 1089
#44 indomet?acin 2798
#45 indomethacin:TI,AB,KY 2582
#46 indometacin:TI,AB,KY 651
#47 #25 OR #26 OR #27 OR #28 OR #29 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 26563
#48 #24 AND #47 112
Appendix 3. MEDLINE search strategy
Searched from 1946 to 20 February 2019
Ovid platform
1 exp insemination, artificial/ or exp reproductive techniques, assisted/ (65428) 2 assisted reproduct$.tw. (13455) 3 iui.tw. (1625) 4 (artificial adj5 insemination).tw. (6270) 5 exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp gamete intrafallopian transfer/ or exp zygote intrafallopian transfer/ (34282) 6 (ivf or icsi).tw. (25447) 7 in vitro fertili$.tw. (22266) 8 intracytoplasmic sperm injection$.tw. (6664) 9 ART.tw. (87483) 10 intra uterine insemination$.tw. (210) 11 intrauterine insemination$.tw. (2341) 12 exp Embryo Transfer/ (15262) 13 (embryo$ adj5 transfer$).tw. (17787) 14 or/1‐13 (167529) 15 exp anti‐inflammatory agents, non‐steroidal/ (196084) 16 exp diclofenac/ or exp ibuprofen/ or exp mefenamic acid/ or exp naproxen/ or exp piroxicam/ or exp cyclooxygenase inhibitors/ or exp cyclooxygenase 2 inhibitors/ (124304) 17 non‐steroidal anti‐inflammator$.tw. (14804) 18 nsaid$.tw. (23170) 19 cyclooxygenase inhibitor$.tw. (4689) 20 cox‐2.tw. (28657) 21 (etoricoxib$ or lumiracoxib$ or parecoxib$).tw. (1287) 22 (rofecoxib$ or valdecoxib$).tw. (2367) 23 sulphonanilide$.tw. (5) 24 (diclofenac or voltaren).tw. (10874) 25 ibuprofen.tw. (12313) 26 (mefenamic acid or naproxen).tw. (6852) 27 piroxicam.tw. (2906) 28 or/15‐27 (232643) 29 randomized controlled trial.pt. (476303) 30 controlled clinical trial.pt. (92914) 31 randomized.ab. (434520) 32 placebo.tw. (200681) 33 clinical trials as topic.sh. (186040) 34 randomly.ab. (305364) 35 trial.ti. (194121) 36 (crossover or cross‐over or cross over).tw. (79187) 37 or/29‐36 (1226241) 38 (animals not (humans and animals)).sh. (4515460) 39 37 not 38 (1126639) 40 14 and 28 and 39 (102)
Appendix 4. Embase search strategy
Searched from 1980 to 20 February 2019
Ovid platform
1 exp insemination, artificial/ or exp reproductive techniques, assisted/ (95530) 2 assisted reproduct$.tw. (21554) 3 iui.tw. (3171) 4 (artificial adj5 insemination).tw. (5739) 5 exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp gamete intrafallopian transfer/ or exp zygote intrafallopian transfer/ (67553) 6 (ivf or icsi).tw. (46602) 7 in vitro fertili$.tw. (30079) 8 intracytoplasmic sperm injection$.tw. (9517) 9 ART.tw. (107832) 10 intra uterine insemination$.tw. (418) 11 intrauterine insemination$.tw. (3697) 12 exp Embryo Transfer/ (30406) 13 (embryo$ adj5 transfer$).tw. (28687) 14 or/1‐13 (223906) 15 non‐steroidal anti‐inflammator$.tw. (20051) 16 nsaid$.tw. (39766) 17 cyclooxygenase inhibitor$.tw. (5138) 18 cox‐2.tw. (37935) 19 (etoricoxib$ or lumiracoxib$ or parecoxib$).tw. (2047) 20 (rofecoxib$ or valdecoxib$).tw. (3102) 21 sulphonanilide$.tw. (6) 22 (diclofenac or voltaren).tw. (17611) 23 ibuprofen.tw. (17054) 24 (mefenamic acid or naproxen).tw. (8997) 25 piroxicam.tw. (4102) 26 antiinflammatory agent/ or exp nonsteroid antiinflammatory agent/ (698871) 27 exp celecoxib/ or exp diclofenac/ or exp etoricoxib/ or exp ibuprofen/ or exp lumiracoxib/ or exp mefenamic acid/ or exp naproxen/ or exp parecoxib/ or exp piroxicam/ or exp rofecoxib/ or exp valdecoxib/ (111136) 28 nonsteroidal antiinflammator$.tw. (5300) 29 exp prostaglandin synthase inhibitor/ or cyclooxygenase 1 inhibitor/ or exp cyclooxygenase 2 inhibitor/ (480461) 30 or/15‐29 (751866) 31 30 and 14 (4244) 32 Clinical Trial/ (944481) 33 Randomized Controlled Trial/ (532332) 34 exp randomization/ (81275) 35 Single Blind Procedure/ (33910) 36 Double Blind Procedure/ (155141) 37 Crossover Procedure/ (58138) 38 Placebo/ (316628) 39 Randomi?ed controlled trial$.tw. (196797) 40 Rct.tw. (31286) 41 random allocation.tw. (1859) 42 randomly allocated.tw. (31650) 43 allocated randomly.tw. (2397) 44 (allocated adj2 random).tw. (799) 45 Single blind$.tw. (22086) 46 Double blind$.tw. (188006) 47 ((treble or triple) adj blind$).tw. (898) 48 placebo$.tw. (279124) 49 prospective study/ (500624) 50 or/32‐49 (1980595) 51 case study/ (59038) 52 case report.tw. (362915) 53 abstract report/ or letter/ (1048196) 54 or/51‐53 (1460847) 55 50 not 54 (1930559) 56 31 and 55 (952)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 20 February 2019
Ovid platform
1 exp reproductive technology/ (1725) 2 assisted reproduct$.tw. (885) 3 iui.tw. (35) 4 (artificial adj5 insemination).tw. (258) 5 (ivf or icsi).tw. (567) 6 in vitro fertili$.tw. (714) 7 intracytoplasmic sperm injection$.tw. (54) 8 ART.tw. (42540) 9 intra uterine insemination$.tw. (2) 10 intrauterine insemination$.tw. (26) 11 (embryo$ adj5 transfer$).tw. (170) 12 or/1‐11 (44793) 13 exp anti inflammatory drugs/ or exp aspirin/ (5516) 14 non‐steroidal anti‐inflammator$.tw. (521) 15 nsaid$.tw. (901) 16 cyclooxygenase inhibitor$.tw. (105) 17 cox‐2.tw. (832) 18 (etoricoxib$ or lumiracoxib$ or parecoxib$).tw. (50) 19 (rofecoxib$ or valdecoxib$).tw. (111) 20 sulphonanilide$.tw. (0) 21 (diclofenac or voltaren).tw. (225) 22 ibuprofen.tw. (456) 23 (mefenamic acid or naproxen).tw. (185) 24 piroxicam.tw. (45) 25 or/13‐24 (7411) 26 12 and 25 (15)
Appendix 6. CINAHL search strategy
Searched from 1961 to 20 February 2019
Ebsco platform
| # | Query | Results |
| S52 | S39 AND S51 | 17 |
| S51 | S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 | 1,305,398 |
| S50 | TX allocat* random* | 9,845 |
| S49 | (MH "Quantitative Studies") | 21,856 |
| S48 | (MH "Placebos") | 11,138 |
| S47 | TX placebo* | 55,343 |
| S46 | TX random* allocat* | 9,845 |
| S45 | (MH "Random Assignment") | 53,424 |
| S44 | TX randomi* control* trial* | 163,491 |
| S43 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 1,004,486 |
| S42 | TX clinic* n1 trial* | 238,780 |
| S41 | PT Clinical trial | 86,749 |
| S40 | (MH "Clinical Trials+") | 254,291 |
| S39 | S23 AND S38 | 42 |
| S38 | S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 | 22,238 |
| S37 | TX piroxicam | 248 |
| S36 | TX (mefenamic acid or naproxen) | 1,034 |
| S35 | TX ibuprofen | 2,681 |
| S34 | TX (diclofenac or voltaren) | 1,676 |
| S33 | TX (rofecoxib* or valdecoxib*) | 563 |
| S32 | TX (etoricoxib* or lumiracoxib* or parecoxib*) | 378 |
| S31 | TX Cox 2 | 994 |
| S30 | TX cyclooxygenase inhibitor* | 485 |
| S29 | (MM "Cox‐2 Inhibitors") | 1,993 |
| S28 | TX nsaid* | 4,967 |
| S27 | TX nonsteroidal antiinflammator* | 884 |
| S26 | TX nonsteroidal anti‐inflammator* | 3,253 |
| S25 | TX non‐steroidal anti‐inflammator* | 1,861 |
| S24 | (MM "Antiinflammatory Agents, Non‐Steroidal+") | 14,164 |
| S23 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 | 14,827 |
| S22 | TX intra‐uterine insemination | 27 |
| S21 | TX natural cycle* | 337 |
| S20 | TX timed intercourse | 36 |
| S19 | TX (ovari* N2 induction) | 31 |
| S18 | TX COH | 217 |
| S17 | TX ovarian hyperstimulation | 773 |
| S16 | TX superovulat* | 77 |
| S15 | TX ovulation induc* | 1,604 |
| S14 | TX intrauterine insemination | 438 |
| S13 | TX IUI | 314 |
| S12 | TX artificial insemination | 742 |
| S11 | TX assisted reproduct* | 3,460 |
| S10 | (MM "Insemination, Artificial") | 411 |
| S9 | (MM "Reproduction Techniques+") | 8,246 |
| S8 | TX intracytoplasmic sperm injection* | 802 |
| S7 | TX embryo* N3 transfer* | 2,782 |
| S6 | TX ovar* N3 hyperstimulat* | 778 |
| S5 | TX ovari* N3 stimulat* | 895 |
| S4 | TX IVF or TX ICSI | 4,535 |
| S3 | (MM "Fertilization in Vitro") | 3,166 |
| S2 | TX vitro fertilization | 6,418 |
| S1 | TX vitro fertilisation | 6,418 |
Appendix 7. Trials registry search terms
Searched 20 February 2019
Web platform
"NSAIDs" AND "IVF", and "anti‐inflammatory agents, non‐steroidal" AND "IVF"
Appendix 8. Grey literature search terms
Searched 20 February 2019
Web platform
"NSAIDs" AND "IVF" OR "ART", and "anti‐inflammatory agents, non‐steroidal" AND "IVF" OR "ART"
Appendix 9. Study eligibility
| Date | |
| Extractor | |
| Trial authors | |
| Publication year | |
| Journal | |
| 1) Design | |
| Described as randomised? If no then exclude . If yes go to questions 2 |
Yes No Unclear |
| 2) Participants | |
| (a) Are participants subfertile women (subfertile for any cause) who were undergoing any form of assisted reproductive therapy (ART) which will included IVF, intracytoplasmic sperm injection, ovulation induction and intrauterine insemination? | Yes No Unclear |
| (b) Are participants on NSAIDs? | Yes No Unclear |
| If 'no', exclude. Otherwise go to question (3). | |
| 3) Interventions | |
| NSAIDs versus placebo/no treatment? Two NSAIDS versus each other? |
Yes No Unclear |
| NSAIDS versus aspirin? | Yes No Unclear |
| If 'no' to (a) or (b), exclude. | |
| Final decision | |
| Include (if all 'yes') Exclude (if any 'no') Unclear |
|
| Excluded or unclear because: | |
| If 'unclear', action taken: |
Appendix 10. Data extraction form
| Date: | ||
| Extractor (initials): | ||
| Trial authors: | ||
| Year of publication: | ||
| Journal: | ||
| Study setting: | ||
| (1) Participants | ||
| Inclusion criteria: | Exclusion criteria: | |
| Median or mean age: | Ethnicity | |
| Age range: | Gravidity | |
| Were all treatment groups comparable at baseline: | Yes No Unclear |
|
| If no or unclear, describe any differences: | ||
| Notes: | ||
| 2) Interventions | Tx 1 | Tx2 |
| Tx used | ||
| Formulation used | ||
| Route | ||
| Dose | ||
| Duration | ||
| Timing and frequency | ||
| Notes | ||
| (3) Outcomes | ||
Primary outcomes
|
||
Secondary outcomes
|
||
| Further information: | ||
| Trialists contacted for more information: | yes | no |
| Address | ||
| Ph | ||
| Data | ||
| Comments |
Data and analyses
Comparison 1. NSAID versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ongoing pregnancy | 4 | 1159 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.71, 1.59] |
| 1.1 Piroxicam versus Placebo/no treatment | 3 | 508 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.70, 2.39] |
| 1.2 Indomethacin versus placebo/no treatment | 2 | 651 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.25] |
| 2 Miscarriage | 4 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.33, 1.16] |
| 2.1 Piroxicam versus Placebo/no treatment | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.15, 1.91] |
| 2.2 Flurbiprofen Axetil vs Placebo/ No treatment | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.36, 2.71] |
| 2.3 Ibuprofen vs Placebo/ no treatment | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.24, 0.87] |
| 3 Clinical pregnancy | 7 | 1570 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.00, 1.52] |
| 3.1 Diclofenac vs placebo/no treatment | 1 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.92, 1.58] |
| 3.2 Piroxicam vs placebo/no treatment | 4 | 696 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.89, 2.08] |
| 3.3 Indomethacin vs placebo/no treatment | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.56, 1.62] |
| 3.4 Flurbiprofen Axetil vs Placebo/ No treatment | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.73, 1.37] |
| 3.5 Ibuprofen vs Placebo/ no treatment | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.02, 2.86] |
| 4 Adverse effects | 5 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.21, 6.95] |
| 4.1 Ectopic pregnancy | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.05, 5.89] |
| 4.2 Multiple pregnancy | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.18, 21.67] |
| 4.3 Side effects | 3 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.02, 119.35] |
Comparison 2. One NSAID vs another NSAID.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ongoing pregnancy | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.63, 2.00] |
| 1.1 Piroxicam vs Indomethacin | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.63, 2.00] |
| 2 Miscarriage | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.44, 2.28] |
| 2.1 Piroxicam vs Indomethacin | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.44, 2.28] |
| 3 Clinical pregnancy | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.71, 1.63] |
| 3.1 Piroxicam vs Indomethacin | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.71, 1.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asgharnia 2007.
| Methods | Study type: randomised study Country: Iran Setting: women attending IVF/ICSI clinic Duration of recruitment: not stated Duration of trial: not stated Follow‐up: clinical pregnancy at least 4 weeks after embryo transfer |
|
| Participants | Inclusion: all of the couples with male factor, tubal factor, ovulatory factor and unexplained Exclusion: all women with uterine problems like myoma, Asherman syndrome #Randomised: n = 500 Baseline characteristics: not stated, but authors stated that there were no differences among groups |
|
| Interventions | Intervention: the treatment group (250 cycles) received an oral dose of 10 mg of piroxicam, and the control group (250 cycles) received a placebo, 1 to 2 hours before fresh ET Co‐intervention: long protocol |
|
| Outcomes | Primary outcomes: pregnancy rate Secondary outcomes: mean number of oocyte retrieval (metaphase II), 2 pn, embryo cleaved, embryo transferred |
|
| Notes | Ethics: an informed consent form was obtained from each participant Funding: supported by Azad Islamic university Rasht; Mehr Infertility Institute Sample size: not provided No adverse effects reported due to treatment Protocol of the study not provided, the study was published as an abstract in a conference meeting |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Data not provided in the abstract |
| Allocation concealment (selection bias) | High risk | Data not provided in the abstract |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Data not provided in the abstract |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Data not provided in the abstract |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data not provided in the abstract |
| Selective reporting (reporting bias) | High risk | Data not provided in the abstract |
| Other bias | High risk | Data not provided in the abstract |
Dal Prato 2009.
| Methods | Study type: randomised study Country: Italy Setting: women attending IVF/ICSI clinic Duration of recruitment: June 2005 to June 2007 Duration of trial: 2 years Follow‐up: clinical pregnancy at least 4 weeks after embryo transfer |
|
| Participants | Inclusion: age < 44 years; regular ovulatory menstrual cycles of 25 to 33 days; infertility due to tubal, idopathic or male factors, or endometriosis, no more than 2 previous embryo transfers Exclusion: FSH concentration > 15 IU/l on day 3 of the menstrual cycle; those who previously showed poor response to gonadotrophins #Randomised: n = 200 Baseline characteristics: all women; age 28 to 43 years; causes of infertility included factors such as tubal (26.1% vs. 44%), male (36.1% vs. 42.1%), endometriosis (31.3% vs. 28.6%) or unexplained (45.5% vs. 35%), respectively for piroxicam vs control. |
|
| Interventions | Intervention: treatment group (n = 100) received single oral dose of 10 mg piroxicam 1 to 2 hours before embryo transfer vs. control (no treatment; n = 100) Co‐intervention: long luteal protocol |
|
| Outcomes | Primary outcomes: clinical pregnancy rate Secondary outcomes: the number of participants with a positive b‐HCG test, miscarriages, implantation rate |
|
| Notes | Ethics: informed consent; approved by Institutional Review Board Funding: not stated Sample size provided No adverse effects reported due to treatment; ectopic pregnancies were reported Protocol of the study not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was provided by an external statistician |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, dark envelopes (opened by a nurse not involved in the trial) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding performed ("because it was not possible to provide a placebo", as authors stated) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant was excluded from the final analysis. Primary outcomes of the review were addressed |
| Selective reporting (reporting bias) | Unclear risk | All data were reported and discussed according to the initially stated objectives of the study authors. No protocol provided |
| Other bias | Low risk | No apparent evidence of other bias |
Fekih 2013.
| Methods | Study type: randomised study ("Prospective, randomized, double‐blinded placebo‐controlled
clinical study") Country: Tunisia Setting: 166 women attending an IVF/ICSI clinic in a university hospital Duration of recruitment: September 2010 to January 2011 Duration of trial: 5 months Follow‐up: up to 12 weeks of gestation |
|
| Participants | Inclusion: women undergoing IVF because of tubal, male infertility, unexplained, or endometriosis factors Exclusion: not stated Baseline characteristics: not stated |
|
| Interventions | Intervention: 83 women included in fresh ET cycles received an oral dose of 200 mg of ibuprofen (10 drops); while in the control group, the same number cycles corresponding to the treatment group were treated with placebo. Both groups started ibuprofen or placebo treatment 90 minutes before embryo transfer Co‐intervention: IVF protocol not stated |
|
| Outcomes | Outcomes reported: pregnancy and implantation rates Secondary outcomes: abortion rates |
|
| Notes | Ethics: not stated Funding: not stated Protocol: not stated The study was performed in Farhat Hached University Hospital, Sousse, Tunisia The article was published as an abstract in a conference (ASRM ‐ Fertility and Sterility Vol. 100, No. 3, Supplement, September 2013) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors state that "They were randomly divided into treatment and control group" in their abstract, but no further details were provided |
| Allocation concealment (selection bias) | High risk | Not stated, not able to get more information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors state that "Patients and staff were blinded to the treatment" in their abstract |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated, not able to get more information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not stated; the primary outcomes of the review were not addressed/measured |
| Selective reporting (reporting bias) | High risk | Not stated; not able to get more information |
| Other bias | Unclear risk | There is no further information |
Firouzabadi 2007.
| Methods | Study type: prospective randomised clinical trial Country: Iran Setting: women attending IVF/ICSI clinic Duration of recruitment: February to October 2006 Duration of trial: 10 months Follow‐up: up to 20 weeks of pregnancy |
|
| Participants | Inclusion and exclusion not mentioned #Randomised: n = 180 Baseline characteristics: all women who underwent fresh IVF; mean age (years): control 29.79 ± 5.1, piroxicam 30.28 ± 4.3; duration of infertility (years): control 7.8 ± 4.4, piroxicam 8.4 ± 4.3 |
|
| Interventions | Intervention: treatment group (n = 90) received single oral dose of 10mg piroxicam 1 to 2 hours before embryo transfer vs. control (placebo; n = 90) Co‐intervention: 21‐long agonist protocol |
|
| Outcomes | Pirmary outcomes: clinical pregnancy rate (fetal heart at 8 weeks) Secondary outcomes: implantation rates, multiple pregnancy, biochemical pregnancy, miscarriage rates |
|
| Notes | Ethics: written informed consent; approved by Institutional Review Board Funding: not stated Protocol: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a computer‐generated random table |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and staff were blinded to the treatment, authors stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant was lost and all data were reported. Primary outcomes of the review were addressed |
| Selective reporting (reporting bias) | Unclear risk | No protocol provided |
| Other bias | Low risk | No evidence of any other source of bias |
Kailasam 2008.
| Methods | Study type: randomised prospective double‐blind study Country: UK Setting: IVF centre (Centre for Reproductive Medicine) Duration of recruitment: February 2003 to December 2006 Duration of trial: 3 years and 10 months Follow‐up: not mentioned |
|
| Participants | Inclusion: women < 40 years old with early follicular FSH ≤ 10 lU/l and no medical contraindications to receiving NSAIDs Exclusion: women ≥ 40 years old, and those with early follicular FSH of > 10 lU/l; past history of allergy to NSAID, women with asthma, peptic ulcer disease or inflammatory bowel disease #Randomised; 381; baseline characteristics: mean age (years): control 35, diclofenac sodium 34.1 |
|
| Interventions | Intervention: diclofenac sodium (100 mg) orally (n = 185); control/no treatment (n = 190). Specifically, a single dose of diclofenac sodium 100 mg was administered as suppository at the end of oocyte retrieval (Voltarol®, Novartis Pharmaceuticals, Surrey, UK) or nothing. Authors stated that it was not considered ethical to administer a placebo as a suppository Co‐interventions: dexamethasone (1 mg; Organon, UK) was commenced on the first day of gonadotrophin administration and continued until the night before oocyte retrieval to optimize ovarian response. Norethisterone (Pharmacia, UK) 5 mg twice daily was administered orally for 7 days from day 19 of the preceding cycle to reduce the incidence of functional ovarian cysts Long luteal phase protocol |
|
| Outcomes | Primary outcomes: implantation rate and clinical pregnancy rate Secondary outcomes: median pain scores prior to discharge were measured using the linear visual analogue pain scores (0 = no pain, 100 = worst pain) |
|
| Notes | Ethics: the study was conducted following approval from the local research ethics committee and participants gave written informed consent for all clinical procedures Funding: not mentioned Protocol: not mentioned The study was performed at Centre for Reproductive Medicine, University of Bristol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was done as per computer‐generated model |
| Allocation concealment (selection bias) | Low risk | A block randomisation list was used in order to balance the number receiving diclofenac sodium versus no treatment (controls) after every 20 women enrolled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and nurses were blinded as regards diclofenac sodium administration |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No loss to follow‐up. Not examined the primary outcome of interest of this review |
| Selective reporting (reporting bias) | Low risk | None. Authors report all initially planned outcomes, including side effects |
| Other bias | Low risk | No other sources of bias identified. The study was initially powered as a ‘non‐inferiority’ study for the pregnancy rate |
Kumbasar 2017.
| Methods | Study type: prospective randomised clinical trial Country: Turkey Setting: 255 women diagnosed with primary or secondary infertility Duration of recruitment: not stated Duration of trial: not stated Follow‐up: not stated |
|
| Participants | Inclusion: women with male or tubal related factor, endometriosis or no detectable cause was the reason for infertility in all participants Exclusion: women with space‐occupying lesions, such as submucous myomas or polyps in the endometrial cavity, a congenital cavity disorder, such as uterine septum, or an acquired cavity disorder, such as Asherman syndrome |
|
| Interventions | Intervention: the participants were divided randomly into 3 groups. Group 1 (n = 85) received sublingual piroxicam (10 mg Felden flash capsules) and group 2 (n = 85) indomethacin (Endol 100 mg suppositories) 1 to 2 h before ET. Group 3 (n = 85), the control, did not receive any form of treatment before ET Co‐intevention: long‐term standard GnRH agonist (long protocol), a microdose protocol or an antagonist protocol. The protocol and GnRH doses were chosen according to the participant’s age, BMI, ovarian grade, basal FSH and E2 levels, history and response to treatment. In all participants, ETs were performed on day 2 following OR |
|
| Outcomes | The rates of implantation, ongoing (authors define it after 24 weeks) and clinical pregnancy and miscarriage. Also, authors reported adverse effects resulting from the use of piroxicam or indomethacin, and congenital anomalies observed during antenatal ultrasound screening | |
| Notes | Ethics: approval for the study was obtained from the hospital’s Training and Planning Coordination Committee. The participants were informed in detail about the study, and their consent was also obtained Funding: not mentioned Protocol: not mentioned The study was performed at Department of Obstetrics and Gynecology, Sakarya Research and Education Hospital, Adapazarı, Sakarya, Turkey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Auhtors stated that the participants were divided randomly into 3 groups, but no further information was available |
| Allocation concealment (selection bias) | High risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes of the review were addressed |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned |
| Other bias | Unclear risk | No protocol or power calculation provided |
Moon 2004.
| Methods | Study type: prospective, randomised, double‐blinded, placebo‐controlled clinical study Country: Korea Setting: large urban medical centre Duration of recruitment: March 1998 to February 2000 Duration of trial: 2 years Follow‐up: not mentioned |
|
| Participants | 188 consecutive cycles of fresh IVF–ET and 78 cycles of frozen–thawed ET Inclusion: all women who underwent IVF because of tubal, male infertility, unexplained or endometriosis factor Exclusion: all not included in the above group #Randomised; baseline characteristics: mean age: control 32.7 years, beta cyclodextrin piroxicam 33.2 years |
|
| Interventions | Intervention: the 188 fresh IVF–ET cycles and 78 frozen–thawed ET cycles were randomly divided into treatment and control groups. In the fresh ET cycles, the treatment group (94 cycles) received an oral dose of 10 mg of piroxicam (Brexin; Kolon Inc., Daejeon, Korea), and the control group (94 cycles) received a placebo. In the frozen–thawed ET cycles, 39 cycles received 10 mg of piroxicam orally (treatment group) and 39 cycles were treated with placebo (control group). Both groups started piroxicam or placebo treatment 1 to 2 hours before ET Co‐intervention: long standard protocol with GnRH agonist |
|
| Outcomes | Primary outcomes: implantation rate and clinical pregnancy rate Secondary outcomes: not reported |
|
| Notes | Ethics: approved by Institutional Review Board of the Human Investigation Committee of Good Moon‐Hwa Hospital; written informed consent from all participants Funding: not mentioned No adverse effects due to piroxicam reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author's response: centralized randomisation process; computer generated |
| Allocation concealment (selection bias) | High risk | Not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and staff were blinded to the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors did not address the primary outcomes of interest of this review |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | No protocol or power calculation provided |
Rijken‐Zijlstra 2013.
| Methods | Study type: double blind, randomised, placebo‐controlled trial on the effectiveness of indomethacin to prevent ovulation in modified natural cycle Country: the Netherlands Setting: tertiary fertility centre, University Medical Centre Groningen, the Netherlands Duration of recruitment: December 2005 to April 2007 Duration of trial: 1 year and 4 months Follow‐up: upto 12 weeks of pregnancy |
|
| Participants | Inclusion: women qualifying for IVF between 18 and 36 years and who had an ovulatory cycle between 26 and 35 days; no ovarian cysts; no previous IVF treatments (except when this treatment had resulted in an ongoing pregnancy); no contraindications for the use of indometacin (e.g. asthma or hypersensitivity) Exclusion: all not included in the above group #Randomised; baseline characteristics: n = 120 women (age 18 to 36 years) qualifying for IVF and with regular ovulatory cycles |
|
| Interventions | Participants were randomly assigned to undergo a maximum of 6 cycles of modified natural‐cycle IVF, either with the
addition of indometacin (60 women) or of matching placebo capsules (60 women) Intervention: group n = 60 i.e. 250 cycles (indomethacin; 50 mg 3 times a day from the day of ovulation trigger until the morning of oocyte retrieval) vs control group n = 60 i.e 274 cycles (placebo capsules 3 times a day from the day of ovulation trigger until the morning of oocyte retrieval) Co‐intervention: modified natural‐cycle IVF. Embryo transfer was performed on the 3rd day after OR |
|
| Outcomes | Primary outcomes: the percentage of women without premature ovulation at the scheduled time of oocyte retrieval in a maximum of 6 modified natural‐cycle IVF cycles Secondary outcomes: LH concentrations on the day of ovulation triggering and the numbers of oocyte retrievals, oocytes obtained, fertilized oocytes, embryo transfers, clinical pregnancies and ongoing pregnancies |
|
| Notes | Ethics: approved by the Institutional Review Board (IRB reference METc 2005/074, approved 24 June 2005) and written informed consent was obtained from participants Funding: Merck Sharp & Dohme who also provided the drug under study; Ferring Pharmaceuticals; Merck Serono The trial was registered under Current Controlled Trials Number ISRCTN11805686 (www.controlled‐trials.com) The sample size was calculated for the primary endpoint |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done using SPSS statistical software with block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and physicians unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was unclear whether the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors include primary endpoints of this review and side effects |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported including adverse events. Intention‐to‐treat analysis |
| Other bias | High risk | Trial funded by pharmaceutical company and the role of the funder was not described The sample size was calculated for the primary endpoint |
Sohrabvand 2009.
| Methods | Study type: double blind clinical trial (pilot study) Country: Iran Setting: Vali‐e‐Asr hospital Duration of recruitment: August 2005 to December 2006 Duration of trial: 1 year 3 months Follow‐up: Up to 2 weeks after embryo transfer |
|
| Participants | Inclusion: ART was recommended by an infertility specialist due to tubal factors, ovulation disorders, or severe male factor, a 3rd‐day FSH serum level of < 10 IU/L Exclusion: systemic diseases or endometriosis #Randomised; baseline characteristics: 66 women with mean age of 29.12 ± 4.9 referring to Vali‐e‐asr hospital (Iran) who underwent ART. Of the couples, 77% had primary infertility, 59% of which were due to male factor. Duration of infertility was 6.33 ± 3.61 years |
|
| Interventions | Intervention: Group A (n = 22): indomethacin 10 mg/rectal; Group B (n = 22): hyoscine 100 mg/rectal (not NSAID), 30 minutes before embryo transfer; Group C (n = 22): no treatment Co‐intervention: long GnRH analogue protocol |
|
| Outcomes | Primary outcomes: biochemical pregnancy, abdominal muscle cramps Secondary outcomes: not mentioned |
|
| Notes | Ethics: not mentioned Protocol: not mentioned Funding: Tehran University of Medical Sciences Study performed in Department of Infertility, Vali‐e‐Asr Reproductive Health Research Center, Vali‐e‐Asr Hospital, Tehran University of Medical Sciences, Tehran, Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described and no response from authors on random sequence generation |
| Allocation concealment (selection bias) | Low risk | Author's response: sealed, opaque, sequentially numbered, identical envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind mentioned though not described in detail how it was done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No missing participants, but the primary outcomes of the review were not included |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective outcome reporting |
| Other bias | High risk | Small sample size, no protocol used, no power calculation |
Sohrabvand 2014.
| Methods | Study type: double blind clinical trial (pilot study) Country: Iran Setting: Vali‐e‐Asr hospital Duration of recruitment: August 2010 to December 2011 Duration of trial: 1 year 4 months Follow‐up: up to 2 weeks from embryo transfer |
|
| Participants | Inclusion: age group of 20 to 35 years old and with ART indication due to tubal factors, ovulation disorders or severe male factor Exclusion: systemic diseases and endometriosis 50 women randomised |
|
| Interventions | Intervention: Group A received piroxicam (10 mg, Tolid Daru, Iran) orally 30 minutes before embryo transfer and group B did not use any form of medication which is the conventional method used (control group). Co‐intervention: long GnRH analogue protocol |
|
| Outcomes | Outcome parameters: abdominal muscle cramps, biochemical pregnancy (β‐hCG positive) | |
| Notes | Ethics: approval from the Ethical Committee of Tehran University of Medical Sciences; written informed
consent from participants Funding: Tehran University of Medical Sciences Protocol: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Mentioned in the text, but no further data are available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No missing participants, but the primary outcomes of the review were not addressed |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective outcome reporting |
| Other bias | High risk | Small sample size, no protocol used, no power calculation |
Zhao 2017.
| Methods | Study type: single‐centre, prospective, double‐blind, randomised, placebo‐controlled study Country: China Setting: university hospital in Beijing, China Duration of recruitment: March to April 2016 Duration of trial: 2 months Follow‐up: up to 2 weeks from embryo transfer |
|
| Participants | Inclusion: age group of 20 to 35 years old and with ART indication due to tubal factors, ovulation disorders or severe male factor Exclusion: women allergic to any anaesthetic, analgesic, or NSAID, had a history of asthma, peptic ulcer disease, or inflammatory bowel disease Age in the study group 34 ± 4 vs. 32 ± 4 in the control group 200 women randomised |
|
| Interventions | Participants were prospectively allocated to 2 groups, the flurbiprofen axetil group (FA group) or the placebo group (control group). 1 single dose of FA given 30 minutes before ultrasound‐guided transvaginal oocyte retrieval Co‐intervention: long luteal down‐regulation protocol, a flare‐up agonist protocol, or an antagonist protocol according to physician evaluation of participants’ potential response to stimulation protocol |
|
| Outcomes | Primary outcome parameters: pregnancy rate (clinical) Secondary outcomes: consumption of analgesic rescue, the number and grading of body movements during the procedure, postoperative pain score, and biomarker (PGE2) concentration in follicular fluid, consumption of tramadol after being discharged |
|
| Notes | Ethics: approval from the institutional review board of the Peking University People’s Hospital; written informed consent
was obtained from all participants on admission Funding: department research funding, 2016‐01 Protocol: Clinical trials registration number is ChiCTR‐IPR‐16008133 (www.chictr.org.cn, 22 March 2016; Yi Feng, MD) Study conducted at Department of Anesthesiology and Reproductive Medical Center, Peking University People’s Hospital, Beijing, China The study was initially powered as a non‐inferiority study for the pregnancy rate |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list that was stored in sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Provided by sealed envelopes from a nurse who was not involved in the actual conduct of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study coordinator, attending anaesthesiologist, data collection resident, and the participants were all unaware of treatment group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No missing participants, but the primary outcomes of the review were not addressed |
| Selective reporting (reporting bias) | Low risk | No evidence for such bias |
| Other bias | Low risk | Protocol provided, power calculation provided, side effects mentioned |
Abbreviations:
NSAID: nonsteroidal anti‐inflammatory drug FSH: follicle stimulating hormone LH: luteinizing hormone E2: estradiol BMI: Body Mass Index ART: assisted reproductive technology IVF/ICSI: in vitro fertilization / intracytoplasmic sperm injection OR: oocyte retrieval ET: embryo transfer 2 pn: 2 pronuclei IU/l: international units per litre Mg; milligrams b‐HCG: human chorionic gonadotropin GnRH: gonadotropin‐releasing hormone
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akande 2006 | Not true RCT |
| Aldeery 2006 | Non‐randomised trial |
| Bernabeu 2006 | A pilot trial on oocyte recipients |
| Bou Nemer 2017 | Non‐randomised trial |
| Bou Nemer 2019 | Study on follicular fluid levels of interleukins after a single‐dose of ibuprofen |
| Kadoch 2008 | Retrospective study |
| Kawachiya 2012 | Retrospective cohort study |
| Lier 2015 | Comparison of opioids |
| Matsota 2012 | Comparison of an opioid |
| Mesen 2013 | Retrospective study |
| Mialon 2011 | Retrospective study |
| NCT02571543 | Non‐randomised trial ‒ protocol |
| Sallam 1999 | Inadequate and vague description of methodology and results |
| Sarhan 2015 | Study on IUI |
| Seidler 2018 | Non RCT |
| Shah Nawaz 2014 | Non‐randomised, comparing standard GnRH analogues with aromatase inhibitors, indomethacine and gonadotrophins |
| Zarei 2016 | RCT on piroxicam in IUI cycles |
Characteristics of ongoing studies [ordered by study ID]
NCT02642601.
| Trial name or title | Role of Indomethacin in Difficult Embryo Transfer |
| Methods | Study type: interventional (clinical trial) Allocation: randomised Intervention model: parallel assignment Masking: none (open label) Primary purpose: prevention |
| Participants | Estimated enrolment: 100 participants Ages eligible for study: 20 years to 38 years Inclusion criteria: infertile women undergoing ICSI cycle with difficult mock transfer done on day of ovum pick‐up Exclusion criteria: repeated ICSI failure Easy mock embryo transfer on day of ovum pick‐up Early follicular FSH > 10 IU/l Past history of allergy to NSAID Past history of asthma, peptic ulcer disease or inflammatory bowel disease. Endometrial pathology |
| Interventions | Experimental: indomethacin 1 dose of indomethacin 100 mg rectal suppository will be administered 1 to 2 hours before embryo transfer No intervention: no medication No indomethacine before embryo transfer |
| Outcomes | Primary outcome measures: clinical pregnancy rate +ve pregnancy test + gestational sac with fetal pulsation by ultrasound Secondary outcome measures: implantation rate, ongoing pregnancy rate |
| Starting date | March 2014 |
| Contact information | Dr Mona M Shaban, Cairo University IVF center, Cairo University Hospital, Cairo, Egypt Contact: Sherin H Gad Allah, MD +201097665573 sherinehosny@gmail.com Contact: Mona M Shaban, MD +201001078586 drmonashaban@gmail.com Kamal Shoeir private IVF center, Cairo, Egypt |
| Notes | Mỹ Đức Hospital Recruitment Status: recruiting ClinicalTrials.gov identifier: NCT02642601 |
Abbreviations:
NSAID: nonsteroidal anti‐inflammatory drug FSH: follicle stimulating hormone ICSI: intracytoplasmic sperm injection
Differences between protocol and review
We have updated the Methods section to current Cochrane standards.
Miscarriage rate was moved to the primary outcomes and clinical pregnancy rate was added to the secondary outcomes to provide a more meaningful approach to the research question. Adverse effects of the drugs administered were considered to have had a temporary effect on the quality of life of the patient, thus these were analysed in the relevant section.
We conducted analyses using RR instead of OR because this is our preferred analysis method, as we estimate probability and not odds for the specific question of the review; notably, results were not affected using ORs.
Contributions of authors
AN lead author: writing of the protocol, extracting data of included studies, conducting the analysis and drafting the manuscript.
CSS: re‐writing of most sections, participating in the selection of studies, and final drafting of manuscript.
DV: extracting data of included studies, conducting the analysis and developing tables and figures.
Sources of support
Internal sources
-
Marian Showell, New Zealand.
The review authors wish to acknowledge Marian Showell (CGF Information Specialist)
External sources
Cochrane South Africa, South Africa.
Declarations of interest
AN, DV and CSS have no conflicts of interests to disclose.
New
References
References to studies included in this review
Asgharnia 2007 {published data only}
- Asgharnia M, Mehrafza M, Oudi M, Hoseeieni A. Effect of piroxicam treatment on pregnancy rate in intracytoplasmic sperm injection (ICSI). Fertility and Sterility 2007;88(Suppl 1):S159. [DOI: ] [Google Scholar]
Dal Prato 2009 {published data only}
- Dal Prato L, Borini A. Effect of piroxicam administration before embryo transfer on IVF outcome: a randomized controlled trial. Reproductive Biomedicine Online 2009;19(4):604‐9. [DOI] [PubMed] [Google Scholar]
Fekih 2013 {published data only}
- Fekih M, Chachia S, Zarrouk W, Khairi H. Effect of ibuprophen administration before embryo transfer on IVF outcome: a randomized controlled trial. Fertility and Sterility 2013;100 Suppl(3):S122. [Google Scholar]
Firouzabadi 2007 {published data only}
- Firouzabadi R, Ghandi S, Tayebi N. Effect of administration of single dose piroxicam before embryo transfer on implantation and pregnancy rates in IVF cycles. Journal of Biological Sciences 2007;7(1):123‐6. [Google Scholar]
Kailasam 2008 {published data only}
- Kailasam C, Hunt L, Ryder I, Bhakri I, Gordon U. Safety and effectiveness of diclofenac sodium in assisted reproduction treatment: A randomized prospective double blind study. Reproductive Biomedicine Online 2008;16(5):724‐9. [DOI] [PubMed] [Google Scholar]
Kumbasar 2017 {published data only}
- Kumbasar S, Gül Ö, Şık A. Evaluation of the effect of indomethacin and piroxicam administration before embryo transfer on pregnancy rate. Journal of Obstetrics and Gynaecology Research 2017;43(3):536‐42. [DOI] [PubMed] [Google Scholar]
Moon 2004 {published data only}
- Moon H, Park S, Lee J, Kim K, Joo B. Treatment with piroxicam before embryo transfer increases the pregnancy rate after in vitro fertilization and embryo transfer. Fertility and Sterility 2004;82(4):816‐20. [DOI] [PubMed] [Google Scholar]
Rijken‐Zijlstra 2013 {published data only}
- Hammer C, Haadmsa M, Pelinck M, Hoel A, Simons A, Land J. The effectiveness of indomethacin to prevent ovulation in modified natural cycle IVF; a randomized controlled trial. Human Reproduction 2008;23(Suppl 1):i23‐4. [DOI] [PubMed] [Google Scholar]
- Rijken‐Zijlstra TM, Haadsma ML, Hammer C, Burgerhof JG, Pelinck MJ, Simons AH, et al. Effectiveness of indometacin to prevent ovulation in modified natural‐cycle IVF: a randomized controlled trial. Reproductive Biomedicine Online 2013;27(3):297‐304. [PUBMED: 23876971] [DOI] [PubMed] [Google Scholar]
Sohrabvand 2009 {published data only}
- Sohrabvand F, Haghollahi F, Maasomi M, Asgarpoor L, Shariat M, Hamedani M. The effect of administrating indomethacin or hyoscine before embryo transfer on ART outcome (a pilot study). Iranian Journal of Reproductive Medicine 2009;7(4):169‐73. [Google Scholar]
Sohrabvand 2014 {published data only}
- Sohrabvand F, Haghollahi F, Maasomi M, Shariat M. Effect of piroxicam on ART outcome: a pilot study. International Journal of Fertility and Sterility 2014;8(3):143‐248. [PUBMED: PMC4221509] [PMC free article] [PubMed] [Google Scholar]
Zhao 2017 {published data only}
- Zhao H, Feng Y, Jiang Y, Lu Q. Flurbiprofen axetil provides effective analgesia without changing the pregnancy rate in ultrasound‐guided transvaginal oocyte retrieval: a double‐blind randomized controlled trial. Anesthesia and Analgesia 2017;125(4):1269‐74. [DOI: 10.1213/ANE.0000000000002025] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akande 2006 {published data only}
- Akande V, Garas A, Cahill D. The effect of diclofenac and paracetamol on pregnancy and implantation rates in infertile women undergoing IVF treatment. Journal of Obstetrics and Gynaecology 2006;26(8):785‐7. [DOI] [PubMed] [Google Scholar]
Aldeery 2006 {published data only}
- Al Deery M, Jaroudi K, Al Hassan S, Bazzi R, Al Shahrani A, Awartani K. Diclofenac for analgesia in assisted conception: a prospective cohort study. Middle East Fertility Society Journal 2006;11(1):48‐52. [Google Scholar]
Bernabeu 2006 {published data only}
- Bernabeu R, Roca M, Torres A, Ten J. Indomethacin effect on implantation rates in oocyte recipients. Human Reproduction 2006;21(2):364‐9. [DOI] [PubMed] [Google Scholar]
Bou Nemer 2017 {published data only}
- Bou Nemer L, Word A, Carr B, Bukulmez O. One dose of ibuprofen decreases levels of interleukins involved in ovulation in the follicular fluid of women undergoing minimal stimulation in‐vitro fertilization. Fertility and Sterility 2017;108(3 Supplement 1):e258. [Google Scholar]
Bou Nemer 2019 {published data only}
- Bou Nemer L, Shi H, Carr BR, Word RA, Bukulmez O. Effect of single‐dose ibuprofen on follicular fluid levels of interleukins in poor responders undergoing in vitro fertilization. Systems Biology in Reproductive Medicine 2019;65(1):48‐53. [DOI] [PubMed] [Google Scholar]
Kadoch 2008 {published data only}
- Kadoch IJ, Al‐Khaduri M, Phillips SJ, Lapensée L, Couturier B, Hemmings R, et al. Spontaneous ovulation rate before oocyte retrieval in modified natural cycle IVF with and without indomethacin. Reproductive Biomedicine Online 2008;16(2):245‐9. [DOI] [PubMed] [Google Scholar]
Kawachiya 2012 {published data only}
- Kawachiya S, Matsumoto T, Bodri D, Kato K, Takehara Y, Kato O. Short‐term, low‐dose, non‐steroidal anti‐inflammatory drug application diminishes premature ovulation in natural‐cycle IVF. Reproductive Biomedicine Online 2012;24(3):308‐13. [DOI] [PubMed] [Google Scholar]
Lier 2015 {published data only}
- Lier MC, Douwenga WM, Yilmaz F, Schats R, Hompes PG, Boer C, et al. Patient‐Controlled Remifentanil Analgesia as Alternative for Pethidine with Midazolam During Oocyte Retrieval in IVF/ICSI Procedures: A Randomized Controlled Trial. Pain Practice 2015;15(5):487‐95. [DOI: 10.1111/papr.12189] [DOI] [PubMed] [Google Scholar]
Matsota 2012 {published data only}
- Matsota P, Sidiropoulou T, Batistaki C, Giannaris D, Pandazi A, Krepi H, et al. Analgesia with remifentanil versus anesthesia with propofol‐alfentanil for transvaginal oocyte retrieval: a randomized trial on their impact on in vitro fertilization outcome. Middle East Journal of Anaesthesiology 2012;21(5):685‐92. [PUBMED: 23265031] [PubMed] [Google Scholar]
Mesen 2013 {published data only}
- Mesen TB, Kacemi‐Bourhim L, Marshburn PB, Usadi RS, Matthews M, Norton HJ, et al. The effect of ketorolac on pregnancy rates when used immediately after oocyte retrieval. Fertility and Sterility 2013;100(3):725‐8. [DOI: 10.1016/j.fertnstert.2013.04.048] [DOI] [PubMed] [Google Scholar]
Mialon 2011 {published data only}
- Mialon O, Delotte J, Lehert P, Donzeau M, Drici M, Isnard V, et al. Comparison between two analgesic protocols on IVF success rates [Comparaison de deux protocoles antalgiques utilisés au cours de la ponction folliculaire sur les taux de réussite en fécondation in vitro]. Journal of Gynecology Obstetrics and Human Reproduction 2011;40(2):137‐43. [DOI: 10.1016/j.jgyn.2010.08.005] [DOI] [PubMed] [Google Scholar]
NCT02571543 {published data only}
- NCT02571543. Can Ibuprofen Delay Ovulation in Natural Cycle‐IVF? [Role of Indomethacin in Cases of Difficult Embryo Transfer in Intra Cytoplasmic Sperm Injection Cycles]. clinicaltrials.gov/ct2/show/NCT02571543 (first posted 8 October 2015).
Sallam 1999 {published data only}
- Sallam HN. Cyclofenil and human reproduction. Assisted Reproduction 1999;9(1):39‐44. [Google Scholar]
Sarhan 2015 {published data only}
- Sarhan A. Indometacin administration can prevent premature follicular repture before timed intra uterine insemination (IUI) and improve the cycle outcome. Human Reproduction (Oxford, England) 2015;30(Suppl 1):i121 Abstract no: O‐276. [Google Scholar]
Seidler 2018 {published data only}
- Seidler E, Sakkas D, Murphya L, Vaughana D, Penzias AS. Routine ketorolac following oocyte retrieval reduces post‐operative narcotic use by more than 50%. Fertility and Sterility 2018;110(4, Supplement):e400. [DOI: ] [Google Scholar]
Shah Nawaz 2014 {published data only}
- Shah Nawaz S, Azhar A. Amazing low cost IVF/ICSI induction protocol in developing countries. Human Reproduction 2014;29(suppl_1):i53‐54. [DOI: ] [Google Scholar]
Zarei 2016 {published data only}
- Zarei A, Mahboubi M, Parsanezhad ME, Alborzi S, Younesi M, Madadi G. Effects of piroxicam administration on pregnancy outcome in intrauterine insemination (IUI) cycles: a randomized clinical trial. Clinical and Experimental Obstetrics & Gynecology 2016;43(2):225‐9. [PubMed] [Google Scholar]
References to ongoing studies
NCT02642601 {published data only}
- NCT02642601. Role of Indomethacin in Difficult Embryo Transfer. clinicaltrials.gov/show/NCT02642601 (first received 30 December 2015).
Additional references
Akhtar 2013
- Akhtar MA, Sur S, Raine‐Fenning N, Jayaprakasan K, Thornton JG, Quenby S. Heparin for assisted reproduction. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD009452.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Ghamdi 2008
- Al‐Ghamdi A, Coskun S, Al‐Hassan S, Al‐Rejjal R, Awartani K. The correlation between endometrial thickness and outcome of in vitro fertilization and embryo transfer (IVF‐ET) outcome. Reproductive Biology and Endocrinology 2008;2(6):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baigent 2003
- Baigent C, Patrono C. Selective cyclooxygenase 2 inhibitors, aspirin, and cardiovascular disease: a reappraisal. Arthritis and Rheumatism 2003;48(1):12‐20. [DOI] [PubMed] [Google Scholar]
Brown 2017
- Brown J, Crawford TJ, Allen C, Hopewell S, Prentice A. Nonsteroidal anti‐inflammatory drugs for pain in women with endometriosis. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD004753.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical Tools for Network Meta‐Analysis in STATA. PLoS One 2013;8(10):e76654. [DOI: 10.1371/journal.pone.0076654] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaouat 2002
- Chaouat G, Zourbas S, Ostojic S, Lappree‐Delage G, Dubanchet S, Ledee N, et al. A brief review of recent data on some cytokine expressions at the materno‐foetal interface which might challenge the classical Th1/Th2 dichotomy. Journal of Reproductive Immunology 2002;53(1‐2):241‐56. [DOI] [PubMed] [Google Scholar]
Cheong 2013
- Cheong YC, Dix S, Hung Yu Ng E, Ledger WL, Farquhar C. Acupuncture and assisted reproductive technology. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD006920.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fanchin 1998
- Fanchin R, Righini C, Olivennes F, Taylor S, Ziegler D, Frydman R. Uterine contractions at the time of embryo transfer alter pregnancy rates after in‐vitro fertilization. Human Reproduction 1998;13(7):1968‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fanchin 2001
- Fanchin R. Assessing uterine receptivity in 2001: ultrasonographic glances at the new millennium. Annals of the New York Academy of Sciences 2001;943:185‐202. [PUBMED: 11594540] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to November 2016. Hamilton (ON): McMaster University (developed by Evidence Prime).
Green 2001
- Green GA. Understanding NSAIDS: From aspirin to COX‐2. Clinical Cornerstone 2001;3(5):50‐9. [DOI] [PubMed] [Google Scholar]
Hawkey 2003
- Hawkey CJ, Langman MJ. Non‐steroidal anti‐inflammatory drugs: overall risks and management. Complementary roles for COX‐2 inhibitors and proton pump inhibitors. Gut 2003;52(4):600‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kuczynski 2001
- Kuczynski W, Dhont M, Grygoruk C, Grochowski D, Wolczynski S, Szamatowicz M. The outcome of intracytoplasmic injection of fresh and cryopreserved ejaculated spermatozoa‐‐a prospective randomized study. Human Reproduction (Oxford, England) 2001;16(10):2109‐13. [DOI: 10.1093/humrep/16.10.2109] [DOI] [PubMed] [Google Scholar]
Marjoribanks 2015
- Marjoribanks J, Ayeleke R, Farquhar C, Proctor M. Nonsteroidal anti‐inflammatory drugs for dysmenorrhoea. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD001751.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Motteram 2015
- Motteram C, Vollenhoven B, Hope N, Osianlis T, Rombauts LJ. Live birth rates after combined adjuvant therapy in IVF‐ICSI cycles: a matched case‐control study. Reproductive Biomedicine Online 2015;30(4):340‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Siristatidis 2016
- Siristatidis CS, Basios G, Pergialiotis V, Vogiatzi P. Aspirin for in vitro fertilisation. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD004832.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Speroff 2005
- Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th Edition. Philadelphia: Lippincott Williams and Wilkins, 2005. [Google Scholar]
Stone 2002
- Stone S, Khamashta MA, Nelson‐Piercy C. Nonsteroidal anti‐inflammatory drugs and reversible female infertility: is there a link?. Drug Safety 2002;25(8):545‐51. [DOI: ] [DOI] [PubMed] [Google Scholar]
Vannuccini 2018
- Vannuccini S, Luisi S, Tosti C, Sorbi F, Petraglia F. Role of medical therapy in the management of uterine adenomyosis. Fertility and Sterility 2018;109(3):398‐405. [DOI: 10.1016/j.fertnstert.2018.01.013] [DOI] [PubMed] [Google Scholar]


