Abstract
Cfr is a radical S-adenosylmethionine (SAM) RNA methylase linked to multi-drug antibiotic resistance in bacterial pathogens. It catalyzes a chemically challenging C-C-bond forming reaction to methylate C8 of A2503 [Escherichia coli (Ec) numbering) of 23S ribosomal RNA during ribosome assembly. The cfr gene has been identified as a mobile genetic element in diverse bacteria and in the genome of select Bacillales and Clostridiales species. Despite the importance of Cfr, few representatives have been purified and characterized in vitro. Here we show that Cfr homologs from Bacillus amyloliquefaciens, Enterococcus faecalis, Paenibacillus lautus, and Clostridioides difficile act as C8 adenine RNA methylases in biochemical assays. C. difficile Cfr contains an additional Cys-rich C-terminal domain that binds a mononuclear Fe2+ ion in a rubredoxin-type Cys4 motif. The C-terminal domain can be truncated with minimal impact on C. difficile Cfr activity, but turnover is diminished upon disruption of the Fe2+ binding site by Zn2+ substitution or ligand mutation. These findings indicate an important purpose for the observed C-terminal iron in the native fusion protein. Bioinformatic analysis of the C. difficile Cfr Cys-rich domain shows that it is widespread (~1000 homologs) as a stand-alone gene in pathogenic or commensal bacilli and clostridia, with >10% encoded adjacent to a predicted radical-SAM RNA methylase. Although the domain is not essential for in vitro C. difficile Cfr activity, genomic co-occurrence and high abundance in the human microbiome suggests a possible functional role for a specialized rubredoxin in certain radical-SAM RNA methylases with relevance to human health.
Graphical Abstract

INTRODUCTION
Radical S-adenosylmethionine (SAM) enzymes compose one of the largest enzyme superfamilies defined to date, with more than 400,000 members.1 Radical SAM enzymes are essential in primary anaerobic metabolism and in the synthesis of diverse secondary metabolites.2, 3 Nucleic acid modification is a third central function of radical SAM proteins, and this activity has been demonstrated in all three domains of life.4, 5 A pair of adenine RNA methylases, RlmN and Cfr, are among the most widespread and well-characterized radical SAM nucleic acid modification enzymes in bacteria.6–8 Although originally discovered to methylate C2 of A2503 in ribosomal RNA (rRNA), a base located in the peptide exit tunnel of the peptidyltransferase center of the bacterial ribosome, RlmN also modifies six additional tRNA substrates at A37.9, 10 In Escherichia coli, RlmN is non-essential, but its activity improves translational fidelity and organism fitness.10 More than 20,000 RlmN homologs have been identified in the sequenced genomes of prokaryotes of diverse origin.1 Related Cfr enzymes are smaller in number, with approximately 200 homologs annotated in Interpro and other protein sequence databases. Cfrs were initially found encoded as mobile genetic elements in staphylococcal pathogens,9 and Staphylococcus aureus (Sa) Cfr has been characterized extensively in vitro. The enzyme methylates C8 of A2503 in 23S rRNA, and this modification confers resistance to multiple classes of antibiotics.11 Although quite relevant to human health, few of the other Cfrs have been characterized in vitro, and the structure of the enzyme is not known.
The common methyl donor SAM is the source of the newly appended methyl carbon in RlmN and Cfr.6 In their function as C-methylases, RlmN and Cfr perform a chemically challenging C-C bond forming reaction involving functionalization of inert sp2-hybridized C-H bonds.12 This activity cannot be accomplished by the standard polar mechanism characteristic of SAM- dependent methyl transferases. Instead, radical chemistry is required and enabled here by a second SAM-dependent activity in RlmN and Cfr – transient formation of a potently oxidizing 5’-deoxyadenosyl radical (5’-dA•).6, 7, 15 Nearly all enzymes in the radical SAM superfamily share this intermediate,13, 16 generated by reduction of a SAM-coordinated [4Fe4S]2+ cluster that, in turn, triggers reductive cleavage of the coordinated cosubstrate. In RlmN and Cfr, the 5’-dA• abstracts a hydrogen atom from a Cys-appended −CH3 group (mC338, Sa Cfr numbering) to yield a methylene radical (Figure 1).7 Radical addition to the adenine nucleobase at either C2 or C8 generates a transient protein-RNA crosslink containing an unpaired electron on the adenine nucleobase.17 Proton transfer from C2/C8 to an active site base, a second conserved Cys residue (C105, Sa Cfr numbering), resolves the crosslink.14, 18, 19 This step is proposed to yield a thiyl radical at the C-terminal C338, which must be reduced prior to subsequent turnover.14 Interestingly, several other redox-neutral radical SAM C-C bond forming enzymes also oxidize an active site Cys side chain12 to balance the initial two-electron reductive cleavage of SAM.12, 20 In these systems, auxiliary iron-sulfur cluster or cobalamin-binding domains are often present and proposed to provide necessary reducing equivalents.12 The RlmN and Cfr homologs characterized to date are distinct in that they do not harbor an obvious universal dedicated thiyl radical reductant.
Figure 1. Proposed mechanism of C8 adenine methylation by Cfr.7, 13, 14.
Schematic diagram of the catalytic Cys residues located in the Cfr RNA binding pocket (gray box) and the A2503 rRNA target nucleobase.
The proposed mechanism of the RNA methylases has been validated through spectroscopic and biochemical analysis of E. coli RlmN and S. aureus Cfr.7, 14, 17, 21 The two key catalytic Cys residues (C338 and C105) are strictly conserved in these homologs and all others identified to date. X-ray crystal structures of E. coli RlmN have been solved, both alone22 and in complex with tRNA,18 the latter obtained by trapping the covalent protein-RNA intermediate via substitution of C118 with a non-functional alanine (C105A equivalent).18, 22 To date, no structures have been reported of either enzyme in complex with a ribosomal RNA substrate. Additionally, Cfr homologs have not been structurally characterized in any form, even though such information would be useful in the design of inhibitors that could rescue the activities of existing antibiotics to which Cfr currently confers bacterial resistance.
To identify Cfr candidates suitable for ongoing structural studies, we performed phylogenetic analyses of Sa Cfr homologs identified in database searches. As reported previously, at least four distinct groups of enzymes emerge, including a clostridial clade classified as Cfr-like.23 Cfr-like homologs from Clostridium sporogenes23 and Clostridium phytofermantas24 have been proposed to harbor a different, still undefined, activity because they fail to confer antibiotic resistance and lack C8 methylation activity in primer extension assays when overexpressed in E. coli.23, 24 Here we show that heterologously expressed and purified Cfr homologs from Bacillus amyloliquefaciens (Ba), Enterococcus faecalis (Ef), Paenibacillus lautus (Pl), and Clostridioides difficile (Cd) are capable of rRNA methylation in vitro. All four enzymes contain a full occupancy [4Fe-4S] cluster in their wild-type (wt) forms, as established by Mössbauer spectroscopy and other analytical techniques. All four homologs modify A2503 at C8 but with different rates. The enzymes also vary in their capacity for subsequent C2 methylation of the same base. C. difficile Cfr is active as a C8 methylase with limiting RNA substrate but exhibits distinctly slower methylation when challenged with excess RNA. Interestingly, this Cfr homolog contains a unique ~50-amino-acid Cys-rich C-terminal extension related by sequence to a much larger family of clostridial and streptococcal proteins of unknown function. In Cd Cfr, this domain binds a mononuclear Fe2+ ion in a 4-Cys binding site with a midpoint potential [Fe2+/Fe3+ couple] of +105 mV vs. NHE, similar to those of rubredoxin electron transfer proteins. Substitution of the C-terminal metal binding site with Zn2+, or Cys ➔ Ala mutation of one of the predicted Fe2+ ligands, significantly impedes turnover. However, complete removal of the C-terminal domain has no effect on in vitro activity. While only a few select Cfr homologs contain a Cys-rich C-terminal fusion, genome neighborhood analysis of the larger family of stand-alone domains reveals that >10% are encoded next to clostridial radical SAM enzymes. The findings suggest that these iron-binding rubredoxin-like proteins are widespread in Clostridiales and, to a lesser extent, Bacillales organisms, including pathogens and human commensals. While the metal-binding domain is dispensable for in vitro Cfr activity in C. difficile, an essential role for this specialized rubredoxin (or another protein partner) in vivo could explain the reported lack of RNA methylation activity in other clostridial Cfr enzymes.
MATERIALS AND METHODS
Materials.
Kanamycin was obtained from Teknova (Hollister, CA). Ampicillin was purchased from Dot Scientific (Burton, MI). N-(2-hydroxyethyl)piperazine-N′−2-ethanesulfonic acid (HEPES), DNaseI (RNase-free), Bradford reagent, and His-Pur Co2+-TALON resin were obtained from Thermo Fisher Scientific (Waltham, MA). Sodium sulfide, 2-mercaptoethanol, sodium dithionite, glucose, S-adenosylmethionine, and p1 nuclease from Penicillium citrinum were purchased from Sigma Aldrich (St. Louis, MS). Iron (III) chloride hexahydrate was acquired from BDH Analytical Chemicals, VWR (Radnor, PA). DL-dithiothreitol (DTT) was obtained from Alfa Aesar (Tewksbury, MA). 57Fe metal (98%) was purchased from Isoflex USA (San Francisco, CA). Bovine serum albumin (BSA), XhoI, NdeI, antarctic phosphatase, Q5 DNA polymerase, and T4 DNA ligase were obtained from New England Biolabs (Ipswich, MA). PD-10 and HiPrep Sephacryl 16/60 S200 columns were purchased from GE Healthcare (Little Chalfont, United Kingdom).
Phylogenetic analysis of Cfrs.
Homologs were identified by BLASTp analysis using Sa Cfr as the query against the non-redundant protein sequence database (NCBI, accessed March 2018) with default search parameters. The top 500 matches were aligned in MUSCLE after consolidation with ExPASy (<98% minimum identity filter in the decrease redundancy tool) to remove duplicate sequences. The final pool contained 361 unique sequences.25, 26 The program MEGA727 was used to compute a phylogenetic tree using the minimum evolution method with the JTT model of substitution, allowance of pairwise deletions, and closest neighbor set to level = 2. Bootstrap values were computed from 1000 replicates.
Cloning and overexpression of full-length Cfr proteins.
DNA sequences encoding genes for the Cfrs selected for study (Table S1) (with the exception of Cd Cfr) were codon-optimized for over-expression in E. coli, synthesized, and inserted into a pMK-T vector (GeneArt). PCR primers (Table S1) were designed with Primer3 and appended at the 5’-end with NdeI and XhoI restriction sites. The coding sequences were amplified by PCR using Q5 DNA polymerase. The PCR products were digested using NdeI and XhoI restriction enzymes, purified by gel electrophoresis, re-isolated using an Omega Biotek gel extraction kit, and ligated into pET26b. The C. difficile cfr overexpression plasmid was constructed using the Gibson assembly method.28 The gene was codon optimized for overexpression in E. coli and synthesized in two pieces, each containing 20 base-pair regions that overlap with the pET26b vector and with each other. The gene and linearized vector were combined with a 5’-exonuclease, DNA polymerase, and DNA ligase to allow for pET26b-cfr assembly. All four pET26b-cfr sequences were verified by Sanger sequencing at the Pennsylvania State University Nucleic Acid Facility, and the coding regions can be found in Table S1 of the Supporting Information. All plasmids additionally encode a C-terminal His6-tag with a short two amino acid (-Leu-Glu-) linker.
The C363A Cd Cfr variant was generated by inverse PCR using a new forward primer containing the relevant substitution in the annealing region (Table S1). Amplification was performed using the pET26b/Cfr (Cd) plasmid (generated as described above) as the DNA template. DpnI digestion was used to remove the original template DNA and the vector was circularized by ligation with T4 DNA ligase.
Overexpression of all constructs was accomplished in E. coli BL21(DE3) cells co-transformed with pET26b-cfr and pDB1282,29 the latter a vector containing inducible iron-sulfur cluster assembly machinery. Successful transformants were selected on kanamycin (50 μg/mL) and ampicillin (100 μg/mL) supplemented LB plates. 1.5 L cultures of the overexpression strains were grown shaking (180 rpm) at 37 °C in M9 minimal medium supplemented with kanamycin (50 μg/mL), ampicillin (100 μg/mL), iron (III) chloride (30 μM), and L-cysteine (300 μM) to maximize iron-sulfur cluster incorporation.30 Growths for samples suitable for Mössbauer analysis utilized 57Fe dissolved in 1 M H2SO4. At an OD600 = 0.3, L-arabinose was added [final concentration 0.08 % (w/v)] to induce overexpression of the genes harbored on the pDB1282 plasmid. At an OD600 = 0.6, IPTG was added to a final concentration of 100 μM to induce overexpression of the pET26b-cfr. After incubation overnight at 18 °C at 180 rpm, cells were pelleted by centrifugation at 5000g for 10 min at 4 °C. Approximately 3.2 g cell paste was obtained per liter of culture. Cell paste was flash-frozen in liquid nitrogen and stored at −80 °C. Flavodoxin and flavodoxin reductase were purified as described previously.30
Purification of full-length Cfrs.
All purification steps were performed anaerobically in an anoxic chamber with a mixed H2/N2 atmosphere (Coy Laboratory Products). Cells were resuspended in lysis buffer (50 mM HEPES pH 7.5, 300 mM KCl, 4 mM imidazole, 10 mM MgCl2, 10% (v/v) glycerol, 10 mM β-mercaptoethanol) in a nickel-coated beaker containing 1 mg/mL lysozyme, 100 μg/mL DNase, and 1 mM phenylmethylsulfonyl fluoride (PMSF). The suspension was stirred at room temperature for 30 min and lysed by sonication (QSonica Q500, 10 s pulse, 30 s rest, 60% amplitude) on ice. The resulting cell debris was pelleted by centrifugation at 11,300g at 4 °C for 1.25 h in a sealed centrifuge bottle. The supernatant was applied to Co2+-TALON resin. Protein fractions were eluted in 50 mM HEPES, pH 7.5, 300 mM KCl, 300 mM imidazole, 10 mM MgCl2, 30% (v/v) glycerol, 10 mM β-mercaptoethanol. Reconstitution of [4Fe4S]2+ clusters was performed on ice for all samples except Cs Cfr. Protein samples (200 μM) were exchanged into gel-filtration chromatography buffer [10 mM HEPES pH 7.5, 500 mM KCl, 5 mM DTT, 10 mM MgCl2, 25% (v/v) glycerol] and incubated for 30 min at room temperature. Iron (III) chloride (800 μM) was added to the solution, which was subsequently incubated for 1 h at room temperature. Sodium sulfide was added to the solution (to a final concentration of 800 μM) in six equal volumes over the course of 2 h. The solution was allowed to incubate on ice overnight. A PD-10 column was used to remove excess iron and sulfide. Size-exclusion chromatography was performed using a Sephacryl S200 column. Protein concentrations were determined by Bradford analysis with correction factors (Molecular Structural Facility at UC Davis) of 0.891 (Cd), 0.627 (Ba), 0.828 (Ef), and 0.687 (Pl). Iron-sulfur cluster incorporation (Table 2) was assessed by measuring the number of iron equiv per protein with the ferrozine assay.31
Table 2.
Iron incorporation for Cfr homologs analyzed by Mössbauer spectroscopy.
| Homolog | Fe/protein | Cluster | Area of subspectrum (%) | Cofactor/protein |
|---|---|---|---|---|
| Clostridioides difficile | 4.4 | [4Fe-4S]2+ Fe2+ Cys4 |
77 22 |
0.8 1.0 |
| Enterococcus faecalis | 4.2 | [4Fe-4S]2+ | 97 | 1.0 |
| Paenibacillus lautus | 3.9 | [4Fe-4S]2+ | 94 | 0.9 |
| Bacillus amyloliquefaciens | 4.6 | [4Fe-4S]2+ | 100 | 1.2 |
Overexpression and purification of truncated and single-site Cd Cfr variant proteins.
Truncated Cd Cfr variants consisting of either the radical SAM domain (Cd-N), residues 1–345, or the rubredoxin/KTR domain (Cd-C), residues 346–416, were generated using the approach described above (see Table S1 for primer sequences). A C363A variant (M) of full-length Cd Cfr was generated as described. Overexpression and purification of all variant proteins was performed as described for the wt enzymes. Zinc replacement of the C-terminal metal binding site in Cd-C was accomplished by addition of ZnCl2 (7 mM) to minimal medium cultures both upon initial inoculation and at OD600 ~ 0.6 in place of iron supplementation. Both iron- and zinc-bound versions of Cd-C were overexpressed without pDB1282 present, and the iron-sulfur cluster reconstitution step was omitted. Iron and zinc occupancies were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) at the Pennsylvania State University Laboratory for Isotopes and Metals in the Environment (LIME). Cd-C contains 0.45 equiv iron, Cd-Z contains 0.36 equiv zinc, and full-length Cd C363A contains 2.53 equiv iron.
Synthesis and purification of RNA.
To generate a 155 nucleotide (nt) fragment of Ec 23S rRNA corresponding to A2454-G2608 (Figure S1), previously shown to be efficiently methylated by Sa Cfr,5 a DNA template was amplified using primers 2454 and 2608 (Table S1), and transcribed in a reaction with T7 RNA polymerase (500 μg/mL). The transcription reaction was performed in 30 mM Tris, pH 8.4, 20 mM DTT, 36 mM MgCl2, 0.01% (w/v) Triton X-100, 2 mM spermidine, 2 mM NTPs (4 mM GTP). The template DNA PCR product was diluted to a final concentration of 5 ng/uL and incubated for 3 h at 37 °C with the components above. DNaseI was added to the solution (to a final concentration of 0.005 U/μL) and incubated at 37 °C for an additional hour. EDTA (pH 8.5) was added to a final concentration of 50 mM, and the solution was frozen at −20 °C for at least 2 h. Full-length 155mer RNA transcripts were purified under anaerobic conditions (Coy Laboratory Products) by PD-10 column equilibrated in filtered nanopure water. RNA concentrations were determined by UV-visible spectrophotometry (ε260 = 1.87 μm−1 cm−1).17 The A2503G variant RNA was prepared similarly, except the first PCR step was performed with a synthetic template (Integrated DNA Technologies, see Supporting information for sequence) containing the desired base change.
RNA methylation activity assays with limiting substrate.
In experiments using a protein-based low-potential reducing system [flavodoxin (Flv), flavodoxin reductase (Flx), and NADPH], RNA methylation was assayed in a total volume of 150 μL at room temperature in an anoxic glovebox (Coy Laboratory Products) over a time course of 2.75 h. Samples contained 48 μM Cfr and 50 μM 155mer RNA in 100 mM sodium EPPS, pH 8.0, 10 mM MgCl2, 12.5% (v/v) glycerol, 250 mM KCl, 200 μM Flv, 20 μM Flx, and 2 mM NADPH. The solution was incubated at room temperature for 5 min before initiating the reaction by addition of SAM to a final concentration of 1 mM. At designated time points, a 10 μL aliquot of the reaction solution was removed and added to 10 μL of quench solution containing 250 μM tryptophan and 95 mM H2SO4. Quenched reactions were diluted 1:1 in P1 nuclease buffer (250 mM sodium acetate, pH 6.0, 45 mM NaCl, 4 mM ZnCl2). Antarctic phosphatase (10 U) and P1 nuclease (1 U) were added to digest the RNA into individual nucleosides. The digestion reactions were incubated at 37 °C overnight and subsequently analyzed by liquid chromatography (Agilent 1960/1990) coupled to mass spectrometry (LC-MS) (Agilent 6460 QQQ) (LC-MS). Assay mixtures were separated on an Agilent Extend-C18 column (4.6 × 50 mm) equilibrated in a 40 mM ammonium acetate, 5% (v/v) methanol (solvent A) / 100% methanol (solvent B) system using the conditions listed in Table 3 below.
Table 3.
Liquid chromatography elution gradient.
| Time (min) | A | B |
|---|---|---|
| 0.0 – 5.0 | 95% | 5% |
| 5.0 – 6.5 | 87% | 13% |
| 6.5 – 7.0 | 73% | 27% |
| 7.0 – 8.0 | 47% | 53% |
| 8.0 – 12.0 | 95% | 5% |
MS detection of products (Figure S2) and assignment of peaks were accomplished as described previously.21 Results were analyzed with the MassHunter software package, and quantitative analyses were facilitated by comparison to a standard curve calculated from data acquired from the tryptophan internal standard. Unproductive cleavage of SAM was determined by dividing the number of SAM cleavage events, obtained from the concentration of 5’-dA in LC-MS samples, by the estimated number of methylation events ([m8A] + 2 • [m2,8A]). Six full-length and four variant Cfr proteins were tested for methylase activity. Assays using Cd Cfr in which the C-terminal domain of Cd Cfr (Cd-C) was removed (giving Cd-N) are indicated as such in activity measurement plots (48 μM protein). Cd-C was also added back separately at a 1:1 ratio. These samples are renamed Cd Fe res (iron-bound Cd-C) or Cd Zn res (zinc-bound Cd-C) in plots. All assays were performed at least three times on different days. Figures show the average product concentration for three selected experiments, with an error bar showing the standard deviation in these values. The assay metrics in Table S2 are the average ± standard deviation over the selected trials.
Time courses for experiments using limiting substrate for Ba, Ef, and Pl Cfrs were initially simulated in KinTek Explorer52 with the following model.
| (1) |
In this model, enzyme (E) and substrate (RNA) bind and react to form the first product, m8a (EM). This monomethylated compound can be further converted to m2,8a (EP), as shown above. Both steps are considered to be irreversible. In initial simulations, we found that the best fit for k1 (kobs m8a) falls below a lower limit set by the steady-state turnover rate (v/[E]) determined in subsequent experiments with excess RNA. This discrepancy indicated that the simple kinetic model in (1) is not sufficient. However, in the absence of additional experimental data to constrain the rates of other steps (substrate binding, product release, etc.), we instead fixed k1 to the lower limit stated above, obtained from fits of m8a production with excess RNA to a single exponential function (described below). We then used KinTek Explorer to simulate the reaction to obtain the apparent rate constant (k2 or kobs m2,8a) for the second methylation step. Note that in our model, the rate constant for the second dimethylation reaction includes, in addition to the catalysis step, m8a product release and substrate rebinding steps. The fits we obtained are reasonable but, given these complexities, this k2 value should be viewed as a lower limit for the rate of the second methylation reaction. For Ba/Ef/Pl Cfr, the active enzyme fraction was allowed to vary and fell below 48 μM for ideal performance in the simulation. Because the limiting-substrate time course of Cd Cfr and its variants yields little dimethylated product, product vs. time traces were fit to a single exponential function to extract the apparent reaction rate (kobs m8a) with equation (2), in which a is the product concentration at completion and k is equivalent to kobs for m8a formation. We assumed a fully active enzyme pool in this analysis.
| (2) |
RNA methylation activity assays with excess substrate.
Activity assays with excess substrate were performed as described above but with 100 μM 155mer RNA substrate and 3 μM Cfr enzyme. The reaction solutions were prepared in 150 μL total volume with 100 mM sodium EPPS, pH 8, 10 mM MgCl2, 12.5% (v/v) glycerol, 250 mM KCl, 200 μM Flv, 20 μM Flx, and 2 mM NADPH. Reactions were initiated by addition of 1 mM SAM. Time courses were analyzed via linear fit to the initial portion of the progression curve for m8a formation to obtain the initial velocities of the reactions. This approach allowed us to obtain a reaction rate, v/[E], for each Cfr enzyme and variant (Table S3). A single exponential fit, using equation (2), to the full m8a time course allowed us to obtain the amplitude of each enzymatic reaction. This value reflects the total amount of methylated product generated by each enzyme for the entire experiment. The information was used to determine a total turnover number (TON, Table S3), after division by the initial enzyme concentration (3 μM). The goal of the latter analysis was to ascertain whether each enzyme is capable of performing more than one full substrate conversion.
Spectroscopic analysis of Cfr proteins.
Reconstituted 57Fe protein samples were transferred to EPR tubes or Mössbauer cups and flash-frozen in liquid nitrogen in an anoxic chamber (Coy Laboratory Products). Mössbauer spectra were recorded on constant acceleration Mössbauer spectrometers (SEE Co., Edina, MN) equipped either with a Janis SVT-400 variable-temperature cryostat (weak-field) or a Janis 8TMOSS-OM-12SVT variable-temperature cryostat (strong-field) (Janis, Woburn, MA). All isomer shifts are quoted relative to the centroid of the spectrum of α-iron metal at room temperature. Simulations of Mössbauer spectra were carried out using WMOSS (SEE Co., Edina, MN). Some of the simulations are based on the spin Hamiltonian formalism (see equation below), in which the first term describes the electron Zeeman effect, the second and third terms describe the axial and rhombic zero-field splitting of the total electron spin ground state, the fourth term represents the interaction between the electric field gradient and the nuclear quadrupole moment, the fifth term describes the magnetic hyperfine interactions of the 57Fe nucleus with respect to the total electron spin ground state, and the last term represents the nuclear Zeeman interactions of 57Fe. All symbols are conventional.32 Spectra were calculated in the slow relaxation limit.
EPR samples were analyzed on a Bruker ESP 300 spectrometer (Billerica, MA) equipped with an ER 041 MR microwave bridge and an ER 4116DM resonator.
UV-visible spectrophotometry.
Oxidation of the Cd Cfr C-terminal domain was analyzed spectrophotometrically using an Agilent 8453 G1103A spectrometer. In an anoxic glovebox, a 50 μL sample of 9.4 mM Cd-C [10 mM HEPES pH 7.5, 500 mM KCl, 5 mM DTT, 10 mM MgCl2, 25% (v/v) glycerol] was diluted to 1 mL in water and sealed in a screw cap quartz cuvette (Starna Cells, Inc). The anoxic protein solution was scanned from 190 nm to 1100 nm with a baseline correction at 800 nm. The sample was oxidized by opening the cuvette and pipetting the sample intermittently to introduce oxygen into the solution. The cuvette remained open for the duration of the experiment. The solution was scanned at the following times: 1 min, 2.5 min, 5 min, 7.5 min, 10 min, 20 min, and 30 min. A difference spectrum was calculated by subtracting the initial anoxic spectrum from the final spectrum.
Protein film voltammetry.
Square-wave (SW) voltammetry experiments were performed on a VersaSTAT 3 (Princeton Applied Research) attached to a three-electrode electrochemical cell housed in an anaerobic chamber (Coy Laboratory Products). Protein films were generated via incubation of 9.4 mM Cd-C [10 mM HEPES pH 7.5, 500 mM KCl, 5 mM DTT, 10 mM MgCl2, 25% (v/v) glycerol] on a pyrolytic graphite edge (PGE) working electrode for 10 min at room temperature. The working electrode surface was prepared by polishing the electrode surface with 0.3 μm alumina slurry (Allied High Tech) followed by washing in nanopure H2O in a sonication bath for 6 min. The electrode was dried and brought into the glovebox. All subsequent steps were performed under anoxic conditions. The background current was measured with the bare PGE working electrode, Ag/AgCl reference electrode (Pine Research), and Pt wire auxiliary electrode in 50 mM HEPES, pH 7.5, 300 mM KCl. SW voltammograms were collected using a step potential of 10 mV, a pulse amplitude of 25 mV, and a SW frequency of 10 Hz. A current range gate of 20 μA was applied and a 1 kHz filter was used as a background filter. A significant decrease in current amplitude was observed between the first and second scans with smaller decreases evident upon successive scans.
Genome neighborhood network (GNN) and isoelectric point analysis of cysteine-rich KTR proteins.
The complete FASTA file for the cysteine-rich KTR family was downloaded from Interpro (IPR025957) and used to generate sequence similarity networks (SSNs) with the EFIEST server33 via default parameters. An SSN calculated with an alignment score of 31 was used to make a genome neighborhood network with the EFI-GNT server34 (±10 ORFs, ≥ 5% co-occurrence). Results were visualized and analyzed in Cytoscape.
To calculate the predicted isoelectric points of cysteine-rich KTRs, the full FASTA file for IPR025957 was pruned with the ExPASy decrease redundancy tool (<98% minimum identity), and the resulting FASTA file was used as input to the protein isoelectric point calculator web server (http://isoelectric.org/).35 The results for each individual KTR were averaged to obtain the overall predicted isoelectric point for the entire family.
Calculating enzyme abundances from metagenomic analysis.
The EFI-CGFP/shortBRED webtool was used to determine the abundance of KTRs in metagenomic samples obtained as part of the Human Microbiome Project (HMP).33, 36–40 The KTR SSN described above (alignment score = 31) was used as the input file for marker identification (UniRef90 database, CD-hit identity = 85, DIAMOND pairwise comparison algorithm). Marker quantification with USEARCH in 380 healthy microbiome datasets yielded a genome-normalized median protein abundance heatmap. The dataset compares the relative quantity of KTR coding sequences (separated by cluster) per microbial genome at six different body sites. To highlight the relative abundance of radical SAM-adjacent KTR proteins in gut microbiome samples, boxplots for clusters 1–3 were generated in Kaleidagraph using the genome-normalized median protein abundance. The shaded area represents the median quartile (Q2), and the error bars extend to the first (Q1) and third (Q3) quartiles. Data points outside of these quartile limits are plotted as open circles.
RESULTS
Selection of phylogenetically diverse Cfr candidates for biochemical and biophysical characterization.
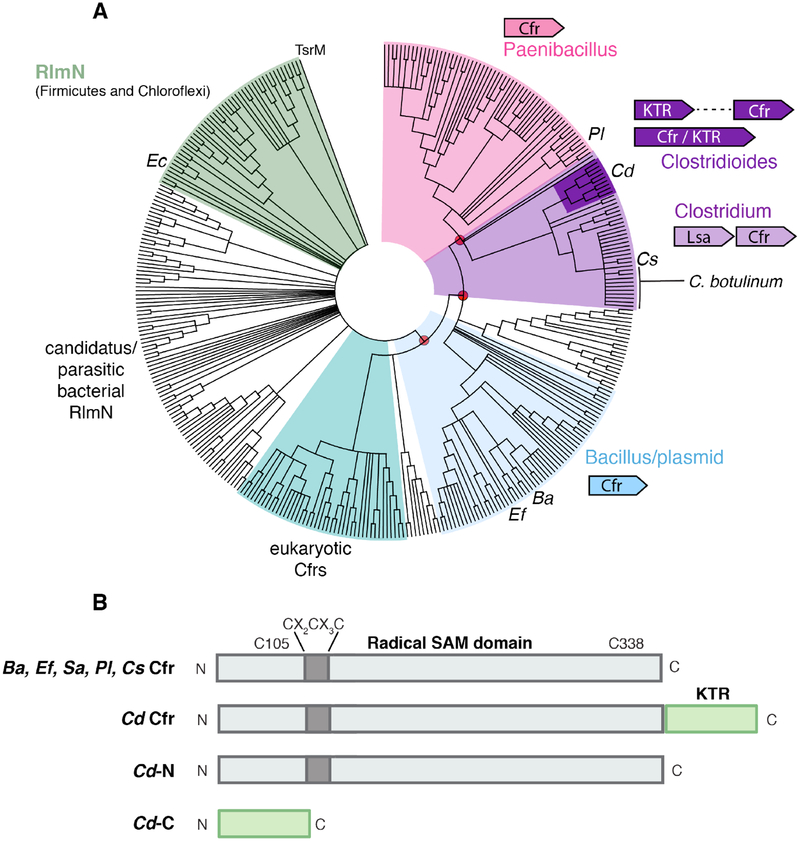
To identify Cfrs of diverse origin for biochemical and biophysical studies, we performed BLAST searches of the NCBI non-redundant protein sequence database using Sa Cfr as a query. The resulting set of 361 representative sequences was used to construct a minimum evolution phylogenetic tree (Figure 2A) with an unrelated radical SAM class B methylase, TsrM,13 as the outgroup. The initial search included two groups of annotated RlmN enzymes that share 37% sequence identity with Sa Cfr but belong to distinct sequence clusters. The more distantly branched RlmN group is composed of sequences from Firmicutes and Chloroflexi organisms. The second group includes putative RlmN sequences from Candidatus phyla and obligate intracellular parasites. Annotated Cfrs cluster into a eukaryotic group and two different bacterial clades, as described previously.24, 41 Plasmid-encoded Cfrs with validated C8 methylation activity from staphylococcal clinical isolates9 belong to a group that also includes genomically-encoded Bacillales sequences42 and plasmid-encoded Cfr proteins from Enterococcus facaelis (Ef) and other enterococcal pathogens. From the latter group, we selected a Ef plasmid-borne Cfr and a genomic sequence from a mesophilic strain of B. amyloliquefaciens (Ba) for study here. The similarity (~75%, Table 1) of these sequences to Sa Cfr suggests that the Ef and Ba proteins are likely to have the same activity, methylation of rRNA at A2503 C8. In support of this hypothesis, several antibiotic-resistant enterococcal clinical isolates have recently been demonstrated to harbor a transposable element with a Cfr that is 99% sequence identical to Ef Cfr from strain TX0635 analyzed here.43–46 Cfr homologs from an alternative Ba strain (92% identity to the Cfr from DSM-7 ATCC 23350 targeted in this work), B. clausii, and B. brevis all confer multidrug antibiotic resistance when expressed heterologously in E. coli AS19, a strain with high antibiotic susceptibility.42 In the latter systems, the mechanism of drug resistance has been directly linked to methylation of A2503, confirmed in a primer extension analysis of total rRNA from an rlmN knockout of E. coli following induction of Bacillales Cfr expression.42 In all of these studies, conversion of A2503 to the singly- and di- methylated forms was not confirmed in vitro with purified proteins or analyzed quantitatively.
Figure 2. Selection of phylogenetically diverse Cfr enzymes for biochemical characterization.
(A) Minimum evolution phylogenetic tree containing 361 representative sequences obtained by NCBI BLAST. The sequence of Sa Cfr was the initial query. Bootstrap values (1000 replicates) were calculated for branch support and red circles denote values > 85% in selected branches. Dominant genera and the genomic context in each cluster are indicated in colored text/schematics. (B) Cartoon diagram of the domain architecture in Cfrs selected for study. Locations of conserved Cys residues (Sa numbering) in the radical SAM domain are shown.
Table 1.
Sequence identity for Cfr homologs tested for RNA methylation.
| Homolog | Bacillus amyloliquefaciens (Ba) | Enterococcus faecalis (Ef) | Paenibacillus lautus (Pl) | Clostridioides difficile (Cd) | Clostridium sporogenes (Cs) |
|---|---|---|---|---|---|
| % ID (Sa Cfr) | 75.8 | 74.1 | 51.5 | 55.7 | 57.2 |
The second bacterial clade is dominated by genomic sequences from Firmicutes and partitioned into three distinct groups. One of these clusters is composed largely of sequences from the Paenibacillus genus.23 It includes a representative [Paenibacillus lautus sp. Y412MC10 (Pl)] originally isolated from the Obsidian hot spring at Yellowstone National Park.47 This Pl Cfr sequence was selected for characterization, owing to the utility of thermotolerant proteins in structural and biophysical studies. Many of the remaining sequences are found in Clostridial organisms. Some of these proteins have been annotated as Cfr-like and are postulated to be functionally divergent from Sa Cfr.24 Our analysis shows that this subset breaks down into two smaller groups that consist of genomically-encoded Cfrs from a set of pathogenic Clostridium difficile (Cd) strains, now reclassified to the Clostridioides genus, and a larger group of Cfrs from more diverse clostridial organisms. The second group includes Cfr sequences from C. sporogenes and C. phytofermentas recently demonstrated to lack rRNA methylation activity.23, 24 We purified a Cfr from C. sporogenes and validated this finding but further characterization of the enzyme was not feasible owing to low soluble expression in E. coli.
Intriguingly, five members of the Cd Cfr cluster have an extended C-terminal motif (Fig. 1B). A BLAST search of the corresponding amino acid sequence shows similarity to an InterPro family (IPR025957)1 designated “cysteine-rich KTR” with more than 1400 members identified as of May 2019. The group was initially named for a common internal Arg-Thr-Lys sequence, although among the current set of IPR025957 sequences, this motif is not universally conserved. Members are most often found as stand-alone proteins approximately 60 amino acids in length, with two additional strictly conserved CXXC motifs. To date, none of these proteins has been functionally characterized in purified form. Cys-rich KTRs are found almost exclusively in Firmicutes, most in the order Clostridales (968 annotated genes). A Cfr-KTR fusion sequence from Clostridioides difficile str. QCD-63q4248 was selected for in vitro characterization in this study. In evaluating the activity and properties of this fusion enzyme, we sought to understand whether it lacks known Cfr activity upon heterologous production in E. coli, as shown for the C. sporogenes and C. phytofermentans homologs. In work by other laboratories, a C. difficile Cfr homolog has been shown to confer antibiotic resistance via A2503 modification, but the activity was promoted by a cfr on a mobile genetic element that shares only 52% sequence identity with our chromosomally-encoded Cd homolog.49, 50 A Clostridium boltae Cfr-KTR fusion has also been shown to confer moderate drug resistance in cell-based assays.51 Neither of these proteins has been purified and characterized in vitro, however. In our work, we also aim to elucidate the function and properties of the distinctive C-terminal Cys-rich domain in our selected Cd enzyme.
Diverse Cfr homologs exhibit RNA methylation activity.
To test for activity in the new Cfr homologs and probe possible functions of the fused Cys-rich domain, we viewed two types of activity measurements as likely to be most informative. We assessed (i) sequential mono- and di-methylation of the relevant adenine in a previously reported 155mer RNA substrate derived from 23S rRNA under conditions of high enzyme concentration and limiting substrate and (ii) catalytic monomethylation of the same base/substrate under conditions of low enzyme concentration and excess RNA. The RNA substrate is a transcribed 155mer of 23S rRNA substrate (nt 2454–2608) from an Ec rRNA template. Ec rRNA has been used to characterize the activity of heterologous Cfr enzymes, both in assays with purified protein/RNA components7 and in cells.11 We elected to use the same substrate to facilitate comparison to Sa Cfr enzymes. The sequence identities of these rRNA fragments to the corresponding region of native host organism 23S rRNA range from 87 to 90% (Figure S1). Reactions were initiated by addition of excess SAM to reaction mixtures containing NADPH and a protein-based Ec flavodoxin/flavodoxin reductase (Flv/Flx) system for reduction of the [4Fe-4S]2+ cluster of Cfr.52, 53 In LC-MS analyses of the digested RNA products in both assays, new peak(s) can be assigned to C8-methyladenosine (m8a) or C2,C8-dimethyl-adenosine (m2,8a) based on retention times, m/z values, and MS/MS fragmentation (Figure 3A and Figure S2).6, 7 Assignment of the methylated adenine products to A2503 was confirmed by comparison to an A2503G RNA substrate in Ba and Cd Cfr assays. The AàG variant yields negligible turnover (Figure S3). Methylation of A2503 by Pl Cfr was demonstrated in a previous study.23
Figure 3. Methylation of a 155mer rRNA substrate by Cfr enzymes under limiting substrate conditions initiated with a protein-based reductant.
Reactions used the Ec Flv/Flx/NADPH reducing system in mixtures containing 100 mM EPPS, pH 8, 10 mM MgCl2, 12.5 % glycerol, 250 mM KCl, 200 μM Flv, 20 μM Flx, 2 mM NADPH, 1 mM SAM, 50 μM 155mer RNA, and 48 μM protein. (A) LC-MS analysis of methylated RNA products via single-ion monitoring at m/z = 282.1 (bottom trace, m8A) and m/z = 296.1 (top trace, m2,8A). Time course experiment monitoring formation and decay of singly-methylated m8A (solid bars) and doubly methylated m2,8a (patterned bars) with (B) Ba Cfr, (C) Ef Cfr, (D) Pl Cfr, and (E) Cd Cfr. Error bars correspond to standard deviation of three averaged trials.
HPLC-MS analysis of the limiting substrate reactions (i) revealed detectable levels of mono-methylated and dimethylated products for three of the four enzymes (Figure 3B–D). The quantities of the two products were consistent with sequential reactions. Build-up of the m2,8a product initially lagged while the m8a product grew in, and the m2,8a product then accumulated significantly as the m8a product was depleted (Figure 3B–D). In general, these enzymes resemble Sa Cfr in their ability to catalyze sequential mono- and dimethylation reactions, albeit with varying efficiencies. The Cd homolog is an outlier in that it produced very little of the dimethylated product (Figure 3E).
In the limiting substrate time course, Ba Cfr accumulates the greatest amount of singly and dimethylated product and exhibits the fastest initial rate of catalysis for both reactions (Figure 3B). The kinetic profiles of the Ba, Ef, and Pl enzymes were analyzed54 to extract kobs for the first methylation step (Figure S4 and Table S2). The rate (0.23 min−1) and extent of Ba Cfr C8 methylation (~80 % substrate consumption within 20 min of initiation) is comparable to previously reported values for Sa Cfr analyzed under similar (limiting RNA) conditions (0.7 ± 0.2 min−1 with >80% of the substrate consumed within the first 5 min of the reaction).55 By contrast, the Pl and Ef enzymes exhibit rates of C8 methylation that are 5- to 10-fold lower (0.07–0.14 min−1), while the Cd Cfr reaction proceeds even more slowly (0.02 min−1) (Figure 3E, Table S2). To understand whether uncoupled SAM consumption could account for these differences, we quantitatively analyzed the 5’-dA and SAH byproducts (Table S2). All enzymes exhibited a near 1:1 ratio of SAH:methylated product. Abortive SAM cleavage was assessed by comparing the amount of methylated RNA produced to the 5’-dA coproduct (Figure S5). All of the homologs tested exhibited slightly elevated levels of 5’-dA (1.3–1.7), suggesting modest uncoupling under these conditions. However, the similarities between the enzymes indicate that abortive SAM cleavage cannot account for the differences in initial methylation rate. Nevertheless, the Ba enzyme is the most efficiently coupled with a 1.3:1 ratio of 5’-dA:methylated product. Under limiting substrate conditions, with the exception of Ba Cfr, none of the enzymes consumed the rRNA substrate completely (Table S2). This outcome could be due to a number of different factors. It may arise from use of a non-native RNA substrate in our assays. It could also reflect a propensity for enzyme inactivation or product inhibition.
In the second type of activity assay (ii), we incubated low concentrations of enzyme (3 μM) with excess RNA substrate (100 μM) at room temperature (Figure 4A–D). In these reactions, the m2,8a dimethylated product was barely detectable (Figure S6), implying that none of the Cfr homologs methylate the RNA processively and that the first C8 methylation site is vastly preferred over the second, C2. The excess substrate assays show that all enzymes exhibit the capacity to undergo more than one m8 methylation event, with 2–14 conversions observed within the ~3 h assay window (Table S3). All are appreciably slower than Sa Cfr (0.24 ± 0.06 min−1 when assayed under similar multi-turnover conditions)19 with the exception of Ba Cfr (v/[E] = 0.23 min−1) (Figure S6, Table S3). The Cd enzyme is the slowest catalyst in this assay, with an m8 methylation rate (v/[E]) that is 5–30% that of the other homologs and the lowest level (~ 5%) of total RNA substrate consumption. In Cd Cfr, we also considered that the low rate of in vitro turnover with excess substrate could be linked to the presence of the extra C-terminal domain. To address this question, we characterized the function and properties of this domain and analyzed the activity of truncated variants.
Figure 4. Methylation of a 155mer rRNA by Cfr enzymes with excess substrate in reactions initiated with a protein-based reductant.
Reactions used the Ec Flv/Flx/NADPH reducing system in mixtures containing 100 mM EPPS, pH 8, 10 mM MgCl2, 12.5 % glycerol, 250mM KCl, 200 μM Flv, 20 μM Flx, 2 mM NADPH, 1 mM SAM, 100 μM 155mer RNA, and 3 μM protein. Time course experiment monitoring formation and decay of singly-methylated m8A with (A) Ba Cfr, (B) Ef Cfr, (C) Pl Cfr, and (D) Cd Cfr. Error bars correspond to standard deviation of three averaged trials. The inset in panel D shows that Cd Cfr can promote multiple turnovers (red line).
Spectroscopic analysis of Cfrs reveals an additional iron-binding site in Cd Cfr.
In order to verify the [4Fe4S] cluster content and interrogate the capacity of the Cd Cfr C-terminal domain to bind iron, we examined Cfr homologs by Mössbauer spectroscopy (Figure 5). Spectra from Ba, Ef, and Pl Cfr are dominated by a single quadrupole doublet with parameters consistent with [4Fe4S]2+ clusters (δ = 0.44–0.45 mm/s and ΔEQ = 1.11 mm/s in each case).56 From the relative area of the subspectrum associated with [4Fe-4S]2+ clusters and the amount of Fe/protein after reconstitution, the number of clusters per protein can be estimated.30 After reconstitution, Ba, Ef, and Pl Cfr contain 4.6, 4.2, and 3.9 Fe per protein, corresponding to 1.1, 1.0, and 0.9 [4Fe4S]2+ clusters per protein, respectively (Table 2). In addition to the features of a [4Fe4S] cluster (δ = 0.45 mm/s and ΔEQ = 1.14 mm/s), the Mössbauer spectrum of Cd Cfr contains a second quadrupole doublet feature with parameters (δ = 0.70 mm/s and ΔEQ = 3.08 mm/s), similar to those of a Fe2+-Cys4 rubredoxin center.56 We propose that this Fe2+ center is coordinated by the four conserved cysteine residues in the C-terminal KTR region of the protein (vide supra). Analysis of all four Cfrs by electron paramagnetic resonance spectroscopy did not result in any detectable signals for the as-isolated reconstituted proteins, thus ruling out assembly of any iron-sulfur clusters with an S = 1/2 ground state in these samples.
Figure 5. Mössbauer spectra of Cfr enzymes.
Left panel: comparison of reconstituted samples of Cfr from Bacillus amyloliquefaciens (Ba), Enterococcus faecalis (Ef), Paenibacillus lautus (Pl), and Clostridioides difficile (Cd). Spectra of N-terminal and C-terminal truncations of Cd Cfr are also shown; features of the [4Fe4S]2+ cluster and ferrous rubredoxin-like site are denoted by red and blue dashed lines, respectively. Right panel: Mössbauer spectra of the C-terminal domain of Cd Cfr in varying applied magnetic fields. Experimental data are displayed as black vertical bars, with simulations overlaid (blue solid lines).
To test the hypothesis that the [4Fe4S]2+ cluster and rubredoxin-like center in Cd Cfr reside in the N-terminal and C-terminal domains, respectively, N-terminal (residues 1–345, Cd-N) and C-terminal (residues 346–416, Cd-C) fragments of Cd Cfr were produced. Mössbauer spectra of these truncated proteins (Figure 5) unambiguously demonstrate that the [4Fe4S] cluster resides in the N-terminal radical SAM domain, whereas the ferrous rubredoxin-like center resides in the C-terminal cysteine-rich KTR domain. In order to gain additional insight into the electronic structure of the rubredoxin-like species in Cd Cfr, we examined Cd-C using Mössbauer spectroscopy at 4.2 K in variable externally applied magnetic fields (Figure 5). The spectra can be simulated with the following parameters and assuming a S = 2 electron spin ground state: D = 5.7 cm−1, E/D = 0.2, δ = 0.70 mm/s, ΔEQ = 3.08 mm/s, η = 0.9, and A/gNβN = [−14.4, −8.3, −23.3] T, similar to those previously reported for ferrous rubredoxin centers coordinated by four cysteines.56, 57
The Cd Cfr C-terminal domain is not essential for RNA methylation activity but functions less efficiently in the absence of Fe2+.
The truncated version of Cd Cfr, Cd-N, lacking the C-terminal Cys-rich domain, is as efficient in m8a production as full-length Cd Cfr in experiments using the Flv/Flx/NADPH reducing system with limiting RNA (Figure 6A–B, Figure S7A). Attempts to modulate activity by addition of the isolated Fe2+-loaded C-terminal domain in trans (Cd Fe res) did not yield any significant difference in product formation. To investigate the functional role of the Fe2+ center in the Cd Cfr C-terminal domain, a variant of one of the putative metal-binding residues in the CXXC motif, a Cys363 ➔ Ala substitution (C363A, renamed Cd M), was generated in the full-length enzyme. Unexpectedly, this substitution alters iron-sulfur cluster stability, giving a sample with < 3 equiv Fe/protein after reconstitution, as determined by ICP-OES analysis. Similar cluster instability is precedented in [4Fe4S]2+ DNA repair enzymes upon mutation of an auxiliary metal binding site Cys ligand.58, 59 As a consequence, the C363A Cd Cfr enzyme exhibits negligible activity. A Zn2+-loaded version of the C-terminal domain was also tested (Cd Zn res) for rescue of RNA methylation activity. Inclusion of the mismetallated Cd-C domain gives a slight decrease in activity in limiting substrate assays (Figure 6B, Figure S7A). We also evaluated the activity of variant forms of Cd Cfr with excess RNA (3 μM enzyme, 100 μM 155mer rRNA) (Figure 6C). The trends observed in the limiting substrate assays are largely maintained, with little effect on endpoint product formation upon truncation of Cd Cfr. However, at early time points, a detectable lag in product formation is evident in the rescue experiment (Figure S8). Interestingly, the impact of Zn2+-substitution in the C-terminal domain is magnified with excess substrate. We observe only a small amount of product after 2.75 h (Figure 6C, Figure S7B), underscoring the initial findings from single turnover assays. Disruption of the C-terminal domain iron-binding site via ligand mutation or metal ion substitution impedes turnover.
Figure 6. Methylation of a 155mer rRNA substrate by variant Cd Cfr enzymes under limiting substrate and excess substrate conditions and initiated by a protein-based reductant.
Reactions were performed with the Ec Flv/Flx/NADPH reducing system in mixtures containing 100 mM EPPS, pH 8, 10 mM MgCl2, 12.5 % glycerol, 250 mM KCl, 200 μM Flv, 20 μM Flx, and 2mM NADPH. (A) Limiting substrate LC-MS analysis of methylated RNA products via single-ion monitoring at m/z = 282.1 (bottom trace, m8A) and m/z = 296.1 (top trace, m2,8A). Cd-N = radical SAM domain only, Cd-C = C-terminal KTR domain with Fe2+ or Zn2+, Cd-M = C363A variant (full-length), rescue (res) experiments contain Cd-C added to Cd-N in trans. Endpoint analysis (165 min) of the formation of singly-methylated m8A under (B) limiting substrate and (C) excess substrate conditions. Error bars correspond to standard deviation of three averaged trials.
The isolated Cd Cfr C-terminal domain exhibits rubredoxin-like redox properties.
Although our activity assays do not indicate an essential role in in vitro catalysis for the metal-bound C-terminal domain in Cd Cfr, particularly under limiting substrate conditions, we speculated that the domain could mediate electron transfer (ET) steps in the rRNA methylation reaction. This function may be dispensable with a non-native reductant in vitro but more critical in vivo (e.g., with a clostridial protein-based redox donor). Our Mössbauer experiments revealed a Rdx-type Cys4-coordinated Fe2+ center in the version of this protein isolated from an E. coli heterologous host and expressed under iron-replete conditions in minimal medium. Given that Rdx proteins typically serve as high-potential electron-transfer shuttles that operate by cycling between Fe2+ and Fe3+ oxidation states,60 we sought to characterize the redox properties of as-isolated Cd-C. Anaerobic isolation of Cd-C yields a clear/bluish protein solution that is exclusively Fe2+ by Mössbauer analysis. Exposure of the domain to O2 results in a visible color change to a red solution that, when monitored spectrophotometrically, exhibits absorption bands at 370, 485, and 582 nm (Figure 7A). These spectral features resemble those of oxidized stand-alone Rdx proteins.61
Figure 7. Electrochemical and UV-visible characterization of Cd C-terminal domain.
(A) UV-visible difference spectrum of the oxidation of the C-terminal domain of Cd Cfr. Absorbance maxima are visible at 370 nm, 485 nm, and 582 nm. (B) Square-wave voltammogram of KTR domain of Cd Cfr. The midpoint potential is +106 mV. (C) Size exclusion chromatogram illustrating the interaction of Cd-N and Cd-C through co-elution. (D) Comparison of the C-terminal domain of Cd Cfr (Cd-C), the KTR from Cb, and the rubredoxin from Cp. Metal coordinating cysteine pairs are highlighted in yellow, the structurally important residues directly following are highlighted in green, the positively charged residues are colored blue, and the negatively charged residues are colored red. The number of positively and negatively charged residues (bottom, numbers in parentheses) differentiates cys-rich KTRs from typical rubredoxins.
Previously characterized Rdx proteins also exhibit reduction potentials in the −50 to +50 mV range (vs. NHE).60 To measure the Fe2+/3+ midpoint potential of the isolated Cd-C domain, protein films were generated on pyrolytic edge graphite (PGE) electrodes under anaerobic conditions by incubation of protein [9.4 mM in 10 mM HEPES pH 7.5, 500 mM KCl, 5 mM DTT, 10 mM MgCl2, 25% (v/v) glycerol] on the electrode surface for 10 min. Modified electrodes were transferred to an electrochemical cell (50 mM HEPES, pH 7.5, and 300 mM KCl buffer), and evaluated by square-wave (SW) voltammetry. Voltammograms obtained at a SW frequency of 10 Hz showed a quasi-reversible waveform with a midpoint potential of +106 mV vs. NHE (Figure 7B). The relatively high midpoint potential compared to prototypical Rdx proteins can be rationalized by differences in the predicted charge of Cd-C at pH 7.5. Cd-C has a calculated pI of 9.2, compared to ~4.0 for a canonical Rdx. At physiological pH, Rdx would be largely negative while Cd-C would have a net positive charge. This scenario would destabilize the Fe3+ state in Cd-C, rendering the protein more difficult to oxidize and resulting in a higher midpoint potential. Interestingly, subsequent electrochemical scans of the isolated domain show loss of current amplitude over time, suggesting that the domain may not be stable in the oxidized form. In the presence of O2, Cd-C visibly precipitated over the 30 min time period in which the protein was monitored electrochemically. These observations could preclude a role for the Fe2+ center as an explicit electron donor in Cfr catalysis. However, the stability/reduction potential may be further modulated via interaction with the radical SAM domain of Cd Cfr and/or the RNA substrate. The protein could also require binding to the protein reducing system prior to ET to ensure rapid re-reduction to the Fe2+ oxidation state.
Homologs of the Cd C-terminal domain are widespread as stand-alone proteins in Clostridiales.
Spectroscopic and electrochemical analyses of the isolated Cd Cfr C-terminal domain suggest properties similar to Rdx ET proteins. Although traditional Rdx proteins are similar in length (~60 aa) and contain identical CXXC metal binding motifs separated by ~20–30 amino acids, the overall sequence identity between the Cd-C domain and a model Rdx from C. pasteurianum is only 30% (Figure 7D). Cp Rdx (and other Rdx proteins with validated function/properties) additionally contain a conserved Gly immediately adjacent to the C-terminal Cys in each metal binding motif. This sequence pattern enables key H-bonds to form between peptide backbone N-H groups and the metal-coordinated thiolates of Cys residues. These interactions can influence Rdx reduction potential.62 Interestingly, Cd-C deviates from this sequence pattern significantly because it contains a Lys residue immediately adjacent to each CXXC motif. In general, Cd-C has a high proportion of positively charged residues, accounting for its aforementioned pI > 9, while Cp Rdx has a net negative charge at neutral pH. These observations leave open the possibility that while Cd-C functionally resembles Rdx proteins, it may adopt a distinct structure.
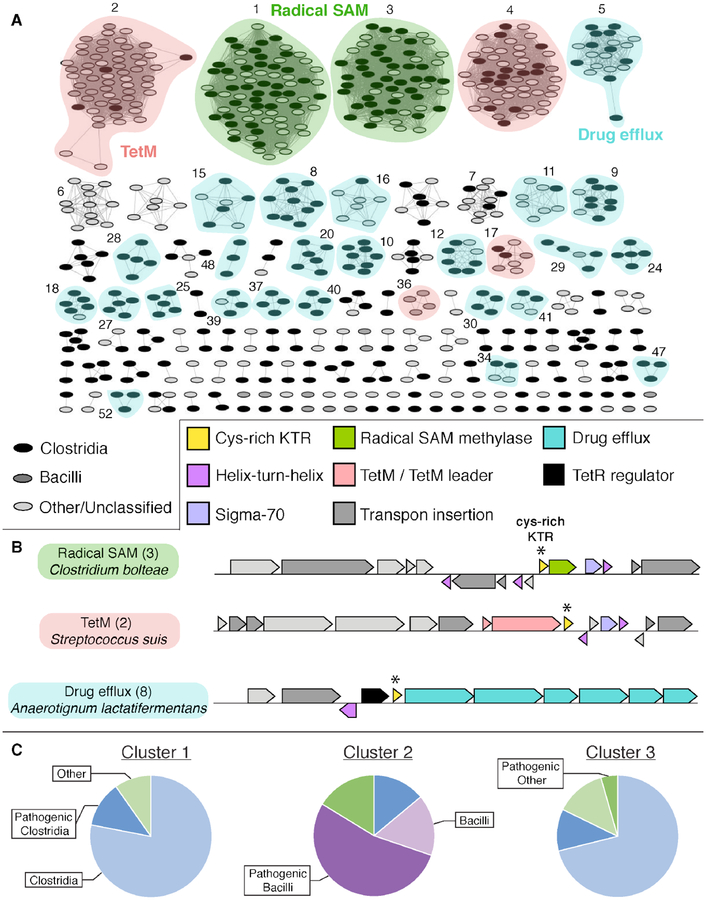
Our initial identification of the Cd-C Fe2+-binding domain via sequence inspection revealed homology to a large group of bacterial stand-alone proteins annotated as Cys-rich KTR proteins. The latter naming scheme comes from an Arg-Thr-Lys sequence that resides in between the two strictly conserved CXXC motifs. Additionally, analysis of the Cys-rich KTR sequence group shows that the stand-alone proteins are also dominated by positively charged residues, resulting in an average isoelectric point of 8.6. Our discovery of an essential Fe2+ binding site in the Cfr-fused version of this domain raises the possibility that the larger family of stand-alone Cys-rich KTR proteins might have the same property, with widespread functional significance for the metal-bound form. To gain insight into this possibility, we analyzed patterns in genome neighbors (± 10 open reading frames) for the entire InterPro family.33 The resulting sequence similarity network revealed that 13% of the Cys-rich KTR family can be found in two large clusters (133 sequences, clusters 1 and 3 in Fig. 8A). For the 85 of the 133 sequences from organisms with fully sequenced genomes, 84 are encoded directly adjacent to a putative RNA methylase. As in the Cd Cfr fusion enzyme studied here, nearly all of these Cys-rich KTRs are from organisms belonging to the clostridiales order (Table S4). This subset of Cys-rich KTR proteins may function similarly to Cd-C, sharing its requirement for Fe2+ and exhibiting Rdx-type redox properties. Interestingly, the organisms with paired Cfr-KTR gene clusters identified in this analysis are commonly found as commensal human gut microbiome constituents (Table S4). We further validated this observation by analyzing the healthy human microbiome metagenome abundance of Cys-rich KTR genes via the Enzyme Function Initiative chemically-guided functional profiling (CGFP) web tool (Figure 9).35–37 The KTR genes associated with clusters 1 and 3 are significantly enriched in human stool microbiome metagenomes. These patterns could indicate a reservoir of Cys-rich KTR-Cfr genes in the beneficial gut flora to protect against antibiotic exposure with the possibility of transfer to pathogens.
Figure 8. Sequence similarity and genome neighborhood network analyses of stand-alone Cys-rich KTR proteins.
(A) SSN of IPR025957 constructed with an alignment score of 31. Nodes are arranged in an organic layout and each represents a single sequence. Nodes are shaded by host organism and colored by co-occurring gene type. Clusters without numbering represent groups that do not fall into one of the three major gene type categories. Additional clusters numbering two or less are not shown. (B) Representative gene clusters from each major category as determined by GNN analysis (±10 ORFs, ≥5% co-occurrence). Host organism and cluster number are displayed (left). KTR proteins are marked by a “*” symbol. (C) Breakdown by organism type in each of the first three SSN clusters. Clostridia = blue, Bacilli = purple, and other = green. Pathogenic organisms are highlighted with dark shading and non-pathogens are shown in light shading.
Figure 9. Metagenome abundance of KTR proteins in healthy human microbiome samples.
(A) Heatmap for clusters 1–10 in the KTR SSN (see Fig. 8) showing marker abundance in 380 HMP datasets collected from six different areas of the body. Cluster 4 is omitted due to low abundance in the metagenomic sample set. (B-D) Box plots of the per-site abundance for the top three clusters. This analysis shows that the KTR proteins encoded adjacent to radical SAM enzymes are abundant in the gut microbiome.
DISCUSSION
In this study, we establish that four different Cfr enzymes from distinct phylogenetic groups assemble a necessary [4Fe4S]2+ cofactor and exhibit adenine C8 RNA methylation activity in vitro, similar to the well-characterized Cfr homolog from S. aureus. Under the assay conditions tested, three of the four enzymes analyzed exhibit both mono- and di-methylation activity. We also show that a genomically encoded Cfr from a Bacillales organism consumes substrate at a comparable rate to Sa Cfr with both limiting and excess RNA. These results establish that diverse Cfr homologs are viable targets for structural and biophysical study.
In vitro characterization of a clostridial Cfr homolog fused to a Cys-rich C-terminal domain also reveals an active mono-methylase with limiting substrate. However, with a >30-fold excess of RNA substrate, the clostridial fusion enzyme is markedly less active. Combined with the unexpected detection of a rubredoxin-like Fe2+ binding site in the C-terminal domain of Cd Cfr, these findings prompted a more detailed investigation of the properties of this module. Complete removal of the domain fails to either increase or decrease the rate of RNA methylation in reactions initiated by a protein-based reducing system, but disruption of the Fe2+ binding site by ligand mutagenesis or Zn2+ substitution decreases turnover. While the effect of Zn2+ is modest with limiting substrate, the mismetallated enzyme is nearly inactive when challenged with excess RNA. Our spectroscopic and electrochemical analysis of the stand-alone domain suggests a similarity to rubredoxin-type electron transfer cofactors, often described as a particularly minimalist member of the iron-sulfur cluster cofactor family.
Many radical SAM enzymes harbor additional iron-sulfur clusters with diverse functions, including electron transfer.63, 64 The redox-neutral carbon methylation catalyzed by RlmN/Cfr enzymes shares in common with other radical SAM-mediated C-C bond forming systems a requirement for reductive quenching of a substrate radical at the end of the reaction.12 In other superfamily members, auxiliary cofactors or redox-active amino acids in the active site can mediate controlled provision of redox equivalents.65, 66 In Cfr and RlmN, a conserved C-terminal Cys residue is oxidized to a thiyl radical to facilitate product release (Figure 1).14, 18 The midpoint potential of the Fe2+ cofactor in the Cd Cfr C-terminal rubredoxin-like domain could enable its use as an electron donor to regenerate the Cys 355 thiolate for subsequent methylation.12 We note, however, that the domain is not universally conserved as a Cfr neighbor. Therefore, a KTR-rubredoxin cannot be the sole mechanism of C355 reduction and other means to quench the radical must exist in other Cfr/RlmNs. Diversity in reductant usage has precedent in other oxidoreductase enzymes. For example, class III ribonucleotide reductases have adapted to use either protein-based (thioredoxin or ferredoxin) or small molecule (formate) reducing systems to resolve an oxidized active site Cys disulfide or a thiosulfuranyl radical.67–69 Also, we cannot rule out other roles for the KTR-rubredoxin such as RNA-binding or structural stabilization. However, our work shows that the Cd-N truncated protein is stable and active in the absence of the C-terminal domain in vitro.
The Cd Cfr rubredoxin-like domain may also serve as an intermediary or coordinator of Cfr reaction initiation via interaction with protein reducing partners. The reduction potential of the Cd-C domain is likely too high (+100 mV vs NHE) to donate an electron to the [4Fe4S]2+ radical SAM cluster directly, even though a similar role was recently proposed for a stand-alone CSL-family Zn finger protein, Dph3, encoded adjacent to a non-canonical radical SAM enzyme, diphthamide biosynthesis protein Dph2.70, 71 Dph3 is the only other example of a biochemically characterized mononuclear metal binding protein found as a genome neighbor to a radical SAM enzyme. The Fe2+-bound form of Dph3 was reported to function in iron-sulfur cluster reduction in Dph2, initiating the reaction in the absence of reductant. However, the redox potential of Dph3 has not been experimentally determined, and no known rubredoxin-type electron transfer protein has a sufficiently low reduction potential (< −400 mV) for activation of a radical SAM [4Fe4S] cluster.72
Our findings also raise the possibility that reported inactivity in RNA methylation in heterologously expressed Cfrs from clostridial clades could be due to lack of a necessary accessory factor or redox partner. The Cys-rich KTR domain is a candidate for such a factor, although this protein is not essential for activity in Cd Cfr and it is not found universally adjacent to the inactive clostridial enzymes. Genome analysis of the remainder of the clostridial Cfr subgroup reveals a second conserved neighbor, an Lsa ABC-F ribosomal protection protein. Our query shows 100% co-occurrence of clostridial cfr genes with either a KTR or an Lsa neighbor. Lsa has not been structurally characterized in complex with rRNA, but another ABC-F protein, VmlR, binds to the fully-assembled 70S ribosome to prompt a structural change that drives drug release from the peptidyl transferase center.73 While Sa-type Cfrs methylate A2503 solely in a protein-free 23S rRNA context,5 clostridial Cfrs could instead modify fully assembled 70S ribosomal rRNA following Lsa remodeling. Such an approach would be particularly efficient, eliminating the fitness cost of pre-emptively methylating nascent ribosomes.
The genome context of other Cys-rich KTR orthologs suggests the protein may additionally play a more generic role in nucleic acid binding or antibiotic resistance. Apart from the clostridial Cys-rich KTR group found adjacent to Cfr radical SAM enzymes, the second most frequent gene neighbor is TetM, a ribosomal protection protein that confers antibiotic resistance by triggering a conformational change in the tetracycline binding site to release the drug.74 Interestingly, this group of KTR homologs is almost exclusively associated with known bacterial pathogens originating from Clostridiales and Bacillales. The remaining KTR proteins are often found near drug efflux systems, with the majority annotated as ABC family transporters or MATE/MFS effluxers. All Cys-rich KTR genes are proximal to transposable elements including helix-turn-helix proteins (HTH3/HTH16), RNA polymerase σ70 components, and TetR-like repressors.75 These patterns suggest KTRs are transferable elements, consistent with a general role in antibiotic resistance. In the other GNN clusters, a functional role for Fe2+ and Rdx-like activity is not immediately evident, although it is interesting to note that the protein families listed above all exhibit some overlap with the biological function of Cfr in drug resistance. The prevalence of Cys-rich KTR genes in pathogens and in members of the human microbiome warrants further investigation of their biological function and metal ion requirements.
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by the National Institutes of Health (GM10011 and GM119707 to A.K.B. and GM122595 to S.J.B.) and the National Science Foundation (Grant No. DGE1255832 to R.J.M.) We thank Erica Schwalm for technical assistance/provision of reagents and J. Martin Bollinger Jr. for assistance with data analysis and critical reading of the manuscript. We also acknowledge the resources of the EFI-CGFP and thank Lauren Rajakovich for assistance with metagenome abundance data analysis.
Footnotes
ACCESSION CODES
WP_002578771 - Clostridioides difficile str. QCD63q42
WP_015735625 - Paenibacillus lautus sp. Y412MC10
WP_013351126 - Bacillus amyloliquefaciens str. DSM-7 (ATCC 23350)
WP_002405682 - Enterococcus faecalis str. TX0635
AIT41446 - Staphylococcus aureus (plasmid)
WP_003483120 - Clostridium sporogenes str. ATCC 15579
Supporting information includes supplementary tables S1–S4, and supplementary figures S1–9.
REFERENCES
- [1].Finn RD, Attwood TK, Babbitt PC, Bateman A, Bork P, Bridge AJ, Chang HY, Dosztanyi Z, El-Gebali S, Fraser M, Gough J, Haft D, Holliday GL, Huang H, Huang X, Letunic I, Lopez R, Lu S, Marchler-Bauer A, Mi H, Mistry J, Natale DA, Necci M, Nuka G, Orengo CA, Park Y, Pesseat S, Piovesan D, Potter SC, Rawlings ND, Redaschi N, Richardson L, Rivoire C, Sangrador-Vegas A, Sigrist C, Sillitoe I, Smithers B, Squizzato S, Sutton G, Thanki N, Thomas PD, Tosatto SC, Wu CH, Xenarios I, Yeh LS, Young SY, and Mitchell AL (2017) InterPro in 2017-beyond protein family and domain annotations, Nucleic Acids Res 45, D190–D199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Frey PA, Hegeman AD, and Ruzicka FJ (2008) The radical SAM superfamily, Crit Rev Biochem Mol Biol 43, 63–88. [DOI] [PubMed] [Google Scholar]
- [3].Broderick JB, Duffus BR, Duschene KS, and Shepard EM (2014) Radical S-adenosylmethionine enzymes, Chem Rev 114, 4229–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Landgraf BJ, McCarthy EL, and Booker SJ (2016) Radical S-adenosylmethionine enzymes in human health and disease, Annu Rev Biochem 85, 485–514. [DOI] [PubMed] [Google Scholar]
- [5].Kimura S, and Suzuki T (2015) Iron-sulfur proteins responsible for RNA modifications, Biochim Biophys Acta 1853, 1272–1283. [DOI] [PubMed] [Google Scholar]
- [6].Yan F, LaMarre JM, Röhrich R, Wiesner J, Jomaa H, Mankin AS, and Fujimori DG (2010) RlmN and Cfr are radical SAM enzymes involved in methylation of ribosomal RNA, J Am Chem Soc 132, 3953–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Grove TL, Benner JS, Radle MI, Ahlum JH, Landgraf BJ, Krebs C, and Booker SJ (2011) A radically different mechanism for S-adenosylmethionine-dependent methyltransferases, Science 332, 604–607. [DOI] [PubMed] [Google Scholar]
- [8].Bauerle MR, Schwalm EL, and Booker SJ (2015) Mechanistic diversity of radical S-adenosylmethionine (SAM)-dependent methylation, J Biol Chem 290, 3995–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kehrenberg C, Schwarz S, Jacobsen L, Hansen LH, and Vester B (2005) A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503, Mol Microbiol 57, 1064–1073. [DOI] [PubMed] [Google Scholar]
- [10].Benítez-Páez A, Villarroya M, and Eugenia Armengod M (2012) The Escherichia coli RlmN methyltransferase is a dual-specificity enzyme that modifies both rRNA and tRNA and controls translational accuracy, RNA 18, 1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, and Vester B (2006) The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics, Antimicrob Agents Chemother 50, 2500–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yokoyama K, and Lilla EA (2018) C–C bond forming radical SAM enzymes involved in the construction of carbon skeletons of cofactors and natural products, Nat Prod Rep 35, 660–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Blaszczyk AJ, Silakov A, Zhang B, Maiocco SJ, Lanz ND, Kelly WL, Elliott SJ, Krebs C, and Booker SJ (2016) Spectroscopic and Electrochemical Characterization of the Iron-Sulfur and Cobalamin Cofactors of TsrM, an Unusual Radical S-Adenosylmethionine Methylase, J Am Chem Soc 138, 3416–3426. [DOI] [PubMed] [Google Scholar]
- [14].Silakov A, Grove TL, Radle MI, Bauerle MR, Green MT, Rosenzweig AC, Boal AK, and Booker SJ (2014) Characterization of a cross-linked protein-nucleic acid substrate radical in the reaction catalyzed by RlmN, J Am Chem Soc 136, 8221–8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yan F, and Fujimori DG (2011) RNA methylation by radical SAM enzymes RlmN and Cfr proceeds via methylene transfer and hydride shift, Proc Natl Acad Sci U S A 108, 3930–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Broderick WE, Hoffman BM, and Broderick JB (2018) Mechanism of radical initiation in the radical S-adenosyl-L-methionine superfamily, Acc Chem Res 51, 2611–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grove TL, Livada J, Schwalm EL, Green MT, Booker SJ, and Silakov A (2013) A substrate radical intermediate in catalysis by the antibiotic resistance protein Cfr, Nat. Chem. Biol 9, 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schwalm EL, Grove TL, Booker SJ, and Boal AK (2016) Crystallographic capture of a radical S-adenosylmethionine enzyme in the act of modifying tRNA, Science 352, 309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bauerle MR, Grove TL, and Booker SJ (2018) Investigation of Solvent Hydron Exchange in the Reaction Catalyzed by the Antibiotic Resistance Protein Cfr, Biochemistry 57, 4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ruszczycky MW, Zhong A, and Liu HW (2018) Following the electrons: peculiarities in the catalytic cycles of radical SAM enzymes, Nat Prod Rep 35, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grove TL, Radle MI, Krebs C, and Booker SJ (2011) Cfr and RlmN contain a single [4Fe-4S] cluster, which directs two distinct reactivities for S-adenosylmethionine: methyl transfer by SN2 displacement and radical generation, J Am Chem Soc 133, 19586–19589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boal AK, Grove TL, McLaughlin MI, Yennawar NH, Booker SJ, and Rosenzweig AC (2011) Structural basis for methyl transfer by a radical SAM enzyme, Science 332, 1089–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Atkinson GC, Hansen LH, Tenson T, Rasmussen A, Kirpekar F, and Vester B (2013) Distinction between the Cfr methyltransferase conferring antibiotic resistance and the housekeeping RlmN methyltransferase, Antimicrob Agents Chemother 57, 4019–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stojkovic V, Noda-Garcia L, Tawfik DS, and Fujimori DG (2016) Antibiotic resistance evolved via inactivation of a ribosomal RNA methylating enzyme, Nucleic Acids Res 44, 8897–8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity, BMC Bioinformatics 5, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaguir K, Redaschi N, Rossier G, Xenarios I, and Stockinger H (2012) ExPASy: SIB bioinformatics resource portal, Nucleic Acids Res 40, W597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kumar S, Stecher G, and Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets, Mol Biol Evol 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gibson DG (2011) Enzymatic assembly of overlapping DNA fragments, Methods Enzymol 498, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Frazzon J, and Dean DR (2003) Formation of iron-sulfur clusters in bacteria: an emerging field in bioinorganic chemistry, Curr Opin Chem Biol 7, 166–173. [DOI] [PubMed] [Google Scholar]
- [30].Lanz ND, Grove TL, Gogonea CB, Lee KH, Krebs C, and Booker SJ (2012) RlmN and AtsB as models for the overproduction and characterization of radical SAM proteins, Methods Enzymol 516, 125–152. [DOI] [PubMed] [Google Scholar]
- [31].Stookey LL (1970) Ferrozine — a new spectrophotometric reagent for iron, Anal Chem 42, 779–781. [Google Scholar]
- [32].Münck E (2000) Physical methods in bioinorganic chemistry: spectroscopy and magnetism, University Science Books, Sausalito, CA. [Google Scholar]
- [33].Gerlt JA, Bouvier JT, Davidson DB, Imker HJ, Sadkhin B, Slater DR, and Whalen KL (2015) Enzyme Function Initiative-Enzyme Similarity Tool (EFI-EST): A web tool for generating protein sequence similarity networks, Biochim Biophys Acta 1854, 1019–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhao S, Kumar R, Sakai A, Vetting MW, Wood BM, Brown S, Bonanno JB, Hillerich BS, Seidel RD, Babbitt PC, Almo SC, Sweedler JV, Gerlt JA, Cronan JE, and Jacobson MP (2013) Discovery of new enzymes and metabolic pathways by using structure and genome context, Nature 502, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kozlowski LP (2016) IPC - Isoelectric Point Calculator, Biology Direct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Human Microbiome Project C (2012) A framework for human microbiome research, Nature 486, 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Human Microbiome Project C (2012) Structure, function and diversity of the healthy human microbiome, Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zallot R, Oberg NO, and Gerlt JA (2018) ‘Democratized’ genomic enzymology web tools for functional assignment, Current Opinion in Chemical Biology 47, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Levin BJ, Huang YY, Peck SC, Wei Y, Martinez-Del Campo A, Marks JA, Franzosa EA, Huttenhower C, and Balskus EP (2017) A prominent glycyl radical enzyme in human gut microbiomes metabolizes trans-4-hydroxy-l-proline, Science 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kaminski J, Gibson MK, Franzosa EA, Segata N, Dantas G, and Huttenhower C (2015) High-Specificity Targeted Functional Profiling in Microbial Communities with ShortBRED, PLoS Comput Biol 11, e1004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kaminska KH, Purta E, Hansen LH, Bujnicki JM, Vester B, and Long KS (2010) Insights into the structure, function and evolution of the radical-SAM 23S rRNA methyltransferase Cfr that confers antibiotic resistance in bacteria, Nucleic Acids Res 38, 1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hansen LH, Planellas MH, Long KS, and Vester B (2012) The order Bacillales hosts functional homologs of the worrisome cfr antibiotic resistance gene, Antimicrob Agents Chemother 56, 3563–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Deshpande LM, Ashcraft DS, Kahn HP, Pankey G, Jones RN, Farrell DJ, and Mendes RE (2015) Detection of a new cfr-Like gene, cfr(B), in Enterococcus faecium isolates recovered from human specimens in the United States as part of the SENTRY Antimicrobial Surveillance Program, Antimicrob Agents Chemother 59, 6256–6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bender JK, Fleige C, Klare I, Fiedler S, Mischnik A, Mutters NT, Dingle KE, and Werner G (2016) Detection of a cfr(B) Variant in German Enterococcus faecium Clinical Isolates and the Impact on Linezolid Resistance in Enterococcus spp, PLoS One 11, e0167042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Morroni G, Brenciani A, Antonelli A, D’Andrea MM, Di Pilato V, Fioriti S, Mingoia M, Vignaroli C, Cirioni O, Biavasco F, Varaldo PE, Rossolini GM, and Giovanetti E (2018) Characterization of a Multiresistance Plasmid Carrying the optrA and cfr Resistance Genes From an Enterococcus faecium Clinical Isolate, Front Microbiol 9, 2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kuroda M, Sekizuka T, Matsui H, Suzuki K, Seki H, Saito M, and Hanaki H (2018) Complete Genome Sequence and Characterization of Linezolid-Resistant Enterococcus faecalis Clinical Isolate KUB3006 Carrying a cfr(B)-Transposon on Its Chromosome and optrA-Plasmid, Front Microbiol 9, 2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mead DA, Lucas S, Copeland A, Lapidus A, Cheng JF, Bruce DC, Goodwin LA, Pitluck S, Chertkov O, Zhang X, Detter JC, Han CS, Tapia R, Land M, Hauser LJ, Chang YJ, Kyrpides NC, Ivanova NN, Ovchinnikova G, Woyke T, Brumm C, Hochstein R, Schoenfeld T, and Brumm P (2012) Complete Genome Sequence of Paenibacillus strain Y4.12MC10, a Novel Paenibacillus lautus strain Isolated from Obsidian Hot Spring in Yellowstone National Park, Stand Genomic Sci 6, 381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Brouwer MS, Warburton PJ, Roberts AP, Mullany P, and Allan E (2011) Genetic organisation, mobility and predicted functions of genes on integrated, mobile genetic elements in sequenced strains of Clostridium difficile, PLoS One 6, e23014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Marin M, Martin A, Alcala L, Cercenado E, Iglesias C, Reigadas E, and Bouza E (2015) Clostridium difficile isolates with high linezolid MICs harbor the multiresistance gene cfr, Antimicrob Agents Chemother 59, 586–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hansen LH, and Vester B (2015) A cfr-like gene from Clostridium difficile confers multiple antibiotic resistance by the same mechanism as the cfr gene, Antimicrob Agents Chemother 59, 5841–5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Candela T, Marvaud JC, Nguyen TK, and Lambert T (2017) A cfr-like gene cfr(C) conferring linezolid resistance is common in Clostridium difficile, Int J Antimicrob Agents 50, 496–500. [DOI] [PubMed] [Google Scholar]
- [52].Bianchi V, Eliasson R, Fontecave M, Mulliez E, Hoover DM, Matthews RG, and Reichard P (1993) Flavodoxin is required for the activation of the anaerobic ribonucleotide reductase, Biochem Biophys Res Commun 197, 792–797. [DOI] [PubMed] [Google Scholar]
- [53].Bianchi V, Reichard P, Eliasson R, Pontis E, Krook M, Jornvall H, and Haggard-Ljungquist E (1993) Escherichia coli ferredoxin NADP+ reductase: activation of E. coli anaerobic ribonucleotide reduction, cloning of the gene (fpr), and overexpression of the protein, J Bacteriol 175, 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].(2017) Kintek Explorer Chemical Kinetics Software, Kintek Corporation, www.kintekcorp.com.
- [55].Schwalm EL (2018) Investigation of the radical SAM methylases RlmN and Cfr, Ph.D. dissertation, The Pennsylvania State University, 68–85. [Google Scholar]
- [56].Pandelia M-E, Lanz ND, Booker SJ, and Krebs C (2015) Mössbauer spectroscopy of Fe/S proteins, Biochimica et Biophysica Acta BBA Molecular Cell Research 1853, 1395–1405. [DOI] [PubMed] [Google Scholar]
- [57].Moura I, Huynh BH, Hausinger RP, Le Gall J, Xavier AV, and Münck E (1980) Mossbauer and EPR Studies of Desulforedoxin from Desulfouibrio gigas * J. Biol. Chem 255, 2493–2498. [PubMed] [Google Scholar]
- [58].Engstrom LM, Brinkmeyer MK, Ha Y, Raetz AG, Hedman B, Hodgson KO, Solomon EI, and David SS (2014) A zinc linchpin motif in the MUTYH glycosylase interdomain connector is required for efficient repair of DNA damage, J Am Chem Soc 136, 7829–7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nuñez NN, Khuu C, Babu CS, Bertolani SJ, Rajavel AN, Spear JE, Armas JA, Wright JD, Siegel JB, Lim C, and David SS (2018) The Zinc Linchpin Motif in the DNA Repair Glycosylase MUTYH: Identifying the Zn2+ Ligands and Roles in Damage Recognition and Repair, J Am Chem Soc 140, 13260–13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Holm RH, Kennepohl P, and Solomon EI (1996) Structural and functional aspects of metal sites in biology, Chem Rev 96, 2239–2314. [DOI] [PubMed] [Google Scholar]
- [61].Czernuszewicz RS, Legall J, Moura I, and Spiro TG (1986) Resonance raman spectra of rubredoxin - new assignments and vibrational coupling mechanism from Fe-54 Fe-56 isotope shifts and variable-wavelength excitation, Inorg Chem 25, 696–700. [Google Scholar]
- [62].Lin IJ, Gebel EB, Machonkin TE, Westler WM, and Markley JL (2005) Changes in hydrogen-bond strengths explain reduction potentials in 10 rubredoxin variants, Proc Natl Acad Sci U S A 102, 14581–14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lanz ND, and Booker SJ (2012) Identification and function of auxiliary iron-sulfur clusters in radical SAM enzymes, Biochim Biophys Acta 1824, 1196–1212. [DOI] [PubMed] [Google Scholar]
- [64].Lanz ND, and Booker SJ (2015) Auxiliary iron-sulfur cofactors in radical SAM enzymes, Biochim Biophys Acta 1853, 1316–1334. [DOI] [PubMed] [Google Scholar]
- [65].Davis KM, Schramma KR, Hansen WA, Bacik JP, Khare SD, Seyedsayamdost MR, and Ando N (2017) Structures of the peptide-modifying radical SAM enzyme SuiB elucidate the basis of substrate recognition, PNAS 114, 10420–10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lilla EA, and Yokoyama K (2016) Carbon extension in peptidylnucleoside biosynthesis by radical SAM enzymes, Nat Chem Biol 12, 905–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wei Y, Funk MA, Rosado LA, Baek J, Drennan CL, and Stubbe J (2014) The class III ribonucleotide reductase from Neisseria bacilliformis can utilize thioredoxin as a reductant, Proc Natl Acad Sci U S A 111, E3756–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wei Y, Li B, Prakash D, Ferry JG, Elliott SJ, and Stubbe J (2015) A Ferredoxin Disulfide Reductase Delivers Electrons to the Methanosarcina barkeri Class III Ribonucleotide Reductase, Biochemistry 54, 7019–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wei Y, Mathies G, Yokoyama K, Chen J, Griffin RG, and Stubbe J (2014) A chemically competent thiosulfuranyl radical on the Escherichia coli class III ribonucleotide reductase, J Am Chem Soc 136, 9001–9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Dong M, Su X, Dzikovski B, Dando EE, Zhu X, Du J, Freed JH, and Lin H (2014) Dph3 is an electron donor for Dph1-Dph2 in the first step of eukaryotic diphthamide biosynthesis, J Am Chem Soc 136, 1754–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhang Y, Zhu X, Torelli AT, Lee M, Dzikovski B, Koralewski RM, Wang E, Freed J, Krebs C, Ealick SE, and Lin H (2010) Diphthamide biosynthesis requires an organic radical generated by an iron-sulphur enzyme, Nature 465, 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Maiocco SJ, Grove TL, Booker SJ, and Elliott SJ (2015) Electrochemical Resolution of the [4Fe-4S] Centers of the AdoMet Radical Enzyme BtrN: Evidence of Proton Coupling and an Unusual, Low-Potential Auxiliary Cluster, J Am Chem Soc 137, 8664–8667. [DOI] [PubMed] [Google Scholar]
- [73].Crowe-McAuliffe C, Graf M, Huter P, Takada H, Abdelshahid M, Nováček J, Murina V, Atkinson GC, Hauryliuk V, and Wilson DN (2018) Structural basis for antibiotic resistance mediated by the Bacillus subtilis ABCF ATPase VmlR, Proc Natl Acad Sci U S A 115, 8978–8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dönhöfer A, Franckenberg S, Wickles S, Berninghausen O, Beckmann R, and Wilson DN (2012) Structural basis for TetM-mediated tetracycline resistance, Proc Natl Acad Sci U S A 109, 16900–16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dingle KE, Elliot B, Robinson E, Griffiths D, Eyre DW, Stoesser N, Vaughan A, Golubchik T, Fawley WN, Wilcox MH, Peto TE, Walker AS, Riley TV, Crook DW, and Didelot X (2013) Evolutionary history of the Clostridium difficile pathogenicity locus, Genome Biol Evol. 6, 36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.