Abstract
Objective
More recent studies suggested that defects in autophagy contribute to the pathogenesis of SLE, especially in adaptive immunity. Occurrence and progression of lupus nephritis (LN) is the end result of complex interactions between regulation of immune responses and pathological process by renal resident cells, but there is still a lot of missing information for an establishment on the role of autophagy in pathogenesis of LN and as a therapy target.
Methods
Systemic and organ specific etiologies of autophagy were firstly evaluated by autophagy protein quantification in tissue homogenates in MRLlpr/lpr lupus prone and female C57BL mice. Analysis of gene expression was also adopted in human blood and urine sediments. Then, some key mediators of the disease, including complement inactivated serum, IgG from patients with LN (IgG-LN) and IFN-α were chosen to induce podocyte autophagy. Podocyte injuries including apoptosis, podocin derangement, albumin filtration, and wound healing were monitored simultaneously with autophagy steady-state and flux.
Results
Elevated LC3B in kidney homogenates and increased autophagosomes in podocyte from MRLlpr/lpr were observed. In humans, mRNA levels of some key autophagy genes were increased in blood and urinary sediments, and podocyte autophagosomes were observed in renal biopsies from patients with LN. Complement inactivated serum, IgG-LN and IFN-α could induce podocyte autophagy in a time and dosage dependent manner, and by reactive oxygen species production and mTORC1 inhibition, respectively. Autophagy inhibition aggravated podocyte damage whereas its inducer relieved the injury.
Conclusion
Podocyte autophagy is activated in lupus-prone mice and patients with lupus nephritis. Increased autophagy is cytoprotective against antibody and interferon-α induced podocyte injury.
Keywords: autophagy, lupus nephritis, podocyte
Introduction
Systemic lupus erythematosus (SLE) is a spectrum of autoimmune disease in which defects can occur at multiple points of the immune cascade, resulting in a striking heterogeneity of clinical presentations1–4. The central pathology is the recognition of, and reaction to, self-antigens by the immune system, leading to type I interferon signaling and the production of pathogenic auto-antibodies, which mediate much of the disease 1–4.
More recent genetic and cellular studies suggested that defects in the evolutionarily conserved catabolic pathway of autophagy contributed to the pathogenesis of SLE, especially in the adaptive immune response 5–12. Hypothesis-free genome-wide association studies (GWAS) and further follow-up studies revealed that variants locating in autophagy related genes associated with susceptibility to SLE 13–22. Increased ATG5, MAP1LC3B and BECN1 expressions were observed in blood of lupus patients 13, 18, 23. And exposure to serum from patients with active SLE in SH-SY5Y cells was associated with increased formation of autophagic vacuoles 24. It was observed that autophagy was upregulated in B cells during plasma cell differentiation in SLE 25, and macrophages from patients with SLE also exhibited increased levels of autophagy 26. In a toll like receptor (TLR) 7 transgenic mouse model of lupus, ATG5-dependent B-cell autophagy was necessary for the induction of lupus-like disease 27. In addition, autophagy in T cells from both lupus prone mice and patients with SLE was deregulated and not distributed homogeneously 28–30. More recently, it was observed that, in transgenic mouse models, disruption of LC3-associated phagocytosis (LAP) resulted in defective clearance of exogenous dead cells, leading to increased serum levels of anti-dsDNA antibodies, anti-nuclear antigens and inflammatory cytokines — all of which were hallmarks of SLE 10, 31. However, to what extent autophagy contributes to the organ damage in human SLE remains obscure.
Lupus nephritis (LN) is one of the most serious manifestations of SLE and is an important predictor of poor outcome. Occurrence and progression of LN is the end result of complex interactions between regulation of immune responses and pathological process involved by renal resident cells. Similar to other diseases, autophagy is also implicated in the pathological process of renal diseases. Studies from renal cells in culture, human kidney tissues, and experimental animal models implicate that autophagy regulates many critical aspects of normal and disease conditions in the kidney 32–35. By specific gene knockout mice, it is indicated that constitutive and induced autophagy is a major protective mechanism against podocyte aging and glomerular injury 36. Also, LAP-deficient animals displayed indications of kidney damage, and exhibited increased functional markers of kidney injury 10, such as increased serum creatinine, blood urea nitrogen, and proteinuria. And variants of APOL1 and MTMR3, key drivers in autophagy, have been associated with lupus nephritis 37–39.
Rapamycin /sirolimus, as an inhibitor of TOR would activate autophagy, and was observed to prolong survival, maintain normal renal function, normalize proteinuria, restore protein levels of podocyte-specific markers including nephrin and podocin, reduce anti-dsDNA titers, ameliorate histological lesions, and reduce Akt and mammalian target of rapamycin (mTOR) activation in lupus nephritis 11, 40–45.But there is still a lot of missing information necessary for a full establishment on the role of autophagy in pathogenesis of LN and as a therapy target. Thus the aim for the current study is to reveal the role and function of autophagy in kidney pathology in LN, checking whether modulating autophagic activity in certain cell type may be therapeutically beneficial.
Methods
Patients and healthy donors
All the 44 patients, recruited from Peking University First Hospital, fulfilled the American College of Rheumatology revised criteria for the classification of SLE and were diagnosed with lupus nephritis by renal biopsy. 37 volunteer controls were reported to be healthy by medical examination. This study was approved by the Institutional Review Board of the Peking University First Hospital.
Quantification of autophagy related genes expression
The quantifications of autophagy related gene expressions were conducted using both peripheral blood mononuclear cells (PBMCs, 13 patients with LN and 12 healthy donors) and urinary sediments (16 patients with LN, and 15 healthy donors) (Supplementary Methods and Supplementary Table 1).
Purification of IgG from patient sera
IgG were purified from sera of 5 LN patients and 5 healthy donors by protein G affinity chromatography (GE Healthcare).
Mice
10 female MRLlpr/lpr lupus prone mice and 10 control female C57BL mice were acquired from Nanjing Medical University. Thymus, spleen, inguinal lymph nodes, and kidneys were harvested at 6 months46. Lupus nephritis was proven by pathology (Supplementary Figure 1). All the procedures were approved by the Institutional Animal Care and Use Committee of Peking University First Hospital.
Electron microscopy
Kidney tissues for electron microscopy was fixed in 3% glutaraldehyde and 1% osmium tetroxide (OsO4), followed by dehydration in graded ethanol series and washed with acetone, and finally embedded in Epon 812. Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a transmission electron microscope (JEM-1230, JEOL, Japan).
A total of 20 human renal biopsy specimens were examined by light microscopy, immunofluorescence (IF) microscopy and transmission electron microscopy (TEM). The average number of autophagosomes per podocyte was counted.
Cell culture and treatments
The immortalized human podocyte cell line was a gift from Moin A. Saleem 47 and the mouse podocyte was purchased from Cell Resource Center of Peking Union Medical College (3111C0001CCC000230). Both human and mouse podocytes were cultured and differentiated as described in Supplementary Methods.
Podocytes were maintained and exposed to complement inactivated sera from patients with LN 24, purified IgG from patients with LN, purified IgG from healthy donors, and commercial IgG (Sigma, I4506) and interferon (IFN)-α 2 (PBL Interferon Source, 11105–1) for human podocyte or interferon α (PBL Interferon Source, 12100–1) for mouse podocyte. For autophagy inhibition, 5 mM 3-Methyladenine (3-MA, M9281, Enzo), and 20 μM bafilomycin A1 (BafA1, BML-CM110, Enzo) were used. And for autophagy induction, 400 μM rapamycin (BML-A275, Enzo) was added.
Lentiviral vector infection
The immortalized human podocyte cell line was seeded at 1 × 105 cells/well in 6-well plates and infected with lentivirus vector ATG5 shRNA (HANBIO, Shanghai, China) for 12 h after 30min incubation with polybrene. Then, change the media regularly and experiments were performed after 10 days in differentiation condition. An empty lentiviral vector (HANBIO, Shanghai, China) was used as the negative control (Supplementary Figure 2).
Western blotting
The primary antibodies against SQSTM1/p62 protein (5114, CST), LC3B (L7543, Sigma) and ACTB/β-actin (sc-47778, Santa Cruz), podocin (ab50339, Abcam), RPS6KB (2708P, CST), phospho-RPS6KB (Thr389, 9205S, CST), RPS6 (2317S, CST), phospho-RPS6 (Ser240/244, 2215S, CST), EIF4B (3592P), phospho-EIF4B (Ser422, 3591, CST), and the horseradish peroxidase (HRP)-conjugated secondary antibodies (sc-2004 and sc-2005, Santa) were used.
Immunocytochemical staining
Indirect immunofluorescence staining was performed as described previously 48, 49. The primary antibodies included 1:100 anti-LC3B (L7543, Sigma) or 1:100 podocin (ab50339, Abcam). Images were captured by immunofluorescence microscopy (Zeiss, Oberkochen, Germany).
mRFP-GFP-LC3
To analyze autophagy flux, cells were infected with dual-tagged LC3 (mRFP-GFP-LC3) adenovirus (Hanbio Biotechnology) according to the manufacturer’s instructions. GFP and mRFP dots/cell and the ratio of mRFP/GFP were used for quantitation of autophagy flux, and twenty cells were counted in each group.
Flow cytometry assay
Podocyte apoptosis was determined by flow cytometry using an Annexin V-PE apoptosis detection kit (559763, BD Biosciences, USA) following the manufacturer’s protocol. 10,000 cells were analyzed by FACScan flow cytometer (BD Biosciences) to analyze cellular apoptosis. The levels of intracellular reactive oxygen species (ROS) were determined by 2′, 7′-Dichlorofluorescin diacetate (DCFH-DA, Sigma) assay.
In Vitro “Wound Healing” assay
It is assumed that foot process retraction is a migration event caused by podocyte injury50. Podocytes were thus seeded in 6-well tissue culture dishes 10 days in 37°C for differentiation. At the time point 0 h the cell monolayer was wounded. Afterward the cell culture medium was replaced by test medium containing either standard medium or medium supplemented with IFN-α with 3-MA or rapamycin or shATG5, or IFN-α, 3-MA, rapamycin, shATG5 alone. The wounds closure were observed at 12h by phase contrast microscopy and documented by photography. Each condition was examined in triplicates.
Albumin influx assay
The filtration barrier function of podocyte monolayer was evaluated by a simple albumin influx assay as described previously 51.
Statistical analysis
All the analysis was done using SPSS 14.0 (SPSS Inc., Chicago, IL, USA). All the results are presented as mean ± standard deviation. Two group comparisons were assessed by two-tailed t test. Values of p < 0.05 were considered statistically significant.
(Please refer to Supplementary Methods for detailed information).
Results
Elevated LC3 quantification in kidney homogenates of MRLlpr/lpr mice
We firstly examined the LC3B protein quantification in the thymus, spleen, inguinal lymph node and kidney tissue homogenate from 6-month-old lupus-prone MRLlpr/lpr mice and C57BL6/J mice. It was observed that LC3B-II was decreased in MRLlpr/lpr spleen homogenate but increased in fraction of lymph nodes (Supplementary Figure 3). No significant difference was observed in the thymus. 5 (50%) MRLlpr/lpr murine lupus mice were histologically-proven with glomerulonephritis at 6 months, and the level of LC3B-II significantly increased in kidney tissue homogenate from MRLlpr/lpr mice with nephritis, compared to MRLlpr/lpr mice without nephritis or C57BL6/J mice (Figure 1A).
Figure 1: Up-regulated autophagy in MRLlpr/lpr mice, LN patients and in podocytes treated by sera from patients with LN.
(A) Western blot analysis of autophagy marker LC3B was shown with long and short exposure in kidney tissue homogenate from MRLlpr/lpr mice. Quantification of LC3B-II levels relative to ACTB or LC3B-I was present with mean and range.
(B) A representative human podocyte autophagosome by transmission electron micrographs (TEM) was shown (scale bar 1μm, 200nm, and enlarged image).
(C) Heatmap of genes in autophagy pathway (pathway: hsa04140) expression in 13 LN patients and 12 healthy donors. A total of 128 genes were included in autophagy pathway according to KEGG in which 11 genes were not included in the Illumina HT-12 v4 Expression BeadChip. Among them, MTMR3 and RRAGD showed the most significance (D).
(E) Scatter plot from selected autophagy gene expressions in PBMC and urinary sediments. (Data were expressed relative to their respective 18S expression with means ± SEM).
(F) A representative human podocyte autophagosome by transmission electron micrographs (TEM) was shown (scale bar 2μm and 0.5μm).
(G) Sera from patients with lupus nephritis could induce podocyte autophagy in vitro. Densitometry analysis of LC3B-II levels relative to GAPDH was also shown. Values are expressed as mean. P-value was indicated in the figure.
Increased frequency of observed autophagosome in MRLlpr/lpr podocyte
Autophagy level detected from homogenates of the whole organ included organelles from infiltrated cells as well as from renal resident cells. We thus further directly checked the presence of autophagosomes in renal resident cells using TEM (Supplementary Figure 4). It was observed that autophagic vacuoles were present in podocytes instead of endothelial cells, mesangial cells or tubular epithelial cells. And the observation was present in 4 out of 5 MRLlpr/lpr mice with nephritis, but absent in MRLlpr/lpr mice without renal impairment or C57BL6/J mice (Figure 1B).
Increased autophagy mRNA levels in PBMC and urinary sediment from human patients with LN
Compared to healthy controls, 31 out of 117 Kyoto Encyclopedia of Genes and Genomes/KEGG autophagy genes (26%) were differentially expressed in PBMC from patients with lupus nephritis. 23 genes were significantly up-regulated and 8 genes were significantly down-regulated in LN patients (Figure 1C, p < 0.05; details in Supplementary Table 2). MTMR3 (myotubularin related protein 3) (case vs. controls, 7.55±0.12 vs. 7.12±0.26; p= 1.75×10−5) and RRAGD (Ras related GTP binding D) (8.58±0.30 vs. 7.56±0.36; p= 7.28×10−8) showed the most significance and can survive from multiple testing (p threshold 0.05/117= 4.27×10−4) (Figure 1D).
Urinary sediment analysis indicated significantly increased mRNA levels of ULK1 (p = 0.035), BECN1 (p = 0.021), and MAP1LC3B (p = 0.04) (Figure 1E) in patients with lupus nephritis compared to healthy controls. But ATG5, ATG12, ATG16 showed non-significantly differed expression (data not shown).
Observed podocyte autophagosome in renal biopsy from human patients with LN
Autophagosomes in podocyte were further analyzed by TEM in 20 patients with lupus nephritis (each 5 patients were selected in every subgroup of type II, III, IV and V according to 2003 ISN/RPS classification of LN). Podocyte autophagosomes could be observed in 17 out of 20 patients (85%) with lupus nephritis (Figure 1F). And only 3 patients showed no podocyte autophagosome and were all belonging to subclass II. However, the number of autophagosomes per podocyte (ranged from 0 to 9) correlated with no clinical data (data not shown).
Sera from patients with lupus nephritis could induce podocyte autophagy in vitro
We hypothesized that a serum factor present with lupus nephritis may induce podocyte autophagy. To test for the presence of such a factor, we incubated human podocytes in the presence of complement inactivated sera from healthy donors or lupus nephritis. It indicated that autophagy was stimulated by sera from patients with LN compared to sera from healthy donors (1:10 dilution) (Figure 1G). As sera contained multiple mediators in disease pathogenesis, we then focused on IFN-α and IgG from patients with lupus nephritis (IgG-LN).
IFN-α could induce podocyte autophagy in vitro
IFN-α, a central cytokine in the pathogenesis of SLE, can induce and accelerate SLE tissue damage in patients and mice. It remained unknown whether IFN-α can induce autophagy in human and murine podocytes. We observed that treatment with IFN-α induced autophagy in both human and murine podocyte, as indicated by an increase of LC3B-II, and a decrease of p62 expression. And this kind of induction was time-and dose-dependent (Figure 2A–2D). Our results also showed enhanced LC3-II and p62 expression levels in IFN (-) podocytes from 6h to 48h, indicating that autophagic vacuole accumulation was partially attributed to impaired autophagic degradation.
Figure 2: Autophagy was induced by IFN-α in podocyte.
(A-D) Western blots analysis of autophagy markers LC3B and p62 in human and mouse podocytes after exposure to 1000 U/ml IFN-α at various time points and after exposure to different concentrations of IFN-α for 48h.
(E-H) Immunofluorescence staining and quantative changes of LC3B (green) in human and mouse podocytes after exposure to 1000 U/ml IFN-α for 48h. Quantative of autophagy vacuoles (green dots) were shown in histogram with mean ±SEM from 3 experiments. GFP-LC3B displayed a diffuse cytoplasmic distribution in the absence of autophagy. In contrast, after the induction of autophagy, LC3B forms autophagic membranes and can be observed as visible puncta representing autophagic vacuoles.
(I and K) Fluorescence imaging of mRFP-GFP-LC3 acquired by confocal microscopy, (J and L) and quantitation of dots per cell and mRFP / GFP ratio in podocytes with or without bafilomycin A1 treatment. The GFP signal (green) can be quenched in a lysosomal environment; in contrast, the RFP signal (red) is more stable in an acidic environment. Therefore, autophagosomes (GFP+RFP+LC3 puncta) was labeled with a yellow (merge from green and red) and autolysosomes (GFP-RFP+LC3 puncta) red color. By detecting and analyzing the two different fluorescent signals, the autophagic flux can be monitored. Scale bar 20,000nm. *p < 0.05. **p < 0.01.
(M-P) Western blotting of LC3B-II with co-treatment of bafilomycin A and IFN-α, compared to single or no treatment (untreated vs. BafA1; IFN-a vs. IFN-a+BafA1). **p < 0.01.
By indirect immunofluorescence, a large number of GFP-LC3B puncta formed in the cytoplasm and the average number of GFP-LC3B puncta per cell was significantly increased after exposure to IFN-α (Figure 2E–2H) compared to untreated cells. And by checking autophagic flux by GFP-RFP-LC3 system, there were more GFP+RFP+LC3 puncta, (autophagosomes) as well as GFP-RFP+LC3 puncta (autophagolysosomes) in podocytes under IFN-α treatment than in untreated controls, indicative of accumulation of LC3. When co-treatment with the lysosomal inhibitor (bafilomycin A) to block autophagic flux, more autophagosomes (GFP+RFP+LC3) were observed (Figure 2I–2L). By Western blotting, it was observed LC3B-II protein quantification was significantly increased with co-treatment of bafilomycin A and IFN-α, compared to single (IFN-α treatment alone or bafilomycin A alone) or no treatment. It suggested that the treatment increased the synthesis of autophagy-related membranes instead of autophagic flux block (Figure 2M–2P).
IgG isolated from patients with lupus nephritis could induce podocyte autophagy in vitro
Because most of the pathogenic autoantibodies were of IgG isotype, to examine the likely link between autoantibodies and the induction of autophagy in podocytes, immunoglobulins (IgG) were isolated. IgG from the sera of human LN patients (IgG-LN), enrolled healthy donors (IgG-Control) and commercial immunoglobulin from healthy donor, were adopted to stimulate podocytes in vitro. Podocytes exposed to IgG-LN displayed significantly elevated LC3B-II levels compared to exposure to IgG-Control and commercial IgG. This increase occurred in both a time-and dose-dependent manner, peaking at 6 hours post-treatment (Figure 3A–3F). Simultaneously, the levels of p62 decreased in time-and dose-dependent manner in IgG-LN-treated podocytes. It was thus indicative of potent induction of autophagy.
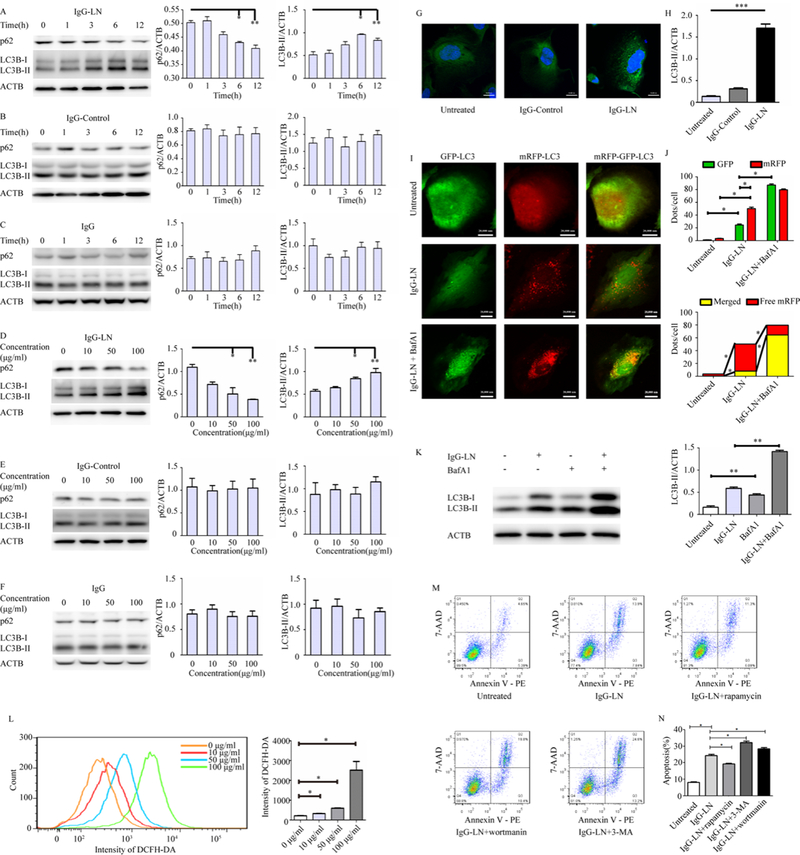
Figure 3: Autophagy induced by IgG extracted from lupus nephritis patients played a protective role in podocyte apoptosis.
(A-F) Western blot analysis of autophagy markers LC3B and p62 at various time points (100 μg/ml IgG) and at different IgG concentrations (exposure to IgG for 6h).
(G-H) Immunofluorescence staining and quantative changes of LC3B (green) of untreated podocytes and after exposure to100 μg/ml IgG from patients with lupus nephritis and healthy donors for 6h. Quantative of autophagy vacuoles (green dots in G) were shown in histogram with mean ±SEM from 3 experiments.
(I-J) Fluorescence imaging of mRFP-GFP-LC3 acquired by confocal microscopy and quantitation of dots per cell and mRFP / GFP ratio in podocytes with or without bafilomycin A1 treatment.
(K) Western blotting of LC3B-II with co-treatment of bafilomycin A and IgG-LN, compared to single or no treatment (untreated vs. BafA1; IFN-a vs. IFN-a+BafA1). **p < 0.01
(L) ROS levels in podocytes after treatment with various concentrations IgG-LN for 6h by flow cytometric analysis and quantative of geometric means were shown in histogram with mean ± SEM from 3 experiments.
(M-N) Representatives of Annexin V-PE / 7-AAD double-staining in different groups and quantative of apoptosis was shown in histogram (N) with mean ± SEM from 3 experiments. Scale bar 20,000nm. *p < 0.05, **p < 0.01, ***p < 0.001.
Indirect immunofluorescence experiments also demonstrated an increased number of LC3-positive dots in podocytes with increased autophagic activity in response to IgG-LN. In untreated or IgG-Control-treated cells, only a few LC3 puncta were detected, but increased number of LC3 positive puncta could be observed after exposure to IgG-LN addition (Figure 3G–3H). Assays using GFP-RFP-LC3 further confirmed this. Podocytes treated with IgG-LN displayed increased mature GFP-RFP+ autophagolysosomes. An additive effect in increased levels of GFP+RFP+ LC3 puncta (autophagosomes) in the presence of both IgG-LN and lysosomal inhibition (with bafilomycin A1) indicated that IgG-LN enhanced autophagic flux (Figure 3I–3J). Increased levels of LC3-II in the presence of lysosomal inhibition were also confirmed by Western blotting, which increased the confidence that an induction of autophagic process was observed (Figure 3K).
IgG-LN regulated generation of ROS and podocyte apoptosis
ROS production was observed to be considerably increased in podocytes treated with IgG-LN in a dose-dependent manner (Figure 3L). To study the effect of IgG-LN on podocyte apoptosis in vitro, podocytes were further treated with IgG-LN. Flow cytometry revealed that apoptosis rates significantly increased to three-fold when treated with IgG-LN (13.9%) compared to no treatment (4.65%) (Figure 3M–3N).
IFN-α treatment linked mTORC1 inhibition and podocyte damage
It was reported that type I interferon induced autophagy in certain human cancer cell lines involving mTORC1 signaling. The activity of mTORC1 can be monitored by phosphorylation changes in downstream target proteins like RPS6KB and EIF4EBP1.
To examine the effect of IFN-α on mTORC1, human and mouse podocytes were treated with 1000U for 6, 24 and 48 h. We observed decreased phosphorylation of RPS6KB (Thr389) and decreased phosphorylation changes of two RPS6KB-dependent proteins, RPS6 (Ser240/Ser242) and EIF4B (Ser422) (Figure 4A–4B), indicating that IFN-α attenuated mTORC1 signaling. Direct inhibition of mTORC1 activity can lead to induction of autophagy.
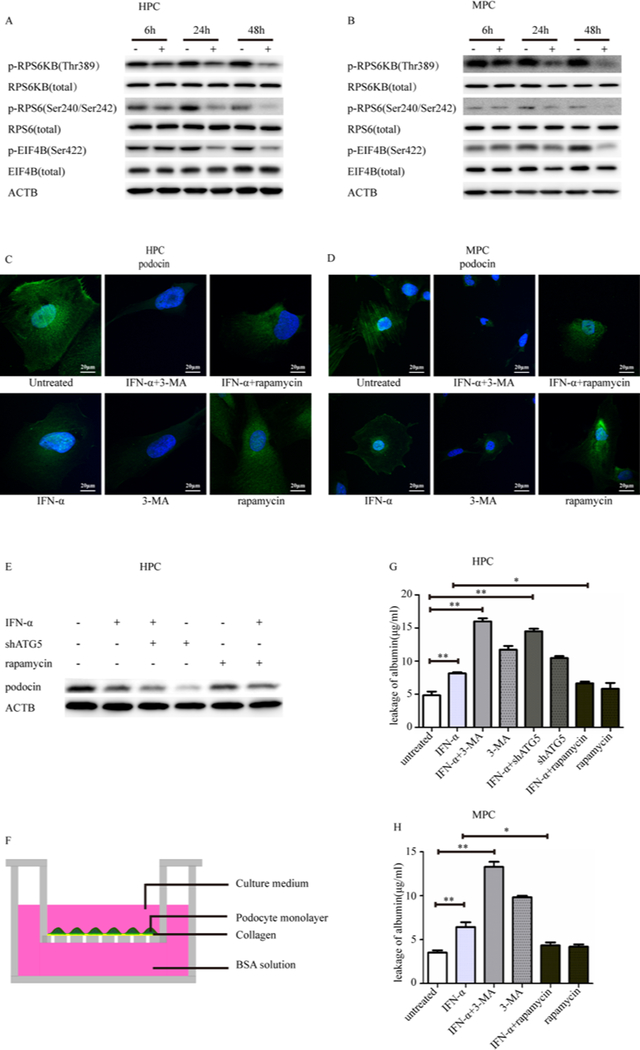
Figure 4: Detection of mTORC1 activity and autophagy protected podocytes from IFN-α induced injury.
(A-B) Western blot analysis of mTORC1 downstream phosphorylation of RPS6 (Ser240/Ser242), EIF4B (Ser422), RPS6KB (Thr389) in human and mouse podocytes after exposure to 1000 U/ml IFN-α for 48h.
(C-D) Immunofluorescence staining showed the localization of podocin (green) in human and mouse podocytes after exposure to 1000 U/ml IFN-α for 48h. Scale bar 20μm.
(E) Western blotting of podocin under different treatments.
(F) Podocytes monolayer on collagen-coated transwell filters and albumin permeability across podocyte monolayer was determined in human and mouse podocytes after exposure to 1000 U/ml IFN-α for 48h and was shown in histogram
(G-H) with mean ± SEM from 3 experiments. Scale bar 20,000nm. *p < 0.05, **p < 0.01.
IFN-α treatment was not observed to affect cell apoptosis (Supplementary Figure 5). Thus, maintenance of integrity of the structure and function of podocytes were further checked. By immunofluorescence staining, it was observed that podocin was in derangement (Figure 4C–4D). Expression of podocin, a podocyte‑specific marker, was detected to be decreased in IFN-α using western blotting analysis(Figure 4E). The scratch gap dimension with IFN-α treatment for 12 hours was larger than with no treatment (Supplementary Figure 6), implying some compromise of the migration capability by IFN-α treatment. To assess the functional consequence, we examined the filtration barrier function of podocyte by using a paracellular permeability influx assay, which measured the albumin flux rate across the differentiated podocyte monolayer. Differentiated podocytes were incubated with IFN-α for 12 hours, and then subjected to albumin influx assay (Figure 4F). As shown in Figure 4G–4H, addition of IFN-α increased albumin influx across the podocyte monolayer from 4.84 ± 1.00μg/ml to 8.14 ± 0.32μg/ml in the human podocyte and from 3.52 ± 0.45μg/ml to 6.43±0.96μg/ml in the mouse podocyte. These results indicated that the filtration barrier function of podocytes was severely impaired by IFN-α.
Autophagy inhibition aggravated podocyte damage whereas its inducer relieved the injury
It was important to demonstrate an effect resulting from inhibition or stimulation of autophagy. We thus applied chemical sequestration inhibitors of autophagy, including 3-MA, and wortmannin, which inhibited class I phosphoinositide 3-kinases (PI3Ks) as well as class III PtdIns3Ks. As they were not entirely specific, some data were confirmed using RNA silencing of ATG5. On the contrary, the most commonly used inducer of autophagy was rapamycin, an allosteric inhibitor of mTORC1, and was adopted as autophagy activator.
In IgG-LN induced podocyte apoptosis, addition of autophagy inhibitor further increased frequencies of podocyte apoptosis, from 13.9% with IgG-LN alone to 19.8% and 24.6% with addition of wortmannin and 3-MA respectively. Whereas it seemed that addition of rapamycin could moderately decrease apoptosis induced by IgG-LN, from 13.9% to 11.3% (Figure 3M). It suggested that induction of autophagy partly protected podocyte from IgG-LN induced apoptosis and the inhibition of autophagy may aggravate.
It was observed that both IFN-α treatment and shATG5 could decrease podocin quantification, whereas rapamycin could partially restore its expression in IFN-a treated cells. Inhibition of autophagy by 3-MA made podocin derangement more pronounced. In contrast, upregulation of autophagy with rapamycin could ameliorate podocin derangement to some extent (Figure 4C–D). Compared with untreated cells, all IFN-a, 3-MA and shATG5 12-hours treatments could weaken the scratch healing potential to some extent. Combination of IFN-α and shATG5 and 3-MA made worse impaired podocyte migration. However, rapamycin can restore podocyte migration to some extent (Supplementary Figure 6). In paracellular permeability influx assay, it was observed that inhibition of autophagy by 3-MA or shATG5 alone can significantly increase podocyte layer permeability, even more significant than addition of IFN-α. However, when combinational treatment was applied, a higher loss of podocyte layer permeability was observed. Inhibition of autophagy by adding of 3-MA (16.00 ± 0.80μg/ml in human podocyte, 13.30 ± 0.99μg/ml in mouse podocyte) and shAtg5 (14.50 ± 0.68μg/ml in human podocyte) in IFN-α worsened podocyte barrier function with increased albumin influx. Induction of autophagy by rapamycin (6.64 ± 0.48μg/ml in human podocyte, 4.34 ± 0.60μg/ml in mouse podocyte) ameliorated this kind of podocyte injury (Figure 4G–4H).
Discussion
Autophagy, an intracellular degrading system, is highly dependent on lysosomes and has existed universally in eukaryotic cells52. As a “nonselective” degradation system for long-lived cytoplasmic proteins and dysfunctional organelles, it has been repeatedly confirmed to be actively involved in pathogenesis in SLE, especially in systemic immunity10, 25–28, 30, 36, 38, 53, 54. The pathogenesis of lupus nephritis involves patho-mechanisms outside and inside the kidney. But there is a lack of investigation on autophagy inside the kidney in lupus nephritis. Although some observational studies indicated autophagy involved in some other immune-related nephritis in recent years35, 36, inconsistent conclusions have been made. There have been studies that provide evidence to support a cytoprotective role of autophagy, and others that support deleterious effects of autophagy. It is plausible that it is context dependent.
In the current study, we firstly checked whether autophagy is involved in pathogenesis of lupus nephritis. The data indicated that autophagy is activated in LN, especially in podocyte. In lupus prone MRLlpr/lpr.mice, we observed that the levels of autophagy varied in different immune organs of MRLlpr/lpr comparing with C57BL mice. In kidney tissues, the levels of autophagy were significantly elevated in MRLlpr/lpr mice with renal injury comparing with MRLlpr/lpr mice without renal injury and C57BL control mice. In addition, in LN patients, we observed that the expression of autophagy related genes were increased in PBMC and urinary sediments. It reinforced the notion that both aberrant systemic immunity and intrarenal immunopathology contribute to lupus nephritis, in which autophagy may exert an important effect. However, as PBMC and urinary sediments were all mixtures of different cell types, they may be not optimal specimens, but they both shed light on autophagy upregulation, which was also consistent with all the previous reports in SLE 28–30. Isolation of blood lineage specific lymphocytes and urine podocytes surely will be more illuminating in future non-invasive biomarker studies in the field of autophagy.
To understand the precise role of autophagy activation in renal injury response and the pathogenesis of LN, we further checked relations between autophagy induction and podocyte injury.
We observed that many of the most important mediators of the disease—patients’ sera, patients’ IgG and IFN-α could induce autophagy in both murine and human podocytes. In general, baseline autophagy in mammalian systems occurs under normal conditions, but can be stimulated by various pathologies including by ischemic, toxic, immunological, and oxidative insults. Other autophagy stimulators are pharmacological agents like the macrolide antibiotic rapamycin, the best-known trigger for autophagy, which acts by inhibiting mTOR. Although previous studies showed that deregulated autophagy plays an essential role for the induction of SLE, the precise nature of the stimulus leading to increased autophagic activity in lupus is not defined. We checked the effect of key known mediators of lupus, including sera from patients, IgG-LN, and interferon α. Although complement was inactivated, sera contain multiple layers of mediators in disease pathogenesis, so we further focused on IgG from patients with lupus nephritis (IgG-LN) and IFN-α. The production of pathogenic autoantibodies and type I interferon signaling are key pathogenic mechanisms in the development of SLE and LN 2–4, 55. It was reported that autoimmune mice with systemic inflammation but impaired autoantibody production were protected from lupus nephritis 55. It has been estimated that anti-dsDNA deposition in lupus nephritis accounts for no more than 10–20% of overall eluted IgG overall, implying that IgG not recognizing DNA represents the vast majority of antibodies in glomeruli. And it was reported that anti-podocyte antibodies in lupus nephritis were multiple and secondary events after autoantibody deposition included complement deposition, and activation of podocyte intracellular mediators such as phosphorylated protein kinase C and free oxygen radicals. And ROS played a key role in mediating autophagy formation in podocytes and determined podocyte depletion. We thus speculated that increased generation of ROS was associated with the IgG-induced autophagy in podocytes. Exposure of NZB/W mice to IFN-α can accelerate pathogenic autoantibody production, proteinuria development, and glomerular IgG deposition 56. And type I IFNs synthesized by resident renal cells (mesangial and endothelial cells) induced renal dysfunction, glomerulonephritis, crescent formation, and tubulointerstitial nephritis57.
We observed that autophagy activation negatively associated with podocyte injury. Terminally differentiated podocytes are vulnerable to various kinds of injury, and the loss of podocytes is considered a key feature of progressive glomerular disease. Podocyte injury is responsible for proteinuria, and loss of podocytes by cell death or detachment is a critical step for the progression of glomerular diseases. In lupus nephritis, podocytes are injured at the structural and molecular level 58. In the study, autophagosomes were observed in the podocyte but not in other glomerular resident cells (such as mesangial cells or endothelial cells), suggesting the regulation and function of autophagy is likely cell type and context specific. In clinical situations it is difficult to demonstrate autophagy clearly in tissues of formalin-fixed and paraffin-embedded biopsy samples retrospectively, because tissues fixed in formalin have low or no LC3 detectable. More recently, LC3B, or GFP-LC3 has been used to monitor autophagy through indirect immunofluorescence (IIF) or direct fluorescence microscopy. However, it was reported that IF assay pointing to LC3 could not differentiate cell types in kidney tissues35, 36. Thus confirmatory data on involved cell types may warrant future transgenic mice study. We observed that autophagy levels negatively associated with podocyte apoptosis, derangement of podocin, paracellular permeability influx, and albumin influx, which were all indicators of podocyte injury. And we observed autophagosomes in all proliferative classes except from some patients with class II. As type class II was characterized by fewer immune deposits and less podocyte injury compared to class III-V, which may be the underlying cause for the difference in which autophagy was cytoprotective against damage caused by IgG-LN. Some observational studies also suggested increased autophagosomes were detected in podocytes in FSGS, membranous nephropathy, and IgA nephropathy35, 36. It thus highlighted the fundamental role of autophagy for the long-time maintenance of glomerular podocytes in lupus nephritis and other immune related glomerulonephritis. But we observed no clinical correlation. The reasons may be several. Firstly, the occurrence of lupus nephritis is the interaction outcome between systemic/local immunity and renal intrinsic mechanism by resident cells. Every patient has his own key pathophysiological pathway. Such is also the fact that no single histological lesion or score could precisely predict clinical findings. Secondly, to correlate every single histological lesion with clinical parameters, it may need hundreds of patients. Lastly but not the least, the formation of autophagosomes is to protect podocyte against damage, which is a feed-back or compensatory mechanism instead of direct damage. The production of autoantibodies to self-antigens followed by formation of immune complexes (ICs) in glomeruli was the core step in leading to nephritis in SLE. Serum IgG from SLE patient could induce the apoptosis of endothelial cell 59 and induce autophagy of T lymphocytes 29. And anti-dsDNA antibodies could bind to podocytes in vitro and in vivo, resulting in segmental effacement of podocyte foot processes and proteinuria 60, 61. Our data provided evidence that serum IgG from SLE patient could not only induce apoptosis but also autophagy in podocyte. With the increase of the concentration of serum IgG from SLE patient, more ROS produced and levels of LC3B increased in podocytes, suggesting that ROS mediated podocyte autophagy induction by IgG from SLE patients. In contrast, consistent to previous report 62, we observed that IFN-α was not involved in podocyte loss, but impairment of cell structure and function. We observed that the induction of autophagy correlated with mTORC1 inhibition in human and mouse podocytes and that autophagy ameliorated IFN-α induced podocyte injury. Taken together, our data indicated that activation of podocyte autophagy protected from cell injury at least under conditions of IFN and IgG-LN, which was in accordance with the data that LAP deficient mice showed nephritis and loss of autophagy in podocytes resulted in a markedly increased susceptibility to glomerulosclerosis. Similarly, based on reported in vivo models of kidney injury, autophagy is often regarded as a renoprotective factor in the development and progression of renal diseases. But a defect in autophagy regulation itself is not (yet) proven to be able to cause human kidney diseases. In this study, we also observed association of autophagy with LN and the identified some factors that could lead to its occurrence, in which autophagy played protective role in feedback. This explains some mechanism of autophagy and its role in LN. Future investigations, for instance by using cell-type specific targeted autophagic gene knockout mice in LN, are necessary to elucidate and clarify the precise functional role of autophagy in renal injuries. As autoantibodies and IFN play an important role in a variety of cell populations, autophagy may also be relevant for biomarker, translational and therapeutic studies in several other diseases.
Last but not the least, in interventional study, we observed autophagy activator could protect podocyte injury whereas its inhibitor aggravated it. Rapamycin, also known as sirolimus, is considered to be non-nephrotoxic in the normal kidney. In most pathophysiological cases, rapamycin showed renal protection, including decreasing proteinuria in lupus prone mice63. However, it may also produce renal injury, including increasing proteinuria in long term therapy. The possible reason is, inhibition of mTOR activates autophagy, but prolonged starvation reactivates mTOR with degradation of autolysosomal products, and mTOR reactivation is required to regenerate functional lysosomes and completion of autophagic process64. In Mtor pod-KO mice or prolonged rapamycin treatment, mTOR activity is blocked at both points65. Thus, inhibition of mTOR activates autophagy, but mTOR reactivation is also blocked, resulting in disruption of autophagic flux. Development of de novo or worsening proteinuria is well-recognized in patients with long-term use of rapamycin. Given that disruption of the autophagic pathway may play a role in the pathogenesis of proteinuria, therapy with mTOR inhibitors can be a double-edged sword with both favorable and unfavorable consequences. In our current assay, rapamycin was added in quite a short time period, and it supported its effects on autophagy activation and renal protection. Future more specific and selective regulators of the autophagic machinery (without off-target effects) is urgently needed. Intermittent upregulation of autophagy may be more effective with fewer side effects than chronic use of such strategy. And studies using targeted autophagic gene knockout mice or transgenic mice, are necessary to elucidate and clarify the precise therapeutic role of autophagy in lupus nephritis.
We systemically checked autophagy in both lupus-prone mice and human patients, confirming the involvement of autophagy in LN. As the exact effect that autophagy might exert in renal disease is, multifaceted and complex: depending on different stimulations, types of disease or cells. We thus further taken multiple mediators of the disease in checking their effect in two different podocyte cell lines, and by combining different autophagy detection system, different types of injuries and different drug interventions. Combining assays with multiple confirmation, we concluded that increased autophagy is cytoprotective against podocyte injury induced by antibody and interferon-α in lupus nephritis. However, there were also some limitations of the current study. Firstly, we checked different autophagy levels in lupus prone mice, similar to previous reports 28, 53, it was observed different tissues showed different autophagy state. But different mice models, metabolic state, and time of assay may be all confounding factors. Future gene knockout or transgenic mice study will be more informative in explaining cause effect and intervention value. Secondly, we observed only IgG from patients with LN could induce autophagy, but how differences in the type of autoantibodies or glycosylation affect autophagy is not clear. Thirdly, an optimal condition and therapeutic window in which induction of autophagy would yield protective effects is still not clear. Fourthly, whether autophagy levels could be monitored as disease biomarker is still an open question.
In the current study, we evaluated the levels of autophagy in kidney in MRLlpr/lpr mice and LN patients and further explored the role of autophagy in podocyte injury of LN. Notably, we found that induction of autophagy (rapamycin) protected podocyte from IgG-LN induced apoptosis and IFN-α induced derangement of podocin and increased albumin influx. Likewise, drugs acting as autophagy inducers such as rapamycin were shown to be beneficial in LN treatment. Our findings, proved the protective role of autophagy in LN, especially in the podocyte (Figure 5). New insights into the molecular mechanisms of podocyte autophagy in LN may shed promising therapeutic strategies.
Figure 5:
Schematic of the key observations of the current study.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the members of the laboratory for technical assistance. We also thank the patients, their families and healthy donors for their cooperation and for giving consent to participate in this study.
FUNDINGS
This work was supported by grants from the National Science Foundation of China (Grant 81570629); National Key Research and Development Program of China (2016YFC0904102); the Training Program of the Major Research Plan of the National Natural Science Foundation of China (91642120); Beijing Nova Program (Z171100001117023); Beijing Youth Top-notch Talent Support Program (2017000021223ZK31); Natural Science Foundation for Innovation Research Group of China (81621092); Beijing Natural Science Foundation (7152148); the University of Michigan Health System–Peking University Health Science Center Joint Institute for Translational and Clinical Research (BMU2017JI007); and Chinese Society of Nephrology (15020030591). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICT OF INTEREST STATEMENT
None declared.
References:
- 1.Deng Y, Tsao BP. Updates in Lupus Genetics. Curr Rheumatol Rep. 2017;19(11):68. [DOI] [PubMed] [Google Scholar]
- 2.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–2121. [DOI] [PubMed] [Google Scholar]
- 3.Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2:16039. [DOI] [PubMed] [Google Scholar]
- 4.Tsokos GC, Lo MS, Costa RP, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12(12):716–730. [DOI] [PubMed] [Google Scholar]
- 5.Wu DJ, Adamopoulos IE. Autophagy and autoimmunity. Clin Immunol. 2017;176:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou XJ, Zhang H. Autophagy in immunity: implications in etiology of autoimmune/autoinflammatory diseases. Autophagy. 2012;8(9):1286–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rockel JS, Kapoor M. Autophagy: controlling cell fate in rheumatic diseases. Nat Rev Rheumatol. 2016;12(9):517–531. [DOI] [PubMed] [Google Scholar]
- 8.Mistry P, Kaplan MJ. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin Immunol. 2017;185:59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemp MG. Crosstalk Between Apoptosis and Autophagy: Environmental Genotoxins, Infection, and Innate Immunity. J Cell Death. 2017;9:2114263837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez J, Cunha LD, Park S, et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature. 2016;533(7601):115–119. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Gros F, Muller S. Pharmacological regulators of autophagy and their link with modulators of lupus disease. Br J Pharmacol. 2014;171(19):4337–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Q, Gao C, Chen Y, Feng Y, Liu WJ, Liu HF. Update on the role of autophagy in systemic lupus erythematosus: A novel therapeutic target. Biomed Pharmacother. 2015;71:190–193. [DOI] [PubMed] [Google Scholar]
- 13.Qi YY, Zhou XJ, Nath SK, et al. A rare variant (rs933717) at FBXO31-MAP1LC3B in Chinese is associated with systemic lupus erythematosus. Arthritis Rheumatol. 2018;70(2):287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YM, Zhou XJ, Cheng FJ, et al. Autophagy-related gene LRRK2 is likely a susceptibility gene for systemic lupus erythematosus in northern Han Chinese. Oncotarget. 2017;8(8):13754–13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou XJ, Nath SK, Qi YY, et al. Brief Report: identification of MTMR3 as a novel susceptibility gene for lupus nephritis in northern Han Chinese by shared-gene analysis with IgA nephropathy. Arthritis Rheumatol. 2014;66(10):2842–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molineros JE, Yang W, Zhou XJ, et al. Confirmation of five novel susceptibility loci for systemic lupus erythematosus (SLE) and integrated network analysis of 82 SLE susceptibility loci. Hum Mol Genet. 2017;26(6):1205–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YM, Cheng FJ, Zhou XJ, et al. Detecting Genetic Associations between ATG5 and Lupus Nephritis by trans-eQTL. J Immunol Res. 2015;2015:153132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou XJ, Lu XL, Lv JC, et al. Genetic association of PRDM1-ATG5 intergenic region and autophagy with systemic lupus erythematosus in a Chinese population. Ann Rheum Dis. 2011;70(7):1330–1337. [DOI] [PubMed] [Google Scholar]
- 19.Sun C, Molineros JE, Looger LL, et al. High-density genotyping of immune-related loci identifies new SLE risk variants in individuals with Asian ancestry. Nat Genet. 2016;48(3):323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang YM, Cheng FJ, Zhou XJ, Qi YY, Zhao MH, Zhang H. Rare Variants of ATG5 Are Likely to Be Associated With Chinese Patients With Systemic Lupus Erythematosus. Medicine (Baltimore). 2015;94(22):e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster C, Gerold KD, Schober K, et al. The Autoimmunity-Associated Gene CLEC16A Modulates Thymic Epithelial Cell Autophagy and Alters T Cell Selection. Immunity. 2015;42(5):942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang W, Tang H, Zhang Y, et al. Meta-analysis followed by replication identifies loci in or near CDKN1B, TET3, CD80, DRAM1, and ARID5B as associated with systemic lupus erythematosus in Asians. Am J Hum Genet. 2013;92(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu ZZ, Zhang JJ, Gao CC, et al. Expression of autophagy related genes mTOR, Becline-1, LC3 and p62 in the peripheral blood mononuclear cells of systemic lupus erythematosus. Am J Clin Exp Immunol. 2017;6(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Towns R, Kabeya Y, Yoshimori T, et al. Sera from patients with type 2 diabetes and neuropathy induce autophagy and colocalization with mitochondria in SY5Y cells. Autophagy. 2005;1(3):163–170. [DOI] [PubMed] [Google Scholar]
- 25.Clarke AJ, Ellinghaus U, Cortini A, et al. Autophagy is activated in systemic lupus erythematosus and required for plasmablast development. Ann Rheum Dis. 2015;74(5):912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Yue Y, Dong C, Shi Y, Xiong S. Blockade of macrophage autophagy ameliorates activated lymphocytes-derived DNA induced murine lupus possibly via inhibition of proinflammatory cytokine production. Clin Exp Rheumatol. 2014;32(5):705–714. [PubMed] [Google Scholar]
- 27.Weindel CG, Richey LJ, Bolland S, Mehta AJ, Kearney JF, Huber BT. B cell autophagy mediates TLR7-dependent autoimmunity and inflammation. Autophagy. 2015;11(7):1010–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gros F, Arnold J, Page N, et al. Macroautophagy is deregulated in murine and human lupus T lymphocytes. Autophagy. 2012;8(7):1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alessandri C, Barbati C, Vacirca D, et al. T lymphocytes from patients with systemic lupus erythematosus are resistant to induction of autophagy. Faseb J. 2012;26(11):4722–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierdominici M, Vomero M, Barbati C, et al. Role of autophagy in immunity and autoimmunity, with a special focus on systemic lupus erythematosus. Faseb J. 2012;26(4):1400–1412. [DOI] [PubMed] [Google Scholar]
- 31.Heckmann BL, Boada-Romero E, Cunha LD, Magne J, Green DR. LC3-Associated Phagocytosis and Inflammation. J Mol Biol. 2017. 24;429(23):3561–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Choi ME. Autophagy in kidney health and disease. Antioxid Redox Signal. 2014;20(3):519–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huber TB, Edelstein CL, Hartleben B, et al. Emerging role of autophagy in kidney function, diseases and aging. Autophagy. 2012;8(7):1009–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anders HJ, Schlondorff DO. Innate immune receptors and autophagy: implications for autoimmune kidney injury. Kidney Int. 2010;78(1):29–37. [DOI] [PubMed] [Google Scholar]
- 35.Hartleben B, Wanner N, Huber TB. Autophagy in glomerular health and disease. Semin Nephrol. 2014;34(1):42–52. [DOI] [PubMed] [Google Scholar]
- 36.Hartleben B, Godel M, Meyer-Schwesinger C, et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120(4):1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freedman BI, Langefeld CD, Andringa KK, et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol. 2014;66(2):390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Law HK. The Role of Autophagy in Lupus Nephritis. Int J Mol Sci. 2015;16(10):25154–25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwamoto T, Niewold TB. Genetics of human lupus nephritis. Clin Immunol. 2017;185:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stylianou K, Petrakis I, Mavroeidi V, et al. The PI3K/Akt/mTOR pathway is activated in murine lupus nephritis and downregulated by rapamycin. Nephrol Dial Transplant. 2011;26(2):498–508. [DOI] [PubMed] [Google Scholar]
- 41.Reddy PS, Legault HM, Sypek JP, et al. Mapping similarities in mTOR pathway perturbations in mouse lupus nephritis models and human lupus nephritis. Arthritis Res Ther. 2008;10(6):R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagai K, Tominaga T, Ueda S, et al. Mesangial Cell Mammalian Target of Rapamycin Complex 1 Activation Results in Mesangial Expansion. J Am Soc Nephrol. 2017;28(10):2879–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oaks Z, Winans T, Huang N, Banki K, Perl A. Activation of the Mechanistic Target of Rapamycin in SLE: Explosion of Evidence in the Last Five Years. Curr Rheumatol Rep. 2016;18(12):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bride KL, Vincent T, Smith-Whitley K, et al. Sirolimus is effective in relapsed/refractory autoimmune cytopenias: results of a prospective multi-institutional trial. Blood. 2016;127(1):17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou XJ, Cheng FJ, Zhang H. Emerging view of autophagy in systemic lupus erythematosus. Int Rev Immunol. 2015;34(3):280–292. [DOI] [PubMed] [Google Scholar]
- 46.Andrews BS, Eisenberg RA, Theofilopoulos AN, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148(5):1198–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13(3):630–638. [DOI] [PubMed] [Google Scholar]
- 48.Liu WJ, Luo MN, Tan J, et al. Autophagy activation reduces renal tubular injury induced by urinary proteins. Autophagy. 2014;10(2):243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang L, Zhou Y, Cao H, et al. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. Plos One. 2013;8(4):e60546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiser J, Oh J, Shirato I, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279(33):34827–34832. [DOI] [PubMed] [Google Scholar]
- 51.Rico M, Mukherjee A, Konieczkowski M, et al. WT1-interacting protein and ZO-1 translocate into podocyte nuclei after puromycin aminonucleoside treatment. Am J Physiol Renal Physiol. 2005;289(2):F431–F441. [DOI] [PubMed] [Google Scholar]
- 52.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368(7):651–662. [DOI] [PubMed] [Google Scholar]
- 53.Wang F, Li B, Schall N, Wilhelm M, Muller S. Assessing Autophagy in Mouse Models and Patients with Systemic Autoimmune Diseases. Cells. 2017;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weindel CG, Richey LJ, Mehta AJ, Shah M, Huber BT. Autophagy in Dendritic Cells and B Cells Is Critical for the Inflammatory State of TLR7- Mediated Autoimmunity. J Immunol. 2017;198(3):1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorenz G, Desai J, Anders HJ. Lupus nephritis: update on mechanisms of systemic autoimmunity and kidney immunopathology. Curr Opin Nephrol Hypertens. 2014;23(3):211–217. [DOI] [PubMed] [Google Scholar]
- 56.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174(5):2499–2506. [DOI] [PubMed] [Google Scholar]
- 57.Fairhurst AM, Xie C, Fu Y, et al. Type I interferons produced by resident renal cells may promote end-organ disease in autoantibody-mediated glomerulonephritis. J Immunol. 2009;183(10):6831–6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dos SM, Poletti PT, Milhoransa P, Monticielo OA, Veronese FV. Unraveling the podocyte injury in lupus nephritis: Clinical and experimental approaches. Semin Arthritis Rheum. 2017;46(5):632–641. [DOI] [PubMed] [Google Scholar]
- 59.van Paassen P, Duijvestijn A, Debrus-Palmans L, Damoiseaux J, Vroomen M, Tervaert JW. Induction of endothelial cell apoptosis by IgG antibodies from SLE patients with nephropathy: a potential role for anti-endothelial cell antibodies. Ann N Y Acad Sci. 2007;1108:147–156. [DOI] [PubMed] [Google Scholar]
- 60.Chan TM, Leung JK, Ho SK, Yung S. Mesangial cell-binding anti-DNA antibodies in patients with systemic lupus erythematosus. J Am Soc Nephrol. 2002;13(5):1219–1229. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Z, Weinstein E, Tuzova M, et al. Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum. 2005;52(2):522–530. [DOI] [PubMed] [Google Scholar]
- 62.Migliorini A, Angelotti ML, Mulay SR, et al. The antiviral cytokines IFN-alpha and IFN-beta modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am J Pathol. 2013;183(2):431–440. [DOI] [PubMed] [Google Scholar]
- 63.Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis Rheum. 1994;37(2):289–297. [DOI] [PubMed] [Google Scholar]
- 64.Yu L, McPhee CK, Zheng L, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465(7300):942–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cina DP, Onay T, Paltoo A, et al. MTOR regulates autophagic flux in the glomerulus. Autophagy. 2012;8(4):696–698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.