Abstract
Sleep disruption has been associated with increased risks for several major chronic diseases that develop over decades. Differences in sleep/wake timing between work and free days can result in the development of social jetlag (SJL), a chronic misalignment between a person’s preferred sleep/wake schedule and sleep/wake timing imposed by his/her work schedule. Only a few studies have examined the persistence of SJL or sleep disruption over time. This prospective investigation examined SJL and sleep characteristics over a two-year period to evaluate whether SJL or poor sleep were chronic conditions during the study period. SJL and sleep measures (total sleep time [TST], sleep onset latency [SOL], wake after sleep onset [WASO]), and sleep efficiency [SE]), were derived from armband monitoring among 390 healthy men and women 21–35 years old. Participants wore the armband for periods of 4–10 days at 6-month intervals during the follow-up period (N=1,431 repeated observations).
The consistency of SJL or sleep disruption over time was analyzed using generalized linear mixed models (GLMMs) for repeated measures. Repeated measures latent class analysis (RMLCA) was then used to identify subgroups among the study participants with different sleep trajectories over time. Individuals in each latent group were compared using GLMMs to identify personal characteristics that differed among the latent groups.
Minor changes in mean SJL, chronotype, or TST were observed over time, whereas no statistically significant changes in SOL, WASO, or SE were observed during the study period. The RMLCA identified two groups of SJL that remained consistent throughout the study (low SJL, mean±SE: 0.4±0.04 h, 42% of the study population; and high SJL, 1.4±0.03 h, 58%). Those in the SJL group with higher values tended to be employed and have an evening chronotype.
Similarly, two distinct subgroups were observed for SOL, WASO, and SE; one group with a pattern suggesting disrupted sleep over time, and another with a consistently normal sleep pattern. Analyses of TST identified three latent groups with relatively short (5.6±1.0 h, 21%), intermediate (6.5±1.0 h, 44%) and long (7.3±1.0 h, 36%) sleep durations, all with temporally stable, linear trajectories. The results from this study suggest that sleep disturbances among young adults can persist over a two-year period. Latent groups with poor sleep tended to be male, African American, lower income, and have an evening chronotype relative to those with more normal sleep characteristics. Characterizing the persistence of sleep disruption over time and its contributing factors could be important for understanding the role of poor sleep as a chronic disease risk factor.
Keywords: actigraphy, chronotype, social jetlag, repeated measures latent class analysis
INTRODUCTION
Sleep disturbances have been associated with increased risks for major chronic diseases such as obesity (Littner et al., 2003), hypertension and cardiovascular disease (Vgontzas et al., 2009; Cappuccio et al., 2011), diabetes (Ayas et al., 2003; Cespedes et al., 2016), and cancer (Sigurdardottir et al., 2012; Gu et al., 2016). The biological processes associated with these relationships may include immunologic, metabolic, and genetic factors (Vgontzas et al., 2002; Taheri et al., 2004; Bass & Takahashi, 2010; Mullington et al., 2010; Stamatakis & Punjabi, 2010; Archer et al., 2014; Rutters et al., 2014; Morris et al., 2015).
An association of sleep disruption with pathophysiological changes that can lead to chronic disease is more plausible for chronic rather than transitory sleep disruption. However, the persistence of sleep disruption over time has not been studied extensively. Among US adolescents ages 13–16 years, 88% of those with the history of insomnia also had current insomnia symptoms (Johnson et al., 2006).
In longitudinal studies that have examined relationships between sleep duration and weight gain, obesity or arterial hypertension, information about sleep was obtained only at a single time point via questionnaire (Gangwisch et al., 2005; Gangwisch et al., 2006; Patel et al., 2006; Chaput et al., 2008). Furthermore, it was assumed that self-reported data accurately described an individual’s sleep characteristics over time. However, sleep assessed by self-report can differ from sleep assessed using polysomnography or actigraphy (Baker et al., 1999; Kushida et al., 2001).
A few studies have investigated the variability of sleep characteristics over prolonged periods of time using objective measures, such as polysomnography or actigraphy (Hoch et al., 1994; Hoch et al., 1997; Knutson et al., 2007; Gaines et al., 2015). In middle-aged people, sleep duration, efficiency, latency and time in bed showed considerable day-to-day variability, although they were stable during a 1- to 3-year follow-up period (Hoch et al., 1994; Hoch et al., 1997; Knutson et al., 2007; Gaines et al., 2015). No comparable studies have been conducted among young adults. The importance of such investigations is based on the relatively high prevalence of sleep disturbances in the general population (Ohayon, 2002; Gaultney, 2010) and a common knowledge that many chronic diseases that manifest in middle age usually develop over decades.
Most physiological processes and behaviors in humans exhibit a circadian rhythm (Roenneberg & Foster, 1997; Mistlberger & Rusak, 2000). Intrinsic variations in biological timing have led to the categorization of individuals as morning, intermediate, or evening chronotypes (Horne & Ostberg, 1977; Roenneberg et al., 2003). People with a morning chronotype, the so-called “larks”, wake up and go to bed relatively early on free days (approximate wake-up time 5:30–7:30 a.m. and bedtime 10 p.m.−12:00 a.m.), whereas evening chronotypes (“owls”), prefer to wake up and go to bed late (approximate wake-up time 9:00–11:00 a.m. and bedtime between 1:00–3:00 a.m.) (Roenneberg et al., 2003). As much as 50% of chronotype may be genetically determined (Vink et al., 2001).
Chronotype may vary by age and sex (Roenneberg et al., 2007) (Roenneberg et al., 2007). For instance, chronotype tends to be relatively early in childhood, to gradually delay reaching the peak of “lateness” in early adulthood and to become progressively earlier with advanced age. Women generally reach the peak of their eveningness slightly earlier than men, and, on average, tend to have earlier chronotypes than men until menopause. After age 60, chronotype becomes very similar for men and women (Roenneberg et al., 2007). Since the above observations were based on the analyses of cross-sectional population surveys, they may have been affected by unaccounted cohort effects. For instance, some evidence suggests that eveningness has become more prevalent over the last three decades (Broms et al., 2014).
The temporal stability of chronotype within the same individuals has been scarcely explored. To address this question, the Munich Chronotype Questionnaire (MCTQ) was used to examine whether a respondent’s chronotype changed over time (Roenneberg et al., 2007). In that study, temporal changes in chronotype of individuals were similar to age-dependent chronotype changes in a cross-sectional population survey. Compared to their current chronotype, participants recalled being delayed in teenage years and less advanced in their childhood (Roenneberg et al., 2007). The MCTQ was validated against sleep logs (Kühnle, 2006), Morningness-Eveningness Questionnaire (MEQ) (Zavada et al., 2005), actigraphy and diurnal rhythms of cortisol and melatonin (Roenneberg et al., 2007); however, it also had a tendency to overestimate extreme chronotypes compared to sleep logs (Kühnle, 2006). In another study that defined an individual’s chronotype based on a single question, those who had evening chronotypes in 1985 were found to have different circadian preference 23 years later (Broms et al., 2014). Others have reported that Delayed Sleep Phase Disorder, which is characterized by extreme evening preference tends to start in adolescence, persist in adulthood, but can decrease with age (Crowley et al., 2007; Paine et al., 2014).
A conflict between one’s sleep/wake preference and social demands can result in a misalignment in sleep/wake timing, or “social jetlag” (SJL) (Wittmann et al., 2006). SJL is calculated as a difference between mid-sleep on free and work days (Wittmann et al., 2006). It reflects the potential magnitude and direction of time shift for daily activities as well as physiological and psychological processes (Valdez et al., 1996; Bass & Takahashi, 2010; Roenneberg et al., 2012). Earlier studies have described manifestations similar to this phenomenon as a weekend bedtime delay (Wolfson & Carskadon, 1998; Moore & Meltzer, 2008). For example, adolescents and college students had delayed sleep and wake times on weekends compared to school days, which coincided with a delayed circadian phase (Valdez et al., 1996; Wolfson & Carskadon, 1998; Crowley et al., 2007). Late wake up times on the weekend reflected an attempt to compensate for a sleep debt accumulated on school days (Valdez et al., 1996).
Circadian desynchronization has been associated with metabolic disturbances, altered melatonin or cortisol secretion, and inflammation (Bass & Takahashi, 2010; Leproult et al., 2014; Rutters et al., 2014; Morris et al., 2015). These impacts are commonly observed among shift workers, although some studies suggest that this relationship may extend to populations not involved in shift work, including those with SJL (Valdez et al., 1996; Antunes et al., 2010; Levandovski et al., 2011; Roenneberg et al., 2012; Kantermann et al., 2013; Wirth et al., 2014; Wong et al., 2015).
Although clinically relevant cut-points for SJL have not been established, those with ≥2 h of SJL had shorter sleep duration, higher 5-hour blood cortisol levels, and a higher resting heart rate relative to those with ≤1 h of SJL (Rutters et al., 2014). In addition, those with >2 h of SJL had higher depression scores than those with ≤2 h (Levandovski et al., 2011). Similarly, adolescents with sleep durations on school nights <6 h 45 min and/or weekend bedtime delays of >2 h had depressive mood, daytime sleepiness and sleep/wake behavior problems more often than those who slept >8 h 15 min, or had a weekend delay of <60 min (Wolfson & Carskadon, 1998). Consistent with SJL are the associations of eveningness in adolescents or young adults with poor subjective sleep quality, poor academic performance and behavioral problems (Giannotti et al., 2002; Merikanto et al., 2016; Merikanto et al., 2017).
To date, no prospective studies have examined the persistence of SJL over time. The objective of this study was to quantify sleep/wake characteristics among young adults over a 2-year period to examine the persistence of sleep disturbances and SJL over time, and to identify demographic or other factors that differed among those with and without persistent SJL or sleep disruption.
METHODS
Study population
Eligible individuals were 21–35 years of age with a body mass index (BMI) of 20–35 kg/m2 and no major health conditions or large changes in health behaviors or body composition during the previous 6 months, or a pregnancy or childbirth during past 12 months (Hand et al., 2013). Part of the rationale for recruitment from this age group was the potential for decreasing metabolic rate and an increasing weight and percent body fat that may be encountered among individuals in this age range (Ogden et al., 2014; Ogden et al., 2016). Individuals with major depression, anxiety disorder and panic attacks, including those who took selective serotonin reuptake inhibitors, were excluded at baseline. Participants also were excluded at baseline if they had: systolic blood pressure ≥150 mmHg, and/or diastolic blood pressure ≥90 mmHg, or a blood glucose level >145 mg/dl, which are used as diagnostic criteria for hypertension and diabetes, respectively (Hand et al., 2013).
Prospective participants were asked about their demographic and health characteristics via the internet, followed by a telephone interview to establish eligibility. Those who were considered eligible were invited for an orientation session and three baseline screenings. Out of 1,831 persons contacted, 1,116 were considered ineligible, 66 declined to participate, and 169 were lost to follow-up or dropped out prior to or at baseline (Hand et al., 2013). In 2011, 430 participants residing in the Columbia, SC region were enrolled.
After a comprehensive baseline assessment (demographics, health history, anthropometrics, diet, and actigraphy), participants were re-examined every 6 months to assess their anthropometric characteristics, as well as their sleep/wake patterns via armband actigraphy. Each participant completed 1–5 assessments over the 2-year study period (2011–2013). The study was approved by the University of South Carolina Institutional Review Board (report #00000240), and all participants provided written informed consent.
Sleep, chronotype, and social jet lag
Sleep measures were obtained using a SenseWear® Mini Armband worn by study participants over the triceps muscle of the left arm (Sharif & Bahammam, 2013). A sensor in the armband recorded limb movements at a sampling frequency of 32 times per second averaged over 1-minute intervals. All participants wore the armband for 4–10 days (minimum of three weekdays and one weekend day) and kept a log of their activities during non-wear periods. Information on sleep and physical activity from the log was used to fill-in the non-wear period gaps in the armband minute-by-minute data before calculating summary measures (Hand et al., 2013). Activities reported on the log were assigned Metabolic Equivalent of Task (MET) values from the 2011 Compendium of Physical Activities (Ainsworth et al., 2011) and used with measured resting metabolic rate (RMR) to calculate energy expenditure. Analogously, periods of sleep or lying down for the non-wear periods were assigned either a 0 or a 1 in the minute-by-minute data. A compliant day was defined as having verifiable wear time (actual wear plus data from the log) for at least 80% of the 24-hour day; observations with wear time <80% were excluded from analysis.
Minute-by-minute armband data were used to calculate sleep parameters taking into account individual demographic information (i.e., age, sex, height, weight, smoking status). The following average night-time sleep measures for work and free days were calculated for every 4–10 day period of armband wear: sleep onset and wake-up times, total sleep time (TST), sleep onset latency (SOL), wake after sleep onset (WASO), and sleep efficiency (SE) (Wirth et al., 2015). The sleep characteristics mentioned above quantify different albeit overlapping domains of sleep, and are commonly used for objective assessment of sleep using actigraphy data (Kushida et al., 2001; Ohayon et al., 2004; Natale et al., 2009; Natale et al., 2014), thus facilitating comparisons among studies. Time of sleep onset was defined as the first of three minutes asleep that coincided with ≥10 minutes lying down. SOL was defined as the time between lying down and sleep onset. Wake-up time was defined as the first of 90 consecutive minutes awake following sleep onset. TST was defined as the sum of all minutes asleep from initiation of the sleep period until wake-up time. SE was calculated as a proportion of the total sleep time to the length of time in bed. WASO was calculated as the sum of wake periods of at least two minutes duration between sleep onset and the final wake time. Previous validation studies showed that SenseWear™ armband-derived data for TST, SE and WASO were consistent with data derived from polysomnography (Ramin et al., 2013; Sharif & Bahammam, 2013; Soric et al., 2013).
Chronotype was defined as the time of mid-sleep on free days corrected for “make-up sleep” on free days (Roenneberg et al., 2012). SJL was defined as a difference between unadjusted midpoints of sleep on a weekend and week day (Roenneberg et al., 2012). Analyses were performed on two continuous SJL variables; the actual value (SJL) (which can include negative numbers), and the absolute value of SJL (absolute SJL) (Valdez et al., 1996; Wittmann et al., 2006; Roenneberg et al., 2012). For all measures, the average value for one 4–10 day period of armband use for a single participant is referred to as an ‘observation’.
Observations were excluded from statistical analyses if armband data had any of the following characteristics: missing bed- or wake-time (n=40 observations), <4 days of data in a given assessment period (n=51 observations), missing sleep data on the weekend (n=38 observations), extreme or implausible TST values (<4 hours either on weekdays, free days, or on average (n=71 observations), TST >11 hours on work days or on average (n=1 observation), or mid-sleep on free days occurring in the afternoon (n=2 observations) (Roenneberg et al., 2012). Participants were excluded if they: regularly used sleep-promoting medications (≥3 times per week by prescription or over the counter, n=22), worked night shifts (n=2), or traveled across time zones during periods of armband use (n=1) (Roenneberg et al., 2012; Paine et al., 2014). Information on work schedule was not available and it was assumed that free days occurred only on weekends. Work on weekends could possibly result in a negative SJL value, thus ancillary analyses were performed by excluding individuals with negative SJL values to evaluate the potential influence of those participants relative to results among all participants.
Covariates
Total daily hours of physical activity were defined as a sum of all activities of at least 3 metabolic equivalents (MET). Average daily napping time was calculated from the minute-by-minute armband data. Estimates of dietary intake were derived from random 24-hour dietary recall interviews (24HR), collected on non-consecutive days by telephone interview. Up to 3 interviews (>97% completed at least 2 interviews) were conducted by trained, registered dietitians, two interviews for weekday and one for weekend day consumption (Hand et al., 2013). The 24HR is the method with the lowest overall variance and, therefore, total error (Hebert et al., 1998) and three days is the optimal number of days needed to compute total caloric intake (Ma et al., 2009; Hébert et al., 2010). The Nutrient Data System for Research (version 2012: Nutrition Coordinating Center, University of Minnesota, Minneapolis, Minnesota) was used to estimate intakes of individual foods and nutrients from the 24HR. Forty-three food parameters (including whole foods and nutrients) were used to calculate the dietary inflammatory index (DII)™ score, which expresses the inflammatory potential of each individual’s diet (Shivappa et al., 2014). Lower DII scores indicate that the diet is more anti-inflammatory, while the higher scores indicate that the diet is more proinflammatory (maximum theoretical range: −8.87 to 7.98). To account for individual differences in energy intake, the DII scores were calculated per 1,000 kilocalories (4,184 kJ) (E-DII), as previously described (Wirth et al., 2015). Height and weight were measured using traditional stadiometer and electronic scales with a precision of 0.1 cm and 0.1 kg, respectively (Hand et al., 2013). The average of three measurements was used to calculate an individual’s BMI (weight [kg]/height [m2]).
Statistical analyses
All statistical analyses were performed using SAS® 9.4 software (Cary, NC).
Participants who were excluded from the analyses were compared with those who were included with respect of their baseline demographic characteristics using t-tests for continuous variables and Chi-square tests for categorical variables. The stability of SJL, absolute SJL, and sleep measures (TST, SOL, WASO, SE) over time was examined using generalized linear mixed models (GLMMs) for repeated measures with an unstructured covariance matrix, and normal or log-normal distributions with an identity link function. Time was treated as the explanatory variable of interest in crude and adjusted statistical models. The following covariates were considered for inclusion in adjusted analyses: sex (male, female), race (European American [EA], African American [AA], Other [Hispanic, Asian, Native American and mixed race]), education (high school graduate/GED, or some college vs. college ≥ 4 y.), annual income (<$20,000, $20,000 to <$40,000, $40,000 to <$60,000, $60,000 to <$80,000, ≥$80,000), employment (student/other, employed/self-employed), marital status (married, single), having children (yes/no), physical activity (h/d), caffeine intake (g/d), E-DII, napping (yes [>0 min/d] vs. no [0 min/d]), season (winter [November-January], spring [February-April], summer [May-July], autumn [August-October]), BMI (kg/m2, continuous), current dieting (yes/no), and the number of data collection time points for each person in the analysis.
To select potential covariates, bivariate relationships with each sleep characteristic were summarized, and variables with p-value <0.20 were selected for further evaluation. Variable selection was performed by identifying covariates for inclusion in the final model that changed the effect estimate of the main exposure variable by ±10%. Variables that were statistically significant (p≤0.05) also were included in the final model. SJL, absolute SJL and each sleep characteristic were modeled separately as a continuous variable. These analyses included all eligible participants with valid data (N=390, 1,431 observations). PROC GLIMMIX in SAS® was used to compute least squares means of continuous sleep variables, SJL, or chronotype, which were then compared among different time points using F-tests to evaluate the overall time trend.
Repeated measures latent class analysis (RMLCA) was performed using PROC TRAJ in SAS® to identify latent groups for each continuous outcome variable (SJL, absolute SJL, TST, SOL, SE, WASO) and chronotype. This analysis assumes a mixture model to define the trajectories of unique subgroups that do not change their group membership within a population over time (Nagin, 2005; Jones & Nagin, 2007). The best fitting crude model was selected using the Bayesian Information Criterion (Jones & Nagin, 2007). Final selection of the number of latent groups was based on the model fit and minimum group sizes containing ≥10% of the study population. Analyses for chronotype, TST and SE were adjusted for race (EA vs. AA or Other) and sex, which were assumed not to change over time. WASO was adjusted for race only. SJL, absolute SJL and SOL were not adjusted for race or sex because the latent groups were not influenced by these variables. Because the group trajectories for each measure were linear, the potential influence of covariates that changed over time was not examined. Only participants with at least three assessments during the study period were included in these analyses (N=312, 1,297 observations). Finally, GLMMs were estimated to identify demographic and other characteristics that differed among the latent classes for absolute SJL and each of the sleep outcomes. For continuous variables, relationships between latent groups were analyzed using an identity link and a normal distribution. For categorical variables, this analysis was performed using a binary distribution with logit link, or a multinomial distribution with a cumulative logit link. Relationships between SJL (continuous, independent variable) and each sleep measure (continuous, dependent variable) were analyzed using GLMMs with adjustment for confounding variables. Finally, the difference between each sleep measure on week days versus weekends was calculated at baseline and then correlated with SJL values at baseline using Spearman correlation coefficients.
RESULTS
The study population was comprised of 390 participants with a total of 1,431 repeated observations. A majority of the participants had at least three (26%), four (15%), or five (39%) assessments at 6-month intervals. The average age at baseline was 28±4 years and the sex distribution was approximately equal (51% women, Table 1). Most participants were EA (68%) and had at least 4 years of college education (84%). College students comprised 45% of participants (19% undergraduate, 81% graduate), and another 55% were employed. A majority of the participants’ annual income was below $60,000 (71%). Thirty-two percent were married, and 14% had children (Table 1). Overall compliance with the armband protocol was very high; the median time of armband use was 23.5 h/day. Most participants wore the armband for 9 or more days (6 days for 0.3% of all time points; 7 days, 1.5%; 8 days, 4%; 9 days, 13%; 10 days, 66%; 11–16 days, 15%). Participants who were excluded (n=40) were not different from those included in the analyses with respect to demographic characteristics: age (p=0.06), sex (p=0.93), race (p=0.27), education (p=0.50), income (p=0.53), employment (p=0.99), or marital status (p=0.60). There were modest differences with respect of having children (14% among participants vs. 25% among those excluded, p=0.05).
Table 1.
Demographic Characteristics of Eligible Energy Balance Study Participants at Baseline, Columbia, SC, 2011–2014
| Variable | All Participants (n=390) |
Women (n=198) |
Men (n=192) |
|---|---|---|---|
| Age (yrs) | 27.6±3.8 | 27.7±3.7 | 27.4±3.9 |
| Race, n (%) | |||
| European American | 264 (68) | 131 (66) | 133 (70) |
| African American | 47 (12) | 31 (16) | 16 (8) |
| Hispanic/Latino | 11 (3) | 7 (3) | 4 (2) |
| Asian | 42 (11) | 15 (8) | 27 (14) |
| Native American | 12 (3) | 8 (4) | 4 (2) |
| Mixed race | 14 (3) | 6 (3) | 8 (4) |
| Education, n (%) | |||
| HS Graduate/GED Some College |
62 (16) | 18 (9) | 44 (23) |
| College (4+ years) | 328 (84) | 180 (91) | 148 (77) |
| Income ($), n (%) | |||
| < 20,000 | 65 (17) | 34 (17) | 31 (16) |
| 20,000 to < 40,000 | 137 (35) | 76 (39) | 61 (32) |
| 40,000 to < 60,000 | 74 (19) | 37 (19) | 37 (19) |
| 60,000 to < 80,000 | 50 (13) | 23 (12) | 27 (14) |
| ≥ 80,000 | 62 (16) | 26 (13) | 36 (19) |
| Employment, n (%) | |||
| Employed and self employed | 214 (55) | 113 (57) | 101 (53) |
| Student/Other1 | 176 (45) | 85 (43) | 91 (47) |
| Marital status, n (%) | |||
| Married2 | 178 (46) | 90 (45) | 88 (46) |
| Single3 | 212 (54) | 108 (55) | 104 (54) |
| Children, age | |||
| <18 years, n (%) | |||
| 0 | 336 (86) | 173 (88) | 163 (85) |
| 1 | 29 (7) | 13 (7) | 16 (8) |
| ≥ 2 | 24 (7) | 11 (5) | 13 (7) |
Out of work (<1 year), homemaker, unable to work (n=4, 1%).
Includes members of unmarried couples (n=55, 14%).
Includes divorced and separated (n=7, 1.7%).
At baseline, 14.3% of participants had negative SJL values (≤1 h 11.5%, >1 but <2 h 2.1%; ≥2 h 0.7%), indicating that they advanced their sleep schedule on their free days. A majority (85.7%) had positive SJL values with the following distribution: ≤1 h: 37.7%; >1 but <2 h: 32.8%; and ≥2 h: 15.2%. Also at baseline, ~50% of participants had ≤1 h of absolute SJL, 33% had >1 but <2 h, and 17% had ≥2 h of absolute SJL. At baseline, SJL was moderately correlated with chronotype (Spearman r=0.42, p<0.001).
Adjusted mean SJL values at 6, 12, 18 and 24 months exceeded the baseline average by 6 min, and there was no linear trend for SJL over two years (Table 2). A 10-min increase from 1 h 6 min to 1 h 16 min was observed for absolute SJL across the study period (p=0.03, data not shown). Chronotype was 24 min earlier by the end of two years (p<0.01). TST, SOL, WASO and SE were generally stable during the study period, although a difference in mean TST was observed at 12 months relative to 6 months (Table 2).
Table 2.
Objective Sleep Measures Over Time (n=390, 1,431 observations), Energy Balance Study, Columbia, SC, 2011–2014
| Sleep measure | Follow-up Time (Months) | p-value: Time Period1 | ||||
|---|---|---|---|---|---|---|
| Baseline (n=390) |
6 (n=341) |
12 (n=317) |
18 (n=206) |
24 (n=177) |
||
| Mean±SE | Mean±SE | Mean±SE | Mean±SE | Mean±SE | ||
| Social Jet Lag (h) | ||||||
| Crude | 1.2±0.04 | 1.1±0.04 | 1.1±0.05 | 1.2±0.06 | 1.2±0.06 | 0.78 |
| Adjusted2 | 0.8±0.06 | 0.9±0.06 | 0.9±0.06 | 0.9±0.07 | 0.9±0.07 | 0.40 |
| Chronotype (h) | ||||||
| Crude | 4.4±0.1 | 4.2±0.1* | 4.1±0.1* | 4.1±0.1* | 4.0±0.1* | <0.01 |
| Adjusted3 | 4.4±0.1 | 4.2±0.1* | 4.1±0.1* | 4.0±0.1* | 4.0±0.1* | <0.01 |
| Total Sleep Time (h) | ||||||
| Crude | 6.6±0.04 | 6.6±0.04 | 6.5±0.04† | 6.6±0.05 | 6.6±0.05 | 0.10 |
| Adjusted4 | 6.5±0.05 | 6.5±0.05 | 6.4±0.05† | 6.5±0.06 | 6.5±0.06 | 0.05 |
| Sleep Onset Latency (min) | ||||||
| Crude | 12.9±0.3 | 13.0±0.3 | 12.9±0.3 | 13.8±0.4 | 12.6±0.4 | 0.15 |
| Adjusted5 | 12.8±0.4 | 12.8±0.4 | 12.7±0.4 | 13.6±0.4 | 12.4±0.5 | 0.16 |
| Wake After Sleep Onset (min) | ||||||
| Crude | 53.8±1.5 | 54.2±1.6 | 53.3±1.6 | 54.0±1.8 | 51.9±1.9 | 0.75 |
| Adjusted6 | 54.0±1.5 | 54.3±1.6 | 53.2±1.6 | 53.9±1.8 | 51.7±1.9 | 0.68 |
| Sleep Efficiency (%) | ||||||
| Crude | 82.3±0.3 | 82.4±0.3 | 82.4±0.4 | 82.1±0.4 | 82.6±0.4 | 0.76 |
| Adjusted7 | 81.2±0.4 | 81.3±0.4 | 80.3±0.4 | 82.0±0.5 | 81.5±0.5 | 0.77 |
F-test p-value for time period as a categorical variable.
Adjusted for time, chronotype, race, education, employment status, current dieting, number of time points.
Adjusted for time, age, sex and season.
Adjusted for time, chronotype, sex, race, napping, physical activity, season, BMI.
Adjusted for time, season, income, caffeine, nap, physical activity, BMI and number of time points.
Adjusted for time, race, BMI.
Adjusted for race, sex, income, energy-adjusted Dietary Inflammatory Index, physical activity.
p<0.05 relative to baseline.
p<0.05 relative to 6-month period.
Abbreviations: SE: Standard Error
Exclusion of observations with negative SJL values (i.e., earlier sleep schedule on weekends) did not change time trends for chronotype, SJL or other sleep measures. By the end of 2 years, chronotype advanced by 18 min from 4:30 to 4:12 a.m (p<0.01) (data not shown). Similar to the results for the entire sample (Table 2), SJL increased 6 min by the end of 2 years after exclusion of observations with negative values (p=0.45) (data not shown).
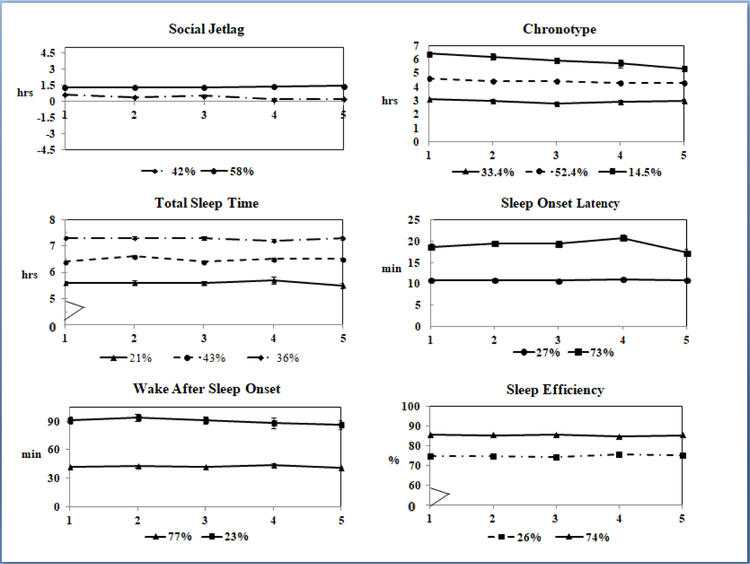
Results from the RMLCA, which only included individuals with ≥3 observations, indicated that latent groups for SJL and each sleep parameter had linear trajectories that remained stable over time (Figure 1). SJL had 2 latent groups, one with relatively low (mean±SE, 0.4±0.04 h, 42%) and another with higher values (1.4±0.03 h, 58%, Figure 1). Absolute SJL also had 2 latent groups with lower (1.1±0.02 h, 88%) and higher (1.7±0.2 h, 12%) values at baseline and linear trajectories (not shown). If the RMLCA sample was analyzed using the methods that generated results for Table 2, absolute SJL remained stable over the study period (overall p-value for time period: p=0.07, data not shown).
Figure 1.
Latent group trajectories for social jetlag, chronotype and objective sleep measures at five time points (baseline, 6 m, 12 m, 18 m, and 24 m) obtained with repeated measures latent class analysis (n=312, 1,297 observations). Only participants with ≥3 time points were included.
Three latent groups were apparent for chronotype: with relatively early (3.0±0.9 h, 33%), intermediate (4.4±0.9 h, 52%) and late (6.0±1.3 h, 14%) times. By the end of the study, a 23-minute shift to an earlier time was observed in the late chronotype group, whereas no changes were observed in the other groups (Figure 1). TST had three latent groups with relatively short (5.6±1.0 h, 21%), intermediate (6.5±1.0, 44%) and long (7.3±1.0 h, 36%) durations, all with temporally stable linear trajectories. SOL was generally low in this population (<30 minutes for 98% of observations, median: 13 min); two latent groups were identified with relatively lower (9.7±1.0 min, 73%) and higher (18.3±1.0 min, 27%) values. Two latent groups were observed for WASO: low (42.1±18.6 min, 77%) and high (90.4±31.0 min, 23%), and for SE: low (74.6±1.0%, 27%) and high SE (85.3±1.0%, 73%).
In bivariate analyses, those in the high SJL group tended to be employed and had later chronotypes relative to those in the low SJL group (Table 3). Participants in the short TST group were younger, male, of AA or Other race, had lower incomes, were married, consumed less caffeine, and tended to have a more proinflammatory diet (i.e., higher E-DII scores) compared with those in the intermediate TST group (Table 3). Those in the long TST group tended to be female, less physically active, have higher incomes and no children relative to those with an intermediate TST (Table 3). The SOL latent groups differed only by employment status; students had a higher SOL relative to those who were employed (Table 4). The two WASO groups differed only by race (Table 4); a higher proportion of those with elevated WASO values were of AA or Other race. Among those with low SE, a higher proportion was male, AA or Other race, had lower incomes and consumed less caffeine (Table 4). Overall, participants with sleep disturbances (e.g., high SOL, high WASO, short TST, or low SE) tended to have an evening chronotype.
Table 3.
Characteristics of Latent Groups: Social Jetlag, Total Sleep Time (n=312 participants; 1,297 observations), Energy Balance Study, Columbia, SC, 2011–2014
| Variable | Social Jet Lag (h) | p-value1 | Total Sleep Time (h) | p-value1 | |||
|---|---|---|---|---|---|---|---|
| Low | High | Short | Intermediate | Long | |||
| (0.4±0.04)2 | (1.4±0.03)2 | (5.6±0.04)2 | (6.5±0.02)2 | (7.3±0.03)2 | |||
| % | % | % | % | % | |||
| Sex | |||||||
| Male | 55 | 48 | 0.20 | 71* | 54 | 35* | <0.01 |
| Female | 45 | 52 | 29* | 46 | 65* | ||
| Race | |||||||
| European American | 64 | 68 | 27* | 71 | 82 | ||
| African American | 12 | 10 | 0.50 | 30* | 9 | 2 | <0.01 |
| Other3 | 24 | 22 | 43* | 20 | 16 | ||
| Employment | |||||||
| Student/Other4 | 52 | 41 | 0.05 | 50 | 46 | 43 | 0.70 |
| Employed | 48 | 59 | 50 | 54 | 57 | ||
| Income ($) | |||||||
| < 20,000 | 19 | 16 | 24* | 16 | 16 | ||
| 20,000 to < 40,000 | 36 | 38 | 43* | 38 | 30 | ||
| 40,000 to < 60,000 | 19 | 19 | 0.80 | 23* | 21 | 16 | 0.02 |
| 60,000 to < 80,000 | 13 | 14 | 4* | 16 | 16 | ||
| ≥ 80,000 | 13 | 13 | 6* | 9 | 22 | ||
| Marital status | |||||||
| Married5 | 46 | 46 | 0.96 | 70* | 50 | 50 | 0.02 |
| Single6 | 54 | 54 | 30* | 50 | 50 | ||
| Children | |||||||
| Yes | 17 | 11 | 0.10 | 13 | 18 | 8* | 0.10 |
| No | 83 | 89 | 87 | 82 | 92* | ||
| Mean±SE | Mean±SE | Mean±SE | Mean±SE | Mean±SE | |||
| Chronotype (h) | 3.8±0.1 | 4.3±0.05 | 0.01 | 4.6±0.1* | 4.1±0.1 | 3.9±0.05 | <0.01 |
| Age (yrs) | 27.8±0.2 | 27.6±0.1 | 0.30 | 27.0±0.2* | 28.0±0.2 | 27.7±0.2 | <0.01 |
| Physical activity (h/d) | 2.2±0.1 | 2.2±0.1 | 1.00 | 2.3±0.2 | 2.3±1.0 | 2.0±0.1* | 0.04 |
| Caffeine (g/d) | 109.0±8.6 | 117.4±6.8 | 0.50 | 79.3±11.7* | 120.2±7.9 | 126.5±8.8 | <0.01 |
| E-DII | 0.4±0.2 | 0.6±0.1 | 0.40 | 1.1±0.2* | 0.4±0.1 | 0.3±0.2 | 0.01 |
| BMI (kg/m2) | 25.3±0.3 | 25.3±0.3 | 0.94 | 26.0±0.5 | 25.2±.3 | 24.9±0.4 | 0.22 |
Overall F-test p-value for variable of interest.
Mean±SE in parentheses.
Hispanic/Latino, Asian, Native American, mixed race.
Out of work (<1 year), homemaker, unable to work.
Includes members of unmarried couples.
Includes divorced and separated.
p<0.05 versus intermediate group.
Abbreviations: SE - standard error, E-DII - energy-adjusted Dietary Inflammatory Index.
Table 4.
Characteristics of Latent Groups: Sleep Onset Latency, Wake After Sleep Onset, Sleep Efficiency (n=312 participants, 1,297 of observations), Energy Balance Study, Columbia, SC, 2011–2014
| Variable | Sleep Onset Latency | Wake After Sleep Onset | Sleep Efficiency | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | p-value1 | Low | High | p-value1 | High | Low | p-value1 | |
| 10.8±0.2 | 19.2±0.3 | 42.1±0.9 | 90.7±1.7 | 85.4±0.1 | 74.8±0.3 | ||||
| % | % | % | % | % | % | ||||
| Sex | |||||||||
| Male | 49 | 54 | 0.50 | 51 | 50 | 0.99 | 47 | 60 | 0.04 |
| Female | 51 | 46 | 49 | 50 | 53 | 40 | |||
| Race | |||||||||
| European American | 68 | 61 | 0.50 | 75 | 36 | <0.01 | 76 | 40 | <0.01 |
| African American | 11 | 11 | 7 | 24 | 7 | 20 | |||
| Other1 | 21 | 28 | 18 | 40 | 17 | 40 | |||
| Employment | |||||||||
| Student/other2 | 41 | 58 | 0.01 | 43 | 54 | 0.10 | 43 | 51 | 0.20 |
| Employed | 59 | 42 | 57 | 46 | 57 | 49 | |||
| Income ($) | |||||||||
| < 20,000 | 8 | 17 | 0.20 | 16 | 26 | 0.10 | 16 | 20 | 0.04 |
| 20,000 to < 40,000 | 33 | 46 | 34 | 45 | 32 | 48 | |||
| 40,000 to < 60,000 | 22 | 12 | 19 | 19 | 21 | 15 | |||
| 60,000 to < 80,000 | 14 | 13 | 16 | 5 | 14 | 11 | |||
| ≥ 80,000 | 13 | 12 | 15 | 5 | 16 | 6 | |||
| Marital status | |||||||||
| Married3 | 47 | 42 | 0.30 | 47 | 41 | 0.40 | 48 | 40 | 0.20 |
| Single4 | 53 | 58 | 53 | 59 | 52 | 60 | |||
| Children | |||||||||
| Yes | 12 | 18 | 0.30 | 14 | 12 | 0.60 | 14 | 11 | 0.40 |
| No | 88 | 82 | 86 | 88 | 86 | 89 | |||
| Mean±SE | Mean±SE | Mean±SE | Mean±SE | Mean±SE | Mean±SE | ||||
| Chronotype (h) | 4.0±0.04 | 4.3±0.01 | <0.01 | 4.0±0.04 | 4.4±0.1 | 0.01 | 4.0±0.1 | 4.4±0.1 | <0.01 |
| Age (yrs) | 27.64±0.1 | 27.8±0.2 | 0.40 | 27.7±3.6 | 27.6±4.2 | 0.30 | 27.9±0.1 | 27.1±0.2 | <0.01 |
| Physical activity (h/d) | 2.3±0.1 | 2.0±0.1 | 0.10 | 2.2±0.1 | 2.1±0.1 | 0.60 | 2.2±0.1 | 2.1±0.1 | 0.60 |
| Caffeine (g/d) | 114.3±3.8 | 114.2±6.4 | 0.80 | 119.9±6.1 | 94.6±11.2 | 0.05 | 124.2±6.2 | 87.2±10.1 | 0.01 |
| E-DII | 0.5±0.1 | 0.5±0.2 | 0.90 | 0.4±0.1 | 0.6±0.2 | 0.40 | 0.4±0.1 | 0.6±0.2 | 0.40 |
| BMI (kg/m2) | 25.0±0.2 | 26.0±0.4 | 0.05 | 25.2±0.2 | 25.5±0.5 | 0.64 | 25.0±0.3 | 25.9±0.4 | 0.06 |
Overall F-test p-value for variable of interest.
Mean±SE in parentheses.
Hispanic/Latino, Asian, Native American, mixed race.
Out of work (<1 year), homemaker, unable to work.
Includes members of unmarried couples.
Includes divorced and separated. Abbreviations: WASO stands for Wake After Sleep Onset, SE - Standard Error, E-DII - energy-adjusted Dietary Inflammatory Index.
Relationships between SJL and sleep characteristics are presented in Table 5. When all time points were included (time points 1–5, n=390, 1,431 observations), SJL was inversely associated with TST on week days (p=0.001) or with WASO on week days (p=0.04). An increase in SJL was associated with an increase in SE on weekends (p=0.001) or decrease in SOL on weekends (p=0.0001). At baseline, the difference between values on week day and weekend for TST was (mean±STD) −0.5±1.2 h, for SOL 2.6±9.4 min, for WASO −7.9±34.0 min, and for SE 0.5±6.6%. SJL was negatively correlated with week day-to-weekend difference in TST (Spearman r=−0.18, p<0.0005) and positively correlated with the week day-to-weekend difference in SOL (Spearman r=0.11, p=0.03). There were no relationships between SJL and the week day-to-weekend differences in WASO (Spearman r=−0.06, p=0.20) or in SE (Spearman r=0.02, p=0.63).
Table 5.
Relationships between Social Jetlag (predictor) and sleep measures (dependent variable) (n=390, 1,431 obs.), Energy Balance Study, Columbia, SC, 2011–2014
| Sleep measure | Crude1 | Adjusted | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | p | Estimate | SE | p | |
| Total Sleep Hours, week days2 (h) | −0.06 | 0.02 | <0.01 | −0.06 | 0.02 | <0.01 |
| Total Sleep Hours, weekend3 (h) | 0.04 | 0.03 | 0.19 | 0.03 | 0.03 | 0.30 |
| Sleep Onset Latency, week days4 (min) | 0.22 | 0.17 | 0.19 | 0.19 | 0.17 | 0.27 |
| Sleep Onset Latency, weekend5 (min) | −1.24 | 0.22 | <0.01 | −1.23 | 0.22 | <0.01 |
| WASO on week days6 (min) | −1.50 | 0.65 | 0.02 | −1.35 | 0.64 | 0.04 |
| WASO on weekends6 (min) | −1.06 | 0.93 | 0.26 | −0.80 | 0.93 | 0.39 |
| Sleep Efficiency, week days7 (%) | 0.14 | 0.13 | 0.28 | 0.22 | 0.12 | 0.08 |
| Sleep Efficiency, weekends7 (%) | 0.56 | 0.18 | <0.01 | 0.61 | 0.17 | <0.01 |
p - linear regression F-test p-value for the variable of interest;
Adjusted for time.
Adjusted for time, BMI, sex, race, physical activity (cont., hour), naps (yes/no).
Adjusted for time, BMI, sex, race, and physical activity (cont., hour).
Adjusted for time, sex, alcohol, physical activity (cont., hour).
Adjusted for time, sex, physical activity (cont., hour).
Adjusted for time, race.
Adjusted for time, race, average total sleep hours.
Abbreviations: SE - Standard Error, WASO - Wake After Sleep Onset.
DISCUSSION
SJL has been associated with symptoms of depression, as well as risk factors for metabolic syndrome, obesity, and cardiovascular disease (Valdez et al., 1996; Scheer et al., 2009; Levandovski et al., 2011; Roenneberg et al., 2012; Kantermann et al., 2013; Rutters et al., 2014; Wong et al., 2015). Thus, understanding the dynamics of SJL and factors that contribute to increased SJL risk may be important for developing disease prevention strategies targeting SJL or the disruption of sleep/wake timing.
To our knowledge, this was the first study that used actigraphy to prospectively quantify SJL in a non-clinical population of young adults. The baseline prevalence of SJL in the current study (>1 h: 50% of participants; ≥2 h: 17%) was somewhat lower than in previous studies of SJL among adults (≥1 h: 63–69%; ≥2 h: 26–33%) (Roenneberg et al., 2012; Rutters et al., 2014). This lower prevalence may have been due to a larger proportion of students (45%) in the current study, who can have somewhat different schedules relative to those who work full-time. Most participants (58%) were in the latent class of SJL that exceeded one hour (mean: 1.4 h at baseline; mean: 1.5 h at 24 months). SJL was more common among those with an evening chronotype and was higher among those who were employed compared to students or those not working. These observations agree with the concept that SJL results from misalignment between an individual’s social and biological timing (Wittmann et al., 2006), and with previous observations that adults with later chronotypes had greater SJL (Valdez et al., 1996; Levandovski et al., 2011; Roenneberg et al., 2012; Rutters et al., 2014; Parsons et al., 2015).
SJL in this study was inversely associated with TST on week days, which is in agreement with previous studies (Roenneberg et al., 2003; Rutters et al., 2014). Increases in SJL were associated with increased SE and decreased SOL on weekends. These findings suggest that sleep occurring within an individual’s preferred circadian window may have a higher quality, and that individuals who accumulated SJL during the week may have compensated for it on weekends (Roenneberg et al., 2007; Roenneberg et al., 2012). The inverse relationship between SJL and WASO on work days seems to contradict previous studies that showed decreased sleep quality and a higher number of nocturnal awakenings among participants with high SJL (Rutters et al., 2014). In the present study, once participants with high SJL fell asleep on work nights, they tended to stay asleep.
In a series of large cross-sectional internet surveys, SJL gradually decreased across older age groups and was larger among males than females (Wittmann et al., 2006; Roenneberg et al., 2012). In the present study, SJL tended to remain stable within the same individuals over time, whereas by the end of the two-year follow-up, chronotype shifted an average of 23 minutes earlier. Because the heritability of chronotype may be as high as 50% (Vink et al., 2001), one might not expect changes within a two-year span. One plausible explanation for this is that by the end of this study some student participants may have completed their education and started jobs with earlier schedules than they had while in school. However, no data were available to test this possibility.
The shift in chronotype observed in the present study is consistent with results from a large cross-sectional study that observed a shift in chronotype to earlier times among people in older age groups beginning at 20 years (Roenneberg et al., 2007). However, it is unclear whether this represented age-related changes, some form of adaptation, random variation, or unaccounted cohort effects (Roenneberg et al., 2012; Broms et al., 2014). Despite the phase advance by the end of two years, a corresponding decrease in SJL was not observed. The reason for this discrepancy is unclear and may be related to the fact that there was only a moderate correlation between chronotype and SJL (Spearman r=0.42 at baseline) and SJL increased only by 6–10 min.
To the authors’ knowledge, this also was the first longitudinal study to examine actigraphic sleep measures among young adults (21–35 years old) over an extended period. The linear trajectories of the sleep patterns observed in this study suggest that a relatively large proportion of this population had sleep disturbances that persisted for the entire study period. Based on the RMLCA, 21% of study participants consistently had a short TST, whereas 36% had long TST, 27% had elevated SOL, 32% had elevated WASO, and 27% had low SE. A previous cross-sectional study among college students (n=536) also identified a relatively high proportion of participants who had disrupted sleep: 33% had SE ≤85%, 41% had average sleep duration <7 hr, and 76% had sleep latency >15 min (Vargas et al., 2014).
Using TST values, three latent groups were identified with relatively short (mean: 5.6 h), intermediate (mean: 6.5 h) and long (mean: 7.3 h) sleep durations. The mean TST value in the short duration group was consistent with a cut-off value (<6 h) used to define disrupted sleep in prior studies (Lauderdale et al., 2009; Bailey et al., 2014; Mezick et al., 2014; Vgontzas et al., 2014), whereas the mean TST value in the long sleep duration group (7.3±0.03 h) was shorter than cut-points used in previous studies (Gottlieb et al., 2006; Cappuccio et al., 2011). However, it was comparable with cut-points of 6.5 h (Kripke et al., 2011) and 440 min (7.3 h) in other actigraphy studies (Natale et al., 2009). Individuals tend to overestimate their sleep duration relative to actigraphic measurements by up to one hour (Lauderdale et al., 2008; Mezick et al., 2014). The mean values for latent groups with low and high SOL in this study (11 and 19 min, respectively) allowed for segregation based on a previously suggested, actigraphy-based cut-point of 12 min (Natale et al., 2009), but were lower than the 30-minute value that has been used previously to characterize insomnia in clinical studies (Buysse et al., 2006; Schutte-Rodin et al., 2008). Mean values in the low and high latent groups for WASO (means: 42 and 91 min, respectively) were both above previously described cut-off points of 25 (Natale et al., 2009) or 30 minutes (Buysse et al., 2006; Schutte-Rodin et al., 2008) for insomnia. Finally, the mean values among latent groups of SE (low: 75%; high: 85%) identified two groups that were above and below a previously used clinical cut-point of 80% for insomnia (Buysse et al., 2006; Schutte-Rodin et al., 2008); however, they were both below suggested cut-points from two other actigraphy studies (87% and 92%) (Natale et al., 2009; Natale et al., 2014).
Some limitations of this investigation are noteworthy. Information on work schedules and alarm clock use on free days was not available and it was assumed that participants worked on week days and had free days only on weekends. This assumption also was used in a recent, nationally representative, cross-sectional study of the US population (Fischer et al., 2017). Approximately 45% of participants in the current study were students (81% at a graduate level), who typically attend classes and/or work part-time during the day. Large surveys indicate that 15–21% of adults ages 18–34 years work on weekends (Marucci-Wellman et al., 2016), and 70–80% use alarm clock on work days (Roenneberg et al., 2012; Weil, 2017). Thus, assuming that perhaps 50% of young adults may start their weekend job in the morning, we estimate that approximately 5–7% of sample may have used an alarm clock on a weekend. In addition, study subjects were excluded from the analyses if they had extreme or implausible TST values, regularly used sleep-promoting medications, worked night shifts, or traveled across time zones; thus, the potential impacts of these limitations may have been minimized. Mean (±STD) chronotype in this study (males: 4.3±1.6 h, females: 4.0±1.2 h) was within the range of estimates for the same age group in several European populations (Estonia, Germany, Scotland) (Allebrandt et al., 2014). Moreover, mean sex-specific chronotypes were generally in agreement with ranges for the same ages (20–34 y.) in a cross-sectional study among US adults for 2003–2014 (males: 4.9±2.4 to 3.6±2.2 h; females: 4.9±2.4 to 3.4±1.7 h) (Fischer et al., 2017). That study included participants who reported activities on a Friday or Saturday and it was assumed that the following day would be free, although no information was provided on whether the next day was actually free or if an alarm clock was used (Fischer et al., 2017). In another large study in the Finnish population, participants reported their chronotype based on a single question, and no data on work schedules and alarm clock use were collected (Broms et al., 2014). Those observations are consistent with other studies that found good agreement between instruments that used a single or limited number of questions to assess chronotype, and did not explicitly target alarm clock use, relative to more comprehensive measures (Chelminski et al., 2000; Zavada et al., 2005; Megdal & Schernhammer, 2007; Roenneberg et al., 2007; Erren, 2013; Levandovski et al., 2013). In the current study, exclusion of observations with negative SJL values (14.3% of participants at baseline), which could indicate that those individuals had an early chronotype, but could also have included those who worked and had to get up relatively early on the weekend, did not change the interpretation of the results presented.
Although this was a longitudinal study, the data were collected cross-sectionally; thus, differences in demographic or other characteristics between the latent groups do not necessarily imply a cause and effect relationship. Also, the possibility of reverse causality cannot be excluded for some variables. For example, one might expect elevated caffeine consumption in latent groups with disrupted sleep. However, reductions in caffeine consumption were observed over time, which may have occurred in response to the sleep disturbances encountered among individuals with persistent, suboptimal sleep. Another limitation is that the generalizability of the results is limited to healthy adults ages 21–35 years, approximately half of whom were college students, or had incomes <$40,000 per year. Finally, it remains to be determined whether findings from the present study extend to more chronic sleep disruption beyond two years.
A major strength of this study is the use of objective measures to characterize SJL, sleep and chronotype, which minimizes issues related to self-reported sleep quality or sleep/wake timing. Armband actigraphy is a valid, non-invasive method for obtaining ‘real-life’ sleep/wake characteristics that are comparable to polysomnography (Sharif & Bahammam, 2013). Armband data were collected minute-by-minute on at least 4 days (including at least 1 weekend day, up to 10 days total; 81% had ≥10 days). Information for non-use periods was obtained using logs completed by participants. Days with less than 80% of verifiable time were excluded (based on data from armband and activity logs). Thus, measurement error is expected to be lower than self-reported information. The American Academy of Sleep Medicine practice parameters for the use of actigraphy in the clinical assessment of sleep and circadian rhythm disorders recommend using at least three consecutive 24-hour periods (Littner et al., 2003). A more recent study showed that reliability of sleep and circadian measures increased with the number of consecutive days of actigraphy, arguing in favor of ≥7 days, but the difference between mean TST for those with 5 and 7 days of actigraphy data was only about 5–7 min (van Someren, 2007). In the same study, the differences between 5- and 7-day means for SE also were relatively small. After all exclusions, there were few observations in the present study based on 6 (N=5, 0.35% of all observations) or 7 days of wear (N=21, 1.5%), and thus a strong impact on the results due to length of armband use would not be expected. Note that the long time of daily average armband use (median 23.5 h) may, in part, explain the relatively long physical activity times observed in this study. Participants did not have any major acute or chronic health conditions that potentially influenced the sleep measures, and those who regularly used sleep-promoting medications were excluded. Therefore, confounding by somatic conditions or sleep disorders was unlikely to have biased the results.
Results from cross-sectional studies indicate that sleep tends to deteriorate with age (e.g., TST and SE decrease, and WASO and SOL increase) (Ohayon et al., 2004; Roenneberg et al., 2007). In middle-aged and elderly adults, prospective studies using repeated actigraphic measures reported that sleep characteristics remained generally stable within a 1- to 2.5-year time frame, although insomnia subtypes changed over time (Hoch et al., 1994; Hohagen et al., 1994; Hoch et al., 1997; Gaines et al., 2015). Prospective studies among college students that used questionnaires to assess sleep produced conflicting results with respect to the temporal stability of sleep duration and the number of nocturnal awakenings during a one-semester time frame (Hawkins & Shaw, 1992; Pilcher & Ott, 1998). Among undergraduate college students, sleep duration decreased and the number of awakenings increased as the semester progressed (Hawkins & Shaw, 1992). However, in another study, sleep duration and quality increased during a 15-week spring semester (Pilcher & Ott, 1998). In a cohort of 591 young adults surveyed for over 20 years, 35% of those with incident one-month insomnia still had it at the next interview (Buysse et al., 2008). Forty percent of those with incident insomnia subsequently developed chronic insomnia, and 17–50% of those with insomnia had a subsequent major depressive episode (Buysse et al., 2008). Results from these studies suggest that sleep disruption tends to be persistent, although some inconsistencies have been observed in both younger and older age groups.
Although actigraphic sleep characteristics are not sufficient to determine a person’s overall health status or the presence of a sleep disorder, those in a subgroup with persistent sleep disruption over time could be at greater risk for deleterious health consequences. Insomnia and other sleep disturbances have been associated with adverse health conditions such as depression (Buysse et al., 2008; Levandovski et al., 2011; Natale et al., 2014), obesity (Littner et al., 2003; Hasler et al., 2004; Theorell-Haglow et al., 2012), diabetes (Ayas et al., 2003; Kühnle, 2006), hypertension (Gangwisch et al., 2006; Javaheri et al., 2008; Vgontzas et al., 2009), cardiovascular disease (Cappuccio et al., 2011) and cancer (Moore & Meltzer, 2008; Sigurdardottir et al., 2012; Merikanto et al., 2017). In the present study, demographic characteristics that were associated with persistent sleep disruption included: male sex, being a student, non-White race, low income, and evening chronotype. The finding that “eveningness” was associated with disrupted sleep is in agreement with previous studies (Giannotti et al., 2002; Merikanto et al., 2012; Merikanto et al., 2015). Evening chronotypes tend to have shorter sleep on week nights, delayed bed- and wake-time on weekends, insomnia, and to report poor quality or insufficient sleep relative to morning chronotypes (Wolfson & Carskadon, 1998; Koskenvuo et al., 2007; Merikanto et al., 2012). Eveningness also has been associated with increased substance use, alcohol consumption and smoking (Koskenvuo et al., 2007; Broms et al., 2011; Merikanto et al., 2017). Results from this study add to knowledge concerning the temporal patterns of SJL and actigraphic sleep characteristics among healthy young adults. The identification of susceptible subgroups of young adults with persistent sleep disruption may aid in chronic disease prevention.
Acknowledgments:
The authors wish to thank the study participants and the Energy Balance Study team.
DECLARATION OF INTEREST: J.B.B. was supported by a Dept. of Veterans Affairs Office of Research and Development grant (Merit Award: I01BX007080). J.R.H. was supported by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute (K05 CA136975). M.D.W. and J.R.H. were supported by grant number R44DK103377 [N. Shivappa and M.D.W., multiple PIs] from the National Institute of Diabetes and Digestive and Kidney Diseases. S.D.Y. was supported by an NIH grant (R01HL095799). S.N.B. has served on the scientific advisory boards of Technogym, Clarity, and Santech. He has received research funding from BodyMedia, Technogym, the U.S. Department of Defense, and the NIH; and he receives book royalties from Human Kinetics. The Energy Balance study was funded by an unrestricted grant from Coca-Cola Company. Coca-Cola representatives did not participate in the development of study protocol, data analyses, interpretation of the results, or manuscript preparation.
REFERENCES
- Ainsworth BE, Haskell WL, Herrmann SD, et al. (2011). 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 43:1575–1581. [DOI] [PubMed] [Google Scholar]
- Allebrandt KV, Teder-Laving M, Kantermann T, et al. (2014). Chronotype and sleep duration: the influence of season of assessment. Chronobiol Int. 31:731–740. [DOI] [PubMed] [Google Scholar]
- Antunes LC, Levandovski R, Dantas G, et al. (2010). Obesity and shift work: chronobiological aspects. Nutr Res Rev. 23:155–168. [DOI] [PubMed] [Google Scholar]
- Archer SN, Laing EE, Moller-Levet CS, et al. (2014). Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci U S A. 111:E682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayas NT, White DP, Al-Delaimy WK, et al. (2003). A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 26:380–384. [DOI] [PubMed] [Google Scholar]
- Bailey BW, Allen MD, LeCheminant JD, et al. (2014). Objectively measured sleep patterns in young adult women and the relationship to adiposity. Am J Health Promot. 29:46–54. [DOI] [PubMed] [Google Scholar]
- Baker FC, Maloney S, Driver HS. (1999). A comparison of subjective estimates of sleep with objective polysomnographic data in healthy men and women. J Psychosom Res. 47:335–341. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. (2010). Circadian integration of metabolism and energetics. Science. 330:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Kaprio J, Hublin C, et al. (2011). Evening types are more often current smokers and nicotine-dependent-a study of Finnish adult twins. Addiction. 106:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Pitkaniemi J, Backmand H, et al. (2014). Long-term consistency of diurnal-type preferences among men. Chronobiol Int. 31:182–188. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, et al. (2006). Recommendations for a standard research assessment of insomnia. Sleep. 29:1155–1173. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, et al. (2008). Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 31:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, et al. (2011). Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 32:1484–1492. [DOI] [PubMed] [Google Scholar]
- Cespedes EM, Dudley KA, Sotres-Alvarez D, et al. (2016). Joint associations of insomnia and sleep duration with prevalent diabetes: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Journal of diabetes. 8:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, et al. (2008). The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 31:517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelminski I, Petros TV, Plaud JJ, et al. (2000). Psychometric properties of the reduced Horne and Ostberg questionnaire. Personality and Individual Differences. 29:469–478. [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. (2007). Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 8:602–612. [DOI] [PubMed] [Google Scholar]
- Erren TC. (2013). Shift work and cancer research: can chronotype predict susceptibility in night-shift and rotating-shift workers? Occup Environ Med. [DOI] [PubMed] [Google Scholar]
- Fischer D, Lombardi DA, Marucci-Wellman H, et al. (2017). Chronotypes in the US–Influence of age and sex. PloS one. 12:e0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines J, Vgontzas AN, Fernandez-Mendoza J, et al. (2015). Short- and Long-Term Sleep Stability in Insomniacs and Healthy Controls. Sleep. 38:1727–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. (2006). Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 47:833–839. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Malaspina D, Boden-Albala B, et al. (2005). Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 28:1289–1296. [DOI] [PubMed] [Google Scholar]
- Gaultney JF. (2010). The prevalence of sleep disorders in college students: impact on academic performance. Journal of American College Health. 59:91–97. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, et al. (2002). Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 11:191–199. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Redline S, Nieto FJ, et al. (2006). Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 29:1009–1014. [DOI] [PubMed] [Google Scholar]
- Gu F, Xiao Q, Chu LW, et al. (2016). Sleep Duration and Cancer in the NIH-AARP Diet and Health Study Cohort. PLoS One. 11:e0161561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand GA, Shook RP, Paluch AE, et al. (2013). The energy balance study: the design and baseline results for a longitudinal study of energy balance. Res Q Exerc Sport. 84:275–286. [DOI] [PubMed] [Google Scholar]
- Hasler G, Buysse DJ, Klaghofer R, et al. (2004). The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 27:661–666. [DOI] [PubMed] [Google Scholar]
- Hawkins J, Shaw P. (1992). Self-reported sleep quality in college students: a repeated measures approach. Sleep. 15:545–549. [DOI] [PubMed] [Google Scholar]
- Hébert JR, Hurley TG, Cavicchia P, et al. (2010). Response to Dr. Arab et al on “Number of 24-hour diet recalls needed to estimate energy intake”. Annals of Epidemiology. 20:87–88. [Google Scholar]
- Hebert JR, Hurley TG, Chiriboga DE, et al. (1998). A comparison of selected nutrient intakes derived from three diet assessment methods used in a low-fat maintenance trial. Public health nutrition. 1:207–214. [DOI] [PubMed] [Google Scholar]
- Hoch CC, Dew MA, Reynolds CF 3rd, et al. (1997). Longitudinal changes in diary- and laboratory-based sleep measures in healthy “old old” and “young old” subjects: a three-year follow-up. Sleep. 20:192–202. [DOI] [PubMed] [Google Scholar]
- Hoch CC, Dew MA, Reynolds CF 3rd, et al. (1994). A longitudinal study of laboratory- and diary-based sleep measures in healthy “old old” and “young old” volunteers. Sleep. 17:489–496. [DOI] [PubMed] [Google Scholar]
- Hohagen F, Kappler C, Schramm E, et al. (1994). Sleep onset insomnia, sleep maintaining insomnia and insomnia with early morning awakening--temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep. 17:551–554. [PubMed] [Google Scholar]
- Horne JA, Ostberg O. (1977). Individual differences in human circadian rhythms. Biol Psychol. 5:179–190. [DOI] [PubMed] [Google Scholar]
- Javaheri S, Storfer-Isser A, Rosen CL, et al. (2008). Sleep quality and elevated blood pressure in adolescents. Circulation. 118:1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Roth T, Schultz L, et al. (2006). Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 117:e247–256. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS. (2007). Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them. Sociological Methods & Research. 35:542–571. [Google Scholar]
- Kantermann T, Duboutay F, Haubruge D, et al. (2013). Atherosclerotic risk and social jetlag in rotating shift-workers: first evidence from a pilot study. Work. 46:273–282. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Rathouz PJ, Yan LL, et al. (2007). Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep. 30:793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskenvuo M, Hublin C, Partinen M, et al. (2007). Heritability of diurnal type: a nationwide study of 8753 adult twin pairs. J Sleep Res. 16:156–162. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Langer RD, Elliott JA, et al. (2011). Mortality related to actigraphic long and short sleep. Sleep Med. 12:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnle T. (2006). Quantitative analysis of human chronotypes Dissertation, LMU München: Faculty of Biology; Available at http://nbn-resolving.de/urn:nbn:de:bvb:19-51686, accessed on 11/08/2017. [Google Scholar]
- Kushida CA, Chang A, Gadkary C, et al. (2001). Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2:389–396. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Rathouz PJ, et al. (2009). Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 170:805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, et al. (2008). Self-reported and measured sleep duration: how similar are they? Epidemiology. 19:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, Holmback U, Van Cauter E. (2014). Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 63:1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandovski R, Dantas G, Fernandes LC, et al. (2011). Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int. 28:771–778. [DOI] [PubMed] [Google Scholar]
- Levandovski R, Sasso E, Hidalgo MP. (2013). Chronotype: a review of the advances, limits and applicability of the main instruments used in the literature to assess human phenotype. Trends in psychiatry and psychotherapy. 35:3–11. [DOI] [PubMed] [Google Scholar]
- Littner M, Kushida CA, Anderson WM, et al. (2003). Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 26:337–341. [DOI] [PubMed] [Google Scholar]
- Ma Y, Olendzki BC, Pagoto SL, et al. (2009). Number of 24-hour diet recalls needed to estimate energy intake. Ann Epidemiol. 19:553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marucci-Wellman HR, Lombardi DA, Willetts JL. (2016). Working multiple jobs over a day or a week: Short-term effects on sleep duration. Chronobiol Int. 33:630–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megdal SP, Schernhammer ES. (2007). Correlates for poor sleepers in a Los Angeles high school. Sleep Med. 9:60–63. [DOI] [PubMed] [Google Scholar]
- Merikanto I, Kronholm E, Peltonen M, et al. (2012). Relation of chronotype to sleep complaints in the general Finnish population. Chronobiol Int. 29:311–317. [DOI] [PubMed] [Google Scholar]
- Merikanto I, Pesonen AK, Kuula L, et al. (2017). Eveningness as a risk for behavioral problems in late adolescence. Chronobiol Int. 34:225–234. [DOI] [PubMed] [Google Scholar]
- Merikanto I, Suvisaari J, Lahti T, et al. (2015). Eveningness relates to burnout and seasonal sleep and mood problems among young adults. Nord J Psychiatry.1–9. [DOI] [PubMed] [Google Scholar]
- Merikanto I, Suvisaari J, Lahti T, et al. (2016). Eveningness relates to burnout and seasonal sleep and mood problems among young adults. Nord J Psychiatry. 70:72–80. [DOI] [PubMed] [Google Scholar]
- Mezick EJ, Wing RR, McCaffery JM. (2014). Associations of self-reported and actigraphy-assessed sleep characteristics with body mass index and waist circumference in adults: moderation by gender. Sleep Med. 15:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Rusak B. (2000). Circadian rhythms in mammals: formal properties and environmental influences In Kryger MH, Roth T, Dement WC (Eds). Principles and Practice of Sleep Medicine. Philadelphia: WB Saunders, pp. 321–333. [Google Scholar]
- Moore M, Meltzer LJ. (2008). The sleepy adolescent: causes and consequences of sleepiness in teens. Paediatric respiratory reviews. 9:114–120; quiz 120–111. [DOI] [PubMed] [Google Scholar]
- Morris CJ, Yang JN, Garcia JI, et al. (2015). Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proceedings of the National Academy of Sciences. 112:E2225–E2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullington JM, Simpson NS, Meier-Ewert HK, et al. (2010). Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 24:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin D. (2005). Group-based modeling of development. Cambridge, Mass.: Harvard University Press. [Google Scholar]
- Natale V, Leger D, Martoni M, et al. (2014). The role of actigraphy in the assessment of primary insomnia: a retrospective study. Sleep Med. 15:111–115. [DOI] [PubMed] [Google Scholar]
- Natale V, Plazzi G, Martoni M. (2009). Actigraphy in the assessment of insomnia: a quantitative approach. Sleep. 32:767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, et al. (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Lawman HG, et al. (2016). Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 315:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM. (2002). Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 6:97–111. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, et al. (2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 27:1255–1273. [DOI] [PubMed] [Google Scholar]
- Paine SJ, Fink J, Gander PH, et al. (2014). Identifying advanced and delayed sleep phase disorders in the general population: a national survey of New Zealand adults. Chronobiol Int. 31:627–636. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Moffitt TE, Gregory AM, et al. (2015). Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Malhotra A, White DP, et al. (2006). Association between reduced sleep and weight gain in women. American journal of epidemiology. 164:947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher JJ, Ott ES. (1998). The relationships between sleep and measures of health and well-being in college students: a repeated measures approach. Behav Med. 23:170–178. [DOI] [PubMed] [Google Scholar]
- Ramin C, Devore EE, Pierre-Paul J, et al. (2013). Chronotype and breast cancer risk in a cohort of US nurses. Chronobiol Int. 30:1181–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, et al. (2012). Social jetlag and obesity. Curr Biol. 22:939–943. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Foster RG. (1997). Twilight times: light and the circadian system. Photochem Photobiol. 66:549–561. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, et al. (2007). Epidemiology of the human circadian clock. Sleep Med Rev. 11:429–438. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. (2003). Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 18:80–90. [DOI] [PubMed] [Google Scholar]
- Rutters F, Lemmens SG, Adam TC, et al. (2014). Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J Biol Rhythms. 29:377–383. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, et al. (2009). Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, et al. (2008). Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 4:487–504. [PMC free article] [PubMed] [Google Scholar]
- Sharif MM, Bahammam AS. (2013). Sleep estimation using BodyMedia’s SenseWear armband in patients with obstructive sleep apnea. Ann Thorac Med. 8:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Steck SE, Hurley TG, et al. (2014). Designing and developing a literature-derived, population-based dietary inflammatory index. Public health nutrition. 17:1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdardottir LG, Valdimarsdottir UA, Fall K, et al. (2012). Circadian disruption, sleep loss, and prostate cancer risk: a systematic review of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 21:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soric M, Turkalj M, Kucic D, et al. (2013). Validation of a multi-sensor activity monitor for assessing sleep in children and adolescents. Sleep Med. 14:201–205. [DOI] [PubMed] [Google Scholar]
- Stamatakis KA, Punjabi NM. (2010). Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 137:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, et al. (2004). Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theorell-Haglow J, Berglund L, Janson C, et al. (2012). Sleep duration and central obesity in women - differences between short sleepers and long sleepers. Sleep Med. 13:1079–1085. [DOI] [PubMed] [Google Scholar]
- Valdez P, Ramirez C, Garcia A. (1996). Delaying and extending sleep during weekends: sleep recovery or circadian effect? Chronobiol Int. 13:191–198. [DOI] [PubMed] [Google Scholar]
- van Someren EJW. (2007). Improving actigraphic sleep estimates in insomnia and dementia: how many nights? J Sleep Res. 16:269–275. [DOI] [PubMed] [Google Scholar]
- Vargas PA, Flores M, Robles E. (2014). Sleep quality and body mass index in college students: the role of sleep disturbances. J Am Coll Health. 62:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Miksiewicz T, et al. (2014). Unveiling the longitudinal association between short sleep duration and the incidence of obesity: the Penn State Cohort. International journal of obesity. 38:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Bixler EO, et al. (2009). Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 32:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. (2002). Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism: clinical and experimental. 51:887–892. [DOI] [PubMed] [Google Scholar]
- Vink JM, Groot AS, Kerkhof GA, et al. (2001). Genetic analysis of morningness and eveningness. Chronobiol Int. 18:809–822. [DOI] [PubMed] [Google Scholar]
- Weil A. (2017). Alarm Clocks: Alarmed in the A.M. Available at https://www.drweil.com/health-wellness/body-mind-spirit/sleep-issues/alarm-clocks-alarmed-in-the-a-m/, accessed on 11/08/2017.
- Wirth MD, Burch JB, Hebert JR, et al. (2014). Case-control study of breast cancer in India: Role of PERIOD3 clock gene length polymorphism and chronotype. Cancer Invest. 32:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MD, Hebert JR, Hand GA, et al. (2015). Association between actigraphic sleep metrics and body composition. Ann Epidemiol. 25:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, et al. (2006). Social jetlag: Misalignment of biological and social time. Chronobiology International. 23:497–509. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. (1998). Sleep schedules and daytime functioning in adolescents. Child development. 69:875–887. [PubMed] [Google Scholar]
- Wong PM, Hasler BP, Kamarck TW, et al. (2015). Social Jetlag, Chronotype, and Cardiometabolic Risk. The Journal of clinical endocrinology and metabolism. 100:4612–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavada A, Gordijn MC, Beersma DG, et al. (2005). Comparison of the Munich Chronotype Questionnaire with the Horne-Ostberg’s Morningness-Eveningness Score. Chronobiol Int. 22:267–278. [DOI] [PubMed] [Google Scholar]