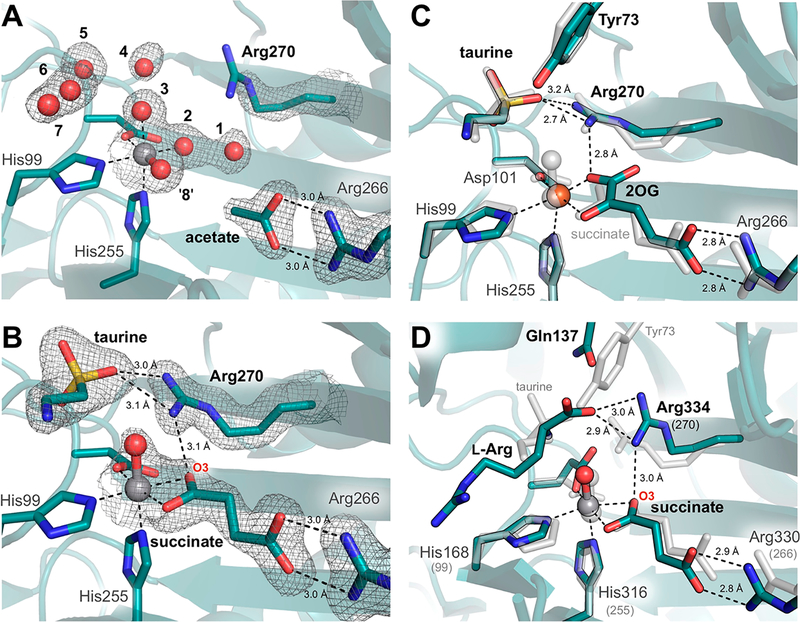
Figure 2.
Influence of substrate binding on the metal-oxo orientation in TauD. Active site structures are shown for chains (A) A and (B) B with corresponding 2F0 − Fc electron density maps contoured at 1.0σ around key residues and substrates. Density surrounding spheres (e.g., vanadyl) is contoured at 0.7σ for the sake of clarity. See Figure S1A,B for ligand omit maps. Minimal changes are observed between the vanadyl complex with taurine and succinate bound (gray) and (C) the reactant complex10 (turquoise, PDB entry 1OS7, chain A). However, ordering of Arg270 upon taurine binding appears to promote in-line oxo formation via H-bonding interactions similar to those observed in (D) the VioC·(VIVO)·l-Arg·succinate structure13 (turquoise, PDB entry 6ALR). Sequence-independent alignments were performed over (C) 237 and (D) 116 Cα atoms and resulted in root-mean-square deviations of 0.26 and 3.21 Å, respectively.