Abstract
Autoreactive B cells can promote autoimmunity through antigen presentation to autoreactive T cells, production of autoantibodies, generation of cytokines promoting T cell activation and differentiation, and inhibition of regulatory T cells and B cells. Here, the authors highlight studies pertaining to B cell mechanisms associated with disease pathogenesis and outcomes in autoimmune hepatitis and the immune-mediated cholangiopathies (primary biliary cholangitis, primary sclerosing cholangitis, and biliary atresia). The vast majority of investigations focus on autoantibodies and future research endeavors should include deciphering the role of the B cell in T cell activation (through antigen presentation, cytokine/chemokine production, and inhibition of regulation). Targeting B cell mechanisms in the treatment of autoimmune liver diseases is also highlighted.
Keywords: immunity, autoantibody, antigen presentation, autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cholangitis, biliary atresia
B cell development from hematopoietic stem cells is initiated in the fetal liver and maintained as a regenerative process within the bone marrow throughout life. B cells populate the secondary lymphoid organs, such as the lymph nodes and spleen, where B-cell-mediated immune responses are initiated by interaction of antigen (Ag) with the B cell receptor (BCR) and direct cell contact with CD4+ T cells. The BCR is composed of a membrane-bound form of immunoglobulin M (IgM) that binds Ag and the signal transduction moiety Ig-α/Ig-β that is necessary for activation. BCR engagement by Ag and co-stimulatory molecules leads to activation and proliferation of Ag-specific B cell clones that differentiate into either plasmablasts or germinal center B cells, which then give rise to plasma cells or memory B cells, respectively (►Fig. 1A).1 Autoreactive B cells, generated upon engagement with auto-antigens, can promote autoimmunity in numerous ways: (1) Ag presentation to autoreactive T cells, (2) production of autoantibodies with Ag/antibody formation and activation of complement or phagocytosis, (3) generation of cytokines promoting Th1 or Th17 pathways,2–5 and (4) inhibition of regulatory T and B cells6 (►Fig. 1B). Autoantibodies are generated in the majority of autoimmune diseases and may function as biomarkers of disease or directly contribute to the pathogenicity through antibody-mediated cytotoxicity or complement activation. Experimental models of autoimmune diseases have shown the importance of B cells as Ag-presenting cells (APCs) in disease pathogenesis, including type 1 diabetes,7 lupus,8 and arthritis.9 More recent discoveries include the role of the B cell as an activator of the adaptive immune response through generation of cytokines associated with innate immunity, as well as chemokines.4,10,11 In this review we highlight research pertaining to the contribution of B cells to disease pathogenesis in immune-mediated liver diseases. These diseases include autoimmune hepatitis (AIH) and the immune-mediated cholangiopathies primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and biliary atresia (BA). Luo et al recently described that the immune-mediated cholangiopathies (PSC, PBC, and BA) share 34 functionally related immunity/inflammation genes that may be linked to disease pathogenesis.12
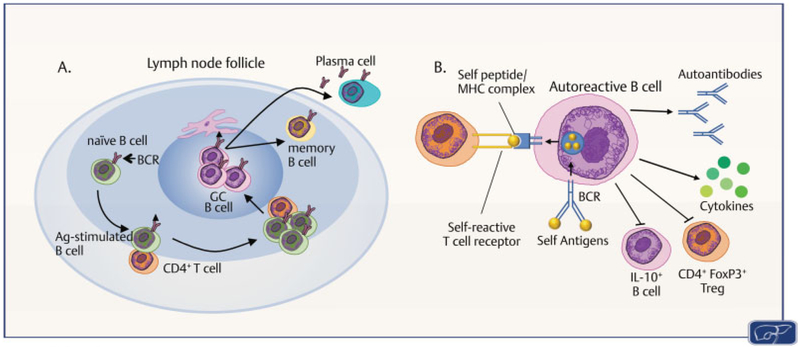
Fig. 1. Fate of the B cell.
(A) B cells in the lymph node or spleen activated by antigen (Ag) can differentiate into either germinal center (GC) B cells, memory B cells, or antibody-secreting plasma cells. (BCR, B cell receptor); (B) Autoreactive B cells are generated upon autoantigen binding to BCR and B cell activation. Mechanisms of B cell autoreactivity include: (1) B cell presentation of Ag to autoreactive T cells; (2) plasma cell differentiation with autoantibody production; (3) B cell production of proinflammatory cytokines/chemokines; and (4) inhibition of anti-inflammatory regulators (FoxP3+ regulatory T cells (Tregs) and IL-10-producing regulatory B cells). (Illustration by Maura Mack, College of Veterinary Medicineand Biological Sciences-ColoradoState University. Adapted with permission from Goodnow et al1 and Bour-Jordan and Bluestone6.)
Autoimmune Hepatitis
Autoimmune hepatitis is a chronic inflammatory liver disease thought to be due to a break in immune tolerance against liver autoantigens. AIH is characterized clinically by detection of autoantibodies, hypergammaglobulinemia, and a lymphoplasmocytic infiltrate with interface hepatitis on liver histology. Historically, AIH has been thought to be a T-cell-mediated disease with disease onset driven by T helper cells directing attack against autoantigens and chronic disease mediated by impaired regulatory T cells. Notably, however, anti-CD20 (B cell depleting antibody) may be an effective treatment for AIH patients refractory to conventional therapy, supporting a key role for B cells in disease pathogenesis.13,14 Through the generation of auto-antibodies, and regulation of T cell responses through Ag presentation and cytokine production, B cells are integral to disease pathogenesis in AIH and are an important therapeutic target that warrants further research.
Generation of Autoantibodies
Serologic autoantibody testing supports classification into two subgroups of AIH in combination with differences in clinical and genetic findings.15 A list of autoantibodies in all autoimmune liver diseases and the associated autoantigens is provided in ►Table 1. AIH type I (AIH-I) is characterized by the detection of antinuclear antibodies (ANAs) and/or anti-smooth muscle autoantibodies. Additional positive autoantibodies in AIH-I may include antineutrophil cytoplasmic autoantibodies (ANCAs), anti-asialoglycoprotein receptor autoantibodies, and antibodies against soluble liver or liver–pancreas Ags. Patients with AIH type II (AIH-II) are commonly younger at diagnosis and have more severe disease than patients with AIH-I. Autoantibodies characteristic of AIH-II include autoantibodies against liver and kidney microsomal Ags (anti-LKM type 1 or type 3) and/or auto-antibodies against liver cytosol I Ag (anti-LCI).
Table 1.
Autoantibodies and autoantigens in autoimmune liver diseases
| Autoantibody | Autoantigen | |
|---|---|---|
| AIH-I | ANA | Chromatin, histones, centromere, ds-DNA and ss-DNA, cyclin A, ribonucleoproteins, other extractable nuclear antigens (ENAs) |
| Anti-SMA/anti-F-actin | Actin, tubulin, or intermediate filaments | |
| Anti-SLA/LP | O-phosphoseryl-tRNA:selenocysteine-tRNA synthase (SepSecS) | |
| p-ANCA | Possible tubulin-β chain | |
| Anti-ASGPR | ASGPR | |
| AIH-II | Anti-LKM-1 | Cytochrome P450 2D6 (CYP2D6) |
| Anti-LKM-3 | Family I of UDP-glucuronosyltransferases | |
| Anti-LC1 | Formiminotransferase cyclodeaminase | |
| p-ANCA | Possible tubulin-β chain | |
| Anti-ASGPR | ASGPR | |
| PBC | ANA (including anti-Sp100, anti-gp210, anticentromere) | Centromere, lamin-B-receptor, Sp100 nuclear antigen, nuclear envelope protein gp210 |
| AMA | Family of mitochondrial enzymes: pyruvate dehydrogenase (PDC-E2), branched chain 2-oxo-acid dehydrogenase (BCOADC-E2), and 2-oxo-glutaric acid dehydrogenase (OADC-E2) | |
| Anti-ASGPR | Asialoglycoprotein receptor (ASGPR) | |
| Anti-Kelch-like 12 | Kelch-like 12 | |
| Antihexokinase1 | Hexokinase E1 | |
| PSC | ANA | Ds-DNA and ss-DNA, anti-Ro/SSA and La/SSB, ribonucleoproteins, topoisomerase-1 (SCL70), other ENAs |
| p-ANCA | Possible β-tubulin isoform 5 | |
| Anti-GP2 IgA | Glycoprotein 2 | |
| Anti-bile duct epithelium | Unknown | |
| BA | Anti-α-enolase | α-Enolase |
| Anti-BPO | Fusion proteins of E2 subunits from 2-oxo-acid dehydrogenase complex targeted by inner mitochondrial membrane | |
| Anti-M2–3E | BPO plus pyruvate dehydrogenase complex | |
| ANCA | Unknown |
Abbreviations: AMA, antimitochondrial antibody; ANA, antinuclear antibody; ASGPR, asialoglycoprotein receptor; ds, double-stranded; LC, liver cytosol; LKM, liver and kidney microsomal; LP, liver–pancreas; p-ANCA, perinuclear antineutrophil cytoplasmic antibody; SLA, soluble liver antigen; SMA, smooth muscle antibody; ss, single-stranded.
Source: Adapted from Sebode M, Weiler-Normann C, Liwinski T, Schramm C. Autoantibodies in autoimmune liver disease-clinical and diagnostic relevance. Front Immunol 2018;9:609.59
While titers of some autoantibodies and total immunoglobulin levels have been shown to correlate with biochemical markers of disease activity in AIH,16,17 only anti-LKM-1 and anti-LCI autoantibodies are thought to play a pathogenic role in AIH-II.18–22 Expression of CYP2D6 on the surface of patients’ hepatocytes triggers anti-LKM-1 production and either direct antibody-mediated damage or activation of liver-infiltrating T lymphocytes. Anti-LCI autoantibodies also correlate with disease activity suggesting a pathogenic role for these antibodies in liver injury in AIH-II.22
Despite the limited data of direct antibody-mediated cytotoxicity in AIH, circumstantial evidence regarding interleukin (IL)-21 suggests that autoantibody production is important in AIH outcome. IL-21 is a cytokine produced by follicular helper T (Tfh) cells that drives B cell activation, plasma cell differentiation, and immunoglobulin production in germinal centers of secondary lymphoid organs.23 Serum levels of IL-21 in AIH patients reflect severity of disease as evidenced by a positive correlation with grade of necroinflammatory activity and total serum bilirubin levels, and a negative correlation with serum albumin levels.24 Furthermore, increased numbers of plasma cells, activated B cells, and Tfh cells are accompanied by elevated serum IL-21 and increased serum immunoglobulins in new onset AIH patients.25 In a murine model of AIH, blockade of IL-21 suppressed Tfh cell generation and prevented development of murine AIH.26 Taken together, evidence supports a role for activated B cells in disease activity of AIH. Further research is needed to clearly define the correlation between disease activity, autoantibodies, and immunoglobulin levels.
B Cell Antigen-Presenting Cell Function
Recently, an increased body of literature supporting a key role for B cells as APCs in AIH has emerged from mechanistic studies evaluating improved disease activity after anti-CD20 antibody treatment in murine models of AIH. B cell depletion with anti-CD20 in a murine model of AIH both prevented disease initiation and ameliorated disease after onset, suggesting a key role for B cells in both induction and progression of disease.27 B cell depletion was accompanied by significantly decreased Ag-experienced CD4+ and CD8+ splenic T cells and less cytotoxic activity of CD8+ T cells, as demonstrated by decreased CD107a and granzyme B expression. Furthermore, decreased T cell proliferation occurred following B cell depletion and functional assays supported a key role for B cells serving as APCs to CD4+ T cells. Demonstration of increased expression of the co-stimulation molecules CD86 and CD95 on B cells in patients with new onset AIH provides clinical evidence for a role of Ag presentation by B cells in AIH.25 In contrast, adoptive transfer of IL-10-dependent CD11b+ regulatory B cells effectively inhibited CD4+ T cell responses and ameliorated murine AIH.28 This suggests that the B cell contribution to disease is characterized by excessive pathogenic B cells and deficiencies in regulatory B cell subsets.
B Cell Cytokine Production
B cells in AIH also regulate the immune response through both direct production of proinflammatory cytokines as well as through cytokine-directed recruitment of other immune cells. Increased numbers of polyfunctional B cells secreting the proinflammatory cytokines interferon (IFN)-γ and tumor necrosis factor (TNF)-α and decreased IL-10-secreting B cells were demonstrated in the spleen and liver of a murine model of AIH.27 CXCL10, or IFN-γ-inducible protein 10, is a chemokine produced largely by monocytes but has also been shown to be produced by B cells.29 CXCL10 stimulates the recruitment of lymphocytes (e.g., monocytes, natural killer cells, T lymphocytes) in the setting of inflammation and correlates with hepatic inflammation and fibrosis.30 Successful treatment of AIH with B cell depletion is associated with a decrease in CXCL10 levels in both a murine model of AIH27 as well as patients after rituximab treatment.13 Additional evidence linking B cells to CXCL10 levels and lymphocyte recruitment is provided by the finding that B cell activating factor (BAFF) levels significantly correlated with CXCL10 in AIH patients, irrespective of degree of fibrosis.31 BAFF is a member of the TNF superfamily and is critical for B cell maturation and immunoglobulin class-switch recombination.32 Excessive BAFF can lead to the development of auto-reactive B cells and stimulation of the adaptive immune response, which may contribute to autoimmunity in various diseases.33 In summary, evidence exists for multiple roles of B cells in AIH pathogenesis and the mechanisms of autoantibody generation, Ag presentation, and cytokine/chemokine production are likely not mutually exclusive.
Primary Biliary Cholangitis
Primary biliary cholangitis, formerly named primary biliary cirrhosis, is a chronic cholestatic disease characterized by destruction of the small intrahepatic bile ducts resulting in inflammation and progressive fibrosis. PBC is a prototypical autoimmune disease in that it is female predominant, characterized by loss of tolerance with both environmental and genetic risk factors, is frequently associated with concurrent autoimmune disorders such as Sjogren’s syndrome and chronic thyroiditis, has a disease-specific antimitochondrial antibody (AMA), and can recur after liver transplantation.34 Despite this clear autoimmune profile, therapeutic approaches using standard immunosuppression have not been successful to date and therefore the ongoing focus on the role of B cells in PBC has significant value.
T cells are the predominant inflammatory cell type found in the portal tract in PBC; however, B cells notably account for up to 10% of the cellular infiltrate and are highly relevant due to autoantibody production, cross-presentation of Ag, and production of pathogenic cytokines.35,36 The role of B cells in PBC is also highlighted by the discovery of several risk loci with genes that encode proteins important for B cell function including CD80, CXCR5, and POU2AF1.37 Furthermore, elevated AMA titers correlate with specific disease symptoms such as fatigue and exercise intolerance, suggesting a possible relationship between B cells, immunoglobulin production, and extra-hepatic manifestations of the disease.38 However, despite the fact that B cell depletion with rituximab resulted in improved anaerobic threshold in patients with PBC, B cell depletion did not improve fatigue.39 To further examine the role of anti-B cell therapy as an alternative approach for the treatment of patients with PBC, several preclinical and clinical studies are currently being performed.39–41
Generation of Autoantibodies
Approximately 95% of patients with PBC have measurable AMAs, which detects a family of mitochondrial enzymes known as 2-oxo-acid dehydrogenase complexes, including pyruvate dehydrogenase (PDC-E2), branched chain 2-oxo-acid dehydrogenase (BCOADC-E2), and 2-oxo-glutaric acid dehydrogenase (OADC-E2).42,43 The PDC-E2 subunit is the principal target of AMAs, and though it is normally located on the inner membrane of mitochondria, in PBC this molecule is aberrantly expressed on the surface of biliary epithelial cells and may be the earliest pathologic manifestation of PBC.44 Indeed, there is a high frequency of AMA-generating B cells in the peripheral circulation of patients with PBC and the frequency increases with fibrosis progression in the early stage of the disease, though there is a subsequent reduction in circulating B cells in the late stage.45 This observed change in B cell abundance supports the hypothesis that immune-mediated injury characterizes early stages of disease whereas toxic injury predominates in later stages.46 In addition, autoantibody-producing B cells have been isolated from explant PBC liver tissue and they clearly sustain immunoglobulin production and reactivity to PDC-E2 in vitro.47 Interestingly, both AMA-positive and -negative PBCs have similar clinical features, and there is evidence that PDC-E2 plays a role even in AMA-negative PBC. This is due to the finding that CD4+ T cells can universally react to this auto-antigen even in AMA-negative conditions, and therefore it is possible that B cell-generated AMAs may not be strictly necessary for pathogenicity in PBC.48
In addition to AMAs, additional relevant autoantibodies in PBC include anti-Sp100 and anti-gp210 which are highly specific for PBC and can indicate a more aggressive clinical course.49–51 These autoantibodies may be clinically useful in the diagnosis of PBC in AMA-negative patients as recommended in recent society guidelines.52 The anticentromere antibody is also elevated in patients with PBC and is associated with a higher risk of progression to cirrhosis.49
Disruption of the B cell repertoire has an impact on both B cell function and IgM production. Recent data from high-throughput sequencing of B cells from PBC patients revealed a clonal expansion and increased IgM production compared with healthy controls; however, there was also a loss of clonal diversity which suggests a relative immunocompromised status.53 Notably, treatment with ursodeoxycholic acid resulted in reduced clonal expansion and increased diversity, suggesting a benefit of the drug with respect to the immune balance in PBC as related to B cell functionality.
B Cell APC Function
A common feature of the portal tract in PBC is the presence of a lymph follicle, known as a tertiary lymphoid organ, which typically surrounds the injured bile duct. B cells are found in the central portion of the follicle and are surrounded by T cells.36 Interestingly, recent data have suggested that CD38+ plasma cells, and not the precursor CD20+ B cells, may be directly involved in the bile duct destruction itself.54 However, it is also well known that B cells can present Ag toT cells in the context of peripheral lymphoid organs.
One of the most robust models of PBC is the recently described ARE-Del knockout mouse, where chronic overexpression of IFN-γ through deletion of the 3′-untranslated region results in female-predominant cholestasis with IgM, AMAs, and gp210 production.55 In addition to these features, the effect of IFN-γ production includes a significant increase in B cells within the lymphoid follicle in the portal tracts. Furthermore, gene deletion of type 1 IFN receptor α chain can undo this enrichment of B cells and significantly reduce cholestatic damage in the mouse.56 Therefore, the ability of B cells to interact productively with T cells in the context of hepatic lymphoid follicles in PBC may additionally depend on key proinflammatory cytokines that are typically associated with T cells, namely IFN-γ. Lastly, previous studies demonstrated not only an elevation of IFN-γ in the serum of patients with PBC but also a correlation of this cytokine with soluble levels of CD30.57 It should be highlighted that CD30+-activated T cells play a major role in helper B cell function.57 Therefore, there are emerging data to suggest that IFN-γ may serve as a critical link between pathogenic B and T cell interactions in the context of PBC.
B Cell Cytokine Production
B cells can produce inflammatory cytokines that promote further injury to the liver in the context of PBC. The CD19+ B cell population is enriched in the liver of patients with PBC and can be induced by CD40L co-stimulation to significantly increase the secretion of IL-6, IL-10, IFN-γ, and TNF-α.58 Furthermore, treatment with ursodeoxycholic acid was able to reduce the frequency of circulating CD19+ B cells in these patients.
In addition, serum levels of BAFF have been detected in PBC59 similarly to AIH and could lead to the impairment of B cell tolerance and development of autoimmunity. Interestingly, the degree of peripheral BAFF elevation may correlate with serum liver enzymes in these patients.60 Furthermore, BAFF has been evaluated in the context of B and T cell interactions in PBC and shown to directly promote B cell-induced apoptosis of regulatory T cells, which would allow autoreactive B cells to promote further biliary damage. In addition, BAFF was found to reduce the production of IL-10 and TGF-β by regulatory B cells.59 Therefore, reduction of regulatory T cell populations and alteration of B-cell-generated cytokines make BAFF a relevant factor in the immune pathways in PBC and a therapeutic target for B-cell-directed immunomodulation.
Primary Sclerosing Cholangitis
Primary sclerosing cholangitis is a rare progressive disease characterized by fibrosis of the extrahepatic and/or intrahepatic biliary tree, leading ultimately to cirrhosis.61,62 PSC is closely linked with inflammatory bowel disease, most commonly with ulcerative colitis (UC). An emerging theory of pathogenesis is that an altered intestinal microbiota results in microbial byproducts entering the portal circulation and triggering liver innate immune activation, with subsequent autoimmune responses and biliary fibrosis.63,64 An important clue to the “gut–liver axis” hypothesis of PSC pathogenesis lies in the discovery of B cell clones within the liver and colonic tissue of PSC patients.65 BCR sequencing revealed that 8.3% of B cell clones overlapped with paired colon and liver samples, suggesting that the same Ag(s) was driving B cell activation in both organs.
Generation of Autoantibodies
Various serum autoantibodies are detectable in PSC, including ANCA, ANA, and antiglycoprotein 2 (anti-GP2); however, none are of diagnostic value.66 ANCA is present in 26 to 93% of PSC patients, but is not specific to PSC, as ANCA is also found in UC without PSC, AIH, and chronic infectious hepatitis. Interestingly, of the many Ag targets of p-ANCA, β-tubulin isoform 5 (TBB-5) and FtsZ (related to TBB-5) are abundant proteins in intestinal bacteria. This provides indirect evidence of the role of the microbiota and abnormal antibody responses to commensal bacteria in disease pathogenesis.66,67 Furthermore, ANCA detected in bile (IgG isotype) was associated with a 10-fold risk of having PSC and positively correlated with presence of dominant strictures, cholangiographic severity, and number of interventions performed.68 This suggests that ANCA may be directly pathogenic; however, mechanistic studies have not been performed to verify this.
Similar to ANCA, the presence ofanti-GP2 IgA in PSC patients has been linked with poor outcomes, including a higher Mayo risk score, serum bilirubin levels, survival rates, and risk of cholangiocarcinoma.69 This secretory antibody is known to react with bacteria-binding Ag GP2, again suggesting a role for the microbiota in the aberrant immune responses in PSC.
With the goal of discovering disease-specific antibodies in PSC, an eloquent study analyzing serum antibodies reactive to bile duct epithelial (BDE) proteins was performed.70 Human BDEs were isolated from normal healthy liver donors and used to probe serum antibodies for reactivity. Approximately 65% of PSC patients had anti-BDE IgG and IgA antibodies above the level of detection of normal controls. Testing positive for anti-BDE IgA was associated with significantly lower rates of transplant-free survival. This provocative study should be followed up with research to discover the specific BDE auto-antigen(s) responsible for the generation of autoantibodies.
B Cell APC Function and Cytokine Production
There is a lack of data on the role of the B cell in Ag presentation and cytokine production in PSC. This area of research is ripe for exploration in the future.
Biliary Atresia
Biliary atresia is an inflammatory sclerosing cholangiopathy that uniquely presents in the neonatal time period with extrahepatic biliary obstruction. Treatment includes Kasai portoenterostomy at the time of diagnosis; however, ongoing intrahepatic biliary injury and cirrhosis result in the need for liver transplantation in the majority of patients. Controversy exists regarding the pathogenesis of BA; however, abundant research in the last decade supports a role for autoinflammatory or autoimmune mechanisms of biliary disease.71 Evidence for B cell activation includes the finding of increased intrahepatic periductal B cell infiltrates at the time of BA diagnosis and at transplant.72 Analysis of hilar lymph nodes contiguous with the most proximal biliary remnants in BA patients revealed that 51% of BA infants (and none of controls) had one or more well-formed reactive germinal centers, suggesting Ag-driven B cell activation.73 The source of the Ag(s) and why only half of the BA patients had mature germinal centers are not known.
Generation of Autoantibodies
Based on the neonatal presentation of BA, a brief review of development of immunoglobulin production is warranted. In humans, nearly all of the IgG at birth is maternally derived and reaches a nadir between 1.5 and 3 months of age. At this point, IgG synthesis by the newborn begins, maternally derived IgG is absent by 1.5 years, and peak IgG levels are attained by 5 years.74 On the other hand, IgM levels rise rapidly during the first months of life, attaining 75% of adult levels by 1 year. A landmark study detailed the presence of IgM autoantibodies to defined self-molecules in newborn humans.75 Termed “natural autoantibodies,” these neonatal IgMs reacted to a selective set of autoantigens, many of which were among the target autoantigens associated with autoimmune diseases. Based on the presence of some major disease-associated autoantigens within the IgM repertoire, pathologic autoimmune disease could arise through a lapse in the regulation of otherwise benign “natural” autoimmunity.
There is limited research regarding autoantibodies in BA. Early studies found periductal IgM and IgG deposits along the basement membrane of bile duct epitheliawithin extrahepatic bile duct remnants in approximately 40% of BA patients at diagnosis.76 Periductal immunoglobulin deposits and serum autoantibodies reactive to BDE proteins have also been described in the rotavirus-induced mouse model of BA (“murine BA”).77 One of these autoantibodies was determined to be anti-α-enolase, an autoantibody present in all BA mice (IgG subtype) and in a subset of human infants (IgM) and older children (IgG) with BA.78 A recent study tested for known autoimmune liver disease in the sera of 124 BA patients and 140 other liver disease or healthy controls.79 The overall rate of autoantibody positivity in BA was 56.5% and the majority of these patients had persistence of autoantibody positivity at 6 months follow-up. The greater part of positive autoantibodies was within the PBC-specific profile, with significantly increased frequency of anti-BPO (fusion proteins of E2 subunits from 2-oxo-acid dehydrogenase complex targeted by inner mitochondrial membrane), anti-M2–3E (BPO plus pyruvate dehydrogenase complex), and ANA in BA compared with controls. In addition, there was a significantly increased frequency of ANCA positivity in BA compared with healthy controls, but not when compared with other liver disease controls. Interestingly, a minor Ag target of ANCA is α-enolase, supporting the previous findings of α-enolase antibodies in BA. ANCA positivity significantly correlated with the risk of cholangitis postportoenterostomy, suggesting that ANCA may be a biomarker for cholangitis. Future research in the role of autoantibodies (IgM and IgG isotypes) as biomarkers of disease and relation to pathogenicity are warranted.
B Cell APC Function
The role of B cells as APCs in murine BA was recently investigated. Ig-α deficient mice (Ig-α−/−), which bear no B cells because of nonfunctional BCRs, are protected from developing murine BA. Importantly, the absence of functional B cells was associated with a lack of T cell activation, suggesting that B cells play a critical role in T cell-mediated bile duct injury in murine BA.80 However, subsequent mechanistic studies showed that B cell production of cytokines, and not APC function, was essential to elicit T cell activation.72 The Ag presentation function of B cells has not been investigated in human BA and based on the presence of persistent periductal B cell infiltrates,72 this area of research should be a future focus.
B Cell Cytokine Production
To date, one study has analyzed the cytokine-producing capacity of B cells in murine BA.72 The investigators first analyzed cytokines known to be produced by B cells and found significantly increased amounts of cytokines involved in Th1 adaptive immune responses (IFN-γ, IL-2, and TNF-α) and innate immune responses (IL-1 and IL-6) in B cells from BA mice compared with controls. RNA-seq technology was utilized to discover additional novel liver B cell molecules associated with immune activation. Numerous pathways involved in B cell and innate immune activation were upregulated in murine BA, including toll-like receptor pathways, cytokine signaling, and myeloid differentiation protein 88 signaling. Trans-well experiments of B cells with naïve T cells revealed that cytokines from B cells of BA mice led to T cell activation. RNA-seq technology should be employed in the future to discover cytokines produced by liver B cells in human BA.
Therapeutic Interventions Targeting B Cells in Autoimmune Liver Diseases
Therapies to target B cells in autoimmune liver diseases include nonspecific, general immunosuppressive agents or B cell-specific agents (►Table 2). Current agents tested in autoimmune liver diseases specifically directed toward B cells include rituximab, an anti-CD20 monoclonal antibody, and intravenous immunoglobulin (IVIG). As increased levels of BAFF have been implicated in both AIH31 and PBC,59,60 the use of the BAFF inhibitor ianalumab is now under investigation for AIH.81 Ianalumab is a human monoclonal antibody against the BAFF receptor that causes B cell depletion and is currently being tested for dosing to achieve alanine aminotransferase (ALT) normalization (primary endpoint) in patients with incomplete response or intolerance to first-line treatment of AIH.81 Once dosing is determined, a placebo-controlled study of the selected dose will begin and may result in new therapeutic options for refractory AIH.
Table 2.
Current/future therapies to target B cells in autoimmune liver diseases
| Tested immunotherapies | |
|---|---|
| Therapy | Mechanism |
| Corticosteroids | Binding to steroid receptors to promote transcriptional regulation of inflammatory and immune pathways |
| Budesonide | Exhibits immune modulatory effects of steroids but with 90% first-pass hepatic metabolism to limit systemic side effects |
| Antiproliferative agents: azathioprine, mycophenolate mofetil | Interferes with DNA synthesis and inhibits T and B cell proliferation |
| Calcineurin inhibitors: cyclosporine, tacrolimus | Inhibits calcineurin phosphatase and prevents T cell activation; inhibits T-cell-dependent B cell activation |
| mTOR inhibitors: sirolimus | Binds FKBP, inhibits mTOR, prevents cell-cycle progression, thereby inhibiting effector T and B cell proliferation |
| Rituximab | Monoclonal antibody directed against the cell surface antigen CD20 to specifically deplete CD20+ B cells |
| Intravenous immunoglobulin (IVIC) | Downregulation of antibody production; Fc-receptor blockade to prevent progression of the complement cascade and activation of innate immunity |
| Future B-cell-specific immunotherapies | |
| Therapy | Mechanism |
| Bortezomib | Proteasome inhibitor that promotes plasma cell apoptosis |
| Anti-B cell activating factor (BAFF) antibody | Inhibit B cell activating factor (BAFF) to decrease B cell activation, reduce immunoglobulin production, and deplete B cells |
| CXCL10 monoclonal antibody | Block CXCL10, a chemokine produced in part by B cells, to prevent recruitment of lymphocytes |
Abbreviation: mTOR, mechanistic target of rapamycin.
General immunosuppressive agents include steroids and antimetabolites, such as azathioprine and mycophenolate mofetil. Agents that modulate B cell activity indirectly through targeted T cell inhibition include the calcineurin inhibitors, tacrolimus, and cyclosporine, which inhibit the T cell help necessary for B cell differentiation and antibody isotype switching.82 Among the above-described autoimmune liver diseases, immunosuppressive treatment is currently recommended only for AIH. First-line treatment in AIH includes corticosteroids and azathioprine.83–86 Second-line therapy for AIH in the setting of treatment failure includes mycophenolate mofetil,87,88 calcineurin inhibitors,89–95 m-TOR inhibitors,96,97 and rituximab.13,14,98,99 With regard to BA, general immunosuppressive therapy with corticosteroids or IVIG did not improve bilirubin levels or transplant-free survival rates.100,101
While B cell depletion with rituximab carries the risk of hypogammaglobulinemia and increased susceptibility to infection particularly with repeated doses,2 rituximab in AIH has generally been well tolerated. Two cases of pediatric AIH treated with rituximab resulted in biochemical remission, although one experienced a flare 2 years later which was controlled with repeat rituximab infusion.14 Six adult patients with AIH either intolerant or refractory to standard therapy received two doses of rituximab given in addition to azathioprine and prednisone.13 All patients had rapid improvement in liver biochemistry by week 12 and four of the six that underwent a repeat liver biopsy at week 48 after rituximab demonstrated improved inflammation compared with their baseline biopsy. The success and safety profile of rituximab in AIH patients refractory to standard regimens suggests that additional studies on the use of rituximab in other autoimmune liver diseases should be considered. Future research to target the multifaceted role of B cells in autoimmune liver diseases may lessen disease activity and improve prognosis.102–104
Main Concepts and Learning Points
B cells contribute to disease pathogenesis through multiple mechanisms, including antigen presentation/cytokine production leading to T cell activation and generation of antigen/autoantibody complexes resulting in complement activation or phagocytosis.
The majority of autoantibodies in autoimmune hepatitis (AIH) function as diagnostic biomarkers; however, a growing body of literature in animal models and humans suggests that B cell antigen presentation and cytokine production contribute to disease pathogenesis.
In PBC, the disease-specific antimitochondrial antibody (AMA) binds to key autoantigens in disease pathogenesis, and the interaction between B and T cells in the lymphoid follicle can be altered by molecules such as IFN-γ and BAFF.
Some autoantibodies in primary sclerosing cholangitis (PSC) are cross-reactive with intestinal bacteria, suggesting a role of the microbiota in activating autoimmunity.
Future research should focus on the role of B cell antigen presentation and cytokine/chemokine synthesis as it relates to disease pathogenesis, as well the efficacy of therapeutic agents that specifically inhibit B cell function.
Footnotes
Conflict of Interest
None declared.
References
- 1.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol 2010;11(08):681–688 [DOI] [PubMed] [Google Scholar]
- 2.Cooper N, Arnold DM. The effect of rituximab on humoral and cell mediated immunity and infection in the treatment of autoimmune diseases. Br J Haematol 2010;149(01):3–13 [DOI] [PubMed] [Google Scholar]
- 3.Anolik JH, Looney RJ, Lund FE, Randall TD, Sanz I. Insights into the heterogeneity of human B cells: diverse functions, roles in autoimmunity, and use as therapeutic targets. Immunol Res 2009;45(2–3):144–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao Y, Cao X. The immune potential and immunopathology of cytokine-producing B cell subsets: a comprehensive review. J Autoimmun 2014;55:10–23 [DOI] [PubMed] [Google Scholar]
- 5.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol 2008;20(03):332–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bour-Jordan H, Bluestone JA. B cell depletion: a novel therapy for autoimmune diabetes? J Clin Invest 2007;117(12):3642–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD. Ig mu null mice. J Exp Med 1996;184(05):2049–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol 1998;160(01):51–59 [PubMed] [Google Scholar]
- 9.O’Neill SK, Shlomchik MJ, Glant TT, Cao Y, Doodes PD, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol 2005;174(06):3781–3788 [DOI] [PubMed] [Google Scholar]
- 10.Luu VP, Vazquez MI, Zlotnik A. B cells participate in tolerance and autoimmunity through cytokine production. Autoimmunity 2014;47(01):1–12 [DOI] [PubMed] [Google Scholar]
- 11.Vazquez MI, Catalan-Dibene J, Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015;74(02):318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Z, Jegga AG, Bezerra JA. Gene-disease associations identify a connectome with shared molecular pathways in human cholangiopathies. Hepatology 2018;67(02):676–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burak KW, Swain MG, Santodomingo-Garzon T, et al. Rituximab for the treatment of patients with autoimmune hepatitis who are refractory or intolerant to standard therapy. Can J Gastroenterol 2013;27(05):273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Agostino D, Costaguta A, Álvarez F. Successful treatment of refractory autoimmune hepatitis with rituximab. Pediatrics 2013;132(02):e526–e530 [DOI] [PubMed] [Google Scholar]
- 15.Mieli-Vergani G, Vergani D, Czaja AJ, et al. Autoimmune hepatitis. Nat Rev Dis Primers 2018;4:18017. [DOI] [PubMed] [Google Scholar]
- 16.Zachou K, Rigopoulou E, Dalekos GN. Autoantibodies and auto-antigens in autoimmune hepatitis: important tools in clinical practice and to study pathogenesis of the disease. J Autoimmune Dis 2004;1(01):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregorio GV, McFarlane B, Bracken P, Vergani D, Mieli-Vergani G. Organ and non-organ specific autoantibody titres and IgG levels as markers of disease activity: a longitudinal study in childhood autoimmune liver disease. Autoimmunity 2002;35(08):515–519 [DOI] [PubMed] [Google Scholar]
- 18.Muratori L, Parola M, Ripalti A, et al. Liver/kidney microsomal antibody type 1 targets CYP2D6 on hepatocyte plasma membrane. Gut 2000;46(04):553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Löhr HF, Schlaak JF, Lohse AW, et al. Autoreactive CD4+ LKM-specific and anticlonotypic T-cell responses in LKM-1 antibody-positive autoimmune hepatitis. Hepatology 1996;24(06): 1416–1421 [DOI] [PubMed] [Google Scholar]
- 20.Arenz M, Pingel S, Schirmacher P, Meyer zum Büschenfelde KH, Löhr HF. T cell receptor Vbeta chain restriction and preferred CDR3 motifs of liver-kidney microsomal antigen (LKM-1)-reactive T cells from autoimmune hepatitis patients. Liver 2001;21(01):18–25 [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Thomas MG, Okamoto M, et al. Key residues of a major cytochrome P4502D6 epitope are located on the surface of the molecule. J Immunol 2002;169(01):277–285 [DOI] [PubMed] [Google Scholar]
- 22.Muratori L, Cataleta M, Muratori P, Lenzi M, Bianchi FB. Liver/kidney microsomal antibody type 1 and liver cytosol antibody type 1 concentrations in type 2 autoimmune hepatitis. Gut 1998; 42(05):721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunol Cell Biol 2014;92(01):64–71 [DOI] [PubMed] [Google Scholar]
- 24.Abe K, Takahashi A, Imaizumi H, et al. Interleukin-21 plays a critical role in the pathogenesis and severity of type I autoimmune hepatitis. Springerplus 2016;5(01):777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L, Qin J, Ji H, Zhao P, Jiang Y. Tfh and plasma cells are correlated with hypergammaglobulinaemia in patients with autoimmune hepatitis. Liver Int 2014;34(03):405–415 [DOI] [PubMed] [Google Scholar]
- 26.Aoki N, Kido M, Iwamoto S, et al. Dysregulated generation of follicular helper T cells in the spleen triggers fatal autoimmune hepatitis in mice. Gastroenterology 2011;140(04):1322–1333.e1, 5 [DOI] [PubMed] [Google Scholar]
- 27.Béland K, Marceau G, Labardy A, Bourbonnais S, Alvarez F. Depletion of B cells induces remission of autoimmune hepatitis in mice through reduced antigen presentation and help to T cells. Hepatology 2015;62(05):1511–1523 [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Jiang X, Liu R, et al. B cells expressing CD11b effectively inhibit CD4+ T-cell responses and ameliorate experimental autoimmune hepatitis in mice. Hepatology 2015;62(05):1563–1575 [DOI] [PubMed] [Google Scholar]
- 29.Hennig C, Ilginus C, Boztug K, et al. High-content cytometry and transcriptomic biomarker profiling of human B-cell activation. J Allergy Clin Immunol 2014;133(01):172–80.e1, 10 [DOI] [PubMed] [Google Scholar]
- 30.Nishioji K, Okanoue T, Itoh Y, et al. Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clin Exp Immunol 2001;123(02):271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishikawa H, Enomoto H, Iwata Y, et al. B-cell activating factor belonging to the tumor necrosis factor family and interferon-γ-inducible protein-10 in autoimmune hepatitis. Medicine (Baltimore) 2016;95(12):e3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lied GA, Berstad A. Functional and clinical aspects of the B-cell-activating factor (BAFF): a narrative review. Scand J Immunol 2011;73(01):1–7 [DOI] [PubMed] [Google Scholar]
- 33.Moisini I, Davidson A. BAFF: a local and systemic target in autoimmune diseases. Clin Exp Immunol 2009;158(02):155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet 2015;386(10003):1565–1575 [DOI] [PubMed] [Google Scholar]
- 35.Krams SM, Van de Water J, Coppel RL, et al. Analysis of hepatic T lymphocyte and immunoglobulin deposits in patients with primary biliary cirrhosis. Hepatology 1990;12(02):306–313 [DOI] [PubMed] [Google Scholar]
- 36.Tsuneyama K, Baba H, Morimoto Y, Tsunematsu T, Ogawa H. Primary biliary cholangitis: its pathological characteristics and immunopathological mechanisms. J Med Invest 2017;64(1.2):7–13 [DOI] [PubMed] [Google Scholar]
- 37.Webb GJ, Siminovitch KA, Hirschfield GM. The immunogenetics of primary biliary cirrhosis: a comprehensive review. J Autoimmun 2015;64:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollingsworth KG, Newton JL, Taylor R, et al. Pilot study of peripheral muscle function in primary biliary cirrhosis: potential implications for fatigue pathogenesis. Clin Gastroenterol Hepatol 2008;6(09):1041–1048 [DOI] [PubMed] [Google Scholar]
- 39.Khanna A, Jopson L, Howel D, et al. Rituximab is ineffective for treatment of fatigue in primary biliary cholangitis: a phase 2 randomized controlled trial. Hepatology 2018. ( 10.1002/hep.30099 [DOI] [PubMed] [Google Scholar]
- 40.Tsuda M, Moritoki Y, Lian ZX, et al. Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Hepatology 2012;55(02):512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moritoki Y, Tsuneyama K, Nakamura Y, et al. Anti-drug antibodies against a novel humanized anti-CD20 antibody impair its therapeutic effect on primary biliary cholangitis in human CD20- and FcγR-expressing mice. Front Immunol 2018;9:2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bassendine MF, Jones DE, Yeaman SJ. Biochemistry and autoimmune response to the 2-oxoacid dehydrogenase complexes in primary biliary cirrhosis. Semin Liver Dis 1997;17(01):49–60 [DOI] [PubMed] [Google Scholar]
- 43.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol 1987; 138(10):3525–3531 [PubMed] [Google Scholar]
- 44.Tsuneyama K, Van de Water J, Leung PS, et al. Abnormal expression of the E2 component of the pyruvate dehydrogenase complex on the luminal surface of biliary epithelium occurs before major histocompatibility complex class II and BB1/B7 expression. Hepatology 1995;21(04):1031–1037 [PubMed] [Google Scholar]
- 45.Nakamura M, Ishibashi H, Matsui M, et al. Peripheral B lymphocyte repertoire to mitochondrial antigen in primary biliary cirrhosis–positive correlation between the disease activity and the frequency of circulating B lymphocytes specific for pyruvate dehydrogenase complex. Autoimmunity 1995;21(04):253–262 [DOI] [PubMed] [Google Scholar]
- 46.Jansen PL, Ghallab A, Vartak N, et al. The ascending pathophysiology of cholestatic liver disease. Hepatology 2017;65(02): 722–738 [DOI] [PubMed] [Google Scholar]
- 47.Chung BK, Guevel BT, Reynolds GM, et al. Phenotyping and autoantibody production by liver-infiltrating B cells in primary sclerosing cholangitis and primary biliary cholangitis. J Autoimmun 2017;77:45–54 [DOI] [PubMed] [Google Scholar]
- 48.Shimoda S, Miyakawa H, Nakamura M, et al. CD4 T-cell autoreactivity to the mitochondrial autoantigen PDC-E2 in AMA-negative primary biliary cirrhosis. J Autoimmun 2008;31(02):110–115 [DOI] [PubMed] [Google Scholar]
- 49.Nakamura M, Kondo H, Mori T, et al. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology 2007;45(01):118–127 [DOI] [PubMed] [Google Scholar]
- 50.Nakamura M Clinical significance of autoantibodies in primary biliary cirrhosis. Semin Liver Dis 2014;34(03):334–340 [DOI] [PubMed] [Google Scholar]
- 51.Wesierska-Gadek J, Penner E, Battezzati PM, et al. Correlation of initial autoantibody profile and clinical outcome in primary biliary cirrhosis. Hepatology 2006;43(05):1135–1144 [DOI] [PubMed] [Google Scholar]
- 52.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2019;69(01): 394–419 [DOI] [PubMed] [Google Scholar]
- 53.Tan YG, Wang YQ, Zhang M, et al. Clonal characteristics of circulating B lymphocyte repertoire in primary biliary cholangitis. J Immunol 2016;197(05):1609–1620 [DOI] [PubMed] [Google Scholar]
- 54.Takahashi T, Miura T, Nakamura J, et al. Plasma cells and the chronic nonsuppurative destructive cholangitis of primary biliary cirrhosis. Hepatology 2012;55(03):846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bae HR, Leung PS, Tsuneyama K, et al. Chronic expression of interferon-gamma leads to murine autoimmune cholangitis with a female predominance. Hepatology 2016;64(04):1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bae HR, Hodge DL, Yang GX, et al. The interplay of type I and type II interferons in murine autoimmune cholangitis as a basis for sex-biased autoimmunity. Hepatology 2018;67(04):1408–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krams SM, Cao S, Hayashi M, Villanueva JC, Martinez OM. Elevations in IFN-gamma, IL-5, and IL-10 in patients with the autoimmune disease primary biliary cirrhosis: association with autoantibodies and soluble CD30. Clin Immunol Immunopathol 1996;80(3, Pt 1):311–320 [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Wang W, Tang L, et al. Chemokine (C-X-C motif) ligand 13 promotes intrahepatic chemokine (C-X-C motif) receptor 5+ lymphocyte homing and aberrant B-cell immune responses in primary biliary cirrhosis. Hepatology 2015;61(06):1998–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Migita K, Ilyassova B, Kovzel EF, et al. Serum BAFF and APRIL levels in patients with PBC. Clin Immunol 2010;134(02):217–225 [DOI] [PubMed] [Google Scholar]
- 60.Zhang B, Hu M, Zhang P, et al. BAFF promotes regulatory T-cell apoptosis and blocks cytokine production by activating B cells in primary biliary cirrhosis. Braz J Med Biol Res 2013;46(05):433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet 2013;382(9904):1587–1599 [DOI] [PubMed] [Google Scholar]
- 62.Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol 2017;67(06):1298–1323 [DOI] [PubMed] [Google Scholar]
- 63.Tabibian JH, O’Hara SP, Larusso NF. Primary sclerosing cholangitis: the gut-liver axis. Clin Gastroenterol Hepatol 2012;10(07): 819, author reply 819–820 [DOI] [PubMed] [Google Scholar]
- 64.Tabibian JH, O’Hara SP, Lindor KD. Primary sclerosing cholangitis and the microbiota: current knowledge and perspectives on etiopathogenesis and emerging therapies. Scand J Gastroenterol 2014;49(08):901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chung BK, Henriksen EKK, Jørgensen KK, Karlsen TH, Hirschfield GM, Liaskou E. Gut and liver B cells of common clonal origin in primary sclerosing cholangitis-inflammatory bowel disease. Hepatol Commun 2018;2(08):956–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sebode M, Weiler-Normann C, Liwinski T, Schramm C. Auto-antibodies in autoimmune liver disease-clinical and diagnostic relevance. Front Immunol 2018;9:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marzorati S, Invernizzi P, Lleo A. Making sense of autoantibodies in cholestatic liver diseases. Clin Liver Dis 2016;20(01):33–46 [DOI] [PubMed] [Google Scholar]
- 68.Lenzen H, Weismüller TJ, Negm AA, et al. Antineutrophil cytoplasmic antibodies in bile are associated with disease activity in primary sclerosing cholangitis. Scand J Gastroenterol 2013;48(10):1205–1212 [DOI] [PubMed] [Google Scholar]
- 69.Jendrek ST, Gotthardt D, Nitzsche T, et al. Anti-GP2 IgA auto-antibodies are associated with poor survival and cholangiocarcinoma in primary sclerosing cholangitis. Gut 2017;66(01): 137–144 [DOI] [PubMed] [Google Scholar]
- 70.Berglin L, Björkström NK, Bergquist A. Primary sclerosing cholangitis is associated with autoreactive IgA antibodies against biliary epithelial cells. Scand J Gastroenterol 2013;48(06): 719–728 [DOI] [PubMed] [Google Scholar]
- 71.Bezerra JA, Wells RG, Mack CL, et al. Biliary atresia: clinical and research challenges for the 21st century. Hepatology 2018. ( 10.1002/hep.29905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bednarek J, Traxinger B, Brigham D, et al. Cytokine-producing B cells promote immune-mediated bile duct injury in murine biliary atresia. Hepatology 2018;68(05):1890–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bove KE, Sheridan R, Fei L, et al. Hepatic hilar lymph node reactivity at Kasai portoenterostomy for biliary atresia: correlations with age, outcome, and histology of proximal biliary remnant. Pediatr Dev Pathol 2018;21(01):29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holladay SD, Smialowicz RJ. Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environ Health Perspect 2000;108 (Suppl 3):463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merbl Y, Zucker-Toledano M, Quintana FJ, Cohen IR. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest 2007; 117(03):712–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hadchouel M, Hugon RN, Odievre M. Immunoglobulin deposits in the biliary remnants of extrahepatic biliary atresia: a study by immunoperoxidase staining in 128 infants. Histopathology 1981;5(02):217–221 [DOI] [PubMed] [Google Scholar]
- 77.Mack CL, Tucker RM, Lu BR, et al. Cellular and humoral auto-immunity directed at bile duct epithelia in murine biliary atresia. Hepatology 2006;44(05):1231–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu BR, Brindley SM, Tucker RM, Lambert CL, Mack CL. α-Enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastroenterology 2010;139(05):1753–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pang SY, Dai YM, Zhang RZ, et al. Autoimmune liver disease-related autoantibodies in patients with biliary atresia. World J Gastroenterol 2018;24(03):387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feldman AG, Tucker RM, Fenner EK, Pelanda R, Mack CL. B cell deficient mice are protected from biliary obstruction in the rotavirus-induced mouse model of biliary atresia. PLoS One 2013;8(08):e73644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones D, Manns MP, Hexham M, Pedrosa MC, Vierling JM. AMBER-a novel phase 2/3 trial of ianalumab, a human anti-BAFF receptor antibody, in autoimmune hepatitis, NCT03217422. Hepatology 2018;68:1120A [Google Scholar]
- 82.Heidt S, Roelen DL, Eijsink C, et al. Calcineurin inhibitors affect B cell antibody responses indirectly by interfering with T cell help. Clin Exp Immunol 2010;159(02):199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis–update 2015. J Hepatol 2015;62(1, Suppl):S100–S111 [DOI] [PubMed] [Google Scholar]
- 84.European Association for the Study of the Liver. Autoimmune hepatitis. J Hepatol 2015;63(04):971–1004 [DOI] [PubMed] [Google Scholar]
- 85.Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D.Autoimmune hepatitis: Standard treatment and systematic review of alternative treatments. World J Gastroenterol 2017;23(33):6030–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manns MP, Czaja AJ, Gorham JD, et al. ; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51(06):2193–2213 [DOI] [PubMed] [Google Scholar]
- 87.Inductivo-Yu I, Adams A, Gish RG, et al. Mycophenolate mofetil in autoimmune hepatitis patients not responsive or intolerant to standard immunosuppressive therapy. Clin Gastroenterol Hepatol 2007;5(07):799–802 [DOI] [PubMed] [Google Scholar]
- 88.Aw MM, Dhawan A, Samyn M, Bargiota A, Mieli-Vergani G. Mycophenolate mofetil as rescuetreatment forautoimmuneliverdisease in children: a 5-year follow-up. J Hepatol 2009;51(01):156–160 [DOI] [PubMed] [Google Scholar]
- 89.Mistilis SP, Vickers CR, Darroch MH, McCarthy SW. Cyclosporin, a new treatment for autoimmune chronic active hepatitis. Med J Aust 1985;143(10):463–465 [DOI] [PubMed] [Google Scholar]
- 90.Person JL, McHutchison JG, Fong TL, Redeker AG. A case of cyclosporine-sensitive, steroid-resistant, autoimmune chronic active hepatitis. J Clin Gastroenterol 1993;17(04):317–320 [DOI] [PubMed] [Google Scholar]
- 91.Sherman KE, Narkewicz M, Pinto PC. Cyclosporine in the management of corticosteroid-resistant type I autoimmune chronic active hepatitis. J Hepatol 1994;21(06):1040–1047 [DOI] [PubMed] [Google Scholar]
- 92.Aqel BA, Machicao V, Rosser B, Satyanarayana R, Harnois DM, Dickson RC. Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol 2004;38(09):805–809 [DOI] [PubMed] [Google Scholar]
- 93.Larsen FS, Vainer B, Eefsen M, Bjerring PN, Adel Hansen B. Low-dose tacrolimus ameliorates liver inflammation and fibrosis in steroid refractory autoimmune hepatitis. World J Gastroenterol 2007;13(23):3232–3236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al Taii H, Hanouneh MA, Hanouneh I, Lopez R, Zein N, Alkhouri N. The use of tacrolimus in refractory autoimmune hepatitis in children and adults: a single center experience. Scand J Gastroenterol 2017;52(02):157–158 [DOI] [PubMed] [Google Scholar]
- 95.Than NN, Wiegard C, Weiler-Normann C, et al. Long-term follow-up of patients with difficult to treat type 1 autoimmune hepatitis on tacrolimus therapy. Scand J Gastroenterol 2016;51(03):329–336 [DOI] [PubMed] [Google Scholar]
- 96.Chatrath H, Allen L, Boyer TD. Use of sirolimus in the treatment of refractory autoimmune hepatitis. Am J Med 2014;127(11): 1128–1131 [DOI] [PubMed] [Google Scholar]
- 97.Kurowski J, Melin-Aldana H, Bass L, Alonso EM, Ekong UD. Sirolimus as rescue therapy in pediatric autoimmune hepatitis. J Pediatr Gastroenterol Nutr 2014;58(01):e4–e6 [DOI] [PubMed] [Google Scholar]
- 98.Rubin JN, Te HS. Refractory autoimmune hepatitis: beyond standard therapy. Dig Dis Sci 2016;61(06):1757–1762 [DOI] [PubMed] [Google Scholar]
- 99.Roberts SK, Kemp W. Salvage therapies for autoimmune hepatitis: a critical review. Semin Liver Dis 2017;37(04):343–362 [DOI] [PubMed] [Google Scholar]
- 100.Bezerra JA, Spino C, Magee JC, et al. ; Childhood Liver Disease Research and Education Network (ChiLDREN). Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. JAMA 2014;311(17):1750–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mack CL, Spino C, Moore J. A phase I/IIa trial of intravenous immunoglobulin (IVIG) therapy following portoenterostomy in infants with biliary atresia. J Ped Gastroenterol Nutr 2019;68(04):495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dörner T, Isenberg D, Jayne D, Wiendl H, Zillikens D, Burmester G; International Roundtable on B cells as Therapeutic Target for Intervention. Current status on B-cell depletion therapy in autoimmune diseases other than rheumatoid arthritis. Autoimmun Rev 2009;9(02):82–89 [DOI] [PubMed] [Google Scholar]
- 103.Hofmann K, Clauder AK, Manz RA. Targeting B cells and plasma cells in autoimmune diseases. Front Immunol 2018;9:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trivedi PJ, Hirschfield GM. Treatment of autoimmune liver disease: current and future therapeutic options. Ther Adv Chronic Dis 2013;4(03):119–141 [DOI] [PMC free article] [PubMed] [Google Scholar]