Abstract
Transition metal ions are essential to bacterial pathogens and their hosts alike but are harmful in excess. In an effort to curtail the replication of intracellular bacteria, host phagocytes exploit both the essentiality and toxicity of transition metals. In the paradigmatic description of nutritional immunity, iron and manganese are withheld from phagosomes to starve microbial invaders of these nutrients. Conversely, the destructive properties of copper and zinc appear to be harnessed by phagocytes, where these metals are delivered in excess to phagosomes to intoxicate internalized bacteria. Here, we briefly summarize key players in metal withholding from intracellular pathogens, before focusing on recent findings supporting the function of copper and zinc as phagocyte antimicrobial effectors. The mechanisms of copper and zinc toxicity are explored, along with strategies employed by intracellular bacterial pathogens to avoid killing by these metals.
Introduction
The six 3d-block transition metals manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn) are essential micronutrients to both hosts and bacterial pathogens. Metals are required for the functionality of nearly half of all enzymes and are involved in virtually all biological processes, from transcription through respiration [1–3]. Within metalloproteins, metals fulfill three main functions: providing structural stability, serving as enzymatic cofactors, and facilitating electron transport reactions through their redox potential [3]. Despite their essentiality, the characteristics of transition metals that make them useful biological catalysts also cause them to be toxic in excess. Uncontrolled cycling by redox active metals may facilitate production of toxic reactive oxygen species (ROS), and the affinity of these metals for the functional groups of proteins may lead to population of enzymes with a non-cognate metal cofactor. As such, both hosts and pathogens possess elaborate mechanisms to maintain homeostatic concentrations of transition metals [4].
In addition to tightly regulating metal availability to protect against damage, vertebrate hosts also restrict access to these metals during infection to curtail bacterial proliferation. This process, known as nutritional immunity, has traditionally focused on how metal limitation inhibits bacterial growth [4,5]. Within the last five to ten years, however, increasing evidence has mounted to suggest that the host also exploits the toxicity of metals to directly poison intracellular bacteria. In this review, we discuss the role of transition metals in controlling intracellular pathogens, with a particular focus on the newly emerging roles for Cu and Zn as phagocyte antimicrobial effectors. We discuss proposed mechanisms of Cu and Zn toxicity in bacterial pathogens, and methods they employ to subvert this toxicity.
Metal withholding as a defense against intracellular pathogens
During infection, the availability and distribution of Fe, Mn, and Zn are profoundly altered through local and systemic changes that restrict the availability of these metals to pathogens. Several recent reviews have been written on metal-withholding as a facet of nutritional immunity against both extracellular and intracellular pathogens, and the reader is referred to these reviews for a comprehensive discussion of the topic [4,6,7]. The importance of nutrient metal withholding as a strategy to control intracellular pathogens, however, emerges from the observation that perturbations to host metal homeostasis can enhance susceptibility to infection by pathogens such as Salmonella and Mycobacteria sp. [8,9]. Below we have summarized major players in restricting access of nutrient metals to intracellular pathogens.
IRON
One of the key mechanisms limiting Fe availability to extracellular pathogens during infection is the maintenance of Fe in the reticuloendothelial system, particularly in macrophages, hepatocytes, and intestinal enterocytes [10,11]. In response to bacterial infection, interleukin-6 induces expression of hepcidin, which in turn binds the only known Fe exporter in eukaryotes, ferroportin (Fpn), and targets it for degradation. Whilst Fpn degradation leads to decreased circulating Fe, it presumably increases the cytosolic pool of Fe available to support growth of intracellular pathogens [12–14]. Fortunately, in the presence of intracellular pathogens such as Salmonella enterica serovar Typhimurium (S. Typhimurium), transcription of the gene encoding Fpn, SLC40A1, is increased, offsetting the hepcidin-induced Fpn protein degradation. Transcription of SLC40A1 is induced by the redox-sensitive host transcription factor Nrf2 (nuclear factor erythroid 2) in the presence of nitric oxide, which helps to maintain macrophage iron homeostasis during intracellular infection [15,16]. Additionally, for bacteria residing in phagosomes, the availability of Fe and Mn (and perhaps other divalent metals) is further depleted through the activity of NRAMP1 (natural resistance-associated macrophage protein 1), a transporter which extrudes these metals from the phagosomal lumen [8,9,17]. To combat host Fe sequestration, bacteria express a plethora of acquisition systems that have been discussed in-depth in other recent reviews [4,18].
MANGANESE AND ZINC
Aside from the function of NRAMP1 in depleting phagosomes of divalent metals, surprisingly little is known about how the host restricts Mn and Zn availability to intracellular pathogens. Neutrophil-derived calprotectin (CP) has materialized as a key facet of nutritional immunity against extracellular pathogens, where it sequesters divalent metals such as Mn, Zn, and likely Fe [19,20]. To date, CP is the only known host Mn sequestration complex that inhibits microbial growth [21]. Notably, CP is unlikely to contribute to the nutritional defense against intracellular pathogens, as it is not thought to bind metals within the cell. Metallothioneins (MTs), however, are emerging as possible players in maintaining Zn homeostasis during infection, functioning to bind Zn (and other heavy metals) in the cytosol following uptake by Zrt- and Irt- like proteins (ZIP). In this manner, MTs may function to sequester Zn from both extracellular and intracellular pathogens and indeed, host MT expression increases during infection [22,23]. Investigating the mechanisms employed by phagocytes to restrict Mn and Zn availability to intracellular pathogens is yet in its infancy and represents a promising area of future research.
Metal intoxication as a defense against intracellular pathogens
COPPER
Model for Cu as a phagocyte antimicrobial effector
Unlike with Fe and Mn, the role of Cu in the innate immune defense against intracellular pathogens is thought to function not through starvation, but rather through intoxication [24]. Early evidence for Cu functioning as a potential phagocyte antimicrobial effector arose from elemental analysis of phagosomes, where Mycobacterium avium-infected macrophages treated with interferon-γ (IFN-γ) or tumor necrosis factor-α (TNF-α) exhibited a trend towards increased accumulation of phagosomal Cu [25]. In seminal work by Petris and colleagues, treatment of RAW264.7 macrophage-like cells with IFN-γ or lipopolysaccharide (LPS) was shown to increase Cu uptake and to stimulate expression of the high affinity copper importer, CTR1 [26]. Similarly, these proinflammatory molecules also enhanced expression of the P1B-type ATPase ATP7A, a transporter usually responsible for delivering Cu from the cytosolic Cu-chaperone ATOX1 to the Golgi apparatus for population of cuproproteins. Interestingly, IFN-γ activation of macrophages also appeared to promote trafficking of ATP7A to post-Golgi vesicles, including redistribution to phagosomal compartments, suggesting that ATP7A may facilitate delivery of Cu to phagocytosed bacteria. Consistent with this notion, disruption of ATP7A expression reduces killing of non-pathogenic Escherichia coli and S. Typhimurium, whereas bacterial mutants that are defective for Cu export are hypersensitive to macrophage killing in an ATP7A-dependent manner [26,27]. Together, these findings provide evidence for intracellular Cu-mediated bacterial killing, and support a model whereby macrophages increase uptake of Cu through CTR1, and subsequently redistribute ATP7A to the phagosomal membrane to drive influx of Cu to kill engulfed bacteria (Figure 1).
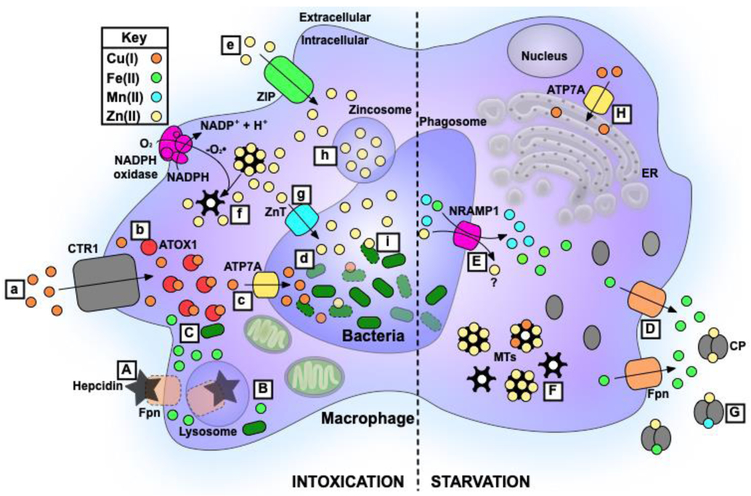
Figure 1. Key metal-dependent strategies employed by phagocytes to inhibit survival of intracellular bacteria.
A schematic cartoon of the metal intoxication (left and lowercase letters) and starvation (right and uppercase letters) tactics used by the host to control the survival and replication of intracellular bacterial pathogens (green bacilli). During infection, ferroportin (Fpn; peach) is bound by hepcidin (black star), which targets Fpn for lysosomal degradation (A,B). Decreased Fpn expression leads to increased accumulation of Fe(II) within the phagocyte cytosol which, may promote growth of intracellular bacteria (C). To counteract Fe accumulation, Fpn expression is increased during intracellular infection, promoting extrusion of Fe (green circles) from the host cytosol (D). Additionally, Fe, Mn (cyan circles), and perhaps Zn (yellow circles) are effluxed from the phagosome by NRAMP1 (pink) (E). Free Zn, Cu (orange circles), and other heavy metals within the cytosol are sequestered by metallothioneins (MTs; black flowers) (F). Divalent metals in the external milieu are sequestered by calprotectin (CP; grey shapes) (G). ATP7A (yellow) associates primarily with the endoplasmic reticulum (ER) to populate cuproproteins with Cu (H).
In using metal toxicity in the killing of intracellular bacteria, expression of the Cu importer CTR1 (grey) is increased, leading to Cu accumulation within the phagocyte (a). Cytosolic Cu is sequestered by the metallochaperone ATOX1 (salmon; b) and shuttled to the phagosomal membrane for transport into the vesicle by ATP7A (c). Once within the phagosome, free Cu poisons bacteria, in part through mismetallation of key metalloproteins (d). Similarly, Zn is likely transported into the phagocyte by a ZIP family importer (green; e). Cytosolic Zn is thought to be liberated from MTs through oxidative release, facilitated by phagocyte NADPH oxidase (pink; f). Zn is delivered to the phagosome by way of a ZnT family transporter (cyan; g) or through fusion with a zincosome (purple; h), where it intoxicates bacteria through mismetallation of key proteins/processes (i).
Mechanisms of Cu toxicity
Whilst the exact mechanism of Cu toxicity is unknown, a number of modes of action have been proposed. A commonly cited means of Cu poisoning hinges upon the redox potential of the metal. Under aerobic conditions, it has been hypothesized that Cu catalyzes the formation of ROS through the Haber-Weiss reaction and Fenton-like chemistry, in a manner analogous to Fe(II) [28,29]:
In addition to being byproducts of normal aerobic respiration, •O2− and H2O2are also components of the phagocyte oxidative burst, produced by NADPH oxidase and superoxide dismutase (SOD), respectively. Through the above reactions, Cu may thus further potentiate the production of phagosomal ROS, including facilitating the production of highly toxic hydroxyl radicals (•OH). Hydroxyl radicals react indiscriminately with macromolecules such as nucleic acids, proteins, and lipids, ultimately compromising bacterial viability. Although this mechanism of toxicity is well-defined for Fe, it is important to note that bacterial killing by Cu-derived ROS has not been conclusively demonstrated in vivo.
Interestingly, and in contrast to the above hypothesis, E. coli mutants that are debilitated for Cu detoxification and export are less sensitive to killing by H2O2, do not exhibit increased nucleic acid damage, and are most inhibited for growth in the presence of Cu under anaerobic conditions [30]. The aforementioned findings are incongruent with the idea that Cu poisons bacteria solely through the generation of ROS. In an effort to reconcile these inconsistencies, Macomber and Imlay investigated the route of Cu toxicity in E. coli and found that Cu appears to target solvent-exposed [Fe-S] cluster enzymes in branched-chain amino acid biosynthesis [31]. Cu is a highly competitive metal for protein binding, as described by the Irving-Williams series (Mn2+ < Fe2+< Co2+ < Ni2+ < Cu2+ > Zn2+) and has the potential to stably displace all other transition metals from metalloenzymes [32]. Mismetallation of key biosynthetic enzymes in the production of heme, nucleotides, and [Fe-S] clusters has since been identified as a mechanism of Cu toxicity in Neisseria gonorrhoeae [33], Streptococcus pneumoniae [34], and E. coli and Bacillus subtilis [35–37], respectively. Additionally, in the case where Cu displaces Fe from metalloproteins, it is possible that these liberated Fe atoms may further enhance the generation of ROS. These findings suggest that the mechanism of Cu-mediated killing by macrophages is likely to be multifaceted and may be specific to a given pathogen.
Bacterial strategies for avoiding Cu toxicity
To avoid poisoning, bacteria possess many, often redundant, mechanisms to avoid or detoxify Cu (see Table 1 for examples). Broadly, strategies to mitigate Cu toxicity include: 1) minimizing the nutritional requirement for Cu, 2) extruding free Cu from the cytoplasm, 3) sequestering free Cu, and 4) oxidizing Cu(I) to less toxic Cu(II). Whilst an extensive examination of these mechanisms is outside the scope of this review, they are briefly summarized below [for more comprehensive reviews see 24,38].
Table 1.
Copper detoxification strategies of intracellular pathogens and their association with survival and virulence.
| Pathogen | Locus/Gene | Function | Infection model | Phenotype of mutant during infection, relative to wild-type |
Reference |
|---|---|---|---|---|---|
| Transportation | |||||
| M. tuberculosis | mctB | Mycomembrane channel; efflux | Inhalational; guinea pig | Reduced bacterial burdens in lungs and lymph nodes | [63] |
| ctpV | Putative inner membrane protein; efflux | Inhalational; mouse, guinea pig | Reduced lung damage and immune response, decreased mortality of mice | [64] | |
| ricR | Transcriptional repressor of Cu responsive regulon; Cu homeostasis | Inhalational; mouse | Mutant with constitutive repression of ricR regulon has reduced bacterial burden in lungs | [65] | |
| S. Typhimurium | copA and golT | Inner membrane P1B-type ATPases; efflux | Peritoneal macrophages | Decreased survival of ΔcopA ΔgolTin wildtype but not ATP7aLysMcre macrophages | [27] |
| RAW264.7 macrophages | Decreased survival of ΔcopA ΔgolT | [48] | |||
| Competitive intraperitoneal challenge; mouse | Decreased survival of ΔcopA ΔgolT in livers and spleens | [27] | |||
| *E. coli | copA | Inner membrane P1B− type ATPase; efflux | RAW264.7 macrophages | Decreased survival of mutant | [26] |
| S. pneumoniae | copA | Inner membrane P1B− type ATPase; efflux | Intratracheal, intraperitoneal, and intravenous; mouse | Decreased survival in blood, decreased mortality of mice | [49,66] |
| A549 lung epithelial cells | Decreased adherence and invasion | [49] | |||
| Sequestration | |||||
| Uropathogenic E. coli | ybt | Cu-binding siderophore (yersiniabactin); sequestration, detoxification | Urinary tract infection; mouse and human | Uropathogenic E. coli expressing ybt during infection has enhanced Cu resistance Cu(II)-Ybt forms during infection | [45] |
| RAW264.7 macrophages | Decreased survival of mutant in Cu-replete macrophages | [47] | |||
| Oxidation | |||||
| S. Typhimurium | cueO | Multicopper oxidase; detoxification | Oral gavage; mouse | Decreased burdens in liver and spleen | [67] |
Non-pathogenic, but provided as a model Gram-negative organism.
Unlike eukaryotes, bacteria have not evolved an extensive repertoire of Cu-requiring proteins. Bacterial cuproenzymes primarily include Cu/Zn SOD, and cytochrome and multicopper oxidases [39,40]. Cu-containing proteins in bacteria are localized to the cytoplasmic membrane or the periplasm, which obviates the need to uptake Cu into the cytosol, and maintains free Cu concentrations in the attomolar range (10−18) [41]. Removal of excess Cu from the cytoplasm is performed by heavy metal efflux pumps of the P1B-type ATPase superfamily, often designated as CopA, that function antagonistically to ATP7A [27]. In Gram-negative bacteria, Cu(I) is subsequently exported from the periplasm by way of additional transport systems, such as the well-characterized and cell envelope-spanning CusCBA complex in E. coli [42].
Notably, simply exporting Cu from the cell is not sufficient to fully protect against toxicity, and bacteria also possess many unique mechanisms to sequester or detoxify Cu within the cell. Examples of this include the Cu chaperone CusF of E. coli, which helps to shuttle Cu to the CusCBA complex for efflux [43]. In M. tuberculosis, one of the first bacterial MTs was identified (MymT), which can sequester up to seven cytoplasmic Cu(I) ions, with a preference for four to six Cu(I) ions [44]. Recently, yersiniabactin of E. coli was found not only to function in Fe uptake, but also in the delivery Cu to a cuproenzyme under low Cu conditions and as a SOD mimic to help protect against the phagocyte oxidative burst [45–47]. It appears that yersiniabactin functions both to supply nutritional Cu, whilst at the same time guarding against its toxicity, a strategy the authors refer to as “nutritional passivation” [46]. Lastly, many bacteria, including E. coli, M. tuberculosis, and S. Typhimurium, express a multicopper oxidase that confers Cu tolerance, purportedly through oxidizing Cu(I) to less toxic Cu(II) [38 and references therein].
Role of Cu detoxification in intracellular survival
The abundance and diversity of Cu detoxification machinery in bacterial pathogens suggests that these organisms must encounter harmful levels of Cu during the course of infection. Indeed, such systems have increasingly been shown to impact bacterial virulence in various models of infection (Table 1). Importantly, and relevant to this issue specifically, disruption to Cu homeostasis mechanisms decreases intracellular survival of a number of pathogens including E. coli [26,47], S. Typhimurium [27,48], and S. pneumoniae [49]. These findings, coupled with the observation of increased trafficking of Cu to the phagosome during infection, suggest that death by phagosomal Cu overload may be a common but underappreciated mechanism of bacterial killing within macrophages.
ZINC
Model for Zn as a phagocyte antimicrobial effector
The role of Zn in nutritional immunity is unique, with both Zn starvation and Zn intoxication posited as mechanisms of inhibiting bacterial proliferation. Bioavailable levels of Zn within vertebrates are sufficiently low as to necessitate the expression of Zn importers in virtually all bacteria, and oftentimes these transporters are associated with bacterial virulence [4,50]. Despite growing appreciation forZn sequestration as an important facet of nutritional immunity, this means of inhibiting bacterial survival and proliferation appears to apply primarily to extracellular bacterial pathogens. However, as with Cu, increasing evidence suggests that excess Zn may also be used as an antimicrobial agent against intracellular pathogens.
In the same elemental analysis as discussed above for Cu, Zn was also shown to increase over time in activated macrophages infected with mycobacteria [34]. Similarly, Botella et al. observed an increase in intraphagosomal Zn that colocalized with M. tuberculosis internalized in human macrophages [22]. Transcriptional analyses revealed upregulation of known heavy metal detoxification genes in both host macrophages and the pathogen, and disruption of a Zn-responsive P1B-type ATPase in M. tuberculosis (CtpC) lead to impaired survival of the mutant intracellularly and under Zn stress [22]. Together these data suggest that the host employs Zn poisoning to eradicate the phagocytosed mycobacteria. The authors propose a model whereby NADPH phagocyte oxidase facilitates the oxidative release of metal from cytosolic Zn(II)-MT complexes. From there, Zn is thought to be delivered to host endocytic compartments and phagosomes by an as-yet-unidentified member of the Zn transporter (ZnT (SLC30)) family, or through fusion with Zn-containing vesicles (“zincosomes”) (Figure 1) [22]. This model is supported by the observation that E. coli also upregulates Zn detoxification genes within human macrophages and promotes intraphagosomal Zn accumulation, where bacteria lacking the well-characterized Zn efflux pump, ZntA, are debilitated for intracellular survival [22,51].
Mobilization of Zn in response to intracellular pathogens does not appearto be limited to macrophages, as neutrophils have also been shown to increase intracellular Zn levels in response to Group A Streptococcus (GAS) infection [52]. In an important study working to dissect the role of Zn homeostasis during GAS infection, Ong et al. provided additional support to the hypothesis that Zn intoxication of bacteria occurs intracellularly, whilst Zn starvation occurs extracellularly [53]. The authors observed that GAS Zn efflux genes were expressed upon exposure to human neutrophils and the corresponding bacterial mutants exhibited decreased intracellular survival, whereas Zn uptake mutants were impaired for extracellular survival. Additionally, increased Zn was observed at sites of infection due to an influx of Zn-rich neutrophils, where the metal was found to colocalize with lysosomes and azurophilic granules in both naive and infected cells. In support of the zincosome model proposed above, upregulation of ZnTs was not observed in infected neutrophils, and instead it was reasoned that preformed Zn-loaded granules fuse with the phagosome to deliver a toxic load of Zn to phagocytosed bacteria [53].
Mechanisms of Zn toxicity
As highlighted in the Irving-Williams series, Zn(II) is a highly competitive metal for protein binding but due to a stably filled 3d orbital, is redox-inert [32,50]. From the outset, early studies have supported the notion that Zn is toxic due to its ability to mismetallate key proteins and enzymes. In S. pneumoniae, Zn can irreversibly bind the extracellular substrate binding protein PsaA, blocking Mn(II) uptake by the bacterium and increasing its susceptibility to oxidative stress [54,55]. Excess Zn inhibits glycolytic enzymes and phosphoglucomutase in GAS, resulting in reduced growth on glucose and diminished capsule production, respectively [56]. In the Gram-positive model organism, B. subtilis, excess extracellular Zn inhibits the electron transport chain through inactivation of the heme-Cu-dependent cytochrome aa3 oxidase. In the absence of Zn-efflux proteins, Zn accumulates intracellularly and mismetallates cytosolic proteins involved in the regulation of heme biosynthesis resulting in toxicity [57]. Together these studies provide important insights into the molecular targets of Zn toxicity; however, further investigation is required to identify if similar proteins/pathways are targeted in phagocytosed bacteria.
Bacterial strategies for avoiding Zn toxicity
Relative to Cu, bacterial mechanisms of avoiding Zn toxicity appear fairly simplistic (see Table 2 for examples). Generally, cytosolic Zn concentrations are sensed by a Zn-dependent transcriptional regulator, and excess Zn is extruded from the cytoplasm by one or more P1B-type ATPases, cation diffusion facilitators (CDFs) and/or transporters of the resistance-nodulation-cell division (RND) superfamily [33]. Zn detoxification is best characterized in E. coli, which expresses multiple efflux systems with varying specificities for Zn(II) and other divalent metals such as lead and cadmium. Under conditions of excess Zn, the transcriptional regulator ZntR binds Zn(II) and induces transcription of the ATPase ZntA, as well as the CDFs ZitB and YiiP [50]. Zn detoxification machinery homologous to that found in E. coli is also used by professional intracellular pathogens such as S. Typhimurium [58]. Some Gram-negative bacteria also appear to possess a tripartite cobalt-zinc-cadmium/nickel RND-driven detoxification system that spans the periplasm and efficiently expels these metals from the bacteria [50,59]. Additionally, Zn within the cytosol is protected from aberrant population of metalloproteins through buffering by MTs, histidine, and/or low molecular weight thiols [50].
Table 2.
Zinc detoxification strategies of intracellular pathogens and their association with survival and virulence.
| Pathogen | Locus/Gene | Function | Infection model |
Phenotype | Reference |
|---|---|---|---|---|---|
| Transportation | |||||
| M. tuberculosis | ctpC | Inner membrane P1B-type ATPase, efflux | Human monocyte-derived macrophages | Decreased survival of mutant | [22] |
| *E. coli | zntA | Inner membrane P1B-type ATPase, efflux | Human monocyte-derived macrophages | Decreased survival of mutant | [22] |
| Neutrophils | Decreased survival of mutant | [51] | |||
| Group A Streptococcus | czcD | CDF family transporter; efflux | Neutrophils | Decreased survival of mutant | [52,53] |
| Invasive subcutaneous; humanized plasminogen transgenic mouse | Decreased initial lesion size, dissemination in blood, mortality of mice Decreased survival relative to wild-type in competitive infection | [52,53] | |||
| S. Typhimurium | Salmonella pathogenicity island-1 | Delivery of bacterial effector molecules; unknown mechanism | Human monocyte-derived macrophages | Prevents co-localization of phagosomes with Zn-containing vesicles | [51] |
| zntA and zitB | Inner membrane P1B-type ATPase and CDF family transporters, respectively; efflux | Competitive intraperitoneal challenge; mouse | Reduced recovery of ΔzntA ΔzitB mutant from liver and spleen | [58] | |
| Regulation | |||||
| S. pneumoniae | sczA | Transcriptional activator of Zn efflux; regulation of Zn homeostasis | Human monocyte-derived macrophages | Decreased survival of mutant | [61] |
| Group A Streptococcus | gczA (sczA) | Transcriptional activator of Zn efflux; regulation of Zn homeostasis | Neutrophils | Decreased survival of mutant | [52] |
| Invasive subcutaneous; humanized plasminogen transgenic mouse | Decreased mortality of mice | [52] | |||
Non-pathogenic, but providec as a model Gram-negative organism.
As our appreciation of Zn intoxication as a mechanism of bacterial killing grows, so too does our knowledge of the subversion tactics employed by bacteria to escape. In addition to efflux, S. Typhimurium was recently shown to avoid Zn poisoning by subverting trafficking of the metal within macrophages [51]. In brief, although S. Typhimurium infection induces the formation of Zn-containing vesicles within human macrophages, unlike E. coli and M. tuberculosis, it does not localize with these vesicles [22,51]. Although the exact mechanism used by S. Typhimurium to evade Zn-containing vesicles was not determined, it is likely to involve effector molecules encoded from Salmonella pathogenicity island-1, as bacteria lacking this genetic locus were found to associate with Zn-containing vesicles [51]. Identifying how bacteria such as S. Typhimurium actively avoid metal toxicity within phagocytes will provide important insights into how to counteract these evasion strategies and enhance metal-associated killing.
Role of Zn detoxification in intracellular survival
Zn deficiency is associated with increased susceptibility to diarrheal diseases and pneumonia, suggesting that Zn plays an important role in the host defense against pathogens [60]. As highlighted in Table 2, a loss of Zn detoxification machinery results in impaired intracellular survival of M. tuberculosis, non-pathogenic E. coli, S. pneumoniae, and GAS [22,51-53,61], and in the case of GAS also reduces bacterial survival in murine models of infection [52]. Conversely, disruption to Zn uptake machinery has a profound impact on the in vivo survival of intracellular pathogens such as Brucella abortus, S. Typhimurium, and L. monocytogenes [62, and references therein]. The utility of both bacterial Zn efflux and uptake machinery within the host highlights the complex nature of Zn homeostasis at the host-pathogen interface. Although it is tempting to speculate that all intracellular pathogens face Zn starvation extracellularly and Zn poisoning within the confines of phagocytes, further studies are required to determine the extent to which intracellular Zn-mediated killing is used against these microbial invaders.
Concluding remarks and future directions
The role of nutritional immunity in controlling bacterial infections to date has largely focused on metal withholding as a means of inhibiting pathogen growth. As highlighted in this review, there is a growing appreciation for the role of metals functioning as phagocyte antimicrobial effectors, where overloading the phagosome with toxic metals is employed as a strategy to directly poison intracellular pathogens. Although we specifically highlighted Cu and Zn, all transition metals are toxic in excess and it is not yet known if all other metals may similarly be employed in the control of intracellular pathogens. Further, how the host decides whether to starve or intoxicate internalized bacteria is not known and represents an exciting area of future research. Understanding how phagocytes target and deliver toxic metals to intracellular bacteria may provide avenues for the development of novel therapeutics that enhance or exploit these natural pathways to control bacterial replication.
Highlights.
Iron and manganese are withheld from the phagosome to inhibit bacterial growth
Excess copper and zinc are used to directly intoxicate intracellular pathogens
Copper toxicity is not fully understood but is likely multifactorial
Zinc toxicity results from mismetallation of key metalloproteins
Bacteria defective for detoxifying metals are hypersusceptible to macrophage killing
Acknowledgements
The authors thank members of the Skaar Lab for critical review of the manuscript. Work in the Skaar Lab is supported by grants from the National Institutes of Health R01AI069233, R01 Al073843, R01 AI101171, R01 AI138581.
Footnotes
Declarations of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
*special interest
**outstanding interest
- 1.Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM: Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem 2008, 13:1205–1218. [DOI] [PubMed] [Google Scholar]
- 2.Waldron KJ, Rutherford JC, Ford D, Robinson NJ: Metalloproteins and metal sensing. Nature 2009, 460:823–830. [DOI] [PubMed] [Google Scholar]
- 3.Maret W: Metalloproteomics, metalloproteomes, and the annotation of metalloproteins. Metallomics 2010, 2:117–125. [DOI] [PubMed] [Google Scholar]
- 4.Palmer LD, Skaar EP: Transition metals and virulence in bacteria. Annu Rev Genet 2016, 50:67–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg ED: Iron and susceptibility to infectious disease. Science 1974, 184:952–956. [DOI] [PubMed] [Google Scholar]
- 6.Lopez CA, Skaar EP: The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe 2018, 23:737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheldon JR, Flannagan RS, Hannauer M, Heinrichs DE: Transition metal ion homeostasis In Staphylococcus: Genetics and Physiology. Edited by Somerville GA: Caister Academic Press; 2016:171–220. [Google Scholar]
- 8.Wessling-Resnick M: Nramp1 and other transporters involved in metal withholding during infection. J Biol Chem 2015, 290:18984–18990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruenheid S, Pinner E, Desjardins M, Gros P: Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J Exp Med 1997, 185:717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J: Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306:2090–2093. [DOI] [PubMed] [Google Scholar]
- 11.Kim DK, Jeong JH, Lee JM, Kim KS, Park SH, Kim YD, Koh M, Shin M, Jung YS, Kim HS, et al. : Inverse agonist of estrogen-related receptor gamma controls Salmonella Typhimurium infection by modulating host iron homeostasis. Nat Med 2014, 20:419–424. [DOI] [PubMed] [Google Scholar]
- 12.Paradkar PN, De Domenico I, Durchfort N, Zohn I, Kaplan J, Ward DM: Iron depletion limits intracellular bacterial growth in macrophages. Blood 2008, 112:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chlosta S, Fishman DS, Harrington L, Johnson EE, Knutson MD, Wessling-Resnick M, Cherayil BJ: The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect Immun 2006, 74:3065–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, Weiss G: The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella Typhimurium. Cell Microbiol 2007, 9:2126–2140. [DOI] [PubMed] [Google Scholar]
- 15.Harada N, Kanayama M, Maruyama A, Yoshida A, Tazumi K, Hosoya T, Mimura J, Toki T, Maher JM, Yamamoto M, et al. : Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch Biochem Biophys 2011, 508:101–109. [DOI] [PubMed] [Google Scholar]
- 16.Nairz M, Schleicher U, Schroll A, Sonnweber T, Theurl I, Ludwiczek S, Talasz H, Brandacher G, Moser PL, Muckenthaler MU, et al. : Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med 2013, 210:855–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P: Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med 2000, 192:1237–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheldon JR, Laakso HA, Heinrichs DE: Iron acquisition strategies of bacterial pathogens. Microbiol Spectr 2016, 4: doi: 10.1128/microbiolspec.VMBF-0010-2015. [DOI] [PubMed] [Google Scholar]
- 19.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, et al. : Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319:962–965. [DOI] [PubMed] [Google Scholar]
- *20.Zygiel EM, Nelson CE, Brewer LK, Oglesby-Sherrouse AG, Nolan EM: The human innate immune protein calprotectin induces iron starvation responses in Pseudomonas aeruginosa. J Biol Chem 2019, doi: 10.1074/jbc.RA118.006819.*Provides the first evidence of calprotectin functioning as a biologically relevant chelator of Fe(II) to inhibit bacterial growth.
- 21.Brophy MB, Nolan EM: Manganese and microbial pathogenesis: sequestration by the Mammalian immune system and utilization by microorganisms. ACS Chem Biol 2015, 10:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charrière GM, et al. : Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 2011, 10:248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian Vignesh K, Deepe GS Jr.: Metallothioneins: Emerging modulators in immunity and infection. Int J MolSci 2017, 18: doi: 10.3390/ijms18102197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ml Samanovic, Ding C, Thiele D, Darwin KH: Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe 2012, 11:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner D, Maser J, Lai B, Cai Z, Barry CE 3rd, Honer Zu, Bentrup K, Russell DG, Bermudez LE: Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J Immunol 2005, 174:1491–1500. [DOI] [PubMed] [Google Scholar]
- **26.White C, Lee J, Kambe T, Fritsche K, Petris MJ: A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem 2009, 284:33949–33956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Ladomersky E, Khan A, Shanbhag V, Cavet JS, Chan J, Weisman GA, Petris MJ: Host and pathogen copper-transporting P-type ATPases function antagonistically during Salmonella Infection. Infect Immun 2017, 85: doi: 10.1128/IAI.00351-17.**Together with their seminal paper above (reference 26), Petris and colleagues identify ATP7A as a key player in the Cu-mediated defense against intracellular microbes and show that bacteria use Cu exporters of the same superfamily to avoid Cu intoxication.
- 28.Fenton HJH: LXXIII.—Oxidation of tartaric acid in presence of iron. J Chem Soc, Trans 1894, 65:899–910. [Google Scholar]
- 29.Haber F, Weiss J: Über die Katalyse des Hydroperoxydes. Naturwissenschaften 1932, 20:948–950. [Google Scholar]
- 30.Macomber L, Rensing C, Imlay JA: Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J Bacteriol 2007, 189:1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macomber L, Imlay JA: The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci 2009, 106:8344–8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irving H, Williams RJP: Order of stability of metal complexes. Nature 1948, 162: 746–747. [Google Scholar]
- 33.Djoko KY, McEwan AG: Antimicrobial action of copper is amplified via inhibition of heme biosynthesis. ACS Chemical Biology 2013, 8:2217–2223. [DOI] [PubMed] [Google Scholar]
- 34.Johnson MD, Kehl-Fie TE, Rosch JW: Copper intoxication inhibits aerobic nucleotide synthesis in Streptococcus pneumoniae. Metallomics 2015, 7:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan G, Yang J, Li T, Zhao J, Sun S, Li X, Lin C, Li J, Zhou H, Lyu J, et al. : Anaerobic copper toxicity and iron-sulfur cluster biogenesis in Escherichia coli. Appl Environ Microbiol 2017, 83: doi: 10.1128/AEM.00867-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fung DK, Lau WY, Chan WT, Yan A: Copper efflux is induced during anaerobic amino acid limitation in Escherichia coli to protect iron-sulfur cluster enzymes and biogenesis. J Bacteriol 2013, 195:4556–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chillappagari S, Seubert A, Trip H, Kuipers OP, Marahiel MA, Miethke M: Copper stress affects iron homeostasis by destabilizing iron-sulfur cluster formation in Bacillus subtilis. J Bacteriol 2010, 192:2512–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ladomersky E, Petris MJ: Copper tolerance and virulence in bacteria. Metallomics 2015, 7:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Besold AN, Culbertson EM, Culotta VC: The Yin and Yang of copper during infection. J Biol Inorg Chem 2016, 21:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rensing C, McDevitt SF: The copper metallome in prokaryotic cells. Met Ions Life Sci 2013, 12:417–450. [DOI] [PubMed] [Google Scholar]
- 41.Braymer JJ, Giedroc DP: Recent developments in copper and zinc homeostasis in bacterial pathogens. Curr Opin Chem Biol 2014, 19:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Outten FW, Huffman DL, Hale JA, O’Halloran TV: The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J Biol Chem 2001, 276:30670–30677. [DOI] [PubMed] [Google Scholar]
- 43.Chacon KN, Mealman TD, McEvoy MM, Blackburn NJ: Tracking metal ions through a Cu/Ag efflux pump assigns the functional roles of the periplasmic proteins. Proc Natl Acad Sci 2014, 111:15373–15378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gold B, Deng H, Bryk R, Vargas D, Eliezer D, Roberts J, Jiang X, Nathan C: Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat Chem Biol 2008, 4:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP: The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol 2012, 8:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.EI Koh, Robinson AE, Bandara N, Rogers BE, Henderson JP: Copper import in Escherichia coli by the yersiniabactin metallophore system. Nat Chem Biol 2017, 13:1016–1021.* Coined the term “nutritional passivation” to describe efforts by bacteria to maintain nutritional availability of a metal, whilst guarding against its toxicity through chelation. Passivation refers to shielding or coating a metal to reduce reactivity. In this case, yersiniabactin serves to passivate Cu through binding, but releases the metal to a cuproenzyme to fulfill metabolic requirements.
- 47.Chaturvedi KS, Hung CS, Giblin DE, Urushidani S, Austin AM, Dinauer MC, Henderson JP: Cupric yersiniabactin is a virulence-associated superoxide dismutase mimic. ACS Chem Biol 2014, 9:551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osman D, Waldron KJ, Denton H, Taylor CM, Grant AJ, Mastroeni P, Robinson NJ, Cavet JS: Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J Biol Chem 2010, 285:25259–25268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson MD, Kehl-Fie TE, Klein R, Kelly J, Burnham C, Mann B, Rosch JW: Role of copper efflux in pneumococcal pathogenesis and resistance to macrophage-mediated immune clearance. Infect Immun 2015, 83:1684–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capdevila DA, Wang J, Giedroc DP: Bacterial strategies to maintain zinc metallostasis at the host-pathogen interface. J Biol Chem 2016, 291:20858–20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *51.Kapetanovic R, Bokil NJ, Achard ME, Ong CL, Peters KM, Stocks CJ, Phan MD, Monteleone M, Schroder K, Irvine KM, et al. : Salmonella employs multiple mechanisms to subvert the TLR-inducible zinc-mediated antimicrobial response of human macrophages. FASEB J 2016, 30:1901–1912.*Demonstrates that Salmonella can evade co-localization with TLR4-inducible Zn-containing vesicles by multiple mechanisms. This finding highlighting the complexity of the strategies employed by bacterial pathogens to escape metal intoxication, and suggests many undiscovered mechanisms likely exist.
- 52.Ong CL, Gillen CM, Barnett TC, Walker MJ, McEwan AG: An antimicrobial role for zinc in innate immune defense against group A Streptococcus. J Infect Dis 2014, 209:1500–1508. [DOI] [PubMed] [Google Scholar]
- **53.Ong CY, Berking O, Walker MJ, McEwan AG: New insights into the role of zinc acquisition and zinc tolerance in group A streptococcal infection. Infect Immun 2018, 86: doi: 10.1128/IAI.00048-18.**Provides the first direct support of the model that extracellular Zn starvation and intracellular Zn intoxication can be employed by innate immune cells to inhibit survival of the same bacterial pathogen.
- 54.McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC: A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathogens 2011, 7:e1002357–e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eijkelkamp BA, Morey JR, Ween MP, Ong C-IY, McEwan AG, Paton JC, McDevitt CA: Extracellular zinc competitively inhibits manganese uptake and compromises oxidative stress management in Streptococcus pneumoniae. PLoS One 2014, 9:e89427–e89427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ong CL, Walker MJ, McEwan AG: Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes. Sci Rep 2015, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chandrangsu P, Helmann JD: Intracellular Zn(II) intoxication leads to dysregulation of the PerR regulon resulting in heme toxicity in Bacillus subtilis. PLoS Genet 2016, 12:e1006515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frawley ER, Karlinsey JE, Singhal A, Libby SJ, Doulias P-T, Ischiropoulos H, Fang FC: Nitric oxide disrupts zinc homeostasis in Salmonella enterica Serovar Typhimurium. mBio 2018, 9:e01040–01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Djoko KY, Ong CL, Walker MJ, McEwan AG: The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J Biol Chem 2015, 290:18954–18961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fischer Walker C, Black RE: Zinc and the risk for infectious disease. Annu Rev Nutr 2004, 24:255–275. [DOI] [PubMed] [Google Scholar]
- 61.Martin JE, Edmonds KA, Bruce KE, Campanello GC, Eijkelkamp BA, Brazel EB, McDevitt CA, Winkler ME, Giedroc DP: The zinc efflux activator SczA protects Streptococcus pneumoniae serotype 2 D39 from intracellular zinc toxicity. Mol Microbiol 2017, 104:636–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma L, Terwilliger A, Maresso AW: Iron and zinc exploitation during bacterial pathogenesis. Metallomics : integrated biometal science 2015, 7:1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolschendorf F, Ackart D, Shrestha TB, Hascall-Dove L, Nolan S, Lamichhane G, Wang Y, Bossmann SH, Basaraba RJ, Niederweis M: Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci 2011, 108:1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ward SK, Abomoelak B, Hoye EA, Steinberg H, Talaat AM: CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Molecular microbiology 2010, 77:1096–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi X, Festa RA, Ioerger TR, Butler-Wu S, Sacchettini JC, Darwin KH, Samanovic MI: The copper-responsive RicR regulon contributes to Mycobacterium tuberculosis virulence. MBio 2014, 5: doi: 10.1128/mBio.00876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shafeeq S, Yesilkaya H, Kloosterman TG, Narayanan G, Wandel M, Andrew PW, Kuipers OP, Morrissey JA: The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol Microbiol 2011, 81:1255–1270. [DOI] [PubMed] [Google Scholar]
- 67.Achard ME, Tree JJ, Holden JA, Simpfendorfer KR, Wijburg OL, Strugnell RA, Schembri MA, Sweet MJ, Jennings MP, McEwan AG: The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect Immun 2010, 78:2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]