Abstract
The phagocyte NADPH oxidase possesses a transmembrane electron transferase comprised of gp91phox (aka NOX2) and p22phox and two multicomponent cytosolic complexes, which in stimulated phagocytes translocate to assemble a functional enzyme complex at plasma or phagosomal membranes. The NOX2-centered NADPH oxidase shuttles electrons from cytoplasmic NADPH to molecular oxygen in phagosomes or the extracellular space to produce oxidants that support optimal antimicrobial activity by phagocytes. Additionally, NOX2-generated oxidants have been implicated in both autocrine and paracrine signaling in a variety of biological contexts. However, when interpreting experimental results, investigators must recognize the complexity inherent in the biochemistry of oxidant-mediated attack of microbial targets and the technical limitations of the probes currently used to detect intracellular oxidants.
Keywords: phagocytes, neutrophils, macrophages, oxidants, NADPH oxidase, NOX proteins
Introduction
For decades, the NADPH oxidase was considered an oxidant-generating system uniquely associated with phagocytes and dedicated exclusively to microbicidal action. However, the discovery of homologues of gp91phox in non-phagocytic cells [1,2] catalyzed an appreciation that nearly all cells in the plant and animal kingdoms possess at least one NADPH oxidase [3]. The family of NADPH oxidase (NOX) proteins includes NOX1, NOX2 (aka gp91phox), NOX3, NOX4, NOX5, DUOX1, and DUOX2. Although some, such as NOX2 and DUOX, contribute to host defense against infection, most serve critical functions related to signaling in many different contexts, ranging from biosynthesis of otoconia and normal vestibular function in the inner ear [4] to physiologic functions in the cardiovascular system [5]. In contrast to activities of the non-phagocyte NOX proteins, NOX2 in the phagocyte NADPH oxidase constitutes a high turnover enzyme complex that in human neutrophils generates ~ 10 nmoles superoxide anion/min/106 cells, most of which targets ingested microbes. However, H2O2 from the NADPH oxidase acts in both an autocrine and paracrine manner to promote signaling, directly or indirectly, in a wide variety of biological settings [5–7]
The phagocyte NADPH oxidase is a multicomponent enzyme complex
Unassembled and inactive in resting phagocytes, the NADPH oxidase complex includes at least five components distributed in membranes and in cytoplasm (reviewed in [8]). Flavocytochrome b558, the heterodimeric integral membrane protein composed of gp91phox and p22phox, serves as the primary electron transferase and exists in the plasma membrane and membranes of secretory vesicles and secondary granules [Figure 1]. Two protein complexes in the cytoplasm of resting phagocytes are essential for optimal oxidase activity. A ternary complex of p47phox, p67phox, and p40phox exists in a 1:1:1 stoichiometry, stabilized by multiple intramolecular and intermolecular interactions between complementary binding domains within individual components (reviewed in [8]). Although strong biochemical data generated over several decades suggested the presence of a multicomponent complex that translocates en masse to target membranes [9–12]. Ziegler et al. have recently integrated analytical data from several sources with crystal structures of the isolated subunits and functional domains to construct a 3D model of this complex [13].
Figure 1. Flavocytochrome b558.
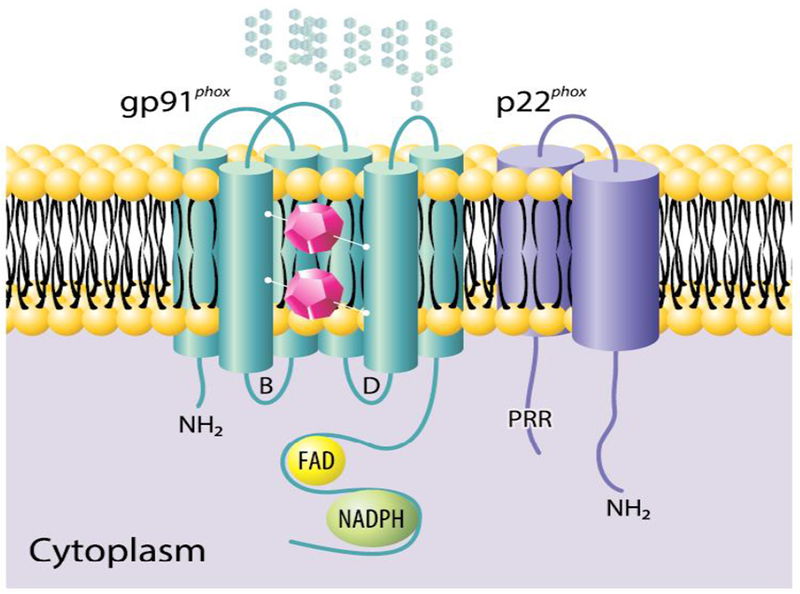
Flavocytochrome b558, composed of gp91phox (aka NOX2) and p22phox, acts as the redox center of the phagocyte NADPH oxidase. The current model for human gp91phox includes six transmembrane helices and both amino (NH2) and carboxy termini on the cytoplasmic face of the membrane. Residing between two intramembrane helices, two inequivalent heme groups (purple polygons) transport electrons across the membrane from cytosolic NADPH. There are three N-linked carbohydrate chains extracellularly and two cytoplasmic loops (B and D). The carboxy terminal region includes both FAD and NADPH binding sites. The current model for human p22phox has both amino and carboxy termini on the cytoplasmic side of the membrane and lacks glycosylation. The carboxy region of p22phox contains a proline-rich region (PRR) that associates with p47phox during oxidase assembly. Figure from [8] and used with permission.
Additional proteins copurify with the NADPH oxidase components under a variety of experimental conditions [reviewed in [8]], although the functional importance of many is not yet established. Zhou et al. recently reported that protein arginine deaminase (PAD) 4 associates with p47phox and p67phox [14], adding to the complex and incompletely understood relationship between the NADPH oxidase and neutrophil extracellular trap (NET) formation. Oxidant-dependent changes in the cytoskeletal network reflect finely tuned coordination of glutathionylation and deglutathionylation of actin and tubulin and likely figure in NET formation [15].
When phagocytes are activated during phagocytosis or exposure to soluble agonists, phosphorylation of p47phox on specific serine residues prompts conformational changes that expose otherwise cryptic domains in p47phox and p40phox that bind to specific phosphoinositides in the plasma and phagosomal membranes, respectively, associate with flavocytochrome b558, and drive superoxide anion generation [Figure 2]. Concomitantly, a second complex, composed of Rac2 in neutrophils (Rac1 in macrophages) and the GDP dissociation inhibitor for Rho, RhoGDI, independently translocates to membranes to promote oxidase activation and activity.
Figure 2. Oxidase assembly.
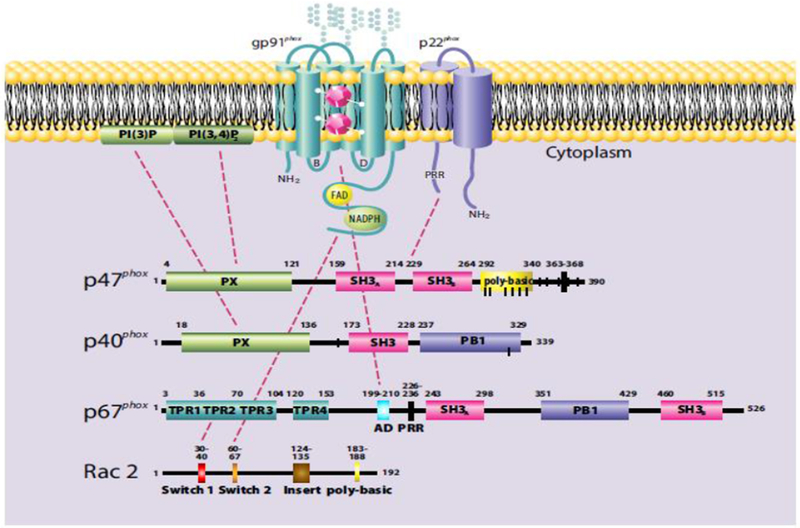
Multiple intermolecular interactions among the individual components and between the PX domains of p47phox and p40phox with PI(3,4)P2 in plasma membrane and PI(3)P in phagosomal membranes, respectively, mediate assembly of the NADPH oxidase in stimulated neutrophils. Shown are some of the interactions, including those between proline rich regions (PRR) and src homology domains (SH3), activation domain of p67phox (AD), and tetratricopeptide repeats (TPR) on p67phox with their binding partners (reviewed in detail in [8]) that mediate assembly. For ease of illustration, interactions of the cytoplasmic complexes present in resting neutrophils are omitted. Figure from [8] and used with permission.
Activity of the phagocyte NADPH oxidase is not bimodal, i.e. either off or on. Immunological modifiers, such as TNFα or GM-CSF, or microbial components, such as lipopolysaccharides, can enhance responsiveness of the phagocyte NADPH oxidase, a process known as priming. Intricate mechanisms underlying priming of the oxidase include phosphorylation of serine 345 of p47phox and participation of the proline isomerase Pin1 (reviewed in [16]}. A reversible process, priming provides a mechanism to modulate NADPH oxidase responsiveness to meet the demands during or after an inflammatory response.
Lessons in NADPH oxidase biology from chronic granulomatous disease
Explication of the molecular basis of chronic granulomatous disease (CGD), the clinical syndrome of enhanced susceptibility to specific microbial infections [17] and exuberant sterile granulomatous inflammation has informed much of our current understanding of the composition and activity of the phagocyte NADPH oxidase [18,19]. Mutations in the genes encoding any one of the five known components of the NADPH oxidase complex results in CGD, although phenotypes vary somewhat as a function of residual oxidase activity [20] and the specific oxidase component affected. Mutations in p40phox compromise oxidase assembly only on phagosomal membranes; oxidase translocation to plasma membrane is normal [21]. The similar but different phosphoinositide recognition and binding by the PX domains of p47phox and p40phox serve to discriminate between plasma and phagosomal membranes, respectively [22,23]. Furthermore, the clinical phenotype of CGD due to p40phox deficiency or dysfunction differs significantly from that of classic CGD, as recently demonstrated in a report of 24 individuals from 12 families [24]. In contrast to patients with other genotypes of CGD, p40phox-deficient individuals do not experience invasive infections but rather suffer from exaggerated inflammation, such as lupus-like skin lesions and inflammatory bowel disease-like colitis, and superficial infections [24].
In addition to defective NADPH oxidase components resulting in CGD, inherited abnormalities in a transmembrane protein essential for flavocytochrome b558 expression [25] have been implicated as a cause of CGD. Two independent reports describe patients with the CGD phenotype and normal NADPH components but with loss of function mutations in CYBC1 [26,27], the gene encoding Eros (essential for reactive oxidant species) [25]. The contrasting clinical phenotypes of p40phox deficiency with that of other genotypes of CGD and the discovery of a CYBC1 mutation as a cause of CGD demonstrate that much about the composition and regulation of the phagocyte NADPH oxidase remains to be elucidated.
Dynamic events within neutrophil phagomes
Once assembled and activated on phagsomal membranes, the NADPH oxidase mediates rapid transfer of electrons from cytoplasmic NADPH into the lumen of phagosomes (Figure 3). Redistribution of electrons creates a charge inequity across the phagosomal membrane and drives membrane depolarization unless corrected. The failure to compensate charge created by electron transfer would terminate NADPH oxidase activity within ~100 milliseconds [reviewed in [28]]. A longstanding controversy as to the direct role of the NADPH oxidase in charge compensation resolved upon discovery of the presence of the voltage-gated proton channel Hv1 in phagocytes [29]. Activated in parallel with the NADPH oxidase, each molecule of Hv1 compensates the electrons transferred by 100 molecules of the NADPH oxidase, thereby efficiently correcting charge inequity. Murine neutrophils that lack Hv1 activate NADPH oxidase activity normally but fail to sustain superoxide generation because of the inability to compensate charge and offset membrane depolarization [29]. Unrelated to properties of the oxidase but germane to neutrophil adhesion and chemotaxis, Hv1-dependent charge compensation support the calcium influx required for calcium-dependent cell responses [30].
Figure 3. MPO-dependent events in human neutrophil phagosomes.
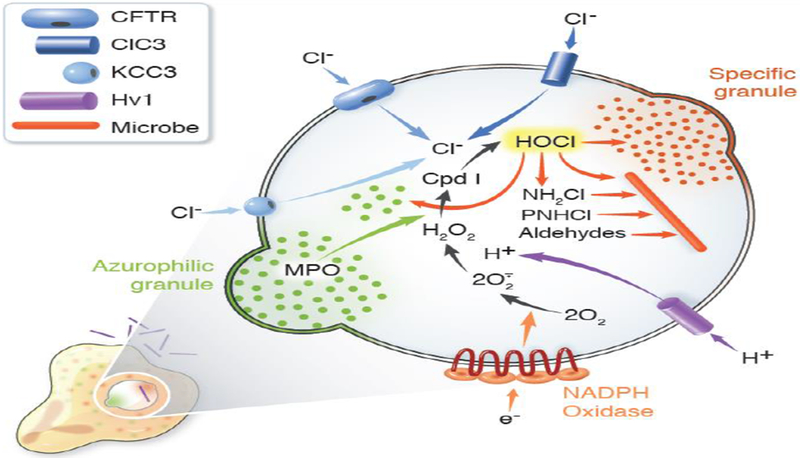
During phagocytosis, human neutrophils assemble and activate the NADPH oxidase and recruit granules to fuse with nascent phagosomes. Electrons transferred into the phagosome by the NADPH oxidase reduce molecular oxygen to superoxide anion (O2•−) and H2O2. The voltage-gated proton channel Hv1 delivers protons into the phagosome to compensate the charge generated by electron transfer from cytoplasm and thus sustain oxidase activity. The cystic fibrosis transmembrane conductance regulator (CFTR), with some contribution from chloride channel 3 (ClC3) and the potassium-chloride cotransporter (KCC3), provides chloride (Cl−) from the neutrophil cytoplasm. MPO, delivered by fusion of azurophilic granules, and H2O2, generated by the oxidase, react to yield Compound I (Cpd I) that in turn catalyzes the oxidation of Cl− to produce hypochlorous acid (HOCl) or bleach. HOCl reacts with targets both from host and microbe to generate a variety of reactive products, including monochloramines (NH2Cl) and protein chloramines (PNHCl), which can then decompose to form aldehydes. Collectively, the granule proteins, acting in both their native and oxidant-modified forms, along with HOCl and its derivatives act synergistically to attack microbial targets. Figure from [40] and used with permission.
The immediate product of the NADPH oxidase, superoxide anion (O2•−), has a pKa of 4.8 in equilibrium with its protonated form, the hydroperoxyl radical (OH2•−) [31]. Although it may directly contribute to antimicrobial action [reviewed in [32]], superoxide anion figures predominantly serves as a precursor to H2O2, either by spontaneous dismutation (i.e. O2•− + OH2•− → H2O2 + O2) or by an enzyme-catalyzed reaction. Dismutation occurs most readily near pH 4.8, when concentrations of superoxide and hydroperoxyl radical are the same. Given that the pH of neutrophil phagosomes is neutral or higher [33,34], equilibrium favors the hydroperoxyl radical and little spontaneous dismutation occurs.
Although the superoxide dismutase (SOD) endogenously expressed in neutrophil cytoplasm does not access the phagosomal lumen, recent evidence that extracellular SOD internalized from plasma resides in secretory vesicles [35] provides a potential mechanism for catalyzed generation of H2O2 if secretory vesicles fuse with nascent phagosomes during phagocytosis. However, the ferric form of myeloperoxidase, aka Compound III, rapidly reacts with O2•− to generate H2O2 + O2 [36,37] and may be the predominant mechanism for H2O2 production from the phagocyte NADPH oxidase, although many variables influence the overall biochemistry [reviewed in [32]].
Oxidants in antimicrobial activity within phagosomes
The infectious morbidity and mortality in patients with CGD demonstrate that oxidants from the NADPH oxidase support optimal antimicrobial activity against many, but not all, ingested microorganisms [17]. For example, both normal and CGD neutrophils kill E.coli equally well, but CGD neutrophils have ~ no microbicidal activity against S.aureus.
Given the small volume of phagosomes, high levels of oxidants and granule proteins can be achieved, some, such as myeloperoxidase, reaching millimolar concentration [32,38]. Consequently, most phagocyte-mediated killing occurs in phagosomes and relatively little in the extracellular space. The overriding theme for intraphagosomal antimicrobial activity is synergy: synergy between oxidants and granule proteins as well as among individual granule proteins [38,39]. The collaborative interactions among phagosome contents culminate in a potent system effective against the varied microbial threats a host may encounter in a lifetime. However, microbes do not passively experience hostile attack in phagosomes, and several organisms apply specific tactics to evade, endure, or neutralize agents of host defense and thus succeed as pathogens. Elsewhere in this issue, Criss et al. describe in detail some of these remarkable microbial adaptations to the harsh conditions within phagosomes.
Neutrophils and macrophages differ with respect to both NADPH oxidase activity (neutrophils >> macrophages), phagosomal pH (neutral in neutrophils, acidic in macrophages) and granule constituents, which together influence the overall capacity for and nature of intraphagosomal antimicrobial activity. For example, whereas most of the H2O2 generated in neutrophil phagosomes will be consumed by the azurophilic granule protein myeloperoxidase (MPO) to generate HOCl [40] (see below), the absence of MPO from macrophages will leave their phagosomes without HOCl but with more H2O2. Furthermore, macrophages in some settings supplement intraphagosomal NOX2-dependent H2O2 with oxidants derived from mitochondria. Ingestion of S.aureus elicits oxidant stress in macrophages and generation of mitochondria-derived vesicles that deliver H2O2 to phagosomes and thereby augment H2O2-dependent action [41]. In light of the many substantive differences in antimicrobial agents in neutrophils and macrophages and for ease of presentation, the discussion that follows uses human neutrophils to illustrate the principles at play in phagosomes.
In the presence of H2O2, generated de novo by the NADPH oxidase, MPO catalyzes the two electron oxidation of chloride to produce HOCl, which is far more potent than is H2O2 both as an oxidant and as an antimicrobial agent [42]. Furthermore, HOCl reacts with both host and microbial proteins in phagosomes to yield chloramines and aldehydes whose reactivity extends the spectrum and duration of antimicrobial action [Figure 3]. HOCl can inactivate susceptible granule proteins, as occurs with elastase, and thereby eliminate their contribution to antimicrobial milieu. The number and complexity of interactions that can occur among both host and microbial targets are difficult to replicate with individual components in reductionist experimental systems in vitro. Consequently, identification of a precise and single mechanism for killing ingested organisms presents a daunting challenge to those who study host defense.
Both host- and microbe-based variables compound the challenge to our understanding of phagocyte antimicrobial action. Within a given neutrophil, phagosomes are remarkably heterogenous with respect to HOCl production [43]. This heterogeneity likely reflects variability both in the extent of granule fusion with and oxidase assembly on any given phagosome. Equally variable are the microbial substrates present in a particular phagosome. The biochemical composition and structural organization of microbes differ; e.g. contrast the cell wall of S.aureus with the endotoxin on the surface of E.coli or the waxy mycolic acids on M.tuberculosis. Many of these features vary with the growth phase of the organism, and some bacteria actively secrete biomolecules under stress conditions, thereby providing competing substrates for interaction with host defense molecules. In light of all the variables of host and microbial origin that influence events in phagosomes, the articulation of a single mechanism by which all ingested microbes are killed becomes a formidable challenge. A detailed description of events under limited and explicitly defined experimental conditions may be the most that can be expected.
The challenges facing the phagocyte NADPH oxidase driving signal transduction
Oxidants participate in a broad spectrum of biological phenomena other than those directed to killing microbes, ranging from mediating tissue damage in a variety of clinical settings to regulating complex physiologic responses [44–46]. However, the attribution of oxidants as the causative agents of observed cellular events frequently rests on weak data or unlikely chemistry [47]. Concerned about the lack of rigor in studies of redox events in experimental systems, experts in oxidant biology have provided investigators with guidelines to optimize evidence before assigning a given oxidant as cause of a particular biological phenomenon [47,48]. These guidelines apply as well to the study of NOX2-dependent oxidants in leukocyte signaling. It is especially important to know the chemistry and to recognize the limitations of detection systems being used to assess oxidant production in experimental systems. Redox-responsive fluorescent probes such as dichlorofluorescein (DCFH) are widely employed to assess intracellular oxidant generation but are problematic as detectors of H2O2 [49]. H2O2 does not react directly with DCFH but requires a peroxidase or transition metal as a catalyst [50]. In addition, cytoplasmic proteins can directly oxidize DCFH independent of reactive oxygen species and thereby foster incorrect interpretations of results. For example, DCFH fluorescence in dying neuronal cells was interpreted as evidence that reactive oxygen species prompts apoptosis [51] until subsequent work demonstrated that mitochondrial cytochrome C released during apoptosis directly oxidizes DCFH [52]. Detection of DCFH fluorescence in cells reflects changes in the intracellular redox state but should not be attributed to the generation of specific oxidants. In addition to technical issues, certain conceptual hurdles to linking NOX2-generated oxidants directly to intracellular signaling need to be recognized.
Situated in lipid-enriched domains in the plasma membrane, the NOX2-centered NADPH oxidase operates as a nodal point for inter- and intracellular signaling [53], but several hurdles need to be surmounted to effect results. First, oxidants need to reach the relevant targets. Superoxide anion is short-lived and rapidly converted to H2O2, which has been established as a signaling molecule in a wide variety of biological systems [44,54]. The topology of the NADPH oxidase in phagocytes results in generation of superoxide anion and its downstream products either in the extracellular space (across the plasma membrane) or in the phagosomal lumen (across the phagosomal membrane) [Figure 1]. Uncharged and unreactive at neutral pH, NOX2-generated H2O2 must traverse the plasma or phagosomal membrane to access cytoplasmic targets in order to drive intracellular signaling cascades. Facilitated transport of H2O2 occurs through aquaporins (AQPs), integral membrane proteins that bidirectionally transport H2O and H2O2 across membranes [44,55]. Isoforms of AQPs are expressed in plasma membranes and membranes of intracellular organelles in cells throughout the plant and animal kingdoms and support paracrine as well as autocrine H2O2-dependent signaling. Varied distribution of particular isotypes in specific cellular locations allows for fine tuning of H2O2 flux and thereby modulation of oxidant-dependent cellular responses [56].
The second major challenge for H2O2 is to react with intracellular targets that initiate signaling cascades. A potent oxidant, H2O2 reacts directly with thiols but only slowly with cysteine residues in many proteins because of its high activation energy [54,57]. In contrast, proteins that support the catabolism of H2O2, most notably catalases, peroxiredoxins (Prxs), and glutathione peroxidases (Gpxs), readily react with H2O2 and thereby protect critical and vulnerable intracellular proteins from detrimental oxidation. For example, the rate constant of the reaction of H2O2 with Prx 2 (108 M−1sec−1) is more than 106-fold greater than that with the signaling cascade protein tyrosine phosphatase 1B (20 M−1sec−1), a difference that underscores the challenge for signaling molecules to react with H2O2 entering the cytoplasm [44]. Compounding the disadvantage of relatively low reactivity with H2O2, redox-sensitive signaling molecules exist in much lower abundance in cytoplasm than do other potential targets. In fact, excluding organelle-compartmentalized catalase and heme-peroxidases, reduced Gpxs and Prxs predominate as cytosolic substrates for H2O2 [44].
Taken together, the greater abundance and reactivity of Prxs favor their reaction with and consumption of nearly all the H2O2 that enters cytoplasm [58]. Two mechanisms, not mutually exclusive, have been proposed to explain how H2O2 overcomes its competitive disadvantages compared with Prxs and succeeds in supporting the oxidation of target proteins in signaling cascades [59]. Agonist-triggered phosphorylation can inactivate Prx and thereby allow for accumulation of H2O2 locally in sufficient concentrations to oxidize target cysteines in signaling molecules. In this way, localized H2O2 supports signaling without promoting more widespread and deleterious oxidation. Such may be the case for B cell receptor (BCR)- and T cell receptor (TCR)-dependent stimulation, whereby membrane-associated Prx1 is transiently phosphorylated and inactivated, thereby allowing local accumulation of H2O2 intracellularly [60]. Alternatively, oxidation of target cysteines can be indirect, mediated by oxidized Prx, which relays the oxidizing reactivity of H2O2 to downstream targets. In this scheme, Prx acts as both sensor of H2O2 and transducer of oxidation, thereby providing the cell with an efficient mechanism for transmitting signals with spatiotemporal precision.
NOX2-generated H2O2 in signaling
Just as the early studies of CGD proved seminal in the discovery of the NADPH oxidase in normal neutrophils [18], the hyperinflammation characteristic of this disease has revealed previously unrecognized links between the phagocyte oxidase and noninfectious inflammatory diseases. Studies of murine models of arthritis and autoimmune diseases have implicated the lack of a functional NADPH oxidase in the etiology of the increased inflammatory phenotypes associated with inflammatory arthritis, systemic lupus erythematosus (SLE), atopic dermatitis, and inflammatory bowel disease (reviewed in [7,61]). The increased inflammation seen in the setting of NOX2 deficiency reflects both augmented proinflammatory activity and also dysregulation of responses that normally terminate inflammation. NADPH oxidase activity influences cell death pathways in neutrophils [62], and efferocytosis, the process by which macrophages engulf apoptotic and spent cells in inflammatory sites, is impaired in the absence of the phagocyte NADPH oxidase. Murine peritoneal exudate macrophages that lack gp91phox fail to remove apoptotic cells [63].
There are intriguing differences in the phenotypes observed in murine models and in humans with CGD. Polymorphisms in NCF1 (gene encoding p47phox) are associated with autoimmune chronic inflammatory diseases in rats and mice. In contrast, the association of NCF1 polymorphisms with a predisposition to inflammatory human diseases has been difficult to make [64], and data from genome-wide association studies link mutations in NCF2 (gene encoding p67phox) and NCF4 (gene encoding p40phox) with autoimmune diseases such as SLE [65] and Crohns disease [66]. Furthermore, the phenotypes in murine models reflect in large part the genetic context in which mutations in NADPH oxidase are expressed. For example, p67phox-deficient NZM2328 mice develop accelerated SLE kidney disease, whereas NCF2 null mice in a B6 background exhibit the expected CGD phenotype but no increased susceptibility to SLE disease [67]. Thus, excessive oxidants promote autoimmune events in hosts genetically predisposed to such diseases and not in all subjects.
Whereas most patients with CGD do not have autoimmune diseases, female carriers of X-linked CGD experience more discoid lupus erythematosus, granulomatous colitis, SLE, and immune-mediated thyroid disease than does the control population [68]. The latter data represent another puzzling observation: the presence of some cells without normal NADPH oxidase activity predisposes affected humans to autoimmune disorders but the proclivity of exaggerated inflammation is independent of the percentage of impaired cells [68]. Reconstitution of NOX2 activity in macrophages and dendritic cells rescues Ncf1-deficient mice from the exuberant tissue inflammation elicited by intradermal injection of β 1,3-glucan, a sterile agonist of microbial origin [69]. Although the responsible mechanisms are not understood, such observations underscore the idea that the phagocyte NADPH oxidase links innate and adaptive arms of the immune system [70] but in ways that are incompletely understood.
Complementing observations derived from animal models of NADPH oxidase-deficiency, in vitro studies likewise support the notion that NOX2-generated oxidants contribute in some way to cell signaling, with both neutrophils and macrophages as the likely sources of H2O2. NOX2 activation by human neutrophils augments NOX2 activity in a positive feedback loop, whereby H2O2 drives uptake of extracellular calcium and subsequent activation of c-Abl tyrosine kinase and protein kinase Cε [71]. Neutrophil NOX2 activity also acts in a paracrine fashion, contributing to myelopoiesis by progenitors and tissue repair in murine bone marrow after ischemia [72]. Maintenance of the integrity of immune receptor regulation depends on the presence of a functional NADPH oxidase. Although the underlying mechanisms have not been eluciated, CGD neutrophils display dysregulation of several receptors important for optimal innate immunity, including TLR5, TLR9, CD11b, CD18, CD35, and CXCR1 [73].
NOX2 activity and redox signaling play a central role in macrophage programing and contributes to changes in macrophage phenotype over the course of an inflammatory response [46]. Murine resident peritoneal macrophages normally express AQP3 in their plasma membrane. AQP3-deficient mice exhibit decreased survival in an experimental peritonitis model, with isolated macrophages defective in phagocytosis and other critical cell functions [74], presumably reflecting defective H2O2 influx during activation in the absence of AQP3 [74]. NOX2-generated H2O2 transported via AQP8 supports tyrosine phosphorylation triggered by BCR for optimal activation and differentiation of murine B cells [75]. H2O2 from dendritic cells or macrophages, operating as antigen-presenting cells, may oxidize susceptible cysteine residues near the TCR and thereby alter signaling [76], although the specific targets of oxidation have not been identified. Human B cells lacking either gp91phox or p40phox exhibit defective MHC II-presentation of exogenous antigens [77], whereas TLR7 and TLR9 in p40phox- or p47phox-deficient human B cells are upregulated and support augmented cytokine secretion and p38 MAPK activation when stimulated [78]. In the absence of p47phox, mice experience greater alveolar inflammation and damage after intratracheal instillation of lipopolysaccharide (LPS), secondary to increased LPS-induced NF-κB activity in the absence of NADPH oxidase activity [79].
The presence of NOX2 influences the activity of the NLRP3 inflammasome, but the nature of that influence depends on the agonist, cell, and species studied. Murine macrophages lacking gp91phox respond to M.tuberculosis with exaggerated caspase-1 activity and IL-1β secretion [80]. A study of human neutrophils (albeit not populations of ultrapure neutrophils) demonstrated that the NADPH oxidase is not required for caspase-1 activity but is necessary for optimal secretion of mature IL-1β in response to LPS priming and stimulation with ATP [81]. However, uric acid treatment of CGD monocytes increases both caspase-1 activity and IL-1β secretion compared to responses of normal monocytes stimulated in parallel [82]. How these apparently disparate findings fit into a coherent model of the role of NOX2 in cell signaling remains to be determined [83].
Conclusions and perspectives
From this brief overview of contributions of the phagocyte NADPH oxidase to two physiologic functions of phagocytes, namely supporting antimicrobial action against ingested microbes and initiating signaling cascades, several “take home” points emerge. In both activities, the outcomes are clear: a significant fraction of ingested microbes are killed and in certain circumstances cellular responses to agonists occur in a NOX2-dependent fashion. However, there are impressive gaps in our current knowledge that leave the underlying mechanisms responsible for antimicrobial action and for cell signaling incompletely elucidated.
In the case of microbial killing within phagocytes, the complex biochemistry, reactive interactions among individual components, both host and microbial, and dynamic responses to the assault by ingested organisms render reductionist approaches to the study of neutrophil-mediated antimicrobial mechanisms in phagosomes as crude approximations of reality. The inherent complexity of events within phagosomes exhibits features of an emergent system, whereby the overall effect of the complete system differs qualitatively from the sum of activities of its individual components. Depending on the specific contributions from both host and microbe and the synergies ongoing among components that may exist in a given phagosome, the overall impact of antimicrobial action in human neutrophils may be greater or lesser than the sum of the individual activities of oxidants, granule proteins or other elements. This perspective fails to provide a unifying theory of neutrophil antimicrobial action and leaves instead, in my opinion with resignation that neutrophils “operate as they do, in a way wholly above our weak understanding” [84]. Going forward, investigators should be circumspect about conclusions drawn from studies of a particular phagocyte-microbe interaction. For certain, rigorous analysis of how, for example, murine peritoneal macrophages kill a particular fungus will yield accurate and informative insights into the mechanisms of those events in those well-defined conditions. However, those mechanisms may not apply to other phagocytes from different species challenged with unrelated microbes (e.g. human neutrophils fed N. gonorrhoeae).
With respect to elucidation of NOX2-mediated oxidant signaling, technical limitations, most glaringly the lack of sensitive and specific probes to report unambiguously intracellular oxidants, slow meaningful progress. In light of these limitations, care should be taken not to extrapolate observations. NOX2-dependent oxidant production may alter the redox state of target cells in ways that profoundly alter phenotype but may not be directly the consequence of H2O2 attack on a specific constituent in a signaling pathway. For example, the absence of superoxide dismutase in murine macrophages results in increased DCF fluorescence in response to ATP and reduced caspase-1-dependent cytokine production [85]. The overall redox potential of the deficient mice is reduced, with subsequent glutathionylation of caspase-1 and decrease in activity, without oxidant-mediated changes in a specific signaling target. Investigations of NOX2-dependent oxidant signaling need to incorporate into their consideration the complex biochemistry that regulates cellular redox status [86].
Gaps in our current knowledge about the consequences of NOX2-dependent oxidant production provide fantastic opportunities for exploration of phagocyte biology and beyond.
Highlights.
Phagocyte NOX2 NADPH oxidase generates oxidants
Optimal antimicrobial action in phagosomes depends on oxidants
Precise mechanisms of oxidant-dependent killing by phagocytes are unknown
NOX2 NADPH-generated oxidants participate in cell signaling
More rigor in studies of oxidant signaling by NOX2 NADPH oxidase is needed
Acknowledgments
The Nauseef laboratory is supported by National Institute of Health grants R01-AI116546 and R01-AI132335, Merit Review award BX000513 from the Veterans Affairs, and use of facilities at the Iowa City Department of Veterans Affairs Medical Center, Iowa City, IA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: none
Literature Cited:
- 1.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD: Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401:79–82. [DOI] [PubMed] [Google Scholar]
- 2.Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, Krause KH: A mammalian H + channel generated through alternative splicing of the NADPH oxidase homolog NOH-1 Science 2000, 287:138–142. [DOI] [PubMed] [Google Scholar]
- 3.Bedard K, Krause KH: The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007, 87:245–313. [DOI] [PubMed] [Google Scholar]
- 4.Paffenholz R, Bergstrom RA, Pasutto F, Wabnitz P, Munroe RJ, Jagla W, Heinzmann Y, Marquardt A, Bareiss A, Laufs J, et al. : Vestibular defects in head-tilt mice result from mutations in nox3 , encoding an NADPH oxidase. Genes and Development 2004, 18 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambeth JD, Neish AS: Nox Enzymes and New Thinking on Reactive Oxygen: A Double-Edged Sword Revisited. Annu Rev Pathol 2014, 9:119–145. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann MH, Griffiths HR: The dual role of ROS in autoimmune and inflammatory diseases: Evidence from preclinical models. Free Radic Biol Med 2018, 125:62–71. [DOI] [PubMed] [Google Scholar]
- 7.Breitenbach M, Rinnerthaler M, Weber M, Breitenbach-Koller H, Karl T, Cullen P, Basu S, Haskova D, Hasek J: The defense and signaling role of NADPH oxidases in eukaryotic cells : Review. Wien Med Wochenschr 2018, 168:286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nauseef WM: The Neutrophil NADPH Oxidase In Hydrogen Peroxide Metabolism in Health and Disease. Edited by Vissers M, Hampton MB, Kettle AJ: CRC Press; 2018:237–277. [Google Scholar]
- 9.Heyworth PG, Curnutte JT, Nauseef WM, Volpp BD, Pearson DW, Rosen H, Clark RA: Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47- phox and p67- phox requires interaction between p47- phox and cytochrome b 558 J. Clin. Invest 1991, 87:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JW, Ma M, Ruedi JM, Smith RM, Babior BM: The cytosolic components of the respiratory burst oxidase exist as a Mr −240,000 complex that acquires a membrane-binding site during activation of the oxidase in a cell-free system. Journal of Biological Chemistry 1992, 267:17327–17332. [PubMed] [Google Scholar]
- 11.Park JW, El Benna J, Scott KE, Christensen BL, Chanock SJ, Babior BM: Isolation of a complex of respiratory burst oxidase components from resting neutrophil cytosol. Biochemistry 1994, 33:2907–2911. [DOI] [PubMed] [Google Scholar]
- 12.Iyer SS, Pearson DW, Nauseef WM, Clark RA: Evidence for a readily dissociable complex of p47phox and p67phox in cytosol of unstimulated human neutrophils. Journal of Biological Chemistry 1994, 269:22405–22411. [PubMed] [Google Scholar]
- 13.Ziegler CS, Bouchab L, Tramier M, Durand D, Fieschi F, Dupre-Crochet S, Merola F, Nusse O, Erard M: Quantitative live-cell imaging and 3D modeling reveal critical functional features in the cytosolic complex of phagocyte NADPH oxidase. J Biol Chem 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Inter- and intramolecular interactions are critical determinants in regulation of the cytosolic ternary complex of p47phox-p67phox-p40phox. The authors use Förster resonance energy transfer (FRET), fluorescence lifetime imaging (FLIM), and fluorescence cross-correlation spectroscopy (FCCS) to create a three-dimensional model of the complex that complements available structural data.
- 14.Zhou Y, An LL, Chaerkady R, Mittereder N, Clarke L, Cohen TS, Chen B, Hess S, Sims GP, Mustelin T: Evidence for a direct link between PAD4-mediated citrullination and the oxidative burst in human neutrophils. Sci Rep 2018, 8:15228. [DOI] [PMC free article] [PubMed] [Google Scholar]; * In their studies of human neutrophils and the human promyelocytic cell line HL-60, Zhou et al. describe a set of novel observations related to protein arginine deiminase (PAD) 4 and cytosolic components of the NADPH oxidase. PAD4, p47phox, and p67phox coprecipitate from unstimulated neutrophils, but stimulation with the calcium ionophore ionomycin activates PAD4, which promotes citrullination of p47phox and its dissociation from the complex, with subsequent augmentation of oxidase activity. How the citrullination of p47phox by PAD4 at very high intracellular calcium concentrations fits into our understanding of normal neutrophil biology or bears on the biogenesis of NETs awaits further exploration.
- 15.Stojkov D, Amini P, Oberson K, Sokollik C, Duppenthaler A, Simon HU, Yousefi S: ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. J Cell Biol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Biochemical and structural determinants of NET formatioin remain incompletely defined. Stojker et al. provide data on the complex balance between NADPH oxidase-dependent polymerization and glutathionylation of actin and tubulin and their glutaredoxin 1-dependent deglutathionylation that is a prerequisite for NET formation. In addition to observations germane to NETs, the authors convincingly demonstrate that NOX2-deficient human and murine neutrophils have defective degranulation, an issue that has been controversial since the mid-1970s.
- 16.El Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM: Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev 2016, 273:180–193. [DOI] [PubMed] [Google Scholar]; * Priming of the phagocyte NADPH oxidase provides a reversible means by which to modulate oxidant production to meet demand. The authors summarize current understanding of the molecular underpinnings of oxidase priming and present unanswered questions that need to be addressed.
- 17.Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, Yockey L, Darnell DN, Barnhart L, Daub J, et al. : Common severe infections in chronic granulomatous disease. Clin Infect Dis 2015, 60:1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nauseef WM, Clark RA: Intersecting Stories of the Phagocyte NADPH Oxidase and Chronic Granulomatous Disease In Methods in Molecular Biology. Edited by Knaus UG, Leto TL; 2019. vol NADPH Oxidases.] [DOI] [PubMed] [Google Scholar]
- 19.Holland SM: Chronic granulomatous disease. Hematol Oncol Clin North Am 2013, 27:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, Uzel G, DeRavin SS, Priel DAL, Soule BP, et al. : Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med 2010, 363:2600–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matute JD, Arias AA, Wright NAM, Wrobel I, Waterhouse CCM, Li XJ, archal CC, Stull ND, Lewis DB, Steele M, et al. : A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood 2009, 114:3309–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaffe MB: The p47phox PX domain: two heads are better than one! Structure 2002, 10:1288–1290. [DOI] [PubMed] [Google Scholar]
- 23.Kanai F, Liu H, Field S, Akbary H, Matsuo T, Brown G, Cantley L, Yaffe M: The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat. Cell Biol. 2001, 3:675–678. [DOI] [PubMed] [Google Scholar]
- 24.van de Geer A, Nieto-Patlan A, Kuhns DB, Tool AT, Arias AA, Bouaziz M, de Boer M, Franco JL, Gazendam RP, van Hamme JL, et al. : Inherited p40phox deficiency differs from classic chronic granulomatous disease. J Clin Invest 2018, 128:3957–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Deficiency of p40phox had been reported in only one patient prior to this report, wherein 24 patients from 12 families are described in detail. Eight different mutations in NCF4 result in selective loss of function of NADPH oxidase activity after phagocyte ingestion of particles, but not in response to the soluble agonist phorbol myristate acetate. The contrasting phenotypes of p40phox deficiency and classic CGD belies links between oxidase activity and dysregulated inflammation that remain undefined.
- 25.Thomas DC, Clare S, Sowerby JM, Pardo M, Juss JK, Goulding DA, van der Weyden L, Storisteanu D, Prakash A, Espeli M, et al. : Eros is a novel transmembrane protein that controls the phagocyte respiratory burst and is essential for innate immunity. J Exp Med 2017, 214:1111–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Thomas et al. identified Eros (essential for reactive oxygen species) as a previously unrecognized transmembrane protein in the endoplasmic reticulum that is required for stability of the gp91phox-p22phox heterodimer during its biosynthesis. The plant ortholog of Eros, Ycf4, is required for expression of photosystem I, an NADPH oxioreductase essential for photosynthesis, and its properties may hint at how Eros operates in production of functional gp91phox-p22phox. Two independent reports of patients with mutations in CYBC1, the gene encoding Eros, and the clinical phenotype of CGD underscores the biomedical importance of Eros in innate immune cells.
- 26.Arnadottir GA, Norddahl GL, Gudmundsdottir S, Agustsdottir AB, Sigurdsson S, Jensson BO, Bjarnadottir K, Theodors F, Benonisdottir S, Ivarsdottir EV, et al. : A homozygous loss-of-function mutation leading to CYBC1 deficiency causes chronic granulomatous disease. Nat Commun 2018, 9:4447. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Thomas et al. identified Eros (essential for reactive oxygen species) as a previously unrecognized transmembrane protein in the endoplasmic reticulum that is required for stability of the gp91phox-p22phox heterodimer during its biosynthesis. The plant ortholog of Eros, Ycf4, is required for expression of photosystem I, an NADPH oxioreductase essential for photosynthesis, and its properties may hint at how Eros operates in production of functional gp91phox-p22phox. Two independent reports of patients with mutations in CYBC1, the gene encoding Eros, and the clinical phenotype of CGD underscores the biomedical importance of Eros in innate immune cells.
- 27.Thomas DC, Charbonnier LM, Schejtman A, Aldhekri H, Coomber EL, Dufficy ER, Beenken AE, Lee JC, Clare S, Speak AO, et al. : EROS mutations: decreased NADPH oxidase function and chronic granulomatous disease. J Allergy Clin Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Thomas et al. identified Eros (essential for reactive oxygen species) as a previously unrecognized transmembrane protein in the endoplasmic reticulum that is required for stability of the gp91phox-p22phox heterodimer during its biosynthesis. The plant ortholog of Eros, Ycf4, is required for expression of photosystem I, an NADPH oxioreductase essential for photosynthesis, and its properties may hint at how Eros operates in production of functional gp91phox-p22phox. Two independent reports of patients with mutations in CYBC1, the gene encoding Eros, and the clinical phenotype of CGD underscores the biomedical importance of Eros in innate immune cells.
- 28.DeCoursey TE: The intimate and controversial relationship between voltage-gated proton channels and the phagocyte NADPH oxidase. Immunol Rev 2016, 273:194–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okochi Y, Saski M Iwasaki H Okamura Y: Voltage-gated proton channel is expressed on phagosomes. Biochem Biophys Res Commun. 2009, 382:274–279. [DOI] [PubMed] [Google Scholar]
- 30.El Chemaly A, Okochi Y, Sasaki M, Arnaudeau S, Okamura Y, Demaurex N: VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. Journal of Experimental Medicine 2010, 207:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller AF, Teixeira M, Valentine JS: Superoxide dismutases and superoxide reductases. Chem Rev 2014, 114:3854–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winterbourn CC, Kettle AJ, Hampton MB: Reactive Oxygen Species and Neutrophil Function. Annu Rev Biochem 2016, 85:765–792. [DOI] [PubMed] [Google Scholar]
- 33.Jankowski A, Scott CC, Grinstein S: Determinants of the phagosomal pH in neutrophils. Journal of Biological Chemistry 2002, 277:6059–6066. [DOI] [PubMed] [Google Scholar]
- 34.Levine AP, Duchen MR, de Villiers S, Rich PR, Segal AW: Alkalinity of neutrophil phagocytic vacuoles is modulated by HVCN1 and has consequences for myeloperoxidase activity. PLoS One 2015, 10:e0125906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iversen MB, Gottfredsen RH, Larsen UG, Enghild JJ, Praetorius J, Borregaard N, Petersen SV: Extracellular superoxide dismutase is present in secretory vesicles of human neutrophils and released upon stimulation. Free Radio Biol Med 2016, 97:478–488. [DOI] [PubMed] [Google Scholar]
- 36.Kettle AJ, Winterbourn CC: Superoxide modulates the activity of myeloperoxidase and optimizes the production of hypochlorous acid. Biochemical Journal 1988, 252:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kettle AJ, Anderson RF, Hampton MB, Winterbourn CC: Reactions of superoxide with myeloperoxidase. Biochemistry 2007, 46:4888–4897. [DOI] [PubMed] [Google Scholar]
- 38.Winterbourn CC, Kettle AJ: Redox reactions and microbial killing in the neutrophil phagosome. Antioxid Redox Signal 2013, 18:642–660. [DOI] [PubMed] [Google Scholar]
- 39.Nauseef WM: How human neutrophils kill and degrade microbes: an integrated view. Immunological Reviews 2007, 219:88–102. [DOI] [PubMed] [Google Scholar]
- 40.Nauseef WM: Myeloperoxidase in human neutrophil host defence. Cell Microbiol 2014, 16:1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abuaita BH, Schultz TL, O’Riordan MX: Mitochondria-Derived Vesicles Deliver Antimicrobial Reactive Oxygen Species to Control Phagosome-Localized Staphylococcus aureus. Cell Host Microbe 2018, 24:625–636. e625. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Whereas ~all oxygen consumed by stimulated neutrophils reflects the activity of the NOX2 NADPH oxidase, macrophages are rich in mitochondria, which provide an additional source of reactive oxygen species. Abuaita et al. demonstrate that murine bone marrow-derived macrophages fed methicillin-resistant S.aureus activate an endoplasmic reticulum stress response that triggers mitochondrial H2O2 production and generation of mitochondria-derived membrane vesicles, which transport H2O2 to microbe-containing phagosomes. Whether similar events occur in human macrophages remains undetermined.
- 42.Forbes LV, Kettle AJ: Myeloperoxidase: Unleashing the Power of Hydrogen Peroxide In Hydrogen Peroxide Metabolism in Health and Disease. Edited by Vissers MCM, Hampton MB, Kettle AJ: CRC Press; 2018:281–304. [Packer L, Cadenas E (Series Editor): Oxidative Stress and Disease [Google Scholar]
- 43.Albrett AM, Ashby LV, Dickerhof N, Kettle AJ, Winterbourn CC: Heterogeneity of hypochlorous acid production in individual neutrophil phagosomes revealed by a rhodamine-based probe. J Biol Chem 2018, 293:15715–15724. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Successful production of HOCl within phagosomes requires coordination at the phagosomal membrane of two events: azurophil granule fusion, to deliver myeloperoxidase, and NADPH oxidase assembly and activation, to generate intraphagosomal H2O2. Using R19-S, a rhodamine-based dye that fluoresces when oxidized by HOCl, and live cell imaging, Albrett et al. demonstrate that phagosomes within individual neutrophils are remarkably heterogeneous. Presumably, heterogeneity in microbicidal parallels that in HOCl generation, providing a possible explanation for the ability of subpopulations of ingested microbes to survive within neutrophils and potentially foster refractory or recurrent infection.
- 44.Winterbourn CC: Biological Production, Detection, and Fate of Hydrogen Peroxide. Antioxid Redox Signal 2018, 29:541–551. [DOI] [PubMed] [Google Scholar]
- 45.Burg AR, Tse HM: Redox-Sensitive Innate Immune Pathways During Macrophage Activation in Type 1 Diabetes. Antioxid Redox Signal 2018, 29:1373–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griffiths HR, Gao D, Pararasa C: Redox regulation in metabolic programming and inflammation. Redox Biol 2017, 12:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gerns D, et al. : Unraveling the biological roles of reactive oxygen species. Cell Metabolism 2011, 13:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forman HJ, Augusto O, Brigelius-Flohe R, Dennery PA, Kalyanaraman B, Ischiropoulos H, Mann GE, Radi R, Roberts LJ 2nd, Vina J, et al. : Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radic Biol Med 2015, 78:233–235. [DOI] [PubMed] [Google Scholar]
- 49.Winterbourn CC: The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim Biophys Acta 2014, 1840:730–738. [DOI] [PubMed] [Google Scholar]; * Although the association of H2O2 production with cell signaling in many biological settings is established, details are often lacking. Winterbourn lucidly reviews what is known about H2O2 generation and reactivity in biological settings and provides an up-to-date summary of the current gaps in our knowledge., including the cellular sites of H2O2 production and the actions of regulatory proteins that operate during oxidant signaling.
- 50.Wardman P: Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med 2007, 43:995–1022. [DOI] [PubMed] [Google Scholar]
- 51.Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Ord T, Bredesen DE: Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science 1993, 262:1274–1277. [DOI] [PubMed] [Google Scholar]
- 52.Burkitt MJ, Wardman P: Cytochrome C is a potent catalyst of dichlorofluorescin oxidation: implications for the role of reactive oxygen species in apoptosis. Biochem Biophys Res Commun 2001, 282:329–333. [DOI] [PubMed] [Google Scholar]
- 53.Ganini D, Leinisch F, Kumar A, Jiang J, Tokar EJ, Malone CC, Petrovich RM, Mason RP: Fluorescent proteins such as eGFP lead to catalytic oxidative stress in cells. Redox Biol 2017, 12:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winterbourn CC: The biological chemistry of hydrogen peroxide. Methods Enzymol 2013, 528:3–25. [DOI] [PubMed] [Google Scholar]
- 55.Tamassia N, Bianchetto-Aguilera F, Arruda-Silva F, Gardiman E, Gasperini S, Calzetti F, Cassatella MA: Cytokine production by human neutrophils: Revisiting the “dark side of the moon”. Eur J Clin Invest 2018, 48 Suppl2:e12952. [DOI] [PubMed] [Google Scholar]
- 56.Lindskog C, Asplund A, Catrina A, Nielsen S, Rutzler M: A Systematic Characterization of Aquaporin-9 Expression in Human Normal and Pathological Tissues. J Histochem Cytochem 2016, 64:287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poole LB: The basics of thiols and cysteines in redox biology and chemistry. Free Radic Biol Med 2015, 80:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stöcker S, Van Laer K, Mijuskovic A, Dick TP: The Conundrum of Hydrogen Peroxide Signaling and the Emerging Role of Peroxiredoxins as Redox Relay Hubs. Antioxid Redox Signal 2018, 28:558–573. [DOI] [PubMed] [Google Scholar]; * Stöcker et al. discuss how peroxiredoxins particpate in redox signaling and thereby provide one solution to the conundrum of how H2O2 succeeds to drive intracellular signaling.
- 59.Rhee SG, Woo HA, Kang D: The Role of Peroxiredoxins in the Transduction of H2O2 Signals. Antioxid Redox Signal 2018, 28:537–557. [DOI] [PubMed] [Google Scholar]
- 60.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG: Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 2010, 140:517–528. [DOI] [PubMed] [Google Scholar]
- 61.Zhong J, Olsson LM, Urbonaviciute V, Yang M, Backdahl L, Holmdahl R: Association of NOX2 subunits genetic variants with autoimmune diseases. Free Radic Biol Med 2018, 125:72–80. [DOI] [PubMed] [Google Scholar]; * The Holmdahl laboratory has demonstrated how mutations in genes encoding cytosolic components of the NOX2 NADPH oxidase predispose to chronic inflammation in a variety of different rat and mouse systems. Here they review the extensive literature linking specific mutations in the phagocyte NADPH oxidase components with autoimmune disease models and contrast those results with studies in humans.
- 62.Geering B, Simon HU: Peculiarities of cell death mechanisms in neutrophils. Cell Death & Differentiation 2011, 18:1457–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bagaitkar J, Huang J, Zeng MY, Pech NK, Monlish DA, Perez-Zapata LJ, Miralda I, Schuettpelz LG, Dinauer MC: NADPH oxidase activation regulates apoptotic neutrophil clearance by murine macrophages. Blood 2018, 131:2367–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Evidence of increased inflammation can reflect excessive initiation, failed termination, or both. Using a murine model, Bagaitkar et al. demonstrate that efferocytosis of apoptotic cells is delayed in gp91phox-deficient peritoneal exudate macrophages (PEMs). Furthermore, CGD PEMs have increased presentation of apoptotic cell-associated antigens to CD8 T cells. These observations have important implications for the observed links between defects in the NOX2 NADPH oxidase and the clinical findings of hyperinflammation and autoimmune abnormalities.
- 64.Oakley FD, Abbott D, Li Q, Engelhardt JF: Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal 2009, 11:1313–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacob CO, Eisenstein M, Dinauer MC, Ming W, Liu Q, John S, Quismorio FP Jr., Reiff A, Myones BL, Kaufman KM, et al. : Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc.Nat.Acad.Sci.USA 2012, 109:E59–E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, barmada MM, Datta LW, et al. : Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nature Genetics 2007, 39:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacob CO, Yu N, Yoo DG, Perez-Zapata LJ, Barbu EA, Kaplan MJ, Purmalek M, Pingel JT, Idol RA, Dinauer MC: Haploinsufficiency of NADPH Oxidase Subunit Neutrophil Cytosolic Factor 2 Is Sufficient to Accelerate Full-Blown Lupus in NZM 2328 Mice. Arthritis Rheumatol 2017, 69:1647–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marciano BE, Zerbe CS, Falcone EL, Ding L, DeRavin SS, Daub J, Kreuzburg S, Yockey L, Hunsberger S, Foruraghi L, et al. : X-linked carriers of chronic granulomatous disease: Illness, lyonization, and stability. J Allergy Clin Immunol 2018, 141:365–371. [DOI] [PubMed] [Google Scholar]
- 69.Deffert C, Carnesecchi S, Yuan H, Rougemont AL, Kelkka T, Holmdahl R, Krause KH, Schappi M: Hyperinflammation of chronic granulomatous disease is abolished by NOX2 reconstitution in macrophages and dendritic cells. J.Pathol. 2012, 228:341–350. [DOI] [PubMed] [Google Scholar]
- 70.Cachat J, Deffert C, Hugues S, Krause KH: Phagocyte NADPH oxidase and specific immunity. Clin Sci (Lond) 2015, 128:635–648. [DOI] [PubMed] [Google Scholar]
- 71.El Jamali A, Valente AJ, Clark RA: Regulation of phagocyte NADPH oxidase by hydrogen peroxide through a Ca 2+ /c-Abl signaling pathway. Free Rad.Biol.& Med. 2010, 48:798–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang MM, Barman PK, Thiruppathi M, Mirza RE, McKinney RD, Deng J, Christman JW, Du X, Fukai T, Ennis WJ, et al. : Oxidant Signaling Mediated by Nox2 in Neutrophils Promotes Regenerative Myelopoiesis and Tissue Recovery following Ischemic Damage. J Immunol 2018, 201:2414–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hartl D, Lehmann N, Hoffmann F, Jansson A, Hector A, Notheis G, Roos D, Belohradsky BH, Wintergerst U: Dysregulation of innate immune receptors on neutrophils in chronic granulomatous disease. Journal of Allergy and Clinical Immunology 2008, 121:375–382. [DOI] [PubMed] [Google Scholar]
- 74.Zhu N, Feng X, He C, Gao H, Yang L, Ma Q, Guo L, Qiao Y, Yang H, Ma T: Defective macrophage function in aquaporin-3 deficiency. Faseb j 2011, 25:4233–4239. [DOI] [PubMed] [Google Scholar]
- 75.Bertolotti M, Farinelli G, Galli M, Aiuti A, Sitia R: AQP8 transports NOX2-generated H2O2 across the plasma membrane to promote signaling in B cells. J Leukoc Biol 2016, 100:1071–1079. [DOI] [PubMed] [Google Scholar]
- 76.Holmdahl R, Sareila O, Pizzolla A, Winter S, Hagert C, Jaakkola N, Kelkka T, Olsson LM, Wing K, Backdahl L: Hydrogen peroxide as an immunological transmitter regulating autoreactive T cells. Antioxid Redox Signal 2013, 18:1463–1474. [DOI] [PubMed] [Google Scholar]
- 77.Crotzer VL, Matute JD, Arias AA, Zhao H, Quilliam LA, Dinauer MC, Blum JS: Cutting Edge: NADPH Oxidase Modulates MHC Class II Antigen Presentation by B Cells. J Immunol 2012, 189:3800–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McLetchie S, Volpp BD, Dinauer MC, Blum JS: Hyper-responsive Toll-like receptor 7 and 9 activation in NADPH oxidase-deficient B lymphoblasts. Immunology 2015, 146:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han W, Li H, Cai J, Gleaves LA, Polosukhin VV, Segal BH, Yull FE, Blackwell TS: NADPH oxidase limits lipopolysaccharide-induced lung inflammation and injury in mice through reduction-oxidation regulation of NF-kappaB activity. J Immunol 2013, 190:4786–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olive AJ, Smith CM, Kiritsy MC, Sassetti CM: The Phagocyte Oxidase Controls Tolerance to Mycobacterium tuberculosis Infection. J Immunol 2018, 201:1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gabelloni ML, Sabbione F, Jancic C, Fuxman Bass J, Keitelman I, Iula L, Oleastro M, Geffner JR, Trevani AS: NADPH oxidase derived reactive oxygen species are involved in human neutrophil IL-1beta secretion but not in inflammasome activation. Eur J Immunol 2013, 43:3324–3335. [DOI] [PubMed] [Google Scholar]
- 82.van de Veerdonk FL, Smeekens SP, Joosten LAB, Kullberg BJ, Dinarello CA, Van der Meer JWM, Netea MG: Reactive oxygen species-independent activation of the IL-16 inflammasome in cells from patients with chronic granulomatous disease. Proc.Nat.Acad.Sci.USA 2010, 107:3030–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abais JM, Xia M, Zhang Y, Boini KM, Li PL: Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal 2015, 22:1111–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Locke J: Complete Essay on Human Understanding: Independently; 1690.
- 85.Meissner F, Molawi K, Zychlinsky A: Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nature Immunology 2008, 9:866–872. [DOI] [PubMed] [Google Scholar]
- 86.Winterbourn CC, Hampton MB: Thiol chemistry and specificity in redox signaling. Free Radical Biology and Medicine 2008, 45:549–561. [DOI] [PubMed] [Google Scholar]


