Abstract
Studies in recent years have strengthened the notion that neural mechanisms are involved in the control of immune responses. From initial studies that highlighted the vagus nerve control of inflammatory responses in vertebrates, many advances have been made, including the dissection of specific neural circuits that are involved in controlling immunity. Part of this has been facilitated by the use of a tractable model animal, Caenorhabditis elegans, in which individual neurons involved in sensing pathogens and controlling the immune response have been identified. Importantly, some of the underlying mechanisms involved in the neural control of immune pathways appear to be present in evolutionarily diverse species. This review focuses on some major developments in vertebrates and C. elegans, and how these discoveries may lead to advances in understanding neural-immune connections that govern inflammatory responses.
Graphical Abstract
Introduction
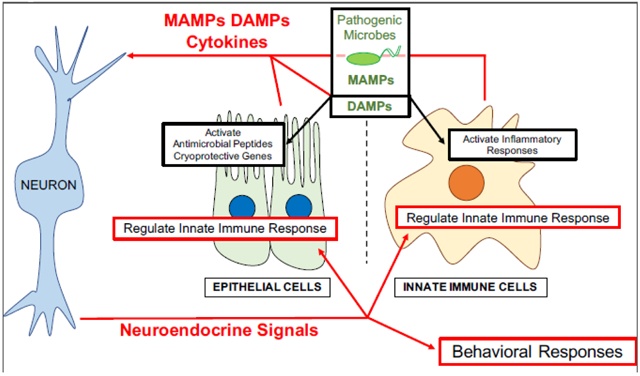
The mechanisms by which individual cells sense and respond to pathogens varies, but in general, cell-autonomous innate immune responses involve surface-displayed receptors, which recognize microbial-associated molecular patterns and/or damage associated molecular patterns, to activate innate immune signaling pathways. However, cells do not operate in isolation and are in direct and indirect contact with a variety of other cells in the body. Paracrine signals from neighboring cells and cytokines in the circulation alter the physiology of cells involved in the innate immune response. It also appears that neuroendocrine signals from the nervous system have the potential to alter different cellular pathways in response to pathogen infection.
In recent years, the connection between the immune and the nervous systems to orchestrate appropriate immune responses has become more apparent, and it appears that a cross-talk between the two systems is evolutionarily conserved [1]. Neuropeptide receptors are present in immune cells, and cytokine receptors are present in neurons. Neurons in the central nervous system produce cytokines and cells of the immune system produce neuropeptides [1-3]. It has been shown that cytokines can regulate diverse physiological processes, including sleep cycles, neuroendocrine function, neuronal development, and responsiveness of the autonomic nervous system [1]. Neuropeptides and neuronal signaling are able to control inflammation and innate immune responses [4]. Herein we describe recent evidence for non-cell-autonomous control of immune responses by the nervous system.
Vagus Nerve Stimulation of the Immune System
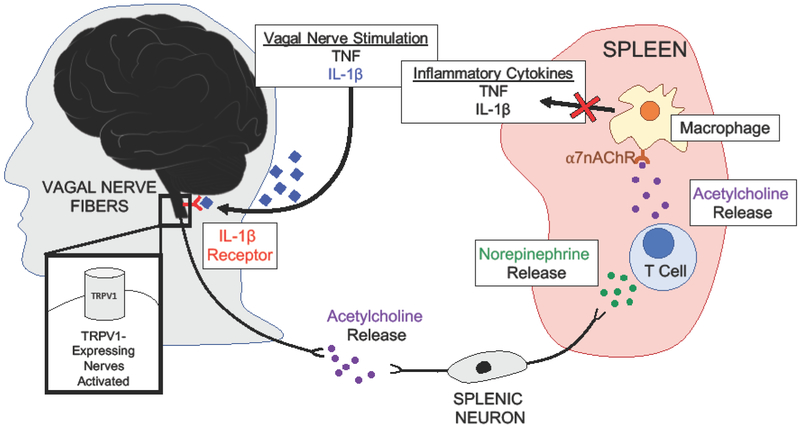
A seminal study by Borovikova et al. demonstrated that vagus nerve stimulation suppresses inflammation [5], solidifying earlier observations that there is communication between the immune and the nervous system. The nervous system seems to sense inflammation in peripheral tissues by the binding of IL-1β ligand to the IL-1β receptor on some or all sensory vagal fibers expressing TRPV1, a ligand-gated non-selective calcium-permeable ion channel [6]. This results in the activation of vagal neurons and subsequent signaling for a downstream anti-inflammatory pathway, deemed the vagal nerve reflex [7]. The vagal nerve reflex was originally discovered when the vagus nerve was directly stimulated in vivo during lethal endotoxemia in rats. This stimulation inhibited the production of tumor necrosis factor (TNFα) and ultimately prevented the development of systemic shock [8]. Vagus nerve stimulation results in the release of the principle vagal neurotransmitter, acetylcholine, which activates adrenergic splenic neurons to produce and secrete norepinephrine [8]. Norepinephrine binds and activates lymphocytes, specifically splenic T cells. These splenic T cells then produce acetylcholine, which was demonstrated in vitro to attenuate the release of inflammatory cytokines from macrophages activated by endotoxin (lipopolysaccharide) [5]. Mice devoid of T cells (nude mice) are unable to reduce TNFα levels upon vagal nerve stimulation. Subsequent work showed that vagus nerve suppression of the inflammatory response requires the α-7 nicotinic acetylcholine receptor-dependent pathway in splenic macrophages [9].
These observations, outlined in Figure 1, were consistent with reports that showed cells from both the nervous and the immune systems expressed ligands and receptors from the other. Although these neural-immune pathways have been dissected primarily in vitro and in vivo in murine models, vagal stimulation through pharmacological agents, such as CNI-1493, has been used to inhibit TNFα synthesis in humans. CNI-1493 has undergone phase 1 and phase 2 clinical trials for the treatment of inflammatory diseases, including Crohn’s Disease [10,11].
Figure 1. The Vagus Nerve Controls Inflammatory Responses.
Electrical vagal nerve stimulation was shown to prevent death and systemic shock in mice and humans. During endotoxemia, inflammatory cytokines are elevated systemically, including TNF and IL-1b. These cytokines are able to activate vagal nerve fibers expressing TRPV1, which triggers the release of acetylcholine. It was shown that electrical vagal nerve stimulation also resulted in the production of acetylcholine, which signals via splenic neurons. These splenic neurons release norepinephrine, which stimulates T cell release of acetylcholine. Acetylcholine binds to a7AChR (a-7 nicotinic acetylcholine receptor) on splenic macrophages to block the release of TNF and IL-1b.
Lessons from C. elegans, a Simple Model System
The aforementioned studies in mammalian systems indicate that the nervous system senses and regulates the immune system to maintain immunological homeostasis. However, understanding the functions of individual neurons in this process is challenging because of the complexity and size of the nervous system of humans and most model animals. The adult human brain contains approximately 86 billion neurons, with some estimates higher. With current technology, it is difficult to imagine scientists thoroughly dissecting neural-immune signaling at the neuronal, single cell level in vertebrates. The complex systems present in vertebrates and shortcomings in current technology create a barrier for understanding neural-immune connections in higher-order organisms. With advances in understanding the nervous systems of vertebrates and the development of genetic tools, dissecting neural circuits and individual neurons that control immune responses in higher-order organisms will become possible. Other model organisms may currently provide better alternatives to understanding these neural-immune connections.
Some of the first pieces of evidence showing that specific neurons are capable of controlling innate immune pathways came from studies performed in the nematode, C. elegans. The soildwelling bacterivore serves as a model organism for understanding the functions of individual neurons and their roles in the immune response. C. elegans has a simple, well-defined nervous system containing only 302 neurons, and the synaptic connectivity of each neuron has been mapped. In addition, the innate immune system of C. elegans resembles some aspects of the human innate immune system, allowing for the study of conserved mechanisms of neural-immune connections.
One of the initial studies linking specific neurons to immune responses in C. elegans demonstrated that NPR-1, a neuronally-expressed G-protein coupled receptor (GPCR) is required for immune responses to a variety of pathogens. C. elegans mutants in npr-1 show enhanced susceptibility to Pseudomonas aeruginosa, Salmonella enterica, and Enterococcus faecalis. To identify the specific neurons that coordinate neural-immune regulation, genetic ablation of sensory neurons expressing npr-1, AQR, PQR, and URX, was performed. This ablation resulted in increased survival during infection with P. aeruginosa and rescued the enhanced susceptibility of npr-1 mutants, suggesting that npr-1 plays an inhibitory role in the immune response [12]. Further characterization showed that npr-1 downregulates innate immune genes, partially via post-translational control of the p38 mitogen-activated protein kinase, PMK-1, as well as other PMK-1 independent pathways [12]. The GPCR, NPR-1, was also shown to play a role in pathogen avoidance behavior [12,13], which will be discussed in more detail below.
Neurotransmitters and Neuropeptides
A variety of neurotransmitters and neuropeptides have been identified to play a role in the control of immune pathways in both humans and C. elegans. Herein some of the neuropeptides and neurotransmitters that regulate immune defense during infection in both C. elegans and higher organisms will be described. Several of these findings have been briefly summarized in Table 1.
Table 1. Identified roles for neurotransmitters in mice and humans.
Neurotransmitters that have been identified in C. elegans have also been implicated in the control of inflammatory responses in vertebrates. The roles of selected neurotransmitters are discussed and referenced in Table 1.
| Neurotransmitter | Role in Immune Response Control | References |
|---|---|---|
| Serotonin | Serotonin activates innate immune cells, increases immune cell trafficking, regulates chemotaxis and proliferation. | [48,49] |
| Serotonin levels are increased in the intestinal mucosa during inflammatory diseases. Reduction in serotonin levels reduces severity of intestinal inflammation and down-regulates inflammatory cytokines. | [50-53] | |
| Dopamine | Macrophages express receptors for dopamine and are able to produce dopamine. Dopamine decreases TNFα production and up-regulates IL-6 and IL-10 production. | [54,55] |
| Receptors are present on neutrophils. Dopamine inhibits superoxide production, increases adhesion molecule expression, influences cell migration and phagocytic activity. | [54,56-59] | |
| Acetylcholine | Prevents tumor necrosis factor production in macrophages. | [5] |
Neuromedin U in Mammals
Mast cells are at the interface between the neuroendocrine system and the immune system because these cells can be activated by neuropeptides. In addition, chemical mediators, such as serotonin and histamine, produced by mast cells can activate neural cells. Neuromedin U (NMU) is a neuropeptide produced by the central nervous system, that binds receptors NMU-R1 (expressed in peripheral tissues) and NMU-R2 (expressed on nerve cells only) [14]. NMU-R1/2 are GPCRs, that mobilize intracellular calcium when activated. NMU was known to have roles in regulating smooth muscle contractions in the uterus, reducing food intake and weight, regulating stress responses, and modifying ion transport.
It was recently shown that NMU stimulates the secretion of cytokines in mouse group 2 lymphoid cells and that these type 2 cytokines (IL-4 IL-5 IL-9 IL-13) activate and mobilize mast cells. NMU-deficient mice were unable to activate mast cells [15]. Group 2 lymphoid cells, expressing NMU-R1, in the gastrointestinal tract co-localize with cholinergic neurons that express NMU [14,16]. These enteric neurons can sense and respond to helminth infection. Enteric neuron, neurosphere-derived neuronal organoids, when stimulated with alarmin IL-33 or with Nippostrongylus brasiliensis excretory and secretory products, induced neuron-derived expression of NMU. Moreover, supernatants from the stimulated neuronal organoids applied to group 2 lymphoid cells resulted in type 2 cytokine production [14].
In vitro NMU stimulation of these group 2 lymphoid cells resulted in an influx of Ca2+, which activates the calcium-dependent serine/threonine phosphatase (calcineurin), leading to NFAT nuclear translocation [14]. NMU administration increased secretion of type 2 cytokines, activation of the group 2 lymphoid cells, and proliferation [14,16]. In vivo administration of NMU resulted in potent type 2 cytokine response [14,16]. During infection, NMU enhanced expulsion of the gastrointestinal nematode N. brasiliensis and NMU-R1 deficient mice had a higher burden of pathogen than WT. These studies outline a specific pathway in which enteric neurons sense and respond to N. brasiliensis infection, to then regulate the local immune response.
Octopamine
Sellegounder et al. recently reported that octopamine, a neurotransmitter closely related to norepinephrine, functions in immune regulation in C. elegans [17]. Under steady state conditions, RIC neurons produce octopamine, which serves as the endogenous ligand for OCTR-1, a neuronally expressed GPCR. OCTR-1 functions in sensory neurons ASH and ASI to suppress innate immune responses in non-neural [18-20]. Octopamine signaling suppresses unwanted innate immune responses by preventing infection triggered protein synthesis and the unfolded protein response, resulting in immunological homeostasis [17]. In the presence of pathogenic bacteria, RIC neurons are deactivated, decreasing octopamine production and allowing for enhanced innate immunity [17]. It is hypothesized that the octopamine-OCTR-1 pathway functions to suppress excessive responses to infection and to restore protein homeostasis after infection [19].
Acetylcholine
Acetylcholine is released from the nervous system in C. elegans. The neurotransmitter functions in a neuroendocrine fashion to activate muscarinic receptors in intestinal epithelial cells, leading to increased expression of Wnt and Frizzled in the intestinal epithelium. The activation of the canonical Wnt pathway increases the expression of host defense genes, such as C-type lectin (clec-60) and lysozymes [21].
Dopamine
The immune inhibitory function of dopamine in C. elegans originates in CEP neurons and requires active DOP-4 (dopamine receptor) in downstream ASG neurons. Using pharmacological techniques, it was shown that the PMK-1/p38 mitogen-activated protein kinase signaling pathway can be activated by chlorpromazine, a phenothiazine antipsychotic drug. The drug signals through DOP-4 to enhance host resistance against bacterial infections [22].
Serotonin
Serotonin, produced and secreted by chemosensory neurons in C. elegans, was also found to modulate the immune response, possibly in response to changes in the availability and/or quality of food. Serotonin released by the chemosensory ADF neuron binds to serotonin receptors SER-1 and SER-7. This directly or indirectly activates G-protein, GOA-1 (Gαo) in rectal epithelial cells, resulting in the suppression of the immune response to M. nematophilum [23].
Neuronal Control of Pathogen Avoidance Responses
Behavioral responses are complementary to other forms of immune defenses, such as immunological resistance and tolerance. Avoidance behavior has been demonstrated in a range of animals, including nematodes [24-39], crustaceans [40], mice [41], bonobos (pygmy chimpanzee) [42], and humans. All of these animals are able to sense and respond to environmental cues of pathogenic contamination.
Panulirus argu, the Caribbean spiny lobster, can be infected by the lethal Panulirus argus virus 1. This species of lobster shows avoidance behavior toward infected individuals, and are able to determine that others are infected before visual signs of disease are present [40]. Bonobos are able to sense and avoid olfactory cues linked to infection and contamination (such as feces or soil). These pygmy chimpanzees prefer food items lacking contaminants and actively move away from areas with contamination [42]. In humans, avoidance behavior is frequently classified as disgust and although little is known about the neural circuitry that controls this phenomenon, it was recently shown that serotonin may play a role. Serotonin levels were elevated during the induction of vomiting and are possibly involved in the development of learned aversion to contamination [43].
The mechanisms by which these avoidance behaviors are controlled are not well known in vertebrates, but some of the specific neuronal signaling pathways that govern avoidance behavior in mice have been mapped. For example, the vomeronasal circuit was previously described to mediate male sexual preference, female sexual behavior, and predator avoidance. It was recently demonstrated that the vomeronasal organ also senses olfactory cues emitted by parasitized or infected conspecifics [41]. Mice typically spend more time investigating healthy individuals in comparison to sick, and this was found to be dependent on specific signaling molecules present in infected individuals’ urine. To better understand the mechanisms by which mice use the vomeronasal organ to sense and avoid contamination, four neuronally expressed formyl-peptide GPCRs were studied for their role in sensing disease-related ligands. Expression of these GPCRs in vitro in non-neuronal cells conferred sensitivity to pathogen and inflammation related compounds [44].
In C. elegans, several neural networks that control avoidance behavior and learned avoidance behavior have been mapped. Interestingly, certain cues are sensed by C. elegans and trigger both a molecular innate immune response and avoidance behavior. The GPCR receptor, OCTR-1 functions in at least ASH and ASI neurons to control immunity [18]. While ASI neurons are required for pathogen avoidance behavior, ASH neurons coordinate an immune response that consists of the activation of microbial-killing and cellular-homeostatic pathways [18,20]. This provides an example of the integration of multiple sensory stimuli that result in a global immune response that involves the activation of both immune-related pathways and pathogen avoidance. Yet to be answered is the timing of each of these responses and if these two distinct responses occur at the same time.
Other neuronal pathways potentially involved in sensing bacterial metabolites have been identified in C. elegans. P. aeruginosa metabolites, phenazine-1-carboxamide, and pyochelin, were shown to activate G-protein signaling in ASJ neurons, leading to expression of daf-7 by ASJ neurons. Secreted DAF-7 binds to the TGF-β receptors in adjacent interneurons to activate the canonical TGF-β signaling pathway, promoting the avoidance of P. aeruginosa [24]. C. elegans is also able to avoid P. aeruginosa by detecting nitric oxide produced by the bacteria. Both the presence and removal of nitric oxide stimulates intracellular calcium increases in ASJ neurons. These changes (transients) in calcium are mediated by the redox sensing protein, TRX-1/thioredoxin, which switches between trans-nitrosylation activity and de-nitrosylation activity to promote the ON and OFF calcium transients respectively [45].
In addition to the roles of neuropeptides in controlling the molecular immune response to pathogens, neuropeptides have been demonstrated to signal for avoidance behavior in C. elegans. INS-6, an insulin-like neuropeptide expressed in ASI neurons, promotes aversive behavior by blocking ins-7 expression in URX neurons [30]. This circuit was further dissected to show that INS-11, a neuropeptide expressed in the intestine is increased upon infection. This results in the downregulation of ins-6, ultimately decreasing aversive behavior, possibly finetuning the avoidance of contaminated and non-contaminated food sources. INS-11 was also shown to regulate serotonin and insulin-like signaling in neurons to control avoidance behavior [35].
Conclusions
We have presented recent and some landmark studies that provide evidence of the roles of neural signals in the control of immune response to pathogens. The neural tractability of C. elegans permitted the identification of specific neurons that control immune pathways. Some of these pathways, particularly those in which neurotransmitters control immune responses, appear to be evolutionarily conserved and thus could be further explored in higher organisms. Advances in technology have led to the mapping of the complete volume of the adult Drosophila melanogaster brain [46] and over 300 neurons were traced in a brain wiring map in mice [47]. Using these tools, with other technologies that have made studying individual neurons possible in higher order organisms, the identification of specific neurons and signaling pathways that govern neural-immune control is foreseeable. It was recently predicted that the next revolution in immunology would come by integrating the neural circuits that regulate innate and adaptive immunity, including the mechanisms by which the nervous system senses microbes, to ultimately fine-tune the immune response [1]. One of the benefits of understanding these neural-immune connections is that they can be employed to control excess inflammation and/or stimulate proper immune responses during infection and inflammatory diseases. Some of the compounds that alter neuronal-immune signaling are being used to treat human diseases in which excess inflammation causes damage.
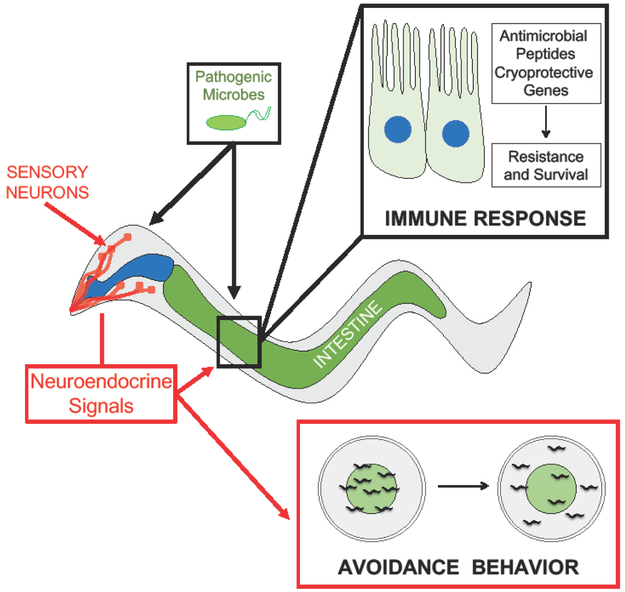
Figure 2. Neural-Immune Connections in C. elegans.
The nematode, C. elegans, has a simple, well-defined nervous system which includes sensory neurons located in the head of the animal. Pathogenic microbes activate and/or regulate innate immune pathways that increase resistance and survival. Additionally, neurons are able to sense the presence of pathogenic microbes to signal for avoidance behavior, or the movement of the animals away from pathogens (marked green in the figure).
Highlights.
Vagus nerve stimulation prevents TNFα production during endotoxemia and is being explored as a therapeutic for septic shock.
The identification of specific neuronal mechanisms that control immune responses in C. elegans provide clues for neural-immune pathways in vertebrates.
One of the first lines of immune defense could be considered pathogen avoidance. These behavioral responses to pathogens are controlled by neurons, that have been stimulated by pathogenic/environmental cues.
Acknowledgments
Funding: This work was supported by the National Institute of Allergy and Infectious Diseases grant number AA -- AI117911, the National Institute of General Medical Sciences grant number AA -- GM070977, and National Institute of Health CH -- T32HL083808
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tracey KJ: Approaching the next revolution? Evolutionary integration of neural and immune pathogen sensing and response. Cold Spring Harb Perspect.Biol 2014, 7: a016360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Breder CD, Dinarello CA, Saper CB: Interleukin-1 immunoreactive innervation of the human hypothalamus. Science 1988, 240: 321–324. [DOI] [PubMed] [Google Scholar]
- [3].Breder CD, Hazuka C, Ghayur T, Klug C, Huginin M, Yasuda K, Teng M, Saper CB: Regional induction of tumor necrosis factor alpha expression in the mouse brain after systemic lipopolysaccharide administration. Proc.Natl.Acad.Sci.U.S.A 1994, 91: 11393–11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tracey KJ: Physiology and immunology of the cholinergic antiinflammatory pathway. J.Clin.Invest 2007, 117: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ: Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405: 458–462. [DOI] [PubMed] [Google Scholar]
- [6].Steinberg BE, Silverman HA, Robbiati S, Gunasekaran MK, Tsaava T, Battinelli E, Stiegler A, Bouton CE, Chavan SS, Tracey KJ, Huerta PT: Cytokine-specific Neurograms in the Sensory Vagus Nerve. Bioelectron.Med 2016, 3: 7–17. [PMC free article] [PubMed] [Google Scholar]
- [7].Zanos TP, Silverman HA, Levy T, Tsaava T, Battinelli E, Lorraine PW, Ashe JM, Chavan SS, Tracey KJ, Bouton CE: Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. Proc.Natl.Acad.Sci.U.S.A 2018, 115: E4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, et al. : Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011, 334: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, et al. : Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J.Exp.Med 2006, 203: 1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Identifier: N: Long-term Study of Semapimod (CNI-1493) for Treatment of Crohn's Disease (CD05). National Library of Medicine (Us) 2008. [Google Scholar]
- [11].Identifier: N: Single ascending dose study of oral CPSI-2364 (Semapimod). National Library of Medicine (US) 2009. [Google Scholar]
- [12].Styer KL, Singh V, Macosko E, Steele SE, Bargmann CI, Aballay A: Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science 2008, 322: 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reddy KC, Andersen EC, Kruglyak L, Kim DH: A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science 2009, 323: 382–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, Veiga-Fernandes H: Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 2017, 549: 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moriyama M, Sato T, Inoue H, Fukuyama S, Teranishi H, Kangawa K, Kano T, Yoshimura A, Kojima M: The neuropeptide neuromedin U promotes inflammation by direct activation of mast cells. J.Exp.Med 2005, 202: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Klose CSN, Mahlakoiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, et al. : The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 2017, 549: 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sellegounder D, Yuan CH, Wibisono P, Liu Y, Sun J: Octopaminergic signaling mediates neural regulation of innate immunity in Caenorhabditis elegans. MBio 2018, 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cao X, Kajino-Sakamoto R, Doss A, Aballay A: Distinct roles of sensory neurons in mediating pathogen avoidance and neuropeptide-dependent immune regulation. Cell.Rep 2017, 21: 1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu Y, Sellegounder D, Sun J: Neuronal GPCR OCTR-1 regulates innate immunity by controlling protein synthesis in Caenorhabditis elegans. Sci.Rep. 2016, 6: 36832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun J, Singh V, Kajino-Sakamoto R, Aballay A: Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science 2011, 332: 729–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Labed SA, Wani KA, Jagadeesan S, Hakkim A, Najibi M, Irazoqui JE: Intestinal Epithelial Wnt signaling mediates acetylcholine-triggered host defense against Infection. Immunity 2018. 48: 978.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cao X, Aballay A: Neural inhibition of dopaminergic signaling enhances immunity in a cell-non-autonomous manner. Curr.Biol 2016, 26: 2329–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Anderson A, Laurenson-Schafer H, Partridge FA, Hodgkin J, McMullan R: Serotonergic chemosensory neurons modify the C. elegans immune response by regulating G-protein signaling in epithelial cells. PLoS Pathog. 2013, 9: e1003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Meisel JD, Panda O, Mahanti P, Schroeder FC, Kim DH: Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell 2014, 159: 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Meisel JD, Kim DH: Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol. 2014, 35: 465–470. [DOI] [PubMed] [Google Scholar]
- [26].Anderson A, McMullan R: Neuronal and non-neuronal signals regulate Caernorhabditis elegans avoidance of contaminated food. Philos.Trans.R.Soc.Lond.B.Biol.Sci 2018, 373: 10.1098/rstb.2017.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Beale E, Li G, Tan MW, Rumbaugh KP: Caenorhabditis elegans senses bacterial autoinducers. Appl.Environ.Microbiol 2006, 72: 5135–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bretscher AJ, Kodama-Namba E, Busch KE, Murphy RJ, Soltesz Z, Laurent P, de Bono M: Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron 2011,69: 1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chang HC, Paek J, Kim DH: Natural polymorphisms in C. elegans HECW-1 E3 ligase affect pathogen avoidance behaviour. Nature 2011, 480: 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen Z, Hendricks M, Cornils A, Maier W, Alcedo J, Zhang Y: Two insulin-like peptides antagonistically regulate aversive olfactory learning in C. elegans. Neuron 2013, 77: 572–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ha HI, Hendricks M, Shen Y, Gabel CV, Fang-Yen C, Qin Y, Colon-Ramos D, Shen K, Samuel AD, Zhang Y: Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron 2010, 68: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Harris G, Shen Y, Ha H, Donato A, Wallis S, Zhang X, Zhang Y: Dissecting the signaling mechanisms underlying recognition and preference of food odors. J.Neurosci 2014, 34: 9389–9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jin X, Pokala N, Bargmann CI: Distinct circuits for the formation and retrieval of an imprinted olfactory memory. Cell 2016, 164: 632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Krzyzanowski MC, Brueggemann C, Ezak MJ, Wood JF, Michaels KL, Jackson CA, Juang BT, Collins KD, Yu MC, L'etoile ND, Ferkey DM: The C. elegans cGMP-dependent protein kinase EGL-4 regulates nociceptive behavioral sensitivity. PLoS Genet. 2013, 9: e1003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lee K, Mylonakis E: An intestine-derived neuropeptide controls avoidance behavior in Caenorhabditis elegans. Cell.Rep. 2017, 20: 2501–2512. [DOI] [PubMed] [Google Scholar]
- [36].Pradel E, Zhang Y, Pujol N, Matsuyama T, Bargmann CI, Ewbank JJ: Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc.Natl.Acad.Sci.U.S.A 2007, 104: 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sawin ER, Ranganathan R, Horvitz HR: C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 2000, 26: 619–631. [DOI] [PubMed] [Google Scholar]
- [38].Stein GM, Murphy CT: C. elegans positive olfactory associative memory is a molecularly conserved behavioral paradigm. Neurobiol.Learn.Mem 2014, 115: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tran A, Tang A, O'Loughlin CT, Balistreri A, Chang E, Coto Villa D, Li J, Varshney A, Jimenez V, Pyle J, et al. : C. elegans avoids toxin-producing Streptomyces using a seven transmembrane domain chemosensory receptor. Elife 2017, 6: 10.7554/eLife.23770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Behringer DC, Butler MJ, Shields JD: Ecology: avoidance of disease by social lobsters. Nature 2006, 441: 421. [DOI] [PubMed] [Google Scholar]
- [41].Boillat M, Challet L, Rossier D, Kan C, Carleton A, Rodriguez I: The vomeronasal system mediates sick conspecific avoidance. Curr.Biol 2015, 25: 251–255. [DOI] [PubMed] [Google Scholar]
- [42].Sarabian C, Curtis V, McMullan R: Evolution of pathogen and parasite avoidance behaviours. Philos.Trans.R.Soc.Lond.B.Biol.Sci 2018, 373: 10.1098/rstb.2017.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rubio-Godoy M, Aunger R, Curtis V: Serotonin--a link between disgust and immunity?. Med.Hypotheses 2007, 68: 61–66. [DOI] [PubMed] [Google Scholar]
- [44].Riviere S, Challet L, Fluegge D, Spehr M, Rodriguez I: Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature 2009, 459: 574–577. [DOI] [PubMed] [Google Scholar]
- [45].Hao Y, Yang W, Ren J, Hall Q, Zhang Y, Kaplan JM: Thioredoxin shapes the C. elegans sensory response to Pseudomonas produced nitric oxide. Elife 2018, 7: 10.7554/eLife.36833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zheng Z, Lauritzen JS, Perlman E, Robinson CG, Nichols M, Milkie D, Torrens O, Price J, Fisher CB, Sharifi N, et al. : A Complete Electron Microscopy Volume of the Brain of Adult Drosophila melanogaster. Cell 2018, 174: 743.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Economo MN, Clack NG, Lavis LD, Gerfen CR, Svoboda K, Myers EW, Chandrashekar J: A platform for brain-wide imaging and reconstruction of individual neurons. Elife 2016, 5: e10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Baganz NL, Blakely RD: A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem.Neurosci 2013, 4: 48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velazquez MA, Garces-Alvarez ME, Hurtado-Alvarado G, Quintero-Fabian S, Pavon L: Immunomodulatory effects mediated by serotonin. J.Immunol.Res 2015, 2015: 354957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Costedio MM, Coates MD, Danielson AB, Buttolph TR 3rd, Blaszyk HJ, Mawe GM, Hyman NH: Serotonin signaling in diverticular disease. J.Gastrointest.Surg 2008, 12: 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Margolis KG, Stevanovic K, Li Z, Yang QM, Oravecz T, Zambrowicz B, Jhaver KG, Diacou A, Gershon MD: Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 2014, 63: 928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA, Mawe GM: Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol.Motil 2005, 17: 565–574. [DOI] [PubMed] [Google Scholar]
- [53].Coleman NS, Foley S, Dunlop SP, Wheatcroft J, Blackshaw E, Perkins AC, Singh G, Marsden CA, Holmes GK, Spiller RC: Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin.Gastroenterol.Hepatol 2006, 4: 874–881. [DOI] [PubMed] [Google Scholar]
- [54].McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ: Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J.Neuroimmunol 2002, 132: 34–40. [DOI] [PubMed] [Google Scholar]
- [55].Gaskill PJ, Carvallo L, Eugenin EA, Berman JW: Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. J.Neuroinflammation 2012, 9: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yamazaki M, Matsuoka T, Yasui K, Komiyama A, Akabane T: Dopamine inhibition of superoxide anion production by polymorphonuclear leukocytes. J.Allergy Clin.Immunol 1989, 83: 967–972. [DOI] [PubMed] [Google Scholar]
- [57].Wenisch C, Parschalk B, Weiss A, Zedwitz-Liebenstein K, Hahsler B, Wenisch H, Georgopoulos A, Graninger W: High-dose catecholamine treatment decreases polymorphonuclear leukocyte phagocytic capacity and reactive oxygen production. Clin.Diagn.Lab.Immunol 1996, 3: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sookhai S, Wang JH, Winter D, Power C, Kirwan W, Redmond HP: Dopamine attenuates the chemoattractant effect of interleukin-8: a novel role in the systemic inflammatory response syndrome. Shock 2000, 14: 295–299. [DOI] [PubMed] [Google Scholar]
- [59].Trabold B, Gruber M, Frohlich D: Functional and phenotypic changes in polymorphonuclear neutrophils induced by catecholamines. Scand.Cardiovasc.J. 2007, 41: 59–64. [DOI] [PubMed] [Google Scholar]