SUMMARY
Molecular mimicry is a common mechanism used by many bacteria to evade immune responses. In recent years, it has become evident that bacteria also decorate the extracellular matrix of their biofilms with molecules that resemble those of the host. These molecules include amyloids and other proteins, polysaccharides, and extracellular DNA. Bacterial amyloids, like curli, and extracellular DNA are found in the biofilms of many species. Recent work demonstrated that curli and DNA form unique molecular structures that are recognized by the immune system causing activation of autoimmune pathways. Although a variety of mechanisms have been suggested as the means by which infections initiate and/or exacerbate autoimmune diseases, the mechanism remains unknown. In this article, we discuss recent work on biofilms that highlight the role of amyloids as a carrier for DNA and potentiator of autoimmune responses and propose a novel link between bacterial infections and autoimmune diseases.
Keywords: amyloid, curli, biofilm, autoimmune response, infection, lupus, SLE
Infections and autoimmune diseases
Chronic autoimmune diseases occur when the immune system recognizes self-antigens as foreign, leading to inflammation and destruction of specific tissues and organs. Autoimmunity was first recognized by a famous physician, Paul Ehrlich, in the early 1900s. He realized that the powerful effector mechanisms used in host defense could, if turned against the host, cause severe tissue damage; Ehrlich termed this horror autotoxicus. To protect itself from an autoimmune response, the body uses a technique called self-tolerance in which T cells go through a negative selection process. Not all self-reactive T cells are removed, however, and the remaining self-reactive T cells must be regulated to avoid an autoimmune response. Dysregulation of these T cells in the periphery can lead to autoimmune responses throughout the body, the reaction first described by Ehrlich.
There is evidence that infectious agents can trigger gut or systemic autoimmune diseases. However, opposing evidence, primarily from animal models of autoimmunity, suggest that many autoimmune disorders occur through internal dysregulation of the immune system without the participation of infectious agents. It is now accepted that susceptibility to autoimmune diseases is multifactorial and that the interaction between genetic and environmental factors underlies disease progression. Different genetic factors are associated with susceptibility to autoimmune disease, and various environmental factors feed into breaking tolerance to self-antigens in the susceptible host. Infections are considered to be one of these environmental factors. How infections contribute to the disease onset or flares of many autoimmune diseases remains a mystery, however. Within this review, we will discuss how amyloid-expressing enteric bacteria drive autoimmune reactions during infections. Amyloids and DNA that potentially trigger autoimmune responses are expressed by many important human pathogens such as Escherichia coli, Borrelia burgdorferi, Salmonella enterica serovar Typhimurium, Mycobacterium tuberculosis, Pseudomonas aeruginosa, and Staphylococcus aureus. It is important to note that the bacteria discussed here with the exception of curli-producing E. coli and S. Typhimurium as well as PSM producing S. aureus have not been shown to produce amyloid/DNA complexes. Many of these bacteria produce amyloids and eDNA in their biofilms in vitro. However, the production of amyloids as part of a biofilm during the human infection and their interactions with DNA leading to autoimmunity needs further investigation.
Autoimmune sequelae following bacterial infections
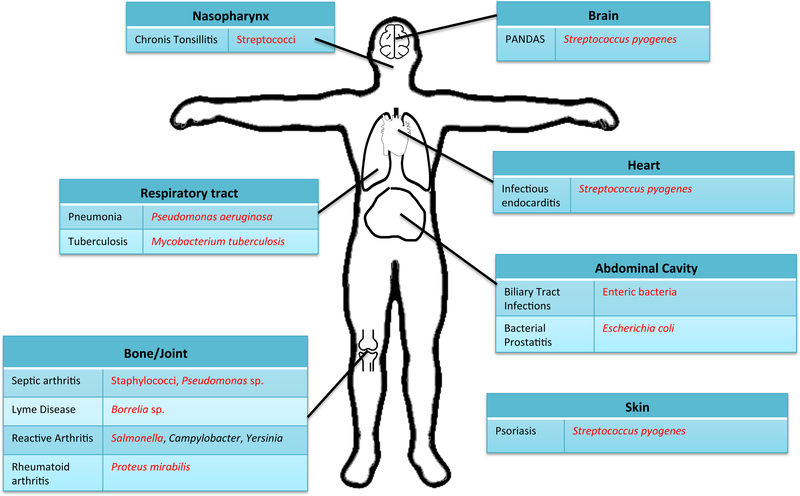
There are many examples of diseases in which bacterial infections initiate or exacerbate autoimmune responses (Figure 1). One of the well-described autoimmune conditions that develop in response to an infection is reactive arthritis (ReA), also known as post-infectious arthritis or ankylosing spondylitis. ReA is a painful form of inflammatory arthritis caused after gastrointestinal infections with enteric pathogens such as Salmonella, Shigella, Yersinia, or Campylobacter or after genital infection with Chlamydia trachomatis (1). ReA usually starts one to four weeks post infection. ReA affect the joints, and many patients also develop eye inflammation (conjunctivitis) and urinary problems (urethritis). ReA affects approximately 5% of patients following gastrointestinal infections, and some patients remain symptomatic for 5 years or longer. ReA has traditionally been thought of as a “sterile” arthritis because antibiotic treatment in individuals with ReA is ineffective (2, 3) and cultures of joint fluids yield no growth of organisms. Recent evidence suggests, however, that Chlamydia species can become persistent in joint and synovial tissue (4, 5) of patients with ReA, and immunocytochemical staining and mass spectrometry has demonstrated the presence of enteric bacterial products in the synovial fluid, further implicating microorganisms in the inflammatory process (6–8).
Figure 1:
Human infections followed by autoimmune sequelae. Shown are human infections with their associated causative bacteria. Each line links the infection to the appropriate site of infection on the body. Amyloid-producing bacteria are marked in red.
Genetic variations and differences in etiology make treatment of ReA difficult. For example, the histocompatibility leukocyte antigen (HLA)-B27 genotype is a risk factor for ReA, and over two-thirds of the patients with ReA carry the HLA-B27 genotype. Those patients negative for HLA-B27 are frequently positive for cross-reacting antigens such as HLA-B7, HLA-B22, HLA-B40, or HLA-B42 (9). It has been suggested that the bacteria express antigens that react with HLA-B27, but it is still unclear why HLA-B27 predisposes individuals to ReA.
In addition to ReA, there are other examples of arthritis likely induced by bacterial infection that are not associated with HLA-B27. Post-streptococcal arthritis is a poorly understood clinical syndrome. It is not clear whether post-streptococcal arthritis is a separate entity or a condition that presents with rheumatic fever. Group A streptococci colonize the throat or skin and are responsible for various infections and post-streptococcal immune sequelae. These bacteria are the most common cause of pharyngitis in school-aged children. Rheumatic fever or rheumatic heart disease is an important autoimmune sequelae that follow pharyngitis. Molecular mimicry by M-protein and the production of cross-reactive antibodies are important for the pathogenesis. Antibodies that recognize the M protein and the N-acetyl-β-D-glucosamine of S. pyogenes cross-react with myosin leading to heart damage (10, 11). Some of the rheumatic heart disease patients also experience arthritis post-infection. However, the mechanisms that trigger arthritis is not known.
Recently, streptococcal carriage has been proposed to have a role in a human skin autoimmune disease, psoriasis. An interesting connection was discovered when researchers found that tonsillectomy provided relief of symptoms in psoriasis patients (12). Streptococcal biofilms that cause chronic infections of the tonsils are thought to exacerbate psoriatic symptoms. This was supported by data showing that patients with moderate to severe chronic plaque psoriasis have shown improvement in skin lesions upon long-term penicillin treatment (12, 13).
The pediatric autoimmune neuropsychiatric disorders associated with streptococci, or PANDAS, is a neurological response seen in children after they have been treated for a group A streptococcal infection (strep throat) (14). Patients present with an obsessive compulsive or tic disorder and express anti-neuronal antibodies against dopamine receptors (14). This condition is one of very few examples of an autoimmune reaction within the brain, most often thought of as an immune privileged area of the body. This provides evidence for an axis of interaction between the peripheral streptococcal infection and the neurological response.
Another example of autoimmunity induced by bacterial infections is Lyme disease. Lyme disease is a tick-borne disease caused mainly by the B. burgdorferi bacterium though other Borrelia species have been found to cause the disease in rare cases. If untreated, Borrelia species spread throughout the body and cause arthritis and nervous system problems as well as pain and fatigue. Although quite controversial, biofilms of Borrelia were detected in a recent study and proposed to be responsible for the persistent infection and continuation of disease symptoms (15). Intriguingly, many Lyme disease symptoms overlap with those of systemic lupus erythematosus (SLE), a chronic human autoimmune disease. Furthermore, autoantibodies detected in sera from Lyme disease patents are the same as those observed in SLE patients. For example, Lyme patients, like SLE patients, test positive for auto-nuclear antigens (ANA)(16, 17). Molecular mimicry of γ enolase by Borrelia enolase has been suggested to drive the autoimmune response and more chronic symptoms (18).
Mysteriously, most individuals positive for ANA do not have an autoimmune disease. This brings up the question of whether the generation of autoantibodies post-infection is a common response to bacterial infection. Consistent with this idea, anti-double-stranded DNA antibodies and antibodies to other autoantigens are seen in patients who have had lung infections caused by Pseudomonas aeruginosa, biliary tract infections caused by enteric bacteria, and bacterial prostatitis caused by E. coli (19–21). The most controversial infection that has been proposed to be an infection-initiated autoimmune disease is tuberculosis (22).
Mycobacterium tuberculosis infections cause granuloma formation, and patients have a depressed immune response (23). Further, 40% of tuberculosis patients present with autoantibodies normally associated with SLE and Wegener’s granulomatosis (22). Additionally, inflammatory arthritis occurs in some patients even without detectable bacteria within the joint space. Tuberculosis shares symptoms with multiple autoimmune diseases including irritable bowel disease, Crohn’s disease, ankylosing spondylitis, and sarcoidosis to name a few (22, 24, 25). Sarcoidosis diagnoses often mirror those of tuberculosis, the only difference being a lack of mycobacterium presence in tissues (22, 25). The similarities between these disease states indicate that similar host molecules are at play in these conditions.
Intriguingly, of all the bacteria implicated in the diseases described above, most have been shown to express amyloids, a type of molecule that the immune system recognizes as a conserved molecular pattern (Figure 1). Though the production of amyloids during human infection remains speculative, increasing evidence suggest that amyloids maybe the link between infections and autoimmunity. Regardless of their amino acid sequence, amyloids are characterized by their conserved beta-sheet fibrillar structure(26). It is this conserved structure that is recognized by the innate immune receptors such as the toll-like receptor (TLR)2/TLR1 heterocomplex and Nod-like receptor protein 3 (NLRP3) (27, 28). Bacteria produce amyloids as part of their biofilm introducing a common molecular pattern for host recognition. In most cases, it remains unclear whether bacterial amyloids are expressed during the infections that trigger autoimmune reactions.
Bacterial amyloids: survival strategy and molecular mimicry
In a biofilm, a group of bacteria are encapsulated in a three-dimensional, self-produced extracellular matrix (ECM) that adheres to abiotic or biotic surfaces (29, 30). Bacteria produce biofilms to protect the population from environmental insults including antimicrobials, physical sheer stress, or the immune response. Intriguingly, the biofilm ECM makes up 85% of the biofilm mass. Bacterial amyloid proteins that possess a conserved cross beta-sheet structure serve as a strong scaffold for the ECM (31, 32). Amyloids are highly resistant to chemical, proteolytic, and enzymatic degradation, and therefore provide a strong scaffold for the community and act almost like a shield under stress (33, 34). Moreover, amyloids are expressed also by humans (35–37); therefore, bacteria may use these molecules to hide the bacterial community from the immune system.
Depending on the habitat, between 5 and 40% of species isolated from environmental biofilms produce amyloids, demonstrating the widespread distribution of this extracellular structure (38). Curli fibers are amyloids produced in the biofilms of enteric bacteria in the environment as well as during infections (39). Curli has high structural and functional similarity to human amyloids (33, 39–41). Although expression, function, and immune activation by E. coli and Salmonella curli species are well characterized, in vivo expression of curli by clinical enteric strains has yet to be described. Phylogenetic analysis suggests that the curli assembly machinery is widespread, as homologs of the csg genes, which encode curli and are responsible for its biosynthesis and secretion, are found within four phylum, Bacteroidetes, Proteobacter, Firmicutes, and Thermodesulfobacteria, as well as nine different classes of sequenced bacteria (42). Consistent with these findings many pathogenic bacteria associated with autoimmune sequelae post-infection produce amyloid proteins. Staphylococci produce phenol-soluble modulins and Bap amyloids; Streptococcus mutans produce amyloidogenic P1, WapA, and SMU_63c; Pseudomonas sp. produce FapC amyloid; and B. burgdorferi produces OspA amyloid (43–49). As S. mutans produces amyloid, it is thought that other Streptococcus sp. may be producing amyloids as well. Intriguingly, M. tuberculosis has been reported to express a curli-like amyloid during human infection (48); however, not much is known about this protein.
OspA protein is an amyloid protein and a major virulence factor expressed by B. burgdorferi, the pathogen that causes Lyme disease. The importance of this protein during infection is highlighted by studies showing anti-OspA treatment is protective against infection in mice (50). Not only is this protein necessary for infection, but it is also used by Borrelia sp. as a molecular mimic for adhesion molecule LFA-1α (51). LFA-1α is a partial agonist of antibodies against OspA, and it exacerbates autoimmune symptoms (52). Finally, sequence similarity between B. burgdorferi OspA and S. pyogenes M5 proteins have been reported (53). These findings suggest that bacteria produce molecules similar to host molecules to mitigate the immune onslaught.
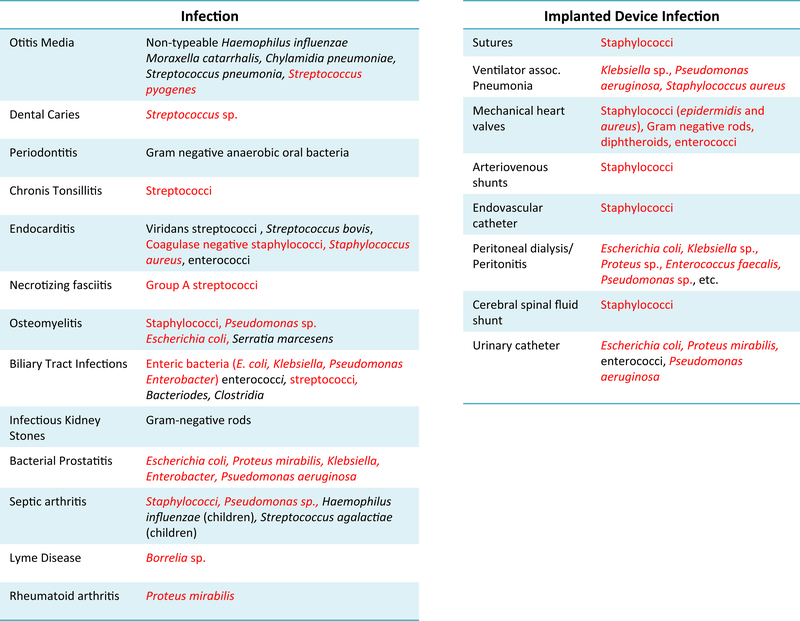
In the bigger picture of infections involving biofilms, the National Institutes of Health reported that among acute and chronic microbial infections, 65% and 80%, respectively, are associated with biofilm formation(54). Infections associated with biofilms include urinary tract infections, middle-ear infections, bone and joint infections, dental caries, periodontitis, endocarditis, chronic tonsillitis, cystic fibrosis, bacterial prostitis, biliary tract infections, and infections of persistent indwelling devices such as heart valves and joint prostheses. Many urinary tract infections and bloodstream infections are caused due to biofilms associated with indwelling medical devices (55–57). Among the long list of bacteria that cause biofilm-associated infections, amyloid-producing bacteria make up a significant number (Figure 2), and the number of amyloid-producing bacteria may be underestimated. Amyloids do not share a conserved amino acid sequence, therefore it is hard to determine if a bacterial species produces an amyloid by analysis of gene sequences. One common method to screen for amyloid production by bacteria is to supplement growth media with Congo Red, an amyloid indicator dye. Amyloid production is indicated by a red colony phenotype. Congo red, however has the potential to stain other polysaccharide components of the biofilm and therefore amyloid production can be confirmed with the addition of Coomassie brilliant blue to the plates or with the use of another amyloid specific dye Thioflavin T (38, 58–60). It must also be noted that for most of the bacterial species that are amyloid-producers, the presence of amyloid during infection has not been definitely demonstrated. For instance, although S. aureus produces phenol-soluble modulins or Bap in biofilms generated in vitro in laboratory conditions (49, 61, 62), it is not known whether staphylococci produce these amyloids during infections or whether the repertoire of amyloids influences the type of immune response.
Figure 2:
Biofilm-related infections in humans. Infections of native body tissue are shown in the left table while bodily infections associated with implanted devices are shown in the table on the right. Amyloid-producing bacteria are marked in red text.
Enteric bacterial biofilms: curli, extracellular DNA, and autoimmunity
Although the bacteria produce amyloids as a way to hide from the insults of the immune cells, the immune system in turn recognizes the amyloids as conserved molecular signatures to detect bacterial activity. For instance, curli is recognized by the TLR2/TLR1 complex and the NLRP3 inflammasome (27, 28, 63, 64). It is important to note that toll-like receptors also recognize human amyloids including beta-amyloid, which is present in plaques in the brains of patients with Alzheimer’s disease, and serum amyloid A (SAA). Studies suggest that the TLR2/TLR1 heterocomplex is a major innate immune receptor that recognizes the fibrillar beta sheet structure of bacterial amyloid curli, human beta-amyloid, and human SAA. The NLRP3 inflammasome also recognizes beta-amyloid and SAA (64–68). Amyloids produced by other bacteria are likely also bound by the TLR2/TLR1 complex and NLRP3 inflammasome due to their common fibrillar beta-sheet structure. Thus, amyloids may mimic host molecules to hide from the immune system. In return, the immune system recognizes these molecules regardless of their origin, which may initiate the autoimmune responses.
In addition to amyloids, carbohydrates and extracellular DNA are major constituents of the ECM of biofilms (32, 39, 69). For example, cellulose produced by enteric bacteria is integrated into the biofilm ECM providing additional structural support and protection. A recent study demonstrated that curli and bacterial DNA form complexes in enteric biofilms that are highly proinflammatory compared to curli alone or DNA alone (70). In fact, curli/DNA complexes activate multiple innate immune receptors in various compartments of an immune cell: First, curli activates the TLR2/TLR1 heterocomplex on the membrane (28, 63, 64), and then internalization of this complex brings curli/DNA into the endosome to activate the DNA receptor TLR9 (71), followed by cytosolic escape of fibrillar curli, which activates NLRP3 (27). Structural analysis suggests that the curli fibers organize DNA with an inter-DNA spacing that promotes effective, multivalent TLR9 binding and activation (71).
Lipopolysaccharide (LPS) and flagellin are thought to be the dominant molecular signatures of enteric bacteria recognized by the immune system. However, recent studies show that in the case of a biofilm, curli/DNA complexes serve as the dominant molecular signature that triggers the autoimmune response (70). Since bacteria decorate the ECM with molecules that resemble the host, it is not surprising that bacterial biofilms that harbor curli and DNA elicit an autoimmune response. In murine models, infection with either commensal E. coli or pathogenic S. enterica serovar Typhimurium leads to elevated levels of antibodies to double-stranded DNA and chromatin as well as positive ANA results, and these responses require expression of curli by the bacteria (70). Injection of purified curli/DNA complexes triggers similar responses in mice that are dependent on the activation of TLR2 and TLR9. Thus, amyloid curli serves as a carrier for DNA and the generation of subsequent DNA-related responses (71).
The elevation of autoantibodies induced by curli/DNA complexes occurs both in lupus-prone and in wild-type mice (70). Therefore, it is possible that the autoimmunity is triggered by biofilm-related infections even in individuals without an autoimmune disease, although this autoimmunity may be resolved over time by the competent immune system. If, however, the individual is already predisposed to an autoimmune disease by genetic factors and there is exposure to the biofilm for a long period of time, biofilm-related infections may contribute to disease progression or flares. We speculate that an autoimmune response driven by curli/DNA complexes contributes to the development of ReA following enteric infections. It is possible that during a gastroenteritis outbreak caused by enteric bacteria, individuals with the HLA-B27 genotype will develop an exacerbated immune response and ReA, whereas those with other HLA genotypes experience an autoimmune response that is moderate or transient. Although these connections are speculative, they warrant further investigation.
Systemic lupus erythematosus (SLE)
Generation of autoantibodies, including anti-double-stranded DNA antibodies, and the type I interferon response are hallmarks of the human autoimmune disease SLE (70). SLE is considered a prototypical autoimmune disease; 80% of SLE patients develop joint and muscle pain, skin rashes, fatigue, and a general feeling of being unwell. SLE is often mistaken for other diseases. An anti-nuclear autoantibody (ANA) test is used to confirm the presence of autoantibodies to aid in diagnosis (72). Intriguingly, patients who eventually develop SLE show positivity for anti-double-stranded DNA and anti-chromatin years before the diagnosis (73).
Infections are the most common causes of hospitalization and mortality in SLE patients. The frequencies of infections in SLE patients, such as pneumonia, sepsis, skin infections, and urinary tract infections, are similar to those of the general population and are caused by both Gram-positive and Gram-negative bacteria (74). However, infections (both viral and bacterial) trigger flares in SLE patients. During a lupus flare the most common complaints are of flu-like symptoms (with or without fever), fatigue, and muscle and joint pains. However, continual flare-ups negatively affect various organs, including kidneys and lungs, and can eventually lead to organ failure and death (75).
Genome-wide association studies suggest an involvement of toll-like receptors in the pathogenesis of SLE (76). It is well established that DNA triggers autoimmune responses and contributes to the pathogenesis of SLE via the activation of TLR9 (77). Although polymorphisms of TLR2 have no correlation with predisposition to SLE, peripheral blood mononuclear cells (PBMCs) from lupus patients express higher levels of TLR2 than PBMCs from healthy controls (76), suggesting that SLE patients may react more strongly to TLR2 ligands than healthy individuals.
SLE patients have elevated levels of LPS in systemic circulation, suggesting that the immune systems of these patients are frequently exposed to bacterial products (78). Consistent with this, infection with S. Typhimurium or E. coli causes bacteremia, sepsis, or soft tissue infections in SLE patients instead of the gastroenteritis and urinary tract infections common in otherwise healthy individuals. This shows how the altered immune system of these patients can influence the location where bacteria establish an infection. The fact that curli/DNA complexes from enteric biofilms as well as several other infections by amyloid-producing bacteria lead to positive ANA results suggests that there may be a link between the infections and autoimmunity.
Biofilms on implanted devices and autoimmunity
Bacterial biofilms on implanted devices are difficult to treat, often requiring additional surgery or implant removal. Many bacteria that colonize implanted devices have the capacity to produce amyloids (Figure 2). There have been recent reports of patients who have undergone transvaginal mesh surgeries or hernia surgeries who have developed autoimmune diseases, including SLE, following surgery. It has been speculated that biofilms associated with the mesh break tolerance and trigger autoimmunity in these patients (79–82). Consistent with this, recent work showed the presence of multiple species in biofilms on implanted mesh (83, 84). The connection between implants colonized with biofilms and autoimmune disease remains controversial, as some studies have shown no direct correlation between infected mesh implants and autoimmune disease onset. More mechanistic studies as well as epidemiological studies are needed to determine the complex immune reactions to biofilms on implanted devices in the future.
Concluding Remarks
Bacteria use biofilms as a hideout from the immune system to establish successful infections. Independent studies show that resolution of many infections are complicated by autoimmune sequelae for unknown reasons. Recent evidence showed that the molecules used by bacteria to decorate the extracellular matrix of the biofilms, e.g. amyloid/DNA complexes, generates autoimmune responses during infections in animal models. It is thought that while biofilm-associated infections trigger a transient autoimmune response by bacterial amyloid/DNA complexes in healthy individuals, this response may be sustained and detrimental in individuals genetically predisposed to an autoimmune disease. However, at this point the role of biofilm-associated infections as a trigger and modulator for human autoimmune diseases remains to be formally proven (see Outstanding Questions). Epidemiological studies that delve into the clinical history of patients and consider biofilm associated-infections or possible exposure to bacterial amyloids as a risk factor to develop autoimmune diseases are needed. More importantly, new practices to ensure the eradication of biofilms are needed to prevent the chronic exposure to biofilms. This will prevent the long-term exposure of the immune system to the biofilm products such as amyloid/DNA complexes that are capable of breaking immune tolerance.
Outstanding Questions.
Are bacterial biofilms responsible for autoimmune sequelae post-infection?
Do biofilm associated bacterial infections accelerate autoimmune disease onset and contribute to flares?
Do both Gram-negative and Gram-positive bacterial amyloids complex with DNA that trigger autoimmune reactions?
Are bacterial amyloid-like proteins expressed during many bacterial infections?
Do amyloids carry bacterial molecules other than DNA that can trigger autoimmunity?
Highlights.
Autoimmune responses are generated during many bacterial infections. Most bacteria that cause autoimmune sequelae post-infection produce amyloid proteins.
Amyloids are detected in biofilms produced by many bacterial species.
The incorporation of amyloid and DNA into the biofilm extracellular matrix enhances biofilm stability.
Amyloid/DNA complexes are dominant molecular signatures that the immune system recognizes.
Systemic exposure to amyloid/DNA complexes lead to an autoimmune response via TLR2 and TLR9.
Biofilm associated infections have the potential to generate autoimmunity via presentation of amyloid/DNA complexes to the immune system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carter JD, Hudson AP. 2009. Reactive arthritis: clinical aspects and medical management. Rheum Dis Clin North Am 35:21–44. [DOI] [PubMed] [Google Scholar]
- 2.Carter JD, Espinoza LR, Inman RD, Sneed KB, Ricca LR, Vasey FB, Valeriano J, Stanich JA, Oszust C, Gerard HC, Hudson AP. 2010. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum 62:1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kvien TK, Gaston JS, Bardin T, Butrimiene I, Dijkmans BA, Leirisalo-Repo M, Solakov P, Altwegg M, Mowinckel P, Plan PA, Vischer T, Eular. 2004. Three month treatment of reactive arthritis with azithromycin: a EULAR double blind, placebo controlled study. Ann Rheum Dis 63:1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter JD, Hudson AP. 2010. The evolving story of Chlamydia-induced reactive arthritis. Curr Opin Rheumatol 22:424–430. [DOI] [PubMed] [Google Scholar]
- 5.Gerard HC, Branigan PJ, Schumacher HR Jr., Hudson AP. 1998. Synovial Chlamydia trachomatis in patients with reactive arthritis/Reiter’s syndrome are viable but show aberrant gene expression. J Rheumatol 25:734–742. [PubMed] [Google Scholar]
- 6.Nikkari S, Merilahti-Palo R, Saario R, Soderstrom KO, Granfors K, Skurnik M, Toivanen P. 1992. Yersinia-triggered reactive arthritis. Use of polymerase chain reaction and immunocytochemical staining in the detection of bacterial components from synovial specimens. Arthritis Rheum 35:682–687. [DOI] [PubMed] [Google Scholar]
- 7.Nikkari S, Rantakokko K, Ekman P, Mottonen T, Leirisalo-Repo M, Virtala M, Lehtonen L, Jalava J, Kotilainen P, Granfors K, Toivanen P. 1999. Salmonella-triggered reactive arthritis: use of polymerase chain reaction, immunocytochemical staining, and gas chromatography-mass spectrometry in the detection of bacterial components from synovial fluid. Arthritis Rheum 42:84–89. [DOI] [PubMed] [Google Scholar]
- 8.Sibilia J, Limbach FX. 2002. Reactive arthritis or chronic infectious arthritis? Ann Rheum Dis 61:580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasution AR, Mardjuadi A, Kunmartini S, Suryadhana NG, Setyohadi B, Sudarsono D, Lardy NM, Feltkamp TE. 1997. HLA-B27 subtypes positively and negatively associated with spondyloarthropathy. J Rheumatol 24:1111–1114. [PubMed] [Google Scholar]
- 10.Cunningham MW. 2012. Streptococcus and rheumatic fever. Curr Opin Rheumatol 24:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham MW, McCormack JM, Fenderson PG, Ho MK, Beachey EH, Dale JB. 1989. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J Immunol 143:2677–2683. [PubMed] [Google Scholar]
- 12.Allen HB, Jadeja S, Allawh RM, Goyal K. 2018. Psoriasis, chronic tonsillitis, and biofilms: Tonsillar pathologic findings supporting a microbial hypothesis. Ear Nose Throat J 97:79–82. [DOI] [PubMed] [Google Scholar]
- 13.Saxena VN, Dogra J. 2005. Long-term use of penicillin for the treatment of chronic plaque psoriasis. Eur J Dermatol 15:359–362. [PubMed] [Google Scholar]
- 14.Cunningham MW. 2016. Post-Streptococcal Autoimmune Sequelae: Rheumatic Fever and Beyond. In Ferretti JJ, Stevens DL, Fischetti VA (ed), Streptococcus pyogenes : Basic Biology to Clinical Manifestations, Oklahoma City (OK). [Google Scholar]
- 15.Sapi E, Balasubramanian K, Poruri A, Maghsoudlou JS, Socarras KM, Timmaraju AV, Filush KR, Gupta K, Shaikh S, Theophilus PA, Luecke DF, MacDonald A, Zelger B. 2016. Evidence of In Vivo Existence of Borrelia Biofilm in Borrelial Lymphocytomas. Eur J Microbiol Immunol (Bp) 6:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Federlin K, Becker H. 1989. [Borrelia infection and systemic lupus erythematosus]. Immun Infekt 17:195–198. [PubMed] [Google Scholar]
- 17.Singh SK, Girschick HJ. 2004. Lyme borreliosis: from infection to autoimmunity. Clin Microbiol Infect 10:598–614. [DOI] [PubMed] [Google Scholar]
- 18.Maccallini P, Bonin S, Trevisan G. 2018. Autoimmunity against a glycolytic enzyme as a possible cause for persistent symptoms in Lyme disease. Med Hypotheses 110:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Carter CJ. 2011. Pathogen and autoantigen homologous regions within the cystic fibrosis transmembrane conductance regulator (CFTR) protein suggest an autoimmune treatable component of cystic fibrosis. FEMS Immunol Med Microbiol 62:197–214. [DOI] [PubMed] [Google Scholar]
- 20.Gershwin ME, Mackay IR. 2008. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology 47:737–745. [DOI] [PubMed] [Google Scholar]
- 21.Quick ML, Wong L, Mukherjee S, Done JD, Schaeffer AJ, Thumbikat P. 2013. Th1-Th17 cells contribute to the development of uropathogenic Escherichia coli-induced chronic pelvic pain. PLoS One 8:e60987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkington P, Tebruegge M, Mansour S. 2016. Tuberculosis: An Infection-Initiated Autoimmune Disease? Trends Immunol 37:815–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagan AJ, Ramakrishnan L. 2014. Immunity and Immunopathology in the Tuberculous Granuloma. Cold Spring Harb Perspect Med 5: a018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chodisetti SB, Rai PK, Gowthaman U, Pahari S, Agrewala JN. 2012. Potential T cell epitopes of Mycobacterium tuberculosis that can instigate molecular mimicry against host: implications in autoimmune pathogenesis. BMC Immunol 13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maertzdorf J, Weiner J 3rd, Mollenkopf HJ, Network TB, Bauer T, Prasse A, Muller-Quernheim J, Kaufmann SH. 2012. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci U S A 109:7853–7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toyama BH, Weissman JS. 2011. Amyloid structure: conformational diversity and consequences. Annu Rev Biochem 80:557–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rapsinski GJ, Wynosky-Dolfi MA, Oppong GO, Tursi SA, Wilson RP, Brodsky IE, Tukel C. 2015. Toll-Like Receptor 2 and NLRP3 Cooperate To Recognize a Functional Bacterial Amyloid, Curli. Infect Immun 83:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tukel C, Nishimori JH, Wilson RP, Winter MG, Keestra AM, van Putten JP, Baumler AJ. 2010. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cell Microbiol 12:1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costerton JW. 1999. Introduction to biofilm. Int J Antimicrob Agents 11:217–221; discussion 237–219. [DOI] [PubMed] [Google Scholar]
- 30.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. 1995. Microbial biofilms. Annu Rev Microbiol 49:711–745. [DOI] [PubMed] [Google Scholar]
- 31.Hung C, Zhou Y, Pinkner JS, Dodson KW, Crowley JR, Heuser J, Chapman MR, Hadjifrangiskou M, Henderson JP, Hultgren SJ. 2014. Escherichia coli biofilms have an organized and complex extracellular matrix structure. MBio 4:e00645–00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCrate OA, Zhou X, Reichhardt C, Cegelski L. 2013. Sum of the parts: composition and architecture of the bacterial extracellular matrix. J Mol Biol 425:4286–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hufnagel DA, Tukel C, Chapman MR. 2013. Disease to dirt: the biology of microbial amyloids. PLoS Pathog 9:e1003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collinson SK, Emody L, Muller KH, Trust TJ, Kay WW. 1991. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J Bacteriol 173:4773–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knowles TP, Vendruscolo M, Dobson CM. 2014. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol 15:384–396. [DOI] [PubMed] [Google Scholar]
- 36.Westermark GT, Johnson KH, Westermark P. 1999. Staining methods for identification of amyloid in tissue. Methods Enzymol 309:3–25. [DOI] [PubMed] [Google Scholar]
- 37.Maury CP. 2009. The emerging concept of functional amyloid. J Intern Med 265:329–334. [DOI] [PubMed] [Google Scholar]
- 38.Larsen P, Nielsen JL, Dueholm MS, Wetzel R, Otzen D, Nielsen PH. 2007. Amyloid adhesins are abundant in natural biofilms. Environ Microbiol 9:3077–3090. [DOI] [PubMed] [Google Scholar]
- 39.Tursi SA, Tukel C. 2018. Curli-Containing Enteric Biofilms Inside and Out: Matrix Composition, Immune Recognition, and Disease Implications. Microbiol Mol Biol Rev 82: e00028–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnhart MM, Chapman MR. 2006. Curli biogenesis and function. Annu Rev Microbiol 60:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ. 2002. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295:851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dueholm MS, Albertsen M, Otzen D, Nielsen PH. 2012. Curli functional amyloid systems are phylogenetically widespread and display large diversity in operon and protein structure. PLoS One 7:e51274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehlin C, Headley CM, Klebanoff SJ. 1999. An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J Exp Med 189:907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lasa I, Penades JR. 2006. Bap: a family of surface proteins involved in biofilm formation. Res Microbiol 157:99–107. [DOI] [PubMed] [Google Scholar]
- 45.Besingi RN, Wenderska IB, Senadheera DB, Cvitkovitch DG, Long JR, Wen ZT, Brady LJ. 2017. Functional amyloids in Streptococcus mutans, their use as targets of biofilm inhibition and initial characterization of SMU_63c. Microbiology 163:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dueholm MS, Petersen SV, Sonderkaer M, Larsen P, Christiansen G, Hein KL, Enghild JJ, Nielsen JL, Nielsen KL, Nielsen PH, Otzen DE. 2010. Functional amyloid in Pseudomonas. Mol Microbiol 77:1009–1020. [DOI] [PubMed] [Google Scholar]
- 47.Ohnishi S, Koide A, Koide S. 2001. The roles of turn formation and cross-strand interactions in fibrillization of peptides derived from the OspA single-layer beta-sheet. Protein Sci 10:2083–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alteri CJ, Xicohtencatl-Cortes J, Hess S, Caballero-Olin G, Giron JA, Friedman RL. 2007. Mycobacterium tuberculosis produces pili during human infection. Proc Natl Acad Sci U S A 104:5145–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. 2012. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog 8:e1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Esquivel R, Flingai S, Schiller ZA, Kern A, Agarwal S, Chu J, Patel A, Sullivan K, Wise MC, Broderick KE, Hu L, Weiner DB, Klempner MS. 2019. Anti-OspA DNA-Encoded Monoclonal Antibody Prevents Transmission of Spirochetes in Tick Challenge Providing Sterilizing Immunity in Mice. J Infect Dis 219:1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pancewicz SA, Rutkowski R, Rutkowski K, Zajkowska JM, Kondrusik M. 2007. [Immunopathology of Lyme arthritis]. Pol Merkur Lekarski 23:141–144. [PubMed] [Google Scholar]
- 52.Trollmo C, Meyer AL, Steere AC, Hafler DA, Huber BT. 2001. Molecular mimicry in Lyme arthritis demonstrated at the single cell level: LFA-1 alpha L is a partial agonist for outer surface protein A-reactive T cells. J Immunol 166:5286–5291. [DOI] [PubMed] [Google Scholar]
- 53.Raveche ES, Schutzer SE, Fernandes H, Bateman H, McCarthy BA, Nickell SP, Cunningham MW. 2005. Evidence of Borrelia autoimmunity-induced component of Lyme carditis and arthritis. J Clin Microbiol 43:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Nawaz MA, Hussain T, Ali M, Rafiq M, Kamil MA. 2018. Bacterial biofilm and associated infections. J Chin Med Assoc 81:7–11. [DOI] [PubMed] [Google Scholar]
- 55.Donlan RM. 2001. Biofilms and device-associated infections. Emerg Infect Dis 7:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fux CA, Costerton JW, Stewart PS, Stoodley P. 2005. Survival strategies of infectious biofilms. Trends Microbiol 13:34–40. [DOI] [PubMed] [Google Scholar]
- 57.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. [DOI] [PubMed] [Google Scholar]
- 58.Biesecker SG, Nicastro LK, Wilson RP, Tukel C. 2018. The Functional Amyloid Curli Protects Escherichia coli against Complement-Mediated Bactericidal Activity. Biomolecules 8: E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jonas K, Tomenius H, Kader A, Normark S, Romling U, Belova LM, Melefors O. 2007. Roles of curli, cellulose and BapA in Salmonella biofilm morphology studied by atomic force microscopy. BMC Microbiol 7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larsen P, Nielsen JL, Otzen D, Nielsen PH. 2008. Amyloid-like adhesins produced by floc-forming and filamentous bacteria in activated sludge. Appl Environ Microbiol 74:1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Latasa C, Roux A, Toledo-Arana A, Ghigo JM, Gamazo C, Penades JR, Lasa I. 2005. BapA, a large secreted protein required for biofilm formation and host colonization of Salmonella enterica serovar Enteritidis. Mol Microbiol 58:1322–1339. [DOI] [PubMed] [Google Scholar]
- 62.Peschel A, Otto M. 2013. Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol 11:667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tukel C, Raffatellu M, Humphries AD, Wilson RP, Andrews-Polymenis HL, Gull T, Figueiredo JF, Wong MH, Michelsen KS, Akcelik M, Adams LG, Baumler AJ. 2005. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol Microbiol 58:289–304. [DOI] [PubMed] [Google Scholar]
- 64.Tukel C, Wilson RP, Nishimori JH, Pezeshki M, Chromy BA, Baumler AJ. 2009. Responses to amyloids of microbial and host origin are mediated through Toll-like receptor 2. Cell Host and Microbe 6(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ather JL, Ckless K, Martin R, Foley KL, Suratt BT, Boyson JE, Fitzgerald KA, Flavell RA, Eisenbarth SC, Poynter ME. 2011. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol 187:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng N, He R, Tian J, Ye PP, Ye RD. 2008. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol 181:22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rube CE, Walter J, Heneka MT, Hartmann T, Menger MD, Fassbender K. 2012. TLR2 is a primary receptor for Alzheimer’s amyloid beta peptide to trigger neuroinflammatory activation. J Immunol 188:1098–1107. [DOI] [PubMed] [Google Scholar]
- 68.Niemi K, Teirila L, Lappalainen J, Rajamaki K, Baumann MH, Oorni K, Wolff H, Kovanen PT, Matikainen S, Eklund KK. 2011. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol 186:6119–6128. [DOI] [PubMed] [Google Scholar]
- 69.Thongsomboon W, Serra DO, Possling A, Hadjineophytou C, Hengge R, Cegelski L. 2018. Phosphoethanolamine cellulose: A naturally produced chemically modified cellulose. Science 359:334–338. [DOI] [PubMed] [Google Scholar]
- 70.Gallo PM, Rapsinski GJ, Wilson RP, Oppong GO, Sriram U, Goulian M, Buttaro B, Caricchio R, Gallucci S, Tukel C. 2015. Amyloid-DNA Composites of Bacterial Biofilms Stimulate Autoimmunity. Immunity 42:1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tursi SA, Lee EY, Medeiros NJ, Lee MH, Nicastro LK, Buttaro B, Gallucci S, Wilson RP, Wong GCL, Tukel C. 2017. Bacterial amyloid curli acts as a carrier for DNA to elicit an autoimmune response via TLR2 and TLR9. PLoS Pathog 13:e1006315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sanchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG Jr., Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, et al. 2012. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64:2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munroe ME, Lu R, Zhao YD, Fife DA, Robertson JM, Guthridge JM, Niewold TB, Tsokos GC, Keith MP, Harley JB, James JA. 2016. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis 75:2014–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zandman-Goddard G, Shoenfeld Y. 2003. SLE and infections. Clin Rev Allergy Immunol 25:29–40. [DOI] [PubMed] [Google Scholar]
- 75.Jung JY, Suh CH. 2017. Infection in systemic lupus erythematosus, similarities, and differences with lupus flare. Korean J Intern Med 32:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu YW, Tang W, Zuo JP. 2015. Toll-like receptors: potential targets for lupus treatment. Acta Pharmacol Sin 36:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Celhar T, Magalhaes R, Fairhurst AM. 2012. TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunol Res 53:58–77. [DOI] [PubMed] [Google Scholar]
- 78.Shi L, Zhang Z, Yu AM, Wang W, Wei Z, Akhter E, Maurer K, Costa Reis P, Song L, Petri M, Sullivan KE. 2014. The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLoS One 9:e93846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chughtai B, Sedrakyan A, Mao J, Eilber KS, Anger JT, Clemens JQ. 2017. Is vaginal mesh a stimulus of autoimmune disease? Am J Obstet Gynecol 216:495 e491–495 e497. [DOI] [PubMed] [Google Scholar]
- 80.Chughtai B, Thomas D, Mao J, Eilber K, Anger J, Clemens JQ, Sedrakyan A. 2017. Hernia repair with polypropylene mesh is not associated with an increased risk of autoimmune disease in adult men. Hernia 21:637–642. [DOI] [PubMed] [Google Scholar]
- 81.Clancy C, Jordan P, Ridgway PF. 2019. Polypropylene mesh and systemic side effects in inguinal hernia repair: current evidence. Ir J Med Sci doi: 10.1007/s11845-019-02008-5. [DOI] [PubMed] [Google Scholar]
- 82.Strietzel FP, Schmidt-Westhausen AM, Neumann K, Reichart PA, Jackowski J. 2019. Implants in patients with oral manifestations of autoimmune or muco-cutaneous diseases - A systematic review. Med Oral Patol Oral Cir Bucal 24:e217–e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kathju S, Nistico L, Melton-Kreft R, Lasko LA, Stoodley P. 2015. Direct demonstration of bacterial biofilms on prosthetic mesh after ventral herniorrhaphy. Surg Infect (Larchmt) 16:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Langbach O, Kristoffersen AK, Abesha-Belay E, Enersen M, Rokke O, Olsen I. 2016. Oral, intestinal, and skin bacteria in ventral hernia mesh implants. J Oral Microbiol 8:31854. [DOI] [PMC free article] [PubMed] [Google Scholar]