Abstract
Reactive nitrogen species play diverse and essential roles in host-pathogen interactions. Here we review selected recent discoveries regarding nitric oxide (NO) in host defense and the pathogenesis of infection, mechanisms of bacterial NO resistance, production of NO by human macrophages, NO-based antimicrobial therapeutics and NO interactions with the gut microbiota.
Introduction
Since the discovery that mammalian cells can produce large quantitites of nitric oxide (NO) in reponse to inflammatory stimuli, the role of reactive nitrogen species in bacterial infections has been a focus of intensive investigation.
NO in host defense
NO generated enzymatically by host NOS2 or abiotically in the gastric lumen by acidification of salivary nitrite exerts antimicrobial activity against diverse pathogens (figure 1), including Clostridioides (Clostridium) difficile, Mycobacterium tuberculosis and Salmonella enterica [*1–3]. NO and congeners arising from its reaction with O2.−, O2, iron and low-molecular weight thiols have high affinity for Fe3+, Fe2+ and Cu++ in terminal cytochromes of the electron transport chain, [4Fe-4S] cluster-containing dehydratases, redox-active protein cysteine residues, and tyrosyl and glycyl radicals in ribonucleotide reductase [4]. Metabolism is a particularly salient target of this diatomic radical. Dihydroxyacid dehydratase, lipoamide dehydrogenase, methionine synthase, aconitase, and fructose bisphosphate aldolase are prominent targets of RNS [4–6]. Strict aerobes such as M. tuberculosis, Burkholderia spp, and Pseudomonas aeruginosa, which overwhelmingly rely on the electron transport chain to satisfy their energy needs, are particularly vulnerable to nitrosylation of terminal respiratory cytochromes [5, 7]. Conversely, Staphylococcus aureus and S. enterica, which can generate ATP via both oxidative and substrate-level phosphorylation, are relatively resistant to the antimicrobial actions of NO engendered by the innate immune response [8*, 9]. Detrimental effects of NO on bacterial metabolism, DNA replication and repair, and protein quality control underlie the robust and broad-spectrum antimicrobial actions of RNS.
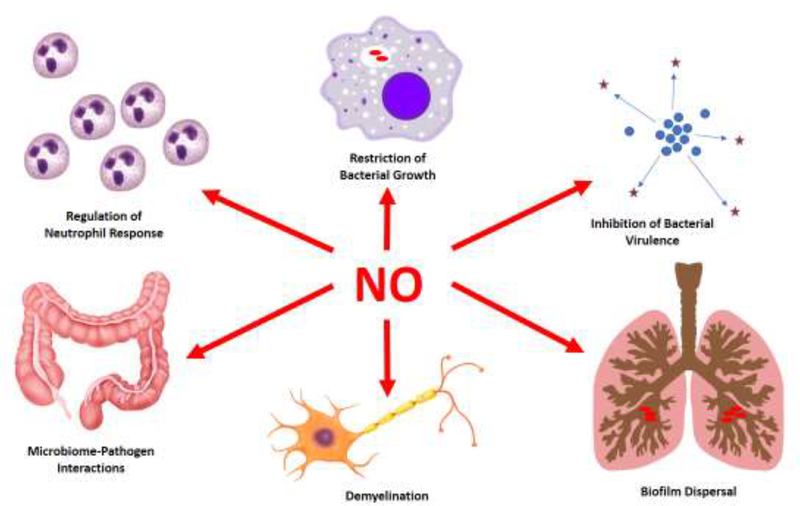
Figure 1. Diverse Actions of Nitric Oxide in Host-Bacterial Interactions.
Some important actions of NO are shown. Please refer to the text for details.
NO also targets regulatory proteins that coordinate bacterial virulence gene expression. For example, S-nitrosylation of AgrA Cys199 interferes with the quorum sensing-dependent S. aureus virulence program [10*] (figure 1). Similarly, S-nitrosylation or oxidation of SsrB Cys208 prevents transcription of genes encoding the Salmonella pathogenicity island-2 (SPI-2) type III secretion system, which is of key importance for the pathogenesis of nontyphoidal Salmonella infections [11].
In addition to exerting direct antimicrobial activity, NO participates in host defense by modulating immunity [12]. It has recently been suggested that NO-mediated resistance to tuberculosis results in part from the negative effect of NO on neutrophil recruitment [13**] (figure 1). Paradoxically, NOS2 may promote host defense against S. aureus by stimulating the accumulation of granulocytes in lungs [10*]. It is presently unclear why NO exerts opposing effects on the granulocytic responses to M. tuberculosis and S. aureus.
Bacterial NO resistance and evasion
Given the profound antimicrobial activity of NO, it is not surprising that pathogenic bacteria possess a variety of anti-nitrosative defenses [4, 5]. The intracellular pathogens S. enterica and M. tuberculosis downregulate NOS2 expression via RNA interference or PPE2-binding to the TATA box of the Nos2 gene [14, 15], and Francisella tularensis inhibits NOS2 expression by suppressing IP-10 production and IFNγ-induced STAT-1 signaling [16]. Salmonella also ameliorates RNS exposure by interfering with trafficking of NOS2-containing vacuoles in a SPI2-dependent manner [5]. NO crosses membranes and enters bacterial cells where low molecular-weight thiols such as glutathione and mycothiol, as well as cytochrome bd, provide a first line of defense against nitrosative stress [4, 17]. Expression of the hmp-encoded flavohemoglobin and cytochrome bd allows the adaptive detoxification of NO in E. coli, M. tuberculosis, Salmonella, S. aureus and Yersinia pseudotuberculosis [17–21]. Mechanisms of hmp regulation vary among pathogenic bacteria. The hmp gene is de-repressed in Gram-negative bacteria such as Salmonella and E. coli upon nitrosylation of the NsrR [2Fe-2S] cluster, whereas hmp transcription in S. aureus is under positive regulation by the SrrAB two-component regulatory system that monitors the redox status of the respiratory chain [18, 22]. Hmp is selectively expressed by bacteria in microenvironments where NO is present. Detoxification of NO by Hmp in marginal zones protects Yersinia within microcolonies, thereby orchestrating a spatially-dependent functional specialization of bacterial cells within infected foci [21].
Pathogenic bacteria reprogram their metabolism under nitrosative stress. A reduction in branched-chain amino acids that follows the nitrosylation of dihydroxyacid dehydratase is sensed by ribosomally-bound RelA monitoring the aminoacylation state of incoming tRNAs [23*]. Guanosine tetraphosphate synthesized by RelA activates transcription of branched-chain amino acid biosynthetic genes, not only reestablishing amino acid homeostasis but also allowing the translation of NO-consuming Hmp [23*]. Although terminal cytochromes of the electron transport chain are some of the most exquisitely-sensitive targets of NO [24], the electron transport chain is still important for the anti-nitrosative defenses of pathogenic bacteria. By working in reverse, the respiratory F1F0 ATPase maintains an alkaline cytoplasm, thereby promoting skin and soft tissue S. aureus infections under conditions such as nitrosative stress and hypoxia that limit respiration [19*]. The F1F0 ATPase may serve a similar function in Salmonella pathogenesis [8*]. In addition, the acquisition of manganese adds to the anti-nitrosative defenses of S. aureus and S. enterica [8*, 19*]. Analogous to its antioxidant role [25], manganese may substitute for iron, which is prone to NO toxicity.
S-nitrosylation of cysteine residues mobilizes Zn2+ from zinc metalloproteins involved in DNA replication and repair, protein synthesis, and metabolism [26*]. Zn2+ mobilized by NO can be detrimental to the cell. Thus, the ZntB and ZitB zinc efflux systems protect Salmonella from nitrosative stress [26*]. Nevertheless, Salmonella must reacquire zinc to resume growth, and the high-affinity ZnuABC zinc uptake system promotes Salmonella pathogenesis during the nitrosative stress engendered by the innate response of macrophages and mice [8*]. Given the widespread utilization of zinc metalloproteins, it is somewhat surprising that zinc-starved Salmonella tolerates nitrosative stress rather well as long as glycolytic fructose bisphosphate aldolase is functional. The metabolism of glucose satisfies cellular energy requirements of S. aureus and S. enterica by allowing the synthesis of ATP by substrate level phosphorylation in the payoff phase of glycolysis and acetate fermentation, while maintaining redox balance by the NADH-consuming fermentation of pyruvate to lactate [8*, 9].
NO in infection pathogenesis
Much effort has been focused on elucidating the multiple ways in which NO contributes to host defense. However, it is becoming increasingly clear that NO produced endogenously by bacteria or exogenously by host phagocytes may also promote bacterial pathogenesis in certain settings. NO produced endogenously by bacterial NOS plays a critical role in the pathogenesis of S. aureus and Bacillus anthracis infections [27**, 28]. Endogenously-synthesized NO by bacterial NOS inhibits aerobic respiration while promoting the utilization of the oxidative branch of the tricarboxylic acid cycle [27**, 29]. NADH generated by the tricarboxylic acid cycle powers reduction of the alternative electron acceptor nitrate, thus maintaining the membrane potential during microaerobiosis, which is essential for nasal colonization by S. aureus [27, 29]. As bacterial NOS is structurally similar to the oxygenase domain of mammalian NOS [30], the regulation of electron transport may represent the primordial role of NOS in biology. As with exogenous NO [31–33], NO produced endogenously prevents oxidative stress while tolerizing Gram-positive pathogens to antibiotics [29, 34, 35].
NO generated by the host can also paradoxically worsen bacterial infection. E. coli takes advantage of the energetic properties of nitrate derived from NOS2-expressing inflammatory cells to outcompete members of the resident gut microbiota [36**] (figure 1), whereas migration of Salmonella toward nitrate generated constitutively by host cells in the lamina propria promotes invasion of Peyer’s patches [37]. Alternatively, NO produced by the innate immune response can directly mediate immunopathology. For example, NO synthesized by infiltrating macrophages in response to Mycobacterium leprae phenolic glycolipids damages mitochondria of nerve cells, triggering demyelination that is pathognomonic of leprosy [38**] (figure 1).
NO production by human macrophages
After lipopolysaccharide and IFNγ were shown to stimulate high-output NO production by murine macrophages [39, 40], it soon became apparent the human peripheral blood mononuclear cell (PBMC)-derived macrophages do not respond similarly. Although some studies have found varying levels of NOS2 mRNA, NOS2 protein, NO production or NO-dependent actions in human macrophages in vitro [41], marked quantitative differences compared to mice have suggested that human macrophages may not produce NO as an antimicrobial mediator [42].
However, analysis of NOS expression in macrophages from humans with active infections suggests that this is not the case [43]. For example, NOS2 protein is visualized within macrophages and epithelioid cells in most patients with leprosy [44, 45] where, as mentioned above, it may also play an important role in nerve damage [38**]. NOS2 protein and mRNA are also observed within submucosal bladder macrophages, in association with increased NO formation, in patients undergoing BCG immunotherapy of bladder cancer [46]. NOS2 mRNA is found in PBMCs in children with moderately severe falciparum malaria but not in those with severe disease [47], suggesting that alternative macrophage polarization with loss of NOS expression may contribute to insufficient NO production in severe cases, resulting in poorer clinical outcomes [48].
Granulomas from patients with active tuberculosis exhibit a complex spatial organization of NO-producing cells, which is also seen in experimentally infected macaques. NOS2 is found within macrophages, epithelioid macrophages and neutrophils centrally situated within TB granulomas. Epithelioid macrophages, some containing bacteria, express high levels of NOS2 and low levels of arginase (Arg1), suggesting that NO is an important component of the antimicrobial response [49**]. In contrast, higher arginase expression is evident in the surrounding lymphocyte cuff region, consistent with an immunoregulatory function of these cells. Even quiescent fibrocalcific granulomas contain NOS-positive cells, suggesting that NO may help to maintain microbial latency, as suggested in experimental models [50, 51].
Expression of NOS2 in human macrophages is not limited to infectious conditions. Macrophages containing NOS2 are seen in rheumatologic diseases and cancer [52–56]. NO production by tumor-associated macrophages can enhance or restrict tumor progression, depending upon the NO concentration and redox environment [57]. Whereas NO production by tumor cells is associated with cancer progression and metastasis, NOS2 production by macrophages may be required for effective responses to chemotherapy [58, 59].
The recent discovery that the NOS2 gene promoter is highly methylated around the transcription start site in human macrophages [60*] suggests that epigenetic regulatory mechanisms are of particular importance. This may account for the difficulty in eliciting high output NO production in human macrophages from healthy subjects. A better understanding of the mechanisms by which NOS2 promoter silencing is relieved during infection will be an important focus of future research.
Therapeutic applications of NO in infections
High NO concentrations are inhibitory for a broad range of bacteria, viruses, fungi and parasites. Efforts to develop NO-based antimicrobial therapies have primarily focused on topical application or local delivery, to avoid unwanted physiological effects of systemic administration. Results are promising but still preliminary. A variety of NO-releasing scaffolds are in development [61]. Small clinical studies as well as animal and in vitro models have demonstrated efficacy of S-nitrosothiols, acidified nitrite or NO-releasing drugs in such diverse infections as cutaneous leishmaniasis, bacterial pneumonia and tinea pedis [62–65]. Acidified nitrite showed some efficacy in the treatment of viral warts but also caused local irritation [66].
The ability of NO to disperse bacterial biofilms by triggering specific sensor proteins [67] suggests that NO-based therapies may be useful for difficult-to-treat chronic infections involving biofilms, such as wound infections and cystic fibrosis-related lung infections [68] (figure 1). Inhaled NO was well tolerated in a phase I study of patients with cystic fibrosis and chronic resistant pulmonary infections, with a reduction in microbial burden and improved lung function after only 5 days of treatment [69]. Another trial of inhaled NO in cystic fibrosis patients observed a reduction in biofilm after 7 days’ treatment [70**]. As biofilm bacteria are more resistant to antibiotics [71], NO might be useful in combination with conventional antimicrobial agents. Synergy has also been shown against drug-resistant enteric bacteria treated with an NO donor, an antimicrobial peptide, and miconazole to inhibit the bacterial NO-detoxifying flavohemoglobin [72], although in other settings NO has been found to promote antibiotic tolerance [73].
Novel NO-charged materials have been developed as a strategy to create implantable catheters that are resistant to infection [74]. This approach could also reduce the incidence of thrombotic complications as a result of NO-mediated anti-platelet actions [75]. Yet another treatment approach is to stimulate the endogenous production of NO by host cells. Infergen, a synthetic interferon, enhances the ability of macrophages to restrict the growth of M. tuberculosis, in part via NO production [76]. L-arginine supplementation also augments NO production during infection [77] and might be a useful adjunctive therapy.
NO and the gut microbiota
The development of new tools to analyze complex microbial communities is yielding exciting new insights into the role of the microbiome in metabolism and immunity. Reduction of dietary nitrate by oral bacteria and reduction of nitrite by the intestinal microbiota play important roles in the enterosalivary circulation of nitrogen oxides and cardiovascular health [78]. Dysbiosis may contribute to the pathogenesis of hypertension, obesity and atherosclerosis. Dietary nitrate supplementation not only reduces systemic blood pressure but also alters the composition of the microbiome [79].
Complex interactions between enzymatic NO production and the microbiota have also been observed. NOS2 and reactive oxygen species maintain bacterial homeostasis in the gut and may limit overgrowth [80]. However, NOS2-driven carbohydrate oxidation [81*] and NOx derived from NOS2 [36**] provide a competitive advantage to enteric pathogens (figure 1). In turn, organic acids produced by gut bacteria modulate NOS expression to prevent the production of nitrate that can be exploited by pathogenic bacteria as a respiratory substrate [82**]. Fecal microbiota transplantation is being utilized to restore gut homeostasis in an increasing number of conditions and may work in part by effects on NO production [83].
Conclusions
Recent studies have provide new and interesting insights into the role of NO and other reactive nitrogen species in host defense and the pathogenesis of infection, as well as mechanisms of bacterial NO resistance, the production of NO by human macrophages, NO-based antimicrobial therapeutics and NO interactions with the gut microbiota.
Acknowledgments
The authors are grateful for support from the National Institutes of Health to FCF (AI112640, AI118962, AI123124) and AVT (AI36520, AI54959, BX02073).
Abbreviations
- NO
nitric oxide
- NOS2
nitric oxide synthase isoform 2, iNOS
- NOx
nitrate and nitrite
- RNS
reactive nitrogen species
- SPI-2
Salmonella Pathogenicity Island-2
Footnotes
Declarations of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cunningham R, Mustoe E, Spiller L, Lewis S Benjamin N: Acidified nitrite: a host defence against colonization with C. difficile spores. J Hosp Infect 2014, 86:155–157.* Clostridioides (Clostridium) difficile spores are resistant to acid but susceptible to acidified nitrite generated in the stomach, which may account for the increased susceptibility of individuals receiving proton pump inhibitors.
- 2.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK Nathan CF: Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A 1997, 94:5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mastroeni P, Vazquez-Torres A, Fang FC, Xu Y, Khan S, Hormaeche CE Dougan G: Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med 2000, 192:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang FC: Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2004, 2:820–832. [DOI] [PubMed] [Google Scholar]
- 5.Henard CA Vázquez-Torres A: Nitric oxide and Salmonella pathogenesis. Front Microbiol 2011, 2:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyduke DR, Jarboe LR, Tran LM, Chou KJ Liao JC: Integrated network analysis identifies nitric oxide response networks and dihydroxyacid dehydratase as a crucial target in Escherichia coli. Proc Natl Acad Sci U S A 2007, 104:8484–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones-Carson J, Laughlin JR, Stewart AL, Voskuil MI Vázquez-Torres A: Nitric oxide-dependent killing of aerobic, anaerobic and persistent Burkholderia pseudomallei. Nitric Oxide 2012, 27:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzsimmons L, Liu L, Porwollik S, Chakraborty S, Desai P, Tapscott T, Henard C, McClelland M, Vazquez-Torres A: Zinc-dependent substrate-level phosphorylation powers Salmonella growth under nitrosative stress of the innate host response. PLoS Pathog 2018, 14:e1007388.* Glucose utilization through substrate-level phosphorylation and fermentation is key to the metabolic adaptation of Salmonella to NOS2-dependent host defense.
- 9.Vitko NP, Spahich NA Richardson AR: Glycolytic dependency of high-level nitric oxide resistance and virulence in Staphylococcus aureus. MBio 2015, 6:e00045–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbano R, Karlinsey JE, Libby SJ, Doulias PT, Ischiropoulos H, Warheit-Niemi HI, Liggitt DH, Horswill AR, Fang FC: Host nitric oxide disrupts microbial cell-to-cell communication to inhibit Staphylococcal virulence. Cell Host Microbe 2018, 23:594–606.e7.* Host NO modulates S. aureus virulence by targeting the quorum sensing AgrA regulatory protein.
- 11.Husain M, Jones-Carson J, Song M, McCollister BD, Bourret TJ Vázquez-Torres A: Redox sensor SsrB Cys203 enhances Salmonella fitness against nitric oxide generated in the host immune response to oral infection. Proc Natl Acad Sci U S A 2010, 107:14396–14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrt S, Schnappinger D, Bekiranov S, Drenkow J, Shi S, Gingeras TR, Gaasterland T, Schoolnik G Nathan C: Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J Exp Med 2001, 194:1123–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra BB, Lovewell RR, Olive AJ, Zhang G, Wang W, Eugenin E, Smith CM, Phuah JY, Long JE, Dubuke ML et al. : Nitric oxide prevents a pathogen-permissive granulocytic inflammation during tuberculosis. Nat Microbiol 2017, 2:17072.** Suppression of the granulocytic response is identified as a dominant aspect of the NO-mediated host response to M. tuberculosis.
- 14.Zhao C, Zhou Z, Zhang T, Liu F, Zhang CY, Zen K Gu H: Salmonella small RNA fragment Sal-1 facilitates bacterial survival in infected cells via suppressing iNOS induction in a microRNA manner. Sci Rep 2017, 7:16979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhat KH, Srivastava S, Kotturu SK, Ghosh S Mukhopadhyay S: The PPE2 protein of Mycobacterium tuberculosis translocates to host nucleus and inhibits nitric oxide production. Sci Rep 2017, 7:39706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsa KV, Butchar JP, Rajaram MV, Cremer TJ, Gunn JS, Schlesinger LS Tridandapani S: Francisella gains a survival advantage within mononuclear phagocytes by suppressing the host IFNgamma response. Mol Immunol 2008, 45:3428–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones-Carson J, Husain M, Liu L, Orlicky DJ Vázquez-Torres A: Cytochrome bd-dependent bioenergetics and antinitrosative defenses in Salmonella pathogenesis. MBio 2016, 7:e02052–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bang IS, Liu L, Vazquez-Torres A, Crouch ML, Stamler JS Fang FC: Maintenance of nitric oxide and redox homeostasis by the Salmonella flavohemoglobin Hmp. J Biol Chem 2006, 281:28039–28047. [DOI] [PubMed] [Google Scholar]
- 19.Grosser MR, Paluscio E, Thurlow LR, Dillon MM, Cooper VS, Kawula TH Richardson AR: Genetic requirements for Staphylococcus aureus nitric oxide resistance and virulence. PLoS Pathog 2018, 14:e1006907.* The F0/F1 ATP synthase working in reverse can maintain proton motive force in S. aureus during nitrosative stress.
- 20.Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V Gennaro ML: Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc Natl Acad Sci U S A 2005, 102:15629–15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis KM, Mohammadi S Isberg RR: Community behavior and spatial regulation within a bacterial microcolony in deep tissue sites serves to protect against host attack. Cell Host Microbe 2015, 17:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinkel TL, Roux CM, Dunman PM Fang FC: The Staphylococcus aureus SrrAB two-component system promotes resistance to nitrosative stress and hypoxia. MBio 2013, 4:e00696–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzsimmons LF, Liu L, Kim JS, Jones-Carson J Vázquez-Torres A: Salmonella reprograms nucleotide metabolism in its adaptation to nitrosative stress. MBio 2018, 9:e00211–18.* The nucleotide alarmone guanosine tetraphosphate activates transcription of branch chain amino acid genes, driving translation of NO-consuming bacterial anti-nitrosative defenses.
- 24.Mason MG, Shepherd M, Nicholls P, Dobbin PS, Dodsworth KS, Poole RK Cooper CE: Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nat Chem Biol 2009, 5:94–96. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Ochoa VE, Lam D, Lee CS, Klaus S, Behnsen J, Liu JZ, Chim N, Nuccio SP, Rathi SG, Mastroianni JR et al. : Salmonella mitigates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration. Cell Host Microbe 2016, 19:814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frawley ER, Karlinsey JE, Singhal A, Libby SJ, Doulias PT, Ischiropoulos H Fang FC: Nitric oxide disrupts zinc homeostasis in Salmonella enterica serovar Typhimurium. MBio 2018, 9:e01040–18.* Efflux of zinc limits mismetallation and malfunction of proteins during the nitrosative stress generated by the innate host response.
- 27.Kinkel TL, Ramos-Montañez S, Pando JM, Tadeo DV, Strom EN, Libby SJ Fang FC: An essential role for bacterial nitric oxide synthase in Staphylococcus aureus electron transfer and colonization. Nat Microbiol 2016, 2:16224.** A fundamental physiological role of bacterial NO synthase in to regulate respiration and maintaining membrane energetics under oxygen-limited conditions.
- 28.Shatalin K, Gusarov I, Avetissova E, Shatalina Y, McQuade LE, Lippard SJ Nudler E: Bacillus anthracis-derived nitric oxide is essential for pathogen virulence and survival in macrophages. Proc Natl Acad Sci U S A 2008, 105:1009–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mogen AB, Carroll RK, James KL, Lima G, Silva D, Culver JA, Petucci C, Shaw LN Rice KC: Staphylococcus aureus nitric oxide synthase (saNOS) modulates aerobic respiratory metabolism and cell physiology. Mol Microbiol 2017, 105:139–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crane BR, Sudhamsu J Patel BA: Bacterial nitric oxide synthases. Annu Rev Biochem 2010, 79:445–470. [DOI] [PubMed] [Google Scholar]
- 31.Husain M, Bourret TJ, McCollister BD, Jones-Carson J, Laughlin J Vázquez-Torres A: Nitric oxide evokes an adaptive response to oxidative stress by arresting respiration. J Biol Chem 2008, 283:7682–7689. [DOI] [PubMed] [Google Scholar]
- 32.Jones-Carson J, Zweifel AE, Tapscott T, Austin C, Brown JM, Jones KL, Voskuil MI Vázquez-Torres A: Nitric oxide from IFNγ-primed macrophages modulates the antimicrobial activity of β-lactams against the intracellular pathogens Burkholderia pseudomallei and nontyphoidal Salmonella. PLoS Negl Trop Dis 2014, 8:e3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCollister BD, Hoffman M, Husain M Vázquez-Torres A: Nitric oxide protects bacteria from aminoglycosides by blocking the energy-dependent phases of drug uptake. Antimicrob Agents Chemother 2011, 55:2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gusarov I Nudler E: NO-mediated cytoprotection: instant adaptation to oxidative stress in bacteria. Proc Natl Acad Sci U S A 2005, 102:13855–13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gusarov I, Shatalin K, Starodubtseva M Nudler E: Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science 2009, 325:1380–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Bäumler AJ: Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2013, 339:708–711.** Nitrate, an oxidation product of NO, is used as an electron acceptor by pathogenic bacteria to gain a growth advantage over the commensal gut microbiota.
- 37.Rivera-Chávez F, Lopez CA, Zhang LF, García-Pastor L, Chávez-Arroyo A, Lokken KL, Tsolis RM, Winter SE Bäumler AJ: Energy taxis toward host-derived nitrate supports a Salmonella pathogenicity island 1-independent mechanism of invasion. MBio 2016, 7:e00960–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madigan CA, Cambier CJ, Kelly-Scumpia KM, Scumpia PO, Cheng TY, Zailaa J, Bloom BR, Moody DB, Smale ST, Sagasti A, Modlin RL, Ramakrishnan L: A macrophage response to Mycobacterium leprae phenolic glycolipid Initiates nerve damage in leprosy. Cell 2017, 170:973–985.e10.** Inhibition of mitochondrial function by host-derived NO leads to demyelination in an animal model of leprosy.
- 39.Stuehr DJ Marletta MA: Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol 1987, 139:518–525. [PubMed] [Google Scholar]
- 40.Stuehr DJ Marletta MA: Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A 1985, 82:7738–7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinberg JB: Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol Med 1998, 4:557–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L Schaffner A: Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis 1993, 167:1358–1363. [DOI] [PubMed] [Google Scholar]
- 43.Fang FC Nathan CF: Man is not a mouse: reply. J Leukoc Biol 2007, 81:580. [DOI] [PubMed] [Google Scholar]
- 44.Schön T, Hernandez-Pando RH, Negesse Y, Leekassa R, Sundqvist T Britton S: Expression of inducible nitric oxide synthase and nitrotyrosine in borderline leprosy lesions. Br J Dermatol 2001, 145:809–815. [DOI] [PubMed] [Google Scholar]
- 45.Lockwood DN, Suneetha L, Sagili KD, Chaduvula MV, Mohammed I, van Brakel W, Smith WC, Nicholls P Suneetha S: Cytokine and protein markers of leprosy reactions in skin and nerves: baseline results for the North Indian INFIR cohort. PLoS Negl Trop Dis 2011, 5:e1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koskela LR, Poljakovic M, Ehrén I, Wiklund NP de Verdier PJ: Localization and expression of inducible nitric oxide synthase in patients after BCG treatment for bladder cancer. Nitric Oxide 2012, 27:185–191. [DOI] [PubMed] [Google Scholar]
- 47.Weinberg JB, Volkheimer AD, Rubach MP, Florence SM, Mukemba JP, Kalingonji AR, Langelier C, Chen Y, Bush M, Yeo TW et al. : Monocyte polarization in children with falciparum malaria: relationship to nitric oxide insufficiency and disease severity. Sci Rep 2016, 6:29151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anstey NM, Weinberg JB, Hassanali MY, Mwaikambo ED, Manyenga D, Misukonis MA, Arnelle DR, Hollis D, McDonald MI Granger DL: Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med 1996, 184:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, Eum SY, Via LE, Barry CE, Klein E, Kirschner DE et al. : Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol 2013, 191:773–784.** NOS2 is found in macrophages, epithelioid macrophages and neutrophils in TB granulomas of humans and experimentally-infected macaques.
- 50.Stenger S, Donhauser N, Thüring H, Röllinghoff M Bogdan C: Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med 1996, 183:1501–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arriaga AK, Orozco EH, Aguilar LD, Rook GA Hernández Pando R: Immunological and pathological comparative analysis between experimental latent tuberculous infection and progressive pulmonary tuberculosis. Clin Exp Immunol 2002, 128:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McInnes IB, Leung BP, Field M, Wei XQ, Huang FP, Sturrock RD, Kinninmonth A, Weidner J, Mumford R Liew FY: Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med 1996, 184:1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grabowski PS, Wright PK, Van ‘t Hof RJ, Helfrich MH, Ohshima H Ralston SH: Immunolocalization of inducible nitric oxide synthase in synovium and cartilage in rheumatoid arthritis and osteoarthritis. Br J Rheumatol 1997, 36:651–655. [DOI] [PubMed] [Google Scholar]
- 54.Klimp AH, Hollema H, Kempinga C, van der Zee AG, de Vries EG Daemen T: Expression of cyclooxygenase-2 and inducible nitric oxide synthase in human ovarian tumors and tumor-associated macrophages. Cancer Res 2001, 61:7305–7309. [PubMed] [Google Scholar]
- 55.Massi D, Marconi C, Franchi A, Bianchini F, Paglierani M, Ketabchi S, Miracco C, Santucci M Calorini L: Arginine metabolism in tumor-associated macrophages in cutaneous malignant melanoma: evidence from human and experimental tumors. Hum Pathol 2007, 38:1516–1525. [DOI] [PubMed] [Google Scholar]
- 56.Edin S, Wikberg ML, Dahlin AM, Rutegård J, Öberg Å, Oldenborg PA Palmqvist R: The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One 2012, 7:e47045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brüne B, Courtial N, Dehne N, Syed SN Weigert A: Macrophage NOS2 in tumor leukocytes. Antioxid Redox Signal 2017, 26:1023–1043. [DOI] [PubMed] [Google Scholar]
- 58.Basudhar D, Somasundaram V, de Oliveira GA, Kesarwala A, Heinecke JL, Cheng RY, Glynn SA, Ambs S, Wink DA Ridnour LA: Nitric oxide synthase-2-derived nitric oxide drives multiple pathways of breast cancer progression. Antioxid Redox Signal 2017, 26:1044–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Somasundaram V, Basudhar D, Bharadwaj G, No JH, Ridnour LA, Cheng RYS, Fujita M, Thomas DD, Anderson SK, McVicar DW et al. : Molecular mechanisms of nitric oxide in cancer progression, signal transduction, and metabolism. Antioxid Redox Signal 2018, 2 May 2018. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gross TJ, Kremens K, Powers LS, Brink B, Knutson T, Domann FE, Philibert RA, Milhem MM Monick MM: Epigenetic silencing of the human NOS2 gene: rethinking the role of nitric oxide in human macrophage inflammatory responses. J Immunol 2014, 192:2326–2338.* The human NOS gene is highly methylated near the transcription start site, suggesting an important role of epigenetic regulatory mechanisms.
- 61.Yang L, Feura ES, Ahonen MJR Schoenfisch MH: Nitric oxide-releasing macromolecular scaffolds for antibacterial applications. Adv Healthc Mater 2018, 7:e1800155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jean D, Maître B, Tankovic J, Meignan M, Adnot S, Brun-Buisson C, Harf A Delclaux C: Beneficial effects of nitric oxide inhalation on pulmonary bacterial clearance. Crit Care Med 2002, 30:442–447. [DOI] [PubMed] [Google Scholar]
- 63.Costa IS, de Souza GF, de Oliveira MG Abrahamsohn IA: S-nitrosoglutathione (GSNO) is cytotoxic to intracellular amastigotes and promotes healing of topically treated Leishmania major or Leishmania braziliensis skin lesions. J Antimicrob Chemother 2013, 68:2561–2568. [DOI] [PubMed] [Google Scholar]
- 64.Elewski BE, Kircik LH, Stasko N, De Leon E, Enloe C, Durham T Maeda-Chubachi T: A phase 2, controlled, dose-ranging study of SB208, an investigational topical nitric oxide-releasing drug, for the treatment of tinea pedis. J Drugs Dermatol 2018, 17:888–893. [PubMed] [Google Scholar]
- 65.Stasko N, McHale K, Hollenbach SJ, Martin M Doxey R: Nitric oxide-releasing macromolecule exhibits broad-spectrum antifungal activity and utility as a topical treatment for superficial fungal infections. Antimicrob Agents Chemother 2018, 62:e01026–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ormerod AD, van Voorst Vader PC, Majewski S, Vanscheidt W, Benjamin N van der Meijden W: Evaluation of the efficacy, safety, and tolerability of 3 dose regimens of topical sodium nitrite with citric acid in patients with anogenital warts: a randomized clinical trial. JAMA Dermatol 2015, 151:854–861. [DOI] [PubMed] [Google Scholar]
- 67.Williams DE Boon EM: Towards understanding the molecular basis of nitric oxide-regulated group behaviors in pathogenic bacteria. J Innate Immun 2018, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barraud N, Kelso MJ, Rice SA Kjelleberg S: Nitric oxide: a key mediator of biofilm dispersal with applications in infectious diseases. Curr Pharm Des 2015, 21:31–42. [DOI] [PubMed] [Google Scholar]
- 69.Deppisch C, Herrmann G, Graepler-Mainka U, Wirtz H, Heyder S, Engel C, Marschal M, Miller CC Riethmüller J: Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase I clinical study. Infection 2016, 44:513–520. [DOI] [PubMed] [Google Scholar]
- 70.Howlin RP, Cathie K, Hall-Stoodley L, Cornelius V, Duignan C, Allan RN, Fernandez BO, Barraud N, Bruce KD, Jefferies J et al. : Low-dose nitric oxide as targeted anti-biofilm adjunctive therapy to treat chronic Pseudomonas aeruginosa infection in cystic fibrosis. Mol Ther 2017, 25:2104–2116.** NO disrupts biofilms and was able to reduce bacterial biofilms in patients with cystic fibrosis, suggesting a potential adjunctive role in combination with antibiotics.
- 71.Venkatesan N, Perumal G Doble M: Bacterial resistance in biofilm-associated bacteria. Future Microbiol 2015, 10:1743–1750. [DOI] [PubMed] [Google Scholar]
- 72.Bang CS, Kinnunen A, Karlsson M, Önnberg A, Söderquist B Persson K: The antibacterial effect of nitric oxide against ESBL-producing uropathogenic E. coli is improved by combination with miconazole and polymyxin B nonapeptide. BMC Microbiol 2014, 14:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vázquez-Torres A Bäumler AJ: Nitrate, nitrite and nitric oxide reductases: from the last universal common ancestor to modern bacterial pathogens. Curr Opin Microbiol 2016, 29:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Margel D, Mizrahi M, Regev-Shoshani G, Ko M, Moshe M, Ozalvo R, Shavit-Grievink L, Baniel J, Kedar D, Yossepowitch O et al. : Nitric oxide charged catheters as a potential strategy for prevention of hospital acquired infections. PLoS One 2017, 12:e0174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wo Y, Brisbois EJ, Wu J, Li Z, Major TC, Mohammed A, Wang X, Colletta A, Bull JL, Matzger AJ et al. : Reduction of thrombosis and bacterial infection via controlled nitric oxide (NO) release from S-nitroso-N-acetylpenicillamine (SNAP) impregnated carbosil intravascular catheters. ACS Biomater Sci Eng 2017, 3:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pahari S, Khan N, Aqdas M, Negi S, Kaur J Agrewala JN: Infergen stimulated macrophages restrict Mycobacterium tuberculosis growth by autophagy and release of nitric oxide. Sci Rep 2016, 6:39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carbajosa S, Rodríguez-Angulo HO, Gea S, Chillón-Marinas C, Poveda C, Maza MC, Colombet D, Fresno M Gironès N : L-arginine supplementation reduces mortality and improves disease outcome in mice infected with Trypanosoma cruzi. PLoS Negl Trop Dis 2018, 12:e0006179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E Morris A: Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med 2017, 105:48–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vanhatalo A, Blackwell JR, L’Heureux JE, Williams DW, Smith A, van der Giezen M, Winyard PG, Kelly J Jones AM: Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med 2018, 124:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matziouridou C, Rocha SDC, Haabeth OA, Rudi K, Carlsen H Kielland A: iNOS- and NOX1dependent ROS production maintains bacterial homeostasis in the ileum of mice. Mucosal Immunol 2018, 11:774–784. [DOI] [PubMed] [Google Scholar]
- 81.Faber F, Tran L, Byndloss MX, Lopez CA, Velazquez EM, Kerrinnes T, Nuccio SP, Wangdi T, Fiehn O, Tsolis RM et al. : Host-mediated sugar oxidation promotes post-antibiotic pathogen expansion. Nature 2016, 534:697–699.* Disruption of the microbiota leads to increased NOS expression and the production of oxidized sugars that provide a substrate for pathogenic bacteria.
- 82.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y et al. : Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017, 357:570–575.** Butyrate-producing commensal bacteria induce PPAR-γ signaling which limits oxygen availability, negatively regulates NOS2 and limits the growth of pathogenic bacteria.
- 83.Li X, Shang Q, Gao Z, Hao F, Guo H Guo C: Fecal microbiota transplantation (FMT) could reverse the severity of experimental necrotizing enterocolitis (NEC) via oxidative stress modulation. Free Radic Biol Med 2017, 108:32–43. [DOI] [PubMed] [Google Scholar]