Abstract
Studies using rodent models of neuropathic pain employ sham surgery control procedures that cause deep tissue damage. Sham surgeries would thus be expected to induce potentially long-lasting postsurgical pain, but little evidence for such pain has been reported. Operant tests of voluntary behavior can reveal negative motivational and cognitive aspects of pain that may provide sensitive tools for detecting pain-related alterations. In a previously described operant mechanical conflict (MC) test involving lengthy familiarization and training, rodents freely choose to either escape from a brightly lit chamber by crossing sharp probes or refuse to cross. Here, we describe a brief (2-day) MC protocol that exploits rats’ innate exploratory response to a novel environment in order to detect persistently enhanced pain-avoidance behavior after sham surgeries for two neural injury models: thoracic spinal cord injury (SCI) and chronic constriction injury (CCI) of the sciatic nerve. Pitting the combined motivations to avoid the bright light and to explore the novel device against pain from crossing noxious probes disclosed a conflicting, hyperalgesia-related reluctance to repeatedly cross the probes after injury. Rats receiving standard sham surgeries demonstrated enhanced pain-like avoidance behavior compared to naive controls, and this behavior was similar to that of corresponding CCI or SCI rats weeks or months after injury. In the case of sham surgery for SCI, video analysis of voluntary exploratory behavior directed at the probes revealed enhanced forepaw withdrawal responses. These findings have important implications for preclinical investigations into behavioral alterations and physiological mechanisms associated with postsurgical and neuropathic pain.
Keywords: Evoked pain, affective pain, exploratory drive, reflex hypersensitivity, nociceptors
1. Introduction
Alterations of reflexive responses to hand-delivered test stimuli are the most common measures of pain in animal studies 5, 41. However, reflex assays such as widely used von Frey and Hargreaves tests do not assess motivational and cognitive aspects of pain 69, 84, 86, 96, and they are prone to unconscious tester bias 12. Better indicators of the motivational-affective properties of pain could increase the predictive value of preclinical models of chronic pain for translational drug discovery 10, 95. In particular, operant behavioral tasks dependent on voluntary behavior, such as conditioned place preference (CPP), 4, 57, 94, place-escape/avoidance paradigm (PEAP) 1, 13, 36, 61, thermal escape 85 and passive avoidance 90 tests can capture negative motivational components of pain that are particularly important clinically 75, 96.
A mechanical conflict (MC) test developed by Harte and colleagues 48 is an operant paradigm in which an animal’s voluntary behavior reveals alterations in the aversiveness of sharp probes the animal must cross to escape bright light. Rats’ latencies to escape from a brightly lit chamber increase as probe height increases, while escape latencies are decreased by treatment with drugs known to reduce pain in humans 48. This test assesses motivational and cognitive processing of pain-related information, and it removes the potential for unconscious experimenter bias inherent in reflex tests that utilize hand-delivered stimuli 43, 48, 64, 78. Activation of brain regions associated with motivational-affective components of pain during the MC test 73, as during the PEAP 62, 63, 98, support the view that these operant tests measure pain avoidance.
In principle, an operant test in which an animal’s voluntary behavior discloses the aversiveness of a test stimulus might reveal evoked pain that has not been evident in reflex tests. Humans often experience painful postoperative hypersensitivity long after surgical procedures similar to those used to expose peripheral nerves or the spinal cord in rodent neuropathic pain models 21. Therefore, we asked whether an operant MC test could detect postoperative enhancement of evoked pain caused by the sham surgeries typically used as control procedures in rat models of long-lasting neuropathic pain caused by thoracic spinal cord injury (SCI) and chronic constriction injury (CCI) of the sciatic nerve. We modified the MC test to take advantage of rats’ innate drive to explore novel environments 11, 32, 52, and measured pain-related changes in a rat’s motivation to repeatedly cross noxious probes. Prior studies using the same device habituated exploratory behavior prior to testing and permitted only a single crossing of the probes, using increased latency to escape a brightly lit chamber to measure enhancement of evoked pain 23, 43, 48, 64, 78. Here we report that a brief MC protocol, which pits the motivation to continue exploring a novel environment against injury-enhanced pain during repeated crossings of noxious probes, reveals persistent enhancement of pain-avoidance behavior following sham surgeries for central SCI and for peripheral CCI models of neuropathic pain. Some of the work reported here has been described in a published Ph.D. dissertation 71.
2. Methods
2.1. Animals
All procedures followed the guidelines of the International Association for the Study of Pain and were approved by the McGovern Medical School at UTHealth and University of Texas MD Anderson Cancer Center Animal Care and Use Committees. Male, Sprague-Dawley rats (Envigo, USA) were used at both institutions. At McGovern Medical School, the rats (250–300 g, 2 per cage) were acclimated to a controlled environment (12-hour reverse light/dark cycle, 21 ± 1°C) for ≥4 days before beginning experiments. The corn cob bedding was replaced 2–3 times per week while food and water were provided ad libitum. At MD Anderson Cancer Center, the rats (10 weeks old, 2–3 per cage) were acclimated to the controlled laboratory environment (12-hour light/dark cycle, lights on at 07:00 h, 22 ± 1°C) for at least 7 days before beginning experiments. The corn cob bedding was replaced once per week. Food and water were provided ad libitum.
2.2. Spinal cord injury (SCI) and sham surgeries
Surgeries were performed at McGovern Medical School as previously described 7, 8, 72, 92. Anesthesia in most studies (Fig. 1A–B) was by isoflurane (induction 4–5%; maintenance 1–2%). In a subset (Fig. 1C), intraperitoneal (i.p.) injection of ketamine (60 mg/kg), xylazine (10 mg/kg), and acepromazine (1 mg/kg) was used. Rats were determined to be deeply anesthetized and areflexic before proceeding. Local anesthetic (bupivacaine, 2 mg/kg) was delivered subcutaneously (s.c.) before incising the skin from T8-T12. Laminectomy of the T10 vertebrae was followed by a contusive impact (150 kdyne, 1 s dwell time) using an Infinite Horizon Spinal Cord Impactor (Precision Systems and Instrumentation, LLC, Fairfax Station, VA, USA). Following impact, the paravertebral muscles were closed with vicryl-coated, absorbable suture and the skin incision was closed with 9 mm wound clips. Rats were returned to their home cage and placed on a heating pad maintained at 37°C. The analgesic buprenorphine hydrochloride (0.02 mg/kg; Buprenex, Reckitt Benckiser Healthcare Ltd., Hull, England, UK) was administered in 0.9% saline (2 mL/kg, i.p.) twice, daily up to 5 days post-surgery. The antibiotic enrofloxacin (0.3 mL; Enroflox, Norbrook, Inc., Overland Park, KS, USA), was also administered in 0.9% saline daily up to 10 days post-surgery. Manual bladder evacuations were performed twice daily until rats recovered neurogenic bladder voiding. The day after surgery, hindlimb locomotion was assessed using the Basso, Beattie, and Bresnahan (BBB) Locomotor Rating Scale 6. Sham surgery procedures were identical except for the spinal contusion. Only sham-operated rats with BBB scores of 21 for both hindlimbs were accepted. The majority of SCI rats had scores of 0 or 1 for both hindlimbs 1 day after injury. At the time of post-injury testing, SCI rats had BBB scores >9. Naive rats were transported to the surgical suite at the same time as SCI and sham-operated rats but were otherwise left undisturbed in their home cages.
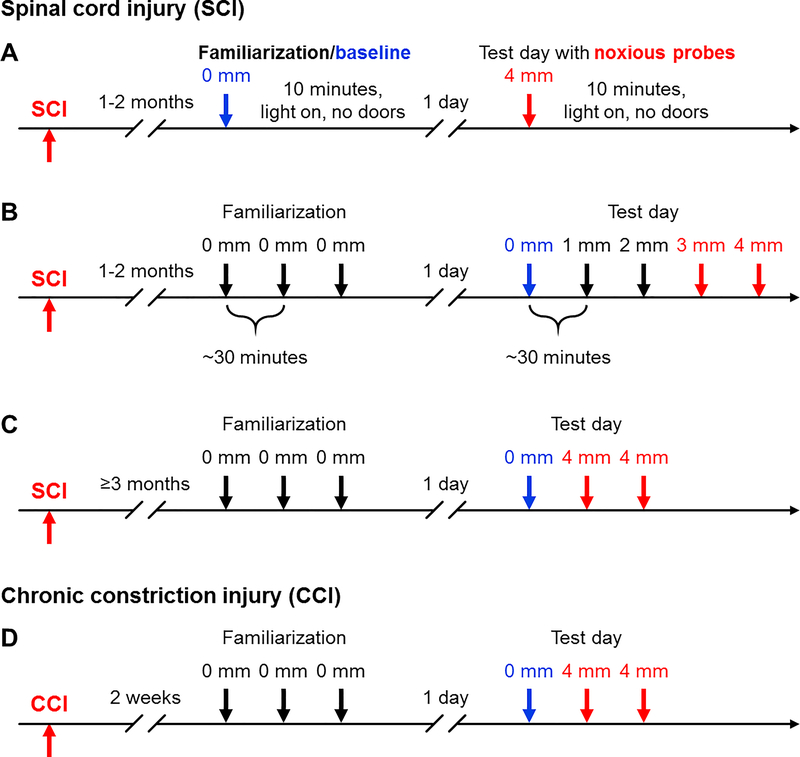
Figure 1.
Timelines of the operant mechanical conflict (MC) test used to measure changes in avoidance of noxious probes. (A) Assessments in the SCI model, providing initial familiarization during a 10-minute session without probes, followed by a test with high probes. (B) Assessments in the SCI model, using a standardized familiarization sequence followed by trials with progressively higher probes. (C) Assessments in the SCI model, with the familiarization sessions and baseline trial followed by trials with high probes. (D) Assessments in the CCI model using the same sequence as in part C. Numbers (in mm) indicate elevation of the sharp probes above the floor of the middle chamber. During the 5-minute familiarization sessions and baseline trial the rat is free to explore the 3-chamber MC device in the absence of elevated probes (0 mm). CCI, chronic constriction injury of the sciatic nerve; SCI, spinal cord injury.
2.3. Chronic constriction injury (CCI) and sham surgeries
Surgeries were performed at MD Anderson Cancer Center. Neuropathic pain from peripheral injury was induced using the CCI model of unilateral sciatic nerve injury 9 as previously described 39, 40. Rats receiving CCI or sham surgeries were anesthetized with isoflurane (4% in oxygen for induction; 2–3% maintenance) and placed on an electric heating pad. Skin at the mid-thigh level of the left leg was shaved with an electric razor and cleansed with povidone-iodine and 70% ethanol. An incision of the skin was made with a scalpel blade and the sciatic nerve was exposed through blunt dissection of the biceps femoris muscle. Using glass nerve hooks, a segment of the sciatic nerve was gently liberated from the surrounding connective tissue. For CCI surgeries, 4 ligatures (4–0 chromic gut; Ethicon, USA) were loosely tied around the sciatic nerve approximately 1 mm apart. For sham surgeries, the sciatic nerve was manipulated with nerve hooks and isolated in an identical fashion, but no chromic gut ligations were sutured around the nerve. The muscle layer was closed with non-absorbable sutures (4–0 silk; Ethicon, USA), 9 mm wound clips were applied to close the skin, and rats were then returned to their home cage and monitored post-operatively until fully ambulatory. Naive rats were transported to the surgical suite at the same time as CCI and sham surgeries were performed, but they were otherwise left undisturbed in their home cages.
2.4. Habituation to ambient testing conditions
At McGovern Medical School, rats were acclimated to the behavioral testing room each morning for 1 hour under red light and constant background white noise generated by a TaskMasking speaker (K.R. Moeller Associates Ltd., Burlington, Ontario, Canada). Several days prior to testing the rats were acclimated to the presence of an experimenter and the acrylic chambers (IITC Life Science Inc., Woodland Hills, CA, USA) used to isolate rats for hindpaw reflex tests. During gentling the rats were placed in the chambers on raised wire-mesh platforms for 20 minutes and periodically fed sweetened cereal. Two experimenters were female (<30 years old) and one experimenter was male (>30 years old). Male and female experimenters did not perform tests on the same days in order to limit male-induced stress and analgesia 80. At MD Anderson Cancer Center, rats were acclimated to the behavioral testing room for at least 1 hour under red light illumination prior to each behavioral test. Rats were handled by the experimenter in 5 min sessions over 3 days. Habituation to hindpaw reflex testing occurred by placing rats in acrylic chambers on raised wire-mesh platforms for 60 min sessions over 3 days. One female and one male experimenter (>30 years old) performed all experiments, and each rat was always manipulated by the same experimenter.
2.5. Tests of hindpaw reflex sensitivity
Threshold for paw withdrawal to mechanical stimulation
At McGovern Medical School, hindpaw sensitivity to mechanical stimuli was measured by two female experimenters at 1–2 months post-surgery for naive, sham-operated, and SCI rats. Following habituation and gentling procedures, the rats were placed in acrylic chambers and the 50% mechanical withdrawal threshold was assessed using the “up-down” method 20, 25, 29, 30 using calibrated von Frey filaments (Stoelting Co., Wood Dale, IL, USA). The set of filaments had logarithmically increasing bending forces (in grams): 0.4, 0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0, 26.0, 60.0. Filaments (starting with 6.0 g) were presented perpendicularly to the plantar surface of the hindpaw between the footpads at a constant speed until the filament bent. In some SCI experiments filaments were applied for ~1 second, and in others the filaments were applied for ~5 seconds. Each hindpaw was presented with a series of 10 stimuli, spaced 30 s apart to provide consistent testing durations and treatment. A rapid, obvious withdrawal of the hindpaw from the filament was considered a positive response, and care was taken to withhold stimulation during rats’ ambulatory movements. In SCI experiments, the withdrawal thresholds for the left and right hindpaws were calculated separately and then averaged together for a single score per rat. All withdrawal thresholds were log transformed to account for Weber’s Law 67. Mills and colleagues demonstrated that the original equation used to calculate the 50% paw withdrawal threshold (in grams) described by Chaplan et al., 20 can be reduced to the following:
where Xf = the final filament used (i.e., the filament handle number), Ƙ = the tabular value for the given sequence of test stimuli 20, and δ = the mean difference between the given sequence of test stimuli (calculated using the filament handle numbers). The filament handle number = Log10 of (10 x filament force in milligrams) (Stoelting Co. Touch Test™ Sensory Evaluators Operation Manual), indicating the handle numbers can be used in the Chaplan or Mills equations without converting gram forces into log units. Also, δ is not a fixed value as not every rat receives the same series of filaments. For CCI experiments at MD Anderson Cancer Center, the naive, sham-operated, and CCI rats were tested 2 weeks post-surgery using the “up-down” method. Rats were placed in acrylic chambers on raised wire-mesh platforms and habituated for at least 30 min before testing. The set of filaments had bending forces (in grams) of 0.4, 0.6, 1.2, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0. Notice that when log transformed, forces between 1 and 0 g yield negative numbers (see Results). Filaments were administered to the distal portion of the heel 39, 40 and applied for ~5 seconds. The mechanical thresholds for the ipsilateral (injured) and contralateral (uninjured) hindpaws were scored separately.
Latency for paw withdrawal to radiant heat stimulation
At McGovern Medical School, hindpaw sensitivity to radiant heat 47 was measured by two female experimenters at 1–2 months post-surgery for naive, sham-operated, and SCI rats 8. Once habituated, the rats were placed in acrylic chambers on a glass platform (Plantar Analgesia Meter; IITC Life Science Inc., Woodland Hills, CA, USA) and acclimated to the 30°C temperature-controlled surface 28 for 20 minutes. Settings for the radiant heat stimulus: idle beam intensity = 10%, active beam intensity = 45%, active beam cutoff = 20 seconds. While idle, the light beam was positioned between the footpads of either the left or right hindpaw, and once positioned the active beam was turned on. A rapid, obvious withdrawal of the hindpaw was considered a positive response to the radiant heat stimulus. Rats that did not exhibit a withdrawal by the time of the automatic active beam cutoff were given a score of 20 seconds. Tests continued until the withdrawal latency was recorded 5 times for each hindpaw, switching between the hindpaws every 30 seconds. If ambulatory movements occurred during presentation of the stimulus, the active light beam was turned off, the experimenter waited 30 seconds, and the other hindpaw was tested. The average withdrawal latency for each hindpaw was calculated using the 3 middle latencies; the highest and lowest latencies were omitted. The two hindpaw latencies were then averaged together for a single score per rat.
2.6. Operant mechanical conflict (MC) test
Voluntary, pain-related aversion to a noxious stimulus was assessed using a commercial 3-chambered device, the Mechanical Conflict System (Coy Laboratory Products, Inc., Grass Lake, MI, USA). The basic MC test presents rats with a choice in responding to two aversive stimuli – either to remain exposed to an aversive bright light in one chamber or to escape the light by crossing a middle chamber having a floor covered by a dense array of sharp probes, in order to reach a dark, safe chamber. Longer latencies to leave the light chamber indicate increased motivation to avoid the probes, and this escape latency is currently the most common measure of pain-related behavior in the MC test 23, 43, 48, 73, 78. We found in pilot studies that, when the MC device is still relatively novel, naive and injured rats cross the noxious probes multiple times. These experiments were extended to assess prolonged escape and crossing behavior under conditions of high novelty using two single trial, 10-minute exposures to the MC device, first without and then with noxious probes (Fig. 1A). The rats’ repeated return to the presumably aversive brightly lit chamber across the noxious probes indicates the presence of a second motivation to cross the probes, likely the rats’ exploratory drive, which might be modulated by pain and other emotional states such as anxiety (see Results and Discussion). We took advantage of this second motivation to investigate additional effects of sham surgery, SCI, and CCI. To do this, we shortened the conventional MC paradigm 48 to a 2-day protocol so that both motivations to cross -- 1) to escape the light and 2) to explore the MC device -- were in conflict with the aversiveness of the sharp probes.
Familiarization sessions
Harte et al. describe a lengthy procedure for familiarization to the MC device and escape training lasting 4–7 days, with a total of 10 to 19 opportunities (each 5 minutes duration) for the rat to explore the MC device before experiencing the sharp probes 48. During this training the rats learn that when the exit door opens they can escape from the brightly lit room to reach the dark room. We shortened the conventional MC test protocol (Fig. 1B) by combining the familiarization and training procedures into three 5-minute familiarization sessions without the probes on a single day, repeated 3 times, spaced 30–60 minutes apart. In each session 1) a rat was placed inside the light chamber with the lid closed, the light off, and the exit door closed; 2) after 20 seconds the light was turned on; 3) after 15 seconds the exit door was opened when (or if) the rat faced the exit; 4) the rat freely explored all 3 chambers in the MC device for 5 minutes, 5) the rat was returned to its home cage, and 6) the device was thoroughly cleaned with 70% ethanol (in distilled water) in preparation for the next session. The rats rapidly learned to escape the light room as soon as the exit door was opened. Indeed, rats sometimes attempted to lift the door on their own by the second or third sessions.
Noxious probe trials
After the familiarization sessions on Day 1, rats underwent one of three different sequences of trials in which they were challenged with sharp probes. In each case, the first trial (baseline) was without probes to reacquaint the rats with the MC device and provide the basis for comparison to noxious probe trials. In the first study (Fig. 1B), probe height was successively increased from 0 to 1, 2, 3, and 4 mm in 3-minute trials spaced ~30 minutes apart. Probe heights were presented in ascending order to minimize possible sensitizing effects from higher probes and to permit testing multiple probe heights per rat on a single day. In subsequent SCI and CCI studies, a 5-minute baseline trial was followed by 2 trials at 4 mm, 5 minutes per trial, spaced ~30 minutes apart (Fig. 1C–D). All SCI and CCI rats were capable of weight-supported plantar stepping (SCI rats had BBB scores ≥10 by the time of testing) and all were observed to readily traverse the probes without bodily harm.
Video scoring and data collection
All sessions and trials were video-recorded in 1080i resolution at 30 frames per second using a Panasonic HC-V750 camcorder (Panasonic Corporation, Osaka, Japan) or 1080p resolution at 30 frames per second using an Apple iPhone (Apple Inc., Cupertino, CA, USA) and scored by a blinded experimenter. The following measures were collected for post-hoc analysis: 1) the escape latency during the first crossing, 2) the number of crossings of the probe chamber, and 3) the total time elapsed to the completion of the second crossing. A subset of videos was scored for behavioral measures that might reveal above-level mechanical hypersensitivity in the forepaws after SCI 8. The number of times each rat withdrew a forepaw after voluntary contact with the probes was scored. No formal definitions of a crossing currently exist for the MC test, and measures other than escape latency depend upon this definition. We defined the first crossing as the rat placing all 4 paws inside the dark chamber after leaving the light chamber. Every subsequent crossing was defined as the rat placing its head and two forepaws inside the light or dark chamber.
2.7. Data analysis and experimental design
Statistical analyses were performed using Prism v7.03 (Graphpad Software, Inc., La Jolla, CA, USA). Data are presented as mean ± SD or SEM or as median with interquartile range. The Shapiro-Wilk test was used to assess normality for continuous measures. Planned comparisons were used to test the overriding hypothesis that sham surgeries induce persistent pain-related behavior. Thus, overall differences were assessed using 1-way ANOVA or Kruskal-Wallis tests followed by planned comparisons of sham versus naive groups, as well as sham versus SCI groups using Dunnett’s or Dunn’s post-hoc tests to gauge the similarity of postoperative effects versus effects of the SCI and CCI procedures (see Table 1). Within-group comparisons of crossings measured during probe trials to each groups’ 0-mm baseline trial were performed using repeated measures 1-way ANOVA or Friedman tests followed by Dunnett’s or Dunn’s post-hoc test (see Table 2). Sphericity was not assumed when using the repeated measures 1-way ANOVA and the Geisser-Greenhouse correction was applied. Planned comparisons between groups in Figure 1B were performed for trials with probe heights found to significantly reduce crossings within groups. Subsequent SCI and CCI experiments were performed with just two trials, using the 4 mm probes. Planned comparisons between groups in subsequent SCI and CCI experiments (Fig. 1C–D) were performed for both probe trials. Significance for all statistical tests was set at P < 0.05 and all reported P values are two-tailed.
Table 1.
Summary of planned comparisons
| Figure | Graph | Experiment | MC Test Probe Height | Behavioral Measure | Statistical Test, P | Post-hoc Test | Naïve vs Sham, P | Neural Injury vs Sham, P |
|---|---|---|---|---|---|---|---|---|
| 2 | B1 | MC 10 minute | 0 mm | Crossings | KW, = 0.0011 | Dunn’s | = 0.0005 | = 0.5238 |
| Time of final crossing | KW, = 0.0072 | Dunn’s | = 0.0051 | > 0.9999 | ||||
| B2 | 4 mm | Crossings | KW, = 0.4345 | Dunn’s | = 0.3963 | > 0.9999 | ||
| Time of final crossing | KW, = 0.2762 | Dunn’s | = 0.4715 | = 0.2652 | ||||
| 3 | A | MC Baseline | Day 2, 0 mm | Escape latency | KW, = 0.4853 | Dunn’s | > 0.9999 | = 0.5572 |
| B | Crossings | 1-way ANOVA, = 0.3896 | Dunnett’s | = 0.6786 | = 0.2911 | |||
| C | Crossings | KW, = 0.7867 | Dunn’s | > 0.9999 | > 0.9999 | |||
| Time of final crossing | KW, = 0.9234 | Dunn’s | > 0.9999 | > 0.9999 | ||||
| 4 | A2 | MC 01234 | Day 2, 0 mm | Crossings | 1-way ANOVA, = 0.4791 | Dunnett’s | = 0.5286 | = 0.4353 |
| Time of final crossing | KW, = 0.7001 | Dunn’s | > 0.9999 | > 0.9999 | ||||
| B2 | 3 mm | Crossings | KW, = 0.0417 | Dunn’s | = 0.1720 | = 0.8256 | ||
| 4 mm | Crossings | KW, = 0.1530 | Dunn’s | = 0.3126 | > 0.9999 | |||
| C1 | 3 mm | Escape latency | KW, = 0.2243 | Dunn’s | = 0.7884 | = 0.7096 | ||
| 4 mm | Escape latency | KW, = 0.7483 | Dunn’s | > 0.9999 | > 0.9999 | |||
| D1 | 3 mm | Time to second crossing | KW, = 0.0606 | Dunn’s | = 0.2935 | = 0.6679 | ||
| 4 mm | Time to second crossing | KW, = 0.1509 | Dunn’s | = 0.2053 | > 0.9999 | |||
| 5 | A2 | MC 044 | Day 2, 0 mm | Crossings | KW, = 0.9386 | Dunn’s | > 0.9999 | > 0.9999 |
| Time of final crossing | KW, = 0.9246 | Dunn’s | > 0.9999 | > 0.9999 | ||||
| B2 | First 4 mm | Crossings | KW, = 0.0114 | Dunn’s | = 0.0094 | > 0.9999 | ||
| Second 4 mm | Crossings | KW, = 0.0347 | Dunn’s | = 0.1423 | = 0.7728 | |||
| C1 | First 4 mm | Escape latency | KW, = 0.1340 | Dunn’s | > 0.9999 | = 0.1081 | ||
| Second 4 mm | Escape latency | KW, = 0.0435 | Dunn’s | > 0.9999 | = 0.0924 | |||
| 6 | A | von Frey | - | 50% PWT – 1 s application | 1-way ANOVA, = 0.0792 | Dunnett’s | = 0.9689 | = 0.0860 |
| B | - | 50% PWT – 5 s application | KW, = 0.0646 | Dunn’s | = 0.6118 | = 0.0388 | ||
| C | Hargreaves | - | Withdrawal latency | 1-way ANOVA, < 0.0001 | Dunnett’s | = 0.0044 | = 0.0587 | |
| 7 | B | MC 01234 | 1 mm | Forepaw withdrawals | KW, = 0.0368 | Dunn’s | = 0.7793 | = 0.1670 |
| 2 mm | Forepaw withdrawals | KW, = 0.0113 | Dunn’s | = 0.1744 | = 0.3461 | |||
| 3 mm | Forepaw withdrawals | KW, = 0.1987 | Dunn’s | = 0.1547 | > 0.9999 | |||
| 4 mm | Forepaw withdrawals | KW, = 0.1243 | Dunn’s | = 0.0852 | = 0.9160 | |||
| Average of 1, 2, 3, 4 mm | Forepaw withdrawals | KW, = 0.0144 | Dunn’s | = 0.0986 | = 0.6665 | |||
| C | MC 044 | First 4 mm | Forepaw withdrawals | KW, = 0.0079 | Dunn’s | = 0.0252 | > 0.9999 | |
| 8 | A2 | MC 044 | First 4 mm | Crossings | KW, = 0.1167 | Dunn’s | = 0.6330 | = 0.7078 |
| Second 4 mm | Crossings | KW, = 0.0035 | Dunn’s | = 0.0079 | > 0.9999 | |||
| B1 | First 4 mm | Escape latency | KW, = 0.6266 | Dunn’s | > 0.9999 | = 0.6671 | ||
| Second 4 mm | Escape latency | KW, = 0.0031 | Dunn’s | = 0.0178 | > 0.9999 | |||
| C | von Frey | - | Ipsilateral 50% PWT – 5 s application | KW, = 0.0002 | Dunn’s | > 0.9999 | = 0.0063 | |
| - | Contralateral 50% PWT – 5 s application | KW, > 0.9999 | Dunn’s | > 0.9999 | > 0.9999 |
Summary of planned comparisons between naïve and neural injury groups to sham controls. The experiment column refers to timelines of mechanical conflict tests shown in Figure 1 (corresponding figure label, experiment: 1A, MC 10 minute; 1B-D, MC baseline; 1B, MC 01234; 1C-D, MC 044) or to standard reflex tests (von Frey or Hargreaves). The statistical test and corresponding post-hoc test was chosen based on whether data was normally distributed (Shapiro-Wilk test, results not shown). ANOVA, analysis of variance; KW, Kruskal-Wallis; MC, mechanical conflict; PWT, paw withdrawal threshold.
Table 2.
Summary of results comparing crossings within groups during probe trials
| Figure | Graph | Experiment | Probe Height | Group | Statistical Test, P | Post-hoc Test | Probe Height vs 0 mm Baseline | P |
|---|---|---|---|---|---|---|---|---|
| 4 | B1 | MC 01234 | 0, 1, 2, 3, 4 mm | Naïve | RM 1-way ANOVA, = 0.0804 | Dunnett’s | 1 mm | = 0.9783 |
| 2 mm | = 0.7718 | |||||||
| 3 mm | = 0.9998 | |||||||
| 4 mm | = 0.1088 | |||||||
| Sham | Friedman, < 0.0001 | Dunn’s | 1 mm | = 0.4792 | ||||
| 2 mm | = 0.1356 | |||||||
| 3 mm | = 0.0002 | |||||||
| 4 mm | < 0.0001 | |||||||
| SCI | Friedman, = 0.0009 | Dunn’s | 1 mm | = 0.8997 | ||||
| 2 mm | = 0.0504 | |||||||
| 3 mm | = 0.0185 | |||||||
| 4 mm | = 0.0150 | |||||||
| 5 | B1 | MC 044 | 0, first 4, second 4 mm | Naïve | RM 1-way ANOVA, = 0.0043 | Dunnett’s | First 4 mm | = 0.0454 |
| Second 4 mm | = 0.0142 | |||||||
| Sham | Friedman, < 0.0001 | Dunn’s | First 4 mm | = 0.0203 | ||||
| Second 4 mm | = 0.0011 | |||||||
| SCI | Friedman, < 0.0001 | Dunn’s | First 4 mm | = 0.0910 | ||||
| Second 4 mm | = 0.0006 | |||||||
| 8 | A1 | MC 044 | 0, first 4, second 4 mm | Naïve | Friedman, < 0.0001 | Dunn’s | First 4 mm | = 0.0003 |
| Second 4 mm | < 0.0001 | |||||||
| Sham | Friedman, < 0.0001 | Dunn’s | First 4 mm | = 0.0203 | ||||
| Second 4 mm | < 0.0001 | |||||||
| CCI | Friedman, < 0.0001 | Dunn’s | First 4 mm | = 0.0203 | ||||
| Second 4 mm | = 0.0011 |
3. Results
3.1. Multiple returns to the brightly lit chamber in the absence of probes and repeated crossing of noxious probes by uninjured, sham-operated, and SCI rats indicate that exploratory drive is very strong when the MC device is unfamiliar
Previous studies with the MC device used conditions in which escape from a bright light was assumed to be the primary motivation to cross noxious probes. We explored metrics in addition to the latency to escape the bright chamber to extract more information about probe-avoidance behavior. We first asked whether, upon introduction to the novel MC device, rats voluntarily crossed the middle chamber repeatedly when the bright light was on and when the noxious probes were raised. This study also allowed us to see if SCI rats >1 month post-injury exhibited deficiencies in mobility that might otherwise confound comparisons to uninjured controls. During their first 10-minute exposure to the MC device the probes were not elevated. Naive, sham-operated, and SCI rats made repeated crossings of the middle chamber (Fig. 2A1), indicating that the bright light was not sufficiently aversive to suppress exploratory behavior during the initial exposure to the MC device. All groups also repeatedly crossed the middle chamber when the sharp probes were subsequently raised to a high 4 mm level (Fig. 2A2), indicating that addition of the noxious probes failed to suppress exploratory behavior under these conditions. Summarized data (Figures 2B1, B2) confirm that 1) the bright light did not prevent multiple crossings of the middle chamber in the absence of high probes, 2) the sham group, but not the SCI group, made significantly fewer crossings and stopped crossing sooner than the naive group when no probes were present, 3) exposure to the 4 mm probes greatly increased crossing variability within all groups, and 4) rats in the SCI group made a comparable number of crossings to those in the naive and sham groups, demonstrating that a contusive T10 SCI does not hinder their mobility. These results suggest there is a strong motivation to repeatedly cross the probes, likely from exploratory drive, which can dominate other influences (which may be complex) on crossing behavior when the MC device is novel. This further suggests that, with a limited degree of familiarization (only partial habituation of exploratory drive), counting crossings could serve as a useful metric for assessing pain-related changes in probe-avoidance behavior in conflict with exploratory behavior.
Figure 2.
The first exposures to the mechanical conflict device reveal a strong motivation to repeatedly explore the device despite an aversive bright light and (in the second session) sharp probes. Naive, sham-operated, and SCI rats were exposed to the MC device twice for 10 minutes, without probes on the first day and with 4-mm probes on the second day (see Figure 1A timeline). The bright light was on during both sessions. (A1, A2) The timing of every crossing in each session is shown for each rat. (B1, B2) Summary results for the number of crossings and timing of the final crossing indicate significant differences between sham and naive groups when no probes are present, and large variability among groups when the 4-mm probes are present. Planned comparisons (B1, B2) were performed using the Kruskal-Wallis test followed by Dunn’s post-hoc test. Data in (B1, B2) shown as mean ± SD for the vertical and horizontal axis. *P < 0.05, **P < 0.01, ***P < 0.001. MC, mechanical conflict; SCI, spinal cord injury.
When given three 5-minute familiarization sessions without probes in the MC device, a majority of rats in the naive, sham, and SCI groups quickly learned to exit the light chamber. Escape latencies measured 24 hours later during the single baseline trial without probes were not significantly different among the groups (Fig. 3A), and these values were similar to but slightly longer than the escape latencies without probes reported for rats that underwent more extensive familiarization and escape training 48. Many rats voluntarily crossed back into the light chamber as they explored the MC device during each familiarization session. The median number of crossings of the middle chamber measured 24 hours later during the single baseline trial without probes was not significantly different among groups (Fig. 3B), and most rats made repeated crossings for the duration of the 5 min baseline trial (Fig. 3C). This indicates that exploratory behavior of the MC apparatus in the baseline trial on Day 2 is not altered by surgical history, and suggests that, after the 3 standardized familiarization sessions, states such as general anxiety don’t differentially influence the behavior of rats in the naïve, sham, and SCI groups in the absence of elevated probes.
Figure 3.
Exploratory behavior in naive, sham-operated, and spinal cord injury rats in the mechanical conflict device during three familiarization sessions without noxious probes. Each session was 5 minutes long, spaced ~30 minutes apart, with a 5-minute test session 24 hours later serving as baseline for escape latencies and crossings for subsequent trials with elevated probes (see Figures 1B, C timelines). Escape latencies (A) and crossings (B) were not significantly different among naive, sham, and SCI groups. (C) Summary results for the number of crossings and time of the final crossing also do not reveal any significant differences among groups. (D) Two independent measures of SCI severity, the displacement of contused tissue (in μm) measured by the spinal impactor device (x-axis) and the BBB motor score measured 24 h after SCI (y-axis), indicate a majority of SCI rats had received a moderately severe contusive SCI, but this SCI did not prevent injured rats from exploring the MC device 1 month or longer after the SCI. Planned comparisons between groups in (A-C) performed using 1-way ANOVA or Kruskal-Wallis test followed by Dunnett’s or Dunn’s post-hoc test. Data in (A, B) shown as median with interquartile range, and data in (C) shown as mean ± SD. ANOVA, analysis of variance; BBB, Basso, Beatie, and Bresnahan locomotor rating scale; MC, mechanical conflict; SCI, spinal cord injury.
In principle, the lack of differences in crossing behavior found in the SCI group compared to the sham (see subsequent sections) and sometimes the naive groups (Figs. 2–3) might be the result of less spinal injury than produced in previous SCI studies. Two independent measures were used to assess SCI severity 19, 25, 58, tissue displacement during spinal impact recorded by the impactor device (in μm) and the BBB motor score measured 1 day after injury. Contusion displacements were typical for a 150 kdyne impact 19 and internally consistent with impact data collected by the prior surgeon in the lab (data not shown). The mean displacement of 947 ± 25 μm and the mean BBB score of 1.3 ± 0.3 one day after SCI were also consistent with a moderate SCI 19, 58. All rats in the sham group exhibited BBB scores of 21 for each hindpaw the day after surgery, indicating that unintended damage to the spinal cord during the T10 laminectomy had not occurred. Injury assessments indicate a majority of SCI rats (76%, 37/49 rats with BBB scores of a 0 or 1) used had moderately severe spinal injuries (Fig. 3D). Data shown in Figs. 3A–C are from SCI experiments using the 2-day protocols shown in Figs. 1B and 1C. Multiple returns to the light chamber occurred during familiarization sessions in all experiments (SCI and CCI; see below), suggesting that the motivation to continue exploring the MC device in the absence of noxious probes remained high enough to offset the aversiveness of the bright light, even after 3 exposures to the device, and that this motivation was not diminished by SCI.
3.2. Sham surgery for the SCI model persistently enhances pain-avoidance behavior in the MC test
Having found that 3 familiarization trials without elevated probes elicited similar exploratory behavior in naive, sham-operated, and SCI rats, we then addressed two questions: 1) how would exploratory behavior (as indicated by multiple crossings) be affected by noxious probes in the middle chamber, and 2) would the response to the probes be altered by prior surgical tissue injury or neural injury plus surgical injury? We began by giving rats in naive, sham, and SCI groups a series of trials (3 minutes each) with progressively greater probe heights (0 to 4 mm), spaced 30 minutes apart. No significant differences were found among the groups in the rats’ exploration during the baseline trial (0 mm) prior to elevating the probes (Figs 4A1, A2. However, rats in the SCI and sham, but not the naive, groups crossed the 3 and/or 4 mm probes significantly fewer times than they had crossed the middle chamber during the 0 mm baseline trial (Fig. 4B1). There was a trend for rats in the SCI group to cross the 1- and 2-mm probes fewer times than rats in the naive and sham groups, but post-hoc comparisons did not reveal significant differences (Kruskal-Wallis P = 0.09 for 1-mm and 1-way ANOVA P = 0.07 for 2-mm probes). No significant differences among groups were observed on the 3- and 4-mm probes for number of crossings (Fig. 4B2) or the escape latency during the first crossing (Fig. 4C1). While some SCI and sham-operated rats showed much longer latencies, and a few refused to cross the probes even once, ~60–80% of the sham-operated and SCI rats had latencies that were comparable to naive rats, as shown in a plot of the fraction of rats in each group exhibiting an escape latency equal to or less than the latency indicated on the x-axis (Fig. 4C2). No rats were excluded based on deviant latencies to cross the probes. Rats in the sham and SCI groups tended to cross the probes fewer times than naive rats (Fig. 4D1, D2), with ~40–60% of the SCI and sham-operated rats refusing to cross the 3 and 4 mm probes a second time. Altogether, the results shown in Figure 4 indicate that 1) exploratory behavior (indicated by multiple crossings) in naive rats shows little or no reduction by the noxious probes in the middle chamber and 2) surgical injury used as a sham control for the SCI procedure results in a possible tendency to increase avoidance of the noxious probes (reduced crossings) similar to that apparent in rats with SCI.
Figure 4.
Sham surgery for spinal cord injury enhances pain-avoidance behavior in the MC test. Rats were exposed successively to ascending probe heights from 0 to 4 mm (see Figure 1B timeline). (A1, A2) Times of successive crossings during the 0 mm baseline trial shown for each rat. Summary results show similar numbers and patterns of crossings in naive, sham, and SCI groups. (B1) Crossings on 3- and 4-mm probes were decreased in the sham and SCI groups. Crossings at each probe height were compared to the 0 mm baseline using a repeated measures 1-way ANOVA or Friedman test. Significance levels for sham (stars) and SCI (pound sign) groups shown for Dunn’s post-hoc tests. (B2) Planned comparisons on 3- and 4-mm probe trials revealed no significant reductions in crossings in sham and SCI groups. (C1, C2) Escape latencies were not different among groups. C2 plots the fraction of rats in each group exhibiting an escape latency equal to or less than the latency indicated on the x-axis. (D1, D2) Some sham and SCI rats refused to cross back into the light chamber, as indicated by 180-second second crossing latencies. D2 plots the fraction of rats in each group showing a latency to their second crossing of the probes equal to or less than the time indicated on the x-axis. Planned comparisons in (A2, B2, C1, and D1) were performed using 1-way ANOVA or Kruskal-Wallis followed by Tukey’s or Dunn’s post-hoc test. Data shown as mean ± SD (A2), mean ± SEM (B1) and median with interquartile range (B2, C1, and D1). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ANOVA, analysis of variance; MC, mechanical conflict; SCI, spinal cord injury.
We then modified the 2-day MC protocol to test the effects of two exposures to high probes without prior exposure to less noxious lower probe heights 48. There was no difference among the groups on the 0 mm baseline trial on the day of probe exposure (Fig. 5A1, A2). All groups showed decreases in the number of crossings on the probes compared to their baseline crossings without the probes, and this effect was significant for each group during the second 4 mm probe exposure (Fig. 5B1). Planned comparisons showed that rats in the sham group crossed the 4 mm probes fewer times than rats in the naive group during the first exposure to the probes (Fig. 5B2). Although, no statistically significant differences in crossings were observed during the second exposure, comparison of escape latencies (Fig. 5C1, C2) shows that a majority of rats in the sham and SCI groups (sham: 70%, 7/10 rats; SCI: 75%, 6/8 rats) refused to cross the probes even once during the second probe exposure, whereas only 1 of 8 naive rats (13%) refused to cross the probes. This suggests that sham-operated and SCI rats continued to display greater pain-avoidance behavior than naive rats on the second probe exposure, even though the naive rats showed some apparent sensitization from the first noxious probe exposure. The escape latencies on the second 4-mm trials showed a distinct bimodal distribution (Fig. 5C1), with a tendency for SCI latencies to be greater than those in the sham group (Fig. 5C1, C2). These observations indicate that sham surgery for the SCI procedure is sufficient to persistently increase the aversiveness of the probes and enhance pain-avoidance behavior, and that the 2-day MC test reveals similarly increased aversion in the sham-operated and SCI rats.
Figure 5.
Sham surgery for spinal cord injury enhances pain-avoidance behavior compared to naive, uninjured rats in an abbreviated mechanical conflict test. Rats were exposed to the 4-mm probes, twice (see Figure 1C timeline). (A1, A2) Times of successive crossings during the 0 mm baseline trial shown for each rat. Summary results show similar numbers and patterns of crossings in naive, sham, and SCI groups. (B1) Significantly reduced crossings in naive and sham groups during the first probe trial, and all groups during the second probe trial. (B2) Planned comparisons between groups show sham rats made fewer crossings than naive rats on the first probe trial, and SCI rats were not different from shams. A majority of SCI rats (75%, 6 out of 8) refused to cross during the second probe trial, while only 30% of shams (3 out of 6) and 13% of naive rats (1 out of 8) refused. (C1, C2) Escape latencies on the second probe trial reflected crossing results in (B2), but no significant differences were observed. C2 plots the cumulative percentage of rats in each group exhibiting an escape latency equal to or less than the latency indicated on the x-axis. Crossings for groups during both probe trials in (B1) were compared to the 0-mm baseline using a repeated-measures 1-way ANOVA or Friedman test. Significance levels for naive (stars), sham (pound sign), and SCI group (plus sign) shown for Dunnett’s or Dunn’s post-hoc tests. Planned comparisons between groups (A2, B2, and C1) were performed using a 1-way ANOVA or Kruskal-Wallis test followed by Dunnett’s or Dunn’s post-hoc tests. Data shown as mean ± SD (A2), mean ± SEM (B1) and median with interquartile range (B2, C1). *P < 0.05, **P < 0.01. ANOVA, analysis of variance; SCI, spinal cord injury.
3.3. Standard tests of reflex sensitivity indicate that sham surgery for SCI increases hindpaw heat sensitivity without decreasing mechanical threshold
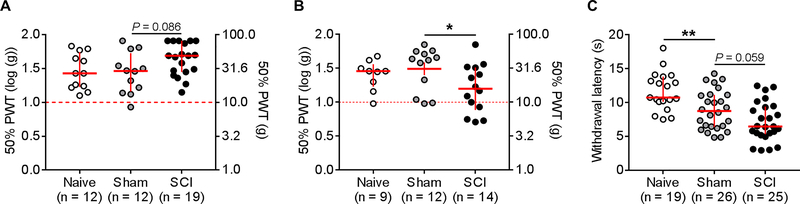
The sham surgery effect in the MC test raised the question of whether commonly used assays of reflex sensitivity, the von Frey mechanical threshold test and the Hargreaves radiant heat test, can also detect persistent differences between naive and sham-operated rats. Hindpaw sensitivity to von Frey filaments was tested using the Chaplan/Dixon up-down method to determine 50% threshold 20, 29, 30, using an extended range of filaments compared to many other studies (0.4–60.0 grams, starting filament = 6 grams, modified from 25) and log transformed for analysis and display (see 67). In the first set of experiments von Frey filaments were applied for ~1 second. Unexpectedly, we observed a trend for SCI rats to exhibit reduced mechanical sensitivity of the hindpaws when compared to sham-operated rats (Fig. 6A). Prior studies by several groups, including the Walters group, had reported below-level hypersensitivity after similar mid- to lower thoracic spinal contusion injuries 1, 2, 8, 19, 22, 25, 26, 38, 44, 46, 49, 58, 70, 92, 94, 97. Many groups, including the Grace group (see Section 3.5), use applications of von Frey filaments that are much longer than 1 second. Thus, we performed another study using ~5-second applications of each filament. The longer application method revealed mechanical hypersensitivity in the SCI group compared to the sham group (Fig. 6B), similar to what had been found previously using brief applications after SCI 8, 92, 94. Because filament applications were not timed precisely, it is possible that the tester using ~1-second applications in our recent experiments applied the filament more briefly or with a different velocity than did the testers in our published studies utilizing brief applications. Regardless of the cause, these divergent results show that major differences in test responses can result from what appear to be small differences in von Frey test procedures.
Figure 6.
Standard tests of reflex sensitivity show postsurgical enhancements to heat but not weak mechanical stimuli. (A) Using the Chaplan “up-down” method and applying von Frey filaments for ~1 second does not reveal significant enhancements of the 50% paw withdrawal threshold 1–2 months following spinal cord injury. Thresholds were similar in naive and sham groups, and SCI exhibited a trend for a reduction in mechanical sensitivity. Planned comparisons were made using a 1-way ANOVA. Corresponding gram forces shown on right axis. (B) Increasing the application time of the von Frey filaments to ~5 seconds reveals a significant enhancement of the 50% PWT in SCI rats 1–2 months after injury. Planned comparisons made using a Kruskal-Wallis test followed by Dunn’s post-hoc test. Corresponding gram forces shown on right axis. (C) Withdrawal latency to a heat stimulus was significantly lower in sham rats and there was a trend for SCI rats to exhibit shorter latencies than shams. Groups were compared using a 1-way ANOVA followed by Dunnett’s post-hoc test. Data in (A-C) shown as median with interquartile range. *P < 0.05, **P < 0.01. ANOVA, analysis of variance; PWT, paw withdrawal threshold; SCI, spinal cord injury.
Randomly selected subsets of the naive, sham-operated, and SCI rats were tested using the Hargreaves radiant heat test. The sham group exhibited a significant reduction in withdrawal latency, and there was a trend for the SCI group to exhibit an even greater reduction than found in the sham group (Fig. 6C). Together, these results suggest that sham surgery can induce a modest increase in hindpaw sensitivity to noxious heat without increasing mechanical sensitivity, and that passive reflex tests of mechanical threshold may fail to disclose persistent pain-like alterations after sham surgery that can be revealed by an appropriate operant test.
3.4. Sham surgery for SCI increases forepaw sensitivity as assessed by withdrawals evoked by sharp probes during the MC test
Rats with SCI exhibit at- and above-level mechanical hypersensitivity 8, 18 as shown by increased responsiveness to von Frey filaments applied to forepaws and a rostral region of the back. Although one study found no correlation between SCI-induced paw hypersensitivity measured with von Frey filaments and escape latency measured in the MC test 23, our observations suggested that forepaw hypersensitivity after injury might be expressed during voluntary behavior. Video analysis showed that the rats often pause before crossing and use their forepaws to investigate the probes. Attempts to establish weight support on the probes with their forepaws often produced a rapid withdrawal response (Fig. 7A). To test whether prior injury produced forepaw hypersensitivity expressed during voluntary behavior, the numbers of rapid forepaw withdrawals from the 1, 2, 3, and 4 mm probes made during initial investigation of the probes and immediately after the first crossing were counted. There was a trend for sham and SCI rats to increase the number of forepaw withdrawals as probe height increased, but there were no statistically significant differences between naive vs sham and sham vs SCI rats at any probe height (Fig. 7B, left panel). Averaging across all probe heights to increase statistical power yielded a trend for sham rats to increase the number of forepaw withdrawals compared to the naive group, and there was little difference between the sham and SCI rats (Fig. 7B, right panel).
Figure 7.
Sham surgery for SCI sensitizes forepaw withdrawals evoked during voluntary contact with noxious probes. (A, B) Sham-operated rats and SCI rats exhibited a trend to withdraw their forepaws from the noxious probes more than naive rats when tested successively on 1-, 2-, 3- and 4-mm probes (see Figure 1B timeline). (A) Example sequence of paw movements during probe investigation by a SCI rat. Rapid forepaw withdrawal – middle image, red arrow. (B) Median number withdrawals on each probe (left) and across all 4 probes (right). (C) Forepaw withdrawals were increased in the sham group during the first noxious probe trial, while the SCI group was not significantly different from the sham group (see Figure 1C timeline). A majority of rats in (C) received two 4-mm probe trials after the 0-mm baseline trial, but no comparisons were made during the second probe trial because a majority of injured rats refused to approach the probes. Data in (B, C) shown as median with interquartile range. Planned comparisons were performed using a Kruskal-Wallis test followed by Dunn’s post-hoc test. *P < 0.05. SCI, spinal cord injury.
Persistent sensitizing effects of sham surgery on forepaw responses to the probes were particularly evident in the 2-day protocol that tested sensitivity to high 4-mm probes without prior exposure to less elevated probes. During their first exposure to the probes, the sham group exhibited significantly more forepaw withdrawals than the naive group (Fig. 7C), but there was no difference between sham and SCI groups. Statistical comparisons of forepaw withdrawals during the second exposure to the 4 mm probes were not performed because many of the rats in the SCI group completely avoided the probes, staying on the opposite side of the light chamber and facing away from the probes. These results suggest that, when challenged with a novel, moderately noxious substrate, injured rats investigate the substrate more carefully than uninjured rats do before deciding to cross, and this active, voluntary behavior can disclose heightened sensitivity of the forepaws to noxious stimuli (hyperalgesia) long after surgical injury.
3.5. Probe-avoidance behavior is also enhanced by sham surgery for the CCI peripheral nerve injury model
The studies above show that surgical damage to tissues, including muscle and bone, required to expose the spinal cord for controlled contusive injury is sufficient to persistently enhance probe-avoidance behavior. This hyperalgesic effect of sham surgery was shown in the operant MC test but not the von Frey reflex test, suggesting that the MC test may be a more sensitive test for pain evoked by mechanical stimuli. We asked whether the MC test might also reveal hyperalgesia produced by the sham surgery used for a common peripheral nerve injury model. While one previous study used an MC test to show that rats with a CCI of the sciatic nerve exhibit prolonged escape latencies 48, this study did not compare MC tests and von Frey tests, nor did it include sham controls. Mice with apparent allodynia (as assessed with von Frey tests) induced by spared nerve injury (SNI) were also found to exhibit prolonged escape latencies 78, but this study did not compare the SNI group to an to a sham group lacking drug treatment in the MC test. Thus, whether peripheral sham surgery is sufficient to enhance pain-avoidance behavior in the MC test and whether the von Frey test is a good predictor of pain-avoidance behavior after hindlimb surgery are unknown. To address these questions we used the sciatic nerve CCI model along with its sham surgery control 9, 39, 40. Rats in naive, sham, and CCI groups were tested 14 days post-surgery, a time when reported mechanical allodynia is well established 31, 39, 40. All groups showed significantly reduced crossings during both 4-mm probe trials compared to their baseline crossings (Fig. 8A1). Note the strong similarity in crossings between groups on the 0 mm baseline trial, indicating the prior familiarization trials had eliminated obvious effects of injury on crossing behavior in the absence of noxious probes. While no significant differences among the groups were observed on the first 4-mm trial, the sham group crossed significantly fewer times than the naive group during the second 4-mm trial (Fig. 8A2). Differences in crossings between sham and CCI groups were statistically indistinguishable. Prolonged escape latencies were often observed in the sham and CCI groups (Fig. 8B1, B2), with a majority of injured rats (60%, 6/10 rats in both sham and CCI groups) refusing to cross even once in the second 4-mm trial. In contrast to the robust hyperalgesic effects found in the sham group in the MC test, no evidence of mechanical hypersensitivity (allodynia) was found in the sham group using the von Frey test (Fig. 8C). In contrast and as expected, CCI produced strong mechanical hypersensitivity in the hindpaw ipsilateral but not contralateral to the injury (Fig. 8C, left panel). In sum, sham-operated rats for the CCI peripheral nerve injury model failed to exhibit mechanical hypersensitivity in von Frey tests that provided evidence for allodynia in CCI rats, yet the sham group showed clear evidence of enhanced pain avoidance in the operant MC test.
Figure 8.
Sham surgery for the chronic constriction injury (CCI) of the sciatic nerve enhances pain-avoidance behavior in a mechanical conflict test. Rats were exposed to the 4-mm probes twice (see Figure 1D timeline). (A1) All groups exhibited a reduction in crossings during both 4-mm probe trials compared to the 0-mm baseline (Friedman test). Significance levels for naive (stars), sham (pound sign), and CCI group (plus sign) shown for Dunn’s post-hoc tests. (A2) No significant differences in crossings were observed on the first probe trial, but sham-operated rats crossed fewer times than naive rats during the second probe trial. A majority of sham and CCI rats (60%, 6 out of 10 in both groups) refused to cross during the second probe trial. (B1, B2) Escape latencies reflected the crossing results and revealed a significant difference between naive and sham groups. B2 plots the cumulative percentage of rats in each group exhibiting an escape latency equal to or less than the latency indicated on the x-axis. (C, left panel) In a subset of rats that were tested using the standard reflex test of mechanical sensitivity, the 50% paw withdrawal threshold of the hindpaw ipsilateral to the side of injury was reduced in rats with CCI, but not on the contralateral (uninjured) hindpaw (C, right panel). Note that 50% PWT measures <1 gram are negative after log transformation. Corresponding gram forces shown on right axis. Planned comparisons between groups in (A2, B1, and C) were performed using a Kruskal-Wallis test followed by Dunn’s post-hoc tests. Data shown as mean ± SEM (A1) and median with interquartile range (A2, B1, and C). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. CCI, chronic constriction injury; PWT, paw withdrawal threshold.
4. Discussion
A novel variant of an operant conflict test 48 that pits rats’ exploratory drive plus their aversion to bright light against pain elicited by voluntary crossing of noxious probes has revealed that sham surgeries used as controls for neuropathic pain models (SCI and CCI) can increase pain-avoidance behavior for long periods after surgery. This finding has significant implications for preclinical investigations of postsurgical and neuropathic pain.
4.1. Implications of a sensitive measure of pain-avoidance behavior for preclinical investigations of postsurgical pain
Postsurgical pain is a pervasive clinical problem 24, 53, 77, 82, 88. Patients often develop severe postsurgical pain 33, 37 that can become chronic 81, 89. Nonetheless, investigations of postsurgical pain are underrepresented in animal research 21, 24, 33, 56. Both clinical and animal data show that postsurgical pain is greater for deeper, more extensive incisions 21, 56. Sham surgeries for most nerve injury models produce deep tissue damage. Furthermore, relatively mild incision models and more severe postsurgical models in rodents can produce transient to chronic signs of behavioral hypersensitivity, ongoing pain, and nociceptor hyperexcitability 3, 14, 16, 17, 40, 55, 59, 65, 66, 93. Our contusive SCI model involves substantial tissue injury, requiring removal of bone (the T10 dorsal process and lamina) and blunt dissection of paravertebral muscles. A recent study demonstrates that sham surgery for SCI increases immune cell trafficking in surrounding muscle and bone 48 hours post-surgery 15, suggesting that prolonged immune cell activation may play a role in postsurgical pain near the lesion site. Surgery for the CCI model requires exposure of the sciatic nerve and retraction of neighboring skin, muscles, and nerves 34, which may induce postsurgical pain 35. Considering the degree of tissue damage associated with sham surgery procedures for neuropathic pain models, it is not surprising that persistent pain-like alterations are revealed by interaction with noxious probes in the MC device. Postsurgical alterations may involve direct effects on nociceptive pathways and indirect stimulation from movements of inflamed, previously damaged tissues during behavioral tests. Direct alterations of nociceptive pathways have been shown by increased excitability of primary nociceptors observed months after sham surgery for SCI, and expressed as decreased rheobase, increased membrane resistance, and increased incidence of spontaneous activity 8, 72.
Why has so little evidence been reported previously for long-lasting pain following sham surgeries for neuropathic pain models (for exceptions, see 51, 54, 83)? One reason may be that motivational states such as pain compete with many other complicating motivational influences, such as fear of a human tester. Appropriate adjustment of competing motivational influences on voluntary behavior, as we did with exploratory drive in the present study, may permit operant responses to reveal otherwise masked states such as persistent postoperative pain. Another reason may be limitations of the reflex tests investigators largely rely on as indicators of allodynia and hyperalgesia. These tests may only be weakly related to motivational and cognitive dimensions of pain 68, 86, 96, and can be highly sensitive to minor variations in procedure, such as the duration of von Frey filament application (Fig. 6A–B). Moreover, because these tests usually depend on hand-delivered stimuli and subjective assessment of reflex responses, they are prone to investigator-dependent sources of variability including unconscious bias and differential exposure to human pheromones 12, 80. Furthermore, results from naive (uninjured) or sham controls are not always reported, and the sham surgery employed (e.g., skin incision alone) may not match the severity of deep surgical procedures used to produce neural injury (e.g., 74, 90).
Previous studies using the MC test reported no evidence for postoperative pain in sham controls for central or peripheral neural injury. In several of these studies, sham controls were not included 23, 48, 64. A spared nerve injury (SNI) study in mice 78 demonstrated that SNI, like CCI in rats (which involves a similar surgery) 48, increased escape latency, which was attenuated by an analgesic. However, this study did not include a vehicle-treated sham group or naive group so potential effects on pain-avoidance behavior after sham surgery could not be assessed. Interestingly, this mouse study hinted at a bimodal distribution of escape latencies, raising the possibility that an MC protocol like that described here might reveal increased pain avoidance after sham surgeries in mice.
4.2. Implications of enhanced pain avoidance behavior for preclinical investigations of neuropathic pain
The overriding goal of this study was to test the hypothesis that appropriate operant conditions can reveal a persistent pain-like state induced by sham surgeries in common neuropathic pain models, which was tested statistically by planned comparisons between naive and sham-operated groups. We also used planned comparisons to ask whether these conditions would distinguish pain-like behavior measured in the sham versus the SCI or CCI groups. In general, no significant differences were found in the MC tests between sham and SCI or CCI groups, although a weak trend for greater pain-avoidance behavior in the SCI than sham group was sometimes seen (Fig. 5C1–C2). The similarity in operant behavior between sham and neuropathic models indicates that SCI and CCI procedures, like the corresponding surgery that is part of the SCI and CCI procedures, induce persistent pain-avoidance behavior, as reported previously for SCI 23, 64 and CCI 48. The surprising similarity between sham groups and both the SCI and CCI groups in the 2-day MC protocol suggests that this test is very sensitive for detecting persistent pain-related states, but in its present form it is not optimal for distinguishing qualities or degrees of different types of pain. In contrast to the similar pain-avoidance behavior of SCI and sham groups described here, many studies have found differences between sham and SCI groups in allodynia-like, hyperalgesia-like, and spontaneous pain-like behaviors 27, 38, 45, 60, 79, 87, and their associated neuronal correlates 7, 8, 18, 72, 94.
The open question of whether neuropathic pain plus postsurgical pain is more severe than postsurgical pain alone in these models is an important one that might be addressed using the 2-day MC protocol by adjusting exploratory drive and/or the aversiveness of the noxious probes to a level that prevents multiple crossings by rats with neural injury but not by rats with sham surgery. Although unlikely in light of clinical observations 56, the possibility exists that the motivation to avoid evoked pain might not be substantially greater for these models of neuropathic pain than for the postsurgical pain produced by their sham surgeries. Even if evoked pain turns out to be similar after sham surgery and neural injury, another distinction between sham-operated and neuropathic rats is emerging: SCI and some peripheral neuropathic pain models, but not their sham surgeries, can induce persistent ongoing/spontaneous pain as measured by the CPP test 4, 42, 50, 57, 76, 91, 94. Thus, enhancement of evoked pain sensitivity may be relatively similar in animals with postsurgical pain and animals with neuropathic pain, whereas injury involving damage to large nerve branches may be more likely to induce persistent ongoing pain than is tissue injury that spares nerve branches.
Clues about possible mechanistic differences between postsurgical and neuropathic pain might be found in properties of nociceptors likely to be activated by noxious probes in the MC test. Pain-related behavior after thoracic SCI has been correlated with hyperactivity in widespread primary nociceptors 8. Antisense knockdown of a Na+ channel (Nav1.8) preferentially expressed in nociceptors and required for SCI-induced nociceptor hyperactivity reverses reflex hypersensitivity and prevents CPP after SCI 94. This indicates that nociceptor hyperactivity can play a large role in promoting both pain hypersensitivity and ongoing pain after SCI. While nociceptors from SCI rats show dramatic, persistent increases in excitability and spontaneous activity (SA), similar but much weaker alterations were found in nociceptors isolated from sham-operated rats 8, 72. If the altered nociceptor rheobase and membrane resistance produced by sham surgery also occur in nociceptors’ peripheral terminals, firing evoked by noxious stimuli such as the sharp probes in the MC test should be enhanced, contributing to enhanced postsurgical pain-avoidance behavior. Sham-operated rats pooled across these two published electrophysiological studies also showed an increase in the fraction of isolated nociceptors exhibiting SA (20% of neurons in the sham versus 9% in the naive groups), but this incidence was far lower than the 54% of the pooled SCI group showing SA. Given the evidence that SA in nociceptors can drive ongoing pain 94, these results plus indications from various neuropathic pain and incision models 21, 33, 75, 81 suggest that postsurgical pain may be manifested primarily as enhanced pain evoked by extrinsic stimuli (hyperalgesia and allodynia) whereas neuropathic pain in these models can be manifested both as hypersensitivity to extrinsic stimuli and as ongoing pain. While nociceptor alterations produced by tissue and nerve injury may be indirectly related to allodynia, they probably contribute directly to hyperalgesia and ongoing pain 72. Investigation of the effects on sham-surgery-induced pain-avoidance behavior produced by analgesic drugs selective for nerve injury pain and for inflammatory pain should be useful for distinguishing persistent postsurgical pain from related effects of injury, such as anxiety, and for guiding continuing mechanistic analysis.
Acknowledgements
We thank Carmen W. Dessauer and Alexis G. Bavencoffe for very helpful feedback, as well as Daniel Liaou for his insightful observations of rats in the MC test and for technical assistance. We also thank Sabina Lorca, Tamara McGhee, Kendra Wicks, Guo-Ying Xu, Anibal Carbajal Garza, and Elia Lopez for technical assistance and animal care.
Funding and Conflicts of Interest
This work was supported by National Institute of Neurological Diseases and Stroke Grant NS091759 to C.W.D. and E.T.W.; a subcontract to E.T.W. from Manzanita Pharmaceuticals Inc. and US Army Medical Research Grant W81XWH-15-1-0702 to E.T.W.; a Zilkha Family Fellowship to M.A.O; U.S. Army Medical Research and Materiel Command Grant W81XWH-16-1-0717 to P.M.G.; University of Texas System Rising STARs Award to P.M.G.; and start-up funds, University of Texas MD Anderson Cancer Center. The authors declare that 1 of 3 MC devices used for this study was provided without charge to E.T.W. by Coy Laboratory Products, Inc.
References
- [1].Baastrup C, Jensen TS, Finnerup NB. Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res 2011;1370:129–135. [DOI] [PubMed] [Google Scholar]
- [2].Baastrup C, Maersk-Moller CC, Nyengaard JR, Jensen TS, Finnerup NB. Spinal-, brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: evaluation of pain-like behavior. Pain 2010;151:670–679. [DOI] [PubMed] [Google Scholar]
- [3].Banik RK, Brennan TJ. Sensitization of primary afferents to mechanical and heat stimuli after incision in a novel in vitro mouse glabrous skin-nerve preparation. Pain 2008;138:380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bannister K, Qu C, Navratilova E, Oyarzo J, Xie JY, King T, Dickenson AH, Porreca F. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain 2017;158:2386–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tests Barrot M. and models of nociception and pain in rodents. Neuroscience 2012;211:39–50. [DOI] [PubMed] [Google Scholar]
- [6].Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995;12:1–21. [DOI] [PubMed] [Google Scholar]
- [7].Bavencoffe A, Li Y, Wu Z, Yang Q, Herrera J, Kennedy EJ, Walters ET, Dessauer CW. Persistent Electrical Activity in Primary Nociceptors after Spinal Cord Injury Is Maintained by Scaffolded Adenylyl Cyclase and Protein Kinase A and Is Associated with Altered Adenylyl Cyclase Regulation. J Neurosci 2016;36:1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Grill RJ, Carlton SM, Walters ET. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci 2010;30:14870–14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988;33:87–107. [DOI] [PubMed] [Google Scholar]
- [10].Berge OG. Predictive validity of behavioural animal models for chronic pain. Br J Pharmacol 2011;164:1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Birke LIA, Archer J: Some issues and problems in the study of animal exploration In Exploration in Animals and Humans (Archer J, Birke LIA, eds). Cambridge, UK: Van Nostrand Reinhold, 1983, pp 1–21 [Google Scholar]
- [12].Bove G Mechanical sensory threshold testing using nylon monofilaments: the pain field’s “tin standard”. Pain 2006;124:13–17. [DOI] [PubMed] [Google Scholar]
- [13].Bravo L, Alba-Delgado C, Torres-Sanchez S, Mico JA, Neto FL, Berrocoso E. Social stress exacerbates the aversion to painful experiences in rats exposed to chronic pain: the role of the locus coeruleus. Pain 2013;154:2014–2023. [DOI] [PubMed] [Google Scholar]
- [14].Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain 1996;64:493–501. [DOI] [PubMed] [Google Scholar]
- [15].Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Förstera B, Zhao S, Bhatia HS, Parra-Damas A, Mrowka L, Theodorou D, Rempfler M, Xavier ALR, Kress BT, Benakis C, Steinke H, Liebscher S, Bechmann I, Liesz A, Menze B, Kerschensteiner M, Nedergaard M, Ertürk A. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat Neurosci 2019;22:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Campillo A, Cabañero D, Romero A, García-Nogales P, Puig MM. Delayed postoperative latent pain sensitization revealed by the systemic administration of opioid antagonists in mice. Eur J Pharmacol 2011;657:89–96. [DOI] [PubMed] [Google Scholar]
- [17].Cao J, Wang PK, Tiwari V, Liang L, Lutz BM, Shieh KR, Zang WD, Kaufman AG, Bekker A, Gao XQ, Tao YX. Short-term pre- and post-operative stress prolongs incision-induced pain hypersensitivity without changing basal pain perception. Mol Pain 2015;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 2009;147:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Carter MW, Johnson KM, Lee JY, Hulsebosch CE, Gwak YS. Comparison of Mechanical Allodynia and Recovery of Locomotion and Bladder Function by Different Parameters of Low Thoracic Spinal Contusion Injury in Rats. Korean J Pain 2016;29:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [21].Chapman CR, Vierck CJ. The Transition of Acute Postoperative Pain to Chronic Pain: An Integrative Overview of Research on Mechanisms. J Pain 2017;18:359.e1–359.e38. [DOI] [PubMed] [Google Scholar]
- [22].Chen Z, Park J, Butler B, Acosta G, Vega-Alvarez S, Zheng L, Tang J, McCain R, Zhang W, Ouyang Z, Cao P, Shi R. Mitigation of sensory and motor deficits by acrolein scavenger phenelzine in a rat model of spinal cord contusive injury. J Neurochem 2016;138:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chhaya SJ, Quiros-Molina D, Tamashiro-Orrego AD, Houlé JD, Detloff MR. Exercise-Induced Changes to the Macrophage Response in the Dorsal Root Ganglia Prevent Neuropathic Pain after Spinal Cord Injury. J Neurotrauma 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Clarke H, Poon M, Weinrib A, Katznelson R, Wentlandt K, Katz J. Preventive analgesia and novel strategies for the prevention of chronic post-surgical pain. Drugs 2015;75:339–351. [DOI] [PubMed] [Google Scholar]
- [25].Detloff MR, Clark LM, Hutchinson KJ, Kloos AD, Fisher LC, Basso DM. Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Exp Neurol 2010;225:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol 2008;212:337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Detloff MR, Wade RE, Houlé JD. Chronic at- and below-level pain after moderate unilateral cervical spinal cord contusion in rats. J Neurotrauma 2013;30:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods 1997;76:183–191. [DOI] [PubMed] [Google Scholar]
- [29].Dixon WJ. The up-and-down method for small samples. Journal of the American Statistical Association 1965;60:967–978. [Google Scholar]
- [30].Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev 1991;15:47–50. [DOI] [PubMed] [Google Scholar]
- [31].Dowdall T, Robinson I, Meert TF. Comparison of five different rat models of peripheral nerve injury. Pharmacol Biochem Behav 2005;80:93–108. [DOI] [PubMed] [Google Scholar]
- [32].Eilam D Of mice and men: building blocks in cognitive mapping. Neurosci Biobehav Rev 2014;47:393–409. [DOI] [PubMed] [Google Scholar]
- [33].Eisenach JC, Brennan TJ. Pain after surgery. Pain 2018;159:1010–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Flatters SJ. Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR). Pain 2008;135:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Flatters SJ. Effect of analgesic standards on persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR). Neurosci Lett 2010;477:43–47. [DOI] [PubMed] [Google Scholar]
- [36].Fuchs PN, McNabb CT. The place escape/avoidance paradigm: a novel method to assess nociceptive processing. J Integr Neurosci 2012;11:61–72. [DOI] [PubMed] [Google Scholar]
- [37].Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin 2014;30:149–160. [DOI] [PubMed] [Google Scholar]
- [38].Garraway SM, Woller SA, Huie JR, Hartman JJ, Hook MA, Miranda RC, Huang YJ, Ferguson AR, Grau JW. Peripheral noxious stimulation reduces withdrawal threshold to mechanical stimuli after spinal cord injury: role of tumor necrosis factor alpha and apoptosis. Pain 2014;155:2344–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Grace PM, Strand KA, Galer EL, Rice KC, Maier SF, Watkins LR. Protraction of neuropathic pain by morphine is mediated by spinal damage associated molecular patterns (DAMPs) in male rats. Brain Behav Immun 2018;72:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 2016;113:E3441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. J Pain 2013;14:1255–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Griggs RB, Bardo MT, Taylor BK. Gabapentin alleviates affective pain after traumatic nerve injury. Neuroreport 2015;26:522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Griggs RB, Donahue RR, Adkins BG, Anderson KL, Thibault O, Taylor BK. Pioglitazone Inhibits the Development of Hyperalgesia and Sensitization of Spinal Nociresponsive Neurons in Type 2 Diabetes. J Pain 2016;17:359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gwak YS, Hassler SE, Hulsebosch CE. Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain 2013;154:1699–1708. [DOI] [PubMed] [Google Scholar]
- [45].Gwak YS, Hulsebosch CE, Leem JW. Neuronal-Glial Interactions Maintain Chronic Neuropathic Pain after Spinal Cord Injury. Neural Plast 2017;2017:2480689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hama AT, Plum AW, Sagen J. Antinociceptive effect of ambroxol in rats with neuropathic spinal cord injury pain. Pharmacol Biochem Behav 2010;97:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988;32:77–88. [DOI] [PubMed] [Google Scholar]
- [48].Harte SE, Meyers JB, Donahue RR, Taylor BK, Morrow TJ. Mechanical Conflict System: A Novel Operant Method for the Assessment of Nociceptive Behavior. PLoS One 2016;11:e0150164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hassler SN, Johnson KM, Hulsebosch CE. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. J Neurochem 2014;131:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].He Y, Tian X, Hu X, Porreca F, Wang ZJ. Negative reinforcement reveals non-evoked ongoing pain in mice with tissue or nerve injury. J Pain 2012;13:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology 2004;101:476–487. [DOI] [PubMed] [Google Scholar]
- [52].Hughes RN. Intrinsic exploration in animals: motives and measurement. Behav Processes 1997;41:213–226. [DOI] [PubMed] [Google Scholar]
- [53].Humble SR, Dalton AJ, Li L. A systematic review of therapeutic interventions to reduce acute and chronic post-surgical pain after amputation, thoracotomy or mastectomy. Eur J Pain 2015;19:451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Inyang KE, Szabo-Pardi T, Wentworth E, McDougal TA, Dussor G, Burton MD, Price TJ. The antidiabetic drug metformin prevents and reverses neuropathic pain and spinal cord microglial activation in male but not female mice. Pharmacol Res 2019;139:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kajita Y, Suetomi K, Okada T, Ikeuchi M, Arai YC, Sato K, Ushida T. Behavioral and neuropathological changes in animal models of chronic painful scar. J Orthop Sci 2013;18:1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–1625. [DOI] [PubMed] [Google Scholar]
- [57].King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci 2009;12:1364–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kloos AD, Fisher LC, Detloff MR, Hassenzahl DL, Basso DM. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp Neurol 2005;191:251–265. [DOI] [PubMed] [Google Scholar]
- [59].Kosta V, Kojundzić SL, Sapunar LC, Sapunar D. The extent of laminectomy affects pain-related behavior in a rat model of neuropathic pain. Eur J Pain 2009;13:243–248. [DOI] [PubMed] [Google Scholar]
- [60].Kramer JL, Minhas NK, Jutzeler CR, Erskine EL, Liu LJ, Ramer MS. Neuropathic pain following traumatic spinal cord injury: Models, measurement, and mechanisms. J Neurosci Res 2017;95:1295–1306. [DOI] [PubMed] [Google Scholar]
- [61].LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol 2000;163:490–494. [DOI] [PubMed] [Google Scholar]
- [62].LaGraize SC, Borzan J, Peng YB, Fuchs PN. Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system. Exp Neurol 2006;197:22–30. [DOI] [PubMed] [Google Scholar]
- [63].LaGraize SC, Fuchs PN. GABAA but not GABAB receptors in the rostral anterior cingulate cortex selectively modulate pain-induced escape/avoidance behavior. Exp Neurol 2007;204:182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lau D, Harte SE, Morrow TJ, Wang S, Mata M, Fink DJ. Herpes simplex virus vector-mediated expression of interleukin-10 reduces below-level central neuropathic pain after spinal cord injury. Neurorehabil Neural Repair 2012;26:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology 2004;101:191–203. [DOI] [PubMed] [Google Scholar]
- [66].Massie JB, Huang B, Malkmus S, Yaksh TL, Kim CW, Garfin SR, Akeson WH. A preclinical post laminectomy rat model mimics the human post laminectomy syndrome. J Neurosci Methods 2004;137:283–289. [DOI] [PubMed] [Google Scholar]
- [67].Mills C, Leblond D, Joshi S, Zhu C, Hsieh G, Jacobson P, Meyer M, Decker M. Estimating efficacy and drug ED50’s using von Frey thresholds: impact of weber’s law and log transformation. J Pain 2012;13:519–523. [DOI] [PubMed] [Google Scholar]
- [68].Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci 2009;10:283–294. [DOI] [PubMed] [Google Scholar]
- [69].Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals. Pain 2004;112:12–15. [DOI] [PubMed] [Google Scholar]
- [70].Nesic O, Lee J, Johnson KM, Ye Z, Xu GY, Unabia GC, Wood TG, McAdoo DJ, Westlund KN, Hulsebosch CE, Regino Perez-Polo J. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J Neurochem 2005;95:998–1014. [DOI] [PubMed] [Google Scholar]
- [71].Odem MA. Behavioral insights into nociceptor function: A systematic approach to understanding postsurgical and neuropathic pain mechanisms in rats. Ph.D. Dissertation The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences, Program in Neuroscience, Houston, Texas: 2018; 194 pages. https://digitalcommons.library.tmc.edu/utgsbs_dissertations/909/ [Google Scholar]
- [72].Odem MA, Bavencoffe AG, Cassidy RM, Lopez ER, Tian J, Dessauer CW, Walters ET. Isolated nociceptors reveal multiple specializations for generating irregular ongoing activity associated with ongoing pain. Pain 2018;159:2347–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pahng AR, Paulsen RI, McGinn MA, Edwards KN, Edwards S. Neurobiological Correlates of Pain Avoidance-Like Behavior in Morphine-Dependent and Non-Dependent Rats. Neuroscience 2017;366:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pan B, Yu H, Park J, Yu YP, Luo ZD, Hogan QH. Painful nerve injury upregulates thrombospondin-4 expression in dorsal root ganglia. J Neurosci Res 2015;93:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Porreca F, Navratilova E. Reward, motivation, and emotion of pain and its relief. Pain 2017;158 Suppl 1:S43–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain 2011;152:1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rashiq S, Dick BD. Post-surgical pain syndromes: a review for the non-pain specialist. Can J Anaesth 2014;61:123–130. [DOI] [PubMed] [Google Scholar]
- [78].Shepherd AJ, Mohapatra DP. Pharmacological validation of voluntary gait and mechanical sensitivity assays associated with inflammatory and neuropathic pain in mice. Neuropharmacology 2018;130:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shiao R, Lee-Kubli CA. Neuropathic Pain After Spinal Cord Injury: Challenges and Research Perspectives. Neurotherapeutics 2018;15:635–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 2014;11:629–632. [DOI] [PubMed] [Google Scholar]
- [81].Tappe-Theodor A, Kuner R. Studying ongoing and spontaneous pain in rodents--challenges and opportunities. Eur J Neurosci 2014;39:1881–1890. [DOI] [PubMed] [Google Scholar]
- [82].Thomazeau J, Rouquette A, Martinez V, Rabuel C, Prince N, Laplanche JL, Nizard R, Bergmann JF, Perrot S, Lloret-Linares C. Predictive Factors of Chronic Post-Surgical Pain at 6 Months Following Knee Replacement: Influence of Postoperative Pain Trajectory and Genetics. Pain Physician 2016;19:E729–41. [PubMed] [Google Scholar]
- [83].Vierck CJ, Acosta-Rua AJ, Johnson RD. Bilateral chronic constriction of the sciatic nerve: a model of long-term cold hyperalgesia. J Pain 2005;6:507–517. [DOI] [PubMed] [Google Scholar]
- [84].Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing. Pain 2008;135:7–10. [DOI] [PubMed] [Google Scholar]
- [85].Vierck CJ, Kline R, Wiley RG. Comparison of operant escape and innate reflex responses to nociceptive skin temperatures produced by heat and cold stimulation of rats. Behav Neurosci 2004;118:627–635. [DOI] [PubMed] [Google Scholar]
- [86].Vierck CJ, Yezierski RP. Comparison of operant escape and reflex tests of nociceptive sensitivity. Neurosci Biobehav Rev 2015;51:223–242. [DOI] [PubMed] [Google Scholar]
- [87].Walters ET. Neuroinflammatory contributions to pain after SCI: roles for central glial mechanisms and nociceptor-mediated host defense. Exp Neurol 2014;258:48–61. [DOI] [PubMed] [Google Scholar]
- [88].Wang SJ, Jiang SD, Jiang LS, Dai LY. Axial pain after posterior cervical spine surgery: a systematic review. Eur Spine J 2011;20:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Werner MU, Kongsgaard UE. I. Defining persistent post-surgical pain: is an update required. Br J Anaesth 2014;113:1–4. [DOI] [PubMed] [Google Scholar]
- [90].Wu HE, Gemes G, Zoga V, Kawano T, Hogan QH. Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain 2010;11:280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wu Z, Li L, Xie F, Du J, Zuo Y, Frost JA, Carlton SM, Walters ET, Yang Q. Activation of KCNQ Channels Suppresses Spontaneous Activity in Dorsal Root Ganglion Neurons and Reduces Chronic Pain after Spinal Cord Injury. J Neurotrauma 2017;34:1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wu Z, Yang Q, Crook RJ, O’Neil RG, Walters ET. TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury. Pain 2013;154:2130–2141. [DOI] [PubMed] [Google Scholar]
- [93].Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology 2010;112:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Yang Q, Wu Z, Hadden JK, Odem MA, Zuo Y, Crook RJ, Frost JA, Walters ET. Persistent pain after spinal cord injury is maintained by primary afferent activity. J Neurosci 2014;34:10765–10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yekkirala AS, Roberson DP, Bean BP, Woolf CJ. Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov 2017;16:545–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yezierski RP, Hansson P. Inflammatory and Neuropathic Pain From Bench to Bedside: What Went Wrong. J Pain 2018;19:571–588. [DOI] [PubMed] [Google Scholar]
- [97].Zeng J, Kim D, Li KW, Sharp K, Steward O, Zaucke F, Luo ZD. Thrombospondin-4 contributes to spinal cord injury-induced changes in nociception. Eur J Pain 2013;17:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, Zamponi GW. Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell Rep 2015;12:752–759. [DOI] [PubMed] [Google Scholar]