Abstract
White matter damage is an important contributor to long-term neurological deficit after stroke. Our previous study has shown that inhibition of CD147 ameliorates acute ischemic stroke in mice. In this study, we aimed to investigate whether inhibition of CD147 promotes white matter repair and long-term functional recovery after ischemic stroke. Male adult C57BL/6 mice were subjected to transient (1-hour) middle cerebral artery occlusion (tMCAO). Anti-CD147 function–blocking antibody (αCD147) was injected intravenously once daily for 3 days beginning 4 hours after onset of ischemia. Sensorimotor and cognitive functions were evaluated up to 28 days after stroke. We found that αCD147 treatment not only prevented neuronal and oligodendrocyte cell death in the acute phase, but also profoundly protected white matter integrity and reduced brain atrophy and tissue loss in the late phase, leading to improved sensorimotor and cognitive functions for at least 28 days after stroke. Mechanistically, we found that αCD147 treatment increased the number of proliferating NG2(+)/PDGFRα(+) oligodendrocyte precursor cells (OPCs) and newly generated mature APC(+)/Sox10(+) oligodendrocytes after stroke, possibly through upregulation of SDF-1/CXCR4 axis in OPCs. In conclusion, inhibition of CD147 promotes long-term functional recovery after stroke, at least in part, by enhancing oligodendrogenesis and white matter repair.
Keywords: CD147, oligodendrocyte precursor cells, oligodendrocytes, white matter, stroke
Introduction
Stroke is a leading cause of permanent disability and mortality worldwide. It is well known that ischemic stroke can cause gray matter injury; however, white matter injury occupies nearly half of the average infarct volume and contributes to poor neurological outcomes in human ischemic stroke (Ho et al., 2005; Pantoni et al., 1996; Wang et al., 2016). White matter consists mainly of myelinated axons, oligodendrocytes, and other glial cells. During normal brain development, mature oligodendrocytes myelinate axons by wrapping around the internodes of axons, thereby facilitating saltatory conduction of nerve impulses and protecting axons from damage. White matter injury following stroke manifest as impaired oligodendrocyte remyelination, axonal injury, and eventually neurological deficits. Focal cerebral ischemia can induce the proliferation of endogenous oligodendrocyte precursor cells (OPCs), which attempt to restore OLGs and subsequently remyelination in a process known as oligodendrogenesis. However, most of these OPCs fail to differentiate into mature OLGs, resulting in insufficient remyelination and unsuccessful white matter repair (Goldman and Osorio, 2014). Therefore, promoting oligodendrogenesis may represent a potential therapeutic strategy to increase white matter repair and neurological recovery after stroke.
CD147 (cluster of differentiation 147), a cell surface glycoprotein that belongs to the IgG superfamily, is broadly expressed on the surface of various cell types including leukocytes, platelets, and endothelial cells. These three cell types that are integrally involved in stroke-induced neuroinflammation (Zhu et al., 2014). Increased expression of CD147 has been implicated in many human diseases including cancer, cardiovascular diseases, and neurological disorders. Therapeutic targeting of CD147 has been shown to attenuate the inflammatory response and disease severity in experimental models of rheumatoid arthritis, myocardial ischemia/reperfusion injury, multiple sclerosis, and autoimmune encephalomyelitis (Agrawal et al., 2011; Agrawal et al., 2012; Damsker et al., 2009; Gwinn et al., 2006; Seizer et al., 2011). Our recent study has shown that inhibition of CD147 ameliorates acute ischemic stroke in mice (Jin et al., 2017), but whether this effect extends to long-term neuroprotection has not been investigated. In this study, we aimed to investigate the effects of CD147 inhibition on white matter repair and long-term functional recovery after ischemic stroke.
Materials and Methods
Animals
All animal experiments were approved by the Penn State College of Medicine Institutional Animal Care and Use Committee, performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animal and reported in accordance with the ARRIVE guidelines (Animal Research: Reporting in Vivo Experiments). C57BL/6 mice (8–10 weeks old, male) were obtained from the Jackson Laboratory. All mice were housed in a specific pathogen-free facility with a 12-h light/dark cycle. All efforts were made to minimize animal suffering and the number of animals used.
Stroke Model and Antibody Treatment
Focal cerebral ischemia was induced in C57BL/6 mice (8–10 weeks, Jackson Laboratories, Bar Harbor, ME) by a 60-minute transient middle cerebral artery occlusion (tMCAO) as described previously (Jin et al., 2017; Jin et al., 2010). Four hours after tMCAO, the mice were randomly assigned to the following treatment groups: a rat anti–mouse CD147 monoclonal antibody (RL73.2, eBbioscience, named αCD147 mAb throughout this article) or isotype control antibody (rat IgG2a) administered via tail vein injection in 100 µL volume of PBS. This anti-CD147 antibody has been well characterized to block CD147 function in various mouse models (Agrawal et al., 2011; Agrawal et al., 2012; Damsker et al., 2009; Gwinn et al., 2006; Jin et al., 2017; Seizer et al., 2011). Antibody treatment was initiated at 4 hours and repeated at 24 and 48 hours after onset of ischemia as we described previously (Jin et al., 2017). A diagram of the experimental design is shown in Fig. 1A.
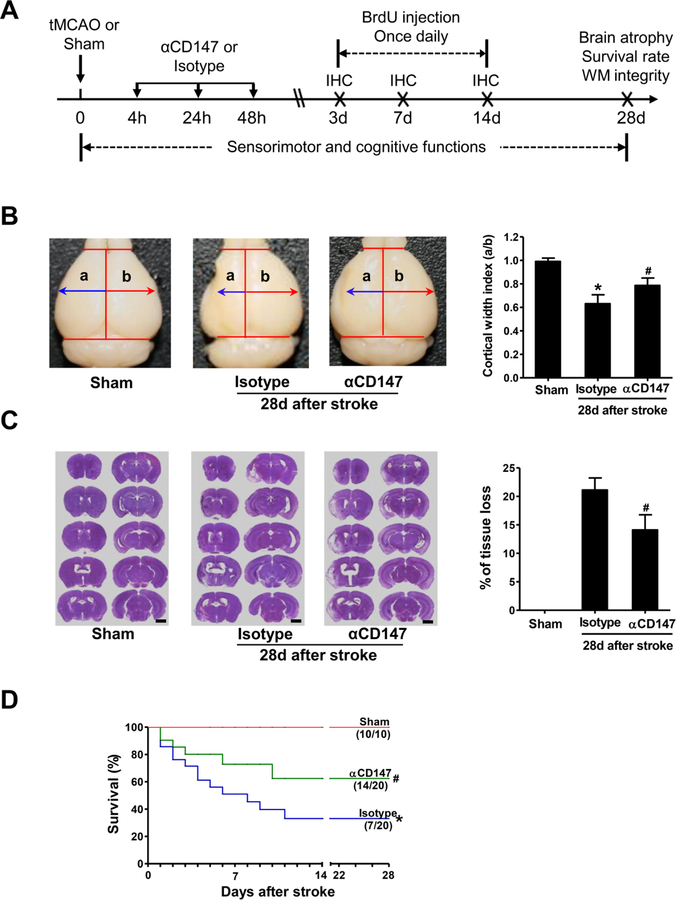
Figure 1.
Inhibition of CD147 improves histological outcome and survival rate 28 days after stroke. A, Experimental design. tMCAO, transient middle cerebral artery occlusion; αCD147, treated with anti-CD147 antibody; isotype, treated with isotype-matched control antibody; BrdU, 5-bromo-2’-deoxyuridine; IHC, immunohistochemistry; WM, white matter. B, Representative pictures (left panel) showing whole brain atrophy and quantitative analysis (right panel) of cortical width index, n=7–14 mice per group, *p < 0.05 vs. Sham; #p < 0.05 vs. Isotype. C, Representative images (left panel) showing coronal sections (Scale bars=2 mm) stained with cresyl violet and quantitative analysis (right panel) of brain tissue loss, n=7–14 mice per group. D, Survival curves of mice in the indicated groups up to 28 days after stroke. Survived/total animals: n= 10/10 (Sham), 7/20 (isotype) and 14/20 (αCD147). *p < 0.05 vs. Sham; #p < 0.05 vs. Isotype.
BrdU Injections
To label proliferating cells, animals were intraperitoneally injected with the thymidine analogue 5′-bromo-2′-deoxy-uridine (BrdU, 50 mg/kg, once daily) for 10 days beginning at 3 days after tMCAO.
Functional Tests
Sensorimotor function was determined using the modified neurological severity score (mNSS: motor, sensory, reflex, and balance) and the foot fault test (forelimb placement) (Schaar et al., 2010). Cognitive function was determined using the Morris water maze test (spatial learning and memory) and novel object recognition (recognition memory) (Schaar et al., 2010). All tests were performed before tMCAO and at the indicated time points after tMCAO by a blinded investigator. Detailed methods for each test are available in the online-only Supplemental Material.
Brain Atrophy and Tissue Loss Measurement
Brain atrophy was determined by measuring cortical width index (Taguchi et al., 2004). Brain tissue loss was measured in 10 cresyl violet-stained coronal sections (from +1 to −3 mm from bregma, 40 μm thick, at 500 μm intervals) on day 28 after tMCAo (Jin et al., 2013; Liu et al., 2019).
Histological and Immunohistochemical Assessment
Immunohistochemistry was performed as described previously (Jin et al., 2017). Bielschowsky silver staining (VB-3015, GeneCopoeia) and luxol fast blue staining (VB-3006, GeneCopoeia) were used to identify axons and myelin, respectively. All data were analyzed by a blinded investigator using Image Pro plus software (version 5.1, Media Cybernetics). The regions of interests (ROIs) selected for image acquisition and quantitative analysis are depicted in Suppl. Fig. 1. Detailed methods are provided in the online-only Supplemental Material.
Statistical Analysis
Data are expressed as mean ± SD. The GraphPad Prism 5 software package was used for statistical analysis. Normality was assessed by the D’Agostino-Pearson omnibus test. One-way analysis of variance (ANOVA) and Bonferroni’s test as post hoc were performed to analyze differences between groups in most of the parameters. If only 2 groups were compared, an unpaired, 2-tailed Student’s t test was used. For functional scores (ordinal in nature), we used the Mann–Whitney U test/Kruskal–Wallis test for statistical analysis. Sample size calculation (power = 0.8, α= 0.05) was performed using an online calculator (http://www.lasec.cuhk.edu.hk/sample-size-calculation.html). For comparison of survival data, the log-rank test was used. p < 0.05 was considered statistically significant.
Results
Inhibition of CD147 improves long-term histological and functional outcomes
Histological examination showed significant brain atrophy and tissue loss at 28 days after stroke in both αCD147- and isotype antibody-treated groups, as measured by cortical width index (Fig. 1B) and tissue loss (Fig. 1C). However, the extent of both brain atrophy and tissue loss were significantly attenuated in the αCD147-treated group compared to the isotype antibody-treated group (Fig. 1B and C). Of note, the value of the cortical width index not only depends on stroke size in the acute phase but also on cortical expansion during the recovery period, thus it is commonly used as a measure of cerebral cortical expansion to determine the effect of post-ischemic treatments during longer periods of time after stroke in rodents (Taguchi et al., 2004; Zhao et al., 2006). Moreover, the 28-day survival rate was significantly increased in the αCD147-treated group (70%, 14 of 20 mice) compared to that of isotype antibody-treated group (35%, 7 of 20 mice) (p=0.026) (Fig. 1D). The mortality rate of 65% (13/20) in the isotyple-treated group was similar to previous reports (Hirt et al., 2017; Xia et al., 2006; Zhao et al., 2018).
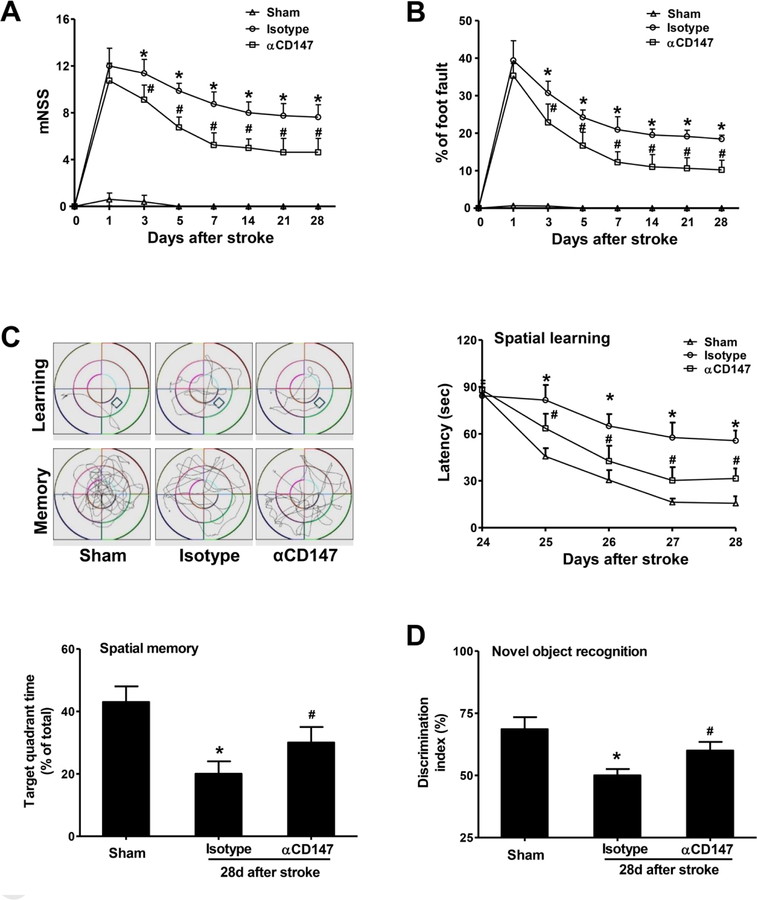
In human stroke studies, functional outcome is considered as the most relevant clinical endpoint. Therefore, we examined if αCD147 treatment can improve long-term recovery of neurological function up to 28 days after stroke. We found that αCD147 treatment significantly improved functional recovery, including sensorimotor function as tested by the modified neurological severity score (motor, sensory, reflex, and balance) (Fig. 2A) and foot fault test (forelimb placement) (Fig. 2B), and cognitive function as tested by Morris water maze test (spatial learning and memory) (Fig. 2C) and novel object recognition (recognition memory) (Fig. 2D).
Figure 2.
Inhibition of CD147 improves sensorimotor and cognitive functions 28 days after stroke. All behavioral tests were performed on the same mice used in Figure 1. A and B, The modified neurological severity score (mNSS) test (A) and foot-fault test (B) measured before (day 0) and up to 28 days after stroke, n=7–14 mice per group. C, The Morris water maze test was performed to measure cognitive deficits, n=7–14 mice per group. (upper, left panel) Representative images showing swim paths of mice during the learning and memory tests. (upper, right panel) Latency to locate the hidden platform in the cued learning test from 24 to 28 days after stroke. (lower, left panel) Spatial memory, defined as the percentage of time spent in the target quadrant after the platform was removed, was measured at 28 d after stroke. D, Novel objective recognition (NOR) was measured on day 28 after stroke, n=7–14 mice per group. Discrimination index=time spent exploring novel object / (time exploring novel object + time exploring familiar object) x 100%. *p < 0.05 vs. Sham; #p < 0.05 vs. Isotype.
Inhibition of CD147 improves white matter integrity in the late phase after stroke
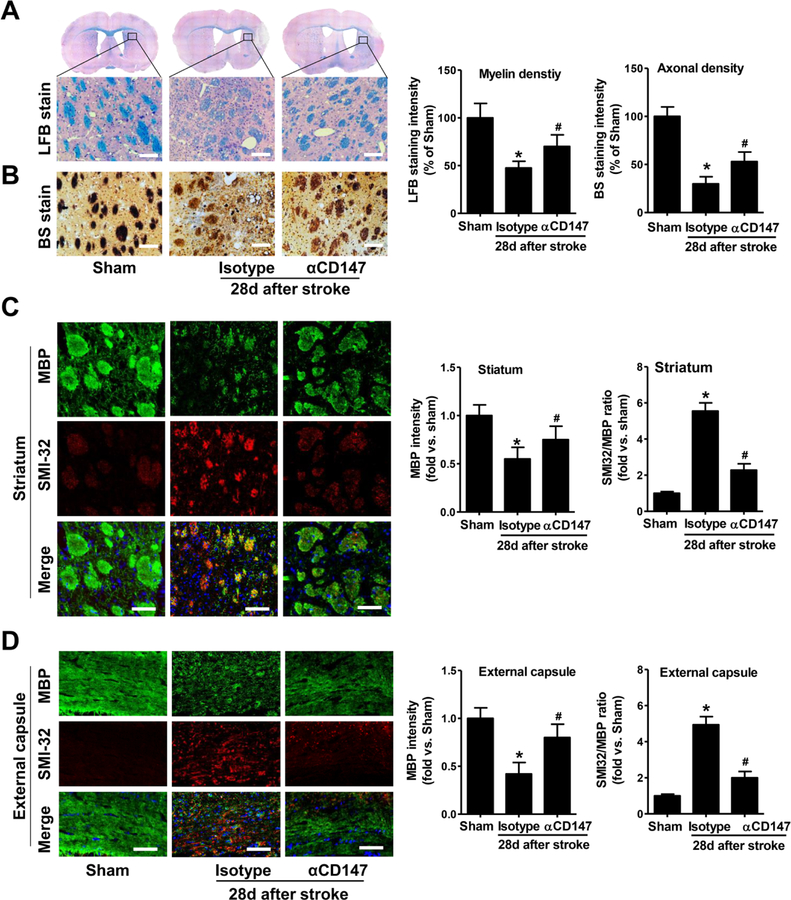
White matter damage is a critical component of the progression of ischemic brain injury. Therefore, we further examined white matter injury 28 days after stroke. The αCD147 treatment significantly reduced tMCAO-induced loss of myelin and axons as determined respectively by Luxol Fast Blue (LFB) staining (Fig. 3A) and Bielschowsky’s silver (BS) staining (Fig. 3B) in the peri-infarct striatum. White matter injury was also assessed by the mean ratio of SMI32 (a marker for axonal damage) to MBP (a marker of myelin integrity). The results of double immunostaining showed a marked loss of myelin accompanied by severe axonal damage in both the striatum (Fig. 3C) and external capsule (Fig. 3D) in the isotype-treated mice. MBP+ myelin structures were almost absent around SMI32-immunoreactive injured axons in the isotype-treated mice. αCD147 treatment significantly improved white matter integrity, as determined by decreased ratio of SMI32 to MBP staining intensity relative to isotype-treated controls (Fig. 3C and D).
Figure 3.
Inhibition of CD147 improves white matter integrity 28 days after ischemic stroke. A and B, Representative images of Luxol Fast Blue staining (LFB, A) and Bielschowsky silver staining (BS, B) in the peri-infarct striatum. Quantitative analysis of myelin density (LFB stain) and axonal density (BS stain) were expressed as percentage of the immunostaining intensity relative to Sham (set at 100%). Scale bars=100 μm. C and D, Representative images of double immunostaining for myelin basic protein (MBP; green) and SMI-32 (red) in the striatum (C) and external capsule (D). Quantitative analysis of the immunostaining intensity of MBP and SMI-32 in the ischemic hemisphere was expressed as fold changes relative to Sham control. Scale bars=50 μm. A through D, n=5–8 mice per group; *p < 0.05 vs. Sham, #p < 0.05 vs. Isotype.
Inhibition of CD147 prevents neuronal and oligodendrocyte cell death and improves white matter integrity in the acute phase after stroke
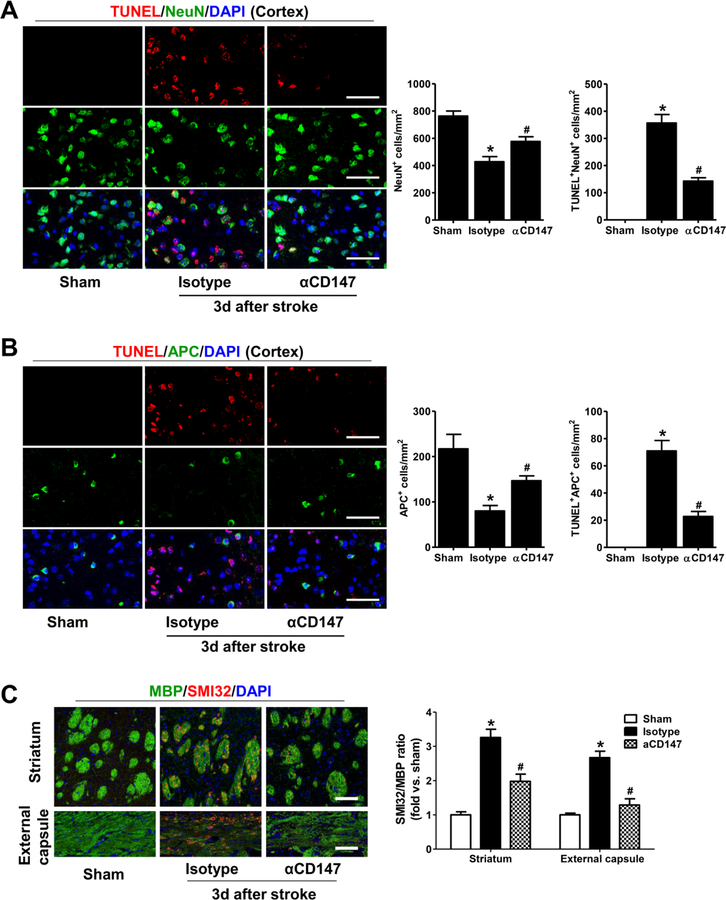
The findings that αCD147 treatment (initiated at 4 h after stroke) not only reduced stroke size in the acute phase (3 d) (Jin et al. 2017) but also improved long-term (28 d) white matter integrity and functional recovery strongly suggest that prevention of brain cell death is likely an important contributor to the long-term beneficial effects. Here, we further determined the effects of αCD147 treatment on neuronal and oligodendrocyte cell death in the acute phase (3 d) of stroke. As expected, tMCAO significantly increased neuronal cell death (marked by TUNEL+/NeuN+ staining). There was also an increase in oligodendroglial cell death (marked by TUNEL+/APC+ staining) in the peri-infarct cortex (Fig. 4A and B) as well as a significant loss of APC+ oligodendrocytes in the the peri-infarct cortex, external capsule, and striatum (Suppl. Fig. 2). Furthermore, we found that tMCAO caused significant initial damage of white matter within the ipsilateral external capsule and striatum (determined by the ratio of SMI32 to MBP immunofluorescence intensity) (Fig. 4C). Importantly, we found that all these stroke-induced changes were markedly prevented by the αCD147 treatment (initiated at 4 h after stroke).
Figure 4.
Inhibition of CD147 prevents neuronal and oligodendrocyte cell death and improves white matter integrity in the acute phase after stroke. A, Representative images (left panel) and quantitative analysis (right panel) of immunostaining for TUNEL (a cell death marker) and NeuN (neuronal cell marker) in the peri-infarct cortex in the indicated groups 3 days after stroke. DAPI (blue) is used as a counterstain to detect nuclei. B, Representative images (left panel) and quantitative analysis (right panel) of immunostaining for TUNEL (a cell death marker) and APC (a marker for mature oligodendrocytes) in the peri-infarct cortex in the indicated groups 3 days after stroke. DAPI (blue) is used as a counterstain to detect nuclei. C, Representative images (left panel) and quantitative analysis (right panel) of double immunostaining for myelin basic protein (MBP; green) and SMI-32 (red) in the striatum and external capsule at 3 days after stroke. Ratio of the immunofluoresece staining intensity of SMI-32 to MBP was expressed as fold changes relative to Sham control. A through C, Images were acquired from the peri-infarct areas; Scale bars=50 μm. n=5–8 mice per group; *p < 0.05 vs. Sham, #p < 0.05 vs. Isotype.
Inhibition of CD147 promotes OPCs proliferation after ischemic stroke.
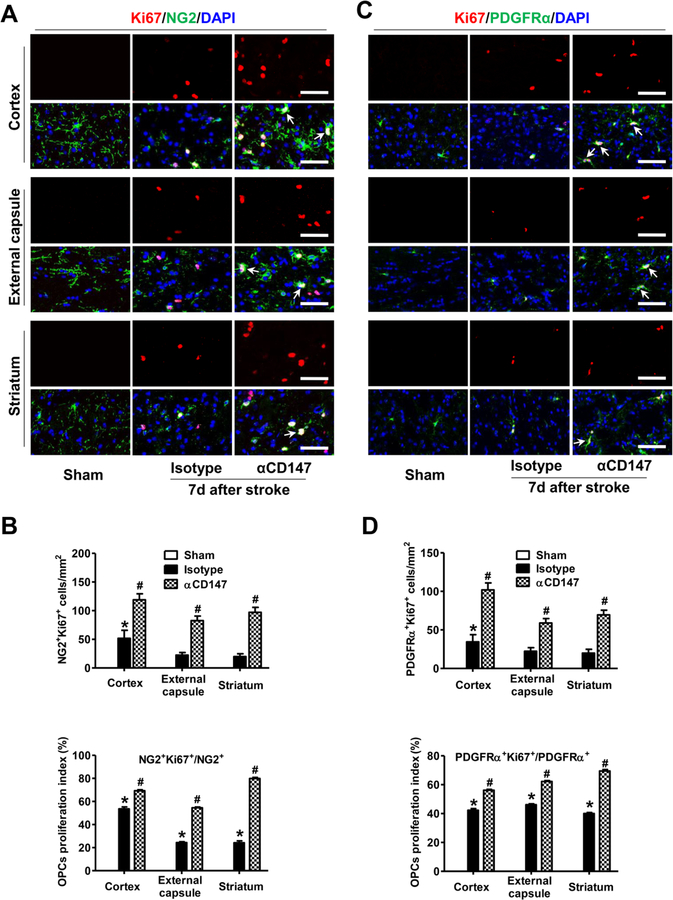
Oligodendrogenesis is essential for white/gray matter repair after stroke. First, we determined whether αCD147 treatment affects OPCs proliferation at 7 days after stroke. This time point was chosen because previous studies showed that the proliferative response of OPCs peaks at 5–7 days after stroke onset and declines thereafter (Iwai et al., 2010; Sozmen et al., 2009). Ki67 is widely accepted as a cell proliferation marker that is present during all active phases of the cell cycle (G1, S, G2, and mitosis). OPCs were identified by immunostaining of two markers: NG2 and PDGFRα (platelet-derived growth factor receptor alpha) (Fernández-López et al., 2010; Han et al., 2015; Kassis et al., 2014; Liu et al., 2017). tMCAO induced the proliferation of OPCs, as indicated by a substantial number of Ki67+NG2+ cells detected over a wide peri-infarct area including the cortex (gray matter), external capsule (white matter), and striatum (both white/gray matter) in the isotype-treated tMCAO mice (Fig. 5A and B). Of note, similar results were obtained using PDGFRα as an alternative cell marker for OPCs (Fig. 5C and D). As expected, no Ki67+ proliferating cells were detected in the non-ischemic (Sham) mice. More importantly, we found that the α CD147 treatment further enhanced the tMCAO-induced OPCs proliferation, as evidenced by increased percentage of either NG2+ or PDGFRα + cells that are Ki67 positive, in the same regions (Fig. 5). In addition, a considerable number of OPC doublets (arrows in Fig. 5A and C), indicating active in situ OPCs proliferation (Kassis et al., 2014), were observed in the αCD147-treated group within the peri-infarct areas. Collectively, these data indicate that the αCD147 treatment actually increased the proliferative index of OPCs in the peri-infarct areas of the ipsilateral hemisphere after stroke.
Figure 5.
Inhibition of CD147 promotes OPCs proliferation after ischemic stroke. A and B, Representative images (A) and quantitative analysis (B) of immunostaining for Ki67 (a cell proliferation marker, red) and NG2 (an OPC marker, green) in the indicated groups 7 days after stroke. C and D, Representative images (C) and quantitative analysis (D) of immunostaining for Ki67 (red) and PDGFRα (an OPC marker, green) in the indicated groups 7 days after stroke. Ki67+NG2+ and Ki67+PDGFRα+ cells indicate actively proliferating OPCs. Doublet OPCs (arrows in A and C) indicate active in situ OPC proliferation. DAPI is used as a counterstain to detect nuclei. Percentage of NG2+ or PDGFRα+ OPCs that are Ki67 positive represents OPCs proliferative index. A through D: Images were acquired from the peri-infarct areas; Scale bars=50 µm; n=5–8 mice per group. *p < 0.05 vs. Sham, #p < 0.05 vs. Isotype.
Inhibition of CD147 promotes generation of newly matured OLGs after ischemic stroke.
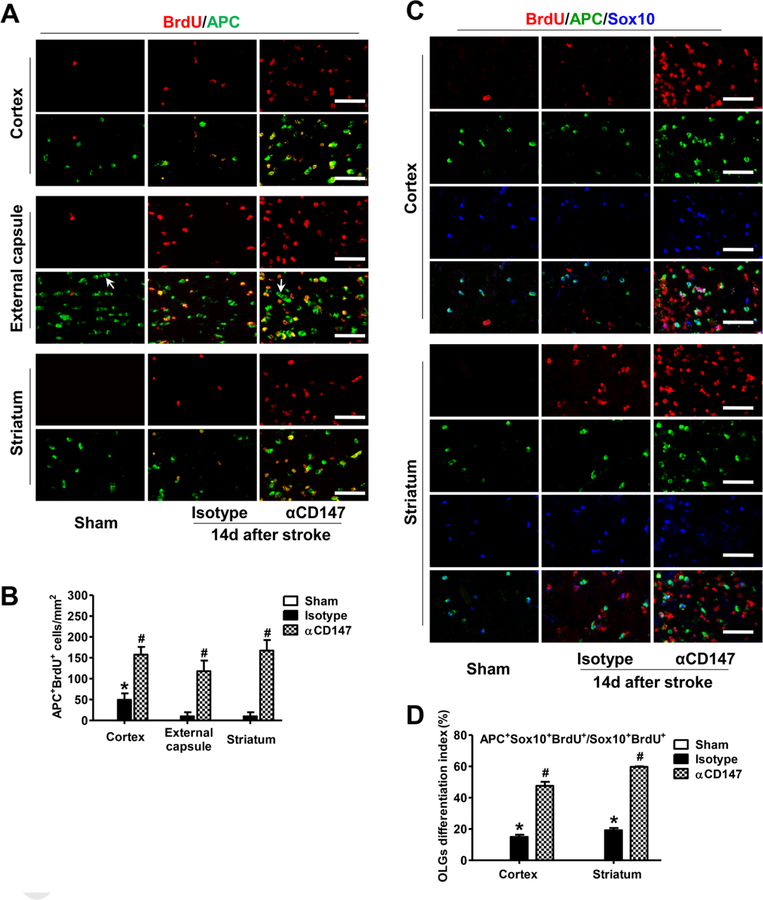
Next, we determined whether the αCD147 treatment enhanced generation of newly matured oligodendrocytes (OLGs) at a later time point (14 d) after stroke. Cumulative BrdU labeling method is used to track all BrdU-incorporated proliferative cells throughout the entire period post BrdU injections. APC (CC1) and Sox10 (SRY-Box 10) were used to identify mature OLGs and oligodendrocyte lineage cells, respectively (Fernández-López et al., 2010; Han et al., 2015; Pozniak et al., 2010). As expected, BrdU+ proliferative cells were absent or minimally detectable in the non-ischemic (Sham) mice. However, a substantial number of BrdU+ cells and BrdU+APC+ OLGs were detected in the peri-infarct cortex, external capsule, and striatum in the isotype-treated mice, and the numbers of these cells were further increased in the αCD147-treated mice (Fig. 6A and B). Of note, the BrdU+APC+ cells were rarely observed within the equivalent areas in the contralateral hemisphere of αCD147-treated mice (Suppl. Fig. 3) indicating that the αCD147 treatment had minimal effect on OLGs proliferation in remote regions. Furthermore, we performed triple staining (BrdU/APC/Sox10) and analyzed the ratio of the BrdU+APC+Sox10+ cells to BrdU+Sox10+ cells as an index of newly generated OLGs. This index was significantly increased in the αCD147-treated group compared with the isotype-treated group (Fig. 6C and D). In addition, the characteristic linear alignment of OLGs (Patel et al., 2010) (arrows in Fig. 6A) within the external capsule was clearly shown in the normal (Sham) mice indicating healthy myelination, but it was absent in the isotype-treated mice. However, linear alignment was greatly restored by the αCD147 treatment. Since mature OLGs do not proliferate, we did not observe any APC+ mature OLGs co-stained with Ki67 within the ipsilateral external capsule in either the isotype- or αCD147- treated group at 7 and 14 days after stroke (Suppl. Fig. 4). This further supports the hypothesis that the detected BrdU+APC+ cells represent newly generated mature OLGs. Collectively, the above data show that the αCD147 treatment improved white matter integrity through promoting OPCs proliferation and oligodendrocytes maturation after stroke.
Figure 6.
Inhibition of CD147 promotes generation of newly matured oligodendrocytes (OLGs) after ischemic stroke. A and B, Representative images (A) and quantitative analysis (B) of double immunostaining for bromodeoxyuridine (BrdU, red) and APC (a marker for mature OLGs, green) in the indicated groups 14 days after stroke. C and D, Representative images (C) and quantitative analysis (D) of triple immunostaining for bromodeoxyuridine (BrdU, red), APC (a marker for mature OLGs, green), and Sox10 (a marker for oligodendrocyte lineage cells, blue) in the indicated groups 14 days after stroke. BrdU+APC+Sox10+ cells indicate newly generated mature oligodendrocytes. Percentage of Sox10+BrdU+ OLGs that are APC positive represents OLGs differentiation index. Characteristic linear alignment of OLGs (arrows in A) indicates normal myelination. A through D, Images were acquired from the peri-infarct areas; Scale bars=50 µm; n=5–8 mice per group. *p < 0.05 vs. Sham, #p < 0.05 vs. Isotype.
Inhibition of CD147 upregulated CXCR4 expression in OPCs after ischemic stroke.
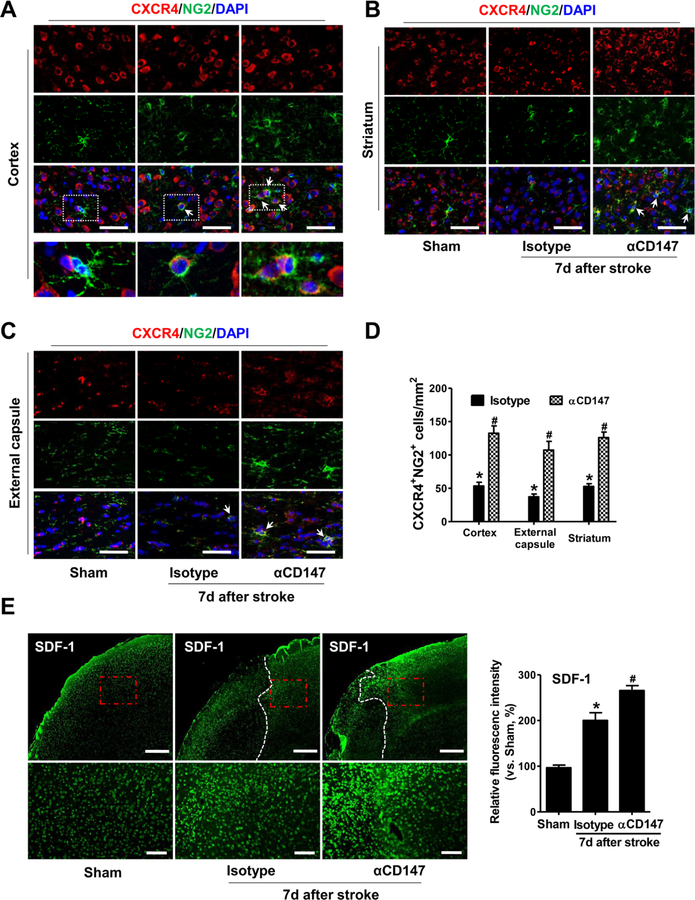
The chemokine receptor CXCR4 is normally expressed by a variety of cells in the brain including oligodendrocyte progenitors. It has been implicated in promoting differentiation of oligodendrocyte progenitors and remyelination (Banisadr et al., 2011; Chu et al., 2017). CXCR4 and its ligand SDF-1 are up-regulated in the peri-ischemic regions following stroke (Hill et al., 2004; Stumm et al., 2002; Wang et al., 2012). We therefore investigated whether promoting oligodendrogenesis with αCD147 treatment is involved in regulating CXCR4 signaling. Immunohistochemistry was performed to detect the expression and distribution of CXCR4 in the peri-infact areas at 7 days after stroke. In the Sham-operated mice, CXCR4 is highly expressed in a wide area (cortex, striatum, external capsule) but is rarely colocalized with NG2-positive OPCs (Fig. 7A through C). Howevwer, tMCAO significantly induced the expression of CXCR4 in OPCs (marked by CXCR4+NG2+ cells, arrows in Fig. 7A through C) in these regions in the isotype-treated mice, and this induction was further enhanced in the αCD147-treated mice. In addition, we observed that the expression of SDF-1 (the ligand of CXCR4) in the peri-infarct cortex was increased in the isotype-treated mice, and this increase was further enhanced by αCD147 treatment (Fig. 7E). Double immunostaining showed that SDF-1 was co-localized with NeuN (neuronal cell marker) and CD31 (endothelial cell marker) (Suppl. Fig. 6). The increased expression of SDF-1 in the perilesional endothelial cells and neurons might be involved in regulating the OPC-endothelial and OPC-neuron interactions, thereby promoting OPCs recruitment to the lesion after stroke (Chu et al., 2017; Patel et al., 2010). Collectively, our data suggest that αCD147 treatment promoted oligodendrogenesis and white matter repair, possibly through modulating the CXCR4 signaling after ischemic stroke.
Figure 7.
Inhibition of CD147 upregulates the expression of CXCR4 in OPCs and SDF-1 in perilesional cortex after ischemic stroke. A, B and C, Representative images of immunostaining for CXCR4 (red) and NG2 (an OPC marker, green) in the indicated groups within the peri-infarct cortex (A), striatum (B) and external capsule (C) 7 days after stroke. Scale bars=50 µm. Higher magnification of images in white dotted boxes from cortex are also shown. CXCR4/NG2 double-positive cells (arrows) indicate CXCR4-expressing OPCs. D, Quantitative analysis of immunostaining for CXCR4 (red) and NG2 (an OPCs marker, green) in the indicated groups. Data were expressed as the number of CXCR4/NG2-double positive cells per mm2 in the above peri-infarct areas. A through D, n=5–8 mice per group. *p < 0.05 vs. Sham, #p < 0.05 vs. Isotype. E, Representative images and quantitative analysis of immunostaining for SDF-1 in the peri-infarct cortex 7 days after stroke. Data were expressed as percentage changes relative to sham control. n=5–8 mice per group. *p < 0.05 vs. Sham, #p < 0.05 vs. Isotype. Scale bars=400 μm in the upper panel, and 100 μm in the lower panel.
Discussion
In the present study, we demonstrate, for the first time, that therapeutic inhibition of CD147 improves long-term stroke outcome. Two major findings from this study contribute to our understanding of the role of CD147 in the recovery process after cerebral ischemia and reperfusion. First, αCD147 treatment promotes post-ischemic oligodendrogenesis and white matter repair. Second, αCD147 treatment ameliorates recovery of sensorimotor function and spatial learning and memory after ischemic stroke.
White matter (WM) is mainly composed of bundles of myelinated axons. WM/axonal damage, characterized by demyelination and loss of axonal integrity, induces disturbance of nerve impulse transport between neurons derived from different brain regions, which is associated with long-term neurological deficits after stroke (Wang et al., 2016). WM repair, including oligodendrogenesis and the myelination of demyelinated or newly generated axons, could help rebuild neuronal connectivity and re-establish axonal signal conduction. Unfortunately, the adult brain has limited capacity for remyelination, at least in part due to the failure of differentiation of OPCs into mature, myelinating oligodendrocytes. Thus, interventions that promote OPCs differentiation may facilitate axonal remyelination, WM repair, and long-term neurological recovery in stroke patients. Consistent with previous studies (McIver et al., 2010), we found that tMCAO induced severe myelin loss (using LFB stain) and axonal damage (using BS stain) in the peri-infarct areas such as external capsule and striatum at 28 days after stroke. These pathological changes in the WM were significantly ameliorated by inhibition of CD147, thereby facilitating recovery of sensorimotor and cognitive functions. In addition, it should be noted that the long-term (28 d) protective effects conferred by the αCD147 treatment (initiated at 4 h after stroke onset) is, at least in part, associated with prevention of stroke-induced neuronal and oligodendrocyte cell death and initial white matter injury (using SMI-32 and MBP stain) in the acute phase (3 d) after stroke.
Oligodendrogenesis is the process by which mature oligodendrocytes are generated from OPCs. Oligodendrocytes are known to be highly vulnerable to ischemic injury (Back et al., 2002; Pantoni et al., 1996), and damage to oligodendrocytes results in myelin loss and axonal injury. Because injured mature oligodendrocytes no longer produce functional myelin and mature oligodendrocytes do not proliferate (Zhang et al., 2013), successful replacement of damaged and lost oligodendrocytes with newly generated oligodendrocytes is essential for remyelination after brain injuries. OPCs are distributed throughout the CNS and encompass approximately 7–8% of cells in the white matter and 3–4% in grey matter (Dawson et al., 2003). In response to oligodendrocyte damage, resident OPCs adjacent to the damaged areas and those originating from the subventricular zone (SVZ) actively proliferate and migrate to the peri-infarct areas and contribute concertedly towards myelinating oligodendrocyte restoration (Maki et al., 2013). Although OPCs may actively proliferate after stroke, very few OPCs can mature into myelin-producing oligodendrocytes (Gonzalez-Perez and Alvarez-Buylla, 2011), thus resulting in poor white matter recovery (Chu et al., 2012). In this study, we found that αCD147 treatment (initiated at 4 h after stroke onset) enhanced proliferation of OPCs (at 7 d) and maturation of new oligodendrocytes (at 14 d) in the late phase of stroke in the peri-infarct areas including the cortex (gray matter), external capsule (white matter), and striatum (white matter/gray matter). In order to determine whether increased OPC proliferation and oligodendrocyte maturation improves white matter repair at 28 days after stroke, we performed an additional experiment with delayed αCD147 treatment (initiated at day 3 after stroke onset) when brain infarct has been fully developed. We found that delayed αCD147 treatment significantly promoted white matter integrity by measuring the mean ratio of SMI32 (a marker for axonal damage) to MBP (a marker of myelin integrity) at 28 days after stroke (Suppl. Fig. 5), although the delayed treatment was relatively less effective when compared with the early αCD147 treatment (initiated at 4 h after stroke). Taking our previous study (Jin et al., 2017) and data presented in this study together, we speculate that the protective effects of the αCD147 treatment (initiated at 4 h after stroke) likely involve multiple mechanisms of action including prevention of early cell death (neuronal and oligodendroglial) and initial damage of white matter in the acute phase (3 d) as well as subsequent enhancement of oligodendrogenesis and white matter repair in the late stages (7–14 d post-stroke), thereby increasing long-term functional recovery.
Although oligodendrogenesis is essential for remyelination and white matter repair after stroke, the underlying molecular mechanism remain poorly understood. Upregulation of CXCR4 has been shown to promote OPC differentiation and remyelination in demyelinating diseases (Carbajal et al., 2011; Patel et al., 2010). In this study, we found that αCD147 treatment upregulates CXCR4 in OPCs and its ligand SDF-1 in endothelial cells and neurons in the peri-infarct areas. This suggests that oligodendrogenesis following αCD147 treatment may be involved in regulating OPC-endothelial and OPC-neuron interactions through the SDF-1/CXCR4-dependent mechanism (Chu et al., 2017; Patel et al., 2010). The detailed mechanisms by which inhibition of CD147 directly or indirectly modulates the SDF-1/CXCR4 axis in specific cell types remain unclear and warrant further investigation. It should also be noted that many other mechanisms may also contribute to improvements in white matter repair mediated by the αCD147 treatment under ischemic conditions such as neuroinflammation, oxidative stress, and excitotoxicity (Dewar et al., 2003). We have previously demonstrated that αCD147 treatment (initiated at 4 h and repeated at 24 h and 48 h after onset of ischemia) profoundly reduced inflammatory cell infiltration including neutrophils, T cells, and macrophages/activated microglia in the ischemic brain 72 h after tMCAO (Jin et al., 2017). These inflammatory cells are involved in stroke progression by producing reactive oxygen species, pro-inflammatory cytokines and chemokines, and other neurotoxic factors (Jin et al., 2017). Because the same mouse stroke model and the same αCD147 treatment protocol were used in the present study, it is likely that the αCD147 treatment promotes white matter repair through attenuating neuroinflammation, thereby providing a permissive environment for oligodendrogenesis after stroke. More recent studies have highlighted the protective role of microglia/macrophage polarization to the M2 phenotype in promoting white matter integrity and repair after cerebral ischemia (Hu et al., 2015; Suenaga et al., 2015). Our previous study has shown that the expression of CD147 is minimally detected in resting microglia but is robustly induced in reactive microglia at 72 h after tMCAO (Jin et al., 2017). Whether and how the the αCD147 treatment resolves chronic neuroinflammation, in particular, promoting microglia/macrophage polarization to the M2 phenotype warrants further investigation.
In summary, therapeutic inhibition of CD147 improves long-term functional recovery possibly by promoting white/gray matter repair and might represent a promising therapeutic strategy for stroke and possibly other demyelinating disorders.
Future Direction
Stroke Therapy Academic Industry Roundtable recommends that after initial evaluations in young, healthy male animals, further studies should be performed in females, aged animals, and animals with comorbid conditions such as hypertension and diabetes (Fisher et al., 2009). The therapeutic effects of αCD147 treatment in aged mice in both sexes and mice with comorbid conditions (hypertension, diabetes) are currently under investigation in our laboratory.
Supplementary Material
Supplemental Figure 1. Schematic diagram showing the regions of interest (ROIs) in the peri-infarct areas (cortex, striatum, and external capsule), with 4 microscopic fields in each region for image acquisition and quantitative analysis. LV, lateral ventricle; EC, external capsule.
Supplemental Figure 2. Inhibition of CD147 (initiated at 4 h after stroke onset) reduces the loss of oligodendrocytes (OLGs) detected at 3 days after stroke. Representative images (upper panel) and quantitative analysis (lower panel) of OLGs (stained by APC marker, green) in the indicated groups. DAPI is used as a counterstain to detect nuclei. Data are expressed as fold changes relative to sham control. Scale bars=50 µm; n=5–8 mice per group. *p < 0.05 vs. Sham, #p < 0.05 vs. Isotype.
Supplemental Figure 3. Inhibition of CD147 has no effects on the number of either total oligodendrocytes (marked by APC staining) or newly generated (BrdU-positive) oligodendrocytes in the contralateral hemisphere. Antibody treatment was performed as described in “Supplemental Method” section. A, Representative images of whole coronal section of the αCD147-treated mouse brain immunostained for BrdU and APC 14 days after stroke. B and C, Representative images (B) and quantitative analysis (C) of double immunostaining for BrdU and APC in the equivalent areas (white dotted boxes) of both hemispheres. Scale bars=50 µm. n=5–8 mice per group, *p < 0.05 vs. Ctrl. Ctrl: contralateral; Ipsi: ipsilateral.
Supplemental Figure 4. Double immunostaining of Ki67 (a cell proliferation marker) and APC ( a cell marker for mature oligodendrocytes) in the indicated groups at 7 and 14 days after stroke. No colocalization of Ki67 and APC confirmed that mature oligodendrocytes do not proliferate. Scale bars= 50 µm; n= 5–8 mice per group. Note that: Ki67 is widely accepted as a cell proliferation marker that is present during all active phases of the cell cycle (G1, S, G2, and mitosis). The monoclonal antibody anti-adenomatous polyposis coli (APC, clone CC1) is the antibody most commonly used to specifically label the cell bodies of mature oligodendrocytes.
Supplemental Figure 5. The delayed αCD147 treatment remains effective in improving white matter integrity at 28 days after stroke. A and B, Representative images (left panel) and quantitative analysis (right panel) of double immunostaining for MBP (myelin basic protein; green) and SMI-32 (red) in the striatum (A) and external capsule (B) at 28 days after stroke. Ratio of the immunofluorescence intensity of SMI-32 to MBP was expressed as fold changes relative to sham control. Scale bars=50 μm; n= 5–8 mice per group; *p < 0.05. Note that: Early treatment: Antibody treatment was initiated at 4 h and repeated at 24 and 48h after stoke; Delayed treatment: Antibody treatment was initiated at day 3 and repeated at day 4 and day 5 after stroke.
Supplemental Figure 6. Representative images of double immunostaining for SDF-1/CD31 and SDF-1/NeuN in the ipsilateral cortex of the αCD147-treated mice 7 days after stroke. Data showed that SDF-1 was co-localized with CD31 (endothelial cell marker) and NeuN (neuronal cell marker). Scale bars= 50 µm; n=5–8 mice per group.
Supplemental Figure 7. Antibody (αCD147 or isotype) treatment has no detectable effects in the Sham-operated mice. A and B, OPCs proliferation was evaluated by immunostaining for Ki67-positive NG2 cells at 7 days after stroke; White matter damage (B) was evaluated by immunostaining for SMI32 and MBP at 28 days after stroke. C, Brain atrophy was measured at 28 days after stroke. D, The modified neurological severity score (mNSS) was measured up to 28 days after stroke. Scale bars= 50 µm; n=5 mice per group.
Highlights.
Oligodendrogenesis is essential for white matter repair and long-term functional recovery after stroke.
Inhibition of CD147 promotes oligodendrogenesis and white matter integrity after ischemic stroke, at least in part, through upregulating the SDF-1/CXCR4 axis in OPCs.
Inhibition of CD147 improves long-term sensorimotor and cognitive functions after ischemic stroke.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grants NS088719 and NS089991 (Dr. Li).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- Agrawal SM, Silva C, Tourtellotte WW, Yong VW, 2011. EMMPRIN: a novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci 31, 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal SM, Silva C, Wang J, Tong JP, Yong VW, 2012. A novel anti-EMMPRIN function-blocking antibody reduces T cell proliferation and neurotoxicity: relevance to multiple sclerosis. J Neuroinflammation 9, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM, 2002. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci 22, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Frederick TJ, Freitag C, Ren D, Jung H, Miller SD, Miller RJ, 2011. The role of CXCR4 signaling in the migration of transplanted oligodendrocyte progenitors into the cerebral white matter. Neurobiol Dis 44, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajal KS, Miranda JL, Tsukamoto MR, Lane TE, 2011. CXCR4 signaling regulates remyelination by endogenous oligodendrocyte progenitor cells in a viral model of demyelination. Glia 59, 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M, Hu X, Lu S, Gan Y, Li P, Guo Y, Zhang J, Chen J, Gao Y, 2012. Focal cerebral ischemia activates neurovascular restorative dynamics in mouse brain. Front Biosci (Elite Ed) 4, 1926–1936. [DOI] [PubMed] [Google Scholar]
- Chu T, Shields LBE, Zhang YP, Feng SQ, Shields CB, Cai J, 2017. CXCL12/CXCR4/CXCR7 Chemokine Axis in the Central Nervous System: Therapeutic Targets for Remyelination in Demyelinating Diseases. Neuroscientist 23, 627–648. [DOI] [PubMed] [Google Scholar]
- Damsker JM, Okwumabua I, Pushkarsky T, Arora K, Bukrinsky MI, Constant SL, 2009. Targeting the chemotactic function of CD147 reduces collagen-induced arthritis. Immunology 126, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R, 2003. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24, 476–488. [DOI] [PubMed] [Google Scholar]
- Dewar D, Underhill SM, Goldberg MP, 2003. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab 23, 263–274. [DOI] [PubMed] [Google Scholar]
- Fernández-López D, Pradillo JM, García-Yébenes I, Martínez-Orgado JA, Moro MA, Lizasoain I, 2010. The cannabinoid WIN55212–2 promotes neural repair after neonatal hypoxia-ischemia. Stroke 41, 2956–2964. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH, Group, S., 2009. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40, 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Osorio J, 2014. So many progenitors, so little myelin. Nat Neurosci 17, 483–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez O, Alvarez-Buylla A, 2011. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res Rev 67, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn WM, Damsker JM, Falahati R, Okwumabua I, Kelly-Welch A, Keegan AD, Vanpouille C, Lee JJ, Dent LA, Leitenberg D, Bukrinsky MI, Constant SL, 2006. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J Immunol 177, 4870–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Cai W, Mao L, Liu J, Li P, Leak RK, Xu Y, Hu X, Chen J, 2015. Rosiglitazone Promotes White Matter Integrity and Long-Term Functional Recovery After Focal Cerebral Ischemia. Stroke 46, 2628–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ, 2004. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol 63, 84–96. [DOI] [PubMed] [Google Scholar]
- Hirt L, Fukuda AM, Ambadipudi K, Rashid F, Binder D, Verkman A, Ashwal S, Obenaus A, Badaut J, 2017. Improved long-term outcome after transient cerebral ischemia in aquaporin-4 knockout mice. J Cereb Blood Flow Metab 37, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PW, Reutens DC, Phan TG, Wright PM, Markus R, Indra I, Young D, Donnan GA, 2005. Is white matter involved in patients entered into typical trials of neuroprotection? Stroke 36, 2742–2744. [DOI] [PubMed] [Google Scholar]
- Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J, 2015. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol 11, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, Chen J, Cao G, 2010. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke 41, 1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Xiao AY, Chen R, Granger DN, Li G, 2017. Inhibition of CD147 (Cluster of Differentiation 147) Ameliorates Acute Ischemic Stroke in Mice by Reducing Thromboinflammation. Stroke 48, 3356–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yu S, Song Z, Quillin JW, Deasis DP, Penninger JM, Nanda A, Granger DN, Li G, 2010. Phosphoinositide 3-kinase-gamma expression is upregulated in brain microglia and contributes to ischemia-induced microglial activation in acute experimental stroke. Biochem Biophys Res Commun 399, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Zhu X, Liu L, Nanda A, Granger DN, Li G, 2013. Simvastatin attenuates stroke-induced splenic atrophy and lung susceptibility to spontaneous bacterial infection in mice. Stroke 44, 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis H, Chopp M, Liu XS, Shehadah A, Roberts C, Zhang ZG, 2014. Histone deacetylase expression in white matter oligodendrocytes after stroke. Neurochem Int 77, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Jin R, Xiao AY, Chen R, Li J, Zhong W, Feng X, Li G, 2019. Induction of Neuronal PI3Kγ Contributes to Endoplasmic Reticulum Stress and Long-Term Functional Impairment in a Murine Model of Traumatic Brain Injury. Neurotherapeutics [DOI] [PMC free article] [PubMed]
- Liu XS, Chopp M, Pan WL, Wang XL, Fan BY, Zhang Y, Kassis H, Zhang RL, Zhang XM, Zhang ZG, 2017. MicroRNA-146a Promotes Oligodendrogenesis in Stroke. Mol Neurobiol 54, 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki T, Liang AC, Miyamoto N, Lo EH, Arai K, 2013. Mechanisms of oligodendrocyte regeneration from ventricular-subventricular zone-derived progenitor cells in white matter diseases. Front Cell Neurosci 7, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver SR, Muccigrosso M, Gonzales ER, Lee JM, Roberts MS, Sands MS, Goldberg MP, 2010. Oligodendrocyte degeneration and recovery after focal cerebral ischemia. Neuroscience 169, 1364–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA, 1996. Cerebral white matter is highly vulnerable to ischemia. Stroke 27, 1641–1646; discussion 1647. [DOI] [PubMed] [Google Scholar]
- Patel JR, McCandless EE, Dorsey D, Klein RS, 2010. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci U S A 107, 11062–11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozniak CD, Langseth AJ, Dijkgraaf GJ, Choe Y, Werb Z, Pleasure SJ, 2010. Sox10 directs neural stem cells toward the oligodendrocyte lineage by decreasing Suppressor of Fused expression. Proc Natl Acad Sci U S A 107, 21795–21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar KL, Brenneman MM, Savitz SI, 2010. Functional assessments in the rodent stroke model. Exp Transl Stroke Med 2, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizer P, Ochmann C, Schönberger T, Zach S, Rose M, Borst O, Klingel K, Kandolf R, MacDonald HR, Nowak RA, Engelhardt S, Lang F, Gawaz M, May AE, 2011. Disrupting the EMMPRIN (CD147)-cyclophilin A interaction reduces infarct size and preserves systolic function after myocardial ischemia and reperfusion. Arterioscler Thromb Vasc Biol 31, 1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozmen EG, Kolekar A, Havton LA, Carmichael ST, 2009. A white matter stroke model in the mouse: axonal damage, progenitor responses and MRI correlates. J Neurosci Methods 180, 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Höllt V, Schulz S, 2002. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci 22, 5865–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga J, Hu X, Pu H, Shi Y, Hassan SH, Xu M, Leak RK, Stetler RA, Gao Y, Chen J, 2015. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol 272, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T, 2004. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 114, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang J, Li Y, Yang GY, 2012. Roles of chemokine CXCL12 and its receptors in ischemic stroke. Curr Drug Targets 13, 166–172. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu G, Hong D, Chen F, Ji X, Cao G, 2016. White matter injury in ischemic stroke. Prog Neurobiol 141, 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CF, Smith RS, Shen B, Yang ZR, Borlongan CV, Chao L, Chao J, 2006. Postischemic brain injury is exacerbated in mice lacking the kinin B2 receptor. Hypertension 47, 752–761. [DOI] [PubMed] [Google Scholar]
- Zhang R, Chopp M, Zhang ZG, 2013. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci 7, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH, 2006. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 12, 441–445. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liu C, Liu J, Kong Q, Mao Y, Cheng H, Li N, Zhang X, Li C, Li Y, Liu L, Ding Z, 2018. HSPA12B promotes functional recovery after ischaemic stroke through an eNOS-dependent mechanism. J Cell Mol Med 22, 2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Song Z, Zhang S, Nanda A, Li G, 2014. CD147: a novel modulator of inflammatory and immune disorders. Curr Med Chem 21, 2138–2145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Schematic diagram showing the regions of interest (ROIs) in the peri-infarct areas (cortex, striatum, and external capsule), with 4 microscopic fields in each region for image acquisition and quantitative analysis. LV, lateral ventricle; EC, external capsule.
Supplemental Figure 2. Inhibition of CD147 (initiated at 4 h after stroke onset) reduces the loss of oligodendrocytes (OLGs) detected at 3 days after stroke. Representative images (upper panel) and quantitative analysis (lower panel) of OLGs (stained by APC marker, green) in the indicated groups. DAPI is used as a counterstain to detect nuclei. Data are expressed as fold changes relative to sham control. Scale bars=50 µm; n=5–8 mice per group. *p < 0.05 vs. Sham, #p < 0.05 vs. Isotype.
Supplemental Figure 3. Inhibition of CD147 has no effects on the number of either total oligodendrocytes (marked by APC staining) or newly generated (BrdU-positive) oligodendrocytes in the contralateral hemisphere. Antibody treatment was performed as described in “Supplemental Method” section. A, Representative images of whole coronal section of the αCD147-treated mouse brain immunostained for BrdU and APC 14 days after stroke. B and C, Representative images (B) and quantitative analysis (C) of double immunostaining for BrdU and APC in the equivalent areas (white dotted boxes) of both hemispheres. Scale bars=50 µm. n=5–8 mice per group, *p < 0.05 vs. Ctrl. Ctrl: contralateral; Ipsi: ipsilateral.
Supplemental Figure 4. Double immunostaining of Ki67 (a cell proliferation marker) and APC ( a cell marker for mature oligodendrocytes) in the indicated groups at 7 and 14 days after stroke. No colocalization of Ki67 and APC confirmed that mature oligodendrocytes do not proliferate. Scale bars= 50 µm; n= 5–8 mice per group. Note that: Ki67 is widely accepted as a cell proliferation marker that is present during all active phases of the cell cycle (G1, S, G2, and mitosis). The monoclonal antibody anti-adenomatous polyposis coli (APC, clone CC1) is the antibody most commonly used to specifically label the cell bodies of mature oligodendrocytes.
Supplemental Figure 5. The delayed αCD147 treatment remains effective in improving white matter integrity at 28 days after stroke. A and B, Representative images (left panel) and quantitative analysis (right panel) of double immunostaining for MBP (myelin basic protein; green) and SMI-32 (red) in the striatum (A) and external capsule (B) at 28 days after stroke. Ratio of the immunofluorescence intensity of SMI-32 to MBP was expressed as fold changes relative to sham control. Scale bars=50 μm; n= 5–8 mice per group; *p < 0.05. Note that: Early treatment: Antibody treatment was initiated at 4 h and repeated at 24 and 48h after stoke; Delayed treatment: Antibody treatment was initiated at day 3 and repeated at day 4 and day 5 after stroke.
Supplemental Figure 6. Representative images of double immunostaining for SDF-1/CD31 and SDF-1/NeuN in the ipsilateral cortex of the αCD147-treated mice 7 days after stroke. Data showed that SDF-1 was co-localized with CD31 (endothelial cell marker) and NeuN (neuronal cell marker). Scale bars= 50 µm; n=5–8 mice per group.
Supplemental Figure 7. Antibody (αCD147 or isotype) treatment has no detectable effects in the Sham-operated mice. A and B, OPCs proliferation was evaluated by immunostaining for Ki67-positive NG2 cells at 7 days after stroke; White matter damage (B) was evaluated by immunostaining for SMI32 and MBP at 28 days after stroke. C, Brain atrophy was measured at 28 days after stroke. D, The modified neurological severity score (mNSS) was measured up to 28 days after stroke. Scale bars= 50 µm; n=5 mice per group.