Abstract
Objective:
For marginalized populations, county health departments may be important PrEP access points; however, there are little data on successful PrEP programs at these venues outside of incentivized demonstration projects. Therefore, we implemented an open-access, free PrEP clinic at a county health department in Atlanta, GA to promote PrEP uptake among high-risk clients.
Methods:
The Fulton County Board of Health (FCBOH) PrEP clinic launched in October 2015, and eligible clients who expressed interest initiated PrEP and attended follow-up visits per CDC guidelines. Clients engaged in quarterly follow-up and seen within the last 6 months were defined as “persistent”, whereas clients with a lapse in follow-up of > 6 months were defined as “not persistent.” Factors associated with PrEP persistence were assessed with unadjusted odds ratios.
Results:
Between October 2015 and June 2017, 399 clients were screened for PrEP, almost all were eligible [392/399 (98%)]; however, 158/392 (40%) did not return to start PrEP after screening. Of 234 patients, 216 (92%) received a prescription for PrEP. As of June 2017, only 69/216 (32%) clients remained persistent in PrEP care, and the only evaluated factor significantly associated with PrEP persistence was age≥ 30 years (OR 1.86, 95% CI 1.02, 3.42).
Conclusions:
Implementation of PrEP in the county health department setting is feasible; however, we have identified significant challenges with PrEP uptake and persistence in our setting. Further research is needed to fully understand mediators of PrEP persistence and inform interventions to optimize health department-based PrEP services.
Keywords: PrEP implementation, health department, HIV prevention, PrEP persistence, PrEP adherence, Atlanta
Introduction
Blacks/African-Americans and men who have sex with men (MSM) bear the largest burden of new HIV infections in the US, and the majority of new infections occur in the southern US [1]. In 2015, the state of Georgia had the third highest lifetime risk of HIV diagnosis in the country, and most new infections occurred in metro-Atlanta [2, 3].
Daily oral Pre-Exposure Prophylaxis (PrEP) with tenofovir disoproxil fumarate/emtrictabine is an effective HIV prevention method [4]; however, widespread implementation may be challenged by structural barriers such as lack of insurance and healthcare access [5] in addition to other obstacles such as fear of side effects, stigma, and low risk perception [6, 7]. Recent CDC data demonstrate that only 4.7% of Atlanta MSM have used PrEP compared to 11.3% of San Francisco MSM, suggesting these barriers may play a larger role in the South [8]. Other studies examining PrEP uptake have confirmed marked geographical disparities [9–11]; findings from the IQVIA Real World Data-Longitudinal Prescriptions database revealed that although 52% of new HIV diagnoses in 2016 occurred in the Southern US, only 27% of PrEP users resided in this region [11]. Data on spatial access to PrEP clinics suggests that though the South experiences the largest burden of new infections, these areas have far fewer than expected PrEP clinics (52% of new diagnoses compared to only 26% of PrEP clinics) [12]. At the city-level, these trends remained the same with lower PrEP clinic availability in Birmingham (19.0 clinics/1000 new diagnoses), Atlanta (14.5 clinics/1000 new diagnoses) and Jacksonville (17.8 clinics/1000 new diagnoses) compared to Philadelphia (58.8 clinics/1000 new diagnoses) Seattle (261.1 clinics/1000 new diagnoses) and New York (58.8 clinics/1000 new diagnoses) [12]. These findings are critical given that a recent study conducted among 787 Black/African Americans, 54% of whom lived in the South, found that living in areas with higher PrEP clinic density was significantly associated with willingness to use PrEP [13]. Hence among the many barriers which contribute to poor PrEP uptake in the South, inadequate geographical access to PrEP providers is likely a significant contributing factor among high-risk minority populations.
While structural barriers in the South are indeed magnified, they do not solely explain the geographical disparities in PrEP uptake. One study conducted among high-risk young black MSM in Atlanta, GA found that only 34% of men initiated PrEP despite amelioration of several key structural barriers as PrEP was offered to all HIV-negative study participants with financial coverage of provider visits and labs and intensive navigation assistance to obtain free or low cost PrEP [14]. This suggests that cultural and social barriers in the South such as racism, stigma and homophobia may be heightened and likely play a significant role in the delayed PrEP uptake observed.
Additionally, few studies have examined factors affecting persistence in PrEP care and have used varying definitions of persistence with estimates ranging between 43–83% [7, 15–21]. Previously identified factors associated with PrEP persistence include commercial insurance, male gender, and low medication co-pays whereas female gender, Black/African American race, uninsured status and younger age have been associated with non-persistence [7, 16, 17, 20, 21]. One study which examined factors influencing PrEP discontinuations in a sample of young black MSM in Atlanta found that marijuana use, age<22 years and having fewer than 3 sex partners were significant predictors of PrEP discontinuation suggesting that tailored interventions for young and marijuana-using black MSM would be needed to improve PrEP persistence in this population [22]. Data from a qualitative analysis of primarily African American young MSM in Jackson, Mississippi revealed that there were several structural, social, behavioral and clinical factors that affected PrEP use and retention in care including access to payment assistance programs, medication copayments and deductibles, HIV stigma and homophobia, relationship status changes, changes in sexual risk behaviors and perceived vs. actual medication side effects [7]. Further research on culturally appropriate interventions to address these barriers which may be uniquely exacerbated among high-risk communities in the South are urgently needed to inform the successful scale-up of PrEP delivery programs.
For economically disadvantaged urban populations, county health departments may be important PrEP access points; however, there are little data on successful PrEP programs at these venues outside of incentivized demonstration projects. The National HIV/AIDS Strategy for the US: Updated to 2020 [23] calls for a 25% reduction in the number of new HIV diagnoses by 2020 (compared to 2010) and highlights “full access to daily oral PrEP services” for those in need by clinical and public health organizations as a key means of achieving this goal. County health departments are expected to be natural partners in the effort to improve community awareness and use of PrEP among high-risk populations given their role in HIV testing and counseling, STD care and partner services and funding services for high-risk populations through community-based and AIDS service organizations [24]. These activities allow for the identification of HIV-negative individuals who may have significant PrEP indications but who would not otherwise have access to adequate healthcare because of poverty and lack of health insurance. Hence county health departments may be the only PrEP access point for such key populations but challenges to PrEP implementation in these settings are difficult and according to a 2015 national survey of local health departments (LHDs) include lack of PrEP awareness and knowledge among staff, lack of staff capacity to support PrEP implementation activities, lack of health care providers willing to prescribe PrEP and concerns about financial access to PrEP for interested individuals [24]. This study also found that among responding LHDs, only 29% were currently engaged in PrEP implementation and of those, 33% were in the Southern US [24]. Making referrals to PrEP providers, conducting PrEP education and outreach to community members and developing local PrEP provider directories were among the most frequent activities LHDs reported being engaged in for PrEP implementation, less frequently reported were delivering PrEP via a health department clinic and monitoring and evaluating PrEP uptake and impact [24]. LHDs in the South were also the least likely to report intent to expand their PrEP implementation services when compared to LHDs from other regions [24]. Despite these barriers there is a strong demand for LHDs in the Southern US to overcome these PrEP implementation challenges and develop innovative models of PrEP delivery that target key populations given their unique access to underserved, high-risk groups.
Given concerns about lack of PrEP access among high-risk populations in metro-Atlanta, the Fulton County Board of Health (FCBOH) opened a free PrEP clinic in October 2015. Here we describe the early implementation and PrEP persistence estimates from this program with the goal of identifying facilitators and barriers to PrEP persistence and to provide local recommendations to improve outreach, access to, and persistence in PrEP care.
Methods
Clients were referred to FCBOH PrEP clinic staff for eligibility screening from several sources including self-referral, the FCBOH Sexual Health (SH) Clinic, community-based organizations, and partner referrals from local HIV clinics. Clients were deemed eligible for PrEP if they met risk criteria outlined in the CDC summary of guidance for PrEP use [4], had a negative HIV antigen/antibody test and HIV viral load, and creatinine clearance >60 ml/min. The FCBOH covered all costs associated with PrEP provider visits and lab monitoring; clients used their health insurance and/or manufacturer assistance program to obtain the drug. Manufacturer co-pay cards were provided for clients with health insurance to minimize associated prescription costs. A 30-day PrEP prescription was given at the initial visit and clients returned in one month for early follow-up. Subsequently, a 90-day prescription was provided during quarterly follow-up visits. At these visits, clients were asked about PrEP adherence (whether they were still taking PrEP and the number of missed doses in the preceding month), and ongoing risk behavior (number of sexual partners since last visit and frequency of condom use since last visit which was assessed as “always, sometimes or never”).
As of June 2017, clients engaged in quarterly follow-up and seen within the last 6 months were defined as “persistent” in PrEP care; clients with a lapse of > 6 months were deemed “not persistent”. This definition of PrEP persistence was based on prior research which examined PrEP retention as remaining in PrEP care for at least 6 months [17]. The primary analysis was limited to PrEP clients seen between October 2015 and June 2017 to allow for at least 6 months of follow-up for each client. Data on demographics and HIV risk behavior were collected via retrospective chart review. In August 2016, we attempted one-time phone contact with all non-persistent clients to date. If the client was interested in returning to PrEP clinic, an appointment was made; and if they were no longer interested in PrEP, they were asked to provide reasons why.
Descriptive statistics were calculated for demographics, HIV risk behaviors, referral sources, and reasons for PrEP interest. We examined PrEP uptake and persistence over time among all clients seen between October 2015-June 2017 to describe the PrEP care cascade [5, 25]. PrEP uptake and persistence among MSM was also stratified by race to determine differences between Black and non-Black MSM. Factors associated with PrEP persistence were assessed with unadjusted odds ratios. McNemar’s test was used to assess change in condom use from baseline to follow-up among PrEP users. The Emory Institutional Review Board (IRB) approved all study procedures.
Results
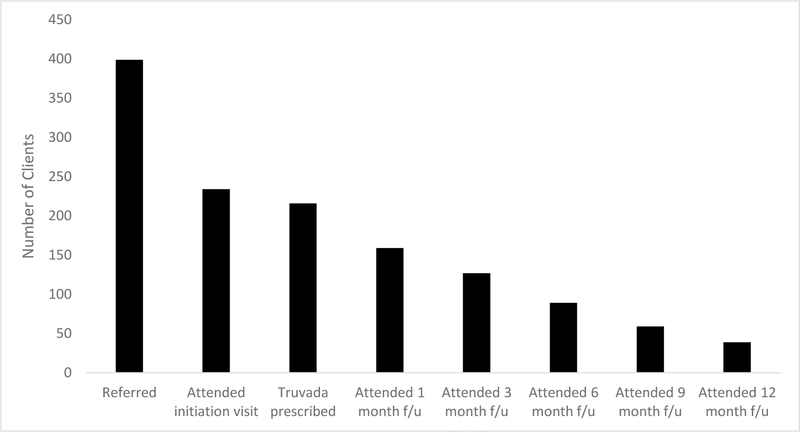
From October 2015-June 2017, 399 clients were screened for PrEP eligibility in accordance with CDC guidelines. Seven had a positive 4th generation HIV test and 392/399 (98%) were found to be eligible; however, 158/392 (40%) did not return to start PrEP after screening. Over half [234/392 (60%)] of PrEP eligible clients attended an enrollment visit, and 216/234 (92%) received a PrEP prescription (Figure 1). Reasons for not receiving a PrEP prescription among the 18/234 (8%) who attended an enrollment visit include a positive 4th generation HIV test at the visit (3/18), a lack of desire to start PrEP due to low self-perceived risk (3/18), no pregnancy test on file prior to PrEP prescription (3/18), and 9/18 had no reason documented. Of 216 clients who started PrEP, 86% were male, 66% were Black, 40% were younger than 30 years, and 70% were uninsured. Eighty-three percent self-identified as MSM, 77% reported inconsistent condom use and 76% self-reported a prior lifetime sexually transmitted infection (STI) diagnosis. While PrEP uptake overall was significantly higher among non-Black MSM compared to Black MSM (67 vs. 52%, p=0.008), uptake among non-Black MSM did not change over time (66% in 2015–16 to 69% in 2016–17) but significantly increased over time among Black MSM (40% in 2015–16 to 67% in 2016–17, p=0.003.) The most common PrEP referral source was the FCBOH SH Clinic (56%), and the most common reasons for PrEP interest reported by clients at the enrollment visit were identifying as MSM (45%), history of an STI (22%) and having an HIV positive partner (21%).
Figure 1.
The PrEP Care Cascade at the FBCOH PrEP clinic (October 2015-June 2017)
As of June 2017, only 69/216 (32%) clients remained persistent in PrEP care, and the only evaluated factor significantly associated with PrEP persistence was age≥ 30 years (OR 1.86, 95% CI 1.02, 3.42; Table 1). There was no significant difference in PrEP persistence among Black and non-Black MSM (40/107 (37%) vs. 19/72 (26%), p=0.12). Among persistent clients who have started PrEP, there have been no HIV seroconversions thus far.
Table 1.
Factors associated with PrEP persistence among PrEP clinic enrollees at the FCBOH in Atlanta, GA 2015–2017
| Characteristic | PrEP Persistent (n=69) n (%) | PrEP non-persistent (n=147) n (%) | Unadjusted OR (95% CI) |
|---|---|---|---|
| Sex | |||
| Female | 6 (9) | 19 (13) | Ref |
| Male | 63 (91) | 128 (87) | 1.56 (0.59, 4.09) |
| Age | |||
| <30 y.o. | 21 (30) | 66 (45) | Ref |
| ≥30 y.o. | 48 (70) | 81 (55) | 1.86 (1.02, 3.42) |
| Race | |||
| Black | 50 (72) | 92 (63) | Ref |
| White | 11 (16) | 37 (25) | 0.55 (0.26, 1.17) |
| Hispanic | 7 (10) | 12 (8) | 1.07 (0.40, 2.90) |
| Other* | 1 (1) | 6 (4) | 0.31 (0.04, 2.62) |
| Education | |||
| Pre-college/Vocational | 11 (20) | 38 (34) | Ref |
| College | 39 (71) | 65 (58) | 2.07 (0.95, 4.52) |
| Post-College/Professional | 5 (9) | 9 (8) | 1.91 (0.53, 6.92) |
| Income | |||
| <20,000 annually | 68 (61) | 34 (64) | Ref |
| ≥20,000 annually | 43 (39) | 19 (36) | 0.88 (0.45, 1.74) |
| Insurance | |||
| Yes | 16 (23) | 49 (34) | Ref |
| No | 53 (77) | 97 (66) | 1.67 (0.87, 3.23) |
| Sexual Orientation | |||
| Homosexual | 59 (86) | 120 (82) | 1.33 (0.60, 2.92) |
| Bisexual/Heterosexual | 10 (14) | 27 (18) | Ref |
| Relationship status | |||
| Committed relationship | 15 (22) | 27 (18) | 1.16 (0.55, 2.44) |
| Single | 36 (52) | 75 (51) | Ref |
| Referral Source | |||
| STI clinic | 30 (51) | 63 (50) | Ref |
| Friend | 12 (20) | 31 (25) | 0.81 (0.37, 1.80) |
| CBO/ASO/External partners | 12 (20) | 20 (16) | 1.26 (0.55, 2.91) |
| Internet/social media | 4 (7) | 11 (9) | 0.76 (0.23, 2.60) |
| Condom usea | |||
| Always | 17 (28) | 23 (19) | Ref |
| Sometimes | 41 (67) | 84 (71) | 0.66 (0.32, 1.37) |
| Never | 3 (5) | 12 (10) | 0.34 (0.08, 1.39) |
| Prior reported STI Diagnosis | |||
| Yes | 47 (72) | 99 (77) | 0.77 (0.39, 1.51) |
| No | 18 (28) | 29 (23) | Ref |
| Number of partners | |||
| ≤5 partners | 43 (75) | 90 (76) | Ref |
| ≥6 partners | 14 (25) | 28 (24) | 1.05 (0.50, 2.19) |
Note. Significant p-values (<0.05) have been bolded for ease of interpretation.
Abbreviations. PrEP, Pre-Exposure Prophylaxis; OR, Odds Ratio; HS, High School; STI, Sexually Transmitted Infection; CBO, Community-based organization; ASO, AIDS Service organization
Other includes Asian, Hawaiian and Pacific-islander
Condom use was self-reported
As of the most recent follow-up visit, the median number of months on PrEP was 5.7 (range 0–25), and time to first follow-up appointment was 1–3 months. At the most recent follow-up visit, 138/159 (89%) reported still taking the medication, 88/155 (57%) reported missing zero doses in the prior month, 113/150 (75%) reported using condoms “sometimes or never” and 14/152 (9%) reported having greater than 6 sexual partners in the past 6 months. There was no statistical change in the percentage of people reporting never using condoms between enrollment and the most recent follow up visit.
In August 2016, we attempted one-time phone follow-up with 123 non-persistent clients and were able to contact 43/123 (35%). Fifteen (35%) were no longer interested in taking PrEP, 13 (30%) had difficulty scheduling an appointment, and 4 (12%) had never started PrEP due to fear of experiencing side effects.
Discussion
Our data demonstrate that PrEP implementation in the county health department setting in the urban South is feasible despite multiple challenges that have been described including drug costs, lab costs, staff availability and education, concerns of risk compensation, and the need for longitudinal follow-up [7, 18, 24, 26, 27]. The FCBOH developed a sustainable and affordable PrEP delivery program by utilizing existing funding sources such as STI program funds to cover labs, and non-clinical staff to perform PrEP monitoring and PrEP navigation including enrollment in the manufacturer’s medication assistance program. Similar strategies may be useful for other health departments seeking to initiate PrEP programs in high incidence, low resourced settings but will require support from health department leadership, willingness of providers to prescribe PrEP and willingness of staff to support implementation and delivery efforts. Though previous studies have demonstrated that Southern health departments have less intent to expand their engagement in PrEP services [24], it is our hope that the successful implementation of our PrEP program in a Southern jurisdiction plagued by poor funding, an overburdened public health system and high rates of poverty [28] serve as a call to action for the delivery of PrEP by other county health department clinics in the South where private PrEP clinic availability is the lowest [12] yet HIV diagnoses rates and need for adequate prevention services remain high.
Only 32% of clients were persistent in PrEP care at the FCBOH and the only factor associated with persistence was age≥ 30 years. This is consistent with prior studies which confirm challenges with PrEP persistence, especially amongst younger patients [7, 15–18, 21, 22, 26, 27, 29, 30]. Demedicalization of PrEP services may be an important intervention to improve persistence among high-risk Southern youth as this group may have more challenges accessing and navigating PrEP services given their inexperience with the healthcare system and fear of PrEP costs [21]. Low or no-cost mobile PrEP delivery is a promising implementation strategy that has been piloted among high-risk youth in the Southern US and resulted in many initiating PrEP with high acceptability ratings [31, 32]. These and other remote care interventions for PrEP delivery should be considered for utilization by Southern public health organizations given the known geographical sparsity of PrEP clinics in this region [12], and increased ease and convenience of accessing services, which is especially relevant for youth and other groups who may be disproportionately impacted by factors such as lack of transportation and financial barriers.
Notably, our PrEP persistence estimate of 32% at 6 months is lower than that previously reported (60% at 6 months) from a clinic-based sample of ethnically diverse, highly-educated MSM utilizing 3 PrEP programs in Rhode Island, Mississippi and Missouri [17] where factors such as refill accessibility, good social support, higher health literacy, and higher PrEP awareness and community acceptance may have contributed to higher PrEP persistence [17, 26]. In our sample, we identified lack of ongoing PrEP interest, difficulty making appointments, and side effect concerns as reasons for non-persistence. This suggests that intensifying community outreach to improve PrEP awareness, providing targeted PrEP education with a focus on high-risk youth, and streamlining the follow-up process including facilitation of medication refills, especially in the setting of delayed or missed visits, may improve PrEP persistence at the FCBOH.
A follow-up study from the US PrEP demonstration project which included data from two municipal STI clinics (one located in Florida) and a community health center in Washington, DC also demonstrated higher persistence rates with 66.1% of participants retained for all study visits [16]. Factors contributing to higher persistence in this study likely included on-site medication dispensation, aggressive follow-up for those who missed study visits (up to 4 phone calls) and study incentives [16]. Though incentivized PrEP uptake and persistence is unlikely to be feasible outside of funded studies and demonstration projects, other strategies which make obtaining PrEP more convenient such as on-site pharmacy access and intensified outreach for those lost to follow-up may lead to improved PrEP persistence in populations experiencing barriers to care. Research to examine these and other factors which may promote PrEP persistence in the South is urgently needed and may be accomplished through the implementation of more PrEP demonstration projects in this region. All implementation efforts should consider the need to include the collection of in-depth qualitative feedback to obtain more insight on social and cultural factors such as stigma, homophobia and racism which likely have a significant influence on retention in PrEP care among Southern communities. This data is critical to inform the development of effective PrEP delivery programs as many of these factors are poorly understood and culturally-appropriate interventions to address them have the ability to significantly enhance PrEP uptake among “difficult to reach” populations. Prior data have demonstrated higher PrEP uptake among White MSM and lower PrEP persistence among Black MSM and uninsured patients [8, 15, 16], however our study demonstrated higher PrEP uptake among Black MSM in the second half of the study period, and similar PrEP persistence among Black and White MSM and insured vs. uninsured clients. Given the observed racial and geographical disparities in access to healthcare, and PrEP awareness and usage, several studies have suggested that PrEP may lead to worsening disparities in HIV incidence if current uptake trends persist [11, 33–35]. Our findings re-emphasize that for many socioeconomically disadvantaged minority populations, health departments continue to serve as venues for the provision of sexual health services and preventative care and, thus, may be an important component in closing the HIV disparity gap. This is especially true in the Southern US where low PrEP clinic density [12], high uninsured rates, and lack of Medicaid expansion [28] may make county health departments the only possible PrEP access point for a significant number in need of effective HIV prevention services. This reinforces the crucial need to direct state and federal funding for HIV prevention services to Southern county health departments to support the implementation and sustainability of health department-based PrEP services to ensure that this important prevention tool reaches communities most impacted by the epidemic.
Though female gender has previously been associated with non-persistence in PrEP care [20, 21], we did not observe this association in our analysis. However, women only made up 12% of those prescribed PrEP in our sample, and this number is likely insufficient to detect associations. In 2016 at the FCBOH, there were over 4000 visits conducted for HIV-uninfected women in the SH clinic; however, the vast majority of these encounters were for complaints found to be due to bacterial vaginosis (>75%) with few cases of confirmed bacterial STIs i.e. gonorrhea, chlamydia and syphilis (<20%) despite frequent testing [internal data]. This suggests that female clients were likely not considered by staff or themselves as “high-risk” if no other easily recognizable HIV risk factor was reported which may have resulted in fewer women undergoing PrEP eligibility screening (50/399, 13%) in our cohort. This finding has been observed in other studies examining PrEP uptake in women, where it has been hypothesized that PrEP appeals to women with an identifiable HIV risk (i.e. HIV-infected partner), leading many women and their providers to assume reduced risk if no obvious risk factor is present [29, 36]. This can be misleading as HIV risk in women is often related to “indirect factors” not accounted for by CDC guidance criteria such as partner risk behavior and HIV density in sexual networks, which can be difficult for women and providers to identify and discuss [36–38]. This is especially relevant for US Black women who have an increased risk of HIV acquisition despite a low number of partners due to higher prevalence of HIV in their sexual networks [37].
Additionally, studies have highlighted lack of PrEP awareness and access as a key challenge to PrEP uptake in women [39, 40] and this in combination with low risk perception, and inadequate provider risk assessment likely contributes to the marked gender disparities observed in PrEP uptake [11]. These challenges are likely greater for minority women, particularly those in the South given the disproportionately higher number of new infections observed in Southern Black women compared to non-Black women residing in other regions [1]. These data suggest that ours and other PrEP programs which serve high-risk Southern women would likely benefit from targeted PrEP education efforts, and the use of expanded risk assessment tools which consider local HIV epidemiology to engage and maintain women in PrEP care.
Our study was limited by a small sample size, particularly for women, limited follow-up time, and missing data, which restricted our ability to perform certain comparisons. We also utilized a definition of PrEP persistence that may not realistically capture the diverse patterns of PrEP use observed in real-world settings [15, 16, 18, 25]. However, this is the first report of PrEP implementation in a Southern county health department outside of funded demonstration projects, and we believe these data can serve to improve PrEP services at FCBOH and inform the development of PrEP programs in other similar jurisdictions.
Here we show that PrEP implementation in a Southern county health department setting is not only feasible but also effectively reaches key populations in need of HIV prevention services and may be an important access point for minority MSM experiencing barriers to PrEP care. Nonetheless, PrEP uptake and persistence was suboptimal despite amelioration of several structural barriers which may limit PrEP use suggesting that additional social and cultural factors may impact engagement in PrEP care, particularly in the South. Further research is needed to fully understand mediators of PrEP persistence among high-risk Southern men and women and inform interventions to optimize health department-based PrEP services, as successful and effective public health delivery models may be a key tool in reducing the significant racial and geographical disparities in HIV infection observed in the US.
Acknowledgements
We thank Diakima Thomas, and the FCBOH PrEP clinic staff for their contributions and feedback.
Source of Funding
This work was supported by the National Institutes of Health: K23AI108335 (PI: Kelley) and K23AI114407 (PI: Sheth)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest:
There are no conflicts of interest or financial disclosures for any author.
References
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, 2016; vol. 28 Available at http://www.cdc.gov/hiv/library/reports/surveillance/. Published November 2017. Accessed 6/15/18. [Google Scholar]
- 2.AIDSVu (www.aidsvu.org). Emory University, Rollins School of Public Health Retrieved from https://aidsvu.org/state/georgia/atlanta/. Accessed 1/9/2017.
- 3.Hess KL, et al. , Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol, 2017. 27(4): p. 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Public Health Service. Pre-exposure prophylaxis for the prevention of HIV infection in the United States – 2014, A Clinical Practice Guideline Available from http://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf. Accessed 12 December 2016.
- 5.Kelley CF, et al. , Applying a PrEP Continuum of Care for Men Who Have Sex With Men in Atlanta, Georgia. Clin Infect Dis, 2015. 61(10): p. 1590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rendina HJ, et al. , Distinguishing hypothetical willingness from behavioral intentions to initiate HIV pre-exposure prophylaxis (PrEP): Findings from a large cohort of gay and bisexual men in the U.S. Soc Sci Med, 2017. 172: p. 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold T, et al. , Social, structural, behavioral and clinical factors influencing retention in Pre-Exposure Prophylaxis (PrEP) care in Mississippi. PLoS One, 2017. 12(2): p. e0172354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoots BE, et al. , Willingness to Take, Use of, and Indications for Pre-exposure Prophylaxis Among Men Who Have Sex With Men-20 US Cities, 2014. Clin Infect Dis, 2016. 63(5): p. 672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuehn B, PrEP Disparities. JAMA, 2018. 320(22): p. 2304. [DOI] [PubMed] [Google Scholar]
- 10.Siegler AJ, et al. , The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol, 2018. 28(12): p. 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YA, et al. , HIV Preexposure Prophylaxis, by Race and Ethnicity - United States, 2014–2016. MMWR Morb Mortal Wkly Rep, 2018. 67(41): p. 1147–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegler AJ, et al. , Location location location: an exploration of disparities in access to publicly listed pre-exposure prophylaxis clinics in the United States. Ann Epidemiol, 2018. 28(12): p. 858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ojikutu BO, et al. , Spatial Access and Willingness to Use Pre-Exposure Prophylaxis Among Black/African American Individuals in the United States: Cross-Sectional Survey. JMIR Public Health Surveill, 2019. 5(1): p. e12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolle CP, et al. , Challenges in Translating PrEP Interest Into Uptake in an Observational Study of Young Black MSM. J Acquir Immune Defic Syndr, 2017. 76(3): p. 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rusie LK, et al. , PrEP Initiation and Retention in care over five years, 2012–2017: Are quarterly visits too much? Clin Infect Dis, 2018. [DOI] [PMC free article] [PubMed]
- 16.Doblecki-Lewis S, et al. , Patterns and Correlates of Participant Retention in a Multi-City Pre-Exposure Prophylaxis Demonstration Project. J Acquir Immune Defic Syndr, 2018. [DOI] [PMC free article] [PubMed]
- 17.Chan PA, et al. , Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc, 2016. 19(1): p. 20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hojilla JC, et al. , HIV Pre-exposure Prophylaxis (PrEP) Uptake and Retention Among Men Who Have Sex with Men in a Community-Based Sexual Health Clinic. AIDS Behav, 2018. 22(4): p. 1096–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grinsztejn B, et al. , Retention, engagement, and adherence to pre-exposure prophylaxis for men who have sex with men and transgender women in PrEP Brasil: 48 week results of a demonstration study. Lancet HIV, 2018. 5(3): p. e136–e145. [DOI] [PubMed] [Google Scholar]
- 20.Huang YA, Tao G, Smith DK, Hoover KW. Persistence with HIV Preexposure Prophylaxis in the United States, 2012–2016. Abstract 106. Conference on Retroviruses and Opportunistic Infections, March 4–7, 2019 Seattle, WA. [Google Scholar]
- 21.Coy KC, et al. , Persistence on HIV preexposure prophylaxis medication over a 2-year period among a national sample of 7148 PrEP users, United States, 2015 to 2017. J Int AIDS Soc, 2019. 22(2): p. e25252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serota DP, Rosenberg ES, Thorne AL, del Rio C, Luisi N, et al. PrEP Persistence and Discontinuation in a Cohort of Young Black Men who have Sex with Men in Atlanta, GA. Abstract 963. Conference on Retroviruses and Opportunistic Infections (CROI). March 4–7, 2019 Seattle, WA. [Google Scholar]
- 23.White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States updated to 2020 2015.
- 24.Weiss G, et al. , PrEP implementation by local health departments in US cities and counties: Findings from a 2015 assessment of local health departments. PLoS One, 2018. 13(7): p. e0200338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunn AS, et al. , Defining the HIV pre-exposure prophylaxis care continuum. AIDS, 2017. 31(5): p. 731–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu AY, et al. , Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med, 2016. 176(1): p. 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcus JL, et al. , Preexposure Prophylaxis for HIV Prevention in a Large Integrated Health Care System: Adherence, Renal Safety, and Discontinuation. J Acquir Immune Defic Syndr, 2016. 73(5): p. 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Social determinants of health and selected HIV care outcomes among adults with diagnosed HIV infection in 32 states and the District of Columbia, 2014. HIV Surveillance Supplemental Report 2016;21(7):1–62. [Google Scholar]
- 29.Blackstock OJ, et al. , Pre-exposure prophylaxis prescribing and retention in care among heterosexual women at a community-based comprehensive sexual health clinic. AIDS Care, 2017. 29(7): p. 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosek S, et al. , An HIV Pre-Exposure Prophylaxis (PrEP) Demonstration Project and Safety Study for Young MSM. J Acquir Immune Defic Syndr, 2017. 74(1): 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan PS, et al. , Implementation Strategies to Increase PrEP Uptake in the South. Curr HIV/AIDS Rep, 2019. [DOI] [PMC free article] [PubMed]
- 32.Siegler AJ, Brock JB, Kelley CF, Ahlschlager LA, Rolle CPM, et al. Pilot test of a PrEP Telmedicine system for Young Black MSM in the rural US South. Abstract 955. Conference of Retroviruses and Opportunistic Infections (CROI). March 4–7, 2019 Seatlle, WA. [Google Scholar]
- 33.Marcus JL, et al. , Disparities in Uptake of HIV Preexposure Prophylaxis in a Large Integrated Health Care System. Am J Public Health, 2016. 106(10): p. e2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenness S, Maloney K, Smith DK, Hoover KW, Goodreau SM, Weiss K, Rosenberg ES, Sullivan PS. The PrEP Care Continuum and HIV Racial Disparities Among Men who have Sex with Men. Conference on Retroviruses and Opportunistic Infections (CROI). Abstract 1149. March 4–7, 2018 Boston, Massachusetts. [Google Scholar]
- 35.Giler Mera R, Magnuson D, Trevor H, Bush S, Rawlings K, McCallister S. Changes in truvada (TVD) for HIV pre-exposure prophylaxis (PrEP) utilization in the United States: (2012–2016). Abstract 1614. 19th IAS Conference on HIV Science, 23–26 July 2017, Paris, France. [Google Scholar]
- 36.Sheth AN, Rolle CP, and Gandhi M, HIV pre-exposure prophylaxis for women. J Virus Erad, 2016. 2(3): p. 149–55. [PMC free article] [PubMed] [Google Scholar]
- 37.Flash CA, et al. , Perspectives on HIV prevention among urban black women: a potential role for HIV pre-exposure prophylaxis. AIDS Patient Care STDS, 2014. 28(12): p. 635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong JN, Farel CE, and Rahangdale L, Pharmacologic prevention of human immunodeficiency virus in women: practical approaches for the obstetrician and gynecologist. Obstet Gynecol Surv, 2015. 70(4): p. 284–90. [DOI] [PubMed] [Google Scholar]
- 39.Doblecki-Lewis S, et al. , HIV risk and awareness and interest in pre-exposure and post-exposure prophylaxis among sheltered women in Miami. Int J STD AIDS, 2016. 27(10): p. 873–81. [DOI] [PubMed] [Google Scholar]
- 40.Auerbach JD, et al. , Knowledge, attitudes, and likelihood of pre-exposure prophylaxis (PrEP) use among US women at risk of acquiring HIV. AIDS Patient Care STDS, 2015. 29(2): p. 102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]