Abstract
Purpose:
To characterize the effects of disease type and clinical characteristics on the pharmacokinetics of siltuximab, an IL-6 inhibiting monoclonal antibody.
Methods:
Siltuximab pharmacokinetic data were combined from 7 phase I/II clinical trials. A population pharmacokinetic model was developed to characterize changes in siltuximab disposition with disease type, albumin, liver and renal function, and patient demographics.
Results:
A total of 7761 concentrations from 460 participants were used in the study. The data were well described by a two-compartment model. Castleman’s disease, healthy volunteer status, albumin, and ALT were independent predictors of clearance. Monte Carlo simulations of the final model for an 11 mg/kg dose resulted in a longer median half-life for healthy volunteers (24.5 days) as compared to Castleman’s disease (19.1 days) and other tumor types (22.2 days). Clearance varied 1.8-fold over the range of albumin values seen in the study (1.5-5.2 g/dL), while ALT resulted in minimal changes in clearance.
Conclusions:
Albumin and disease state are important factors for siltuximab disposition and will likely need to be considered for dosing in future therapeutic applications.
Keywords: siltuximab, IL-6, population pharmacokinetics, oncology, Castleman’s disease
INTRODUCTION
Siltuximab is a chimeric mouse-human monoclonal antibody which binds with high affinity and specificity to interleukin 6 (IL-6). IL-6 mediates signaling via CD130 through the JAK/STAT pathway leading to pro-inflammatory signaling which promotes inflammatory states including cancer. Tumor necrosis factor alpha induces expression of IL-6 and other inflammatory cytokines to promote the inflammatory response. IL-6 is the primary inducer of C-reactive protein (CRP) synthesis in the liver [1]. Elevated CRP levels have been reported to correlate with serum IL-6 in multiple tumor types [2,3]. CRP suppression has previously been used as a surrogate for inhibition of IL-6 signaling [4].
IL-6 is an important therapeutic target in oncology and rheumatology. IL-6 has previously been suggested to correlate with tumor metastasis, disease, stage, and shortened survival in multiple cancer types. It has been found to be elevated in serum and tumor tissue for a variety of cancers including colorectal, breast, prostate, ovarian, pancreatic, lung, renal cell, cervical, and multiple myeloma [5]. IL-6 is also elevated in many autoimmune conditions. The IL-6 directed therapy tocilizumab targets the IL-6 receptor and is FDA approved for treatment of multiple rheumatologic conditions including rheumatoid arthritis, giant cell arteritis, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis. It is also used for cytokine release syndrome due to CAR T-cell directed therapy [6].
Siltuximab is FDA-approved for treatment of multi-centric Castleman’s disease which is characterized by high IL-6 levels and targets IL-6. IL-6 elevation has previously been linked to pathogenesis of the disease and treatment with IL-6 inhibition led to objective tumor responses in over half of patients [7]. It has also been tested in a wide variety of solid tumors and hematologic malignancies as part of therapeutic development including renal cell carcinoma, ovarian cancer, multiple myeloma, and smoldering multiple myeloma along with healthy volunteers [4,8–13].
Differences between siltuximab disposition based on disease state have not been well characterized and may lead to altered clearance which can affect dosing requirements. The current study developed a population PK model across multiple studies to determine how disease state affects siltuximab disposition. It also evaluated the effects of demographics and laboratory values on pharmacokinetic parameters.
MATERIALS AND METHODS
Patient population, drug administration, and pharmacokinetic sampling:
Siltuximab data was combined from 7 previously published clinical trials in adults [4,8,10,11,9,13,12]. Informed consent was obtained from all participants in the study. All studies were approved by the institutional review boards of the participating sites and have been performed in accordance with the ethical standard as laid down in the 1964 Declaration of Helsinki.
T01 was a Phase 1/2 study of patients with metastatic renal cell carcinoma. Participants received siltuximab intravenously (IV) over 2 hours at 1-12 mg/kg every 21 days. PK sampling consisted of up to 10 samples after the dose between the completion of the infusion and 42 days after the end of the infusion [4].
T03 was a Phase 1 study of patients with non-Hodgkin’s lymphoma, multiple myeloma, and Castleman’s disease. Siltuximab was administered intravenously between 2.8 and 11 mg/kg every 1-3 weeks. Up to 8 PK samples were obtained starting mid-infusion until a maximum of 21 days after the dose [8]
MCD2001 was a Phase 2 study of patients with multicentric Castleman’s disease. Participants received siltuximab intravenously over 1 hour at 11 mg/kg every 21 days. Up to 6 PK samples were obtained between the end of the infusion and 21 days after the dose [12].
SMM1001 was a phase 1 study in patients with monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and multiple myeloma. Siltuximab was administrated at 15 mg/kg IV every 21 days over 1 hour. Up to 4 PK samples were obtained after the dose between completion of the infusion and 24 hours after the dose [10].
SMM2001 was a phase 2 study in patients with high-risk smoldering multiple myeloma. Participants received 15 mg/kg IV siltuximab every 28 days over 1 hour. Up to 2 PK samples per cycle were obtained post-dose and prior to the next dose [11].
STM2001 was a Phase 1/2 study of patients with advanced cancer and included separate cohorts of ovarian and KRAS mutated tumors. Patients received 2.8-15 mg/kg of siltuximab IV over 1 hour every 21-28 days. PK sampling consisted of up to 9 samples after the dose between the completion of the infusion and 22 days [9].
T08 was a Phase 1 clinical trial in healthy volunteers. Siltuximab was given at 1.4-2.8 mg/kg IV over 1 hour as a single dose. PK sampling consisted of up to 16 samples after the dose between the completion of the infusion and 85 days [13].
Siltuximab serum concentrations were measured using validated ELISA. The lower limit of detection for siltuximab was 0.05 mcg/mL [4].
Pharmacokinetic analysis:
Using the computer program NONMEM (version 7.3) with a GNU Fortran G77 compiler, concentration time data was fitted using first-order conditional estimation methods (FOCE) with interaction. A two-compartment pharmacokinetic structural model (ADVAN3, TRANS4 subroutine) with first-order absorption was used to describe the data. The two-compartment model had the following parameters: clearance (CL), inter-compartmental clearance (Q), volume of distribution for the central compartment (V1), and volume of the peripheral compartment (V2). An exponential-normal distribution error model was used for inter-subject variability.
Age, weight, serum creatinine, AST, ALT, total bilirubin, albumin, gender, dose, race (white, Asian, African-American, other), and disease state (healthy volunteer, Castleman’s disease, smoldering multiple myeloma, renal cell carcinoma, other solid tumors, multiple myeloma, monoclonal gammopathy of undetermined significance) were evaluated as potential covariates for CL and the combined volumes of distribution (Vss). Potential covariates were added to the model one at a time as a linear function, with covariates that improved the model fitting by a change in the objective function of at least 4.0 (p<~0.05) being retained in the initial covariate screen. A forward selection approach was utilized for the multivariate assessment. Covariates found to improve the objective function by 10.8 (p<~0.001) or greater were retained in the final model.
Empiric Bayesian estimates of the individual pharmacokinetic parameters were generated from the final model using the POSTHOC routine. A 1000 sample bootstrap assessment of the final model was performed using Wings for NONMEM (v. 7.4.1). Monte Carlo simulations were performed using the final population PK model to compare differences between tumor types with standard dosing. Concentration profiles were generated for 1000 patients with the median serum creatinine, liver function, and albumin from the study. A dosage of 11 mg/kg and 1 hour infusion every 3 weeks was used for all simulated subjects.
RESULTS
Patients:
Raw concentration data and dosing were reviewed for completeness. Records with incomplete dosing information were removed from the data set. In total, 15 subjects were excluded from the analysis. Pharmacokinetic data for 460 subjects (7,761 siltuximab concentrations) were available for population PK modeling. Demographics and laboratory values from the first cycle of therapy are shown in Table 1. Siltuximab raw data is summarized in Figure 1. The majority of sampling occurred in the first 2 days after the dose (Figure 1A–B) and the majority of concentrations are less than 50 mcg/mL (Figures 1C–1D).
Table 1:
Demographics from First Visit
| Study | Total subjects (Number of concentrations) | Dose (mg/kg)* | Disease (N) | Age (years)* | Gender | Weight (kg)* | SCR* (mg/dL) | ALT* (U/L) | Total bilirubin* (mg/dL) | Albumin* (g/dL) |
|---|---|---|---|---|---|---|---|---|---|---|
| T01 | 66 (1400) | 5.4 (0.15 – 12.0) | 6 (66) | 59 (26 – 82) | Male: 52 Female: 14 |
83 (42 – 132) | 1.2 (0.8 – 2.1) | 18 (8 – 96) | 0.6 (0.2 – 1.8) | 4.1 (3.1 – 4.0) |
| T03 | 50 (1230) | 11.8 (2.9 – 13.6) | 2 (30) 4 (12) 5 (8) |
54 (18 – 82) | Male:0 Female: 50 |
78 (40 – 170) | 1.0 (0.5 – 2.4) | 18 (6 – 75) | 0.4 (0.2 – 1.8) | 3.8 (1.6 – 3.1) |
| MCD 2001 | 52 (929) | 11.0 (11.0 – 11.3) | 2 (52) | 47 (20 – 74) | Male: 32 Female: 20 |
67 (42 – 136) | 0.8 (0.5 – 1.7) | 15 (4 – 84) | 0.4 (0.2 – 1.7) | 3.6 (1.5 – 4.9) |
| SMM 1001 | 25 (204) | 15 (14.8 – 15.1) | 3 (11) 5 (14) |
58 (24 – 79) | Male: 9 Female: 16 |
69 (52 – 127) | 0.9 (0.5 – 1.9) | 20 (10 – 57) | 0.5 (0.2 – 1.0) | 3.9 (2.7 – 4.8) |
| SMM 2001 | 43 (352) | 15 (14.4 – 15.3) | 3 (43) | 61 (46 – 84) | Male: 26 Female: 17 |
78 (47 – 127) | 0.9 (0.5 – 1.6) | 19 (5 – 66) | 0.5 (0.2 – 1.8) | 3.7 2.7 – 4.7) |
| STM 2001 | 83 (791) | 15 (2.8 – 15.6) | 4 (83) | 60 (32 – 81) | Male: 29 Female: 54 |
69 (40 – 117) | 0.8 (0.4 – 1.9) | 23 (5 – 77) | 0.5 (0.1 – 1.8) | 3.8 (2.1 – 5) |
| T08 | 141 (2855) | 1.4 (0.2 – 2.8) | 1 (141) | 26 (19 – 45) | Male: 92 Female: 49 |
69 (51 – 92) | 0.8 (0.4 – 1.3) | 18 (9 – 73) | 0.7 (0.3 – 2.5) | 4.5 (3.8 – 5.2) |
| Overall | 460 (7761) | 9.2 (0.15 – 15.6) | 1 (141) 2 (82) 3 (54) 4 (95) 5 (22) 6 (66) |
51 (18 – 84) | Male: 240 Female: 220 |
73 (40 – 170) | 0.9 (0.4 – 2.4) | 19 (4 – 96) | 0.5 (0.1 – 2.5) | 4.1 (1.5 – 5.2) |
Data represent median (25-75% interquartile range)
Abbreviations: Disease: 1: Healthy volunteers; 2: Castleman’s disease; 3: smoldering multiple myeloma, 4: KRAS mutated/ovarian cancer/other solid tumors; 5: multiple myeloma/MGUS; 6: renal cell carcinoma
Figure 1:
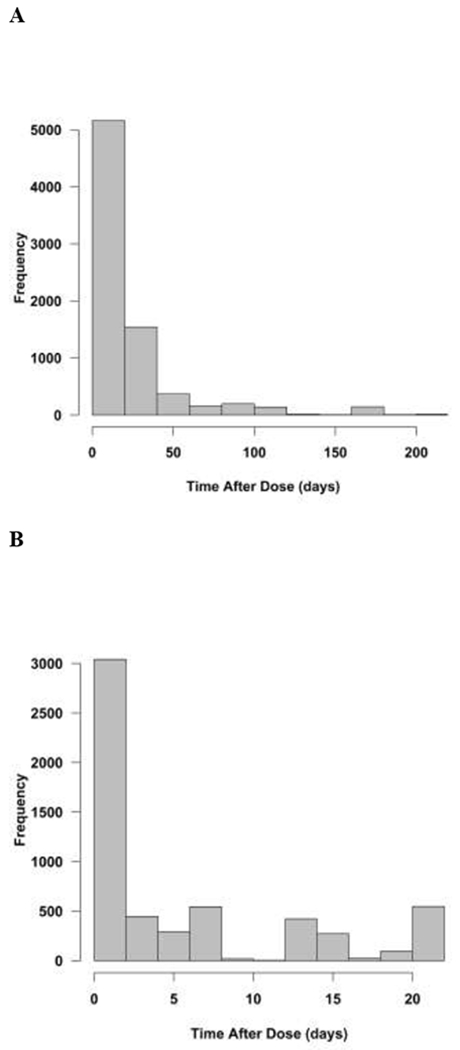
Histogram Summary of Raw Data. A. Frequency of time after dose (days). B. Frequency of time after dose (days) within the standard 21-day dosing interval. C. Frequency of siltuximab concentrations (mcg/mL). D. Frequency of concentrations less than 50 mcg/mL. The data set represented a broad range of concentrations and time after doses.
Population pharmacokinetic analysis: covariate analysis:
The base 2-compartment model adequately described the data thus more complicated models were not pursued. The following were identified in the univariate screen as potential covariates for clearance: age, AST, ALT, total bilirubin, albumin, race, and disease state. Age, weight, serum creatinine, total bilirubin, albumin, gender, race, and disease state were identified as potential covariates for the combined volume of distribution. Following the multivariate assessment, albumin, ALT, and disease status (healthy and Castleman’s disease) remained as significant covariates on CL, while weight, albumin, serum creatinine, and disease status (healthy volunteer and smoldering multiple myeloma) were significant on the volume of distribution. Inter-subject variability (ETA) was assessed on CL and a single inter-subject variability was used for VI and V2. A combined additive and proportional error were used to characterize residual error.
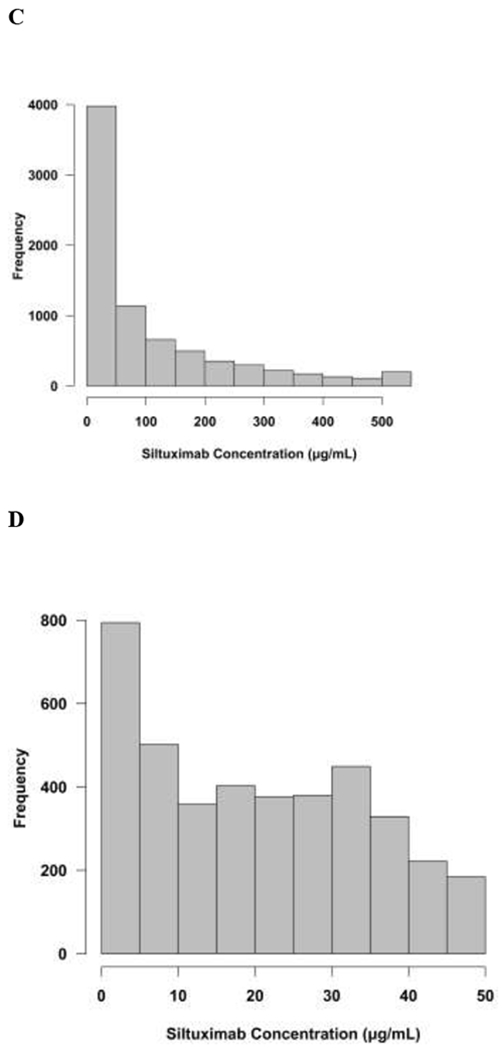
The final population pharmacokinetic model described the data without significant bias as shown in Figure 2A–B. Shrinkage estimates for inter-subject variability were low: 8.4% (CL) and 3.5% (V1/V2). Final model parameter and variance estimates are shown in Table 2. Bootstrap evaluation of the final model successfully converged 98.9% of the time and estimation results are summarized in Table 2. The final parameter estimates of the model fall well within the 95% confidence interval and are close in value to the median estimates which suggests that the final model represents the population well. CL varied 1.8-fold over the range of albumin values seen in the study. CL decreased by 23% for healthy volunteers and increased 24% for patients with Castleman’s disease.
Figure 2:
Diagnostic plots for final population PK model. A. Conditional weight residuals (CWRES) vs. time after dose (hrs). B. Individual predicted siltuximab concentrations vs. measured concentrations. Dashed line represents line of unity. Solid line represents linear regression line. Overall the model represents the data without bias.
Table 2:
Final Population PK Model Parameters and Bootstrap Estimate
| Final Parameter | Standard Error | Bootstrap Estimates* (Median and 95% CI) | |
|---|---|---|---|
| Θ1 (VI) | 3.66 | 0.064 | 3.67 (3.55 – 3.78) |
| Θ2 (CL) | 0.214 | 0.00816 | 0.216 (0.20 – 0.23) |
| Θ3 (V2) | 3.13 | 0.088 | 3.14 (2.97 – 3.31) |
| Θ4 (Q) | 0.624 | 0.0456 | 0.62 (0.55 – 0.72) |
| Θ5 (Albumin CL) | −0.84 | 0.17 | −0.86 (−1.14 - −0.53) |
| Θ6 (Weight Vss) | 0.65 | 0.056 | 0.64 (0.54 – 0.75) |
| Θ7 (Albumin Vss) | −0.35 | 0.11 | −0.32 (−0.53 - −0.15) |
| Θ8 (HV on Vss) | 0.83 | 0.025 | 0.82 (0.78 – 0.87) |
| Θ9 (SMM on Vss) | 0.77 | 0.025 | 0.77 (0.72 – 0.83) |
| Θ10 (HV on CL) | 0.77 | 0.041 | 0.77 (0.70 – 0.83) |
| Θ11 (ALT CL) | −0.096 | 0.041 | −0.097 (−0.18 - −0.013) |
| Θ12 (SCR Vss) | 0.16 | 0.051 | 0.15 (0.062 – 0.25) |
| Θ13 (CD on CL) | 1.24 | 0.081 | 1.24 (1.11 – 1.45) |
| Variability (η) | |||
| Between-subject (CL) | 20.0% | 0.11% | 19.9% (17.8% - 22.1%) |
| Between-subject (Vss) | 42.0% | 1.71% | 41.0% (37.8% - 44.5%) |
| Error (ε) | |||
| Proportional | 22.4% | 0.79% | 22.2% (20.8% - 23.8%) |
| Additive | 0.0217 | 0.0028 | 0.022 (0.0005 – 0.056) |
Bootstrap successfully converged 98.9%
Abbreviations: ALB=albumin, ALT=alanine aminotransferase, CD=Castleman’s disease, CL=clearance, HV=healthy volunteers, SCR= serum creatinine, SMM=smoldering multiple myeloma, V1=central volume of distribution, V2=peripheral volume of distribution, Vss=covariate assessed on both V1 and V2
CL (L/day) = 0.214 x (ALB/4.1)−0.84 x (0.77 if HV) x (ALT/17)−0.096 x (1.24 if CD)
Vss (L) = (3.66 + 3.13) x (WT/73)0.65 x (ALB/4.1)−0.35 x (0.83 if HV) x (0.77 if SMM) x (SCR/0.894)0.16
Monte Carlo simulations:
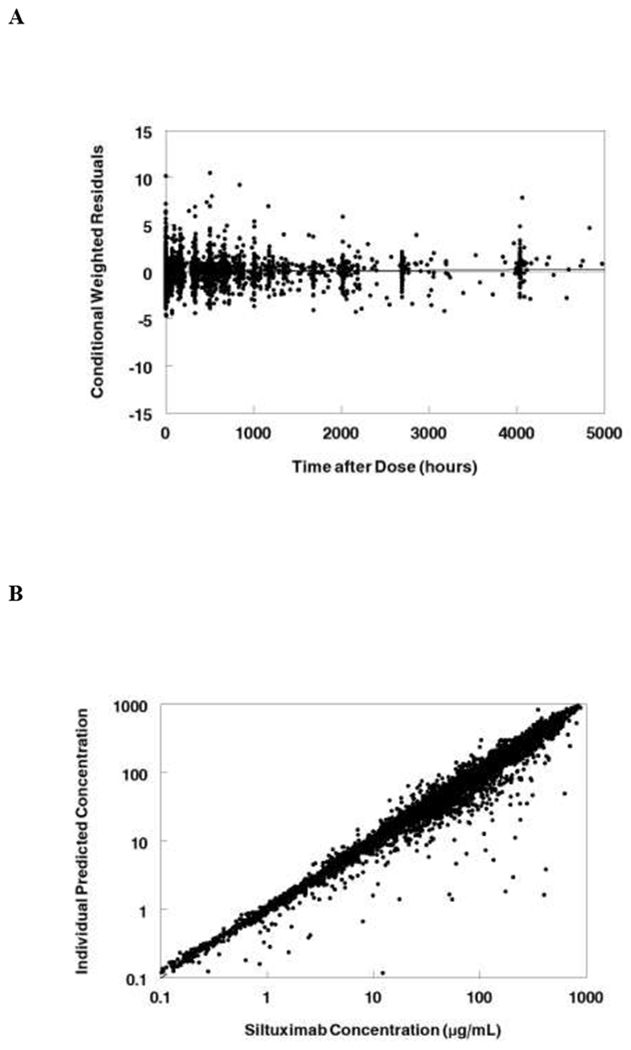
The Monte Carlo simulations used the FDA approved dose for Castleman’s disease (11 mg/kg IV every 3 weeks). Median and 25-75% interquartile range CL, AUC, and beta half-life for healthy volunteers, Castleman’s disease, and other tumors following a single dose of siltuximab are shown in Table 2. The median clearance is 0.16 L/day for healthy volunteers as compared to 0.27 for Castleman’s disease, and 0.22 for other tumor types. Median half-life varies from 19.1 days is Castleman’s disease to 24.5 days in healthy volunteers. Median concentrations from the simulation are shown in Figure 3A. The median and interquartile range steady state concentrations for the 11 mg/kg dose are shown for Castleman’s disease (Figure 3B) and other tumors (Figure 3C).
Figure 3:
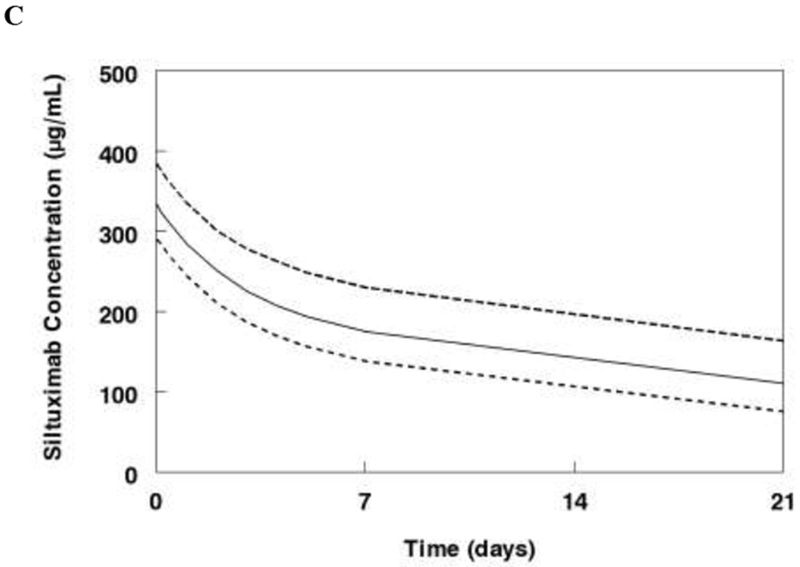
Monte Carlo simulations of the final model. A. Median concentrations following single dose of siltuximab (11 mg/kg) for a 70 kg subject healthy subject, with Castleman’s disease, or with other tumors. B. Steady state concentrations for Castleman’s disease patients receiving siltuximab 11 mg/kg every 3 weeks. C. Steady state concentrations for patients with other cancers receiving siltuximab 11 mg/kg every 3 weeks. Data represent median and interquartile range (25-75%).
DISCUSSION
Monoclonal antibodies are increasingly being utilized as oncology targeted therapies [14]. Antibodies have a benefit over small molecule inhibitors in their high specificity and affinity for a target. These therapies generally do not require dose adjustments for renal and hepatic dysfunction and instead are cleared primarily via intracellular catabolism. Following receptor mediated endocytosis, they can escape lysosomal degradation by binding to the FcRN in the endosome which leads to recycling back to the cell surface and the long half-life characteristic of antibody therapeutics. Antibodies can compete with endogenous IgG and other proteins which may impact monoclonal antibody PK. Thus, clearance can vary significantly between individuals and factors affecting antibody clearance are important to understand.
Siltuximab was FDA approved for multi centric Castleman’s disease, however had limited therapeutic efficacy as a single agent in other oncology disease states [15]. Renal cell carcinoma showed disease stabilization in 50% of patients [16], but otherwise no single agent benefit was seen for other cancer types. While IL-6 has been shown to be elevated in other tumor types, these tumors may be more dependent on additional pathways beyond the IL-6 JAK2/STAT signaling pathway. Thus, siltuximab may still have a role in combination therapy for these cancers and understanding differences in clearance may be important for dosing in future therapeutic treatment options.
The current study developed a composite population PK model of siltuximab, a monoclonal antibody against IL-6. It utilized a broad data set of over 7700 siltuximab concentrations with 460 patients from seven phase I and II clinical trials and evaluated multiple disease types. We utilized a standard two compartment model to characterize siltuximab disposition. Siltuximab clearance was found to be slow with a half-life of close to 3 weeks. Albumin levels, healthy volunteer status, and Castleman’s disease had the most significant effects on clearance.
IL-6 is the primary factor the drives CRP expression [1] and CRP has previously been shown to be a biomarker for IL-6 activity [4]. CRP levels are highly related to monoclonal antibody clearance [17,18] and higher CRP levels were correlated with higher levels of monoclonal antibody clearance. Castleman’s disease has high levels of IL-6 and thus had the highest clearance seen in the study. Healthy volunteers generally have lower IL-6 and CRP levels, as compared to patients with cancer, thus had the slowest clearance and longest half-life of siltuximab. CRP levels have been shown to be elevated in other cancers including multiple myeloma, renal cell carcinoma, prostate cancer, and lymphoma. Interestingly, while Castleman’s disease was previously reported to have a median CRP level of 17.6 mg/L, lymphomas had a median of 29.2 mg/L [15]. We tested lymphoma alone as a separate covariate for clearance, but it was not signficiant in the multivariate screens. This is likely due to the fact that only 12 non-Hodgkin’s lymphoma patients were represented in the dataset, thus making it difficult to assess differences in clearance.
Albumin concentrations have previously been shown to be inversely correlated with monoclonal antibody clearance; thus decreased albumin levels are correlated with increased clearance [17,19]. This is consistent with the results seen in the current study where albumin was the most significant covariate in the analysis and was inversely related to albumin levels. The albumin changed by 1.8-fold for the range of albumin values seen in the study. An increase in albumin from 3 to 4 g/dL was associated with a 21% decrease in clearance. The endogenous catabolic rate for albumin is correlated with the catabolic turnover of IgG. The increased protein turnover that occurs with low albumin results in increased degradation of IgG, increased clearance, and reduced systemic exposure of monoclonal antibodies. The clinical trials patient population is likely a healthier population which has higher albumin levels than the standard oncology patient seen in clinic. Thus, consideration of alterations of dosing for albumin levels may be important.
There was no clear evidence of targeted mediated drug disposition for siltuximab. The drug concentrations from a wide variety of dose ranges were well-characterized by a standard two compartment model without any evidence of non-linear kinetics or dose levels effects. While dose was significant in the univariate screens, it was not significant in the multivariate screens. There was less than a 25% reduction in clearance over a 10-fold dose range when a dose effect was added to the model and lower clearance with higher doses would not be consistent with target mediated drug disposition. A steady state concentration of 100 mcg/mL of siltuximab is 690 μM while a mean IL-6 level of 34 pg/mL [20] is 0.0016 μM, which is a ratio of over 400,000. Thus, siltuximab is present in significantly higher amounts than plasma IL-6, making targeted mediated drug disposition highly unlikely to occur.
The current model was limited as we were not able to link pharmacokinetics to CRP or IL-6 levels due to the lack of availability of CRP and IL-6 data for our analysis. Puchalski et al [4] previously developed a PK-PD model linking siltuximab PK and CRP suppression using data from a phase I/II study in metastatic renal cell carcinoma. The model was used to simulate the siltuximab dosing regimens that would maintain CRP suppression below the lower limit of quantification and recommended doses of 6 mg/kg every 2 weeks or 9 mg/kg every 3 weeks.
In conclusion, we have developed a composite population PK model of siltuximab which describes antibody disposition for a wide variety of oncology disease states. This model found differences in clearance between the disease states and will be useful in helping dose siltuximab for future applications.
Table 3:
Monte Carlo Simulation of Tumor Type*
| CL (L/day)** | AUC (mcg*day/mL)** | Half-life β (days)** | |
|---|---|---|---|
| Healthy volunteers | 0.16 (0.12 – 0.21) | 4,856 (3,717 - 6,426) | 24.5 (19.2 – 33. 6) |
| Castleman’s Disease | 0.27 (0.20 – 0.34) | 2,884 (2,234 - 3,783) | 19.1 (14.6 – 25.0) |
| Other tumor types | 0.22 (0.17 – 0.28) | 3,515 (2,786 – 4,627) | 22.2 (17.5 – 30.5) |
Simulations were conducted using a single dose of siltuximab at 11 mg/kg for a 70 kg patient with the average laboratory values from the study
Median (25-75% interquartile range)
Acknowledgements:
The clinical trials presented in the study were funded by Janssen Pharmaceuticals. Funding support was provided by a Research in Pediatric and Developmental Pharmacology NIH grant (1U54HD090259-01, Dr. Capparelli).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Disclosures: Dr. Capparelli serves on the data safety and monitoring board for Cempra Pharmaceuticals and Celltrion. Dr. Nikanjam and Dr. Yang have no potential conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
REFERENCES
- 1.Heinrich PC, Castell JV, Andus T (1990) Interleukin-6 and the acute phase response. Biochem J 265 (3):621–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelliniemi TT, Irjala K, Mattila K, Pulkki K, Rajamaki A, Tienhaara A, Laakso M, Lahtinen R (1995) Immunoreactive interleukin-6 and acute phase proteins as prognostic factors in multiple myeloma. Finnish Leukemia Group. Blood 85 (3):765–771 [PubMed] [Google Scholar]
- 3.Ljungberg B, Grankvist K, Rasmuson T (1997) Serum interleukin-6 in relation to acute-phase reactants and survival in patients with renal cell carcinoma. Eur J Cancer 33 (11):1794–1798 [DOI] [PubMed] [Google Scholar]
- 4.Puchalski T, Prabhakar U, Jiao Q, Berns B, Davis HM (2010) Pharmacokinetic and pharmacodynamic modeling of an anti-interleukin-6 chimeric monoclonal antibody (siltuximab) in patients with metastatic renal cell carcinoma. Clin Cancer Res 16 (5):1652–1661. doi: 10.1158/1078-0432.CCR-09-2581 [DOI] [PubMed] [Google Scholar]
- 5.Kumari N, Dwarakanath BS, Das A, Bhatt AN (2016) Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol 37 (9): 11553–11572. doi: 10.1007/sl3277-016-5098-7 [DOI] [PubMed] [Google Scholar]
- 6.Tocilizumab Package Insert, https://www.gene.com/download/pdf/actemra_prescribing.pdf. Accessed January 26, 2019. [Google Scholar]
- 7.El-Osta HE, Kurzrock R (2011) Castleman’s disease: from basic mechanisms to molecular therapeutics. Oncologist 16 (4):497–511. doi: 10.1634/theoncologist.2010-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie L, Li LY, Kurzrock R, van Rhee F, Qin X, Reddy M, Qi M, Davis HM, Zhou H, Puchalski TA Population Pharmacokinetic and Pharmacodynamic Modeling of an Anti-Interleukin-6 Chimeric Monoclonal Antibody, Siltuximab (CNTO 328), in Patients with B-Cell Non-Hodgkin’s Lymphoma, Multiple Myeloma, or Castleman’s Disease. In: American Society of Hematology Annual Meeting, Atlanta, Georgia, 2012. p 1365 [Google Scholar]
- 9.A Safety, Efficacy and Pharmacokinetic Study of Siltuximab (CNTO 328) in Participants With Solid Tumors. https://clinicaltrials.gov/ct2/show/NCT00841191?term=siltuximab&draw=2&rank=14. Accessed January 12, 2019. [Google Scholar]
- 10.A Study Evaluating the Effects of Siltuximab on the Heart in Patients With Monoclonal Gammopathy of Undetermined Significance, Smoldering Multiple Myeloma, or Indolent Multiple Myeloma. https://clinicaltrials.gov/ct2/show/NCT01219010?term=siltuximab&draw=2&rank=8. Accessed January 12, 2019. [Google Scholar]
- 11.A Study of Siltuximab (Anti-IL 6 Monoclonal Antibody) in Patients With High-risk Smoldering Multiple Myeloma. https://clinicaltrials.gov/ct2/show/NCT01484275?term=siltuximab&rank=7. Accessed January 12 2019. [Google Scholar]
- 12.A Study to Evaluate the Efficacy and Safety of CNTO328 Plus Best Supportive Care in Multicentric Castleman’s Disease. https://clinicaltrials.gov/ct2/show/NCT01024036?term=siltuximab&draw=2&rank=15. Accessed: January 12, 2019. [Google Scholar]
- 13.A study to assess the safety and pharmacokinetics of a single intravenous administration of CNTO 328 derived from 2 different cell lines in healthy participants. https://clinicaltrials.gov/ct2/show/NCT02074800?term=NCT+02074800&rank=1. Accessed: January 12, 2019. [Google Scholar]
- 14.Pento JT (2017) Monoclonal Antibodies for the Treatment of Cancer. Anticancer Res 37 (11):5935–5939. doi: 10.21873/anticanres.12040 [DOI] [PubMed] [Google Scholar]
- 15.Rossi JF, Lu ZY, Jourdan M, Klein B (2015) Interleukin-6 as a therapeutic target. Clin Cancer Res 21 (6):1248–1257. doi: 10.1158/1078-0432.CCR-14-2291 [DOI] [PubMed] [Google Scholar]
- 16.Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H, Prabhakar U, Qin X, Mulders P, Berns B (2010) A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer 103 (8):1154–1162. doi: 10.1038/sj.bjc.6605872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryman JT, Meibohm B (2017) Pharmacokinetics of Monoclonal Antibodies. CPT Pharmacometrics Syst Pharmacol 6 (9):576–588. doi: 10.1002/psp4.12224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ordas I, Mould DR, Feagan BG, Sandborn WJ (2012) Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 91 (4):635–646. doi: 10.1038/clpt.2011.328 [DOI] [PubMed] [Google Scholar]
- 19.Fasanmade AA, Adedokun OJ, Ford J, Hernandez D, Johanns J, Hu C, Davis HM, Zhou H (2009) Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol 65 (12):1211–1228. doi: 10.1007/s00228-009-0718-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimoto N, Sasai M, Shima Y, Nakagawa M, Matsumoto T, Shirai T, Kishimoto T, Yoshizaki K (2000) Improvement in Castleman’s disease by humanized anti-interleukin-6 receptor antibody therapy. Blood 95 (1):56–61 [PubMed] [Google Scholar]


