Abstract
Accumulating evidence from preclinical and clinical studies has implicated a role for the cytokine IL-6 in a variety of CNS diseases including anxiety-like and depressive-like behaviors, as well as alcohol use disorders. Here we use homozygous and heterozygous transgenic mice expressing elevated levels of IL-6 in the CNS due to increased astrocyte expression and non-transgenic littermates to examine a role for astrocyte-produced IL-6 in emotionality (response to novelty, anxiety-like, and depressive-like behaviors). Our results from homozygous IL-6 mice in a variety of behavioral tests (light/dark transfer, open field, digging, tail suspension, and forced swim tests) support a role for IL-6 in stress-coping behaviors. Ex vivo electrophysiological studies of neuronal excitability and inhibitory spontaneous GABAergic synaptic transmission in the central nucleus of the amygdala (CeA) of the homozygous transgenic mice revealed increased inhibitory GABAergic signaling and increased excitability of CeA neurons, suggesting a role for astrocyte produced IL-6 in the amygdala in exploratory drive and depressive-like behavior. Furthermore, studies in the hippocampus of activation/expression of proteins associated with IL-6 signal transduction and inhibitory GABAergic mechanisms support a role for astrocyte produced IL-6 in depressive-like behaviors. Our studies indicate a complex and dose-dependent relationship between IL-6 and behavior and implicate IL-6 induced neuroadaptive changes in neuronal excitability and the inhibitory GABAergic system as important contributors to altered behavior associated with IL-6 expression in the CNS.
1. Introduction
The cytokine interleukin-6 (IL-6) is an important neuroimmune signaling protein in the CNS. IL-6 is produced within the CNS, primarily by glial cells, although some neurons also express IL-6 (Gadient and Otten, 1997; Gruol and Nelson, 1997; Ringheim et al., 1995; Sallmann et al., 2000). CNS levels of IL-6 are low under physiological conditions, with elevations occurring during pathophysiological conditions, such as neuropsychological disorders and CNS disease and injury (Campbell and Chiang, 1995; Erta et al., 2012; Gruol and Nelson, 1997; Hodes et al., 2016; Rothaug et al., 2016). Studies using genetically modified mice indicate that IL-6 plays a role in a number of important physiological and pathophysiological behaviors. Specifically, IL-6 knockout mice (IL-6 KO) show alterations in anxiety-like and depression-like behaviors (Aniszewska et al., 2015; Armario et al., 1998; Butterweck et al., 2003; Chourbaji et al., 2006), aggressive behavior (Alleva et al., 1998), and deficits in memory and learning (Baier et al., 2009; Sparkman et al., 2006; Yang et al., 2014), implicating a role for IL-6 in these behaviors. Central or intra-hippocampal administration of IL-6 also supports a role for IL-6 in depressive-like behavior (Sukoff Rizzo et al., 2012; Wu and Lin, 2008). An involvement of IL-6 in anxiety-like and depression-like behaviors is consistent with clinical studies in humans (Hodes et al., 2016). IL-6 KO mice also exhibit altered responses to drugs of abuse including alcohol (Blednov et al., 2012) and opioids (Bianchi et al., 1999), and to drugs that induce seizures (Penkowa et al., 2001) and fever (Chai et al., 1996).
IL-6 KO mice lack IL-6 in the CNS and periphery. In the CNS, astrocytes are known to be a primary source of IL-6 under physiological and pathophysiological conditions (Gruol and Nelson, 1997). Thus, to identify CNS actions of IL-6, transgenic mice with altered astrocyte expression of IL-6 have been constructed, such as mice that produce elevated astrocyte levels of IL-6 (IL-6 tg) (Campbell et al., 1993), and conditional astrocyte IL-6 KO mice (Ast IL-6 KO) with reduced astrocyte IL-6 expression (Quintana et al., 2013). Adult Ast IL-6 KO mice show increased anxiety-like behavior, altered social behavior, and impaired learning (Erta et al., 2015). IL-6 tg mice exhibit altered cognitive function (Heyser et al., 1997). Overall, these findings strongly suggest that IL-6 produced by astrocytes plays a role in a several important mouse behaviors.
Many of the behaviors identified in IL-6 tg, Ast IL-6 KO or IL-6 KO mice are also symptoms of excessive alcohol use, including anxiety-like and depressive-like behavior, and impaired memory and learning (Gong et al., 2017; Martinez et al., 2018; Wills et al., 2009). Interestingly, there is evidence that alcohol consumption and/or withdrawal increased levels of IL-6 in the CNS (Alfonso-Loeches et al., 2010; Baxter-Potter et al., 2017; Doremus-Fitzwater et al., 2014; Emanuele et al., 2005; Gano et al., 2016; Kane et al., 2013; Zhang et al., 2014). These findings raise the possibility of a role for IL-6 in the behavioral effects of alcohol.
Given that the behavioral phenotype of IL-6 tg mice has not been well characterized, here we tested IL-6 homozygous and heterozygous mice and their non-transgenic littermates in several mouse behaviors, including those that capture stress coping reactions (i.e. anxiety-like and depressive-like tests), with the goal of advancing the understanding of the specific role of astrocyte-produced IL-6 in these behaviors. In addition, to identify potential mechanisms underlying effects of IL-6 on behavior we performed ex vivo electrophysiological recordings of inhibitory GABAergic transmission and neuronal excitability in the central amygdala (CeA). We also examined activation/expression of IL-6 signal transduction partners and proteins associated with inhibitory GABAergic signaling in the hippocampus. These brain regions (amygdala and hippocampus) are known to play a role in anxiety-like and depressive-like behaviors (Adhikari, 2014; Caliskan and Stork, 2018), with dysregulated GABAergic signaling being a major contributor/player/component (Gilpin et al., 2015; Kalueff and Nutt, 2007; Smith and Rudolph, 2012). Altered GABAergic transmission and increased CNS excitability are also important alcohol withdrawal symptoms (Kumar et al., 2004; Little, 1999; Olsen and Spigelman, 2012), and can contribute to excessive alcohol drinking associated with alcohol dependence (Enoch, 2008). Therefore, we also examined the role of IL-6 in changes of CNS excitability during alcohol withdrawal, as well as changes in hippocampal protein activation/expression as a consequence of alcohol exposure/withdrawal.
Our results strongly support a regulatory role of astrocyte produced IL-6 in several important mouse behaviors and have identified potential targets altered by IL-6 that could contribute to effects of IL-6 on behavior through the regulation of neuronal network activity. These results have important implications for conditions associated with elevated levels of IL-6 in the CNS, including CNS disease and injury, neuropsychological disorders and alcohol abuse disorders.
2. Materials and Methods
2.1. Animals
Adult (9–12 weeks at beginning of experiment) male and female IL-6 homozygous (IL-6 tg +/+)(25 females, 27 males), IL-6 heterozygous (IL-6 tg +/−)(23 females, 27 males) and non-transgenic littermates (IL-6 tg −/−)(24 females, 26 males) were used for these studies. The IL-6 tg +/− mice were obtained by breeding IL-6 tg +/− tg mice with wild-type C57BL/6J mice, or for the IL-6 tg +/+ mice, by breeding IL-6 tg +/− mice with IL-6 tg +/− mice. The IL-6 tg −/− were littermates from the two breeding approaches. Mice were genotyped by PCR analysis of tail DNA using standard methods and the PreciGene 2x HotStart Taq Master Mix with loading dye (BioPioneer Inc., San Diego, CA). Construction of the transgenic mice was described previously (Campbell et al., 1993). The transgenic mice were modified to express elevated levels of IL-6 through increased astrocyte production using an expression vector derived from the murine glial fibrillary acidic protein (GFAP) gene. All animal procedures were performed in accordance with the National Institutes of Health Guideline for the Care and Use of Laboratory Animals and approved by The Scripps Research Institute’s IACUC. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care.
2.2. Behavioral tests
2.2.1. Light/dark transfer test
This procedure has been used to assess anxiety-like behavior and exploratory drive in mice by capitalizing on the conflict between exploration of a novel environment and the avoidance of a brightly lit open field (Holmes et al., 2002; Li et al., 2013; Rodgers et al., 1997). The apparatus is a rectangular box made of Plexiglas divided by a partition into two environments. One compartment (14.5×27×26.5 cm) is dark (8–16 lux) and the other compartment (28.5×27×26.5 cm) is highly illuminated (400–600 lux) by a 60 W light source located above it. The compartments are connected by an opening (7.5×7.5 cm) located at floor level in the center of the partition. Mice were placed in the dark compartment to start the 5-minute test. The time spent in the light compartment was used as a predictor of anxiety-like behavior, i.e. a greater amount of time in the light compartment is indicative of decreased anxiety-like behavior.
2.2.2. Open field test
This test predicts how animals respond when introduced into a brightly illuminated open arena (Crawley, 1999). It is a classical test of “emotionality” used to measure anxiety-like responses of rodents exposed to stressful environmental stimuli (brightly illuminated open spaces) as well as to capture spontaneous activity measures under novel conditions. The apparatus is a square white Plexiglas (50W × 50L × 22H cm) open field illuminated to 400 lux in the center. Each animal was placed in the center of the field and several behavioral parameters (distance traveled, velocity, center time, entries to center) were recorded during a 60-minute observation period and analyzed using Noldus Ethovision XT software. In addition, rearing numbers and grooming time were analyzed from videos off line.
2.2.3. Digging test
Digging is a naturalistic rodent behavior that has been hypothesized to reflect response to novelty (Deacon, 2006), which is sensitive to hippocampal damage (Deacon and Rawlins, 2005) and is decreased in a mouse model of Alzheimer’s Disease (Janus et al., 2015). Mice were placed individually in a standard mouse cage containing bedding that was 5 cm in depth and the number of digging bouts in a 3-min session was recorded.
2.2.4. Tail suspension test
The tail suspension test is a classic test for examining depressive-like behavior in mice (Sarkisyan et al., 2010; Steru et al., 1985). Each mouse is suspended from its tail using adhesive tape on a metal bar located 30 cm above a flat surface for 6 minutes. Immobility is quantified by measuring the amount of time when no whole-body movement is observed. Increased time spent immobile is indicative of increased helpless (depressive)-like behavior. Data from 7 mice (mostly female IL-6 tg −/−) were removed as mistrials due to repeated tail climbing.
2.2.5. Forced swim test
The forced swim test is a classic test used as a predictive animal model for antidepressant actions of drugs (Cryan et al., 2002; Porsolt, 2000). We used a modification of the test originally described by Porsolt and colleagues (1977)(Porsolt et al., 1977) and adapted by Lucki (1997)(Lucki, 1997). Mice were individually placed into clear polypropylene cylinders containing 23–25 °C water, 15 cm deep, for 6 min. An observer blinded to the treatment recorded the number of seconds per minute each mouse was immobile. Immobility was measured when no activity was observed other than that required for the mouse to keep its head above the water.
2.2.6. Alcohol exposure
A single i.p. injection of 4 g/kg alcohol (ethanol; 20%w/v in 0.9% saline) was administered to 37–38 mice per genotype (equal males and females) two hours into the dark phase of the circadian rhythm (additional 12–14 mice per genotype were used for the protein assays and electrophysiological studies requiring alcohol naïve mice. This dose of alcohol in mice has been associated with increased handling-induced convulsions (HIC), albeit mild, in C57BL/6J mice 6–12 hr following alcohol injection (Crabbe, 1998; Crabbe et al., 1983; Roberts et al., 1992) and increased seizure thresholds 8 hrs following injection (McQuarrie and Fingl, 1958). HIC were assessed using a scale developed by Goldstein and Pal (Goldstein and Pal, 1971), which is used extensively in the field (e.g. (Crabbe et al., 1980; Farook et al., 2008; Finn et al., 2007; Ghozland et al., 2005; Metten et al., 2010; Olive and Becker, 2008)). Briefly, this procedure involves lifting the mouse by the tail and observing it for possible convulsions. If none occur, the mouse is gently spun 180 degrees by rubbing the tail between the thumb and forefinger. Convulsions are scored on a 6 point scale ranging from facial grimace to severe tonic-clonic convulsions (higher numbers reflect more severe behavioral signs). Baseline HIC was assessed prior to alcohol injection. Mice were scored for HIC at 2, 4, 6, 8, 12 and 24 hours following alcohol injection by a single experimenter blind to the animals’ experimental history. HIC scores at 2, 4, 6, 8, 12 and 24 hours were normalized to baseline control scores for each animal to adjust for individual differences.
2.3. Protein assays
Hippocampi from alcohol naïve (IL-6 tg −/−, 5 females, 4 males), IL-6 tg +/− (4 females, 7 males) and IL-6 tg +/+ (6 females, 3 males) mice, and alcohol exposed IL-6 tg −/−, IL-6 tg +/−, and IL-6 tg +/+ female (7 of each genotype) and male (7 of each genotype) mice were used for Western blot studies to determine the relative levels of specific cellular proteins in the CNS of the IL-6 tg +/+. IL-6 tg +/− and IL-6 tg−/− mice. The alcohol treated mice were sacrificed after the 24 hour alcohol withdrawal period. Protein samples were prepared using standard methods as described previously (Gruol et al., 2018). Mice were anesthetized with isoflurane, decapitated, and the hippocampus dissected. The hippocampus was snap frozen in liquid nitrogen and stored at −80 °C until processing. To extract the proteins, the hippocampi were sonicated individually in cold lysis buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.5% NP-40, a Complete Protease Inhibitor Cocktail Tablet (Roche Diagnostics, Mannheim, Germany), and a cocktail of phosphatase inhibitors (Na+ pyrophosphate, β-glycerophosphate, NaF, Na+ orthovanadate; all from Sigma-Aldrich). The samples were incubated on ice for 30 minutes, centrifuged at 13,860g for 30 minutes at 4°C, and the supernatants were collected. Protein concentration in the supernatants was determined using the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA). Aliquots were stored at −80°C.
Protein samples were separated by SDS-PAGE electrophoresis using 4–12% Novex NuPAGE Bis-Tris gels (Invitrogen Life Technologies, Grand Island, NY) following standard methods as described previously (Gruol et al., 2018). Typically, samples from all three genotypes were run on the same gel to enable comparisons across genotypes. Proteins were transferred to Immobilon-P membranes (Millipore, Billerica, MA) and uniform transfer assessed by Ponceau S staining (Pierce, Rockford, IL). After washing, membranes were blocked in a 5% casein solution (Pierce), incubated in primary antibody overnight (4°C), washed, and then incubated (room temperature) in secondary antibody coupled to horseradish peroxidase (HRP). Protein bands were identified by chemiluminescence and quantified by densitometry measurements using NIH Image software (http://rsb.info.nih.gov/nih-image/). Membranes were stripped and reprobed for beta-actin. To standardize results, for each animal tested on a gel the density of band for the protein of interest was first normalized to the density of the band for beta-actin in the same lane. This normalized value was then normalized to the average normalized value for the same protein in alcohol exposed IL-6 tg −/− hippocampus run on the same gel. The alcohol exposed IL-6 tg −/− samples were used for standardization because they were run on all gels. Samples were tested for the same protein multiple times and a final average for each animal determined. Data from all gels were combined according to protein, animal, genotype, and treatment and analyzed statistically.
Antibodies to the following proteins were used in these studies: a monoclonal rabbit antibody produced in rabbits by immunizing with a fusion protein corresponding to a sequence in the carboxy-terminal of mouse STAT3 protein (AB#4904; 1:1000; Cell Signaling Technologies); a purified rabbit polyclonal antibody produced in rabbits by immunizing with a synthetic phospho-peptide corresponding to the residues surrounding Tyr705 of mouse STAT3 (AB#9131; 1:1000; Cell Signaling Technologies); A purified rabbit polyclonal antibody produced by immunizing rabbits with a synthetic peptide derived from human GAD65/GAD67 (#PA5–38102, 1–2000, Invitrogen); a purified mouse monoclonal antibody to gamma-aminobutyric acid receptor (GABAAR) subunit alpha-5 (GABAAR alpha-5) produced by immunizing mice with a fusion protein containing a sequence from the cytoplasmic domain (amino acids 368–419) of human GABAAR alpha-5 (Clone N415/24, 1–500, UC Davis/NIH NeuroMab Facility); a purified mouse monoclonal antibody to gamma-aminobutyric acid receptor subunit alpha-1 (GABAAR alpha-1) produced by immunizing mice with a fusion protein (amino acids 355–394) from mouse GABAAR alpha-1 (clone N95/35, 1–500, UC Davis/NIH NeuroMab Facility); a purified mouse monoclonal antibody to a synthetic peptide (amino acids 411–422) of human glial fibrillary acidic protein (GFAP) (#75–240, 1–10,000, UC Davis/NIH NeuroMab Facility); a purified rabbit polyclonal antibody produced by immunizing rabbits with a synthetic peptide derived from the sequence of human enolase-2 (neuron specific enolase) (AB#9536, 1–1000, Cell Signaling Technology); a monoclonal antibody to beta-actin produced by immunizing mice with a synthetic peptide corresponding to a sequences in the amino-terminal of human beta-actin (AB#3700, 1:10,000; Cell Signaling Technology); a monoclonal antibody to beta-actin produced by immunizing rabbits with a synthetic peptide corresponding to a sequences in the amino-terminal of human beta-actin (AB#4970, 1:5,000; Cell Signaling Technology).
IL-6 levels in the cerebellum of the three genotypes were determined using the DuoSet mouse IL-6 ELISA kit (DY406, R&D Systems, Minneapolis, Minn) following manufacturer’s instructions. Protein samples from the cerebellum of alcohol naïve and acute alcohol treated IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+ mice were prepared following the same protocol used for the hippocampus. For alcohol-exposed mice, the cerebellum was obtained at the end of the 24 hr withdrawal period.
2.4. Electrophysiological studies
2.4.1. Slice preparation
Slices were prepared from three mice of each genotype (IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+) using previously described protocols (Bajo et al., 2015; Patel et al., 2019). Briefly, the mice were anaesthetized with 3% isoflurane, decapitated and the brain quickly removed and placed in ice-cold oxygenated high-sucrose cutting solution (composition in mM: 206 sucrose, 2.5 KCl, 0.5 CaCl2, 7 MgCl2, 1.2 NaH2PO4, 26 NaHCO3, 5 glucose, and 5 HEPES). Coronal slices (300 μm) containing the central amygdala (CeA) were cut using a Leica 1200S vibratome (Leica Microsystems, Buffalo Grove, IL). The slices were incubated for 30 min at 37 °C in O2/CO2-gassed artificial cerebrospinal fluid (ACSF: composition in mM: NaCl, 130; KCl, 3.5; NaH2PO4, 1.25; MgSO4.7H2O, 1.5; CaCl2, 2.0; NaHCO3, 24; glucose, 10) followed by a 30 min incubation at room temperature. Within the next 2–8 hours the slices were transferred to a recording chamber (RC-26 Warner Instruments, Hamden, CT), and continuously perfused with O2/CO2-gassed ACSF at a flow rate of 2–3 ml/min.
2.4.2. Whole cell patch-clamp recordings
CeA neurons were visualized using infrared-differential interference contrast (IR-DIC) optics, using either w60 or w40 water immersion objectives (Olympus B×51WI) and a CCD camera (EXi Aqua, QImaging). For data acquisition, a Multiclamp 700B amplifier, Digidata 1440A and pClamp 11 software package (Molecular Devices, Sunnyvale, CA) were used.
Passive and active (spike firing) membrane properties of CeA neurons were determined by a standard current step protocol consisting of incremental hyperpolarizing to depolarizing current injections. Current-Voltage (I-V) relationships were calculated for 51 neurons from IL-6 tg −/−, for 36 neurons from IL-6 tg +/− and 63 neurons from IL-6 tg +/+ mice ~5 min after a whole-cell configuration was established. They commenced at membrane potentials Vm= −70 mV and were generated by 600 ms current injection at steps starting from −120 pA with 10 pA current increments.
Pharmacologically-isolated action-potential dependent GABAA receptor-mediated spontaneous inhibitory postsynaptic currents (sIPSCs) and action-potential independent GABAAR -mediated miniature IPSCs (mIPSCs) were recorded from CeA neurons (total of 86 neurons from the 3 different genotypes) in a gap-free acquisition mode at a 10 kHz sampling rate. Neurons were held at −60 mV and sIPSCs and mIPSCs were isolated by adding the GABAB-receptor blocker CGP 55845A (1μM) and the glutamate receptor blockers DNQX (20 μM) and DL-AP-5 (30 μM) to the ACSF; mIPSCs were recorded in the presence of tetrodotoxin (TTX; 0.5 μM).
Patch pipettes were pulled of borosilicate glass to a resistance of 3–5 MΩ and filled with K-gluconate-based internal solution to measure membrane excitability and containing (in mM): 145 K-Gluconate, 10 HEPES, 2 MgCl2, 1 EGTA, 2 Mg-ATP, 0.2 Na-GTP (300 mOsm; pH adjusted to 7.2 – 7.4 using 1M KOH) or with a KCl-based internal solution to record m/sIPSCs and containing (in mM): 145 KCl, 10 HEPES, 2 MgCl2, 1 EGTA, 2 Mg-ATP, and 0.2 Na-GTP (290–310 mOsm, pH adjusted to 7.2–7.4 using 1M KOH) to record m/sIPSCs.
Cells with more than 20% change in access resistance (Ra ≤15 MΩ, monitored with frequent 10 mV pulses) were not included in the final data analysis. We analyzed frequencies, amplitudes and kinetics (rise and decay times) of s/mIPSCs using Mini Analysis software (Synaptosoft Inc., Fort Lee, NJ) followed by a visual inspection of each individual event. Averages of s/mIPSC characteristics were calculated based on a minimum time interval of 5 mins or a minimum of 50 events. Passive and active membrane properties were analyzed using the software NeuroExpress (version 18.c.13) developed and provided by Dr. Szucs.
2.5. Statistics
Data from behavioral and protein studies were analyzed statistically using ANOVA for parametric data and the Mann-Whitney test for non-parametric data. Normality was assessed using the Kolmogorov-Smirnov (K-S) test, and variance was assessed by the F-test. For data analyzed by ANOVA, compiled data were expressed as the mean ± SEM. For non-parametric data, median, maximum, and minimum values are shown in box plots. For studies involving the repeated measurements from the same animal, repeated measures ANOVA was used. Statistical significance was set at p ≤ 0.05. n = number of animals tested. These analyses were carried out using Statview (Abacus Corporation).
Data from electrophysiological studies were analyzed and graphed in Prism 7.01 (GraphPad, San Diego, CA). Data are presented as means ± SEM, and n represents number of cells. Statistical difference between groups was calculated using one-way ANOVA followed by Tukey post-hoc test. In all cases, p < 0.05 was the criterion for statistical significance.
3. Results
3.1. Emotionality
Five behavioral tests were carried out to assess emotionality (response to novelty, anxiety- and depressive-like behaviors, and stress coping behaviors) in the IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+ mice. The IL-6 tg −/− mice are littermates that do not express the transgene and are considered to express normal levels of IL-6 mRNA and protein in the CNS, whereas the IL-6 +/+ and IL-6 +/− mice express increased levels of IL-6 mRNA in the CNS in a transgene-dose respective manner (Campbell et al., 1993). In the light/dark transfer and open field tests several different measures were made that are thought to be relevant to anxiety-like behavior and exploratory drive. In the forced swim and tail suspension tests, immobility was measured, which is thought to reflect a stress-induced loss of escape effort. Loss of escape effort is considered a depression-like behavior because anti-depressant drugs can modify escape effort. For all behavioral tests, results from males and females were combined for further comparisons when there was no sex or sex x genotype interaction. Unless otherwise noted, repeated measures ANOVA or ANOVA were used for statistical analyses of data. Results are summarized in Table 1.
Table 1.
Summary of behavioral results
| Behavioral Test | Comparisons | ||
|---|---|---|---|
| IL-6 tg+/+ vs IL-6 tg −/− | IL-6 tg+/+ vs IL-6 tg +/− | IL-6 tg+/− vs IL-6 tg −/− | |
| 1. Light/Dark Transfer | |||
| - time spent in light chamber | ns | ns | ns |
| - # of transitions | ↓ | ↓ | ns |
| 2. Open field test | |||
| - distance | ↓ | ↓ | ns |
| - velocity | ↓ | ↓ | ns |
| - center entries | ↓ | ↓ | ns |
| - center time | ns | ns | ns |
| 3. Grooming, rearings | ns | ns | ns |
| 4. Digging bouts | ↓ | ↓ | ns |
| 5. Tail suspension test | ns | ns | ns |
| 6. Forced swim test | |||
| Immobility | ↑ | ↑ | ns |
| 7. HIC score | ↑ | ↑ | ns |
↓= decrease, ↑= increase; ns = no significant difference
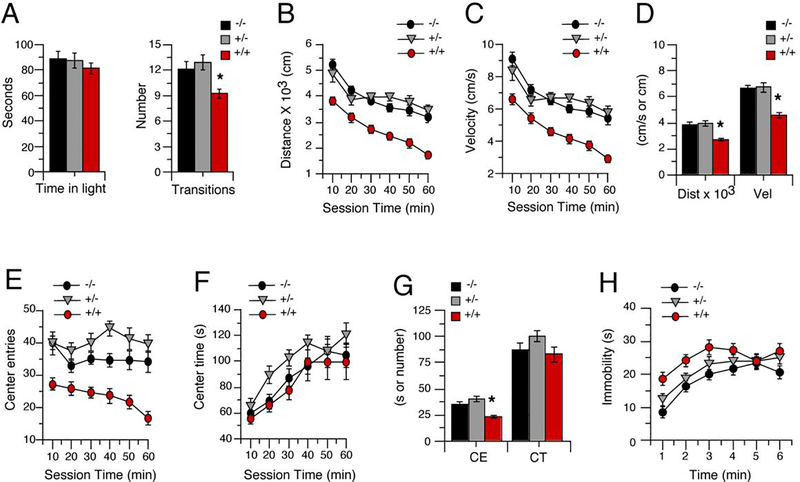
3.1.1. IL-6 tg +/+ mice show decreased exploratory behavior in the light/dark transfer test
There was no significant genotypic or sex difference in the time spent in the light chamber, and no genotype x sex interaction (Fig. 1A). In contrast, the number of transitions between the light and dark chamber was significantly different across genotypes (F(2,66) = 6.25, p = 0.003). However, there was no sex difference or genotype x sex interaction (Fig. 1A). Combined means (female plus male), showed that IL-6 tg +/+ animals had a significantly lower (Tukey’s post hoc tests) number of transitions than IL-6 tg +/− or IL-6 tg −/− mice, indicative of decreased exploratory behavior. There was no significant difference in transitions between IL-6 tg +/− and IL-6 tg −/− mice (Fig. 1A).
Figure 1.
Performance in behavioral tests is altered in IL-6 tg +/+ mice. A. Light/dark transfer test: Mean (± SEM) values for time in light and number of transitions. B-C. Open field test: Mean (± SEM) values during the 60 min test period for distance traveled (B) and movement velocity (C). E-F. Number of center entries during the 60 min test period (E), and time spent in the center of the field (F). G. Mean (± SEM) values for combined data. H. Immobility in the forced swim test: Mean (± SEM) values for immobility during the 6 min test period. For studies in A-H, 24 mice of each genotype were used. * = significantly different from other genotypes. For B,C,E,F,H significance was determined by repeated measures ANOVA; for A,D,G, ANOVA was used.
3.1.2. IL-6 tg +/+ mice show decreased exploratory behavior in the open field test
There were significant genotype effects in the activity measures (Fig. 1B–G). Distance traveled (Fig. 1B) and velocity of movement (Fig. 1C) showed a significant genotypic difference during the 60 min testing period (distance, F(2,66) = 23.73 p < 0.0001; velocity, F(2,66) = 21.47 p < 0.0001), but no sex difference or genotype x sex interaction. Analysis of mean values for combined data followed by post hoc tests showed a significant genotypic difference for velocity (F(2,69) = 19.38, p <0.0001) and distance (F(2,69) = 21.21, p <0.0001), with IL-6 tg +/+ mice having a significantly lower velocity and distance traveled than either IL-6 tg +/− or IL-6 tg −/− mice (Fig. 1D). There was no significant difference in velocity or distance traveled between IL-6 tg +/− and IL-6 tg −/− mice.
Center entries showed a significant genotypic difference (F(2,66) = 20.79, p < 0.001) but no sex difference or genotype x sex interaction during the 60 min testing period (Fig. 2E). The number of center entries were similar for IL-6 tg +/− and IL-6 tg −/− mice. There were no significant genotype differences in the time spent in the field center, and no genotype x sex interaction (Fig. 1F). Analysis of mean values for combined data followed by post hoc tests showed a significant genotypic difference for center entries (F(2,69) = 19.38, p <0.0001) with IL-6 tg +/+ animals having a significantly lower number of center entries than IL-6 tg +/− or IL-6 tg −/− mice (Tukey’s)(Fig. 1G). Taken together, these results suggest that IL-6 affected activity levels rather than anxiety-like behavior in this test.
Figure 2.
Intrinsic membrane and spike properties of CeA neurons are altered in IL-6 tg −/−, IL-6 tg +/−, and IL-6 tg +/+ mice. Sample recordings of membrane voltage responses to 50 pA depolarizing (top traces), 40 pA depolarizing (middle traces) and 120 pA hyperpolarizing (middle traces) current pulses (bottom traces) from neurons from IL-6 tg −/−, IL-6 tg +/−, and IL-6 tg +/+ mice are shown. Inset: Expanded spike shapes derived from spikes recorded at 50 pA current pulse showing genotypic differences in positive and negative spike slopes and half-width.
Grooming and number of wall rearings and open rearings were also assessed. There was no genotypic difference for grooming, wall rearings, or open rearings (data not shown).
3.1.3. IL-6 tg +/+ mice show decreased digging behavior
There was a significant effect of genotype on digging bouts (F(2,66) = 7.7, p = 0.001). IL-6 tg +/+ mice had significantly fewer bouts than either IL-6 tg +/− (p = 0.007) or IL-6 tg −/− mice (p = 0.002). There was no sex difference or genotype x sex interaction. Average numbers of bouts were 7.88 ± 0.81 for IL-6 tg −/− mice, 8.29 ± 0.77 for IL-6 tg +/− mice, and 4.29 ± 0.86 for IL-6 tg +/+ mice.
3.1.4. No genotypic difference in depressive-like behavior in the tail suspension test
There were no genotypic differences in immobility in the tail suspension test. While there was an overall sex difference (F(1,100) = 18.57, p < 0.0001) with females having less immobility than males, there was no genotype x sex interaction, suggesting that the sexes were not differentially affected by IL-6 expression levels (data not shown).
3.1.5. IL-6 tg +/+ mice show greater depressive-like behavior in the forced swim test
Analysis of immobility during the forced swim test showed a significant genotypic difference (F(2,107) = 6.129, p = 0.003), but no sex difference or genotype x sex interaction (Fig. 1H). Post hoc tests (Tukey’s) showed that the IL-6 tg +/+ mice had significantly higher immobility than IL-6 tg −/− mice, indicating enhanced depressive-like behavior and/or passive stress coping strategy in the IL-6 tg +/+ mice. Immobility for the IL-6 tg +/− mice was intermediate and was significantly different from the IL-6 tg +/+ mice but not the IL-6 tg −/− mice.
3.2. Neuronal excitability and GABAergic synaptic transmission are altered in the central amygdala of IL-6 transgenic mice
Results from the above behavioral studies, summarized in Table 1, indicate that elevated expression of IL-6 in the CNS can modulate exploratory and depressive-like behaviors. These results are consistent with recent studies showing that repetitive administration of IL-6 into the amygdala or hippocampus of rats increases the immobility time in the forced swim test (Wu and Lin, 2008), a change that is thought to reflect increased depressive-like behavior. Although, we recently reported significant neuronal alterations in the hippocampus of IL-6 tg mice (Hernandez et al., 2016; Nelson et al., 2012), the functional characterization of the central amygdala in IL-6 tg mice is still lacking. Thus, here we sought to investigate the potential role of IL-6 signaling on intrinsic membrane properties and inhibitory synaptic transmission of neurons in the medial subdivision of the central amygdala (CeA) in brain slices obtained from IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+ mice.
3.2.1. Intrinsic membrane properties are altered in CeA neurons of IL-6 tg +/+ mice
Intrinsic passive and active membrane properties of neurons in the CeA of IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+ mice were determined from I-V relationships in current clamp mode (Fig. 2)(Table 2,3).
Table 2. Intrinsic membrane properties of CeA neurons from IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+ mice.
The voltage sag and afterdepolarization (ADP) values were plotted against the level of the injected current and the corresponding relationships were fitted with linear functions. The slope of the linear fits is proportional with the magnitude of the voltage sag and ADP associated with the action of intrinsic h-current. The data are presented as mean ± SEM. Statistical significance * p < 0.05 was calculated by one-way ANOVA followed with Tukey’s test. For post-hoc group comparisons: * = significant difference for IL-6 tg −/− vs IL-6 tg +/+ (p < 0.01); # = significant difference for IL-6 tg +/− vs IL-6 tg +/+ mice (p < 0.01).
| Animal group (# of cells) | Capacitance (pF) | Resistance (MΩ) | Voltage Sag Slope (mV/pA) | ADP Slope (mV/pA) |
|---|---|---|---|---|
| IL-6 tg −/− (n=51) | 78.6 ± 3.2 | 432.9 ± 28.8 | −0.071 ± 0.007 | −0.074 ± 0.006 |
| IL-6 tg +/− (n=36) | 78.9 ± 6.3 | 516.9 ± 47.8 | −0.080 ± 0.009 | −0.085 ± 0.009 |
| IL-6 tg +/+ (n=63) | 63.6 ± 2.3 (*,#) | 463.2 ± 22.6 | −0.081 ± 0.006 | −0.097 ± 0.007 |
Table 3. Action potential (spike) properties of CeA neurons from IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+ transgenic (tg) mice.
Spike After-hyperpolarization (AHP) after the first spike is calculated relative to the spike threshold. Maximal Positive Slope represents a maximal voltage slope under spike upshot phase (mediated primarily by Na+-channels). Maximal Negative Slope represents a maximal voltage slope during spike falling phase (mediated primarily by K+-channels). The data are presented as mean ± SEM. Statistical significance was calculated with one way ANOVA followed by Tukey’s test. For post-hoc group comparisons: * = significant difference for IL-6 tg −/− vs IL-6 tg +/+; # = significant difference for IL-6 tg +/− vs. IL-6 tg +/+ mice.
| Animal group (# of cells) | Spike Thresh (mV) | Spike Amp (mV) | Spike Half-Width (ms) | Spike AHP (mV) | Spike Latency (ms) | Relat. Cumul. # of Spikes | Maximal Pos. Slope (mV/ms) | Maximal Neg. Slope (mV/ms) |
|---|---|---|---|---|---|---|---|---|
| IL-6 tg −/− (n=51) | −40.6 ± 0.9 | 85.8 ± 1.3 | 1.63 ± 0.05 | 6.9 ± 0.6 | 148 ± 13 | 4.6 ± 0.4 | 162.6 ± 6.0 | 52.0 ± 1.9 |
| IL-6 tg +/− (n=36) | −42.9 ± 0.9 | 84.8 ± 1.3 | 1.50 ± 0.07 | 6.2 ± 0.8 | 123 ± 12 | 4.9 ± 0.5 | 169.7 ± 6.9 | 59.3 ± 2.8 |
| IL-6 tg +/+ (n=63) | −42.2 ± 0.6 | 85.5 ± 1.0 | 1.38 ± 0.05 (*) | 5.7 ± 0.5 | 84 ± 8 (*,#) | 6.1 ± 0.4 (*) | 195.2 ± 8.0 (*) | 64.7 ± 2.7 (*) |
Statistical differences in intrinsic membrane and spike properties between the groups were calculated using one-way ANOVA followed by a post-hoc mean comparison (Tukey’s). Most membrane properties did not differ significantly between genotypes including membrane resistance (Rm), hyperpolarization-activated Ih-currents determined from the membrane voltage sag, and afterdepolarization (ADP) at the initial phase and the end of the hyperpolarizing pulse, respectively (Table 2). However, a significant genotypic difference in membrane capacitance (Cm) (F(2,143) = 7.06, p = 0.001) was observed, with IL-6 tg +/+ CeA neurons showing a significantly decreased Cm compared to CeA neurons from IL-6 tg −/− mice and IL-6 tg +/− mice (Table 2). Significant differences in Cm were not observed between neurons from IL-6 tg +/− and IL-6 tg −/− mice. The reduced membrane capacitance of the CeA neurons in the IL-6 tg +/+ mice suggests that IL-6 exposure may alter neuronal structure (e.g., size, morphology, dendritic arborization, membrane surface area) or the capacity of the membrane to store charge.
Active membrane excitability of CeA neurons was assessed by measurement of spike firing characteristics (Fig. 2). Spike threshold and spike amplitude showed no genotypic difference. However, the spike half-width reflecting the rate of de- and re-polarization was significantly reduced in CeA neurons of IL-6 tg +/+ mice compared to CeA neurons of IL-6 tg −/− mice (F(2,146) = 5.20, p = 0.007; Tukey’s p < 0.05), whereas no differences were observed between IL-6 tg +/− and IL-6 tg −/− mice or between CeA neurons from IL6 tg+/+ and IL-6 tg+/− mice. Significant differences were also observed for spike latency (F(2,109) = 9.99; p = 0.0001), with CeA neurons from IL-6 tg −/− mice showing a significantly longer spike latency than CeA neurons from either the IL-6 tg +/+ or IL-6 tg +/− mice. Additional significant differences between spike characteristics of CeA neurons from IL-6 tg +/+ mice compared to CeA neurons from IL-6 tg −/− mice included an increase in relative cumulative number of spikes (# of spikes/3 current steps) (F(2,141) = 3.75; p = 0.03; Tukey’s p < 0.05), increased positive spike slopes (F(2,146) = 6.08, p = 0.003; Tukey’s p < 0.01 and enhanced negative spike slopes (F(2,146) = 183, p = 0.001, Tukey’s p < 0.001). In general, these spike characteristics of CeA neurons from IL-6 tg +/− mice were intermediate to that of IL-6 tg −/− and IL-6 tg +/+ mice and did not differ significantly from either IL-6 tg +/+ and IL-6 tg −/−. Results are summarized in Table 3. Taken together, these results indicate increased neuronal excitability of the CeA neurons of IL-6 tg +/+ mice compared to CeA neurons from IL-6 tg −/− mice.
3.2.2. Inhibitory synaptic responses are altered in CeA neurons of IL-6 tg +/+ mice
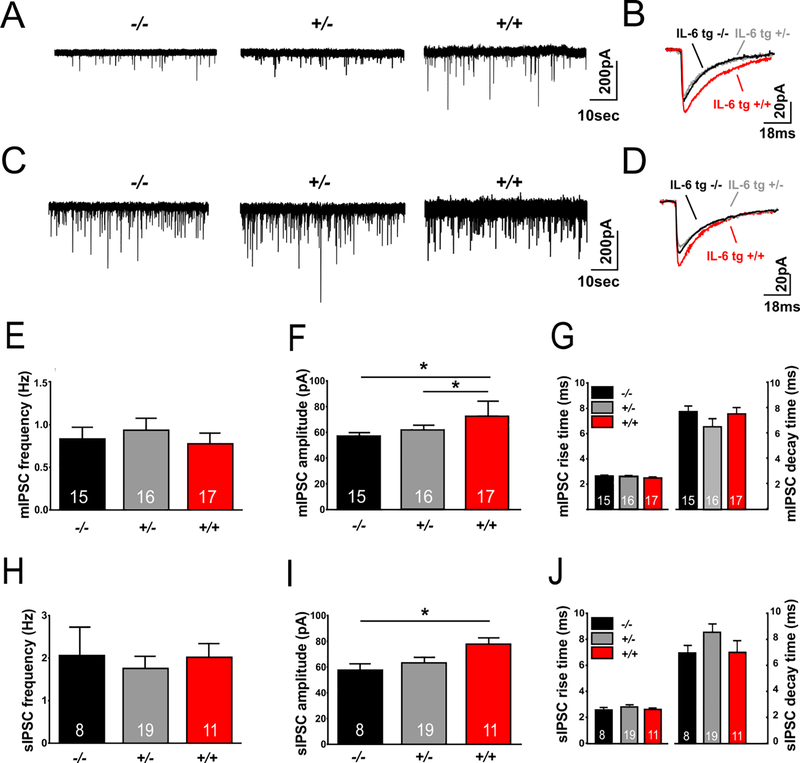
Modulation of inhibitory GABAergic circuits plays an important role in anxiety-like and depressive-like behaviors (Engin et al., 2018; Kalueff and Nutt, 2007; Smith and Rudolph, 2012). To assess whether the increased IL-6 expression in IL-6 tg mice was associated with alterations in GABAA-receptor mediated synaptic transmission in the CeA, we recorded action-potential independent, miniature inhibitory postsynaptic currents (mIPSCs; tetrodotoxin (TTX)-sensitive) and action-potential dependent, spontaneous IPSCs (sIPSCs). Representative mIPSC and sIPSC recordings are shown in Figure 3 A–D. There was no significant genotypic difference in mIPSC frequencies, or in sIPSC frequencies (Fig. 3, E,H)(Table S1) indicating that increased astrocyte IL-6 expression does not alter either spontaneous action-potential independent or action-potential dependent GABA release.
Figure 3.
CeA GABA transmission is increased in IL-6 tg +/+ mice. Representative (A) mIPSC and (C) sIPSC recordings from CeA neurons from IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+ mice and scaled averages of a representative (B) CeA mIPSC and (D) CeA sIPSC recording are depicted. Data are represented as means ± S.E.M (E) of mIPSC frequencies, (F) mIPSC amplitudes, (G) mIPSC kinetics, (H) sIPSC frequencies, (I) sIPSC amplitudes as well as (J) sIPSC kinetics from 8–19 cells per group from 3 mice for each genotype. Statistical differences between the groups were calculated using one-way ANOVA followed by a post-hoc mean comparison (Tukey’s). (*) indicates a significant difference. The number of cells (n) for each individual group is indicated in the respective bars.
In contrast, there was a main effect of genotype on mIPSC amplitudes (F(2,45) = 6.52, p = 0.003); post hoc tests (Tukey’s) revealed that mIPSC amplitudes from IL-6 tg +/+ mice were significantly larger compared to mIPSC amplitudes from IL-6 tg −/− (p = 0.004) and IL-6 tg +/− (p = 0.03) mice (Fig. 3B,F) (Table S1). Similarly, there was a main effect of genotype on sIPSC amplitudes (F(2,35) = 4.17, p = 0.024); post hoc tests (Tukey’s) showed that sIPSC amplitudes were also significantly larger in the CeA of IL-6 tg +/+ mice compared to IL-6 tg −/− (p = 0.04), but not compared to IL-6 tg +/− (p = 0.11) mice (Fig. 3I)(Table S1).
No significant differences in mIPSC and sIPSC current kinetics including rise time and decay times were detected (Fig. 3G,J)(Table S1). These results indicate that increased IL-6 expression enhances postsynaptic GABAA receptor-function in the CeA.
3.3. CNS excitability during alcohol withdrawal is enhanced in IL-6 transgenic mice
The electrophysiological results (Fig. 2,3) from the CeA, a brain region that plays a central role in exploratory response to novelty and depression-like behaviors, show that elevated levels of IL-6 in the CNS affect membrane excitability and GABAergic synaptic transmission. Increased sensitivity to novelty (i.e. decreased exploration) and depression-like behaviors are also symptoms of withdrawal from excessive alcohol use, as are increases in membrane excitability and altered GABAergic transmission. Our previous studies showed that IL-6 tg +/− mice showed increased CNS excitability during acute alcohol withdrawal compared to IL-6 tg −/− mice in the handling-induced convulsions test (HIC) (Hernandez et al., 2016). Alcohol exposure/consumption and/or withdrawal have been shown to increase IL-6 levels in the CNS (Alfonso-Loeches et al., 2010; Baxter-Potter et al., 2017; Doremus-Fitzwater et al., 2014; Emanuele et al., 2005; Gano et al., 2016; Kane et al., 2013; Zhang et al., 2014), which could contribute to the withdrawal symptoms. The IL-6 tg +/+ mice show greater expression of IL-6 and more prominent alterations in behavioral and physiological properties than IL-6 tg +/− and IL-6 tg −/− mice, but it is unknown if they show greater CNS excitability during withdrawal. To address this question, we subjected IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice to a high dose of acute alcohol (4 g/kg i.p.), an alcohol exposure paradigm that produces a mild form of alcohol dependence (Crabbe et al., 1991), and measured their behavior in the HIC test.
Measurements (i.e., HIC scoring) were made prior to administration of alcohol and every 2 hr post-alcohol up to 24 hrs. There were no significant differences between groups in baseline HIC scores. Using the sum of scores between 2 and 24 hr as a measure of the area under the curve, there was an overall effect of genotype (F(2,110) = 6.1, p = 0.003), with both IL-6 tg +/+ and IL-6 tg +/− mice showing a greater acute alcohol withdrawal response than IL-6 tg −/− mice (Fig. 4). Examination of the time course post-alcohol using repeated measures ANOVA revealed a significant effect of genotype (F(2,106) = 6.35, p = 0.0025), but no sex difference or genotype x sex interaction. However, there was a significant time effect (F(11,1166) = 65.16, p < 0.0001) and genotype x time interaction (F(22,1166) = 2.26, p = 0.0008). IL-6 tg +/− mice had higher HIC scores than IL-6 tg −/− mice at 8, 10, 12, 14, 18, 20, and 22 hr post alcohol and IL-6 tg +/+ mice had higher HIC scores than IL-6 tg −/− mice at 18, 20 and 22 hr post alcohol. Taken together, these data indicate that increased IL-6 expression is associated with increased neuronal excitability during acute alcohol withdrawal.
Figure 4.
Increased CNS excitability during alcohol withdrawal is enhanced in IL-6 tg +/− and IL-6 tg +/+ mice. Graph shows mean ± SEM values for HIC scores measured at 2 hr intervals over a 24 hr period. * = IL-6 tg +/− significantly different from IL-6 tg −/−, @ = IL-6 tg +/+ significantly different from IL-6 tg −/−.
3.4. IL-6 levels in the CNS varies according to genotype
IL-6 is expressed in several CNS regions, including the amygdala and hippocampus (Schobitz et al., 1993; Su et al., 2015). However, sufficient tissue was not available from these regions for our ELISA measurement of IL-6 levels. Therefore, IL-6 levels were determined in cerebellum, a CNS region that provided sufficient tissue for the assay, from a cohort of alcohol naïve mice and mice tested in the HIC studies. The cerebellum of the IL-6 tg mice expresses a high level of IL-6 mRNA (Campbell et al., 1993) and presumably protein. For alcohol-exposed mice, the cerebellum was obtained at the end of the 24 hr withdrawal period.
A significant genotype (F(2,61) = 20.97, p < 0.0001) and treatment (F(1,61) = 7.59, p = 0.008) difference in cerebellar IL-6 levels was observed, but there was no genotype x treatment interaction (Fig. S1). Post hoc tests (Fisher’s PLSD) indicated that there was a significant difference between all genotypes (p < 0.0002) and between naïve and alcohol exposed/withdrawn cerebellum (p = 0.02). The highest IL-6 levels were observed in cerebellum from IL-6 tg +/+ mice, under both alcohol naïve and alcohol exposed/withdrawn conditions (Fig. S1). IL-6 levels were significantly higher in cerebellum from alcohol exposed/withdrawn IL-6 tg +/− (U = 44.0, n1 = 16, n2 = 12, p = 0.02, Mann Whitney) and IL-6 tg −/− mice (F(1,23) = 6.86, p = 0.02) mice than alcohol naïve cerebellum of the same genotype. Cerebellum from alcohol exposed/withdrawn IL-6 +/+ mice did not show a significant difference from alcohol naïve cerebellum from IL-6 +/+ mice, probably due to variability in the alcohol-treated samples.
3.5. Protein activation/expression is altered in IL-6 transgenic mice
Results from our studies on anxiety-like and depressive-like behavior show that elevated expression of IL-6 in the CNS can alter these behaviors, primarily in the IL-6 tg +/+ mice. Results from the HIC studies show that elevated expression of IL-6 in the CNS can influence the magnitude of CNS excitability during alcohol withdrawal, with both IL-6 tg +/+ and IL-6 tg +/− mice showing significantly higher HIC scores than IL-6 tg −/− mice during the later phase of withdrawal (12–24 hr). Electrophysiological studies of CeA show that elevated levels of IL-6 in the CNS affect membrane excitability and GABAergic synaptic transmission. To identify potential factors that contributed to the genotypic differences in alcohol naïve and alcohol exposed/withdrawn mice, we examined the levels of protein activation/expression in hippocampus obtained from alcohol naïve and alcohol exposed/withdrawn IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice. IL-6 signal transduction components and proteins involved in inhibitory GABAergic synaptic transmission were examined. The alcohol exposed/withdrawn mice were studied at the end of the 24 hr withdrawal period. The hippocampus was studied because it is known to be a modulator of anxiety-like and depression-like behavior (Collinson et al., 2002). Also, the hippocampus is a sensitive target of alcohol action, and sufficient tissue could be obtained from this brain region for assays. Moreover, IL-6 and IL-6R mRNA are expressed in high levels in the hippocampus (Gadient and Otten, 1994), and a high number of transgene-expressing astrocytes are present in the hippocampus of the IL-6 tg mice (Vallieres et al., 2002). Results are summarized in Table 4.
Table 4.
Summary of protein activation/expression in hippocampus
| Protein | Naïve@ | Acute alcohol& | |||
|---|---|---|---|---|---|
| IL-6 tg +/+ | IL-6 tg +/− | IL-6 tg +/+ | IL-6 tg +/− | IL-6 tg −/− | |
| pSTAT3 | ↑ | ↑ | ↑ | ↑ | ↑ |
| total STAT3 | ↑ | ↑ | ns | ns | ns |
| GABAAR α-1 | ns | ns | ↑ | ns | ns |
| GABAAR α-5 | ↑ | ↑ | ↓ | ↓ | ↓ |
| GAD 65 | ↓ | ↓ | ns | ns | ↓ |
| GAD 67 | ↓ | ↓ | ns | ns | ↓ |
| GFAP | ↑ | ↑ | ↑ | ↑ | ns |
| Enolase | ns | ns | ns | ns | ns |
compared to naïve IL-6 tg −/−
compared to naïve of the same genotype; ↓= decrease, ↑= increase; ns= no significant difference
3.5.1. STAT3
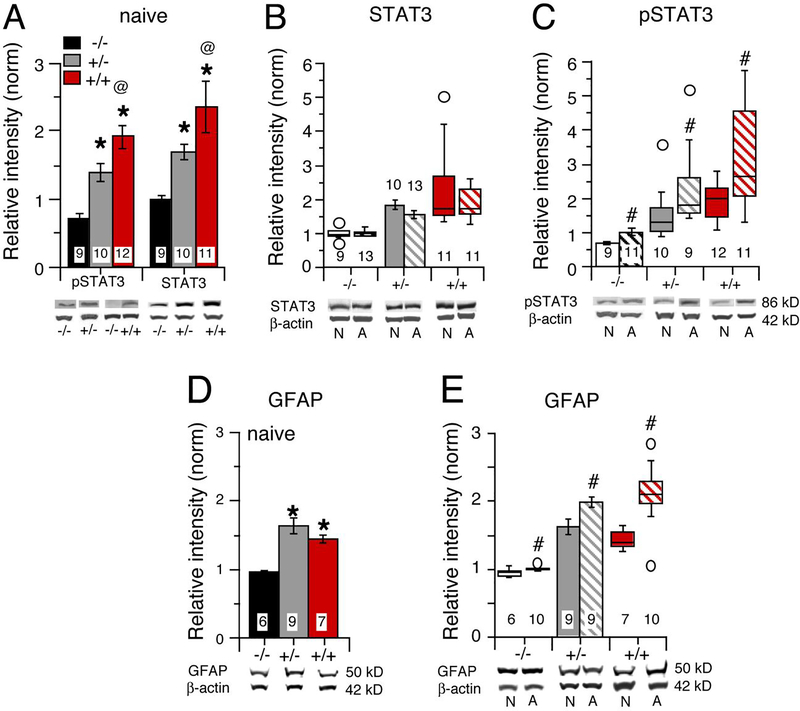
STAT3 is a primary signal transduction partner of the IL-6 receptor and an important regulator of gene transcription. STAT3 levels in hippocampus from alcohol naive IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice showed a significant genotypic difference (F(2,27) = 6.81, p < 0.004), with significantly higher levels of STAT3 in hippocampus from IL-6 tg +/+ (p = 0.001) and IL-6 tg +/− (p = 0.049) mice compared to hippocampus from IL-6 tg −/− mice (Post hoc analysis, Fisher’s PLSD)(Fig. 5A). There was no significant difference in STAT3 levels between hippocampus from IL-6 tg +/+ and IL-6 tg +/− mice (p = 0.12).
Figure 5.
Protein activation/expression is altered in hippocampus from IL-6 tg +/− and IL6 tg +/+ mice. A. Relative levels of pSTAT3 and STAT3 in hippocampus from IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+ mice. B,C. STAT3 (D) and pSTAT3 (C) levels in hippocampus from alcohol naïve and alcohol exposed/withdrawn IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+ mice. D,E. Relative levels of GFAP in hippocampus from alcohol naïve and alcohol exposed/withdrawn IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+ mice. For these and other graphs of Western blot data, parametric data are represented in bar graphs and nonparametric data are represented in box graphs. Representative samples of Western blots are shown below the respective bar or box graph. The top band of the Western blot is the protein of interest and the bottom band is beta-actin. N= naïve, A = exposed/withdrawn. Solid bars represent alcohol naïve samples and stripped bars represent alcohol exposed/withdrawn samples. Data analyzed by ANOVA are expressed as the mean ± SEM (bar graphs). For non-parametric data, median, maximum, and minimum values are shown (box plots; open circles are data points in box plots). * = significantly different from IL-6 tg −/− of the same treatment group., # = significantly from alcohol naïve of the same genotype. @= significantly different from IL-6 +/−.
STAT3 levels in hippocampus from alcohol treated and alcohol naive IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice showed a significant genotypic difference (F(2,61) = 18.58, p < 0.0001) but no treatment effect. Comparisons of STAT3 levels between hippocampus from alcohol treated and alcohol naive mice of the same genotype showed no significant treatment effect for all three genotypes (Fig. 5B).
3.5.2. pSTAT3
Levels of pSTAT3, the activated form of STAT3, in hippocampus from alcohol naïve IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice showed a significant genotypic difference (F(2,28) = 19.81, p = <0.0001). As for STAT3 levels, pSTAT3 levels were significantly higher (p < 0.05, Post hoc analysis, Fisher’s PLSD) in hippocampus from IL-6 tg +/+ and IL-6 tg +/− mice compared with hippocampus from IL-6 tg −/− mice. pSTAT3 levels in hippocampus from IL-6 tg +/+ mice were significantly higher than for hippocampus from IL-6 tg +/− mice (Fig. 5B).
Comparison of pSTAT3 levels in hippocampus from alcohol treated and alcohol naive IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice showed a significant genotypic difference (F(2,52) = 12.59, p = 0.0001) and treatment effect (F(1,51) = 13.58, p = 0.0005). Further analysis within genotypes revealed that pSTAT3 levels were significantly higher in hippocampi from alcohol treated mice compared to the respective alcohol naive mice for all three genotypes (IL-6 tg −/−, F(1,18) = 4.87, p = 0.04; IL-6 tg +/−, U = 14, n1 = 8, n2 = 10, p = 0.02; IL-6 tg +/+, U = 30, n1 = 11, n2 = 12, p = 0.03; Mann-Whitney test) (Fig. 5D). These results suggest that IL-6 levels are higher in alcohol-treated hippocampus than in alcohol naïve hippocampus for all genotypes.
3.5.3. GFAP
GFAP is a downstream target of pSTAT3 gene regulation (Herrmann et al., 2008). In alcohol naïve mice, GFAP levels in hippocampus from IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice were significantly different (F(2,20) = 10.81, p < 0.0007). Post hoc tests (Fisher’s PLSD) indicated that GFAP levels in hippocampus from IL-6 tg +/+ and IL-6 +/− mice were significantly higher (p < 0.05) than in hippocampus from IL-6 −/− mice (Fig. 5D). There was no significant difference in GFAP levels between hippocampus from IL-6 +/+ and IL-6 +/− mice. Thus, although levels of pSTAT3 showed a gene-dose related increase, a gene-dose relationship was not observed for GFAP. This difference could reflect downstream regulatory mechanisms that can affect gene-dose relationships or the involvement of multiple cell types in the increased pSTAT3 levels.
Comparison of GFAP levels in hippocampus from alcohol treated and alcohol naïve IL-6 tg +/+, IL-6 tg +/− and IL-6 tg−/− mice showed a significant genotypic difference (F(2,39) = 45.23, p < 0.0001) and treatment effect (F(1,39) = 22.79, p < 0.0001)(Fig. 5E). Hippocampus from alcohol treated mice showed a significantly higher GFAP level than hippocampus from alcohol naive mice of the same genotype for all genotypes (IL-6 +/+, U = 7.0, n1 = 10, n2 = 7, p = 0.006; IL-6 +/−, F(1,16) = 7.10, p = 0.02; IL-6 −/−, U = 11.0, n1 = 10, n2 = 6, p = 0.04) (Fig. 5E). These results are consistent with the higher level of pSTAT3 (a regulator of GFAP expression), in alcohol-treated mice for all genotypes.
3.5.4. GABAAR alpha-5
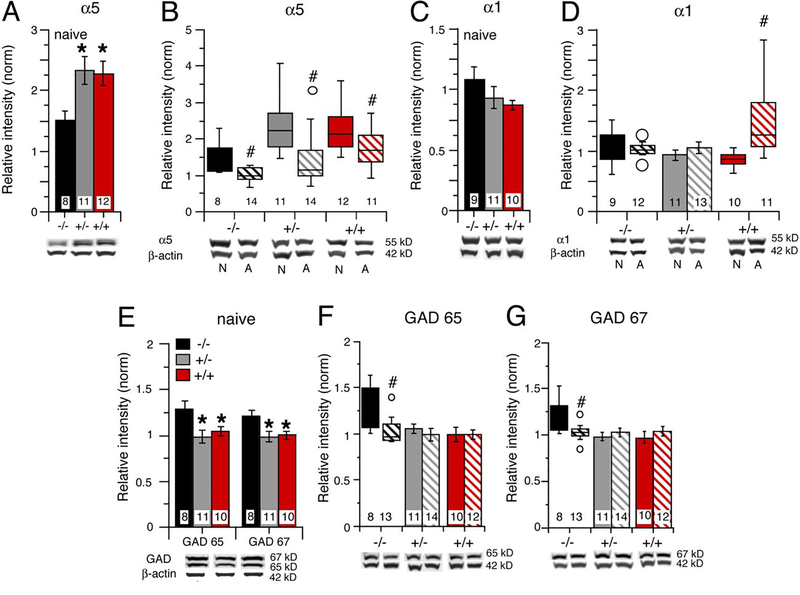
The GABAAR alpha-5 subunit is prominently expressed in CA1 and CA3 regions of the hippocampus (Wisden et al., 1992), where GABAARs incorporating alpha-5 subunits mediate tonic inhibition (Glykys et al., 2008; Luscher and Keller, 2004). For alcohol naive mice, there was a significant genotypic difference in GABAAR alpha-5 levels (F(2,27) = 4.32, p= 0.023) between hippocampus from IL-6 tg +/+, IL-6 tg +/− mice and IL-6 tg −/− mice. GABAAR alpha-5 levels in hippocampus from IL-6 tg +/+ mice and IL-6 tg +/− mice were significantly higher than in hippocampus from IL-6 −/− mice (p < 0.05, Fisher’s PLSD post hoc test). There was no significant difference in GABAAR alpha-5 levels between hippocampus from IL-6 tg +/+ and IL-6 tg +/− mice (Fig. 6A).
Figure 6.
Levels of proteins associated with GABAergic synaptic inhibition are altered in hippocampus from IL-6 tg +/− and IL-6 tg +/+ mice. A-G. Relative levels of GABAAR alpha-5 (A,B), GABAAR alpha-1 (C,D) and GAD 65/67 (E-G) in hippocampus from IL-6 tg −/−, IL-6 tg +/− and IL-6 tg +/+ mice. * = significantly different from IL-6 tg −/−, # = significantly different from alcohol naïve of the same genotype.
Analysis of GABAAR alpha-5 levels in hippocampus from alcohol-treated and alcohol naïve IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice showed a significant genotype difference and treatment effect (genotype, F(2,64) = 9.24, p = 0.0003; treatment, F(1,64) = 18.66, p <0.0001). Within genotype comparison of GABAAR alpha-5 levels between hippocampus from alcohol treated and alcohol naive mice showed a significant decrease in GABAAR alpha-5 levels in hippocampus from alcohol treated mice for all genotypes (IL-6 tg +/+, F(1,21) = 5.49, p = 0.03; IL-6 tg +/−, U = 24.0, n1 = 14, n2 = 11, p = 0.004; IL-6 tg −/−, U = 15.0, n1 = 14, n2 = 8, p = 0.005) (Fig. 6B). These results suggest that reduced tonic inhibition in the hippocampus could contribute to the increased excitability during alcohol withdrawal in all genotypes.
3.5.5. GABAAR alpha-1
GABAAR incorporating alpha-1 subunits mediate phasic inhibitory neurotransmission and are also widely expressed in the hippocampus (Mody, 2001; Wisden et al., 1992). There was no significant genotypic difference in the levels of GABAAR alpha-1 in hippocampus from alcohol naïve IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice (Fig. 6C). Comparison of GABAAR alpha-1 levels between hippocampus from alcohol treated and alcohol naïve IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice also showed no significant genotype differences, but a significant treatment effect (F(1,60) = 6.74, p = 0.012) and genotype x treatment interaction (F(2,60) = 5.18, p = 0.009).
Within genotype comparisons showed a significant increase in GABAAR alpha-1 levels in hippocampus from alcohol treated IL-6 tg +/+ mice compared to alcohol naïve IL-6 tg +/+ mice (U = 12.0, n1 = 11, n2 = 10, p = 0.003). Hippocampus from alcohol treated IL-6 tg +/− and IL-6 tg −/− mice showed no significant treatment effect compared to the respective alcohol naïve mice (Fig. 6D).
3.5.6. GAD 65/67
GAD 65 and GAD 67 are isomers of the synthetic enzyme for the inhibitory transmitter GABA. GAD 65 is primarily located in axon terminals, whereas GAD67 is located in cell bodies. In hippocampus from alcohol naive IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice, GAD 65 levels showed a significant genotypic difference (F(2,26) = 5.03, p = 0.01), with GAD 65 levels in hippocampus from IL-6 tg +/+ and IL-6 tg +/− mice significantly lower than GAD 65 levels in hippocampus from IL-6 tg −/− mice (Fisher’s PLSD post hoc test, (p <0.05). There was no significant difference in GAD 65 levels between hippocampus from IL-6 tg +/+ and IL-6 tg +/− mice (Fig. 6E). Similar results were observed for GAD 67 levels in hippocampus from alcohol naïve mice (F(2,26) = 4.48, p = 0.012), with significantly lower levels of GAD 67 in hippocampus from alcohol naive IL-6 tg +/+ and IL-6 tg +/− mice compared to hippocampus from alcohol naive IL-6 tg −/− mice (p <0.05). There was no significant difference in GAD 67 levels between hippocampus from alcohol naive IL-6 tg +/+ and IL-6 tg +/− mice (Fig. 6E).
GAD 65 levels in hippocampus from alcohol treated and alcohol naïve IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice showed a significant genotype difference (F(2,62) = 5.40, p = 0.007) and treatment effect (F(1,62) = 4.95, p = 0.03). Comparison of GAD 65 levels between hippocampus from alcohol treated and alcohol naive mice of the same genotype showed no significant treatment effect for hippocampus from IL-6 tg +/+ or IL-6 tg +/−mice, but a significant decrease in GAD 65 levels for alcohol treated IL-6 tg −/− mice (U = 15.0, n1 = 13, n2 = 8, p = 0.008)(Fig. 6F).
There was no significant genotype difference or treatment effect for GAD 67 levels in hippocampus from alcohol treated and alcohol naïve IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice, but a significant genotype x treatment interaction (F(2,62) = 3.88, p = 0.03)(Fig. 6E). Within genotype comparison of GAD 67 levels in hippocampus from alcohol treated and alcohol naive mice showed no significant treatment effect for IL-6 tg +/+ and IL-6 tg +/−mice, but a significant decrease in GAD 67 levels for alcohol treated IL-6 tg −/− mice (U = 16.0, n1 = 13, n2 = 8, p = 0.009)(Fig. 6G). Thus, GAD 65/67 levels in hippocampus from IL-6 tg +/+ and IL-6 tg +/− mice were lower than in the hippocampus from IL-6 tg −/− mice and were not sensitive to alcohol, whereas alcohol reduced GAD 65/67 levels in hippocampus from IL-6 tg −/− mice to a value similar to that found in hippocampus from IL-6 tg +/+ and IL-6 tg +/− mice.
3.5.7. Neuron specific enolase (enolase 2)
Enolase 2 is an enzyme that is expressed by neurons and is commonly used a specific marker for neurons. There was no significant genotypic difference or treatment effect between IL-6 tg +/+, IL-6 tg +/− and IL-6 tg −/− mice in the levels of the neuron specific enolase (not shown) The lack of an alcohol-induced change in enolase levels suggests that neither IL-6 nor alcohol treatment had a significant toxic effect on the neuronal population in the hippocampus under the conditions of our experiment.
4. Discussion
The current studies focus on the consequences of increased expression of IL-6 in the CNS of IL-6 transgenic mice with respect to specific mouse behaviors, as well as the function of neurons and synapses, and expression/activation of synaptic and IL-6 signal transduction proteins in brain regions relevant to the behaviors. The increased IL-6 expression in the CNS of the transgenic mice was accomplished through genetic modification of astrocytes, which are a primary source of IL-6 in the CNS (Campbell et al., 1993). Thus, alterations in the IL-6 transgenic mice are presumably initiated by astrocyte secretion of IL-6 in the CNS, although other cell types could be activated by the actions of astrocyte secreted IL-6 and further contribute to IL-6 levels. Results show a prominent effect of IL-6 on behaviors associated with emotionality, and cellular and synaptic mechanisms that underlie the behaviors.
4.1. Emotionality
Results from our behavioral studies of IL-6 tg mice support a role for IL-6 in exploration in novel environments and depressive-like and/or stress-coping behavior. IL-6 tg +/+ mice, which expressed the highest level of IL-6 in the CNS among the three genotypes, showed decreased activity/exploration in the light/dark transfer, open field, and digging tests compared to IL-6 tg −/− mice. There was no significant difference between IL-6 tg +/− and IL-6 tg −/− mice in these tests, perhaps because the animals were too young to express the full heterozygous phenotype (Gyengesi et al., 2019; Heyser et al., 1997). Previous studies of IL-6 tg +/+ and IL-6 tg +/− mice have shown that some behaviors show age dependency. For example, deficits in avoidance learning were age dependent, with IL-6 tg +/+ mice expressing deficits at an earlier age (3–6 months of age) than IL-6 tg +/− (6–12 months of age) (Heyser et al., 1997).
Total IL-6 KO (Armario et al., 1998; Baier et al., 2009) and Ast IL-6 KO mice, which express reduced astrocyte expression of IL-6 (Erta et al., 2015; Quintana et al., 2013), also exhibit decreased activity levels in novel environments (except see Butterweck et al., 2003). Thus, both increased and decreased IL-6 levels can result in a decreased response to novelty, a situation that suggests that astrocyte produced IL-6 may play a role as a homeostatic regulator of responses to novelty. Interestingly, deficits in cognitive function also occur with both an excess and deficit of IL-6 in the CNS (Heyser et al., 1997; Ma and Zhu, 1997; Baier et al., 2009), as does susceptibility to kainate induced seizures (Penkowa et al., 2001; Samland et al., 2003). As these behaviors involve a variety of brain regions and circuitries (sensory, motor, stress, emotion), the increased and decreased IL-6 may produce these effects through different mechanisms.
IL-6 tg +/+ mice showed greater immobility in the forced swim test, but not in the tail suspension test compared to control. Interestingly, Ast-IL-6 KO male mice and IL-6 KO mice exhibited less immobility in the tail suspension test compared to controls (Chourbaji et al., 2006; Erta et al., 2015). These results support a role for IL-6 in the behavioral response to inescapable stressors, although IL-6 involvement may vary across behavioral test. One reason for the variability across these tests is that they were validated as tests of anti-depressant drug effects and not necessary intended for use to reveal genotypic differences in depressive-like behavior. Indeed, the growing consensus is that these tests represent coping strategy upon exposure to inescapable stressors (Commons et al., 2017; Molendijk and de Kloet, 2019) and not, as has historically been the interpretation, differing depressive-like states. However, it has been argued that analysis of genetically modified mice in these tests represents an important and still growing strategy in elucidating new mechanistic approaches for developing novel treatments for various medical disorders, including depression (Cryan and Mombereau, 2004). Others have shown sex, age, and behavioral test-dependent actions of IL-6 (Blednov et al., 2012; Erta et al., 2015), suggesting a complex interaction between IL-6, CNS cells and CNS circuitry.
4.2. Neuronal and synaptic function in the CeA
Both the amygdala and hippocampus play a central role in emotionality (Adhikari, 2014; Caliskan and Stork, 2018). Thus, IL-6 effects on these brain regions could contribute to the genotypic differences in behavior observed in the current studies. One potential action of IL-6 that could affect the function of these brain regions is an alteration in neuronal or synaptic physiology.
The neurocircuits mediating emotionality are complex, but dysregulation of GABAergic inhibition in the amygdala is known to play a key role (Babaev et al., 2018; Gilpin et al., 2015; Herman et al., 2016). The CeA is primarily comprised of inhibitory GABAergic neurons and is a primary output region of the amygdala complex. Thus, we examined excitability and GABAergic synaptic transmission in CeA neurons from IL-6 +/+, IL-6 +/− and IL-6 tg −/− mice.
Results from these physiological studies showed that CeA neurons from IL-6 tg +/+ mice exhibited enhanced neuronal excitability and GABAergic synaptic transmission compared to CeA neurons from IL-6 tg −/− mice. There was no difference in neuronal excitability and GABAergic synaptic transmission between CeA neurons from IL-6 tg +/− and IL-6 tg −/− mice. Thus, results from behavioral and physiological studies are in accordance, with significant genotypic differences between IL-6 tg +/+ and IL-6 tg −/− mice but not between IL-6 +/− and IL-6 tg −/− mice. These results support a role for IL-6 in the amygdala in emotion-related behaviors. These physiological changes presumably involved STAT3, the signal transduction partner of IL-6. STAT3 activation was not measured in the amygdala in the current studies. However, IL-6 has been shown to alter amygdala-dependent behavior (auditory fear conditioning) via STAT3 activation (Hao et al., 2014).
4.3. Proteins involved in inhibitory synaptic transmission in the hippocampus
In addition to physiological studies, we carried out Western blot studies to identify changes in protein expression that could impact synaptic function in neurocircuits involved in emotionality. These studies were carried out in the hippocampus, a brain region that also plays an important role in emotionality. The hippocampus expresses many of the same synaptic proteins as the amygdala (Fritschy and Mohler, 1995), and thus changes in protein levels in the hippocampus can potentially inform on changes in levels in the amygdala and other brain regions. Because of its size and anatomically well-defined boundaries, the hippocampus was technically advantageous to use for our Western blot studies..
No genotypic difference was observed in the level of the GABAAR alpha 1 subunit. However, hippocampus from IL-6 tg +/+ and IL-6 tg +/− mice showed a significant increase in the level of the alpha-5 subunit, which is expressed in GABAA receptors involved in tonic inhibition, and these receptors are prominently expressed in the hippocampus (Pirker et al., 2000).
Several drugs that act at GABAARs containing the alpha-5 subunit are being examined as potential therapeutic agents for anxiety. L-655,708, a selective partial inverse agonist of alpha-5 subunit containing GABAARs increased anxiety-like behavior in mice, indicating reduced levels of the alpha-5 subunit are associated with increased anxiety (Navarro et al., 2002). In our studies IL-6 tg +/+ mice expressed increased levels of the alpha-5 subunit in the hippocampus but showed reduced responses to novelty, suggesting a lack of involvement of hippocampal GABAARs containing alpha-5 subunit in this behavior in the IL-6 tg +/+ mice.
Drugs that act as negative modulators of GABAARs containing the GABAAR alpha-5 subunit also have antidepressant-like activity (Fischell et al., 2015; Xu et al., 2018; Zanos et al., 2017). RY-080, a negative allosteric modulator of GABAARs containing the GABAAR alpha-5 subunit, produced a prominent dose-dependent reduction in immobility in the forced swim test, indicating the ability to reduce depressive-like behavior (Xu et al., 2018). In our studies, IL-6 tg +/+ mice had a higher level of immobility than IL-6 tg −/− mice in the forced swim test, indicative of a higher level of depressive-like behavior. Thus, increased levels of alpha-5 subunit in the hippocampus of IL-6 tg +/+ mice could contribute to the increased depressive-like behavior observed in the forced swim test.
However, IL-6 tg +/− also showed increased levels of alpha-5 subunit but no change in emotionality. These results argue against the increased levels of alpha-5 subunits in the hippocampus as the only mechanism responsible for these behavioral effects, although it could be a contributing factor by changing the inhibition/excitation balance in neurocircuitry involved in these behaviors.
The increased levels of the alpha-5 subunit in the hippocampus of IL-6 tg +/+ and IL-6 tg +/− mice could also contribute to the reduced performance in memory tasks observed in previous studies of these genotypes (Heyser et al., 1997). Reduced levels of the alpha-5 subunit in the hippocampus are associated with increased performance in memory and learning tasks (Collinson et al., 2002), whereas increases in levels of the alpha-5 subunit in response to drugs (Zurek et al., 2014) or inflammation (Wang et al., 2012) are associated with memory deficits.
Alterations in the levels of GAD 65/67, the synthetic enzymes for GABA, are also thought to be involved in emotionality, with reduced levels of GAD67 associated with increased depression and reduced levels of GAD 65 associated with increased anxiety. Lower levels of GAD 67 were observed in postmortem samples from hippocampus and other brain regions of humans with depressive disorders (Gao et al., 2013; Karolewicz et al., 2010; Thompson Ray et al., 2011), whereas GAD 65 levels were increased in the hippocampus of mice after repeated administration of honokiol, a drug with anxiolytic action (Ku et al., 2011). In our studies, the levels of GAD 65/67 were reduced in hippocampus from IL-6 tg +/+ and IL-6 tg +/− mice, which could result in lower levels of synaptic GABA. The reduction in GAD 67 is consistent with the increased depressive-like behavior in the IL-6 tg +/+ mice, although GAD 67 was also reduced in the IL-6 tg +/− mice, which did not show increased depressive-like behavior. Reduced GABA levels have been demonstrated in the hippocampus of animal models of depression (Gronli et al., 2007) and may be a factor in the increased levels of GABAAR-alpha 5 subunit, which could be a compensatory mechanism. Altered GABAARs expression has been reported for humans who suffered from depression (Choudary et al., 2005).
4.5. Handling-induced convulsions (HIC)
A variety of symptoms are associated with alcohol withdrawal in both humans and animals including hyperexcitability, anxiety-like and depressive-like behaviors, and irritability (Becker, 2000; Hall and Zador, 1997). For example, increased excitability is known to occur in the hippocampus during alcohol withdrawal (Becker, 1994; Becker and Mulholland, 2014; Little, 1999; Ripley et al., 1996; Veatch and Gonzalez, 1996), an effect that could involve decreased inhibitory influences mediated by GABAARs (Kang et al., 1996; Liang et al., 2004; Liang et al., 2006). Our Western blot studies showed that GABAAR alpha-5 subunit levels were decreased in the hippocampus of alcohol withdrawn mice for all genotypes. GABAARs containing the alpha-5 subunit mediate tonic inhibition in the CA1 and CA3 region of the hippocampus (Glykys et al., 2008; Luscher and Keller, 2004). These receptors are an important target of alcohol action and have been shown to play a role in alcohol seeking behaviors (June et al., 2001). Previous studies of hippocampal neurons showed that acute alcohol exposure (60 mM) enhanced tonic GABA currents, whereas tonic GABA currents were decreased during withdrawal (Shen et al., 2011). Thus, the decrease in GABAAR alpha-5 subunit levels in the alcohol exposed/withdrawn transgenic mice could result in decreased tonic inhibition, resulting in increased excitability during alcohol withdrawal. The decrease in GABAAR alpha-5 subunit levels in the alcohol exposed/withdrawn transgenic mice could also play a role in other symptoms associated with alcohol withdrawal such as anxiety-like behavior (Navarro et al., 2002).
Measurement of IL-6 levels in the cerebellum showed a gene-dose relationship under both alcohol naïve and alcohol exposed/withdrawn conditions, with the highest IL-6 in the IL-6 tg +/+ mice. Consistent with these results, measurement of the level of activated STAT3 (pSTAT3), a primary downstream signal transduction partner of the IL6 receptor, in the hippocampus showed a gene-dose related increase from IL-6 tg +/+ and IL-6 tg +/− mice compared to hippocampus from IL-6 tg −/− mice, consistent with CNS production and secretion of IL-6 in the transgenic mice. Therefore, we anticipated that IL-6 tg +/+ mice would show the highest level of CNS excitability during alcohol withdrawal, which did not occur. Effects of alcohol exposure/withdrawal on expression of the GABAAR alpha-1 subunit could be a contributing factor. Hippocampus from the alcohol exposed/withdrawn IL-6 tg +/+ mice showed significantly higher levels of the GABAAR alpha-1 subunit than hippocampus from alcohol exposed/withdrawn IL-6 tg +/− mice. The higher levels could have contributed to greater phasic inhibitory influences in IL-6 tg +/+ mice compared to IL-6 tg +/− and IL-6 tg −/− mice, and consequently lower excitability.
4.6. Summary
Taken together, our studies show an involvement of IL-6 in a several mouse behaviors, including a role in the amygdala as a regulator of responses to novelty and stressors, and in the hippocampus as a regulator of depression. Our studies also show an involvement of IL-6 in hyperexcitability caused by alcohol withdrawal. In contrast, IL-6 was not involved in behaviors assessed by the tail suspension test or in grooming and rearing. Potential mechanisms mediating the altered behavior were identified and included neuroadaptive changes in neuronal excitability, inhibitory synaptic transmission and activation/expression of synaptic and signal transduction proteins. Overall these results are consistent with the idea that astrocyte produced IL-6 plays a significant role in CNS function.
Supplementary Material
Highlights.
Persistent expression of IL-6 by astrocytes alters brain biology and behavior
IL-6 plays a role in responses to novelty and depressive-like behavior
GABAergic signaling and neuronal excitability in the amygdala are altered by IL-6
IL-6 regulates expression of proteins involved in synaptic function
Alcohol alters the function of IL-6 signal transduction pathways
Acknowledgements
This work was supported by NIH/NIAAA: AA024484, the Integrated Neuroscience Initiative on Alcoholism (INIA)-West U01 AA020893 and INIA-neuroimmune U01 AA013498, the Austrian Science Fund (FWF J-3942-B30), and The Scripps Research Institute’s Animal Models Core Facility. We thank Kristine Ly, Jasmin Sisouvanthong, and Claudia Melkonian for performing the biochemical assays, and Dr. A. Szucs (Dept. Physiology and Neurobiology, Eötvös Lóránd University, Hungary; and BioCircuits Institute, University of California San Diego, USA) for the NeuroExpress software.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari A, 2014. Distributed circuits underlying anxiety. Front. Behav. Neurosci 8, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C, 2010. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci 30, 8285–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva E, Cirulli F, Bianchi M, Bondiolotti GP, Chiarotti F, De Acetis L, Panerai AE, 1998. Behavioural characterization of interleukin-6 overexpressing or deficient mice during agonistic encounters. Eur. J. Neurosci 10, 3664–3672. [DOI] [PubMed] [Google Scholar]
- Aniszewska A, Chlodzinska N, Bartkowska K, Winnicka MM, Turlejski K, Djavadian RL, 2015. The expression of interleukin-6 and its receptor in various brain regions and their roles in exploratory behavior and stress responses. J. Neuroimmunol 284, 1–9. [DOI] [PubMed] [Google Scholar]
- Armario A, Hernandez J, Bluethmann H, Hidalgo J, 1998. IL-6 deficiency leads to increased emotionality in mice: evidence in transgenic mice carrying a null mutation for IL-6. J. Neuroimmunol 92, 160–169. [DOI] [PubMed] [Google Scholar]
- Babaev O, Piletti Chatain C, Krueger-Burg D, 2018. Inhibition in the amygdala anxiety circuitry. Exp. Mol. Med 50, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier PC, May U, Scheller J, Rose-John S, Schiffelholz T, 2009. Impaired hippocampus-dependent and -independent learning in IL-6 deficient mice. Behav. Brain Res 200, 192–196. [DOI] [PubMed] [Google Scholar]
- Bajo M, Varodayan FP, Madamba SG, Robert AJ, Casal LM, Oleata CS, Siggins GR, Roberto M, 2015. IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala. Front. Pharmacol 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Potter LN, Henricks AM, Berger AL, Bieniasz KV, Lugo JM, McLaughlin RJ, 2017. Alcohol vapor exposure differentially impacts mesocorticolimbic cytokine expression in a sex-, region-, and duration-specific manner. Neuroscience 346, 238–246. [DOI] [PubMed] [Google Scholar]
- Becker HC, 1994. Positive relationship between the number of prior ethanol withdrawal episodes and the severity of subsequent withdrawal seizures. Psychopharmacology (Berl.) 116, 26–32. [DOI] [PubMed] [Google Scholar]
- Becker HC, 2000. Animal models of alcohol withdrawal. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism 24, 105–113. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Mulholland PJ, 2014. Neurochemical mechanisms of alcohol withdrawal. Handb. Clin. Neurol 125, 133–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Maggi R, Pimpinelli F, Rubino T, Parolaro D, Poli V, Ciliberto G, Panerai AE, Sacerdote P, 1999. Presence of a reduced opioid response in interleukin-6 knock out mice. Eur. J. Neurosci 11, 1501–1507. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA, 2012. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict. Biol 17, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterweck V, Prinz S, Schwaninger M, 2003. The role of interleukin-6 in stress-induced hyperthermia and emotional behaviour in mice. Behav. Brain Res 144, 49–56. [DOI] [PubMed] [Google Scholar]
- Caliskan G, Stork O, 2018. Hippocampal network oscillations at the interplay between innate anxiety and learned fear. Psychopharmacology (Berl.). [DOI] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L, 1993. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl. Acad. Sci. U. S. A 90, 10061–10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IL, Chiang CS, 1995. Cytokine involvement in central nervous system disease. Implications from transgenic mice. Ann. N. Y. Acad. Sci 771, 301–312. [DOI] [PubMed] [Google Scholar]
- Chai Z, Gatti S, Toniatti C, Poli V, Bartfai T, 1996. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1 beta: a study on IL-6-deficient mice. J. Exp. Med 183, 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE Jr., Akil H, Watson SJ, Jones EG, 2005. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc. Natl. Acad. Sci. U. S. A 102, 15653–15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, Schwaninger M, Gass P, 2006. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol. Dis 23, 587–594. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW, 2002. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J. Neurosci 22, 5572–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, Ehlinger DG, 2017. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem. Neurosci 8, 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, 1998. Provisional mapping of quantitative trait loci for chronic ethanol withdrawal severity in BXD recombinant inbred mice. J. Pharmacol. Exp. Ther 286, 263–271. [PubMed] [Google Scholar]
- Crabbe JC, Janowsky JS, Young ER, Rigter H, 1980. Handling induced convulsions in twenty inbred strains of mice. Subst. Alcohol Actions Misuse 1, 159–163. [PubMed] [Google Scholar]
- Crabbe JC Jr., Young ER, Kosobud A, 1983. Genetic correlations with ethanol withdrawal severity. Pharmacol. Biochem. Behav 18 Suppl 1, 541–547. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill C, Belknap JK, 1991. Acute dependence on depressant drugs is determined by common genes in mice. J. Pharmacol. Exp. Ther 257, 663–667. [PubMed] [Google Scholar]
- Crawley JN, 1999. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res 835, 18–26. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I, 2002. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci 23, 238–245. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, 2004. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol. Psychiatry 9, 326–357. [DOI] [PubMed] [Google Scholar]
- Deacon RM, 2006. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat. Protoc 1, 122–124. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN, 2005. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav. Brain Res 156, 241–249. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME, Deak T, 2014. Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol. Clin. Exp. Res 38, 2186–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele N, LaPaglia N, Kovacs EJ, Emanuele MA, 2005. Effects of chronic ethanol (EtOH) administration on pro-inflammatory cytokines of the hypothalamic-pituitary-gonadal (HPG) axis in female rats. Endocr. Res 31, 9–16. [DOI] [PubMed] [Google Scholar]
- Engin E, Benham RS, Rudolph U, 2018. An Emerging Circuit Pharmacology of GABAA Receptors. Trends Pharmacol. Sci 39, 710–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, 2008. The role of GABA(A) receptors in the development of alcoholism. Pharmacol. Biochem. Behav 90, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erta M, Giralt M, Esposito FL, Fernandez-Gayol O, Hidalgo J, 2015. Astrocytic IL-6 mediates locomotor activity, exploration, anxiety, learning and social behavior. Horm. Behav 73, 64–74. [DOI] [PubMed] [Google Scholar]
- Erta M, Quintana A, Hidalgo J, 2012. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci 8, 1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farook JM, Krazem A, Lewis B, Morrell DJ, Littleton JM, Barron S, 2008. Acamprosate attenuates the handling induced convulsions during alcohol withdrawal in Swiss Webster mice. Physiol. Behav 95, 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ, 2007. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-PheCRF(12–41). Alcohol. Clin. Exp. Res 31, 939–949. [DOI] [PubMed] [Google Scholar]
- Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM, 2015. Rapid Antidepressant Action and Restoration of Excitatory Synaptic Strength After Chronic Stress by Negative Modulators of Alpha5-Containing GABAA Receptors. Neuropsychopharmacology 40, 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H, 1995. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol 359, 154–194. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten U, 1994. Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development. Brain Res 637, 10–14. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten UH, 1997. Interleukin-6 (IL-6)--a molecule with both beneficial and destructive potentials. Prog. Neurobiol 52, 379–390. [DOI] [PubMed] [Google Scholar]
- Gano A, Doremus-Fitzwater TL, Deak T, 2016. Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Res 1646, 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SF, Klomp A, Wu JL, Swaab DF, Bao AM, 2013. Reduced GAD(65/67) immunoreactivity in the hypothalamic paraventricular nucleus in depression: a postmortem study. J. Affect. Disord 149, 422–425. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Chu K, Kieffer BL, Roberts AJ, 2005. Lack of stimulant and anxiolytic-like effects of ethanol and accelerated development of ethanol dependence in mu-opioid receptor knockout mice. Neuropharmacology 49, 493–501. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M, 2015. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol. Psychiatry 77, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I, 2008. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci 28, 1421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Pal N, 1971. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science 172, 288–290. [DOI] [PubMed] [Google Scholar]
- Gong MF, Wen RT, Xu Y, Pan JC, Fei N, Zhou YM, Xu JP, Liang JH, Zhang HT, 2017. Attenuation of ethanol abstinence-induced anxiety- and depressive-like behavior by the phosphodiesterase-4 inhibitor rolipram in rodents. Psychopharmacology (Berl.) 234, 3143–3151. [DOI] [PubMed] [Google Scholar]
- Gronli J, Fiske E, Murison R, Bjorvatn B, Sorensen E, Ursin R, Portas CM, 2007. Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behav. Brain Res 181, 42–51. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Huitron-Resendiz S, Roberts AJ, 2018. Altered brain activity during withdrawal from chronic alcohol is associated with changes in IL-6 signal transduction and GABAergic mechanisms in transgenic mice with increased astrocyte expression of IL-6. Neuropharmacology 138, 32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Nelson TE, 1997. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol. Neurobiol 15, 307–339. [DOI] [PubMed] [Google Scholar]
- Gyengesi E, Rangel A, Ullah F, Liang H, Niedermayer G, Asgarov R, Venigalla M, Gunawardena D, Karl T, Munch G, 2019. Chronic Microglial Activation in the GFAP-IL6 Mouse Contributes to Age-Dependent Cerebellar Volume Loss and Impairment in Motor Function. Front. Neurosci 13, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Zador D, 1997. The alcohol withdrawal syndrome. Lancet 349, 1897–1900. [DOI] [PubMed] [Google Scholar]
- Hao Y, Jing H, Bi Q, Zhang J, Qin L, Yang P, 2014. Intra-amygdala microinfusion of IL-6 impairs the auditory fear conditioning of rats via JAK/STAT activation. Behav. Brain Res 275, 88–95. [DOI] [PubMed] [Google Scholar]
- Herman MA, Contet C, Roberto M, 2016. A Functional Switch in Tonic GABA Currents Alters the Output of Central Amygdala Corticotropin Releasing Factor Receptor-1 Neurons Following Chronic Ethanol Exposure. J. Neurosci 36, 10729–10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RV, Puro AC, Manos JC, Huitron-Resendiz S, Reyes KC, Liu K, Vo K, Roberts AJ, Gruol DL, 2016. Transgenic mice with increased astrocyte expression of IL-6 show altered effects of acute ethanol on synaptic function. Neuropharmacology 103, 27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV, 2008. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci 28, 7231–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH, 1997. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc. Natl. Acad. Sci. U. S. A 94, 1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Menard C, Russo SJ, 2016. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiology of stress 4, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN, 2002. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes, brain, and behavior 1, 55–69. [DOI] [PubMed] [Google Scholar]
- Janus C, Flores AY, Xu G, Borchelt DR, 2015. Behavioral abnormalities in APPSwe/PS1dE9 mouse model of AD-like pathology: comparative analysis across multiple behavioral domains. Neurobiol. Aging 36, 2519–2532. [DOI] [PubMed] [Google Scholar]
- June HL, Harvey SC, Foster KL, McKay PF, Cummings R, Garcia M, Mason D, Grey C, McCane S, Williams LS, Johnson TB, He X, Rock S, Cook JM, 2001. GABA(A) receptors containing (alpha)5 subunits in the CA1 and CA3 hippocampal fields regulate ethanol-motivated behaviors: an extended ethanol reward circuitry. J. Neurosci 21, 2166–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ, 2007. Role of GABA in anxiety and depression. Depress. Anxiety 24, 495–517. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Drew PD, 2013. Effects of ethanol on immune response in the brain: region-specific changes in aged mice. J. Neuroinflammation 10, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Spigelman I, Sapp DW, Olsen RW, 1996. Persistent reduction of GABA(A) receptor-mediated inhibition in rat hippocampus after chronic intermittent ethanol treatment. Brain Res 709, 221–228. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G, 2010. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int. J. Neuropsychopharmacol 13, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku TH, Lee YJ, Wang SJ, Fan CH, Tien LT, 2011. Effect of honokiol on activity of GAD(65) and GAD(67) in the cortex and hippocampus of mice. Phytomedicine 18, 1126–1129. [DOI] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL, 2004. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol. Ther 101, 211–226. [DOI] [PubMed] [Google Scholar]
- Li X, Risbrough VB, Cates-Gatto C, Kaczanowska K, Finn MG, Roberts AJ, Markou A, 2013. Comparison of the effects of the GABAB receptor positive modulator BHF177 and the GABAB receptor agonist baclofen on anxiety-like behavior, learning, and memory in mice. Neuropharmacology 70, 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I, 2004. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J. Pharmacol. Exp. Ther 310, 1234–1245. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I, 2006. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J. Neurosci 26, 1749–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ, 1999. The contribution of electrophysiology to knowledge of the acute and chronic effects of ethanol. Pharmacol. Ther 84, 333–353. [DOI] [PubMed] [Google Scholar]
- Lucki I, 1997. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav. Pharmacol 8, 523–532. [DOI] [PubMed] [Google Scholar]
- Luscher B, Keller CA, 2004. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol. Ther 102, 195–221. [DOI] [PubMed] [Google Scholar]
- Martinez P, Lien L, Zemore S, Bramness JG, Neupane SP, 2018. Circulating cytokine levels are associated with symptoms of depression and anxiety among people with alcohol and drug use disorders. J. Neuroimmunol 318, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuarrie D, Fingl E, 1958. Effects of single doses and chronic administration of ethanol on experimental seizures in mice. J. Pharmacol. Exp. Ther 124, 264–271. [PubMed] [Google Scholar]
- Metten P, Sorensen ML, Cameron AJ, Yu CH, Crabbe JC, 2010. Withdrawal severity after chronic intermittent ethanol in inbred mouse strains. Alcohol. Clin. Exp. Res 34, 1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, 2001. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem. Res 26, 907–913. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, de Kloet ER, 2019. Coping with the forced swim stressor: Current state-of-the-art. Behav. Brain Res 364, 1–10. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Buron E, Martin-Lopez M, 2002. Anxiogenic-like activity of L-655,708, a selective ligand for the benzodiazepine site of GABA(A) receptors which contain the alpha-5 subunit, in the elevated plus-maze test. Prog. Neuropsychopharmacol. Biol. Psychiatry 26, 1389–1392. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Olde Engberink A, Hernandez R, Puro A, Huitron-Resendiz S, Hao C, De Graan PN, Gruol DL, 2012. Altered synaptic transmission in the hippocampus of transgenic mice with enhanced central nervous systems expression of interleukin-6. Brain. Behav. Immun 26, 959–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Becker HC, 2008. Effects of the mGluR2/3 agonist LY379268 and the mGluR5 antagonist MPEP on handling-induced convulsions during ethanol withdrawal in mice. Alcohol 42, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Spigelman I, 2012. GABAA Receptor Plasticity in Alcohol Withdrawal In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (Eds.), Jasper’s Basic Mechanisms of the Epilepsies National Center for Biotechnology Information (US) [PubMed] [Google Scholar]
- Rogawski Michael A, Delgado-Escueta Antonio V, Noebels Jeffrey L, Massimo Avoli and Olsen. Richard W, Bethesda (MD). [Google Scholar]
- Patel RR, Khom S, Steinman MQ, Varodayan FP, Kiosses WB, Hedges DM, Vlkolinsky R, Nadav T, Polis I, Bajo M, Roberts AJ, Roberto M, 2019. IL-1beta expression is increased and regulates GABA transmission following chronic ethanol in mouse central amygdala. Brain. Behav. Immun 75, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa M, Molinero A, Carrasco J, Hidalgo J, 2001. Interleukin-6 deficiency reduces the brain inflammatory response and increases oxidative stress and neurodegeneration after kainic acid-induced seizures. Neuroscience 102, 805–818. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G, 2000. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101, 815–850. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, 2000. Animal models of depression: utility for transgenic research. Rev. Neurosci 11, 53–58. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M, 1977. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther 229, 327–336. [PubMed] [Google Scholar]
- Quintana A, Erta M, Ferrer B, Comes G, Giralt M, Hidalgo J, 2013. Astrocyte-specific deficiency of interleukin-6 and its receptor reveal specific roles in survival, body weight and behavior. Brain. Behav. Immun 27, 162–173. [DOI] [PubMed] [Google Scholar]
- Ringheim GE, Burgher KL, Heroux JA, 1995. Interleukin-6 mRNA expression by cortical neurons in culture: evidence for neuronal sources of interleukin-6 production in the brain. J. Neuroimmunol 63, 113–123. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Whittington MA, Butterworth AR, Little HJ, 1996. Ethanol withdrawal hyperexcitability in vivo and in isolated mouse hippocampal slices. Alcohol Alcohol 31, 347–357. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Crabbe JC, Keith LD, 1992. Genetic differences in hypothalamic-pituitary-adrenal axis responsiveness to acute ethanol and acute ethanol withdrawal. Brain Res 579, 296–302. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cao BJ, Dalvi A, Holmes A, 1997. Animal models of anxiety: an ethological perspective. Braz. J. Med. Biol. Res 30, 289–304. [DOI] [PubMed] [Google Scholar]
- Rothaug M, Becker-Pauly C, Rose-John S, 2016. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta 1863, 1218–1227. [DOI] [PubMed] [Google Scholar]
- Sallmann S, Juttler E, Prinz S, Petersen N, Knopf U, Weiser T, Schwaninger M, 2000. Induction of interleukin-6 by depolarization of neurons. J. Neurosci 20, 8637–8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisyan G, Roberts AJ, Hedlund PB, 2010. The 5-HT(7) receptor as a mediator and modulator of antidepressant-like behavior. Behav. Brain Res 209, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobitz B, de Kloet ER, Sutanto W, Holsboer F, 1993. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Eur. J. Neurosci 5, 1426–1435. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lindemeyer AK, Spigelman I, Sieghart W, Olsen RW, Liang J, 2011. Plasticity of GABAA receptors after ethanol pre-exposure in cultured hippocampal neurons. Mol. Pharmacol 79, 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Rudolph U, 2012. Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology 62, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW, 2006. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J. Neurosci 26, 10709–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P, 1985. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl.) 85, 367–370. [DOI] [PubMed] [Google Scholar]
- Su J, Yin J, Qin W, Sha S, Xu J, Jiang C, 2015. Role for pro-inflammatory cytokines in regulating expression of GABA transporter type 1 and 3 in specific brain regions of kainic acid-induced status epilepticus. Neurochem. Res 40, 621–627. [DOI] [PubMed] [Google Scholar]
- Sukoff Rizzo SJ, Neal SJ, Hughes ZA, Beyna M, Rosenzweig-Lipson S, Moss SJ, Brandon NJ, 2012. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Translational psychiatry 2, e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Ray M, Weickert CS, Wyatt E, Webster MJ, 2011. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J. Psychiatry Neurosci 36, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch LM, Gonzalez LP, 1996. Repeated ethanol withdrawal produces sitedependent increases in EEG spiking. Alcohol. Clin. Exp. Res 20, 262–267. [DOI] [PubMed] [Google Scholar]
- Wang DS, Zurek AA, Lecker I, Yu J, Abramian AM, Avramescu S, Davies PA, Moss SJ, Lu WY, Orser BA, 2012. Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABAA receptors. Cell reports 2, 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR, 2009. Sensitization, duration, and pharmacological blockade of anxiety-like behavior following repeated ethanol withdrawal in adolescent and adult rats. Alcohol. Clin. Exp. Res 33, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH, 1992. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci 12, 1040–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TH, Lin CH, 2008. IL-6 mediated alterations on immobile behavior of rats in the forced swim test via ERK1/2 activation in specific brain regions. Behav. Brain Res 193, 183–191. [DOI] [PubMed] [Google Scholar]
- Xu NZ, Ernst M, Treven M, Cerne R, Wakulchik M, Li X, Jones TM, Gleason SD, Morrow D, Schkeryantz JM, Rahman MT, Li G, Poe MM, Cook JM, Witkin JM, 2018. Negative allosteric modulation of alpha 5-containing GABAA receptors engenders antidepressant-like effects and selectively prevents age-associated hyperactivity in tau-depositing mice. Psychopharmacology (Berl.) 235, 1151–1161. [DOI] [PubMed] [Google Scholar]
- Yang L, Xin X, Zhang J, Zhang L, Dong Y, Zhang Y, Mao J, Xie Z, 2014. Inflammatory pain may induce cognitive impairment through an interlukin-6-dependent and postsynaptic density-95-associated mechanism. Anesth. Analg 119, 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Nelson ME, Highland JN, Krimmel SR, Georgiou P, Gould TD, Thompson SM, 2017. A Negative Allosteric Modulator for alpha5 Subunit-Containing GABA Receptors Exerts a Rapid and Persistent Antidepressant-Like Action without the Side Effects of the NMDA Receptor Antagonist Ketamine in Mice. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wei G, Di Z, Zhao Q, 2014. miR-339–5p inhibits alcohol-induced brain inflammation through regulating NF-kappaB pathway. Biochem. Biophys. Res. Commun 452, 450–456. [DOI] [PubMed] [Google Scholar]
- Zurek AA, Yu J, Wang DS, Haffey SC, Bridgwater EM, Penna A, Lecker I, Lei G, Chang T, Salter EW, Orser BA, 2014. Sustained increase in alpha5GABAA receptor function impairs memory after anesthesia. J. Clin. Invest 124, 5437–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.